ABSTRACT
EVIDENS is an ongoing, prospective, non-interventional study evaluating the effectiveness and safety of nivolumab in lung cancer patients in France (ClinicalTrials.gov NCT03382496).
Adults with a pathologically confirmed diagnosis of lung cancer and initiating treatment with nivolumab were recruited from 146 sites in France. This analysis included only patients with non-small cell lung cancer (NSCLC) who received ≥1 nivolumab infusion, and evaluated patient characteristics at the time of nivolumab initiation and its effectiveness and safety after a median follow-up of 18 months.
A total of 1,420 patients with NSCLC were included, most of whom had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0–1 (82.9%), non-squamous histology (69.2%) and stage IV disease (91.4%). Brain metastases were present in 19.9% of patients. Nivolumab was a second-line or ≥third-line regimen in 73.6% and 26.1% of patients, respectively. Almost all patients had prior chemotherapy (99.7%). Median overall survival was 11.2 months (95% confidence interval [CI]: 10.0–12.4). ECOG PS, smoking status, corticosteroids at baseline, epidermal growth factor receptor mutation status, presence of symptomatic brain metastases and treatment-related adverse events (TRAEs) were independent predictors of survival. Grade 3 and 4 TRAEs were reported in 105 (7.4%) and 12 (0.8%) patients, respectively; no treatment-related deaths were reported.
Preliminary results of the EVIDENS study confirm the effectiveness and safety of nivolumab, mostly in pre-treated advanced NSCLC patients, with similar benefits to those observed in the phase III randomized clinical trials, despite a broader study population.
KEYWORDS: EVIDENS, france, nivolumab, non-small cell lung cancer, observational study
Introduction
Lung cancer is one of the most commonly diagnosed cancer types and the leading cause of cancer-related deaths, with approximately 470,000 new cases reported in Europe in 2018.1 More than 46,000 people were diagnosed with lung cancer in France in 2018.2 Lung cancer is frequently diagnosed at an advanced stage and 5-year survival rates do not exceed 5%.3 Non-small cell lung cancer (NSCLC) is the most common histological subtype, accounting for 87% of all cases.4
In France, most patients with NSCLC without an actionable oncogenic driver receive platinum-based chemotherapy as first-line treatment.5 In phase III clinical trials, antibodies targeting programmed cell death 1 (PD-1)/programmed cell death ligand 1 (PD-L1) such as nivolumab, pembrolizumab and atezolizumab have shown greater efficacy compared with docetaxel in second-line treatment of NSCLC.6–9 PD-1/PD-L1 blockers also surpassed chemotherapy in first-line treatment, either as monotherapy in patients with PD-L1–expressing tumors or in combination with other systemic treatments (i.e. chemotherapy or other immune checkpoint inhibitors).10
Nivolumab has been available in France since January 2015, at first under the Temporary Authorization for Use program, and then as a marketed drug for locally advanced or metastatic NSCLC patients who have previously received chemotherapy. The pivotal phase III CheckMate 0177 and 0576 randomized clinical trials demonstrated a significantly improved OS benefit with nivolumab over docetaxel among these patients, with a significant improvement in health-related quality of life (HRQoL).11,12
To date, there have been few large-scale prospective real-world studies reporting the effectiveness and safety of nivolumab treatment in advanced NSCLC patients in Europe. The major strength of a prospective cohort study is the accuracy of data collection with regard to exposures, confounders and endpoints.13 Instead, data have been reported from small patient cohorts, retrospective studies and early access programs, for which inclusion/exclusion enrollment criteria may be restrictive.13–27 Due to known variation in cancer survival rates across Europe,28 country-specific data may be more appropriate to describe the real-world experience with nivolumab in the treatment of NSCLC. Furthermore, data collected in the context of a real-world study can also help to address important clinical evidence gaps such as the outcomes in patients who were underrepresented in, or excluded from, pivotal clinical trials of nivolumab due to more severe comorbidities or poor prognostic factors.
EVIDENS (Lung cancer patients trEated with NiVolumab: a longItuDinal, prospecEctive, observatioNal, multicentric Study) is an ongoing, prospective, non-interventional study of lung cancer patients in France who initiated treatment with nivolumab in 2016–2017. Key objectives are to describe the demographic and clinical characteristics and survival outcomes over 3 years. Presented herein are the preliminary results in NSCLC patients.
Materials and methods
Study design and patients
In order to be considered eligible for inclusion in the study, clinical sites had to have ≥40 patients treated with chemotherapy for lung cancer in 2014 (according to the Programme National de Médicalisation des Systèmes d’Information, PMSI). This threshold was the result of a trade-off between representativeness (systematic sampling of any center at which patients started nivolumab for lung cancer) and feasibility (sufficient enrollment). Using this threshold, 47% of centers listed in the PMSI in 2014 were contacted, which covered 91% of patients who received chemotherapy for lung cancer.
Patients ≥18 years old at the time of nivolumab initiation with a pathologically confirmed lung cancer diagnosis were eligible for inclusion. PD-L1 testing was not necessary for enrollment. If performed, PD-L1 expression was tested in tumor cells and/or immune cells at the investigator’s discretion. The test result (expressed vs not expressed) was reported by the investigator regardless of cutoff. Nonetheless, the proportion of tumor cells positive for PD-L1 was captured if available. Patients receiving nivolumab as part of an interventional study were excluded. Patient selection was based on a systematic sampling technique: all consecutive eligible patients were expected to be included in the study, up to 30 patients per investigator.
While physicians could prescribe nivolumab at their own discretion, the recommended dose at the time of study initiation was 3 mg/kg infused every 2 weeks. As of April 23, 2018, a flat dose of 240 mg infused every 2 weeks was approved in Europe.29
Baseline sociodemographic characteristics, disease characteristics and history, and prior treatments were collected. Data were collected at 13 patient visits over 36 months: inclusion visit (index date) and follow-up at day 15 and at 1, 2, 3, 6, 9, 12, 15, 18, 24, 30 and 36 months. However, all study visits were scheduled as per real-life clinical practice; no interventions, extra procedures, or extra visits were mandatory. Patient data were collected by investigators using electronic case report forms (eCRF). Collected data were remotely checked and secured after approval by the National Information Science and Liberties Commission and the Advisory Committee on Information Processing in Material Research in the Field of Health.
The study was approved by the French National Agency for Medicines, conducted according to local ethical standards and registered on ClinicalTrials.gov (NCT03382496). In accordance with local regulations, patients provided either written or oral consent before enrollment into the study.
The present analysis was restricted to patients with NSCLC who received ≥1 nivolumab infusion.
Study outcomes
The primary objectives of the EVIDENS study are: a) to describe the sociodemographic and clinical characteristics of patients at initial diagnosis and after initiation of nivolumab treatment, in the total population and according to histology (squamous or non-squamous NSCLC); and b) to estimate 3-year overall survival (OS) after initiation of nivolumab, both in the total population and according to histology. Secondary objectives include assessment of OS at 1 and 2 years, and progression-free survival (PFS), overall response rate (ORR), health-related quality of life (HRQoL) assessed using the EuroQol-5D-3 Level (EQ-5D-3 L) questionnaires and treatment-related adverse events (TRAEs; incidence, grade and management) at 1, 2 and 3 years of nivolumab treatment.
This analysis presents patient characteristics at inclusion, best ORR at 6 months, PFS, OS and TRAEs for patients who initiated nivolumab and various subgroup analyses (data cutoff: April 5, 2019).
Statistical analysis
Descriptive statistics were used to summarize patient characteristics (proportions and medians were calculated for categorical and continuous variables, respectively). Median follow-up time was determined according to methods described by Schemper and Smith.30 OS, PFS and duration of response were estimated using the Kaplan-Meier method with their 95% confidence intervals (CIs). If an event (progression or death) was not recorded at the time of database lock, patients were censored at the date of the last known visit for which the absence of event was reported. The chi squared test was used ad hoc to test for possible differences in the rates of TRAEs according to age, autoimmune disease, brain metastases and Eastern Cooperative Oncology Group performance status (ECOG PS). Multivariate Cox proportional-hazards regression models, adjusted for variables conventionally included and/or with a known prognostic value (i.e. age, sex, histology and ECOG PS), were used to compute hazard ratios with 95% CIs for the association between patient baseline characteristics and survival with statistical significance assessed at P ≤ 0.05. Each variable was modeled using available cases. Unless otherwise specified, data not reported in the eCRF by the investigator were excluded from the analysis, and thus from percentage calculations. All statistical analyses were performed using SAS version 7.
Results
The study prospectively enrolled 1,462 lung cancer patients at 146 centers in France between October 2016 and November 2017. Of these, 1,420 patients had NSCLC and received ≥1 nivolumab infusion. At the time of analysis, the median follow-up was 18 months (range 0–25.1).
Patient characteristics at nivolumab initiation and treatment pattern
The majority of patients had an ECOG PS of 0 or 1 (82.9%), stage IV disease (91.4%) and a non-squamous histology (69.2%; Table 1). Brain metastases were present in 19.9% of patients, about one-quarter of whom had symptomatic lesions and two-thirds had treated lesions. PD-L1 expression status was assessed in 211 (15.9%) patients, of whom 61.6% were reported to have a PD-L1-positive tumor. An epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) translocation were reported in 44 (4.9%) and four (0.5%) patients out of 904 and 823 tested individuals, respectively. A total of 42 (3.0%) patients had an active autoimmune disease, including rheumatoid arthritis (n = 13), type 1 diabetes (n = 7) and hypothyroidism (n = 5). The majority of patients were previously treated with platinum-based chemotherapy (98.8%) and received nivolumab as second-line treatment (73.6%; Table 2). The median duration of treatment with nivolumab was 72 days (range 1–749). Overall, 45.5% of patients received further treatment after nivolumab (Table 2).
Table 1.
Baseline characteristics of patients included in the study
| Characteristics | Total n = 1,420 |
Non-squamous NSCLC n = 983 |
Squamous NSCLC n = 437 |
|---|---|---|---|
| Sex | |||
| Male | 986 (69.4) | 633 (64.4) | 353 (80.8) |
| Median age, years (range) | 66 (35–91) | 65 (35–91) | 68 (44–91) |
| Patients aged ≥80 years | 116 (8.2) | 69 (7.0) | 47 (10.8) |
| Smoking status1 | |||
| Nonsmoker | 145 (10.2) | 122 (12.4) | 23 (5.3) |
| Former or current smoker | 1,272 (89.8) | 858 (87.6) | 414 (94.7) |
| ECOG PS at inclusion visit2 | |||
| 0 or 1 | 1,172 (82.9) | 829 (84.6) | 343 (79.2) |
| 2 | 192 (13.6) | 122 (12.4) | 70 (16.2) |
| 3 or 4 | 49 (3.5) | 29 (3.0) | 20 (4.6) |
| TNM classification at inclusion visit I–II* |
4 (0.3) | 2 (0.2) | 2 (0.2) |
| IIIA | 24 (1.7) | 11 (1.1) | 13 (3.0) |
| IIIB | 94 (6.6) | 31 (3.2) | 63 (14.1) |
| IV | 1298 (91.4) | 939 (95.5) | 359 (82.2) |
| Median number of metastatic sites, n (range) | 2 (0–8) | 2 (0–7) | 2 (0–8) |
| Patients with brain metastases | 282 (19.9) | 237 (24.1) | 45 (10.3) |
| Symptomatic brain metastases | 78 (5.5) | 68 (6.9) | 10 (2.3) |
| Treated brain metastases | 197 (13.9) | 165 (16.8) | 32 (7.3) |
| Patients with liver metastases | 235 (16.5) | 168 (17.1) | 67 (15.3) |
| Active autoimmune disease | 42 (3.0) | 29 (3.0) | 13 (3.0) |
All values are presented as n (%) unless stated otherwise.
1three missing value;
2seven missing values.
*likely understood as stage at diagnosis instead of stage at nivolumab initiation.
ECOG PS, Eastern Cooperative Oncology Group Performance Status; max, maximum; min, minimum; NSCLC, non-small cell lung cancer; TNM, tumor, nodes, metastasis.
Table 2.
Treatment patterns among patients included in the study
| Treatment | Overall n = 1,420 |
|---|---|
| Nivolumab treatment line | |
| 1st line* | 4 (0.3) |
| 2nd line | 1,045 (73.6) |
| 3rd line or higher | 371 (26.1) |
| Treatment received after nivolumab discontinuation | 646 (45.5) |
| Chemotherapy | 527 (37.1) |
| Docetaxel | 174 (12.3) |
| Gemcitabine | 184 (13.0) |
| Paclitaxel | 199 (14.0) |
| Radiotherapy | 168 (11.8) |
| Targeted therapy | 110 (7.7) |
| Anti-EGFR | 68 (4.8) |
| Immunotherapy | 19 (1.3) |
All values presented as n (%). EGFR, epidermal growth factor receptor.
*Patients likely refractory to previous multimodal treatment given for a non-metastatic disease.
Effectiveness
At 6 months, the investigator-assessed best ORR was 19.6% (95%CI: 17.5–21.6; partial response: 18.5%; complete response: 1.1%). Median duration of response was 13.4 months (95%CI: 11.0–16.0). Overall, median OS was 11.2 months (95%CI: 10.0–12.4; Figure 1) and the 12-month OS rate was 48.6% (95%CI: 45.9–51.3). The median OS estimate in patients with non-squamous and squamous NSCLC was 12.1 months (95%CI: 10.2–13.5) and 10.2 months (95%CI: 8.6–12.1), respectively (Figure S1). No statistical difference was observed between these two subgroups in a multivariate analysis (Table 3). The median OS was 11.8 months (95%CI: 8.9–14.8) in patients with positive PD-L1 expression and 9.1 months (95%CI: 7.6–15.8) in patients with no PD-L1 expression. Median OS in patients with PD-L1 expression of ≥50% was similar to the former group (11.8 months, 95%CI: 7.3–18.4). Univariate analysis did not show any statistically significant effect of positive PD-L1 expression on median OS (Table 3).
Figure 1.
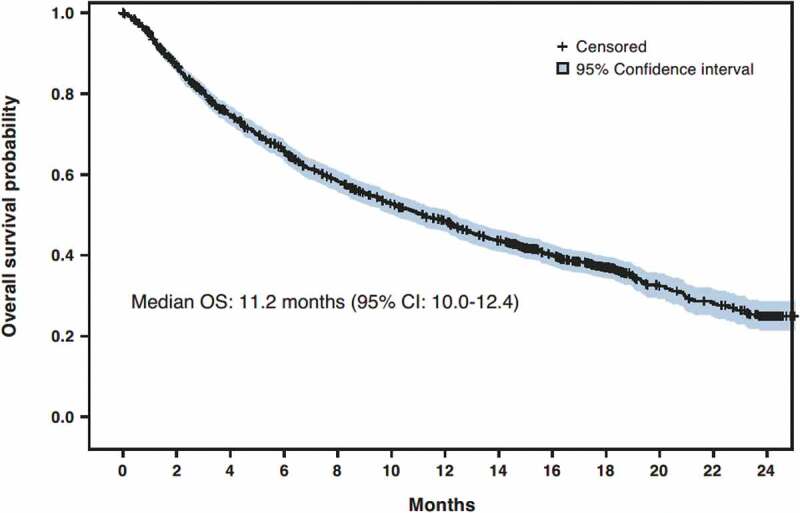
Overall survival. CI, confidence interval; OS, overall survival
Table 3.
Cox proportional-hazards regression model for factors affecting overall survival
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| Variable | Median OS (95% CI), months | HR (95% CI) |
P | HR (95% CI) | P | |
| Histology | Squamous | 10.2 (8.6–12.1) | ref | ref | ||
| Non-squamous | 12.1 (10.2–13.5) | 0.86 (0.75–0.99) | .0367 | 0.88 (0.76–1.02) | .0825 | |
| Any grade TRAEs | No | 8.5 (7.4–9.5) | ref | ref | ||
| Yes | 18.3 (15.8–19.4) | 0.55 (0.48–0.64) | .0001 | 0.55 (0.48–0.64) | .0001 | |
| Grade 3–4 TRAEs | No | 10.8 (9.7–12.3) | ref | |||
| Yes | 12.6 (10.9–19.1) | 0.83 (0.65–1.07) | .145 | |||
| ECOG PS | 0-1 | 13.0 (11.9–14.5) | ref | ref | ||
| 2 | 4.9 (4.0–6.3) | 1.97 (1.65–2.36) | .0001 | 1.98 (1.65–2.38) | .0001 | |
| 3-4 | 3.5 (2.1–7.7) | 2.22 (1.61–3.06) | .0001 | 2.22 (1.61–3.06) | .0001 | |
| Brain metastasis | No | 11.9 (10.2–12.8) | ref | |||
| Yes | 9.8 (7.6–12.2) | 1.07 (0.90–1.27) | .4258 | |||
| Symptomatic brain metastasis | No | 11.5 (10.2–12.6) | ref | ref | ||
| Yes | 9.2 (4.9–10.8) | 1.37 (1.03–1.81) | .0283 | 1.38 (1.04–1.84) | .0277 | |
| Active auto-immune disease | No | 11.1 (10.0–12.4) | ref | |||
| Yes | 11.3 (8.3–16.3) | 1.07 (0.74–1.56) | .7071 | |||
| Age | ≥80 | 9.8 (6.7–13.0) | ref | |||
| <80 | 11.3 (10.2–12.5) | 0.92 (0.72–1.17) | .4755 | |||
| PD-L1 | Not expressed | 9.1 (7.6–15.8) | ref | |||
| Expressed | 11.8 (8.9–14.8) | 0.94 (0.66–1.34) | .7364 | |||
| PD-L1 | <50%* | 11.6 (6.7–14.8) | ref | |||
| ≥50%* | 11.8 (7.3–18.4) | 0.93 (0.61–1.43) | .7478 | |||
| Corticosteroids at inclusion | No | 12.0 (10.5–13.0) | ref | ref | ||
| Yes | 5.8 (4.2–8.4) | 1.62 (1.28–2.05) | .0001 | 1.54 (1.22–1.96) | .0004 | |
| EGFR status | Wildtype | 12.2 (10.2–13.8) | ref | ref | ||
| Mutated | 8.1 (4.5–11.3) | 1.50 (1.03–2.18) | .035 | 1.50 (1.02–2.21) | .041 | |
| Smoking status | Current/former smoker | 11.7 (10.2–12.9) | ref | ref | ||
| Never smoked | 8.9 (6.1–11.5) | 1.26 (1.02–1.56) | .0328 | 1.35 (1.07–1.69) | .0109 | |
p-values in bold are significant.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group Performance Status; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1; ref, reference group; TRAEs, treatment-related adverse events
*% of tumor cells expressing PD-L1
All models were adjusted for age, sex, histology and ECOG PS, except when one of these variables was the main factor of interest, in which case it was not included as an adjustment factor.
The median PFS estimate in patients with squamous and non-squamous histology was 2.8 months (95%CI: 2.6–3.4) and 3.0 months (95%CI: 2.6–3.2), respectively (Figure S2). The 12-month PFS rate was 27.9% (95%CI: 25.0–30.8) in patients with non-squamous NSCLC and 24.4% (95%CI: 20.3–28.6) in patients with squamous NSCLC. Overall median PFS was 2.8 months (95%CI: 2.6–3.2; Figure 2).
Figure 2.
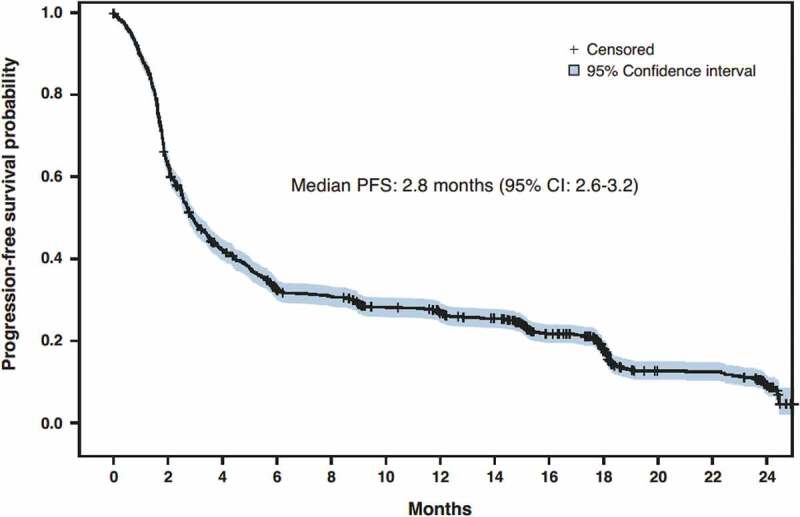
Progression-free survival. CI, confidence interval; PFS, progression-free survival
The multivariate analysis found that patients with an EGFR mutated status, who never smoked, had corticosteroid treatment at baseline, had symptomatic brain metastasis or who had an ECOG PS status 2 or 3–4 had a significantly shorter OS than comparator subgroups (Table 3). OS in subgroups of patients according to baseline ECOG PS are shown in Figure S3. Conversely, age showed no significant relationship with OS in univariate analysis (Table 3).
Safety
A total of 496 (34.9%) patients experienced TRAEs of any grade during the study (Table 4); grade 3 and 4 TRAEs were reported in 105 (7.4%) and 12 (0.8%) patients, respectively. Immune-mediated TRAEs were reported in 14.2% of patients (2.7% were grade 3 and 0.4% were grade 4). The median time to onset of any TRAE was 17 days (range 0–481). A total of 101 (7.1%) patients permanently discontinued the study due to TRAEs. At least one serious TRAE, whether immune-mediated or not, was observed in 137 (9.6%) patients, 18.3% of whom resumed nivolumab treatment after a temporary suspension. No treatment-related deaths were reported. TRAEs of special interest of any grade included interstitial lung disease (1.2%), colitis (0.8%), cardiac disorders (0.1%) and nervous system disorders (2.0%) such as headache (0.6%) and paresthesia (0.5%). Table S1 shows TRAEs reported in patients with active autoimmune disease.
Table 4.
Treatment-related adverse events reported during the study
| Patients experiencing any grade TRAEs*, n (%) | 496 (34.9) |
|---|---|
| Any grade TRAEs reported in ≥1% of patients*, n (%) | |
| Asthenia | 79 (5.6) |
| Diarrhea | 61 (4.3) |
| Pruritus | 55 (3.9) |
| Hypothyroidism | 42 (3.0) |
| Hyperthyroidism | 39 (2.7) |
| Arthralgia | 36 (2.5) |
| Fatigue | 25 (1.8) |
| Decreased appetite | 22 (1.5) |
| Anemia | 20 (1.4) |
| Interstitial lung disease | 17 (1.2) |
| Dry skin | 17 (1.2) |
| Rash | 14 (1.0) |
| Patients experiencing grade 3–4 TRAEs*, n (%) | 117 (8.2) |
| Grade 3–4 TRAEs reported in ≥0.3% of patients*, n (%) | |
| Diarrhea | 11 (0.8) |
| Asthenia | 9 (0.6) |
| General physical health deterioration | 7 (0.5) |
| Colitis | 6 (0.4) |
| Anemia | 5 (0.4) |
| Lung disorder | 5 (0.4) |
| Interstitial lung disease | 5 (0.4) |
| Decreased appetite | 4 (0.3) |
| Dyspnea | 4 (0.3) |
TRAE, treatment-related adverse event
*Malignant neoplasm progression classified as TRAE was reported in 44 patients, including 10 with grade 3–4.
Patients with active autoimmune disease (χ2 = 0.2, P = .627), patients older than 80 years (χ2 = 2.1, P = .148) and patients with brain metastasis (χ2 = 0.67, P = .414) did not have an increased risk of TRAEs. Conversely, patients with an ECOG PS greater than 1 had more TRAEs of any grade than patients with an ECOG PS 0–1 (χ2 = 4.8, P = .028).
TRAE occurrence was found to be an independent predictor of OS in the multivariate analysis (Table 3).
Discussion
Clinical trials evaluate treatments under controlled conditions and in patients who fulfil selective eligibility criteria. Real-world studies are therefore needed to confirm how the trial results transfer into routine practice including the treatment experience among a wider range of patients, particularly those with poor prognostic factors who are often excluded from clinical trials. The EVIDENS study was therefore conducted to describe the real-world experience of nivolumab in the treatment of French patients with NSCLC. Given that cancer outcomes vary significantly across Europe,28 these data, combined with the evidence from phase III clinical studies, may effectively guide treatment decisions for this indication in France.
To our knowledge, EVIDENS is the largest prospective study evaluating the safety and effectiveness of nivolumab for the treatment of lung cancer patients in a real-life setting. An important consideration for this study was to allow recruitment of patients who may have been underrepresented in, or excluded from, pivotal clinical trials of nivolumab. Therefore, the EVIDENS cohort included patients with brain metastases, ECOG PS ≥2 and active autoimmune disease (19.9%, 17.0% and 3.0% of patients, respectively). The results of this interim analysis show that patient characteristics were mostly similar to those reported in the epidemiological KBP-2010-CPHG study of 7,051 adult patients with primary lung cancer treated in France in 2010,31 suggesting high generalizability of results from EVIDENS.
The results of the present analysis confirm the favorable benefit/risk ratio of nivolumab in a real-life cohort in France. The median OS estimate was 12.1 months in patients with non-squamous NSCLC and 10.2 months in patients with squamous NSCLC, the median PFS estimates were 3 months and 2.8 months, respectively, and nivolumab was well tolerated. Despite broader inclusion criteria used in EVIDENS, these effectiveness results are in line with those of the CheckMate 0177 and 0576 phase III trials and other real-world studies of nivolumab conducted in Europe (Table 5).15–27
Table 5.
Published European studies reporting the effectiveness of nivolumab in the treatment of advanced lung cancer
| Smoking status |
ECOG PS |
Histology |
Median survival, months (95%CI) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies | n | Median age (range), years | Sex, Male | Current/former | Never | 0–1 | >1 | Nsq | Sq | Brain metastases | 2nd line | PFS | OS | ORR (95%CI) | |
| Randomized clinical trials | |||||||||||||||
| Vokes et al.29 | 427† | 61 (37–85) |
61 | 82 | NS | 100 | 0 | 68 | 32 | 10 | 91 | 2.6 (2.2–3.5) |
11.1 (9.2–13.1) |
19.0 (16.0–24.0) |
|
| European real-world studies | |||||||||||||||
| EVIDENS | 1,420 | 66 (35–91) |
69 | 90 | 10 | 83 | 17 | 69 | 31 | 20 | 74 | 2.8 (2.6–3.2) |
11.2 (10–12.4) |
19.6 (17.5–21.6) |
|
| Areses Manrique et al.23 | 188 | 58 (45–81) |
77 | 91 | 9 | 90 | 10 | 60‡ | 35 | 22 | 62 | 4.8 (3.7–6.0) |
12.9 (9.1–16.6) |
25.5 (NS) |
|
| Geier et al.22 | 259 | 62 (29–85) |
72 | 86 | 9 | 77 | 23 | 64‡ | 27 | 21 | 61 | 2.3 (1.9–3.3) |
11.0 (8.9–14.0) |
22.4 (17.7–27.9) |
|
| Montana et al.21 | 98 | 66 (42–86) |
71 | NS | NS | 60 | 40 | 79 | 21 | NS | 43 | 1.8 (1.7–2.7) |
6.3 (4.1–10.9) |
4.1 (NS) |
|
| Brustugun et al.20 | 58 | 65 (32–88) |
48 | NS | NS | 76 | 24 | 55‡ | 41 | 0 | 35 | NS | 11.7 (NS) |
NS | |
| Merino Almazán et al.24 | 221 | 65 (NS) |
84 | NS* | NS* | 85 | 14 | 38 | 60 | 10 | 65 | 5.3 (3.2–7.3) |
9.7 (7.6–11.8) |
16.7 (NS) |
|
| Crinò et al.17 | 1,588 | 66 (27–89) |
65 | 71 | 19 | 92 | 7 | 100 | 0 | 26 | 24 | 3.0 (2.9–3.1) |
11.3 (10.2–12.4) |
18.0 (NS) |
|
| Krefting et al.18 | 40 | 65 (59–82) |
75 | 97 | 3 | 73 | 8 | 0 | 100 | NS | 23 | 5.3 (1.1–9.4) |
NS | NS | |
| Schouten et al.15 | 248 | 63 (29–84) |
55 | 81 | 18 | 84 | 16 | 67‡ | 22 | 23 | 75 | 2.6 (2.4–2.8) |
10.0 (6.7–13.4) |
20.2 (NS) |
|
| Grossi et al.19 | 371 | 68 (31–91) |
80 | 83 | 8 | 94 | 6 | 0 | 100 | 10 | 44 | 4.2 (3.4–5.0) |
7.9 (6.2–9.6) |
18 (NS) |
|
| Tournoy et al. 201826 | 267 | 66 (41–86) |
72 | 92 | 6 | 76 | 24 | 73 | 27 | 17 | 52 | 3.7 (2.9–4.5) |
7.8 (6.3–9.3) |
23.2 (NS) |
|
| Costa et al.25 | 107 | 65 (37–83) |
NS | NS | NS | NS | NS | 100** | NS | NS | 30 | 5.3 (2.8–7.9) |
11.4 (11.1–11.7) |
19.7 (NS) |
|
| Giaj Levra et al.27 | 10,452 | NS | 71 | NS | NS | NS | NS | 56 | 44 | 17 | NS | NS | 11.5 (11.1–11.9) | NS | |
†Patients treated with nivolumab; ‡Adenocarcinoma; *27% of never or former smokers and 69% of current smokers; **the analysis was performed on the 107 patients having a non-squamous histology out of the 115 included in the study
All values presented as % unless stated otherwise. Total values that do not equal 100% are either due to missing data or to a proportion of patients who did not fit in the categories presented in this table.
CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; NS, not stated; Nsq, non-squamous; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; Sq, squamous
Examination of the influence of baseline characteristics on OS did not reveal any significant effect of PD-L1 expression, although the analysis may have been underpowered to detect an association given that the sample size of patients in whom PD-L1 expression data were available was small. ECOG PS, smoking status, corticosteroids at baseline, EGFR mutation status, symptomatic brain metastasis and TRAEs significantly influenced OS in nivolumab recipients as seen in our multivariate analysis, even though some of these factors are also well-known prognostic factors in NSCLC.32,33
A pooled analysis of data from the CheckMate 017, 057, 063 and 003 clinical trials reported that the incidence of any-grade TRAEs was 70% and the incidence of grade 3 and 4 TRAEs was 11% and 2%, respectively.34 In the present analysis of the EVIDENS study, TRAEs of any grade were reported in 34.9% of patients, and grade 3 and 4 TRAEs were reported in 7.4% and 0.8% of patients, respectively. The lower frequency of TRAEs reported in the present study may be due to a number of factors, including underreporting of low grade adverse events in the real-life setting or reporting directly to regional pharmacovigilance centers or to the national medicine agency in circumvention of the EVIDENS eCRF.
Of note, 45.5% of patients included in the present study received additional treatment post-nivolumab. This shows that administration of nivolumab did not compromise the use of subsequent lines of treatment upon discontinuation of nivolumab.
Conclusions
The first results of the EVIDENS study confirmed both the effectiveness and safety of nivolumab observed in clinical trials for the treatment of advanced NSCLC in a real-life setting in France. ECOG PS, smoking status, corticosteroids at baseline, EGFR mutation status, symptomatic brain metastasis and TRAEs were independent predictors of survival. No significant difference in survival was found for patients aged less than versus greater than 80 years, suggesting that nivolumab has a role in the treatment of elderly lung cancer patients. Further analysis will provide final readout of OS.
Supplementary Material
Acknowledgments
The authors thank all patients and their families as well as the investigators who made this trial possible. The authors also thank Nishad Parkar, PhD, and Georgii Filatov of Springer Healthcare Communications for medical writing assistance in the preparation of this manuscript. This medical writing assistance was funded by Bristol-Myers Squibb. Finally, the authors thank ICTA for providing help in conducting this study.
Funding Statement
This work was supported by Bristol-Myers Squibb.
Author contributions
FB, AD, DD, CR, JBA, NB, PB, DMS, CAV, BA, PL, FEC, VA, MD, JD, DR, CYC, NO and MP contributed to study design and/or data interpretation. FB, AD, DD, CR, JBA, NB, PB, CAV, TE, AR, JF, MLS, JLL and VW enrolled patients. All authors provided input during preparation of the manuscript, and read and approved the final manuscript before submission
Declaration of interest statement
FB, AD, DD, CR, JBA, NB, PB, DMS, CAV, BA, MP, TE, AR, JF, MLS, JLL, VW received fees from Bristol-Myers Squibb for their contribution to the study (see author contributions section). AD is an advisory board member for Bristol-Myers Squibb, Roche and Novartis, and has participated in congresses for Bristol-Myers Squibb, Roche, AstraZeneca, Boehringer Ingelheim, MSD, Amgen and Eli Lilly. TE has received fees from Amgen, Roche, Eli Lilly, Boehringer Ingelheim, MSD, Bristol-Myers Squibb, Novartis, Pierre Fabre, AstraZeneca and Vifor Pharma for participating in advisory boards and symposia. DD is an advisory board member and speaker for, and has received honoraria from, Roche, Pfizer, MSD and Bristol-Myers Squibb, has received honoraria and institutional research grant from AstraZeneca, Chugai Pharmaceuticals and Eli Lilly, is a speaker for, and has received honoraria and institutional research grant from, Novartis, and has received institutional research grant from Janssen, GlaxoSmithKline, Pierre Fabre and Mundi pharma. JBA is an advisory board member for Bristol-Myers Squibb, Roche, AstraZeneca and Boehringer Ingelheim, and has received grants from Bristol-Myers Squibb, Roche, AstraZeneca, Boehringer Ingelheim, MSD, Amgen and Pfizer. DMS has received honoraria from Bristol-Myers Squibb, MSD, Eli Lilly, Abbvie, AstraZeneca, Boehringer Ingelheim France, Takeda, Roche and Pfizer. CAV is an advisory member for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, MSD, Pfizer and Roche, and is a speaker for AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Eli Lilly, Novartis, Pfizer and Roche. BA is an advisory board member and a consultant for Bristol-Myers Squibb. MP is an advisory board member and has received honoraria from Roche, is an advisory board member for Eli Lilly, Bristol-Myers Squibb, Pfizer, MSD, Boehringer Ingelheim, Novartis, Pierre Fabre, Takeda and Clovis, and is an advisory board member for and has received institutional research grant from AstraZeneca.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Ferlay J, Colombet M, Soerjomataram I, Dyba T, Randi G, Bettio M, Gavin A, Visser O, Bray F.. Cancer incidence and mortality patterns in Europe: estimates for 40 countries and 25 major cancers in 2018. Eur J Cancer. 2018;103:356–8. doi: 10.1016/j.ejca.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute . The essential facts and figures of cancer in France. 2019. [accessed 2019 February26]. http://www.e-cancer.fr/Actualites-et-evenements/Actualites/L-Institut-publie-L-essentiel-des-faits-et-chiffres-des-cancers-en-France-edition-2019.
- 3.Siegel RL, Miller KD, Jemal A.. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 4.Grapatsas K, Leivaditis V, Tsilogianni Z, Haussmann E, Kaplunov V, Dahm M, Zarogoulidis P, Hohenforst-Schmidt W, Tsakiridis K, Foroulis C, et al. Epidemiology, risk factors, symptomatology, TNM classification of non small cell lung cancer. An overview while waiting the 8th TNM classification. Oncomedicine. 2017;2:14–23. doi: 10.7150/oncm.17097. [DOI] [Google Scholar]
- 5.Couraud S, Westeel V, Ranchon F, Toffart A-C, Souquet P-J, Editorial board of the 2019 edition. Non-small cell lung cancer. Auvergne Rhône-Alpes guidelines in thoracic oncology. 2019. update 2019 [accessed 2019 May29]. https://ressources-aura.fr/wp-content/uploads/2018/12/CBNPC_2019_VDEF.pdf.
- 6.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, Molina J, Kim J-H, Arvis CD, Ahn M-J. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 9.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, Von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, Mok TS, Reck M, Van Schil PE, Hellmann MD, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv192–iv237. doi: 10.1093/annonc/mdy275. [DOI] [PubMed] [Google Scholar]
- 11.Reck M, Taylor F, Penrod JR, DeRosa M, Morrissey L, Dastani H, Orsini L, Gralla RJ. Impact of nivolumab versus docetaxel on health-related quality of life and symptoms in patients with advanced squamous non–small cell lung cancer: results from the CheckMate 017 study. J Thorac Oncol. 2018;13:194–204. doi: 10.1016/j.jtho.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 12.Reck M, Brahmer J, Bennett B, Taylor F, Penrod JR, DeRosa M, Dastani H, Spigel DR, Gralla RJ. Evaluation of health-related quality of life and symptoms in patients with advanced non-squamous non-small cell lung cancer treated with nivolumab or docetaxel in CheckMate 057. Eur J Cancer. 2018;102:23–30. doi: 10.1016/j.ejca.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Euser AM, Zoccali C, Jager KJ, Dekker FW. Cohort studies: prospective versus retrospective. Nephron Clin Pract. 2009;113:c214–7. doi: 10.1159/000235241. [DOI] [PubMed] [Google Scholar]
- 14.Molinier O, Audigier-Valette C, Cadranel J, Monnet I, Hureaux J, Hilgers W, Fauchon E, Fabre E, Besse B, OA BP. 17.05 IFCT-1502 CLINIVO: real-life experience with nivolumab in 600 patients (pts) with advanced non-small cell lung cancer (NSCLC). J Thorac Oncol. 2017;12:S1793. doi: 10.1016/j.jtho.2017.09.430. [DOI] [Google Scholar]
- 15.Schouten RD, Muller M, de Gooijer CJ, Baas P, van den Heuvel M. Real life experience with nivolumab for the treatment of non-small cell lung carcinoma: data from the expanded access program and routine clinical care in a tertiary cancer centre-The Netherlands cancer institute. Lung Cancer. 2018;126:210–216. doi: 10.1016/j.lungcan.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Crinò L, Bidoli P, Delmonte A, Grossi F, De Marinis F, Ardizzoni A, Vitiello F, Lo Russo G, Parra HS, Cortesi E, et al. Italian cohort of nivolumab expanded access program in squamous non-small cell lung cancer: results from a real-world population. Oncologist. 2019;24:e1165–e71. doi: 10.1634/theoncologist.2018-0737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Crinò L, Bronte G, Bidoli P, Cravero P, Minenza E, Cortesi E, Garassino MC, Proto C, Cappuzzo F, Grossi F, et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer. 2019;129:35–40. doi: 10.1016/j.lungcan.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 18.Krefting F, Basara N, Schutte W, Spath-Schwalbe E, Alt J, Thiel S, Kimmich M, Fischer JR, Kurz S, Griesinger F, et al. Clinical experience of immunotherapy treatment: efficacy and toxicity analysis of the compassionate use program of nivolumab in patients with advanced squamous cell non-small cell lung cancer. Oncol Res Treat. 2019;42:243–255. doi: 10.1159/000499321. [DOI] [PubMed] [Google Scholar]
- 19.Grossi F, Crino L, Logroscino A, Canova S, Delmonte A, Melotti B, Proto C, Gelibter A, Cappuzzo F, Turci D, et al. Use of nivolumab in elderly patients with advanced squamous non-small-cell lung cancer: results from the Italian cohort of an expanded access programme. Eur J Cancer. 2018;100:126–134. doi: 10.1016/j.ejca.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 20.Brustugun OT, Sprauten M, Helland A. Real-world data on nivolumab treatment of non-small cell lung cancer. Acta Oncol. 2017;56:438–440. doi: 10.1080/0284186X.2016.1253865. [DOI] [PubMed] [Google Scholar]
- 21.Montana M, Garcia ME, Ausias N, Jeanpierre M, Meiffren M, Giorgi R, Vanelle P, Barlesi F. Efficacy and safety of nivolumab in patients with non-small cell lung cancer: a retrospective study in clinical practice. J Chemother. 2018;31:1–5. [DOI] [PubMed] [Google Scholar]
- 22.Geier M, Descourt R, Corre R, Léveiller G, Lamy R, Goarant E, Bizec J, Bernier C, Quéré G, Gaye E, et al. Real life second-line nivolumab in advanced non-small cell lung cancer: a French observational multicenter study of 259 patients (ABCT-IMMUNOBZH). Cancer Rep Rev. 2018;2:1–6. doi: 10.15761/CRR.1000164. [DOI] [Google Scholar]
- 23.Areses Manrique MC, Mosquera Martinez J, Garcia Gonzalez J, Afonso Afonso FJ, Lazaro Quintela M, Fernandez Nunez N, Azpitarte Raposeiras C, Amenedo Gancedo M, Santome Couto L, Garcia Campelo MR, et al. Real world data of nivolumab for previously treated non-small cell lung cancer patients: a Galician lung cancer group clinical experience. Transl Lung Cancer Res. 2018;7:404–415. doi: 10.21037/tlcr.2018.04.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merino Almazan M, Duarte Perez JM, Marin Pozo JF, Ortega Granados AL, Muros De Fuentes B, Quesada SP, Gago Sanchez AI, Rodriguez GP, Jurado Garcia JM, Artime Rodriguez-Hermida F, et al. A multicentre observational study of the effectiveness, safety and economic impact of nivolumab on non-small-cell lung cancer in real clinical practice. Int J Clin Pharm. 2019;41:272–279. doi: 10.1007/s11096-018-0772-z. [DOI] [PubMed] [Google Scholar]
- 25.Costa FA, Ramos C, Murteira R, Almodovar T, Passos-Coelho JL, Carvalho MI, Costa L, Brito MJ, Ramos S, Ferreira M, et al. The cancer registry as an ally in monitoring treatment effectiveness. Pulmonology. 2019;25:3–8. doi: 10.1016/j.pulmoe.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Tournoy KG, Thomeer M, Germonpre P, Derijcke S, De Pauw R, Galdermans D, Govaert K, Govaerts E, Schildermans R, Declercq I, et al. Does nivolumab for progressed metastatic lung cancer fulfill its promises? An efficacy and safety analysis in 20 general hospitals. Lung Cancer. 2018;115:49–55. doi: 10.1016/j.lungcan.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Giaj Levra M, Cotte FE, Corre R, Calvet C, Gaudin AF, Penrod JR, Grumberg V, Jouaneton B, Jolivel R, Assie JB, et al. Immunotherapy rechallenge after nivolumab treatment in advanced non-small cell lung cancer in the real-world setting: A national data base analysis. Lung Cancer. 2019;140:99–106. doi: 10.1016/j.lungcan.2019.12.017. [DOI] [PubMed] [Google Scholar]
- 28.De Angelis R, Sant M, Coleman MP, Francisci S, Baili P, Pierannunzio D, Trama A, Visser O, Brenner H, Ardanaz E, et al. Cancer survival in Europe 1999-2007 by country and age: results of EUROCARE–5-a population-based study. Lancet Oncol. 2014;15:23–34. doi: 10.1016/S1470-2045(13)70546-1. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency . OPDIVO® (Nivolumab) summary of product characteristics. 2019. [accessed February 2019 11]. http://www.ema.europa.eu/documents/product-information/opdivo-epar-product-information_en.pdf.
- 30.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 31.Locher C, Debieuvre D, Coëtmeur D, Goupil F, Molinier O, Collon T, Dayen C, Le Treut J, Asselain B, Martin F. Major changes in lung cancer over the last ten years in France: the KBP-CPHG studies. Lung Cancer. 2013;81:32–38. doi: 10.1016/j.lungcan.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Kawaguchi T, Takada M, Kubo A, Matsumura A, Fukai S, Tamura A, Saito R, Maruyama Y, Kawahara M, Ignatius Ou SH. Performance status and smoking status are independent favorable prognostic factors for survival in non-small cell lung cancer: a comprehensive analysis of 26,957 patients with NSCLC. J Thorac Oncol. 2010;5:620–630. doi: 10.1097/JTO.0b013e3181d2dcd9. [DOI] [PubMed] [Google Scholar]
- 33.Sanchez de Cos J, Sojo GMA, MV M, Perez Calvo MC, MJ V, MH V. Non-small cell lung cancer and silent brain metastasis. Survival and prognostic factors. Lung Cancer. 2009;63:140–145. doi: 10.1016/j.lungcan.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 34.Antonia SJ, Borghaei H, Ramalingam SS, Horn L, De Castro Carpeno J, Pluzanski A, Burgio MA, Garassino M, Chow LQM, Gettinger S, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol. 2019;20:1395–1408. doi: 10.1016/S1470-2045(19)30407-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- National Cancer Institute . The essential facts and figures of cancer in France. 2019. [accessed 2019 February26]. http://www.e-cancer.fr/Actualites-et-evenements/Actualites/L-Institut-publie-L-essentiel-des-faits-et-chiffres-des-cancers-en-France-edition-2019.
- Couraud S, Westeel V, Ranchon F, Toffart A-C, Souquet P-J, Editorial board of the 2019 edition. Non-small cell lung cancer. Auvergne Rhône-Alpes guidelines in thoracic oncology. 2019. update 2019 [accessed 2019 May29]. https://ressources-aura.fr/wp-content/uploads/2018/12/CBNPC_2019_VDEF.pdf.
- European Medicines Agency . OPDIVO® (Nivolumab) summary of product characteristics. 2019. [accessed February 2019 11]. http://www.ema.europa.eu/documents/product-information/opdivo-epar-product-information_en.pdf.


