Abstract
Background:
Older adults with advanced non-dialysis-dependent chronic kidney disease (NDD-CKD) face a high risk of hospitalization and related adverse events.
Methods:
This prospective cohort study followed nephrology clinic patients ≥60 years old with NDD-CKD stages 4-5. After an eligible patient’s office visit, study staff asked the patient’s provider to rate the patient’s risk of death within the next year using the surprise question (“Would you be surprised if this patient died in the next 12 months?”) with a 5-point Likert scale response (1, “definitely not surprised” to 5, “very surprised”). We used a statewide database to ascertain hospitalization during follow-up.
Results:
There were 488 patients (median age 72 years, 51% female, 17% black) with median estimated glomerular filtration rate 22 mL/min/1.73 m2. Over a median follow-up of 2.1 years, the rates of hospitalization per 100 person-years in the respective response groups were 41 (95% confidence interval [CI]: 34–50), “very surprised”; 65 (95% CI: 55–76), “surprised”; 98 (95% CI: 85–113), “neutral”; 125 (95% CI: 107–144), “not surprised”; and 120 (95% CI: 94–151), “definitely not surprised.” In a fully adjusted cumulative probability ordinal regression model for proportion of follow-up time spent hospitalized, patients whose providers indicated that they would be “definitely not surprised” if they died spent a greater proportion of follow-up time hospitalized compared with those whose providers indicated that they would be “very surprised” (odds ratio 2.4, 95% CI: 1.0–5.7). There was a similar association for time to first hospitalization.
Conclusion:
Nephrology providers’ responses to the surprise question for older patients with advanced NDD-CKD were independently associated with proportion of future time spent hospitalized and time to first hospitalization. Additional studies should examine how to use this information to provide patients with anticipatory guidance on their possible clinical trajectory and to target potentially preventable hospitalizations.
Keywords: Chronic kidney disease, Geriatric nephrology, Hospitalization
Introduction
Adults with non-dialysis-dependent chronic kidney disease (NDD-CKD) are at increased risk for hospitalization [1-7], and as their CKD progresses, their risk of hospitalization increases [1,3-5, 7, 8]. Once hospitalized, patients with NDD-CKD are more likely than their non-CKD counterparts to suffer from a preventable hospital-acquired condition [9], which heightens their risk of in-hospital death, prolonged length of stay, or readmission within 90 days [10]. Like patients with NDD-CKD, older adults also face increased risk of adverse events during hospitalization [11], often followed by disability and loss of independence [12], which deserves special attention given that maintaining independence is the top health outcome priority for older adults with advanced NDD-CKD [13].
Given how common and consequential hospitalization is for older adults with NDD-CKD, the ability to identify those whose risk extends above the baseline risk of being older and having diminished kidney function could benefit these patients by directing their healthcare team’s efforts towards prognostication of clinical trajectory and prevention of hospitalization. Patients with advanced CKD and end-stage kidney disease (ESKD) often report that though they want to discuss prognosis with their doctors, they generally have not [14-17]. With regard to hospitalization prevention, prior research suggests that some hospitalizations of patients with NDD-CKD are preventable and that particular patient characteristics, including older age, higher stage of CKD, lower income, and poor attachment to primary care, are associated with these preventable hospitalizations [18, 19].
Recognizing that the ability to both prognosticate and prevent depends upon knowing who is in need of such efforts, we sought a simple means of identifying older NDD-CKD patients at especially high risk of hospitalization. Previous research has demonstrated that a nephrologist’s response to the “surprise question” about a patient’s mortality risk (“Would you be surprised if this patient died in the next 12 months?”, which can be answered with a binary and 5-point Likert scale response) is associated with mortality [20] and has moderate to good reliability in older patients with NDD-CKD stages 4-5 [21]. We hypothesized that a provider’s impression of a patient’s health, as expressed in the 5-point Likert scale response to the surprise question, would be independently associated with hospitalization in this population.
Materials and Methods
As part of an observational prospective study in older adults (aged ≥60 years) with NDD-CKD stages 4-5, we approached all attending physicians and advanced practitioners with a continuity clinic at an academic nephrology practice in the southern United States to participate in this study from June 2014 to February 2016. All providers gave written informed consent. Through these providers, we accrued 488 patients, whom we followed to December 2016. As described in previous studies, we excluded patients who were dialysis-dependent, had a prior kidney transplant, had been referred for acute kidney injury, or were having an initial visit with a particular nephrology provider [21]. Use of the surprise question to identify patients at high risk of hospitalization was a prespecified secondary outcome of the parent study. The Vanderbilt University Institutional Review Board approved this study, including a waiver of patient informed consent (#140402).
Surprise Question Assessment
We conducted a brief didactic session to familiarize providers with the surprise question. Study staff asked providers the question immediately following an eligible patient’s office visit and recorded responses on a paper form. As previously described, in addition to the usual binary response (i.e., “yes” or “no”), providers also answered the question “How surprised would you be if this patient died in the next 12 months?” with a 5-point Likert scale to capture the degree of surprise (i.e., “definitely not surprised,” “not surprised,” “neutral,” “surprised,” and “very surprised”) [21].
Covariates
We performed manual chart reviews and electronic health record (EHR) data abstraction to obtain baseline variables, including sociodemographic information, comorbid conditions, prescription medications, and common clinical measurements such as laboratory values. We reviewed clinical notes, problem lists, medical, surgical, and social histories, and scanned documents for information. We determined the number of medications by counting active prescription medications, not including topical agents or eye drops, on the date of the patient’s baseline visit. With information on comorbidities, we calculated a Charlson Comorbidity Index (CCI) for each patient [22]. We also ascertained the total number and cumulative length of hospitalizations starting in June 2013, at least 1 year prior to the baseline visit (see below).
Hospitalization Ascertainment
We determined dates of hospitalizations from June 2013 (1 year prior to the first patient’s baseline visit) to December 2016 through linkage with Tennessee Department of Health data. Since 1994, all hospitals in the state have been required to submit hospital claims data to the Department of Health. These data include admissions with observation status or for acute rehabilitation; thus, we counted both of these types of admission as hospitalizations. We did not have data on subacute rehabilitation or nursing home admissions. We calculated the length of each hospitalization by enumerating the number of days between the admission and discharge dates.
Death and Renal Replacement Therapy Ascertainment
Through linkage with the Social Security Death Master File, we captured vital status and date of death, if it occurred. To ensure that we minimized the number of deaths missed, we also performed EHR chart review and online obituary searches for all patients. The EHR contains the date of all deaths at all facilities within the health system, as well as communications with bereaved family members (e.g., while canceling an upcoming appointment). In the rare cases in which vital status and/or date of death remained uncertain, we queried the Tennessee Office of Vital Records to provide verification on vital status. Vital status abstractors were blinded to surprise question responses.
In addition, based on EHR review, we recorded if a patient at any point in follow-up had a documented preference for conservative nondialytic management of ESKD, though we did not record whether or not the patient eventually chose to forego renal replacement therapy (RRT). If a patient initiated RRT by the end of follow-up, we recorded both the modality and the date.
Statistical Analysis
We present patients’ characteristics as medians and interquartile ranges (IQR) for continuous variables and counts with percentages for categorical variables. We used the number of hospitalizations and the length of follow-up to determine hospitalization rates per 100 person-years and the proportion of follow-up time spent hospitalized for both the overall cohort and by surprise question response. We calculated the 95% confidence intervals (95% CI) for hospitalization rates using the exact likelihood method.
For our main regression analysis, the primary outcome was proportion of follow-up time spent hospitalized (i.e., the number of follow-up days hospitalized divided by the total number of follow-up days). We chose this primary outcome to account for the occurrence of repeated hospitalizations while minimizing the potential impact of variable follow-up due to death. We analyzed this outcome with the highly flexible cumulative probability ordinal regression model (CPM) [23]. It should be noted that we implemented this more flexible model after a Poisson regression of hospital days with follow-up time incorporated as an offset term did not adequately fit the outcome. Therefore, Poisson regression was abandoned in favor of the CPM model. Model diagnostics indicated that the CPM model adequately described the data. We selected independent variables for this model a priori on the basis of plausibility of an association and prior literature in similar populations [3, 24, 25]: age, sex, race, marital status, insurance type, CCI, number of prescription medications, body mass index (BMI), estimated glomerular filtration rate (eGFR), serum albumin, hemoglobin, days spent hospitalized in the prior year, and 5-point Likert scale surprise question response. To allow for possible nonlinear associations, we included continuous variables in the regression model as restricted cubic splines. For continuous variables, we scaled odds ratios (ORs) to express the odds per an increase equal to the variable’s IQR. For categorical variables, we expressed ORs against specified references.
In addition, to explore the robustness of our findings, we conducted 2 supplemental analyses. In the first supplemental analysis, we re-ran the fully adjusted CPM model using the surprise question with trinary response [21] rather than 5-point Likert scale response. For this model, responses of “definitely not surprised” and “not surprised” were collapsed into “not surprised,” and responses of “surprised” and “very surprised” were collapsed into “surprised”; the response of “neutral” remained on its own. In the second supplemental analysis, we examined time to first hospitalization. We used the Kaplan-Meier estimator to investigate median time to first hospitalization for both the overall cohort and by surprise question response. We then fit a Cox proportional hazards model adjusting for the same covariates described in the primary regression analysis. We used visual diagnostics to check that the proportional hazards assumption was met. Censoring events included death or last date of linkage with the Tennessee Department of Health data in December 2016. We considered p values of <0.05 to be statistically significant and performed all analyses using R (version 3.4.4).
Results
A total of 488 patients, 51% women, with median age 72 years (IQR: 66–78), were available for analysis (Table 1). The median eGFR at enrollment was 22 mL/min/1.73 m2 (IQR: 16–26) by the Modification of Diet in Renal Disease equation [26]. Diabetes mellitus was present in 55% of patients, while 40% had coronary artery disease, 32% had heart failure, 24% had cerebrovascular disease, 21% had peripheral vascular disease, 17% had cancer (excluding nonmelanoma skin cancer), and 22% had chronic lung disease. In general, the patients about whom the providers would be less surprised were older; had higher CCIs; had lower BMIs, eGFRs, and serum albumin and hemoglobin values; and had more comorbidities, particularly heart failure, cerebrovascular disease, cancer, chronic lung disease, and chronic liver disease. Overall, 34% of the patients had been hospitalized at least once in the year prior to study enrollment, with a low of 15% in the “very surprised” response group to a high of 66% in the “not surprised” response group and 45% in the “definitely not surprised” response group.
Table 1.
Demographic and clinical characteristics of the patients stratified by surprise question 5-point Likert scale response
| Definitely not surprised |
Not surprised | Neutral | Surprised | Very surprised | Overall | |
|---|---|---|---|---|---|---|
| n = 40 | n = 84 | n = 111 | n = 114 | n = 139 | N = 488 | |
| Age, years | 75 (70, 83) | 75 (68, 81) | 74 (67, 80) | 71 (66, 75) | 69 (65, 73) | 72 (66, 78) |
| Women | 23 (58) | 35 (42) | 68 (61) | 48 (42) | 75 (54) | 249 (51) |
| Racea | ||||||
| Black | 5 (13) | 14 (17) | 19 (17) | 19 (17) | 25 (18) | 82 (17) |
| White/Other | 35 (88) | 70 (83) | 92 (83) | 93 (83) | 114 (82) | 404 (83) |
| Marital statusb | ||||||
| Married | 23 (61) | 55 (66) | 59 (54) | 70 (62) | 94 (69) | 301 (63) |
| Divorced/separated | 4 (11) | 3 (4) | 15 (14) | 14 (12) | 17 (13) | 53 (11) |
| Widowed | 10 (26) | 22 (27) | 30 (27) | 21 (19) | 22 (16) | 105 (22) |
| Single | 1 (3) | 3 (4) | 6 (6) | 8 (7) | 3 (2) | 21 (4) |
| Insurance type | ||||||
| Private | 3 (8) | 12 (14) | 11 (10) | 21 (18) | 40 (29) | 87 (18) |
| Medicare | 37 (93) | 71 (85) | 99 (89) | 91 (80) | 99 (71) | 397 (81) |
| Medicaid/medical assistance/uninsured | 0 (0) | 1 (1) | 1 (1) | 2 (2) | 0 (0) | 4 (1) |
| Charlson comorbidity index | 6 (5, 8) | 6 (4, 8) | 5 (4, 7) | 4 (4, 6) | 4 (3, 6) | 5 (4, 7) |
| Current prescription medications (excluding topicals and eye drops), n | 9 (7, 13) | 10 (8, 13) | 9 (7, 12) | 9 (7, 11) | 9 (7, 11) | 9 (7, 11) |
| Body mass index, kg/m2 | 26 (21, 30) | 28 (26, 32) | 29 (25, 35) | 30 (26, 35) | 29 (25, 35) | 29 (25, 34) |
| Specific comorbidities | ||||||
| Hypertension | 39 (98) | 84 (100) | 107 (96) | 112 (98) | 138 (99) | 480 (98) |
| Diabetes mellitus | 21 (53) | 53 (63) | 62 (56) | 56 (49) | 76 (55) | 268 (55) |
| Coronary artery disease | 18 (45) | 45 (54) | 55 (50) | 46 (40) | 33 (24) | 197 (40) |
| Heart failure | 22 (55) | 33 (39) | 42 (38) | 31 (27) | 26 (19) | 154 (32) |
| Cerebrovascular disease | 14 (35) | 23 (27) | 33 (30) | 31 (27) | 14 (10) | 115 (24) |
| Peripheral vascular disease | 9 (23) | 25 (30) | 30 (27) | 23 (20) | 17 (12) | 104 (21) |
| Cancer | 9 (23) | 19 (23) | 13 (12) | 22 (19) | 18 (13) | 81 (17) |
| Chronic lung disease | 14 (35) | 23 (27) | 25 (23) | 19 (17) | 25 (18) | 106 (22) |
| Chronic liver disease | 10 (25) | 9 (11) | 13 (12) | 8 (7) | 13 (9) | 53 (11) |
| Laboratory values | ||||||
| eGFR, mL/min/1.73 m2,c | 16 (13, 23) | 21 (15, 25) | 20 (16, 26) | 22 (17, 25) | 23 (17, 28) | 22 (16, 26) |
| Serum creatinine, mg/dL | 2.9 (2.2, 4.0) | 2.6 (2.1, 3.3) | 2.5 (2.1, 3.1) | 2.6 (2.2, 3.2) | 2.4 (2.1, 3.1) | 2.5 (2.1, 3.2) |
| Serum albumin, g/dLd | 3.7 (3.5, 4.0) | 3.8 (3.6, 4.2) | 4.0 (3.7, 4.1) | 4.1 (3.9, 4.2) | 4.0 (3.8, 4.2) | 4.0 (3.7, 4.2) |
| Hemoglobin, g/dLe | 11.0 (9.9, 12.1) | 11.2 (9.8, 12.6) | 11.2 (10.1, 12.1) | 12.0 (10.9, 13.1) | 12.0 (11.0, 12.6) | 11.5 (10.4, 12.7) |
| Any hospitalization in 1 year prior to study entry | 18 (45) | 55 (66) | 44 (40) | 30 (26) | 21 (15) | 168 (34) |
| Time spent hospitalized in 1 year prior to study entry, days | 0 (0, 9) | 4 (0, 9) | 0 (0, 7) | 0 (0, 2) | 0 (0, 0) | 0 (0, 5) |
Continuous variables expressed as median (interquartile range); categorical variables expressed as n (%). Percentages in columns may not add up to 100 due to rounding. eGFR, estimated glomerular filtration rate.
n = 112 in the “surprised” response group. Among the nonblack patients, 396 reported their race as white and 8 reported their race as other.
n = 38 in the “definitely not surprised” response group; n = 83 in the “not surprised” response group; n = 110 in the “neutral” response group; n = 113 in the “surprised” response group; n = 136 in the “very surprised” response group; N = 480 overall.
Calculated using the Modification of Diet in Renal Disease study equation.
n = 462.
n = 487.
As shown in Table 2, the median follow-up was 2.1 years (IQR: 1.7–2.4). The rates of hospitalizations per 100 person-years in the respective response groups were 41 (95% CI: 34–50), “very surprised”; 65 (95% CI: 55–76), “surprised”; 98 (95% CI: 85–113), “neutral”; 125 (95% CI: 107–144), “not surprised”; and 120 (95% CI: 94–151), “definitely not surprised.” The entire cohort spent a median of 0.5% (IQR: 0–2.2%) of follow-up time hospitalized; the group medians ranged from 0% (IQR: 0–0.8%) in the “very surprised” response group to 1.5% (IQR: 0–4.7%) in the “definitely not surprised” response group. The median number of days and follow-up time to which these percentages equate is displayed in Table 2. In the “definitely not surprised” response group, 55% of the patients died during follow-up, more than twice the rate of the “not surprised” response group (27%). In contrast, only 8% of the “very surprised” response group patients died during follow-up.
Table 2.
Patient outcomes stratified by surprise question 5-point Likert scale response
| Definitely not surprised |
Not surprised | Neutral | Surprised | Very surprised | Overall | |
|---|---|---|---|---|---|---|
| n = 40 | n = 84 | n = 111 | n = 114 | n = 139 | N = 488 | |
| Duration of follow-up, years | 1.8 (0.6, 2.4) | 2.1 (1.2, 2.3) | 2.1 (1.6, 2.3) | 2.2 (1.8, 2.4) | 2.2 (1.9, 2.4) | 2.1 (1.7, 2.4) |
| Hospitalizations, n | 1 (0, 3) | 1 (0, 3) | 1 (0, 3) | 1 (0, 2) | 0 (0, 1) | 1 (0, 2) |
| Hospitalizations, per 100 person-yr (95% CI) | 120 (94, 151) | 125 (107, 144) | 98 (85, 113) | 65 (55, 76) | 41 (34, 50) | 78 (73, 84) |
| Total duration of hospitalizations, days | 9 (0, 19) | 8 (0, 18) | 5 (0, 18) | 3 (0, 10) | 0 (0, 6) | 3 (0, 13) |
| Follow-up time spent hospitalized, % | 1.5 (0, 4.7) | 1.2 (0, 3.3) | 0.9 (0, 3.1) | 0.3 (0, 1.4) | 0 (0, 0.8) | 0.5 (0, 2.2) |
| Time to first hospitalization or censoring, days | 126 (49, 417) | 277 (114, 606) | 368 (152, 714) | 522 (265, 785) | 702 (322, 835) | 449 (172, 782) |
| Deaths during follow-up | 22 (55) | 23 (27) | 21 (19) | 11 (10) | 11 (8) | 88 (18) |
| Started RRT during follow-up 1 | 6 (15) | 9 (11) | 22 (20) | 11 (10) | 16 (12) | 64 (13) |
| HD | 3 (8) | 7 (8) | 13 (12) | 7 (6) | 8 (6) | 38 (8) |
| HD, then PD | 1 (3) | 0 (0) | 1 (1) | 1 (1) | 0 (0) | 3 (1) |
| PD | 2 (5) | 2 (8) | 8 (7) | 3 (3) | 5 (4) | 20 (4) |
| Kidney transplant | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (2) | 3 (1) |
| Expressed preference for conservative nondialytic management of ESKD | 14 (35) | 7 (8) | 4 (4) | 4 (4) | 0 (0) | 29 (6) |
Continuous variables expressed as median (interquartile range) except where specified; categorical variables expressed as n (%). Percentages in columns may not add up to 100 due to rounding. CI, confidence interval; RRT, renal replacement therapy; HD, hemodialysis; PD, peritoneal dialysis; ESKD, end-stage kidney disease.
In the multivariable model examining the proportion of follow-up time spent hospitalized, being divorced or separated as opposed to married (OR 2.16, 95% CI: 1.25–3.73), having a CCI of 7 versus 4 (OR 1.31, 95% CI: 1.00–1.73), and being prescribed 11 versus 7 medications (OR 1.58, 95% CI: 1.21–2.06) were significantly associated with spending a greater proportion of follow-up time hospitalized (Table 3). Compared with a response of “very surprised,” a response of “definitely not surprised” (OR 2.44, 95% CI: 1.04–5.72), “not surprised” (OR 2.84, 95% CI: 1.58–5.11), “neutral” (OR 2.58, 95% CI: 1.50–4.45), and “surprised” (OR 1.70, 95% CI: 1.02–2.82) were all associated with spending a greater proportion of follow-up time hospitalized.
Table 3.
Multivariable adjusted odds of proportion of follow-up time spent hospitalized
| Odds ratio (95% CI) |
|
|---|---|
| Age (78 vs. 66), years | 0.99 (0.69–1.42) |
| Women | 1.32 (0.89–1.96) |
| Black | 1.12 (0.68–1.84) |
| Marital status (vs. married) | |
| Divorced/separated | 2.16 (1.25–3.73) |
| Widowed | 1.14 (0.68–1.90) |
| Single | 0.99 (0.38–2.61) |
| Insurance type (private vs. nonprivate) | 1.22 (0.73–2.06) |
| Charlson comorbidity index (7 vs. 4) | 1.31 (1.00–1.73) |
| Current prescription medications (11 vs. 7), n | 1.58 (1.21–2.06) |
| Body mass index (34 vs. 25), kg/m2 | 0.70 (0.52–0.94) |
| Laboratory values | |
| eGFR (26 vs. 16), mL/min/1.73 m2 | 0.79 (0.60–1.04) |
| Serum albumin (4.2 vs. 3.7), g/dL | 0.66 (0.54–0.81) |
| Hemoglobin (12.7 vs. 10.5), g/dL | 0.90 (0.69–1.18) |
| Days spent hospitalized in prior 1 year (5 vs. 0) | 1.09 (0.83–1.43) |
| Surprise question response (vs. very surprised) | |
| Definitely not surprised | 2.44 (1.04–5.72) |
| Not surprised | 2.84 (1.58–5.11) |
| Neutral | 2.58 (1.50–4.45) |
| Surprised | 1.70 (1.02–2.82) |
For continuous variables, odds ratios express the odds per an increase equal to the variable’s interquartile range. CI, confidence interval; eGFR, estimated glomerular filtration rate.
Supplemental Analyses
The multivariable model examining the proportion of follow-up time spent hospitalized using the trinary surprise question response demonstrated results similar to the model using the 5-point Likert scale response, with patients whose providers responded “not surprised” (OR 2.09, 95% CI: 1.28–3.40) and “neutral” (OR 1.98, 95% CI: 1.24–3.18) spending a higher proportion of follow-up time hospitalized compared with patients whose providers responded “surprised” (see online suppl. Table 1; see www.karger.com/doi/10.1159/000509046 for all online suppl. material).
Figure 1 demonstrates Kaplan-Meier curves of time to first hospitalization according to surprise question 5-point Likert scale response. Until the end of follow-up, when the curves for “not surprised” and “definitely not surprised” cross, the respective curves for each surprise question response remain ordered, such that “very surprised” has the lowest proportion of patients with a hospitalization and “definitely not surprised” has the highest proportion of patients with a hospitalization. The multivariable adjusted hazard ratios for time to first hospitalization follow the same pattern as the primary analysis, with a response of “definitely not surprised” (HR 1.99, 95% CI: 1.14–3.49), “not surprised” (OR 2.09, 95% CI: 1.35–3.22), “neutral” (OR 1.72, 95% CI: 1.17–2.51), and “surprised” (OR 1.54, 95% CI: 1.04–2.27) all associated with shorter time to first hospitalization compared with a response of “very surprised” (online suppl. Table 2).
Fig. 1.
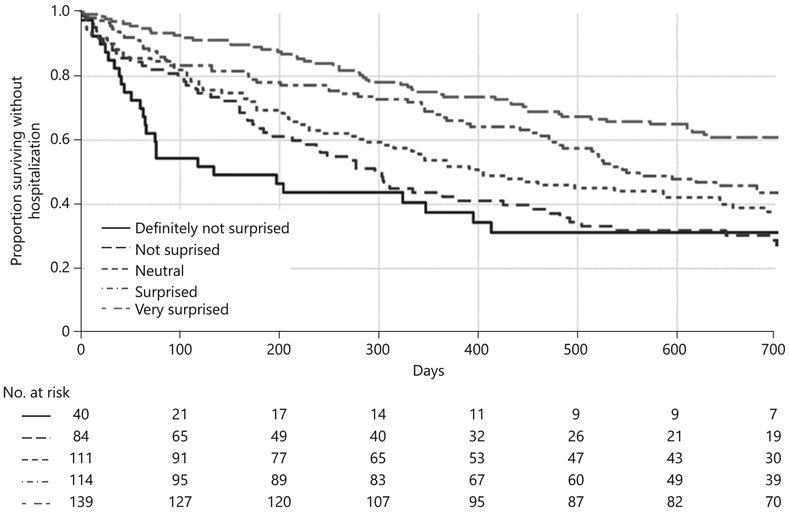
Kaplan-Meier curves of time to first hospitalization by surprise question 5-point Likert scale response.
Discussion
In this study, we found that the nephrology provider response to the surprise question identified older adults with advanced NDD-CKD with higher hospitalization rates and a shorter time to first hospitalization and was independently associated with proportion of follow-up time spent hospitalized and time to first hospitalization. This was especially true in patients whose providers responded “neutral,” “not surprised,” and “definitely not surprised,” as the odds of increased proportion of time spent in the hospital for these patients were approximately 2.5–3 times those of their “very surprised” response counterparts.
Our findings suggest that provider intuition has unique value in anticipating critical patient-centered outcomes besides death. In the past, others have provided estimates of the hospitalization rate in advanced NDD-CKD. Go et al., based on their cohort of adults of all ages from the Kaiser Permanente Renal Registry in 1996–2000, calculated a rate of 87 per 100 person-years in patients with eGFR 15–29 mL/min/1.73 m2 and 145 per 100 person-years in patients with eGFR < 15 mL/min/1.73 m2[1], while the United States Renal Data System, using Medicare data, placed it at 86 per 100 person-years in patients aged 66 and older with CKD stages 4–5 in 2016 [7]. Our cohort’s overall rate of 78 hospitalizations per 100 person-years is similar, but the difference between surprise question response groups – from as low as 41 hospitalizations per 100 person-years in the “very surprised” response group to as high as 125 hospitalizations per 100 person-years in the “not surprised” response group – demonstrates that older adults with advanced NDD-CKD are a heterogeneous group, and nephrology providers recognize patients who are at increased risk of hospitalization when they answer the surprise question.
The issue, then, is how best to employ this provider intuition for the benefit of the patient. One way in which to do it would be in offering prognostic information to facilitate informed shared decision-making. The findings of this study and its precursor [21] demonstrate that nephrologists are able to prognosticate about outcomes that matter to patients with NDD-CKD stages 4-5, even if they often report they cannot [15, 27, 28], and nephrologists’ intuitions are valuable independent of traditional markers of health and disease. Despite this, available data suggest that patients are not receiving prognostic information as much as they would like [14-17]. Though nephrology providers might regard hospitalization as an expected outcome in advanced NDD-CKD, hospitalization can be a sentinel event heralding the onset of new problems or even the imminent end of life. Hospitalization and its complications can also irrevocably alter a patient’s clinical trajectory. Finally, some patients view hospitalization so unfavorably that they would accept a shorter lifespan in exchange for fewer days hospitalized. Whatever the significance of hospitalization to the individual patient, it is worth studying if integrating a nephrology provider’s response to the surprise question into sensitive but honest conversations about the future could help meet the patient’s need for prognostic information while easing the nephrology provider’s work of delivering this information.
Another way in which a nephrology provider could use his or her clinical gestalt of a patient for the patient’s benefit would be in preventing hospitalization. Overall, inquiry into interventions that could prevent hospitalization of NDD-CKD patients, targeting them on the basis of their CKD rather than on the basis of another condition, is still in its infancy, but there have been some promising results. Through the use of nurse care management augmented with a disease-based informatics system, Fishbane and colleagues were successful in lowering the hospitalization rate in patients with NDD-CKD stages 4-5 [29]. The main driver of this lower hospitalization rate was fewer hospitalizations for volume overload or congestive heart failure. Though patients were enrolled in this randomized controlled trial without regard for perceived hospitalization risk, the application of the intervention across 2 academic clinics, a public hospital clinic, and a private practice suggests that the results are likely generalizable. Similarly, in patients with heart failure and chronic obstructive pulmonary disease, the chronic diseases that have so far fostered the most research in hospitalization prevention, case management interventions, and integrated disease management programs have been shown to reduce hospitalizations [30, 31]. The message from the literature in these conditions, as well as the aforementioned NDD-CKD study, is that it requires a team to keep patients out of the hospital. Further research into interventions to avoid hospitalization in NDD-CKD patients might be enriched by efforts to include patients whose providers respond “definitely not surprised,” “not surprised,” or “neutral” to the surprise question.
Our study had notable strengths. First, since all providers in the clinic consented to participation and patients were not required to consent, we did not exclude any patients, greatly reducing potential selection bias and enhancing generalizability. Second, the use of the Tennessee Department of Health hospitalization data allowed us to capture hospitalizations throughout the whole state. This permitted us to adjust for days hospitalized in the year prior to study enrollment and thereby more fully ascertain the primary outcome. We also acknowledge some limitations. First, this was a single-center study of an academic clinic population with some racial but limited ethnic diversity. Second, if any hospitalizations occurred outside Tennessee, we missed them, but this misclassification would likely have been nondifferential and biased our results towards the null. Third, because many of the hospitalizations were not at the hospital affiliated with the clinic at which the study was performed, we were not able to systematically examine the diagnoses prompting hospitalizations and whether or not these hospitalizations could have been prevented. Based on prior work, we know that the leading causes of hospitalization in NDD-CKD patients are cardiovascular events and infections [4, 5]. Fourth, although we were able to capture admissions to acute rehabilitation units and freestanding rehabilitation hospitals, we were not able to capture admissions to skilled nursing facilities for subacute rehabilitation or long-term care. Skilled nursing facility stays probably are also important outcomes for patients; however, their inclusion could have substantially increased the proportion of time spent within a healthcare institution. Fifth, in our analysis we did not distinguish between hospitalizations in which death did and did not occur, as it is not possible at time of admission to know which hospitalizations will end in death. We also do not know which patients eventually chose to forego dialysis, so we cannot account for how decisions to forego dialysis affected the occurrence of hospitalization or death. Finally, we did not specifically validate the 5-point Likert scale surprise question response in our earlier work [21], but the 5-point responses yielded similar predictive ability as the binary and trinary surprise question responses, albeit with somewhat lower interrater reliability.
Although the surprise question was devised to ask about risk of death, our findings demonstrate that nephrology provider response identifies older adults with advanced NDD-CKD at increased risk of hospitalization. This work strengthens the case for using the surprise question in everyday practice to start conversations that extend from risk of hospitalization and anticipated clinical trajectory to shared decision-making about broader treatment preferences in the setting of serious illness.
Supplementary Material
Acknowledgements
The authors thank the patients and providers at the Vanderbilt University Medical Center Nephrology Clinic for their participation during this study.
Funding Sources
This work was supported by National Institutes of Health (NIH) Grant T32AG049666 (S.J.R.), the Satellite Health Norman Coplon Extramural Grant Program (K.A-K.), NIH Grant K23DK090304 (K.A-K.), the Vanderbilt Center for Kidney Disease, and National Center for Advancing Translational Sciences Clinical and Translational Science Awards award UL1TR000445. The funders of this study had no role in study design, data collection, analysis, interpretation, manuscript preparation, or the decision to report the findings.
Footnotes
Statement of Ethics
All providers gave written informed consent to participate in this study. The Vanderbilt University Institutional Review Board approved this study, including a waiver of patient informed consent (#140402).
Disclosure Statement
E.D.S. receives research funding from the Veterans Health Administration and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). He has consulted for Akebia, Inc., and has received an honorarium as an invited speaker at the Da-Vita Annual Physician Leadership Conference in 2019 and royalties as an author for UptoDate. S.P.Y.W. receives research funding from the NIDDK, National Palliative Care Research Center, and the Veterans Affairs National Center for Ethics in Health Care. M.J. receives research funding from the NIDDK. K.A-K. receives research funding from the NIDDK. An abstract including these findings was presented at the American Society of Nephrology Kidney Week 2018, San Diego, CA.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–305. [DOI] [PubMed] [Google Scholar]
- 2.Baumeister SE, Boger CA, Kramer BK, Doring A, Eheberg D, Fischer B, et al. Effect of chronic kidney disease and comorbid conditions on health care costs: a 10-year observational study in a general population. Am J Nephrol. 2010;31:222–9. [DOI] [PubMed] [Google Scholar]
- 3.Nitsch D, Nonyane BA, Smeeth L, Bulpitt CJ, Roderick PJ, Fletcher A. CKD and hospitalization in the elderly: a community-based cohort study in the United Kingdom. Am J Kidney Dis. 2011;57:664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iwagami M, Caplin B, Smeeth L, Tomlinson LA, Nitsch D. Chronic kidney disease and cause-specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract. 2018;68:e512–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong E, Ballew SH, Daya N, Ishigami J, Rebholz CM, Matsushita K, et al. Hospitalization risk among older adults with chronic kidney disease. Am J Nephrol. 2019;50:212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daratha KB, Short RA, Corbett CF, Ring ME, Alicic R, Choka R, et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7:409–16. [DOI] [PubMed] [Google Scholar]
- 7.United States Renal Data System. United States Renal Data System: 2018 USRDS annual data report: epidemiology of kidney disease in the United States. Bethesda (MD): United States Renal Data System; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kent S, Schlackow I, Lozano-Kuhne J, Reith C, Emberson J, Haynes R, et al. Group SC: what is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol. 2015;16:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bohlouli B, Tonelli M, Jackson T, Hemmelgam B, Klarenbach S. Risk of hospital-acquired ccomplications in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2016;11:956–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bohlouli B, Jackson TJ, Tonelli M, Hemmelgarn B, Klarenbach S. Adverse outcomes associated with preventable complications in hospitalized patients with CKD. Clin J Am Soc Nephrol. 2017;12:799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Long SJ, Brown KF, Ames D, Vincent C. What is known about adverse events in older medical hospital inpatients? A systematic review of the literature. Int J Qual Health Care. 2013;25: 542–54. [DOI] [PubMed] [Google Scholar]
- 12.Covinsky KE, Pierluissi E, Johnston CB. Hospitalization-associated disability: “She was probably able to ambulate, but I’m not sure”. JAMA. 2011;306:1782–93. [DOI] [PubMed] [Google Scholar]
- 13.Ramer SJ, McCall NN, Robinson-Cohen C, Siew ED, Salat H, Bian A, et al. Health outcome priorities of older adults with advanced CKD and concordance with their nephrology providers’ perceptions. J Am Soc Nephrol. 2018;29:2870–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davison SN. End-of-life care preferences and needs: perceptions of patients with chronic kidney disease. Clin J Am Soc Nephrol. 2010; 5:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schell JO, Patel UD, Steinhauser KE, Ammarell N, Tulsky JA. Discussions of the kidney disease trajectory by elderly patients and nephrologists: a qualitative study. Am J Kidney Dis. 2012;59:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ladin K, Buttafarro K, Hahn E, Koch-Weser S, Weiner DE. “End-oflifecare? I’m not going to worry about that yet.” Health literacy gaps and end-of-life planning among elderly dialysis patients. Gerontologist. 2018;58:290–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selman LE, Bristowe K, Higginson IJ, Murtagh FEM. The views and experiences of older people with conservatively managed renal failure: a qualitative study of communication, information and decision-making. BMC Nephrol. 2019;20:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiebe N, Klarenbach SW, Allan GM, Manns BJ, Pelletier R, James MT, et al. Potentially preventable hospitalization as a complication of CKD: a cohort study. Am J Kidney Dis. 2014;64:230–8. [DOI] [PubMed] [Google Scholar]
- 19.Ronksley PE, Hemmelgarn BR, Manns BJ, Wick J, James MT, Ravani P, et al. Potentially preventable hopitalization among patients with CKD and high inpatient use. Clin J Am Soc Nephrol. 2016;11:2022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmidt RJ, Landry DL, Cohen L, Moss AH, Dalton C, Nathanson BH, et al. Derivation and validation of a prognostic model to predict mortality in patients with advanced chronic kidney disease. Nephrol Dial Transplant. 2019;34:1517–25. [DOI] [PubMed] [Google Scholar]
- 21.Javier AD, Figueroa R, Siew ED, Salat H, Morse J, Stewart TG, et al. Reliability and utility of the surprise question in CKD Stages 4 to 5. Am J Kidney Dis. 2017;70:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh B, Singh A, Ahmed A, Wilson GA, Pickering BW, Herasevich V, et al. Derivation and validation of automated electronic search strategies to extract Charlson comorbidities from electronic medical records. Mayo Clin Proc. 2012;87:817–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu Q, Shepherd BE, Li C, Harrell FE Jr. Modeling continuous response variables using ordinal regression. Stat Med. 2017;36:4316–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Becker BN, Coomer RW, Fotiadis C, Evanson J, Shyr Y, Hakim RM. Risk factors for hospitalization in well-dialyzed chronic hemodialysis patients. Am J Nephrol. 1999;19:565–70. [DOI] [PubMed] [Google Scholar]
- 25.Yan G, Norris KC, Greene T, Yu AJ, Ma JZ, Yu W, et al. Race/ethnicity, age, and risk of hospital admission and length of stay during the first year of maintenance hemodialysis. Clin J Am Soc Nephrol. 2014;9:1402–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. [DOI] [PubMed] [Google Scholar]
- 27.Eneanya ND, Hailpern SM, O’Hare AM, Kurella Tamura M, Katz R, Kreuter W, et al. Trends in receipt of intensive procedures at the end of life among patients treated with maintenance dialysis. Am J Kidney Dis. 2017; 69:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ladin K, Pandya R, Kannam A, Loke R, Oskoui T, Perrone RD, et al. Discussing conservative management with older patients with CKD: an interview study of nephrologists. Am J Kidney Dis. 2018;71:627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fishbane S, Agoritsas S, Bellucci A, Halinski C, Shah HH, Sakhiya V, et al. Augmented nurse care management in CKD Stages 4 to 5: a randomized trial. Am J Kidney Dis. 2017;70: 498–505. [DOI] [PubMed] [Google Scholar]
- 30.Takeda A, Martin N, Taylor RS, Taylor SJ. Disease management interventions for heart failure. Cochrane Database Syst Rev. 2019;1: CD002752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruis AL, Smidt N, Assendelft WJ, Gussekloo J, Boland MR, Rutten-van Molken M, et al. Integrated disease management interventions for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2013;CD009437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


