Abstract
Background
Night eating has been associated with an elevated risk of obesity, dyslipidemia, and cardiovascular disease. However, there is no longitudinal study on whether habitual night eating, regardless of diet quality and energy intake, is associated with arterial stiffness, a major etiological factor in the development of cardiovascular disease.
Methods and Results
The study included 7771 adult participants without cardiovascular disease, cancer, or diabetes mellitus prior to dietary assessment by a validated food frequency questionnaire in 2014 through 2015. Participants were categorized into 3 groups based on self‐reported night‐eating habits: never or rarely, some days (1–5 times per week), or most days (6+ times per week). Arterial stiffness was assessed by brachial‐ankle pulse wave velocity at baseline and repeatedly during follow‐ups. Mean differences and 95% CIs in the yearly change rate of brachial‐ankle pulse wave velocity across the 3 groups were calculated, adjusting for age, sex, socioeconomic status, total energy intake, diet quality, sleep quality, and other cardiovascular disease risk factors. At baseline, 6625 (85.2%), 610 (7.8%), and 536 (6.9%) participants reported night eating as never or rarely, some days, or most days, respectively. During a mean 3.19 years, we observed a positive association between night‐eating frequency and progression of arterial stiffness (P trend=0.01). The adjusted difference in brachial‐ankle pulse wave velocity change rate between the group that ate at night most days and the group that never or rarely ate at night was 14.1 (95% CI, 0.6–27.5) cm/s per year. This association was only significant in women, but not in men (P interaction=0.03).
Conclusions
In an adult population free of major chronic diseases, habitual night eating was positively associated with the progression of arterial stiffness, a hallmark of arteriosclerosis and biological aging.
Registration
URL: https://www.chictr.org.cn; Unique identifier: ChiCTR‐TNRC‐11001489.
Keywords: arterial stiffness, meal timing, night eating, pulse wave velocity
Subject Categories: Epidemiology
Nonstandard Abbreviations and Acronyms
- baPWV
brachial‐ankle pulse wave velocity
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food frequency questionnaire
Clinical Perspective
What Is New?
The first large‐scale, prospective population study on the association between night eating and preclinical cardiovascular disease.
Habitual night eating was associated with more rapid progression of arterial stiffness in women.
This association was independent of total energy consumption, diet quality, insomnia, menopausal status, and other major risk factors for cardiovascular disease.
What Are the Clinical Implications?
Night eating, independent of other lifestyle behaviors, plays a critical role in the prevention of chronic disease.
Potential underlying biological and physiological mechanisms are warranted in future studies.
Guidelines may consider recommendation for timing of meals in addition to nutrients, foods, and meal patterns.
The association between individual dietary components, as well as overall dietary patterns, and cardiovascular health has been well‐documented. 1 Eating patterns, such as eating late at night, have been associated with higher odds of having cardiovascular risk factors, such as increased visceral adiposity, obesity, and dyslipidemia. 2 In a prospective observational study, men with night‐eating habits had a higher risk of developing coronary heart disease, relative to men who did not eat late at night. 3 The underlying mechanism connecting night eating and coronary heart disease might involve higher energy intake, change in appetite, and disturbed circadian clock. 4 Currently, literature on habitual night eating is sparse, and no prospective population study has examined the association between night eating and preclinical, pathological changes in cardiovascular disease (CVD), such as arterial stiffness.
Arterial stiffness characterizes pathological changes in the artery wall, including an increase in wall thickness and lumen diameter, and a decrease in elasticity and resilience. 5 It is a significant predictor and preclinical sign of CVD, with prognostic values independent of traditional CVD risk factors. 6 Brachial‐ankle pulse wave velocity (baPWV) is a common measure for arterial stiffness, especially in large‐scale cohort studies in the Asian population. 7 It has been validated against carotid‐femoral PWV, the gold standard of arterial stiffness measurement, and has been found to have predictive value for CVD. 8 , 9 , 10 Our aim was to determine, longitudinally, the relationship between habitual night eating and progression of arterial stiffness among ≈8000 adults without major chronic diseases (ie, CVD, diabetes mellitus, and cancer), adjusting for overall diet quality, sleep parameters, and other potential confounders.
Methods
Data used herein and analytic code will be made available from the corresponding author upon reasonable request and approval.
Study Population
The analysis was based on 2 ongoing, population‐based cohorts in Tangshan City, China: the Kailuan study I and the Kailuan study II. A detailed description of this cohort can be found elsewhere. 7 , 11 Briefly, the Kailuan study I spanned from 2006 through 2007 and included 101 510 participants; the Kailuan study II ranged from 2008 to 2010, and included 35 865 participants. Participants from both cohorts were followed biannually. This study was approved by the Ethics Committee of the Kailuan Medical Group, Kailuan Company, Tangshan, China. All participants provided their written informed consent. At baseline and at each follow‐up visit, physical examinations and laboratory analyses were performed, anthropometric measures were assessed, and questionnaires regarding socioeconomic status and lifestyle factors were completed by the participants. Medical records and death certificates were reviewed annually. Starting in 2010, arterial stiffness was measured in a subcohort of participants, as detailed previously. 7 , 12 Dietary data were collected in 2014 using a validated food frequency questionnaire (FFQ), and the data were used for the current analyses. 13 Inclusion criteria in the current study were: (1) participation in the 2014 examination; (2) completion of the dietary assessment; and (3) 2 baPWV measurements ≥3 months apart. Of the 9073 participants who met these criteria, we excluded participants with established CVD or cancer (confirmed by medical record review) or diabetes mellitus (defined as either having fasting blood glucose concentration ≥7.0 mmol/L or using antidiabetic drugs) prior to 2014 14 because participants diagnosed with these conditions were likely counseled to change their dietary habits (n=1302). After applying these exclusion criteria, 7771 participants were included in our analyses (Figure).
Figure 1. Flow chart of this study.
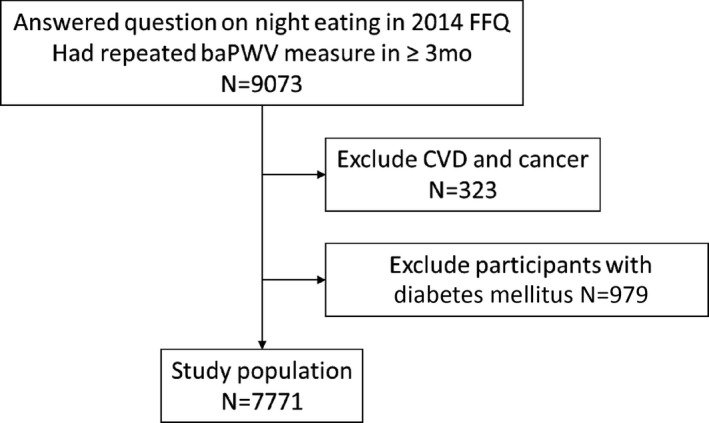
BaPWV indicates brachial‐ankle pulse wave velocity; CVD, cardiovascular disease, including myocardial infarction, stroke, and heart failure; and FFQ, food frequency questionnaire.
Assessment of Night‐Eating Frequency
Night‐eating behavior/frequency was assessed via a multiple‐choice question, “How many days do you usually eat at night in a typical week?” The possible answers were, “never or rarely,” “1 to 2 times weekly,” “3 to 5 times weekly,” and “almost every day.” Night eating was defined as the consumption of food and beverages, excluding water, after 8 pm and before 5 am, in the 2 hours before bedtime, or after going to bed. In the current study, we merged the answers, 1 to 2 times weekly and 3 to 5 times weekly into 1 category “some days” because of the small sample size in each group (n=443 in the 1–2 times weekly group and n=167 in the 3–5 times weekly group). The night‐eating frequency was thus classified on an ordinal scale of “never or rarely,” “some days,” and “most days.”
Assessment of Arterial Stiffness Change
Arterial stiffness was assessed twice at baseline and during follow‐up (mean difference between 2 assessments 3.19 years; interquartile range, 1.73–4.72 years) using baPWV, a simple and validated measurement that has been widely used in Asian cohort studies. 15 A detailed description of the assessment has been described elsewhere. 7 Briefly, participants were instructed to refrain from smoking and alcohol consumption 1 day prior to their visit to the clinic. On the examination day, participants were asked to rest for 5 minutes and then to lie down on the examination coach. Four cuffs were placed, 1 on each side of the brachial area and 1 on each ankle. The pulse transit times from brachial to ankle of both sides were read, and the transit velocities (cm/s) were calculated using a BP‐203RPE III networked arteriosclerosis‐detection device (Omron Healthcare, Pudong, Shanghai, China). Higher velocity indicates greater stiffness of the artery wall. The mean baPWV of 2 repeated readings was recorded. Finally, the mean baPWV of both the right and left sides was used in our analysis.
Assessment of Covariates
Personal and social demographic data, including age, sex, marital status, occupation type, education level, smoking habits, alcohol consumption, physical activity, antihypertensive drug usage, menopause status for women, and sleep quality were collected via self‐reported questionnaire. 13 A validated semiquantitative FFQ was used to obtain habitual dietary intake in the past year. 13 Overall diet quality was assessed via the Dietary Approaches to Stop Hypertension (DASH) diet‐quality score as detailed elsewhere. 16 , 17 Height, weight, and systolic blood pressure were measured on site by trained field workers (ie, physicians and nurses). Sleep parameters, including sleep duration, snoring, and insomnia, were assessed via a separate questionnaire as detailed elsewhere. 18 Physical activity was assessed by the validated Chinese version of the International Physical Activity Questionnaire Short Form. 19 Height and weight were measured by trained nurses, and were used to calculate body mass index (BMI). Fasting blood glucose, low‐density lipoprotein‐cholesterol, and high‐density lipoprotein‐cholesterol were quantified using a Hitachi 747 auto‐analyzer (Hitachi, Tokyo, Japan); a blood sample was collected after an overnight fast (>8 hours). Details of the collection and assessment methods can be found in a previous publication. 13
Statistical Analysis
All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC). Baseline characteristics across the 3 night‐eating groups were compared using a 1‐way ANOVA for continuous variables and a chi‐square test for categorical variables. Data were presented as mean±standard deviation for continuous variables, and percentage for categorical variables.
The outcome in this analysis was the annual change rate of baPWV, as calculated by the difference of 2 values of baPWV (cm/s) divided by the follow‐up duration (years). Greater change rates indicate a faster progression of arterial stiffness. Multiple linear regression models were used to calculate and compare adjusted means of baPWV change rate across night‐eating habits, with the never or rarely group as the reference group. Model 1 adjusted for age and sex; model 2 further adjusted for baseline baPWV, total energy intake, and DASH diet‐quality score; model 3 further adjusted for physical activity (low, moderate, or high), marriage (single or married), occupation (blue‐collar or white‐collar worker), education level (high school and below, or college and above), alcohol consumption (yes/no), smoking status (yes/no), BMI, systolic blood pressure, antihypertensive drug use (yes/no), fasting blood glucose, low‐density lipoprotein‐cholesterol and high‐density lipoprotein‐cholesterol; and model 4 further adjusted for sleep duration, insomnia (yes/no), snoring (yes/no), and breakfast frequency. Mean difference in baPWV annual change rate compared to the reference group with 95% CI was calculated. We further used linear regression to test the trend of arterial stiffness progression with an ordinal increase in night‐eating frequency by including it as a continuous variable. In sensitivity analyses, we further excluded 900 participants who had 2 baPWV measurements in <1 year and 404 participants with insomnia.
We tested multiplicative interaction between night‐eating frequency and age, sex, BMI, hypertension, and DASH diet‐quality score, in relation to baPWV change rate, adjusted for the same set of covariates as our final model (model 4), and conducted relevant subgroup analysis when the interaction term was significant.
Results
The mean age at baseline was 45.7±10.3 years, and 71.4% participants were men. In total, 14.8% of participants reported night eating some days or most days. Compared with those who never or rarely ate at night, participants with night‐eating habits were younger, more likely to have longer sleep duration, higher BMI, and higher low‐density lipoprotein‐cholesterol concentration (Table 1).
Table 1.
Baseline Characteristics Across Night‐Eating Habits
| Night Eating |
Never or Rarely (n=6625) |
Some Days (n=610) |
Most Days (n=536) |
P Value |
|---|---|---|---|---|
| Age, y | 46.1±10.5 | 42.4±8.7 | 44.1±9.3 | <0.001 |
| Male, % | 69.7 | 80.5 | 82.1 | <0.001 |
| College or above, % | 17.6 | 18.4 | 11.6 | 0.03 |
| Manual labor, % | 80.5 | 81.6 | 89.0 | 0.002 |
| Married, % | 95.2 | 91.5 | 94.6 | 0.02 |
| Current smoker, % | 39.7 | 50.3 | 42.6 | <0.001 |
| Current drinker, % | 35.8 | 57.2 | 46.4 | <0.001 |
| Physical exercise, % | 24.1 | 24.3 | 25.6 | 0.009 |
| Antihypertensive drug, % | 10.1 | 6.6 | 7.4 | 0.006 |
| Sleep duration, h | 7.86±1.61 | 7.83±1.68 | 8.05±1.91 | 0.04 |
| Insomnia, % | 4.6 | 11.1 | 6.1 | <0.001 |
| Frequent snoring, % | 11.1 | 14.9 | 11.0 | 0.02 |
| Everyday breakfast, % | 82.9 | 61.5 | 82.8 | <0.001 |
| Total energy intake, kcal/d | 1668±562 | 1724±624 | 1685±559 | 0.08 |
| DASH diet‐quality score | 26.1±4.7 | 26.1±4.5 | 26.1±4.6 | 0.91 |
| baPWV, cm/s | 1408±275 | 1385±241 | 1401±234 | 0.10 |
| Body mass index, kg/m2 | 24.8±3.3 | 24.9±3.3 | 24.4±3.1 | 0.02 |
| Systolic blood pressure, mm Hg | 130±17 | 129±15 | 129±16 | 0.05 |
| Fasting blood glucose, mmol/L | 5.31±0.61 | 5.25±0.63 | 5.30±0.62 | 0.13 |
| Low‐density lipoprotein cholesterol, mmol/L | 2.96±0.76 | 2.86±0.76 | 2.87±0.72 | <0.001 |
| High‐density lipoprotein cholesterol, mmol/L | 1.41±0.43 | 1.40±0.60 | 1.40±0.34 | 0.71 |
baPWV indicates brachial‐ankle pulse wave velocity; and DASH, Dietary Approaches to Stop Hypertension.
During a mean 3.19 years of follow‐up, frequent night‐eating behavior was associated with more rapid progression of arterial stiffness, as indicated by annual change rate of baPWV, after adjustment for age, sex, baseline baPWV, total energy intake, overall diet quality, physical activity, marriage, employment, education level, alcohol consumption, smoking status, BMI, systolic blood pressure, antihypertensive drug use, fasting blood glucose, low‐density lipoprotein‐cholesterol, high‐density lipoprotein‐cholesterol, sleep duration, insomnia, snoring, and breakfast frequency (P trend=0.01; Table 2). The adjusted difference in change rate of baPWV between participants who ate at night most days and those who never or rarely ate at night was 14.1 (95% CI, 0.6–27.5) cm/s per year (Table 2).
Table 2.
Difference of Brachial‐Ankle Pulse Wave Velocity Change Rate (cm/s per year) According to Night‐Eating Frequency in Women, Men, and Total Participants
| Never or Rarely | Some Days | Most Days | P for Trend | |
|---|---|---|---|---|
| Women (n=2222) | ||||
| N | 2007 | 119 | 96 | |
| Model 1 | 0 (reference) | 36.1 (11.3–61.0)* | 27.2 (0–54.5) | 0.003 |
| Model 2 | 0 (reference) | 38.8 (13.1–64.5)* | 29.8 (1.7–57.9)* | 0.002 |
| Model 3 | 0 (reference) | 34.2 (7.5–61.0)* | 34.3 (4.7–64.0)* | 0.002 |
| Model 4 | 0 (reference) | 32.5 (5.0–59.9)* | 35.1 (5.4–64.8)* | 0.002 |
| Further adjusting for menopausal status | 0 (reference) | 32.2 (4.7–59.6)* | 35.1 (5.3–64.8)* | 0.003 |
| Men (n=5549) | ||||
| N | 4618 | 491 | 440 | |
| Model 1 | 0 (reference) | 3.4 (−10.2 to 17.1) | 4.3 (−9.7 to 18.3) | 0.47 |
| Model 2 | 0 (reference) | 1.9 (−11.0 to 15.7) | 4.0 (−10.1 to 18.1) | 0.55 |
| Model 3 | 0 (reference) | −0.1 (−14.8 to 14.5) | 6.9 (−8.2 to 22.0) | 0.43 |
| Model 4 | 0 (reference) | 4.1 (−11.3 to 19.5) | 6.3 (−8.9 to 21.4) | 0.36 |
| Total (n=7771) | 6625 | 610 | 536 | |
| Model 1 | 0 (reference) | 11.0 (−0.9 to 22.9) | 9.8 (−2.6 to 22.3) | 0.04 |
| Model 2 | 0 (reference) | 10.4 (−1.8 to 22.5) | 10.0 (−2.6 to 22.6) | 0.04 |
| Model 3 | 0 (reference) | 8.9 (−4.0 to 21.7) | 14.3 (0.9–27.8)* | 0.02 |
| Model 4 | 0 (reference) | 12.2 (−1.2 to 25.6) | 14.1 (0.6–27.5)* | 0.01 |
Model 1 adjusted for age and sex; model 2 further adjusted for baseline brachial‐ankle pulse wave velocity, total energy intake (quartiles), and Dietary Approaches to Stop Hypertension diet‐quality score; model 3 further adjusted for physical activity (low, moderate, or high), marriage (single or married), employment (blue‐collar or white‐collar worker), education level (high school and below, or college and above), alcohol consumption (yes/no), smoking status (yes/no), antihypertensive drug (yes/no), body mass index (quintile), systolic blood pressure (quintile), fasting blood glucose (quintile), low‐density lipoprotein‐cholesterol (quintile) and high‐density lipoprotein‐cholesterol (quintile); model 4 further adjusted for sleep duration (hours), insomnia (yes/no), snoring (yes/no), and breakfast frequency. Menopause status was categorized as no menopause, developed menopause, or postmenopausal during follow‐up.
P difference <0.05 compared with “never or rarely” ate‐at‐night group.
Values are adjusted mean differences (95% CIs).
This association was not modified by age, BMI, hypertension, or DASH diet‐quality score (P interaction >0.1 for all). We found a significant interaction between night‐eating frequency and sex, in relation to change in baPWV during follow‐up (P interaction=0.03; Table 2 and Table S1). A significant association between night‐eating behavior and annual change rate of baPWV was observed in women (P trend=0.002), but not in men (P trend=0.36). Further adjustment for menopausal status in women did not result in material change in the association between night‐eating behavior and annual change rate of baPWV (Table 2). The observed association also persisted in the subgroup analyses stratified by menopausal status (P interaction=0.92; Table S2). A detailed baseline comparison between men and women is presented in Tables S3 and S4. We performed multivariable analysis separately for men and women, adjusting for the same set of covariates in model 4. For women only, being single and presence of snoring were significantly associated (P<0.05), whereas for men only, higher total energy intake, smoking, and higher fasting blood glucose concentration were significantly associated (P<0.05) with faster progress of arterial stiffness.
Significant associations persisted after we excluded participants with short follow‐up duration (<1 year); the adjusted difference in annual change rate of baPWV comparing the 2 extreme night‐eating groups was 10.4 (95% CI, 1.4–19.4) cm/s per year in the total population (P trend=0.04; Table 3). Further, the trends persisted after we excluded participants with insomnia (P trend=0.02; Table 3).
Table 3.
Sensitivity Analyses of Adjusted Difference of Brachial‐Ankle Pulse Wave Velocity Change Rate (cm/s per year) According to Night‐Eating Frequency
|
Never or Rarely (n=6625) |
Some Days (n=610) |
Most Days (n=536) |
P for Trend | |
|---|---|---|---|---|
| Propensity score adjusted (n=7771) | 0 (reference) | 8.6 (0.9–16.3)* | 8.8 (0.7–17.0)* | 0.01 |
| Excluding repeated measurement in <1 y | 0 (reference) | |||
| Women (n=2062) | 0 (reference) | 4.5 (−17.6 to 26.6) | 23.1 (−0.2 to 46.4) | 0.05 |
| Men (n=4809) | 0 (reference) | −3.2 (−12.7 to 6.4) | 5.9 (−3.1 to 15.0) | 0.35 |
| Total (n=6871) | 0 (reference) | −0.1 (−9.4 to 9.1) | 10.4 (1.4–19.4)* | 0.04 |
| Excluding participants with insomnia | ||||
| Women (n=2026) | 0 (reference) | 33.5 (3.7–63.4)* | 35.0 (3.1–66.9)* | 0.005 |
| Men (n=5341) | 0 (reference) | 3.9 (−12.5 to 20.3) | 6.1 (−9.5 to 21.7) | 0.39 |
| Total (n=7367) | 0 (reference) | 11.6 (−2.8 to 25.9) | 13.6 (−0.4 to 27.7) | 0.02 |
Model adjusted for age, baseline brachial‐ankle pulse wave velocity, total energy intake (quartiles), Dietary Approaches to Stop Hypertension diet‐quality score, physical activity (low, moderate, or high), marriage (single or married), employment (blue‐collar or white‐collar worker), education level (high school and below, or college and above), alcohol consumption (yes/no), smoking status (yes/no), antihypertensive drug (yes/no), body mass index (quintile), systolic blood pressure (quintile), fasting blood glucose quintile), low‐density lipoprotein‐cholesterol (quintile), high‐density lipoprotein‐cholesterol (quintile), sleep duration (hours), insomnia (yes/no), snoring (yes/no), and breakfast frequency.
P difference <0.05 compared with the “never or rarely” ate‐at‐night group.
Values are adjusted mean differences (95% CIs).
Discussion
In a population without major chronic diseases, women, but not men, who reported eating most nights had a more rapid increase in baPWV than those who never or rarely ate at night. The observed association was independent of total energy consumption, overall diet quality, and other major risk factors for CVD.
This is the first large‐scale longitudinal study, to the best of our knowledge, which examined the association between night‐eating habits and arterial stiffness, an important risk factor and preclinical marker for CVD. Physical stiffening of arteries significantly contributes to an increased risk of developing hypertension, coronary heart disease, and stroke. 6 Although recognized as part of the natural process of aging, arterial stiffness progression rates vary among individuals and may be accelerated by lifestyle behaviors such as night eating. A meta‐analysis, including 14 673 participants, showed that the addition of baPWV to a model incorporating the Framingham risk score significantly increases the predictive power for future CVD risk. 10 To date, less attention has been paid to the progression of arterial stiffness. This study took advantage of repeated measures of baPWV to estimate the speed of vascular aging as our main outcome.
Our findings are consistent with previous studies that found night eating was associated with CVD risk factors. In a clinical study including 52 participants who completed 7 days of wrist actigraphy and food logs, energy consumed after 8 pm was positively associated with higher BMI after adjustment for age, sleep duration, and sleep timing. 20 Night eating, defined as having dinner immediately before bed or having snacks after dinner, has been reported to result in a 2.37‐fold (95% CI, 1.71–3.29) higher risk of having obesity, and a 1.49‐fold (95% CI, 1.14–1.94) higher risk of having dyslipidemia, after adjustment for age, smoking habits, alcohol consumption, physical activity, breakfast intake, and hypertension. 2 Similarly, in a large‐scale prospective cohort study, men who reported eating late at night had a 55% (95% CI, 5%–129%) higher risk of developing coronary heart disease, compared with those who did not eat late at night, after adjustment for age, demographic factors, and physical activity. 3 These studies, together with the current findings, suggest that habitual night eating—independent of other lifestyle behaviors, dietary quality, and sleep quality—plays a critical role in the prevention of chronic disease. However, because of the small number of studies, the 2015 Dietary Guidelines for Americans did not make a recommendation for the timing of meals. 21 Future studies with large case numbers and long follow‐up durations are needed to study the direct association between food‐intake distribution patterns, specifically night‐eating habits, and health outcomes.
Somewhat unexpectedly, the significant association between night‐eating habits and baPWV annual change rate was significant in women, but not in men. Menopause status did not significantly modify this association. However, this finding might be limited by the small sample size of postmenopausal women (n=438) in this cohort. We cannot exclude the possibility that the sex difference in association between night‐eating habits and baPWV annual change rate is a chance finding. This result could also be because of the differences in lifestyle and health status between men and women. For example, the prevalence of insomnia was higher in women relative to men. Although we adjusted for these factors in the model, we still cannot totally exclude the possibility of residual confounding. However, these data suggest there may be sex differences in mental and physical adaptation to night eating. Women have been reported to experience higher postmeal satiety and dietary restraint than men. 22 Sex differences are also present in the association between the intake of macronutrients and alcohol and body fat indicators. 23 For example, 1 study reported an inverse association between fat intake and BMI, and between fat intake and waist circumference in men, but not in women. 23 The circadian rhythm of plasma cortisol concentrations also exhibits sex differences: Women have a higher age‐related elevation in the morning, and a more abrupt ending of the quiescent period than men. 24 Early studies found that circadian misalignment—suppressed and delayed melatonin secretion—would increase estrogen concentration and function of the estrogen receptor. 25 Finally, although the findings are limited, certain aspects of jejunal motility during sleep were modulated by sex. 26 These lifestyle and biological differences between women and men may impact patterns associated with arterial stiffness and CVD risk.
Night eating may influence a number of variables that could result in an acceleration of arterial stiffening. Night eating leads to a disturbance or shift in an individual's cardiac clock and overnight‐fasting period. Glucose, epinephrine, and cortisol all exhibited significant impacts on endogenous circadian rhythm. 27 Circadian misalignment could further alter endocrine function. Moreover, eating close to bedtime would necessitate digestion during sleep, contributing to sleep disturbance and imposing an extra burden on the pancreas 28 and adipose tissue. 29 The potentially resulting hyperglycemia, elevated nonesterified fatty acid level, and insulin resistance could result in elevated oxidative stress and inflammation, contributing to endothelial dysfunction and arterial stiffness progression. 30 Previous studies on meal timing (eg, breakfast frequency and overall eating frequency) and CVD and mortality, also reported significant associations. 3 , 31 The observational data support potential underlying biological and physiological mechanisms underpinning the effect of the biological clock on cardiovascular health.
Night eating may indirectly accelerate arterial stiffness as a result of insomnia (eg, difficulty falling sleep). However, controlling for sleep‐quality assessments, including sleep duration, snoring status, insomnia, and breakfast habits had little effect on the results. Night eating may be associated with the intake of excess energy or specific foods associated with less healthy dietary patterns. However, no significant difference was observed in total energy intake or diet quality across different night‐eating groups, and the association observed with arterial stiffness remained significant after adjustment for total energy intake and diet quality. Night eating might be an indicator of other lifestyle characteristics that would promote arterial stiffening, such as sedentary lifestyle, smoking, and alcohol consumption. In our cohort, controlling for these variables had little effect on the statistical significance of the results, although because of the observational study design, residual confounding cannot be avoided.
Limitations
This study focused on a population without CVD, cancer, and diabetes mellitus at the time of recruitment because diagnosis of these diseases would likely change a person's eating habits. Thus, our findings may not be generalizable to individuals with these chronic conditions. Another limitation of this study was the self‐reporting of night‐eating habits. Participants may not be able to correctly recall or may misreport their night‐eating frequencies. The definition of night eating in our study did not consider the number of calories consumed. However, total energy was adjusted in all models. This study also lacked information on participants' stress levels, mood disorders, chronotypes, and the exact timing of each meal, bedtime, and work hours. A systematically delayed biological clock may contribute differently to arterial stiffness than an additional meal or snack at night. Further, only a single measure, baPWV, was used to measure arterial stiffness. Other indicators, such as the carotid‐femoral PWV, which is the current gold standard measure of arterial stiffness, and the augmentation index, which characterizes the amplitude of the reflected blood wave, should be further examined. However, baPWV has been validated for use in large epidemiological cohorts, 7 , 15 and has previously been demonstrated to have significant prediction value for CVD. 10 Data were not available for diet behaviors, such as number of meals per day, snacks, and types of food that were consumed at night; or measures of circadian rhythms, which would have allowed for further exploration of the sex‐specific difference we observed. Another limitation is that only 29% of the study population was women. This is because participants were recruited from the Kailuan Company, a coal‐mining company where the majority of employees are men. Nevertheless, the large sample size gave power for sex‐specified analyses. Strengths of this study include the large sample size, availability of a wide range of behavioral and biological variables, and longitudinal follow‐up of arterial stiffness progression.
To conclude, in this community‐based prospective longitudinal study, habitual night eating was associated with more rapid progression of arterial stiffness in women without CVD, cancer, and diabetes mellitus during the observational period. Future studies with more detailed measures of eating habits, meal timings, and work and leisure patterns, as well as sex‐specific endocrine measures, are needed to further examine the association between food‐intake patterns during waking hours and cardiovascular risk factors, as well as CVD risk.
Sources of Funding
None.
Disclosures
None.
Supporting information
Tables S1–S4
(J Am Heart Assoc. 2020;9:e016455 DOI: 10.1161/JAHA.120.016455.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016455
For Sources of Funding and Disclosures, see page 7.
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Xiang Gao, Email: xxg14@psu.edu.
References
- 1. Yu E, Malik VS, Hu FB. Cardiovascular disease prevention by diet modification: JACC health promotion series. J Am Coll Cardiol. 2018;914–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yoshida J, Eguchi E, Nagaoka K, Ito T, Ogino K. Association of night eating habits with metabolic syndrome and its components: a longitudinal study. BMC Public Health. 2018;1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cahill LE, Chiuve SE, Mekary RA, Jensen MK, Flint AJ, Hu FB, Rimm EB. Prospective study of breakfast eating and incident coronary heart disease in a cohort of male US health professionals. Circulation. 2013;337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. St‐Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris‐Etherton P, Varady K; American Heart Association Obesity Committee of the Council on L, Cardiometabolic H, Council on Cardiovascular Disease in the Y, Council on Clinical C, Stroke C . Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;e96–e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shirwany NA, Zou MH. Arterial stiffness: a brief review. Acta Pharmacol Sin. 2010;1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;932–943. [DOI] [PubMed] [Google Scholar]
- 7. Wu S, Jin C, Li S, Zheng X, Zhang X, Cui L, Gao X. Aging, arterial stiffness, and blood pressure association in Chinese adults. Hypertension. 2019;893–899. [DOI] [PubMed] [Google Scholar]
- 8. Takashima N, Turin TC, Matsui K, Rumana N, Nakamura Y, Kadota A, Saito Y, Sugihara H, Morita Y, Ichikawa M, et al. The relationship of brachial‐ankle pulse wave velocity to future cardiovascular disease events in the general japanese population: the Takashima Study. J Hum Hypertens. 2014;323–327. [DOI] [PubMed] [Google Scholar]
- 9. Munakata M. Brachial‐ankle pulse wave velocity in the measurement of arterial stiffness: recent evidence and clinical applications. Curr Hypertens Rev. 2014;49–57. [DOI] [PubMed] [Google Scholar]
- 10. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, et al.; Collaborative Group for JB . Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;1045–1052. [DOI] [PubMed] [Google Scholar]
- 11. Li S, Cheng J, Cui L, Gurol ME, Bhatt DL, Fonarow GC, Benjamin EJ, Xing A, Xia Y, Wu S, et al. Cohort study of repeated measurements of serum urate and risk of incident atrial fibrillation. J Am Heart Assoc. 2019;9:e012020 DOI: 10.1161/JAHA.119.012020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen S, Li W, Jin C, Vaidya A, Gao J, Yang H, Wu S, Gao X. Resting heart rate trajectory pattern predicts arterial stiffness in a community‐based chinese cohort. Arterioscler Thromb Vasc Biol. 2017;359–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu S, An S, Li W, Lichtenstein AH, Gao J, Kris‐Etherton PM, Wu Y, Jin C, Huang S, Hu FB, et al. Association of trajectory of cardiovascular health score and incident cardiovascular disease. JAMA Netw Open. 2019;9:e194758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris‐Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, et al. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;2022–2027. [DOI] [PubMed] [Google Scholar]
- 16. Gao Y, Cui LF, Sun YY, Yang WH, Wang JR, Wu SL, Gao X. Adherence to the dietary approaches to stop hypertension (DASH) diet and hyperuricemia: a cross‐sectional study. Arthritis Care Res (Hoboken). 2020. Jan 21 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 17. Chiuve SE, Fung TT, Rimm EB, Hu FB, McCullough ML, Wang M, Stampfer MJ, Willett WC. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;1009–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Huang Z, Hou J, Sawyer AM, Wu Z, Cai J, Curhan G, Wu S, Gao X. Sleep and CKD in Chinese adults: a cross‐sectional study. Clin J Am Soc Nephrol. 2017;885–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Macfarlane DJ, Lee CC, Ho EY, Chan KL, Chan DT. Reliability and validity of the Chinese version of IPAQ (short, last 7 days). J Sci Med Sport. 2007;45–51. [DOI] [PubMed] [Google Scholar]
- 20. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring). 2011;1374–1381. [DOI] [PubMed] [Google Scholar]
- 21. U.S. Department of Health and Human Services and U.S. Department of Agriculture . 2015–2020 Dietary Guidelines for Americans. 8th ed December 2015. Available at: https://health.Gov/dietaryguidelines/2015/guidelines/. Accessed July 2019. [Google Scholar]
- 22. Cornier MA, Salzberg AK, Endly DC, Bessesen DH, Tregellas JR. Sex‐based differences in the behavioral and neuronal responses to food. Physiol Behav. 2010;538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brandhagen M, Forslund HB, Lissner L, Winkvist A, Lindroos AK, Carlsson LM, Sjostrom L, Larsson I. Alcohol and macronutrient intake patterns are related to general and central adiposity. Eur J Clin Nutr. 2012;305–313. [DOI] [PubMed] [Google Scholar]
- 24. Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;2468–2473. [DOI] [PubMed] [Google Scholar]
- 25. Sanchez‐Barcelo EJ, Cos S, Mediavilla D, Martinez‐Campa C, Gonzalez A, Alonso‐Gonzalez C. Melatonin‐estrogen interactions in breast cancer. J Pineal Res. 2005;217–222. [DOI] [PubMed] [Google Scholar]
- 26. Aytug N, Giral A, Imeryuz N, Enc FY, Bekiroglu N, Aktas G, Ulusoy NB. Gender influence on jejunal migrating motor complex. Am J Physiol Gastrointest Liver Physiol. 2001;G255–G263. [DOI] [PubMed] [Google Scholar]
- 27. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;4453–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naidoo N, Davis JG, Zhu J, Yabumoto M, Singletary K, Brown M, Galante R, Agarwal B, Baur JA. Aging and sleep deprivation induce the unfolded protein response in the pancreas: implications for metabolism. Aging Cell. 2014;131–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poroyko VA, Carreras A, Khalyfa A, Khalyfa AA, Leone V, Peris E, Almendros I, Gileles‐Hillel A, Qiao Z, Hubert N, et al. Chronic sleep disruption alters gut microbiota, induces systemic and adipose tissue inflammation and insulin resistance in mice. Sci Rep. 2016;35405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beckman JA, Creager MA, Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;2570–2581. [DOI] [PubMed] [Google Scholar]
- 31. Rong S, Snetselaar LG, Xu G, Sun Y, Liu B, Wallace RB, Bao W. Association of skipping breakfast with cardiovascular and all‐cause mortality. J Am Coll Cardiol. 2019;2025–2032. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


