Abstract
Background
Public health policies reflect concerns that certain fruit sources may not have the intended benefits and that vegetables should be preferred to fruit. We assessed the relation of fruit and vegetable sources with cardiovascular outcomes using a systematic review and meta‐analysis of prospective cohort studies.
Methods and Results
MEDLINE, EMBASE, and Cochrane were searched through June 3, 2019. Two independent reviewers extracted data and assessed study quality (Newcastle‐Ottawa Scale). Data were pooled (fixed effects), and heterogeneity (Cochrane‐Q and I2) and certainty of the evidence (Grading of Recommendations Assessment, Development, and Evaluation) were assessed. Eighty‐one cohorts involving 4 031 896 individuals and 125 112 cardiovascular events were included. Total fruit and vegetables, fruit, and vegetables were associated with decreased cardiovascular disease (risk ratio, 0.93 [95% CI, 0.89–0.96]; 0.91 [0.88–0.95]; and 0.94 [0.90–0.97], respectively), coronary heart disease (0.88 [0.83–0.92]; 0.88 [0.84–0.92]; and 0.92 [0.87–0.96], respectively), and stroke (0.82 [0.77–0.88], 0.82 [0.79–0.85]; and 0.88 [0.83–0.93], respectively) incidence. Total fruit and vegetables, fruit, and vegetables were associated with decreased cardiovascular disease (0.89 [0.85–0.93]; 0.88 [0.86–0.91]; and 0.87 [0.85–0.90], respectively), coronary heart disease (0.81 [0.72–0.92]; 0.86 [0.82–0.90]; and 0.86 [0.83–0.89], respectively), and stroke (0.73 [0.65–0.81]; 0.87 [0.84–0.91]; and 0.94 [0.90–0.99], respectively) mortality. There were greater benefits for citrus, 100% fruit juice, and pommes among fruit sources and allium, carrots, cruciferous, and green leafy among vegetable sources. No sources showed an adverse association. The certainty of the evidence was “very low” to “moderate,” with the highest for total fruit and/or vegetables, pommes fruit, and green leafy vegetables.
Conclusions
Fruits and vegetables are associated with cardiovascular benefit, with some sources associated with greater benefit and none showing an adverse association.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03394339.
Keywords: cardiovascular outcomes, cohort, fruit, nutrition, vegetables
Subject Categories: Cerebrovascular Disease/Stroke, Cardiovascular Disease, Diet and Nutrition, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- GRADE
Grading of Recommendations Assessment, Development, and Evaluation
- NOS
Newcastle‐Ottawa Scale
Clinical Perspective
What Is New?
Public health policies discourage the consumption of certain fruit sources (eg, 100% fruit juice, dried fruit, and tropical fruit) because of their sugar content and emphasize vegetable consumption before fruit.
We examined the relation of fruit and vegetable sources with cardiovascular disease outcomes.
What Are the Clinical Implications?
In this systematic review and meta‐analysis of 81 unique cohorts, we identified that fruits and vegetables are associated with cardiovascular benefit and no fruit or vegetable sources are associated with cardiovascular harm.
Certain fruit and vegetable sources showed greater associations with cardiovascular benefit, including citrus, 100% fruit juice, and pommes fruit and allium, carrots, and cruciferous and green leafy vegetables.
Increased fruit and vegetable consumption is the cornerstone of dietary guidance for cardiovascular disease (CVD) prevention. Their benefit as part of heart healthy diets is balanced against an increasing concern of their contribution to an excess intake of sugars. 1 , 2 Some influential commentators have even questioned the value of the proverbial “apple a day.” 3 Public health outlets are emphasizing vegetables before fruit intake and discouraging the intake of certain sources of fruit, such as fruit juice and dried, tropical, and canned fruit, some of which have been reflected in health policies. 4 , 5 , 6 , 7 , 8
Given the longstanding perceived value of fruit and vegetables in reducing global CVD morbidity and mortality 9 and in light of developing efforts to limit dietary sugars, there is a need to reassess the role of different fruit and vegetable sources in CVD prevention. Whether different fruit and vegetable sources show comparable CVD risk reduction is unclear. Systematic reviews and meta‐analyses of prospective cohort studies have shown evidence of a cardiovascular benefit of broad categories of fruits and vegetables, 11 , 12 , 13 , 14 , 15 , 16 , 17 but the relative contributions of specific fruit and vegetable sources and the certainty of the estimates for these sources are underexplored. We, therefore, conducted a systematic review and meta‐analysis of prospective cohort studies using Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach to assess the role of different fruit and vegetable sources in CVD risk reduction and to quantify the certainty of the evidence to inform public health policy.
METHODS
All supporting data are available within the article and its online supplementary files. We followed the Cochrane Handbook for Systematic Reviews and Interventions 17 and reported results in accordance with Meta‐Analysis of Observational Studies in Epidemiology 16 and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses guidelines. 18 The protocol was registered at Clinicaltrials.gov (identifier, NCT03394339).
Search Strategy
We searched MEDLINE, EMBASE, and the Cochrane Library databases through June 3, 2019, using the search strategy presented in Table S1 and restrictions for prospective cohorts. We supplemented the search with manual searches of the references of included studies.
Study Selection
Prospective cohort studies that reported the association of fruit and/or vegetable intake with CVD, coronary heart disease (CHD), or stroke incidence and mortality with a minimum follow‐up time of 1 year in individuals free of disease at baseline were included. Cohorts that presented data on exposures to fruits and vegetables within the context of a dietary index were not included unless fruits and/or vegetables were presented separately from the other components of the diet index.
Data Extraction
Two reviewers (A.Z., F.A.) independently extracted relevant information, including study design, sample size, subject characteristics, exposure, outcomes, assessment method, dose for each quantile, number of events, population, person‐years of follow‐up, duration of follow‐up, covariates adjustments, and risk ratios (RRs; or odds ratios or hazard ratios) with 95% CIs for each quantile of exposure. We contacted authors for missing data. Data on CVD outcomes were extracted for exposures to total fruits and vegetables, fruits, vegetables, and their sources. Potatoes were not included in the present analysis as they are nutritionally classified as a starchy food and are largely omitted in quantifications of exposure to vegetables.
Outcomes
Outcomes were CVD, CHD, and stroke incidence and mortality.
Risk of Bias
Included studies were assessed for risk of bias with the Newcastle‐Ottawa Scale (NOS), 19 which awards up to 9 points based on cohort selection (up to 4 points), outcome ascertainment (up to 3 points), and degree of covariate adjustments (up to 2 points with adjustment for age as the primary confounding variable awarded 1 point and adjustment for ≥7/9 secondary confounding variables, including sex, family history, smoking, markers of adiposity, energy intake, physical activity, presence of diabetes mellitus, hypertension [or related medications], and dyslipidemia [or related medications]). Studies achieving ≥7 points were considered high quality. Disagreements in NOS score between the 2 reviewers were resolved by a third reviewer (J.L.S.).
Statistical Analysis
Review Manager version 5.3 (The Nordic Cochrane Centre, Denmark) and STATA version 13.0 (StataCorp, TX) were used to conduct all analyses. We prespecified in our analysis plan the use of the generic inverse variance method with DerSimonian and Laird random effects models to pool the natural log‐transformed RRs of extreme quantiles, comparing the highest versus the lowest (reference) exposures. 20 On the basis of a deviation from our prespecified analysis plan requested by the statistical reviewer, we present the generic inverse variance with fixed effects models as the primary analysis and the DerSimonian and Laird random effects models as a secondary analysis in the Supplemental Material. Hazard ratios and odds ratios (as cumulative incidence <10%) were considered equivalent to RR. 21 Studies that provided RR on a continuous scale (ie, per dose increment) were scaled to the highest quantile reported for the exposure in the respective cohort as necessary. Test for differences between fruit and vegetable categories were conducted in RevMan, with a test for subgroup differences, with P<0.05 indicating a significant difference between fruit categories or vegetable categories on a given outcome. We also conducted a dose‐response analysis. A random‐effects linear dose‐response was modeled using a generalized least square trend (glst) for estimation of summarized dose‐response data, as per Greenland and Longnecker 22 and Orsini. 23 A 2‐stage multivariate random‐effects method was used to model a nonlinear association using restricted cubic splines with 3 knots. 23 A Wald test was used to evaluate linear and nonlinear dose‐response trends. The median dose of each quantile was used, and when not provided we chose the midpoint of the upper and lower boundaries for each quantile as the assigned dose. For open‐ended lower and upper quantiles, we defined lowest and highest boundary as the same as the adjacent category cutoff. Servings per day were calculated, with one serving defined as 80 g of fruits and/or vegetables and their categories, with the exception of citrus fruit (122 g), fruit juice (125 g), and green leafy vegetables (88 g), or unless otherwise specified. 24 Heterogeneity was assessed by the Cochran Q statistic and quantified by the I2 statistic. An I2≥50% and PQ<0.1 was considered evidence of substantial heterogeneity. 26 , 27 Sensitivity analyses and a priori subgroup analyses were used to explore sources of heterogeneity. We performed sensitivity analyses by systematically removing each study with recalculation of the summary estimates. A priori subgroup analyses were conducted for all comparisons with ≥10 observations. Subgroup analyses included age (less than median versus median or greater), sex (males, females, and mixed), follow‐up years (less than median versus median or greater), number of covariates in extracted model (<8 versus ≥8 covariates), exposure assessment tool (validated Food Frequency Questionnaire [FFQ], unvalidated FFQ, and food record), risk of bias score (<6 versus ≥6), and country of data collection. Wald test in metaregression was used to assess differences within each subgroup. Because of the exploratory intent of our subgroup analyses, we did not prespecify adjustment for the false discovery rate in our prespecified analysis plan. On the basis of a deviation from our prespecified analysis plan requested by the statistical reviewer, we adjust for the false discovery rate in our subgroup analyses using the Holm‐Bonferroni procedure. If ≥10 cohort comparisons were available, then publication bias was assessed by visual inspection of funnel plots for asymmetry and formal testing with the Begg and Egger tests. If publication bias was suspected (P<0.10), the Duval and Tweedie trim and fill method imputed missing study data in attempt to adjust for funnel plot asymmetry. 27
Grading the Evidence
The GRADE method was used to assess the certainty of the evidence for each comparison on a 4‐point scale, ranging from “very low” to “high.” 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 Because of their inherent limitations, observational studies start at a “low” certainty of evidence that can be downgraded or upgraded based on established criteria. Criteria to downgrade included risk of bias (weight of studies shows high risk of bias by NOS), inconsistency (substantial unexplained heterogeneity, I2>50%, and PQ<0.10), indirectness (presence of factors that limit generalizability based on populations, exposures, and outcomes), imprecision (95% CIs cross minimally important difference of 5% [RR, 0.95–1.05]), and publication bias (significant evidence of small study effects). Criteria to upgrade included a large risk estimate (RR <0.5 or >2 in the absence of plausible confounders), a dose‐response gradient, and attenuation by plausible confounders.
Results
Flow of the Literature
Figure 1 illustrates a flow of the literature. Of 4271 reports, we included a total of 117 publications 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 of 81 unique prospective cohort studies of 4 031 896 individuals and 125 112 cardiovascular events.
Figure 1. Summary of evidence search and selection.
CV indicates cardiovascular.
Study Characteristics
The Table shows the characteristics of the included studies. 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 Participants were from 69 countries with cohorts distributed worldwide (36 from Europe, 23 from North America, 1 from South America, 17 from Asia, 4 from Australia, and 1 large global cohort including 18 countries worldwide). The median participant age at baseline was 55 (range, 7–90) years with a median follow‐up of 11 (range, 2–37) years. Median (range) intakes in servings per day in the highest quantiles were 7.4 (2.6–10.4) fruits and vegetables, 2.6 (0.29–11.0) fruits, 2.85 (0.74–11.0) vegetables, 0.4 (0.3–0.5) bananas, 0.27 (0.13–0.7) berries, 0.71 (0.22–2.2) citrus fruit, 0.82 (0.4–2.28) fruit juice, 0.95 (0.29–2.0) pommes, 2.37 (2.1–2.65) watermelon, 0.54 (0.07–2) allium vegetables, 9.5 (5–14) carrots, 0.43 (0.1–3.0) cruciferous vegetables, 0.71 (0.25–1.5) green leafy vegetables, and 0.63 (0.29–2.0) tomatoes. Doses were not available for apricots and celery. Dietary intake was assessed by self‐administered validated food frequency questionnaire (54%), interview administered validated FFQ (10%), unvalidated FFQ (19%), or 24‐hour recalls/food records (17%).
Table 1.
Table of Study Characteristics
| Study | Cohort | Country | Participants (Men:Women) | Age, y | Follow‐Up, y | Dietary Assessment | Exposure | Quantiles | Outcomes | Incidence (Men:Women) |
|---|---|---|---|---|---|---|---|---|---|---|
| Adriouch, 2018 41 | NutriNet‐Sante | France |
84 158 (17 931:66 227) |
44.1±14.5 | 4.9±1.6 | 24‐h recall | Fruit category | 3 |
CVD incidence CHD incidence Stroke incidence |
602 309 293 |
| Appleby, 2002 42 | Health Food Shoppers | United Kingdom |
10 741 (4325:6416) |
16–89 | 18–24 | Unvalidated FFQ | Fruit | 2 |
CVD mortality CHD mortality Stroke mortality |
1202 (591:611) 605 (347:258) 356 (142:214) |
| Atkins, 2014 43 | British Regional Heart | England |
3328 (3328:0) |
60–79 | 11.3 | Validated FFQ | Fruit and/or vegetable | 2 |
CVD incidence CVD mortality CHD incidence |
582 327 307 |
| Bahadoran, 2017 44 | Theran Lipid and Glucose | Iran |
2369 (1047:1322) |
≥19 | 6 | Validated FFQ | Vegetable categories | 3 | CVD risk | 79 |
| Bazzano, 2002 45 | National Health and Nutrition Examination Survey Epidemiologic Follow‐up Study | United States | 9608 | 25–74 |
19 |
Unvalidated FFQ | Fruit and vegetable | 4 |
CVD mortality CVD incidence IHD mortality IHD incidence Stroke mortality Stroke incidence |
1145 N/A 639 1786 218 888 |
| Belin, 2011 46 | WHI‐OS (Women's Health Initiative Observational Study) |
United States |
93 676 (0:93 676) |
50–79 |
10 |
Self‐administered validated FFQ | Fruit, vegetable | 2 | CVD incidence | 6006 |
| Bendinelli, 2011 47 | EPIC | Italy |
29 689 (0:29 689) |
50.0±7.9 |
7.85 |
Validated FFQ | Fruit, vegetable, categories | 4 | CHD incidence | 144 |
| Berard, 2017 48 | MONICA | France | 1311 | 35–64 | 16–18 | Food recall | Fruit, vegetable | 5 | CVD mortality | 41 |
| Bhupathiraju, 2013 49 | NHS (Nurses' Health Study) and HPFS (Health Professionals Follow‐Up Study) | United States |
113 276 (42 135:71 141) |
40–75 (men) 30–55 (women) |
22 (men) 24 (women) |
Validated FFQ | Fruit and/or vegetable, categories | 5 | CHD incidence |
6189 (3607:2582) |
| Bingham, 2008 50 | EPIC | United Kingdom | 11 134 | 45–75 | 4 | Validated FFQ | Fruit and vegetable | 5 | IHD risk | 678 |
| Blekkenhorst, 2017 51 | PLSAW (Perth Longitudinal Study of Aging Women) | Australia |
1226 (0:1226) |
75.1±2.7 | 15 | Validated FFQ | Vegetable, categories | Per 5–75 g/d |
CVD mortality IHD mortality Stroke mortality |
238 128 92 |
| Bos, 2014 52 | Rotterdam Study | The Netherlands |
3570 (1405:2165) |
69.4±6.3 | 12.9 | Unvalidated FFQ | Fruit and vegetable | 3 | Stroke risk | 545 |
| Buijsse, 2008 53 | Zutphen Elderly Study | The Netherlands |
559 (559:0) |
65–84 | 15 | Unvalidated FFQ | Vegetable category | Per 1‐SD increase | CVD mortality | 197 |
| Buil‐Cosiales, 2016 55 | PREDIMED (Prevención con DIeta Mediterránea) | Spain | 7216 | 55–80 | 6 | Validated FFQ | Fruit, vegetable, categories | 5 |
CVD composite score CVD mortality MI incidence Stroke incidence |
342 104 118 169 |
| Buil‐Cosiales, 2017 54 | SUN (Seguimiento University of Navarra) | Spain |
17 007 (6633:10 374) |
38 | 10.3 | Validated FFQ | Fruit, vegetable, categories | 5 | CVD incidence | 112 |
| Cassidy, 2012 56 | NHS | United States |
69 622 (0:69 622) |
30–55 | 14 | Validated FFQ | Fruit, vegetable | 5 | Stroke incidence | 1803 |
| Collin, 2019 57 | REGARDS (Reasons for Geographic and Racial Differences in Stroke) | United States |
13 440 (7972:5469) |
≥45 | 6±1.8 | Validated FFQ | Fruit category | 12 oz/d | CHD mortality | 168 |
| Conrad, 2018 58 | NHANES | United States |
29 133 (13 926:15 207) |
46.3 (95% CI, 45.8–46.7) | 6.5 | 24‐h recall | Vegetable | 3 |
CVD mortality CHD mortality |
726 556 |
| Dauchet, 2004 59 | PRIME | France, North Ireland |
8087 (8087:0) |
50–59 | 5 | Interview | Fruit category | 3 | CHD event | 133 |
| Dauchet, 2010 60 | PRIME | France, North Ireland |
8060 (8060:0) |
50–59 | 10 | Interview‐validated FFQ | Fruit and/or vegetable | 3 |
CVD risk Acute coronary syndrome |
612 367 |
| Du, 2016 61 | China Kadoorie Biobank | China |
451 665 (186 086:265 579) |
50.5±10.4 | N/A, ≈7.14 y | Interview unvalidated FFQ | Fruit | 5 |
Acute coronary event Hemorrhagic stroke event Other CeVD events Ischemic stroke |
2551 14 579 11 054 3523 |
| Du, 2017 62 | China Kadoorie Biobank | China |
462 342 (189 560:272 782) |
51±10.5 | ≈7 | Interview unvalidated FFQ | Fruit | 4 |
CVD mortality IHD mortality Ischemic stroke mortality Hemorrhagic stroke mortality |
6166 2038 585 2351 |
| Elwood, 2013 63 | Carphilly Cohort Study | United Kingdom |
2235 (2235:0) |
45–59 | 30 | Unvalidated FFQ | Fruit and vegetable | 2 | CVD incidence | N/A |
| Eriksen, 2015 64 | SABRE (Southhall and Brent Revised) | United Kingdom | 2096 |
40–69 |
21 |
Validated FFQ | Fruit, vegetable | 2 |
CVD incidence CHD incidence |
571 520 |
| Fitzgerald, 2012 65 | Women's Health Study | United States |
34 827 (0:34 827) |
55 (46–68) (mean [95% CI]) |
14.6 | Validated FFQ | Fruit, vegetable | 5 | CVD risk | 1094 |
| Fraser, 1992 66 | Adventis Health Study | United States |
26 473 (10 003:16 740) |
Men: 51.3±16.0 Women: 53.2±16.6 (mean±SD) |
6 | Validated FFQ | Fruit | 3 |
CHD mortality CHD event |
463 134 |
| Gardener, 2011 67 | NOMAS (Northern Manhattan Study) | United States |
2568 (924:1644) |
69±10 (Mean±SD) |
9 |
Interview validated FFQ |
Fruit, vegetable | Continuous |
CVD mortality CVD incidence MI incidence Ischemic stroke incidence |
314 518 133 171 |
| Gaziano, 1995 68 | Massachusetts Health Care Panel Study | United States |
1299 (494:805) |
≥66 | 4.75 | Unvalidated FFQ | Fruit and vegetable categories | 2 | CVD mortality | 161 |
| Genkinger, 2004 69 | Odyssey | United States |
6151 (2276:3875) |
30–93 | 13 | Validated FFQ | Fruit, vegetable categories | 5 | CVD mortality | 378 |
| Gillman, 1995 70 | Framingham Study | United States |
832 (832:0) |
45–65 | 18–22 | 24‐h recall | Fruit and vegetable | 5 |
Stroke mortality Stroke incidence |
14 97 |
| Goetz, 2016 71 | REGARDS | United States | 16 678 | ≥45 | 6.0±1.9 | Validated FFQ | Fruit categories | 5 | CHD events | 589 |
| Goetz, 2016 72 | REGARDS | United States |
20 024 (9011:11 013) |
≥45 | 6.5 | Validated FFQ | Fruit, vegetable | 5 | Stroke incidence | 524 |
| Gunge, 2017 73 | Danish Diet, Cancer and Health Cohort | Denmark |
57 053 (25 759:28 809) |
50–64 | 13.6 | Validated FFQ | Fruit and vegetable categories | 2 | MI incidence |
2322 (1669:653) |
| Gunnell, 2013 74 | Health and Wellbeing Surveillance System | Australia |
14 890 (6114:8776) |
45–97 | 6 | Validated FFQ | Fruit and vegetable | 2 | IHD hospitalization | 538 |
| Hansen, 2010 76 | Danish Diet, Cancer and Health | Denmark |
53 383 (25 065:28 318) |
50–64 | 7.7 | Validated FFQ | Fruit, vegetable categories | 4 | Acute coronary syndrome | 1075 (820:255) |
| Hansen, 2017 75 | Danish Diet, Cancer and Health | Denmark | 55 338 | 50–64 | 13.5 | Validated FFQ | Fruit and vegetable categories | 2 | Stroke incidence | 2283 |
| Harriss, 2007 77 | Melbourne Collaborative | Australia |
40 653 16 673:23 980 |
40–69 | 10.4 | Validated FFQ | Fruit, vegetable | 4 |
CVD mortality IHD mortality |
697 407 |
| Hertog, 1997 78 | Caerphilly Prospective Study | South Wales |
1900 (1900:0) |
45–59 | 14.6 | Validated FFQ | Vegetable categories | 4 | IHD mortality | 131 |
| Hirvonen, 2000 80 | Finnish Male Smokers in the ATBC Study | Finland |
26 497 (26 497:0) |
50–69 | 6.1 | Validated FFQ | Fruit category | 4 |
Cerebral infarction Subarachnoid hemorrhage Intracerebral hemorrhage |
736 83 95 |
| Hirvonen, 2001 79 | Finnish Male Smokers in the ATBC Study | Finland |
25, 373 (25, 373:0) |
50–69 | 6.1 |
Validated FFQ |
Fruit, vegetable, categories | 5 |
CHD mortality MI event |
815 1122 |
| Hjartaker, 2015 81 | Migrant Study | Norway |
9766 (9766:0) |
42–73 | 20.3 | Unvalidated FFQ | Fruit and/or vegetable, categories | 4 |
CVD mortality CHD mortality Stroke mortality |
4595 2386 1034 |
| Hodgson, 2016 82 | Australian Women aged 70–85 y | Australia |
1456 0:1456 |
>70 | 15 | Validated FFQ | Fruit category | 3 | CVD mortality | 235 |
| Holmberg, 2009 83 | Swedish National Farm Register | Sweden |
1738 (1738:0) |
50±6.0 | 12 | Unvalidated FFQ | Fruit and vegetable | 2 | CHD incidence | 138 |
| Iso, 2007 84 | Japan Collaborative Cohort | Japan | N/A | 40–79 | N/A | Validated FFQ | Fruit or vegetable categories | 3 |
IHD mortality CeVD mortality |
N/A N/A |
| Jacques, 2015 85 | Framingham Offspring | United States |
2880 (1302:1578) |
28–62 (mean=54) | 14.9 | Validated FFQ | Fruit categories | 3 |
CVD incidence CHD incidence |
518 261 |
| Johnsen, 2003 86 | Danish Diet, Cancer and Health | Denmark | 54 506 | 50–64 | 3.09 | Validated FFQ | Fruit and/or vegetable | 5 | Stroke incidence | 266 |
| Joshipura, 1999 87 | NHS and HPFS cohorts | United States |
114 279 (38 683:75 596) |
30–55 (men) 40–75 (women) |
8 (men) 14 (women) |
Validated FFQ | Fruit and/or vegetable, categories | 5 | Ischemic stroke incidence |
570 (366:204) |
| Joshipura, 2009 88 | NHS and HPFS cohorts | United States |
109 788 (38 918:70 870) |
30–55 (men) 40–75 (women) |
14–16 | Validated FFQ | Fruit and/or vegetable, categories | 5 | CVD incidence | 3892 |
| Keli, 1996 89 | Zutphen Elderly | The Netherlands |
552 (552:0) |
50–69 | 15 | Interview | Fruit, vegetable categories | 3 | Stroke risk | 42 |
| Kim, 2013 90 | British Women's Heart and Health Study | United Kingdom |
3080 (0:3080) |
60–79 | 7 | Unvalidated FFQ | Fruit | 2 | CVD incidence | 329 |
| Knekt, 1994 93 | Finnish Mobile Clinic Health | Finland |
5133 (2748:2385) |
30–69 | 14 | Interview unvalidated FFQ | Fruit, vegetable | 3 | CHD mortality | 244 (186:58) |
| Knekt, 1996 92 | Finnish Mobile Clinic Health | Finland |
5133 (2748:2385) |
30–69 | 26 | Interview unvalidated FFQ | Fruit or vegetable categories | 4 | CHD death |
473 (324:149) |
| Knekt, 2000 91 | Finnish Mobile Clinic Health | Finland | 9208 | ≥15 | 28 | Interview unvalidated FFQ | Fruit or vegetable categories | 5 | CeVD incidence | 824 |
| Kobylecki, 2015 94 | Copenhagen City Heart | Denmark | 78 527 | 20–100 | 10 | Self‐reported unvalidated FFQ | Fruit and vegetable | 3 | IHD incidence | 2823 |
| Kondo, 2019 95 | NIPPON DATA80 | Japan |
9115 (4002:5113) |
30–79 | 29 | 3‐d food record | Fruit or vegetable | 3 | CVD mortality | 1070 |
| Kvaavik, 2010 96 | Health and Lifestyle Survey | United Kingdom |
4866 (2509:2377) |
43.7±16.3 | 20 | Interview Unvalidated FFQ | Fruit and vegetable | 2 | CVD mortality | 431 |
| Lai, 2015 97 | UK Women's Cohort | United Kingdom |
30 458 (0:30 458) |
35–69 | 16.7 | Validated FFQ | Fruit, categories | 5–6 |
CVD mortality CHD mortality Stroke mortality |
286 138 148 |
| Larsson, 2009 98 | Finnish Male Smokers in the ATBC Study | Finland |
26 556 (26 556:0) |
50–69 | 13.6 | Validated FFQ | Fruit, vegetable | 5 | Stroke incidence | 2702 |
| Larsson, 2013 99 | Swedish Mammography and Swedish Men Cohorts | Sweden |
74 961 40 291:34 670 |
45–83 | 10.2 | Validated FFQ | Fruit and/or vegetable, categories | 5 | Stroke incidence | 4089 |
| Leenders, 2013 101 | EPIC |
Europe (10 countries)* |
451 151 129 882:321 269 |
25–70 |
12.8 (median) |
Validated FFQ and 7‐d food record | Fruit and/or vegetable | 4 | CVD mortality | 5125 |
| Leenders, 2014 100 | EPIC |
Europe (10 countries)* |
451 151 (129 882:321 269) |
25–70 | 13 | Validated FFQ and 7‐d food record | Fruit and/or vegetable | 4 |
CHD mortality Stroke mortality |
2139 1291 |
| Lin, 2007 102 | NHS | United States |
66 360 (0:66 360) |
30–55 | 12 | Validated FFQ | Fruit, vegetable, categories | 5 |
CHD mortality MI event |
324 938 |
| Lin, 2017 103 | Survey of Health & Living Status of the Elderly | Taiwan | 4176 | ≥50 | 11 | Interview FFQ | Fruit and vegetable | 2 | CVD mortality | N/A |
| Liu, 2000 105 | Women's Health Study | United States |
39 127 (0:39 127) |
45–89 | 5 | Validated FFQ | Fruit and/or vegetable | 5 |
CVD incidence MI event |
418 126 |
| Liu, 2001 104 | The Physician's Health Study | United States |
15 520 (15 520:0) |
40–84 | 6 | Validated FFQ | Vegetable | 5 | CHD incidence | 1148 |
| Mann, 1997 106 | The Oxford Vegetarian Study | United Kingdom |
10 802 (4102:6700) |
16–79 | 13.3 | Validated FFQ | Fruit, vegetable, categories | 3 | IHD mortality | 64 |
| Manuel, 2015 107 | Canadian Community Health Survey | Canada |
82 259 (37 483:44 746) |
20–83 (men: 48.2; women: 49.4) |
8.6 | Interview FFQ | Fruit and vegetable | 3 | Stroke incidence | 1551 |
| Miller, 2017 108 | PURE (Prospective Urban and Rural Epidemiology) | 18 Countries† | 135 335 | 35–70 | 7.4 (Median) | Validated FFQ | Fruit and/or vegetable | 4 |
CVD events MI Stroke Cardiovascular mortality |
4784 N/A N/A N/A |
| Mink, 2007 109 | Iowa Women's Health | United States |
34 492 (0:34 492) |
55–69 | 16 |
Validated FFQ |
Fruit or vegetable categories | 3 |
CVD mortality CHD mortality Stroke mortality |
2316 1329 469 |
| Mizrahi, 2009 110 | Finnish Mobile Clinic Health Examination Survey | Finland | 3932 | 40–74 | 24 | Interview | Fruit, vegetable, categories | 4 | Stroke risk | 625 |
| Mori, 2018 111 | Japan Public Health Center Based Prospective Study | Japan |
88 184 (40 622:47 562) |
45–74 | 16.9 | Validated FFQ | Vegetable categories | 5 |
CHD mortality Stroke mortality |
1968 (1192:776) 1470 (856:614) |
| Mytton, 2018 112 | EPIC‐Norfolk | England |
22 992 (10 002:12 990) |
40–79 | 16.4 | 7‐d food record | Fruit and vegetable | 5 | CVD incidence | 4965 |
| Nagura, 2009 113 | Japan Collaborative | Japan |
59 485 (25 206:34 279) |
40–79 | 12.7 | Validated FFQ | Fruit, vegetable | 4 |
CVD mortality CHD mortality Stroke mortality |
2243 (1207:1036) 452 (258:194) 1053 (559:494) |
| Nakamura, 2008 114 | Takayama | Japan |
29 079 (13 355:15 724) |
≥35 (men: 54.0; women: 55.1) |
7.33 |
Validated FFQ |
Fruit, vegetable | 4 | CVD mortality |
384 (200:184) |
| Nechuta, 2010 115 | Shanghai Women's Health | China |
71 243 (0:71 243) |
40–70 | 9 |
Interview Validated FFQ |
Fruit and vegetable | Daily | CVD mortality | 775 |
| Neelakantan, 2018 116 | Singapore Chinese Health Study | China | 57 078 | 45–74 | 17 | Validated FFQ | Fruit or vegetable | 1 Serving/d | CVD mortality | 4871 |
| Ness, 2005 117 | Boyd Orr Cohort | United Kingdom (England and Scotland) |
4028 (1995:2033) |
3.5–11.2 | 37 | Household survey | Fruit, vegetable | 4 |
CHD mortality Stroke mortality |
298 83 |
| Nothlings, 2008 118 | EPIC |
Europe (10 countries)† |
10 262 | 35–70 | 9 | Validated FFQ | Fruit or vegetable | 80 g/d | CVD mortality | 517 |
| Okuda, 2015 119 | NIPPON DATA80 | Japan |
9112 (4000:5112) |
30–79 | 24 | Household survey | Fruit and/or vegetable | 4 |
CVD mortality CHD mortality Stroke mortality |
823 165 385 |
| Oude Griep, 2010 120 | MORGEN | The Netherlands | 19 819 | 20–59 | 10.5 | Validated FFQ | Fruit, vegetable | 4 | CHD incidences | 245 |
| Oude Griep, 2011 122 | MORGEN | The Netherlands |
20 069 (8988:11 081) |
20–68 | 10.3 | Validated FFQ | Fruit and vegetable | 4 | Stroke incidence | 233 |
| Oude Griep, 2011 121 | MORGEN | The Netherlands |
20 069 (8989:11 081) |
42±11 |
10.5 | Validated FFQ | Fruit or vegetable categories | 3–4 | CHD incidence | 245 |
| Oyebode, 2014 123 | HSE (Health Survey for England) | England |
65 226 (28 960:36 266) |
56.6±14.3 | 7.7 | 24‐h recall | Fruit and/or vegetable | 4 | CVD mortality | 1554 |
| Pham, 2007 124 | Miyako Study | Japan |
9651 (4254:5397) |
Men: 56.5±10.63; women: 57.4±10.89 (mean±SD) |
13.8 | Questionnaire | Fruit, vegetable | 2 | Stroke mortality | 226 |
| Rebello, 2014 125 | Singapore Chinese Health Study | China |
53 469 (23 501:29 968) |
45–7 | 15 | Interview validated FFQ | Fruit and vegetable | 5 | IHD mortality | 1660 (1022:638) |
| Rissanen, 2003 126 | Kuopio Ischaemic Heart Disease Risk Factor | Finland |
1950 (1950:0) |
42–60 | 12.8 | 4‐d food record | Fruit and vegetable | 5 | CVD mortality |
115 |
| Saglimbene, 2017 127 | DIET‐HD | Europe and South America | 9757 | N/A | 1.5 | Validated FFQ | Fruit, categories | 2 | CVD mortality | N/A |
| Sahyoun, 1996 128 | Nutrition Status Study | United States | 680 | 60–101 | 9–12 | 3‐d food record | Fruit, vegetable, categories | 3 | CHD mortality | 101 |
| Sauvaget, 2003 129 | Life Span Study | Japan |
39 337 (14 966:23 471) |
34–103 | 16 | Validated FFQ | Fruit, vegetable, categories | 3 | Stroke mortality |
1926 (692:1234) |
| Scheffers, 2019 130 |
EPIC Netherlands and MORGEN |
The Netherlands |
34 560 (25 574:8986) |
20–69 | 14.6 | Validated FFQ | Fruit and categories | 5 |
CVD incidence CHD incidence Stroke incidence |
3801 2135 1135 |
| Sesso, 2003 131 | WHS (Women's Health Study) | United States |
38 445 (0:38 445) |
45–89 | 6.9 | Validated FFQ |
Fruit or vegetable categories |
4 | CVD incidence | 729 |
| Sesso, 2003 133 | WHS | United States |
38 445 (0:38 445) |
≥45 | 7.2 | Validated FFQ |
Vegetable categories |
5 |
CVD incidence MI incidence Stroke incidence |
729 201 247 |
| Sesso, 2007 132 | WHS | United States |
38 176 (0:38 176) |
54.5 | 10.1 | Validated FFQ | Fruit category | 4 |
CVD mortality CVD incidence MI incidence Stroke incidence |
204 1004 289 339 |
| Shah, 2018 134 | Cooper Center Longitudinal Study | United States |
11 376 (8577:2799) |
47 | 18 | 3‐d food record | Fruit or vegetable | Continuous | CVD mortality | 249 |
| Sharma, 2013 135 | Multi Ethnic Cohort | United States |
174 028 (78 410:95 618) |
45–75 | 7.5 | Validated FFQ | Fruit, vegetable | 5 | Stroke mortality | 860 (434:426) |
| Sharma, 2014 136 | Multi Ethnic Cohort | United States |
164 617 (72 866:91 751) |
45–75 | 5–8 | Validated FFQ | Fruit, vegetable | 5 | IHD mortality |
1951 (1140:811) |
| Simila, 2013 137 | ATBC | Finland |
21 955 (21 955:0) |
50–69 | 19 | Validated FFQ | Fruit, fruit juices | Daily | CHD risk | 4379 |
| Sonestedt, 2015 138 | Malmo Diet and Cancer | Sweden |
26 445 (10 048:16 397) |
44–74 | 14 | Validated FFQ | Fruit, vegetable | 5 |
CVD incidence CHD incidence Stroke incidence |
2921 N/A N/A |
| Sotomayor, 2018 139 | Renal Transplant Recipients | The Netherlands |
400 (217:183) |
52±12 | 7.2 | Unvalidated FFQ | Fruit | 3 | CVD mortality | 49 |
| Steffen, 2003 140 | ARIC (Atherosclerosis Risk in Communities) | United States |
11 940 (5271:6669) |
45–64 (men: 54.4±5.7; women: 54.1±5.7) |
11 |
Interview validated FFQ |
Fruit and vegetable | 5 |
CHD incidence Ischemic stroke incidence |
535 214 |
| Stefler, 2016 141 | HAPIEE (Health, Alcohol and Psychosocial Factors in Eastern Europe) | Poland, Russia, Czech Republic | 19 263 | 57 | 7.1 | Validated FFQ | Fruit and/or vegetable | 4 |
CVD mortality CHD mortality Stroke mortality |
438 226 109 |
| Strandhagen, 2000 142 | Men Born in 1913 | Sweden |
730 (730:0) |
54 | 26 | Interview unvalidated FFQ | Fruit, vegetable | 5 |
CVD mortality CVD incidence |
226 209 |
| Takachi, 2008 143 | Japan Public Health Center Based Prospective Study | Japan |
77 891 (35 909:41 982) |
45–74 | 5.9 | Validated FFQ | Fruit and/or vegetable, categories | 4 | CVD incidence | 1386 (830:556) |
| Tanaka, 2013 144 | Japan Diabetes Complications Study | Japan | 1414 | 40–70 | 8.1 (Median) | Validated FFQ | Fruit and vegetable | 4 |
CHD incidence Stroke incidence |
96 68 |
| Tognon, 2014 145 | MONICA | Denmark |
1849 (901:948) |
30–59 | 11 | Food record | Fruit, vegetable | 2 |
CVD mortality CVD incidence MI mortality MI incidence Stroke mortality Stroke incidence |
223 755 64 161 40 167 |
| Tucker, 2005 146 | Baltimore Longitudinal Study of Aging | United States |
501 (501:0) |
34–80 | 18 | 7‐d food record | Fruit and/or vegetable | 2 | CHD mortality | 71 |
| Von Ruesten, 2013 147 | EPIC | Germany |
23 531 (9098:14 433) |
35–65 | 8 | Validated FFQ | Fruit, vegetable, categories | Daily | CVD incidence | 363 |
| Vormund, 2015 148 | MONICA | Switzerland |
17 861 (8663:9198) |
16–92 | 21.4 (Mean) | 24‐h recall |
Fruit, vegetable |
Daily | CVD mortality | 1385 |
| Wang, 2016 149 | Linxian Nutrition Intervention Trials | China |
2455 (1105:1340) |
40–69 | 19–26 | Unvalidated FFQ | Fruit and/or vegetable, categories | 2 |
CHD mortality Stroke mortality |
355 (men) 452 (women) |
| Watkins, 2000 150 | CPS‐11 (Cancer Prevention Study 11) | United States |
1 063 023 (453 962:609 061) |
≥30 | 7 | Unvalidated FFQ | Vegetable | CHD mortality |
13 761 (9156:4605) |
|
| Whiteman, 1999 151 | OXCHECK | United Kingdom |
10 522 (4929:5593) |
35–64 | 9 | Unvalidated FFQ | Fruit, green vegetables | 2–3 | IHD mortality | 144 |
| Yamada, 2011 152 | Jidni Medical School Cohort | Japan |
10 623 (4147:6476) |
N/A | 10.7 | Validated FFQ | Fruit category | 5 |
CVD event MI event Stroke event |
758 (270:488) 565 (383:182) 99 (76:23) |
| Yokoyama, 2000 153 | Shibata Study | Japan |
2121 (880:1241) |
≥40 | 20 | Unvalidated FFQ | Fruit, vegetable | 3 | Stroke incidence | 196 (91:105) |
| Yoshizaki, 2019 154 | Japan Public Health Centre Based Prospective Study | Japan |
16 498 (7726:8772) |
45–74 | 14 | Validated FFQ | Fruit and/or vegetable | 3 |
CHD incidence Stroke incidence |
839 197 |
| Yu, 2014 155 | Shanghai Men and Women's Health Study | China |
122 635 (55 424:67 211) |
40–74 | 5.4–9.8 | Interview validated FFQ | Fruit and/or vegetable, categories | 4 | CHD incidence | 365 (217:148) |
| Zhang, 2011 156 | Shanghai Men and Women's Health Study | China |
134 796 (61 436:73 360) |
40–74 40–70 |
4.5 10.2 |
Interview validated FFQ |
Fruit, vegetable, and categories |
5 | CVD mortality | 5393 (1951:3442) |
| Zhang, 2011 157 | MONICA | Finland |
36 686 (17 287:19 399) |
25–74 | 13.7 | 24‐h recall | Fruit, veg | 4 | Stroke incidence | 1478 |
ATBC, alpha‐tocopherol, beta‐carotene cancer prevention; CeVD indicates cerebrovascular disease; CHD, coronary heart disease; CVD, cardiovascular disease; DIET‐HD, dietary intake, death and hospitalization in adult with early stage kidney disease treated with hemodialysis; EPIC, European Prospective Investigation Into Cancer and Nutrition; FFQ, Food Frequency Questionnaire; IHD, ischemic heart disease; MI, myocardial infarction; MONICA, monitoring of trends and determinants in cardiovascular disease; MORGEN, monitoring project on risk factors for chronic diseases; N/A, Not Available; NHANES, National Health and Nutrition Examination Survey; NIPPON DATA80, National Integrated Project for Prospective Observation of non‐communicable disease and its trends in aged; and OXCHECK, Oxford and Collaborators health check.
The EPIC cohort represented the following countries: France, Germany, Greece, Italy, the Netherlands, Spain, United Kingdom, Sweden, Denmark, and Norway.
The PURE cohort represented the following countries: high income: Canada, Sweden, United Arab Emirates; middle income: Argentina, Brazil, Chile, China, Colombia, Iran, Malaysia, Poland, South Africa, Turkey, Occupied Palestinian Territory; low income: Bangladesh, India, Pakistan, Zimbabwe.
Table S2 lists the variables that were statistically adjusted in the included studies. Age, the prespecified primary confounding variable, was adjusted for in 95% of included studies, of which 55% also adjusted for all 9 of the prespecified secondary confounding variables.
Study Quality
Table S3 summarizes the NOS assessment of included studies. There was a high risk of bias in associations between fruit juice, cruciferous, green leafy, and tomato vegetables, and CHD and stroke mortality and citrus and stroke mortality as >35% of the pooled risk estimate was derived from Iso et al, 84 which was scored 5 on the NOS. The association between apricots and CVD mortality was derived from one study, Saglimbene et al, 158 which was scored 1 on the NOS. Although most studies had scores reduced because of self‐administered ascertainment of exposure, 88% of studies received a total score ≥6, which was considered high quality.
Cardiovascular Disease
CVD Incidence
Figure 2 and Figures S1 through S11 show the relation of total and specific fruit and vegetables with CVD incidence. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.93 [95% CI, 0.89–0.96], no significant heterogeneity), fruits (RR, 0.91 [95% CI, 0.88–0.95], no significant heterogeneity), and vegetables (RR, 0.94 [95% CI, 0.90–0.97], no significant heterogeneity). Figures S12 and S13 summarize the relation of sources of fruit or vegetables with CVD incidence. A significant interaction by fruit source was observed (P<0.001), with significant associations with lower risk limited to citrus (RR, 0.88 [95% CI, 0.80–0.86], no significant heterogeneity) and pommes (RR, 0.76 [95% CI, 0.66–0.88], no significant heterogeneity). We found no significant associations from the highest versus lowest intakes of berries (RR, 1.27 [95% CI, 0.95–1.71], heterogeneity not applicable) and juice (RR, 1.00 [95% CI, 0.93–1.07], no significant heterogeneity) fruit. No interaction by vegetable source was observed (P=0.227).
Figure 2. Relation between intake of fruits and vegetables and total incident cardiovascular disease (CVD) (highest vs lowest level of intake).
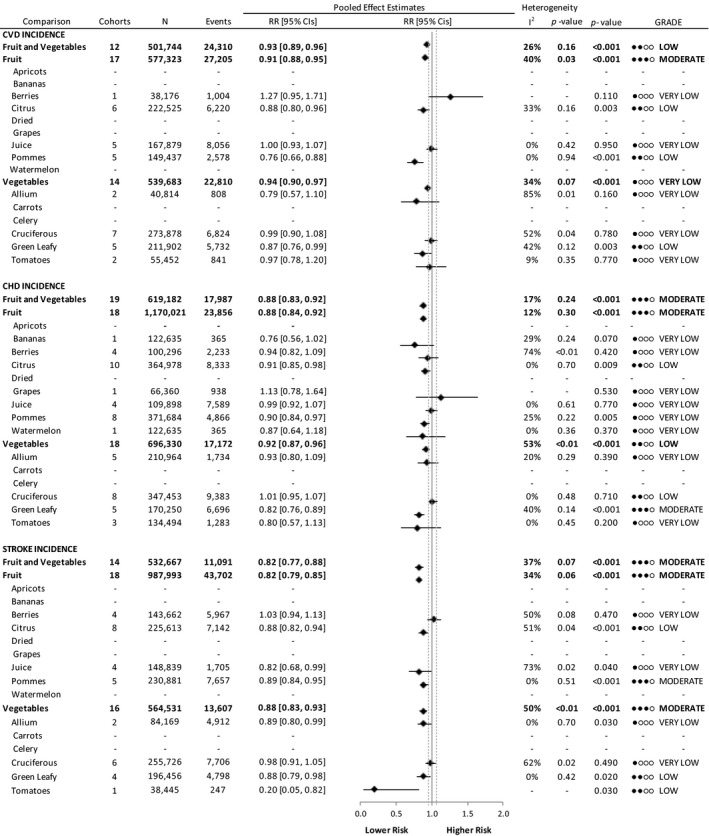
Pooled risk estimates are represented by the black diamond, with principal exposures highlighted in bold. Principal exposures (fruits and vegetables, fruits, and vegetables) represent the pooled data of the risk estimates reported for these exposures and were not tabulated by pooling fruit and vegetable varieties. Values of I2≥50% indicate substantial heterogeneity, with significance at P>0.10. The mean important difference of 5% change in relative risk, indicating a clinically relevant association with lower or higher risk, is indicated by the dashed gray lines. CHD indicates coronary heart disease; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; and RR, risk ratio.
CVD Mortality
Figure 3 and Figures S14 through S30 show the relation of total and specific fruit and vegetables with CVD mortality. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.89 [95% CI, 0.85–0.93], substantial heterogeneity [I2=68%, P<0.001]), fruits (RR, 0.88 [95% CI, 0.86–0.91], substantial heterogeneity [I2=79%, P<0.001]), and vegetables (RR, 0.87 [95% CI, 0.85–0.90], substantial heterogeneity [I2=59%, P<0.001]). Figures S31 and S32 summarize the association of sources of fruits or vegetables with CVD mortality. A significant interaction by fruit (P=0.001) and vegetable sources (P<0.001) was observed with significant associations with lower risk limited to pommes fruit (RR, 0.86 [95% CI, 0.80–0.92], no significant heterogeneity) and to allium (RR, 0.33 [95% CI, 0.22–0.49], heterogeneity not applicable), cruciferous (RR, 0.85 [95% CI, 0.82–0.89], no significant heterogeneity), and green leafy (RR, 0.87 [95% CI, 0.81–0.94], substantial heterogeneity [I2=88%, P<0.001]) vegetables. There was a significant increased risk with CVD mortality from the highest versus lowest intake of apricots (RR, 1.84 [95% CI, 1.27–2.67], heterogeneity not applicable). We found no significant associations from the highest versus lowest intakes of bananas (RR, 1.06 [95% CI, 0.87–1.29], heterogeneity not applicable), berries (RR, 0.97 [95% CI, 0.92–1.03], no significant heterogeneity), citrus (RR, 0.95 [95% CI, 0.90–1.02], substantial heterogeneity [I2=62%, P=0.049]), juice (RR, 0.81 [95% CI, 0.58–1.13], heterogeneity not applicable), and grapes (RR, 0.90 [95% CI, 0.81–1.01], substantial heterogeneity [I2=61%, P=0.077) fruit and carrots (RR, 0.92 [95% CI, 0.85–1.01], no significant heterogeneity), celery (RR, 0.91 [95% CI, 0.83–1.01], heterogeneity not applicable), and tomato (RR, 0.98 [95% CI, 0.93–1.04], no significant heterogeneity) vegetables.
Figure 3. Relation between intake of fruits and vegetables and cardiovascular mortality (highest vs lowest level of intake).
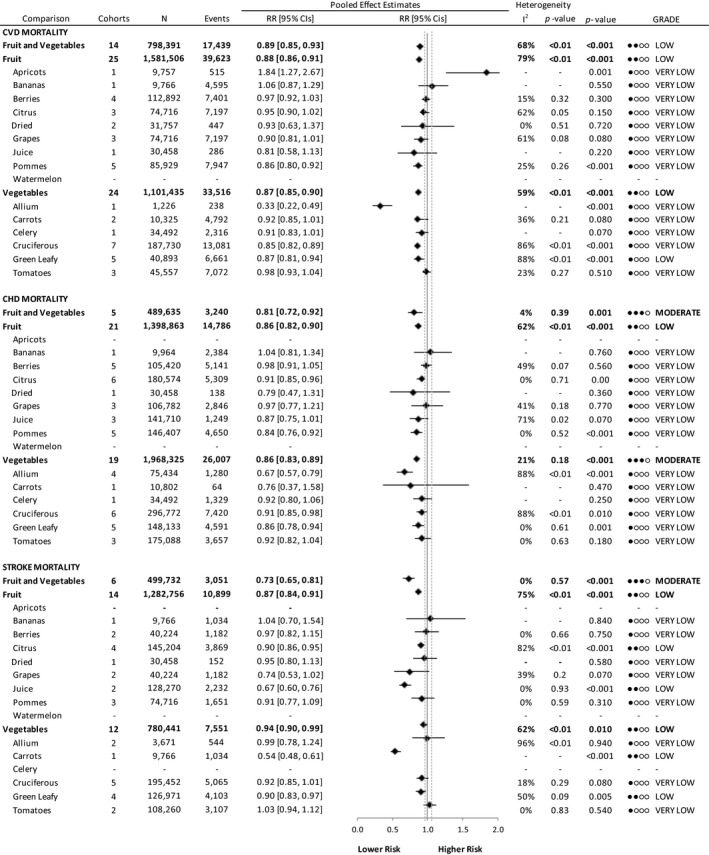
Pooled risk estimates are represented by the black diamond, with principal exposures highlighted in bold. Principal exposures (fruits and vegetables, fruits, and vegetables) represent the pooled data of the risk estimates reported for these exposures and were not tabulated by pooling fruit and vegetable varieties. Values of I2≥50% indicate substantial heterogeneity, with significance at P>0.10. The mean important difference of 5% change in relative risk, indicating a clinically relevant association with lower or higher risk, is indicated by the dashed gray lines. CHD indicates coronary heart disease; CVD, cardiovascular disease; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; and RR, risk ratio.
Figures S33 through S55 show the dose‐response analyses for total and specific fruit and vegetables and CVD incidence and mortality. A nonlinear model best fit the data for citrus fruit and incident CVD (P=0.033), with a plateau at 0.5 servings/day, total fruits and vegetables with CVD mortality (P<0.001), with a plateau at 4 daily servings, and fruits and CVD mortality (P=0.003), with a plateau in risk reduction after 2 daily servings. An inverse dose‐response gradient was found for the following associations: total fruits and vegetables (RR, 0.97 [95% CI, 0.96–0.99] per serving/day), fruits (RR, 0.97 [95% CI, 0.95–0.99] per serving/day), pommes (RR, 0.87 [95% CI, 0.75–0.99] per serving/day), and green leafy vegetables (RR, 0.72 [95% CI, 0.56–0.93]) with CVD incidence and total fruits and vegetables (RR, 0.72 [95% CI, 0.56–0.93] per serving/day), fruits (RR, 0.92 [95% CI, 0.89–0.96] per serving/day), and vegetables (RR, 0.94 [95% CI, 0.92–0.97] per serving/day) with CVD mortality.
Coronary Heart Disease
CHD Incidence
Figure 2 and Figures S56 through S69 show the relation of total and specific fruit and vegetables with CHD incidence. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.88 [95% CI, 0.83–0.92], no significant heterogeneity), fruits (RR, 0.88 [95% CI, 0.84–0.92], no significant heterogeneity), and vegetables (RR, 0.92 [95% CI, 0.87–0.96], substantial heterogeneity [I2=53%, P=0.002]). Figures S70 and S71 summarize the relation of sources of fruits or vegetables with CHD incidence. No interaction by fruit source was observed (P=0.375). A significant interaction by vegetable sources was seen (P<0.001) with significant associations with lower risk limited to green leafy vegetables (RR, 0.82 [95% CI, 0.76–0.89], no significant heterogeneity). We found no significant associations from the highest versus lowest intakes of allium (RR, 0.93 [95% CI, 0.80–1.09], no significant heterogeneity), cruciferous (RR, 1.01 [95% CI, 0.95–1.07], no significant heterogeneity), and tomato (RR, 0.80 [95% CI, 0.57–1.13], no significant heterogeneity) vegetables.
CHD Mortality
Figure 3 and Figures S72 through S87 show the relation of total and specific fruit and vegetables with CHD mortality. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.81 [95% CI, 0.72–0.92], no significant heterogeneity), fruits (RR, 0.86 [95% CI, 0.82–0.90], substantial heterogeneity [I2=62%, P<0.001]), and vegetables (RR, 0.86 [95% CI, 0.83–0.89], no significant heterogeneity). Figures S88 and S89 summarize the relation of sources of fruits or vegetables with CHD mortality. No significant interaction was found by fruit sources (P=0.144). A significant interaction by vegetable source was seen (P=0.023), with significant associations with lower risk limited to allium (RR, 0.67 [95% CI, 0.57–0.79], substantial heterogeneity [I2=88%, P<0.001]), cruciferous (RR, 0.91 [95% CI, 0.85–0.98], substantial heterogeneity [I2=88%, P<0.001]), and green leafy (RR, 0.86 [95% CI, 0.78–0.94], no significant heterogeneity) vegetables. We found no significant associations from the highest versus lowest intakes of carrots (RR, 0.76 [95% CI, 0.37–1.58], heterogeneity not applicable), celery (RR, 0.92 [95% CI, 0.80–1.06], heterogeneity not applicable), and tomato (RR, 0.92 [95% CI, 0.82–1.04], no significant heterogeneity) vegetables.
Figures S90 through S116 show the dose‐response analyses for fruit and vegetables and CHD incidence and mortality. A nonlinear model best fit the data for citrus fruit (P=0.005) and green leafy vegetables (P=0.004) and incident CHD and total fruits and vegetables and CHD mortality (P=0.044), with plateaus in risk reductions following 0.5, 0.5, and 3 daily servings, respectively. An inverse dose‐response was found in the associations between total fruits and vegetables (RR, 0.97 [95% CI, 0.96–0.98] per serving/day), fruits (RR, 0.96 [95% CI, 0.93–0.99] per serving/day), vegetables (RR, 0.98 [95% CI, 0.95–0.99] per serving/day), and green leafy vegetables (RR, 0.85 [95% CI, 0.76–0.94] per serving/day) with CHD incidence and fruits (RR, 0.94 [95% CI, 0.90–0.97] per serving/day) and vegetables (RR, 0.89 [95% CI, 0.83–0.96] per serving/day) with CHD mortality.
Stroke
Stroke Incidence
Figure 2 and Figures S117 through S127 show the relation of total and specific fruit and vegetables with stroke incidence. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.82 [95% CI, 0.77–0.88], no significant heterogeneity), fruits (RR, 0.82 [95% CI, 0.79–0.85], no significant heterogeneity), and vegetables (RR, 0.88 [95% CI, 0.83–0.93], substantial heterogeneity [I2=50%, P=0.006]). Figures S128 and S129 summarize the relation of sources of fruits or vegetables with stroke incidence. A significant interaction by fruit (P=0.017) and vegetable sources (P=0.044) was observed with significant associations with lower risk limited to citrus (RR, 0.88 [95% CI, 0.82–0.94], substantial heterogeneity [I2=51%, P=0.04]), juice (RR, 0.82 [95% CI, 0.68–0.99], substantial heterogeneity [I2=73%, P=0.02]), and pommes (RR, 0.89 [95% CI, 0.84–0.95], no significant heterogeneity) fruit and to allium (RR, 0.89 [95% CI, 0.80–0.99], no significant heterogeneity), green leafy (RR, 0.88 [95% CI, 0.79–0.98], no significant heterogeneity), and tomato (RR, 0.20 [95% CI, 0.05–0.82], heterogeneity not applicable) vegetables. We found no significant associations from the highest versus lowest intakes of berries (RR, 1.03 [95% CI, 0.94–1.13], substantial heterogeneity [I2=50%, P=0.078]) fruit and cruciferous (RR, 0.98 [95% CI, 0.91–1.05], substantial heterogeneity [I2=62%, P=0.022]) vegetables.
Stroke Mortality
Figure 3 and Figures S130 through S144 show the relation of total and specific fruits and vegetables with stroke mortality. We found a lower risk associated with the highest versus the lowest intakes of fruits and vegetables (RR, 0.73 [95% CI, 0.65–0.81], no significant heterogeneity), fruits (RR, 0.87 [95% CI, 0.84–0.91], substantial heterogeneity [I2=75%, P<0.001]), and vegetables (RR, 0.94 [95% CI, 0.90–0.99], substantial heterogeneity [I2=62%, P=0.001]). Figures S145 and S146 summarize the relation of sources of fruit or vegetables with stroke mortality. A significant interaction by fruit (P<0.001) and vegetable sources (P<0.001) was observed with significant associations, with lower risk limited to citrus (RR, 0.90 [95% CI, 0.86–0.95], substantial heterogeneity [I2=82%, P<0.001]) and juice (RR, 0.67 [95% CI, 0.60–0.76], no significant heterogeneity) fruit and carrots (RR, 0.54 [95% CI, 0.48–0.61], heterogeneity not applicable) and green leafy (RR, 0.90 [95% CI, 0.83–0.97], substantial heterogeneity [I2=50%, P=0.09]) vegetables. We found no significant associations from the highest versus lowest intakes of bananas (RR, 1.04 [95% CI, 0.70–1.54], heterogeneity not applicable), berries (RR, 0.97 [95% CI, 0.82–1.15], no significant heterogeneity), grapes (RR, 0.74 [95% CI, 0.53–1.02], no significant heterogeneity), and pommes (RR, 0.91 [95% CI, 0.77–1.09], no significant heterogeneity) fruit and allium (RR, 0.99 [95% CI, 0.79–1.24], substantial heterogeneity [I2=96%, P<0.001]), cruciferous (RR, 0.92 [95% CI, 0.85–1.01], no significant heterogeneity), and tomato (RR, 1.03 [95% CI, 0.94–1.12], no significant heterogeneity) vegetables.
Figures S147 through S171 show the dose‐response analyses for fruit and vegetables and stroke mortality and incidence. A nonlinear model best fit the data for citrus fruit (P=0.039) and vegetables (P=0.012) and stroke incidence and fruit (P<0.001) and green leafy (P=0.043) vegetables and stroke mortality, with plateaus in risk reductions following 0.5, 1, 2, and >0.7 daily servings, respectively. An inverse dose‐response gradient was found in the associations between total fruits and vegetables (RR, 0.95 [95% CI, 0.92–0.98] per serving/day), fruits (RR, 0.92 [95% CI, 0.88–0.96] per serving/day), citrus fruit (RR, 0.83 [95% CI, 0.69–0.98] per serving/day), pommes (RR, 0.87 [95% CI, 0.79–0.96] per serving/day), green leafy vegetables (RR, 0.88 [95% CI, 0.79–0.97] per serving/day), and tomatoes (RR, 0.67 [95% CI, 0.52–0.87] per serving/day) with stroke incidence and fruits and vegetables (RR, 0.93 [95% CI, 0.88–0.98] per serving/day), fruits (RR, 0.85 [95% CI, 0.78–0.92] per serving/day), vegetables (RR, 0.93 [95% CI, 0.87–0.99] per serving/day), citrus fruit (RR, 0.67 [95% CI, 0.57–0.80] per serving/day), fruit juice (RR, 0.54 [95% CI, 0.36–0.89] per serving/day), carrots (RR, 0.44 [95% CI, 0.28–0.69] per serving/day), and green leafy vegetables (RR, 0.85 [95% CI, 0.73–0.98] per serving/day) with stroke mortality.
Sensitivity Analyses
The systematic removal of each study did not modify the direction or significance of the association estimates or the evidence for heterogeneity (data not shown).
Subgroup Analyses
Figures S172 through S188 illustrate a priori categorical subgroup analyses. There were no statistically significant subgroup differences. Inverse associations were predominately limited to studies with statistical adjustments of ≥8 potential confounders. Confining analyses to studies using validated exposure assessment techniques did not alter the associations. No effect modification was seen by sex, age, follow‐up duration, NOS, or study location.
Publication Bias
Figures S189 through S205 illustrate publication bias analyses for comparisons with at least 10 observations. Visual inspection and formal analysis with the Begg and Egger test did not show evidence of publication bias in any comparison, except for vegetable intake with CVD (PBegg=0.015, PEgger=0.004), CHD (PBegg=0.018, PEgger=0.004), and stroke (PBegg=0.545, PEgger=0.018) mortality and fruit intake with stroke mortality (PBegg=0.820, PEgger=0.031), which were subsequently unsupported by the trim and fill test.
Grading of Recommendations Assessment, Development, and Evaluation
Figures 2 and 3 and Tables S4 through S9 summarize the GRADE assessments. The certainty of the evidence was rated as “moderate” for 11, “low” for 21, and “very low” for 52 of the exposure‐outcome relationships. Our certainty in the evidence was strongest for the associations of total fruits and vegetables with lower risks of CHD incidence and CHD and stroke mortality; fruits with lower risks of CVD, CHD, and stroke incidence; vegetables with lower risks of CHD mortality and stroke incidence; pommes fruit with lower risks of stroke incidence; and green leafy vegetables with lower risks of CHD incidence. The evidence was rated as “moderate” in each case, because of an upgrade for dose‐response gradient in the absence of any downgrades. The associations for specific types of fruits and vegetables were rated largely as “very low,” because of downgrades for imprecision, risk of bias, indirectness, and/or inconsistency. The fixed effects model improved our certainty in the evidence for fruit and CVD incidence by improving precision of the pooled risk estimate. There were no other marked differences between the random effects and fixed effects models.
DISCUSSION
We conducted a systematic review and meta‐analysis of 81 unique prospective cohorts involving 4 031 896 individuals and 125 112 cardiovascular events to assess the relation of total and specific fruit and vegetable consumption on CVD incidence and mortality outcomes. Pooled analyses of highest versus lowest consumption illustrate a lower risk in CVD, CHD, and stroke incidence or mortality by 7% to 27% from total fruit and vegetable intake, 9% to 18% from fruit intake, and 5% to 14% from vegetable intake. Of the specific fruit sources, highest versus lowest intakes of citrus and pommes fruit showed significant risk reductions in most CVD outcomes, from 9% to 12% and from 10% to 24%, respectively, and fruit juice showed a significant risk reduction in stroke incidence and mortality by 18% and 33%, respectively. Most notably of the vegetable categories, one daily serving of green leafy vegetables was associated with 12% to 18% risk reduction in CVD, CHD, and stroke incidence and CHD mortality. There was a consistent linear dose‐response between fruits and vegetables and CHD, with a maximum daily intake of 7 fruit and 7 vegetable servings showing a risk reduction of ≈20% and ≈30% in CHD incidence and mortality, respectively.
Findings in the Context of Existing Literature
Our findings are consistent with those of previous systematic review and meta‐analyses, which also detected inverse associations between fruits and/or vegetables and CVD mortality and incident outcomes. 11 , 15 , 160 Our analyses were in line with those reported most recently by Aune et al, who observed the lowest risk on CVD, CHD, and stroke from maximum intakes of total fruits and vegetables. 10 This is despite our division of CVD outcomes differing significantly, with the present study distinguishing between mortality and incidence data. Our findings on individual fruits and vegetables were also relatively consistent, highlighting a high versus low intake of citrus and pommes fruit, fruit juice, and green leafy vegetables as protective on CVD outcomes, suggesting they may independently play a valuable role in the diet. Nonetheless, the current study benefited from the inclusion of updated and novel large prospective cohorts, namely, the SUN (Seguimiento University of Navarra) 160 and PURE (Prospective Urban and Rural Epidemiology) 161 cohorts, which combined contributed an additional 152 342 individuals and 4896 events to our analyses.
Numerous mechanisms have been proposed to explain the benefits of fruit and vegetable consumption on the cardiovascular system. Perhaps the most supported hypothesis is through their essential contribution to total dietary fiber, an established modifier of CVD risk factors. 163 , 164
Fruits with highlighted benefits in the present review tend to be of low glycemic index, a characteristic with demonstrated CVD risk factor reductions. 164 Their consumption has also been associated with improved weight management 165 and decreased prevalence of obesity, 166 a risk factor attributed to 7% to 44% of CVD incidence, 167 likely because of their low energy density and displacement of high calorie foods in the diet. The relationships between the extensive list of micronutrients offered by fruits and vegetables and CVD risk reduction has also been widely explored. They are a key source of antioxidants in the diet, necessary for eradicating free radicals, and may defend against damaging lipid oxidation. 168 Individual sources may offer distinct benefits, such as green leafy vegetables, which are dense in dietary nitrates, a compound linked to reductions in early prognostic markers of CVD. 170 , 171 , 172 Interestingly, however, we did not observe a benefit from high consumption of berries as the most concentrated fruit source of antioxidants. Several vasoactive minerals, such as potassium, magnesium, and calcium, are also obtained from fruits and vegetables in the diet. 173 , 174 , 175 Although each mechanism may be individually biologically plausible, the complexity of the nutrient combinations cannot be underestimated. A whole food approach is necessary to evaluate their efficacy in CVD risk reduction as it can account for additive and multiplicative mechanisms.
Strengths and Limitations
Our systematic review and meta‐analysis has several strengths. It provides a comprehensive synthesis of the available knowledge on consumption of fruits, vegetables, and their varieties and CVD outcomes of importance to public health and clinical practice. We included a systematic search strategy to ensure all published prospective cohort data were identified and used a priori established approaches to explore the pooled risk estimates, including dose‐response analyses. Finally, the certainty of the evidence was assessed using the GRADE approach with the evidence upgraded in several cases for the presence of a protective inverse dose‐response gradient for the association of total fruits and vegetables, fruits, vegetables, and green leafy vegetables with CVD outcomes.
There are also several limitations of our systematic review and meta‐analysis. Although ≈90% of the included prospective cohort studies were of high quality, residual confounding (measured and unmeasured) cannot be ruled out in observational studies. This issue is addressed in the GRADE assessment, which starts observational studies as “low” certainty. We downgraded the certainty of evidence because of imprecision in 55 of the 84 associations as the upper 95% CI crossed the minimal clinically important difference of a 5% reduction in relative risk, from which evidence of harm could not be excluded in 30 associations. Because of limited number of observations, indirectness was also present in several cases and the lack of reported exposures for different tropical fruit limited our exploration of this fruit category. Another source of uncertainty leading to downgrades in the evidence was the presence of high risk of bias in several of the studies that presented data on specific sources of fruits and vegetables. Last, the evidence was downgraded for inconsistency based on the presence of substantial unexplained heterogeneity in 19 of the 84 associations.
Balancing the strengths and limitations, the certainty of the evidence was rated as “very low” to “low” for most of the exposure‐outcome relationships for the association of fruits and vegetables with cardiovascular outcomes. The highest (“moderate”) rated evidence was for the cardiovascular benefit of total fruits and vegetables, fruits, vegetables, pommes fruit, and green leafy vegetables. The least certainty was for other specific fruit and vegetable sources.
Implications
Addressing the low prevalence of adequate fruit and vegetable consumption remains an important global health target. 175 With average intakes of 1 and 1.7 servings of fruit and vegetables per day, respectively, in developed countries, such as the United States, 150 there is an opportunity to increase intakes to meet the established minimum recommendations of 5 daily servings and realize the cardiovascular benefits. 176 We observed a linear dose relationship between fruits and vegetables and CHD and stroke risk, suggesting an increased cardiovascular benefit with additional servings and that targets beyond “5 a day” should also be considered. Successful strategies for increasing fruit and vegetable intake, nevertheless, are lacking and may benefit from emphasizing a larger variety of sources. Our synthesis highlighted that different sources of fruit, including 100% fruit juice, are associated with comparable CVD risk reduction as that of vegetables. Public health guidance to limit the intake of certain fruit sources because of concerns related to their contribution to sugars may have unintended harm in preventing people from meeting fruit and vegetable targets for CVD risk reduction.
Conclusions
Current evidence supports the role of a variety of fruits and vegetables for CVD prevention. Higher intakes of fruits and/or vegetables are associated with improvements in all CVD outcomes, with fruit associated with the largest risk reductions. Greater benefits may be seen for some fruits, including citrus, pommes, and 100% fruit juice, and vegetables, including allium, cruciferous, and green leafy vegetables, supporting recommendations for emphasizing specific fruit and vegetable sources in dietary guidelines. No fruit and vegetable sources were adversely associated with CVD, including fruit sources of concern, such as 100% fruit juice and dried fruit. Our certainty in the evidence ranges from “very low” to “moderate,” with the least certainty for specific sources of fruits and vegetables and the highest certainty for broad categories. More research of specific food sources of fruits and vegetables is needed to improve our estimates.
Sources of Funding
This work was funded by the Canadian Institutes of Health Research (funding reference number, 129920). The Diet, Digestive Tract, and Disease Centre, funded through the Canada Foundation for Innovation and the Ministry of Research and Innovation's Ontario Research Fund, provided the infrastructure for the conduct of this project. Zurbau was funded by the Banting & Best Diabetes Centre Tamarack Graduate Award. Sievenpiper was funded by a PSI Graham Farquharson Knowledge Translation Fellowship, Diabetes Canada Clinician Scientist Award, Canadian Institutes of Health Research Institute of Nutrition, Metabolism and Diabetes (INMD) and the Canadian Nutrition Society (CNS) New Investigator Partnership Prize, and Banting & Best Diabetes Centre Sun Life Financial New Investigator Award.
Disclosures
Zurbau is a part‐time employee at INQUIS Clinical Research Ltd., a contract research organization. Khan reports he has received research support from the Canadian Institutes of Health Research (CIHR), the International Life Science Institute (ILSI), and National Honey Board. He has been an invited speaker at the Calorie Control Council Annual meeting for which he has received an honorarium. Vuksan has a Canadian (2410556) and American (7326.404) patent on the medical use of viscous fiber blend for reducing blood glucose for treatment of diabetes mellitus, increasing insulin sensitivity, and reduction in systolic blood pressure and blood lipids issued. Kendall has received grants or research support from the Advanced Food Materials Network, Agriculture and Agri‐Foods Canada, Almond Board of California, American Peanut Council, Barilla, CIHR, Canola Council of Canada, International Nut and Dried Fruit Council, International Tree Nut Council Research and Education Foundation, Loblaw Brands Ltd, Pulse Canada, and Unilever. He has received in‐kind research support from the Almond Board of California, American Peanut Council, Barilla, California Walnut Commission, Kellogg Canada, Loblaw Companies, Quaker (Pepsico), Primo, Unico, Unilever, and White Wave Foods/Danone. He has received travel support and/or honoraria from the American Peanut Council, Barilla, California Walnut Commission, Canola Council of Canada, General Mills, International Nut and Dried Fruit Council, International Pasta Organization, Loblaw Brands Ltd, Nutrition Foundation of Italy, Oldways Preservation Trust, Paramount Farms, Peanut Institute, Pulse Canada, Sun‐Maid, Tate & Lyle, Unilever, and White Wave Foods. He has served on the scientific advisory board for the International Tree Nut Council, International Pasta Organization, McCormick Science Institute, and Oldways Preservation Trust. He is a member of the International Carbohydrate Quality Consortium, Executive Board Member of the Diabetes and Nutrition Study Group of the European Association for the Study of Diabetes (EASD), is on the Clinical Practice Guidelines Expert Committee for Nutrition Therapy of the EASD, and is a Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. Jenkins has received research grants from Saskatchewan Pulse Growers, the Agricultural Bioproducts Innovation Program through the Pulse Research Network, the Advanced Foods and Material Network, Loblaw Companies Ltd, Unilever, Barilla, the Almond Board of California, Agriculture and Agri‐food Canada, Pulse Canada, Kellogg's Company, Canada, Quaker Oats, Canada, Procter & Gamble Technical Centre Ltd, Bayer Consumer Care, Springfield, NJ, Pepsi/Quaker, International Nut & Dried Fruit (INC), Soy Foods Association of North America, the Coca‐Cola Company (investigator‐initiated, unrestricted grant), Solae, Haine Celestial, the Sanitarium Company, Orafti, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Soy Nutrition Institute, the Canola and Flax Councils of Canada, the Calorie Control Council, the CIHR, the Canada Foundation for Innovation, and the Ontario Research Fund. He has received in‐kind supplies for trials as a research support from the Almond Board of California, Walnut Council of California, American Peanut Council, Barilla, Unilever, Unico, Primo, Loblaw Companies, Quaker (Pepsico), Pristine Gourmet, Bunge Limited, Kellogg Canada, and White Wave Foods. He has been on the speaker's panel, served on the scientific advisory board, and/or received travel support and/or honoraria from the Almond Board of California, Canadian Agriculture Policy Institute, Loblaw Companies Ltd, the Griffin Hospital (for the development of the NuVal scoring system), the Coca‐Cola Company, EPICURE, Danone, Diet Quality Photo Navigation, Better Therapeutics (FareWell), Verywell, True Health Initiative, Institute of Food Technologists, Soy Nutrition Institute, Herbalife Nutrition Institute, Saskatchewan Pulse Growers, Sanitarium Company, Orafti, the Almond Board of California, the American Peanut Council, the International Tree Nut Council Nutrition Research and Education Foundation, the Peanut Institute, Herbalife International, Pacific Health Laboratories, Nutritional Fundamentals for Health, Barilla, Metagenics, Bayer Consumer Care, Unilever Canada and Netherlands, Solae, Kellogg, Quaker Oats, Procter & Gamble, the Coca‐Cola Company, the Griffin Hospital, Abbott Laboratories, the Canola Council of Canada, Dean Foods, the California Strawberry Commission, Haine Celestial, PepsiCo, the Alpro Foundation, Pioneer Hi‐Bred International, DuPont Nutrition and Health, Spherix Consulting and White Wave Foods, the Advanced Foods and Material Network, the Canola and Flax Councils of Canada, the Nutritional Fundamentals for Health, Agri‐Culture and Agri‐Food Canada, the Canadian Agri‐Food Policy Institute, Pulse Canada, the Saskatchewan Pulse Growers, the Soy Foods Association of North America, the Nutrition Foundation of Italy, Nutra‐Source Diagnostics, the McDougall Program, the Toronto Knowledge Translation Group (St. Michael's Hospital), the Canadian College of Naturopathic Medicine, The Hospital for Sick Children, the Canadian Nutrition Society, the American Society of Nutrition, Arizona State University, Paolo Sorbini Foundation, and the Institute of Nutrition, Metabolism and Diabetes. He received an honorarium from the US Department of Agriculture to present the 2013 W.O. Atwater Memorial Lecture. He received the 2013 Award for Excellence in Research from the International Nut and Dried Fruit Council. He received funding and travel support from the Canadian Society of Endocrinology and Metabolism to produce mini cases for the Canadian Diabetes Association. He is a member of the International Carbohydrate Quality Consortium. His wife, Alexandra L Jenkins, is a director and partner of INQUIS Clinical Research for the Food Industry, his 2 daughters, Wendy Jenkins and Amy Jenkins, have published a vegetarian book that promotes the use of the low glycemic index plant foods advocated here, The Portfolio Diet for Cardiovascular Risk Reduction (Academic Press/Elsevier 2020 ISBN:978‐0‐12‐810510‐8) and and his sister, Caroline Brydson, received funding through a grant from the St. Michael's Hospital Foundation to develop a cookbook for one of his studies. Sievenpiper has received research support from the Canadian Foundation for Innovation, Ontario Research Fund, Province of Ontario Ministry of Research and Innovation and Science, Canadian Institutes of health Research (CIHR), Diabetes Canada, PSI Foundation, Banting and Best Diabetes Centre (BBDC), American Society for Nutrition (ASN), INC International Nut and Dried Fruit Council Foundation, National Dried Fruit Trade Association, National Honey Board, International Life Sciences Institute (ILSI), The Tate and Lyle Nutritional Research Fund at the University of Toronto, The Glycemic Control and Cardiovascular Disease in Type 2 Diabetes Fund at the University of Toronto (a fund established by the Alberta Pulse Growers), and the Nutrition Trialists Fund at the University of Toronto (a fund established by an inaugural donation from the Calorie Control Council). He has received in‐kind food donations to support a randomized controlled trial from the Almond Board of California, California Walnut Commission, American Peanut Council, Barilla, Unilever, Upfield, Unico/Primo, Loblaw Companies, Quaker, Kellogg Canada, WhiteWave Foods, and Nutrartis. He has received travel support, speaker fees and/or honoraria from Diabetes Canada, Dairy Farmers of Canada, FoodMinds LLC, International Sweeteners Association, Nestlé, Pulse Canada, Canadian Society for Endocrinology and Metabolism (CSEM), GI Foundation, Abbott, Biofortis, ASN, Northern Ontario School of Medicine, INC Nutrition Research & Education Foundation, European Food Safety Authority (EFSA), Comité Européen des Fabricants de Sucre (CEFS), and Physicians Committee for Responsible Medicine. He has or has had ad hoc consulting arrangements with Perkins Coie LLP, Tate & Lyle, Wirtschaftliche Vereinigung Zucker e.V., and Inquis Clinical Research. He is a member of the European Fruit Juice Association Scientific Expert Panel and Soy Nutrition Institute (SNI) Scientific Advisory Committee. He is on the Clinical Practice Guidelines Expert Committees of Diabetes Canada, European Association for the study of Diabetes (EASD), Canadian Cardiovascular Society (CCS), and Obesity Canada. He serves or has served as an unpaid scientific advisor for the Food, Nutrition, and Safety Program (FNSP) and the Technical Committee on Carbohydrates of ILSI North America. He is a member of the International Carbohydrate Quality Consortium (ICQC), Executive Board Member of the Diabetes and Nutrition Study Group (DNSG) of the EASD, and Director of the Toronto 3D Knowledge Synthesis and Clinical Trials foundation. His wife is an employee of AB InBev. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S9
Figures S1–S205
(J Am Heart Assoc. 2020;9:e017728 DOI: 10.1161/JAHA.120.017728.)
Supplementary Materials for this article are available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017728
For Sources of Funding and Disclosures, see page 21.
References
- 1. Lustig RH. Fructose: it's “alcohol without the buzz.” Adv Nutr. 2013;4:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lustig R, Schmidt L, Brindis C. Public health: the toxic truth about sugar. Nature. 2012;482:27–29. [DOI] [PubMed] [Google Scholar]
- 3. Zurger A. A diet manifesto: drop the apple and walk away. The New York Times 6. 2010. Accessed December 27.
- 4. Government of Canada . Canada’s food guide consultations: guiding principles. Government of Canada; 2017. https://www.foodguideconsultation.ca/guiding-principles-detailed. Accessed August 5, 2020. [Google Scholar]
- 5. International Diabetes Federation . International Diabetes Federation framework for action on sugar. 2015. https://www.idf.org/images/site1/content/Framework-for-Action-on-Sugar-010615.pdf. Accessed April 24, 2018.
- 6. Mcmurray S. Sugar content in fruit: is it damaging to your health and waistline? 2018. University Health News Daily. Available at: https://universityhealthnews.com/daily/nutrition/high-sugar-content-fruit-damaging-health-waistline/. Accessed August 31, 2020.
- 7. World Health Organization . Guideline: Sugars Intake for Adults and Children. 2015. Available at: https://www.who.int/publications/i/item/9789241549028. Accessed August 16, 2019. [PubMed] [Google Scholar]
- 8. Villines Z, Butler N. What to know about sugar in fruit. 2019.
- 9. Law MR, Morris JK. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? Eur J Clin Nutr. 1998;52:549–556. [DOI] [PubMed] [Google Scholar]
- 10. World Health Organization . The world health report: reducing risks, promoting healthy life. 2002. Available at: https://www.who.int/dietphysicalactivity/publications/f&v_promotion_initiative_report.pdf?ua=1. Accessed August 31, 2020.
- 11. Aune D, Giovannucci E, Boffetta P, Fadnes LT, Keum N, Norat T, Greenwood DC, Riboli E, Vatten LJ, Tonstad S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int J Epidemiol. 2017;46:1029–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dauchet L, Amouyel P, Hercberg S, Dallongeville J. Fruit and vegetable consumption and risk of coronary heart disease: a meta-analysis of cohort studies. J Nutr. 2006;136:2588–2593. [DOI] [PubMed] [Google Scholar]
- 13. Gan Y, Tong X, Li L, Cao S, Yin X, Gao C, Herath C, Li W, Jin Z, Chen Y, et al. Consumption of fruit and vegetable and risk of coronary heart disease: a meta-analysis of prospective cohort studies. Int J Cardiol. 2015;183:129–137. [DOI] [PubMed] [Google Scholar]
- 14. He FJ, Nowson CA, Lucas M, Macgregor GA. Increased consumption of fruit and vegetables is related to a reduced risk of coronary heart disease: meta-analysis of cohort studies. J Hum Hypertens. 2007;21:717–728. [DOI] [PubMed] [Google Scholar]
- 15. Hu D, Huang J, Wang Y, Zhang D, Qu Y. Fruits and vegetables consumption and risk of stroke: a meta-analysis of prospective cohort studies. Stroke. 2014;45:1613–1619. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Ouyang Y, Liu J, Zhu M, Zhao G, Bao W, Hu FB. Fruit and vegetable consumption and mortality from all causes, cardiovascular disease, and cancer: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2014;349:g4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhan J, Liu YJ, Cai LB, Xu FR, Xie T, He QQ. Fruit and vegetable consumption and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr. 2017;57:1650–1663. [DOI] [PubMed] [Google Scholar]
- 18. Higgins J, Green S. Cochrane handbook for systematic reviews of interventions. Cochrane Collaboration; 2011. https://training.cochrane.org/handbook/archive/v5.1/. Accessed October 10, 2016. [Google Scholar]
- 19. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269; w264. [DOI] [PubMed] [Google Scholar]
- 20. Wells G, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2000. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed August 31, 2020.
- 21. Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 22. Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280:1690–1691. [DOI] [PubMed] [Google Scholar]
- 23. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. [DOI] [PubMed] [Google Scholar]
- 24. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2011;175:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee JE, Mannisto S, Spiegelman D, Hunter DJ, Bernstein L, Van Den Brandt PA, Buring JE, Cho E, English DR, Flood A, et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: a pooled analysis of 13 prospective studies. Cancer Epidemiol Biomarkers Prev. 2009;18:1730–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Deeks J, Higgins J, Altman D. Chapter 10: analysing data and undertaking meta-analyses. 2011. http://handbook.cochrane.org. Accessed September 25, 2014.
- 27. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, et al. Grade guidelines: 7: rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 28. Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 29. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, Devereaux PJ, Montori VM, Freyschuss B, Vist G, et al. Grade guidelines 6: rating the quality of evidence—imprecision. J Clin Epidemiol. 2011;64:1283–1293. [DOI] [PubMed] [Google Scholar]
- 30. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, Debeer H, et al. GRADE guidelines: 1: introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64:383–394. [DOI] [PubMed] [Google Scholar]
- 31. Guyatt GHO, Kunz AD, Atkins R, Brozek D, Vist J, Alderson G, Glasziou P, Falck-Ytter P, Schunemann HJ. GRADE guidelines: 2: framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64:395–400. [DOI] [PubMed] [Google Scholar]
- 32. Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris S, et al. GRADE guidelines: 3: rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. [DOI] [PubMed] [Google Scholar]
- 33. Guyatt GH, Oxman AD, Vist G, Kunz R, Brozek J, Alonso-Coello P, Montori V, Akl EA, Djulbegovic B, Falck-Ytter Y, et al. GRADE guidelines: 4: rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol. 2011;64:407–415. [DOI] [PubMed] [Google Scholar]
- 34. Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y, et al. GRADE guidelines: 5: rating the quality of evidence—publication bias. J Clin Epidemiol. 2011;64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 35. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Glasziou P, Jaeschke R, Akl EA, et al. GRADE guidelines: 7: rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–1302. [DOI] [PubMed] [Google Scholar]
- 36. Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, Alonso-Coello P, Falck-Ytter Y, Jaeschke R, Vist G, et al. GRADE guidelines: 8: rating the quality of evidence—indirectness. J Clin Epidemiol. 2011;64:1303–1310. [DOI] [PubMed] [Google Scholar]
- 37. Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, et al. GRADE guidelines: 9: rating up the quality of evidence. J Clin Epidemiol. 2011;64:1311–1316. [DOI] [PubMed] [Google Scholar]
- 38. Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, Sisk J, Ruiz F, Hill S, Guyatt GH, et al. GRADE guidelines: 10: considering resource use and rating the quality of economic evidence. J Clin Epidemiol. 2013;66:140–150. [DOI] [PubMed] [Google Scholar]
- 39. Guyatt G, Oxman AD, Sultan S, Brozek J, Glasziou P, Alonso-Coello P, Atkins D, Kunz R, Montori V, Jaeschke R, et al. GRADE guidelines: 11: making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol. 2013;66:151–157. [DOI] [PubMed] [Google Scholar]
- 40. Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, Brozek J, Norris S, Meerpohl J, Djulbegovic B, et al. GRADE guidelines: 12: preparing summary of findings tables-binary outcomes. J Clin Epidemiol. 2013;66:158–172. [DOI] [PubMed] [Google Scholar]
- 41. Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, Johnston BC, Karanicolas P, Akl EA, Vist G, et al. GRADE guidelines: 13: preparing summary of findings tables and evidence profiles-continuous outcomes. J Clin Epidemiol. 2013;66:173–183. [DOI] [PubMed] [Google Scholar]
- 42. Adriouch S, Lampure A, Nechba A, Baudry J, Assmann K, Kesse-Guyot E, Hercberg S, Scalbert A, Touvier M, Fezeu LK. Prospective association between total and specific dietary polyphenol intakes and cardiovascular disease risk in the Nutrinet-Sante French cohort. Nutrients. 2018;10:1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Appleby PN, Key TJ, Burr ML, Thorogood M. Mortality and fresh fruit consumption. IARC Sci Publ. 2002;156:131–133. [PubMed] [Google Scholar]
- 44. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. High diet quality is associated with a lower risk of cardiovascular disease and all-cause mortality in older men. J Nutr. 2014;144:673–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bahadoran Z, Mirmiran P, Momenan AA, Azizi F. Allium vegetable intakes and the incidence of cardiovascular disease, hypertension, chronic kidney disease, and type 2 diabetes in adults: a longitudinal follow-up study. J Hypertens. 2017;35:1909–1916. [DOI] [PubMed] [Google Scholar]
- 46. Bazzano LA, He J, Ogden LG, Loria CM, Vupputuri S, Myers L, Whelton PK. Fruit and vegetable intake and risk of cardiovascular disease in US adults: the first National Health and Nutrition Examination Survey Epidemiologic Follow-Up Study. Am J Clin Nutr. 2002;76:93–99. [DOI] [PubMed] [Google Scholar]
- 47. Belin RJ, Greenland P, Allison M, Martin L, Shikany JM, Larson J, Tinker L, Howard BV, Lloyd-Jones D, Van Horn L. Diet quality and the risk of cardiovascular disease: the Women's Health Initiative (WHI). Am J Clin Nutr. 2011;94:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bendinelli B, Masala G, Saieva C, Salvini S, Calonico C, Sacerdote C, Agnoli C, Grioni S, Frasca G, Mattiello A, et al. Fruit, vegetables, and olive oil and risk of coronary heart disease in Italian women: the EPICOR study. Am J Clin Nutr. 2011;93:275–283. [DOI] [PubMed] [Google Scholar]
- 49. Berard E, Bongard V, Haas B, Dallongeville J, Moitry M, Cottel D, Ruidavets JB, Ferrieres J. Score of adherence to 2016 European cardiovascular prevention guidelines is an independent determinant of cardiovascular and all-cause mortality in a French general population. Eur Heart J. 2017;38:1064. Conference: European Society of Cardiology, ESC congress. Spain. [DOI] [PubMed] [Google Scholar]
- 50. Bhupathiraju SN, Wedick NM, Pan A, Manson JE, Rexrode KM, Willett WC, Rimm EB, Hu FB. Quantity and variety in fruit and vegetable intake and risk of coronary heart disease. Am J Clin Nutr. 2013;98:1514–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bingham S, Luben R, Welch A, Low YL, Khaw KT, Wareham N, Day N. Associations between dietary methods and biomarkers, and between fruits and vegetables and risk of ischaemic heart disease, in the EPIC Norfolk Cohort Study. Int J Epidemiol. 2008;37:978–987. [DOI] [PubMed] [Google Scholar]
- 52. Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Zhu K, Lim WH, Woodman RJ, Beilin LJ, Prince RL, Hodgson JM. Cruciferous and allium vegetable intakes are inversely associated with 15-year atherosclerotic vascular disease deaths in older adult women. J Am Heart Assoc. 2017;6:e006558 DOI: 10.1161/JAHA.117.006558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bos MJ, Koudstaal PJ, Hofman A, Ikram MA. Modifiable etiological factors and the burden of stroke from the Rotterdam study: a population-based cohort study. PLoS Med. 2014;11:e1001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Buijsse B, Feskens EJ, Kwape L, Kok FJ, Kromhout D. Both alpha- and beta-carotene, but not tocopherols and vitamin C, are inversely related to 15-year cardiovascular mortality in Dutch elderly men. J Nutr. 2008;138:344–350. [DOI] [PubMed] [Google Scholar]
- 55. Buil-Cosiales P, Martinez-Gonzalez MA, Ruiz-Canela M, Diez-Espino J, Garcia-Arellano A, Toledo E. Consumption of fruit or fiber-fruit decreases the risk of cardiovascular disease in a Mediterranean young cohort. Nutrients. 2017;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Buil-Cosiales P, Toledo E, Salas-Salvado J, Zazpe I, Farras M, Basterra-Gortari FJ, Diez-Espino J, Estruch R, Corella D, Ros E, et al. Association between dietary fibre intake and fruit, vegetable or whole-grain consumption and the risk of CVD: results from the prevencion con dieta Mediterranea (PREDIMED) trial. Br J Nutr. 2016;116:534–546. [DOI] [PubMed] [Google Scholar]
- 57. Cassidy A, Rimm EB, O'Reilly EJ, Logroscino G, Kay C, Chiuve SE, Rexrode KM. Dietary flavonoids and risk of stroke in women. Stroke. 2012;43:946–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA. Association of sugary beverage consumption with mortality risk in US adults: a secondary analysis of data from the REGARDS study. JAMA Netw Open. 2019;2:e193121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Conrad Z, Thomson J, Jahns L. Prospective analysis of vegetable amount and variety on the risk of all-cause and cause-specific mortality among US adults, 1999–2011. Nutrients. 2018;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Dauchet L, Ferrieres J, Arveiler D, Yarnell JW, Gey F, Ducimetiere P, Ruidavets JB, Haas B, Evans A, Bingham A, et al. Frequency of fruit and vegetable consumption and coronary heart disease in France and Northern Ireland: the PRIME study. Br J Nutr. 2004;92:963–972. [DOI] [PubMed] [Google Scholar]
- 61. Dauchet L, Montaye M, Ruidavets JB, Arveiler D, Kee F, Bingham A, Ferrieres J, Haas B, Evans A, Ducimetiere P, et al. Association between the frequency of fruit and vegetable consumption and cardiovascular disease in male smokers and non-smokers. Eur J Clin Nutr. 2010;64:578–586. [DOI] [PubMed] [Google Scholar]
- 62. Du H, Li L, Bennett D, Guo Y, Key TJ, Bian Z, Sherliker P, Gao H, Chen Y, Yang L, et al. Fresh fruit consumption and major cardiovascular disease in China. N Engl J Med. 2016;374:1332–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Du H, Li L, Bennett D, Yang L, Guo Y, Key TJ, Bian Z, Chen Y, Walters RG, Millwood IY, et al. Fresh fruit consumption and all-cause and cause-specific mortality: findings from the China Kadoorie Biobank. Int J Epidemiol. 2017;46:1444–1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Elwood P, Galante J, Pickering J, Palmer S, Bayer A, Ben-Shlomo Y, Longley M, Gallacher J. Healthy lifestyles reduce the incidence of chronic diseases and dementia: evidence from the caerphilly cohort study. PLoS One. 2013;8:e81877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Eriksen A, Tillin T, O’Connor L, Brage S, Hughes A, Mayet J, McKeigue P, Whincup P, Chaturvedi N, Forouhi NG. The impact of health behaviours on incident cardiovascular disease in Europeans and South Asians—a prospective analysis in the UK SABRE study. PLoS One. 2015;10:e0117364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fitzgerald KC, Chiuve SE, Buring JE, Ridker PM, Glynn RJ. Comparison of associations of adherence to a Dietary Approaches to Stop Hypertension (DASH)-style diet with risks of cardiovascular disease and venous thromboembolism. J Thromb Haemost. 2012;10:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fraser GE, Sabate J, Beeson WL, Strahan TM. A possible protective effect of nut consumption on risk of coronary heart disease: the Adventist Health Study. Arch Intern Med. 1992;152:1416–1424. [PubMed] [Google Scholar]
- 68. Gardener H, Wright CB, Gu Y, Demmer RT, Boden-Albala B, Elkind MSV, Sacco RL, Scarmeas N. Mediterranean-style diet and risk of ischemic stroke, myocardial infarction, and vascular death: the Northern Manhattan Study. Am J Clin Nutr. 2011;94:1458–1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Gaziano JM, Manson JE, Branch LG, Colditz GA, Willett WC, Buring JE. A prospective study of consumption of carotenoids in fruits and vegetables and decreased cardiovascular mortality in the elderly. Ann Epidemiol. 1995;5:255–260. [DOI] [PubMed] [Google Scholar]
- 70. Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–1233. [DOI] [PubMed] [Google Scholar]
- 71. Gillman MW, Cupples LA, Gagnon D, Posner BM, Ellison RC, Castelli WP, Wolf PA. Protective effect of fruits and vegetables on development of stroke in men. JAMA. 1995;273:1113–1117. [DOI] [PubMed] [Google Scholar]
- 72. Goetz ME, Judd SE, Safford MM, Hartman TJ, Mcclellan WM, Vaccarino V. Dietary flavonoid intake and incident coronary heart disease: the reasons for geographic and racial differences in stroke (REGARDS) study. Am J Clin Nutr. 2016;104:1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Goetz ME, Judd SE, Hartman TJ, Mcclellan W, Anderson A, Vaccarino V. Flavanone intake is inversely associated with risk of incident ischemic stroke in the reasons for geographic and racial differences in stroke (REGARDS) study. J Nutr. 2016;146:2233–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gunge VB, Andersen I, Kyro C, Hansen CP, Dahm CC, Christensen J, Tjonneland A, Olsen A. Adherence to a healthy Nordic food index and risk of myocardial infarction in middle-aged Danes: the diet, cancer and health cohort study. Eur J Clin Nutr. 2017;71:652–658. [DOI] [PubMed] [Google Scholar]
- 75. Gunnell AS, Einarsdottir K, Galvao DA, Joyce S, Tomlin S, Graham V, Mcintyre C, Newton RU, Briffa T. Lifestyle factors, medication use and risk for ischaemic heart disease hospitalisation: a longitudinal population-based study. PLoS One. 2013;8:e77833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hansen CP, Overvad K, Kyro C, Olsen A, Tjonneland A, Johnsen SP, Jakobsen MU, Dahm CC. Adherence to a healthy Nordic diet and risk of stroke: a Danish cohort study. Stroke. 2017;48:259–264. [DOI] [PubMed] [Google Scholar]
- 77. Hansen L, Dragsted LO, Olsen A, Christensen J, Tjønneland A, Schmidt EB, Overvad K. Fruit and vegetable intake and risk of acute coronary syndrome. Br J Nutr. 2010;104:248–255. [DOI] [PubMed] [Google Scholar]
- 78. Harriss LR, English DR, Powles J, Giles GG, Tonkin AM, Hodge AM, Brazionis L, O'Dea K. Dietary patterns and cardiovascular mortality in the Melbourne Collaborative Cohort Study. Am J Clin Nutr. 2007;86:221–229. [DOI] [PubMed] [Google Scholar]
- 79. Hertog MG, Sweetnam PM, Fehily AM, Elwood PC, Kromhout D. Antioxidant flavonols and ischemic heart disease in a Welsh population of men: the Caerphilly Study. Am J Clin Nutr. 1997;65:1489–1494. [DOI] [PubMed] [Google Scholar]
- 80. Hirvonen T, Pietinen P, Virtanen M, Ovaskainen ML, Hakkinen S, Albanes D, Virtamo J. Intake of flavonols and flavones and risk of coronary heart disease in male smokers. Epidemiology. 2001;12:62–67. [DOI] [PubMed] [Google Scholar]
- 81. Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000;31:2301–2306. [DOI] [PubMed] [Google Scholar]
- 82. Hjartaker A, Knudsen MD, Tretli S, Weiderpass E. Consumption of berries, fruits and vegetables and mortality among 10,000 Norwegian men followed for four decades. Eur J Nutr. 2015;54:599–608. [DOI] [PubMed] [Google Scholar]
- 83. Hodgson JM, Prince RL, Woodman RJ, Bondonno CP, Ivey KL, Bondonno N, Rimm EB, Ward NC, Croft KD, Lewis JR. Apple intake is inversely associated with all-cause and disease-specific mortality in elderly women. Br J Nutr. 2016;115:860–867. [DOI] [PubMed] [Google Scholar]
- 84. Holmberg S, Thelin A, Stiernstrom EL. Food choices and coronary heart disease: a population based cohort study of rural Swedish men with 12 years of follow-up. Int J Environ Res Public Health. 2009;6:2626–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Iso H, Kubota Y; Japan Collaborative Cohort Study for Evaluation of Cancer . Nutrition and disease in the Japan Collaborative Cohort Study for Evaluation of Cancer (JACC). Asian Pac J Cancer Prev. 2007;8(suppl):35–80. [PubMed] [Google Scholar]
- 86. Jacques PF, Cassidy A, Rogers G, Peterson JJ, Dwyer JT. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Johnsen SP, Overvad K, Stripp C, Tjonneland A, Husted SE, Sorensen HT. Intake of fruit and vegetables and the risk of ischemic stroke in a cohort of Danish men and women. Am J Clin Nutr. 2003;78:57–64. [DOI] [PubMed] [Google Scholar]
- 88. Joshipura KJ, Ascherio A, Manson JE, Stampfer MJ, Rimm EB, Speizer FE, Hennekens CH, Spiegelman D, Willett WC. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–1239. [DOI] [PubMed] [Google Scholar]
- 89. Joshipura KJ, Hung HC, Li TY, Hu FB, Rimm EB, Stampfer MJ, Colditz G, Willett WC. Intakes of fruits, vegetables and carbohydrate and the risk of CVD. Public Health Nutr. 2009;12:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996;156:637–642. [PubMed] [Google Scholar]
- 91. Kim LG, Adamson J, Ebrahim S. Influence of life-style choices on locomotor disability, arthritis and cardiovascular disease in older women: prospective cohort study. Age Ageing. 2013;42:696–701. [DOI] [PubMed] [Google Scholar]
- 92. Knekt P, Isotupa S, Rissanen H, Heliovaara M, Jarvinen R, Hakkinen S, Aromaa A, Reunanen A. Quercetin intake and the incidence of cerebrovascular disease. Eur J Clin Nutr. 2000;54:415–417. [DOI] [PubMed] [Google Scholar]
- 93. Knekt P, Jarvinen R, Reunanen A, Maatela J. Flavonoid intake and coronary mortality in Finland: a cohort study. BMJ. 1996;312:478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Knekt P, Reunanen A, Jarvinen R, Seppanen R, Heliovaara M, Aromaa A. Antioxidant vitamin intake and coronary mortality in a longitudinal population study. Am J Epidemiol. 1994;139:1180–1189. [DOI] [PubMed] [Google Scholar]
- 95. Kobylecki CJ, Afzal S, Davey Smith G, Nordestgaard BG. Genetically high plasma vitamin C, intake of fruit and vegetables, and risk of ischemic heart disease and all-cause mortality: a Mendelian randomization study. Am J Clin Nutr. 2015;101:1135–1143. [DOI] [PubMed] [Google Scholar]
- 96. Kondo K, Miura K, Tanaka-Mizuno S, Kadota A, Arima H, Okuda N, Fujiyoshi A, Miyagawa N, Yoshita K, Okamura T, et al. Cardiovascular risk assessment chart by dietary factors in Japan—NIPPON DATA80. Circ J. 2019;83:1254–1260. [DOI] [PubMed] [Google Scholar]
- 97. Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom Health and Lifestyle Survey [erratum appears in Arch Intern Med. 2010;170:998]. Arch Intern Med. 2010;170:711–718. [DOI] [PubMed] [Google Scholar]
- 98. Lai HT, Threapleton DE, Day AJ, Williamson G, Cade JE, Burley VJ. Fruit intake and cardiovascular disease mortality in the UK Women's Cohort Study. Eur J Epidemiol. 2015;30:1035–1048. [DOI] [PubMed] [Google Scholar]
- 99. Larsson SC, Mannisto S, Virtanen MJ, Kontto J, Albanes D, Virtamo J. Dietary fiber and fiber-rich food intake in relation to risk of stroke in male smokers. Eur J Clin Nutr. 2009;63:1016–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Larsson SC, Virtamo J, Wolk A. Total and specific fruit and vegetable consumption and risk of stroke: a prospective study. Atherosclerosis. 2013;227:147–152. [DOI] [PubMed] [Google Scholar]
- 101. Leenders M, Boshuizen HC, Ferrari P, Siersema PD, Overvad K, Tjonneland A, Olsen A, Boutron-Ruault MC, Dossus L, Dartois L, et al. Fruit and vegetable intake and cause-specific mortality in the EPIC study. Eur J Epidemiol. 2014;29:639–652. [DOI] [PubMed] [Google Scholar]
- 102. Leenders M, Sluijs I, Ros MM, Boshuizen HC, Siersema PD, Ferrari P, Weikert C, Tjonneland A, Olsen A, Boutron-Ruault MC, et al. Fruit and vegetable consumption and mortality: European prospective investigation into cancer and nutrition. Am J Epidemiol. 2013;178:590–602. [DOI] [PubMed] [Google Scholar]
- 103. Lin J, Rexrode KM, Hu F, Albert CM, Chae CU, Rimm EB, Stampfer MJ, Manson JE. Dietary intakes of flavonols and flavones and coronary heart disease in US women. Am J Epidemiol. 2007;165:1305–1313. [DOI] [PubMed] [Google Scholar]
- 104. Lin YH, Ku PW, Chou P. Lifestyles and mortality in Taiwan: an 11-year follow-up study. Asia Pac J Public Health. 2017;29:259–267. [DOI] [PubMed] [Google Scholar]
- 105. Liu S, Lee IM, Ajani U, Cole SR, Buring JE, Manson JEP; Physicians' Health Study . Intake of vegetables rich in carotenoids and risk of coronary heart disease in men: the Physicians' Health Study. Int J Epidemiol. 2001;30:130–135. [DOI] [PubMed] [Google Scholar]
- 106. Liu S, Manson JE, Lee IM, Cole SR, Hennekens CH, Willett WC, Buring JE. Fruit and vegetable intake and risk of cardiovascular disease: the Women's Health Study. Am J Clin Nutr. 2000;72:922–928. [DOI] [PubMed] [Google Scholar]
- 107. Mann JI, Appleby PN, Key TJ, Thorogood M. Dietary determinants of ischaemic heart disease in health conscious individuals. Heart. 1997;78:450–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Manuel DG, Tuna M, Perez R, Tanuseputro P, Hennessy D, Bennett C, Rosella L, Sanmartin C, Van Walraven C, Tu JV. Predicting stroke risk based on health behaviours: development of the stroke population risk tool (SPoRT). PLoS One. 2015;10:e0143342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S, et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037–2049. [DOI] [PubMed] [Google Scholar]
- 110. Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong C-P, Nettleton JA, Jacobs DR. Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. [DOI] [PubMed] [Google Scholar]
- 111. Mizrahi A, Knekt P, Montonen J, Laaksonen MA, Heliovaara M, Jarvinen R. Plant foods and the risk of cerebrovascular diseases: a potential protection of fruit consumption. Br J Nutr. 2009;102:1075–1083. [DOI] [PubMed] [Google Scholar]
- 112. Mori N, Shimazu T, Charvat H, Mutoh M, Sawada N, Iwasaki M, Yamaji T, Inoue M, Goto A, Takachi R, et al. Cruciferous vegetable intake and mortality in middle-aged adults: a prospective cohort study. Clin Nutr. 2018;24:24. [DOI] [PubMed] [Google Scholar]
- 113. Mytton OT, Forouhi NG, Scarborough P, Lentjes M, Luben R, Rayner M, Khaw KT, Wareham NJ, Monsivais P. Association between intake of less-healthy foods defined by the United Kingdom's nutrient profile model and cardiovascular disease: a population-based cohort study. PLoS Med. 2018;15:e1002484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nagura J, Iso H, Watanabe Y, Maruyama K, Date C, Toyoshima H, Yamamoto A, Kikuchi S, Koizumi A, Kondo T, et al. Fruit, vegetable and bean intake and mortality from cardiovascular disease among Japanese men and women: the JACC study. Br J Nutr. 2009;102:285–292. [DOI] [PubMed] [Google Scholar]
- 115. Nakamura K, Nagata C, Oba S, Takatsuka N, Shimizu H. Fruit and vegetable intake and mortality from cardiovascular disease are inversely associated in Japanese women but not in men. J Nutr. 2008;138:1129–1134. [DOI] [PubMed] [Google Scholar]
- 116. Nechuta SJ, Shu XO, Li HL, Yang G, Xiang YB, Cai H, Chow WH, Ji B, Zhang X, Wen W, et al. Combined impact of lifestyle-related factors on total and cause-specific mortality among Chinese women: prospective cohort study. PLoS Med. 2010;7:e1000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Neelakantan N, Koh WP, Yuan JM, Van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among Chinese adults. J Nutr. 2018;148:1323–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ness AR, Maynard M, Frankel S, Smith GD, Frobisher C, Leary SD, Emmett PM, Gunnell D. Diet in childhood and adult cardiovascular and all cause mortality: the Boyd Orr cohort. Heart. 2005;91:894–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Nothlings U, Schulze MB, Weikert C, Boeing H, Van Der Schouw YT, Bamia C, Benetou V, Lagiou P, Krogh V, Beulens JW, et al. Intake of vegetables, legumes, and fruit, and risk for all-cause, cardiovascular, and cancer mortality in a European diabetic population. J Nutr. 2008;138:775–781. [DOI] [PubMed] [Google Scholar]
- 120. Okuda N, Miura K, Okayama A, Okamura T, Abbott RD, Nishi N, Fujiyoshi A, Kita Y, Nakamura Y, Miyagawa N, et al. Fruit and vegetable intake and mortality from cardiovascular disease in Japan: a 24-year follow-up of the NIPPON DATA80 study. Eur J Clin Nutr. 2015;69:482–488. [DOI] [PubMed] [Google Scholar]
- 121. Oude Griep LM, Geleijnse JM, Kromhout D, Ocke MC, Verschuren WM. Raw and processed fruit and vegetable consumption and 10-year coronary heart disease incidence in a population-based cohort study in the Netherlands. PLoS One. 2010;5:e13609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Colours of fruit and vegetables and 10-year incidence of CHD. Br J Nutr. 2011;106:1562–1569. [DOI] [PubMed] [Google Scholar]
- 123. Oude Griep LM, Verschuren WM, Kromhout D, Ocke MC, Geleijnse JM. Raw and processed fruit and vegetable consumption and 10-year stroke incidence in a population-based cohort study in the Netherlands. Eur J Clin Nutr. 2011;65:791–799. [DOI] [PubMed] [Google Scholar]
- 124. Oyebode O, Gordon-Dseagu V, Walker A, Mindell JS. Fruit and vegetable consumption and all-cause, cancer and CVD mortality: analysis of Health Survey for England data. J Epidemiol Community Health. 2014;68:856–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Pham TM, Fujino Y, Tokui N, Ide R, Kubo T, Shirane K, Mizoue T, Ogimoto I, Yoshimura T. Mortality and risk factors for stroke and its subtypes in a cohort study in Japan. Prev Med. 2007;44:526–530. [DOI] [PubMed] [Google Scholar]
- 126. Rebello SA, Koh H, Chen C, Naidoo N, Odegaard AO, Koh WP, Butler LM, Yuan JM, Van Dam RM. Amount, type, and sources of carbohydrates in relation to ischemic heart disease mortality in a Chinese population: a prospective cohort study. Am J Clin Nutr. 2014;100:53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Rissanen TH, Voutilainen S, Virtanen JK, Venho B, Vanharanta M, Mursu J, Salonen JT. Low intake of fruits, berries and vegetables is associated with excess mortality in men: the Kuopio Ischaemic Heart Disease Risk Factor (KIHD) study. J Nutr. 2003;133:199–204. [DOI] [PubMed] [Google Scholar]
- 128. Saglimbene V, Wong G, Bondonno N, Ruospo M, Palmer SC, Campbell K, Garcia Larsen V, Natale P, Teixeirapinto A, Gargano L, et al. Fruit intake and cardiovascular and all-cause mortality in adults on hemodialysis: the DIET-HD multinational cohort study. Nephrology. 2017;22(suppl 3):25.26718476 [Google Scholar]
- 129. Sahyoun NR, Jacques PF, Russell RM. Carotenoids, vitamins C and E, and mortality in an elderly population. Am J Epidemiol. 1996;144:501–511. [DOI] [PubMed] [Google Scholar]
- 130. Sauvaget C, Nagano J, Allen N, Kodama K. Vegetable and fruit intake and stroke mortality in the Hiroshima/Nagasaki Life Span Study. Stroke. 2003;34:2355–2360. [DOI] [PubMed] [Google Scholar]
- 131. Scheffers FR, Boer JMA, Verschuren WMM, Verheus M, Van Der Schouw YT, Sluijs I, Smit HA, Wijga AH. Pure fruit juice and fruit consumption and the risk of CVD: the European Prospective Investigation into Cancer and Nutrition-Netherlands (EPIC-NL) study. Br J Nutr. 2019;121:351–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sesso HD, Gaziano JM, Liu S, Buring JE. Flavonoid intake and the risk of cardiovascular disease in women. Am J Clin Nutr. 2003;77:1400–1408. [DOI] [PubMed] [Google Scholar]
- 133. Sesso HD, Gaziano JM, Jenkins DJ, Buring JE. Strawberry intake, lipids, C-reactive protein, and the risk of cardiovascular disease in women. J Am Coll Nutr. 2007;26:303–310. [DOI] [PubMed] [Google Scholar]
- 134. Sesso HD, Liu S, Gaziano JM, Buring JE. Dietary lycopene, tomato-based food products and cardiovascular disease in women. J Nutr. 2003;133:2336–2341. [DOI] [PubMed] [Google Scholar]
- 135. Shah NS, Leonard D, Finley CE, Rodriguez F, Sarraju A, Barlow CE, Defina LF, Willis BL, Haskell WL, Maron DJ. Dietary patterns and long-term survival: a retrospective study of healthy primary care patients. Am J Med. 2018;131:48–55. [DOI] [PubMed] [Google Scholar]
- 136. Sharma S, Cruickshank JK, Green DM, Vik S, Tome A, Kolonel LN. Impact of diet on mortality from stroke: results from the U.S. multiethnic cohort study. J Am Coll Nutr. 2013;32:151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Sharma S, Vik S, Kolonel LN. Fruit and vegetable consumption, ethnicity and risk of fatal ischemic heart disease. J Nutr Health Aging. 2014;18:573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Simila ME, Kontto JP, Mannisto S, Valsta LM, Virtamo J. Glycaemic index, carbohydrate substitution for fat and risk of CHD in men. Br J Nutr. 2013;110:1704–1711. [DOI] [PubMed] [Google Scholar]
- 139. Sonestedt E, Hellstrand S, Schulz CA, Wallstrom P, Drake I, Ericson U, Gullberg B, Hedblad B, Orho-Melander M. The association between carbohydrate-rich foods and risk of cardiovascular disease is not modified by genetic susceptibility to dyslipidemia as determined by 80 validated variants. PLoS One. 2015;10:e0126104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sotomayor CG, Gomes-Neto AW, Eisenga MF, Nolte IM, Anderson JLC, De Borst MH, Oste MCJ, Rodrigo R, Gans ROB, Berger SP, et al. Consumption of fruits and vegetables and cardiovascular mortality in renal transplant recipients: a prospective cohort study. Nephrol Dial Transplant. 2018;27:27. [DOI] [PubMed] [Google Scholar]
- 141. Steffen LM, Jacobs DR Jr, Stevens J, Shahar E, Carithers T, Folsom AR. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;78:383–390. [DOI] [PubMed] [Google Scholar]
- 142. Stefler D, Pikhart H, Kubinova R, Pajak A, Stepaniak U, Malyutina S, Simonova G, Peasey A, Marmot MG, Bobak M. Fruit and vegetable consumption and mortality in Eastern Europe: longitudinal results from the Health, Alcohol and Psychosocial Factors in Eastern Europe study. Eur J Prev Cardiol. 2016;23:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Strandhagen E, Hansson PO, Bosaeus I, Isaksson B, Eriksson H. High fruit intake may reduce mortality among middle-aged and elderly men: the Study of Men Born in 1913. Eur J Clin Nutr. 2000;54:337–341. [DOI] [PubMed] [Google Scholar]
- 144. Takachi R, Inoue M, Ishihara J, Kurahashi N, Iwasaki M, Sasazuki S, Iso H, Tsubono Y, Tsugane S; JPHC Study Group . Fruit and vegetable intake and risk of total cancer and cardiovascular disease: Japan Public Health Center-Based Prospective Study. Am J Epidemiol. 2008;167:59–70. [DOI] [PubMed] [Google Scholar]
- 145. Tanaka S, Yoshimura Y, Kamada C, Tanaka S, Horikawa C, Okumura R, Ito H, Ohashi Y, Akanuma Y, Yamada N, et al. Intakes of dietary fiber, vegetables, and fruits and incidence of cardiovascular disease in Japanese patients with type 2 diabetes. Diabetes Care. 2013;36:3916–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tognon G, Lissner L, Saebye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. Br J Nutr. 2014;111:151–159. [DOI] [PubMed] [Google Scholar]
- 147. Tucker KL, Hallfrisch J, Qiao N, Muller D, Andres R, Fleg JL. The combination of high fruit and vegetable and low saturated fat intakes is more protective against mortality in aging men than is either alone: the Baltimore Longitudinal Study of Aging. J Nutr. 2005;135:556–561. [DOI] [PubMed] [Google Scholar]
- 148. Von Ruesten A, Feller S, Bergmann MM, Boeing H. Diet and risk of chronic diseases: results from the first 8 years of follow-up in the EPIC-Potsdam study. Eur J Clin Nutr. 2013;67:412–419. [DOI] [PubMed] [Google Scholar]
- 149. Vormund K, Braun J, Rohrmann S, Bopp M, Ballmer P, Faeh D. Mediterranean diet and mortality in Switzerland: an alpine paradox? Eur J Nutr. 2015;54:139–148. [DOI] [PubMed] [Google Scholar]
- 150. Wang JB, Fan JH, Dawsey SM, Sinha R, Freedman ND, Taylor PR, Qiao YL, Abnet CC. Dietary components and risk of total, cancer and cardiovascular disease mortality in the Linxian Nutrition Intervention Trials cohort in China. Sci Rep. 2016;6:22619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Watkins ML, Erickson JD, Thun MJ, Mulinare J, Heath CW Jr. Multivitamin use and mortality in a large prospective study. Am J Epidemiol. 2000;152:149–162. [DOI] [PubMed] [Google Scholar]
- 152. Whiteman D, Muir J, Jones L, Murphy M, Key T. Dietary questions as determinants of mortality: the OXCHECK experience. Public Health Nutr. 1999;2:477–487. [DOI] [PubMed] [Google Scholar]
- 153. Yamada T, Hayasaka S, Shibata Y, Ojima T, Saegusa T, Gotoh T, Ishikawa S, Nakamura Y, Kayaba K; Jichi Medical School Cohort Study Group . Frequency of citrus fruit intake is associated with the incidence of cardiovascular disease: the Jichi Medical School Cohort Study. J Epidemiol. 2011;21:169–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20-year incidence of stroke in a Japanese rural community: the Shibata study. Stroke. 2000;31:2287–2294. [DOI] [PubMed] [Google Scholar]
- 155. Yoshizaki T, Ishihara J, Kotemori A, Yamamoto J, Kokubo Y, Saito I, Yatsuya H, Yamagishi K, Sawada N, Iwasaki M, et al. Association of vegetable, fruit, and okinawan vegetable consumption with incident stroke and coronary heart disease. J Epidemiol. 2019;12:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Yu D, Zhang X, Gao YT, Li H, Yang G, Huang J, Zheng W, Xiang YB, Shu XO. Fruit and vegetable intake and risk of CHD: results from prospective cohort studies of Chinese adults in Shanghai. Br J Nutr. 2014;111:353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Zhang X, Shu XO, Xiang YB, Yang G, Li H, Gao J, Cai H, Gao YT, Zheng W. Cruciferous vegetable consumption is associated with a reduced risk of total and cardiovascular disease mortality. Am J Clin Nutr. 2011;94:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhang Y, Tuomilehto J, Jousilahti P, Wang Y, Antikainen R, Hu G. Lifestyle factors on the risks of ischemic and hemorrhagic stroke. Arch Intern Med. 2011;171:1811–1818. [DOI] [PubMed] [Google Scholar]
- 159. Saglimbene VW, Bondonno G, Ruospo N, Palmer M, Campbell SC, Garcia Larsen K, Natale V, Teixeirapinto P, Gargano A, Murgo L, et al. Fruit intake and cardiovascular and all-cause mortality in adults on hemodialysis: the DIET-HD multinational cohort study. Nephrology. 2017;22(suppl 3):25.26718476 [Google Scholar]
- 160. Gan YT, Li X, Cao L, Yin S, Gao X, Herath C, Li C, Jin W, Chen Z, Lu Y, et al. Consumption of fruit and vegetable and risk of coronary heart disease: a meta-analysis of prospective cohort studies. Int J Cardiol. 2015;183:129–137. [DOI] [PubMed] [Google Scholar]
- 161. Buil-Cosiales P, Martinez-Gonzalez M, Ruiz-Canela M, Díez-Espino J, García-Arellano A, Toledo E. Consumption of fruit or fiber-fruit decreases the risk of cardiovascular disease in a Mediterranean young cohort. Nutrients. 2017;9:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Miller V, Mente A, Dehghan M, Rangarajan S, Zhang X, Swaminathan S, Dagenais G, Gupta R, Mohan V, Lear S, et al. Fruit, vegetable, and legume intake, and cardiovascular disease and deaths in 18 countries (PURE): a prospective cohort study. Lancet. 2017;390:2037–2049. [DOI] [PubMed] [Google Scholar]
- 163. Hartley L, May MD, Loveman E, Colquitt JL, Rees K. Dietary fibre for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2016;2016:Cd011472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kim Y, Je Y. Dietary fibre intake and mortality from cardiovascular disease and all cancers: a meta-analysis of prospective cohort studies. Arch Cardiovasc Dis. 2016;109:39–54. [DOI] [PubMed] [Google Scholar]
- 165. Jenkins DJ, Srichaikul K, Kendall CW, Sievenpiper JL, Abdulnour S, Mirrahimi A, Meneses C, Nishi S, He X, Lee S, et al. The relation of low glycaemic index fruit consumption to glycaemic control and risk factors for coronary heart disease in type 2 diabetes. Diabetologia. 2011;54:271–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Mytton OT, Nnoaham K, Eyles H, Scarborough P, Ni MC. Systematic review and meta-analysis of the effect of increased vegetable and fruit consumption on body weight and energy intake. BMC Public Health. 2014;14:886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Fardet A, Boirie Y. Associations between food and beverage groups and major diet-related chronic diseases: an exhaustive review of pooled/meta-analyses and systematic reviews. Nutr Rev. 2014;72:741–762. [DOI] [PubMed] [Google Scholar]
- 168. Flegal KM, Panagiotou OA, Graubard BI. Estimating population attributable fractions to quantify the health burden of obesity. Ann Epidemiol. 2015;25:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994;344:793–795. [DOI] [PubMed] [Google Scholar]
- 170. Ashor AW, Lara J, Siervo M. Medium-term effects of dietary nitrate supplementation on systolic and diastolic blood pressure in adults: a systematic review and meta-analysis. J Hypertens. 2017;35:1353–1359. [DOI] [PubMed] [Google Scholar]
- 171. Blekkenhorst LC, Bondonno CP, Lewis JR, Devine A, Woodman RJ, Croft KD, Lim WH, Wong G, Beilin LJ, Prince RL, et al. Association of dietary nitrate with atherosclerotic vascular disease mortality: a prospective cohort study of older adult women. Am J Clin Nutr. 2017;106:207–216. [DOI] [PubMed] [Google Scholar]
- 172. Jovanovski E, Bosco L, Khan K, Au-Yeung F, Ho H, Zurbau A, Jenkins AL, Vuksan V. Effect of spinach, a high dietary nitrate source, on arterial stiffness and related hemodynamic measures: a randomized, controlled trial in healthy adults. Clin Nutr Res. 2015;4:160–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Filippini T, Violi F, D'Amico R, Vinceti M. The effect of potassium supplementation on blood pressure in hypertensive subjects: a systematic review and meta-analysis. Int J Cardiol. 2017;230:127–135. [DOI] [PubMed] [Google Scholar]
- 174. Van Mierlo LA, Arends LR, Streppel MT, Zeegers MP, Kok FJ, Grobbee DE, Geleijnse JM. Blood pressure response to calcium supplementation: a meta-analysis of randomized controlled trials. J Hum Hypertens. 2006;20:571–580. [DOI] [PubMed] [Google Scholar]
- 175. Zhang X, Li Y, Del Gobbo LC, Rosanoff A, Wang J, Zhang W, Song Y. Effects of magnesium supplementation on blood pressure: a meta-analysis of randomized double-blind placebo-controlled trials. Hypertension. 2016;68:324–333. [DOI] [PubMed] [Google Scholar]
- 176. World Heath Organization . Fruit and vegetable promotion initiative—report of the meeting. 2003. Available at: https://www.who.int/dietphysicalactivity/publications/f&v_promotion_initiative_report.pdf?ua=1. Accessed August 31, 2020.
- 177. World Health Organization . Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation. Geneva: WHO Technical Report Series; 2003. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S9
Figures S1–S205


