Abstract
Objectives:
Abacavir’s potential to cause cardiovascular disease (CVD) among people living with HIV (PLWH) is debated. We conduct a systematic review and meta-analyses to assess CVD risk from recent and cumulative abacavir exposure.
Methods:
We searched Medline, Embase, Web of Science, abstracts from Conference on Retroviruses and Opportunistic Infections, and International AIDS Society/AIDS Conferences and bibliographies of review articles to identify research studies published through 2018 on CVD risk associated with abacavir exposure among PLWH. Studies assessing risk of CVD associated with recent (exposure within last 6 months) or cumulative abacavir exposure across all age-groups were eligible. Risks were quantified using fixed- and random-effects models.
Results:
Of 378 unique citations, 68 full-text research articles and abstracts were reviewed. Seventeen studies assessed risk of CVD from recent or cumulative abacavir exposure. Summary relative risk (sRR) is increased for recent exposure (n=16 studies, sRR=1.61; 95% confidence interval: 1.48–1.75), higher in antiretroviral-therapy-naive population (n=5, 1.91; 1.48–2.46) and all studies reported RR> 1. The sRR for recent exposure was similarly increased for the outcome of acute myocardial infarction, and for studies that adjusted for substance abuse, smoking, prior CVD, traditional CVD risk factors, and CD4 cell-count/HIV viral load. The sRR was increased for cumulative abacavir exposure (per year) (n=4, 1.12; 1.05–1.20) but no increase was seen after adjusting for recent exposure (n=5, 1.00; 0.93–1.08).
Conclusions:
Our findings suggest an increased risk of CVD from recent abacavir exposure. The risk remained elevated after adjusting for potential confounders. Further investigations are needed to understand CVD risk from cumulative exposure.
Keywords: Abacavir, Human immunodeficiency virus, Cardiovascular disease
1. Introduction
In 2008, the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study groups first reported that abacavir use is associated with an increased risk of acute myocardial infarction (AMI) among people living with HIV (PLWH) [1]. Subsequent studies conducted to investigate this risk have yielded conflicting results [2–10]. Although most recent studies have shown an increased risk of cardiovascular disease (CVD) associated with use of abacavir, many of the results did not reach statistical significance [3,11–14]. The studies that assessed the risk of CVD associated with exposure to abacavir were mostly observational, and there is a possibility that the study results are confounded and biased. For instance, PLWH may be preferentially prescribed abacavir or tenofovir based on the presence or absence of risk factors for CVD (i.e. hypertension, diabetes mellitus, renal dysfunction, dyslipidemia, and lipodystrophy), their cardiovascular safety profile, and the safety profile of companion antiretroviral (ARV) agents used. The results of epidemiologic studies also differed based on whether the study populations were antiretroviral therapy (ART) -naive or experienced, and using injection drugs. A previously conducted meta-analysis reported an increased risk of CVD exposure by pooling results across two studies and no conclusion was made on CVD risk from cumulative abacavir exposure due to inadequacy of studies [15]. Eight studies investigating the risk of CVD associated with exposure to abacavir have been published since that meta-analysis [10–14,16–18], one of which assessed the risk from specific abacavir-based ARV drug combinations. Therefore, we performed an updated systematic review and meta-analysis to summarize the relationship between recent (including current exposure) and cumulative exposure to abacavir or abacavir-based drug combinations and the risk of CVD among HIV-infected individuals. We also discuss the plausible biological mechanisms underlying the observed risk of CVD associated with exposure to abacavir. Finally, we address the biases potentially affecting the study results, the methodological challenges and inconsistencies we observed with regard to study design and analysis, and interpretation of these results.
2. Methods
2.1. Literature Search
Two authors (K.D. and T.C.) independently searched Medline, Embase, Web of Science, abstract books from the 2014–2018 Conference on Retroviruses and Opportunistic Infections (CROI) and 2014–2017 International AIDS Society (IAS) Conference and International AIDS Conference, and the bibliographies of five published reviews [15,19–22] to identify studies published through May 2018 that investigated the risk of CVD associated with exposure to abacavir. We used the keywords, ‘abacavir’, ‘cardiovascular disease’, ‘myocardial infarction’, and ‘heart disease’ for the search. PRISMA guidelines were followed in conducting the review.
2.2. Study Selection
We included randomized controlled trials (RCTs), and cohort and case-control studies, published in peer-reviewed journals or presented in conference proceedings that assessed abacavir exposure, either as an individual agent or in specific ARV drug combinations, and provided an estimate of a relative risk and variance. Outcome was defined as a new episode of CVD after the start of exposure. We required CVD to be defined as an ischemia-driven cardiac event/procedure such as AMI, angina pectoris, percutaneous coronary intervention or coronary artery bypass grafting. We included studies across all age-groups that assessed ischemic stroke as a component of the CVD definition, but excluded results that assessed only stroke as an outcome. We included conference abstracts if the data were unique (i.e. not included in the research articles chosen for the meta-analysis). We excluded from the meta-analysis studies that (1) assessed a class of ARV agents but did not specify abacavir as the exposure, (2) assessed possible risk factors for CVD including biomarkers as outcome but did not measure CVD, or (3) that reported only crude estimates and did not adjust for basic confounders, such as age. Two authors (K.D. and T.C.) independently evaluated each study and abstracted relative risk estimates. Discrepancies were resolved through open deliberation.
2.3. Data abstraction and definitions
We reviewed each study in detail and categorized them based on the type of exposure, outcome, study design, study population, data year, comparator ARV agent/group used, and adjustment for potential causal mediators and specific covariates. We performed meta-analyses of the results for the risk of CVD associated with (1) recent exposure to abacavir, defined as exposure within the last 6 months, including current exposure, and (2) cumulative exposure, defined as the accumulated sum of the total duration of exposure at pre-specified time points. CVD risk from cumulative abacavir exposure was calculated separately for models that did and did not adjust for recent exposure. We performed pertinent sub-group analyses for the risk of CVD from recent exposure. For studies that reported multiple risk estimates using marginal structural models and traditional models, we chose the marginal structural model estimates because of its potential to address channeling bias and difference in the exposure groups [23]. In a sensitivity analysis, we assessed the risk of CVD associated with recent exposure by excluding the 2008 D:A:D study that initiated the other studies.
2.4. Statistical analysis and evaluation of bias
We have calculated and reported summary estimates from both fixed- and random-effects models [24]. We assessed heterogeneity across studies using Cochran’s Q-test (χ2 P<0.10) [25] and I2 statistics (I2 >30%) [26]. Some believe that the random-effects model is more conservative than the fixed-effects model because it accounts for variance between studies. However, unlike the fixed- effects model, the random-effects model does not weight studies directly on precision; it assigns smaller, less precise studies greater relative weight than does the fixed-effect model. Fixed-effects model weight studies directly on precision while still incorporating between-study variance. Therefore, we used the fixed-effects model to calculate the sRRs and then adjusted their 95% confidence intervals (CIs) for between-study heterogeneity using the method described by Shore et al. [27]. Random effects model may also be used in the event of heterogeneity [28]. The calculations were performed in Microsoft Office Excel 2016 (Microsoft corporation, Redmond, WA, USA). The P-values are two-sided. We analysed publication bias using funnel plots and Begg’s and Egger’s test. Quality of each cohort and case-control study was assessed using the Newcastle-Ottawa assessment scales and each RCT using the PRISMA guidelines.
3. Results
We identified 377 unique articles/abstracts from the searches of Medline, Embase, Web of Science, and abstract books of 2014–2018 CROI, and 2014–2017 IAS/AIDS conferences. Of these, we reviewed the full texts of 65 research articles (Fig. 1). Seventeen studies meeting our inclusion criteria were used for the meta-analysis. Six studies assessed the risk associated with cumulative exposure to abacavir. There were 13 cohort studies, three case-control studies, and one RCT. Table 1 summarizes these studies.
Fig. 1.
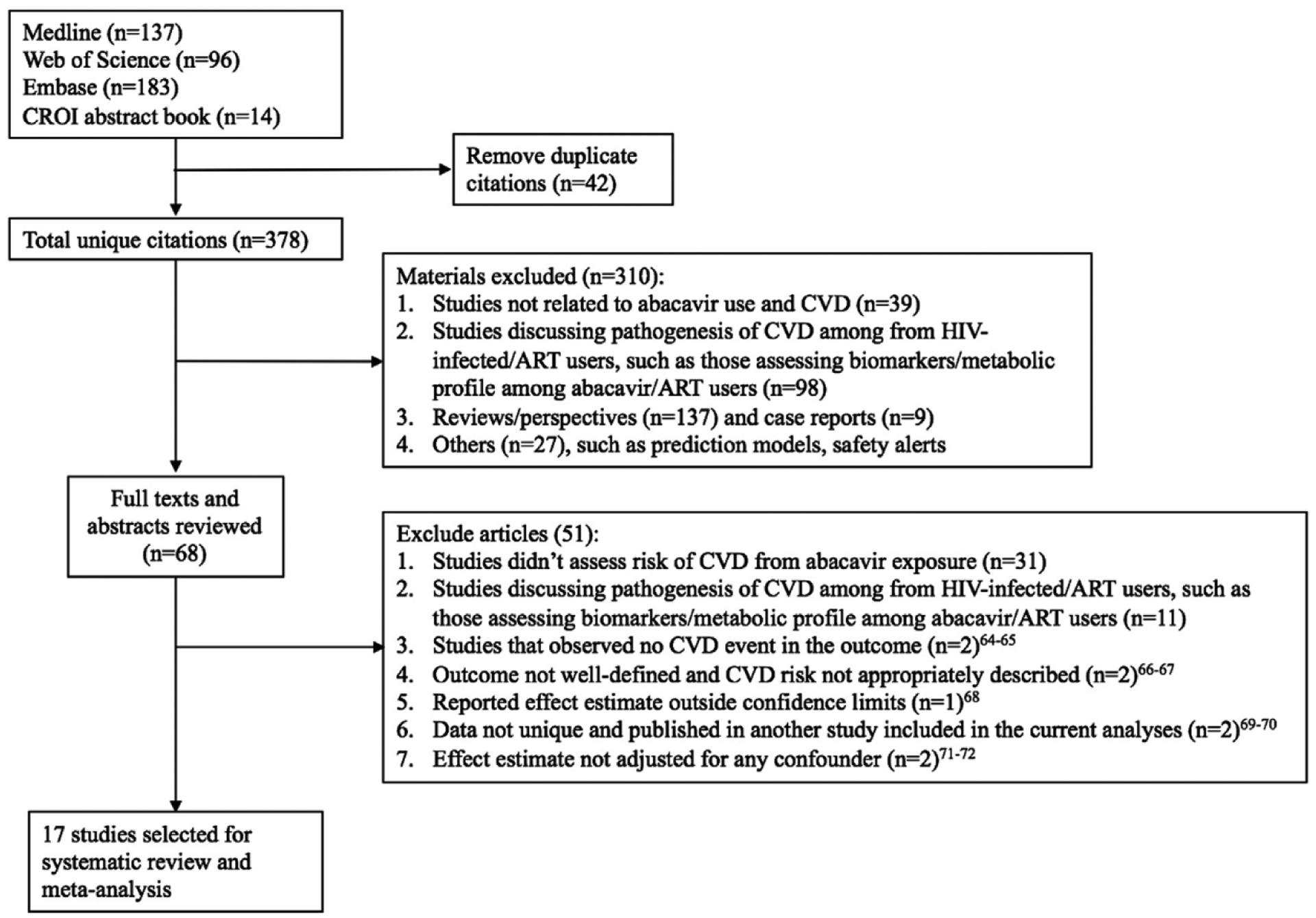
Flow diagram showing the search strategy and algorithm for identification of studies. ART, antiretroviral therapy; CVD, cardiovascular disease (See Refs. [64–72]).
Table 1.
Studies assessing the risk of acute myocardial infarction (AMI) or cardiovascular disease (CVD) from recent or cumulative exposure to abacavir.
| Author, year of publication | Study period | Cohort/Location | Study Design | Sample size (n) | Outcome (n) | Exposure (recent or cumulative) | Reference group | Statistics (95% CI) | Covariates included in the model and comments |
|---|---|---|---|---|---|---|---|---|---|
| D:A:D Study Groups, 2008 | 1999–2007 | Multinational D:A:D cohort | Prospective cohort | 33 347 | AMI (517) AMI (517) | Recent Cumulative | Other ARV agents Other ARV agents |
RR: 1.90 (1.47, 2.45) RR: 1.14 (1.08, 1.21) |
Age, sex, risk group, ethnicity, calendar year, cohort, smoking, family history of CVD, prior CVD, BMI, cumulative exposure to other ARV drugs. Further adjustment for CD4 cell count, viral load, lipid and glucose levels, blood pressure, diabetes, and lipodystrophy made little difference to the result. |
| D:A:D study Groups, 2016 | 1999–2013 | Multinational D:A:D cohort | Prospective cohort | 49 717 | AMI (941) | Current | Other ARV agents | RR: 1.98 (1.72–2.29) | Time-fixed covariates: gender, mode of HIV acquisition, ethnicity, participating clinical cohort: time-updated covariates: age, smoking status, family history of CVD, previous CVD event, BMI, cumulative exposure to the main protease inhibitors and non-NRTIs, and cumulative and current exposure to other NRTIs. |
| 1999-Feb 2008 | Multinational D:A:D cohort | Prospective cohort | 210250 person-years | AMI (672) | Current | Other ARV agents | RR: 1.97 (1.68–2.33) | Sensitivity analyses separately carried out adjusting potential causal mediators: diabetes mellitus, total and high-density lipoprotein cholesterol, triglycerides, blood pressure, antihypertensive drugs, blood glucose, Framingham score, body weight, and renal function | |
| March 2008–2013 | Multinational D:A:D cohort | Prospective cohort | 157 309 person-years | AMI (269) | Current | Other ARV agents | RR: 1.97 (1.43–2.72) | ||
| Lundgren et al., 2008 | 2002–2007 | Multinational (SMART study) | Prospective cohort | 4544 | AMI (19) CVD (70) |
Recent Recent |
Other NRTIs Other NRTIs |
HR: 4.25 (1.39, 13) HR: 1.80 (1.04, 3.11) |
Age, sex, race, baseline viral load and CD4 cell count, smoking, prior CVD, diabetes, BP-lowering drugs, hepatitis B or C virus infection, baseline use of NNRTI and PI. |
| Martin et al., 2009 | 2005–2008 | STEAL study, Australia | Randomized controlled trial | 357 | CVD (9) | Recent | Tenofovir-Emtricitabir | HR: 8.33 (1.40, 1649.58) | |
| Lang et al., 2010 | 2000–2006 | French Hospital Database | Case-control | 1173 | AMI (289) | Recent Cumulative | Not exposed to abacavir Not exposed to abacavir |
OR: 1.62 (0.93, 2.81) OR: 0.97 (0.87, 1.10) |
Cases and controls were matched for age and sex and the models were adjusted for hypertension, smoking, family history of premature coronary artery disease, cocaine or IVDU, HIV viral load, CD4:CD8 cell ratio, and exposure to other ARV drugs. |
| Obel et al., 2010 | 1995–2005 | Danish HIV Cohort Study | Prospective cohort | 2952 | AMI (67) | Current | Not exposed to abacavir | HR: 2.00 (1.10, 3.64) | Age, sex, year of diagnosis, year of HAART initiation, CD4 cell count, viral load, race, injection drug use, use of other ARV drugs, diabetes, alcoholism, COLD, hypertension, liver disease, and kidney disease. |
| Choi et al., 2011 | 1997–2007 | VA HIV Clinical Case Registry, USA | Retrospective cohort | 10 931 | CVD (501) CVD (501) AMI (NS) |
Recent Cumulative Recent |
ARV agents other than tenofovir and abacavir | HR: 1.48 (1.08, 2.04) HR: 0.93 (0.79, 1.10) HR: 1.64 (0.88, 3.08) |
Age, sex, race, calendar year, diabetes, hypertension, dyslipidemia, prevalent CVD, smoking, drug abuse, hepatitis B and C virus infection, cancer, eGFR, proteinuria, BMI, CD4 cell count, viral load, and cumulative exposure to other ARV drugs. |
| Bedimo et al., 2011 | 1996–2004 | VA HIV Clinical Case Registry, USA | Retrospective cohort | 19 424 | AMI (267) AMI (278) |
Current Cumulative | ARV agents other than tenofovir and abacavir Not exposed to abacavir |
HR: 0.67 (0.43, 1.03) HR: 1.18 (0.92, 1.50) |
Model for current exposure was adjusted for only chronic kidney disease. Model for cumulative exposure was adjusted for age, hypercholesterolemia, hypertension, diabetes, and smoking. |
| Durand et al., 2011 | 1985–2007 | RAMQ and Med-Echo Databases, Quebec | Case-control | 1209 | AMI (125) | Recent | Not exposed to abacavir | OR: 1.72 (1.10, 2.71) | Age, sex, past AMI, past stroke, chronic kidney disease, anti-diabetic drug use, lipid-lowering drug use, anti-platelet or warfarin use, hepatitis C infection, illicit drug use. |
| Rotger et al., 2013 | 2000–2009 | MAGNIFICENT Consortium | Case-control | 1875 | CAD (571) | Current | Not specified | OR: 1.56 (1.17, 2.11) | Sex-matched study and restricted to people without prior CAD. Adjusted for age, smoking, family history of CAD, cholesterol levels, hypertension or anti-hypertensives, diabetes mellitus and anti-diabetic medication, current ART exposure, cumulative exposure to lopinavir and indinavir, CD4 cell count, and HIV viral load. |
| Brouwer et al., 2014 | 2002–2008 | North Carolina Medicaid Beneficiaries | Retrospective cohort | 3481 | AMI (38) | Recent | Tenofovir | HR: 2.05 (0.72, 5.86) | Age, sex, race, calendar year, cardiovascular medication use (β-blockers, ACEI, ARB, CCB, statins), hospitalization, comorbidities including heart failure, diabetes, renal disease, cerebrovascular disease, peripheral vascular disease, and ART-regimen type. |
| Desai et al., 2015 | 1996–2009 | VA HIV Clinical Case Registry, USA | Retrospective cohort | 24 510 | CVD (934) | Current | All other ARV agents |
HR: 1.50 (1.26, 1.79) | Age, year of exposure, viral load, CD4 cell count, exposure to statins, and follow up time. |
| Young et al., 2015 | 2000–2012 | Swiss HIV Cohort Study | Prospective cohort | 11856 | CVD (365) | Recent Cumulative |
Not exposed to abacavir |
HR: 1.63 (1.14, 2.32) HR: 1.22 (0.98, 1.52) |
Age, sex, injection drug use, Caucasian ethnicity, family history of CAD, prior CVD, smoking, BMI, calendar year, cumulative exposure to other ARV drugs, hypertension, diabetes, Framingham risk score categories, CD4 cell count and viral load. |
| Palella et al., 2015 | 1995–2010 | NA-ACCORD, North America | Prospective cohort | 16733 (full study population) 6485 (ART-naive) |
AMI (301) AMI (93) |
Recent Recent |
Not recently exposed Not recently exposed |
HR: 1.34 (0.96, 1.88) HR: 1.95 (1.18, 3.45) |
Model for full study population (ART-naive and ART-experienced) was adjusted for age, sex, race, risk group, calendar year, smoking, hypertension, diabetes, renal impairment, cholesterol and triglyceride levels, statin use, CD4 count, viral load, previous PI use, and cohort. In addition to above covariates, the model for restricted study population (ART-naive) was adjusted for hepatitis C infection and history of clinical AIDS diagnosis. |
| Marcus et al., 2016 | 1998–2011 | Kaiser (KPNC&KPSC), California | Retrospective cohort | 8154 | CVD (178) | Recent | All other ARV agents |
HR: 2.2 (1.4, 3.5) | Age, sex, calendar era, years known to be HIV-infected at ART initiation, race/ethnicity, CD4 cell count, HIV viral load, risk group, previous ART experience, alcohol use, smoking, overweight/obesity, diabetes, lipid-lowering therapy, renal function, and indicator for pre-2008 and post-2008. |
| Dorjee et al., 2017 | 2009–2014 | US Insurance Claims Data | Retrospective cohort | 72 733 | CVD (714) CVD (714) |
Recent Cumulative | All other ARV agents All other ARV agents |
HR: 1.40 (1.16, 1.69) HR: 1.08 (0.89, 1.30) |
Age, sex, calendar year, smoking, body weight, substance abuse, alcohol abuse, hepatitis B and C infection, prior CVD, chronic kidney disease, hypertension, diabetes, dyslipidemia, anti-hyperglycemic agents, cardiovascular medications (ACEI, ARB, CCB, β-blockers, statins, aspirin), stroke, cancer, symptomatic HIV-disease, and exposure to specific ARV drugs. |
| Elion et al., 2018 | 2001–2013 | NA-ACCORD, North America | Prospective cohort | 8265 | AMI (123) | Recent | All other ARV agents |
HR: 1.84 (1.17, 2.91) | Age, sex, race/ethnicity, acquisition, calendar year, smoking, hepatitis C infection, hypertension, diabetes, renal function, lipid levels, statin use, CD4 cell count, HIV viral load, history of clinical AIDS |
ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; ARV, antiretroviral; BMI, body mass index; BP, blood pressure; CAD, coronary artery disease; CCB, calcium channel blocker; Cl, confidence interval; COLD, chronic obstructive lung disease; eGFR, estimated glomerular filtration rate; HAART, highly active antiretroviral agent; IVDU, intravenous drug use; NNRTI, non nucleoside reverse transcriptase inhibitor; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor.
3.1. Recent exposure
All 16 studies that met our inclusion criteria for recent exposure showed an increased risk of CVD from recent exposure to abacavir; 13 were statistically significant (Fig. 2). We obtained an sRR (95% Cl) of 1.61 (1.48, 1.75) for recent exposure (Table 2). We excluded the study by Bedimo et al. [2] in the above analysis because the estimate adjusted only for chronic kidney disease and not for any other variable including age and gender. In a sensitivity analysis, the risk continued to be elevated (sRR: 1.56; 95% Cl; 1.40, 1.74) when Bedimo et al.’s study was included in the meta-analysis (Table 2). In addition to reporting risk of CVD from abacavir as an individual antiretroviral agent, Desai et al. obtained the following results (hazard ratio (HR) (95% Cl)) for CVD risk from exposure to abacavir-based ARV drug combinations: abacavir + atazanavir + lamivudine: 2.08 (1.41–3.06); abacavir + efavirenz + lamivudine: 1.94 (1.34–2.79); abacavir + Iamivudine + zidovudine: 1.60 (1.21–2.11); abacavir + lamivudine + lopinavir: 1.44 (0.91–2.28); and abacavir + lamivudine + nevirapine: 1.49 (0.81–2.73) [11]. We have not included the above results of Desai et al. into our analyses because each of the abacavir-based combinations represents a unique exposure, and it would be misleading to pool the study results of specific abacavir-based drug combinations with studies that assessed abacavir’s risk as an individual agent. Table 2 lists the results of our group and sub-group analyses for recent and cumulative exposure to abacavir. The summary estimate for risk of CVD associated with recent abacavir exposure remained elevated for ART-naive populations (n=5 studies) (sRR: 1.91; 95% Cl: 1.48, 2.46) and for studies conducted in the pre-2008 (n=8; sRR:1.77; 95% Cl: 1.51, 2.07) and post-2008 periods (n=2; sRR: 1.53; 95% Cl: 1.14, 2.06) (Fig. 4). Meta-analysis of two studies that used tenofovir as the comparator ARV agent showed a greater risk of CVD in the abacavir groups (sRR: 2.94; 95% Cl: 0.88, 9.76) as compared to the result for all studies, but the sRR was not statistically significant (Fig. 4). The results for recent exposure in subgroup analyses based on outcome, study design, adjustment for causal intermediates, and adjustment of other specific covariates including substance abuse/HIV acquisition through injection drug use are similar to the result for all studies (Table 2, Fig. 3). In a sensitivity analysis for recent exposure, the risk remained elevated after excluding the 2008 D:A:D study (HR: 1.58; 95% Cl: 1.45, 1.72). Egger’s (P=0.648) and Begg’s (P=0.153) tests for publication bias were not statistically significant (funnel plot in Fig. 5). The Newcastle-Ottawa scores (highest possible score=9) for qualitative bias analyses for cohort and case-control studies ranged from 6 to 9, with a median score of 9 (Supplementary Tables S1, S2).
Fig. 2.
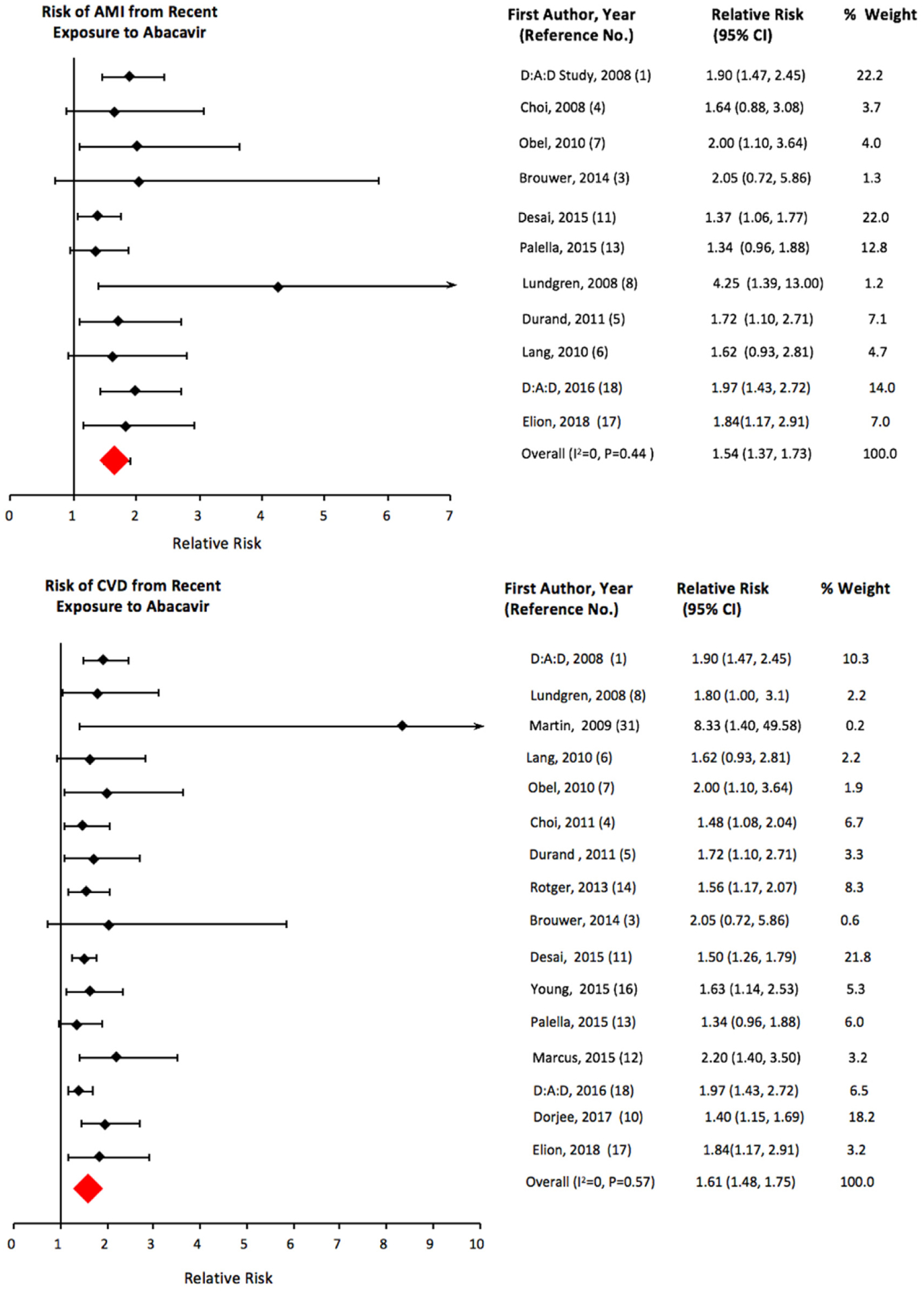
Pooled risk of cardiovascular disease and acute myocardial infarction from recent exposure to abacavir.
Table 2.
Results of meta-analyses for the risk of cardiovascular disease (CVD) from exposure to abacavir in HIV-infected individuals.
| Group and subgroup$ (uutcome = CVD, unless specified) | No. of studies | Fixed effects | Shore-adjusted# | Random effects# | Heterogeneity | ||||
|---|---|---|---|---|---|---|---|---|---|
| sRR | 95% CI | 95% CI | sRR | 95% CI | χ2 | P | I2 value (%) | ||
| Recent exposure | |||||||||
| All studies | 17 | 1.56 | 1.44, 1.69 | 1.40, 1.74 | 1.35 | 1.20, 1.53 | 28.5 | 0.03 | 44 |
| Excluding Bedimo et al., 2011 | 16 | 1.61 | 1.48, 1.75 | NA | NA | NA | 13.6 | 0.57 | 0 |
| Outcome | |||||||||
| AMI | 11 | 1.68 | 1.49, 1.90 | NA | NA | NA | 9.30 | 0.50 | 0 |
| Study population | |||||||||
| ART-naïve | 5 | 1.91 | 1.48, 2.46 | NA | NA | NA | 0.79 | 0.94 | 0 |
| Study design | |||||||||
| Observational studies | 15 | 1.60 | 1.48, 1.74 | NA | NA | NA | 10.30 | 0.74 | 0 |
| Data year$ | |||||||||
| Pre-2008 | 8 | 1.85 | 1.63, 2.10 | NA | NA | NA | 5.63 | 0.58 | 0 |
| Post-2008 | 2 | 1.53 | 1.30, 1.81 | 1.14, 2.06 | 1.62 | 1.16, 2.25 | 3.19 | 0.07 | 69 |
| Comparator agent | |||||||||
| Tenofovir | 2 | 2.94 | 1.19, 7.25 | 0.88, 9.76 | 3.41 | 0.91,12.76 | 1.76 | 0.18 | 43 |
| Confounder adjustment | |||||||||
| Potential causal mediators or traditional CVD risk factors* | 15 | 1.56 | 1.44, 1.70 | NA | NA | NA | 13.84 | 0.46 | 0 |
| Substance abuse | 12 | 1.64 | 1.49, 1.82 | 1.48, 1.83 | 1.26 | 1.13, 1.41 | 12.39 | 0.33 | 11 |
| Smoking status | 12 | 1.55 | 1.42, 1.68 | 1.41, 1.69 | 1.37 | 1.25, 1.51 | 12.32 | 0.34 | 11 |
| Prior CVD | 12 | 1.57 | 1.43, 1.71 | 1.41, 1.73 | 1.41 | 1.26, 1.57 | 14.87 | 0.19 | 26 |
| CD4 cell count and HIV viral load | 11 | 1.56 | 1.42, 1.72 | NA | NA | NA | 9.78 | 0.46 | 0 |
| Cumulative exposure (per year) | |||||||||
| Model not adjusted for recent exposure | 4 | 1.12 | 1.07, 1.18 | 1.05, 1.20 | 1.11 | 1.02, 1.21 | 6.71 | 0.08 | 55 |
| Model adjusted for recent abacavir exposure | 5 | 1.00 | 0.94, 1.06 | 0.93, 1.08 | 1.00 | 0.92, 1.10 | 6.75 | 0.15 | 41 |
AMI, acute myocardial infarction; ART, antiretroviral therapy; Cl, confidence interval; sRR, summary relative risk.
Studies included in this group adjusted for at least three of five potential factors (hypertension, diabetes mellitus, renal dysfunction, dyslipidemia, and lipodystrophy) that may lie on the causal pathway between abacavir exposure and risk of CVD.
Shore-adjusted confidence intervals and random-effects models may be used when the χ2 statistic was greater than the degrees of freedom (number of studies minus 1).
Fig. 4.
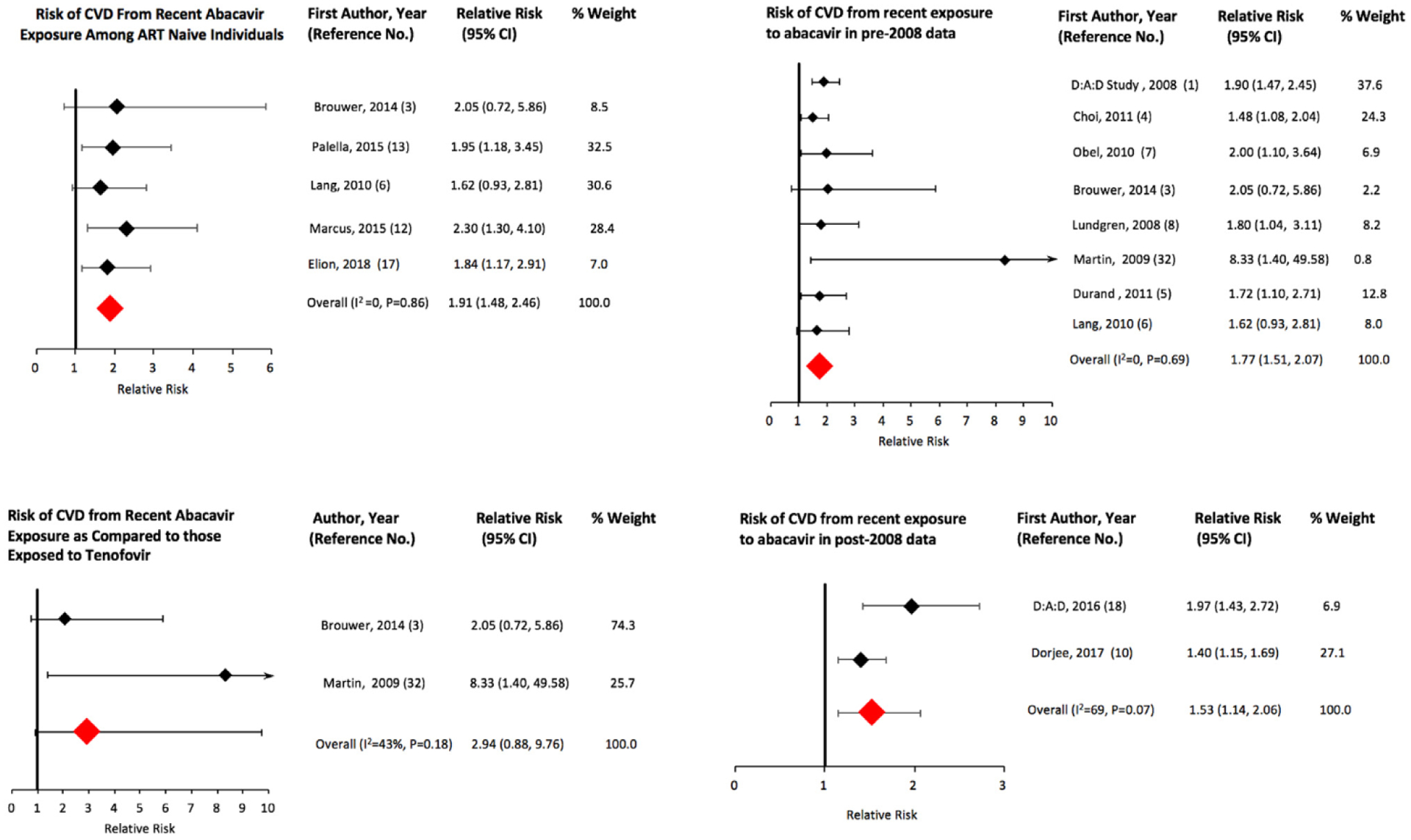
Pooled risk of cardiovascular disease from recent exposure to abacavir in sub-groups of HIV-infected individuals.
Fig. 3.
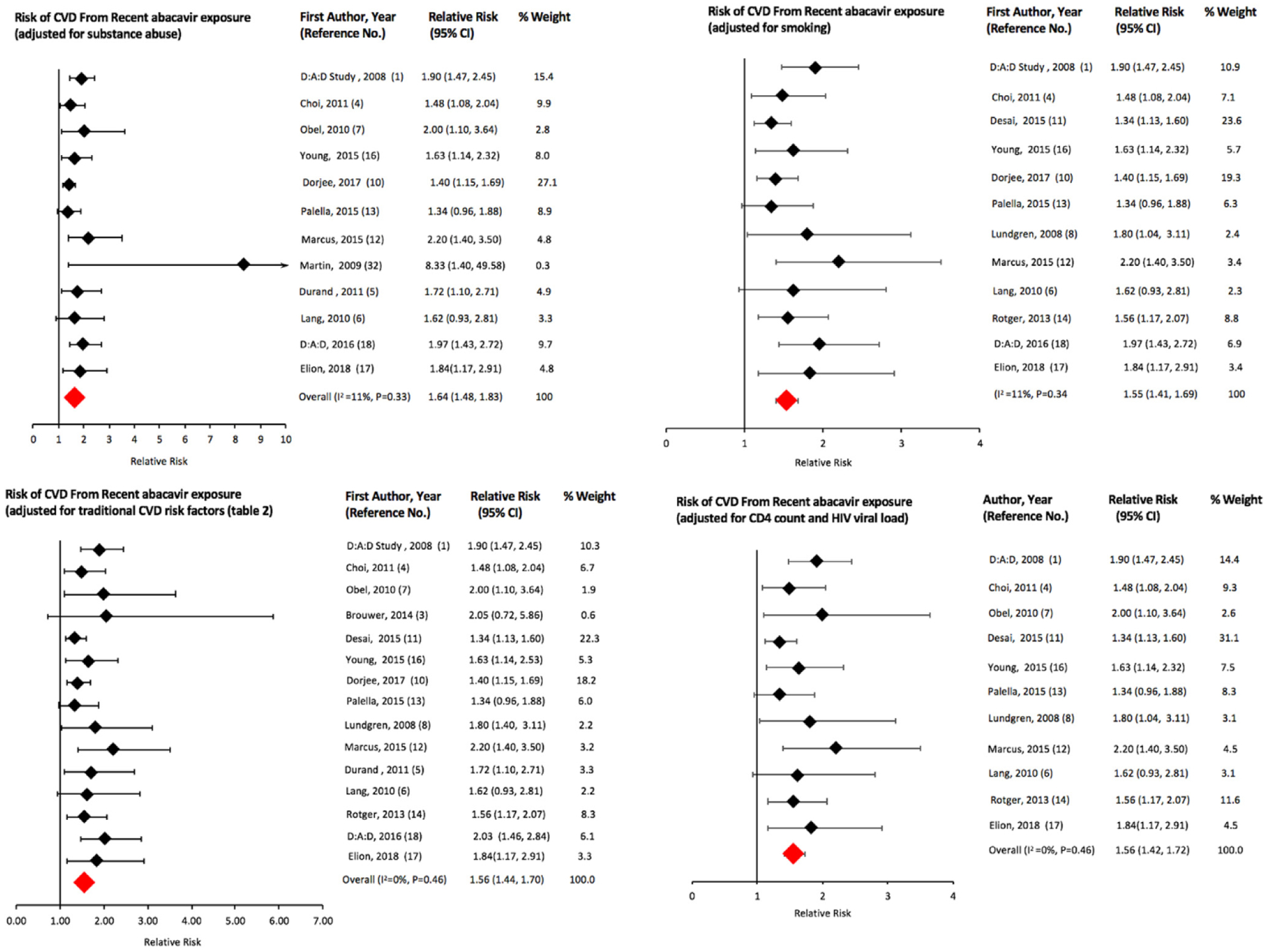
Pooled risk of cardiovascular disease from recent exposure to abacavir in various sub-groups of HIV-infected individuals.
Fig. 5.
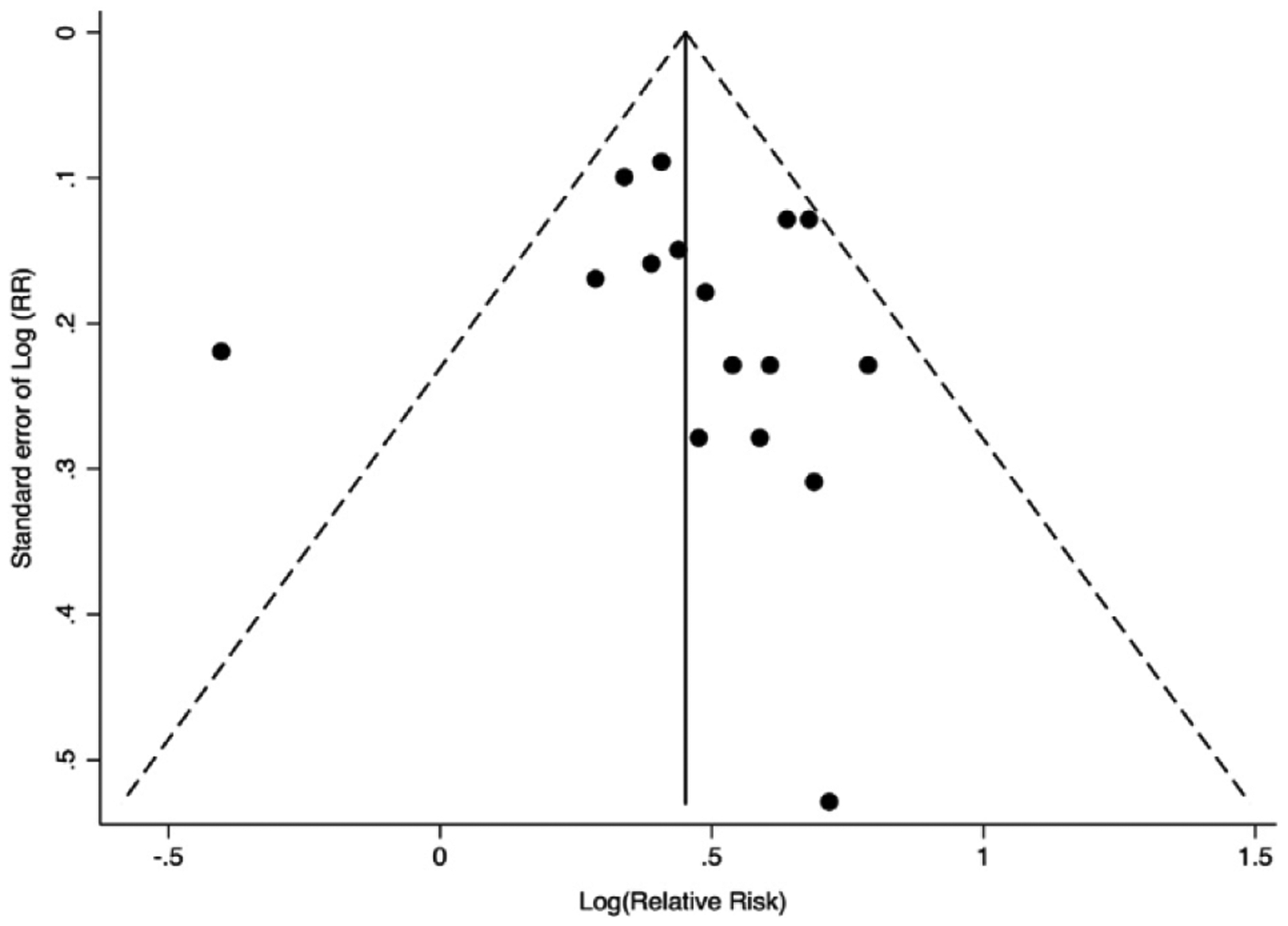
Funnel plot of 16 observational studies to assess publication bias for risk of CVD associated with recent exposure to abacavir.
3.2. Cumulative exposure
Results for CVD risk from cumulative abacavir exposure have varied across studies based on whether the model was adjusted for recent exposure. We obtained an increased sRR (95% Cl) of CVD of 1.12 (1.05, 1.20) from cumulative abacavir exposure (per year) for studies that did not adjust for recent exposure and no increased risk (sRR: 1.00; 95% Cl: 0.93, 1.08) for studies that adjusted for recent exposure (Fig. 6). The D:A:D study groups reported an increased risk (RR: 1.14; 95% Cl: 1.08, 1.21) of AMI in the cumulative exposure only model but the risk normalized after adjusting for recent exposure [1]. However, Dorjee et al. recently reported that the risk of CVD from abacavir exposure followed a dose-response pattern, with the risk peaking between 13 and 24 months and leveling off thereafter, after adjusting for recent exposure [10]. Earlier, Young et al. had also reported an increased risk of AMI (HR: 1.22; 95% Cl: 0.98, 1.52) beyond 6 months from a cumulative exposure for up to 36 months after adjusting for recent exposure [16]. Findings by Dorjee et al. and Young et al. highlight the importance of continued investigation into the risk of CVD from a cumulative abacavir exposure beyond 6 months, despite a pooled estimate showing no risk for CVD after adjusting for recent exposure.
Fig. 6.
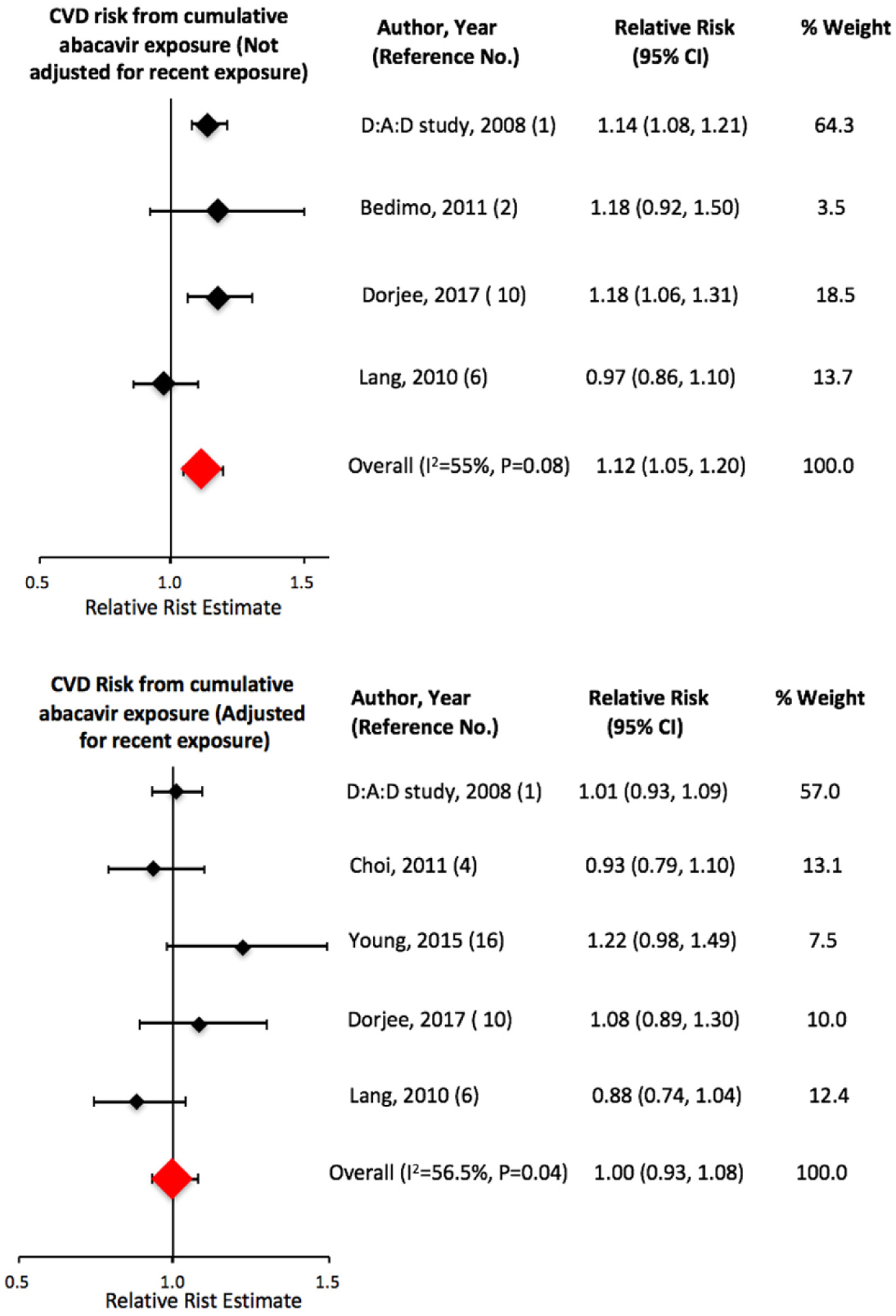
Pooled risk of cardiovascular disease from cumulative exposure to abacavir.
4. Discussion
We calculated a 61% increased pooled risk of CVD among PLWH who were recently exposed to abacavir. A higher summary estimate calculated for ART-naive PLWH or individuals observed to initiate ART (sRR: 1.91), that was not affected by confounding by prior ART use, may more closely estimate the causal effect from exposure to abacavir. Earlier, Bavinger et al. had also reported an increased CVD risk (HR: 1.92; 95% Cl: 1.50, 2.42) from recent abacavir exposure by pooling results across two studies; however, fewer studies were available for them to review as part of that meta-analysis. [15] Two studies had reported results using patient data post-March 2008 when the D:A:D study associating abacavir with AMI was first published; both the studies observed increased risk with a sRR of 1.53. This may refute the argument that CVD risk from abacavir exposure could be due to a channeling bias wherein PLWH having renal dysfunction, a risk factor for CVD, may have been preferentially prescribed abacavir as compared to tenofovir, a phenomenon described as confounding by indication, which we have further discussed below. The prescription of abacavir declined after March 2008, especially among individuals with moderate to severe risk factors for CVD [13,18,29], leading to the possibility of a reverse channeling bias.
Desai et al. [11] have reported increased risk of CVD associated with three specific abacavir based ARV drug combinations: abacavir + lamivudine + atazanavir, abacavir + lamivudine + efavirenz, and abacavir + lamivudine + zidovudine. They observed different CVD risk estimates for specific drug combinations as compared to the risk calculated for the drugs individually, suggesting interaction between the drugs. While it is important to understand CVD risk from ARV drug combinations, especially as ARTs are prescribed in combinations, all the above three abacavir-based combinations contained lamivudine. Therefore, teasing apart the individual drug effects is necessary.
We noted that the results across studies for cumulative exposure to abacavir were inconsistent in terms of magnitude and direction of association. There is ongoing controversy regarding whether the CVD risk from abacavir is limited to individuals who were recently exposed in the previous 6 months and studies have reported results for cumulative exposure by including and not including a recent exposure variable in the estimating model. As such, we conducted meta-analyses for cumulative exposure risk separately for models that did and did not adjust for recent exposure. In our pooled analysis, we observed no increased risk of CVD associated with cumulative exposure after adjusting for recent exposure, i.e. no increased risk in PLWH exposed to abacavir prior to 6 months ago and not recently exposed. However, we note that recent studies by Dorjee et al. and Young et al. found an increased risk of CVD from a cumulative abacavir exposure beyond the last 6 months in an inverted U-shaped dose-response pattern after adjusting for recent exposure [10,16]. Dorjee et al. reported that CVD risk peaked between 13 and 24 months of exposure and leveled off thereafter. Such results could suggest a reversible but more gradual underlying mechanism with a longer lasting effect that regresses slowly after removal of the exposure rather than an acute underlying mechanism [10]. We note here, as was previously explained, that when a model contains both cumulative exposure and recent exposure, the parameter for cumulative exposure captures the risk only among the exposed group [15]. Additional studies are needed to better understand this relationship.
Three meta-analyses with significant overlap in the data they included, have assessed the risk of CVD using RCT data and all showed no increase in risk of CVD associated with abacavir exposure [9,30,31]. However, these studies were of limited duration, lacking in generalizability because of healthier study populations, and had low power, owing to their primary objective being to assess the efficacies of various ARV drugs, instead of measuring CVD as an outcome. Therefore, we did not include the results from these trial data in our meta-analyses. An RCT conducted by Martin et al. (STEAL trial) assessed the risk of CVD as a study outcome and reported an increased risk of CVD in the abacavir group [32]. However, it was a small RCT and lacked adequate discriminatory power to detect a difference in effects [18]. Therefore, we conducted a subgroup analysis of only the observational studies excluding the study by Martin et al.; the summary estimate did not change. Lang et al., in a case-control study using a French hospital database, reported a significantly increased risk of AMI (HR: 1.62, 95% Cl: 0.93, 2.81) associated with exposure to abacavir; however, they did not see the effect when the study population was restricted to those not using cocaine or injection drugs (HR: 1.27, 95% Cl: 0.64, 2.49) [6]. This finding by Lang et al. was not re-produced in other studies, which continued to show an increased risk of CVD in association with abacavir exposure after adjusting for substance use [4,5,10,16,17]. A recent study conducted in the NA-ACCORD cohort by Elion et al. with robust ascertainment and adjudication of outcomes has also shown an increased risk of Type I (adjusted hazard ratio (aHR): 1.62) and Type II AMI (aHR: 2.11) associated with abacavir exposure. The risk persisted even after adjusting for injection drug use warranting screening for not just traditional CVD risk factors associated with Type I MI, but also for risk factors of type II AMI such as sepsis and illicit substance use including cocaine [17]. In our meta-analysis, the summary risk pooled from studies that adjusted for substance abuse as a confounder (n=12) remained elevated (sRR: 1.64). Elion et al. observed a statistically significant association between AMI and CD4 cell-count <200 cells/mm3 whereas the 2008 D:A:D study found that CD4 cell-count did not influence the association between abacavir use and AMI risk. In our analysis, the summary estimate for CVD risk did not significantly change in the sub-group of studies that adjusted for CD4 cell-count.
In 2011, Bedimo et al. argued that the 2008 D:A:D study results linking abacavir exposure to AMI could be due to a channeling bias, whereby individuals having renal dysfunction were preferentially put on abacavir to avoid additional nephrotoxicity from tenofovir. In a study in a US Veteran population, they reported that renal dysfunction is a significant risk factor for AMI (HR: 3.85; 95% Cl: 2.74, 5.42) and that the HR for AMI associated with current exposure to abacavir decreased from 0.73 (P = 0.013) to 0.67 (P = 0.07) after adjusting for renal dysfunction [2]. We note that this is only a slight decrease in risk and, moreover, the model adjusted for only renal dysfunction as a covariate. The result may be less vulnerable to confounding if the model had been adjusted for additional covariates including age and gender, which are two of several important risk factors for CVD. Subsequently, the D:A:D study groups showed through separate pre- and post-March 2008 analyses that the risk of CVD continued to remain elevated (HR: 1.98; 95% Cl: 1.72–2.29) after adjusting for pertinent covariates, including chronic kidney disease (CKD) [18,29]. They demonstrated that individuals at moderate and high risk for CVD were, in fact, channeled away from abacavir use after 2008. Other studies, including two studies in the veteran population [4,11], have confirmed elevated risk of CVD associated with abacavir use after adjusting for renal dysfunction [4,5,7,10–12,16,32].
Although we observed variations across studies in terms of study designs, confounder adjustment set of confounders, statistical methods, and populations, all 16 studies (100%) that met our inclusion criteria for recent exposure reported RR>1, suggesting homogeneity in the data. We explored whether a number of factors were related to heterogeneity, including difference in study design (observational vs. interventional), outcomes assessed (AMI only vs. all CVD), study populations (ART naive vs not), year of publication (pre- vs. post-2008), comparison group (tenofovir vs. other), the extent of adjustment for potential confounding, and other factors (Table 2). Analysis of all studies combined showed moderate heterogeneity (I2 value =44%, P-value for heterogeneity =0.03). However, when removing the study by Bedimo [2], or when limiting studies to those that only assessed AMI (vs. all CVD combined), heterogeneity was markedly reduced (I2 values of 0%). Heterogeneity was more marked for results from cumulative abacavir exposure. In our analyses, we combined odds ratios and rate ratios because the former approximate the latter for rare outcomes such as AMI [33]. We combined rate ratio and hazard ratio because most studies had discrete time data and the estimate from a logistic or a Poisson model for such data yielding ORs or RRs is an approximation of a hazard ratio from a Cox model [34,35]. Additionally, we thoroughly reviewed each study, and where we saw important differences between any two studies in terms of definition of exposure, study population, covariates, and outcome, we performed appropriate subgroup analyses and accordingly reported the results. Where heterogeneity P-values (Table 2) were <0.20, we recommend using the Shore-adjusted confidence interval for the estimate from the fixed-effects model or the estimates from the random-effects model.
4.1. Plausible biological mechanisms
The D:A:D study result showing a reversal of risk of CVD within 6 months of discontinuation of abacavir prompted investigators to search for a relatively rapidly acting underlying biological mechanism for the risk of CVD associated with abacavir exposure. While the SMART/INSIGHT study investigators, Kristoffersen et al. and Hileman et al. [8,36,37], showed evidence for a possible role of inflammatory biomarkers (e.g. increased levels of high sensitivity c-reactive protein (hsCRP) and interleukin-6 (IL-6)) in association with CVD among abacavir users, several other studies showed that levels of biomarkers such as hsCRP, IL-6, selectin P and E, D-dimer, vascular adhesion molecule-1, intercellular adhesion molecule-1, and tumor necrosis factor alpha are not elevated in the setting of abacavir exposure [38–50].
Studies that evaluated abacavir’s role in causing endothelial dysfunction have also yielded mixed results [46,51,52]. Endothelial dysfunction, induced by both traditional cardiovascular risk factors and chronic inflammation [53], increases the risk of CVD by promoting atherosclerosis [54]. Hsue et al. reported that abacavir use independently predicts lower brachial artery flow mediated vasodilation, a measure of endothelial dysfunction [52]. Sinn et al. observed lower arterial stiffness and improvement in Framingham risk score when individuals on abacavir were switched to tenofovir [51]. However, in an RCT, Wohl et al. found no evidence of endothelial dysfunction from abacavir use as compared to tenofovir [46].
Baum et al., Satchell et al., and Falcinelli et al. showed that abacavir increases platelet aggregation and reactivity, that could potentially lead to thrombosis and myocardial infarction [55–57]. Satchell et al. demonstrated that among abacavir recipients, platelet aggregation increased upon exposure to various platelet agonists, such as, adenosine diphosphate (ADP), collagen, epinephrine, and thrombin receptor-activating peptide [57]. Baum et al. further showed that abacavir causes platelet hyper-reactivity by competitive inhibition of a nitric oxide-induced soluble guanylyl cyclase via its active metabolite, carbovir-triphosphate, leading to a decreased production of cyclic guanosine monophosphate, an inhibitor of platelet aggregation and secretion [53,55]. Falcinelli et al., confirmed these findings in both in vivo and ex vivo settings [56]. In an RCT, switching from abacavir-lamivudine to tenofovir-emtricitabine resulted in decreased platelet reactivity to thrombin receptor-activating peptide (TRAP), ADP, and collagen, suggesting that the endothelial-platelet pathway may be a possible underlying mechanism for the AMI risk associated with abacavir exposure [58].
A recent review has discussed in more detail the existing literature on plausible underlying biological mechanisms [21]. The review summarizes that results from in vivo and in vitro experiments and abacavir’s structural similarity to endogenous purines possessing pro-inflammatory and pro-thrombotic potential may stand to support abacavir’s role as an inducer of vascular inflammation via leucocyte-endothelia cell interactions with resultant cardiovascular implications [21,59–61]. It is unclear how such mechanisms and accompanying effects, which are acute and reversible, may reconcile with recent clinical findings of CVD risk from cumulative exposure beyond 6 months [10,16]. With the literature demonstrating that abacavir does not affect lipid profile, insulin sensitivity, limb fat mass, or traditional cardiovascular risk factors [21,62,63], the search for plausible biological underpinnings continues.
Recent exposure to abacavir is associated with ~60% increased risk of CVD that was not attenuated after adjusting for substance use, renal dysfunction, and several other potential confounders. The finding of an increased risk of CVD associated with abacavir use among ART-naive individuals may be more suggestive of a causal relationship. We note that because of the observational nature of most studies in our analysis, the study results may be subject to confounding from unmeasured risk factors. While this risk appears to be reversible upon discontinuation of abacavir, further research is necessary to confirm this in view of recent studies that showed evidence of increased risk from cumulative exposure beyond 6 months.
5. Conclusion
In view of the increased risk of CVD associated with exposure to abacavir among HIV-infected individuals, risks and benefits for PLWH must be carefully weighed in prescribing abacavir-based regimens taking into account existing risk factors for CVD, a detailed history of prior exposure to ART, the patient’s clinical status, and the availability of other ARV drugs.
Supplementary Material
Research in context.
Evidence before this study
Abacavir, a nucleoside reverse transcriptase inhibitor, is a backbone antiretroviral drug for people living with HIV (PLWH). Its prescription had declined after reports showed that people receiving abacavir experience increased risk of acute myocardial infarction (AMI). Most recent studies have shown that abacavir use is associated with an increased risk of AML. However, it has been strongly argued that this observed risk is due to a higher prevalence of risk factors for cardiovascular disease (CVD), such as renal dysfunction and substance abuse, among abacavir recipients. There is ongoing confusion about whether the risk of CVD is for exposure to abacavir within the recent time (~last 6 months) only or if there is cumulative risk beyond 6 months of exposure. Inability to identify an underlying biological mechanism for such a risk has added to the dilemma.
Added value of this study
To our knowledge, this is the first systematic review and meta-analyses to investigate and summarize all existing evidence to date on the CVD risk associated with recent and cumulative abacavir exposure. We found an approximately 60% increased risk of CVD from recent exposure to abacavir as compared to PLWH not receiving abacavir. We found a higher summary risk among antiretroviral therapy naive PLWH who were recently exposed to abacavir. The pooled risk remained significantly elevated after studies adjusted for risk factors of CVD including renal dysfunction and substance abuse. The risk remained similarly elevated when studies adjusted for smoking, prior CVD, CD4 cell-count, and HIV viral load. Summary risk for cumulative exposure to abacavir was elevated for studies that did not adjust for recent exposure, but no increased cumulative risk was seen when the studies adjusted for recent exposure. This may suggest a reversible acute underlying biological mechanism. However, there are fewer studies that have investigated the CVD risk from cumulative abacavir exposure and two recent studies have argued with findings that risk of CVD from abacavir exposure may persist beyond 6 months of exposure.
Implications of all the available evidence
Risk and benefits should be weighed in prescribing abacavir-based antiretroviral regimens to PLWH. Research is needed to identify a clear underlying biological mechanism that corroborates the clinical evidence. The majority of evidence on CVD risk from abacavir exposure to date is among PLWH in the high-income countries.
Footnotes
Declarations
None.
Competing Interests
None.
Ethical Approval
This study received ethical approval from the University of California, Berkeley Office of Protection of Human Subjects.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2018.07.010.
References
- 1.Sabin CA, Worm SW, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet 2008;371 (9622): 1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedimo RJ, Westfall AO, Drechsler H, Vidiella G, Tebas P. Abacavir use and risk of acute myocardial infarction and cerebrovascular events in the highly active antiretroviral therapy era. Clin Infect Dis 2011; 53(1):84–91. [DOI] [PubMed] [Google Scholar]
- 3.Brouwer ES, Napravnik S, Eron JJ Jr, et al. Effects of combination antiretroviral therapies on the risk of myocardial infarction among HIV patients. Epidemiology 2014;25(3):406–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi Al, Vittinghoff E, Deeks SG, Weekley CC, Li YM, Shlipak MG. Cardiovascular risks associated with abacavir and tenofovir exposure in HIV-infected persons. AIDS 2011;25(10):1289–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Durand M, Sheehy O, Lelorier J, Tremblay CL. Association between use of antiretroviral therapy and risk of acute myocardial infarction: A nested case control study using Quebec’s Public Health Insurance Database (RAMQ). J Popul Ther Clin Pharmacol 2011;18(2):el78–9. [DOI] [PubMed] [Google Scholar]
- 6.Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, et al. Impact of individual antiretroviral drugs on the risk of myocardial infarction in human immunodeficiency virus-infected patients: A case-control study nested within the French hospital database on HIV ANRS cohort C04. Arch Intern Med 2010;170(14): 1228–38. [DOI] [PubMed] [Google Scholar]
- 7.Obel N, Farkas DK, Kronborg G, Larsen CS, Pedersen G, Riis A, et al. Abacavir and risk of myocardial infarction in HIV-infected patients on highly active antiretroviral therapy: a population-based nationwide cohort study. HIV Med 2010;ll(2):130–6. [DOI] [PubMed] [Google Scholar]
- 8.Strategies for Management of Anti-Retroviral Therapy/INSIGHT, DAD Study Groups Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS 2008;22(14):F17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding X, Andraca-Carrera E, Cooper C, Miele P, Kornegay C, Soukup M. No association of abacavir use with myocardial infarction: findings of an FDA meta-analysis. J Acquir Immune Defic Syndr 2012;61(4):441–7. [DOI] [PubMed] [Google Scholar]
- 10.Dorjee K, Baxi SM, Reingold AL, Hubbard A. Risk of cardiovascular events from current, recent, and cumulative exposure to abacavir among persons living with HIV who were receiving antiretroviral therapy in the United States: a cohort study. BMC Infect Dis 2017;17(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Desai M, Joyce V, Bendavid E, Olshen RA, Hlatky M, Chow A, et al. Risk of cardiovascular events associated with current exposure to HIV antiretroviral therapies in a US veteran population. Clin Infect Dis 2015;61(3):445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcus JL, Neugebauer RS, Leyden WA, Chao CR, Xu L, Quesenberry CP Jr, et al. Use of abacavir and risk of cardiovascular disease among HIV-infected individuals. J Acquir Immune Defic Syndr 2016;71(4):413–19. [DOI] [PubMed] [Google Scholar]
- 13.Palella FJ Jr, Althoff K, Moore R, Zhang J, Kitahata M, Gange SJ, et al. Abacavir Use and Risk for Myocardial Infarction in the NA-ACCORD. CROI 2015, Seattle, USA; 2015. February 23–26. [Google Scholar]
- 14.Rotger M, Glass TR, Junier T, Lundgren J, Neaton JD, Poloni ES, et al. Contribution of genetic background, traditional risk factors, and HIV-related factors to coronary artery disease events in HIV-positive persons. Clin Infect Dis 2013;57(1):112–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bavinger C, Bendavid E, Niehaus K, Olshen RA, Olkin I, Sundaram V, et al. Risk of cardiovascular disease from antiretroviral therapy for HIV: a systematic review. PLoS One 2013;8(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young J, Xiao Y, Moodie EEM, Abrahamowicz M, Klein MB, Bernasconi E, et al. Effect of cumulating exposure to abacavir on the risk of cardiovascular disease events in patients from the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2015;69(4):413–21. [DOI] [PubMed] [Google Scholar]
- 17.Elion RA, Althoff KN, Zhang J, Moore RD, Gange SJ, Kitahata MM, et al. Recent abacavir use increases risk of type 1 and type 2 myocardial infarctions among adults with HIV. J Acquir Immune Defic Syndr 2018;78(l):62–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sabin CA, Reiss P, Ryom L, Phillips AN, Weber R, Law M, et al. Is there continued evidence for an association between abacavir usage and myocardial infarction risk in individuals with HIV? A cohort collaboration. BMC Med 2016; 14:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Costagliola D, Lang S, Mary-Krause M, Boccara F. Abacavir and cardiovascular risk:reviewing the evidence. Curr HIV/AIDS Rep 2010;7(3): 127–33. [DOI] [PubMed] [Google Scholar]
- 20.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med 2012; 13(8):453–68. [DOI] [PubMed] [Google Scholar]
- 21.Alvarez A, Orden S, Andujar I, Collado-Diaz V, Nüñez-Delgado S, Galindo MJ, et al. Cardiovascular toxicity of abacavir: a clinical controversy in need of a pharmacological explanation. AIDS 2017;31(13):1781–95. [DOI] [PubMed] [Google Scholar]
- 22.Llibre JM, Hill A. Abacavir and cardiovascular disease: A critical look at the data. Antiviral Res 2016;132:116–21. [DOI] [PubMed] [Google Scholar]
- 23.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;ll(5):561–70. [DOI] [PubMed] [Google Scholar]
- 24.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7(3): 177–88. [DOI] [PubMed] [Google Scholar]
- 25.Cochran WG. The combination of estimates from different experiments. Biometrics 1954; 10(1): 101–29. [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21(ll):1539–58. [DOI] [PubMed] [Google Scholar]
- 27.Shore RE, Gardner MJ, Pannett B. Ethylene oxide: an assessment of the epidemiological evidence on carcinogenicity. Br J Ind Med 1993;50(ll):971–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroll JB, Moustgaard R, Gotzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol 2011;11:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sabin C, Reiss P, Ryom P, de Wit S, Kirk O, Weber R, et al. In: Is there continued evidence for an association between abacavir and myocardial infarction risk? CROI 2014; March 3–6 2014. p. 483. [Google Scholar]
- 30.Cruciani M, Zanichelli V, Serpelloni G, Bosco O, Malena M, Mazzi R, et al. Abacavir use and cardiovascular disease events: a meta-analysis of published and unpublished data. AIDS 2011; 25(16): 1993–2004. [DOI] [PubMed] [Google Scholar]
- 31.Ribaudo HJ, Benson CA, Zheng Y, Koletar SL, Collier AC, Lok JJ, et al. No risk of myocardial infarction associated with initial antiretroviral treatment containing abacavir: Short and long-term results from ACTG A5001/ALLRT. Clin Infect Dis 2011; 52(7):929–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin A, Bloch M, Amin J, Baker D, Cooper DA, Emery S, et al. Simplification of Antiretroviral Therapy with Tenofovir-Emtricitabine or Abacavir-Lamivudine: A Randomized, 96-Week Trial. Clin Infect Dis 2009;49(10):1591–601. [DOI] [PubMed] [Google Scholar]
- 33.Greenland S, Thomas DC. On the need for the rare disease assumption in case-control studies. Am J Epidemiol 1982;116(3):547–53. [DOI] [PubMed] [Google Scholar]
- 34.D’Agostino RB, Lee ML, Belanger AJ, Cupples LA, Anderson K, Kannel WB. Relation of pooled logistic regression to time dependent Cox regression analysis: the Framingham Heart Study. Stat Med 1990;9(12):1501–15. [DOI] [PubMed] [Google Scholar]
- 35.Thompson WA Jr. On the treatment of grouped observations in life studies. Biometrics 1977;33(3):463–70. [PubMed] [Google Scholar]
- 36.Kristoffersen US, Kofoed K, Kronborg G, Benfield T, Kjaer A, Lebech AM. Changes in biomarkers of cardiovascular risk after a switch to abacavir in HIV-1-infected individuals receiving combination antiretroviral therapy. HIV Med 2009;10(10):627–33. [DOI] [PubMed] [Google Scholar]
- 37.Hileman CO, Wohl DA, Tisch DJ, Debanne SM, McComsey GA. Short communication: Initiation of an abacavir-containing regimen in HIV-infected adults is associated with a smaller decrease in inflammation and endothelial activation markers compared to non-abacavir-containing regimens. AIDS Res Hum Retroviruses 2012;28(12):1561–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young B, Squires KE, Ross LL, Santiago L, Sloan LM, Zhao HH. Inflammatory biomarker changes and their correlation with Framingham cardiovascular risk and lipid changes in antiretroviral-naive HIV-infected patients treated for 144 weeks with abacavir/lamivudine/atazanavir with or without ritonavir in ARIES. AIDS Res Hum Retroviruses 2013;29(2):350–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez E, Larrousse M, Podzamczer D, Pérez I, Gutiérrez F, Loncá M, et al. Abacavir-based therapy does not affect biological mechanisms associated with cardiovascular dysfunction. AIDS 2010;24(3):Fl–9. [DOI] [PubMed] [Google Scholar]
- 40.Palella FJ, Gange SJ, Benning L, Jacobson L, Kaplan RC, Landay AL, et al. Inflammatory biomarkers and abacavir use in the Women’s Interagency HIV Study and the Multicenter AIDS Cohort Study. AIDS 2010;24(ll):1657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Padilla S, Masia M, Garcia N, Jarrin I, Tormo C, Gutierrez F. Early changes in inflammatory and pro-thrombotic biomarkers in patients initiating antiretroviral therapy with abacavir or tenofovir. BMC Infect Dis 2011;11:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Luca A, de Gaetano Donati K, Cozzi-Lepri A, Colaflgli M, De Curtis A, Capobianchi MR, et al. Exposure to abacavir and biomarkers of cardiovascular disease in HIV-1-infected patients on suppressive antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr 2012;60(3):E98–E101. [DOI] [PubMed] [Google Scholar]
- 43.Kim C, Gupta SK, Green L, Taylor BM, Deuter-Reinhard M, Desta Z, et al. Abacavir, didanosine and tenofovir do not induce inflammatory, apop-totic or oxidative stress genes in coronary endothelial cells. Antivir Ther 2011;16(8):1335–9. [DOI] [PubMed] [Google Scholar]
- 44.Patel P, Bush T, Overton T, Baker J, Hammer J, Kojic E, et al. Effect of abacavir on acute changes in biomarkers associated with cardiovascular dysfunction. Antivir Ther 2012;17(4):755–61. [DOI] [PubMed] [Google Scholar]
- 45.Rasmussen TA, Tolstrup M, Melchjorsen J, Frederiksen CA, Nielsen US, Lang-dahl BL, et al. Evaluation of cardiovascular biomarkers In HIV-infected patients switching to abacavir or tenofovir based therapy. BMC Infect Dis 2011:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wohl DA, Arnoczy G, Fichtenbaum CJ, Campbell T, Taiwo B, Hicks C, et al. Comparison of cardiovascular disease risk markers in HIV-infected patients receiving abacavir and tenofovir: the nucleoside inflammation, coagulation and endothelial function (NICE) study. Antivir Ther 2014;19(2):141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hammond E, McKinnon E, Mallal S, Nolan D. Longitudinal evaluation of cardiovascular disease-associated biomarkers in relation to abacavir therapy. AIDS 2008;22(18):2540–3. [DOI] [PubMed] [Google Scholar]
- 48.O’Halloran J, Dunne E, Tinago W, Denieffe S, Kenny D, Mallon P. In: Effect of switch from abacavir to tenofovir DF on platelet function markers: a SWIFT Trial Substudy. CROI 2014; March 3–6 2014. p. 484. [Google Scholar]
- 49.Martin A, Amin J, Cooper DA, Carr A, Kelleher AD, Bloch M, et al. Abacavir does not affect circulating levels of inflammatory or coagulopathic biomarkers in suppressed HIV: a randomized clinical trial. AIDS 2010;24(17):2657–63. [DOI] [PubMed] [Google Scholar]
- 50.De Luca A, de Gaetano Donati K, Cozzi-Lepri A, Colafigli M, De Curtis A, Capobianchi MR, et al. Exposure to abacavir and biomarkers of cardiovascular disease in HIV-1-infected patients on suppressive antiretroviral therapy: a longitudinal study. J Acquir Immune Defic Syndr 2012;60:e98–101. [DOI] [PubMed] [Google Scholar]
- 51.Sinn K, Richardson R, Carr A. Lower arterial stiffness and Framingham score after switching abacavir to tenofovir in men at high cardiovascular risk. AIDS 2010;24(15):2403–5. [DOI] [PubMed] [Google Scholar]
- 52.Hsue PY, Hunt PW, Wu Y, Schnell A, Ho JE, Hatano H, et al. Association of abacavir and impaired endothelial function in treated and suppressed HlV-infected patients. AIDS 2009;23(15):2021–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gresele P, Falcinelli E, Momi S, Francisci D, Baldelli F. Highly active antiretroviral therapy-related mechanisms of endothelial and platelet function alterations. Rev Cardiovasc Med 2014;15(Suppl l):S9–20. [PubMed] [Google Scholar]
- 54.Gresele P, Momi S, Migliacci R Endothelium, venous thromboembolism and ischaemic cardiovascular events. Thromb Haemost 2010;103(1):56–61. [DOI] [PubMed] [Google Scholar]
- 55.Baum PD, Sullam PM, Stoddart CA, McCune JM. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. AIDS 2011; 25(18):2243–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Falcinelli E, Francisci D, Belfiori B, Petito E, Guglielmini G, Malincarne L, et al. In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients. Thromb Haemost 2013;110(2):349–57. [DOI] [PubMed] [Google Scholar]
- 57.Satchell CS, O’Halloran JA, Cotter AG, Peace AJ, O’Connor EF, Tedesco AF, et al. Increased platelet reactivity in HIV-1-infected patients receiving abacavir-containing antiretroviral therapy. J Infect Dis 2011; 204(8): 1202–10. [DOI] [PubMed] [Google Scholar]
- 58.Mallon WP, Winston A, Post F, Kenny D, Bergin C, Maughan RT, et al. Platelet function upon switching to TAF vs continuing ABC: a randomized substudy. CROI 2018, Boston, USA; March 4–7 2018. [Google Scholar]
- 59.De Pablo C, Orden S, Apostolova N, Blanquer A, Esplugues JV, Alvarez A. Abacavir and didanosine induce the interaction between human leukocytes and endothelial cells through Mac-1 upregulation. AIDS 2010;24(9): 1259–66. [DOI] [PubMed] [Google Scholar]
- 60.De Pablo C, Orden S, Calatayud S, Marti-Cabrera M, Esplugues JV, Alvarez A. Differential effects of tenofovir/emtricitabine and abacavir/lamivudine on human leukocyte recruitment. Antivir Ther 2012;17(8):1615–19. [DOI] [PubMed] [Google Scholar]
- 61.De Pablo C, Orden S, Peris JE, Barrachina MD, Esplugues JV, Alvarez A. Profile of leukocyte-endothelial cell interactions induced in venules and arterioles by nucleoside reverse-transcriptase inhibitors in vivo. J Infect Dis 2013; 208(9): 1448–53. [DOI] [PubMed] [Google Scholar]
- 62.Podzamczer D, Ferrer E, Sanchez P, Gatell JM, Crespo M, Fisac C, et al. Less lipoatrophy and better lipid profile with abacavir as compared to stavudine: 96-week results of a randomized study. J Acquir Immune Defic Syndr 2007;44(2): 139–47. [DOI] [PubMed] [Google Scholar]
- 63.Behrens GMN, Reiss P. Abacavir and cardiovascular risk. Curr Opin Infect Dis 2010;23(1):9–14. [DOI] [PubMed] [Google Scholar]
- 64.Trevillyan JM, Cheng AC, Hoy J. Abacavir exposure and cardiovascular risk factors in HIV-positive patients with coronary heart disease: A retrospective case-control study. Sexual Health 2013;10(2):97–101. [DOI] [PubMed] [Google Scholar]
- 65.Belloso WH, Orellana LC, Grinsztejn B, Madero JS, La Rosa A Veloso VG, et al. Analysis of serious non-AIDS events among HIV-infected adults at Latin American sites. HIV Med 2010;ll(9):554–64. [DOI] [PubMed] [Google Scholar]
- 66.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV Outpatient Study. Clin Infect Dis 2010;51(4):435–47. [DOI] [PubMed] [Google Scholar]
- 67.Kurita T, Kitaichi T, Nagao T, Miura T, Kitazono Y. Safety analysis of Epzicom(R) (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan. Pharma-coepidemiol Drug Saf 2014;23(4):372–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith KY, Patel P. Fine D, Bellos N, Sloan L, Lackey P, et al. Randomized, double-blind, placebo-matched, multicenter trial of abacavir/lamivudine or tenofovir/emtricitabine with lopinavir/ritonavir for initial HIV treatment. AIDS 2009;23(12):1547–56. [DOI] [PubMed] [Google Scholar]
- 69.Das S, Arumainayagam J, Carlin E, Bilakanti K, Riddle L, Pammi M, et al. Five-year follow up of safety and efficacy of Truvada Or Kivexa in combination with Efavirenz in treatment Naive HIV patients-Multicenter prospective cohort study. HIV Med 2012; 13:71. [Google Scholar]
- 70.Pammi M, Arumainayagam J, Kumari B, Ahmed-Jushuf I, Carlin EM, Chan-dramani S, et al. Safety and efficacy of tenofovir/emtricitabine or abacavir/lamivudine in combination with efavirenz in treatment naive HIV patients: a 5 year retrospective observational cohort study (the TOKEN Study). Int J Clin Pract 2013;67(9):922–3. [DOI] [PubMed] [Google Scholar]
- 71.Bucher HC, Richter W, Glass TR, Magenta L, Wang Q, Cavassini M, et al. Small dense lipoproteins, apolipoprotein B, and risk of coronary events in HlV-infected patients on antiretroviral therapy: the Swiss HIV Cohort Study. J Acquir Immune Defic Syndr 2012;60(2): 135–42. [DOI] [PubMed] [Google Scholar]
- 72.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: The Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) Study. J Infect Dis 2010;201(3):318–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


