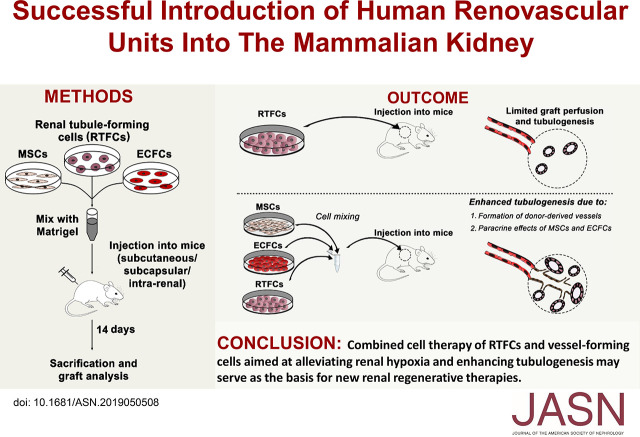
Significance Statement
Most cell-based renal regenerative strategies are limited by an inability to generate donor-derived vascular networks upon in vivo transplantation, which is especially problematic in hypoxic CKD kidneys. The authors report that coadministering human renal tubule–forming cells and vessel-forming cells (mesenchymal stromal cells and endothelial colony-forming cells) into mice generates in vivo vascularized grafts comprising renal tubules of different nephron segments and donor-derived vessels connecting to host vasculature. The vessel-forming cells enhanced tubulogenic capacity of renal tubule–forming cells by improving graft perfusion and by inducing a protubulogenic state via paracrine mechanisms. These effects occurred with injection of cells into either the subcapsular renal or intraparenchymatic space. The findings suggest that augmenting the regenerative potential of renal cell–based methods through use of vessel-forming cells hold promise.
Keywords: renal stem cell, vascular endothelial growth factor, renal tubular epithelial cells, kidney spheres, organoids, mesenchymal stem cell (MSC)
Visual Abstract
Abstract
Background
Cell-based therapies aimed at replenishing renal parenchyma have been proposed as an approach for treating CKD. However, pathogenic mechanisms involved in CKD such as renal hypoxia result in loss of kidney function and limit engraftment and therapeutic effects of renal epithelial progenitors. Jointly administering vessel-forming cells (human mesenchymal stromal cells [MSCs] and endothelial colony-forming cells [ECFCs]) may potentially result in in vivo formation of vascular networks.
Methods
We administered renal tubule–forming cells derived from human adult and fetal kidneys (previously shown to exert a functional effect in CKD mice) into mice, alongside MSCs and ECFCs. We then assessed whether this would result in generation of “renovascular units” comprising both vessels and tubules with potential interaction.
Results
Directly injecting vessel-forming cells and renal tubule–forming cells into the subcutaneous and subrenal capsular space resulted in self-organization of donor-derived vascular networks that connected to host vasculature, alongside renal tubules comprising tubular epithelia of different nephron segments. Vessels derived from MSCs and ECFCs augmented in vivo tubulogenesis by the renal tubule–forming cells. In vitro coculture experiments showed that MSCs and ECFCs induced self-renewal and genes associated with mesenchymal–epithelial transition in renal tubule–forming cells, indicating paracrine effects. Notably, after renal injury, renal tubule–forming cells and vessel-forming cells infused into the renal artery did not penetrate the renal vascular network to generate vessels; only administering them into the kidney parenchyma resulted in similar generation of human renovascular units in vivo.
Conclusions
Combined cell therapy of vessel-forming cells and renal tubule–forming cells aimed at alleviating renal hypoxia and enhancing tubulogenesis holds promise as the basis for new renal regenerative therapies.
CKD is a growing global problem, with an estimated incidence of 11%–13%.1–3 The lack of true regenerative potential of the postnatal kidney (i.e., generation of new nephrons)4 makes CKD a progressive disease, relentlessly advancing toward ESKD.5,6 Currently, patients with ESKD can only be offered one of two therapeutic options: renal transplantation or dialysis. However, transplantation is limited by shortage of donor organs and entails lifelong immunosuppression, whereas dialysis severely compromises the patient’s quality of life. These shortcomings have prompted the search for cell-based therapies that can replenish damaged renal parenchyma with new nephrons.
In recent years, several cell-based strategies have been proposed as a means to regenerate damaged kidneys.3 These include transplantation of whole embryonic kidney grafts,7 isolation of progenitor populations from human fetal kidneys (hFKs),8–11 differentiation of pluripotent stem cells into renal progenitors,12–14 and modification of human adult kidney (hAK) cells into a regenerative state.15–17 For instance, we recently showed that hAK epithelial cells derived from both normal and ESKD kidneys can be grown as three-dimensional nephrospheres (nSPHs) that, upon transplantation into NOD-SCID mice, demonstrate long-term engraftment, form renal tubules, and improve kidney function in CKD mice.17 Although the significant progress in generating nephron components in vivo (e.g., glomeruli and tubules) is a major step toward regenerating damaged kidneys, CKD is a complex process, involving other pathogenic processes besides nephron loss. Among these, one of the most important is renal hypoxia, which is closely related to capillary rarefaction, and considered a final common pathway in the progression to ESKD.18 Specifically, decreased expression of VEGF, a key regulator of angiogenesis, mediates capillary rarefaction and hypoxia via impaired angiogenesis.19 Importantly, in addition to its cardinal role in CKD progression, renal hypoxia also represents a major impediment to most cell-based strategies aimed at generating new tubular units, by limiting the survival of donor renal tubule–forming cells (RTFCs),20 which require timely development of vascular networks to efficiently engraft and differentiate. Moreover, not only does the hypoxic environment typical of CKD limit RTFC survival, but it has been previously shown that most renal cell–based regenerative strategies (e.g., renal organoids) fail to develop donor-derived vessels and are only capable of recruiting host vessels to a certain extent, and are thus dependent on the poor vascularization of the host. Indeed, a key determinant of the regenerative effect exerted by nSPHs was the long-term survival of transplanted human cells within the host tissue in order to allow the formation of tubules, and consequently, improvement in renal function.17 Hence, addressing renal hypoxia is likely to be a crucial step in any cell-based regenerative strategy, by facilitating regeneration both directly (by preventing hypoxia) and indirectly (by allowing RTFCs to survive, integrate, and differentiate).
Endothelial colony-forming cells (ECFCs) and multipotent mesenchymal stromal cells (MSCs) have been proposed as effective cell types in promoting renal angiogenesis.21,22 ECFCs have potent angiogenic capacity and are capable of contributing to the repair of injured endothelium, as well as de novo vessel formation.23–25 In the context of renal injury, ECFCs have been shown to exert a therapeutic effect in AKI.21 MSCs promote regeneration of various tissues, including kidneys, via a myriad of paracrine mechanisms,26 including promotion of angiogenesis, apoptosis inhibition, and reduction of inflammation.27–29 These effects are mediated by various secreted factors, including VEGF.28,30,31 Of note, coadministration of ECFCs and MSCs results in the formation of stable vascular networks in vivo.32 These networks can be transplanted to other sites, demonstrating that the nascent vessels can reconnect with neighboring vasculature.33 MSCs also stabilize newly formed vessels in vivo by functioning as perivascular cells, resulting in the formation of long-lasting vessels.34 Notably, despite their well established renoprotective effect, and contrary to early reports,35,36 ECFCs and MSCs do not differentiate into tubular epithelial cells.37 Indeed, lineage restriction among vascular, tubular, and mesenchymal cell types is the rule in the adult kidney.4
Herein, we hypothesized that the survival and tubulogenic potential of administered human RTFCs could be augmented by injecting them alongside MSCs and ECFCs, speculating that the latter would form donor-derived vessels that would feed the graft and improve tubulogenesis. We show that upon in vivo injection into immunodeficient mice, this cell combination forms “renovascular units,” consisting of well developed donor-derived vessels that connect to the host vasculature, as well as renal tubules of different nephron segments. Importantly, we demonstrate that the presence of accompanying donor-derived vessels in these units results in enhanced tubulogenesis by the RTFCs. Notably, vascular infusion of cells precludes the generation of renal vascular units, as cells fail to penetrate the vascular wall. These results demonstrate the importance of achieving vascularization in protocols using combined renal regenerative strategies.
Methods
hFKs and hAKs
hFKs were collected from elective abortions. Fetal gestational age ranged from 15 to 19 weeks. hAK samples were retrieved from borders of renal cell carcinoma tumors from patients who underwent partial nephrectomy. Fetal and adult kidney tissues were handled within 1 hour after the curettage or nephrectomy procedures, respectively. Collected tissues were washed with cold HBSS (Invitrogen, Carlsbad, CA) and cut into 0.5 cm cubes by sterile surgical scalpels. hAK and hFK cells were grown as previously described.8,38,39 All studies were approved by the local ethical committee and informed consent was given by the legal guardians of the patients involved, according to the Declaration of Helsinki.
Isolation, Culture, and Characterization of Human MSCs and ECFCs
ECFCs were isolated as previously described.40 Briefly, human umbilical cord blood mononuclear cells (MNCs) were collected and plated onto 1% gelatin‐coated plates in endothelial‐specific growth media. Nonadherent cells were discarded every 48 hours and endothelial celllike colonies emerged from the adherent cell population 5–7 days after plating the cells. Upon reaching confluence, magnetic bead purification was performed to select for CD31‐positive ECFCs. ECFC were expanded on fibronectin-coated plates (1 μg/cm2; Millipore) using EGM-2 (without hydrocortisone; Lonza) supplemented with 20% FBS (Hyclone) and 1× glutamine-penicillin-streptomycin (GPS; Cellgro). The cells exhibited typical endothelial morphology, expressed vWF CD31, and stained negative for SMA. ECFCs between passages ten and 12 were used for all experiments. Expanded ECFCs could be cryopreserved using standard methods (using 90% FBS and 10% DMSO as freezing medium and liquid nitrogen for storage). We have tested endothelial progenitor cells (EPCs) cryopreserved at different passages (up to passage 15), and their phenotype as well as their in vitro and in vivo functions were properly maintained.24
MSCs were isolated from the MNC fraction of human adult bone marrow. MNC fraction isolated using Ficoll-Paque (GE Healthcare) was seeded on 1% gelatin-coated plates using MSCGM medium (Lonza) supplemented with 10% FBS and 1× GPS. Unbound cells were removed at 48 hours, and the adherent cell fraction was maintained in culture until 70% confluence. Cells were propagated using MSC medium (EGM-2 without hydrocortisone, VEGF, bFGF, and heparin, and supplemented with 20% FBS, 1× GPS). Passages five to ten were used for all experiments.
For validation of the proper MSC phenotype, cells were analyzed for the expression of characteristic surface markers41 via flow cytometry, demonstrating lack of expression of CD3, CD14, CD34, and CD45, alongside expression of CD73, CD90, CD105, and CD166.
Both MSCs and ECFCs were a kind gift from Prof. Joyce Bischoff.
Differentiation Assays of MSCs
Differentiation was induced at passage five of MSCs. Cells were plated in tissue culture slides with fresh medium at a density of 8000 cells per well. Fresh medium was added after 4 days. After 8 days, the medium was changed to differentiation media, each of which was composed of αMEM supplemented with 10% FCS. Osteogenic medium contained 100 μM dexamethasone, 0.1 mM ascorbic acid, and 10 nM β-glycerophosphate. Adipogenic medium contained 1 mM dexamethasone, 5 μl/ml insulin, and 1 μl/ml 3-isobutyl-1-methylxanthine. Fresh differentiation medium was given to the cells every 4 days for a total of 16 days, at which point the cells were fixed with 100% ethanol for 15 minutes at room temperature and stained with 0.25% Alizarin Red (for osteogenic differentiation) and Oil Red O (for adipogenic differentiation).
In Vivo Differentiation Assays
Cells from NOD-SCID mice (Harlan Laboratories) were suspended in 200 μl liquid Matrigel (BD Biosciences) at 4°C, transferred to a syringe on ice, and injected into the subcutis; alternatively, they were suspended in 20 ml and injected into two injection site in the renal subcapsule or the renal parenchyma adjacent to the resection area. At 14 days after injection, the grafts, which were typically 0.1–0.3 cm in size, were removed, paraffin-embedded, and serially sectioned at 5 μm for histologic and immunohistochemical (IHC) analyses. hFK cells were injected into the subcutis, using cells from four different donors (each injected into the flanks of one mouse). hAK cells from three different donors were injected both subcutaneously (four mice) and into the subcapsular space (five mice), yielding the same results compared with the control group. The combination of MSCs and EPCs was injected (1) subcutaneously into four mice, each at two flanks (i.e., eight injections); (2) into the renal subcapsular space in four mice; and (3) intraparenchymally in two additional mice (out of the ten used in these experiments).
Hematoxylin and Eosin Staining
Paraffin-embedded Matrigel grafts (5 µm sections) were mounted on SuperFrost Plus glass and incubated at 60°C for 40 minutes. After deparaffinization, slides were incubated in Mayer hematoxylin solution (Sigma-Aldrich) and incubated with 1% hydrochloric acid in 70% ethanol for 1 minute. Slides were then incubated for 10 seconds in eosin (Sigma-Aldrich). Images were produced using Olympus BX51TF fluorescence microscope with Olympus DP72 camera and cellSens standard software.
IHC and Immunofluorescence Staining of Paraffin-Embedded Grafts
Sections were pretreated using OmniPrep solution (Zytomed Systems) in 95°C for 1 hour according to the manufacturer’s protocol. For IHC staining, blocking was performed using Cas‐Block solution (Invitrogen) for 20 minutes followed by 1 hour of incubation at room temperature with primary antibodies for HLA, CD31, Cytokeratin, and VEGFA. Samples were incubated with secondary antibodies (ImmPRESS anti-mouse/rabbit reagent peroxidase; Vector Laboratories) for 30 minutes at room temperature and detected using ImmPACT DAB kit (Vector Laboratories) according to the manufacturer’s protocol. Hematoxylin was used for counterstaining. For immunofluorescence staining, sections were blocked with Cas‐Block solution (Invitrogen) for 1 hour, followed by incubation with primary antibodies for Cytokeratin and CD31 (Supplemental Table 1), and then washed and incubated with secondary antibodies (Supplemental Table 2) for 1 hour. Mounting containing DAPI (Southern Biotech) was applied. Photos were obtained using Olympus BX51TF fluorescence microscope with Olympus DP72 camera and cellSens standard software.
Generation of nSPHs from hAKs
nSPHs were established from primary hAK cell culture as previously described.17
Intrarenal Injection of Cells into Rats
Human cells were first infected with a retroviral vector expressing mCherry. Sprague–Dawley rats were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (15 mg/kg). Next, the rat was shaved and a 4-cm longitudinal incision was performed, the kidney was separated from the perinephric fat, and the main vessels were exposed, with the cells injected into the renal artery. The rats were euthanized 2 hours later, and their two kidneys were removed and visualized.
Results
Coadministration of hFK Cells alongside MSCs and ECFCs Results in the Generation of Vascularized Grafts
We were first interested in assessing whether administration of human RTFCs alongside human MSCs and ECFCs would result in formation of tubules and/or blood vessels. For this purpose, we used a previously described in vivo differentiation model, whereby human cells are injected subcutaneously into NOD-SCID mice within Matrigel, with subsequent removal of the grafts after 2 weeks for histologic analysis.38 Initially, we were interested in confirming whether combined injection of MSCs and ECFC can generate vessels in this model, similar to previous reports.32,40 After characterization of both MSCs and ECFCs for morphology, surface marker expression, and in vitro differentiation capacity (Supplemental Figures 1 and 2), we injected 106 MSCs and 7.5×105 ECFCs within Matrigel into NOD-SCID mice (n=4). After 7 days, the grafts were removed (Figure 1A). Hematoxylin and eosin (H&E) staining of the grafts revealed patent, functional vascular networks filled with erythrocytes (Figure 1B). To confirm that the vessels were donor derived, we first investigated the human specificity of the CD31 antibody. Indeed, we invariably found that it only stained human vessels and not mouse vessels (Supplemental Figure 3), consistent with previous reports.24 Similarly, the HLA antibody was seen to mark only human-derived structures (Supplemental Figure 3). Staining of the grafts using the human-specific CD31 antibody confirmed that the vessels originated from the injected MSCs/ECFCs (Figure 1B). Subsequently, we were interested in determining whether injection of hFK cells, alongside the MSC and ECFC mix, would result in formation of both renal tubules and vascular structures. To test this hypothesis, we subcutaneously injected NOD-SCID mice (n=4) with 106 primary hFK cells to one flank or a mixture of 106 hFK cells, 7.5×105 human ECFCs, and 106 human MSCs to the contralateral flank, and analyzed the grafts after 14 days. Mice injected with Matrigel served as a control (Figure 1C). Upon macroscopic inspection, hFK-only grafts exhibited scarce presence of miniature vessels, an appearance highly similar to Matrigel-only grafts. In contrast, mixed grafts were highly vascularized, demonstrating a rich network of well developed vessels (Figure 1D). In order to better characterize the grafts in terms of vascularity and tubule formation, we next carried out H&E staining. In accordance with the macroscopic appearance, the hFK-only grafts, although harboring numerous, well developed tubular structures, were devoid of vessels (Figure 1E). In contrast, mixed grafts demonstrated not only a similar number of tubular structures, but also abundant blood vessels, exhibiting clear lumens and the presence of erythrocytes, indicating successful integration into the host vasculature (Figure 1E). Notably, the tubules expressed cytokeratin whereas the vessels expressed CD31, indicating proper differentiation into the two lineages (Figure 1, F–H). Interestingly, the tubular structures and vessels were seen to reside in close proximity to one another within the grafts, indicating possible interdependence between the two elements in these renovascular units (Figure 1I). Within the established renovascular units, the tubular and vascular compartments were mutually exclusive, with only rare mosaic structures harboring both CD31- and cytokeratin-expressing cells. Taken together, these results indicate that although hFK cells can generate tubular structures in vivo, the established grafts are avascular. The addition of human MSCs and ECFCs results in formation of highly vascularized grafts, characterized by the presence of well developed renovascular units, harboring donor-derived structures that are both tubular and vascular.
Figure 1.
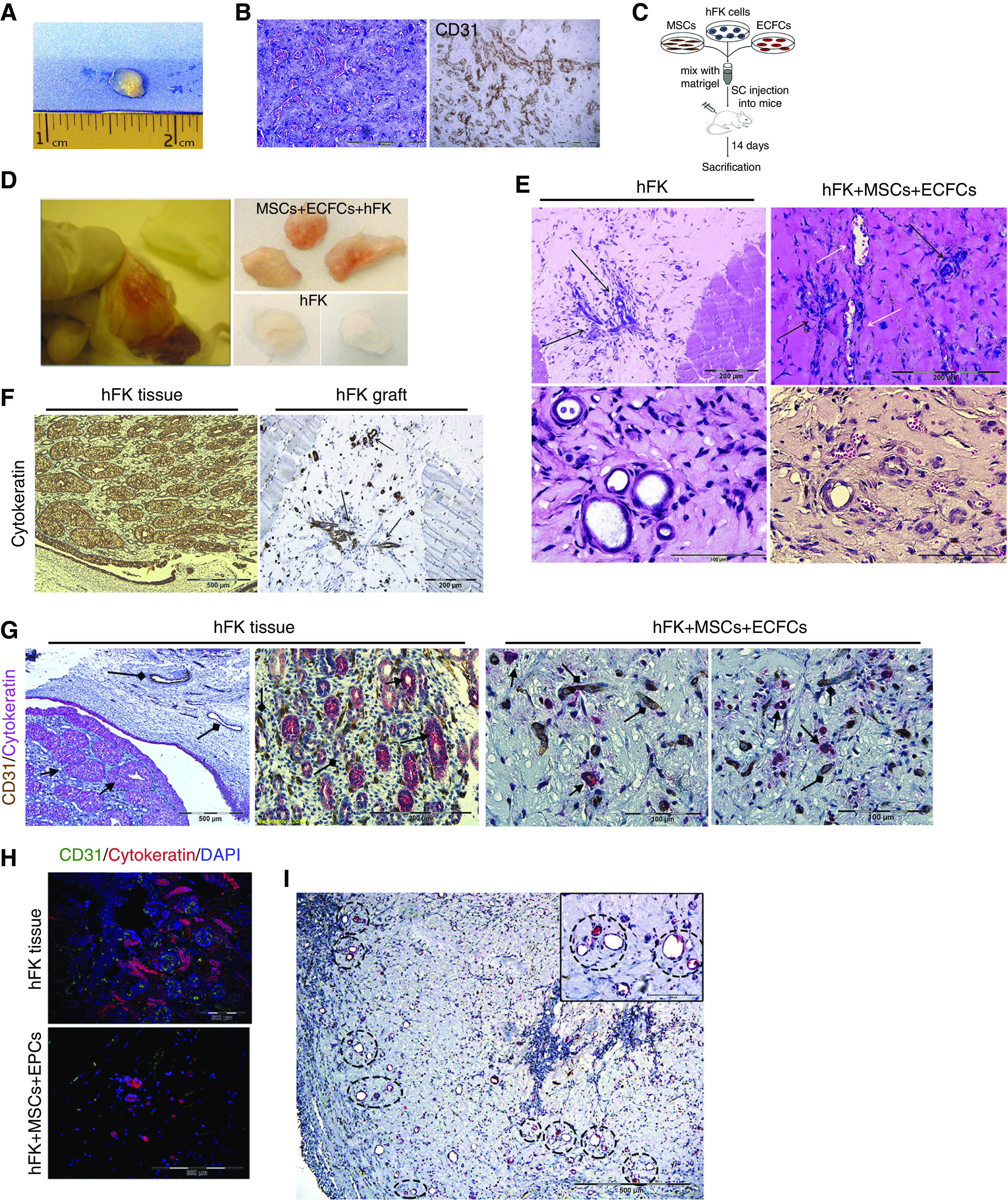
Subcutaneous coadministration of hFK cells, MSCs, and ECFCs results in the formation of renovascular units. (A and B) First, MSCs and ECFCs (106 and 7.5×105, respectively) were mixed with Matrigel and injected into NOD-SCID mice. (A) Seven days later, the grafts were removed for analysis. (B) H&E staining showed patent vascular networks filled with erythrocytes (left). Anti-human CD31 staining confirmed that the vessels’ origin was from the injected MSCs/ECFCs (right). Scale bars, 200 μm. (C) Experiment scheme: to test whether adding hFK cells would allow generation of renal tubules, NOD-SCID mice were injected with either hFK cells alone (106 cells/graft) or with a mix of hFK cells, MSCs, and ECFCs, within Matrigel, and euthanized 14 days later. (D) Macroscopically, mixed grafts exhibited a vascularized appearance (left and top right), compared with hFK only (bottom right). (E) H&E staining showed the presence of tubular structures in both groups (black arrows). However, implants from the mixed group also harbored blood vessels (white arrows). Scale bars: top, 200 μm; bottom, 100 μm. (F) Anti-human cytokeratin was used to track hFK cells in the grafts. hFK tissue served as positive control (left). We detected cytokeratin positive tubular structures (arrows). Scale bars: left, 500 μm; right, 200 μm. (G) Staining of mixed grafts (right) for human CD31 (brown) and cytokeratin (red), demonstrating the presence of both blood vessels (diamond arrows) and tubular structures (arrows). hFK tissue (left) served as a positive control. Scale bars: left, 500 μm; middle left, 200 μm; middle right and right, 100 μm. (H) Immunofluorescence staining of mixed grafts (bottom) for human CD31 (green) and cytokeratin (red), confirming the copresence of human blood vessels with hFK cell–derived tubular structures. hFK tissue (top) served as a positive control. Scale bars, 200 μm. (I) Within the grafts, donor-derived vessels and tubules formed in close proximity (H&E staining with renovascular units marked by circles, inset showing larger magnification of two such units). Scale bar, 500 μm; inset, 100 μm. SC, subcutaneous.
Coadministration of hAK Cells alongside MSCs and ECFCs Augments Their Tubulogenic Capacity
Having shown that combined administration of hFK cells, MSCs, and ECFCs gives rise to well developed tubules and vessels, we next queried whether similar results could be obtained using another type of RTFCs, hAK cells, which is important from the translational perspective because hAKs, in contrast to hFKs, represent an autologous source of RTFCs. In addition, hAK cells are much more limited in their tubulogenic potential compared with hFK cells, and we thus speculated that injecting them alongside vasculogenic cells could increase their tubule-formation capacity. Therefore, we carried out a similar experiment, using hAKs, whereby NOD-SCID mice were subcutaneously injected with 3×106 or 1×106 hAK cells, alone or combined with 106 MSCs and 7.5×105 ECFCs within Matrigel (Figure 2A). Similar to the results obtained using hFKs, macroscopic evaluation of 14-day grafts generated from the combination of hAK cells, MSCs, and ECFCs revealed highly vascularized grafts, compared with the poorly vascularized appearance of hAK-only grafts (Figure 2B). Interestingly, injection of 106 cells generated grafts that were more vascularized (Figure 2B). Accordingly, H&E staining of the grafts revealed that although hAK-only grafts were composed mostly of tubular structures, mixed grafts exhibited both tubules and a multitude of erythrocyte-containing, patent vessels (Figure 2C). HLA staining of the grafts demonstrated that both the tubules and vessels were human derived (Figure 2C). So as to confirm successful connection of the human-derived vessels to the host vasculature, we injected the mice with high-molecular-weight dextran via the tail vein. Indeed, we were able to detect the presence of dextran within the human HLA+ vessels (Figure 2D). Notably, the tubular and vascular structures were again seen to form close to one another (Figure 2E). Indeed, quantification of vessel number demonstrated that grafts derived from hAK cells contained significantly less blood vessels than those derived from the cell mixture (2.3±3.2 versus 7.8±3.3, respectively) (Figure 2F). In order to further validate that the established vascular structures were of human origin, we carried out IHC staining of the grafts using a human-specific CD31 antibody (Figure 2E). The vast majority of vessels demonstrated positive staining for human CD31, indicating that they were donor derived and that successful integration into the host vasculature was achieved. Concomitantly, the tubular structures demonstrated positive staining for the epithelial marker cytokeratin (Figure 2E), indicating proper epithelial differentiation. Importantly, a significantly higher number of tubular structures was detected in grafts generated from the mixture of hAK cells, MSCs, and ECFCs, as compared with those generated from hAK alone (13.8±3.6 versus 9.9±3.1, respectively) (Figure 2F). Immunofluorescence staining confirmed the presence of CD31+ vessels and demonstrated the expression of both cytokeratin and EMA by the tubules, validating their epithelial nature (Figure 2G). Notably, the vessels harbored αSMA+ perivascular cells (Figure 2G), indicating the formation of mature, donor-derived vessels composed of both endothelium and perivascular cells, which likely originate from the injected ECFCs and MSCs,24 respectively. Interestingly, the grafts also contained a large number of HLA− cells, representing mouse cells that invaded the grafts (Figure 2C), as previously observed in this assay.17 We were next interested in further characterizing the established tubular structures with respect to their renal lineage. Hence, we carried out immunofluorescence staining of the grafts for the proximal tubule marker Megalin and the distal tubule markers DBA and EMA. Notably, we detected both tubules of the proximal lineage and tubules of the distal lineage (Figure 3A). Taken together, these results indicate that although human RTFC-derived grafts harbor low numbers of tubules and host-derived vessels, grafts generated from RTFCs mixed with vessel-forming cells are rich in donor-derived vessels, leading to enhanced tubulogenesis.
Figure 2.
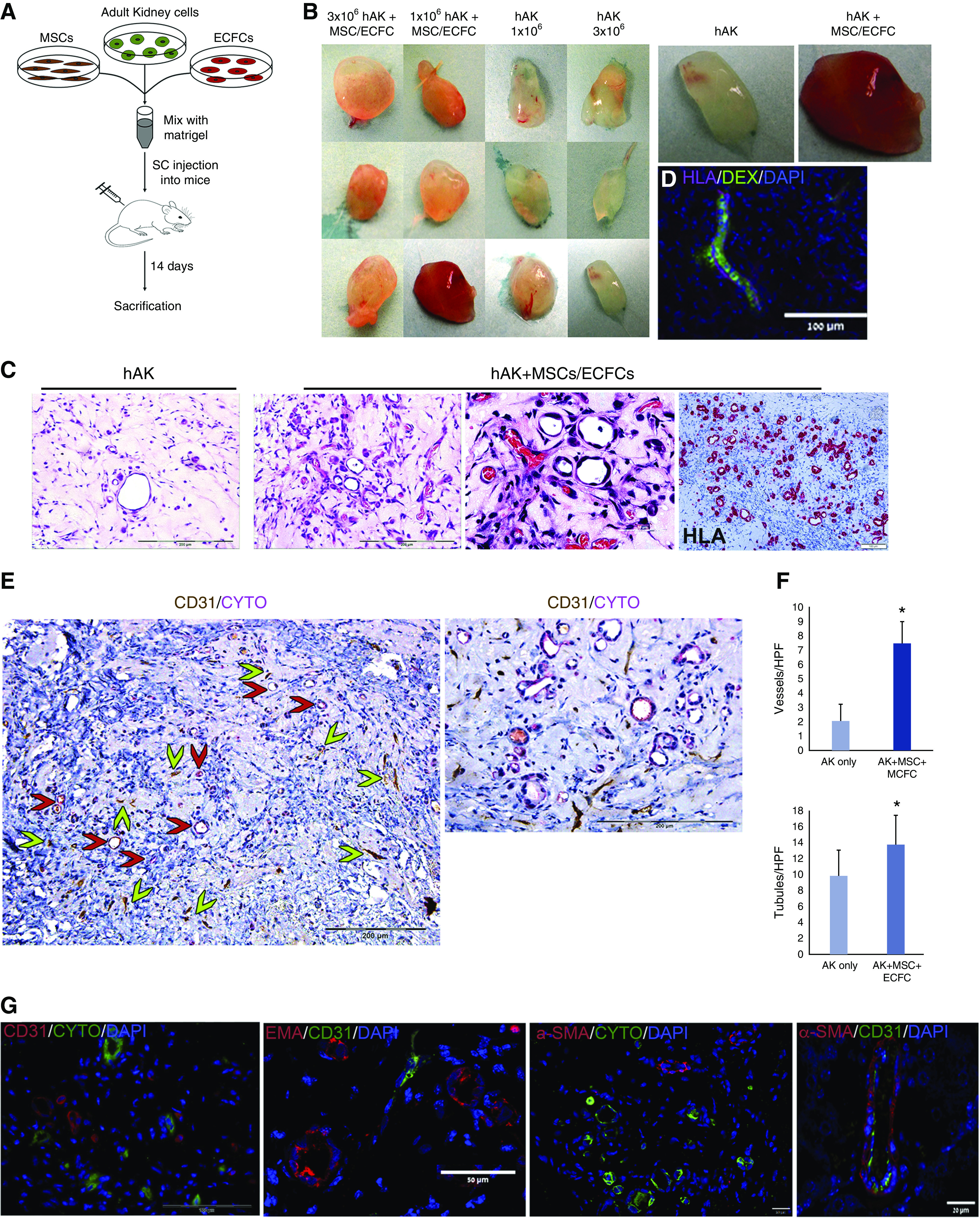
Subcutaneous coadministration of hAK cells, MSCs, and ECFCs results in the formation of renovascular units. (A) Experimental scheme. (B) Initially, hAK cells (106 or 3×106) were injected alone or with a mix of MSCs and ECFCs. Shown is the macroscopic appearance of the different experimental groups. Mixed grafts exhibit a significantly more vascular appearance. (C) H&E staining of grafts generated from hAK alone (left) or mixed grafts (right), demonstrating formation of both tubules and patent, erythrocyte-containing vessels in the mixed grafts compared with only tubules in the hAK-only grafts. HLA staining demonstrated that all formed structures were donor derived (right). Scale bars: left and middle left, 200 μm; middle right, 50 μm; right, 100 μm. (D) Staining of mixed grafts from mice intravenously injected with high-molecular-weight dextran (DEX; green) for HLA (purple). Seen is a donor-derived HLA+ vessel with intraluminal dextran, indicating successful connection of the human-derived vessels to the host vasculature. Scale bar, 100 μm. (E) Left: IHC staining of mixed grafts for human CD31 (brown) and cytokeratin (purple), demonstrating formation of both vessels (green arrowheads) and tubular structures (red arrowheads). Right: high-power magnification of left panel. Scale bars, 200 μm. (F) Top: number of vessels in hAK-only grafts versus mixed grafts, shown as mean±SD; *P=0.02, two-tailed t test. Bottom: number of tubular structures in hAK-only grafts versus mixed grafts, shown as mean±SD; *P=0.03, two-tailed t test. (G) Immunofluorescence staining of mixed grafts. Left: staining for human CD31 (red) and cytokeratin (CYTO; green), confirming the formation of both vessels and tubules; middle left: staining for human CD31 (green) and the epithelial marker EMA (red); middle right: staining for cytokeratin (CYTO; green) and the perivascular marker αSMA (red); right: staining for human CD31 (green) and αSMA (red), demonstrating that the formed vessels are well developed, containing both endothelium and perivascular cells. Scale bars: left, 100 μm; middle left, 20 μm; middle right, 50 μm; right, 20 μm. SC, subcutaneous.
Figure 3.
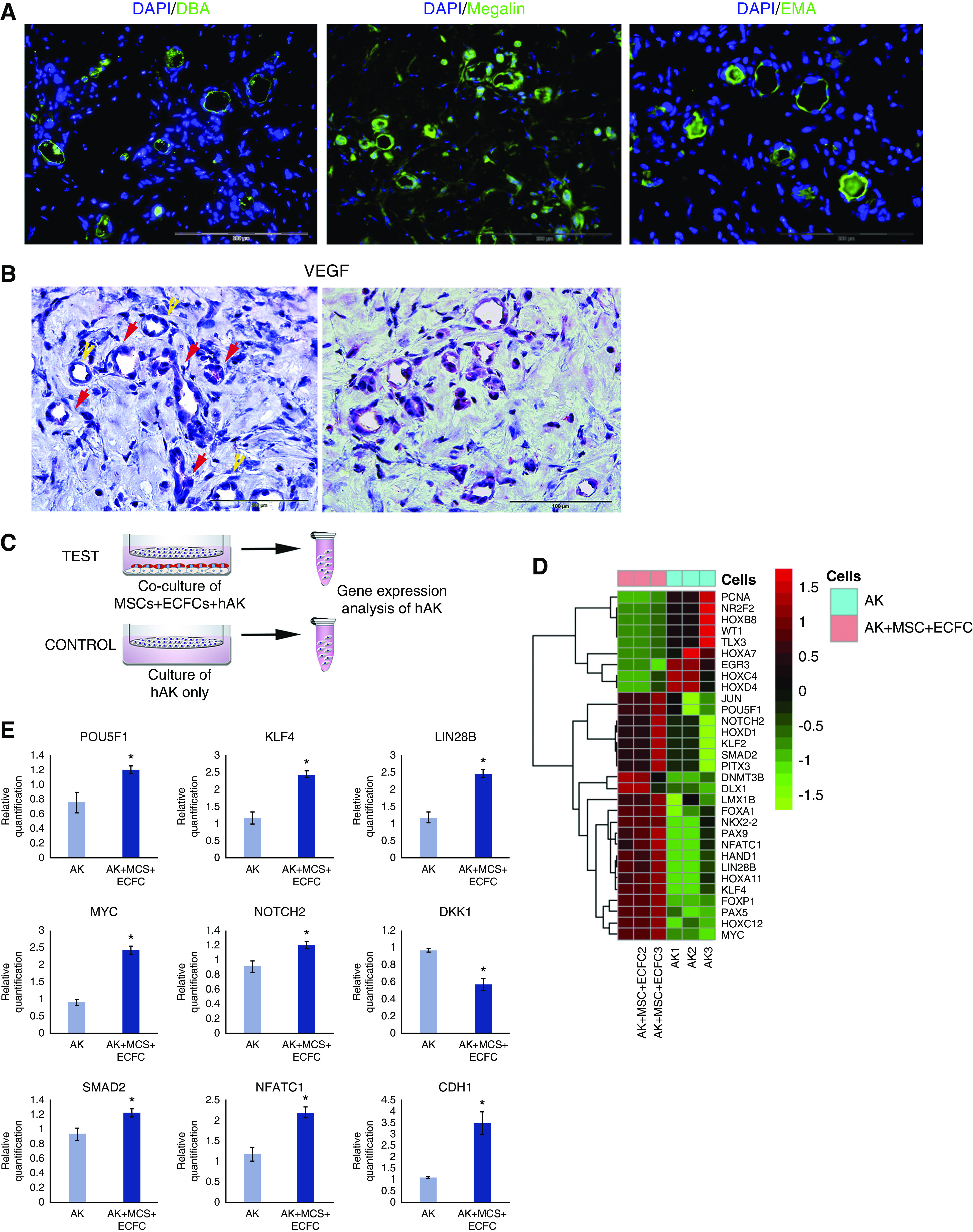
Characterization of hAK cell–derived tubules and analysis of paracrine effects of MSCs and ECFCs on hAK cells. (A) Immunofluorescence staining of grafts generated from hAK cells, MSCs, and ECFCs injected subcutaneously into NOD-SCID mice for the proximal tubule marker Megalin and distal tubule markers DBA and EMA. Scale bars, 200 μm. (B) IHC staining of mixed grafts for VEGFA, showing strong VEGFA expression in virtually all nascent vessels, demonstrating a perivascular distribution (red arrows), in proximity to renal tubules (yellow arrows). Scale bars, 100 μm. (C) Experimental scheme: hAK cells were cultured either alone (CONTROL), or with MSCs and ECFCs (TEST) in a Transwell setting for 72 hours, after which the gene expression of the two groups was compared via a real time PCR array of stem related transcription factors. (D) Heat map of gene expression. Colors indicate z-score of RQ values. (E) Relative expression of selected genes in hAK cells grown alone or alongside MSCs and ECFCs. *P<0.05 (two-tailed t test).
The Generation of Renovascular Units Involves VEGFA Expression and Induction of Self-Renewal and Mesenchymal–Epithelial Transition in hAK Cells
Subsequently, in order to assess the mechanisms underlying vasculogenesis within the grafts, we stained them for VEGFA, previously shown to participate in neo-vasculogenesis during kidney regeneration.42 We found strong VEGFA expression in virtually all nascent vessels, demonstrating a perivascular distribution (Figure 3B). Next, we were interested in exploring the mechanisms responsible for the enhanced survival of transplanted RTFCs and greater number of tubules formed thereof when injected alongside ECFCs and MSCs, as compared with hAK cells alone. To this end, we cultured hAK cells alone or in a Transwell system alongside MSCs and ECFCs, allowing free passage of secreted factors, but not direct cell contact, for 72 hours. Subsequently, hAK cells were collected and analyzed for the expression of a panel of 84 stem cell–associated genes (Figure 3C). Hierarchical clustering revealed significant differences in gene expression between the two culture settings (Figure 3D). Remarkably, hAK cells grown together with MSCs and ECFCs showed activation of several major regulators of self-renewal, including OCT4,43 KLF4,44 LIN28B,45 and MYC,46,47 compared with hAK cells grown in isolation (Figure 3E). Moreover, we noted activation of NOTCH2, which has been shown to regulate the regeneration and proliferation of renal tubules,48 as well as reduced expression of DKK1, an inhibitor of the Wnt pathway, which has been shown to promote expansion of adult kidney tubular cells during homeostasis and after injury4 (Figure 3E). These observations suggest that when mixed with MSCs and ECFCs, the administered RTFCs gain self-renewal capacity, which enhances their ability to survive and proliferate within the host tissue, and consequently form more tubular structures. Concomitantly, hAK cells grown together with MSCs and ECFCs exhibited upregulation of SMAD2 and NFATC1, both of which have been found to promote tubular epithelial regeneration by attenuating or reversing the acquisition of a mesenchymal fibrotic phenotype.49,50 Accordingly, we found increased expression of CDH1, the gene encoding the epithelial marker E-Cadherin in hAK cells (Figure 3E). These results indicate that formation of the vascular compartment in the grafts entails VEGFA expression in the perivascular niche, which implies recruitment and differentiation of ECFCs into endothelial cells by MSCs/perivascular cells, as previously described.51 The continued VEGFA expression after 14 days indicates ongoing vasculogenic processes. Moreover, the increased number of tubules formed from hAK cells when MSCs and ECFCs are injected alongside them seems to result not only from improved survival of hAK cells because of the presence of accompanying vessels, but also from paracrine effects exerted by the MSCs and ECFCs, which support their self-renewal and acquisition of a proper epithelial phenotype.
Coadministration of hAK Cells, MSCs, and ECFCs Results in the Generation of Renovascular Units within a Renal Niche
Having shown the potential of the mixture of hAK cells, MSCs, and ECFCs to generate tubular and vascular structures after subcutaneous administration, we subsequently asked whether similar results could be obtained in a renal niche, which is more relevant from a translational standpoint. We therefore injected hAK cells, MSCs, and ECFCs within Matrigel under the kidney capsule of NOD-SCID mice (n=5) and analyzed the grafts 14 days later (Figure 4A). Upon macroscopic inspection, vascularized grafts were detected (Figure 4B). Accordingly, H&E staining of the grafts uncovered formation of numerous vessels, exhibiting well developed lumens. Importantly, erythrocytes were observed within the vessels, indicating that the newly formed vessels connected to the host renal vasculature (Figure 4C). IHC staining using a human-specific CD31 antibody confirmed that the vessels were indeed of donor origin (Figure 4C). Furthermore, the grafts also demonstrated the presence of tubular structures, expressing cytokeratin (Figure 4D). In addition, we found tubules of both the proximal and, to a much greater extent, distal lineage, as evident by the expression of CD13 and EMA, respectively (Figure 4E). Altogether, these results indicate that coadministration of hAK cells, MSCs, and ECFCs is effective in generating renovascular units when applied within a renal niche.
Figure 4.
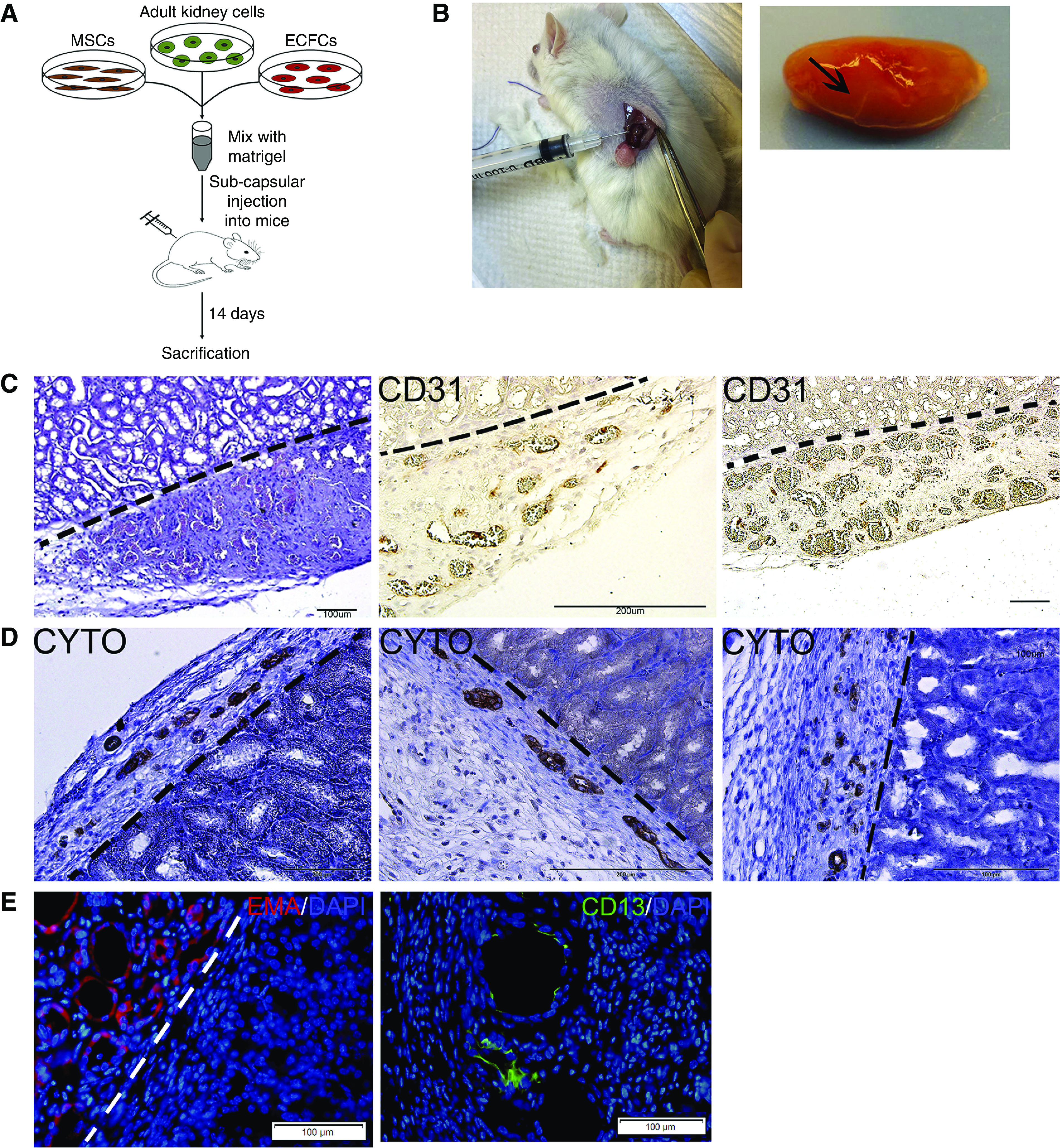
Subcapsular coadministration of hAK cells, MSCs, and ECFCs results in formation of renovascular units. (A) Experimental scheme. (B) Macroscopic appearance of the grafts in situ (left) and after removal (right, graft marked by arrow on the whole resected kidney). (C) H&E staining (left) and staining for human CD31 (middle and right) demonstrate the formation of erythrocyte-filled, donor-derived vessels. Scale bars: left and right, 100 μm; middle, 200 μm. (D) Staining for cytokeratin demonstrates the formation of tubular epithelial structures. Scale bars: left and middle, 200 μm; right: 100 μm. (E) Immunofluorescence staining for the distal tubule marker EMA (left) and proximal tubule marker CD13 (right), demonstrating the formation of renal tubules of both lineages. Dotted lines mark the border between the graft and mouse kidney tissue.
Coadministration of Human nSPHs, MSCs, and ECFCs Results in the Generation of Renovascular Units within Damaged Kidneys
Lastly, we were interested in assessing whether coadministered RTFCs, MSCs, and ECFCs could effectively generate renovascular units within a damaged kidney. First, we tested whether intravascular infusion would allow RFTCs to home and integrate into the renal parenchyma. To this end, we injected Sprague–Dawley rats with mCherry-labeled RTFCs alongside MSCs and ECFCs into the renal artery (n=3). Upon histologic analysis, we found that vascular infusion did not result in the cells arriving at the renal parenchyma. Instead, the cells were shown by HLA staining to accumulate within blood vessels and glomeruli (Figure 5A). Thus, although they arrived at the kidney (Supplemental Figure 4), they could not be detected within the renal parenchyma. Hence, we decided to proceed with direct intraparenchymal cell injection. For this experiment, we used human nSPHs, generated by culturing passage-one hAK cells in low-adherence conditions, and previously shown to harbor tubulogenic and regenerative properties.16,17 First, NOD-SCID mice (n=10) underwent left partial nephrectomy. After 48 hours, the mice were randomly divided into three treatment groups: (1) intrarenal injection of MSCs and ECFCs; (2) intrarenal injection of nSPHs; and (3) intrarenal injection of nSPHs, MSCs, and ECFCs. All cells were injected within Matrigel into the renal parenchyma adjacent to the resection area. After 14 days, the mice were euthanized, and their kidneys were removed for analysis (Figure 5B). Although kidney sections of mice injected with MSCs and ECFCs harbored only CD31+ vessels and no human-derived tubular structures, as evident by IHC for HLA (Figure 5C), tubular structures could be observed in kidneys injected with nSPHs (Figure 5D), consistent with previous reports.17 However, the grafts remained avascular, with no evidence of human blood vessels (Figure 5D). Lastly, kidneys derived from mice injected with nSPHs, MSCs, and ECFCs demonstrated the presence of numerous tubular structures, as well as donor-derived blood vessels (Figure 5E), expressing human cytokeratin and CD31, respectively. The newly formed vessels were seen to include αSMA+ perivascular cells, indicating proper vascular differentiation. Taken together, these results indicate that combined direct administration of human nSPHs, MSCs, and ECFCs into injured kidneys is superior to any of the cell types in isolation, resulting in the formation of rich networks of tubules and vessels, even in a hostile environment that characterizes injured kidneys.
Figure 5.
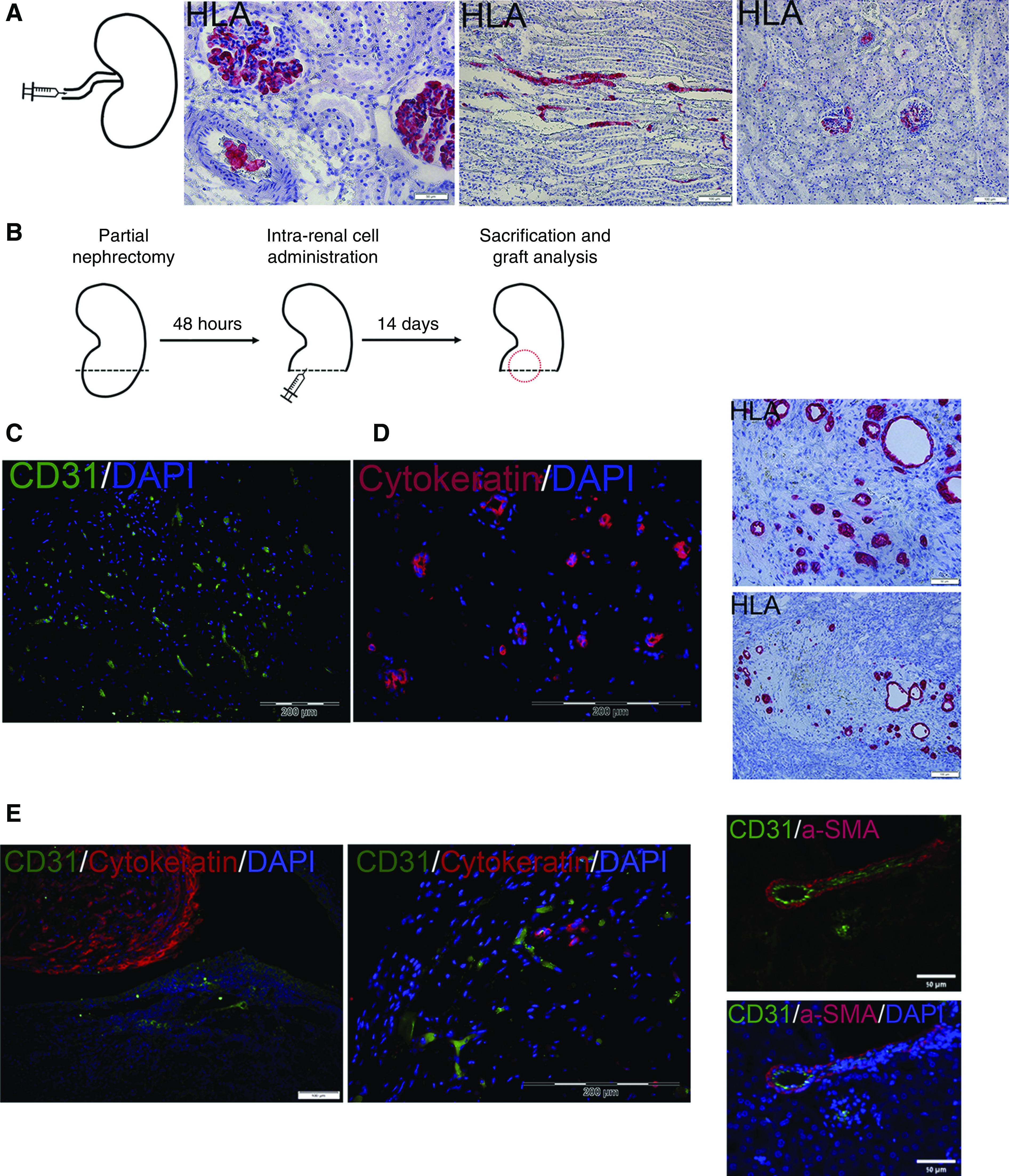
Coadministration of three-dimensional human nSPHs, MSCs, and ECFCs results in formation of renovascular units within damaged kidneys. (A) Initially, the intra-arterial route was tested (left, experimental scheme). Administration of donor nSPHs failed at localizing the cells to the renal parenchyma, as evident by the staining for HLA, which demonstrated the presence of human cells only within blood vessels and glomeruli. Scale bars: middle, 50 μm; right, 100 μm. (B) Experimental scheme: NOD-SCID mice underwent left partial nephrectomy. Forty eight hours later, they were divided into three groups: (1) intrarenal injection of MSCs and ECFCs; (2) intrarenal injection of human nSPHs; and (3) intrarenal injection of human nSPHs, MSCs, and ECFCs. All cells were injected within Matrigel into the renal parenchyma adjacent to the resection area. After 14 days, the mice were euthanized, and their kidneys were removed for analysis. (C) Grafts generated from injection of MSCs and ECFCs: immunofluorescence staining for human CD31 (green) demonstrates the presence of donor-derived blood vessels but no tubular structures. Scale bar, 200 μm. (D) Grafts generated from injection of nSPHs: immunofluorescence staining for cytokeratin (red, left) and IHC staining for HLA (red, right) demonstrated the presence of tubular structures but no blood vessels. Scale bars: top left, 50 μm; bottom left, 100 μm; right, 200 μm. (E) Grafts generated from injection of nSPHs, MSCs, and ECFCs. Left and middle: immunofluorescence staining for human CD31 (green) and cytokeratin (red), demonstrating the presence of both numerous tubular structures and donor-derived blood vessels; right: immunofluorescence staining for human CD31 (green) and the pericyte marker αSMA (red), demonstrating the formation of well developed donor-derived vessels, containing both endothelium and pericytes. Scale bars: left, 50 μm; middle, 200 μM; right, 50 μm.
Discussion
Significant progress has been achieved in recent years in the field of renal regenerative medicine, with the emergence of various protocols, allowing both in vitro and in vivo generation of different parts of the nephron (e.g., podocytes, glomeruli, and renal tubules) from both fetal and adult tissues8,12–14,17,52–54. However, a major barrier toward successful implementation of these strategies in renal regeneration is the need for proper in vivo vascularization of implanted cell types to facilitate their survival and tubulogenesis, a critical need in the hostile and hypoxic kidneys of patients with CKD, which are poorly vascularized. Indeed, most types of renal cell types have been shown to lack the ability to form donor-derived vessels upon transplantation, which would undoubtedly limit their regenerative potential considering the hypoxic conditions of the host’s kidneys. For instance, when transplanted into mice, induced pluripotent stem cell–derived renal organoids, despite their ability to recruit host-derived vessels to a certain extent, do not have the ability to actively develop human vascular networks.55,56 Similarly, when we transplanted human nSPHs into mice with CKD, we did not detect formation of human vessels, although numerous well developed tubules formed and an improvement in kidney function was noted.17
Furthermore, these findings reinforce the notion of clear lineage boundaries within the metanephric kidney, not only between epithelial cell types in the renal tubule,4 but also between the epithelial (SIX2+CITED1+), endothelial (Flk1+/Scl/Tal1+), and stromal (Foxd1+) lineages. Accordingly, grafts of early human metanephric kidneys, harboring epithelial, endothelial, and stromal progenitors in their niches, generate upon transplantation into mice fully vascularized renal structures with donor human vessels.7 Similarly, cell suspensions of embryonic kidneys, which likely contain all metanephric progenitor types, were shown to form vascularized glomeruli of donor origin in vivo.57 These observations indicate that for RTFCs to be able to achieve efficient blood supply in vivo, the presence of vessel-forming cells is required. Relying on these studies, we show herein that the addition of vessel-forming cells (i.e., MSCs and ECFCs) to RTFCs results in a synergistic effect, culminating both in formation of vascularized grafts harboring donor-derived vessels, and enhanced tubulogenesis.
In this study, we again show that RTFCs can only give rise to tubules, whereas vessel-forming cells organize only into blood vessels. In our system, injected MSCs most likely take the form of pericytes in nascent vessels, consistent with the perivascular identity of MSCs.58 Indeed, Melero-Martin et al.24 reported that when human MSCs and ECFCs are administered to mice, both cell types are needed to form vessels, with ECFCs differentiating into CD31+ endothelium and MSCs into αSMA+ pericytes, which surround and stabilize nascent vessels, a process involving VEGFA-mediated recruitment of endothelial cells. Moreover, in 2014, Lin et al.59 reported that aside from the support provided by MSCs, endothelial cells mediate MSC engraftment via paracrine mechanisms, thereby establishing a mechanism for mutual cooperation between the two cell types. Notably, both cell types are critical for vessel formation, and implants containing only ECFCs or MSCs do not yield vessels. This was also evident in our experiments, which revealed the presence of perivascular αSMA+ cells in the outer part of donor-derived vessels. Interestingly, we also detected a large number of “interstitial” cells present between donor-derived tubular and vascular structures. Virtually all of these cells were host cells that invaded the graft, as evident by HLA staining, and in accordance with our recent work, which found that upon injection into mice, practically all injected human cells in the grafts are located within organized structures or cell clusters, and only rarely are single donor cells detected.17 Nonetheless, we cannot exclude the possibility that a small fraction of this interstitial population represents MSCs that did not engraft as pericytes. Indeed, it has been reported that upon in vivo transplantation of MSCs and ECFCs, MSCs that fail to engraft as pericytes become interstitial cells, although this phenomenon is limited by the presence of ECFCs, which act via paracrine mechanisms to support MSC differentiation into pericytes.59
Our results suggest that coadministering human RTFCs with a combination of MSCs and ECFCs could improve the tubulogenic potential of RTFCs in at least two ways (Figure 6): (1) by generating donor-derived vessels, which serve as a bridge to the host circulation, thereby improving graft survival and differentiation; and (2) by exerting paracrine effects on RTFCs, thereby priming them into a protubulogenic state. Regarding the first mechanism, our results indicate that although grafts derived solely from RTFCs are fed by few host-derived vessels, grafts generated from RTFCs combined with MSCs and ECFCs harbor multiple donor-derived vessels that effectively connect with the host vasculature. Consequently, grafts derived from a mixture of RTFCs and vessel-forming cells consist of both more tubules and more vessels (which are primarily donor derived). As for the second mechanism, our gene expression analysis of hAK exposed to MSCs and ECFCs uncovered two main molecular pathways potentially underlying the acquisition of a protubulogenic state. The first is enhanced self-renewal, as evident by activation of OCT4, KLF4, LIN28B, and MYC, all factors favoring cellular self-renewal. Although robust ectopic upregulation of these factors leads to pluripotency,60,61 they have also been shown to be expressed in lower levels in tissue stem cells, where they regulate self-renewal and stemness 43–47,62. Thus, it is plausible that by injecting RTFCs alongside MSCs and ECFCs, they gain enhanced self-renewal capacity, manifesting in prolonged survival and stronger proliferative ability within the host kidney, which results in the larger number of tubules observed as compared with injection of only RTFCs. This was supported by the activation of NOTCH2, which as part of the Delta-1/Notch-2/Hes-1 pathway promotes proliferation of tubular epithelia after damage,48 as well as by the repression of DKK1, a Wnt pathway inhibitor, which plays a key role in expansion of tubular cells.4 Interestingly, renal epithelia in CKD kidneys show increased DKK1 production, which was shown to play a major role in vascular calcification characteristic of CKD, and its dampening may therefore improve vascularization. The second molecular process detected in our analysis was re-acquisition of a normal tubular epithelial phenotype. We previously demonstrated17 that culturing of hAK cells results in partial loss of their epithelial phenotype, thereby limiting their in vivo tubular differentiation potential.17 However, as suggested by the activation of SMAD2 and NFATC1, two factors which act to revert postinjury fibrotic tubular cells into a normal epithelial phenotype49,50 as well as by the induction of E-Cadherin, hAK cells exposed to MSCs and ECFCs undergo mesenchymal–epithelial transition and reacquire a renal epithelial phenotype.
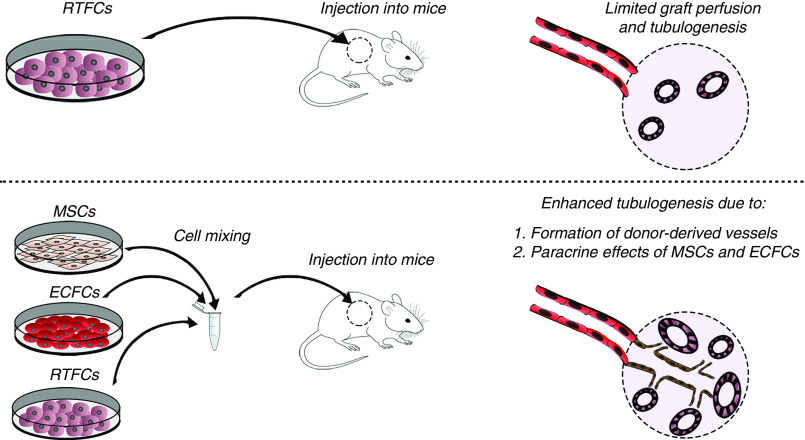
Figure 6.
Summarizing scheme. Top: RTFCs injected into mice are supplied by host-derived blood vessels, resulting in limited graft perfusion and low tubulogenic potential. Bottom: In contrast, when RTFCs are injected alongside MSCs and ECFCs, the latter augment the RTFCs tubulogenic potential via two mechanisms: 1. Generation of a large number of donor-derived vessels, which provide the grafts with rich blood supply, thereby promoting tubulogenesis. 2. The exertion of pro-tubulogenic paracrine effects on RTFCs.
Aside from improved tubulogenesis achieved by the addition of vasculogenic cell types to RTFCs, the generation of vascular networks within damaged kidneys could in itself have a positive effect, potentially alleviating renal hypoxia by contributing to the diminished vascularization of CKD kidneys. These are characterized by tissue hypoxia accompanying the loss of renal parenchyma, which not only results in further tissue damage but also impairs the normal reparative mechanisms of the kidney and enhances fibrosis.63,64 In this regard, the continued in vivo expression of VEGFA by the newly formed vessels in our system, previously shown to be essential for the maintenance of peritubular capillaries, could also potentially be advantageous in CKD, which is characterized by rarefication of peritubular capillaries.19 Importantly, the strategy proposed herein was successful in generating both tubules and donor-derived vessels in injured kidneys, which represent a hostile environment for exogeneous cells to survive, organize and differentiate. Nonetheless, this would have to be further tested in different models of renal damage, to assess whether these findings represent a consistent phenomenon.
An important point observed in our study is the importance of the cell administration route. Although intra-arterial infusion resulted in complete failure of the cells to integrate into the kidney, the intraparenchymatic route was effective and allowed formation of both renal tubules and vessels. This is consistent with a recent report, which demonstrated that after vascular infusion, cardiac progenitors fail to penetrate cardiac capillaries, which precludes them from exerting a regenerative effect. In contrast, direct intracardiac injection led to a significant functional improvement.65
A different, ex vivo approach to improving vascularization of RTFCs was recently proposed by Homan et al.,66 who cultured renal organoids with flow over their top surface, which resulted in higher numbers of endothelial cells that formed networks that invaded organoid glomerular structures. However, the study did not provide definite proof that the vascular networks were fully perfusable. In addition, Taniguchi’s group reported an ex vivo method for generating vascularized organ buds by combining pluripotent stem cell–derived tissue progenitors (from various organs, including kidney),67 as well as tissue fragments,68 with MSCs and endothelial cells. Upon intracranial transplantation in mice, the organ buds successfully integrated into the host.67 Notably, similar to our observations, the authors found that fetal renal progenitors transplanted in isolation (i.e., not within organ buds) failed to vascularize.67 These findings corroborate with our results, highlighting the importance of proper vascularization of transplanted RTFCs.
The experiments described herein that used hAK cells involved early passage proliferative hAK cells. Nonetheless, for renal regenerative medicine, scaling up is needed. Because expansion of hAK cells leads to loss of renal potency upon in vivo grafting, they are less relevant at higher passages. A means to regain renal potency in higher passages after scale-up of hAK cells is to generate three-dimensional nSPHs.17 Thus, to examine the ability of scaled-up RTFCs to participate in generation of renovascular units once grafted with MSCs/EPCs, we utilized nSPHs. The use of nSPHs and hAK cells as renal precursors in this system is important from the translational perspective, as it implies that this strategy can rely on autologous cells, with hAK cells/nSPHs derived from a renal biopsy and expanded in vitro, and MSCs and ECFCs derived from peripheral blood or other sources, such as bone marrow. Moreover, it is possible that RTFCs from other sources (e.g., induced pluripotent stem cell–derived renal progenitors) could serve the “nephrogenic” role in this strategy.12–14 Our results provide a proof of concept for the potential benefit of the joint usage of RTFCs and vasculogenic cells.
Future studies using animal models of kidney damage are needed to determine whether the addition of MSCs and ECFCs to nSPHs, which manifests in enhanced tubulogenesis, would result in an improved therapeutic effect compared with using nSPHs without additional cell types.17 Lastly, the renovascular units generated in vivo, which include well developed renal tubules as well as blood vessels, could also potentially serve as a novel tool for toxicity studies.
Disclosure
B. Dekel is a co-founder and board member at KidneyCure Ltd. O. Harari-Steinberg is a shareholder at KidneyCure Ltd. and has a patent 16/045,784 pending. All remaining authors have nothing to disclose.
Funding
This study was supported by the Israel Medical Association Society for Research, Prevention and Treatment of Atherosclerosis (S. Greenberger), Israel Society of Atherosclerosis and Vascular Biology (O. Harari-Steinberg and S. Greenberger), Israel Cancer Association grant 20140134 (to S. Greenberger and O. Harari-Steinberg), Tel Aviv University Shauder Fund for Biomedical Research (B. Dekel), Tel Aviv University Brettler Family Foundation (B. Dekel), Israel Science Foundation grant 2071/17 (to B. Dekel and O. Harari-Steinberg), Euro-Asian Jewish Congress (B. Dekel), and the Lisa and David Pulver Family Foundation (B. Dekel).
Supplementary Material
Acknowledgments
Dr. Oren Pleniceanu, Dr. Orit Harari-Steinberg, Dr. Dorit Omer, Prof. Benjamin Dekel, and Dr. Shoshana Greenberger designed the experiment. Dr. Dorit Omer, Dr. Bat-El Lachmi, Dr. Osnat Cohen-Zontag, Dr. Eugenia Manevitz-Mendelson, Prof. Aviv Barzilai, Mr. Matan Yampolsky, Dr. Yaron Fuchs, Dr. Barak Rosenzweig, Dr. Alon Eisner, Prof. Zohar Dotan, and Dr. Shoshana Greenberger performed the experiments. Dr. Oren Pleniceanu, Dr. Orit Harari-Steinberg, Dr. Dorit Omer, Prof. Leon G. Fine, Prof. Benjamin Dekel, and Dr. Shoshana Greenberger were responsible for data analysis and wrote the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Mix for Regeneration: Nephron Replacement by Transplanted Cells,” on pages 2743–2745.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050508/-/DCSupplemental.
Supplemental Figure 1. Characterization of MSCs.
Supplemental Figure 2. Characterization of ECFCs.
Supplemental Figure 3. Validation of human specificity of anti-CD31 antibody.
Supplemental Figure 4. In vivo tracing of intra-arterially injected hAK cells.
Supplemental Table 1. Antibodies used in the study.
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, et al. : Global prevalence of chronic kidney disease - a systematic review and meta-analysis. PLoS One 11: e0158765, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pleniceanu O, Harari-Steinberg O, Dekel B: Concise review: Kidney stem/progenitor cells: Differentiate, sort out, or reprogram? Stem Cells 28: 1649–1660, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pleniceanu O, Omer D, Harari-Steinberg O, Dekel B: Renal lineage cells as a source for renal regeneration. Pediatr Res 83: 267–274, 2018. [DOI] [PubMed] [Google Scholar]
- 4.Rinkevich Y, Montoro DT, Contreras-Trujillo H, Harari-Steinberg O, Newman AM, Tsai JM, et al. : In vivo clonal analysis reveals lineage-restricted progenitor characteristics in mammalian kidney development, maintenance, and regeneration. Cell Rep 7: 1270–1283, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dziedzic K, Pleniceanu O, Dekel B: Kidney stem cells in development, regeneration and cancer. Semin Cell Dev Biol 36: 57–65, 2014. [DOI] [PubMed] [Google Scholar]
- 6.Harari-Steinberg O, Pleniceanu O, Dekel B: Selecting the optimal cell for kidney regeneration: Fetal, adult or reprogrammed stem cells. Organogenesis 7: 123–134, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekel B, Burakova T, Arditti FD, Reich-Zeliger S, Milstein O, Aviel-Ronen S, et al. : Human and porcine early kidney precursors as a new source for transplantation. Nat Med 9: 53–60, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Harari-Steinberg O, Metsuyanim S, Omer D, Gnatek Y, Gershon R, Pri-Chen S, et al. : Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med 5: 1556–1568, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pode-Shakked N, Gershon R, Tam G, Omer D, Gnatek Y, Kanter I, et al. : Evidence of in vitro preservation of human nephrogenesis at the single-cell level. Stem Cell Reports 9: 279–291, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pode-Shakked N, Pleniceanu O, Gershon R, Shukrun R, Kanter I, Bucris E, et al. : Dissecting stages of human kidney development and tumorigenesis with surface markers affords simple prospective purification of nephron stem cells. Sci Rep 6: 23562, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, et al. : Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol 18: 3128–3138, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, et al. : Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taguchi A, Kaku Y, Ohmori T, Sharmin S, Ogawa M, Sasaki H, et al. : Redefining the in vivo origin of metanephric nephron progenitors enables generation of complex kidney structures from pluripotent stem cells. Cell Stem Cell 14: 53–67, 2014. [DOI] [PubMed] [Google Scholar]
- 14.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, et al. : Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis [published correction appears in Nature 26: 564–568, 2015]. Nature 536: 238, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Hendry CE, Vanslambrouck JM, Ineson J, Suhaimi N, Takasato M, Rae F, et al. : Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J Am Soc Nephrol 24: 1424–1434, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buzhor E, Harari-Steinberg O, Omer D, Metsuyanim S, Jacob-Hirsch J, Noiman T, et al. : Kidney spheroids recapitulate tubular organoids leading to enhanced tubulogenic potency of human kidney-derived cells. Tissue Eng Part A 17: 2305–2319, 2011. [DOI] [PubMed] [Google Scholar]
- 17.Harari-Steinberg O, Omer D, Gnatek Y, Pleniceanu O, Goldberg S, Cohen-Zontag O, et al. : Ex vivo expanded 3D human kidney spheres engraft long term and repair chronic renal injury in mice. Cell Rep 30: 852–869.e4, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Nangaku M: Chronic hypoxia and tubulointerstitial injury: A final common pathway to end-stage renal failure. J Am Soc Nephrol 17: 17–25, 2006. [DOI] [PubMed] [Google Scholar]
- 19.Dimke H, Sparks MA, Thomson BR, Frische S, Coffman TM, Quaggin SE: Tubulovascular cross-talk by vascular endothelial growth factor a maintains peritubular microvasculature in kidney. J Am Soc Nephrol 26: 1027–1038, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Long DA, Norman JT, Fine LG: Restoring the renal microvasculature to treat chronic kidney disease. Nat Rev Nephrol 8: 244–250, 2012. [DOI] [PubMed] [Google Scholar]
- 21.Collett JA, Mehrotra P, Crone A, Shelley WC, Yoder MC, Basile DP: Endothelial colony-forming cells ameliorate endothelial dysfunction via secreted factors following ischemia-reperfusion injury. Am J Physiol Renal Physiol 312: F897–F907, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saad A, Dietz AB, Herrmann SMS, Hickson LJ, Glockner JF, McKusick MA, et al. : Autologous mesenchymal stem cells increase cortical perfusion in renovascular disease. J Am Soc Nephrol 28: 2777–2785, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP: Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105: 71–77, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J: In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood 109: 4761–4768, 2007. [DOI] [PubMed] [Google Scholar]
- 25.Tasev D, Koolwijk P, van Hinsbergh VW: Therapeutic potential of human-derived endothelial colony-forming cells in animal models. Tissue Eng Part B Rev 22: 371–382, 2016. [DOI] [PubMed] [Google Scholar]
- 26.Humphreys BD, Bonventre JV: Mesenchymal stem cells in acute kidney injury. Annu Rev Med 59: 311–325, 2008. [DOI] [PubMed] [Google Scholar]
- 27.Plotkin MD, Goligorsky MS: Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol 291: F902–F912, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Tögel F, Weiss K, Yang Y, Hu Z, Zhang P, Westenfelder C: Vasculotropic, paracrine actions of infused mesenchymal stem cells are important to the recovery from acute kidney injury. Am J Physiol Renal Physiol 292: F1626–F1635, 2007. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal S, Pittenger MF: Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105: 1815–1822, 2005. [DOI] [PubMed] [Google Scholar]
- 30.Jiang MH, Li G, Liu J, Liu L, Wu B, Huang W, et al. : Nestin(+) kidney resident mesenchymal stem cells for the treatment of acute kidney ischemia injury. Biomaterials 50: 56–66, 2015. [DOI] [PubMed] [Google Scholar]
- 31.Jang HR, Park JH, Kwon GY, Lee JE, Huh W, Jin HJ, et al. : Effect of preemptive treatment with human umbilical cord blood-derived mesenchymal stem cells on the development of renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol 307: F1149–F1161, 2014. [DOI] [PubMed] [Google Scholar]
- 32.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, et al. : Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res 103: 194–202, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kang KT, Allen P, Bischoff J: Bioengineered human vascular networks transplanted into secondary mice reconnect with the host vasculature and re-establish perfusion. Blood 118: 6718–6721, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Au P, Tam J, Fukumura D, Jain RK: Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood 111: 4551–4558, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta S, Verfaillie C, Chmielewski D, Kim Y, Rosenberg ME: A role for extrarenal cells in the regeneration following acute renal failure. Kidney Int 62: 1285–1290, 2002. [DOI] [PubMed] [Google Scholar]
- 36.Poulsom R, Forbes SJ, Hodivala-Dilke K, Ryan E, Wyles S, Navaratnarasah S, et al. : Bone marrow contributes to renal parenchymal turnover and regeneration. J Pathol 195: 229–235, 2001. [DOI] [PubMed] [Google Scholar]
- 37.Dekel B, Shezen E, Even-Tov-Friedman S, Katchman H, Margalit R, Nagler A, et al. : Transplantation of human hematopoietic stem cells into ischemic and growing kidneys suggests a role in vasculogenesis but not tubulogenesis. Stem Cells 24: 1185–1193, 2006. [DOI] [PubMed] [Google Scholar]
- 38.Buzhor E, Omer D, Harari-Steinberg O, Dotan Z, Vax E, Pri-Chen S, et al. : Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol 183: 1621–1633, 2013. [DOI] [PubMed] [Google Scholar]
- 39.Pode-Shakked N, Shukrun R, Mark-Danieli M, Tsvetkov P, Bahar S, Pri-Chen S, et al. : The isolation and characterization of renal cancer initiating cells from human Wilms’ tumour xenografts unveils new therapeutic targets. EMBO Mol Med 5: 18–37, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Melero-Martin JM, Bischoff J: Chapter 13. An in vivo experimental model for postnatal vasculogenesis. Methods Enzymol 445: 303–329, 2008. [DOI] [PubMed] [Google Scholar]
- 41.Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, et al. : Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8: 315–317, 2006. [DOI] [PubMed] [Google Scholar]
- 42.Tögel F, Zhang P, Hu Z, Westenfelder C: VEGF is a mediator of the renoprotective effects of multipotent marrow stromal cells in acute kidney injury. J Cell Mol Med 13: 2109–2114, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE: Oct4 expression in adult human stem cells: Evidence in support of the stem cell theory of carcinogenesis [published correction appears in Carcinogenesis 26: 1316, 2005]. Carcinogenesis 26: 495–502, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Schuetz A, Nana D, Rose C, Zocher G, Milanovic M, Koenigsmann J, et al. : The structure of the Klf4 DNA-binding domain links to self-renewal and macrophage differentiation. Cell Mol Life Sci 68: 3121–3131, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shyh-Chang N, Daley GQ: Lin28: Primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12: 395–406, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dong J, Sutor S, Jiang G, Cao Y, Asmann YW, Wigle DA: c-Myc regulates self-renewal in bronchoalveolar stem cells. PLoS One 6: e23707, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerosuo L, Piltti K, Fox H, Angers-Loustau A, Häyry V, Eilers M, et al. : Myc increases self-renewal in neural progenitor cells through Miz-1. J Cell Sci 121: 3941–3950, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Kobayashi T, Terada Y, Kuwana H, Tanaka H, Okado T, Kuwahara M, et al. : Expression and function of the Delta-1/Notch-2/Hes-1 pathway during experimental acute kidney injury. Kidney Int 73: 1240–1250, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, et al. : Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Langworthy M, Zhou B, de Caestecker M, Moeckel G, Baldwin HS: NFATc1 identifies a population of proximal tubule cell progenitors. J Am Soc Nephrol 20: 311–321, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hoch AI, Binder BY, Genetos DC, Leach JK: Differentiation-dependent secretion of proangiogenic factors by mesenchymal stem cells. PLoS One 7: e35579, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaminski MM, Tosic J, Kresbach C, Engel H, Klockenbusch J, Müller AL, et al. : Direct reprogramming of fibroblasts into renal tubular epithelial cells by defined transcription factors. Nat Cell Biol 18: 1269–1280, 2016. [DOI] [PubMed] [Google Scholar]
- 53.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshimura Y, Taguchi A, Tanigawa S, Yatsuda J, Kamba T, Takahashi S, et al. : Manipulation of nephron-patterning signals enables selective induction of podocytes from human pluripotent stem cells. J Am Soc Nephrol 30: 304–321, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van den Berg CW, Ritsma L, Avramut MC, Wiersma LE, van den Berg BM, Leuning DG, et al. : Renal subcapsular transplantation of PSC-derived kidney organoids induces neo-vasculogenesis and significant glomerular and tubular maturation in vivo. Stem Cell Reports 10: 751–765, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sharmin S, Taguchi A, Kaku Y, Yoshimura Y, Ohmori T, Sakuma T, et al. : Human induced pluripotent stem cell-derived podocytes mature into vascularized glomeruli upon experimental transplantation. J Am Soc Nephrol 27: 1778–1791, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xinaris C, Benedetti V, Rizzo P, Abbate M, Corna D, Azzollini N, et al. : In vivo maturation of functional renal organoids formed from embryonic cell suspensions. J Am Soc Nephrol 23: 1857–1868, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, et al. : A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3: 301–313, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Lin RZ, Moreno-Luna R, Li D, Jaminet SC, Greene AK, Melero-Martin JM: Human endothelial colony-forming cells serve as trophic mediators for mesenchymal stem cell engraftment via paracrine signaling. Proc Natl Acad Sci U S A 111: 10137–10142, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. : Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131: 861–872, 2007. [DOI] [PubMed] [Google Scholar]
- 61.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. : Induced pluripotent stem cell lines derived from human somatic cells. Science 318: 1917–1920, 2007. [DOI] [PubMed] [Google Scholar]
- 62.Kuruvilla JG, Kim CK, Ghaleb AM, Bialkowska AB, Kuo CJ, Yang VW: Krüppel-like factor 4 modulates development of BMI1(+) intestinal stem cell-derived lineage following γ-radiation-induced gut injury in mice. Stem Cell Reports 6: 815–824, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orphanides C, Fine LG, Norman JT: Hypoxia stimulates proximal tubular cell matrix production via a TGF-beta1-independent mechanism. Kidney Int 52: 637–647, 1997. [DOI] [PubMed] [Google Scholar]
- 64.Fine LG, Bandyopadhay D, Norman JT: Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl 75: S22–S26, 2000. [PubMed] [Google Scholar]
- 65.Vagnozzi RJ, Sargent MA, Molkentin JD: Cardiac cell therapy rejuvenates the infarcted rodent heart via direct injection but not by vascular infusion. Circulation 141: 1037–1039, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Homan KA, Gupta N, Kroll KT, Kolesky DB, Skylar-Scott M, Miyoshi T, et al. : Flow-enhanced vascularization and maturation of kidney organoids in vitro. Nat Methods 16: 255–262, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Takebe T, Enomura M, Yoshizawa E, Kimura M, Koike H, Ueno Y, et al. : Vascularized and complex organ buds from diverse tissues via mesenchymal cell-driven condensation. Cell Stem Cell 16: 556–565, 2015. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi Y, Sekine K, Kin T, Takebe T, Taniguchi H: Self-condensation culture enables vascularization of tissue fragments for efficient therapeutic transplantation. Cell Rep 23: 1620–1629, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.