Abstract
Background
We previously investigated the association between 5 “first-generation” measures of epigenetic aging and cancer risk in the Melbourne Collaborative Cohort Study. This study assessed cancer risk associations for 3 recently developed methylation-based biomarkers of aging: PhenoAge, GrimAge, and predicted telomere length.
Methods
We estimated rate ratios (RRs) for the association between these 3 age-adjusted measures and risk of colorectal (N = 813), gastric (N = 165), kidney (N = 139), lung (N = 327), mature B-cell (N = 423), prostate (N = 846), and urothelial (N = 404) cancer using conditional logistic regression models. We also assessed associations by time since blood draw and by cancer subtype, and we investigated potential nonlinearity.
Results
We observed relatively strong associations of age-adjusted PhenoAge with risk of colorectal, kidney, lung, mature B-cell, and urothelial cancers (RR per SD was approximately 1.2-1.3). Similar findings were obtained for age-adjusted GrimAge, but the association with lung cancer risk was much larger (RR per SD = 1.82, 95% confidence interval [CI] = 1.44 to 2.30), after adjustment for smoking status, pack-years, starting age, time since quitting, and other cancer risk factors. Most associations appeared linear, larger than for the first-generation measures, and were virtually unchanged after adjustment for a large set of sociodemographic, lifestyle, and anthropometric variables. For cancer overall, the comprehensively adjusted rate ratio per SD was 1.13 (95% CI = 1.07 to 1.19) for PhenoAge and 1.12 (95% CI = 1.05 to 1.20) for GrimAge and appeared larger within 5 years of blood draw (RR = 1.29, 95% CI = 1.15 to 1.44 and 1.19, 95% CI = 1.06 to 1.33, respectively).
Conclusions
The methylation-based measures PhenoAge and GrimAge may provide insights into the relationship between biological aging and cancer and be useful to predict cancer risk, particularly for lung cancer.
DNA methylation is one of the key mechanisms thought to underlie the association between aging and cancer (1,2). Biological aging measures derived from blood DNA methylation—taking advantage of varying rates of aging-associated methylation changes between individuals—have gained considerable popularity as tools to better understand and predict disease (3-6). We previously investigated the association between 5 “first-generation” measures of epigenetic aging (7-9) and the risk of 7 cancer types using data from the Melbourne Collaborative Cohort Study (MCCS) (10). The observed associations were relatively weak compared with those obtained for all-cause mortality (9); cancer risk overall was increased by 4%-9% per 5-year increase in methylation “age acceleration,” although these estimates varied by cancer type.
Two novel methylation-based measures of biological aging, called PhenoAge (11) and GrimAge (12), have been developed based on associations of DNA methylation with, for PhenoAge, age, mortality, and clinical biomarkers; and for GrimAge, smoking pack-years and plasma concentrations of adrenomedullin, beta-2 microglobulin, cystatin C, growth differentiation factor 15, leptin, plasminogen activation inhibitor 1, and tissue inhibitor metalloproteinase 1. These new measures have proved to be more strongly associated with mortality (11) than the first-generation measures. Telomere length is another widely used biomarker of aging, which shows unclear associations with cancer risk (2,13,14). A methylation-based predictor of telomere length was recently developed (15).
In this study, we aimed to assess the association of the 3 aforementioned measures of biological aging, calculated using DNA methylation data from the Infinium HumanMethylation450 assay, and the risk of 7 cancer types: colorectal, gastric, kidney, lung, prostate, and urothelial, and mature B-cell neoplasms. We used a prospective design, and 3117 incident cancer cases and matched controls were included in the analysis.
Methods
Study Sample and Blood Collection
We used data collected from participants in the MCCS, a prospective study of 41 513 adult volunteers (24 469 women) aged between 27 and 76 years (99.3% aged 40-69 years) when recruited between 1990 and 1994 (16). DNA samples were collected from peripheral blood drawn at the time of recruitment (1990-1994) or at the wave 2 follow-up visit (2003-2007). The DNA source was dried blood spots, peripheral blood mononuclear cells, or buffy coats for 70%, 28%, and 2% of participants, respectively (Supplementary Methods, available online).
Study participants provided informed consent in accordance with the Declaration of Helsinki, and the study was approved by Cancer Council Victoria’s Human Research Ethics Committee.
Cancer Case-Control Studies Nested in the MCCS
A series of case-control studies nested within the MCCS of colorectal (N = 835 pairs), gastric (N = 170), kidney (N = 143), lung (N = 332), prostate (N = 869), and urothelial cancers (N = 428) and mature B-cell neoplasms (N = 439) were conducted (17-20). Cancer diagnoses were identified by linkage with the Victorian Cancer Registry and the Australian Cancer Database (Australian Institute of Health and Welfare). For each nested case-control study, controls were individually matched to incident cases (diagnosed after blood sample collection) on age using incidence density sampling (ie, they had to be free of the cancer of interest up to the age at diagnosis of the corresponding case), sex, country of birth (Australia or New Zealand, southern Europe, northern Europe), blood DNA source (dried blood spots, peripheral blood mononuclear cells, or buffy coat), and collection period (baseline or wave 2, the latter applicable to 151 case-control pairs of the urothelial cancer study). Controls were also matched to cases on year of birth, except for the colorectal cancer study, where controls were matched on year of baseline attendance. For the lung cancer study, controls were also matched on smoking history (never; former, quitting <10 years; former, quitting ≥10 years; current, smoking <15 cigarettes per day; current smoking ≥15 cigarettes per day) at the time of blood collection. For each study, matched cases and controls were placed next to each other, but allocated randomly, on the same slide.
DNA Extraction and Bisulfite Conversion, and DNA Methylation Data Processing
Methods relating to DNA extraction and bisulfite conversion and to DNA methylation data processing have been described previously (21) and are detailed in the Supplementary Material (available online).
Methylation-Based Measures of Biological Aging
PhenoAge, GrimAge, methylation-predicted telomere length, and their respective age-adjusted measures (the residual from the regression of biological age on chronological age) were obtained using Horvath’s online calculator at https://dnamage.genetics.ucla.edu/new (7,11,12).
Statistical Analysis
Pearson correlations of the 3 aging measures with each other and with age were calculated for participants selected as controls. We used conditional logistic regression to calculate odds ratios, which are estimates of the rate ratios (RRs) when incidence density sampling matching is used (22), for the associations between age-adjusted biological aging measures, per standard deviation (SD), and the risk of cancer. In Model 1, no covariates were included. In Model 2, we adjusted for smoking status (current, former, or never), smoking pack-years, age at starting smoking (never smoked, aged 16 years or younger, aged 17-21 years, older than 21 years), years since quitting smoking (never smoked, >10 years without smoking, between 5 and 10 years without smoking, <5 years without smoking), body mass index (in kg/m2), height (in meters), alcohol intake in the past week (in grams per day), physical activity (categorized score based on time spent doing vigorous or less vigorous activities) (23), dietary quality (Alternative Healthy Eating Index 2010) (24), socioeconomic status (deciles of the relative socioeconomic disadvantage of area of residence index) (25), education (ordinal variable ranging from 1, primary school only, to 8, tertiary or higher university degree) (Table 1). In Model 3, we added to Model 2 the white blood cell proportions estimated using the Houseman algorithm (26). These models were used to analyze each cancer type separately, and all 7 cancers combined; for the combined analysis, where an individual was diagnosed with several cancers, we included the first diagnosis only (respecting the incidence density sampling procedure) so that participants did not contribute twice to the pooled estimate. Analyses were additionally stratified (Model 1) by time between blood draw and diagnosis of the case (≤5 years, 5-10 years, and >10 years), and effect modification was examined using likelihood ratio tests of the interaction between each measure and the time-to-diagnosis variable, used as either categorical (Pheterogeneity) or continuous (Plinearity). Potential nonlinearity in the associations between methylation-based measures and cancer risk was assessed using penalized regression splines, specifically P-splines, which are based on cubic B-splines and a large number of equidistant knots (27), with 3 degrees of freedom. This type of spline was chosen because results are numerically stable, not sensitive to the location and number of knots (28). These were represented graphically, and nonlinearity was assessed by comparing the P-spline and linear models using a likelihood ratio test. Case-control pairs with any missing values for the confounders (Model 2) measured at baseline were excluded, and missing values at follow-up (urothelial cancers) were replaced by baseline values; 3% of the initial sample was excluded because of missing values. We also excluded 6 case-control pairs (0.2%) for which a participant had an outlying value (>5 or ≤ −5) for any of the 3 age-adjusted methylation-based measures (Supplementary Figure 1, available online). The same models were used to calculate associations: expressed for a 5-year increase for PhenoAge and GrimAge (Supplementary Table 4, available online); expressed per a 1 SD increase for the first-generation measures (Supplementary Table 5, available online).
Table 1.
Characteristics of the study sample, 7 case-control studies nested within the Melbourne Collaborative Cohort Study (N = 41 513)
| Variable of interest | Controls | Cases |
|---|---|---|
| Cancer type, No. | ||
| Colorectal cancer | 813 | 813 |
| Gastric cancer | 165 | 165 |
| Kidney cancer | 139 | 139 |
| Lung cancer | 327 | 327 |
| Mature B-cell neoplasms | 423 | 423 |
| Prostate cancer | 846 | 846 |
| Urothelial cancers | 404 | 404 |
| Matching variables | ||
| Age at blood draw, median (IQR), y | 61 (54-66) | 61 (54-66) |
| Sex, No. (%) | ||
| Male | 2159 (69.3) | 2159 (69.3) |
| Female | 958 (30.7) | 958 (30.7) |
| Country of birth, No. (%) | ||
| Australia/New Zealand | 2,079 (66.7) | 2,094 (67.2) |
| Northern Europe | 211 (6.8) | 205 (6.6) |
| Southern Europe | 827 (26.5) | 818 (26.2) |
| Blood sample type, No. (%) | ||
| Dried blood spots | 2142 (68.7) | 2142 (68.7) |
| Peripheral blood mononuclear cells | 794 (25.5) | 794 (25.5) |
| Buffy coats | 181 (5.8) | 181 (5.8) |
| Potential confounders | ||
| Smoking, No. (%) | ||
| Current | 458 (14.7) | 485 (15.8) |
| Former | 1230 (39.5) | 1294 (41.5) |
| Never | 1429 (45.8) | 1338 (42.9) |
| Pack-years, median (IQR) | 2.4 (0-27.2) | 4.5 (0-31.1) |
| Age at starting, No. (%) | ||
| Never smoked | 1429 (45.8) | 1338 (42.9) |
| ≤16 y | 639 (20.5) | 672 (21.6) |
| 17-21 y | 749 (24.0) | 834 (26.8) |
| ≥22 y | 300 (9.6) | 273 (8.8) |
| Time since quitting, No. (%) | ||
| Never smoked | 1429 (45.8) | 1338 (42.9) |
| <5 y | 622 (20.0) | 675 (21.7) |
| 5-10 y | 862 (27.7) | 885 (28.4) |
| >10 y | 204 (6.5) | 219 (7.0) |
| Body mass index, median (IQR), kg/m2 | 27 (24-29) | 27 (25-30) |
| Height, median (IQR), m | 168 (161-174) | 169 (162-175) |
| Alcohol consumption, median (IQR), g/d | 4 (0-19) | 5 (0-19.3) |
| Diet quality: AHEI-2010, median (IQR) | 63 (56-71) | 63 (56-71) |
| Physical activity score, median (IQR) | 2 (1-4) | 2 (1-4) |
| Education score, median (IQR) | 4 (4-6) | 4 (4-6) |
| Socioeconomic status: SEIFA-10, median (IQR) | 5 (3-8) | 6 (3-9) |
AHEI-2010 = Alternate Healthy Eating Index 2010; IQR = interquartile range; SEIFA-10 = Socio-Economic Indexes for Areas deciles.
In secondary analyses, we assessed the association between biological aging measures and risk of the following cancer subtypes, as defined in previous publications: colon and rectal cancer; multiple myeloma, follicular lymphoma, low-grade non-Hodgkin lymphoma (including chronic lymphocytic leukemia), and high-grade non-Hodgkin lymphoma (20); aggressive and nonaggressive prostate cancer (18); and invasive and superficial urothelial cancers (19).
As per the Journal guidelines, a P value of less than .05 was considered statistically significant, and P values of less than .001 were written as “<.001.” All statistical tests were 2-sided. All analyses were undertaken using R version 3.6.1.
Results
The correlation with chronological age was 0.70, 0.80, and −0.55 for PhenoAge, GrimAge, and methylation-predicted telomere length, respectively (Supplementary Table 1, available online). For the age-adjusted measures, the correlation between PhenoAge and GrimAge was 0.34, and their correlations with methylation-predicted telomere length were −0.25 and −0.29, respectively. The correlations of the 3 measures with the 5 first-generation measures of epigenetic aging (all age adjusted) were in the same range (Supplementary Table 2, available online).
Hereafter, the age-adjusted measures are referred to as PhenoAge, GrimAge, and telomere length. Their associations with cancer risk are presented in Table 2. In models without adjustment other than that provided by the matching variables, increasing PhenoAge was associated with increased risk of several types of cancer, including colorectal cancer (per 1-SD RR = 1.22, 95% confidence interval [CI] = 1.10 to 1.36), kidney cancer (RR = 1.25, 95% CI = 0.96 to 1.63), lung cancer (RR = 1.23, 95% CI = 1.06 to 1.44), mature B-cell neoplasms (RR = 1.24, 95% CI = 1.07 to 1.43), urothelial cancer (RR = 1.21, 95% CI = 1.05 to 1.40), and cancer overall (RR = 1.14, 95% CI = 1.08 to 1.20). These rate ratios were virtually the same after adjustment for a comprehensive set of cancer risk factors (cancer overall, RR = 1.13, 95% CI = 1.07 to 1.19). GrimAge biological aging showed similar associations to PhenoAge for risk of colorectal, kidney cancer, and cancer overall. The association with risk of lung cancer was much stronger (per 1-SD RR = 1.81, 95% CI = 1.45 to 2.26). A possible inverse association with risk of prostate cancer was also observed (RR = 0.88, 95% CI = 0.79 to 0.98). These associations were virtually the same in comprehensively adjusted models (lung cancer RR = 1.82, 95% CI = 1.44 to 2.30) except for urothelial cancer, for which estimates showed substantial attenuation while remaining quite strong (RR = 1.22, 95% CI = 1.00 to 1.48). The RR also remained similar after additional adjustment for estimated white blood cell proportions for cancer overall (RR = 1.11, 95% CI = 1.05 to 1.18), being somewhat smaller for colorectal cancer risk (PhenoAge: RR = 1.18, 95% CI = 1.05 to 1.32; GrimAge: RR = 1.12, 95% CI = 0.96 to 1.30) but larger for lung cancer risk (GrimAge: RR = 2.03, 95% CI = 1.56 to 2.64) (Table 2). We found no association between methylation-predicted telomere length and risk of any type of cancer or cancer overall (all P > .1).
Table 2.
Association (RRs, 95% CIs) between 3 methylation-based measures of aging (per 1 SD) and cancer risk in the Melbourne Collaborative Cohort Study
| Cancer type | Cases, No. |
PhenoAge
|
GrimAge
|
Telomere length |
||||
|---|---|---|---|---|---|---|---|---|
| Model | RR (95% CI) | P | RR (95% CI) | P | RR (95% CI) | P | ||
| Colorectal cancer | 813 | Model 1a | 1.22 (1.10 to 1.36) | <.001 | 1.20 (1.07 to 1.34) | .001 | 0.98 (0.88 to 1.09) | .68 |
| Model 2b | 1.22 (1.09 to 1.36) | <.001 | 1.19 (1.03 to 1.36) | .02 | 0.99 (0.89 to 1.10) | .90 | ||
| Model 3c | 1.18 (1.05 to 1.32) | .01 | 1.12 (0.96 to 1.30) | .15 | 1.02 (0.90 to 1.14) | .78 | ||
| Gastric cancer | 165 | Model 1 | 0.95 (0.77 to 1.18) | .65 | 1.03 (0.83 to 1.27) | .80 | 1.17 (0.92 to 1.48) | .19 |
| Model 2 | 0.96 (0.76 to 1.22) | .74 | 1.05 (0.78 to 1.41) | .74 | 1.25 (0.96 to 1.63) | .10 | ||
| Model 3 | 0.88 (0.67 to 1.15) | .34 | 0.95 (0.68 to 1.33) | .75 | 1.19 (0.86 to 1.64) | .29 | ||
| Kidney cancer | 139 | Model 1 | 1.25 (0.96 to 1.63) | .09 | 1.27 (0.98 to 1.65) | .07 | 1.07 (0.80 to 1.43) | .65 |
| Model 2 | 1.28 (0.94 to 1.76) | .12 | 1.32 (0.91 to 1.91) | .15 | 1.11 (0.78 to 1.57) | .57 | ||
| Model 3 | 1.25 (0.88 to 1.77) | .21 | 1.28 (0.84 to 1.95) | .25 | 1.19 (0.79 to 1.79) | .40 | ||
| Lung cancer | 327 | Model 1 | 1.23 (1.06 to 1.44) | .007 | 1.81 (1.45 to 2.26) | <.001 | 0.90 (0.76 to 1.06) | .19 |
| Model 2 | 1.23 (1.05 to 1.45) | .01 | 1.82 (1.44 to 2.30) | <.001 | 0.88 (0.74 to 1.04) | .13 | ||
| Model 3 | 1.25 (1.05 to 1.49) | .01 | 2.03 (1.56 to 2.64) | <.001 | 0.88 (0.73 to 1.06) | .19 | ||
| Mature B-cell neoplasms | 423 | Model 1 | 1.24 (1.07 to 1.43) | .003 | 0.95 (0.81 to 1.11) | .49 | 0.92 (0.81 to 1.05) | .24 |
| Model 2 | 1.27 (1.09 to 1.47) | .002 | 0.96 (0.78 to 1.17) | .66 | 0.91 (0.79 to 1.05) | .20 | ||
| Model 3 | 1.23 (1.04 to 1.45) | .02 | 1.03 (0.82 to 1.28) | .81 | 0.95 (0.81 to 1.13) | .57 | ||
| Prostate cancer | 846 | Model 1 | 0.98 (0.88 to 1.08) | .68 | 0.88 (0.79 to 0.98) | .02 | 1.06 (0.95 to 1.18) | .28 |
| Model 2 | 0.99 (0.89 to 1.10) | .85 | 0.88 (0.76 to 1.01) | .07 | 1.05 (0.94 to 1.17) | .43 | ||
| Model 3 | 1.00 (0.89 to 1.11) | .96 | 0.84 (0.72 to 0.98) | .02 | 1.06 (0.94 to 1.20) | .35 | ||
| Urothelial cancers | 404 | Model 1 | 1.21 (1.05 to 1.40) | .01 | 1.39 (1.19 to 1.61) | <.001 | 0.90 (0.77 to 1.04) | .14 |
| Model 2 | 1.17 (1.00 to 1.36) | .05 | 1.22 (1.00 to 1.48) | .05 | 0.95 (0.81 to 1.10) | .48 | ||
| Model 3 | 1.16 (0.99 to 1.37) | .07 | 1.22 (0.98 to 1.52) | .08 | 0.92 (0.78 to 1.09) | .33 | ||
| All types | 2994d | Model 1 | 1.14 (1.08 to 1.20) | <.001 | 1.13 (1.06 to 1.19) | <.001 | 0.98 (0.93 to 1.03) | .46 |
| Model 2 | 1.13 (1.07 to 1.19) | <.001 | 1.12 (1.05 to 1.20) | .001 | 0.98 (0.93 to 1.04) | .58 | ||
| Model 3 | 1.11 (1.05 to 1.18) | <.001 | 1.11 (1.03 to 1.20) | .01 | 1.00 (0.94 to 1.06) | 1.00 | ||
Model 1: No adjustment other than that provided by the matching variables age, sex, country of birth (Australia, northern Europe, or southern Europe), sample type (peripheral blood mononuclear cells, dried blood spots, or buffy coats); lung cancer study: additional matching for smoking status (never; former, quitting less than 10 years; former, quitting 10 years and over; current, smoking less than 15 cigarettes per day; current smoking 15 or more cigarettes per day). CI = confidence interval; RR = rate ratio.
Model 2: Additional adjustment for smoking (current, former, or never), smoking pack-years, age at starting smoking (4 categories), time since quitting smoking (4 categories), body mass index (in kg/m2), height (in meters), alcohol consumption (in grams per day), physical activity (categorized score), dietary quality (24), socioeconomic status score (at the local area level, ranging from 1 to 10), education score (ordinal variable ranging from 1, primary school to 8, postgraduate degree).
Model 3: Model 2 + additional adjustment for white blood cell proportions estimated using the Houseman algorithm.
For the combined analysis, where an individual was diagnosed with several cancers, we included the first diagnosis only (respecting the incidence density sampling procedure), so that participants did not contribute twice to the pooled estimate, resulting in 2994 pairs out of 3117 in total.
The same results expressed per a 5-year increase for PhenoAge and GrimAge and expressed per a 1 SD increase for the first-generation measures are shown in Supplementary Tables 4 and 5 (available online), respectively.
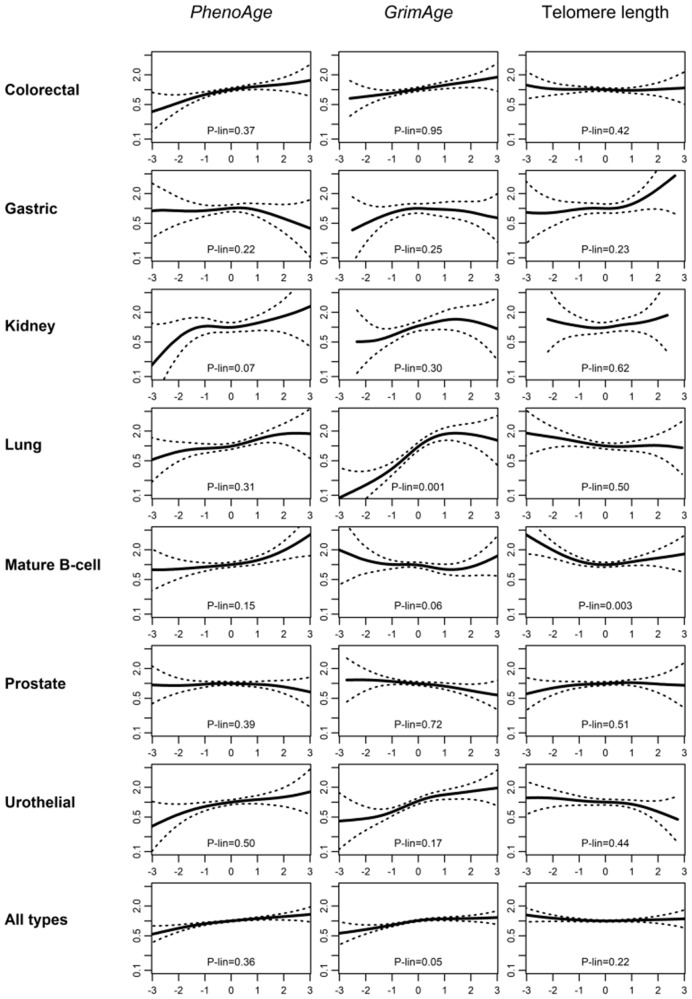
In analyses stratified by time since blood draw (Table 3), associations were somewhat larger within 5 years of blood draw for several cancer types for PhenoAge: colorectal cancer (RR = 1.48, 95% CI = 1.16 to 1.89, Plinearity = .07), lung cancer (RR = 1.51, 95% CI = 1.05 to 2.18, Plinearity = .18), and mature B-cell neoplasms (RR = 1.38, 95% CI = 1.01 to 1.90, Plinearity = .14), and this pattern was even clearer in the overall cancer analysis (RR = 1.29, 95% CI = 1.15 to 1.44; RR = 1.12, 95% CI = 1.01 to 1.23; and RR = 1.09, 95% CI = 1.01 to 1.17 for ≤5, 5-10 years, and >10 years, respectively, Plinearity = .004). A similar trend, albeit weaker, was observed for GrimAge (RR = 1.19, 95% CI = 1.06 to 1.33; RR = 1.15, 95% CI = 1.04 to 1.28; RR = 1.08, 95% CI = 1.00 to 1.17, respectively, Plinearity = .11). As shown in Figure 1, most associations with cancer risk appeared relatively linear. Some evidence of nonlinearity was observed for GrimAge and lung cancer risk (P = .001), with a sharp increase at lower values and a plateau after the 75th percentile. A similar shape of association, while less marked, was also observed for GrimAge and overall cancer risk (P = .05).
Table 3.
Stratification by time since blood draw for the association (RRs, 95% CIs) between 3 methylation-based measures of aging (per 1 SD) and cancer risk in the Melbourne Collaborative Cohort Study
|
PhenoAge
|
GrimAge
|
Telomere length |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | No. cases | Time since blood draw | RRa95% CI | P het b | P lin b | RRa95% CI | P het b | P lin b | RRa95% CI | P het b | P lin b |
| Colorectal cancer | 813 | ≤5 y | 1.48 (1.16 to 1.89) | 1.20 (0.94 to 1.52) | 0.95 (0.75 to 1.20) | ||||||
| 5-10 y | 1.23 (1.02 to 1.50) | .13 | .07 | 1.24 (1.03 to 1.50) | .89 | .32 | 1.05 (0.87 to 1.26) | .68 | .79 | ||
| >10 y | 1.11 (0.95 to 1.30) | 1.17 (0.99 to 1.38) | 0.95 (0.81 to 1.10) | ||||||||
| Gastric cancer | 165 | ≤ 5 y | 0.72 (0.42 to 1.26) | 0.99 (0.54 to 1.84) | 0.98 (0.57 to 1.71) | ||||||
| 5-10 y | 1.06 (0.71 to 1.59) | .52 | .48 | 0.93 (0.61 to 1.42) | .85 | .49 | 1.14 (0.73 to 1.77) | .74 | .60 | ||
| >10 y | 0.97 (0.73 to 1.30) | 1.08 (0.82 to 1.41) | 1.26 (0.91 to 1.74) | ||||||||
| Kidney cancer | 139 | ≤5 y | 1.29 (0.61 to 2.70) | 0.81 (0.46 to 1.42) | 1.68 (0.82 to 3.45) | ||||||
| 5-10 y | 1.33 (0.72 to 2.48) | .97 | .67 | 1.91 (1.06 to 3.44) | .09 | .26 | 1.01 (0.54 to 1.91) | .36 | .13 | ||
| >10 y | 1.22 (0.89 to 1.67) | 1.29 (0.89 to 1.87) | 0.95 (0.66 to 1.38) | ||||||||
| Lung cancer | 327 | ≤5 y | 1.51 (1.05 to 2.18) | 2.15 (1.28 to 3.61) | 0.76 (0.51 to 1.14) | ||||||
| 5-10 y | 1.13 (0.85 to 1.50) | .44 | .18 | 1.46 (1.00 to 2.12) | .40 | .92 | 0.97 (0.71 to 1.31) | .64 | .51 | ||
| >10 y | 1.20 (0.97 to 1.49) | 1.94 (1.40 to 2.69) | 0.91 (0.73 to 1.13) | ||||||||
| Mature B-cell neoplasms | 423 | ≤5 y | 1.38 (1.01 to 1.90) | 0.85 (0.58 to 1.25) | 0.94 (0.72 to 1.22) | ||||||
| 5-10 y | 1.21 (0.93 to 1.57) | .73 | .14 | 1.22 (0.87 to 1.71) | .22 | .30 | 1.03 (0.78 to 1.35) | .64 | .30 | ||
| >10 y | 1.20 (0.98 to 1.46) | 0.88 (0.71 to 1.09) | 0.88 (0.73 to 1.05) | ||||||||
| Prostate cancer | 846 | ≤5 y | 1.23 (0.96 to 1.57) | 0.90 (0.71 to 1.13) | 1.03 (0.82 to 1.30) | ||||||
| 5-10 y | 0.93 (0.77 to 1.13) | .13 | .13 | 0.94 (0.74 to 1.18) | .78 | .95 | 1.09 (0.88 to 1.34) | .95 | .95 | ||
| >10 y | 0.93 (0.81 to 1.07) | 0.85 (0.73 to 0.99) | 1.06 (0.91 to 1.23) | ||||||||
| Urothelial cancers | 404 | ≤5 y | 1.17 (0.94 to 1.47) | 1.70 (1.31 to 2.22) | 0.90 (0.72 to 1.13) | ||||||
| 5-10 y | 1.16 (0.89 to 1.51) | .76 | .93 | 1.07 (0.84 to 1.38) | .04 | .52 | 1.04 (0.80 to 1.36) | .22 | .47 | ||
| >10 y | 1.32 (0.99 to 1.75) | 1.48 (1.10 to 2.00) | 0.74 (0.54 to 1.00) | ||||||||
| All types | 2994 | ≤5 y | 1.29 (1.15 to 1.44) | 1.19 (1.06 to 1.33) | 0.95 (0.85 to 1.06) | ||||||
| 5-10 y | 1.12 (1.01 to 1.23) | .05 | .004 | 1.15 (1.04 to 1.28) | .36 | .11 | 1.05 (0.95 to 1.16) | .31 | .70 | ||
| >10 y | 1.09 (1.01 to 1.17) | 1.08 (1.00 to 1.17) | 0.96 (0.89 to 1.03) | ||||||||
Model 1: No adjustment other than that provided by the matching variables age, sex, country of birth (Australia, northern Europe, or southern Europe), sample type (peripheral blood mononuclear cells, dried blood spots, or buffy coats); lung cancer study: additional matching for smoking status (never; former, quitting less than 10 years; former, quitting 10 years and over; current, smoking less than 15 cigarettes per day; current smoking 15 and more cigarettes per day).
P het (Pheterogeneity) and Plin (Plinearity) were calculated using a likelihood ratio test for the interaction between each methylation-based measure and the time-to-diagnosis variable, taken as categorical and continuous, respectively.
Figure 1.
Assessment of linearity. Relative cancer rates for age-adjusted PhenoAge, GrimAge, and predicted telomere length for 7 cancer types and overall in the Melbourne Collaborative Cohort Study. Model 1 was used (no other adjustment than that provided by the matching variables). x-axis: methylation-based measures of aging. All measures were expressed as Z scores (mean = 0, SD = 1), so that approximately 95% of the values are between –2 and 2. y-axis: Relative cancer rate, using as a reference (y = 1) the median value of the methylation-based measure distribution. P values (P-lin) are from a likelihood ratio test comparing P-spline and linear models.
Associations were generally consistent across cancer subtypes (Supplementary Table 3, available online). Evidence of heterogeneity was observed for the association of PhenoAge with B-cell lymphoma subtypes (P = .05, being stronger for low-grade non-Hodgkin lymphoma: RR = 1.90, 95% CI = 1.37 to 2.62). Weak evidence of heterogeneity was observed for PhenoAge and colorectal cancer risk (P = .16; colon: RR = 1.16, 95% CI = 1.01 to 1.32; rectum: RR = 1.38, 95% CI = 1.12 to 1.69). The inverse association observed between GrimAge and prostate cancer risk was only apparent for nonaggressive disease (RR = 0.79, 95% CI = 0.64 to 0.97, Pheterogeneity = 0.16). No association was found between methylation-predicted telomere length and risk of cancer subtypes.
Discussion
In this prospective study, including 3117 incident cancer cases, we observed relatively strong associations of PhenoAge and GrimAge with risk of several cancer types; these appeared to be greater than in our study of first-generation epigenetic aging measures for risk of colorectal, lung, and urothelial cancer (Supplementary Table 5, available online) (10). For GrimAge, a very strong association was observed with risk of lung cancer independently of several questionnaire-collected variables relating to smoking. An association stronger than for PhenoAge was also observed with risk of urothelial cancer. A possible inverse association was observed between GrimAge and (nonaggressive) prostate cancer. No association was observed between methylation-predicted telomere length and any cancer type or subtype.
PhenoAge and GrimAge integrate methylation measures at CpG sites associated with age, mortality, key disease risk factors, and biomarkers, which are also involved in the aetiology of cancer. That GrimAge is enriched for smoking-associated methylation measures likely explains the very strong association observed with lung cancer risk; of note, however, case-control pairs were matched on smoking history in the lung cancer study, and the estimates were robust to further adjustment for questionnaire-collected variables. In the case of urothelial cancer, for which smoking is a strong risk factor, the association was partially attenuated after adjustment for smoking status, which was not a matching variable in that study. However, for both PhenoAge and GrimAge, there was overall little attenuation of risk estimates after adjustment for a comprehensive set of sociodemographic and lifestyle cancer risk factors, which may indicate that these measures capture information beyond self-reported questionnaires and on many adverse environmental and lifestyle factors that affect the methylome over the life course. To our knowledge, no data exist on the association of PhenoAge and GrimAge with risk of cancers other than pancreatic cancer (29) and breast cancer; in the latter case, the Sister Study revealed a reasonably strong association with PhenoAge (30) (invasive disease; hazard ratio per 5-year increase: 1.13, which is of similar magnitude to our findings for colorectal, kidney, lung, mature B-cell, and urothelial cancers) (Supplementary Table 4, available online) but not with GrimAge (31). Further adjustment for estimated white blood cell proportions slightly attenuated associations with cancer risk overall, although a larger association was observed for GrimAge and lung cancer, similar to observations made for PhenoAge and risk of breast (30) and pancreatic cancer (29).
Although our sample size was quite large, our findings should be replicated by other studies before these methylation-based measures can be used for cancer risk prediction. In the case of lung cancer, our rate ratio estimate of 1.8 per SD for GrimAge is considerably larger than current estimates obtained for polygenic risk scores (32-35). For other cancers, our estimates are lower than for polygenic risk scores for colorectal, gastric, B-cell lymphoma, and prostate cancer and similar or greater for kidney and urothelial cancer (32-35). Combining polygenic and methylation aging scores may therefore be required to summarize risk associated with genetic factors and lifestyle or environmental exposures accumulated over the lifetime. Our findings also suggest that PhenoAge and GrimAge may be more valuable biomarkers than the first-generation aging clocks (10,30) and generally show a linear association with risk. That we observed stronger associations within 5 years of blood draw for PhenoAge, and to a lesser extent for GrimAge, suggests that these aging measures may have more utility for assessment of short-term risk, but corroborating data are required to confirm this. In our previous report on the first-generation measures (10), we found at best weak evidence of effect modification by time since blood draw. Consistent with this, it was observed that Horvath epigenetic aging was largely determined before adulthood (36), and this might not hold true for PhenoAge and GrimAge since these predictors were developed to predict a composite phenotype (age and clinical markers). Finally, although these methylation-based predictors show some degree of correlation with age in other tissues (11), they were developed and validated in blood so at this stage should be considered as biomarkers of future cancer risk and extrapolation to cancer or normal-adjacent tissue made with caution (37), because DNA methylation usually shows substantial variation across tissues (38).
We also used DNA methylation measures at a set of 140 CpGs to estimate telomere length. The correlation of this predictor with measured telomere length in independent data has been shown to be moderate (r = approximately 0.40), but its correlation with age appeared stronger than was the case for measured telomere length (r = approximately −0.75 vs −0.35), which is consistent with our findings (correlation with age r = −0.56). Our findings of no association between telomere length and cancer risk are consistent with those reported in a Danish prospective study of 3142 cancer cases of any type (14). In a Mendelian randomization study and meta-analysis by Haycock et al., which included a larger number of cancer cases and types, genetically predicted telomere length was strongly positively associated with risks of lung and bladder cancers, which is inconsistent with our findings. Our results were nevertheless consistent with Mendelian randomization estimates for other cancer types and with estimates from prospective studies for all cancer types, all showing null or weak associations with cancer risk (13,14).
We conclude that biological aging, as defined by the methylation-based measures PhenoAge and GrimAge, is associated with risk of several cancer types, including colorectal, lung, kidney and urothelial, and mature B-cell neoplasms, independent of key demographic, lifestyle, and socioeconomic variables. These measures, derived using a limited number of methylation sites across the genome, have the potential to improve cancer risk prediction, particularly in contexts where relevant cancer biomarkers have not been extensively measured.
Funding
This work was supported by the Australian National Health and Medical Research Council (NHMRC) grants 1164455. MCCS cohort recruitment was funded by VicHealth and Cancer Council Victoria. The MCCS was further supported by Australian NHMRC grants 209057, 251553, and 504711 and by infrastructure provided by Cancer Council Victoria. The nested case-control methylation studies were supported by the NHMRC grants 1011618, 1026892, 1027505, 1050198, 1043616, and 1074383. S.L. is a Victorian Cancer Agency Early Career Research Fellow (ECRF19020). M.C.S. is an NHMRC Senior Research Fellow (1061177). This work also received funding from Monash University, Melbourne, Australia.
Notes
Role of the funder: The funders had no role in the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: None declared.
Author contributions: Conceptualization: all authors; data collection and curation: JKB, EMW and JEJ; formal analysis and visualization: PAD; methodology: all authors; funding and resources: DS, EM, NWD, DDB, AMH, DRE, GGG, MCS, and RLM, writing—original draft: PAD; writing—review & editing: all authors.
Acknowledgements: Cases were ascertained through the Victorian Cancer Registry (VCR) and the Australian Cancer Database (Australian Institute of Health and Welfare).
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplementary Material
References
- 1. Lopez-Otin C, Blasco MA, Partridge L, et al. The hallmarks of aging. Cell. 2013;153(6):1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aunan JR, Cho WC, Soreide K. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis. 2017;8(5):628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19(6):371–384. [DOI] [PubMed] [Google Scholar]
- 4. Dugué PA, Bassett JK, Joo JE, et al. Association of DNA methylation-based biological age with health risk factors and overall and cause-specific mortality. Am J Epidemiol. 2018;187(3):529–538. [DOI] [PubMed] [Google Scholar]
- 5. Dugué PA, Li S, Hopper JL, et al. DNA methylation-based measures of biological aging In: Tollefsbol TO, ed. Epigenetics in Human Disease. London: Academic Press; 2018:39–64. [Google Scholar]
- 6. Field AE, Robertson NA, Wang T, et al. DNA methylation clocks in aging: categories, causes, and consequences. Mol Cell. 2018;71(6):882–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14(10):R115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hannum G, Guinney J, Zhao L, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49(2):359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen BH, Marioni RE, Colicino E, et al. DNA methylation-based measures of biological age: meta-analysis predicting time to death. Aging (Albany NY). 2016;8(9):1844–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dugué PA, Bassett JK, Joo JE, et al. DNA methylation-based biological aging and cancer risk and survival: pooled analysis of seven prospective studies. Int J Cancer. 2018;142(8):1611–1619. [DOI] [PubMed] [Google Scholar]
- 11. Levine ME, Lu AT, Quach A, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging (Albany NY). 2018;10(4):573–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lu AT, Quach A, Wilson JG, et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging (Albany NY). 2019;11(2):303–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haycock PC, Hemani G, Aviv A. Association between telomere length and risk of cancer and non-neoplastic diseases: a Mendelian randomization study. JAMA Oncol. 2017;3(12):1741–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weischer M, Nordestgaard BG, Cawthon RM, et al. Short telomere length, cancer survival, and cancer risk in 47102 individuals. J Natl Cancer Inst. 2013;105(7):459–468. [DOI] [PubMed] [Google Scholar]
- 15. Lu AT, Seeboth A, Tsai P-C, et al. DNA methylation-based estimator of telomere length. Aging (Albany NY). 2019;11(16):5895–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Milne RL, Fletcher AS, MacInnis RJ, et al. Cohort profile: The Melbourne Collaborative Cohort Study (Health 2020). Int J Epidemiol. 2017;46(6):1757–1757i. [DOI] [PubMed] [Google Scholar]
- 17. Baglietto L, Ponzi E, Haycock P, et al. DNA methylation changes measured in pre-diagnostic peripheral blood samples are associated with smoking and lung cancer risk. Int J Cancer. 2017;140(1):50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. FitzGerald LM, Naeem H, Makalic E, et al. Genome-wide measures of peripheral blood DNA methylation and prostate cancer risk in a prospective nested case-control study. Prostate. 2017;77(5):471–478. [DOI] [PubMed] [Google Scholar]
- 19. Dugué PA, Brinkman MT, Milne RL, et al. Genome-wide measures of DNA methylation in peripheral blood and the risk of urothelial cell carcinoma: a prospective nested case-control study. Br J Cancer. 2016;115(6):664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wong Doo N, Makalic E, Joo JE, et al. Global measures of peripheral blood-derived DNA methylation as a risk factor in the development of mature B-cell neoplasms. Epigenomics. 2016;8(1):55–66. [DOI] [PubMed] [Google Scholar]
- 21. Dugué PA, English DR, MacInnis RJ, et al. Reliability of DNA methylation measures from dried blood spots and mononuclear cells using the HumanMethylation450k BeadArray. Sci Rep. 2016;6(1):30317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pearce N. What does the odds ratio estimate in a case-control study? Int J Epidemiol. 1993;22(6):1189–1192. [DOI] [PubMed] [Google Scholar]
- 23. MacInnis RJ, English DR, Hopper JL, et al. Body size and composition and colon cancer risk in men. Cancer Epidemiol Biomarkers Prev. 2004;13(4):553–559. [PubMed] [Google Scholar]
- 24. Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pink B. Socio-economic indexes for areas (SEIFA). In. 2011 ed. Canberra: Australian Bureau of Statistics; 2013. [Google Scholar]
- 26. Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics. 2012;13(1):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eilers PH, Marx BD. Flexible smoothing with B-splines and penalties. Stat Sci. 1996;11(2):89–102. [Google Scholar]
- 28. Perperoglou A, Sauerbrei W, Abrahamowicz M, et al. A review of spline function procedures in R. BMC Med Res Methodol. 2019;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chung M, Ruan M, Zhao N, et al. DNA methylation aging clocks and pancreatic cancer risk: Pooled analysis of three prospective nested case-control studies. Epigenetics 2020. Dec 14. doi: 10.1080/15592294.2020.1861401. Online ahead of print. [DOI] [PMC free article] [PubMed]
- 30. Kresovich JK, Xu Z, O’Brien KM, et al. Methylation-based biological age and breast cancer risk. J Natl Cancer Inst. 2019;111(10):1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kresovich JK, Xu Z, O’Brien KM, et al. Epigenetic mortality predictors and incidence of breast cancer. Aging (Albany NY). 2019;11(24):11975–11987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jia G, Lu Y, Wen W, et al. Evaluating the utility of polygenic risk scores in identifying high-risk individuals for eight common cancers. JNCI Cancer Spectrum. 4(3):pkaa021. doi: 10.1093/jncics/pkaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kachuri L, Graff RE, Smith-Byrne K, et al. Pan-cancer analysis demonstrates that integrating polygenic risk scores with modifiable risk factors improves risk prediction. Nat Commun 2020;11(1):6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fritsche LG, Gruber SB, Wu Z, et al. Association of polygenic risk scores for multiple cancers in a phenome-wide study: results from the Michigan Genomics Initiative. Am J Hum Genet. 2018;102(6):1048–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fritsche LG, Patil S, Beesley LJ, et al. Cancer PRSweb: an online repository with Polygenic Risk Scores for major cancer traits and their evaluation in two independent biobanks. Am J Hum Genet 2020;107(5):815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li S, Nguyen T, Wong EM, et al. Genetic and environmental causes of variation in epigenetic aging across the lifespan. Clin Epigenetics2020;12(1):158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bell CG, Lowe R, Adams PD, et al. DNA methylation aging clocks: challenges and recommendations. Genome Biol. 2019;20(1):249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang B, Zhou Y, Lin N, et al. Functional DNA methylation differences between tissues, cell types, and across individuals discovered using the M&M algorithm. Genome Re. 2013;23(9):1522–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.