Abstract
Background
Although most newly presenting patients with pulmonary hypertension (PH) have elevated pulmonary artery wedge pressure, identification of so‐called postcapillary PH can be challenging. A noninvasive tool predicting elevated pulmonary artery wedge pressure in patients with incident PH may help avoid unnecessary invasive diagnostic procedures.
Methods and Results
A combination of clinical data, ECG, and echocardiographic parameters was used to refine a previously developed left heart failure risk score in a retrospective cohort of pre‐ and postcapillary PH patients. This updated score (renamed the OPTICS risk score) was externally validated in a prospective cohort of patients from 12 Dutch nonreferral centers the OPTICS network. Using the updated OPTICS risk score, the presence of postcapillary PH could be predicted on the basis of body mass index ≥30, diabetes mellitus, atrial fibrillation, dyslipidemia, history of valvular surgery, sum of SV1 (deflection in V1 in millimeters) and RV6 (deflection in V6 in millimeters) on ECG, and left atrial dilation. The external validation cohort included 81 postcapillary PH patients and 66 precapillary PH patients. Using a predefined cutoff of >104, the OPTICS score had 100% specificity for postcapillary PH (sensitivity, 22%). In addition, we investigated whether a high probability of heart failure with preserved ejection fraction, assessed by the H2 FPEF score (obesity, atrial fibrillation, age >60 yrs, ≥2 antihypertensives, E/e' >9, and pulmonary artery systolic pressure by echo >35 mmHg), similarly predicted the presence of elevated pulmonary artery wedge pressure. High probability of heart failure with preserved ejection fraction (H2 FPEF score ≥6) was less specific for postcapillary PH.
Conclusions
In a community setting, the OPTICS risk score can predict elevated pulmonary artery wedge pressure in PH patients without clear signs of left‐sided heart disease. The OPTICS risk score may be used to tailor the decision to perform invasive diagnostic testing.
Keywords: diagnosis, heart failure, prediction models, pulmonary vasculature, validation study
Subject Categories: Vascular Disease, Clinical Studies, Diagnostic Testing
Nonstandard Abbreviations and Acronyms
- AUC
area under the receiver operating characteristics curve
- CTEPH
chronic thromboembolic pulmonary hypertension
- E/E’
ratio of peak early mitral inflow velocity to peak early diastolic velocity of the septal annulus
- HFpEF
heart failure with preserved ejection fraction
- LA
left atrial
- LHF
left heart failure
- PAH
pulmonary arterial hypertension
- PAWP
pulmonary artery wedge pressure
- PH
pulmonary hypertension
- RHC
right‐heart catheterization
- RV6
R deflection in V6 in millimeters
- SV1
S deflection in V1 in millimeters
Clinical Perspective
What Is New?
The OPTICS risk score, based on a combination of medical history and simple findings on ECG and echocardiography, accurately predicts the presence of postcapillary pulmonary hypertension (PH) in incident patients newly presenting with PH in a nonreferral setting.
In patients with a new diagnosis of PH, a high H2FPEF score (obesity, atrial fibrillation, age >60 yrs, ≥2 antihypertensives, E/e' >9, and pulmonary artery systolic pressure by echo >35 mmHg) does not exclude the possibility of precapillary PH.
What Are the Clinical Implications?
Using the OPTICS risk score can help tailor the decision to perform invasive diagnostic testing.
Treatable precapillary PH can be missed when the likelihood of postcapillary PH is based on the H2FPEF score.
Because of profound differences in management, it is of great clinical importance to make a distinction between pre‐ and postcapillary pulmonary hypertension (PH). Precapillary PH includes treatable conditions such as pulmonary arterial hypertension (PAH) and chronic thromboembolic PH (CTEPH).1, 2 When echocardiography shows clear signs of systolic left ventricular dysfunction or left‐sided valvular disease, postcapillary PH is often diagnosed without performing a right heart catheterization (RHC). The distinction between pre‐ and postcapillary PH is much more challenging in patients without clear signs of left‐sided heart disease. Many of these patients suspected of PH are >60 years old and have multiple comorbidities.3 Particularly in patients with heart failure with preserved ejection fraction (HFpEF), echocardiography can remain inconclusive and further invasive diagnostic steps may be required. In a considerable number of clinics, access to RHC is limited and may require referral to a tertiary PAH center.
Previous studies have indicated that 12% to 19% of patients referred to a tertiary PH center for RHC are found to have elevated pulmonary artery wedge pressure (PAWP)—that is, postcapillary PH—despite a priori suspicion of precapillary PH.4, 5, 6 Because a diagnosis of postcapillary PH usually does not change heart failure management, a considerable proportion of patients undergo RHC without subsequent treatment implications. Conversely, many other patients are likely denied a proper diagnosis of (treatable) precapillary PH because RHC is perceived as too invasive or too costly and because the pretest likelihood of precapillary PH is low. To facilitate noninvasive prediction of postcapillary PH, several scoring models have been developed. These models are typically based on echocardiography or a combination of echocardiography and clinical parameters.7, 8, 9, 10, 11, 12
We previously developed a left heart failure (LHF) risk score to predict elevated PAWP on the basis of medical history, ECG, and echocardiography.11 When this risk score was used with the highest possible specificity for the presence of elevated PAWP in incident PH patients (to ensure recognition of all possible cases of precapillary PH), the score allowed identification of postcapillary PH in 20% of patients. In principle, use of the score would allow prevention of unnecessary RHCs in 20% of patients. However, left valvular heart disease worse than mild on echocardiography was one of the strongest independent predictors of elevated PAWP in PH patients using the LHF risk score, whereas in daily practice, the possibility of precapillary PH is generally not considered in patients with significant left‐sided valve disease. It is thus desirable to refine the predictive algorithm and to tailor the risk score for use in a population of PH patients without significant valve disease but with still high pretest likelihood of postcapillary PH due to HFpEF.3
The H2FPEF score was originally developed in a primarily HFpEF population to discriminate between HFpEF and noncardiac causes of dyspnea and relies on 6 simple clinical characteristics and echocardiographic measurements.12 One of these predictors is pulmonary artery systolic pressure >35 mm Hg. In the setting of HFpEF, an elevated pulmonary artery systolic pressure would usually point to postcapillary PH. Up to 70% of patients with HFpEF may present with postcapillary PH.2 Although the H2FPEF score has been well validated to assess the probability of HFpEF, it is unclear whether the score can also be used to predict the presence of elevated PAWP in patients with incident PH. Because the population of precapillary patients is currently aging and presents with more comorbidities, some patients with precapillary PH may actually have a high probability of HFpEF. In that case, assessment of high probability of HFpEF, using the H2FPEF score, should not obviate the search of other causes of treatable precapillary PH, such as CTEPH.
In this study, we aimed (1) to refine a previously developed LHF risk score for the detection of elevated PAWP in PH patients without clear signs of left‐sided heart disease and (2) to externally validate this risk score (renamed OPTICS risk score) in a prospective cohort of PH patients in nonreferral centers. In addition, we investigated the specificity of a high H2FPEF score for postcapillary PH.
Methods
Development OPTICS Risk Score
Patient Selection
The authors declare that all supporting data are available within the article (and its online supplementary files). The first part of our study refines the previously developed LHF score.11 In a retrospective cohort, we included all patients suspected of PAH who attended our PH referral center between April 1998 and December 2012 and in whom, ultimately, a diagnosis was made of PAH or postcapillary PH. This cohort was 79% similar to the cohort previously studied by Jacobs et al.11 In addition to the previously used exclusion criteria of a likely diagnosis of postcapillary PH due to systolic heart failure (left ventricular ejection fraction <50%), we excluded left valvular disease that was more than mild on echocardiography, according to current guidelines.13
Study Design
Diagnosis of PH and PH classification in all patients followed standard criteria indicated by current guidelines.2 The classical definition of PH as a condition with mean pulmonary artery pressure >25 mm Hg was used, not >20 mm Hg. The reason for using this definition was that specific treatments are currently still proven only when mean pulmonary artery pressure is ≥25 mm Hg. Therefore, the importance of performing an RHC is still defined by the classical cutoff value. If PAWP was >15 mm Hg at rest or increased >18 mm Hg immediately after 500 mL of saline infusion over 5 minutes, a diagnosis was made of postcapillary PH.14, 15 If no reliable wedge was obtained, left ventricular end‐diastolic pressure was measured. RHC was performed at our institution by a team of 3 PH clinicians. PAWP was measured at end‐expiration at rest and over multiple breathing‐cycles. In cases of atrial fibrillation, we measured PAWP after the onset of the QRS complex and just before the v‐wave.
Potential predictors of postcapillary PH were documented from the medical history, ECG, and echocardiography. Potential predictors included age; body mass index >30 (in kg/m2; obesity); sex; a medical history of hypertension, diabetes mellitus, dyslipidemia (nonfasting total cholesterol >5 mmol/L; high‐density lipoprotein cholesterol <1.0 mmol/L and/or low‐density lipoprotein cholesterol >3 mmol/L), atrial fibrillation (paroxysmal or persistent) or left heart disease (either coronary artery disease or history of left valvular surgery without residual left valvular heart disease at PH diagnosis); and smoking history >1 pack‐year. From the ECG, evidence of left ventricular hypertrophy (SV1 [S deflection in V1 in millimeters] and RV6 [R deflection in V6 in millimeters]) and presence or absence of ECG evidence of left atrial (LA) dilation were taken. LA dilation on ECG was defined as prolonged a P‐wave duration of >120 ms in leads I or II with a negative portion of the P‐wave ≥1 mm in depth and ≥40 ms in duration in lead V1.16 From the echocardiographic parameters, only the presence of LA dilation was taken as potential predictor. LA dilatation on echocardiography was defined as indexed LA volume above 34 mL/m2.17 When actual measurements were not available, the description (dilated/nondilated) of LA dilation was used. We did not include other echocardiographic descriptors of left ventricular diastolic function, such as the ratio of peak early mitral inflow velocity to peak early diastolic velocity of the septal annulus (E/E′), to stay as close as possible to the original LHF risk score. Echocardiographic examinations were scored by a cardiologist blinded to the diagnosis; ECG data were measured by an observer blinded to the final diagnosis.
Statistical Analysis
All measurements were graded dichotomously (present or absent), as mentioned in Study Design, except ECG predictors SV1 and RV6. This finding is in line with what was used in the previous LHF risk score. Receiver operating characteristic curve analysis was applied to assess the optimal cutoff point for age. The optimal cutoff point was obtained based on the highest sensitivity and specificity values. We observed that the optimal cutoff point for age was 59 years old, with sensitivity of 75%, specificity of 59%, and area under the receiver operating characteristic curve (AUC) of 0.696 and thus dichotomized it to age ≥59 years old. Univariate logistic regression was used to evaluate the effect of the predictor. Selecting all univariate predictors (P<0.10), a multivariate logistic analysis with stepwise backward elimination determined the final model. Throughout the analyses, a P value of <0.05 was considered statistically significant. The performance of the model was determined by its discriminative abilities, obtained by AUC. The coefficients in the model were transformed into easy‐to‐use risk scores by dividing all regression coefficients by the lowest coefficient value. The clinical performance of the risk score was also evaluated via AUC, sensitivity, specificity, positive and negative predictive values, and probability, at different cutoff values of the OPTICS risk score. Because it is important not to misclassify any treatable precapillary PH, a specificity of 100% is considered most favorable.
External Validation of the OPTICS Risk Score
Patient Selection
In this preplanned prospective external validation study, data directly derived from the OPTICS network of 12 Dutch nonreferral community hospitals were used to validate the refined OPTICS risk score. The OPTICS network was launched in January 2015 and continues to collect data on incident PH patients in participating centers. To improve diagnostic and clinical care for PH patients, all participating centers adhere to the following (diagnostic) workflow. First, a team consisting of at least 1 cardiologist and 1 pulmonologist identifies all patients with signs, symptoms, or risk factors for PH and undertakes further diagnostic steps (eg, computed tomography, pulmonary function test, echocardiography), Second, after multidisciplinary consultation, the local PH team determines whether a RHC is required to rule out precapillary PH. In patients with overt postcapillary PH or PH due to pulmonary disease, RHC is not routinely performed, according to current guidelines.2 All patients discussed by the local PH team are anonymously entered in an online registry (part of PAHtool; Inovoltus). Detailed entry criteria for inclusion in the registry are (1) suspected PH on echocardiography (tricuspid regurgitation velocity >2.8 m/s and/or other echocardiographic signs for PH); (2) uncertainty about the cause of PH (defined as possible precapillary PH, warranting multidisciplinary consultation); or (3) incident cases, defined by either absence of signs of PH on a previous echo or RHC, or no previous investigations for PH performed. Patients with systolic heart failure (left ventricular ejection fraction <50%) or significant valvular heart disease (more than mild at PH diagnosis) are excluded from the registry.13 In addition, patients with scleroderma in whom echocardiography is performed for PH screening purposes are excluded from the registry.
Study Design
The external validation study included all patients with a final diagnosis of PAH, CTEPH, or postcapillary PH, diagnosed between January 2015 and October 2018, in whom hemodynamics were performed because diagnostic doubt persisted after multidisciplinary consultation (end‐point final validation cohort; Figure 11). A final diagnosis of PH was made in the presence of mean pulmonary artery pressure ≥25 mm Hg. The presence of PAWP >15 mm Hg at rest or left ventricular end‐diastolic pressure >15 mm Hg resulted in a diagnosis of postcapillary PH. PAWP tracings were assessed during end‐expiration at rest and over multiple breathing cycles. In cases of atrial fibrillation, we measured PAWP after the onset of the QRS complex and just before the v‐wave. The RHC was reviewed by either PH clinicians from the referral center or local experienced PH physicians specifically trained in RHC performance and evaluating PAWP tracings. In precapillary PH, significant lung disease was ruled out by lung function testing (including spirometry and diffusion capacity) and high‐resolution computed tomography, according to current guidelines.2 CTEPH was diagnosed based on a combination of findings from computed tomography angiography, perfusion scintigraphy, and pulmonary angiography.
Figure 1. Receiver operator characteristic (ROC) curve and optimal cutoff point of the OPTICS risk scoring system for prediction of postcapillary pulmonary hypertension.
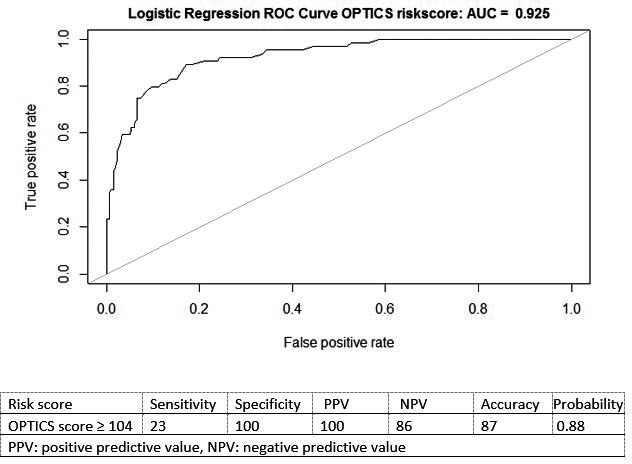
AUC indicates area under the curve.
The registry was approved by the local ethics committee in The Netherlands and was not considered to fall within the scope of the Medical Research Involving Human Subjects (medical research involving human subjects act [WMO]; approval number 2014326). All requirements of the hospital research and ethics review board were met, and no informed consent statement was required.
Statistical Analysis
Patient characteristics were described as either mean±SD for continuous variables or absolute number (percentage) for categorical variables. Student t tests and χ2 tests were performed to compare patient characteristics between patients with pre‐ and postcapillary PH. Throughout the analyses, P<0.05 was considered statistically significant. Because there are no generally accepted approaches to estimate the sample size for a validation study, we used all data available for this study.18 In the external validation cohort, the developed OPTICS risk score was calculated for each patient, and sensitivity, specificity, negative predictive value, positive predictive value, accuracy, and probability were measured at a predefined cutoff value.
Prediction of Postcapillary PH by the H2FPEF Score
The H2FPEF score enables discrimination of HFpEF from noncardiac causes of dyspnea and can assist in determination of the need for further diagnostic testing in the evaluation of patients with unexplained dyspnea.12 A higher score (eg, 6–9) can establish the diagnosis of HFpEF with reasonably high confidence. The H2FPEF score consists of the following components: heavy (body mass index >30) and hypertensive (≥2 antihypertensive medicines); atrial fibrillation (paroxysmal or persistent); PH (pulmonary artery systolic pressure >35 mm Hg); elderly (age >60 years); and filling pressure (Doppler echocardiographic E/e′ >9). For each clinical variable, a number of scoring points are given.12 With these clinical variables, a high probability of HFpEF assessed by the H2FPEF score may also be useful for the prediction of postcapillary PH.
To investigate whether the presence of PH in a patient with a high probability of HFpEF almost exclusively points to the presence of elevated PAWP, we determined the sensitivity, specificity, negative predictive value, positive predictive value, accuracy, and probability of the H2FpEF scores. For the calculation of the probability of HFpEF, a cutoff of ≥6 points and the online tool of the continuous model of the H2FPEF score were used.12 Model discriminatory properties were evaluated using AUC. In addition, change in logistic regression coefficient was measured to explore whether the combined use of the OPTICS risk score and H2FPEF score could improve the prediction of elevated PAWP in patients with incident PH.
Results
Clinical Characteristics and Model Building OPTICS Risk Score
In total, 331 patients with precapillary PH and 75 patients with postcapillary PH were included in the development cohort (Figure S1). Patient characteristics and hemodynamics at diagnosis are shown in Table 11. The mean age of patients with a diagnosis of precapillary or postcapillary PH was 53 or 64 years, respectively (P<0.001).
Table 1.
General Characteristics and Hemodynamics of the Development Cohort
| Precapillary PH | Postcapillary PH | P Value | |
|---|---|---|---|
| Patients, n | 331 | 73 | |
| Age, y | 53±17 | 64±14 | <0.001 |
| Male, n (%) | 95 (29) | 16 (22) | NS |
| BMI, kg/m2 | 25.9±5.8 | 31.7±7.5 | <0.001 |
| BMI ≥30a | 74 (22.4%) | 42 (61%) | <0.001 |
| Medical history of | |||
| Diabetes mellitus, n (%)a | 55 (17) | 44 (60) | <0.001 |
| Atrial fibrillation, n (%)a | 29 (9) | 30 (41) | <0.001 |
| Hypertension, n (%) | 77 (23) | 44 (60) | <0.001 |
| Dyslipidemia, n (%)a | 37 (11) | 22 (30) | <0.001 |
| Smoking >1 pack‐year, n (%) | 159 (49) | 37 (52) | NS |
| Valvular surgery without residual left valvular disease, n (%)a | 4 (1) | 15 (21) | <0.001 |
| Coronary artery disease, n (%) | 24 (7) | 20 (27) | <0.001 |
| Left heart disease, n (%) | 27 (8) | 28 (38) | <0.001 |
| ECG | |||
| LA dilatation, n (%) | 44 (14) | 5 (7) | NS |
| SV1+RV6, mma | 11±6 | 15±8 | <0.001 |
| Echocardiography | |||
| LA dilation, n (%)a | 66 (20) | 49 (70) | <0.001 |
| RHC | |||
| mRAP, mm Hg | 7 (4–12) | 9 (6–14) | 0.001 |
| mPAP, mm Hg | 49±16 | 40±12 | <0.001 |
| Cardiac output, L/min | 5.0±2.0 | 5.4±1.3 | NS |
| Heart rate, beats/min | 80±14 | 74±13 | 0.006 |
| PVR, dyn·s·cm−5 | 655 (407–1014) | 287 (170–412) | <0.001 |
| Wedge pressure, mm Hg | 8 (5–11) | 21 (19–23) | <0.001 |
| Mixed venous O2 saturation, % | 64±10 | 66±9 | NS |
Data are given as mean±SD, median (interquartile range), or percentage. BMI indicates body mass index; LA, left atrial; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; NS, not significant; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; and SV1+RV6, sum of S wave in V1 and R wave in V6 on ECG (in mm).
Independent predictors of the OPTICS risk score.
Univariate logistic regression was performed on predefined potential predictors of postcapillary PH, and results are presented in Table S1. Using backward multivariate logistic regression, the following parameters were identified as predictors of postcapillary PH: body mass index ≥30, a medical history of diabetes mellitus, atrial fibrillation (paroxysmal or persistent), dyslipidemia and history of valvular surgery, the sum of SV1 and RV6 on ECG (in millimeters), and the presence of LA dilation on echocardiography (Table 22).
Table 2.
Results From the Backward Multivariate Logistic Regression Identifying Independent Predictors of Postcapillary PH and the Subsequently Derived OPTICS Risk Scoring System
| OR (95% CI) | P Value | OPTICS Risk Scorea | |
|---|---|---|---|
| BMI ≥30, kg/m2 | 4.64 (2.12–10.15) | <0.001 | 22 |
| Medical history of | |||
| Diabetes mellitus | 6.49 (2.99–14.12) | <0.001 | 26 |
| Atrial fibrillation, any | 4.65 (1.79–12.07) | 0.001 | 21 |
| Dyslipidemia | 3.27 (1.31–8.11) | 0.01 | 17 |
| Valvular surgery without residual left valvular disease | 52.96 (9.23–303.79) | <0.001 | 56 |
| ECG | |||
| SV1+RV6 per mm | 1.07 (1.02–1.13) | 0.006 | 1x (SV1+RV6) |
| Echocardiography | |||
| LA dilation | 4.33 (1.97–9.52) | <0.001 | 21 |
BMI indicates body mass index; OR, odds ratio; PH, pulmonary hypertension; and SV1+RV6, sum of S wave in V1 and R wave in V6 on ECG (in mm).
Total risk score is calculated as follows. For presence of a BMI >30, the patient is attributed 22 points. If a medical history of diabetes mellitus is present, an additional 26 points are scored, and if a history of atrial fibrillation is present (paroxysmal or permanent atrial fibrillation), an additional 21 points are scored. History of Dyslipidemia gets 17 points , and 56 points are scored for a history of valvular surgery without residual left valvular disease. SV1+RV6 on ECG in millimeters is the risk score attributed for the ECG in each patient. For the presence of left atrial dilatation on echocardiography, the patient is attributed 21 points. The total score in each patient constitutes the OPTICS risk score for that individual.
The following model was constructed:
This model had a high predictive value with R 2=0.64 and an AUC of 0.93 (Figure S2). The OPTICS risk score was derived from the model (Table 22). The OPTICS risk score resulted in similar predictive values (R 2=0.64; AUC, 0.93; 95% CI, 0.89–0.96), as shown in Figure 11. Table S2 shows the performance of the OPTICS risk score in terms of sensitivity, specificity, accuracy, positive and negative predictive values, and probability at different risk‐score categories. Figure S3 shows the distribution of the OPTICS risk score for each group (precapillary PH and postcapillary PH). Using a risk score cutoff value of ≥104, postcapillary PH could be noninvasively predicted in 24% of the postcapillary PH patients, with a positive predictive value of 100%, probability of 0.88, and 100% specificity (Figure 1). Using an OPTICS risk score ≥104, no precapillary PH patients were predicted as having postcapillary PH.
External Validation OPTICS Risk Score
Patient Selection and Clinical Characteristics
Between January 2015 and October 2018, 439 patients at community hospitals with signs of PH on echo were included in the OPTICS registry (Figure 2). Overall, 292 patients received a final diagnosis of postcapillary PH or PH due to lung disease, based on multidisciplinary consultation and without a perceived need to perform RHC. The OPTICS validation cohort consisted of the 147 patients for whom the presence of treatable precapillary PH could not be excluded and who thus underwent RHC. Clinical characteristics of these patients are presented in Table 33.
Figure 2. Flowchart representing patients’ numbers and study methods of the external OPTICS cohort.
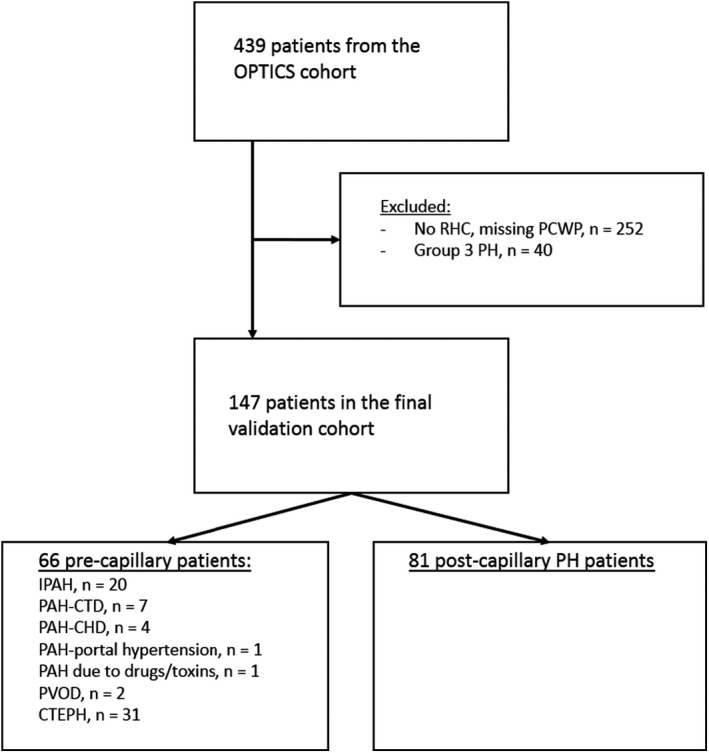
CTEPH indicates chronic thromboembolic pulmonary hypertension; IPAH, idiopathic pulmonary arterial hypertension; LVEDP, left ventricular end‐diastolic pressure; PAH, pulmonary arterial hypertension; PAH‐CHD, pulmonary arterial hypertension due to congenital heart disease; PAH‐CTD, pulmonary arterial hypertension due to connective tissue disease; PCWP, pulmonary capillary wedge pressure; PH, pulmonary hypertension; PVOD, pulmonary veno occlusive disease; and RHC, right heart catheterization.
Table 3.
General Characteristics and Hemodynamics of the Validation Cohort
| Precapillary PH | Postcapillary PH | P Value | |
|---|---|---|---|
| Patients, n | 66 | 81 | |
| Age, y | 66±13 | 69±11 | 0.042 |
| Male, n (%) | 37 (56) | 33 (41) | NS |
| BMI, kg/m2 | 27.3±5.5 | 31.3±7.3 | <0.001 |
| BMI ≥30, n (%)a | 13 (20) | 37 (46) | 0.002 |
| Medical history | |||
| Diabetes mellitus, n (%)a | 10 (15) | 30 (37) | 0.005 |
| Atrial fibrillation, n (%)a | 9 (14) | 34 (42) | <0.001 |
| Hypertension, n (%) | 39 (59) | 71 (88) | <0.001 |
| Dyslipidemia, n (%)a | 15 (23) | 42 (52) | 0.001 |
| Smoking >1 pack‐year, n (%) | 34 (52) | 43 (53) | NS |
| Valvular surgery without residual left valvular disease, n (%)a | 2 (3) | 5 (6.2) | NS |
| Coronary artery disease, n (%) | 12 (18) | 30 (37) | 0.020 |
| Left heart disease, n (%) | 14 (21) | 32 (40) | 0.028 |
| ECG | |||
| LA dilatation, n (%) | 11 (18) | 6 (10) | NS |
| SV1+RV6, mma | 14±6 | 15±6 | NS |
| Echocardiography | |||
| LA dilation, n (%)a | 17 (30) | 56 (70) | <0.001 |
| RHC | |||
| mRAP, mm Hg | 10 (7–11) | 12 (9–16) | 0.001 |
| mPAP, mm Hg | 45±11 | 36±9 | <0.001 |
| Cardiac output, L/min | 4.9±1.7 | 5.5±1.5 | 0.028 |
| Heart rate, beats/min | 80±16 | 73±15 | 0.031 |
| PVR, dyn·s·cm−5 | 551 (390–890) | 204 (147–274) | <0.001 |
| Wedge pressure, mm Hg | 11±3 | 21±6 | <0.001 |
| Mixed venous O2 saturation, % | 63±12 | 64±8 | 0.725 |
| NT‐proBNP, pg/mL | 1244 (320–2881) | 687 (271–1463) | 0.137 |
Data are given as mean±SD, median (interquartile range), or percentages. BMI indicates body mass index; LA, left atrial; mPAP, mean pulmonary arterial pressure; mRAP, mean right atrial pressure; NS, not significant; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; PH, pulmonary hypertension; PVR, pulmonary vascular resistance; RHC, right heart catheterization; and SV1+RV6, sum of S wave in V1 and R wave in V6 on ECG (in mm).
Independent predictors of the OPTICS risk score.
The majority of the population in The Netherlands is of white descent, although race and ethnicity are not routinely documented. The mean age of patients with a final diagnosis of precapillary or postcapillary PH was 66 or 69 years, respectively. Patients with postcapillary PH had significantly higher body mass index, presented more often with LA dilatation, and had more comorbidities, lower mean pulmonary artery pressure, and lower pulmonary vascular resistance (all P<0.05).
External Performance of the OPTICS Risk Score
Using the proposed risk score cutoff of ≥104, among 5 patients in whom the presence of precapillary PH could not be excluded, 1 patient could be noninvasively predicted as postcapillary PH (sensitivity 22%) without missing any precapillary cases (specificity of 100% and positive predictive value of 100%). Sensitivity, specificity, and probability of the prediction of postcapillary PH are presented in Figure 33. The OPTICS risk score could not be obtained in 15 patients (10%) because of missing data. AUC in the external validation cohort for OPTICS risk score was 0.81 (95% CI, 0.73–0.88). A continuous scale of the predicted probabilities according to several cutoff points is shown in Figure 44.
Figure 3. External validation of the OPTICS risk score and H2FPEF score.
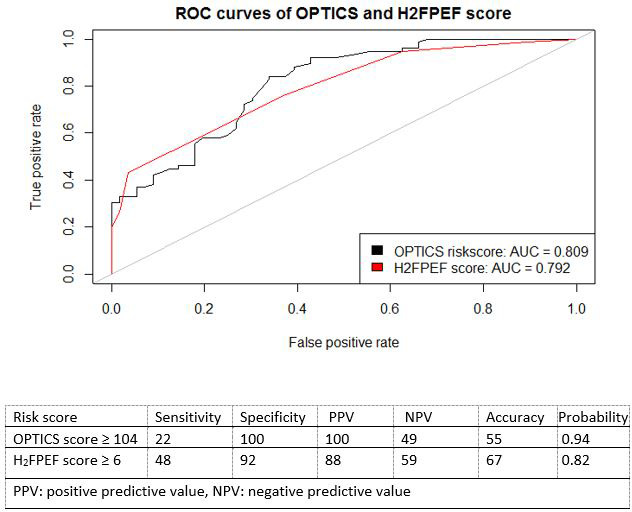
AUC indicates area under the curve.
Figure 4. Description of the OPTICS risk score and point allocation for each clinical parameter, with associated probability of having postcapillary PH, based on the total score as estimated from the model.
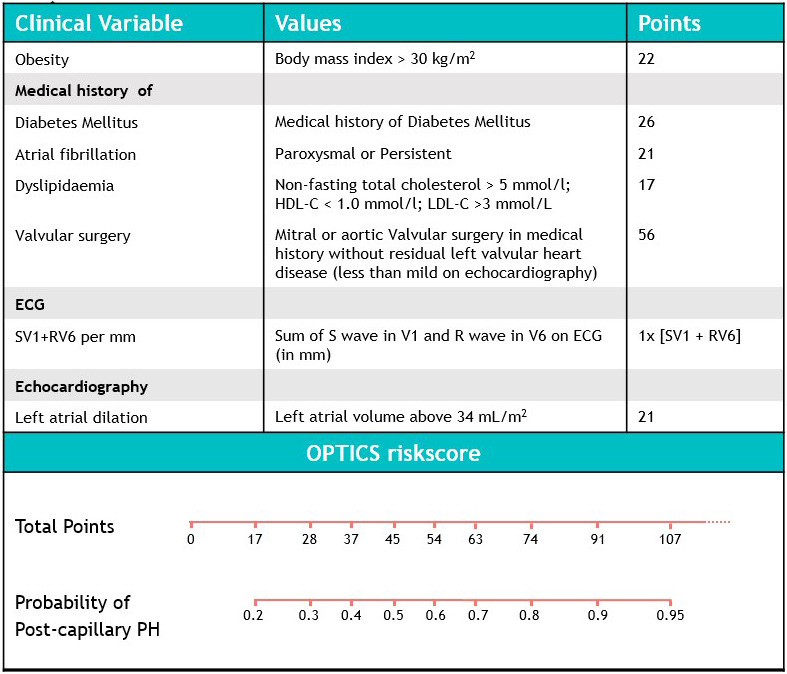
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; PH, pulmonary hypertension; RV6, deflection in V6 in millimeters; and SV1, deflection in V1 in millimeters.
Prediction of Postcapillary PH by the H2FPEF Score
The H2FPEF score was able to predict postcapillary PH when applying a cutoff ≥6, with sensitivity of 48% and specificity of 92% (Figures 33 and 55). A high probability of HFpEF, using the cutoff ≥6 with the H2FPEF score, was present in 5 precapillary PH patients (4 CTEPH and 1 PAH due to congenital heart disease). In addition, using the continuous model of the H2FPEF score (probability >0.9 for the prediction of HFpEF), 14 patients with precapillary PH had a high probability of HFpEF (6 CTEPH, 4 idiopathic PAH, 2 PAH due to congenital heart disease, 1 PAH due to connective tissue disease, and 1 pulmonary veno occlusive disease). The H2FPEF score could not be calculated in 2 patients because of missing data. AUC in the external validation cohort for H2FPEF score was 0.79 (95% CI, 0.72–0.87), as shown in Figure 33.
Figure 5. Pyramid graphs from the validation cohort of patients with postcapillary PH and precapillary PH, divided according to the OPTICS risk score outcomes or H2FPEF score outcomes of individual patients.
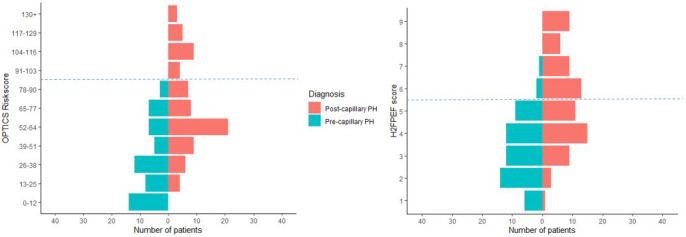
The blue line represents a cutoff of ≥104 for the OPTICS risk score and ≥6 for the H2FPEF score. PH indicates pulmonary hypertension.
We performed a probability analysis to explore whether combined use of the H2FPEF score (continuous model) and the OPTICS risk score could improve the prediction of elevated PAWP in PH patients. Only postcapillary PH was predicted when a probability >0.9 for both scores was present (Figure 66). By using the OPTICS score combined with the H2FpEF score, an additional value was observed in the multivariate analysis for the prediction of postcapillary PH (42% change in logistic regression coefficient for the H2FPEF score, 0.706–0.410).
Figure 6. Probability analysis of postcapillary PH by H2FPEF score and OPTICS score, divided according to PH group.
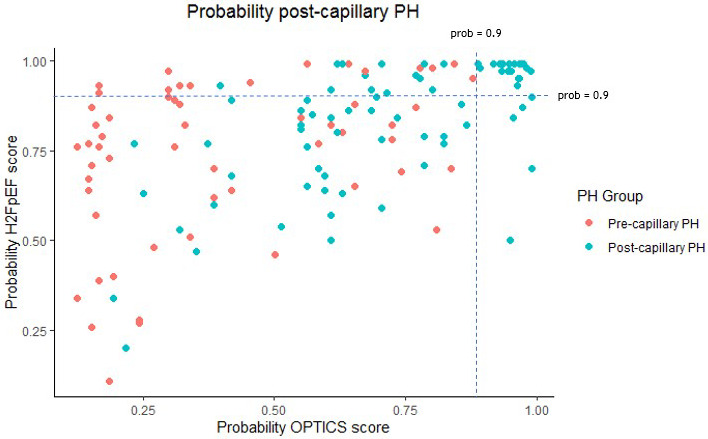
PH indicates pulmonary hypertension.
Discussion
In daily clinical practice, it is often difficult to distinguish between pre‐ and postcapillary PH. RHC is frequently required when echocardiography alone remains inconclusive. Because RHC is invasive and costly and often requires referral to a PH expert center, it is desirable that the procedure be performed only in patients with a high pretest likelihood of treatable precapillary PH. A noninvasive tool for the prediction of elevated PAWP in PH patients with high specificity may help avoid unnecessary diagnostic procedures. In the updated OPTICS risk score, we show that prediction of elevated PAWP in PH patients is possible using easily obtained noninvasive variables. The OPTICS risk score, developed in a tertiary PAH center, showed good performance in the setting of nonreferral community hospitals. The score accurately predicted elevated PAWP in 22% of the PH patients without clear signs of left heart disease, with specificity of 100%. The H2FPEF score, developed to predict HFpEF in patients with unexplained dyspnea, was able to predict postcapillary PH (sensitivity 48%). However, a high probability of HFpEF with the H2FPEF score was also detected in precapillary PH patients (specificity of 92%). This result underscores the fact that a high probability of HFpEF, assessed by the H2FPEF score, should not obviate the search for other causes of treatable precapillary PH, such as CTEPH.
Risk factors for postcapillary PH are well known.15, 19, 20, 21, 22 However, the distinction between pre‐ and postcapillary PH remains challenging. Any effort made to improve this distinction is helpful, but it should not lead to missing treatable precapillary PH. Early diagnosis and treatment of PAH is essential to improve clinical outcomes.2, 23 From our data, histories of valvular surgery, diabetes mellitus, and atrial fibrillation were independent predictors of postcapillary PH. Atrial fibrillation and diabetes mellitus are both well‐known risk factors for postcapillary PH.15, 19, 22 Interestingly, dyslipidemia is an independent predictor for postcapillary PH due to HFpEF. Dyslipidemia is a known risk factor for the development of left heart disease but is also frequently observed in patients with HFpEF.15, 24 Several reports have shown that left valvular heart disease is a risk factor for the development of postcapillary PH.20, 21, 22 The current study showed that prior left valvular surgery without residual left valvular heart disease is still a strong risk factor for the development of postcapillary PH. This risk factor was also reported by Vachiéry et al15 in the series world symposium on PH. Another study has shown that 15% of patients with severe mitral regurgitation and without any complications before valvular surgery (eg, no atrial fibrillation or PH) develop congestive heart failure during 15 years of follow‐up.25 In contrast with previous research, in the current study, older age was not found to be an independent predictor of postcapillary PH.12, 15, 22 This finding is in line with the observation of a changing phenotype of precapillary PH in the current era: PAH patients are older at the time of diagnosis and have more comorbidities.26, 27 For accurate noninvasive prediction of postcapillary PH, the data from medical history, ECG, and echocardiography must be combined, like the OPTICS score, because a single risk factor has insufficient discriminatory power.
Although our updated OPTICS risk score was developed from a retrospective single‐center cohort, we externally validated our risk score in a prospective setting of community hospitals. By excluding patients with overt left valvular disease, our updated risk score differs from the original LHF risk score.11 A history of left heart disease, including a history of coronary artery disease and/or left valvular disease, was not included in the updated OPTICS risk score. Coronary artery disease is well known as a common risk factor for postcapillary PH and LHF; however, a history of coronary artery disease is less common in patients with postcapillary PH due to HFpEF.13 Bonderman et al8 adopted a different strategy to facilitate noninvasive decision‐making by excluding the presence of precapillary PH. A decision tree was developed that combines the absence of right ventricular strain on ECG with low NT‐proBNP (N‐terminal pro‐B‐type natriuretic peptide) levels to effectively exclude precapillary PH.8 Our model differs from the model by Bonderman et al8 because we aimed to identify postcapillary PH as an alternative cause of PH, whereas Bonderman et al aimed to exclude precapillary PH. High NT‐proBNP levels are known to reflect right ventricular dysfunction and dilatation.28 However, not all patients with precapillary PH have elevated NT‐proBNP at diagnosis; in particular, normal NT‐proBNP is frequently found in those with PAH associated with connective tissue disease.29 These precapillary PH patients with normal NT‐proBNP may have relatively mild disease, possibly with adaptive right ventricular remodeling. Nevertheless, the model of Bonderman et al8 and our OPTICS risk score may have additional value in reducing the need for RHC.
The OPTICS risk score and the H2FPEF score similarly integrate clinical and imaging data that are easy to obtain. However, each model adopts a different strategy to facilitate noninvasive diagnostic decision‐making, either by prediction of HFpEF, which includes elevated systolic pulmonary artery pressure (SPAP) as a variable, or by prediction of postcapillary PH. In the present external validation study, we show that in the setting of community hospitals, application of the updated OPTICS risk score ensures that no patients with treatable precapillary PH are labeled as having elevated PAWP. In contrast, a high probability of HFpEF assessed by the H2FPEF score (score ≥6 or probability >0.9 with the continuous model) was not found exclusively in patients with postcapillary PH. In our cohort, 5 precapillary PH patients had a score ≥6, and 14 precapillary PH patients had a high probability of HFpEF >0.9 with the continuous model. A high probability of HFpEF in precapillary PH patients, assessed by the H2FPEF score, could be due to HFpEF as a comorbidity. We observed multiple comorbidities at diagnosis in these patients, and atrial fibrillation was especially prevalent. Elevated right‐sided filling pressures and structural changes can cause primarily dilatation of the right atrium and right ventricle, with subsequent tricuspid regurgitation, and this may trigger atrial fibrillation and atrial flutter.30, 31 In our cohort, we observed atrial fibrillation (predominantly paroxysmal atrial fibrillation) in 9% to 14% of the precapillary PH patients—in line with the COMPERA (Comparative, Prospective Registry of Newly Initiated Therapies for Pulmonary Hypertension) registry.32 In addition, postcapillary PH due to HFpEF or combined pre‐ and postcapillary PH could stay unrecognized in a standard RHC procedure. Invasive hemodynamics measured during exercise RHC may have revealed postcapillary PH in these patients. In contrast, most of these precapillary PH patients with a high probability of HFpEF did have symptoms of chronic thromboembolic disease, which is treatable. Importantly, this result emphasizes the fact that even when there is a high suspicion of HFpEF in a new patient with dyspnea due to PH, other treatable causes of PH such as venous thromboembolic disease should be excluded, preferably by perfusion scanning.2
In the present study we purposely chose not to create a new tool for the detection of PH with a high sensitivity for precapillary PH. Several widely used methods are sensitive enough to detect PH, such as echocardiographic tricuspid regurgitation velocity, dilatation of ventricle or atrium, and midsystolic notching of the pulmonary artery flow in combination with clinical risk factors.2 All patients included in our cohort were suspected of PH and had signs of PH on echocardiography. In addition, we chose not to itemize more complex echocardiographic parameters such as pulse‐wave Doppler of the right ventricle outflow tract.9, 10 Notwithstanding the promising performance of these parameters in distinguishing between pre‐ and postcapillary PH, they are not always easily assessable in busy, nonexpert settings. As such, in the present study we favored testing the use of risk scores incorporating unambiguous and easy‐to‐obtain variables for the detection of postcapillary PH.
Despite the potential reduction of unnecessary patient referrals when applying the OPTICS risk score, about 78% of patients with postcapillary PH in our study still presented with a low OPTICS risk score. These patients typically had no previous history of coronary artery disease, a preserved ejection fraction, and no signs of left ventricular hypertrophy or valvular dysfunction. Therefore, the clinical significance of a low OPTICS risk score seems to be limited, and a low risk score does not appear to be particularly useful in predicting precapillary PH. Perhaps a score that incorporates more sophisticated measures of diastolic dysfunction could improve the noninvasive diagnosis for this specific group of patients with postcapillary PH. For instance, a recent study demonstrated the independent predictive value of LA strain and isovolumetric relaxation time derived from echocardiography as indicators of elevated filling pressures in HFpEF.33 However, the potential incremental value in predicting postcapillary PH in the community setting needs to be addressed in future studies.
Limitations
The development cohort was assembled over a relatively long time period from 1998 to 2012. Therefore, echocardiographic data were heterogeneous and, at times, incomplete. E/e′ data, for example, were often lacking in the development cohort. This is probably explained by the fact that the E/e′ ratio was added to the advised parameters to register in 2016.13, 34 However, we tried to stay as close as possible to the original LHF risk score by using this cohort of patients. For external validation, we included only patients in whom RHC was performed, which may have result in a selection bias. Nevertheless, this bias would not have resulted in better performance of the OPTICS score. In addition, the diagnosis of pre‐ versus postcapillary PH relied primarily on PAWP and left ventricular end‐diastolic pressure tracings at rest or combined with saline infusion, which can be considered an oversimplification of the real‐world setting. In addition, because Doppler echocardiographic E/e′ was not available in 24% of patients, we interpreted the missing values as an absent E/e′ >9. This may have resulted in lower sensitivity of the H2FPEF score but would not have affected the specificity of the score.
Clinical Implications
Based on the current data, the use of the OPTICS risk score in daily clinical practice will enable predicting postcapillary PH in a subset of patients for whom RHC is considered. A high OPTICS score can tailor the diagnostic workup and should prompt further investigation into the possible presence of postcapillary PH, even when echocardiography does not provide obvious evidence of the presence of left‐sided heart disease. A high probability of HFpEF with the H2FPEF score was not found exclusively in postcapillary PH patients. Therefore, a high probability of HFpEF in a new patient with PH does not obviate the search for other causes of treatable precapillary PH, such as CTEPH. Of note, the referral of patients with a high suspicion of postcapillary PH can still be considered if diagnostic doubt persists or when severe PH or RV dysfunction is present and improved prognostication is desirable. In addition, when postcapillary PH with a precapillary component is suspected (ie, combined post‐ and precapillary PH), patients may still benefit from referral to expert centers for trial participation or disease management. Potential applications of the OPTICS score outside daily clinical practice include improving or comparing patient phenotypes and redefining inclusion criteria for clinical trial participation.
Conclusions
This study shows that in the real‐world community hospital setting, the new updated OPTICS score can predict the presence of elevated PAWP in PH patients without clear signs of left‐sided heart disease. For patients in whom precapillary PH cannot be excluded, the OPTICS score could aid in guiding the decision to refer a patient to PH expert centers and may help avoid unnecessary diagnostic procedures.
Sources of Funding
None.
Disclosures
Bogaard and Vonk Noordegraaf received unrestricted research support for the OPTICS registry from Actelion, MSD and Therabel Pharma, and GSK. AV was supported by NWO‐VICI (2002406) and the Dutch Heart Foundation. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S2
Figures S1–S3
(J Am Heart Assoc. 2020;9:e015992 DOI: 10.1161/JAHA.119.015992.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.119.015992
For Sources of Funding and Disclosures, see page 12.
See Editorial by Simon and Vachiery
References
- 1. D'Alonzo GE, Barst RJ, Ayres SM, Bergofsky EH, Brundage BH, Detre KM, Fishman AP, Goldring RM, Groves BM, Kernis JT, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med. 1991;343–349. [DOI] [PubMed] [Google Scholar]
- 2. Galie N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;903–975. [DOI] [PubMed] [Google Scholar]
- 3. Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, Marino P, Smiseth OA, De Keulenaer G, Leite‐Moreira AF, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;2539–2550. [DOI] [PubMed] [Google Scholar]
- 4. Gall H, Felix JF, Schneck FK, Milger K, Sommer N, Voswinckel R, Franco OH, Hofman A, Schermuly RT, Weissmann N, et al. The Giessen Pulmonary Hypertension Registry: survival in pulmonary hypertension subgroups. J Heart Lung Transplant. 2017;957–967. [DOI] [PubMed] [Google Scholar]
- 5. Deano RC, Glassner‐Kolmin C, Rubenfire M, Frost A, Visovatti S, McLaughlin VV, Gomberg‐Maitland M. Referral of patients with pulmonary hypertension diagnoses to tertiary pulmonary hypertension centers: the multicenter RePHerral study. JAMA Intern Med. 2013;887–893. [DOI] [PubMed] [Google Scholar]
- 6. Hurdman J, Condliffe R, Elliot CA, Davies C, Hill C, Wild JM, Capener D, Sephton P, Hamilton N, Armstrong IJ, et al. ASPIRE registry: assessing the spectrum of pulmonary hypertension identified at a REferral centre. Eur Respir J. 2012;945–955. [DOI] [PubMed] [Google Scholar]
- 7. Richter SE, Roberts KE, Preston IR, Hill NS. A simple derived prediction score for the identification of an elevated pulmonary artery wedge pressure using precatheterization clinical data in patients referred to a pulmonary hypertension center. Chest. 2016;1261–1268. [DOI] [PubMed] [Google Scholar]
- 8. Bonderman D, Wexberg P, Martischnig AM, Heinzl H, Lang MB, Sadushi R, Skoro‐Sajer N, Lang IM. A noninvasive algorithm to exclude pre‐capillary pulmonary hypertension. Eur Respir J. 2011;1096–1103. [DOI] [PubMed] [Google Scholar]
- 9. Opotowsky AR, Ojeda J, Rogers F, Prasanna V, Clair M, Moko L, Vaidya A, Afilalo J, Forfia PR. A simple echocardiographic prediction rule for hemodynamics in pulmonary hypertension. Circ Cardiovasc Imaging. 2012;765–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. D'Alto M, Romeo E, Argiento P, Pavelescu A, Melot C, D'Andrea A, Correra A, Bossone E, Calabro R, Russo MG, et al. Echocardiographic prediction of pre‐ versus postcapillary pulmonary hypertension. J Am Soc Echocardiogr. 2015;108–115. [DOI] [PubMed] [Google Scholar]
- 11. Jacobs W, Konings TC, Heymans MW, Boonstra A, Bogaard HJ, van Rossum AC, Vonk Noordegraaf A. Noninvasive identification of left‐sided heart failure in a population suspected of pulmonary arterial hypertension. Eur Respir J. 2015;422–430. [DOI] [PubMed] [Google Scholar]
- 12. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence‐based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation. 2018;861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2016;891–975. [DOI] [PubMed] [Google Scholar]
- 14. D'Alto M, Romeo E, Argiento P, Motoji Y, Correra A, Di Marco GM, Iacono AM, Barracano R, D'Andrea A, Rea G, et al. Clinical relevance of fluid challenge in patients evaluated for pulmonary hypertension. Chest. 2017;119–126. [DOI] [PubMed] [Google Scholar]
- 15. Vachiery JL, Tedford RJ, Rosenkranz S, Palazzini M, Lang I, Guazzi M, Coghlan G, Chazova I, De Marco T. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;1801897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;1466–1480. [DOI] [PubMed] [Google Scholar]
- 17. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;1–39.e14. [DOI] [PubMed] [Google Scholar]
- 18. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;W1–W73. [DOI] [PubMed] [Google Scholar]
- 19. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, Edelmann F, Fu M, Guazzi M, Lam CSP, et al. How to diagnose heart failure with preserved ejection fraction: the HFA‐PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J. 2019;3297–3317. [DOI] [PubMed] [Google Scholar]
- 20. Kannel WB, D'Agostino RB, Silbershatz H, Belanger AJ, Wilson PW, Levy D. Profile for estimating risk of heart failure. Arch Intern Med. 1999;1197–1204. [DOI] [PubMed] [Google Scholar]
- 21. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med. 2001;996–1002. [DOI] [PubMed] [Google Scholar]
- 22. Rosenkranz S, Gibbs JS, Wachter R, De Marco T, Vonk‐Noordegraaf A, Vachiery JL. Left ventricular heart failure and pulmonary hypertension. Eur Heart J. 2016;942–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Humbert M, Gerry Coghlan J, Khanna D. Early detection and management of pulmonary arterial hypertension. Eur Respir Rev. 2012;306–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, Bonow RO, Huang CC, Deo RC. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enriquez‐Sarano M, Suri RM, Clavel MA, Mantovani F, Michelena HI, Pislaru S, Mahoney DW, Schaff HV. Is there an outcome penalty linked to guideline‐based indications for valvular surgery? Early and long‐term analysis of patients with organic mitral regurgitation. J Thorac Cardiovasc Surg. 2015;50–58. [DOI] [PubMed] [Google Scholar]
- 26. Ling Y, Johnson MK, Kiely DG, Condliffe R, Elliot CA, Gibbs JS, Howard LS, Pepke‐Zaba J, Sheares KK, Corris PA, et al. Changing demographics, epidemiology, and survival of incident pulmonary arterial hypertension: results from the pulmonary hypertension registry of the United Kingdom and Ireland. Am J Respir Crit Care Med. 2012;790–796. [DOI] [PubMed] [Google Scholar]
- 27. Hoeper MM, Huscher D, Ghofrani HA, Delcroix M, Distler O, Schweiger C, Grunig E, Staehler G, Rosenkranz S, Halank M, et al. Elderly patients diagnosed with idiopathic pulmonary arterial hypertension: results from the COMPERA registry. Int J Cardiol. 2013;871–880. [DOI] [PubMed] [Google Scholar]
- 28. Gan CT, McCann GP, Marcus JT, van Wolferen SA, Twisk JW, Boonstra A, Postmus PE, Vonk‐Noordegraaf A. NT‐proBNP reflects right ventricular structure and function in pulmonary hypertension. Eur Respir J. 2006;1190–1194. [DOI] [PubMed] [Google Scholar]
- 29. Geenen LW, Baggen VJM, Koudstaal T, Boomars KA, Eindhoven JA, Boersma E, Roos‐Hesselink JW, van den Bosch AE. The prognostic value of various biomarkers in adults with pulmonary hypertension; a multi‐biomarker approach. Am Heart J. 2019;91–99. [DOI] [PubMed] [Google Scholar]
- 30. Olsson KM, Nickel NP, Tongers J, Hoeper MM. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int J Cardiol. 2013;2300–2305. [DOI] [PubMed] [Google Scholar]
- 31. Tongers J, Schwerdtfeger B, Klein G, Kempf T, Schaefer A, Knapp JM, Niehaus M, Korte T, Hoeper MM. Incidence and clinical relevance of supraventricular tachyarrhythmias in pulmonary hypertension. Am Heart J. 2007;127–132. [DOI] [PubMed] [Google Scholar]
- 32. Opitz CF, Hoeper MM, Gibbs JS, Kaemmerer H, Pepke‐Zaba J, Coghlan JG, Scelsi L, D'Alto M, Olsson KM, Ulrich S, et al. Pre‐capillary, combined, and post‐capillary pulmonary hypertension: a pathophysiological continuum. J Am Coll Cardiol. 2016;368–378. [DOI] [PubMed] [Google Scholar]
- 33. Hummel YM, Liu LCY, Lam CSP, Fonseca‐Munoz DF, Damman K, Rienstra M, van der Meer P, Rosenkranz S, van Veldhuisen DJ, Voors AA, et al. Echocardiographic estimation of left ventricular and pulmonary pressures in patients with heart failure and preserved ejection fraction: a study utilizing simultaneous echocardiography and invasive measurements. Eur J Heart Fail. 2017;1651–1660. [DOI] [PubMed] [Google Scholar]
- 34. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the Task Force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur J Heart Fail. 2008;933–989. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S2
Figures S1–S3


