Abstract
In subacute sclerosing panencephalitis (SSPE) the persistence of measles virus (MeV) may be related to the altered immune response. In this study, cytokine responses of lymphocytes and monocytes were evaluated in SSPE compared to controls with non-inflammatory (NICON) and inflammatory (ICON) diseases. Patients with SSPE (n = 120), 78 patients with ICON and 63 patients with NICON were included in this study. Phenotypes of peripheral blood mononuclear cells (PBMC) have been analyzed by flow cytometry. CD3 and CD28, and S. aureus Cowan strain I (SAC) stimulated and unstimulated cells were cultured and IL-2, IL-10, IFN-γ, IL-12p40, IL-12p70 and IL-23 were detected in supernatants by ELISA. MeV peptides were used for MeV-specific stimulation and IFN-γ secretion of PBMC was measured by ELISPOT. Spontaneous and stimulated secretions of IL-10 were lower in SSPE compared to both control groups. T cell stimulation induced lower IFN-γ production than ICON group, but higher IL-2 than NICON group in SSPE. Stimulated PBMC produced lower IL-12p70 in SSPE and had decreased CD46 on the cell surface, suggesting the interaction with the virus. IFN-γ responses against MeV peptides were not prominent and similar to NICON patients. The immune response did not reveal an inflammatory activity to eliminate the virus in SSPE patients. Even IL-10 production was diminished implicating that the response is self-limited in controlling the disease.
Introduction
Subacute sclerosing panencephalitis (SSPE) is a progressive disease of the central nervous system (CNS) affecting mainly children and early adolescents. It is a rare and late complication of measles virus (MeV) infection with fatal outcome. Typically SSPE patients have a history of primary measles infection at an unusually young age followed by a latent period of 6 to 8 years. The incidence of SSPE worldwide is estimated 1 per million [1]. In a recent epidemiological study of Istanbul, the incidence was found as 2 per million with a girl dominance [2].
Both alterations in the host immune system and changes in the MeV have been the subject of investigations on the pathogenesis of SSPE. In early studies, antibodies against nucleocapsid and matrix proteins of the virus were detected in the serum and cerebrospinal fluid (CSF) of patients. Proliferative T cell responses were found comparable to healthy individuals [3]. However, virus-specific cytotoxic activity was impaired while NK cell cytotoxicity was preserved [4]. Suppression of Th1 cytokine production was reported by demonstrating defective IFN-γ response of peripheral blood mononuclear cells (PBMC) to MeV in SSPE patients with severe disease progression [5]. Elevated IL-12p70+p40 and CXCL10 levels in CSF without an accompanying IFN–γ increase compared with other inflammatory and non-inflammatory disease controls were reported [6]. Higher serum IL-2 concentrations and suppressed Th2 cytokines (IL-4, IL-6 and IL-10) were also demonstrated [7]. Lower IL-12 secretion in response to MeV vaccine and PPD, and IFN-γ and IL-10 productions to PPD have also provided evidence for alterations in immune response regulation in a previous study [8]. More recently, activated IL-12/IFN-γ and the IL-23/IL-17/IL-22 pathways were reported in SSPE patients [9].
The virus is anticipated to induce alterations in the immune response of infected cells to MeV. As a costimulatory molecule of the immune system, signaling lymphocyte activating molecule (SLAMF1/SLAM/CD150) is a common receptor for MeV and expressed mainly on activated lymphocytes [10–12]. In MeV infection, SLAM expression is reduced on host cells [13]. On the other hand, CD46 has been shown to act as a receptor mainly for a limited number of MeV strains [14]. The binding of CD46 by MeV has reduced IL-12 production of monocytes thereby regulating the immune response against the virus [15].
In the present study, the immune response to MeV in SSPE patients has been investigated by cytokine secretions of PBMC in response to non-specific and specific stimulations. Possible regulatory changes on the immune response have been evaluated mainly by cytokine measurements.
Materials and methods
Patients and controls
The study was approved by the institutional ethics committee of Istanbul University Istanbul Medical Faculty and written informed consent was taken from the patients and from the parents of the children according to the Declaration of Helsinki. Patients with SSPE (n = 120) were enrolled to the study between the years 2003–2017. Seventy-five of them were male and 45 were female (Median age: 9 years (1–34 years)). All patients fulfilling the criteria for the diagnosis of SSPE are included in this prospective study: Typical clinical presentations (myoclonus, head drops, hemiplegia, deafness, severe mental and behavioral changes, dementia, visual and speech involvement), EEG findings, measles antibody titers in the CSF [1, 16, 17]. Additionally, all 108 patients tested had oligoclonal IgG bands; in 17.6% of patients presented pattern 2, while in 82.4% patients patterns 3 and 4 with concomitant IgG bands in the serum were detected. Only the patients whose parents did not consent are excluded. The patients were referred to the specialized neurology centers of the referral hospitals from Marmara Region and also from Black Sea, East and Southeast Anatolia regions of Turkey. According to the limited information available from the patients, only 56.7% of patients had a natural history of measles disease occurring between 2 months and 9 years of age and 46.7% of the patients had a known history of measles vaccination (Table 1).
Table 1. Characteristics of donors.
| Group | N (M/F) | Age (years) | Disease onset age | Measles vaccination | Measles | Age of infection (months) |
|---|---|---|---|---|---|---|
| SSPE | 120 (75/45) | 9 (1–34) | 9 (3–19) | 46.7% | 56.7% | 18 (2–108) |
| ICON | 78 (41/37) | 8 (1–24) | 6 (2–15) | 43.6% | 7.7% | |
| NICON | 63 (43/20) | 11 (1.5–38) | 6.5 (1–16) | 47.6% | 6.4% |
SSPE: Subacute sclerosing panencephalitis patients, ICON: Controls with inflammatory diseases, NICON: Controls with non-inflammatory diseases. Ages were presented as median values, and minimum and maximum values were given in parentheses. M: male, F: female.
Blood from 141 totally unrelated donors were used as controls: 78 of them had inflammatory diseases (ICON) such as multiple sclerosis, asthma, bronchitis, Miller-Fisher syndrome, type I diabetes, tonsillitis, upper respiratory tract infection and viral infection (37 female and 41 male, median age: 8 years (1–24 years)). As another control group, 63 patients (20 female and 43 male, median age: 11 years (1.5–38 years)) with non-inflammatory diseases (NICON) like afebrile convulsion, anemia, headache, leg pain, fatigue, joint pain, epilepsy, X-linked adenoleucodystrophy, nesioblastosis, vomiting and urticeria were included in this study (Table 1). SSPE patients and control donors were not on immunomodulatory treatment.
Due to the scarcity of the blood obtained from the donors, not all measurements have been performed in all donors. Measles antibodies (IgG) were detected with ELISA kit (2326000, Trinity Biotech, Ireland and ESR102G, Serion, Institut Virion, Germany) in all CSF samples.
Phenotypic staining and reagents
PBMC of donors were isolated from EDTA anti-coagulated blood samples by ficoll density gradient centrifugation. Cells were surface stained with fluorochrome-conjugated mouse anti-human CD3-FITC and -APC (IgG1, A07746, Beckman Coulter, France and C7225, Dako Cytomation, Denmark), CD8-APC (IgG1, C7227, Dako Cytomation, Denmark), CD4-APC and -FITC (IgG1, C7226 and F0766 Dako Cytomation, Denmark), CD19-PC5 (IgG1, A07771, Beckman Coulter, France), CD14-FITC and -PC5 (IgG2a, F0844, Dako Cytomation, Denmark and A07765, Beckman Coulter, France), CD45-FITC/CD14-PE (IgG1/IgG2a, Catalog Nr: 873.032.050, Diaclone, France), CD46-PE (IgG2a, 197–050, Ancell, USA), CD150-PE (IgG1, 12–1509, eBioscience), PD-1-PE (IgG1, 557946, BD Pharmingen, USA) and isotype control antibodies (BD Biosciences Pharmingen, USA and X0950, X0933, X0968, Dako Cytomation, Denmark and A07798, Beckman Coulter, France). Phenotypes have been analyzed by flow cytometry (FACSCalibur, Becton-Dickinson) and compared between groups in respective cell gates.
Cell stimulation and measurements of proliferation and cytokines
To stimulate the T cells, flat-bottomed 96-well plates (TPP, Switzerland) were coated overnight at 4°C with anti-CD3 (10 μg/ml, 854.010.000, Diaclone, France) and anti-CD28 (5 μg/ml, 177–020, Ancell, USA) or with IgG1 isotypic control (5 μg/ml, 857.070.000, Diaclone, France) antibodies. PBMC were seeded as 200.000 cells/well in triplicates. In another set of donors, S. aureus Cowan strain I (SAC, Pansorbin, 507858, Calbiochem, USA) was added to a final dilution of 1:10000. After 72 hours of incubation at 37°C in 5% CO2 with culture medium containing RPMI-1640 (R0883, Sigma, USA), 10% FBS (10082139, Gibco, USA), 100 IU/100 μg/ml penicillin/streptomycin (P4333, Sigma, USA) and 2 mM L-glutamine (25030081, Gibco, USA), supernatants were collected and fresh medium was added. Proliferation was detected by [3H] thymidine incorporation (0.5 μCi/well, 20 Ci/mmol, ART 178C, American Radiolabeled Chemicals, USA) after overnight incubation in culture. Supernatants were stored at -80°C and used for the detection of IL-2, IL-10, IFN-γ (KHC0022, CHC1323, CHC1233, Biosource, USA), IL-12p40 and IL-12p70 (551116 and 559258, BD Biosciences, USA) and IL-23 (BMS2023, Bender MedSystems, Austria) by ELISA according to manufacturer’s protocols.
Peptides from hemagglutinin (H, 30–38), matrix (M, 211–219), C protein (C, 84–92), two of nucleoprotein (N1, 210–218 and N2, 340–348) and the pool of these peptides (MeVp) were used to MeV specific stimulations [18]. All peptides and the MeVp were used as 10 μM (NMI Peptides, Germany). IFN-γ secretion of PBMC was detected with ELISPOT (3420-2AW-Plus, MabTech, Sweden and 874.000.005 Diaclone, France) according to manufacturer’s guidelines. PHA (5 μg/ml, Sigma) was used as positive control and spot counts were presented as spots/200000 cells (CTL Europe GmbH).
Statistical analysis
Statistical analyses were performed by non-parametric tests (Anova and Mann-Whitney U tests) for comparisons between groups using SPSS. Results were presented as median values. A p value < 0.05 was regarded as significant.
Results
Cytokine responses in SSPE
When we evaluated the distributions of T and B cells, and monocytes in the peripheral blood of SSPE patients with two different age-matched control groups with (ICON) or without inflammatory diseases (NICON), the proportion of CD3+ T cells was slightly decreased in SSPE patients compared only to NICON (64.7% vs. 68.9%, p = 0.02), confirming our previous findings [19]. No other alterations were observed in the distribution of CD4+ and CD8+ T cells, CD19+ B cells and CD14+ monocytes in all groups (Table 2).
Table 2. Distribution of CD3+, CD4+ and CD8+ T cells, CD19+ B cells and CD14+ cells among PBMC in the study groups.
| CD3+ cells | CD4+ cells | CD8+ cells | CD19+ cells | CD14+ cells | |
|---|---|---|---|---|---|
| SSPE | 64.7* | 32.4 | 26.1 | 11.6 | 73.9 |
| (15.5–82.7) | (11.4–62.8) | (9.0–45.0) | (1.7–25.7) | (2.4–99.1) | |
| N | 50 | 90 | 89 | 27 | 62 |
| ICON | 66.6 | 34.3 | 25.2 | 10.2 | 76.0 |
| (0.4–82.5) | (11.8–53.7) | (7.9–47.9) | (2.6–17.5) | (12.1–98.8) | |
| N | 44 | 64 | 66 | 22 | 55 |
| NICON | 68.9* | 36.3 | 27.1 | 7.0 | 71.8 |
| (29.2–80.5) | (12.5–59.2) | (15.7–52.8) | (3.8–16.0) | (5.5–96.0) | |
| N | 22 | 33 | 36 | 9 | 26 |
Results are presented as median values, and minimum and maximum values are given in parenthesis. SSPE: Subacute sclerosing panencephalitis patients, ICON: Controls with inflammatory diseases, NICON: Controls with non-inflammatory diseases.
*p = 0.02.
Spontaneous cytokine secretion
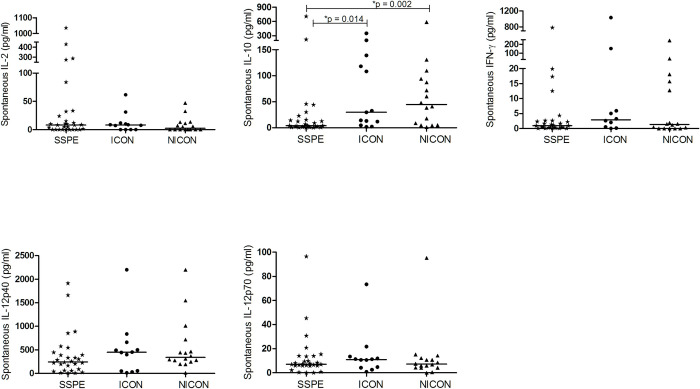
To analyze in vivo stimulated state of PBMC, we firstly measured spontaneous secretion of IL-2, IFN-γ, IL-12 and IL-10 in cell culture. Among all measured cytokines, only IL-10 levels were significantly lower in SSPE compared with both ICON and NICON groups (4.1 vs. 29.8 and 44.5 pg/ml; p = 0.014 and p = 0.002) (Fig 1).
Fig 1. Spontaneous cytokine secretion of PBMC.
Spontaneous in vitro IL-2, IL-10, IFN-γ, IL-12p40 and IL-12p70 secretion of PBMC from subacute sclerosing panencephalitis patients (SSPE, n = 29), controls with inflammatory diseases (ICON, n = 13) and with non-inflammatory diseases (NICON, n = 16) are shown. Horizontal lines depict median values.
T cell receptor mediated stimulation by CD3 and CD28
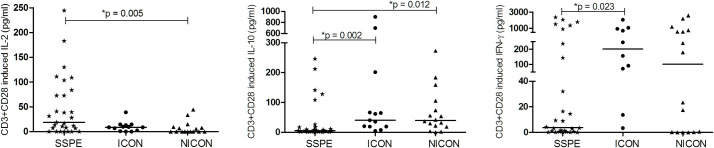
To evaluate the response of T cells by stimulation via T cell receptor, PBMC were incubated with anti-CD3 and anti-CD28 antibodies for 72 hours and cytokine productions were compared between the groups. With stimulation, IL-10 secretion was still lower in patients than in ICON and NICON groups (5.4 vs. 40.2 and 39.5 pg/ml; p = 0.002 and p = 0.012). Similarly, T cell stimulation induced lower levels of IFN-γ production compared with ICON group only (3.8 vs. 201 pg/ml, p = 0.023). However, IL-2 secretion of T cells was higher than that of NICON group (18.7 vs. 0.0 pg/ml; p = 0.005) (Fig 2).
Fig 2. CD3 and CD28 induced cytokine secretion of PBMC.
CD3 and CD28 induced productions of IL-2, IL-10, IFN-γ in subacute sclerosing panencephalitis patients (SSPE, n = 29), controls with inflammatory diseases (ICON, n = 13) and with non-inflammatory diseases (NICON, n = 16) are shown. Horizontal lines depict median values.
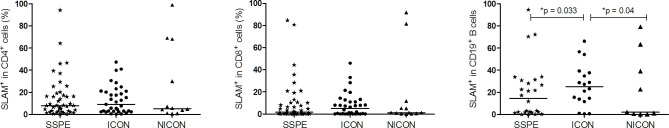
As a costimulatory molecule possibly effecting the cytokine production and a receptor for the MeV, we measured the expression of SLAM on CD4+, CD8+ T cells and B cells. SLAM on CD4+ T cells was slightly higher in SSPE and ICON groups without reaching statistical significance. On CD19+ B cells, SLAM was mainly increased in ICON group (25.29% p = 0.033 and p = 0.04). Relatively higher levels of SLAM in SSPE patients (14.6%) were not significantly different from NICON group (2.1%) (Fig 3, S1 Fig).
Fig 3. Signaling Lymphocyte Activating Molecule (SLAM) expression on T and B lymphocytes.
SLAM expression on CD4+, CD8+ T cells and CD19+ B cells of subacute sclerosing panencephalitis patients (SSPE, n = 44, n = 43 and n = 26), controls with inflammatory diseases (ICON, n = 35, n = 34 and n = 18) and with non-inflammatory diseases (NICON, n = 12 n = 13 and n = 9) are shown. Horizontal lines depict median values.
Despite the differences in cytokine productions, no significant changes between the groups were observed in proliferative responses of T cells to CD3 and CD28 stimulation (S2 Fig).
Stimulation with SAC
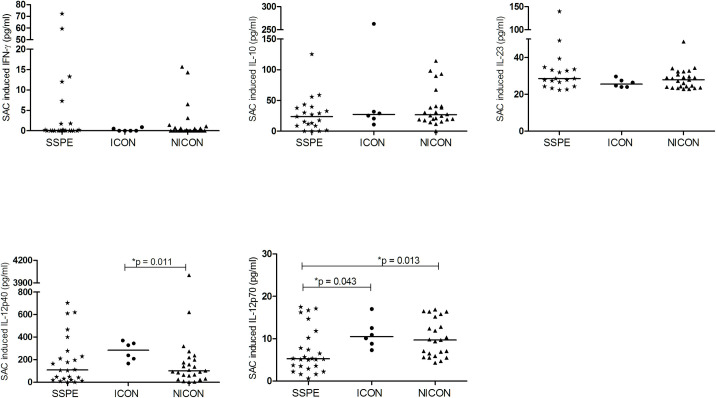
Cytokine production by PBMC was also studied by SAC stimulation to cover a broader group of cell responses [20, 21]. In SSPE patients, SAC induced lower IL-12p70 production compared with ICON and NICON groups (5.3 vs. 10.5 and 9.7 pg/ml, p = 0.043 and p = 0.013). Higher IL-12p40 secretion was detected in ICON patients compared with NICON group (283.4 vs. 101.4 pg/ml, p = 0.011). No other differences in IL-10, IL-23 and IFN-γ productions were detected between the groups in response to SAC (Fig 4).
Fig 4. SAC induced cytokine productions.
IFN-γ, IL-10, IL-23, IL-12p40 and IL-12p70 productions of SAC stimulated PBMC in subacute sclerosing panencephalitis patients (SSPE, n = 26), controls with inflammatory diseases (ICON, n = 6) and with non-inflammatory diseases (NICON, n = 25) are shown. Horizontal lines depict median values.
As the main source of IL-12 is monocytes, the reduced IL-12 production may have been induced by interaction of the virus with cellular receptors/molecules on monocytes [15]. Therefore, we screened the possible MeV receptor, CD46 on monocytes (CD14+). CD46 was lower in SSPE patients than NICON (63.0% vs. 74.4%; p = 0.033), but not than the ICON group (69.3%) (Fig 5, S3 Fig).
Fig 5. CD46 expression on CD14+ monocytes.
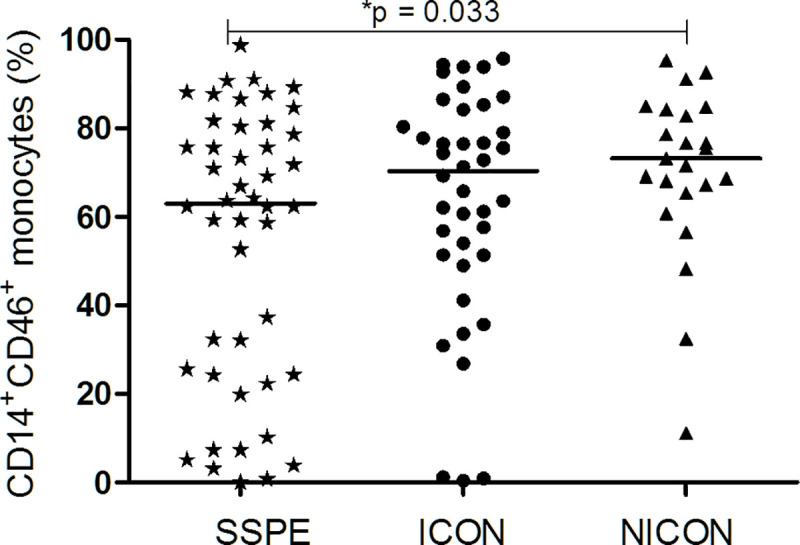
CD46 on CD14+ cell populations was analyzed in subacute sclerosing panencephalitis patients (SSPE, n = 46), controls with inflammatory diseases (ICON, n = 40) and with non-inflammatory diseases (NICON, n = 23). Horizontal lines depict median values.
Antigen-specific T cell stimulation
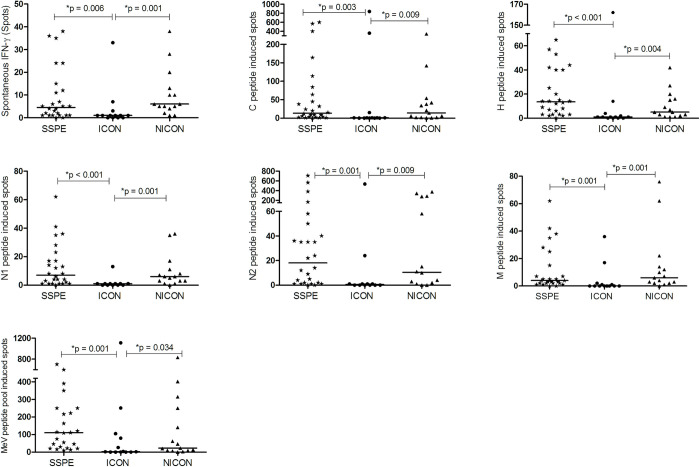
As the etiological agent of SSPE, the specific response against MeV has also been screened and compared with diseased controls. We have evaluated the antigen specific IFN-γ responses against immunodominant MeV peptides which were described previously [18]. SSPE patients had similar reactivity to N1, N2, M, H and C peptides of MeV and MeV peptide pool as in NICON patients. Interestingly, IFN-γ responses to all peptides and peptide pool were reduced in ICON group compared to SSPE, and NICON groups (Fig 6).
Fig 6. MeV peptide induced IFN-γ production of PBMC.
IFN-γ secretion without and with MeV peptide (C, H, N1, N2, M and the pool) induction of PBMC in subacute sclerosing panencephalitis patients (SSPE, n = 26), controls with inflammatory diseases (ICON, n = 13) and with non-inflammatory diseases (NICON, n = 15) are shown. Horizontal lines depict median values of IFN-γ secreting cells per 200000 PMBC.
Expression of programmed cell death-1 (PD-1) on CD8+ T lymphocytes
As a possible mechanism of viral persistence of MeV in SSPE, we have looked at the exhaustion of T cells by analyzing the expression of PD-1 on CD8+ T cells. PD-1 is a member of the CD28 superfamily that delivers negative signals upon interaction with its ligands. However, CD8+ PD-1+ cells were not different between groups in PBMC (S4 Fig).
Discussion
The pathogenesis of the persistence and the late complication of MeV infection in the CNS have not been elucidated. In this study, the immune alterations in the SSPE patients have been compared with two different age-matched control groups, namely patients with other inflammatory- or non-inflammatory-diseases. The immune response against MeV-specific and non-specific stimuli was evaluated in these groups. Changes were detected in cytokine secretion in SSPE compared with both control groups. Notably, cytokine production pattern of SSPE patients was relatively similar to that of NICON group. Thus, the findings of the present study emphasized the moderate immune response in the patients.
MeV infection induces profound and prolonged abnormalities in cellular immune responses in infected hosts causing an immunosuppression [22]. The mechanistic models underlying this suppression have been proposed as altered cytokine profiles, bystander lymphocyte apoptosis and lymphocyte infection and depletion [23]. The immunosuppression associated with MeV could underlay the viral persistence and the development of SSPE [4, 8]. The findings of this study did not provide evidence for an active immunosuppressive mechanism similar to acute measles infection in SSPE development.
In previous evaluations of the immune response, mainly CD4+ T cells, B cells as well as IL-1β, IL-2, IL-6, TNF-α, lymphotoxin and IFN-γ have been detected in brain specimens from SSPE patients, which demonstrated cellular infiltrates, demyelination, gliosis and inflammatory activation [24–27]. Detection of higher IL-10 levels in both CSF [6, 28], and serum of SSPE patients [29], supported a suppressive environment in this disease. In another study, CSF IL-4 and IL-6 concentrations were lower, whereas serum IL-2 concentration was higher in SSPE patients implicating the dominance of Th1 over Th2-type cytokines particularly at the early inflammatory response in SSPE [7]. On the other hand, preserved IL-10 production by PBMC of SSPE patients was reported, whereas defective IFN-γ secretion has been related to the worse progression of disease in response to MeV vaccine [5]. In our previous study, proliferation, as well as IFN-γ, IL-12 and IL-10 productions in response to MeV vaccine were not different from controls, although the response to purified protein derivate (PPD) was impaired in SSPE patients [8]. The decrease in regulatory T cell phenotype and the lower inhibitory NK receptors on CD8+ T cells, and higher activating NK receptors on NK cells indicated also an active state of the immune response possibly caused by the chronic stimulation with viral antigens [19]. A recent report demonstrated increased productions of IL-12, IL-23, IL-17 and IL-22 and higher frequencies of IL-17 and IFN-γ producing cells in response to MeV peptide stimulation in SSPE pointing at both Th1 and Th17 responses [9]. In the present study, production of IL-10 was lower in SSPE compared with both control groups spontaneously and after T cell stimulation. Supporting an immune activity potential, IL-2 response of T cells was higher than in NICON group, whereas IFN-γ production was not as high as in other inflammatory diseases. The MeV peptide specific IFN-γ responses were also similar to NICON patients and not higher. This inefficient IFN-γ response in our cohort is not in accordance with previous findings, but in accordance with the lower IL-12 production. This discrepancy may be related to the selection of our patients with late stages or to the small numbers of patients in different experimental settings in these studies.
Similar to measles infection, a reduction of T cells in SSPE samples was observed in the present and previous studies [19, 30]. This observation may be related to the lymphopenia caused by MeV during the infection. MeV infected monocytes induce apoptosis in uninfected T cells which also may contribute to the pathogenesis of MeV-induced immunosuppression [31].
IL-10 is considered a prototypical anti-inflammatory cytokine, which significantly contributes to the maintenance and reestablishment of immune homeostasis. However, with its pleiotropic roles, it can also promote immune responses by supporting B cell and CD8+ T cell activation. In infectious diseases, it is considered as a master regulator of immunity, as it can mainly help to ameliorate the excessive Th1, Th2 and CD8+ T cell responses in infections [32]. Functionally, reduced production of IL-10 with IFN-γ in response to general T cell stimulation in SSPE may highlight the absence of a highly pro-inflammatory state of the disease. On the other hand, polymorphic features of IL-10 gene may have an effect on susceptibility to SSPE and the decreased production may be related to the low-producer alleles of IL-10 [21], which has not been investigated in SSPE.
The MeV cell entry receptor, SLAM is classified as a costimulatory molecule that favors lymphocyte proliferation, Ig synthesis, and secretion of IFN-γ [33–35]. Therefore, the interaction of MeV with SLAM could have effects on those immune cells leading to virus-mediated activation. In a previous study, SLAM expression was also demonstrated to be increased in lymphocytes, monocytes and brain tissues of two SSPE patients [36]. However, in the present study SLAM on T or B cells was not increased significantly in SSPE.
On the other hand, MeV has been shown specifically to ablate IL-12 production by monocyte/macrophages in vitro through binding to CD46 [15]. Reduced IL-12 production of monocytic cells was also accompanied by the down-regulation of CD46 from the surface of cells infected with vaccine strains of MeV in vitro [37, 38]. Lower expression of CD46 in lesions of SSPE brains suggested also an interaction of CD46 with a SSPE-specific MeV strain [14]. In SSPE patients, the lower frequencies of CD46+ monocytes compared with NICON group in this study also implicated a related interaction of the virus with these cells. The presence of viral RNA has been documented in monocytes during acute measles infection [39]. Although not confirmed in SSPE patients, persistence of viral RNA has been demonstrated in PBMC and lymph nodes of the monkeys experimentally infected with MeV after months [40]. Possibly a low number of MeV present in the body and inducing the stable antibody response may use CD46 to enter the monocytes and internalize these molecules persistently. Moreover, reduced IL-12 production may be related to CD46-downregulation as well as effected by complement and phagocytic receptors on monocytes [41]. As IL-12 is critical for the development of cell mediated immunity and a potent inducer of IFN-γ from T and NK cells, the development of SSPE can pursue in these patients.
On the other hand, a relatively strong immune response generated in the CNS is evident by the anti-MeV antibodies and oligoclonal IgG bands in the CSF and in the serum of majority of SSPE patients. Probably this antibody response is induced by persistent virus in the CNS and with the leakage of the antibodies, some viral antigens can also reach the periphery and cause the subtle changes detected in this study, but not effective in eliminating virus or controlling replication in the CNS. Demonstration of persistent viral RNA in lymphoid cells would have contributed to explain dysfunctional immune response [40].
The study has certain limitations mainly due to the nature of this rare disease. Findings presented here were derived from data obtained throughout many years, which has been a caveat for prospective planning of the experiments in the study. Additionally, the comparisons with two different diseased-control groups have been somehow problematic, as the diseases of the controls were heterogeneous and the donors have been difficult to assign to the respective groups. The two control groups have been composed of children with known diseases with or without inflammatory features.
Conclusions
In SSPE patients, T cells produced lower levels of IL-10 and IFN-γ, but were inducible to produce IL-2, consistent with an altered immune response of T cells, not competent enough to eliminate the virus in SSPE. Monocytic cells of the patients revealed reduced IL-12 production and CD46 surface expression implicating the effect of CD46 binding in SSPE similar to some MeV strains. Reduced production of IL-10 in combination with reduced IFN-γ points at inefficiency of effector functions of T cells. These observations in SSPE pointed at an attenuated inflammatory pattern at a chronic phase of the disease.
Supporting information
SLAM expression on CD19+ cells in a subacute sclerosing panencephalitis patient (SSPE) and in controls with inflammatory diseases (ICON) or non-inflammatory diseases (NICON) are shown.
(TIF)
Spontaneous and CD3+CD28 induced proliferative responses of T cells in subacute sclerosing panencephalitis (SSPE) patients and in controls with inflammatory diseases (ICON) and non-inflammatory diseases (NICON) are shown. Cpm: Counts per minute, SI: Stimulation index (SI = Induced cpm / Spontaneous cpm).
(TIF)
CD14+CD46+ monocytes in a subacute sclerosing panencephalitis patient (SSPE), controls with inflammatory diseases (ICON) and with non-inflammatory diseases (NICON) are shown.
(TIF)
PD-1 on CD8+ T cells in a subacute sclerosing panencephalitis patient (SSPE), controls with inflammatory diseases (ICON) and with non-inflammatory diseases (NICON) are shown. Horizontal lines depict median values.
(TIF)
(PDF)
Acknowledgments
We are grateful to patients and their parents, and to Dr. Erdem Tüzün for critically reading the manuscript.
Data Availability
All relevant data are within the manuscript.
Funding Statement
The study is supported by Istanbul University Research Fund (BAP: ACIP3363-ACIP- 22421-T291).
References
- 1.Graves M. Subacute sclerosing panencephalitis. Neurol Clin. 1984;2: 267–280. [PubMed] [Google Scholar]
- 2.Onal AE, Gurses C, Direskeneli GS, Yılmaz G, Demirbilek V, Yentur SP, et al. Subacute sclerosing panencephalitis surveillance study in Istanbul. Brain Dev. 2006;28: 183–189. 10.1016/j.braindev.2005.07.004 [DOI] [PubMed] [Google Scholar]
- 3.Dhib-Jalbut S, McFarland HF, Mingioli ES, Sever JL, McFarlin DE. Humoral and cellular immune responses to matrix protein of measles virus in subacute sclerosing panencephalitis. J Virol. 1988;62: 2483–2489. 10.1128/JVI.62.7.2483-2489.1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhib-Jalbut S, Jacobson S, McFarlin DE, McFarland HF. Impaired human leukocyte antigen–restricted measles virus–specific cytotoxic T‐cell response in subacute sclerosing panencephalitis. Ann Neurol. 1989;25: 272–280. 10.1002/ana.410250311 [DOI] [PubMed] [Google Scholar]
- 5.Hara T, Yamashita S, Aiba H, Nihei K, Koide N, Good RA, et al. Measles virus-specific T helper 1/T helper 2-cytokine production in subacute sclerosing panencephalitis. J Neurovirol. 2000;6: 121–126. 10.3109/13550280009013155 [DOI] [PubMed] [Google Scholar]
- 6.Saruhan-Direskeneli G, Gürses C, Demirbilek V, Yentür SP, Yilmaz G, Önal E, et al. Elevated interleukin-12 and CXCL10 in subacute sclerosing panencephalitis. Cytokine. 2005;32: 104–110. 10.1016/j.cyto.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 7.Aydin ÖF, Ichiyama T, Anlar B. Serum and cerebrospinal fluid cytokine concentrations in subacute sclerosing panencephalitis. Brain Dev. 2010;32: 463–466. 10.1016/j.braindev.2009.04.018 [DOI] [PubMed] [Google Scholar]
- 8.Yentür SP, Gürses C, Demirbilek V, Yilmaz G, Önal AE, Yapici Z, et al. Alterations in cell-mediated immune response in subacute sclerosing panencephalitis. J Neuroimmunol. 2005;170: 179–185. 10.1016/j.jneuroim.2005.09.002 [DOI] [PubMed] [Google Scholar]
- 9.Uygun DFK, Uygun V, Burgucu D, Ekinci NÇ, Sallakçı N, Filiz S, et al. Role of the Th1 and Th17 Pathway in Subacute Sclerosing Panencephalitis. J Child Neurol. 2019;34: 815–819. 10.1177/0883073819860631 [DOI] [PubMed] [Google Scholar]
- 10.Ono N, Tatsuo H, Tanaka K, Minagawa H YY. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J Virol. 2001;75: 1594–1600. 10.1128/JVI.75.4.1594-1600.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tatsuo H, Yanagi Y. The morbillivirus receptor SLAM (CD150). Microbiol Immunol. 2002;46: 135–142. 10.1111/j.1348-0421.2002.tb02678.x [DOI] [PubMed] [Google Scholar]
- 12.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406: 893–897. 10.1038/35022579 [DOI] [PubMed] [Google Scholar]
- 13.Welstead GG, Hsu EC, Iorio C, Bolotin S, Richardson CD. Mechanism of CD150 (SLAM) Down Regulation from the Host Cell Surface by Measles Virus Hemagglutinin Protein. J Virol. 2004;78: 9666–9674. 10.1128/JVI.78.18.9666-9674.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuaid S, Cosby SL. An immunohistochemical study of the distribution of the measles virus receptors, CD46 and SLAM, in normal human tissues and subacute sclerosing panencephalitis. Lab Investig. 2002;82: 403–409. 10.1038/labinvest.3780434 [DOI] [PubMed] [Google Scholar]
- 15.Atabani SF, Byrnes AA, Jaye A, Kidd IM, Magnusen AF, Whittle H, et al. Natural Measles Causes Prolonged Suppression of Interleukin‐12 Production. J Infect Dis. 2001. 10.1086/321009 [DOI] [PubMed] [Google Scholar]
- 16.Garg RK. Subacute sclerosing panencephalitis. Postgrad Med J. 2002;78: 63–70. 10.1136/pmj.78.916.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gürses C, Öztürk A, Baykan B, Gökyiğit A, Eraksoy M, Barlas M, et al. Correlation between Clinical Stages and EEG Findings of Subacute Sclerosing Panencephalitis. Clin EEG Neurosci. 2000;31: 201–206. 10.1177/155005940003100409 [DOI] [PubMed] [Google Scholar]
- 18.Van Els CACM, Nanan R. T cell responses in acute measles. Viral Immunol. 2002;15: 435–450. 10.1089/088282402760312322 [DOI] [PubMed] [Google Scholar]
- 19.Yentur SP, Gurses C, Demirbilek V, Adin-Cinar S, Kuru U, Uysal S, et al. A decrease of regulatory T cells and altered expression of NK receptors are observed in subacute sclerosing panencephalitis. Viral Immunol. 2014;27: 506–511. 10.1089/vim.2014.0070 [DOI] [PubMed] [Google Scholar]
- 20.Seegers D, Zwiers A, Strober W, Peña AS, Bouma G. A TaqI polymorphism in the 3′ UTR of the IL-12 p40 gene correlates with increased IL-12 secretion. Genes Immun. 2002;3: 419–423. 10.1038/sj.gene.6363919 [DOI] [PubMed] [Google Scholar]
- 21.Yilmaz V, Yentür SP, Saruhan-Direskeneli G. IL-12 and IL-10 polymorphisms and their effects on cytokine production. Cytokine. 2005;30: 188–194. 10.1016/j.cyto.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 22.Griffin DE. Measles virus persistence and its consequences. Curr Opin Virol. 2020;41: 46–51. 10.1016/j.coviro.2020.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Vries RD, de Swart RL. Measles Immune Suppression: Functional Impairment or Numbers Game? PLoS Pathog. 2014;10: 10–13. 10.1371/journal.ppat.1004482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagano I, Nakamura S, Yoshioka M, Onodera J, Kogure K, Itoyama Y. Expression of cytokines in brain lesions in subacute sclerosing panencephalitis. Neurology. 1994;44: 710 LP– 710. 10.1212/wnl.44.4.710 [DOI] [PubMed] [Google Scholar]
- 25.Nagano I, MD SN, Yoshioka M, MD KK. Immunocytochemical analysis of the cellular infiltrate in brain lesions in subacute sclerosing panencephalitis. Neurology. 1991;41: 1639 LP–1639. 10.1212/WNL.41.10.1639 [DOI] [PubMed] [Google Scholar]
- 26.Hofman FM, Hinton DR, Baemayr J, Weil M, Merrill JE. Lymphokines and immunoregulatory molecules in subacute sclerosing panencephalitis. Clin Immunol Immunopathol. 1991;58: 331–342. 10.1016/0090-1229(91)90124-s [DOI] [PubMed] [Google Scholar]
- 27.Anlar B, Söylemezoğlu F, Aysun S, Köse G, Belen D, Yalaz K. Tissue inflammatory response in subacute sclerosing panencephalitis (SSPE). J Child Neurol. 2001;16: 895–900. 10.1177/088307380101601206 [DOI] [PubMed] [Google Scholar]
- 28.Mistchenko AS, Fornari MC, Viegas M, Barrero PR and DR. Detection of interleukin 10 in cerebrospinal fluid of patients with subacute sclerosing panencephalitis. J Neurovirol. 2005;11: 66–69. 10.1080/13550280590901769 [DOI] [PubMed] [Google Scholar]
- 29.Ichiyama T, Siba P, Suarkia D, Reeder J, Takasu T, Miki K, et al. Analysis of serum and cerebrospinal fluid cytokine levels in subacute sclerosing panencephalitis in Papua New Guinea. Cytokine. 2006;33: 17–20. 10.1016/j.cyto.2005.11.009 [DOI] [PubMed] [Google Scholar]
- 30.Sanal O, Başaran M, Aysun S, Yeğin O, Ersoy F BA. Subpopulations of T lymphocytes in subacute sclerosing panencephalitis (SSPE). Turk J Pediatr. 1986;28: 23–9. [PubMed] [Google Scholar]
- 31.Vuorinen T, Peri P, Vainionpää R. Measles virus induces apoptosis in uninfected bystander T cells and leads to granzyme B and caspase activation in peripheral blood mononuclear cell cultures. Eur J Clin Invest. 2003;33: 434–442. 10.1046/j.1365-2362.2003.01164.x [DOI] [PubMed] [Google Scholar]
- 32.Couper KN, Blount DG, Riley EM. IL-10: The Master Regulator of Immunity to Infection. J Immunol. 2008;180: 5771–5777. 10.4049/jimmunol.180.9.5771 [DOI] [PubMed] [Google Scholar]
- 33.Punnonen BJ, Cocks BG, Carballido JM, Bennett B, Peterson D, Aversa G, et al. Lymphocytic Activation Molecule (SLAM) Induce. 1997;185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castro AG, Hauser TM, Cocks BG, Abrams J, Zurawski S, Churakova T, et al. Molecular and functional characterization of mouse signaling lymphocytic activation molecule (SLAM): Differential expression and responsiveness in Th1 and Th2 cells. J Immunol. 1999;163: 5860–5870. [PubMed] [Google Scholar]
- 35.Hsu EC, Iorio C, Sarangi F, Khine AA, Richardson CD. Cdw150(SLAM) is a receptor for a lymphotropic strain of measles virus and may account for the immunosuppressive properties of this virus. Virology. 2001;279: 9–21. 10.1006/viro.2000.0711 [DOI] [PubMed] [Google Scholar]
- 36.Piskin AK, Akpinar P, Muftuoglu S, Anlar B. Signaling lymphocyte activating molecule (SLAM) expression in subacute sclerosing panencephalitis. Brain Dev. 2007;29: 439–442. 10.1016/j.braindev.2006.11.009 [DOI] [PubMed] [Google Scholar]
- 37.Yanagi Y, Takeda M, Ohno S, Hashiguchi T. Measles virus receptors. Curr Top Microbiol Immunol. 2009;329: 13–30. 10.1007/978-3-540-70523-9_2 [DOI] [PubMed] [Google Scholar]
- 38.Tanaka K, Minagawa H, Xie MF, Yanagi Y. The measles virus hemagglutinin downregulates the cellular receptor SLAM (CD150). Arch Virol. 2002;147: 195–203. 10.1007/s705-002-8312-0 [DOI] [PubMed] [Google Scholar]
- 39.Esolen LM, Ward BJ, Moench TR, Griffin DE. Infection of Monocytes during Measles. 2016;168: 47–52. [DOI] [PubMed] [Google Scholar]
- 40.Griffin DE, Lin WHW, Nelson AN. Understanding the causes and consequences of measles virus persistence. F1000Research. 2018;7: 1–8. 10.12688/f1000research.12094.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Karp CL. Measles: Immunosuppression, interleukin-12, and complement receptors. Immunol Rev. 1999;168: 91–101. 10.1111/j.1600-065x.1999.tb01285.x [DOI] [PubMed] [Google Scholar]