Abstract
Background
Inclusion of productivity losses and gains in cost-effectiveness analyses for drugs is recommended by pharmacoeconomic guidelines in some countries and is considered optional in others. Often guidelines recommend analysis based on the payer perspective, but suggest that a supplemental analysis based on the societal perspective may be submitted that includes productivity losses/gains. However, there is no universally recognized framework for the approach to including productivity losses and gains in pharmacoeconomic analyses.
Objectives
This study aimed to systematically review literature on cost-effectiveness analyses of drug interventions that included costs associated with productivity losses/gains and to summarize the types cost elements included and cost calculation methods employed. Moreover, this study examines variations in the calculation of productivity losses/gains by target disease type, geographic region, income group, period of analysis, and analysis time horizon—as well as the impact of their inclusion on the study outcomes.
Methods
A search of three databases was performed, including MEDLINE, Embase, and the Cochrane Library, to identify cost-effectiveness and cost-utility analyses that included indirect costs such as productivity losses/gains. Publications from January 2010 to October 2019 were examined and selected for inclusion by two independent reviewers. In addition to the citation details, data on the country of analysis, country income group, target disease area, study sponsorship, type of analysis, study design, time horizon, analysis perspective, productivity loss/gain elements included, the approach used to estimate productivity losses/gains, and the impact of their inclusion on the study outcome—namely the incremental cost effectiveness ratio—were extracted and summarized.
Results
The search strategy identified 5038 unique studies, and 208 were included in the final analysis. Among the studies reviewed, 165 (79%) were conducted in high-income countries and 160 (77%) were conducted for North American and European/Central Asian countries. The productivity loss/gain elements included in the analysis were reported for 169 studies (81%). Absenteeism only was included for 98 studies (47%), and absenteeism plus presenteeism was included for 29 studies (14%). Absenteeism plus some other element such as costs associated with unemployment and/or early retirement was included for 32 studies (15%) examined. Only one out of four of the studies reviewed included information on the approach used to estimate productivity losses/gains, which was predominantly the human capital approach. One-hundred forty-four studies (69%) reported the impact of including productivity losses/gains on the ICER, with 110 studies (53%) reporting that their inclusion contributed to more favorable cost-effectiveness.
Conclusions
Although inclusion of productivity losses/gains was shown to have a favorable impact on evaluations for many studies, their impact and method of calculation was often not reported or was unclear. Further examination and discussion is needed to consider the optimal framework for considering productivity losses/gains in cost-effectiveness analyses, including the appropriate cost elements to include (e.g., patient absenteeism, caregiver absenteeism, presenteeism, unemployment, etc.) and how those costs should be estimated.
Electronic supplementary material
The online version of this article (10.1007/s40273-020-00986-4) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Inclusion or exclusion of productivity losses/gains in cost-effectiveness analyses (CEAs) of health technologies such as pharmaceutical agents may significantly impact formulary decisions due to their potential to increase or decrease incremental cost-effectiveness ratios (ICERs) of treatment interventions being compared. |
| No systematic review of studies that examined the impact of productivity losses/gains on estimates of cost-effectiveness of drug interventions was available. This study was undertaken to address that information gap, restricting itself to a review of studies of pharmaceutical agents without consideration of medical devices or vaccines. |
| Further examination and discussion is needed to consider the optimal framework for considering productivity losses/gains in cost-effectiveness and cost-utility analyses. |
Introduction
Cost-effectiveness analyses (CEAs) in healthcare assess health outcomes and the costs of interventions implemented to improve health [1, 2]. They compare the costs and effectiveness of two or more therapeutic alternatives to provide an estimate of the value for money provided by new therapeutic interventions, facilitating resource allocation decisions based on the relative value of one or more interventions compared with the standard of care [3]. These analyses directly relate the clinical benefits and economic value of multiple therapeutic interventions and in some cases may help determine their eligibility for inclusion in private, national, or provincial governmental health insurance programs [4]. A cost-utility analysis (CUA) is a type of CEA wherein the unit of outcome measurement is quality-adjusted life years (QALYs) gained or lost [2, 4, 5].
Since universal guidelines for performing CEAs are lacking, parameters concerning the inclusion of costs included in CEAs and CUAs vary by country. Parameters concerning the inclusion of costs may vary based on the perspective of the study design, the method used to estimate productivity costs, and the kinds of productivity costs that should be included. To begin, the perspective of the study may influence the costs included in the analysis. For CEA and CUA studies conducted based on the healthcare or public payer perspective, costs are typically limited to direct healthcare costs such as the costs of treatment intervention, hospitalization, outpatient visits, etc. [6]. Less frequently costs included under the healthcare or public payer perspective may also include costs borne through long-term care insurance and other healthcare programs. In addition to direct healthcare costs, CEA or CUA studies conducted based on the societal perspective or the patient perspective may also include other costs borne by the individual patient or their families. For example, under the societal perspective, direct non-healthcare costs such as transportation or indirect costs such as costs associated with losses or gains in productivity when undergoing treatment may also be included in the analysis [7, 8]. Naturally, the broader scope of costs included can lead to different results in terms of cost-effectiveness. In fact, while several studies have shown that fewer than 10% of economic studies include productivity costs, their inclusion has been shown to lead to more favorable outcomes in terms of cost-effectiveness [6, 7, 9].
Guidelines used in countries with established CEA programs including the United States (US), United Kingdom (UK), Australia, Canada, France, and Japan recommend performing analyses from a healthcare or public payer perspective at least as a baseline analysis [10–15]. In addition to a baseline analysis using the healthcare or public payer perspective, some countries such as the US, the UK, Australia, Canada, and France also allow for or recommend an additional analysis from a societal perspective that takes a broader view of the cost of illness [10–14]. Guidelines for a few countries such as Norway, Portugal, and Sweden specify the use of the societal perspective [5]. The rationale for recommending a healthcare or public payer perspective versus a societal perspective is often not provided in pharmacoeconomic guidelines [10–15].
While studies employing a societal perspective may vary with regard to the additional costs included in the economic analyses, some guidelines refer specifically to the inclusion of indirect costs, defined as productivity lost (or gained) by patients and/or their families due to morbidity or mortality resulting from illness [8, 14]. Absenteeism and presenteeism for the patient and/or their family members are commonly mentioned components for productivity costs [16, 17]. Absenteeism refers to productivity losses or gains associated with the time off required due to an illness or treatment or the ability to forego taking time off due to the benefits of treatment. Presenteeism refers to losses or gains due to loss of productivity while at work due to an illness or treatment without actually taking time off of work. Some studies also consider unemployment and premature mortality, both of which involve a permanent loss of employment. Unemployment refers to loss of productivity from having to stop working altogether due to a condition. Premature mortality refers to productivity loss or gain that may accumulate due to death from an illness [8]. Unemployment and premature mortality-associated productivity losses/gains are similar to absenteeism in that they refer to a loss of work associated with an illness, but some studies clearly differentiate unemployment and premature mortality as a specific type of cost element.
There are two commonly mentioned approaches to estimating productivity losses/gains—namely the human capital approach and the friction cost approach [1, 8, 13]. For the human capital approach, costs associated with productivity losses/gains are estimated by multiplying lost work time by discounted gross wages. For the friction cost approach, costs associated with productivity losses are estimated by multiplying the lost work hours during the period that the patient must take off work and when a new employee can be hired and trained to replace them by the discounted gross wages for the patient. Many guidelines specify the method of estimation that should be used for estimating productivity losses/gains [5, 13, 15, 18–20]. Some guidelines such as those for Sweden, Japan, and Taiwan specify the use of the human capital approach [15, 19, 20]. Guidelines for Canada and The Netherlands, however, specify the use of the friction cost approach [13, 18]. Very few guidelines provide a detailed explanation of the rationale for the method recommended. The guidelines for Taiwan recommend the human capital approach “because it is challenging to collect data for the friction cost method” [20]. Despite apparent challenges in using the friction cost approach, it has been suggested that the human capital approach may overestimate the value of lost production given that it assumes full employment and that loss of employment is not covered by replacing the person with someone else [8].
Despite variations in the inclusion of productivity losses/gains, previous studies have found that their inclusion in economic evaluations may substantially impact cost-effectiveness estimates and may cause different formulary decisions to be made than if they are not incorporated [4, 9, 21, 22]. Analyses incorporating productivity losses/gains have been shown to increase or decrease the incremental cost-effectiveness ratio (ICER) (a measure of the difference in cost between two interventions, divided by the difference in their effect), depending on the direction of their impact on costs. Although multiple reports support the inclusion of productivity losses/gains in CEAs [21–24], a systematic review of all health economic evaluation studies published through May 2014 that employed a patient’s perspective found that only half of the included studies examined indirect costs [25]. Moreover, a study conducted in 2000 that examined CUAs found that among 228 studies conducted between 1975 and 1997, only 19 (8.3%) included productivity costs. While it is clear that historically only a small proportion of CEA and CUA have included productivity losses/gains, no systematic review is currently available that examines the approaches used to include productivity losses/gains and their impact on the outcomes for all CEA and/or CUA studies. To address this knowledge gap, a global systematic review of the peer-reviewed English-language literature from both developed and developing countries was conducted. The objective of this review is to summarize the findings of CEAs and CUAs of drug interventions (except vaccines) that assessed productivity losses/gains, including the types of cost elements included and methods of cost estimation employed, as well as variations in the approach used, by target disease type, region, income group, period of conduct of the evaluation, and the time horizon of the evaluation. Furthermore, the target disease area, the cost elements included, and the estimation method used are examined to determine if they are statistically associated with an impact on the outcomes of CEA and CUA.
Methods
Definition of Indirect Costs
For this systematic review, indirect costs were defined as productivity losses/gains associated with morbidity or mortality that lead to a loss of work time, a definition consistent with that used in multiple guidelines [8, 14].
Systematic Literature Review Methodology
A systematic literature review (SLR) attempts to assemble all available relevant evidence using explicit, valid, and replicable methods to minimize the risk of bias in the selection of studies [26, 27]. This SLR was performed in accordance with recommended international guidelines for the conduct of systematic reviews, including the Centre for Reviews and Dissemination (CRD) guidance [26], the Cochrane Handbook [27], and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [28, 29].
Search Methodology
An experienced team of health economics experts designed the search strategy used to identify publications for inclusion in this systematic review. Keywords were identified from the literature for two concepts: (1) type of health economic evaluation (i.e., CEAs and CUAs) and (2) productivity loss components and methods used for calculation. The search terms included a number of indexing and free-text terms to ensure that a high proportion of the relevant articles were captured. The keywords were combined using Boolean operators to create the search strings listed in Appendix 1 (see the electronic supplementary material). Cost-benefit analyses were excluded from this systematic review since few countries recommend this approach for economic evaluation of new therapeutic interventions [5]. Studies evaluating the cost-effectiveness of vaccines were also excluded since vaccines are not included in reimbursement programs in some countries and decisions pertaining to their use often involve a broader consideration of public health interventions that may fall outside the drug reimbursement system.
Sources
The databases used were (1) MEDLINE (including Epub Ahead of Print, In-Process and Other Non-Indexed Citations, MEDLINE Daily, MEDLINE <1946 to Present>, MEDLINE In-Process Citations and Daily Update) via OvidSP; (2) Embase (via OvidSP); and (3) Cochrane Library (via Cochrane). The search strategies used are presented in Appendix 1 (see the electronic supplementary material). The initial search period included all publications available up to October 22, 2019, with the earliest search year being 1946 for MEDLINE and 1974 for Embase. The Cochrane Library began in 1996, but does not have a specific start year for searches, but no restrictions on the start date were applied for the initial search. In addition, as recommended by the CRD and Cochrane guidelines [26, 27], the lists of references cited in each of the included studies and relevant (but not included) systematic reviews were manually searched to identify any additional relevant primary literature on the topic of interest.
Study Identification, Selection, and Data Extraction
Data Management
Records retrieved during the searches were first added to an Endnote (referencing software) library, and duplicate records were removed (de-duplication) [30]. The records were then exported into Covidence (a systematic review management tool) and Microsoft Excel version 14.0 to carry out the screening exercise [31].
Eligibility Criteria and Selection Process
Inclusion and exclusion criteria were created based on the PICOS criteria (P = Population, I = Intervention, C = Comparator, O = Outcome, S = study type) to screen the retrieved studies (Table 1). To be selected for each research question, a study had to fulfill all inclusion criteria and none of the exclusion criteria. The selected study had to provide evidence for one or more of the outcomes of interest. Non-English studies were excluded from the analysis. Lastly, since methods of analysis and modeling for CEA/CUA as well as the application of CEA/CUA in decision making has changed over time, the review was limited to publications from 2010 to present, to ensure relevance to current methods.
Table 1.
Inclusion and exclusion criteria
| Category | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Intervention/comparator | Intervention or comparator is a drug |
No drug evaluated Health policy programs evaluated |
| Outcomes |
Presenteeism Absenteeism Time off/days off/sick leave Loss of employment Productivity loss associated with premature mortality |
No relevant cost element incorporated in cost assessment |
| Study types |
Cost-effectiveness analyses Cost-utility analyses |
Not original research study (e.g., reviews, commentaries, and editorials) Health economic assessment other than a cost-effectiveness analyses/cost-utility analyses |
| Language | English (full text version) | Full text in language other than English |
| Time period | Studies published in or after 2010 | Studies published before 2010 were excluded to ensure that we only include the most recent studies |
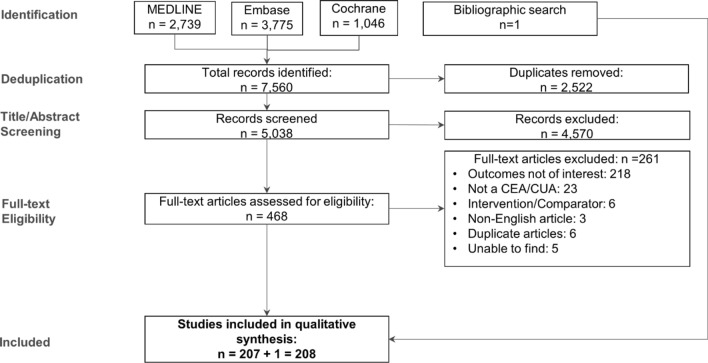
After exclusion of duplicates, each retrieved record was marked as “include” or “exclude” based on a review of the study title and abstract, where the latter was available (‘TiAb’ screening). Full-text articles were obtained for records that met the inclusion criteria. Each record was re-evaluated through a full-text review by two independent analysts. Any disagreements were resolved through discussion until a consensus was reached, failing which a third reviewer was consulted for a final and irrevocable decision. Reason(s) for exclusion of all records excluded based on the review of the full text were documented. A summary of the reasons for exclusion of articles excluded based on the full-text article review is provided in the PRISMA flow diagram shown in Fig. 1.
Fig. 1.
Flow diagram depicting search results and selection of studies for analysis. CEA cost-effectiveness analysis, CUA cost-utility analysis
Data Extraction
Data from the included studies were extracted by the first reviewer into data extraction tables created for this analysis. To identify and rectify any errors in data extraction, a second reviewer checked and validated the outcome data by conducting an independent internal data check once all required data had been collected/extracted. Data extracted included the fields listed in Appendix 2 (see the electronic supplementary material).
Assessment of the Quality of Included Studies
The reporting quality of the studies included in the review was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) guidelines, first published in 2013 by the International Society of Pharmacoeconomics and Outcomes Research (ISPOR) [32, 33]. The CHEERS checklist has since been utilized extensively and helps ensure the consistency of reporting [34–36]. The CHEERS checklist consists of 24 items in six main sections: (1) title and abstract, (2) introduction, (3) methods, (4) results, (5) discussion, and (6) other (i.e., source of funding, conflict of interest). These items focus on key elements of the economic evaluation, each of which has a direct impact on the validity of the overall results of that study. Records for which only an abstract was available were not considered for quality assessment in this systematic review. Each item was rated independently by two reviewers using the following scale: 1 = “fully satisfied,” 0.5 = “partially satisfied,” 0 = “not satisfied,” or NA = “not applicable” [37]. Any disagreements were resolved by a third reviewer. The number (percentage) of included studies fully/partially addressing each item of the CHEERS checklist was calculated according to the criteria they met. Total scores were calculated for each study (maximum score = 24). Each study was then categorized based on the percentage score into one of four categories: excellent (score ≥ 85%), very good (70 ≤ score < 85%), good (55 ≤ score < 70%), and poor (score < 55%) [37].
Synthesis of Extracted Evidence
Data extraction forms were managed in Microsoft Excel version 14.0. Synthesis of the extracted evidence was qualitative in nature. Descriptive study characteristics such as country, disease area, study sponsor, type of study, study design, analysis perspective, etc. were recorded as reported. Country region groups were based on the 2019 list by the World Bank [38]. Time horizon was grouped into five categories: “less than 1 year,” “1–10 years,” “more than 10 years,” “lifetime,” and “data not available.” Types of productivity cost elements included were determined based on a description of the costs included. Cost elements included absenteeism, presenteeism, unemployment/early retirement, and premature mortality as defined above. Studies that stated that productivity loss was incorporated in the model without explicitly specifying the exact elements included were listed as “other.” The impact of the inclusion of productivity costs on the ICER was grouped into four categories depending on their impact: “more favorable,” “no substantial impact,” “less favorable,” or “not reported.” Studies were labeled as having a “more favorable” impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a “less favorable” impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having “no substantial impact” if there was no change in the ICER with the inclusion of productivity costs based on specific figures provided or if their inclusion was reported by the author(s) as having no substantial impact when no specific figures were provided. When the impact of inclusion of productivity losses/gains was not mentioned then “not reported” was recorded.
Statistical Analysis Plan
Following the data extraction process, a statistical analysis of the association between specific study characteristics and the impact of the inclusion of productivity losses/gains on the outcomes of CEA and CUA was examined. Specifically, the influence of the following items on the impact of including productivity costs on the outcomes of CEA and CUA were examined: (1) target disease area, (2) productivity loss/gain cost elements included, and (3) the estimation approach used to estimate productivity costs. Since results concerning study characteristics (e.g., disease area, cost element included) and the impact of inclusion of productivity losses/gains on cost-effectiveness (e.g., “more favorable,” “less favorable”) are presented as categorical data, a Chi-square test was used to consider if there is a statistical difference in the impact on cost-effectiveness based on study characteristics [39]. Specifically, a Chi-square test was conducted using SPSS version 22 to determine whether there is a significant difference between the group of studies that least commonly reported a “more favorable” outcome and the other groups of studies. The null hypothesis in this case was that there is no difference in the impact of inclusion of productivity losses/gains based on the study characteristics considered. This approach was chosen to allow us to identify the strongest tendencies in terms of impact on cost-effectiveness given the limited number of studies included. Studies for which the impact on cost-effectiveness was labeled as “not reported” were excluded from the analysis.
Results
Study Selection
The search strategy facilitated the retrieval of 7560 citations in total, of which 2739, 3775, and 1046 records, respectively, were from the MEDLINE, Embase, and Cochrane databases (Fig. 1). Removal of duplicates brought down the number of records to be screened to 5038. Review of titles and abstracts (‘TiAb’ screening) by two independent reviewers resulted in exclusion of 4570 records, with the full-text versions of the remaining 468 studies being assessed for meeting inclusion/exclusion criteria. This step resulted in exclusion of a further 261 studies for reasons listed in Fig. 1, leaving a total of 207 studies for inclusion in the qualitative analysis. An additional study was identified through a search of the lists of references cited in the included studies and relevant (but not included) systematic reviews, bringing the total number of studies included in the analysis to 208.
Characteristics of Included Studies
The 208 studies meeting inclusion criteria were analyzed with regard to distribution by income groups of the nations in which they had been conducted, global regional groupings, disease area, sponsor type, time period of analysis, type(s) of analysis, and perspective adopted for the economic evaluation (Table 2 and Appendix 3, see the electronic supplementary material). One hundred sixty-five studies (79%) had been conducted in high-income countries based on the World Bank Atlas method, and 160 (77%) of the studies were conducted for countries in North American and Europe/Central Asian regions [38]. One hundred fifty-five studies (75%) included in the analysis focused on immunological disorders, central nervous system (CNS)/psychiatric disorders, cardiovascular and metabolic (CVM) disorders, and oncological disorders. One hundred seven studies (51%) had pharmaceutical company sponsorship. An almost equal number of CEA/CUA studies were published before and after 2016, the year in which the recommendations of the second panel on cost-effectiveness in health and medicine were published [40]. As for the type of pharmacoeconomic analysis, 166 studies (80%) were CUAs, while 30 (14%) were CEAs and 12 studies (6%) had conducted both types of analyses (Table 3).
Table 2.
Distribution of included studies based on global regional grouping, income category of country of study conduct, disease area, study sponsorship, and time period of publication
| Distribution based on | Number of studies | Proportion (%) |
|---|---|---|
| Regiona | 208 | |
| North America | 51 | 25 |
| Europe and Central Asia | 109 | 52 |
| Latin America and Caribbean | 13 | 6 |
| Middle East and North Africa | 6 | 3 |
| South Asia, East Asia, and Pacific | 25 | 12 |
| Sub-Saharan Africa | 2 | 1 |
| Not reported | 2 | 1 |
| Income category | 208 | |
| High income | 165 | 79 |
| Upper-middle income | 32 | 15 |
| Lower-middle income | 9 | 4 |
| Not reported | 2 | 1 |
| Disease area | 208 | |
| Immunology | 55 | 26 |
| Central nervous system/psychiatry | 42 | 20 |
| Cardiovascular and metabolic | 33 | 16 |
| Oncology | 25 | 12 |
| Others | 53 | 25 |
| Study sponsorb | 208 | |
| Pharma | 107 | 51 |
| Non-pharma | 95 | 46 |
| Information not available | 6 | 3 |
| Time period of study publication | 208 | |
| January 2010–December 2012 | 66 | 32 |
| January 2013–December 2014 | 40 | 19 |
| January 2015–December 2016 | 44 | 21 |
| January 2017–December 2018 | 40 | 19 |
| January 2019–October 2019 | 18 | 9 |
aThe region groups for the countries are based on the 2019 list by the World Bank
bType of sponsor was based on the funding disclosures and author affiliations. If a study was funded by a pharmaceutical company or if any of the authors were employees of a pharmaceutical company, it was labeled as pharma sponsored
Table 3.
Distribution of included studies based on study type, study design, time horizon, and analysis perspective
| Distribution based on | Number of studies (%) |
|---|---|
| Study type | 208 |
| Cost-effectiveness analysis | 30 (14%) |
| Cost-utility analysis | 166 (80%) |
| Both | 12 (6%) |
| Study design | 208 |
| Model based | 167 (80%) |
| Decision tree | 19 (9%) |
| Markov | 83 (40%) |
| Decision tree + Markov | 11 (5%) |
| Partitioned survival | 5 (2%) |
| Patient simulation | 30 (14%) |
| Others (micro costing, not described etc.) | 20 (10%) |
| Patient data based | 41 (20%) |
| RCT | 22 (11%) |
| Observational prospective | 9 (4%) |
| Observational retrospective | 8 (4%) |
| Others | 2 (1%) |
| Time horizon | 208 |
| Less than 1 year | 24 (12%) |
| 1–10 years | 78 (38%) |
| More than 10 years | 34 (16%) |
| Lifetime | 55 (26%) |
| Data not available | 17 (8%) |
| Perspective | 208 |
| Societal | 103 (50%) |
| Societal and payer | 41 (20%) |
| Societal and healthcare | 28 (13%) |
| Healthcare | 15 (7%) |
| Patient | 7 (3%) |
| Payer | 6 (3%) |
| Societal, healthcare, and payer | 4 (2%) |
| Hospital | 1 (0%) |
| Patient and payer | 1 (0%) |
| Payer and employer | 1 (0%) |
| Societal and patient | 1 (0%) |
RCT randomized controlled trial
One hundred sixty-seven (80%) of the included studies were model based, with Markov modeling being the most widely employed technique (83 studies [40%]) (Table 3 and Appendix 4, see the electronic supplementary material). The remaining 41 (20%) of included studies had been conducted in conjunction with clinical studies, and about half of those (22 [11%]) were based on randomized controlled trials (RCTs). The proportion of CEAs/CUAs based on modeling varied by disease area, with 31 (94%) of all studies in the CVM disease area employing a modeling approach compared with 29 (69%) in the CNS/psychiatry disease area. Studies in the immunology and CNS/psychiatry disease areas used patient data more frequently. The analysis time horizon varied substantially by disease area, with studies in the CNS/psychiatry disease area more frequently evaluating interventions over smaller time periods (less than a year), while studies in the oncology, CVM, and immunology areas used longer time horizons. Consistently across disease areas, regional groups, and the income group of the countries in which they had been conducted, 103 (50%) of all studies reviewed focused on only the societal perspective, while 74 (36%) included at least one additional perspective (e.g., payer, healthcare, or patient perspectives) over and above the societal perspective.
Productivity Loss/Gain Analysis
Absenteeism was the most widely used productivity loss/gain element used to estimate productivity losses/gains (Table 4). Ninety-eight studies (47%) modeled only absenteeism as the productivity loss/gain element. Presenteeism was modeled in only 30 (14%) of the studies. However, presenteeism was not considered in any of the 33 studies that examined treatments for CVM conditions and oncological conditions. Unemployment/early retirement and costs associated with premature mortality were each modeled in 21 (10%) and 22 (11%) of the CEAs/CUAs included in the review, respectively (Table 4). CEA/CUAs in oncology (nine [36%]) more frequently modeled costs associated with premature mortality (i.e., lost productivity associated with premature death and not being able to continue work until the end of one’s working life), which is consistent with the higher mortality among cancer patients. Studies that included patients with immunological (15 [27%]) and CNS/psychiatric disorders (4 [10%]) more frequently modeled unemployment and early retirement costs, which is reflective both of the population modeled (working adults) and the debilitating nature of those disease states (Table 4). There was no discernible difference between the costs elements for studies included by geographic region, country income group, and period of evaluation (Table 4), especially given that the sample for some groups was too small to identify differences. However, studies whereby the time horizon was less than one year more commonly included only absenteeism and no other cost elements.
Table 4.
Productivity loss analysis by cost elements used
| Cost elements used | |||||||
|---|---|---|---|---|---|---|---|
| Absenteeism only | Presenteeism only | Absenteeism + presenteeism | Absenteeism + others | Unemployment/early retirement | Premature mortality | Other, not specifiedb | |
| Totala (N = 208) | 98 (47%) | 1 (0%) | 29 (14%) | 32 (15%) | 21 (10%) | 22 (11%) | 39 (19%) |
| Cost elements used by disease area | |||||||
| Cardiovascular and metabolic (n = 33) | 25 (76%) | 0 (0%) | 0 (0%) | 2 (6%) | 0 (0%) | 3 (9%) | 4 (12%) |
| Immunology (n = 55) | 22 (40%) | 1 (2%) | 11 (20%) | 12 (22%) | 15 (27%) | 3 (5%) | 11 (20%) |
| Central nervous system/psychiatry (n = 42) | 23 (55%) | 0 (0%) | 8 (19%) | 4 (10%) | 4 (10%) | 1 (2%) | 7 (17%) |
| Oncology (n = 25) | 8 (32%) | 0 (0%) | 0 (0%) | 7 (28%) | 1 (4%) | 9 (36%) | 8 (32%) |
| Respiratory/Infectious (n = 27) | 14 (52%) | 0 (0%) | 5 (19%) | 3 (11%) | 1 (4%) | 3 (11%) | 4 (15%) |
| Others (n = 26) | 6 (23%) | 0 (0%) | 6 (23%) | 4 (15%) | 0 (0%) | 3 (12%) | 12 (46%) |
| Cost elements used by region | |||||||
| North America (n = 51) | 21 (41%) | 0 (0%) | 7 (14%) | 9 (18%) | 3 (6%) | 7 (14%) | 12 (24%) |
| Europe and Central Asia (n = 109) | 50 (46%) | 1 (1%) | 17 (16%) | 18 (17%) | 12 (11%) | 11 (10%) | 19 (17%) |
| Latin America and Caribbean (n = 13) | 8 (62%) | 0 (0%) | 2 (15%) | 1 (8%) | 3 (23%) | 2 (15%) | 0 (0%) |
| Middle East and North Africa (n = 6) | 3 (50%) | 0 (0%) | 1 (17%) | 2 (33%) | 1 (17%) | 0 (0%) | 0 (0%) |
| South Asia, East Asia and Pacific (n = 25) | 14 (56%) | 0 (0%) | 0 (0%) | 2 (8%) | 1 (4%) | 2 (8%) | 6 (24%) |
| Sub-Saharan Africa (n = 2) | 2 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Not reported (n = 2) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) |
| Cost elements used by country income group | |||||||
| High income (n = 165) | 75 (45%) | 1 (1%) | 25 (15%) | 27 (16%) | 15 (9%) | 18 (11%) | 32 (19%) |
| Upper-middle income (n = 32) | 16 (50%) | 0 (0%) | 4 (13%) | 3 (9%) | 4 (13%) | 4 (13%) | 5 (16%) |
| Lower-middle income (n = 9) | 7 (78%) | 0 (0%) | 0 (0%) | 2 (22%) | 1 (11%) | 0 (0%) | 0 (0%) |
| Not reported (n = 2) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (100%) |
| Cost elements used by period of evaluation | |||||||
| 2010.1 to 2012.12 (n = 66) | 31 (47%) | 1 (2%) | 5 (8%) | 8 (12%) | 7 (11%) | 8 (12%) | 15 (23%) |
| 2013.1 to 2014.12 (n = 40) | 17 (43%) | 0 (0%) | 5 (13%) | 8 (20%) | 4 (10%) | 6 (15%) | 10 (25%) |
| 2015.1 to 2016.12 (n = 44) | 22 (50%) | 0 (0%) | 8 (18%) | 9 (20%) | 4 (9%) | 4 (9%) | 5 (11%) |
| 2017.1 to 2018.12 (n = 40) | 18 (45%) | 0 (0%) | 8 (20%) | 5 (13%) | 3 (8%) | 3 (8%) | 7 (18%) |
| 2019.1 to 2019.10 (n = 18) | 10 (56%) | 0 (0%) | 3 (17%) | 2 (11%) | 0 (0%) | 1 (6%) | 2 (11%) |
| Cost elements used by time horizon | |||||||
| Less than 1 year (n = 24) | 16 (67%) | 0 (0%) | 3 (13%) | 1 (4%) | 1 (4%) | 2 (8%) | 2 (8%) |
| 1–10 years (n = 78) | 39 (50%) | 0 (0%) | 11 (14%) | 11 (14%) | 7 (9%) | 5 (6%) | 15 (19%) |
| More than 10 years (n = 34) | 10 (29%) | 0 (0%) | 9 (26%) | 6 (18%) | 6 (18%) | 5 (15%) | 6 (18%) |
| Lifetime (n = 55) | 25 (45%) | 1 (2%) | 4 (7%) | 13 (24%) | 5 (9%) | 8 (15%) | 11 (20%) |
| Not available (n = 17) | 8 (47%) | 0 (0%) | 2 (12%) | 1 (6%) | 2 (12%) | 2 (12%) | 5 (29%) |
aGrand total is higher than the number of studies as some studies included more than one productivity cost element
bThirty-one studies simply state that productivity loss was incorporated in modeling without explicitly specifying the exact elements
Studies which included absenteeism and presenteeism were further analyzed to understand whether these parameters were incorporated for patients alone or for caregivers as well (Table 5). Altogether, 160 studies (77%) had included absenteeism or presenteeism, of which 151 studies (94%) reported whether it was incorporated for patients, caregivers, or both (the information was not clear in nine studies [6%]). Patient absenteeism was the most frequently included productivity loss/gain element, with 90 studies (43%) modeling only patient absenteeism, 21 (10%) modeling the costs of absenteeism for both patients and caregivers, and 11 (5%) modeling the costs of absenteeism for caregivers only. A small proportion of studies (25 [12%]) modeled loss of productivity resulting from both patient absenteeism and patient presenteeism. Presenteeism had been modeled in a relatively smaller proportion of included studies (29 [14%]), with only a single study assessing the impact of caregiver presenteeism. Observations pertaining to absenteeism and presenteeism were fairly consistent across the different disease categories. However, 64% of studies (21 out of 33) included in the CVM disease category assessed the impact of patient absenteeism alone compared to 43% of all studies (90 out of 208). Moreover, 18% of studies (10 out of 55) for immunology diseases assessed the impact of lost productivity based on both patient absenteeism and presenteeism compared to only 12% of all studies (25 out of 208).
Table 5.
Absenteeism/presenteeism-related cost elements used by disease area
| Absenteeism/presenteeism | Cardiovascular and metabolic (n = 33) | Immunology (n = 55) | Central nervous system/psychiatry (n = 42) | Oncology (n = 25) | Respiratory/infectious (n = 27) | Others (n = 26) | Total (N = 208) |
|---|---|---|---|---|---|---|---|
| Absenteeism (patients) | 21 (64%) | 29 (53%) | 18 (43%) | 7 (28%) | 9 (33%) | 6 (23%) | 90 (43%) |
| Absenteeism (caregivers) | 2 (6%) | 1 (2%) | 1 (2%) | 0 (0%) | 4 (15%) | 3 (12%) | 11 (5%) |
| Absenteeism (patients + caregivers) | 3 (9%) | 2 (4%) | 6 (14%) | 5 (20%) | 4 (15%) | 1 (4%) | 21 (10%) |
| Absenteeism + presenteeism (patients) | 0 (0%) | 10 (18%) | 6 (14%) | 0 (0%) | 5 (19%) | 4 (15%) | 25 (12%) |
| Absenteeism + presenteeism (caregivers) | 0 (0%) | 0 (0%) | 1 (2%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (0%) |
| Absenteeism (patients and caregivers) + presenteeism (patients) | 0 (0%) | 1 (2%) | 0 (0%) | 1 (4%) | 0 (0%) | 1 (4%) | 3 (1%) |
| Not reported | 7 (21%) | 12 (22%) | 10 (24%) | 12 (48%) | 5 (19%) | 11 (42%) | 57 (27%) |
The method used to value productivity losses/gains was explicitly reported in only one quarter of all included studies (52 out of 208). Of the studies reporting the method used, more than 80% of studies (43 out of 52) used the human capital approach, while only nine studies used the friction cost approach (Table 6). Studies for which the approach used to estimate productivity losses/gains was not explicitly mentioned were also reviewed in more detail to determine if the description of productivity loss/gain estimations provided could help determine the approach used. However, most of the studies that did not specify the approach used simply mentioned that lost worker productivity costs were included, and the approach used to estimate them was uncertain based on the description provided. Therefore, the analysis of the approach used in those cases was based on the explicit mention of the approach used by the author. Most studies that used the friction cost approach originated from The Netherlands and were conducted after 2016. There was no discernible difference in disease area or other aspects for those studies compared to those that used the human capital approach.
Table 6.
Approach used to estimate productivity losses by disease area
| Approach | Cardiovascular and metabolic (n = 33) | Immunology (n = 55) | Central nervous system/psychiatry (n = 42) | Oncology (n = 25) | Respiratory/infectious (n = 27) | Others (n = 26) | Total (N = 208) |
|---|---|---|---|---|---|---|---|
| Human capital | 10 (30%) | 12 (22%) | 10 (24%) | 1 (4%) | 8 (30%) | 2 (8%) | 43 (21%) |
| Friction cost | 3 (9%) | 2 (4%) | 2 (5%) | 0 (0%) | 0 (0%) | 2 (8%) | 9 (4%) |
| Not reported | 20 (61%) | 41 (75%) | 30 (71%) | 24 (96%) | 19 (70%) | 22 (85%) | 156 (75%) |
Almost 70% of the studies (144 out of 208) reported the impact of including productivity losses/gains on the ICER. While most studies that reported the impact of inclusion of productivity losses/gains on the ICER reported the impact based on specific figures provided in the analysis, about 17% of studies (25 out of 144) reported the impact only as comments by the author(s) about the impact of their inclusion. Overall, the majority of studies (76% [110 of 144]) reported the inclusion of productivity losses/gains as contributing to more favorable cost outcomes and ICERs, meaning that their inclusion was said to lead to a reduction in the ICER. A smaller proportion of included studies (13% [18 of 144]) reported a negative impact due to the inclusion of productivity losses/gains, meaning that their inclusion led to an increase in the ICER (Table 7). Less favorable outcomes were typically due to productivity losses for the comparator being higher relative to the intervention. A similar proportion of studies included (11% [16 of 144]) reported no substantial impact due to the inclusion of productivity losses/gains. As explicitly stated in many of those studies, the limited impact of their inclusion may have been a consequence of productivity losses/gains being a very small fraction of the overall costs. Alternatively, it may be that there were similar impacts on both arms of the studies with their inclusion. The statistical analysis found no differences based on the study characteristics considered in terms of the impact of inclusion of productivity losses/gains on the ICER (Tables 7, 8, 9, 10). (The closest relationship found was based on the inclusion of both absenteeism and presenteeism, with a p value of 0.059 relative to inclusion of absenteeism and some other element. This might suggest that including both absenteeism and presenteeism could lead to more favorable outcomes in terms of cost-effectiveness. But, again, no statistically significant differences were identified.)
Table 7.
Impact of productivity loss inclusion on incremental cost-effectiveness ratio (ICER) by disease area
| Impact1 | Cardiovascular and metabolic (CVM) (n = 33)2 | Immunology (n = 55)3 | Central nervous system (CNS)/psychiatry (n = 42)4 | Oncology (n = 25) | Respiratory/infectious (n = 27)5 | Others (n = 26)6 | Total (N = 208) |
|---|---|---|---|---|---|---|---|
| More favorable | 18 (55%) | 30 (55%) | 23 (55%) | 9 (36%) | 16 (59%) | 14 (54%) | 110 (53%) |
| No substantial impact | 6 (18%) | 5 (9%) | 2 (5%) | 2 (8%) | 0 (0%) | 1 (4%) | 16 (8%) |
| Less favorable | 1 (3%) | 4 (7%) | 6 (14%) | 2 (8%) | 2 (7%) | 3 (12%) | 18 (9%) |
| Not reported | 8 (24%) | 16 (29%) | 11 (26%) | 12 (48%) | 9 (33%) | 8 (31%) | 64 (31%) |
1Three of the included studies stated that productivity costs had a substantial impact without specifying the direction of impact. Studies were labeled as having a “more favorable” impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a “less favorable” impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having “no substantial impact” if there was no change in the ICER with the inclusion of productivity costs or if their inclusion was reported by the author(s) as having no substantial impact and no specific figures were provided
2Chi-square test of oncology and CVM publications excluding “not reported” yielded a p value of 0.424
3Chi-square test of oncology and immunology publications excluding “not reported” yielded a p value of 0.841
4Chi-square test of oncology and CNS/psychiatry publications excluding “not reported” yielded a p value of 0.633
5Chi-square test of oncology and respiratory/infections publications excluding “not reported” yielded a p value of 0.198
6Chi-square test of oncology and other publications excluding “not reported” yielded a p value of 0.6585
Table 8.
Impact of productivity loss inclusion on incremental cost-effectiveness ratio (ICER) by cost element
| Impact1 | Absenteeism only2 (n = 98) | Presenteeism only3 (n = 1) | Absenteeism + presenteeism4 (n = 29) | Absenteeism + others (n = 32) | Unemployment/early retirement5 (n = 21) | Premature mortality6 (n = 22) | Other, not specified (n = 39) | Total7,8 (N = 208) |
|---|---|---|---|---|---|---|---|---|
| More favorable | 50 (51%) | 1 (100%) | 21 (72%) | 16 (50%) | 12 (57%) | 13 (59%) | 17 (44%) | 110 (53%) |
| No substantial impact | 10 (10%) | 0 (0%) | 0 (0%) | 5 (16%) | 4 (19%) | 0 (0%) | 1 (3%) | 16 (8%) |
| Less favorable | 5 (5%) | 0 (0%) | 4 (14%) | 4 (13%) | 2 (10%) | 1 (5%) | 5 (13%) | 18 (9%) |
| Not reported | 33 (34%) | 0 (0%) | 4 (14%) | 7 (22%) | 3 (14%) | 8 (36%) | 15 (38%) | 64 (31%) |
1Three of the included studies stated that productivity costs had a substantial impact without specifying the direction of impact. Studies were labeled as having a “more favorable” impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a “less favorable” impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having “no substantial impact” if there was no change in the ICER with the inclusion of productivity costs or if their inclusion was reported by the author(s) as having no substantial impact and no specific figures were provided
2Chi-square test of “absenteeism + others” and “absenteeism only” excluding “not reported” yielded a p value of 0.389
3Chi-square test of “absenteeism + others” and “presenteeism only” excluding “not reported” yielded a p value of 0.759
4Chi-square test of “absenteeism + others” and “absenteeism + presenteeism” excluding “not reported” yielded a p value of 0.059
5Chi-square test of “absenteeism + others” and “unemployment + early/retirement” excluding “not reported” yielded a p value of 0.898
6Chi-square test of “absenteeism + others” and “premature mortality” excluding “not reported” yielded a p value of 0.113
7Thirty-one studies simply state that productivity loss was incorporated in modeling without explicitly specifying the exact elements
8Grand total is higher than the number of studies as some studies included more than one productivity cost element
Table 9.
Impact of productivity loss inclusion on incremental cost-effectiveness ratio (ICER) with and without inclusion of premature mortality
| Impact1 | Without premature mortality (n = 186) | With premature mortality2 (n = 22) | Total (N = 208) |
|---|---|---|---|
| More favorable | 97 (52%) | 13 (59%) | 110 (53%) |
| No substantial impact | 16 (9%) | 0 (0%) | 16 (8%) |
| Less favorable | 17 (9%) | 1 (5%) | 18 (9%) |
| Not reported | 56 (30%) | 8 (36%) | 64 (31%) |
1Three of the included studies stated that productivity costs had a substantial impact without specifying the direction of impact. Studies were labeled as having a “more favorable” impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a “less favorable” impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having “no substantial impact” if there was no change in the ICER with the inclusion of productivity costs or if their inclusion was reported by the author(s) as having no substantial impact and no specific figures were provided
2Chi-square test of “without premature mortality” and “with premature mortality” excluding “not reported” yielded a p value of 0.2685
Table 10.
Impact of productivity loss inclusion on incremental cost-effectiveness ratio (ICER) by estimation approach used
| Impact1 | Human capital approach (HCA) (n = 43) | Friction cost approach (FCA)2 (n = 9) | Total (n = 52) |
|---|---|---|---|
| More favorable | 32 (75%) | 4 (44%) | 36 (69%) |
| No substantial impact | 3 (7%) | 1 (11%) | 4 (8%) |
| Less favorable | 4 (9%) | 2 (22%) | 6 (12%) |
| Not reported | 4 (9%) | 2 (22%) | 6 (12%) |
1Three of the included studies stated that productivity costs had a substantial impact without specifying the direction of impact. Studies were labeled as having a “more favorable” impact if inclusion of productivity costs resulted in a decrease in the ICER or an increase in the cost savings based on the specific cost figures provided or based on the comments of the author(s) when no specific figures were provided. Similarly, studies were labeled as having a “less favorable” impact if inclusion resulted in an increase in the ICER or a decrease in the cost savings. Studies were labeled as having “no substantial impact” if there was no change in the ICER with the inclusion of productivity costs or if their inclusion was reported by the author(s) as having no substantial impact and no specific figures were provided
2Chi-square test of HCA and FCA excluding “not reported” yielded a p value of 0.3177
Assessment of the Quality of Included Studies
Of the 208 studies included in this review, 148 were included in the quality assessment exercise. The number of the studies appropriately or partially addressing each item of the CHEERS checklist are depicted in Appendix 5 and summarized in Appendix 6 (see the electronic supplementary material). More than 90% of the studies appropriately described the title (99%), setting and location (91%), and measurement of effectiveness (91%). The least appropriately addressed items included analytic methods (30% of the studies included in the exercise did not describe the methods used for handling missing data, pooling or extrapolation, or model validation in detail), discount rates (27% of the studies did not describe the reason for selecting the discount rates), conflicts of interest (26% of the studies did not describe potential conflicts of interest among study contributors in accordance with journal policy), and characterization of heterogeneity (24% of the studies did not describe any subgroup analyses or report differences in costs, outcomes, or cost-effectiveness among various patient subgroups). The most frequent partially addressed items included the time horizon (50% of the studies partially addressed the appropriateness of time horizon over which costs and consequences were evaluated), choice of health outcomes (49% of the studies partially addressed the relevance of outcomes for the type of analysis performed), and choice of model (41% of the studies partially addressed the rationale for selection of the specific type of decision-analytic model). Just over half of the studies met the CHEERS criteria for reporting the currency, price date, and conversion (item 14), the assumptions made in the evaluation (item 16), or details of the study parameters (item 18).
A majority of the studies were deemed to be of good quality, with almost half of them even being considered to be of “excellent” quality (Appendix 7, see the electronic supplementary material). Almost a quarter were considered to have been “very good.” Only 12% of the studies included in this exercise were deemed to be of poor quality.
Discussion
Increasing healthcare costs have necessitated the rigorous economic evaluation of new health technologies such as drugs on the basis of their value proposition to determine the technology that provides the best outcomes for the cost incurred [41]. Indirect costs constitute an important component of the costs of healthcare interventions assessed, especially in CEAs employing a societal perspective [8, 14]. Most studies assessing indirect costs tend to focus on tangible costs outside the healthcare system, such as the imputed value of lost productivity resulting from patients’ reduced efficiency or inability to work due to morbidity or premature mortality. In some disease conditions such as cancer, assessing the impact of reduced productivity of a caregiver may also be appropriate. This systematic review set out to understand the extent of use of costs associated with productivity losses/gains, defined as losses/gains associated with morbidity or mortality leading to a loss of work time, in CEAs and CUAs conducted over the past decade and to estimate their impact on the findings of those studies.
The review identified a few broad themes in the findings of the CEAs/CUAs modeling the costs of productivity loss/gain—themes concerning (1) the origin of the studies that included productivity losses/gains, (2) their perspective, (3) the productivity loss/gain elements included, (4) the approach used to estimate productivity losses/gains, and (5) the impact that inclusion of productivity losses/gains has on the cost-effectiveness of interventions. A decline in the number of studies including productivity costs was observed for January 2013 to December 2015 and later periods relative to the number of studies conducted between January 2010 to December 2012. The reason for this decline is unclear; however, given that this study only included CEA and CUA studies that included productivity losses/gains, it is not possible to generalize about whether this trend applies to only the studies included in this analysis or to CEA and CUA studies as a whole. Most of the studies reviewed originated from high-income countries in Europe and North America based on the World Bank Atlas method [38]. A vast majority of studies were based on mathematical modeling and reported the perspective adopted for economic evaluation as the societal perspective.
Absenteeism, defined as time away from work, was the most widely used productivity loss/gain element used in the analyses, followed by presenteeism (reduced productivity while at work). Presenteeism was not assessed in any of the interventions except CVM and oncological conditions, which may reflect the debilitating nature of these diseases that prevents afflicted individuals from going to work. Analyses in the disease area of oncology modeled premature mortality costs, which are costs associated with loss of productivity due to premature death. This is indicative of a high mortality burden associated with those conditions. Costs associated with loss of productivity due to an outright loss of employment or the need to take early retirement were modeled predominantly in immunological and CNS/psychiatric disorders, reflecting the population modeled (adult working populations) and the debilitating nature of these conditions.
Most of the studies modeling absenteeism and presenteeism only considered patients’ absenteeism as a cost element, while ignoring the time lost by caregivers. Reduced productivity of caregivers was modeled in less than one out of five studies. Some studies have pointed to this as a limitation and cited a lack of data and difficulty in assigning a value to lost productivity as possible reasons for its omission from the analyses [42, 43]. In particular, the difficulty in the valuation of informal care can be challenging. Based on this, greater discussion of approaches to measuring caregiver productivity more readily may be important. This topic was taken up during a special themed edition of PharmacoEconomics in April 2019, with several publications focusing on challenges and approaches to the valuation of caregiver time costs [44–46].
The approach used to estimate productivity losses/gains was not commonly reported. In fact, only 25% of articles reviewed (52 out of 208) explicitly mentioned the approach used to estimate productivity losses/gains. Of the studies reporting the approach used, 83% (43 out of 52) used the human capital approach and only 17% (nine out of 52) used the friction cost approach. This could be because the human capital approach is relatively easier to use compared to the friction cost approach, which is more data intensive. The approach used to estimate productivity losses/gains may also be driven by the guidelines for the country where the studies were conducted. In fact, eight out of the nine studies that used the friction cost approach were conducted in The Netherlands, where the friction cost approach is specified in the local guidelines [18]. Among the studies that reported the use of the human capital approach, at least 60% were for countries that have guidelines that recommend the use of the human capital approach or do not specify the approach that should be used. Again, this suggests that the approach used may be driven by in part the guidelines for the country of origin.
The impact of inclusion of productivity loss/gain elements was reported by most of the studies, with inclusion typically resulting in a decrease in ICER and making the intervention more favorable. This supports previous findings based on a study conducted in 2016 that inclusion of productivity losses/gains in CEA and CUA is important to accurately assess the value of new treatments [9]. The fact that inclusion of productivity losses/gains may have a favorable impact on the ICER is not surprising since, in many cases, any new intervention is expected to result in a favorable clinical outcome and improve the health condition of patients, resulting in improved productivity and lower mortality. Sixteen studies reported that inclusion of productivity losses/gains did not have any significant impact on the ICER, which probably stems from two reasons: either the productivity costs formed a very small fraction of total costs or the costs of productivity loss were similar in both intervention and comparator arms.
No statistical difference was observed between impact on the ICER and most of the study characteristics considered—namely the target disease area, the productivity cost elements included, and the estimation approach used. The closest relationship observed between impact on the ICER and the study characteristics was based on the inclusion of both absenteeism and presenteeism. But those results were also not statistically significant. Inclusion of both absenteeism and presenteeism may yield more favorable outcomes for CEA and CUA when productivity costs are included, but further observation is needed in order to make that determination. Based on studies reporting the impact of the inclusion of productivity costs on the ICER, a trend towards a more favorable ICER was observed for studies that included premature mortality and those for which the productivity costs were estimated using the human capital approach, but those associations were not statistically significant—possibly due to the low number of studies that included premature mortality and those that use the friction cost approach. This suggests that excluding premature mortality may understate the value of new interventions in some cases. It has also been noted in previous studies that the human capital approach has a tendency to yield more favorable cost outcomes relative to the friction cost approach because it does not take into account the replacement of employees that take a long period of time off of work due to illness [7].
Explicit ICER thresholds have been adopted for some countries, including the UK, Ireland, and Thailand. In the UK, an ICER threshold of 20,000–30,000 British pounds (GBP) is considered to be cost-effective, and in Ireland, a threshold of 45,000 EUR has been adopted [47, 48]. In Thailand, an ICER of 1.0–1.5 the per capita gross national income (GNI)/QALY is considered to be cost-effective—or about 160,000 Thai baht per QALY [49]. Among the studies included in this analysis, six studies were conducted for the UK and four studies for Thailand. None of the studies included in the analysis were conducted for Ireland. Among the studies conducted for the UK, consideration of costs associated with productivity loss/gain led to cost-effectiveness based on the explicit threshold of 30,000 GBP for two studies (study #303 and #121), whereby the results would otherwise not have been cost-effective. Moreover, for one study (study #394), dominance was achieved with the inclusion of productivity losses/gains—although the ICER without the inclusion of productivity losses/gains was already considered cost-effective since it was lower than 30,000 GBP. All three of those studies were evaluations of immunology treatments. For the remaining studies, cost-effectiveness was achieved with or without inclusion of productivity losses/gains or the difference was not clearly stated. Among the four studies conducted for Thailand, the impact of inclusion of productivity losses/gains was unclear for three studies and the drug treatment was cost-effective with or without their inclusion for one study (study #197) based on the explicit ICER threshold. These findings suggest that inclusion of productivity losses/gains may be critical to decision making in some cases, but not always clear or impactful in other cases.
Given the increasing number of economic evaluations, it is important to have a standardized reporting framework to allow comparison of multiple evaluations. To address this, the reporting quality of the studies included was evaluated using the CHEERS checklist [32, 33]. While none of the studies met all the criteria set out in the CHEERS checklist, many studies were deemed to be “very good” and “excellent” with regard to the quality of reporting. The areas of deficiency included the degree of detail provided on the analytic methods used in the analysis, not specifying the rationale for applying or not applying the discount rates, and lack of full disclosure on conflicts of interest.
The findings of this review should be viewed in light of its strengths and limitations. This paper presents, to our knowledge, the most exhaustive review to date of the use of productivity losses/gains in CEAs and CUAs. Another strength of this review is the detailed analysis of the various elements being used across different disease areas, which throws some light on how productivity loss/gain elements are used or are being overlooked in pharmacoeconomic analyses. This review has a few limitations as well. Although the review included a large number of studies, the quality of reporting in the studies identified had a bearing on its findings. The extent of details available in the publications varied across studies. For example, only about 75% of the studies were available in full-text or poster formats, precluding the ability to make meaningful observations. Moreover, some studies did not provide sufficient detail for key items such as the productivity loss/gain elements included, the approach used to estimate productivity losses/gains, and their impact on the ICER. In those cases, we relied on the explicit reporting of the study authors, and when the information provided was not sufficiently reported, a label of “not reported” was applied. Ultimately, the approach used to estimate productivity losses/gains was not adequately reported in many publications. As many as 48 out of 208 studies (23%) did not provide the type of productivity loss/gain element (e.g., absenteeism or presenteeism) included in the analysis or provided only limited information. Moreover, only about 25% of the studies reviewed included a clear statement about the approach used to estimate the productivity losses/gains—e.g., the human capital approach and the friction cost approach. Lastly, some studies did not separately present the ICERs with and without inclusion of productivity losses/gains, making it difficult to make direct inferences about the impact of the inclusion of productivity losses/gains on the ICER.
This review was conducted using three online databases. Other databases and gray literature sources including any unpublished reports were not included in the review, which may have led to non-identification of some relevant studies. However, this review had set out to identify CEAs and CUAs for drug interventions only, and the databases searched are widely acknowledged to be very comprehensive with regard to the medical and health economics literature. As such, it does not seem likely that inclusion of more databases would have resulted in a substantially higher number of studies that could have altered the conclusion drawn.
Conclusions
This review revealed that, while the inclusion of productivity losses/gains in CEA and CUA are likely to lead to more favorable cost-effectiveness outcomes, economic evaluations for drug interventions around the globe are not being carried out according to a standardized framework when it comes to the inclusion or exclusion of productivity losses/gains. While the findings from this study provide some direction, further examination and discussion is needed to consider the optimal framework for considering productivity losses/gains in CEA, including the appropriate cost elements to include (e.g., patient absenteeism, caregiver absenteeism, presenteeism, unemployment) and how those costs should be estimated. Moreover, an analysis by country may be important given the different context and background of healthcare systems in each country.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
Assistance in the preparation of a draft manuscript was provided by Asclepius Medical Communications LLC, Ridgewood, New Jersey, USA.
Declarations
Author contributions
This study includes research content that AY has conducted during his doctoral studies at the International University of Health and Welfare. AY and NY were responsible for the study conceptualization. AY and ML were responsible for overseeing the data extraction and reporting of the results. All of the authors contributed to the review, revision, and finalization of the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
Conflict of interest
The authors declare the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Akira Yuasa and Naohiro Yonemoto are full-time employees of Pfizer Japan Inc. Michael LoPresti is a full-time employee of INTAGE Healthcare Inc. Shunya Ikeda declares no conficts of interest associated with this manuscript.
Funding
Pfizer Japan Inc. was the only direct sponsor of this study, and fees were paid to INTAGE Healthcare Inc. Akira Yuasa and Naohiro Yonemoto are employees of Pfizer Japan Inc. and were involved in substantial contributions to the conception, design, analysis of data and the subsequent drafting of this work.
References
- 1.Neumann PJ, Russell LB, Siegel JE, Prosser LA, Krahn M, Mandelblatt JS, et al. Using cost-effectiveness in health and medicine: experiences since the original panel. In: Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, et al., editors. Cost-effectiveness in health and medicine. New York: Oxford University Press; 2017. pp. 1–38. [Google Scholar]
- 2.Shi CR, Nambudiri VE. Research techniques made simple: cost-effectiveness analysis. J Investig Dermatol. 2017;137(7):e143–e147. doi: 10.1016/j.jid.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Thokala P, Ochalek J, Leech AA, Tong T. Cost-effectiveness thresholds: the past, the present and the future. Pharmacoeconomics. 2018;36(5):509–522. doi: 10.1007/s40273-017-0606-1. [DOI] [PubMed] [Google Scholar]
- 4.Zhang W, Sun H, Woodcock S, Anis AH. Valuing productivity loss due to absenteeism: firm-level evidence from a Canadian linked employer-employee survey. Health Econ Rev. 2017;7(1):3. doi: 10.1186/s13561-016-0138-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.International Society for Pharmacoeconomics and Outcomes Research I, (ISPOR). Pharmacoeconomic guidelines around the world. International Society for Pharmacoeconomics and Outcomes Research, Lawrenceville, NJ, USA. 2020. https://www.tools.ispor.org/peguidelines/. Accessed 29 Mar 2020.
- 6.Stone PW, Chapman RH, Sandberg EA, Liljas B, Neumann PJ. Measuring costs in cost-utility analyses: variations in the literature. Int J Technol Assess Health Care. 2000;16(1):111–124. doi: 10.1017/s0266462300161100. [DOI] [PubMed] [Google Scholar]
- 7.Pike J, Grosse SD. Friction cost estimates of productivity costs in cost-of-illness studies in comparison with human capital estimates: a review. Appl Health Econ Health Policy. 2018;16(6):765–778. doi: 10.1007/s40258-018-0416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riewpaiboon A. Measurement of costs for health economic evaluation. J Med Assoc Thai. 2014;97(Suppl 5):S17–26. [PubMed] [Google Scholar]
- 9.Krol M, Papenburg J, Tan SS, Brouwer W, Hakkaart L. A noticeable difference? productivity costs related to paid and unpaid work in economic evaluations on expensive drugs. Eur J Health Econ. 2016;17(4):391–402. doi: 10.1007/s10198-015-0685-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AMCP. AMCP format for formulary submissions—guidance on submission of pre-approval and post-approval clinical and economic information and evidence, Version 4.1. December 23, 2019. https://www.amcp.org/Resource-Center/format-formulary-submissions/AMCP-Format-for-Formulary-Submissions-4.1. Accessed 14 Sept 2020.
- 11.National Institute for Health and Care Excellence (NICE). Guide to the methods of technology appraisal 2013 (PMG9). National Institute for Health and Care Excellence, London, UK. 2013. https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed 29 Mar 2020. [PubMed]
- 12.Commonwealth of Australia DoH. Guidelines for Preparing a Submission to the Pharmaceutical Benefits Advisory Committee (Version 5.0). Commonwealth of Australia, Department of Health, Canberra, ACT, Australia. 2016. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf. Accessed 29 Mar 2020.
- 13.Canadian Agency for Drugs and Technologies in Health (CADTH). Guidelines for the Economic Evaluation of Health Technologies: Canada—4th Edition (Version 1.0). Canadian Agency for Drugs and Technologies in Health (CADTH), Ottawa, ON, Canada. 2017. https://www.cadth.ca/sites/default/files/pdf/guidelines_for_the_economic_evaluation_of_health_technologies_canada_4th_ed.pdf. Accessed 29 Mar 2020.
- 14.Haute Autorité de santé (HAS). Choices in Methods for Economic Evaluation: A Methodological Guide. Department of Economics and Public Health Assessment, HAS, Saint-Denis La Plaine, France. 2012. https://www.has-sante.fr/upload/docs/application/pdf/2012-10/choices_in_methods_for_economic_evaluation.pdf. Accessed 29 Mar 2020.
- 15.Center for Outcomes Research and Economic Evaluation for Health. Guideline for Preparing Cost-Effectiveness Evaluation to the Central Social Insurance Medical Council (Version 2.0). National Institute of Public Health (C2H), Japan, Tokyo, Japan. 2019. https://c2h.niph.go.jp/tools/guideline/guideline_en.pdf. Accessed 29 Mar 2020.
- 16.Krol M, Brouwer W. How to estimate productivity costs in economic evaluations. Pharmacoeconomics. 2014;32(4):335–344. doi: 10.1007/s40273-014-0132-3. [DOI] [PubMed] [Google Scholar]
- 17.Kigozi J, Jowett S, Lewis M, Barton P, Coast J. Estimating productivity costs using the friction cost approach in practice: a systematic review. Eur J Health Econ. 2016;17(1):31–44. doi: 10.1007/s10198-014-0652-y. [DOI] [PubMed] [Google Scholar]
- 18.Zorginstituut Nederland. Richtlijn voor het Uitvoeren van Economische Evaluaties in de Gezondheidszorg [Guideline for Conducting Economic Evaluations in Health Care.] Zorginstituut Nederland, 2015.
- 19.Assessment of Methods in Health Care, The Swedish Agency for Health Technology Assessment and Assessment of Social Services. August 2017.
- 20.Center for Drug Evaluation. Taiwan. Accessed via the ISPOR HTA guidelines website: https://www.tools.ispor.org/PEguidelines/source/HTA_guidelines_Taiwan.pdf.
- 21.Krol M, Papenburg J, Koopmanschap M, Brouwer W. Do productivity costs matter?: the impact of including productivity costs on the incremental costs of interventions targeted at depressive disorders. Pharmacoeconomics. 2011;29(7):601–619. doi: 10.2165/11539970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 22.Koopmanschap MA, Rutten FF. The impact of indirect costs on outcomes of health care programs. Health Econ. 1994;3(6):385–393. doi: 10.1002/hec.4730030606. [DOI] [PubMed] [Google Scholar]
- 23.Johannesson M, Jonsson B, Jönsson L, Kobelt G, Zethraeus N. Why should economic evaluations of medical innovations have a societal perspective? SSRN Electron J. 2009 doi: 10.2139/ssrn.2640123. [DOI] [Google Scholar]
- 24.Ten JB. arguments for a societal perspective in the economic evaluation of medical innovations. Eur J Health Econ. 2009;10(4):357–359. doi: 10.1007/s10198-009-0173-2. [DOI] [PubMed] [Google Scholar]
- 25.Tai BB, Bae YH, Le QA. A systematic review of health economic evaluation studies using the patient's perspective. Value Health. 2016;19(6):903–908. doi: 10.1016/j.jval.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 26.Centre for Reviews and Dissemination (CRD) UoY. Systematic Reviews: CRD’s guidance for undertaking reviews in health care. York: Centre for Reviews and Dissemination (CRD), University of York; 2009.
- 27.Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M et al. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane, London, UK. July 2019. https://training.cochrane.org/handbook. Accessed 29 Mar 2020.
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clarivate Analytics. EndNote. Clarivate Analytics, Philadelphia, PA. 2020. https://endnote.com/. Accessed 30 Mar 2020.
- 31.Covidence. Covidence systematic review software. Covidence, Melbourne, Victoria, Australia. 2020. https://www.covidence.org/home. Accessed 29 Mar 2020.
- 32.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMJ. 2013;346:f1049. doi: 10.1136/bmj.f1049. [DOI] [PubMed] [Google Scholar]
- 33.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Hettiarachchi RM, Kularatna S, Downes MJ, Byrnes J, Kroon J, Lalloo R, et al. The cost-effectiveness of oral health interventions: a systematic review of cost-utility analyses. Community Dent Oral Epidemiol. 2018;46(2):118–124. doi: 10.1111/cdoe.12336. [DOI] [PubMed] [Google Scholar]
- 35.Palfreyman SJ, Stone PW. A systematic review of economic evaluations assessing interventions aimed at preventing or treating pressure ulcers. Int J Nurs Stud. 2015;52(3):769–788. doi: 10.1016/j.ijnurstu.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 36.Rogers HJ, Rodd HD, Vermaire JH, Stevens K, Knapp R, El Yousfi S, et al. A systematic review of the quality and scope of economic evaluations in child oral health research. BMC Oral Health. 2019;19(1):132. doi: 10.1186/s12903-019-0825-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hope SF, Webster J, Trieu K, Pillay A, Ieremia M, Bell C, et al. A systematic review of economic evaluations of population-based sodium reduction interventions. PLoS ONE. 2017;12(3):e0173600. doi: 10.1371/journal.pone.0173600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The World Bank Group, World Bank Country and Lending Groups. Accessed March 18, 2020. https://www.datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups
- 39.Agresti A. An introduction to categorical data analysis. 2. New York: Wiley; 2007. [Google Scholar]
- 40.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093–1103. doi: 10.1001/jama.2016.12195. [DOI] [PubMed] [Google Scholar]
- 41.Sanders GD, Maciejewski ML, Basu A. Overview of cost-effectiveness analysis. JAMA. 2019;321(14):1400–1401. doi: 10.1001/jama.2019.1265. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs JC, Van Houtven CH, Tanielian T, Ramchand R. Economic spillover effects of intensive unpaid caregiving. Pharmacoeconomics. 2019;37(4):553–562. doi: 10.1007/s40273-019-00784-7. [DOI] [PubMed] [Google Scholar]
- 43.Lin PJ, D'Cruz B, Leech AA, Neumann PJ, Aigbogun MS, Oberdhan D, Lavelle TA. Family and caregiver spillover effects in cost-utility analyses of Alzheimer’s disease interventions. Pharmacoeconomics. 2019;37(4):597–608. doi: 10.1007/s40273-019-00788-3. [DOI] [PubMed] [Google Scholar]
- 44.Arora S, Goodall S, Viney R, Einfeld S. Using discrete-choice experiment methods to estimate the value of informal care: the case of children with intellectual disability. Pharmacoeconomics. 2019;37(4):501–511. doi: 10.1007/s40273-018-0637-2. [DOI] [PubMed] [Google Scholar]
- 45.Hoefman RJ, van Exel J, Brouwer WBF. The monetary value of informal care: obtaining pure time valuations using a discrete choice experiment. Pharmacoeconomics. 2019;37(4):531–540. doi: 10.1007/s40273-018-0724-4. [DOI] [PubMed] [Google Scholar]
- 46.Grosse SD, Pike J, Soelaeman R, Tilford JM. Quantifying family spillover effects in economic evaluations: measurement and valuation of informal care time. Pharmacoeconomics. 2019;37(4):461–473. doi: 10.1007/s40273-019-00782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.National Institute for Health and Care Excellence (NICE). Developing NICE guidelines: the manual Process and methods [PMG20]. Last updated: 31 October 2018. https://www.nice.org.uk/process/pmg20/chapter/incorporating-economic-evaluation.
- 48.O'Mahony JF, Coughlan D. The Irish cost-effectiveness threshold: does it support rational rationing or might it lead to unintended harm to Ireland’s health system? Pharmacoeconomics. 2016;34(1):5–11. doi: 10.1007/s40273-015-0336-1. [DOI] [PubMed] [Google Scholar]
- 49.Krittayaphong R, Yadee J, Permsuwan U. Cost-effectiveness analysis of the adjunctive therapy of ivabradine for the treatment of heart failure with reduced ejection fraction. Clinicoecon Outcomes Res. 2019;5(11):767–777. doi: 10.2147/CEOR.S226568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its supplementary information files).