Abstract
Background
Insulin resistance (IR) is a collective clinical entity that exacerbates metabolic syndrome (MetS). As the gold-standard test to quantify IR involves intravenous insulin loading and repeated blood glucose monitoring, many indices have been developed for IR assessment for convenience. This study tested the ideal cut-off values and clinical utility of IR indices in identifying MetS.
Methods
We recruited 150 subjects, 75 MetS patients and 75 healthy controls, then obtained written informed consent to participate in this study. We collected fasting blood samples for glucose and lipid profiles and calculated nineteen indices of IR and insulin secretion using validated formulae. We determined the precision of these IR indices using the area under the curve (AUC) in a receiver operating characteristic analysis.
Results
Subjects with MetS have significantly higher IR coupled with lower insulin sensitivity and beta-cell function than controls. Among the surrogate markers of IR tested, the homeostatic model assessment of insulin resistance (HOMA-IR), HOMA-adiponectin (HOMA-AD), triglyceride-glucose (TyG) index, HOMA-1%S (insulin sensitivity), quantitative insulin sensitivity check index (QUICKI), McAuley index, single-point insulin sensitivity estimator (SPISE), and HOMA-2%B (beta-cell function) showed the highest AUC values for detecting MetS.
Conclusion
Our study results suggest that the ideal cut-off and AUC values identified for HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, the McAuley index, SPISE, and HOMA-2%B offer a clinical approach to the early detection and risk stratification for MetS among people in southern India.
Keywords: Beta-cell function, Homeostatic model assessment of insulin resistance, Insulin sensitivity, Homeostatic model assessment-adiponectin, McAuley index, Single-point insulin sensitivity estimator
INTRODUCTION
During the past decade, technological breakthroughs in healthcare and medical technologies have substantially improved the diagnostic approaches to and treatment options for cardiovascular disease (CVD). Nonetheless, CVD is the primary cause of morbidity and mortality globally.1 Impaired glucose tolerance, atherogenic dyslipidemia, hypertension (HTN), and central or abdominal obesity are well-known CVD risk factors. Metabolic syndrome (MetS) is the collection of all the CVD risk factors related to vascular and metabolic dysfunctions that precede overt CVD and type 2 diabetes mellitus (T2DM).2 The association of MetS and its components with CVD risk is complicated and multifactorial. It ranges from developing resistance to insulin, aggravated glycemic and lipid profiles, and oxidative stress to low-grade inflammation, and all the factors are interconnected and share underlying mechanisms and pathways.3 In addition to those risk factors, the etiology of MetS can also include smoking and drinking habits, a fatty diet, physical inactivity, and heavy work stress. It is essential to improve our knowledge about the onset and pathogenesis of MetS to facilitate practical therapeutic strategies.4 The prevalence of MetS is rising rapidly, which affects public health. According to the evidence, approximately one-fourth of the world’s population can be classified as having MetS.5 In India, a community-based cross-sectional study in 2017 showed that the prevalence of MetS had increased from 24% to 33%.6 Thus, studies on MetS and ways to reduce CVD risk are intended to build better prevention strategies.
Insulin resistance (IR) is a noteworthy risk factor for CVD and T2DM. It appears to be the most predominant of the five clinical risk factors used to diagnose MetS.7 IR is a state in which the insulin hormone no longer has sufficient capability to bind to its receptors and signal anticipated functions, which leads the liver, skeletal muscle, and adipose tissue to have reduced sensitivity to the metabolic effects of insulin. In other words, a known dosage of insulin does not increase the glucose disposal of a person with IR as much as it does in a healthy person, and that can contribute to beta-cell dyfucntion.8 Genetic abnormalities in proteins involved in the insulin pathway and increased visceral fat have been proposed as significant contributors to IR.9 In this study, we focus on IR to enhance our understanding of MetS and find diagnostic markers to identify MetS. Among the various methods available for the estimation of IR, the gold standard and validated technique is the hyperinsulinemic-euglycemic clamp test, which directly measures the insulin-mediated glucose disposal rate in vivo in a steady-state.10 It involves continuously loading and adjusting insulin and glucose intravenously and testing blood glucose at regular intervals over a 2-hour period.11 Its application to quantify IR in clinical practice is limited by its dosage, invasiveness, complexity, time-consumption, and expense. Obviously, surrogate IR markers are needed. In recent years, many studies have focused on finding simpler, non-invasive surrogate IR indices that use equations and software to enable the early diagnosis and risk stratification of MetS.
Currently, three categories of surrogate IR markers are available. The first category involves glucose loading, insulin loading, combined glucose and insulin loading, and measuring glucose and insulin at hourly intervals. The second category comprises markers that address IR in a steady state without a glucose or insulin intervention.12 The third category contains indirect markers that correlate with IR, such as ferritin, insulin-like growth factor binding protein-1, adiponectin, or resistin.13 To preserve simplicity and enhance clinical utility, we designed this study to investigate the ideal cut-off values for IR markers in the second category and compare their detectable accuracy in identifying MetS among the people in southern India.
METHODS
Study participants
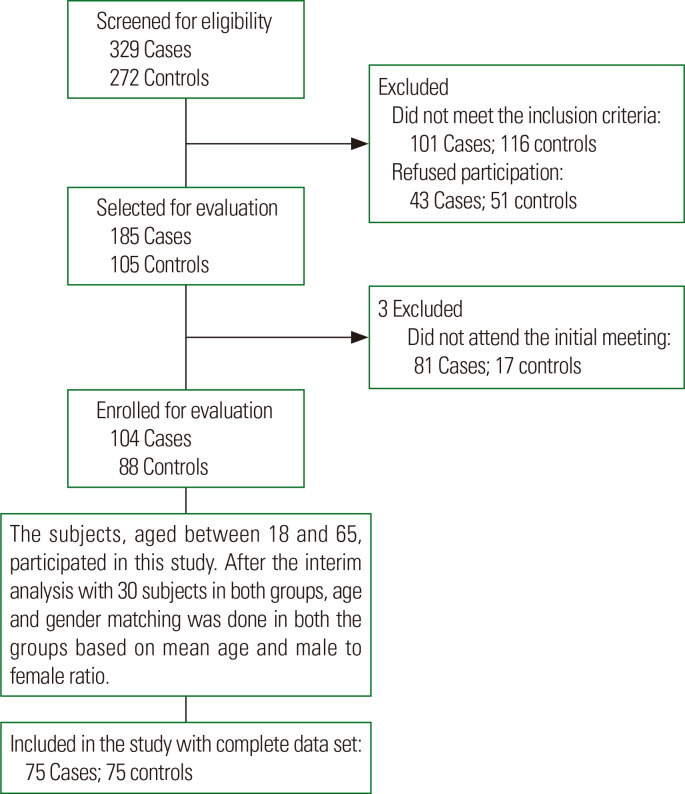
Our subjects, aged 18 to 65 years, participated in this cross-sectional study from November 2018 to February 2020. This study has ethical clearance from the Institutional Ethics Committee (Human research), Jawaharlal Institute of Postgraduate Medical Educa-tion and Research, Puducherry, India (JIP/IEC/2018/0301). All participants provided signed and dated written informed consent before their enrollment. Seventy-five adults diagnosed with MetS were recruited from the Endocrine outpatient department (Fig. 1). Based on the National Cholesterol Education Program Adult Treatment Panel III guidelines, MetS was defined as having any three of the following five conditions3,4: waist circumference (WC) ≥ 90 cm in Asian males, ≥ 80 cm in Asian females; fasting plasma glucose (FPG) ≥ 100 mg/dL (5.6 mmol/L) or receiving medication for T2DM; serum triglycerides (TG) ≥ 150 mg/dL (1.7 mmol/L) or receiving specific treatment for dyslipidemia; high-density-lipoprotein cholesterol (HDL-C) ≤ 40 mg/dL (1.03 mmol/L) in males, ≤ 50 mg/dL (1.29 mmol/L) in females or receiving specific treatment for dyslipidemia; systolic blood pressure (SBP) ≥ 130 mmHg or diastolic blood pressure (DBP) ≥ 85 mmHg or receiving antihypertensive medication. Among the 75 subjects in the MetS group, 34 were receiving oral hypoglycemic agents (metformin, daonil), 20 were receiving lipid-lowering agents (atorvastatin), and 15 were taking antihypertensive agents (envas). Seventy-five healthy volunteers who did not have MetS were included in the study as age and sex-matched controls. Potential subjects with a medical history of ischemic heart disease; cancer; thyroid disorders; neurological, psychological, or other endocrinology disorders; or drug intake for any chronic illness were excluded.
Figure. 1.
Flow diagram of the selection and continuity of study participants.
Sample size calculation
We used the OpenEpi version 3 (Andrew G. Dean and Kevin M. Sullivan, Atlanta, GA, USA), online calculator to estimate the sample size. Based on a previous report,14 the recommended sample size was 108, with 54 subjects in each group, to determine differences of 1.7 between two independent medians with an interquartile range of homeostatic model assessment of insulin resistance (HOMA-IR) values (1.6 [1.1–2.4] vs. 3.3 [2.0–4.8]) with a power of 80% and an alpha error of 0.05. We recruited 150 participants, with 75 in each group, to enable broader and wider coverage of the population, provide more information, and reduce uncertainty.
Brief procedures
Assessment of traditional anthropometric measures
We evaluated subject standing height and weight using a wall-mounted stadiometer and digital weight balance while the subjects were wearing light clothing and barefoot. Body mass index (BMI) was calculated as weight in kg divided by height in m2. WC was measured using a stretchable measuring tape at the narrowest point around the abdomen between the inferior border of the last costal rib and the upper portion of the iliac crest. Hip circumference (HC) was taken around the most comprehensive part of the buttocks. The waist-to-hip ratio (WHR) and waist-to-height ratio (WHtR) of the subjects were calculated. Brachial artery blood pressure (BP) was measured thrice in the subject’s left arm in a sitting position after a rest of five minutes using an Omron (SEM-1; Kyoto, Japan).
Assessment of biochemical profile
We assayed glucose and lipid profiles using an AU680 Analyzer (Beckman Coulter, Atlanta, GA, USA). Serum low-density lipoprotein cholesterol (LDL-C) was calculated based on the Friedewald formula [LDL-C= total cholesterol–(HDL-C+TG/5)]. The Friedewald formula is not valid when the serum TG of an individual is < 400 mg/dL.15 The atherogenic index of plasma (AIP) was calculated using the formula [AIP= log10(TG/ HDL-C)]. Insulin and adiponectin levels were quantified using a commercially available enzyme-linked immunosorbent assay kit (Calbiotech, El Cajon, CA, USA).
Surrogate markers of IR and insulin secretion
IR indices
We used these indices, calculated as follows: HOMA-IR16: fasting plasma insulin (µU/mL) × FPG (mmol/L)/22.5; HOMA2-IR17: the online HOMA Calculator v2.2.2; HOMA-adiponectin (HOMA-AD)18: fasting plasma insulin (µU/mL) × FPG (mmol/L)/adiponectin (µg/mL); HOMA-triglycerides (HOMA-TG)19: fasting plasma insulin (µU/mL) × FPG (mmol/L)/TG (mg/dL); triglyceride-glucose (TyG) index20: FPG (mmol/L)/TG (mmol/L); fasting insulin to glucose ratio (FIGR)21: fasting plasma insulin (µU/mL)/FPG (mg/dL); fasting insulin resistance index (FIRI)10: fasting plasma insulin (µU/mL) × FPG (mmol/L)/25.
Insulin sensitivity indices
We used these indices, calculated as follows: HOMA-1%S (insulin sensitivity)16: 1/HOMA-IR× 100; HOMA-2%S17: the online HOMA calculator v2.2.2; quantitative insulin sensitivity check index (QUICKI)22: 1/[log (fasting insulin in µU/mL)+log (FPG in mg/dL)]; Bennet index23: 1/[log (fasting insulin in µU/mL) ×log (FPG in mmol/L)]; McAuley index24: exp [2.63–0.28 ln (insulin in µU/L)−0.31 ln (TG in mmol/L)]; Raynaud index25: 40/fasting insulin (µU/mL); Reciprocal insulin26: 1/fasting insulin (µU/mL); glucose to insulin (GI) ratio26: FPG (mg/dL)/fasting plasma insulin (µU/mL); fasting insulin sensitivity index (FISI)27: 104/[fasting plasma insulin (µU/mL) ×FPG (mmol/L)]; single-point insulin sensitivity estimator (SPISE)28: 600 ×HDL-C0.185/(TG0.2 × BMI1.338).
Beta cell function indices
HOMA-1%B (beta-cell function)16: [20 × fasting insulin (µU/mL)/[FPG (mmol/L)–3.5]; HOMA-2%B17: the online HOMA calculator v2.2.2.
Statistical analysis
Statistical analyses were carried out using IBM SPSS ver. 20.00 (IBM Corp., Armonk, NY, USA). We use the mean ± standard deviation and median with interquartile range to represent continuous variables, depending on the normality of the data, which was tested by the Kolmogorov-Smirnov test. The Student independent t-test (two-tailed) and Mann-Whitney U-test were used to compare the continuous variables. Categorical findings are presented as percentages, and chi-square test was used to detect differences between the groups. By observing the area under the curve (AUC) in a receiver operating characteristic (ROC) analysis, we calculated the detectable accuracy of the IR indices to identify MetS and its components. MedCalc 11.4.2.0 (Ostend, Belgium) was used for the AUC comparisons. A logistic regression analysis was done using MedCalc software to calculate the odds ratios (ORs) and 95% confidence intervals (CIs) that surrogate IR markers have for MetS and its components. A P-value was considered statistically significant at less than 0.05.
RESULTS
Table 1 depicts the demographic and anthropometric data for the healthy control subjects and MetS subjects. We observed no significant differences in age, sex, smoking, family history of HTN, T2DM, and CVD between the control and MetS group subjects (Table 1). Anthropometric measurements (weight, BMI, WC, WHR, and WHtR) were significantly higher in the MetS group than in the control group (P<0.001).
Table 1.
Comparison of demographic and anthropometric data between healthy control subjects and patients with MetS
| Variable | Control group (n = 75) | MetS group (n = 75) | P |
|---|---|---|---|
| Age (yr) | 44.18 ± 8.32 | 46.13 ± 6.12 | 0.105 |
| Sex (male:female) | 37:38 | 43:32 | 0.326 |
| Smoking | 11 (14.7) | 15 (20) | 0.388 |
| Alcohol intake | 21 (28) | 33 (44) | 0.041 |
| Family H/O HTN | 27 (36) | 36 (48) | 0.317 |
| Family H/O T2DM | 38 (50.7) | 40 (53.3) | 0.744 |
| Family H/O CVD | 8 (10.7) | 12 (16) | 0.337 |
| High WC | 9 (12) | 34 (45.3) | 0.001 |
| Hyperglycemia | 18 (24) | 69 (92) | 0.001 |
| High TG | 14 (18.7) | 40 (53.3) | 0.001 |
| Low HDL-C | 7 (9) | 51 (68) | 0.001 |
| High BP | 15 (20) | 32 (42.7) | 0.003 |
| Height (cm) | 164.04 ± 8.05 | 160.73 ± 9.48 | 0.022 |
| Weight (kg) | 69.17 ± 10.73 | 73.60 ± 7.28 | 0.004 |
| BMI (kg/m2) | 26.25 ± 3.23 | 27.50 ± 3.61 | 0.027 |
| WC (cm) | 90.94 ± 8.9 | 98.63 ± 9.68 | 0.001 |
| HC (cm) | 100.05 ± 9.44 | 102.20 ± 9.36 | 0.164 |
| WHR | 0.90 ± 0.04 | 0.96 ± 0.05 | 0.001 |
| WHtR | 0.55 ± 0.05 | 0.62 ± 0.07 | 0.001 |
Values are presented as mean± standard deviation or number (%). BMI, WHR, and WHtR should be lower to be healthy.
MetS, metabolic syndrome; H/O, history of; HTN, hypertension; T2DM, type 2 diabetes mellitus; CVD, cardiovascular disease; WC, waist circumference; TG, triglycerides; HDLC, high-density lipoprotein cholesterol; BP, blood pressure; BMI, body mass index; HC, hip circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio.
The impaired glycemic state in the MetS group was observed as significantly increased FPG and insulin levels (Table 2). In contrast, adiponectin levels in the MetS group were significantly decreased. Atherogenic dyslipidemia in the MetS group was evident as significantly increased lipid parameters (TC, TG) and lipid ratios (LDL/HDL-C, TG/HDL-C, TC/HDL-C, non-HDL/HDL-C, AIP), except for HDL-C (P<0.001), which was significantly decreased (Table 2). MetS subjects had higher LDL-C levels than controls, but that difference was not statistically significant. Based on the estimated cardiovascular parameters, the MetS group exhibited prehypertension status, with significantly increased systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP) (Table 2).
Table 2.
Comparison of biochemical and cardiometabolic parameters between healthy control subjects and patients with MetS
| Variable | Control group (n = 75) | MetS group (n = 75) | P |
|---|---|---|---|
| FPG (mg/dL) | 93.00 (86–100) | 129 (118–145) | 0.001 |
| Insulin (μU/mL) | 11.7 (8.8–14.9) | 14.9 (12.9–18.6) | 0.001 |
| Adiponectin (µg/mL) | 17.36 (13.26–23.08) | 12.1 (9.13–18.23) | 0.001 |
| BHR (/min) | 72.90 ± 8.73 | 76.37 ± 11.55 | 0.039 |
| SBP (mmHg) | 119.05 ± 12.59 | 127.33 ± 13.79 | 0.001 |
| DBP (mmHg) | 77.29 ± 11.06 | 80.93 ± 7.23 | 0.018 |
| MAP (mmHg) | 91.21 ± 10.81 | 96.39 ± 8.56 | 0.001 |
| TC (mg/dL) | 174.87 ± 22.53 | 187.17 ± 28.89 | 0.004 |
| TG (mg/dL) | 129 (100–147) | 154 (116–180) | 0.001 |
| HDL-C (mg/dL) | 46.05 ± 4.95 | 37.73 ± 6.28 | 0.001 |
| LDL-C (mg/dL) | 105.20 ± 22.78 | 109.81 ± 27.18 | 0.350 |
| VLDL-C (mg/dL) | 24 (16–30) | 27 (19–37) | 0.050 |
| LDL-C/HDL-C | 2.34 ± 0.60 | 2.83 ± 0.91 | 0.001 |
| TG/HDL | 2.78 (2.18–3.17) | 4.17 (3.14–5.08) | 0.001 |
| TC/HDL | 3.82 ± 0.54 | 4.99 ± 1.15 | 0.001 |
| Non-HDL-C/HDL-C | 2.82 ± 0.54 | 3.99 ± 1.15 | 0.001 |
| AIP | 0.431 ± 0.12 | 0.602 ± 0.16 | 0.001 |
Values are presented as median (interquartile range) or mean ± standard deviation. FPG, insulin, BHR, SBP, DBP, MAP, and all the lipid parameters except HDL-C should be lower to be healthy; HDL-C and adiponectin should be higher.
MetS, metabolic syndrome; FPG, fasting plasma glucose; BHR, basal heart rate; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; VLDL-C, very-low-density lipoprotein cholesterol; AIP, atherogenic index of plasma.
Among the IR indices, the HOMA-IR, HOMA2-IR, HOMA-AD, HOMA-TG, TyG index, and FIRI values were significantly higher (P<0.001) in the MetS subjects than in the controls (Table 3). In contrast, the values for the insulin-sensitivity indices and beta-cell function indices (HOMA-1%S, HOMA-2%S, QUICKI, Bennet index, McAuley index, Raynaud index, reciprocal insulin, FISI, SPISE, HOMA-1%B, and HOMA-2%B) were significantly lower in the MetS group than the control group.
Table 3.
Comparison of surrogate markers of IR and insulin secretion between healthy control subjects and patients with MetS
| Variable | Control group (n = 75) | MetS group (n = 75) | P |
|---|---|---|---|
| HOMA-IR | 2.67 (1.98–3.53) | 4.63 (3.69–6.82) | 0.001 |
| HOMA2-IR | 1.51 (1.13–1.96) | 2.06 (1.77–2.72) | 0.001 |
| HOMA-AD | 3.85 (1.82–5.24) | 8.11 (5.24–9.86) | 0.001 |
| HOMA-TG | 0.47 (0.33–0.64) | 0.81 (0.53–1.01) | 0.001 |
| TyG index | 7.55 (5.54–8.92) | 12.26 (9.95–14.44) | 0.001 |
| FIGR | 2.05 (1.89–2.30) | 2.31 (1.73–2.64) | 0.307 |
| FIRI | 2.41 (1.78–3.18) | 4.17 (3.33–6.14) | 0.001 |
| HOMA-1%S | 37.40 (28.30–50.56) | 21.58 (14.65–27.06) | 0.001 |
| HOMA-2%S | 66.10 (51–88.7) | 49 (37.4–56.7) | 0.001 |
| QUICKI | 0.33 (0.31–0.34) | 0.30 (0.29–0.31) | 0.001 |
| Bennet index | 1.32 (1.19–1.52) | 1.01 (0.86–1.11) | 0.001 |
| McAuley index | 7.43 ± 2.70 | 6.01 ± 1.90 | 0.001 |
| Raynaud index | 3.41 (2.68–4.54) | 2.68 (2.15–3.10) | 0.001 |
| 1/Insulin | 0.085 (0.067–0.114) | 0.066 (0.054–0.078) | 0.001 |
| GI ratio | 8.76 (7.81–9.53) | 7.77 (6.81–10.43) | 0.316 |
| FISI | 9.23 (6.98–12.48) | 5.32 (3.61–6.68) | 0.001 |
| SPISE | 6.01 (4.65–7.20) | 3.53 (2.80–5.11) | 0.001 |
| HOMA-1%B | 130.82 (108–164.25) | 80.57 (65.90–96.01) | 0.001 |
| HOMA-2%B | 110.20 (97.30–129.10) | 73.80 (61.30–87.03) | 0.001 |
Values are presented as median (interquartile range) or mean± standard deviation. All the IR indices should be lower to be healthy, and the insulin sensitivity and beta-cell function indices should be higher.
IR, insulin resistance; MetS, metabolic syndrome; HOMA, homeostatic model assessment; AD, adiponectin; TG, triglycerides; TyG, triglyceride-glucose; FIGR, fasting insulin to glucose ratio; FIRI, fasting insulin resistance; %S, insulin sensitivity; QUICKI, quantitative insulin sensitivity check index; 1/Insulin, reciprocal insulin; GI, glucose to insulin; FISI, fasting insulin sensitivity index; SPISE, single-point insulin sensitivity estimator; %B, beta-cell function.
Table 4 explains the detectable accuracy of all the surrogate markers of IR and insulin secretion in identifying MetS among the southern-Indian subjects in our study population. Among the IR and insulin secretion markers, HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, the McAuley index, SPISE, and HOMA-2%B yielded an AUC above 0.8, showing the best detectable accuracy for the identification of MetS. Most of the other indices tested (HOMA2-IR, HOMA-TG, FIRI, HOMA-2%S, the Bennet index, Raynaud index, reciprocal insulin, FISI, and HOMA-1%B) reported an AUC between 0.6 and 0.8. In our results, FIGR and the GI ratio were the least effective, with the lowest AUC in the ROC analysis.
Table 4.
AUCs, ideal cut-offs, sensitivity, and specificity of surrogate markers for IR and insulin secretion for identifying MetS in the ROC analysis
| Variable | AUC (95% CI) | P | Cut-off value | Sensitivity | Specificity | Youden index |
|---|---|---|---|---|---|---|
| HOMA-IR | 0.851 (0.784–0.904) | 0.001 | ≥ 2.86 | 86.67 | 70.67 | 0.5733 |
| HOMA2-IR | 0.787 (0.712–0.849) | 0.001 | ≥ 1.69 | 80.01 | 65.33 | 0.4533 |
| HOMA-AD | 0.846 (0.778–0.899) | 0.001 | ≥ 6.26 | 68.14 | 85.33 | 0.5333 |
| HOMA-TG | 0.755 (0.678–0.822) | 0.001 | ≥ 0.72 | 53.33 | 81.35 | 0.3467 |
| TyG index | 0.836 (0.767–0.891) | 0.001 | ≥ 9.88 | 76.00 | 88.00 | 0.6400 |
| FIGR | 0.548 (0.465–0.630) | 0.335 | ≥ 2.41 | 85.33 | 46.67 | 0.3200 |
| FIRI | 0.768 (0.669–0.850) | 0.001 | ≥ 2.96 | 83.67 | 70.47 | 0.4414 |
| HOMA-1%S | 0.850 (0.783–0.903) | 0.001 | ≤ 28.2 | 82.74 | 77.33 | 0.5731 |
| HOMA-2%S | 0.779 (0.704–0.843) | 0.001 | ≤ 53.9 | 69.33 | 74.67 | 0.4400 |
| QUICKI | 0.844 (0.776–0.898) | 0.001 | ≤ 0.32 | 93.33 | 61.33 | 0.5467 |
| Bennet index | 0.795 (0.721–0.856) | 0.001 | ≤ 1.2 | 89.30 | 74.62 | 0.4534 |
| McAuley index | 0.815 (0.743–0.874) | 0.001 | ≤ 6.05 | 78.76 | 92.12 | 0.4937 |
| Raynaud index | 0.742 (0.664–0.810) | 0.001 | ≤ 3.10 | 76.01 | 62.67 | 0.3867 |
| 1/Insulin | 0.735 (0.657–0.804) | 0.001 | ≤ 0.08 | 86.67 | 50.15 | 0.3733 |
| GI ratio | 0.547 (0.464–0.629) | 0.343 | ≤ 7.45 | 85.33 | 55.81 | 0.2928 |
| FISI | 0.797 (0.724–0.858) | 0.001 | ≤ 6.96 | 83.67 | 70.37 | 0.4414 |
| SPISE | 0.822 (0.751–0.880) | 0.001 | ≤ 3.79 | 73.47 | 90.70 | 0.5067 |
| HOMA-1%B | 0.784 (0.680–0.875) | 0.001 | ≤ 94.74 | 71.43 | 74.42 | 0.4585 |
| HOMA-2%B | 0.842 (0.773–0.896) | 0.001 | ≤ 87.12 | 93.62 | 76.74 | 0.6800 |
Cut-off values correspond to the highest Youden index.
AUC, area under the curve; IR, insulin resistance; MetS, metabolic syndrome; ROC, receiver operating characteristic; HOMA, homeostatic model assessment; AD, adiponectin; TG, triglycerides; TyG, triglyceride-glucose; FIGR, fasting insulin to glucose ratio; FIRI, fasting insulin resistance; %S, insulin sensitivity; QUICKI, quantitative insulin sensitivity check index; 1/Insulin, reciprocal insulin; GI, glucose to insulin; FISI, fasting insulin sensitivity index; SPISE, single-point insulin sensitivity estimator; %B, beta-cell function.
Table 5 provides information about the proportion of subjects with values higher than the cut-off for each index. Compared with the control group, the MetS group had a higher proportion of subjects with higher cut-off values for HOMA-IR and QUICKI (93.3%), indicating increased IR and decreased insulin sensitivity, respectively.
Table 5.
Proportion of subjects with values higher than the cut-off for the IR, insulin sensitivity, and beta-cell function indices
| Variable | Cut-off value | Control group (n = 75) | MetS group (n = 75) | P |
|---|---|---|---|---|
| HOMA-IR | ≥ 2.86 | 34 (45.3) | 70 (93.3) | 0.001 |
| HOMA2-IR | ≥ 1.69 | 27 (36) | 60 (80) | 0.001 |
| HOMA-AD | ≥ 6.26 | 12 (16) | 52 (69.3) | 0.001 |
| HOMA-TG | ≥ 0.72 | 15 (20) | 43 (57.3) | 0.001 |
| TyG index | ≥ 9.88 | 9 (12) | 57 (76) | 0.001 |
| FIGR | ≥ 2.41 | 12 (16) | 35 (46.7) | 0.001 |
| FIRI | ≥ 2.96 | 22 (29.3) | 65 (86.7) | 0.001 |
| HOMA-1%S | ≤ 28.2 | 18 (24) | 59 (78.7) | 0.001 |
| HOMA-2%S | ≤ 53.9 | 19 (25.3) | 52 (69.3) | 0.001 |
| QUICKI | ≤ 0.32 | 29 (38.7) | 70 (93.3) | 0.001 |
| Bennet index | ≤ 1.2 | 19 (25.3) | 67 (89.3) | 0.001 |
| McAuley index | ≤ 6.05 | 11 (14.7) | 46 (61.3) | 0.001 |
| Raynaud index | ≤ 3.10 | 28 (37.3) | 57 (76) | 0.001 |
| 1/Insulin | ≤ 0.08 | 37 (49.3) | 65 (86.7) | 0.001 |
| GI ratio | ≤ 7.45 | 11 (14.7) | 36 (48) | 0.001 |
| FISI | ≤ 6.96 | 17 (22.7) | 60 (80) | 0.001 |
| SPISE | ≤ 3.79 | 5 (6.7) | 43 (57.3) | 0.001 |
| HOMA-1%B | ≤ 94.74 | 7 (9.3) | 56 (74.7) | 0.001 |
| HOMA-2%B | ≤ 87.12 | 6 (8) | 57 (76) | 0.001 |
Values are presented as number (%).
IR, insulin resistance; MetS, metabolic syndrome; HOMA, homeostatic model assessment; AD, adiponectin; TG, triglycerides; TyG, triglyceride-glucose; FIGR, fasting insulin to glucose ratio; FIRI, fasting insulin resistance; %S, insulin sensitivity; QUICKI, quantitative insulin sensitivity check index; 1/Insulin, reciprocal insulin; GI, glucose to insulin; FISI, fasting insulin sensitivity index; SPISE, single-point insulin sensitivity estimator; %B, beta-cell function.
The AUC values for HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, the McAuley index, SPISE, and HOMA-2%B in identifying MetS and its components are shown in Supplementary Table 1. Among those markers of IR and insulin secretion, HOMA-IR has the largest AUC for identifying MetS (AUC, 0.851; 95% CI, 0.784–0.904). SPISE has the highest AUC for predicting central obesity, and HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, and the McAuley index showed similar AUCs for predicting central obesity. An elevated FPG was best predicted by HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, and HOMA-2%B. The TyG index, McAuley index, and SPISE showed the largest AUCs for predicting elevated TG and reduced HDL-C. None of the indices tested showed good predictive ability in identifying high BP.
Among the markers of IR, insulin sensitivity and beta-cell function tested, HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, the McAuley index, SPISE, and HOMA-2%B were associated with increased odds of having MetS and its components after adjustment for age, sex, BMI, smoking, and alcohol status (Supplementary Table 2). The binomial logistic regression analysis showed that subjects with higher HOMA-IR levels were more likely to have MetS and hyperglycemia than those with lower HOMA-IR levels (OR, 2.24; 95% CI, 1.60–3.13 and OR, 16.47; 95% CI, 6.12– 28.44, respectively). Subjects with higher TyG index and lower McAuley index values were more likely to have dyslipidemia (elevated TG) and hyperglycemia (OR, 3.46; 95% CI, 2.32–4.83 and OR, 3.34; 95% CI, 2.84–4.16, respectively).
DISCUSSION
The quantification of IR is of utmost importance, because it is the root factor associated with the clinical and metabolic abnormalities in MetS. It predicts the future risk of developing T2DM and CVD, can remain undiagnosed for a long time, and is established before any signs of disease appear. It is crucial to address IR as soon as possible to reduce the risk of developing life-threatening illnesses. Therefore, in this pioneering regional study, we have focused on understanding the ideal cut-off values and clinical utility of surrogate IR and insulin secretion markers for detecting MetS and its components among subjects near a tertiary care teaching hospital in southern India.
Matthews et al.16 developed the homeostatic model assessment concept in 1985 to quantify IR, insulin sensitivity, and beta-cell functions. In this study, we found that HOMA-IR was significantly higher in the MetS group than in the controls. The World Health Organization29 has defined IR as a HOMA-IR of ≥ 1.8. In the current study, a HOMA-IR cut-off of ≥ 2.86 provided adequate predictive power, sensitivity and specificity in detecting MetS and elevated FPG among the southern Indians (AUC, 0.851; 95% CI, 0.784–0.904 and AUC, 0.963; 95% CI, 0.919–0.987, respectively). Using different criteria to define IR and different approaches to determine cut-off values are the reasons for a notably higher HOMA-IR. Moreover, a high HOMA-IR value correlated strongly with the odds of having MetS and elevated FPG. The HOMA2-IR is a simplified version of HOMA-IR that is calculated using an online calculator.17 HOMA2-IR displays metabolic processes more precisely than the original HOMA-IR because it highlights the feedback interactions between glucose and insulin in various parts of the body. However, it did not detect MetS more efficiently than HOMA-IR. The differences in the predictive ability of HOMA-IR and HOMA2-IR could reflect a drawback of HOMA2-IR: it accepts only a specific range of values for computation.30 Keskin et al.31 observed similar results in the Brazilian Metabolic Syndrome Study; HOMA-IR had a slightly higher AUC than HOMA2-IR in the ROC analysis. IR is a causative agent of MetS, which could indicate that MetS-related metabolic abnormalities are the end result of long-term IR. Excess weight and obesity in the MetS group could be an essential link between IR and MetS.
Recently, HOMA-AD was introduced as a novel, simple, and adipokine-based IR index that is calculated using serum adiponectin levels as a denominator in the HOMA-IR formula.18 To date, few studies have addressed the role of the HOMA-AD in IR assessment. Matsuhisa et al.18 surveyed Japanese respondents and observed that the IR measured by the euglycemic-hyperinsulinemic clamp technique was more significantly associated with HOMA-AD (r = –0.64) than with HOMA-IR (r = –0.59). In the present study, HOMA-AD has shown higher predictive power in discriminating subjects with MetS and hyperglycemia (AUC, 0.846; 95% CI, 0.778–0.899 and AUC, 0.893; 95% CI, 0.832–0.937, respectively). The ideal cut-off value for identifying MetS with the HOMA-AD index was ≥ 6.26. Contrary to our findings, Da Silva et al.32 found that the HOMA-AD (AUC, 0.712) had less detectable accuracy than HOMA-IR (AUC, 0.859). However, they also found that HOMA-AD could discriminate patients with and without IR as well as HOMA-IR. The exact mechanisms connecting adiponectin and IR have not been studied extensively. Recent findings show that insulin could specifically affect adiponectin gene expression and in vitro adiponectin levels.33 Thus, it is reasonable to think that the higher insulin levels found in IR subjects could decrease their adiponectin levels. The primary reason for low adiponectin levels in MetS could be central or abdominal obesity because adiponectin levels are determined mainly by the degree of visceral fat.
Another new surrogate marker of IR, the TyG index, was developed by Simental-Mendía et al.34 in 2008 as a product of FPG and TG. Recent studies have found that the TyG index can predict the future risk of diabetes in men and women and is associated with CVD, obesity, fatty liver, and MetS.35 We found the TyG index to be highly predictive of MetS (AUC, 0.836; 95% CI, 0.779–0.928), elevated FPG and TG (AUC, 0.865; 95% CI, 0.799–0.915; and AUC, 0.906; 95% CI, 0.848–0.948, respectively). In other words, the TyG index could precisely identify around 83.6% of individuals with MetS, 86.5% of individuals with elevated FPG, and 90.6% of individuals with high TG. The TyG index is also associated with increased odds of having MetS, elevated FPG and TG. The ideal cut-off value for the TyG index to detect MetS in our study was ≥ 9.88. However, certain pathological conditions, such as dyslipidemia and abdominal obesity, might influence the TyG index in ways that affect its ability to identify MetS. These results suggest that both glucose and lipid abnormalities could play a crucial role in the pathogenesis of MetS. Similar results have been reported by Zheng and Mao36 They showed that the TyG index independently predicted incident HTN and suggested that including the TyG index in routine check-ups could help to prevent HTN.
In addition to the IR indices, we examined insulin sensitivity and beta-cell function indices derived from equations that use glucose and insulin levels. HOMA-1%S and QUICKI indicate insulin sensitivity, and HOMA-2%B is a method for evaluating beta-cell function. In Mexican subjects, Baez-Duarte et al.37 demonstrated a gradual deterioration of beta-cell function and insulin sensitivity in MetS patients as their number of components of MetS increased. We here found that HOMA-1%S, QUICKI, and HOMA-2%B were significantly lower in MetS patients than in controls, confirming the findings of Baez-Duarte et al.37 and indicating that the function or mass of beta cells was significantly reduced in subjects with MetS, along with decreased insulin sensitivity. This can be explained by the fact that a disproportionate accumulation of fat in the abdominal area is related to decreased insulin-mediated glucose disposal in southern-Indian adults. Increased pancreatic beta-cell apoptosis, necrosis, or autophagy could be involved in beta cell dysfunction in MetS. In this study, HOMA-1%S, QUICKI, and HOMA-2%B showed the best predictive power (AUC, 0.850; 95% CI, 0.783– 0.903; AUC, 0.844; 95% CI, 0.776–0.898 and AUC, 0.842; 95% CI, 0.773–0.896, respectively), with cut-offs of ≤ 28.2, ≤ 0.32, and ≤ 87.12, respectively, to identify MetS patients from southern India.
In 2001, McAuley et al.24 proposed the McAuley index based on serum insulin and TG, to provide information on the pathophysiology of IR and MetS. Patients with MetS have a significantly lower McAuley index than controls. Thus, the dysregulation of serum lipid metabolism, mainly an increase in TG levels, as the result of hyperinsulinemia can directly cause reduced insulin sensitivity and beta-cell dysfunction through the deposition of TG within cells. We also found that the McAuley index showed good predictive power to identify MetS, elevated FPG and TG, and reduced HDL-C (AUC, 0.815; 95% CI, 0.743–0.874; AUC, 0.796; 95% CI, 0.723– 0.857; AUC, 0.895; 95% CI, 0.834–0.939; and AUC, 0.762; 95% CI, 0.680– 0.827, respectively). Furthermore, the McAuley index was strongly associated with the odds of having central obesity and elevated FPG. The mean McAuley index in the MetS group was 6.01± 1.90, with a cut-off of ≤ 6.05. A similar pattern for the McAuley index was reported in a study conducted among Korean adults in 2016 by Kim et al.,38 who concluded that the McAuley index showed the best accuracy in detecting MetS. Contrary to our findings, a 2007 study by Sarafidis et al.39 on a Greek population found that McAuley’s index was the worst predictor of insulin sensitivity among the measures examined. This discrepancy in the results could be due to the normal mean TG levels in the Greek study population.
Paulmichl et al.28 introduced SPISE as a reliable tool for estimating IR based on fasting TG, HDL-C, and BMI measurements. They developed the model using computer-assisted mathematical modeling of their study population data. They also compared the reliability of SPISE to that of the gold standard euglycemic-hyperinsulinemic clamp test, HOMA-IR, and other indices for IR. Compared with control subjects, the SPISE value was significantly lower in the MetS group. SPISE showed a high ability to discriminate MetS cases from controls (AUC, 0.822; 95% CI, 0.751–0.880). SPISE can also predict central obesity, elevated FPG and TG, and reduced HDL-C with excellent discriminating power. We observed a cut-off value of ≤ 3.79 for identifying MetS patients in southern India. Paulmichl et al.28 found a SPISE cut-off value of ≤ 6.61 among a European population. The lower cut-off value of SPISE found in this study indicates higher IR and decreased insulin sensitivity among southern Indians. Dudi et al.40 demonstrated similar results and found SPISE to be a valuable, low-cost measure with high sensitivity and specificity for IR and insulin sensitivity in MetS among a northern Indian population in 2019.
In conclusion, we found increased IR coupled with decreased insulin sensitivity and beta-cell function in the MetS group compared with the control group. Our study results suggest that HOMA-IR, HOMA-AD, the TyG index, HOMA-1%S, QUICKI, the McAuley index, SPISE, and HOMA-2%B show better detectable accuracy in identifying MetS subjects than the other surrogate markers of IR and insulin secretion we tested. We also obtained ideal cut-off points for the surrogate markers of IR and insulin secretion for the detection of MetS, and those numbers are not consistent with those in previous studies. The differences in ideal cut-off points could be due to the study design, ethnic differences, sample size, or criteria for MetS diagnosis. Therefore, using these surrogate measures of IR and insulin secretion, in addition to FPG and lipid profiles, could help with the identification and risk stratification of MetS among southern Indians. Our findings suggest that early treatment options to address IR and intervention strategies to improve insulin sensitivity are the best ways to slow disease development and progression.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1 and 2 can be found via https://doi.org/10.7570/jomes20071.
ACKNOWLEDGMENTS
We sincerely acknowledge the Jawaharlal Institute of Postgraduate Medical Education and Research for providing financial help in the form of an intramural PhD research grant (JIP/Res/Intramural/Phs-1/2018-19/98). We are thankful to all the subjects who took part in this study.
Footnotes
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Study concept and design: CKE, GSG, DY, JS, and BV; acquisition of data: CKE; analysis and interpretation of data: GSG, CKE, and DY; drafting of the manuscript: GSG, CKE, and DY; critical revision of the manuscript: CKE, GSG, DY, JS, and BV; statistical analysis: CKE and DY; obtained funding: CKE; administrative, technical, or material support: GSG; and study supervision: GSG.
REFERENCES
- 1.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–646. doi: 10.1161/CIR.0000000000000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ford ES. Risks for all-cause mortality, cardiovascular disease, and diabetes associated with the metabolic syndrome: a summary of the evidence. Diabetes Care. 2005;28:1769–78. doi: 10.2337/diacare.28.7.1769. [DOI] [PubMed] [Google Scholar]
- 3.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 4.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 5.Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harikrishnan S, Sarma S, Sanjay G, Jeemon P, Krishnan MN, Venugopal K, et al. Prevalence of metabolic syndrome and its risk factors in Kerala, South India: analysis of a community based cross-sectional study. PLoS One. 2018;13:e0192372. doi: 10.1371/journal.pone.0192372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shin JA, Lee JH, Lim SY, Ha HS, Kwon HS, Park YM, et al. Metabolic syndrome as a predictor of type 2 diabetes, and its clinical interpretations and usefulness. J Diabetes Investig. 2013;4:334–43. doi: 10.1111/jdi.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu R, Christoffel KK, Brickman WJ, Liu X, Gadgil M, Wang G, et al. Do static and dynamic insulin resistance indices perform similarly in predicting pre-diabetes and type 2 diabetes? Diabetes Res Clin Pract. 2014;105:245–50. doi: 10.1016/j.diabres.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–48. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 10.Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 12.Park SE, Park CY, Sweeney G. Biomarkers of insulin sensitivity and insulin resistance: past, present and future. Crit Rev Clin Lab Sci. 2015;52:180–90. doi: 10.3109/10408363.2015.1023429. [DOI] [PubMed] [Google Scholar]
- 13.Engin A. Adiponectin-resistance in obesity. Adv Exp Med Biol. 2017;960:415–41. doi: 10.1007/978-3-319-48382-5_18. [DOI] [PubMed] [Google Scholar]
- 14.Antoniolli LP, Nedel BL, Pazinato TC, Gerchman F , de Andrade Mesquita L. Accuracy of insulin resistance indices for metabolic syndrome: a cross-sectional study in adults. Diabetol Metab Syndr. 2018;10:65. doi: 10.1186/s13098-018-0365-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. doi: 10.1093/clinchem/18.6.499. [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 17.Oxford Centre for Diabetes, Endocrinology & Metabolism. HOMA calculator [Internet] Diabetes Trial Unit; Oxford: 2009. [cited 2020 Oct 30]. Available from: http://www.dtu.ox.ac.uk. [Google Scholar]
- 18.Matsuhisa M, Yamasaki Y, Emoto M, Shimabukuro M, Ueda S, Funahashi T, et al. A novel index of insulin resistance determined from the homeostasis model assessment index and adiponectin levels in Japanese subjects. Diabetes Res Clin Pract. 2007;77:151–4. doi: 10.1016/j.diabres.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 19.Khan SH, Khan AN, Chaudhry N, Anwar R, Fazal N, Tariq M. Comparison of various steady state surrogate insulin resistance indices in diagnosing metabolic syndrome. Diabetol Metab Syndr. 2019;11:44. doi: 10.1186/s13098-019-0439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang B, Yang Y, Lee EY, Yang HK, Kim HS, Lim SY, et al. Triglycerides/glucose index is a useful surrogate marker of insulin resistance among adolescents. Int J Obes (Lond) 2017;41:789–92. doi: 10.1038/ijo.2017.14. [DOI] [PubMed] [Google Scholar]
- 21.Nauck MA, Meier JJ. Diagnostic accuracy of an "amended" insulin-glucose ratio for the biochemical diagnosis of insulinomas. Ann Intern Med. 2012;157:767–75. doi: 10.7326/0003-4819-157-11-201212040-00004. [DOI] [PubMed] [Google Scholar]
- 22.Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G, et al. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab. 2000;85:2402–10. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- 23.Anderson RL, Hamman RF, Savage PJ, Saad MF, Laws A, Kades WW, et al. Exploration of simple insulin sensitivity measures derived from frequently sampled intravenous glucose tolerance (FSIGT) tests. The Insulin Resistance Atherosclerosis Study. Am J Epidemiol. 1995;142:724–32. doi: 10.1093/aje/142.7.724. [DOI] [PubMed] [Google Scholar]
- 24.McAuley KA, Williams SM, Mann JI, Walker RJ, Lewis-Barned NJ, Temple LA, et al. Diagnosing insulin resistance in the general population. Diabetes Care. 2001;24:460–4. doi: 10.2337/diacare.24.3.460. [DOI] [PubMed] [Google Scholar]
- 25.Raynaud E, Perez-Martin A, Brun JF, Benhaddad AA, Mercier J. Revised concept for the estimation of insulin sensitivity from a single sample. Diabetes Care. 1999;22:1003–4. doi: 10.2337/diacare.22.6.1003. [DOI] [PubMed] [Google Scholar]
- 26.Hermans MP, Levy JC, Morris RJ, Turner RC. Comparison of insulin sensitivity tests across a range of glucose tolerance from normal to diabetes. Diabetologia. 1999;42:678–87. doi: 10.1007/s001250051215. [DOI] [PubMed] [Google Scholar]
- 27.Sluiter WJ, Erkelens DW, Terpstra P, Reitsma WD, Doorenbos H. Glucose tolerance and insulin release, a mathematical approach. II. Approximation of the peripheral insulin resistance after oral glucose loading. Diabetes. 1976;25:245–9. doi: 10.2337/diab.25.4.245. [DOI] [PubMed] [Google Scholar]
- 28.Paulmichl K, Hatunic M, Højlund K, Jotic A, Krebs M, Mitrakou A, et al. Modification and validation of the triglyceride-to-HDL Cholesterol ratio as a surrogate of insulin sensitivity in white juveniles and adults without diabetes mellitus: the Single Point Insulin Sensitivity Estimator (SPISE) Clin Chem. 2016;62:1211–9. doi: 10.1373/clinchem.2016.257436. [DOI] [PubMed] [Google Scholar]
- 29.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 30.Geloneze B, Vasques AC, Stabe CF, Pareja JC, Rosado LE, Queiroz EC, et al. HOMA1-IR and HOMA2-IR indexes in identifying insulin resistance and metabolic syndrome: Brazilian Metabolic Syndrome Study (BRAMS) Arq Bras Endocrinol Metabol. 2009;53:281–7. doi: 10.1590/S0004-27302009000200020. [DOI] [PubMed] [Google Scholar]
- 31.Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115:e500–3. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 32.Da Silva CC, Zambon MP, Vasques AC, Camilo DF, De Bernardi Rodrigues AM, Antonio MÂ, et al. Homeostatic model assessment of adiponectin (HOMA-Adiponectin) as a surrogate measure of insulin resistance in adolescents: comparison with the hyperglycaemic clamp and homeostatic model assessment of insulin resistance. PLoS One. 2019;14:e0214081. doi: 10.1371/journal.pone.0214081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blaslov K, Bulum T, Zibar K, Duvnjak L. Relationship between adiponectin level, insulin sensitivity, and metabolic syndrome in type 1 diabetic patients. Int J Endocrinol. 2013;2013:535906. doi: 10.1155/2013/535906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034. [DOI] [PubMed] [Google Scholar]
- 35.Sánchez-Íñigo L, Navarro-González D, Fernández-Montero A, Pastrana-Delgado J, Martínez JA. The TyG index may predict the development of cardiovascular events. Eur J Clin Invest. 2016;46:189–97. doi: 10.1111/eci.12583. [DOI] [PubMed] [Google Scholar]
- 36.Zheng R, Mao Y. Triglyceride and glucose (TyG) index as a predictor of incident hypertension: a 9-year longitudinal population-based study. Lipids Health Dis. 2017;16:175. doi: 10.1186/s12944-017-0562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baez-Duarte BG, Sánchez-Guillén Mdel C, Pérez-Fuentes R, Zamora-Ginez I, Leon-Chavez BA, Revilla-Monsalve C, et al. β-cell function is associated with metabolic syndrome in Mexican subjects. Diabetes Metab Syndr Obes. 2010;3:301–9. doi: 10.2147/DMSO.S12375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim TJ, Kim HJ, Kim YB, Lee JY, Lee HS, Hong JH, et al. Comparison of surrogate markers as measures of uncomplicated insulin resistance in Korean adults. Korean J Fam Med. 2016;37:188–96. doi: 10.4082/kjfm.2016.37.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarafidis PA, Lasaridis AN, Nilsson PM, Pikilidou MI, Stafilas PC, Kanaki A, et al. Validity and reproducibility of HOMA-IR, 1/HOMA-IR, QUICKI and McAuley's indices in patients with hypertension and type II diabetes. J Hum Hypertens. 2007;21:709–16. doi: 10.1038/sj.jhh.1002201. [DOI] [PubMed] [Google Scholar]
- 40.Dudi P, Goyal B, Saxena V, Rabari K, Mirza AA, Naithani M, et al. Single point insulin sensitivity estimator as an index for insulin sensitivity for metabolic syndrome: a study in North Indian population. J Lab Physicians. 2019;11:244–8. doi: 10.4103/JLP.JLP_163_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.