Abstract
Whereas the genetic basis of insulin sensitivity is determined by variation in multiple genes, mutations of single genes can give rise to profound changes in such sensitivity. Mutations of the insulin receptor gene (INSR)—which trigger type A insulin resistance, Rabson–Mendenhall, or Donohue syndromes—and those of the gene for the p85α regulatory subunit of phosphoinositide 3-kinase (PIK3R1), which give rise to SHORT syndrome, are the most common and second most common causes, respectively, of single-gene insulin resistance. Loss-of-function mutations of the genes for the protein kinase Akt2 (AKT2) or for TBC1 domain family member 4 (TBC1D4) have been identified in families with severe insulin resistance. Gain-of-function mutations of the gene for protein tyrosine phosphatase nonreceptor type 11 (PTPN11), which negatively regulates insulin receptor signaling, give rise to Noonan syndrome, and some individuals with this syndrome manifest insulin resistance. Gain-of-function mutations of the gene for the p110α catalytic subunit of phosphoinositide 3-kinase (PIK3CA) have been identified in individuals with segmental overgrowth or megalencephaly, some of whom also manifest spontaneous hypoglycemia. A gain-of-function mutation of AKT2 was also found in individuals with recurrent hypoglycemia. Loss-of-function mutations of the gene for phosphatase and tensin homolog (PTEN), another negative regulator of insulin signaling, give rise to Cowden syndrome in association with exaggerated metabolic actions of insulin. Clinical manifestations of individuals with such mutations of genes related to insulin signaling thus provide insight into the essential function of such genes in the human body.
Introduction
The most important biological action of insulin is to regulate the storage and supply of energy sources in the body. Insulin exerts a variety of metabolic and nonmetabolic effects, however, and impairment of insulin action gives rise not only to diabetes mellitus but also to various other pathological conditions including dyslipidemia, atherosclerosis, nonalcoholic fatty liver disease, cognitive disorders, and certain types of cancer.
Impairment of insulin sensitivity, or insulin resistance, has both genetic and environmental causes [1]. In general, genetic predisposition to insulin resistance is determined by variation in multiple genes. However, in rare cases, mutations of a single gene can trigger severe insulin resistance. Defects in the insulin receptor gene (INSR) are the most common cause of such single-gene insulin resistance, but mutations of several other genes related to insulin signaling have also been identified in individuals with severe insulin resistance. In addition, mutations of such genes have been found to give rise to exaggerated insulin action in some individuals.
We here review various conditions triggered by mutations of genes related to insulin signaling (Table 1), with a distinction as to whether the conditions are associated with insulin resistance or exaggerated insulin sensitivity.
Table 1.
Conditions caused by mutations of genes related to insulin signaling
| Gene | Effect on protein function | Effect on insulin action | Frequency | Condition caused | Other characteristics |
|---|---|---|---|---|---|
| INSR | Loss | Resistance | Relatively common (> 200) | Type A insulin resistance, Donohue, and Rabson–Mendenhall syndromes | Acanthosis nigricans, polycystic ovary, hirsutism (in type A insulin resistance); early death (in Donohue and Rabson–Mendenhall) |
| PIK3R1 | Loss | Resistance | Rare (> 30) | SHORT syndrome | Short stature, facial characteristics |
| PIK3R2 | Gain | Sensitivity | Rare (> 10) | None | Segmental overgrowth or megalencephaly |
| PIK3CA | Gain | Sensitivity | Rare (> 60) | None | Segmental overgrowth or megalencephaly |
| AKT1 | Gain | Not known | Rare (> 20) | Proteus syndrome | Overgrowth of various tissues, mosaic mutation |
| AKT2 | Loss | Resistance | Very rare (3) | None | Hypertension |
| Gain | Sensitivity | Very rare (1) | None | Overgrowth, hypoglycemia | |
| AKT3 | Gain | Not known | Very rare (~ 3) | None | Megalencephaly |
| TBC1D4 | Loss | Resistance | Very rare (1) | None | Acanthosis nigricans, postprandial hyperinsulinemia |
| PTEN | Loss | Sensitivity | Relatively common (> 300)a | Cowden syndrome | Hamartoma, cancer predisposition |
| PTPN11 | Gain | Resistance | Relatively common (> 800)b | Noonan syndrome | Short stature, congenital heart disease, skeletal malformation |
| PRKCE | Loss | Nonec | Very rare (1) | SHORT syndrome | Short stature, facial characteristics |
Numbers in parentheses for frequency indicate the number of cases or families described in published reports
aFifteen families have been evaluated for glucose tolerance or insulin sensitivity
bOne family has been evaluated for glucose tolerance or insulin sensitivity
cThe patient might have been too young to develop apparent insulin resistance
Insulin signaling pathway
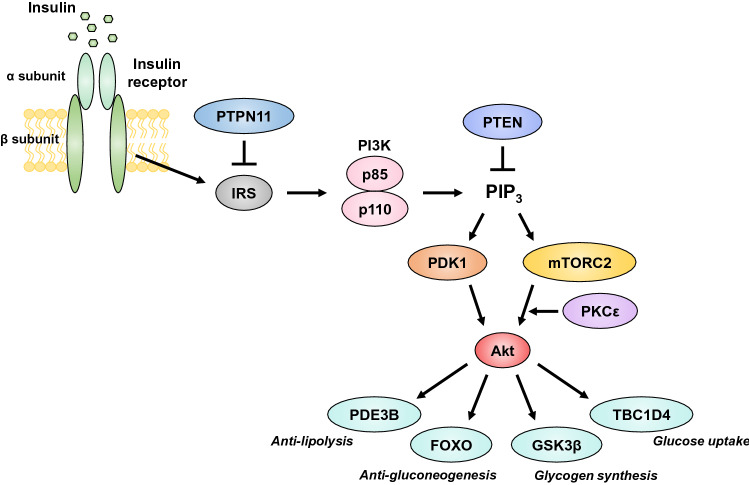
The insulin receptor is a tetrameric structure composed of two extracellular α subunits and two transmembrane β subunits [1] (Fig. 1). The binding of insulin to the α subunits activates the intrinsic tyrosine kinase of the β subunits, resulting in phosphorylation of tyrosine residues of insulin receptor substrate (IRS) proteins and their consequent association with signaling molecules that contain a Src homology 2 (SH2) domain [1]. Among such signaling molecules, class Ia phosphoinositide 3-kinase (PI3K), composed of a 110-kDa catalytic subunit and an 85-kDa regulatory subunit, is a key mediator of the metabolic actions of insulin [1]. The association of PI3K with IRS results in stimulation of its lipid kinase activity and consequent generation of phosphatidylinositol 3,4,5-trisphosphate (PIP3), which promotes the phosphorylation and activation of Akt by phosphoinositide-dependent kinase 1 (PDK1) [1]. PI3K also activates mammalian target of rapamycin complex 2 (mTORC2), which also contributes to the activation of Akt.
Fig. 1.
The insulin signaling pathway
Akt is a serine-threonine kinase that mediates the effects of insulin by phosphorylating various cellular substrates including the serine-threonine kinase GSK3β (glycogen synthase kinase 3β), transcription factors of the forkhead box O (FOXO) family, the cyclic AMP-hydrolyzing enzyme phosphodiesterase 3B (PDE3B), and the Rab GTPase-activating protein TBC1 domain family member 4 (TBC1D4, also known as Akt substrate 160 or AS160) [1].
Protein tyrosine phosphatase nonreceptor type 11 (PTPN11, also known as SH2 domain-containing phosphatase-2 or SHP-2) and phosphatase and tensin homolog (PTEN) negatively regulate insulin signaling by catalyzing the dephosphorylation of IRS and PIP3, respectively.
Mutations of the insulin receptor gene
Mutations of INSR give rise to type A insulin resistance syndrome, which is characterized by bodily features of hirsutism, acanthosis nigricans, or polycystic ovary in addition to insulin resistance [2]. The severity of the disease varies widely depending on the specific gene defect [3]. Rabson–Mendenhall syndrome and Donohue syndrome, which overlap to some extent, are characterized by the development of serious insulin resistance and intractable diabetes in association with specific physical findings and result in infant or pediatric death [3]. Most individuals with either of these two syndromes harbor pathological mutations of INSR in both alleles [3].
Several hundred cases of type A insulin resistance syndrome have been described, and at least 0.05% of the general population in Japan is estimated to harbor a pathological mutation of INSR [4].
Mutations of genes for PI3K
PIK3R1 encodes p85α, a major and ubiquitously expressed regulatory subunit of PI3K. Mutations of PIK3R1 have been identified in individuals with SHORT syndrome [5–7], which is characterized by short stature, hyperextensibility of joints and/or inguinal hernia, ocular depression, Rieger abnormality, and teething delay. Some patients with this syndrome also develop diabetes [8]. Mutations of PIK3R1 have also been identified in individuals with insulin-resistant diabetes who had not been recognized as having SHORT syndrome, although these individuals manifest some, if not all, of the classic bodily characteristics of this syndrome [4, 5, 9].
More than 30 families with PIK3R1 mutations have been described in the context of either SHORT syndrome or insulin-resistant diabetes [4–7, 9–15]. A recent nationwide survey in Japan identified 23 and 5 cases of INSR and PIK3R1 mutations, respectively, in individuals with severe insulin resistance [4], suggesting that PIK3R1 mutations are the second most common cause of single-gene insulin resistance. The most frequent mutation of PIK3R1 observed in individuals with SHORT syndrome or genetic insulin resistance is Arg649Trp, with the mutant p85α protein having been shown to inhibit insulin signaling in a dominant manner [16]. A total of 11 different PIK3R1 mutations have been identified in such patients to date [4–7, 9–15].
Somatic activating mutations of PIK3CA, which encodes the ubiquitously expressed p110α catalytic subunit of PI3K, have been found to occur frequently in cancer cells and less frequently in tissues with segmental overgrowth [17], whereas germline mutations of PIK3CA have been identified in individuals with segmental overgrowth or megalencephaly [17, 18]. Some patients with such germline PIK3CA mutations also manifest recurrent hypoglycemia [19]. Mutations of PIK3R2, which encodes the p85β regulatory subunit of PI3K, have also been found in patients with hypoglycemia and either segmental overgrowth or megalencephaly [18]. Given that the activity of PI3K in cells is determined by a balance between the functions of multiple regulatory and catalytic subunits [20], the mutations of PIK3R2 found in these patients likely result in augmentation of PI3K signaling.
Mutations of the genes for Akt
Among the three isoforms of Akt, Akt1 and Akt2 are ubiquitously expressed, whereas Akt3 is expressed almost exclusively in the central nervous system [1]. A heterozygous Arg274His mutation of AKT2, which is located in the kinase domain of the encoded protein and exerts a dominant inhibitory effect on insulin action, was identified in a family with severe insulin-resistant diabetes [21]. The affected individuals manifested profound hyperinsulinemia of > 100 and > 1000 μU/mL under fasting and postprandial conditions, respectively, together with acanthosis nigricans and hypertension [21]. The population of Finland appears to harbor the partial loss-of-function Pro50Thr variant of AKT2 at a relatively high frequency (1.1%), with this mutation increasing the risk of the development of type 2 diabetes with an odds ratio of 1.05 in this population [22].
An activating mutation of AKT2 (Glu17Lys), either mosaic or nonmosaic, has been identified in individuals with left-sided overgrowth and severe recurrent hypoglycemia from infancy [23], and an activating mosaic mutation affecting the same residue of AKT1 (Glu17Lys) was identified in patients with Proteus syndrome [24], which is characterized by the overgrowth of various tissues including skin, bone, muscle, blood vessels, and the brain. Activating mutations of AKT3 were identified in patients with megalencephaly syndrome [25], which likely reflects the central nervous system-specific expression of this isoform. Information regarding glucose tolerance or insulin sensitivity in individuals with such activating mutations of AKT1 or AKT3 is not available.
Mutations of the gene for TBC1D4
TBC1D4, a substrate of Akt, contributes to insulin-stimulated glucose uptake by promoting the translocation of glucose transporter 4 (GLUT4) [1]. A mutation of TBC1D4 (Arg363Ter) was found in a family in which affected members manifested marked postchallenge hyperinsulinemia with mild glucose intolerance [26]. An Arg684Ter variant of TBC1D4 was also identified with a high allele frequency (~ 17%) in a Greenlandic cohort [27], whereas this variant is rare in other populations [28]. Homozygous carriers of this variant manifested increased circulating concentrations of glucose and insulin at 2 h after an oral glucose load, whereas fasting glucose and insulin levels were not increased, but were instead decreased [27]. Moreover, homozygous carriers showed a markedly increased risk of the development of type 2 diabetes, with an odds ratio of 10.3 [27]. The Arg684Ter variant thus appears to confer insulin resistance exclusively under the postprandial condition as well as a predisposition to type 2 diabetes.
Mutations of other genes related to insulin signaling
Heterozygous loss-of-function mutations of PTEN give rise to Cowden syndrome, which is characterized by multiple hamartomas in various tissues and an increased risk of certain types of cancer including breast, endometrial, and thyroid tumors [29]. Patients with Cowden syndrome manifest lower circulating levels of insulin in spite of a higher body mass index compared with healthy control individuals [30], suggesting that insulin action is augmented in these patients.
Noonan syndrome, characterized by short stature, congenital heart disease, and skeletal malformation, is caused by genetic defects in the Ras-MAPK (mitogen-activated protein kinase) signaling pathway [31]. Gain-of-function mutations of PTPN11, which result in inhibition of tyrosine kinase signaling and downstream Ras-MAPK signaling, therefore trigger Noonan syndrome [31]. Insulin-induced activation of Akt as well as glucose uptake and glycogen synthesis were found to be impaired in cells obtained from individuals harboring gain-of-function mutations of PTPN11, and some of these individuals manifested insulin resistance [32].
A loss-of-function mutation (Glu599Lys) of PRKCE, which encodes the ε isoform of protein kinase C (PKCε), was identified in an individual with clinical features of SHORT syndrome but without a PIK3R1 mutation [33]. Forced expression of a kinase-deficient mutant of PKCε was previously shown to inhibit insulin-induced Akt activity [34] (Fig. 1). The Glu599Lys mutant form of PKCε was found to inhibit Akt activation by the mTORC2 pathway [33]. The patient with this mutation did not manifest insulin resistance or diabetes at the age of 13 years, although diabetes becomes overt in most individuals with SHORT syndrome at an older age [5–7, 9].
Homozygous or compound heterozygous mutations of the insulin-like growth factor-1 (IGF-1) receptor gene (IGF1R) give rise to SHORT syndrome-like bodily features associated with insulin resistance [35–37]. Given that the IGF-1 receptor and the insulin receptor are similar with regard to their structure and intracellular signaling and that the metabolic effects of insulin are likely mediated in part by the IGF-1 receptor, it is possible that the insulin resistance of these patients results directly from impaired function of the IGF-1 receptor. The circulating level of growth hormone is markedly increased in individuals with IGF1R mutations [35, 36], which might also contribute to the development of insulin resistance. Of interest, some patients with IGF1R mutations were found to manifest relatively insulin-deficient diabetes [38], which may be related to the notion that IGF-1 receptor signaling plays an important role in the development of pancreatic β cells [39].
Conclusion
We have here summarized conditions caused by mutations of genes related to insulin signaling. Identification of the responsible genes and mutations in such genetic conditions may contribute to better treatment not only of these rare diseases but also of insulin resistance due to more common causes. In addition to insulin resistance or exaggerated insulin sensitivity, patients with these genetic conditions manifest a variety of disorders or characteristic bodily features, consistent with the many functions of insulin and its downstream signaling mediators. Further detailed investigation of the clinical manifestations of these conditions should provide insight into the essential functions of insulin signaling in the human body.
Author contributions
RK and WO conceived the content of and wrote the manuscript. YH contributed to discussion and collection of information. All authors reviewed the manuscript for intellectual content and approved the final version. This study was supported by a Grant-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (to Y.H., 19K08981). This article does not contain any studies with human or animal subjects performed by any of the authors.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiol Rev. 2018;98:2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn CR, Flier JS, Bar RS, Archer JA, Gorden P, Martin MM, et al. The syndromes of insulin resistance and acanthosis nigricans. Insulin-receptor disorders in man. N Engl J Med. 1976;294:739–745. doi: 10.1056/NEJM197604012941401. [DOI] [PubMed] [Google Scholar]
- 3.Taylor SI, Cama A, Accili D, Barbetti F, Quon MJ, de la Luz SM, et al. Mutations in the insulin receptor gene. Endocr Rev. 1992;13:566–595. doi: 10.1210/edrv-13-3-566. [DOI] [PubMed] [Google Scholar]
- 4.Takeuchi T, Ishigaki Y, Hirota Y, Hasegawa Y, Yorifuji T, Kadowaki H, et al. Clinical characteristics of insulin resistance syndromes: a nationwide survey in Japan. J Diabetes Investig. 2020;11:603–616. doi: 10.1111/jdi.13171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thauvin-Robinet C, Auclair M, Duplomb L, Caron-Debarle M, Avila M, St-Onge J, et al. PIK3R1 mutations cause syndromic insulin resistance with lipoatrophy. Am J Hum Genet. 2013;93:141–149. doi: 10.1016/j.ajhg.2013.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chudasama KK, Winnay J, Johansson S, Claudi T, König R, Haldorsen I, et al. SHORT syndrome with partial lipodystrophy due to impaired phosphatidylinositol 3 kinase signaling. Am J Hum Genet. 2013;93:150–157. doi: 10.1016/j.ajhg.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dyment DA, Smith AC, Alcantara D, Schwartzentruber JA, Basel-Vanagaite L, Curry CJ, et al. Mutations in PIK3R1 cause SHORT syndrome. Am J Hum Genet. 2013;93:158–166. doi: 10.1016/j.ajhg.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Innes AM, Dyment DA. SHORT syndrome. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors. GeneReviews. Seattle: University of Washington; 1993. [PubMed] [Google Scholar]
- 9.Hamaguchi T, Hirota Y, Takeuchi T, Nakagawa Y, Matsuoka A, Matsumoto M, et al. Treatment of a case of severe insulin resistance as a result of a PIK3R1 mutation with a sodium-glucose cotransporter 2 inhibitor. J Diabetes Investig. 2018;9:1224–1227. doi: 10.1111/jdi.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schroeder C, Riess A, Bonin M, Bauer P, Riess O, Döbler-Neumann M, et al. PIK3R1 mutations in SHORT syndrome. Clin Genet. 2014;86:292–294. doi: 10.1111/cge.12263. [DOI] [PubMed] [Google Scholar]
- 11.Bárcena C, Quesada V, De Sandre-Giovannoli A, Puente DA, Fernández-Toral J, Sigaudy S, et al. Exome sequencing identifies a novel mutation in PIK3R1 as the cause of SHORT syndrome. BMC Med Genet. 2014;15:51. doi: 10.1186/1471-2350-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Avila M, Dyment DA, Sagen JV, St-Onge J, Moog U, Chung BHY, et al. Clinical reappraisal of SHORT syndrome with PIK3R1 mutations: toward recommendation for molecular testing and management. Clin Genet. 2016;89:501–506. doi: 10.1111/cge.12688. [DOI] [PubMed] [Google Scholar]
- 13.Petrovski S, Parrott RE, Roberts JL, Huang H, Yang J, Gorentla B, et al. Dominant splice site mutations in PIK3R1 cause hyper IgM syndrome, lymphadenopathy and short stature. J Clin Immunol. 2016;36:462–471. doi: 10.1007/s10875-016-0281-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang-Doran I, Tomlinson P, Payne F, Gast A, Sleigh A, Bottomley W, et al. Insulin resistance uncoupled from dyslipidemia due to C-terminal PIK3R1 mutations. JCI Insight. 2016;1:e88766. doi: 10.1172/jci.insight.88766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klatka M, Rysz I, Kozyra K, Polak A, Kołłątaj W. SHORT syndrome in a two-year-old girl—case report. Ital J Pediatr. 2017;43:44. doi: 10.1186/s13052-017-0362-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winnay JN, Solheim MH, Dirice E, Sakaguchi M, Noh HL, Kang HJ, et al. PI3-kinase mutation linked to insulin and growth factor resistance in vivo. J Clin Invest. 2016;126:1401–1412. doi: 10.1172/JCI84005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindhurst MJ, Parker VE, Payne F, Sapp JC, Rudge S, Harris J, et al. Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat Genet. 2012;44:928–933. doi: 10.1038/ng.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rivière JB, Mirzaa GM, O’Roak BJ, Beddaoui M, Alcantara D, Conway RL, et al. De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat Genet. 2012;44:934–940. doi: 10.1038/ng.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leiter SM, Parker VER, Welters A, Knox R, Rocha N, Clark G, et al. Hypoinsulinaemic, hypoketotic hypoglycaemia due to mosaic genetic activation of PI3-kinase. Eur J Endocrinol. 2017;177:175–186. doi: 10.1530/EJE-17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fruman DA, Chiu H, Hopkins BD, Bagrodia S, Cantley LC, Abraham RT. The PI3K pathway in human disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.George S, Rochford JJ, Wolfrum C, Gray SL, Schinner S, Wilson JC, et al. A family with severe insulin resistance and diabetes due to a mutation in AKT2. Science. 2004;304:1325–1328. doi: 10.1126/science.1096706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manning A, Highland HM, Gasser J, Sim X, Tukiainen T, Fontanillas P, et al. A low-frequency inactivating AKT2 variant enriched in the Finnish population is associated with fasting insulin levels and type 2 diabetes risk. Diabetes. 2017;66:2019–2032. doi: 10.2337/db16-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain K, Challis B, Rocha N, Payne F, Minic M, Thompson A, et al. An activating mutation of AKT2 and human hypoglycemia. Science. 2011;334:474. doi: 10.1126/science.1210878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–619. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, et al. De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet. 2012;44:941–945. doi: 10.1038/ng.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dash S, Sano H, Rochford JJ, Semple RK, Yeo G, Hyden CS, et al. A truncation mutation in TBC1D4 in a family with acanthosis nigricans and postprandial hyperinsulinemia. Proc Natl Acad Sci USA. 2009;106:9350–9355. doi: 10.1073/pnas.0900909106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moltke I, Grarup N, Jørgensen ME, Bjerregaard P, Treebak JT, Fumagalli M, et al. A common Greenlandic TBC1D4 variant confers muscle insulin resistance and type 2 diabetes. Nature. 2014;512:190–193. doi: 10.1038/nature13425. [DOI] [PubMed] [Google Scholar]
- 28.Genome Aggregation Database, https://gnomad.broadinstitute.org/variant/13-75898521-G-C?dataset=gnomad_r2_1. Accessed 24 June 2020
- 29.Blumenthal GM, Dennis PA. PTEN hamartoma tumor syndromes. Eur J Hum Genet. 2008;16:1289–1300. doi: 10.1038/ejhg.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pal A, Barber TM, Van de Bunt M, Rudge SA, Zhang Q, Lachlan KL, et al. PTEN mutations as a cause of constitutive insulin sensitivity and obesity. N Engl J Med. 2012;367:1002–1011. doi: 10.1056/NEJMoa1113966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El Bouchikhi I, Belhassan K, Moufid FZ, IraquiHoussaini M, Bouguenouch L, Samri I, et al. Noonan syndrome-causing genes: molecular update and an assessment of the mutation rate. Int J Pediatr Adolesc Med. 2016;3:133–142. doi: 10.1016/j.ijpam.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranza E, Guimier A, Verloes A, Capri Y, Marques C, Auclair M, et al. Overlapping phenotypes between SHORT and Noonan syndromes in patients with PTPN11 pathogenic variants. Clin Genet. 2020;98:10–18. doi: 10.1111/cge.13746. [DOI] [PubMed] [Google Scholar]
- 33.Alcantara D, Elmslie F, Tetreault M, Bareke E, Hartley T, Care4Rare Consortium et al. SHORT syndrome due to a novel de novo mutation in PRKCE (protein kinase Cɛ) impairing TORC2-dependent AKT activation. Hum Mol Genet. 2017;26:3713–3721. doi: 10.1093/hmg/ddx256. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto M, Ogawa W, Hino Y, Furukawa K, Ono Y, Takahashi M, et al. Inhibition of insulin-induced activation of Akt by a kinase-deficient mutant of the epsilon isozyme of protein kinase C. J Biol Chem. 2001;276:14400–14406. doi: 10.1074/jbc.M011093200. [DOI] [PubMed] [Google Scholar]
- 35.Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med. 1996;335:1363–1367. doi: 10.1056/NEJM199610313351805. [DOI] [PubMed] [Google Scholar]
- 36.Gannagé-Yared MH, Klammt J, Chouery E, Corbani S, Mégarbané H, Abou Ghoch J, et al. Homozygous mutation of the IGF1 receptor gene in a patient with severe pre- and postnatal growth failure and congenital malformations. Eur J Endocrinol. 2012;168:K1–7. doi: 10.1530/EJE-12-0701. [DOI] [PubMed] [Google Scholar]
- 37.Prontera P, Micale L, Verrotti A, Napolioni V, Stangoni G, Merla G. A new homozygous IGF1R variant defines a clinically recognizable incomplete dominant form of SHORT syndrome. Hum Mutat. 2015;36:1043–1047. doi: 10.1002/humu.22853. [DOI] [PubMed] [Google Scholar]
- 38.Fang P, Cho YH, Derr MA, Rosenfeld RG, Hwa V, Cowell CT. Severe short stature caused by novel compound heterozygous mutations of the insulin-like growth factor 1 receptor (IGF1R) J Clin Endocrinol Metab. 2012;97:E243–E247. doi: 10.1210/jc.2011-2142. [DOI] [PubMed] [Google Scholar]
- 39.Ueki K, Okada T, Hu J, Liew CW, Assmann A, Dahlgren GM, et al. Total insulin and IGF-I resistance in pancreatic beta cells causes overt diabetes. Nat Genet. 2006;38:583–588. doi: 10.1038/ng1787. [DOI] [PubMed] [Google Scholar]