Abstract
The arcuate nucleus of the hypothalamus (ARC) plays a key role in linking peripheral metabolic status to the brain melanocortin system, which influences a wide range of physiological processes including the sympathetic nervous system and blood pressure. The importance of the activity of agouti-related peptide (AgRP)- and proopiomelanocortin (POMC)-expressing neurons, two molecularly distinct populations of ARC neurons, for metabolic regulation is well established, but their relevance for sympathetic and cardiovascular control remains unclear. We used designer receptors exclusively activated by designer drug (DREADD) technology to study how activation of AgRP and POMC neurons affect renal sympathetic nerve traffic and blood pressure. In addition to the drastic feeding-stimulatory effect, DREADD-mediated activation of AgRP, but not POMC neurons, induced an acute reduction in renal sympathetic nerve activity in conscious mice. Paradoxically, however, DREADD-mediated chronic activation of AgRP neurons caused a significant increase in blood pressure specifically in the inactive light phase. On the other hand, chronic activation of POMC neurons led to a significant reduction in blood pressure. These results bring new insights to a previously unappreciated role of ARC AgRP and POMC neuronal activity in autonomic and cardiovascular regulation.
NEW & NOTEWORTHY Agouti-related peptide (AgRP)- and proopiomelanocortin (POMC)-expressing neurons of the arcuate nucleus are essential components of the brain melanocortin system that controls various physiological processes. Here, we tested the metabolic and cardiovascular effects of direct activation of these two populations of neurons. Our findings show that, in addition to stimulation of food intake, chemogenetic mediated activation of hypothalamic arcuate nucleus AgRP, but not POMC, neurons reduce renal sympathetic traffic. Despite this, chronic activation of AgRP neurons increased blood pressure. However, chronic activation of POMC neurons led to a significant reduction in blood pressure. Our findings highlight the importance of arcuate nucleus AgRP and POMC neuronal activity in autonomic and cardiovascular regulation.
Keywords: arcuate neurons, blood pressure, chemogenetic, sympathetic nerve activity
INTRODUCTION
Activity of the autonomic nervous system, which is largely determined by hypothalamic circuits, plays a crucial role in maintaining different homeostatic processes, including energy metabolism and blood pressure. Within the hypothalamus, the arcuate nucleus (ARC) is recognized as an important player in the regulation of energy metabolism and glycemic control (3, 34, 37, 42). In addition, ARC neurons have emerged as important regulators of sympathetic traffic and cardiovascular function (8, 37). For instance, electrical or chemical ARC stimulation causes blood pressure elevation and vasoconstriction (7). Strong c-Fos induction was observed in ARC neurons in response to prolonged decrease in arterial pressure induced by sodium nitroprusside (24). Moreover, electrical stimulation of afferent renal nerves causes ARC neuronal activation as indicated by the increase in c-Fos (36).
The ARC contains several neurochemically and functionally distinct populations of neurons that include those expressing small neuropeptides agouti-related protein (AgRP), or proopiomelanocortin (POMC). AgRP is a unique endogenous antagonist that inhibits melanocortin receptors (MCRs), whereas POMC-derived α-melanocyte-stimulating hormone (α-MSH) is an endogenous agonist that activates these same MCRs, including MC4R, located in second-order neurons such as the paraventricular nucleus of the hypothalamus (9). AgRP and POMC neurons regulate a wide range of physiological functions, including food intake, glycemic control, energy expenditure, and sympathetic nerve traffic (20, 22, 44). Adult-stage ablation of AgRP neurons induces aphagia (25), whereas ablation of POMC neurons causes hyperphagia and morbid obesity (45). Moreover, acute optogenetic (1) or chemogenetic (21) activation of AgRP neurons elicits a rapid increase in food intake in satiated mice, whereas chronic, but not acute, optogenetic stimulation of POMC neurons reduces food intake (1, 21).
ARC AgRP and POMC neurons express various receptors that enable them to sense a variety of circulating metabolic hormones in the bloodstream, including leptin, insulin, and ghrelin (42). Action of leptin and other hormones on AgRP and POMC neurons triggers downstream transcriptional and translational events in a cell type-specific manner. In addition, action of the circulating metabolic hormones alters the firing patterns of AgRP and POMC neurons. For example, leptin is known to inhibit AgRP neurons while activating POMC neurons, which leads to a negative energy balance. However, the relative contribution of AgRP versus POMC neuronal activity to arterial pressure and renal sympathetic outflow remain unclear.
In the present study, we hypothesized that activating AgRP and POMC neurons causes contrasting changes in metabolic functions and cardiovascular parameters. For this, we used designer receptor exclusively activated by designer drugs (DREADD) to evaluate the functional consequences of selective activation of AgRP or POMC neurons on body weight, feeding behavior, glucose tolerance, renal sympathetic nerve activity (SNA), and blood pressure.
MATERIAL AND METHODS
Animals.
POMCCre and AgRPCre mice on a mixed background were obtained from the Jackson Laboratory (stock Nos. 005965 and 012899, respectively) and bred to wild-type (WT) female mice to generate experimental cohorts of POMCCre, AgRPCre, and WT littermate mice. It is worth noting that, although both POMCCre and AgRPCre mice were on mixed 129 and B6 background, the POMCCre mice that we used for the experiment were closer to pure B6 background (>93%) than AgRPCre mice (50–75%). Genotyping of the mice was performed using a PCR assay, as described previously (4). Animals were housed at the University of Iowa vivarium in a 23°C temperature-controlled environment with a 12-h:12-h light-dark cycle (lights on: 6 AM–6 PM) with ad libitum access to tap water and standard chow. Ten- to twelve-week-old-male mice were used for stereotaxic microinjection to create experimental cohorts. Animal procedures were approved by the University of Iowa Animal Research Committee.
Stereotaxic microinjection of adeno-associated virus into the ARC.
Stereotactic surgery was performed as previously described (12, 18). Briefly, POMCCre and AgRPCre mice were anesthetized with intraperitoneal (ip) ketamine-xylazine (100 and 10 mg/kg ip, respectively) and placed on a Kopf stereotaxic apparatus. Following standard disinfection, a ∼1.0-cm incision was made to expose the skull, and a small hole was drilled into the skull bilaterally at defined positions (anteroposterior –1.5 mm, mediolateral ± 0.8 mm, dorsoventral –5.9 mm, with 8° angle of injection). A Hamilton 33-gauge needle microsyringe with a small-hub removable needle filled with solution containing an adeno-associated virus (AAV) that drives the expression, in a Cre-dependent manner, of a mutated Gq-coupled muscarinic receptor tagged with the fluorescent mCherry (AAV-hM3Dq-mCherry, 3.8 × 1012 GC/mL; University of North Carolina Vector Core) was slowly injected at a rate of 0.05 μL/min (for a total injection volume of 0.4 µL/side). To ensure a full penetration of AAV into the targeted area, the needle was left in place for 10 min before it was removed, and the incision was closed using wound clips. Mice were then kept on a warming pad until awake. WT mice that received AAV-hM3Dq-mCherry were used as controls. Clozapine-N-oxide (CNO; 1 mg/kg, Sigma-Aldrich) was used to activate hM3Dq-expressing POMC or AgRP neurons.
At the end of each study, mice were anesthetized with ketamine-xylazine (100 and 10 mg/kg ip) and transcardially perfused with phosphate-buffered saline (PBS) followed by cold 4% paraformaldehyde. Brains were then extracted and postfixed in 4% paraformaldehyde and sliced into 30-µm sections. Injection sites were confirmed in every mouse by assessing the mCherry signal under fluorescent microscope (Zeiss Apotome 2.0).
Fluorescent immunohistochemistry.
Ninety minutes after intraperitoneal injection of CNO (1 mg/kg) or saline (vehicle) ARC AAV-hMD3q-mCherry microinfused AgRPCre and POMCCre mice were anesthetized with ketamine-xylazine and perfused and fixed with cold 4% paraformaldehyde. Extracted brains were post-fixed and cryoprotected in 25% sucrose overnight before being sliced on a freezing microtome. The brain sections were blocked and permeabilized with 3% normal donkey serum with 0.3% Triton X-100 in PBS for 30 min at room temperature. Sections were then incubated with rabbit primary antibody against c-Fos (1:5,000, Cat. No. PC38, Calbiochem) overnight at 4°C. Staining was developed by Cy2-conjugated secondary antibody against rabbit IgG (1:400, Jackson ImmunoResearch). Specific fluorescent signals of mCherry and Cy2 were detected and imaged using a confocal fluorescent microscope (Zeiss, Apotome 2.0).
Body weight, food intake, and glucose tolerance test.
Body weight and food intake were measured in the home cages of singly housed mice before and following CNO intraperitoneal injection as indicated. For the glucose tolerance test (GTT), mice were subject to 6-h fasting with free access to water. Intraperitoneal injection of CNO (1 mg/kg) was given 15 min before the GTT: blood obtained from the tail was used to measure glucose at baseline (time 0) and then at 15, 30, 60, and 120 min after intraperitoneal injection of d-glucose (1 g/kg; Cat. No. G8270, Sigma-Aldrich) (30).
Sympathetic nerve recording.
Renal SNA was studied in the conscious state, as described previously (4, 29). Mice were anesthetized with isoflurane (up to 5% for induction, 1.5–2% for maintenance). Next, a microrenathane tube (MRE-40, Braintree Scientific) was inserted into the right jugular vein for intravenous administration of CNO or saline (vehicle). Another MRE-40 catheter was inserted into the left carotid artery for continuous measurement of arterial pressure and heart rate (HR). Next, mice were equipped for direct multifiber recording of renal SNA. The left kidney was accessed through a retroperitoneal incision, and the nerve fascicle was carefully isolated from surrounding connective tissues. A bipolar platinum-iridium electrode (40-gauge, A-M Systems) was then suspended under the nerve. After optimal positioning of the electrode (based on signal-to-noise ratio) was achieved, the electrodes were encased with a minimal amount (∼100 μL) of silicone gel (Kwik-Sil, World Precision Instruments, Inc.) that allowed the movement of the assembly without breaking the nerve. A separate grounding wire was inserted into the nearby muscle to ground the mouse. A 6-0 silk was used to suture the recording electrodes and the ground wire to the spinal muscle and to secure the electrodes along the subcutaneous dorsal surface, exteriorized from the nape of the neck alongside the arterial and venous catheters. All skin incisions at the neck and along the left flank were sutured together with 4-0 silk. Mice were then gradually weaned off the isoflurane and allowed 4–5 h to recover before the experimental protocol was started. Baseline renal SNA was continuously recorded for 30 min before treatment. Renal SNA was then quantified at 15-min intervals.
SNA data were acquired by attaching the electrodes to a high-impedance probe (HIP-511, Grass Instruments), and the nerve signal was amplified 105 times with a Grass P5 AC preamplifier. The amplified nerve signal was filtered at a low-frequency (100 Hz) and high-frequency (1,000 Hz) cutoff with a nerve traffic analysis system (model 706C, University of Iowa Bioengineering). The amplified and filtered nerve signal monitored the audio and visual quality of the SNA recordings and, for quantification purposes, through a speaker system and oscilloscope (model 54501A, Hewlett-Packard Co.). The amplified, filtered nerve signal was also directed to a MacLab analogdigital converter (model 8S, ADInstruments) containing software (MacLab Chart Pro; version 7.0) for recording and data analysis. A cursor to analyze the number of spikes per second that exceeded the background noise threshold was utilized. Any residual nerve activity after death was considered background noise and subtracted from the measured SNA.
SNA was determined over a 30-s range at three distinct time points during the control period and then every 5 min after the treatment until the end of the study. The values obtained during the control period were averaged, and percent change activity was calculated to be 0% for baseline control (time 0). The percent change (∆ in %) in SNA compared with basal control was calculated for each time point during the protocol.
Radiotelemetry measurements.
Arterial pressure, HR, and locomotor activity were recorded continuously using radiotelemetry probes (PA-C10, Data Science Instruments) as described previously (5). Under isoflurane anesthesia and aseptic surgical conditions, the catheter of the telemeter was inserted into the left carotid artery and tied securely using 6-0 silk suture. The transmitter was tunneled subcutaneously from the neck until the unit reached the midabdominal region. The neck incision was sutured closed with 4-0 absorbable cat gut and then further sealed with tissue adhesive (Vet-Bond) along the incision line.
Animals recovered for at least 10 days before arterial pressure, HR, and locomotor activity were recorded continuously in the conscious unrestrained state. To evaluate the acute effect of AgRP and POMC neuron activation, AgRPCre and POMCCre mice were handled and subjected to intraperitoneal injections daily before onset of the dark cycle (around 5:30 PM) for 7 days to reduce handling and injection-induced stress. At day 8, mice received saline (vehicle, ip) before the dark cycle, and blood pressure, HR, and locomotor activity were continuously monitored for 24 h. The next day, the mice were treated with CNO (1 mg/kg ip) before the dark cycle, and the measurement was performed again for 24 h. The responses were analyzed during the first 2 h (2-h period, which corresponded to the SNA measurements), the remaining dark period (10 h), and the light period (12 h).
After 3-days recovery from the acute experiments, baseline arterial pressure, HR, and locomotor activity were recorded for 36 h, and then mice were subcutaneously implanted with osmotic micropumps (Alzet Osmotic pumps 1007D), which infused 2 mg·kg−1·day−1 CNO to achieve slow delivery of CNO for chronic neuronal activation. Food intake, body weight, arterial pressure, HR, and locomotor activity were continuously monitored for an additional 13 days. Hemodynamic parameters were recorded for 10 s every 5 min and stored on a personal computer using Data Science Dataquest software.
Data analysis.
Results are shown as means ± SE. Data were analyzed using Student’s t test and one- or two-way analysis of variance with or without repeated measures. When analysis of variance reached significance, a post hoc comparison was made using Fisher’s test. A P < 0.05 was considered statistically significant.
RESULTS
Validation of DREADD-mediated activation of AgRP and POMC neurons.
To validate the effectiveness of DREADD activation of POMC and AgRP neurons, respectively, we performed stereotaxic microinfusion of AAV-hM3Dq-mCherry into the ARC of POMCCre and AgRPCre mice (Fig. 1, A and B). Two weeks after microinjection of AAV-hM3Dq-mCherry, mCherry expression was detected in the ARC of AgRPCre and POMCCre mice but not in WT littermate controls (Supplemental Fig. S1A; all supplemental material is available at https://doi.org/10.6084/m9.figshare.12425132.v1), indicating selective Cre-dependent expression of hM3Dq-mCherry. In addition, mCherry expression colocalized with AgRP in the ARC of AgRPCre mice (Supplemental Fig. S1B). Effective neuronal activation was confirmed using cFos, an established marker of neuronal activation. Indeed, intraperitoneal administration of CNO caused strong c-Fos induction in the ARC of POMCCre and AgRPCre mice relative to saline treatment (Fig. 1C). A clear overlap of cFos with mCherry was observed in both AgRPCre and POMCCre mice treated with CNO. These data showed the successful activation of AgRP and POMC neurons with Cre-dependent DREADD.
Fig. 1.
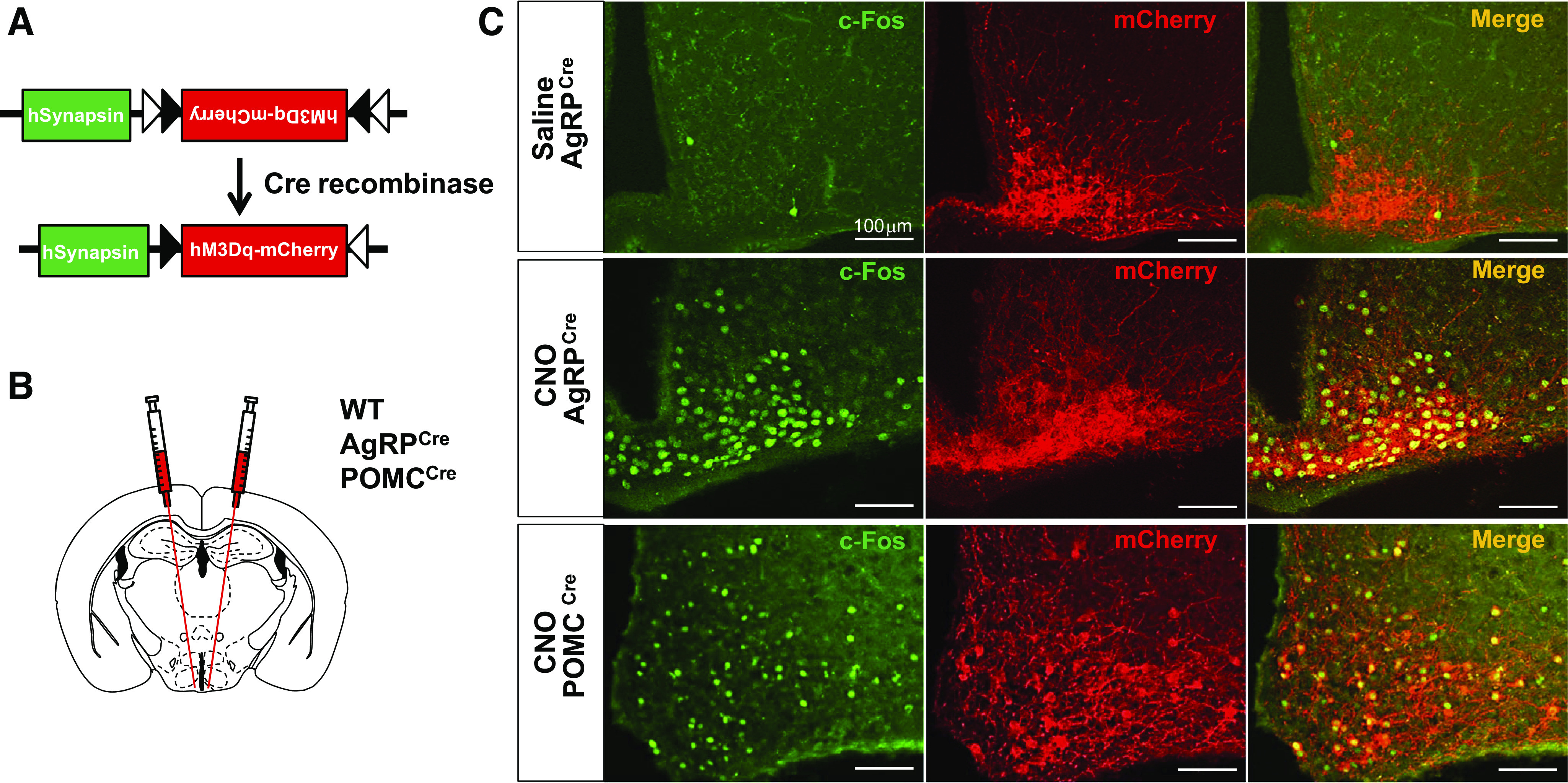
Efficacy of DREADD-mediated activation of ARC of AgRP and POMC neurons. A and B: schematics of the Cre-dependent hM3Dq-mCherry DREADD used (A) and stereotaxic targeted microinjection into hypothalamic ARC (B). C: representative images showing expression of mCherry and c-Fos in the ARC of AgRPCre (n = 13) and POMCCre (n = 9) mice treated intraperitoneally with CNO or vehicle. Scale bar, 100 µm. AgRP, agouti-related peptide; ARC, arcuate nucleus of the hypothalamus; POMC, proopiomelanocortin; CNO, clozapine-N-oxide; DREADD, designer receptor exclusively activated by designer drugs.
Metabolic effects of acute activation of AgRP and POMC neurons.
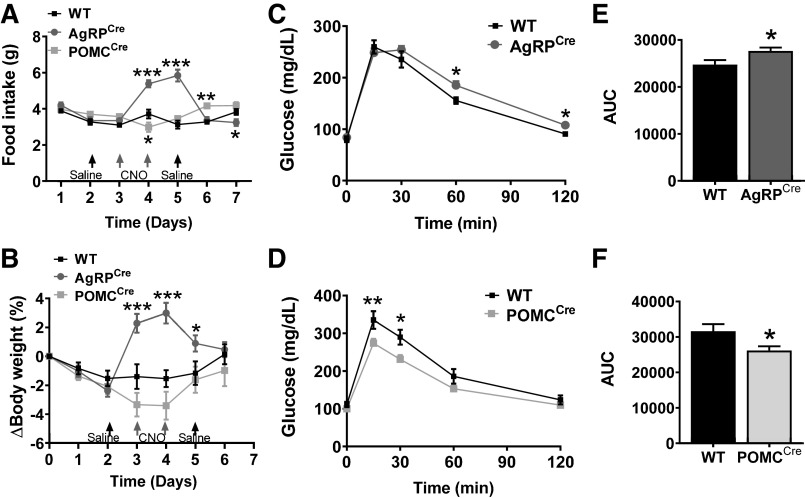
DREADD activation of AgRP neurons through consecutive 2-day intraperitoneal injection of CNO robustly increased food intake and body weight, whereas activation of POMC neurons caused a marginal decrease in food intake and body weight (Fig. 2, A and B). Additionally, we tested how acute activation of AgRP and POMC neurons affects glucose homeostasis. However, basal blood glucose levels did not change up to 2 h after a single dose of CNO in both AgRPCre and POMCCre mice compared with their weight-matched WT littermates (Supplemental Fig. S2, A and B). Next, we performed the GTT during POMC and AgRP neuronal activation, after 15-min treatment with CNO. Slight but significant impairment in glucose tolerance was noticed after AgRP neuron activation (Fig. 2, C and E). Conversely, POMC neuron stimulation improved glucose tolerance (Fig. 2, D and F). These results are consistent with the important role of AgRP and POMC neurons in metabolic regulation. These findings further confirm the effectiveness of Cre-dependent DREADD-induced selective activation of ARC AgRP and POMC neurons.
Fig. 2.
Metabolic effects of DREADD-mediated activation of POMC or AgRP neurons. A and B: food intake (A) and change in body weight (B) of WT (n = 10–12), AgRPCre (n = 13), and POMCCre mice (n = 9) subject to intraperitoneal CNO injection (once a day for 2 days). C–F: GTT in AgRPCre (C and E; n = 11), POMCCre (D and F; n = 6) and WT mice (n = 6–8) that received a single ip injection of CNO 15 min earlier (GTT results shown as AUC in E and F). *P < 0.05, **P < 0.01, and ***P < 0.001 (AgRPcre or POMCCre vs. WT) by two-way ANOVA with repeated measures. AgRP, agouti-related peptide; ARC, arcuate nucleus of the hypothalamus; POMC, proopiomelanocortin; CNO, clozapine-N-oxide; DREADD, designer receptor exclusively activated by designer drugs; GTT, glucose tolerance test; WT, wild type; AU, arbitrary units. Data are presented as means ± SE.
Selective activation of AgRP, but not POMC, neurons alters renal SNA.
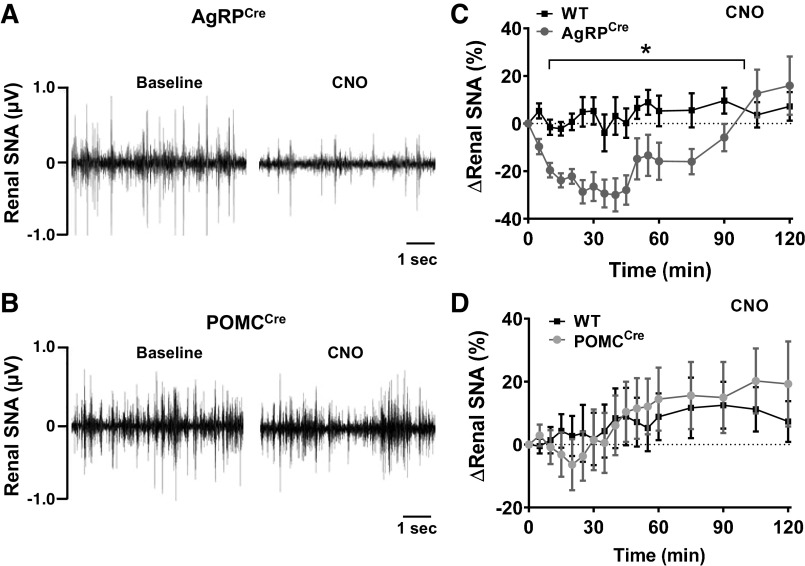
To test the effect of activating AgRP or POMC neurons on sympathetic nerve traffic, direct multifiber recording of SNA subserving the kidney was performed in conscious mice followed by activation of AgRP or POMC neurons by CNO. A single intravenous injection of CNO induced a significant reduction in renal SNA in conscious AgRPCre mice (Fig. 3, A and C). Compared with WT mice, renal SNA in AgRPCre mice started to decline immediately upon CNO injection. Renal SNA reached a peak at 30 min postinjection and then gradually recovered to baseline level at the end of the recording. Surprisingly, DREADD-mediated activation of POMC neurons did not alter renal SNA (Fig. 3, B and D). Renal SNA was not affected by saline injection in any of the groups tested (Supplemental Fig. S3, A and B).
Fig. 3.
Activation of AgRP and POMC neurons differentially affect renal SNA. A and B: representative neurograms showing the effect of CNO (1 mg/kg iv) on renal SNA in conscious AgRPCre (A) and POMCCre mice (B). C and D: effect of CNO on renal SNA in AgRPCre (C; n = 13) and POMCCre mice (D; n = 9) relative to their littermate WT controls (n = 10–12). *P < 0.001 (AgRPCre vs. WT) by two-way ANOVA with repeated measures. AgRP, agouti-related peptide; POMC, proopiomelanocortin; CNO, clozapine-N-oxide; SNA, sympathetic nerve activity; WT, wild type. Data are presented as means ± SE.
Acute activation of AgRP, but not POMC, neurons decreases locomotor activity and HR.
To assess how DREADD-evoked activation of AgRP neurons affects cardiovascular function, we assessed the effect of a single intraperitoneal injection of CNO, administered right before the start of the dark cycle, on 24-h mean arterial pressure (MAP), HR, and locomotor activity measured by telemetry. Locomotor activity was significantly decreased by activation of AgRP neurons during the 2-h period after CNO injection (Fig. 4A). This effect was sustained throughout the remaining 10 h of the dark period. However, no difference was noticed between the groups during the light phase. HR was also significantly decreased, but only during the 10-h dark period (Fig. 4B). Interestingly, MAP was not affected by CNO injection at any given time interval analyzed (Fig. 4C). Consistent with this, systolic and diastolic arterial pressures were not altered by DREADD-mediated activation of AgRP neurons (Supplemental Fig. S4, A and B).
Fig. 4.
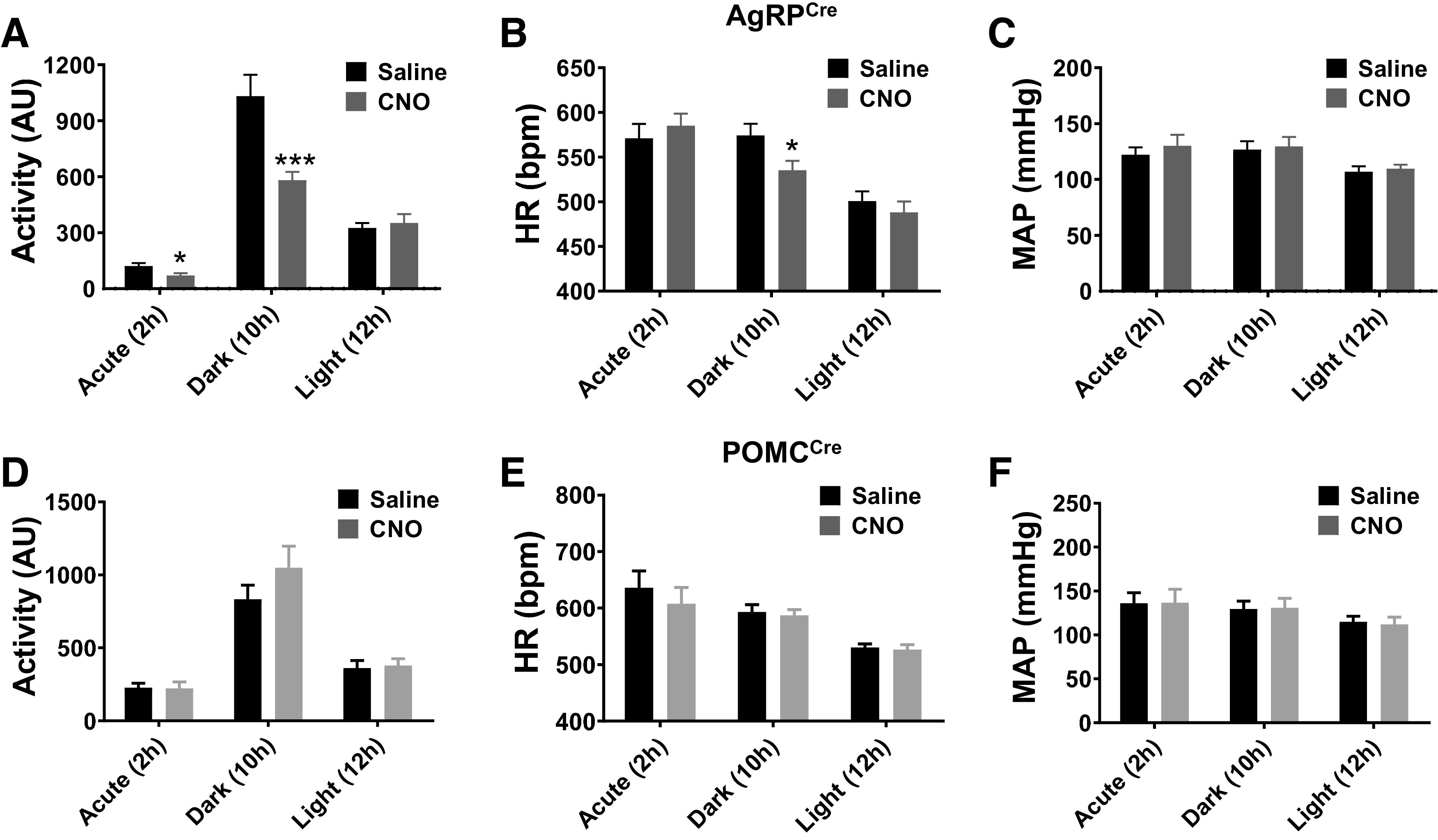
Impact of acute AgRP and POMC neuron activation on locomotor activity, HR, and MAP. Locomotor activity (A and D), HR (Band E), and MAP (C and F) responses to a single dose of vehicle or CNO (1 mg/kg ip) injection, at beginning of the dark period, in AgRPCre (A–C; n = 7) and POMCCre mice (D–F; n = 7). Responses were divided into 3 phases: acute 2 h during dark period (Acute-2h), following 10 h dark period (Dark-10h), and last 12-h light period (Light-12h). *P < 0.05, ***P < 0.001 (CNO vs. saline) by Student’s t test. AgRP, agouti-related peptide; POMC, proopiomelanocortin; CNO, clozapine-N-oxide; HR, heart rate; ip, intraperitoneal; MAP, mean arterial pressure; AU, arbitrary units. Data are presented as means ± SE.
We also evaluated the effect of acute activation of POMC neurons on locomotor activity, MAP, and HR. However, no significant effect was observed for these parameters at any time period following DREADD-mediated activation of POMC neurons (Fig. 4, D–F, and Supplemental Fig. S4, C and D).
Metabolic effects of chronic AgRP and POMC neuron stimulation.
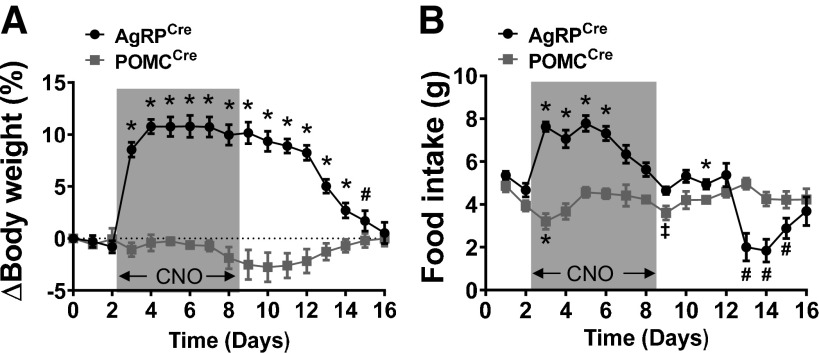
The effect of chronic activation of AgRP and POMC neurons was evaluated by using subcutaneous osmotic minipumps to continuously infuse CNO (2 mg·kg−1·day−1) for up to 6 days (predicted from manufacturer’s instructions). Daily food intake and body weight were continuously monitored during CNO infusion and for an additional 8 days after the expected depletion of osmotic minipump CNO. Activation of AgRP neurons caused a rapid and robust weight gain (∼10%) occurring during the first 2 days of CNO infusion (Fig. 5A). This weight gain was likely due, at least in part, to the dramatic increase in food intake (Fig. 5B). Body weight of AgRPCre mice that received CNO decreased steadily after day 8 and returned to baseline value at day 16.
Fig. 5.
Effects of chronic AgRP and POMC neuron activation on body weight and food intake. Body weight change (A) and food intake (B) of AgRPCre (n = 7) and POMCCre mice (n = 6) treated chronically with CNO (2 mg·kg−1·day−1 via osmotic micropump during the period highlighted in gray) and during recovery (after CNO infusion, days 9–16). AgRP, agouti-related peptide; POMC, proopiomelanocortin; CNO, clozapine-N-oxide. ‡P < 0.05; #P < 0.01; *P < 0.001 vs. baseline (day 0) by Student’s t test. Data are presented as means ± SE.
Activation of POMC neurons produced a marginal body weight reduction particularly around the end of CNO infusion, which returned to baseline level by the end of the study (Fig. 5A). No obvious reduction in food intake was noticed when POMC neurons were chronically activated with CNO (Fig. 5B).
Cardiovascular effects of chronic activation of AgRP and POMC neurons.
Next, we measured MAP, HR, and locomotor activity during chronic DREADD-mediated stimulation of AgRP and POMC neurons as above. Chronic activation of AgRP neurons dramatically decreased locomotor activity especially during the dark period, beginning at the first day of CNO infusion and returning gradually back to baseline level within next 5 days (Fig. 6A and Supplemental Fig. S5A). However, there was no significant change in locomotor activity during the light cycle. Interestingly, MAP was significantly increased during the light cycle period after CNO infusion and then gradually returned to baseline levels (Fig. 6B and Supplemental Fig. S5B). During the dark cycle, MAP trended to decrease during the first day of CNO treatment followed by no significant change throughout the remaining experimental period. Similar effects on systolic and diastolic arterial pressure were evoked by DREADD-mediated activation of AgRP neurons (Supplemental Fig. S6, A and B). HR transiently increased in both dark and light cycles during the first 3 days of CNO infusion and then returned to baseline level (Fig. 6C and Supplemental Fig. S5C).
Fig. 6.
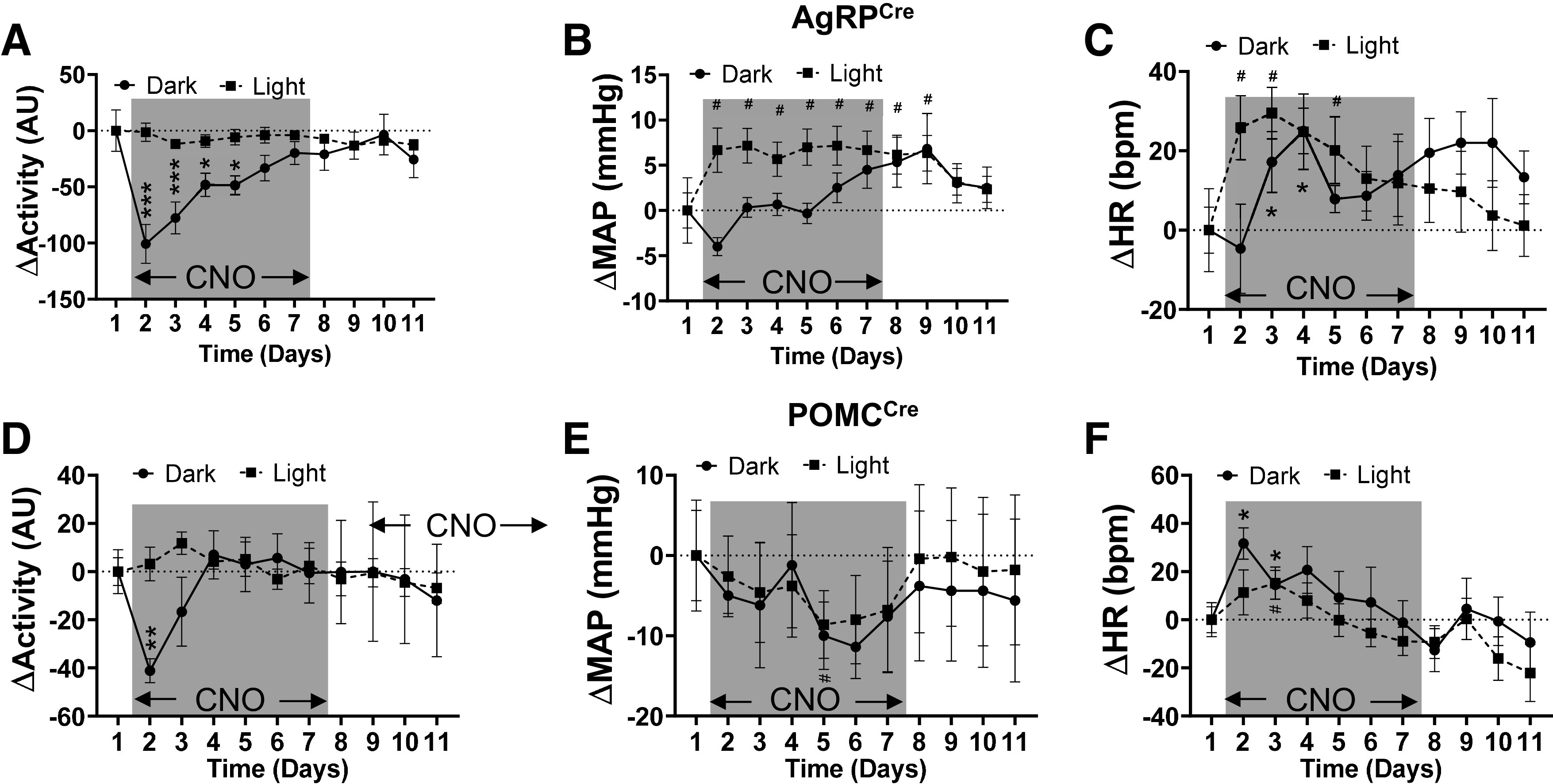
Effects of chronic AgRP and POMC neuron activation on locomotor activity, MAP, and HR. Changes in locomotor activity (A and D), MAP (B and E), and HR (C and F) during light and dark phases in AgRPCre (A–C; n = 6) and POMCCre mice (D–F; n = 6) during chronic infusion of CNO (2 mg·kg−1·day−1 via osmotic micropump during the period highlighted in gray) and during recovery (after CNO infusion, days 9–11). AgRP, agouti-related peptide; ARC, arcuate nucleus of the hypothalamus; POMC, proopiomelanocortin; CNO, clozapine-N-oxide; HR, heart rate; MAP, mean arterial pressure; AU, arbitrary units. *P < 0.05, **P < 0.01, ***P < 0.001 at indicated day vs. baseline (D1) during dark phase; #P < 0.05 at indicated day vs. baseline (D1) during the light phase, by Student’s t test. Data are presented as means ± SE.
POMC activation transiently decreased locomotor activity during the dark cycle of the first day of CNO infusion and then quickly recovered without significant change in the light cycle (Fig. 6D and Supplemental Fig. S5D). Surprisingly, MAP gradually decreased throughout the CNO infusion period during both dark and light cycles (Fig. 6E and Supplemental Fig. S5E). Similar effects on systolic and diastolic arterial pressure were evoked by DREADD-mediated activation of POMC neurons (Supplemental Fig. S6, C and D). However, HR remained largely unchanged except for a slight, but significant, increase in the dark cycle during the first days of CNO infusion (Fig. 6F and Supplemental Fig. S5F).
DISCUSSION
Using DREADD, a chemogenetic approach, we demonstrate that ARC AgRP and POMC neurons differentially regulate metabolism, renal SNA, and cardiovascular function. We show that activation of AgRP and POMC neurons cause contrasting changes in food intake, body weight, and glucose tolerance. Interestingly, activation of AgRP neurons, but not POMC neurons, induces acute reduction in renal SNA. Moreover, chronic activation of AgRP neurons decreases locomotor activity during the dark cycle and increases HR and blood pressure mainly during the light cycle, whereas chronic activation of POMC neurons reduces locomotor activity during the dark cycle and HR in both light and dark cycles without significant change in blood pressure. These results shed new light on circadian-specific roles of ARC AgRP and POMC neurons in the differential regulation of autonomic and cardiovascular functions.
AgRP and POMC neurons are two major ARC neuronal populations that play an important role in metabolic homeostasis (1, 14, 21, 37). Although a previous study has shown that chronic, but not acute, DREADD-mediated POMC neuronal activation tends to decrease food intake and body weight (45), we observed a minimal effect on food intake and body weight when POMC neurons were activated by excitatory DREADD through either daily systemic injection or chronic continuous osmotic CNO delivery. Although the cause of this discrepancy is unclear, variations in the dose and the injection timing and frequency of CNO could account for the differences. It should be noted that we were able to confirm the induction of c-Fos in POMC neurons upon DREADD activation and observed an improved glucose tolerance in mice with DREADD activation of POMC neurons. These data demonstrate the efficacy of DREADD activation of POMC neurons.
Sympathetic premotor neurons located in the lower brain stem and hypothalamus are important mediators in connecting peripheral metabolic signals to the autonomic nervous system. Physiologically, energy depletion and hunger increase and decrease the activity of AgRP and POMC neurons, respectively, and suppress SNA (27, 38, 39). These effects can be reversed by ingestion of food and postprandial rise in glycemia (31, 44). Consistent with this notion, we observed that DREADD-mediated activation of AgRP neurons suppressed renal SNA, which is also in line with a prior study showing that DREADD stimulation of AgRP neurons suppressed SNA subserving brown adipose tissue (37) and the splanchnic bed (45). Strikingly, however, we did not observe any change in renal SNA after DREADD-mediated activation of POMC neurons. This is surprising, given the well-known sympathoexcitatory and pressor effects induced by activation of MC4R, a downstream effector of POMC neurons (13, 17, 33). Of note, we have previously shown that DREADD-mediated POMC neuron activation increases hepatic SNA in mice (6). Together, these findings suggest that POMC neuron activity is differentially involved in the control of regional SNA.
ARC neurons play an important role in mediating the sympathoexcitatory effects of circulating peripheral metabolic hormones such as leptin and insulin (32). Leptin is known to inhibit and activate AgRP and POMC neurons, respectively (2, 10). We previously demonstrated an important role of AgRP and POMC neurons in mediating leptin regulation of the activity of the autonomic nervous system (4, 19). For example, deletion of leptin receptor (LepR) specifically in POMC, but not in AgRP neurons, significantly blunted the leptin-evoked renal, lumbar, and splanchnic SNA. Given that leptin activates POMC neurons and increases renal SNA, it is puzzling that DREADD-mediated activation of this neuronal population did not mimic leptin’s action to increase renal SNA. Possible explanations include 1) the slow increase in SNA evoked by leptin (4), suggesting that this process may requires activation of specific intracellular signaling cascades and subsequent transcriptional and translational processes that may not take place during DREADD-mediated neuronal activation, or 2) the changes of firing pattern (spike frequency and/or amplitude) of POMC neurons triggered by systemic leptin and DREADD could be different, leading to a distinct releasing pattern by POMC neurons of neurotransmitters and neuropeptides such as α-MSH, β-endorphin, adrenocorticotropic hormone, cocaine- and amphetamine-regulated transcript, γ-aminobutyric acid, and glutamate. The latter explanation is especially attractive because it has been shown that different neuropeptides released from POMC neurons trigger different, sometime even opposite, physiological responses (41). However, very little is known about the mechanisms underlying this differential release of neuropeptides from POMC neurons. This represents an important area of research that warrants further investigation.
ARC neurons directly innervate the brain regions that regulate cardiovascular function (23, 28). In contrast to our expectation, we found that chronic, but not acute, activation of POMC neurons tended to decrease blood pressure in both light and dark cycles with transient HR increase during the dark cycle without significant changes in body weight, whereas AgRP neuron activation led to a light cycle-specific increase in blood pressure and HR despite the drastic reduction in locomotor activity. This pressor effect induced by AgRP neuron activation seems independent of weight gain, as blood pressure returned to baseline levels by the 11th day of CNO infusion whereas body weight remained elevated. However, we cannot exclude the possibility that the phase-specific changes in MAP and HR are compensating for decreased dark phase activity. This is unlikely, because changes in MAP and HR generally follow the activity status, and there is no prior literature supporting the idea that circadian change of activity will cause phasic delayed compensatory changes in MAP and HR. Interestingly, the increase in MAP and HR evoked by activation of AgRP neurons in the situation of hunger during the night despite the decrease in activity may indicate the cardiovascular side effect of AgRP activation during circadian cycle. In contrast, the increase in HR after POMC neuron activation is within the range of the physiological compensatory response to a decrease in activity and MAP.
Our observations challenge the generally held view that activity of POMC and AgRP neurons causes contrasting changes in blood pressure based on the antagonistic nature of the neuropeptides (α-MSH and AgRP) that these two neuronal populations produce and release (15, 23). It should be noted, however, that, in addition to antagonist (AgRP) and agonist (α-MSH) of MC4R, AgRP and POMC neurons corelease different neuropeptides and neurotransmitters that could affect blood pressure. For instance, it is known that nearly all AgRP neurons contain neuropeptide Y, that administration of neuropeptide Y can increase blood pressure (35, 40), and that POMC neurons produce and release endogenous opioid β-endorphin, which has been shown to decrease systemic vascular resistance and reduce blood pressure via opioid receptors (11). Future studies are needed to clarify the underlying mechanisms by which chronic DREADD activation of AgRP and POMC neurons increases and decreases blood pressure, respectively.
The use of CNO for DREADD technology has been criticized owing to its potential conversion in vivo to clozapine (16), an atypical antipsychotic that has significant impact on brain monoamine neurotransmission. However, we previously demonstrated that CNO has no effect on SNA in WT mice (37). In addition, in the present study we show that activation of AgRP and POMC neurons by CNO mostly produces opposite responses based on the many parameters measured, including glucose tolerance, renal SNA, and blood pressure. This indicates that the physiological responses we observe are evoked by CNO-induced neuronal activation rather than CNO derivatives. One exception is the locomotor activity that is consistently decreased in the first day of osmotic CNO delivery in both AgRPCre and POMCCre mice. Although previous literature indicates that CNO alone has no profound effect on locomotor activity (26, 43), we cannot fully rule out the possibility that decreased locomotor activity in both AgRP and POMC neuron activation is due to CNO itself or one of its derivatives rather than to CNO-mediated neuronal excitation. This is supported by our previous study showing that CNO decreased locomotor activity in WT mice (37). However, the degree and the time course of suppressed locomotor activity seem to be greater and longer in AgRPCre mice compared with POMCCre mice (Fig. 6, A and D), which is indicative of a locomotion-suppressing effect of AgRP neuronal activation. Additional studies are needed to assess further the role of AgRP neurons in the control of locomotor activity.
Perspectives and significance.
Our data show that acute and chronic chemogenetic activation of ARC AgRP and POMC neurons differentially affects metabolism, renal SNA, and blood pressure. Because the ARC is a key node in relaying peripheral metabolic signals to the brain to coordinate a wide range of physiological and behavioral adaptations, a better understanding of these circuits may help develop novel strategies to combat metabolic diseases and associated cardiovascular complications.
GRANTS
This work was supported by National Institutes of Health Grants 5T32 DK-112751 and 5T32 HL-007344 (to J.J.), HL-127673 and MH-109920 (to H.C.), and HL-084207 (to H.C. and K.R.); Department of Veterans Affairs Grant BX004249 (to K.R.); the University of Iowa Fraternal Order of Eagles Diabetes Research Center; and the Iowa Neuroscience Institute.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.J., H.C. and K.R. conceived and designed research; J.J., D.A.M. and H.C. performed experiments; J.J., D.A.M., H.C. and K.R. analyzed data; J.J., H.C. and K.R. interpreted results of experiments; J.J., D.A.M., H.C. and K.R. prepared figures; J.J. and H.C. drafted manuscript; J.J., D.A.M., H.C. and K.R. edited and revised manuscript; J.J., D.A.M., H.C. and K.R. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Jessica R. Thompson and Clay Rosinski for technical assistance. We also thank Paul J. Casella for editorial assistance.
REFERENCES
- 1.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14: 351–355, 2011. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baver SB, Hope K, Guyot S, Bjørbaek C, Kaczorowski C, O’Connell KM. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci 34: 5486–5496, 2014. doi: 10.1523/JNEUROSCI.4861-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belgardt BF, Okamura T, Brüning JC. Hormone and glucose signalling in POMC and AgRP neurons. J Physiol 587: 5305–5314, 2009. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bell BB, Harlan SM, Morgan DA, Guo DF, Cui H, Rahmouni K. Differential contribution of POMC and AgRP neurons to the regulation of regional autonomic nerve activity by leptin. Mol Metab 8: 1–12, 2018. [Erratum in Mol Metab 14: 158, 2018]. doi: 10.1016/j.molmet.2017.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beyer AM, Guo DF, Rahmouni K. Prolonged treatment with angiotensin 1-7 improves endothelial function in diet-induced obesity. J Hypertens 31: 730–738, 2013. doi: 10.1097/HJH.0b013e32835ecbe5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brandt C, Nolte H, Henschke S, Engström Ruud L, Awazawa M, Morgan DA, Gabel P, Sprenger HG, Hess ME, Günther S, Langer T, Rahmouni K, Fenselau H, Krüger M, Brüning JC. food perception primes hepatic er homeostasis via melanocortin-dependent control of mTOR activation. Cell 175: 1321–1335, 2018. doi: 10.1016/j.cell.2018.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brody MJ. Central nervous system mechanisms of arterial pressure regulation. Fed Proc 45: 2700–2706, 1986. [PubMed] [Google Scholar]
- 8.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 589: 1643–1662, 2011. doi: 10.1113/jphysiol.2011.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci 8: 571–578, 2005. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 10.Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411: 480–484, 2001. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- 11.Cozzolino D, Sasso FC, Cataldo D, Gruosso D, Giammarco A, Cavalli A, Di Maggio C, Renzo G, Salvatore T, Giugliano D, Torella R. Acute pressor and hormonal effects of β-endorphin at high doses in healthy and hypertensive subjects: role of opioid receptor agonism. J Clin Endocrinol Metab 90: 5167–5174, 2005. doi: 10.1210/jc.2004-2554. [DOI] [PubMed] [Google Scholar]
- 12.Cui H, Sohn JW, Gautron L, Funahashi H, Williams KW, Elmquist JK, Lutter M. Neuroanatomy of melanocortin-4 receptor pathway in the lateral hypothalamic area. J Comp Neurol 520: 4168–4183, 2012. doi: 10.1002/cne.23145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.da Silva AA, do Carmo JM, Wang Z, Hall JE. Melanocortin-4 receptors and sympathetic nervous system activation in hypertension. Curr Hypertens Rep 21: 46, 2019. doi: 10.1007/s11906-019-0951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodd GT, Michael NJ, Lee-Young RS, Mangiafico SP, Pryor JT, Munder AC, Simonds SE, Brüning JC, Zhang ZY, Cowley MA, Andrikopoulos S, Horvath TL, Spanswick D, Tiganis T. Insulin regulates POMC neuronal plasticity to control glucose metabolism. eLife 7: e3870, 2018. doi: 10.7554/eLife.38704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunbar JC, Lu H. Proopiomelanocortin (POMC) products in the central regulation of sympathetic and cardiovascular dynamics: studies on melanocortin and opioid interactions. Peptides 21: 211–217, 2000. doi: 10.1016/S0196-9781(99)00192-8. [DOI] [PubMed] [Google Scholar]
- 16.Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M. Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357: 503–507, 2017. doi: 10.1126/science.aan2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, Astruc B, Mayer JP, Brage S, See TC, Lomas DJ, O’Rahilly S, Farooqi IS. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med 360: 44–52, 2009. doi: 10.1056/NEJMoa0803085. [DOI] [PubMed] [Google Scholar]
- 18.Guo DF, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, Grobe JL, Sheffield VC, Rahmouni K. The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet 12: e1005890, 2016. doi: 10.1371/journal.pgen.1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ Res 108: 808–812, 2011. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre neurons in the arcuate nucleus selectively regulate energy expenditure. Cell 151: 645–657, 2012. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121: 1424–1428, 2011. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krashes MJ, Shah BP, Madara JC, Olson DP, Strochlic DE, Garfield AS, Vong L, Pei H, Watabe-Uchida M, Uchida N, Liberles SD, Lowell BB. An excitatory paraventricular nucleus to AgRP neuron circuit that drives hunger. Nature 507: 238–242, 2014. doi: 10.1038/nature12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SJ, Varga K, Archer P, Hruby VJ, Sharma SD, Kesterson RA, Cone RD, Kunos G. Melanocortin antagonists define two distinct pathways of cardiovascular control by alpha- and gamma-melanocyte-stimulating hormones. J Neurosci 16: 5182–5188, 1996. doi: 10.1523/JNEUROSCI.16-16-05182.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li YW, Dampney RA. Expression of Fos-like protein in brain following sustained hypertension and hypotension in conscious rabbits. Neuroscience 61: 613–634, 1994. doi: 10.1016/0306-4522(94)90439-1. [DOI] [PubMed] [Google Scholar]
- 25.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310: 683–685, 2005. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 26.MacLaren DA, Browne RW, Shaw JK, Krishnan Radhakrishnan S, Khare P, España RA, Clark SD. Clozapine N-oxide administration produces behavioral effects in Long-Evans rats: implications for designing DREADD experiments. eNeuro 3: ENEURO.0219-16.2016, 2016. doi: 10.1523/ENEURO.0219-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mandelblat-Cerf Y, Ramesh RN, Burgess CR, Patella P, Yang Z, Lowell BB, Andermann ML. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. eLife 4: e07122, 2015. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mastrianni JA, Palkovits M, Kunos G. Activation of brainstem endorphinergic neurons causes cardiovascular depression and facilitates baroreflex bradycardia. Neuroscience 33: 559–566, 1989. doi: 10.1016/0306-4522(89)90408-9. [DOI] [PubMed] [Google Scholar]
- 29.Morgan DA, Despas F, Rahmouni K. Effects of leptin on sympathetic nerve activity in conscious mice. Physiol Rep 3: e12554, 2015. doi: 10.14814/phy2.12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan DA, McDaniel LN, Yin T, Khan M, Jiang J, Acevedo MR, Walsh SA, Ponto LL, Norris AW, Lutter M, Rahmouni K, Cui H. Regulation of glucose tolerance and sympathetic activity by MC4R signaling in the lateral hypothalamus. Diabetes 64: 1976–1987, 2015. doi: 10.2337/db14-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449: 228–232, 2007. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 32.Rahmouni K. Leptin-induced sympathetic nerve activation: signaling mechanisms and cardiovascular consequences in obesity. Curr Hypertens Rev 6: 104–209, 2010. doi: 10.2174/157340210791170994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rahmouni K, Haynes WG, Morgan DA, Mark AL. Role of melanocortin-4 receptors in mediating renal sympathoactivation to leptin and insulin. J Neurosci 23: 5998–6004, 2003. doi: 10.1523/JNEUROSCI.23-14-05998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sapru HN. Role of the hypothalamic arcuate nucleus in cardiovascular regulation. Auton Neurosci 175: 38–50, 2013. doi: 10.1016/j.autneu.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi Z, Madden CJ, Brooks VL. Arcuate neuropeptide Y inhibits sympathetic nerve activity via multiple neuropathways. J Clin Invest 127: 2868–2880, 2017. doi: 10.1172/JCI92008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Solano-Flores LP, Rosas-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal nerve stimulation. Brain Res 753: 102–119, 1997. doi: 10.1016/S0006-8993(96)01497-7. [DOI] [PubMed] [Google Scholar]
- 37.Steculorum SM, Ruud J, Karakasilioti I, Backes H, Engström Ruud L, Timper K, Hess ME, Tsaousidou E, Mauer J, Vogt MC, Paeger L, Bremser S, Klein AC, Morgan DA, Frommolt P, Brinkkötter PT, Hammerschmidt P, Benzing T, Rahmouni K, Wunderlich FT, Kloppenburg P, Brüning JC. AgRP neurons control systemic insulin sensitivity via myostatin expression in brown adipose tissue. Cell 165: 125–138, 2016. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Su Z, Alhadeff AL, Betley JN. Nutritive, post-ingestive signals are the primary regulators of AgRP neuron activity. Cell Rep 21: 2724–2736, 2017. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology 146: 1043–1047, 2005. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 40.Tan CMJ, Green P, Tapoulal N, Lewandowski AJ, Leeson P, Herring N. The role of neuropeptide Y in cardiovascular health and disease. Front Physiol 9: 1281, 2018. doi: 10.3389/fphys.2018.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toda C, Santoro A, Kim JD, Diano S. POMC neurons: from birth to death. Annu Rev Physiol 79: 209–236, 2017. doi: 10.1146/annurev-physiol-022516-034110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Varela L, Horvath TL. Leptin and insulin pathways in POMC and AgRP neurons that modulate energy balance and glucose homeostasis. EMBO Rep 13: 1079–1086, 2012. doi: 10.1038/embor.2012.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vazey EM, Aston-Jones G. Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci USA 111: 3859–3864, 2014. doi: 10.1073/pnas.1310025111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Williams KW, Elmquist JK. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat Neurosci 15: 1350–1355, 2012. doi: 10.1038/nn.3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhan C, Zhou J, Feng Q, Zhang JE, Lin S, Bao J, Wu P, Luo M. Acute and long-term suppression of feeding behavior by POMC neurons in the brainstem and hypothalamus, respectively. J Neurosci 33: 3624–3632, 2013. doi: 10.1523/JNEUROSCI.2742-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]