Abstract
Background
Nonpharmacologic interventions that modify lifestyle can lower blood pressure (BP) and have been assessed in numerous randomized controlled trials and pairwise meta‐analyses. It is still unclear which intervention would be most efficacious.
Methods and Results
Bayesian network meta‐analyses were performed to estimate the comparative effectiveness of different interventions for lowering BP. From 60 166 potentially relevant articles, 120 eligible articles (14 923 participants) with a median follow‐up of 12 weeks, assessing 22 nonpharmacologic interventions, were included. According to the surface under the cumulative ranking probabilities and Grading of Recommendations Assessment, Development and Evaluation (GRADE) quality of evidence, for adults with prehypertension to established hypertension, high‐quality evidence indicated that the Dietary Approach to Stop Hypertension (DASH) was superior to usual care and all other nonpharmacologic interventions in lowering systolic BP (weighted mean difference, 6.97 mm Hg; 95% credible interval, 4.50–9.47) and diastolic BP (weighted mean difference, 3.54 mm Hg; 95% credible interval, 1.80–5.28). Compared with usual care, moderate‐ to high‐quality evidence indicated that aerobic exercise, isometric training, low‐sodium and high‐potassium salt, comprehensive lifestyle modification, breathing‐control, and meditation could lower systolic BP and diastolic BP. For patients with hypertension, moderate‐ to high‐quality evidence suggested that the interventions listed (except comprehensive lifestyle modification) were associated with greater systolic BP and diastolic BP reduction than usual care; salt restriction was also effective in lowering both systolic BP and diastolic BP. Among overweight and obese participants, low‐calorie diet and low‐calorie diet plus exercise could lower more BP than exercise.
Conclusions
DASH might be the most effective intervention in lowering BP for adults with prehypertension to established hypertension. Aerobic exercise, isometric training, low‐sodium and high‐potassium salt, comprehensive lifestyle modification, salt restriction, breathing‐control, meditation and low‐calorie diet also have obvious effects on BP reduction.
Keywords: hypertension, network meta‐analysis, nonpharmacologic interventions, randomized controlled trial, systematic review
Subject Categories: Epidemiology, Meta Analysis, Hypertension, Treatment
Nonstandard Abbreviations and Acronyms
- CrI
credible interval
- DASH
Dietary Approach to Stop Hypertension
- DBP
diastolic blood pressure
- GRADE
Grading of Recommendations Assessment, Development and Evaluation
- SBP
systolic blood pressure
- SUCRA
surface under the cumulative ranking
- WMD
weighted mean difference
Clinical Perspective
What Is New?
Single interventions including Dietary Approach to Stop Hypertension (DASH; ranked first), aerobic exercise, isometric training, low‐sodium and high‐potassium salt, salt restriction, breathing‐control, and meditation are associated with effective reduction of systolic blood pressure and diastolic blood pressure.
Comprehensive lifestyle modification, as a combined intervention, can lower both systolic and diastolic blood pressure effectively.
For patients who are overweight and obese, low‐calorie diet or low‐calorie diet plus exercise could lower blood pressure levels more than exercise alone.
What Are the Clinical Implications?
High‐quality evidence suggests that DASH might be the most effective intervention to lower blood pressure for adults with prehypertension to established hypertension.
Aerobic exercise, isometric training, low‐sodium and high‐potassium salt, comprehensive lifestyle modification, salt restriction, breathing‐control, and meditation should also be recommended to lower blood pressure for patients with hypertension who are receiving pharmacotherapy.
Weight loss from low‐calorie diet or low‐calorie diet plus exercise could lower blood pressure level more than exercise alone among people who are overweight and obese.
Hypertension is an important worldwide public health problem. As populations age, adopt unhealthy lifestyles, and increase their body weight, the number of people with hypertension will continue to increase, reaching close to 1.5 billion by 2025. 1 Studies have proven that hypertension is a strong risk factor for severe cardiovascular events, including myocardial infarction and stroke, if uncontrolled. 2 , 3 , 4 Compared with people who are normotensive, patients with prehypertension have a higher risk of developing sustained hypertension and cardiovascular disease. 5 , 6 Pharmacotherapy with first‐line antihypertensive agents has significant effects in lowering blood pressure (BP) 7 but also has side effects, treatment resistance, and financial burden. 8 Effective, widely available, low‐cost, and sustainable strategies are needed to prevent and manage hypertension.
Numerous randomized controlled trials (RCT), systematic reviews, and meta‐analyses have assessed the BP‐lowering effects of nonpharmacologic interventions. 9 , 10 , 11 US, Canadian, and European guidelines for hypertension recommend different nonpharmacologic interventions to prevent and manage hypertension. 12 , 13 , 14 However, their suggestions were based on traditional meta‐analysis, which can only compare the relative efficacy of pairs of interventions. A study that can compare the BP‐lowering effects of different nonpharmacologic interventions comprehensively is urgently needed to provide concrete evidence of the practice of nonpharmacologic interventions. Network meta‐analyses can synthesize direct and indirect evidence in a network of studies that compare multiple interventions. This approach has the potential to rank the competing treatments according to the studied outcome and determine the best available option for intervention. 15 , 16 , 17
The aim of our study was to assess the comparative effectiveness of different nonpharmacologic interventions for reducing BP in adults with prehypertension to established hypertension and to determine the most efficacious intervention.
Methods
This network meta‐analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta‐analyses (PRISMA) extension statement for reporting of systematic reviews incorporating network meta‐analyses of healthcare interventions. 18 All supporting data are available within the article and its online supplementary files. The study protocol can be found online (https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=67522). An extended description of the methods is reported in Data S1.
Search Strategies, Eligibility Criteria, and Information Sources
We searched PubMed, Embase, Cochrane Central Register of Controlled Trials, ClinicalTrials.gov, and the EU Clinical Trials Register up to July 1, 2019, to identify eligible studies. We limited searches to English‐language publications and supplemented them by perusing reference lists of reviews and retrieved literature. The search strategies are presented in Data S1.
We included RCTs of at least 4 weeks' duration that compared the BP‐lowering effects of nonpharmacologic interventions for adult patients (aged ≥18years) with primary hypertension or prehypertension. Patients with hypertension were defined as those with office systolic BP (SBP) ≥140 mm Hg and/or diastolic BP (DBP) ≥90 mm Hg without taking antihypertensive medication or those with established hypertension using antihypertensive medication, even if BP was <140/90 mm Hg. 14 Prehypertension was defined as an office SBP of 120 to 139 mm Hg and/or DBP of 80 to 89 mm Hg, according to the Joint National Committee in the Seventh Report. 19 Eligible interventions were different nonpharmacologic therapies. Comparators were other nonpharmacologic therapies or usual care as a control. We excluded studies that enrolled participants who had a history of heart failure, renal disease, stroke, serious mental or physical illness, malignancy, diabetes mellitus, or metabolic syndrome. We also excluded studies that focused on postmenopausal or pregnant women or single‐sex populations. However, studies that focused on participants who used alcohol habitually or who were overweight or obese were included because those features could be modified.
Study Selection and Data Collection Process
Two reviewers (Y.L. and D.L.) independently screened the titles and abstracts of all potentially eligible studies. Three reviewers (J.F., L. Zhang, and L. Zhou) performed full‐text review to identify studies that met all criteria for inclusion in the quantitative synthesis. Disagreements were resolved by discussion.
Pairs of independent reviewers extracted relevant data from each eligible study in duplicate, and discrepancies were resolved by discussion among reviewers. We extracted data on characteristics and demographics of study participants, mean baseline and follow‐up SBP and DBP, dropout, and other information.
End Points and Handling of Missing Data
Reductions of SBP and DBP after intervention were separately evaluated as co–primary end points, and the summary estimates were calculated by using the mean difference and SE. If the SEs of the mean differences were not available from included articles, we either estimated SEs based on the sample size, median, and range 20 or based on the mean difference, sample size, and P value. 21 We also imputed these data by estimsting correlation coefficient values between baseline and follow‐up. 21
Statistical Analysis
Traditional meta‐analyses were conducted using a random‐effects model for every direct comparison. 22 Next, Bayesian random‐effects network meta‐analyses were performed using the GeMTC package (R 3.4.3) based on the Markov‐chain Monte Carlo method. 23 , 24 Comparative effect estimates are presented as the weighted mean difference (WMD) and 95% credible interval (CrI) because all end points were continuous variables. Trace plots and the Brooks–Gelman–Rubin statistic were assessed to ensure convergence. 25 Network consistency between direct and indirect evidence was analyzed by the node‐splitting method, and its bayesian P value was reported. 26 Statistical heterogeneity of studies and the global heterogeneity of network meta‐analysis were also examined using the I2 statistic. 27
The relative rankings of different nonpharmacologic interventions were calculated using surface under the cumulative ranking (SUCRA) probabilities and were presented graphically (WinBUGS 1.4.3 [BUGS Project] and Stata 14.0 [StataCorp]). 28 Sensitivity analyses were conducted by omitting data from specific studies, including studies with high risk of bias, studies started before 1999 (international diagnostic criteria for hypertension were issued by the World Health Organization in 1999), 29 studies with end points of home BP or 24‐hour ambulatory BP, or studies targeted to special population (participants who used alcohol habitually or who were overweight or obese). Metaregression analyses were also performed by adding covariates (mean or median age, mean body mass index [BMI], proportion of participants taking antihypertensive medicine, and proportion of female patients). In addition, subgroup network meta‐analyses were conducted in different subgroups defined by study duration or region of origin of study participants. Because all analyses were based on bayesian framework, no multiplicity was adjusted. Publication bias was assessed using the comparison‐adjusted funnel plot and the netfunnel command (Stata 14.0). 30
Risk‐of‐Bias Assessment and Certainty of Evidence
Two reviewers (J.F. and L. Zhang) assessed the risk of bias separately for each included study using the Cochrane risk‐of‐bias tool (RevMan 5.3). 31 They also assessed the quality of evidence contributing to each direct, indirect, and network estimate independently using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) method of network meta‐analysis. 32 , 33 Discrepancies were resolved by discussion with another reviewer (Y.L.).
Results
A total of 60 166 articles were identified in the initial systematic search, and 888 potentially eligible articles were retrieved as full text. Overall, 120 articles (corresponding to 126 RCTs) with 14 923 participants met the inclusion criteria and were included in the network meta‐analysis (Figure 1).
Figure 1. PRISMA flow chart of the study selection for the network meta‐analysis.
*In case of multiple publications from the same population, only the study with the largest sample size was included. For studies published more than once, only the study with the most informative and complete data was included. Any additional publications were excluded to avoid double counting data from the same trial. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta‐analyses.
Study Characteristics
This network meta‐analysis covered 22 nonpharmacologic interventions including dietary approaches, physical exercise, approaches to reduce stress or lose weight, restriction of alcohol intake, combined interventions, and comprehensive lifestyle modification. All 22 interventions have been practiced in clinical or community trials, and brief descriptions and median intensity of all interventions and usual care are presented in Table 1. 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 Baseline and basic characteristics of included studies are presented in Table S1. Overall, 8530 participants were randomly assigned to intervention groups and 6393 to usual care; the mean age of all participants was 51.2 years; the median proportion of female patients was 0.49 (range, 0.05–0.88); median study duration was 12 weeks (range, 4–144); studies recruited participants from Europe (49.20%), the United States (24.60%), Asia (13.49%), and Africa (3.18%), and included Black Americans (9.53%); of 126 included studies, 91 (72.22%) recruited only patients with hypertension, 27 (21.43%) recruited patients with hypertension and prehypertension, and 8 (6.35%) recruited only patients with prehypertension (Table 2). The mean SBP and DBP levels of adults with prehypertension to established hypertension were 136.74 and 86.27 mm Hg, respectively. The mean SBP and DBP levels of patients with hypertension were 143.80 and 87.51 mm Hg, respectively.
Table 1.
Coding Guide for Components of Nonpharmacologic Interventions
| Intervention/Abbreviation | Brief Descriptions | Median Intensity |
|---|---|---|
| Dietary approach | ||
| DASH 34 , 35 | Participants' diet strictly follows the DASH eating pattern, which recommends a diet rich in fruits, vegetables, whole grains, and low‐fat dairy with reduced sodium and saturated and total fat content | Eating on the DASH pattern every day |
| Low‐sodium and high‐potassium salt 36 , 37 | Participants receive either a salt substitute (25%−30% potassium chloride, 50%−65% sodium chloride, and 5%–10% calcium and magnesium sulfate) to cover all cooking, or test food cooked using salt substitution | 5 g of low‐sodium and high‐potassium salt every day |
| Salt restriction 38 , 39 | The goal is to restrict daily sodium intake <100 mmol (5.85 g salt). Professional instructors give participants detailed advice about how to reduce their salt intake and to avoid foods that contain large amount of salt and also offer metric salt‐spoon or placebo to participants | Restrict sodium intake <100 mmol (5.85 g salt) every day |
| Physical exercise | ||
| Aerobic exercise 40 , 41 | Participants are supervised by project staff to perform exercise (eg, treadmill or brisk walking, jogging, bicycle training, swimming, ball games), at least 30 min/time; almost all were moderate or high intensity (60%–90% of the maximum heart rate or maximum oxygen consumption) | 3 d/wk, 50 min/time |
| Isometric training 42 , 43 | Participants perform isometric training, which involves sustained contraction against an immovable load or resistance with no or minimal change in length of the involved muscle group. Training consisted of four 2‐min isometric contractions at 30% MVC using alternate hands with a programmed handgrip dynamometer, with a 1‐min rest period between each contraction for 3 d per week | 3 d/wk, bilateral contractions at 30% of MVC |
| Resistance training 44 , 45 | Participants perform active movement progress through muscle to overcome external resistance, such as leg press, leg curl, knee extension, chest press, seated row, overhead press, triceps dip, and biceps curl, 50–60 min/d, 2–3 d/wk | 3 d/wk |
| Tai chi 46 | Tai chi js a set of Chinese systematic calisthenic exercises with slow circular movements and requires the muscles to remain relaxed while making sustained movement. Participants are taught by instructors with expertise to finish each session, which includes warm‐up exercises, tai chi practice, and cool‐down exercise | 3 d/wk, 50 min with 50% to 60% Vo 2max |
| Qigong 47 | Qigong, a traditional Chinese health and fitness exercise, includes qi gong ba duan jin, shu xin ping xue gong and dao yin shu qigong. Qigong experts help participants to reconstruct this instrument using a warming‐up exercise, qigong, and cool‐down exercise | Qigong classes 2 d/wk, home practice 2 d/wk |
| Interventions to reduce stress | ||
| Breathing control 48 , 49 | Use of a device guides participants toward slow and regular breathing in the evening (the goal is <10 breaths/min with accumulating ≥40 min of therapeutic breathing per week) | Every day, 15 min/time |
| Meditation 50 | Transcendental meditation is considered the principal approach for stress reduction. Participants are instructed by a professional meditation instructor and then practice 20 min twice a day while sitting comfortably with eyes closed | Practice meditation 20 min twice a day |
| MBSR 51 , 52 | MBSR is a multicomponent group intervention that provides systematic training in mindfulness meditation as a self‐regulation approach to stress reduction and emotion management. It can be explored through activities including but not limited to gentle stretching and mindful yoga, a meditative body scan, mindful breathing, and mindful walking | Practice MBSR techniques 45 min every day |
| PMR 53 , 54 | PMR involves directing the participants' attention to tense and relax various muscle groups throughout the body systematically to achieve deep relaxation | Practice PMR techniques 15–20 min twice a day |
| Yoga 55 | Participants are instructed by a professional yoga instructor through yoga home training or a yoga class and practice yoga at least 30 min/d, 3 d/wk | Practice yoga 3 d/wk, 45 min |
| Interventions to lose weight | ||
| Low‐calorie diet 56 , * | Participants who are overweight or obese using the low‐calorie diet induce weight loss are provided with detailed guidelines on the daily number of servings from each food group and on fat intake to achieve weight loss of ≤10% of each participant's baseline body weight. To enhance compliance with the low‐calorie diet, participants are provided with food diaries that assisted them in recording intake | Low‐caloric diet every day for weight loss |
| Exercise 56 , * | Participants who are overweight or obese in the exercise training group are provided with an individualized exercise prescription consisting of 30–40 min exercise (eg, aerobic exercise or others), at least 3 d/wk, keeping 60%–80% of the maximum heart rate. To enhance compliance, details of each exercise session are recorded in a training diary and reviewed by the study's counselor | Exercise 3 d/wk, reach 60%–80% peak heart rate |
| Low‐calorie diet plus exercise 56 , * | Participants who are overweight or obese using the exercise training plus low‐calorie diet for weight loss are provided with detailed guidelines on a low‐calorie diet to achieve weight loss and decrease BMI. In addition, they perform systematic exercise training, 30–45 min/d, at least 3 d/wk, keeping 60%–80% of the maximum heart rate | Low‐caloric diet for losing weight, with exercise 3 d/wk, reaching 60%–80% peak heart rate |
| Restrict alcohol | ||
| Alcohol restriction 57 , 58 , † | Participants reduce their alcohol consumption to <14 drinks weekly or 50% cut or total abstinence, with education for alcohol restriction provided by investigators | Reduce alcohol intake by half or abstain |
| Combined intervention | ||
| Aerobic exercise+DASH 59 | Participants follow the DASH eating pattern and perform aerobic exercise | At least 5 d/wk, 30–60 min aerobic exercise plus DASH |
| Aerobic exercise+resistance training 60 | Participants attend an aerobic exercise session and a resistance training session at the center at least twice a week | At least 2 d/wk, endurance training and resistance training |
| Salt restriction+DASH 35 | Participants follow the DASH eating pattern with salt restriction (sodium intake <100 mmol/d) | Follow diet every day |
| Salt restriction+low‐calorie diet plus exercise 61 , * | Participants who are overweight or obese follow a low‐sodium (80 mmol/d) diet with low‐calorie intake to achieve weight loss of 4.5 kg | Low‐sodium and low‐calorie diet every day; 3 d/wk, reach 60%–80% peak heart rate |
| Comprehensive lifestyle modification | ||
| Comprehensive lifestyle modification 62 , 63 | Participants are recommended to comprehensively modify their lifestyle, such as lose weight, restrict sodium intake, reduce alcohol consumption, increase physical exercise to a moderate degree, give up cigarette smoking, and learn to manage stress | Use lifestyle modification every day |
| Control group | ||
| Usual care | Participants keep usual lifestyle and do not change during the period of intervention | |
Brief descriptions of 22 interventions plus usual care (as control) are summarized, with 17 nonpharmacologic interventions targeted to the general population with hypertension or prehypertension. BMI indicates body mass index; DASH, Dietary Approaches to Stop Hypertension; MBSR, mindfulness‐based stress reduction; MVC, maximum voluntary contraction; and PMR, progressive muscle relaxation.
Nonpharmacologic intervention targeted only people who were overweight and obese who had hypertension or prehypertension.
Nonpharmacologic intervention targeted only people who used alcohol habitually who had hypertension or prehypertension.
Table 2.
Details of Included Studies (N=126)
| Study Details | n (%) |
|---|---|
| Region of origin of study participants | |
| Europe | 62 (49.20) |
| America (all) | 31 (24.60) |
| Asia | 17 (13.49) |
| America (Black Americans)* | 12 (9.53) |
| Africa | 4 (3.18) |
| Year thestudy started | |
| 1973–1998 | 54 (42.86) |
| 1999–2019 | 72 (57.14) |
| Study design | |
| Parallel | 108 (85.71) |
| Crossover | 18 (14.29) |
| Study duration, wk | |
| <12 | 55 (43.65) |
| 12–24 | 54 (42.86) |
| >24 | 17 (13.49) |
| Usage of antihypertensive medications | |
| Yes | 43 (34.13) |
| No | 65 (51.58) |
| Not reported | 18 (14.29) |
| Health status of recruited participants | |
| Hypertension and prehypertension (mixed) | 27 (21.43) |
| Hypertension only | 91 (72.22) |
| Prehypertension only | 8 (6.35) |
America (Black Americans) studies are those from America that were done in Black participants.
Risk of Bias Within Studies
Of 126 included RCTs, 41 (32.54%) were judged to have low risk of bias, and only 9 (7.14%) were judged to have high risk of bias; all other studies (60.32%) were judged to have moderate risk of bias (Table S2 and Figure S1). All comparison‐adjusted funnel plots of network meta‐analysis for outcomes did not show distinct asymmetry, which suggested no evidence of publication bias in this study (Figure S2).
Network Meta‐Analysis in Adults With Prehypertension to Established Hypertension (BP ≥120/80 mm Hg)
The results of traditional meta‐analyses showed that, compared with usual care, 10 interventions were more effective for lowering both SBP and DBP (Table S3).
Network meta‐analysis included 126 RCTs (14 923 participants) with 22 interventions and usual care. All 22 nonpharmacologic interventions had direct comparison with usual care, and 14 interventions compared directly with at least one other intervention (Figure 2A). Comparative effect estimates of 22 nonpharmacologic interventions in lowering BP are presented in Figure S3. Because indirect comparisons provided observational evidence in network meta‐analysis, we focused on the effective BP‐lowering estimates of interventions that were supported by the combination evidence of direct and indirect comparisons (Figure S3).
Figure 2. Network geometry used to assess the comparative effects of 22 nonpharmacologic interventions.
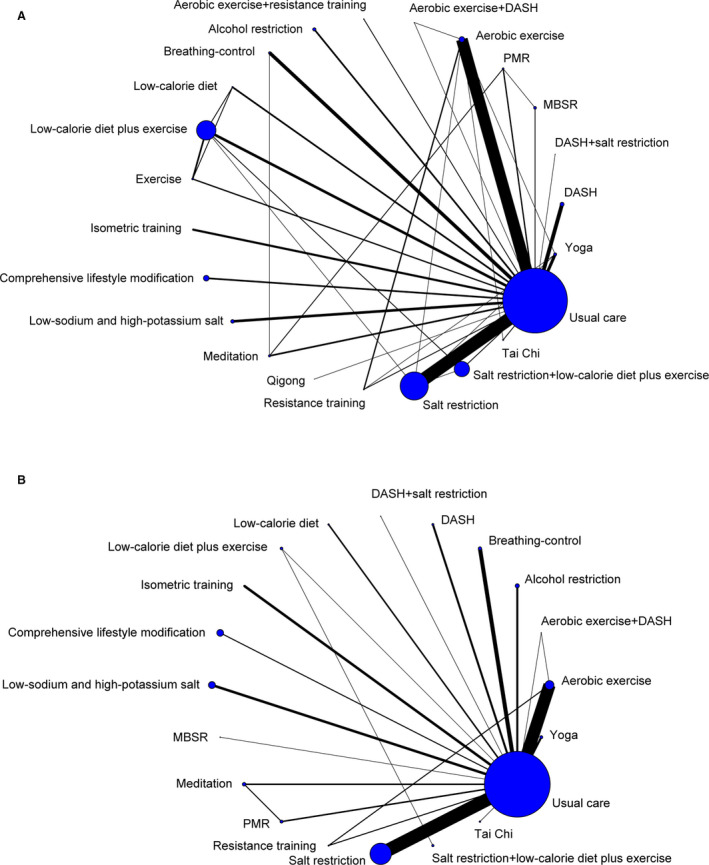
A, Adult with prehypertension to established hypertension. B, Patients with hypertension. The nodes represent 22 nonpharmacologic interventions and usual care. The size of every node is proportional to the number of randomly assigned participants (sample size). Each line represents a direct comparison, and the width of the lines is proportional to the number of studies comparing every pair of interventions. The coding guide, which provides a description of each intervention component, can be found in Table 1. DASH indicates Dietary Approaches to Stop Hypertension; MBSR, mindfulness‐based stress reduction; and PMR, progressive muscle relaxation.
In terms of lowering SBP, 15 interventions were shown to be more effective than usual care (Figure 3A). Based on SUCRA, the following interventions ranked ahead: tai chi (WMD, 13.47 mm Hg [95% CrI, 9.30–17.64]), Dietary Approaches to Stop Hypertension (DASH; WMD, 6.97 mm Hg [95% CrI, 4.50–9.47]), aerobic exercise plus DASH (WMD, 11.20 mm Hg [95% CrI, 2.81–19.61]), low‐calorie diet (WMD, 6.50 mm Hg [95% CrI, 2.78–10.17]), aerobic exercise (WMD, 6.60 mm Hg [95% CrI, 4.98–8.23]), isometric training (WMD, 5.77 mm Hg [95% CrI, 1.41–10.16]), low‐sodium and high‐potassium salt (WMD, 8.21 mm Hg [95% CrI, 4.99–11.43]), comprehensive lifestyle modification (WMD, 4.63 mm Hg [95% CrI, 1.32–7.94]), and the others include salt restriction, salt restriction plus low‐calorie diet and exercise, breathing‐control, low‐calorie diet plus exercise, meditation, yoga, and alcohol restriction (Figure 3A and Figure S4A). In addition, low‐calorie diet lowered SBP level more than exercise (WMD, 5.36 mm Hg [95% CrI, 0.45–10.25]) for participants who were overweight and obese (Figure S3).
Figure 3. Forest plots for mean changes of blood pressure in adults with prehypertension to established hypertension.
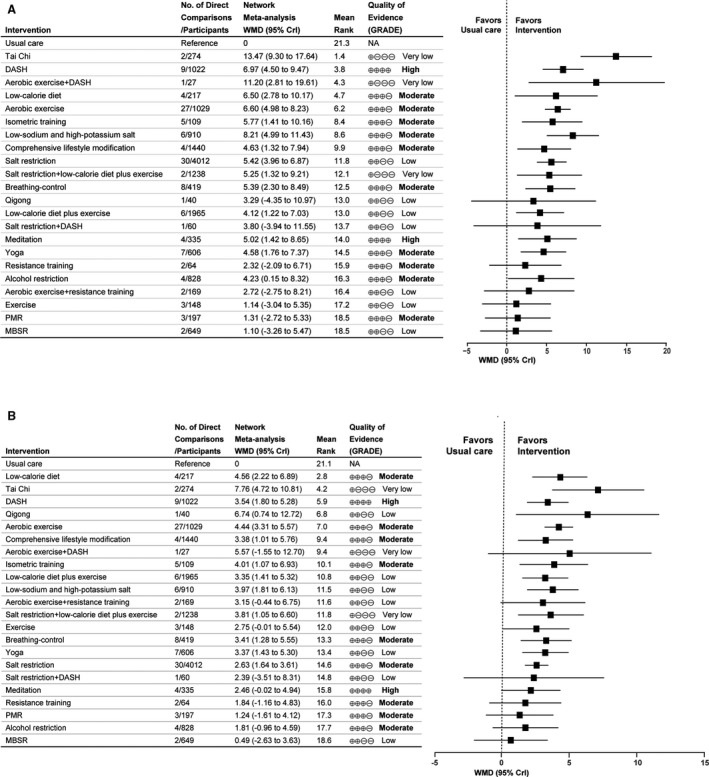
A, Systolic blood pressure. B, Diastolic blood pressure. Mean changes of blood pressure are reported in WMD and 95% CrI for intervention vs usual care. Rectangle represents the point estimate for the effect of each intervention. Horizontal lines indicate 95% CrI. Tables on the left of the forest plot show, for each intervention, the number of direct comparison studies, number of participants, rankings of SUCRA probabilities and quality of evidence. Interventions are ranked according to the rankings of SUCRA. The quality of evidence was classified as high, moderate, low, or very low. CrI indicates credible interval; DASH, Dietary Approaches to Stop Hypertension; MBSR, mindfulness‐based stress reduction; NA, not available; PMR, progressive muscle relaxation; SUCRA, surface under the cumulative ranking; and WMD, weighted mean difference.
In terms of lowering DBP, based on SUCRA, low‐calorie diet (WMD, 4.56 mm Hg [2.22–6.89]), tai chi (WMD, 7.76 mm Hg [95% CrI, 4.72–10.81]), DASH (WMD, 3.54 mm Hg [95% CrI, 1.80–5.28]), qigong (WMD, 6.74 mm Hg [95% CrI, 0.74–12.72]), aerobic exercise (WMD, 4.44 mm Hg [95% CrI, 3.31–5.57]), comprehensive lifestyle modification (WMD, 3.38 mm Hg [95% CrI, 1.01–5.76]), isometric training (WMD, 4.01 mm Hg [95% CrI, 1.07–6.93]), and low‐calorie diet plus exercise (WMD, 3.35 mm Hg [95% CrI, 1.41–5.32]), followed by low‐sodium and high‐potassium salt, salt restriction plus low‐calorie diet and exercise, breathing‐control, yoga, and salt restriction, were more effective than usual care (Figure 3B and Figure S4B). In addition, aerobic exercise was slightly better than salt restriction in lowering DBP (WMD, 1.82 mm Hg [95% CrI, 0.33–3.31]) (Figure S3).
The quality of evidence for interventions in comparisons with usual care are summarized in Table S4. We focused on high‐ or moderate‐quality evidence given the large number of results from the GRADE framework. In terms of lowering SBP and DBP, the quality of evidence for DASH and meditation were rated as high, and as moderate for low‐calorie diet, isometric training, aerobic exercise, comprehensive lifestyle modification, resistance training, alcohol restriction, breathing‐control and progressive muscle relaxation. There was also moderate confidence supporting the use of low‐sodium and high‐potassium salt and yoga in lowering SBP and the use of salt restriction in lowering DBP (Figure 3 and Table S4).
Network Meta‐Analysis in Patients With Hypertension (BP ≥140/90 mm Hg)
In traditional meta‐analyses of patients with hypertension, 10 interventions were more effective in lowering BP compared with usual care (Table S3).
Network meta‐analysis in patients with hypertension included 91 studies (7291 participants) with 19 interventions and usual care (Figure 2B). Comparative effect estimates were presented in Figure S5, and we focused on the effective BP‐lowering estimates of interventions that were supported by the combination evidence of direct and indirect comparisons. Ten interventions were shown to be more effective than usual care in lowering SBP and DBP (Figure 4). Tai chi (WMD, 12.75 mm Hg [95% CrI, 6.54–18.98]), DASH (WMD, 8.69 mm Hg [95% CrI, 5.23–12.19]), and low‐calorie diet (WMD, 7.78 mm Hg [95% CrI, 3.53–11.91]) were ranked first in lowering SBP; low‐calorie diet (WMD, 4.98 mm Hg [95% CrI, 2.03–7.89]) was ranked higher in lowering DBP than DASH (WMD, 4.54 mm Hg [95% CrI, 1.91–7.18]); regardless of lowering either SBP or DBP, aerobic exercise, isometric training, low‐sodium and high‐potassium salt, yoga, meditation, salt restriction, and breathing‐control followed low‐calorie diet and DASH (Figure 4 and Figure S6).
Figure 4. Forest plots for mean changes of blood pressure in patients with hypertension.
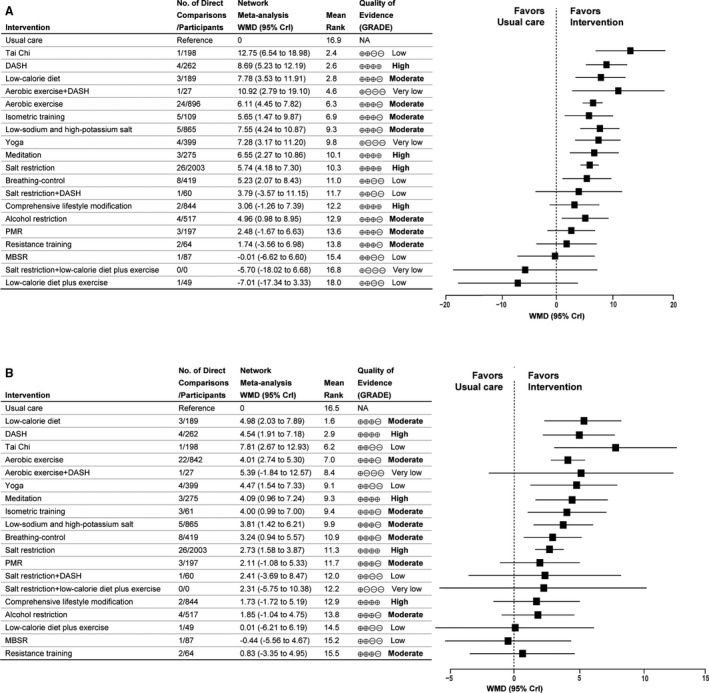
A, Systolic blood pressure. B, Diastolic blood pressure. Mean changes of blood pressure are reported in WMD and 95% CrI for intervention vs usual care. Rectangle represent the point estimate for the effect of each intervention. Horizontal lines indicate 95% CrI. For each intervention, tables on the left of the forest plot show the number of direct comparison studies, number of participants, rankings of SUCRA probabilities, and quality of evidence. Interventions are ranked according to the rankings of SUCRA. The quality of evidence was classified as high, moderate, low, and very low. CrI indicates credible interval; DASH, Dietary Approaches to Stop Hypertension; MBSR, mindfulness‐based stress reduction; NA, not available; PMR, progressive muscle relaxation; SUCRA, surface under the cumulative ranking; and WMD, weighted mean difference.
The quality of evidence for interventions in comparisons with usual care in patients with hypertension was similar to that for adults with prehypertension to established hypertension (Table S5). In addition, the quality of evidence for salt restriction was rated as high regardless of lowering either SBP or DBP (Figure 4 and Table S5).
Assessment of Heterogeneity and Inconsistency
The global I2 values were 74.26% and 76.70% for mean SBP change and mean DBP change, respectively, in network meta‐analysis of adults with prehypertension to established hypertension, and the global I2 values were 63.25% and 74.60% in the analysis of patients with hypertension. The test of inconsistency showed significant differences in only 2 comparisons (tai chi versus usual care, tai chi versus aerobic exercise) between direct and indirect results. Details of heterogeneity and consistency are given in Table S6.
Network Sensitivity, Metaregression, and Subgroup Analyses
We conducted sensitivity, metaregression, and subgroup analyses based on all existing data. Sensitivity analyses omitting studies with high risk of bias, studies started before 1999, or studies with end points of home BP or 24‐hour ambulatory BP did not significantly alter the results of overall network meta‐analysis (Table S7). Sensitivity analysis was carried out by omitting special populations along with specified interventions, and the SUCRA rankings of the other interventions did not change except that the rankings of the deleted interventions were missing (Table S7). The results of metaregression analyses showed that covariates (mean age, mean body mass index, proportion of participants taking antihypertensive medicine, and proportion of female patients) did not affect the results of this study (Table S8). The BP‐lowering effects of interventions among different subgroups defined by study duration and region of origin of study participants were not statistically different (Table S9).
Discussion
To the best of our knowledge, this study is the first to estimate the aggregate BP effects of 22 nonpharmacologic interventions through network meta‐analysis including patients with hypertension and prehypertension. In the results, which combined the SUCRA rankings and GRADE quality of evidence and overcame the lack of head‐to‐head trials, DASH ranked as the most effective intervention for lowering BP, followed by aerobic exercise, isometric training, low‐sodium and high‐potassium salt, and comprehensive lifestyle modification. Meditation and breathing‐control were considered to be relatively better among stress‐reduction interventions but were less effective than the above‐mentioned interventions. Salt restriction was also supported for lowering BP, especially in patients with hypertension.
Nonpharmacologic interventions, including dietary approaches, are a cornerstone for the prevention and treatment of hypertension. 2 The DASH diet promotes consumption of whole grains, vegetables and fruits, lean meat, and fat‐free dairy products and the inclusion of micronutrients in the diet. 64 These foods are also naturally low in sodium and contain nutrients, which may help lower BP. 64 This diet can also decrease concentrations of total cholesterol and LDL (low‐density lipoprotein), which may predict a reduction of ≈13% in the 10‐year Framingham risk score for cardiovascular disease. 65 Our report demonstrates that eating a DASH diet every day has a significant effect on lowering BP compared to usual care, which is in keeping with previous meta‐analysis. 9 In addition, our network meta‐analysis suggests DASH to be the most effective intervention, based on its top SUCRA ranking and high‐quality evidence supporting.
The World Health Organization has proposed that a 30% reduction in salt or sodium intake may reduce the risk of hypertension. 66 In our study, salt restriction (sodium intake <100 mmol, equivalent to 5.85 g salt) can significantly lower SBP, which is consistent with the result of a previously published meta‐analysis. 10 Because the quality of evidence was rated down by risk of bias, inconsistency, and publication bias, there is low confidence supporting the use of salt restriction for lowering SBP in adults with prehypertension to established hypertension. However, the risk of bias was due to a study that was published in 1973 with insufficient information, 67 and high heterogeneity (I2=77.1%) was mainly produced by combining the results of participants with hypertension and prehypertension. In the analysis for only patients with hypertension, the quality of evidence for salt restriction was considered high.
For people with a long‐established habit of high salt intake, it is difficult to attain and maintain long‐term voluntary salt control, and alternative approaches with equivalent effects are needed. 68 A salt substitute with low‐sodium and high‐potassium content and an acceptable salty flavor would be an ideal population‐wide preventative strategy. In our network meta‐analysis, moderate‐quality evidence supports the BP‐lowering effect of low‐sodium and high‐potassium salt (≈5–8 g) in adults with prehypertension to established hypertension.
An extensive body of research suggested that physical activity may have beneficial effects on BP in people with hypertension. Published meta‐analyses have also confirmed the efficacy of physical activity in lowering BP. 11 , 69 , 70 In our study, moderate‐ to high‐intensity aerobic exercise (at least 3 days weekly, 30 minutes per time, achieving 60% to 90% of the maximum heart rate) and isometric training (3 days weekly, bilateral contractions at 30% of maximum voluntary contraction), followed behind DASH in lowering BP significantly.
Tai chi fared relatively better based on SUCRA rankings. However, highly ranked interventions would result in misleading inferences when most evidence is of low or very low quality. 18 Based on the very low quality of evidence (severe inconsistency, imprecision, and publication bias), tai chi disappeared from our recommendations. However, tai chi was still among the interventions with a potentially better effectiveness profile, although the BP‐lowering effect was potentially spurious according to the analysis of existing data, and more RCTs should be conducted to evaluate this result further.
In addition to single nonpharmacologic interventions, comprehensive lifestyle modification is also effective in lowering BP and has been evaluated in several RCTs. 62 , 63 , 71 , 72 In our network meta‐analysis, it is not surprising that moderate‐quality evidence supported comprehensive lifestyle modification for lowering both SBP and DBP. However, based on SUCRA rankings, this intervention did not seem to be the most effective, possibly because different studies have different approaches to modify multiple unhealthy lifestyles, which may bring heterogeneity and affect the results. Benefits of other combined interventions (aerobic exercise plus DASH, salt restriction plus low‐calorie diet and exercise, salt restriction plus DASH, aerobic exercise plus resistance training) could not be judged because of insufficient studies and low quality of evidence until now.
This study also extends findings from previous traditional meta‐analyses that aerobic exercise seems more effective than salt restriction with respect to lowering BP, whereas the differences in comparative effect among other effective nonpharmacologic interventions mentioned above were modest (Figures S3 and S5), signaling potential equivalence of these interventions for lowering BP. Low‐calorie diet and low‐calorie diet plus exercise could lower BP level more than exercise among participants who were overweight and obese because of participants' weight loss with these 2 interventions.
Considering that the BP‐lowering effects of nonpharmacologic interventions may be affected by study duration, we performed subgroup analysis. The results showed that the BP‐lowering effects of interventions among different subgroups were not statistically different. Despite the differences that were not statistically significant, we observed that low‐sodium and high‐potassium salt and aerobic exercise lowered BP more over 12 to 24 weeks, and the BP‐lowering effects of salt restriction, low‐calorie diet, and comprehensive lifestyle modification were decreased with the extension of duration. These results may be caused by different persistence over time. For breathing‐control, DASH, and isometric training, the duration of most studies was <12 weeks. Consequently, more RCTs should be conducted to assess the long‐term effects of nonpharmacologic interventions.
Our network meta‐analysis strictly excluded studies involving adults with resistant hypertension, who are particularly salt‐sensitive 73 and reacted differently from patients with primary hypertension in terms of salt‐related interventions. Studies involving patients with diabetes mellitus or metabolic syndrome were also excluded because these conditions might influence the effects of nonpharmacologic interventions. 74 , 75 The participants who used alcohol habitually or who were overweight or obese in our study were also free of diabetes mellitus and metabolic syndrome; therefore, these special participants were treated equally as patients with hypertension and prehypertension. After omitting these specified interventions along with their corresponding participants, the SUCRA rankings of the other interventions did not change except that the rankings of the deleted interventions were missing.
This analysis has several limitations. First, for the 22 interventions included in the network meta‐analysis, 8 were only directly compared with usual care. The effects of these interventions were estimated with direct evidence; however, this did not affect the evaluation and rankings of these 8 interventions because direct evidence has a higher rating than indirect evidence. In addition, many indirect comparisons were assessed as being of low or very low quality in the GRADE framework, which largely restricts the interpretation of these results. Inconsistency existed in the comparison of tai chi versus aerobic exercise; however, this did not affect the estimates of other interventions seriously. Second, our study reported only the effectiveness of nonpharmacologic interventions in lowering BP, lacking secondary end points such as rate of BP control, incidence of hypertension, and mortality due to complications of hypertension, as most RCTs included in this study provided data of mean BP or changes in BP. Third, smoking cessation as a nonpharmacologic intervention was not included in our study because existing RCTs on smoking cessation in patients with hypertension or prehypertension were not truly intervened. Music therapy was also not included because of a wide variety of music was used, and there was no comparable control group. Fourth, most RCTs included in this study had short‐ or moderate‐term follow‐up. Fifth, we only reviewed publications in English.
Conclusions
This network meta‐analysis showed that, among 22 nonpharmacologic interventions, DASH was the most effective intervention in lowering BP for adults with prehypertension to established hypertension. Aerobic exercise, isometric training, low‐sodium and high‐potassium salt, comprehensive lifestyle modification, breathing control, meditation, and low‐calorie diet also have obvious effects in lowering BP. Moreover, our findings suggest that salt restriction be used for lowering BP, especially in patients with hypertension.
Sources of Funding
Yang, Sun, and Y. Zhao received funding from the Chinese Academy of Engineering advisory project: “International comparative study on strategies for the prevention and control of a major disease (hypertension) in China's cold regions” (2015‐XZ‐32).
Disclosures
None.
Supporting information
(J Am Heart Assoc. 2020;9:e016804 DOI: 10.1161/JAHA.120.016804.)
For Sources of Funding and Disclosures, see page 12.
Contributor Information
Dianjun Sun, Email: hrbmusdj@163.com.
Yashuang Zhao, Email: zhao_yashuang@263.net.
References
- 1. Lip GYH, Coca A, Kahan T, Boriani G, Manolis AS, Olsen MH, Oto A, Potpara TS, Steffel J, Marín F, et al. Hypertension and cardiac arrhythmias: executive summary of a consensus document from the European Heart Rhythm Association (EHRA) and ESC Council on Hypertension, endorsed by the Heart Rhythm Society (HRS), Asia‐Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLEACE). Eur Heart J Cardiovasc Pharmacother. 2017;235–250. [DOI] [PubMed] [Google Scholar]
- 2. James PA, Oparil S, Carter BL, Cushman WC, Dennison‐Himmelfarb C, Handler J, Lackland DT, LeFevre ML, MacKenzie TD, Ogedegbe O, et al. 2014 evidence‐based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;507–520. [DOI] [PubMed] [Google Scholar]
- 3. Oparil S, Acelajado MC, Bakris GL, Berlowitz DR, Cifkova R, Dominiczak AF, Grassi G, Jordan J, Poulter NR, Rodgers A, et al. Hypertension. Nat Rev Dis Primers. 2018;18014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mm Hg, 1990–2015. JAMA. 2017;165–182. [DOI] [PubMed] [Google Scholar]
- 5. Vasan RS, Larson MG, Leip EP, Evans JC, O'Donnell CJ, Kannel WB, Levy D. Impact of high‐normal blood pressure on the risk of cardiovascular disease. N Engl J Med. 2001;1291–1297. [DOI] [PubMed] [Google Scholar]
- 6. Gupta AK, McGlone M, Greenway FL, Johnson WD. Prehypertension in disease‐free adults: a marker for an adverse cardiometabolic risk profile. Hypertens Res. 2010;905–910. [DOI] [PubMed] [Google Scholar]
- 7. Thomopoulos C, Parati G, Zanchetti A. Effects of blood pressure lowering on outcome incidence in hypertension: 4. Effects of various classes of antihypertensive drugs–overview and meta‐analyses. J Hypertens. 2015;195–211. [DOI] [PubMed] [Google Scholar]
- 8. French MT, Mundt MP, Fleming M, Zavala SK. The cost of medical care for patients with diabetes, hypertension and both conditions: does alcohol use play a role? J Intern Med. 2005;45–54. [DOI] [PubMed] [Google Scholar]
- 9. Saneei P, Salehi‐Abargouei A, Esmaillzadeh A, Azadbakht L. Influence of Dietary Approaches to Stop Hypertension (DASH) diet on blood pressure: a systematic review and meta‐analysis on randomized controlled trials. Nutr Metab Cardiovasc Dis. 2014;1253–1261. [DOI] [PubMed] [Google Scholar]
- 10. He FJ, Li J, Macgregor GA. Effect of longer term modest salt reduction on blood pressure: cochrane systematic review and meta‐analysis of randomised trials. BMJ. 2013;f1325. [DOI] [PubMed] [Google Scholar]
- 11. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;493–503. [DOI] [PubMed] [Google Scholar]
- 12. Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2018;e484–e594. [DOI] [PubMed] [Google Scholar]
- 13. Leung AA, Daskalopoulou SS, Dasgupta K, McBrien K, Butalia S, Zarnke KB, Nerenberg K, Harris KC, Nakhla M, Cloutier L, et al. Hypertension Canada's 2017 guidelines for diagnosis, risk assessment, prevention, and treatment of hypertension in adults. Can J Cardiol. 2017;557–576. [DOI] [PubMed] [Google Scholar]
- 14. Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur Heart J. 2018;3021–3104. [DOI] [PubMed] [Google Scholar]
- 15. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta‐analysis. Ann Intern Med. 2013;130–137. [DOI] [PubMed] [Google Scholar]
- 16. Mills EJ, Ioannidis JP, Thorlund K, Schunemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA. 2012;1246–1253. [DOI] [PubMed] [Google Scholar]
- 17. Riley RD, Jackson D, Salanti G, Burke DL, Price M, Kirkham J, White IR. Multivariate and network meta‐analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ. 2017;j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta‐analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;777–784. [DOI] [PubMed] [Google Scholar]
- 19. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al. The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;2560–2572. [DOI] [PubMed] [Google Scholar]
- 20. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 22. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;1–30. [DOI] [PubMed] [Google Scholar]
- 23. Madden LV, Piepho HP, Paul PA. Statistical Models And Methods For Network Meta‐Analysis. Phytopathology. 2016;792–806. [DOI] [PubMed] [Google Scholar]
- 24. Neupane B, Richer D, Bonner AJ, Kibret T, Beyene J. Network meta‐analysis using R: a review of currently available automated packages. PLoS One. 2014;9:e115065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;434–455. [Google Scholar]
- 26. van Valkenhoef G, Dias S, Ades A, Welton N. Automated generation of node‐splitting models for assessment of inconsistency in network meta‐analysis. Res Synth Methods. 2016;80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wei J, Zeng C, Lei G‐H. Heterogeneity, consistency and model fit should be assessed in Bayesian network meta‐analysis. Ann Rheum Dis. 2016;9:e5. [DOI] [PubMed] [Google Scholar]
- 28. Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. J Clin Epidemiol. 2011;163–171. [DOI] [PubMed] [Google Scholar]
- 29. Chalmers J, MacMahon S, Mancia G, Whitworth J, Beilin L, Hansson L, Neal B, Rodgers A, Ni Mhurchu C, Clark T. 1999 World Health Organization‐International Society of Hypertension Guidelines for the management of hypertension. Guidelines sub‐committee of the World Health Organization. Clin Exp Hypertens. 1999;1009–1060. [DOI] [PubMed] [Google Scholar]
- 30. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One. 2013;9:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, Kessels AG, Guyatt GH. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ. 2014;g5630. [DOI] [PubMed] [Google Scholar]
- 34. Svetkey LP, Simons‐Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, Ard J, Kennedy BM. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med. 1999;285–293. [DOI] [PubMed] [Google Scholar]
- 35. Zou P, Dennis CL, Lee R, Parry M. Dietary approach to stop hypertension with sodium reduction for Chinese Canadians (DASHNa‐CC): a pilot randomized controlled trial. J Nutr Health Aging. 2017;1225–1232. [DOI] [PubMed] [Google Scholar]
- 36. Sarkkinen ES, Kastarinen MJ, Niskanen TH, Karjalainen PH, Venalainen TM, Udani JK, Niskanen LK. Feasibility and antihypertensive effect of replacing regular salt with mineral salt ‐rich in magnesium and potassium‐ in subjects with mildly elevated blood pressure. Nutr J. 2011;88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhao X, Yin X, Li X, Yan LL, Lam CT, Li S, He F, Xie W, Sang B, Luobu G, et al. Using a low‐sodium, high‐potassium salt substitute to reduce blood pressure among Tibetans with high blood pressure: a patient‐blinded randomized controlled trial. PLoS One. 2014;9:e110131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cappuccio FP, Markandu ND, Carney C, Sagnella GA, MacGregor GA. Double‐blind randomised trial of modest salt restriction in older people. Lancet. 1997;850–854. [DOI] [PubMed] [Google Scholar]
- 39. Swift PA, Markandu ND, Sagnella GA, He FJ, MacGregor GA. Modest salt reduction reduces blood pressure and urine protein excretion in black hypertensives: a randomized control trial. Hypertension. 2005;308–312. [DOI] [PubMed] [Google Scholar]
- 40. Molmen‐Hansen HE, Stolen T, Tjonna AE, Aamot IL, Ekeberg IS, Tyldum GA, Wisloff U, Ingul CB, Stoylen A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur J Prev Cardiol. 2012;151–160. [DOI] [PubMed] [Google Scholar]
- 41. Rogers MW, Probst MM, Gruber JJ, Berger R, Boone JB Jr. Differential effects of exercise training intensity on blood pressure and cardiovascular responses to stress in borderline hypertensive humans. J Hypertens. 1996;1369–1375. [DOI] [PubMed] [Google Scholar]
- 42. Badrov MB, Horton S, Millar PJ, McGowan CL. Cardiovascular stress reactivity tasks successfully predict the hypotensive response of isometric handgrip training in hypertensives. Psychophysiology. 2013;407–414. [DOI] [PubMed] [Google Scholar]
- 43. Taylor AC, McCartney N, Kamath MV, Wiley RL. Isometric training lowers resting blood pressure and modulates autonomic control. Med Sci Sports Exerc. 2003;251–256. [DOI] [PubMed] [Google Scholar]
- 44. Blumenthal JA, Siegel WC, Appelbaum M. Failure of exercise to reduce blood pressure in patients with mild hypertension. Results of a randomized controlled trial. JAMA. 1991;2098–2104. [PubMed] [Google Scholar]
- 45. Cononie CC, Graves JE, Pollock ML, Phillips MI, Sumners C, Hagberg JM. Effect of exercise training on blood pressure in 70‐ to 79‐yr-old men and women. Med Sci Sports Exerc. 1991;505–511. [PubMed] [Google Scholar]
- 46. Tsai JC, Wang WH, Chan P, Lin LJ, Wang CH, Tomlinson B, Hsieh MH, Yang HY, Liu JC. The beneficial effects of Tai Chi Chuan on blood pressure and lipid profile and anxiety status in a randomized controlled trial. J Altern Complement Med. 2003;747–754. [DOI] [PubMed] [Google Scholar]
- 47. Park JE, Hong S, Lee M, Park T, Kang K, Jung H, Shin KM, Liu Y, Shin M, Choi SM. Randomized, controlled trial of qigong for treatment of prehypertension and mild essential hypertension. Altern Ther Health Med. 2014;21–30. [PubMed] [Google Scholar]
- 48. Elliot WJ, Izzo JLJ, White WB, Rosing DR, Snyder CS, Alter A, Gavish B, Black HR. Graded blood pressure reduction in hypertensive outpatients associated with use of a device to assist with slow breathing. J Clin Hypertens (Greenwich). 2004;553–559; quiz 560–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Grossman E, Grossman A, Schein MH, Zimlichman R, Gavish B. Breathing‐control lowers blood pressure. J Hum Hypertens. 2001;263–269. [DOI] [PubMed] [Google Scholar]
- 50. Castillo‐Richmond A, Schneider RH, Alexander CN, Cook R, Myers H, Nidich S, Haney C, Rainforth M, Salerno J. Effects of stress reduction on carotid atherosclerosis in hypertensive African Americans. Stroke. 2000;568–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Blom K, Baker B, How M, Dai M, Irvine J, Abbey S, Abramson BL, Myers MG, Kiss A, Perkins NJ, et al. Hypertension analysis of stress reduction using mindfulness meditation and yoga: results from the HARMONY randomized controlled trial. Am J Hypertens. 2014;122–129. [DOI] [PubMed] [Google Scholar]
- 52. Hughes JW, Fresco DM, Myerscough R, van Dulmen MH, Carlson LE, Josephson R. Randomized controlled trial of mindfulness‐based stress reduction for prehypertension. Psychosom Med. 2013;721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schneider RH, Staggers F, Alxander CN, Sheppard W, Rainforth M, Kondwani K, Smith S, King CG. A randomised controlled trial of stress reduction for hypertension in older African Americans. Hypertension. 1995;820–827. [DOI] [PubMed] [Google Scholar]
- 54. Schneider RH, Alexander CN, Staggers F, Orme‐Johnson DW, Rainforth M, Salerno JW, Sheppard W, Castillo‐Richmond A, Barnes VA, Nidich SI. A randomized controlled trial of stress reduction in African Americans treated for hypertension for over one year. Am J Hypertens. 2005;88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wolff M, Sundquist K, Larsson Lonn S, Midlov P. Impact of yoga on blood pressure and quality of life in patients with hypertension—a controlled trial in primary care, matched for systolic blood pressure. BMC Cardiovasc Disord. 2013;111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gordon NF, Scott CB, Levine BD. Comparison of single versus multiple lifestyle interventions: are the antihypertensive effects of exercise training and diet‐induced weight loss additive? Am J Cardiol. 1997;763–767. [DOI] [PubMed] [Google Scholar]
- 57. Cushman WC, Cutler JA, Hanna E, Bingham SF, Follmann D, Harford T, Dubbert P, Allender PS, Dufour M, Collins JF, et al. Prevention and Treatment of Hypertension Study (PATHS): effects of an alcohol treatment program on blood pressure. Arch Intern Med. 1998;1197–1207. [DOI] [PubMed] [Google Scholar]
- 58. Lang T, Nicaud V, Darne B, Rueff B. Improving hypertension control among excessive alcohol drinkers: a randomised controlled trial in France. The WALPA Group. J Epidemiol Community Health. 1995;610–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Edwards KM, Wilson KL, Sadja J, Ziegler MG, Mills PJ. Effects on blood pressure and autonomic nervous system function of a 12‐week exercise or exercise plus DASH‐diet intervention in individuals with elevated blood pressure. Acta Physiol (Oxf). 2011;343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stewart KJ, Bacher AC, Turner KL, Fleg JL, Hees PS, Shapiro EP, Tayback M, Ouyang P. Effect of exercise on blood pressure in older persons: a randomized controlled trial. Arch Intern Med. 2005;756–762. [DOI] [PubMed] [Google Scholar]
- 61. The Trials of Hypertension Prevention Collaborative Research Group . Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high‐normal blood pressure. The Trials of Hypertension Prevention, phase II. Arch Intern Med. 1997;657–667. [PubMed] [Google Scholar]
- 62. Elmer PJ, Obarzanek E, Vollmer WM, Simons‐Morton D, Stevens VJ, Young DR, Lin PH, Champagne C, Harsha DW, Svetkey LP, et al. Effects of comprehensive lifestyle modification on diet, weight, physical fitness, and blood pressure control: 18‐month results of a randomized trial. Ann Intern Med. 2006;485–495. [DOI] [PubMed] [Google Scholar]
- 63. Burke V, Beilin LJ, Cutt HE, Mansour J, Wilson A, Mori TA. Effects of a lifestyle programme on ambulatory blood pressure and drug dosage in treated hypertensive patients: a randomized controlled trial. J Hypertens. 2005;1241–1249. [DOI] [PubMed] [Google Scholar]
- 64. Challa HJ, Ameer MA, Uppaluri KR. DASH Diet (Dietary Approaches to Stop Hypertension) In StatPearls. Treasure Island, FL: StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 65. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta‐analysis. Br J Nutr. 2015;1–15. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization . World Health Organization. Global action plan for the prevention and control of noncommunicable diseases 2013–2020. WHO IRIS; 2013. Available at: https://apps.who.int/iris/handle/10665/94384. Accessed November 14, 2013. [Google Scholar]
- 67. Parijs J, Joossens JV, Van der Linden L, Verstreken G, Amery AK. Moderate sodium restriction and diuretics in the treatment of hypertension. Am Heart J. 1973;22–34. [DOI] [PubMed] [Google Scholar]
- 68. Tsunematsu N. The actual amount of reductions in salt intake among those on a restricted salt diet: from the INTERMAP Japan. J Jpn Soc Prev Cardiovasc Dis. 2004;149–155. [Google Scholar]
- 69. Inder JD, Carlson DJ, Dieberg G, McFarlane JR, Hess NC, Smart NA. Isometric exercise training for blood pressure management: a systematic review and meta‐analysis to optimize benefit. Hypertens Res. 2016;88–94. [DOI] [PubMed] [Google Scholar]
- 70. Rossi AM, Moullec G, Lavoie KL, Gour‐Provencal G, Bacon SL. The evolution of a Canadian Hypertension Education Program recommendation: the impact of resistance training on resting blood pressure in adults as an example. Can J Cardiol. 2013;622–627. [DOI] [PubMed] [Google Scholar]
- 71. Marquez‐Celedonio FG, Texon‐Fernandez O, Chavez‐Negrete A, Hernandez‐Lopez S, Marin‐Rendon S, Berlín‐Lascurain S. [Clinical effect of lifestyle modification on cardiovascular risk in prehypertensives: PREHIPER I study]. Rev Esp Cardiol. 2009;86–90. [PubMed] [Google Scholar]
- 72. Mattila R, Malmivaara A, Kastarinen M, Kivela SL, Nissinen A. Effectiveness of multidisciplinary lifestyle intervention for hypertension: a randomised controlled trial. J Hum Hypertens. 2003;199–205. [DOI] [PubMed] [Google Scholar]
- 73. Pimenta E, Gaddam KK, Oparil S, Aban I, Husain S, Dell'Italia LJ, Calhoun DA. Effects of dietary sodium reduction on blood pressure in subjects with resistant hypertension: results from a randomized trial. Hypertension. 2009;475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Cohen JD. Overview of physiology, vascular biology, and mechanisms of hypertension. J Manag Care Pharm. 2007;S6–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. de Jongh RT, Serne EH, Ijzerman RG, de Vries G, Stehouwer CD. Free fatty acid levels modulate microvascular function: relevance for obesity‐associated insulin resistance, hypertension, and microangiopathy. Diabetes. 2004;2873–2882. [DOI] [PubMed] [Google Scholar]
- 76. Altena MR, Kleefstra N, Logtenberg SJ, Groenier KH, Houweling ST, Bilo HJ. Effect of device‐guided breathing exercises on blood pressure in patients with hypertension: a randomized controlled trial. Blood Press. 2009;273–279. [DOI] [PubMed] [Google Scholar]
- 77. Anderson DE, McNeely JD, Windham BG. Regular slow‐breathing exercise effects on blood pressure and breathing patterns at rest. J Hum Hypertens. 2010;807–813. [DOI] [PubMed] [Google Scholar]
- 78. Anderssen S, Holme I, Urdal P, Hjermann I. Diet and exercise intervention have favourable effects on blood pressure in mild hypertensives: the Oslo Diet and Exercise Study (ODES). Blood Press. 1995;343–349. [DOI] [PubMed] [Google Scholar]
- 79. Australian National Health & Medical Research Council Dietary Salt Study Management Committee. Effects of replacing sodium intake in subjects on a low sodium diet: a crossover study. Clin Exp Hypertens A. 1989;1011–1024. [DOI] [PubMed] [Google Scholar]
- 80. Australian National Health and Medical Research Council Dietary Salt Study Management Committee. Fall in blood pressure with modest reduction in dietary salt intake in mild hypertension. Lancet. 1989;399–402. [PubMed] [Google Scholar]
- 81. Appel LJ, Espeland MA, Easter L, Wilson AC, Folmar S, Lacy CR. Effects of reduced sodium intake on hypertension control in older individuals: results from the Trial of Nonpharmacologic Interventions in the Elderly (TONE). Arch Intern Med. 2001;685–693. [DOI] [PubMed] [Google Scholar]
- 82. Baros AM, Wright TM, Latham PK, Miller PM, Anton RF. Alcohol consumption, %CDT, GGT and blood pressure change during alcohol treatment. Alcohol Alcohol. 2008;192–197. [DOI] [PubMed] [Google Scholar]
- 83. Barros CL, Sousa AL, Chinem BM, Rodrigues RB, Jardim TS, Carneiro SB, Souza WK, Jardim PC. Impact of light salt substitution for regular salt on blood pressure of hypertensive patients. Arq Bras Cardiol. 2015;128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Beard TC, Cooke HM, Gray WR, Barge R. Randomised controlled trial of a no‐added‐sodium diet for mild hypertension. Lancet. 1982;455–458. [DOI] [PubMed] [Google Scholar]
- 85. Benetos A, Xiao YY, Cuche JL, Hannaert P, Safar M. Arterial effects of salt restriction in hypertensive patients. A 9‐week, randomized, double‐blind, crossover study. J Hypertens. 1992;355–360. [DOI] [PubMed] [Google Scholar]
- 86. Blumenthal JA, Sherwood A, Gullette EC, Babyak M, Waugh R, Georgiades A, Craighead LW, Tweedy D, Feinglos M, Appelbaum M, et al. Exercise and weight loss reduce blood pressure in men and women with mild hypertension: effects on cardiovascular, metabolic, and hemodynamic functioning. Arch Intern Med. 2000;1947–1958. [DOI] [PubMed] [Google Scholar]
- 87. Chen ST, Maruthur NM, Appel LJ. The effect of dietary patterns on estimated coronary heart disease risk: results from the Dietary Approaches to Stop Hypertension (DASH) trial. Circ Cardiovasc Qual Outcomes. 2010;484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Cohen DL, Bloedon LT, Rothman RL, Farrar JT, Galantino ML, Volger S, Mayor C, Szapary PO, Townsend RR. Iyengar yoga versus enhanced usual care on blood pressure in patients with prehypertension to stage I hypertension: a randomized controlled trial. Evid Based Complement Alternat Med. 2011;546428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Collier SR, Kanaley JA, Carhart R Jr, Frechette V, Tobin MM, Hall AK, Luckenbaugh AN, Fernhall B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre‐ and stage‐1 hypertensives. J Hum Hypertens. 2008;678–686. [DOI] [PubMed] [Google Scholar]
- 90. Conlin PR, Erlinger TP, Bohannon A, Miller ER III, Appel LJ, Svetkey LP, Moore TJ. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens. 2003;337–342. [DOI] [PubMed] [Google Scholar]
- 91. Cooper AR, Moore LA, McKenna J, Riddoch CJ. What is the magnitude of blood pressure response to a programme of moderate intensity exercise? Randomised controlled trial among sedentary adults with unmedicated hypertension. Br J Gen Pract. 2000;958–962. [PMC free article] [PubMed] [Google Scholar]
- 92. Cottier C, Shapiro K, Julius S. Treatment of mild hypertension with progressive muscle relaxation. Predictive value of indexes of sympathetic tone. Arch Intern Med. 1984;1954–1958. [PubMed] [Google Scholar]
- 93. Croft PR, Brigg D, Smith S, Harrison CB, Branthwaite A, Collins MF. How useful is weight reduction in the management of hypertension? J R Coll Gen Pract. 1986;445–448. [PMC free article] [PubMed] [Google Scholar]
- 94. Erlinger TP, Conlin PR, Macko RF, Bohannon AD, Miller ER III, Moore TJ, Svetkey LP, Appel LJ. The impact of angiotensin II receptor blockade and the DASH diet on markers of endogenous fibrinolysis. J Hum Hypertens. 2002;391–397. [DOI] [PubMed] [Google Scholar]
- 95. Erwteman TM, Nagelkerke N, Lubsen J, Koster M, Dunning AJ. Beta blockade, diuretics, and salt restriction for the management of mild hypertension: a randomised double blind trial. Br Med J (Clin Res Ed). 1984;406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Farah BQ, Rodrigues SLC, Silva GO, Pedrosa RP, Correia MA, Barros MVG, Deminice R, Marinello PC, Smart NA, Vianna LC, et al. Supervised, but not home‐based, isometric training improves brachial and central blood pressure in medicated hypertensive patients: a randomized controlled trial. Front Physiol. 2018;961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Farahani AV, Mansournia MA, Asheri H, Fotouhi A, Yunesian M, Jamali M, Ziaee V. The effects of a 10‐week water aerobic exercise on the resting blood pressure in patients with essential hypertension. Asian J Sports Med. 2010;159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Farinatti P, Monteiro WD, Oliveira RB. Long term home‐based exercise is effective to reduce blood pressure in low income Brazilian hypertensive patients: a controlled trial. High Blood Press Cardiovasc Prev. 2016;395–404. [DOI] [PubMed] [Google Scholar]
- 99. Ferreira JB, Plentz RD, Stein C, Casali KR, Arena R, Lago PD. Inspiratory muscle training reduces blood pressure and sympathetic activity in hypertensive patients: a randomized controlled trial. Int J Cardiol. 2013;61–67. [DOI] [PubMed] [Google Scholar]
- 100. Fotherby MD, Potter JF. Effects of moderate sodium restriction on clinic and twenty‐four-hour ambulatory blood pressure in elderly hypertensive subjects. J Hypertens. 1993;657–663. [DOI] [PubMed] [Google Scholar]
- 101. Gates PE, Tanaka H, Hiatt WR, Seals DR. Dietary sodium restriction rapidly improves large elastic artery compliance in older adults with systolic hypertension. Hypertension. 2004;35–41. [DOI] [PubMed] [Google Scholar]
- 102. Geleijnse JM, Witteman JC, Bak AA, den Breeijen JH, Grobbee DE. Reduction in blood pressure with a low sodium, high potassium, high magnesium salt in older subjects with mild to moderate hypertension. BMJ. 1994;436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Grobbee DE, Hofman A, Roelandt JT, Boomsma F, Schalekamp MA, Valkenburg HA. Sodium restriction and potassium supplementation in young people with mildly elevated blood pressure. J Hypertens. 1987;115–119. [DOI] [PubMed] [Google Scholar]
- 104. Guimaraes GV, Ciolac EG, Carvalho VO, D'Avila VM, Bortolotto LA, Bocchi EA. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens Res. 2010;627–632. [DOI] [PubMed] [Google Scholar]
- 105. Hagins M, Rundle A, Consedine NS, Khalsa SB. A randomized controlled trial comparing the effects of yoga with an active control on ambulatory blood pressure in individuals with prehypertension and stage 1 hypertension. J Clin Hypertens (Greenwich). 2014;54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Haynes RB, Harper AC, Costley SR, Johnston M, Logan AG, Flanagan PT, Sackett DL. Failure of weight reduction to reduce mildly elevated blood pressure: a randomized trial. J Hypertens. 1984;535–539. [DOI] [PubMed] [Google Scholar]
- 107. He FJ, Marciniak M, Visagie E, Markandu ND, Anand V, Dalton RN, MacGregor GA. Effect of modest salt reduction on blood pressure, urinary albumin, and pulse wave velocity in white, black, and Asian mild hypertensives. Hypertension. 2009;482–488. [DOI] [PubMed] [Google Scholar]
- 108. Higashi Y, Sasaki S, Sasaki N, Nakagawa K, Ueda T, Yoshimizu A, Kurisu S, Matsuura H, Kajiyama G, Oshima T. Daily aerobic exercise improves reactive hyperemia in patients with essential hypertension. Hypertension. 1999;591–597. [DOI] [PubMed] [Google Scholar]
- 109. Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima T. Regular aerobic exercise augments endothelium‐dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium‐derived nitric oxide. Circulation. 1999;1194–1202. [DOI] [PubMed] [Google Scholar]
- 110. Hikmat F, Appel LJ. Effects of the DASH diet on blood pressure in patients with and without metabolic syndrome: results from the DASH trial. J Hum Hypertens. 2014;170–175. [DOI] [PubMed] [Google Scholar]
- 111. Izadi MR, Ghardashi Afousi A, Asvadi Fard M, Babaee Bigi MA. High‐intensity interval training lowers blood pressure and improves apelin and NOx plasma levels in older treated hypertensive individuals. J Physiol Biochem. 2018;47–55. [DOI] [PubMed] [Google Scholar]
- 112. Jablonski KL, Fedorova OV, Racine ML, Geolfos CJ, Gates PE, Chonchol M, Fleenor BS, Lakatta EG, Bagrov AY, Seals DR. Dietary sodium restriction and association with urinary marinobufagenin, blood pressure, and aortic stiffness. Clin J Am Soc Nephrol. 2013;1952–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Jalkanen L. The effect of a weight reduction program on cardiovascular risk factors among overweight hypertensives in primary health care. Scand J Soc Med. 1991;66–71. [DOI] [PubMed] [Google Scholar]
- 114. Jones CU, Sangthong B, Pachirat O. An inspiratory load enhances the antihypertensive effects of home‐based training with slow deep breathing: a randomised trial. J Physiother. 2010;179–186. [DOI] [PubMed] [Google Scholar]
- 115. MacGregor GA, Markandu ND, Best FE, Elder DM, Cam JM, Sagnella GA, Squires M. Double‐blind randomised crossover trial of moderate sodium restriction in essential hypertension. Lancet. 1982;351–355. [DOI] [PubMed] [Google Scholar]
- 116. MacGregor GA, Markandu ND, Sagnella GA, Singer DR, Cappuccio FP. Double‐blind study of three sodium intakes and long‐term effects of sodium restriction in essential hypertension. Lancet. 1989;1244–1247. [DOI] [PubMed] [Google Scholar]
- 117. MacMahon SW, Macdonald GJ, Bernstein L, Andrews G, Blacket RB. Comparison of weight reduction with metoprolol in treatment of hypertension in young overweight patients. Lancet. 1985;1233–1236. [DOI] [PubMed] [Google Scholar]
- 118. Makela P, Vahlberg T, Kantola I, Vesalainen R, Jula A. The effects of a 6‐month sodium restriction on cardiac autonomic function in patients with mild to moderate essential hypertension. Am J Hypertens. 2008;1183–1187. [DOI] [PubMed] [Google Scholar]
- 119. Malloy‐McFall J, Barkley JE, Gordon KL, Burzminski N, Glickman EL. Effect of the DASH Diet on pre‐ and stage 1 hypertensive individuals in a free‐living environment. Nutr Metab Insights. 2010;15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Maruf FA, Akinpelu AO, Salako BL, Akinyemi JO. Effects of aerobic dance training on blood pressure in individuals with uncontrolled hypertension on two antihypertensive drugs: a randomized clinical trial. J Am Soc Hypertens. 2016;336–345. [DOI] [PubMed] [Google Scholar]
- 121. McCarron DA, Weder AB, Egan BM, Krishna GG, Morris CD, Cohen M, Oparil S. Blood pressure and metabolic responses to moderate sodium restriction in isradipine‐treated hypertensive patients. Am J Hypertens. 1997;68–76. [DOI] [PubMed] [Google Scholar]
- 122. Meland E, Laerum E, Aakvaag A, Ulvik RJ, Hostmark AT. Salt restriction: effects on lipids and insulin production in hypertensive patients. Scand J Clin Lab Invest. 1997;501–505. [DOI] [PubMed] [Google Scholar]
- 123. Meles E, Giannattasio C, Failla M, Gentile G, Capra A, Mancia G. Nonpharmacologic treatment of hypertension by respiratory exercise in the home setting. Am J Hypertens. 2004;370–374. [DOI] [PubMed] [Google Scholar]
- 124. Miller ER III, Erlinger TP, Young DR, Jehn M, Charleston J, Rhodes D, Wasan SK, Appel LJ. Results of the Diet, Exercise, and Weight Loss Intervention Trial (DEW‐IT). Hypertension. 2002;612–618. [DOI] [PubMed] [Google Scholar]
- 125. Modesti PA, Ferrari A, Bazzini C, Costanzo G, Simonetti I, Taddei S, Biggeri A, Parati G, Gensini GF, Sirigatti S. Psychological predictors of the antihypertensive effects of music‐guided slow breathing. J Hypertens. 2010;1097–1103. [DOI] [PubMed] [Google Scholar]
- 126. Moore TJ, Vollmer WM, Appel LJ, Sacks FM, Svetkey LP, Vogt TM, Conlin PR, Simons‐Morton DG, Carter‐Edwards L, Harsha DW. Effect of dietary patterns on ambulatory blood pressure: results from the Dietary Approaches to Stop Hypertension (DASH) Trial. DASH Collaborative Research Group. Hypertension. 1999;472–477. [DOI] [PubMed] [Google Scholar]
- 127. Nakamura M, Aoki N, Yamada T, Kubo N. Feasibility and effect on blood pressure of 6‐week trial of low sodium soy sauce and miso (fermented soybean paste). Circ J. 2003;530–534. [DOI] [PubMed] [Google Scholar]
- 128. Nelson L, Jennings GL, Esler MD, Korner PI. Effect of changing levels of physical activity on blood‐pressure and haemodynamics in essential hypertension. Lancet. 1986;473–476. [DOI] [PubMed] [Google Scholar]
- 129. Nualnim N, Parkhurst K, Dhindsa M, Tarumi T, Vavrek J, Tanaka H. Effects of swimming training on blood pressure and vascular function in adults >50 years of age. Am J Cardiol. 2012;1005–1010. [DOI] [PubMed] [Google Scholar]
- 130. Ohkubo T, Hozawa A, Nagatomi R, Fujita K, Sauvaget C, Watanabe Y, Anzai Y, Tamagawa A, Tsuji I, Imai Y, et al. Effects of exercise training on home blood pressure values in older adults: a randomized controlled trial. J Hypertens. 2001;1045–1052. [DOI] [PubMed] [Google Scholar]
- 131. Ohta Y, Kawano Y, Minami J, Iwashima Y, Hayashi S, Yoshihara F, Nakamura S. Effects of daily walking on office, home and 24‐h blood pressure in hypertensive patients. Clin Exp Hypertens. 2015;433–437. [DOI] [PubMed] [Google Scholar]
- 132. Okumiya K, Matsubayashi K, Wada T, Kimura S, Doi Y, Ozawa T. Effects of exercise on neurobehavioral function in community‐dwelling older people more than 75 years of age. J Am Geriatr Soc. 1996;569–572. [DOI] [PubMed] [Google Scholar]
- 133. Patel C, Marmot M. Can general practitioners use training in relaxation and management of stress to reduce mild hypertension? Br Med J (Clin Res Ed). 1988;21–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Pinjuh Markota N, Rumboldt M, Rumboldt Z. Emphasized warning reduces salt intake: a randomized controlled trial. J Am Soc Hypertens. 2015;214–220. [DOI] [PubMed] [Google Scholar]
- 135. Punita P, Trakroo M, Palamalai S, Subramanian S, Bhavanani A, Madhavan C. Randomized controlled trial of 12‐week yoga therapy as lifestyle intervention in patients of essential hypertension and cardiac autonomic function tests. Natl J Physiol Pharm Pharmacol. 2016;19. [Google Scholar]
- 136. Puska P, Iacono JM, Nissinen A, Korhonen HJ, Vartianinen E, Pietinen P, Dougherty R, Leino U, Mutanen M, Moisio S, et al. Controlled, randomised trial of the effect of dietary fat on blood pressure. Lancet. 1983;1–5. [DOI] [PubMed] [Google Scholar]
- 137. Ramos RM, Coelho‐Júnior HJ. Moderate aerobic training decreases blood pressure but no other cardiovascular risk factors in hypertensive overweight/obese elderly patients. Gerontol Geriatr Med. 2018;2333721418808645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Richards AM, Nicholls MG, Espiner EA, Ikram H, Maslowski AH, Hamilton EJ, Wells JE. Blood‐pressure response to moderate sodium restriction and to potassium supplementation in mild essential hypertension. Lancet. 1984;757–761. [DOI] [PubMed] [Google Scholar]
- 139. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons‐Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;3–10. [DOI] [PubMed] [Google Scholar]
- 140. Schein MH, Gavish B, Herz M, Rosner‐Kahana D, Naveh P, Knishkowy B, Zlotnikov E, Ben‐Zvi N, Melmed RN. Treating hypertension with a device that slows and regularises breathing: a randomised, double‐blind controlled study. J Hum Hypertens. 2001;271–278. [DOI] [PubMed] [Google Scholar]
- 141. Seals DR, Reiling MJ. Effect of regular exercise on 24‐hour arterial pressure in older hypertensive humans. Hypertension. 1991;583–592. [DOI] [PubMed] [Google Scholar]
- 142. Shou XL, Wang L, Jin XQ, Zhu LY, Ren AH, Wang QN. Effect of T'ai Chi exercise on hypertension in young and middle‐aged in‐service staff. J Altern Complement Med. 2019;73–78. [DOI] [PubMed] [Google Scholar]
- 143. Silman AJ, Locke C, Mitchell P, Humpherson P. Evaluation of the effectiveness of a low sodium diet in the treatment of mild to moderate hypertension. Lancet. 1983;1179–1182. [DOI] [PubMed] [Google Scholar]
- 144. Sohn AJ, Hasnain M, Sinacore JM. Impact of exercise (walking) on blood pressure levels in African American adults with newly diagnosed hypertension. Ethn Dis. 2007;503–507. [PubMed] [Google Scholar]
- 145. Steffen PR, Sherwood A, Gullette EC, Georgiades A, Hinderliter A, Blumenthal JA. Effects of exercise and weight loss on blood pressure during daily life. Med Sci Sports Exerc. 2001;1635–1640. [DOI] [PubMed] [Google Scholar]
- 146. Stevens VJ, Corrigan SA, Obarzanek E, Bernauer E, Cook NR, Hebert P, Mattfeldt‐Beman M, Oberman A, Sugars C, Dalcin AT, et al. Weight loss intervention in phase 1 of the Trials of Hypertension Prevention. The TOHP Collaborative Research Group. Arch Intern Med. 1993;849–858. [PubMed] [Google Scholar]
- 147. Stiller‐Moldovan C, Kenno K, McGowan CL. Effects of isometric handgrip training on blood pressure (resting and 24 h ambulatory) and heart rate variability in medicated hypertensive patients. Blood Press Monit. 2012;55–61. [DOI] [PubMed] [Google Scholar]
- 148. Subramanian H, Soudarssanane MB, Jayalakshmy R, Thiruselvakumar D, Navasakthi D, Sahai A, Saptharishi L. Non‐pharmacological interventions in hypertension: a community‐based cross‐over randomized controlled trial. Indian J Community Med. 2011;191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Sujatha T, Judie A. Effectiveness of a 12‐week yoga program on physiopsychological parameters in patients with hypertension. Int J Pharm Clin Res. 2014;329–335. [Google Scholar]
- 150. Suppa G, Pollavini G, Alberti D, Savonitto S. Effects of a low‐sodium high‐potassium salt in hypertensive patients treated with metoprolol: a multicentre study. J Hypertens. 1988;787–790. [PubMed] [Google Scholar]
- 151. Tanaka H, Bassett DR Jr, Howley ET, Thompson DL, Ashraf M, Rawson FL. Swimming training lowers the resting blood pressure in individuals with hypertension. J Hypertens. 1997;651–657. [DOI] [PubMed] [Google Scholar]
- 152. The Trials of Hypertension Prevention Collaborative Research Group . The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels. Results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;1213–1220. [DOI] [PubMed] [Google Scholar]
- 153. Thiyagarajan R, Pal P, Pal GK, Subramanian SK, Trakroo M, Bobby Z, Das AK. Additional benefit of yoga to standard lifestyle modification on blood pressure in prehypertensive subjects: a randomized controlled study. Hypertens Res. 2015;48–55. [DOI] [PubMed] [Google Scholar]
- 154. Tsai JC, Chang WY, Kao CC, Lu MS, Chen YJ, Chan P. Beneficial effect on blood pressure and lipid profile by programmed exercise training in Taiwanese patients with mild hypertension. Clin Exp Hypertens. 2002;315–324. [DOI] [PubMed] [Google Scholar]
- 155. Tsai JC, Yang HY, Wang WH, Hsieh MH, Chen PT, Kao CC, Kao PF, Wang CH, Chan P. The beneficial effect of regular endurance exercise training on blood pressure and quality of life in patients with hypertension. Clin Exp Hypertens. 2004;255–265. [DOI] [PubMed] [Google Scholar]
- 156. Vollmer WM, Sacks FM, Ard J, Appel LJ, Bray GA, Simons‐Morton DG, Conlin PR, Svetkey LP, Erlinger TP, Moore TJ, et al. Effects of diet and sodium intake on blood pressure: subgroup analysis of the DASH‐sodium trial. Ann Intern Med. 2001;1019–1028. [DOI] [PubMed] [Google Scholar]
- 157. Watt GC, Edwards C, Hart JT, Hart M, Walton P, Foy CJ. Dietary sodium restriction for mild hypertension in general practice. Br Med J (Clin Res Ed). 1983;432–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, et al. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA. 1998;839–846. [DOI] [PubMed] [Google Scholar]
- 159. Whitt‐Glover MC, Hunter JC, Foy CG, Quandt SA, Vitolins MZ, Leng I, Hornbuckle LM, Sanya KA, Bertoni AG. Translating the Dietary Approaches to Stop Hypertension (DASH) diet for use in underresourced, urban African American communities, 2010. Prev Chronic Dis. 2013;120088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Wilson GB, Wray C, McGovern R, Newbury‐Birch D, McColl E, Crosland A, Speed C, Cassidy P, Tomson D, Haining S, et al. Intervention to reduce excessive alcohol consumption and improve comorbidity outcomes in hypertensive or depressed primary care patients: two parallel cluster randomized feasibility trials. Trials. 2014;235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Young DR, Appel LJ, Jee S, Miller ER III. The effects of aerobic exercise and T'ai Chi on blood pressure in older people: results of a randomized trial. J Am Geriatr Soc. 1999;277–284. [DOI] [PubMed] [Google Scholar]
- 162. Zhou X, Liu JX, Shi R, Yang N, Song DL, Pang W, Li YM. Compound ion salt, a novel low‐sodium salt substitute: from animal study to community‐based population trial. Am J Hypertens. 2009;934–942. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


