Abstract
The purpose of this investigation was to evaluate the effects of aging and lifelong exercise on skeletal muscle components of the innate immune system. Additionally, the effects of an acute resistance exercise (RE) challenge were explored. Three groups of men were studied: young exercisers (YE: n = 10, 25 ± 1 yr; V̇o2max: 53 ± 3 mL/kg/min; quadriceps size: 78 ± 3 cm2), lifelong aerobic exercisers with a 53 ± 1 yr training history (LLE; n = 21, 74 ± 1 yr; V̇o2max: 34 ± 1 mL/kg/min; quadriceps size: 67 ± 2 cm2), and old healthy nonexercisers (OH: n = 10, 75 ± 1 yr; V̇o2max: 22 ± 1 mL/kg/min, quadriceps size: 56 ± 3 cm2). Vastus lateralis muscle biopsies were obtained in the basal state and 4 h after RE (3 × 10 reps, 70% of 1 repetition maximum) to assess Toll-like receptors (TLR)1–10, TLR adaptors (Myd88 and TRIF), and NF-κB pathway components (IκΒα and IKKβ) mRNA expression. Basal TLR3, TLR6, and TLR7 tended to be higher (P ≤ 0.10) with aging (LLE and OH combined). In general, RE increased expression of TLR1 and TLR8 (P ≤ 0.10) and TLR3 and TLR4 (P < 0.05), although TLR3 did not respond in OH. Both TLR adaptors also responded to the exercise bout; these were primarily (Myd88, main effect P ≤ 0.10) or exclusively (TRIF, P < 0.05) driven by the OH group. In summary, aging appears to increase basal expression of some innate immune components in human skeletal muscle, and lifelong aerobic exercise does not affect this age-related increase. An exercise challenge stimulates the expression of several TLRs, while the TLR adaptor response appears to be dysregulated with aging and maintained with lifelong exercise. Partially preserved muscle mass, coupled with a notable immunity profile, suggests lifelong exercisers are likely better prepared for a stress that challenges the immune system.
NEW & NOTEWORTHY Findings from this investigation provide novel insight into the effect of aging and lifelong aerobic exercise on structural components of the innate immune system in skeletal muscle of humans. Data presented here suggest aging increases basal expression of select Toll-like receptors (TLRs), and lifelong exercise does not impact this age-related increase. Additionally, acute exercise stimulates gene expression of several TLRs, while the adaptor response is likely dysregulated with aging and maintained with lifelong exercise.
Keywords: aging, innate immunity, lifelong exercise, skeletal muscle, TLR
INTRODUCTION
The decline in skeletal muscle health (i.e., size and performance) seen with aging is a challenge for older individuals due to the increased risk of associated negative health outcomes, including disability (18), hospitalization, and susceptibility to disease or infection (2, 8, 56). In fact, individuals with a low amount of skeletal muscle mass are twice as likely to acquire an infection during hospitalization (8). While the quantity of skeletal muscle an individual possesses plays a key role, the quality of the muscle infrastructure related to susceptibility and management of infections may play an equally critical role. For example, muscle may detect and drive a coordinated immune response to infections.
The immune system is a layered defense network, in which initial detection and activation occurs through the innate immune system. Toll-like receptors (TLR) are commonly referred to as sentinels of the innate immune system due to their central role in detecting and mounting a response to danger signals (5). There are currently 10 known TLRs (TLR1–10) in humans organized to detect circulating and intracellular pathogen-associated (PAMPs) and damage-associated molecular patterns (DAMPs) (1, 25, 38, 42). Once activated, most TLRs communicate into the cell via myeloid differentiation primary response 88 (Myd88). This adaptor induces activation of the MAPK, NF-κB, and cyclooxygenase (COX) pathways (25). While nine of the TLRs (TLR1–2, 4–10) signal through Myd88, TLR3 and TLR4 utilize the Toll interleukin (IL)-1 receptor-containing adaptor-inducing interferon gamma (TRIF). Signaling through these pathway systems is well known to produce inflammatory factors, including IL6, IL8, IL10, TNFα, and prostaglandin (PG)E2 (1, 25, 55), which propagate the immune response.
Despite being sentinels of immunity, TLRs and associated machinery have received surprisingly little attention in skeletal muscle. Existing literature suggests aging increases components of the innate immune system in skeletal muscle (14). For example, TLR4 expression in skeletal muscle is reported to increase with age (14). Circulating levels of lipopolysaccharide (LPS), a PAMP ligand for TLR4, and its binding protein (LBP) also increase with age (14, 15). More of these receptor components, PAMPs and DAMPs, in older individuals may promote inflammation through greater basal TLR pathway flux (22). Inflammation regulates protein metabolism, via reduced amino uptake (54) and increased protein degradation (3), and is thus a concern for older adults because this population classically possess low muscle mass (11, 52), the primary protein and amino acid reservoir in the body.
Innate immune components exhibit plasticity to physical activity and exercise training. More specifically, controlled reductions in physical activity patterns activate the innate immune response in older adults (10), whereas exercise training decreases activator levels (i.e., LPS), at least in young individuals (32). Although 1 yr of aerobic training reduces LPS in young (obese) individuals (31), older adults fail to adapt to 4-mo aerobic training with respect to LPS and TLR4 (14). This suggests older individuals may be capable of modifying immunity through exercise training; however, the duration of exercise intervention is likely critical. Therefore, for immunity to adapt, exercise training likely needs to extend years or even the course of the life span.
The innate immune system is the early line of defense against infection. Muscle maintains components of this protective mechanism and is a major target for the inflammatory response, which highlights the importance of maintaining skeletal muscle size into late life. The catastrophic impact of infections on muscle size is clear (43), and when coupled with heightened susceptibility in older individuals, the importance of infection management becomes paramount. This relationship between aging skeletal muscle, infections, and related immune infrastructure components is an emerging knowledge gap. Because innate immune components are not responsive to short-term exercise training in older individuals, long-term training or maintaining an active lifestyle over the life span (i.e., lifelong exercise) may be more effective. Lifelong exercise guards against age-related declines in many facets critical to overall health, including skeletal muscle (6, 9, 16, 48).
We had the unique opportunity to examine men that consistently exercised for more than 50 yr to gain insight into the impact of this lifestyle on innate immune infrastructure in human skeletal muscle. Two groups of successful aging men, lifelong exercisers (LLE) and old healthy individuals (OH), were compared with young exercisers (YE). Through a targeted yet comprehensive approach, analysis of 14 components of the innate immune system was performed. Given the general inflammatory nature of these innate immune components, we hypothesized findings would follow a hierarchical pattern (YE<LLE<OH). Additionally, a novel exploration of the innate immune infrastructure gene response to a resistance exercise challenge was conducted. We selected an exercise challenge that when performed acutely induce growth signals and when completed chronically elicit significant increases in muscle size and strength in young and old individuals (47, 50).
METHODS
Subjects
Old lifelong exercisers (LLE; n = 21 men), old healthy controls (OH; n = 10 men), and young exercisers (YE; n = 10 men) were included in this investigation (Table 1). Subjects were recruited from the greater Muncie, Indiana area by newspaper advertisements, mailed flyers, and personal interaction. More extensive subject characteristics and details regarding the recruitment and screening process, along with cardiovascular and skeletal muscle profiles are presented by our research team elsewhere (7, 16). Enrolled individuals were free from acute or chronic illness (cardiac, pulmonary, liver, or kidney abnormalities, cancer, uncontrolled hypertension, insulin- or noninsulin dependent diabetes, or other known metabolic disorders) and free from orthopedic limitations (including any artificial joints), and they did not smoke or participate in other forms of tobacco use. The study was approved by the Institutional Review Board of Ball State University. All study procedures, risks, and benefits were explained to the subjects before they gave written informed consent to participate.
Table 1.
Subject characteristics
| Lifelong Exercisers |
|||||
|---|---|---|---|---|---|
| YE | Combined | LLE-P | LLE-F | OH | |
| N | 10 | 21 | 14 | 7 | 10 |
| Age, yr | 25 ± 1* | 74 ± 1 | 74 ± 1 | 75 ± 2 | 75 ± 1 |
| Height, cm | 181 ± 2 | 180 ± 2 | 179 ± 2 | 182 ± 3 | 177 ± 2 |
| Weight, kg | 75 ± 3 | 79 ± 2 | 77 ± 2 | 83 ± 5 | 89 ± 3* |
| BMI, kg/m2 | 23 ± 1 | 24 ± 1 | 24 ± 1 | 25 ± 1 | 28 ± 1* |
| Body fat, % | 18 ± 2* | 24 ± 1† | 22 ± 1‡ | 27 ± 1 | 32 ± 1 |
| V̇o2max, mL/kg/min | 53 ± 3* | 34 ± 1† | 38 ± 1‡ | 27 ± 2 | 22 ± 1 |
| Quadriceps size, cm2 | 78 ± 3* | 67 ± 2† | 68 ± 2 | 65 ± 3 | 56 ± 3 |
| Quadriceps strength, Nm | 210 ± 12* | 165 ± 6† | 166 ± 8 | 163 ± 10 | 134 ± 9 |
| Quadriceps power, W | 699 ± 30* | 370 ± 19 | 365 ± 13 | 377 ± 50 | 318 ± 42 |
| Handgrip strength, kg | 51 ± 3 | 46 ± 2 | 48 ± 3 | 43 ± 2 | 44 ± 1 |
| Steps per day | 9,404 ± 635 | 9,560 ± 619 | 9,369 ± 725 | 10,006 ± 1,265 | 5813 ± 488* |
Values are means ± SE. YE, young exercisers; LLE-P, lifelong exercisers-performance; LLE-F, lifelong exercisers-fitness, OH, old healthy. Additional cardiovascular and skeletal muscle data, as well as details of the body fat (DXA), V̇o2max, muscle size (MRI) and function, and steps per day measurements, are presented by us elsewhere (7, 16).
P ≤ 0.05 vs. main groups;
P ≤ 0.05 vs. OH;
P ≤ 0.05 LLE-P vs. LLE-F.
Exercise history of the subjects was carefully evaluated using a comprehensive questionnaire and confirmed through personal interviews (Table 2). The LLE cohort consisted primarily of cyclists and runners that reported ∼50 yr of structured exercise. LLE trained ∼5 days and ∼7 h per week. Exercise history of LLE subjects was extensively reviewed for frequency, duration, intensity, and athletic achievements. As such, two clear LLE subgroups emerged: one group that participated in lower intensity training for physical fitness (Fitness, LLE-F; n = 7) and another group that trained more vigorously and often participated in competitive events (Performance, LLE-P; n = 14).
Table 2.
Exercise training histories
| Lifelong Exercisers |
|||||
|---|---|---|---|---|---|
| YE | Combined | LLE-P | LLE-F | OH | |
| Total training years | 5 ± 1* | 53 ± 1 | 53 ± 1 | 53 ± 3 | |
| Competitive focusa | Yes | Yes | No | ||
| Lifetime average | |||||
| Frequency, days/wk | 4.5 ± 0.2 | 4.4 ± 0.2 | 4.6 ± 0.3 | ||
| Duration, h/wk | 7.3 ± 0.5 | 7.6 ± 0.7 | 6.6 ± 0.9 | ||
| Intensityb | 2.0 ± 0.1 | 2.1 ± 0.1‡ | 1.8 ± 0.1 | ||
| Current decade | |||||
| Frequency, days/wk | 5.1 ± 0.2 | 4.7 ± 0.3 | 4.5 ± 0.3 | 4.9 ± 0.7 | |
| Duration, h/wk | 7.0 ± 0.7 | 8.1 ± 1.1 | 8.5 ± 1.4 | 7.4 ± 1.9 | |
| Intensityb | 2.8 ± 0.1* | 2.0 ± 0.1 | 2.2 ± 0.1‡ | 1.5 ± 0.2 | |
Values are means ± SE. YE, young exercisers; LLE-P, lifelong exercisers-performance; LLE-F, lifelong exercisers-fitness; OH, old healthy. In the case that a subject reported >1 training intensity, values were weighted and averaged (e.g., 80% of training at a 2 and 20% of training at a 3 resulted in an overall intensity of 2.2). More detailed exercise training histories are presented by us elsewhere (16).
Competitive focus indicates exercise training for the purpose of competition was currently or once a primary goal for the majority of the group. Lifetime average reflects current decade exercise habits for YE.
Levels of self-reported training intensity were: 1 (Light), 2 (Moderate), and 3 (Hard).
P ≤ 0.05 vs. LLE Combined;
P ≤ 0.05 LLE-P vs. LLE-F.
Acute Exercise Trial and Skeletal Muscle Biopsies
Subjects completed a resistance exercise challenge of the knee extensors, consisting of 3 sets of 10 repetitions at 70% of 1 repetition maximum, with 2 min rest between each set. This exercise bout was selected to provide a robust exercise challenge that activates protein metabolism pathways and induces skeletal muscle growth when completed chronically in young and old individuals (47, 50). Muscle biopsies (4) of the vastus lateralis were obtained before and 4 h after the resistance exercise challenge. This postexercise timepoint provides an optimal window to assess expression of numerous regulators of muscle adaptation (35, 44, 59). All biopsies were obtained in the fasted state (≥ 10 h), after at least 30 min of supine rest. Subjects remained in the laboratory and rested quietly during the 4-h postexercise period. Subjects also refrained from structured exercise and aspirin consumption for 72 h, alcohol consumption for 24 h, and caffeine the morning of the trial.
Following each muscle biopsy, excess blood, visible fat, and connective tissue were removed, and a portion of the muscle (∼20 mg) to be used for mRNA analysis was immediately frozen and then stored in liquid nitrogen. Before analysis, the muscle was transferred to 0.5 mL RNAlater-ICE (Ambion, Austin, TX) and stored at −20°C until analysis.
Gene Expression Measurements
Innate immune infrastructure targets listed in Table 3 were assessed in vastus lateralis skeletal muscle homogenates using real-time quantitative polymerase chain reaction (qPCR). Gene expression was assessed in muscle homogenates with the intent of representing the in vivo muscle environment more closely. Muscle mRNA analyses were completed on all 41 subjects for basal expression and on 39 subjects for expression 4 h postexercise (i.e., 2 individuals did not undergo the postexercise biopsy, 1 from LLE and 1 from OH) (29).
Table 3.
Nomenclature, gene information, and mRNA primer characteristics
| Common Name | Gene Name | Accession No. | Sequence (5′→3′) | Amplicon Size, bp | mRNA Region, bp | Annealing Temp, °C |
|---|---|---|---|---|---|---|
| Toll-like receptors | ||||||
| TLR1 | TLR1 | NM_003263.3 |
TCCCGGAGGCAATGCTGCTGT ACAGATTCCTTTTGTAGGGGTGCCCA |
145 | 70–214 | 61 |
| TLR2 | TLR2 | NM_003264.4a |
GCAGGATCCAAAGGAGACCT CCAGTGCTTCAACCCACAAC |
129 | 125–253 | 61 |
| TLR3 | TLR3 | NM_003265.2 |
GCAAAAGATTCAAGGTACATCATGC CCTCTTCGCAAACAGAGTGC |
127 | 2581–2707 | 61 |
| TLR4 | TLR4 | NM_003266.3b |
CCGTTTTATCACGGAGGTGGT GGGAGGTTGTCGGGGATTTT |
81 | 496–576 | 61 |
| TLR5 | TLR5 | NM_003268.5 |
ACGAGGATCATGGGAGACCA ATTCGGCCATCAAAGGAGCA |
98 | 633–730 | 61 |
| TLR6 | TLR6 | NM_006068.4 |
GCAGGGGACAATCCATTCCA AGAATCAGGCCAGCCCTCTA |
99 | 1697–1795 | 61 |
| TLR7 | TLR7 | NM_016562.3 |
GGCCCATCTCAAGCTGATCT GTCCACATTGGAAACACCATTT |
106 | 54–159 | 61 |
| TLR8 | TLR8 | NM_016610.3c |
ACTTGACCCAACTTCGATACCT CAGCACCTTCAGATGAGGCA |
95 | 1078–1172 | 61 |
| TLR9 | TLR9 | NM_017442.3 |
TACCTTGCCTGCCTTCCTAC TGACATTGCCACGGGGTG |
116 | 715–830 | 60 |
| TLR10 | TLR10 | NM_030956.3d |
TATACAGGGTTTTGAGCTCATCTT GCATCACCCTCTGCTGTCAT |
131 | 570–700 | 61 |
| Adaptors | ||||||
| Myd88 | MYD88 | NM_001172569.1e |
TCTCCAGGTGCCCATCAGAA AAGGCGAGTCCAGAACCAAG |
138 | 637–774 | 61 |
| TRIF* | TICAM1 | NM_182919.3 |
CATCCTCGGCTTTGCCCTC GGGGACTGGCTGATTTCCAA |
139 | 768–906 | 61 |
| Signaling components | ||||||
| IKK↠| IKBKB | NM_001556.2f |
ATGTCATCCGATGGCACAATCAGG TGGGTCAGCCTTCTCATGATCTGG |
127 | 260–386 | 60 |
| IκBα‡ | NFKBIA | NM_020529.2 |
CCAACTACAATGGCCACACGTGTCTACA GAGCATTGACATCAGCACCCAAGG |
99 | 646–744 | 60 |
For primer sequences, top sequence reflects the forward primer and bottom sequence reflects the reverse primer. Other aliases:
Toll-like receptor adaptor molecule 1;
Inhibitor of nuclear factor kappa B kinase subunit beta;
NF-κB inhibitor alpha.
Primers detect variant 2 (NM_001318789.1), variant 3 (NM_003264.4), variant 4 (NM_001318790.1), and variant 6 (NM_001318793.1).
Primers detect all variants encoding isoform a: variant 1 (NM_030956.3), variant 2 (NM_001017388.2) variant 3 (NM_001195106.1), and variant 4 (NM_001195107.1).
Primers detect all variants: variant 1 isoform 1 (NM_001172567.1), variant 2 isoform 2 (NM_002468.4), variant 3 isoform 3 (NM_001172568.1), variant 4 isoform 4 (NM_001172569.1), and variant 5 isoform 5 (NM_001172566.1).
Primers detect variant 1 isoform 1 (NM_001556.2), variant 2 isoform 2 (NM_001190720.2), and variant 7 isoform 5 (NM_001242778.1).
Total extraction and RNA quality check.
Total RNA was extracted in TRI Reagent RT (Molecular Research Center, Cincinnati, OH). The quality and integrity (RIN = 8.34 ± 0.05) of extracted RNA (94.24 ± 3.97 ng/µL) were evaluated using a RNA 6000 Nano LabChip kit on an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) as previously described (19, 51, 59).
Real-time polymerase chain reaction.
Oligo (dT) primed first-strand cDNA was synthesized (96–144 ng of total RNA, depending on magnitude of gene target expression) using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA). For each target, quantification of mRNA levels was performed in duplicate in a 72-well Rotor-Gene Q Centrifugal Real-Time Cycler (Qiagen, Germantown, MD). Ribosomal protein lateral stalk subunit P0 (RPLP0) was used as a housekeeping/reference gene, as previously done in human muscle (37). RPLP0 was similar among the three groups at baseline [threshold cycle (CT): 19.02 ± 0.03] and stable after exercise (CT: 19.01 ± 0.03). All primers in this study were mRNA specific (on different exons and/or crossing over an intron) and designed for qPCR (Vector NTI Advance 9 software, Invitrogen and Primer Design Tool [Entrez] NCBI/Primer-BLAST program) using SYBR Green chemistry (19). Primer details are presented in Table 3. A melting curve analysis was generated for all PCR runs to validate that only one product was present. For each run, a serial dilution curve was made using cDNA from a known amount (500–2,000 ng) of human skeletal muscle RNA (Ambion, Austin, TX) or from human muscle samples collected in our laboratory. The amplification calculated by the Rotor-Gene software was specific and highly efficient (efficiency = 1.04 ± 0.02; R2 = 0.98 ± 0.00; slope = 3.26 ± 0.04). Basal gene expression among YE, LLE, and OH was compared using the 2−ΔCT (arbitrary units) method. Gene expression before and after the resistance exercise challenge was compared using the 2−ΔΔCT (fold change) relative quantification method, as previously described (33, 53, 59). On the basis of the principle of the calculation, it was determined the preexercise value and associated variability are close to 1 for each group. In the present study, this was true of all genes analyzed, and preexercise 2−ΔΔCT was not statistically different among the three groups and between subgroups (P > 0.05). Therefore, to simplify interpretation, preexercise expression for each gene is graphically represented as a dotted line at 1.0-fold.
Statistical Analyses
Data were analyzed with a one-way ANOVA to compare subject characteristics, training histories, and basal gene expression (2−ΔCT method) among the three main groups (YE, LLE, and OH) and between LLE subgroups (LLE-F and LLE-P). A follow-up one-way ANOVA was used to compare basal gene expression between YE and both old groups combined (LLE and OH). A two-way ANOVA (group × time) was completed to evaluate gene expression (2−ΔΔCT method) in response to exercise among the three main groups and LLE subgroups. Post hoc comparisons were made with Tukey’s test. No adjustments were made to control for potential false positives across the gene targets. Significance was accepted at P < 0.05. Data are presented as means ± SE.
RESULTS
Subject characteristics, maximal aerobic capacity, and muscle size and performance data are shown in Table 1 (7, 16). Exercise training histories are presented in Table 2. A hierarchical pattern (YE>LLE>OH) was observed for V̇o2max (mL/kg/min), quadriceps muscle size (cm2), and quadriceps strength (Nm). More specifically, LLE attenuated (P < 0.05) the age-related decline in V̇o2max by 39% (LLE: −36%, OH: −59%; P < 0.05). For quadriceps muscle size, LLE attenuated (P < 0.05) the age-related decline by 50% (LLE: −14%, OH: −29%; P < 0.05). Similarly, LLE attenuated (P < 0.05) the age-related decline in quadriceps strength by 41% (LLE: −21%, OH: −36%; P < 0.05).
Basal Skeletal Muscle Gene Expression
Toll-like receptors.
Expression of all Toll-like receptors (TLR1–10) was detected in skeletal muscle of the lifelong exercisers (LLE), young exercisers (YE), and old healthy (OH) in the basal state (Supplemental Table S1, available at https://doi.org/10.6084/m9.figshare.12971729.v1). In general, all three main groups followed similar TLR expression patterns. For the YE group alone, relative basal expression of TLR1–10 is presented in Supplemental Fig. S1 (available at https://doi.org/10.6084/m9.figshare.12971669). TLR9 exhibited the greatest expression and was ∼54-fold higher mRNA expression than TLR7, the lowest expressed TLR.
Basal mRNA expression of each of the 10 TLRs (TLR1–10) was not different (P > 0.05) among YE, LLE, and OH (Fig. 1). However, the aging cohorts (LLE and OH combined) tended to have higher (P ≤ 0.10) expression of TLR3, TLR6, and TLR7 than YE (59–73% higher). There were no differences (P > 0.05) in basal expression of any TLR between the LLE fitness (LLE-F) and performance (LLE-P) subgroups (Table 4), suggesting no effect of intensity of lifelong aerobic training in aging muscle.
Fig. 1.
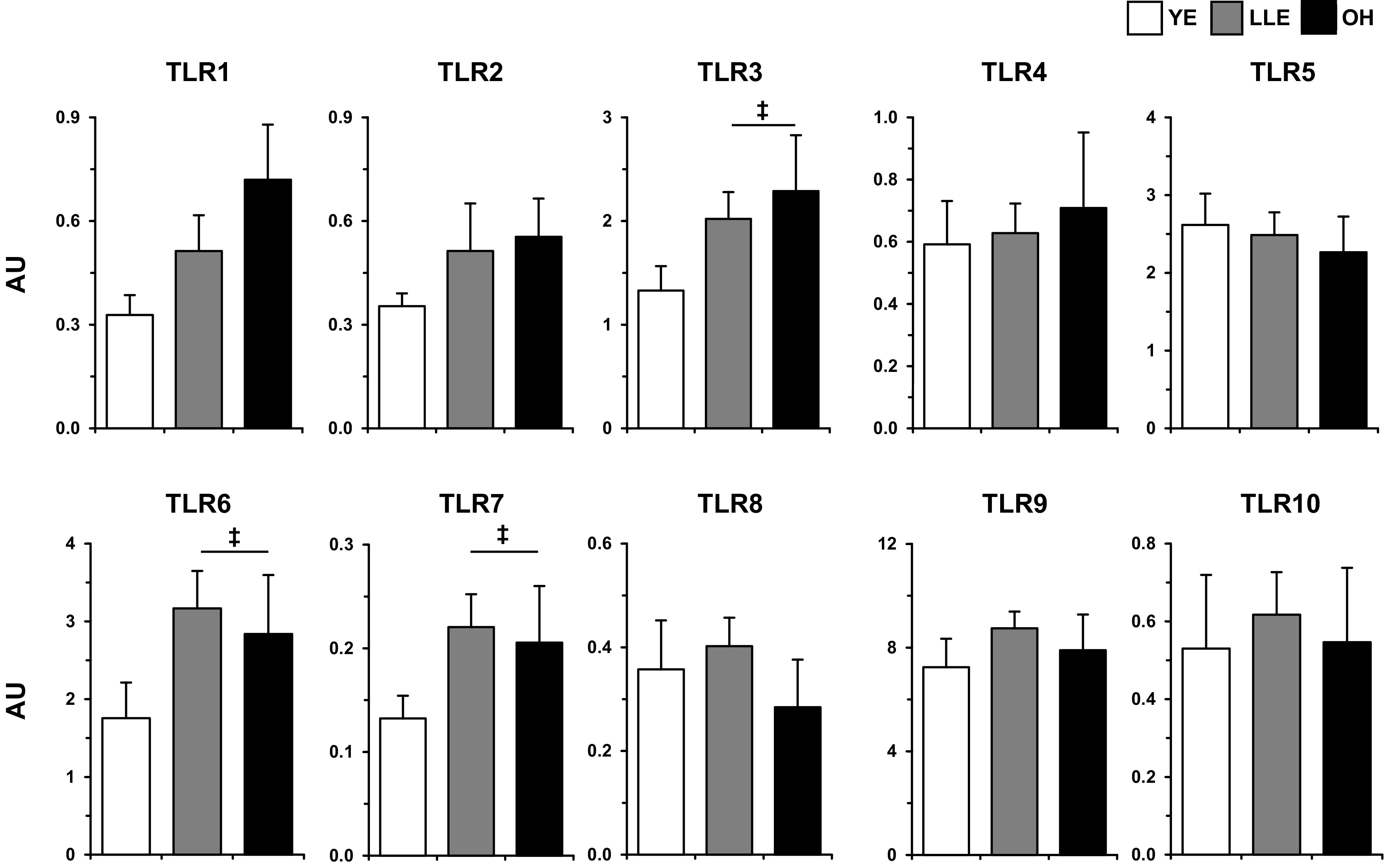
Basal Toll-like receptor (TLR) expression in human skeletal muscle (vastus lateralis) among young exercisers (YE), lifelong exercisers (LLE), and old healthy (OH). ‡P ≤ 0.10 both old groups vs. YE. AU, arbitrary units.
Table 4.
Basal and exercise-induced Toll-like receptor and adaptor gene expression between LLE subgroups
| Acute Exercise Response |
||||||
|---|---|---|---|---|---|---|
| Basal |
LLE-P (Δ) |
LLE-F (Δ) |
||||
| Gene | LLE-F (AU) | LLE-P (AU) | Pre | Post | Pre | Post |
| TLR1§ | 0.26 ± 0.08 | 0.62 ± 0.14 | 1.44 ± 0.27 | 1.87 ± 0.30 | 1.54 ± 0.47 | 3.13 ± 0.90 |
| TLR2 | 0.78 ± 0.40 | 0.38 ± 0.05 | 1.12 ± 0.16 | 2.10 ± 0.40 | 1.59 ± 0.81 | 1.28 ± 0.24 |
| TLR3§ | 1.75 ± 0.50 | 2.16 ± 0.30 | 1.18 ± 0.17 | 1.43 ± 0.18 | 1.30 ± 0.37 | 1.83 ± 0.42 |
| TLR4 | 0.63 ± 0.21 | 0.63 ± 0.10 | 1.14 ± 0.17 | 2.05 ± 0.25** | 1.40 ± 0.46 | 2.47 ± 0.69** |
| TLR5 | 2.53 ± 0.64 | 2.47 ± 0.32 | 1.14 ± 0.16 | 1.28 ± 0.15 | 1.19 ± 0.30 | 0.90 ± 0.21 |
| TLR6 | 3.04 ± 0.86 | 3.23 ± 0.60 | 1.17 ± 0.21 | 1.66 ± 0.23 | 1.21 ± 0.34 | 1.27 ± 0.36 |
| TLR7 | 0.27 ± 0.06 | 0.20 ± 0.04 | 1.35 ± 0.30 | 1.38 ± 0.29 | 1.25 ± 0.28 | 1.06 ± 0.21 |
| TLR8 | 0.32 ± 0.12 | 0.44 ± 0.06 | 1.20 ± 0.17 | 1.38 ± 0.20 | 1.53 ± 0.55 | 1.96 ± 0.37 |
| TLR9 | 9.54 ± 1.45 | 8.35 ± 0.66 | 1.05 ± 0.08 | 0.98 ± 0.04 | 1.07 ± 0.16 | 0.90 ± 0.07 |
| TLR10 | 0.72 ± 0.28 | 0.57 ± 0.09 | 1.21 ± 0.19 | 2.11 ± 0.42 | 1.50 ± 0.59 | 0.90 ± 0.20 |
| Myd88 | 33.8 ± 4.2 | 32.4 ± 1.9 | 1.03 ± 0.07 | 1.16 ± 0.09 | 1.04 ± 0.13 | 1.08 ± 0.07 |
| TRIF | 82.8 ± 11.1* | 50.0 ± 2.1 | 1.01 ± 0.04 | 1.23 ± 0.11 | 1.05 ± 0.14 | 0.97 ± 0.08 |
TLR, Toll-like receptor; Myd88, myeloid differentiation primary response 88; TRIF, Toll interleukin (IL)-1 receptor-containing adaptor-inducing IFN-γ; AU, arbitrary units; LLE-P, lifelong exercisers-performance; LLE-F, lifelong exercisers-fitness.
P < 0.05 vs. LLE-P;
P < 0.05 vs. preexercise;
P < 0.05 main effect for exercise.
Adaptors.
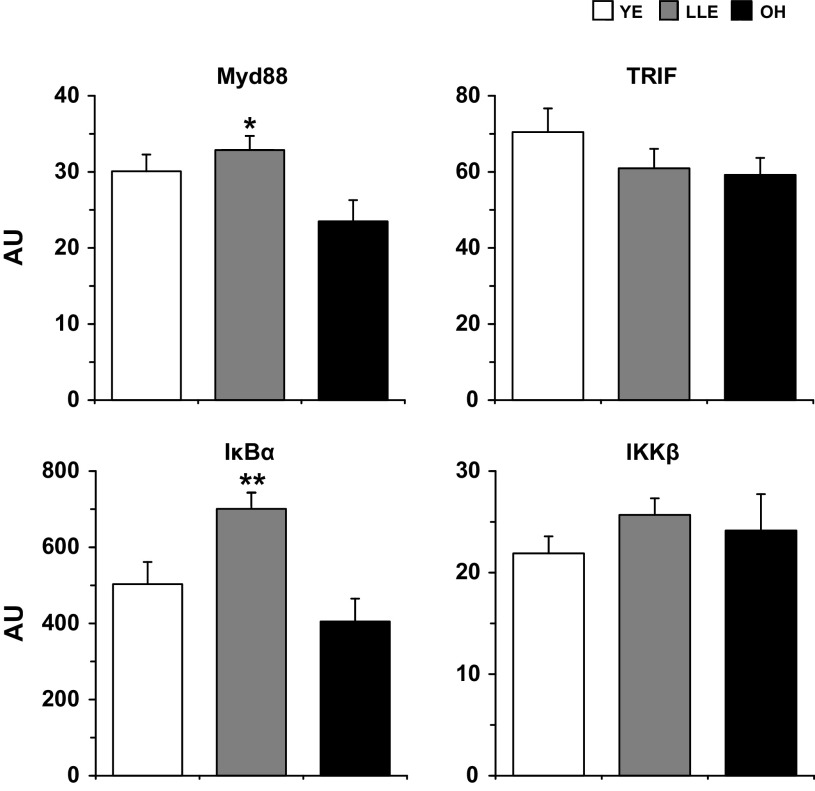
Expression of both adaptors, Myd88 and TRIF were detected basally in all subjects (Supplemental Table S1). Basal Myd88 expression was 40% higher (P < 0.05) in LLE compared with OH, while TRIF was similar (P > 0.05) among groups (Fig. 2). Myd88 was similar (P > 0.05) between LLE subgroups and TRIF was 66% higher (P < 0.05) in LLE-F than LLE-P (Table 4).
Fig. 2.
Basal adaptor and NF-κB (IκBα and IKKβ) expression in human skeletal muscle (vastus lateralis) among young exercisers (YE), lifelong exercisers (LLE), and old healthy (OH). *P < 0.05 vs OH. **P < 0.05 vs. both groups. Myd88, myeloid differentiation primary response 88; TRIF, Toll interleukin (IL)-1 receptor-containing adaptor-inducing IFN-γ; AU, arbitrary units.
NF-κB pathway components.
IκBα and IKKβ expression for the three main groups is presented in Fig. 2. IκBα expression was 39 and 73% higher (P < 0.05) in LLE compared with YE and OH, respectively. IKKβ was similar (P > 0.05) among YE, LLE, and OH. LLE-F [677 ± 74 arbitrary units (AU)] and LLE-P (713 ± 53 AU) exhibited similar (P > 0.05) IκBα expression, while IKKβ was 54% higher (P < 0.05) in LLE-P than LLE-F (LLE-P: 29.1 ± 1.7; LLE-F: 18.9 ± 1.7 AU).
Exercise-Induced Skeletal Muscle Gene Expression
Toll-like receptors.
Fold-changes in TLR expression following exercise for the three main groups and LLE subgroups are found in Fig. 3 and Table 4, respectively. The three main groups together tended to increase TLR1 following exercise (main effect, P ≤ 0.10). Additionally, there was a main effect (2.3-fold, P < 0.05) for exercise to increase TLR1 in the LLE subgroups. TLR3 expression increased (2.2-fold, P < 0.05) in YE, and there was a main effect (1.6-fold, P < 0.05) for exercise with LLE-F and LLE-P. For the three primary groups, TLR4 increased (main effect, P < 0.05) after exercise, and LLE-F and LLE-P also increased TLR4 expression 2.5- and 2.1-fold (P < 0.05), respectively. Together (main effect, P ≤ 0.10), YE, LLE, and OH tended to increase (1.9-fold) TLR8 expression following exercise. This TLR8 response was primarily driven by the OH group.
Fig. 3.
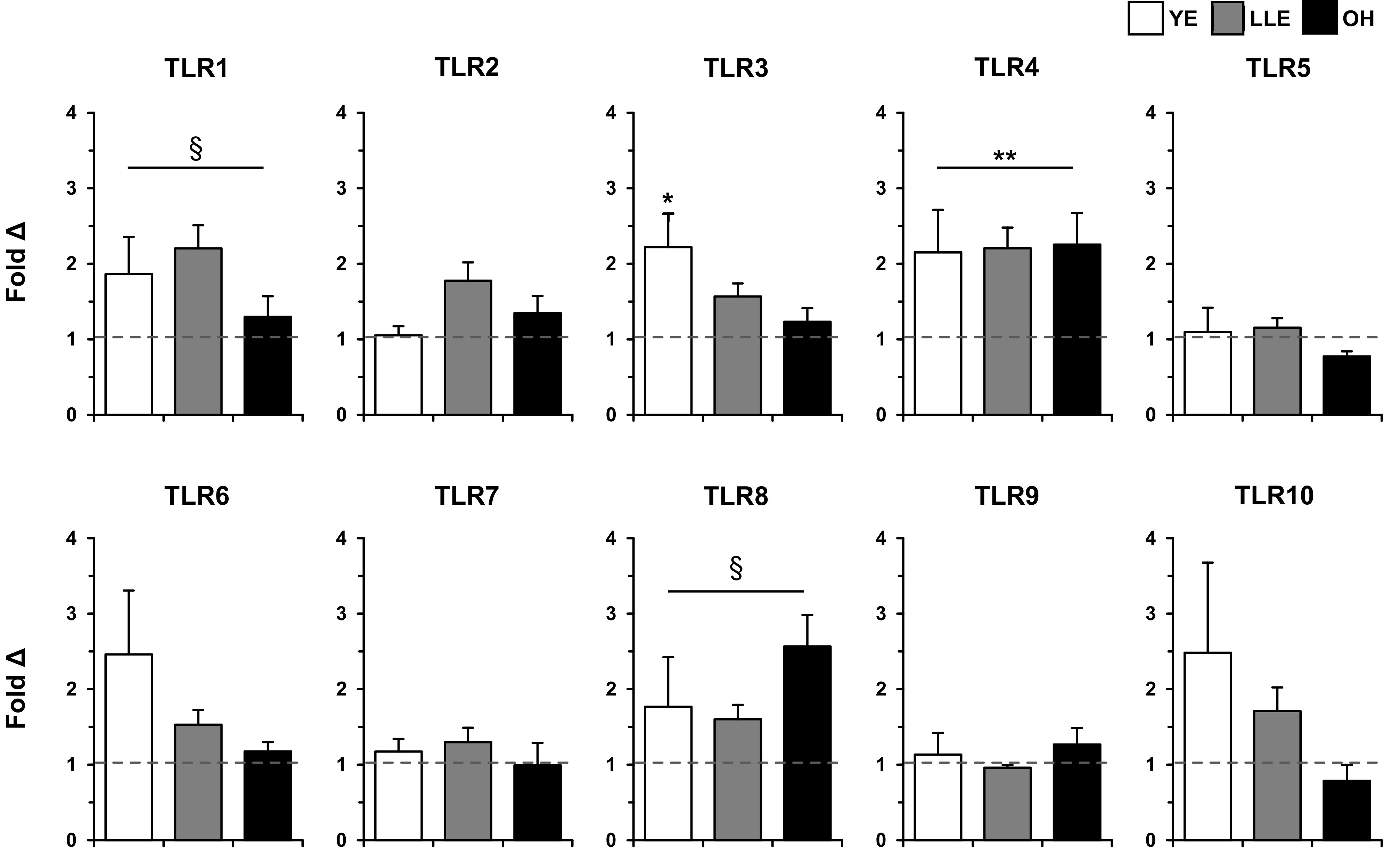
Fold-changes in Toll-like receptor (TLR) expression 4 h following acute resistance exercise in human skeletal muscle (vastus lateralis) among young exercisers (YE), lifelong exercisers (LLE), and old healthy (OH). The dotted line represents the preexercise fold-change for each group, derived from the 2−ΔΔCT calculation (see methods). *P < 0.05 vs preexercise. **P < 0.05 main effect for exercise. §P ≤ 0.10 main effect for exercise.
Adaptors.
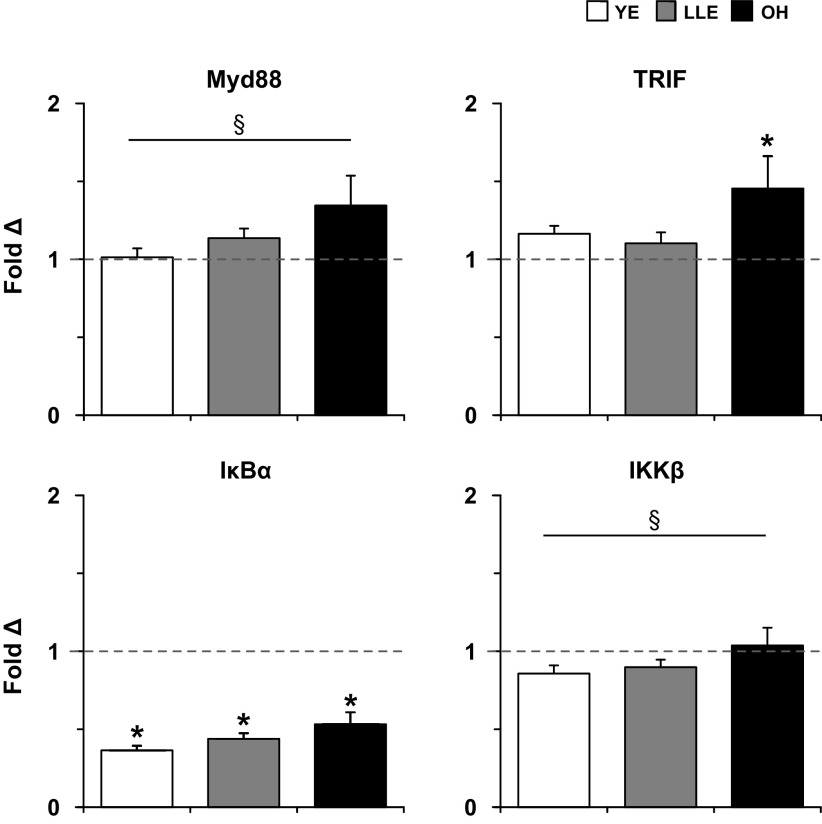
With the three main groups, there was a trend (1.2-fold, P ≤ 0.10) for a main effect to increase Myd88 expression after exercise, primarily driven by the OH group (Fig. 4). OH increased (P < 0.05) TRIF expression in response to exercise 1.5-fold, whereas YE and LLE did not change (P > 0.05). There was no change (P > 0.05) in response to exercise with Myd88 and TRIF expression in LLE-F and LLE-P (Table 4).
Fig. 4.
Fold-changes in adaptor and NF-κB (IκBα and IKKβ) expression 4 h following acute resistance exercise in human skeletal muscle (vastus lateralis) among young exercisers (YE), lifelong exercisers (LLE), and old healthy (OH). The dotted line represents the preexercise fold-change for each group, derived from the 2−ΔΔCT calculation (see methods). Myd88, myeloid differentiation primary response 88; TRIF, Toll interleukin (IL)-1 receptor-containing adaptor-inducing IFN-γ. *P < 0.05 vs. preexercise. §P ≤ 0.10 main effect for exercise.
NF-κB pathway components.
Following exercise, expression of IκBα decreased (P < 0.05) in YE, LLE, and OH (Fig. 4). In addition, there was a trend (P ≤ 0.10) toward a main effect of exercise decreasing IKKβ expression. Both LLE-F (Pre: 1.03 ± 0.11; Post: 0.36 ± 0.03-fold) and LLE-P (Pre: 1.04 ± 0.08, Post: 0.47 ± 0.05-fold) decreased (P < 0.05) IκBα in response to exercise. IKKβ expression in LLE-F (Pre: 1.03 ± 0.09, Post: 1.03 ± 0.09-fold) and LLE-P (Pre: 1.03 ± 0.06, Post: 0.82 ± 0.05-fold) did not change (P > 0.05) following exercise.
DISCUSSION
The primary goals of this investigation were to assess the presence and relative expression of innate immune infrastructure components in healthy human skeletal muscle and to evaluate the effects of aging and lifelong aerobic exercise on these elements. Illuminating this pathway in aging skeletal muscle is important because muscle health declines during aging (11, 36) and may underscore the heightened susceptibility to infection commonly observed in older individuals (2, 17). Due to the absence of data on the effect of acute exercise on constituents of the innate immune system, an additional exploratory aim was addressed to provide insight into the impact of a resistance exercise challenge. The resistance exercise bout utilized in this study was chosen because it represents an effective stimulus to combat age-related muscle decline and presents an unaccustomed mechanical challenge. Our findings show that all Toll-like receptors (TLR1–10) and both intracellular adaptors (Myd88 and TRIF) are constitutively expressed in young and old skeletal muscle in the basal state and 4 h after an acute resistance exercise challenge. Furthermore, aging appears to increase basal expression of select TLRs, and lifelong aerobic exercise does not affect this age-related increase. Acute exercise stimulated the expression of several TLRs, while the adaptor response appeared to be dysregulated with aging and maintained with lifelong exercise.
Ten TLRs are known to exist in human tissues, with primary cell and transcriptomic studies confirming muscle cell-specific expression (1, 25, 38, 42). While it is clear muscle cells express innate immune components, other cell types, including resident or trafficked immune cells and endothelial cells, can contribute to the overall profile. Interestingly, we recently showed similar macrophage abundance (CD68+ cells) among these same groups (29) and lower capillary density in OH than YE and LLE (16). Nonetheless, it is possible that other cell types in addition to muscle participate in the innate immune profile presented here.
Given the sentinel role of TLRs, it is important to understand the relative expression of these essential receptors. TLR9 was the highest expressed of the 10 TLRs, and others have shown skeletal muscle expresses TLR9 to a greater degree than 17 other organs/tissues (38). This is interesting because TLR9 is known to recognize liberated DNA fragments, such as those derived from viruses or the host during conditions involving cellular stress (1, 25, 26). Though no effect of age was found, the very high relative expression of this TLR is particularly relevant for aging individuals. Skeletal muscle fibers are lost over the life span (30) and breakdown of these fibers likely release small DNA fragments from cellular compartments, contributing to the accumulation of circulating DNA (i.e., cf-DNA). Once liberated, cf-DNA may interact with the highly abundant TLR9 in muscle and activate the innate immune response. In fact, cf-DNA has been shown to be higher in older individuals (21) and is associated with frailty and increased risk of mortality (20).
Muscle tissue appears to play a key role in detecting and managing PAMPs and DAMPs and is particularly relevant as LLE is partially protective of muscle mass during aging (∼50%; YE: 78 ± 3; LLE: 67 ± 2; OH: 56 ± 3 cm2) (7). The consequence of possessing reduced muscle mass is evident as individuals with a low amount of skeletal muscle are twice as likely to acquire an infection than those with a larger amount of muscle (8). Because innate immune components are present in muscle, this relationship between muscle mass and infection susceptibility may be due to the abundance of innate immune infrastructure components. This study provides initial evidence that TRIF is expressed far more (∼100%) than Myd88 in muscle. This was unexpected due to Myd88 being the intracellular adaptor utilized by most TLRs for intracellular signal transduction (1, 25). Greater basal TRIF expression indicates that when muscle is presented with a pathogenic insult, such as viral nucleic acids, the capacity to produce interferons is potentially high. Coupling the constitutive mRNA expression of both adaptors with the understanding that their presence is critical to transducing the signal originating from their associated TLR into the cell (24, 58) provides compelling evidence that healthy muscle is likely prepared to participate in the infectious response.
Effects of Aging and Lifelong Exercise
The exploratory nature of this investigation provided novel insight into the effect of aging on basal expression of TLRs and both associated adaptors in human skeletal muscle. To our knowledge, only one other study has examined the impact of age on TLRs in skeletal muscle and found a similar age-related increase in TLR4 mRNA and protein expression (14). Findings presented here are the first to suggest an age-related increase in basal muscle expression of TLR3, TLR6, and TLR7. Each of these receptors tended to be elevated (62–72%) in both old groups combined compared with young. TLR3 and TLR7 aid in recognition of nucleic acids associated with viral infection, while TLR6 participates in detection of many different types of PAMPs and DAMPs (1, 25). Existing literature on circulating cells (e.g., monocytes) has established an age-related decrease in expression of most TLRs (41, 45). Therefore, a tissue-specific effect of human aging may exist: TLR expression decreases on circulating cells and increases in skeletal muscle. The overall effect of this might be an attempt to maintain absolute TLR abundance. Thus, because impaired immunity exists in circulating cells (i.e., immunosenescence), infection preparedness in aging individuals likely becomes increasingly reliant on remaining muscle mass.
Due to its broad range of roles with infection and metabolism, TLR4 is the best studied of the TLR family (42). For example, TLR4 in muscle detects free fatty acids (46) and LPS (13). Despite these well-established roles, little information exists about the effect of aging on TLR4 levels in humans. Limited data show that TLR4 levels are higher in muscle of sedentary old individuals compared with young (14). In contrast, no effect of aging was noted in TLR4 expression in the current study. This divergent finding suggests overall health profile may influence factors of the innate immune system in muscle. The extensive screening process included in this study yielded very healthy old subject groups (16), as all participants were active (exercise-trained in the case of LLE), disease free, and community dwelling. On average, the LLE group was >90th and OH were near the 70th percentile for aerobic capacity for their age (16, 23). Thus the good overall health profile of these older groups may explain the preservation of TLR4.
Lifelong aerobic exercise at least partially mitigated the decline in aerobic capacity, muscle size, and function commonly seen with aging (7, 16). This is particularly interesting because LLE did not appear to rescue the age-related increase in basal TLR levels (TLR3, 6, and 7). Furthermore, no impact of lifelong training intensity was evident, as basal expression of all TLRs was similar between LLE-F and LLE-P. Somewhat similarly, short-term aerobic training (16-wk) has been shown to be ineffective at reducing elevated basal muscle TLR4 levels in healthy, community-dwelling, older individuals (14). However, obese, frail individuals exhibit decreased muscle TLR4 levels following combined aerobic and resistance training (12 wk) (27). These discrepant findings may be due to 1) a mode-dependent adaptation, thus resistance training may be a more potent adaptive stimulus for muscle, or 2) a lower threshold for maintenance in healthy human muscle (the physical activity conducted by YE, LLE, and OH was sufficient for similar adaptation). Further investigation into this area is clearly warranted.
There did not appear to be an effect of age on expression of signaling components downstream of TLRs (Myd88, TRIF, or the NF-κB pathway). It is possible that these components in muscle are less susceptible to change with healthy aging than TLRs. However, lifelong aerobic training does increase basal expression of Myd88. This adaptor is considered the universal TLR adaptor and is critical to activation of the intracellular signaling and inflammatory factor production necessary to combat infection (1, 24, 25). More Myd88 adaptors in LLE compared with OH suggest that when presented with an infectious challenge, LLE may be able to respond more rapidly through greater signal amplification. Findings in young males demonstrate that training, despite similar basal TLR4 levels, enhances the inflammatory response in skeletal muscle to a given innate immune challenge (39), and this may be the result of heightened downstream signaling capacity (i.e., Myd88).
Although LLE increased Myd88 expression and no differences between subgroups were noted, it is possible a threshold for training intensity exists for basal levels of other intracellular signaling components. That is, aging muscle may have a diminished capacity to handle intense training. Within the LLE group, higher lifelong training intensity (LLE-P group) substantially impacted basal TRIF levels. The LLE fitness-oriented subgroup (LLE-F) had 66% higher TRIF expression than the performance focused subgroup. This adaptor is considered relevant in pathological situations due its participation in interferon production and may imply lower infection preparedness in LLE-P than LLE-F. When coupled with higher basal IKKβ in LLE-P (conceivable greater basal NF-κB signaling in LLE-P), an effect of training or delayed recovery from a previous exercise bout may exist. This is potentially problematic because intense training is linked to compromised immunity (57), suggesting exercise intensity and recovery may be particularly important in older individuals.
Effects of Acute Resistance Exercise
Evaluating the effect of an acute bout of exercise on skeletal muscle TLR and adaptor expression was another novel component of this investigation. The type of resistance exercise completed here is known to improve muscle health when performed chronically through increased size and function (47, 49–51, 53). Given the role that the innate immune signaling plays in infection management, assessing the response to an unaccustomed exercise challenge provides critical insight into plasticity of the skeletal muscle innate immune program.
In this study, three different TLRs (TLR1, 4, and 8) increased expression following the resistance exercise challenge across the three main groups. Additionally, YE and both LLE subgroups increased TLR3 expression. The TLR3 response seen only in the exercise-trained groups (YE, LLE-F, and LLE-P) indicates that plasticity of this TLR is lost with sedentary aging and maintained with lifelong training. Exercise-induced expression of TLR1 and TLR4 was also observed in LLE-F and LLE-P. Compared with studies in circulating cell types, the findings presented here suggest a tissue-specific response also likely exists with TLR expression following acute exercise. That is, TLR expression is typically reduced in circulating cells (28, 40) and, based on these new findings, is increased in skeletal muscle following acute exercise. This may be due to the unique metabolic and mechanical demand muscle is subjected to during resistance exercise. Additionally, given other cell types reside within muscle tissue (e.g., immune), it is possible those cells contribute to the overall exercise-induced mRNA expression profile.
The stress muscle experienced with resistance exercise was particularly evident for the NF-κB pathway, as both components responded to the exercise challenge. Provided TLR signaling occurs through this pathway, the observed responsivity of IκBα and IKKβ offers additional support that TLR activation contributes to signaling through these downstream elements (1, 25). Both TLR-related adaptors also responded to the exercise bout; these were primarily (Myd88) or exclusively (TRIF) driven by the OH group. This indicates that OH perceived resistance exercise as more of an innate immune insult than the exercise-trained groups (29). OH may have a lower threshold for innate immune stress and therefore may be more reliant on TRIF signaling than the exercise-trained groups. TRIF-related signaling results in production of interferons, which can inhibit protein translation (12) and upregulate proteolytic gene expression (34), therefore potentially impairing muscle preservation and/or growth.
Summary
This study provides a comprehensive profile of Toll-like receptors, associated adaptors, and downstream signaling components in healthy human skeletal muscle, basally and after unaccustomed acute resistance exercise. These findings provide the first insight into the effect of aging and a lifetime of structured aerobic exercise (>50 yr) on expression of these innate immune elements in muscle tissue. In general, aging appears to increase basal expression of TLRs and LLE does not mitigate this effect. However, LLE appears to modify expression patterns of some of these components in response to an exercise challenge. This provides a platform to better understand the link between the age-related increase in infection susceptibility and muscle health.
GRANTS
This research was supported by National Institute on Aging Grant AG-038576.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.K.P., K.M.L., U.R., B.J., S.W.T., and T.A.T. conceived and designed research; R.K.P., K.M.L., U.R., B.J., S.W.T., and T.A.T. performed experiments; R.K.P., K.M.L., B.J., and T.A.T. analyzed data; R.K.P., K.M.L., U.R., B.J., S.W.T., and T.A.T. interpreted results of experiments; R.K.P., K.M.L., and T.A.T. prepared figures; R.K.P., K.M.L., and T.A.T. drafted manuscript; R.K.P., K.M.L., U.R., B.J., S.W.T., and T.A.T. edited and revised manuscript; R.K.P., K.M.L., U.R., B.J., S.W.T., and T.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank the many HPL graduate students and support staff that were involved with this project. We are grateful to all the volunteers who graciously gave time, energy, and support to this project.
REFERENCES
- 1.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell 124: 783–801, 2006. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med 29: 1303–1310, 2001. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Baracos V, Rodemann HP, Dinarello CA, Goldberg AL. Stimulation of muscle protein degradation and prostaglandin E2 release by leukocytic pyrogen (interleukin-1). A mechanism for the increased degradation of muscle proteins during fever. N Engl J Med 308: 553–558, 1983. doi: 10.1056/NEJM198303103081002. [DOI] [PubMed] [Google Scholar]
- 4.Bergström J Muscle electrolytes in man. Scand J Clin Lab Invest 14: 1–110, 1962. [Google Scholar]
- 5.Beutler B TLR4 as the mammalian endotoxin sensor. Curr Top Microbiol Immunol 270: 109–120, 2002. doi: 10.1007/978-3-642-59430-4_7. [DOI] [PubMed] [Google Scholar]
- 6.Carrick-Ranson G, Hastings JL, Bhella PS, Fujimoto N, Shibata S, Palmer MD, Boyd K, Livingston S, Dijk E, Levine BD. The effect of lifelong exercise dose on cardiovascular function during exercise. J Appl Physiol (1985) 116: 736–745, 2014. doi: 10.1152/japplphysiol.00342.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers TL, Burnett TR, Raue U, Lee GA, Finch WH, Graham BM, Trappe TA, Trappe S. Skeletal muscle size, function, and adiposity with lifelong aerobic exercise. J Appl Physiol (1985) 128: 368–378, 2020. doi: 10.1152/japplphysiol.00426.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cosquéric G, Sebag A, Ducolombier C, Thomas C, Piette F, Weill-Engerer S. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br J Nutr 96: 895–901, 2006. doi: 10.1017/BJN20061943. [DOI] [PubMed] [Google Scholar]
- 9.Crane JD, Macneil LG, Tarnopolsky MA. Long-term aerobic exercise is associated with greater muscle strength throughout the life span. J Gerontol A Biol Sci Med Sci 68: 631–638, 2013. doi: 10.1093/gerona/gls237. [DOI] [PubMed] [Google Scholar]
- 10.Drummond MJ, Timmerman KL, Markofski MM, Walker DK, Dickinson JM, Jamaluddin M, Brasier AR, Rasmussen BB, Volpi E. Short-term bed rest increases TLR4 and IL-6 expression in skeletal muscle of older adults. Am J Physiol Regul Integr Comp Physiol 305: R216–R223, 2013. doi: 10.1152/ajpregu.00072.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frontera WR, Hughes VA, Lutz KJ, Evans WJ. A cross-sectional study of muscle strength and mass in 45- to 78-yr-old men and women. J Appl Physiol (1985) 71: 644–650, 1991. doi: 10.1152/jappl.1991.71.2.644. [DOI] [PubMed] [Google Scholar]
- 12.Frost RA, Nystrom GJ, Lang CH. Endotoxin and interferon-γ inhibit translation in skeletal muscle cells by stimulating nitric oxide synthase activity. Shock 32: 416–426, 2009. doi: 10.1097/SHK.0b013e3181a034d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost RA, Nystrom GJ, Lang CH. Multiple Toll-like receptor ligands induce an IL-6 transcriptional response in skeletal myocytes. Am J Physiol Regul Integr Comp Physiol 290: R773–R784, 2006. doi: 10.1152/ajpregu.00490.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Lertwattanarak R, Garduño JJ, Galeana JJ, Li J, Zamarripa F, Lancaster JL, Mohan S, Hussey S, Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J Gerontol A Biol Sci Med Sci 70: 232–246, 2015. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gonzalez-Quintela A, Alonso M, Campos J, Vizcaino L, Loidi L, Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One 8: e54600–e54608, 2013. doi: 10.1371/journal.pone.0054600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gries KJ, Raue U, Perkins RK, Lavin KM, Overstreet BS, D’Acquisto LJ, Graham B, Finch WH, Kaminsky LA, Trappe TA, Trappe S. Cardiovascular and skeletal muscle health with lifelong exercise. J Appl Physiol (1985) 125: 1636–1645, 2018. doi: 10.1152/japplphysiol.00174.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall MJ, Williams SN, DeFrances CJ, Golosinskiy A. Inpatient care for septicemia or sepsis: a challenge for patients and hospitals. NCHS Data Brief 62: 1–8, 2011. [PubMed] [Google Scholar]
- 18.Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc 50: 889–896, 2002. doi: 10.1046/j.1532-5415.2002.50216.x. [DOI] [PubMed] [Google Scholar]
- 19.Jemiolo B, Trappe S. Single muscle fiber gene expression in human skeletal muscle: validation of internal control with exercise. Biochem Biophys Res Commun 320: 1043–1050, 2004. doi: 10.1016/j.bbrc.2004.05.223. [DOI] [PubMed] [Google Scholar]
- 20.Jylhävä J, Jylhä M, Lehtimäki T, Hervonen A, Hurme M. Circulating cell-free DNA is associated with mortality and inflammatory markers in nonagenarians: the Vitality 90+ Study. Exp Gerontol 47: 372–378, 2012. doi: 10.1016/j.exger.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 21.Jylhävä J, Kotipelto T, Raitala A, Jylhä M, Hervonen A, Hurme M. Aging is associated with quantitative and qualitative changes in circulating cell-free DNA: the Vitality 90+ study. Mech Ageing Dev 132: 20–26, 2011. doi: 10.1016/j.mad.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 22.Kalis C, Kanzler B, Lembo A, Poltorak A, Galanos C, Freudenberg MA. Toll-like receptor 4 expression levels determine the degree of LPS-susceptibility in mice. Eur J Immunol 33: 798–805, 2003. doi: 10.1002/eji.200323431. [DOI] [PubMed] [Google Scholar]
- 23.Kaminsky LA, Imboden MT, Arena R, Myers J. Reference standards for cardiorespiratory fitness measured with cardiopulmonary exercise testing using cycle ergometry: data from the Fitness Registry and the Importance of Exercise National Database (FRIEND) registry. Mayo Clin Proc 92: 228–233, 2017. doi: 10.1016/j.mayocp.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Kawai T, Adachi O, Ogawa T, Takeda K, Akira S. Unresponsiveness of MyD88-deficient mice to endotoxin. Immunity 11: 115–122, 1999. doi: 10.1016/S1074-7613(00)80086-2. [DOI] [PubMed] [Google Scholar]
- 25.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11: 373–384, 2010. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 26.Krieg AM CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20: 709–760, 2002. doi: 10.1146/annurev.immunol.20.100301.064842. [DOI] [PubMed] [Google Scholar]
- 27.Lambert CP, Wright NR, Finck BN, Villareal DT. Exercise but not diet-induced weight loss decreases skeletal muscle inflammatory gene expression in frail obese elderly persons. J Appl Physiol (1985) 105: 473–478, 2008. doi: 10.1152/japplphysiol.00006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lancaster GI, Khan Q, Drysdale P, Wallace F, Jeukendrup AE, Drayson MT, Gleeson M. The physiological regulation of toll-like receptor expression and function in humans. J Physiol 563: 945–955, 2005. doi: 10.1113/jphysiol.2004.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lavin KM, Perkins RK, Jemiolo B, Raue U, Trappe SW, Trappe TA. Effects of aging and lifelong aerobic exercise on basal and exercise-induced inflammation. J Appl Physiol (1985) 128: 87–99, 2020. doi: 10.1152/japplphysiol.00495.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lexell J, Henriksson-Larsén K, Winblad B, Sjöström M. Distribution of different fiber types in human skeletal muscles: effects of aging studied in whole muscle cross sections. Muscle Nerve 6: 588–595, 1983. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 31.Lira FS, Rosa JC, Pimentel GD, Santos RV, Carnier J, Sanches PL, de Piano A, de Souza CT, Tock L, Tufik S, de Mello MT, Seelaender M, Oller do Nascimento CM, Oyama LM, Dâmaso AR. Long-term interdisciplinary therapy reduces endotoxin level and insulin resistance in obese adolescents. Nutr J 11: 74, 2012. doi: 10.1186/1475-2891-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lira FS, Rosa JC, Pimentel GD, Souza HA, Caperuto EC, Carnevali LC Jr, Seelaender M, Damaso AR, Oyama LM, de Mello MT, Santos RV. Endotoxin levels correlate positively with a sedentary lifestyle and negatively with highly trained subjects. Lipids Health Dis 9: 82, 2010. doi: 10.1186/1476-511X-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Schnittger C(T)) method. Methods 25: 402–408, 2001. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Llovera M, Carbó N, López-Soriano J, García-Martínez C, Busquets S, Alvarez B, Agell N, Costelli P, López-Soriano FJ, Celada A, Argilés JM. Different cytokines modulate ubiquitin gene expression in rat skeletal muscle. Cancer Lett 133: 83–87, 1998. doi: 10.1016/S0304-3835(98)00216-X. [DOI] [PubMed] [Google Scholar]
- 35.Louis E, Raue U, Yang Y, Jemiolo B, Trappe S. Time course of proteolytic, cytokine, and myostatin gene expression after acute exercise in human skeletal muscle. J Appl Physiol (1985) 103: 1744–1751, 2007. doi: 10.1152/japplphysiol.00679.2007. [DOI] [PubMed] [Google Scholar]
- 36.Manini TM, Clark BC. Dynapenia and aging: an update. J Gerontol A Biol Sci Med Sci 67A: 28–40, 2012. doi: 10.1093/gerona/glr010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Murach K, Raue U, Wilkerson B, Minchev K, Jemiolo B, Bagley J, Luden N, Trappe S. Single muscle fiber gene expression with run taper. PLoS One 9: e108547, 2014. doi: 10.1371/journal.pone.0108547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human toll-like receptors and related genes. Biol Pharm Bull 28: 886–892, 2005. doi: 10.1248/bpb.28.886. [DOI] [PubMed] [Google Scholar]
- 39.Olesen J, Biensø RS, Meinertz S, van Hauen L, Rasmussen SM, Gliemann L, Plomgaard P, Pilegaard H. Impact of training status on LPS-induced acute inflammation in humans. J Appl Physiol (1985) 118: 818–829, 2015. doi: 10.1152/japplphysiol.00725.2014. [DOI] [PubMed] [Google Scholar]
- 40.Oliveira M, Gleeson M. The influence of prolonged cycling on monocyte Toll-like receptor 2 and 4 expression in healthy men. Eur J Appl Physiol 109: 251–257, 2010. doi: 10.1007/s00421-009-1350-9. [DOI] [PubMed] [Google Scholar]
- 41.Panda A, Qian F, Mohanty S, van Duin D, Newman FK, Zhang L, Chen S, Towle V, Belshe RB, Fikrig E, Allore HG, Montgomery RR, Shaw AC. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J Immunol 184: 2518–2527, 2010. doi: 10.4049/jimmunol.0901022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pillon NJ, Krook A. Innate immune receptors in skeletal muscle metabolism. Exp Cell Res 360: 47–54, 2017. doi: 10.1016/j.yexcr.2017.02.035. [DOI] [PubMed] [Google Scholar]
- 43.Puthucheary ZA, Rawal J, McPhail M, Connolly B, Ratnayake G, Chan P, Hopkinson NS, Phadke R, Dew T, Sidhu PS, Velloso C, Seymour J, Agley CC, Selby A, Limb M, Edwards LM, Smith K, Rowlerson A, Rennie MJ, Moxham J, Harridge SD, Hart N, Montgomery HE. Acute skeletal muscle wasting in critical illness. JAMA 310: 1591–1600, 2013. doi: 10.1001/jama.2013.278481. [DOI] [PubMed] [Google Scholar]
- 44.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol (1985) 112: 1625–1636, 2012. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenstiel P, Derer S, Till A, Häsler R, Eberstein H, Bewig B, Nikolaus S, Nebel A, Schreiber S. Systematic expression profiling of innate immune genes defines a complex pattern of immunosenescence in peripheral and intestinal leukocytes. Genes Immun 9: 103–114, 2008. doi: 10.1038/sj.gene.6364454. [DOI] [PubMed] [Google Scholar]
- 46.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest 116: 3015–3025, 2006. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trappe S, Godard M, Gallagher P, Carroll C, Rowden G, Porter D. Resistance training improves single muscle fiber contractile function in older women. Am J Physiol Cell Physiol 281: C398–C406, 2001. doi: 10.1152/ajpcell.2001.281.2.C398. [DOI] [PubMed] [Google Scholar]
- 48.Trappe S, Hayes E, Galpin A, Kaminsky L, Jemiolo B, Fink W, Trappe T, Jansson A, Gustafsson T, Tesch P. New records in aerobic power among octogenarian lifelong endurance athletes. J Appl Physiol (1985) 114: 3–10, 2013. doi: 10.1152/japplphysiol.01107.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trappe S, Williamson D, Godard M. Maintenance of whole muscle strength and size following resistance training in older men. J Gerontol A Biol Sci Med Sci 57: B138–B143, 2002. doi: 10.1093/gerona/57.4.B138. [DOI] [PubMed] [Google Scholar]
- 50.Trappe S, Williamson D, Godard M, Porter D, Rowden G, Costill D. Effect of resistance training on single muscle fiber contractile function in older men. J Appl Physiol (1985) 89: 143–152, 2000. doi: 10.1152/jappl.2000.89.1.143. [DOI] [PubMed] [Google Scholar]
- 51.Trappe TA, Carroll CC, Dickinson JM, LeMoine JK, Haus JM, Sullivan BE, Lee JD, Jemiolo B, Weinheimer EM, Hollon CJ. Influence of acetaminophen and ibuprofen on skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 300: R655–R662, 2011. doi: 10.1152/ajpregu.00611.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trappe TA, Lindquist DM, Carrithers JA. Muscle-specific atrophy of the quadriceps femoris with aging. J Appl Physiol (1985) 90: 2070–2074, 2001. doi: 10.1152/jappl.2001.90.6.2070. [DOI] [PubMed] [Google Scholar]
- 53.Trappe TA, Standley RA, Jemiolo B, Carroll CC, Trappe SW. Prostaglandin and myokine involvement in the cyclooxygenase-inhibiting drug enhancement of skeletal muscle adaptations to resistance exercise in older adults. Am J Physiol Regul Integr Comp Physiol 304: R198–R205, 2013. doi: 10.1152/ajpregu.00245.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Hall G, Steensberg A, Fischer C, Keller C, Møller K, Moseley P, Pedersen BK. Interleukin-6 markedly decreases skeletal muscle protein turnover and increases nonmuscle amino acid utilization in healthy individuals. J Clin Endocrinol Metab 93: 2851–2858, 2008. doi: 10.1210/jc.2007-2223. [DOI] [PubMed] [Google Scholar]
- 55.Watanabe K Prostaglandin F synthase. Prostaglandins Other Lipid Mediat 68-69: 401–407, 2002. doi: 10.1016/S0090-6980(02)00044-8. [DOI] [PubMed] [Google Scholar]
- 56.Wolfe RR The underappreciated role of muscle in health and disease. Am J Clin Nutr 84: 475–482, 2006. doi: 10.1093/ajcn/84.3.475. [DOI] [PubMed] [Google Scholar]
- 57.Woods JA, Davis JM, Smith JA, Nieman DC. Exercise and cellular innate immune function. Med Sci Sports Exerc 31: 57–66, 1999. doi: 10.1097/00005768-199901000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-beta promoter in the Toll-like receptor signaling. J Immunol 169: 6668–6672, 2002. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- 59.Yang Y, Creer A, Jemiolo B, Trappe S. Time course of myogenic and metabolic gene expression in response to acute exercise in human skeletal muscle. J Appl Physiol (1985) 98: 1745–1752, 2005. doi: 10.1152/japplphysiol.01185.2004. [DOI] [PubMed] [Google Scholar]