Abstract
Background:
The efficacy of double antithrombotic therapy (DAT) vs. triple antithrombotic therapy (TAT) for prevention of bleeding and ischemic events in patients with atrial fibrillation following percutaneous coronary intervention (PCI) is unclear in those subgroups defined by the 5 factors (i.e., sex, age, race, history of diabetes, and type of P2Y12 inhibitor). We aimed to assess the efficacy of DAT vs TAT in these patient subgroups.
Methods:
We searched PubMed and relevant websites to include related randomized controlled trials (RCTs). Two endpoints of interest were clinically significant bleeding and major adverse cardiac events (MACE). Meta-analysis was performed stratified by 5 factors of interest (i.e., sex, age, race, history of diabetes, and type of P2Y12 inhibitor) to obtain pooled hazard ratio (HR) and 95% confidence interval (CI). Meta-regression analysis was conducted to evaluate subgroup effects. We detected publication bias by Egger test and funnel plots.
Results:
We included 4 RCTs for meta-analysis. DAT vs TAT significantly reduced the risk of clinically significant bleeding (HR 0.56, 95% CI 0.50–0.63). This effect of DAT was observed in most subgroups of interest (HR ranged from 0.54 to 0.69), and was consistent across various subgroups defined by each of the 5 factors of interest (Psubgroup ranged from 0.290 to 0.794). DAT vs TAT led to the similar risk of MACE (HR 0.98, 95% CI 0.89–1.08). This effect of DAT was observed in all subgroups of interest (all 95% CIs of HRs were across 1.0), and was consistent across various subgroups defined by each of the 5 factors of interest (Psubgroup ranged from 0.308 to 0.828). Publication bias was found only in one subgroup.
Conclusions:
Compared with TAT, DAT significantly reduces the risk of clinically significant bleeding and leads to the similar risk of MACE in patients with atrial fibrillation following PCI, irrespective of sex, age, race, history of diabetes, and type of P2Y12 inhibitor used at baseline.
Keywords: atrial fibrillation, bleeding, double antithrombotic therapy, major adverse cardiac events, PCI, triple antithrombotic therapy
1. Introduction
The efficacy of double antithrombotic therapy (DAT) vs. triple antithrombotic therapy (TAT) for prevention of bleeding and ischemic events in patients with atrial fibrillation following percutaneous coronary intervention (PCI) is unclear in some subgroups of patients with different clinical characteristics, because different randomized controlled trials (RCTs) reported inconsistent findings and individual trials were underpowered to assess the efficacy of DAT vs TAT in specific patient subgroups. As one of the examples that different studies reported inconsistent findings, 3 trials[1–3] showed that DAT vs TAT significantly reduced the risk of major bleeding in the subgroup of patients with age ≥75 years, whereas 1 other trial[4] did not show the significant reduction in the risk of major bleeding with DAT. As one of the examples that individual trials were underpowered for some patient subgroups, 2 trials[1,3] had the sufficient statistical power to reveal that DAT significantly reduced the risk of major bleeding compared with TAT in the subgroup of White patients whereas none had the sufficient statistical power to reveal that in the subgroups of Asian patients and Black patients.[1,3,4]
Thus, we conducted this meta-analysis to assess the efficacy of DAT vs TAT on bleeding and ischemic endpoints in various patient subgroups pre-defined according to 5 factors (i.e., sex, age, race, history of diabetes, and type of P2Y12 inhibitor).
2. Methods
This meta-analysis was completed in accordance with the PRISMA statement. The PRISMA checklist for this paper is available in Supplementary data 1.
2.1. Inclusion and exclusion criteria
We searched PubMed and relevant websites (i.e., clinicaltrials.gov, and clinical trial result.org) from the inception of databases/websites to August 2019 to obtain relevant RCTs. We used the same search strategies as those used in a meta-analysis[5] recently published in the European Heart Journal.
Original studies we included in this meta-analysis were RCTs in patients with atrial fibrillation who received percutaneous coronary intervention (PCI) in at least 50% of the sample and were allocated to DAT or TAT. Atrial fibrillation considered in this meta-analysis was previous, persistent, permanent, or paroxysmal atrial fibrillation. TAT consisted of any vitamin K antagonist (VKA) in combination with DAT, while DAT consisted of any non-VKA oral anticoagulant in combination with a P2Y12 inhibitor. This study directly focused on patients with atrial fibrillation following PCI, but not patients with atrial fibrillation and acute coronary syndrome (ACS, a composite of ST-elevation myocardial infarction, non-ST-elevation myocardial infarction, or unstable angina), although some of the included patients were patients with atrial fibrillation who underwent PCI for ACS. Included RCTs aimed to compare DAT with TAT for prevention of bleeding and ischemic endpoints. The bleeding endpoint of interest was clinically significant bleeding which was a composite of major bleeding or minor bleeding according to Thrombolysis in Myocardial Infarction criteria or bleeding requiring medical attention,[1] while the ischemic endpoint of interest was major adverse cardiac events (MACE) which was a composite of myocardial infarction, stroke, or death from cardiovascular causes.[1]
Two authors independently completed study selection, data extraction and quality assessment for included studies. Quality assessment for included studies was done using the Cochrane risk of bias tool. Any disagreements about the above assignments were resolved by the involvement of a third author.
2.2. Statistical analysis
Meta-analysis was conducted on 2 outcomes of interest (i.e., clinically significant bleeding and MACE) in various subgroups of interest which were subgroups of patients with different sex (male and female), age (<75 years vs ≥75 years), race (White, Black and Asian), history of diabetes (no and yes), and type of P2Y12 inhibitor used at baseline (clopidogrel, ticagrelor and prasugrel). Relative effect was measured by hazard ratio (HR) and 95% confidence interval (CI) of HR. We conducted meta-regression analysis to explore subgroup effects, and Psubgroup < 0.05 was considered as statistical significance. Meta-analysis and meta-regression analysis was based on the random-effects model. We examined statistical heterogeneity using I2 statistic, and I2 > 50% was considered as substantial heterogeneity. Publication bias was detected by Egger test and funnel plots. Statistical analyses were done and forest and funnel plots were drawn using Stata (version 15.1).
2.3. Ethical statement
The data analyzed in this study were extracted from previously published studies, and therefore ethical approval was not necessary.
3. Results
3.1. Characteristics of included studies
We finally included 4 RCTs[1–4] for meta-analysis. The study selection process is shown in Figure 1. The results of quality assessment for included trials were the same as those in a meta-analysis[5] recently published in the European Heart Journal. All the included 4 RCTs[1–4] reported the subgroup analyses of interest for the outcome of clinically significant bleeding, while 3[1–3] of them reported the subgroup analyses of interest for the outcome of MACE. Across the 4 trials included, DAT and TAT consistently started at the time of randomization, which occurred from immediately after PCI up until 14 days after PCI. The follow-up duration ranged from 6 months to 14 months. The data used for pooled analysis in the study are presented in Supplementary data 2.
Figure 1.
PRISMA Flow Diagram.
3.2. Meta-analyses
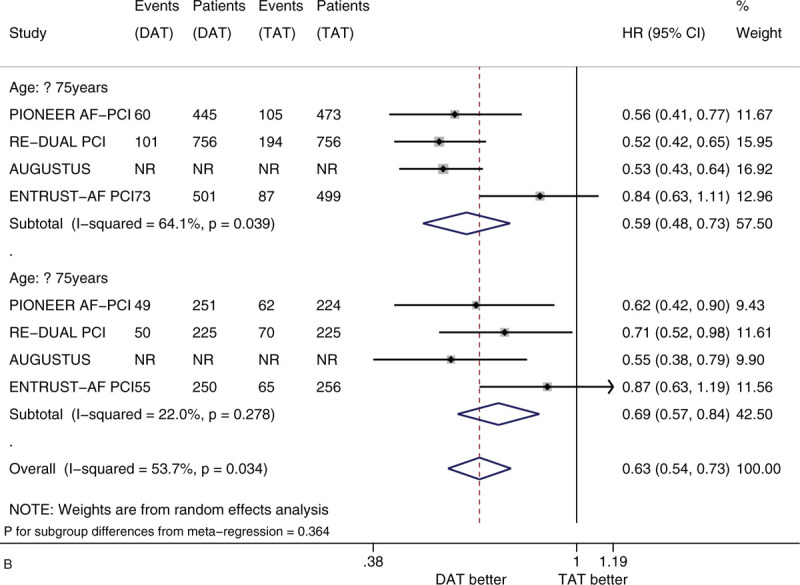
Figure 2 shows the forest plots of meta-analysis on clinically significant bleeding stratified by 5 factors of interest (i.e., sex, age, race, history of diabetes, and type of P2Y12 inhibitor). Compared with TAT, DAT significantly reduced the risk of clinically significant bleeding (HR 0.56, 95% CI 0.50–0.63, I2 0%). DAT vs TAT significantly reduced this risk in the subgroups of male patients (HR 0.54, 95% CI 0.47–0.61) and of female patients (HR 0.63, 95% CI 0.51–0.77) (Fig. 2 A). DAT vs TAT significantly reduced this risk in the subgroups of patients with age <75 years (HR 0.59, 95% CI 0.48–0.73) and of patients with age ≥75 years (HR 0.69, 95% CI 0.57–0.84) (Fig. 2 B). DAT vs TAT significantly reduced this risk in the subgroups of White patients (HR 0.56, 95% CI 0.48–0.65) and of Asian patients (HR 0.56, 95% CI 0.35–0.90) (Fig. 2 C). DAT vs TAT significantly reduced this risk in the subgroups of patients with no diabetes (HR 0.54, 95% CI 0.46–0.63) and of patients with diabetes (HR 0.56, 95% CI 0.46–0.68) (Fig. 2 D). DAT vs TAT significantly reduced this risk in the subgroups of patients with baseline use of clopidogrel (HR 0.64, 95% CI 0.48–0.87) and of patients with baseline use of ticagrelor (HR 0.54, 95% CI 0.39–0.75) (Fig. 2 E). All of these subgroup effects were not statistically significant (P value for subgroup differences ranged from .290 to .794).
Figure 2.
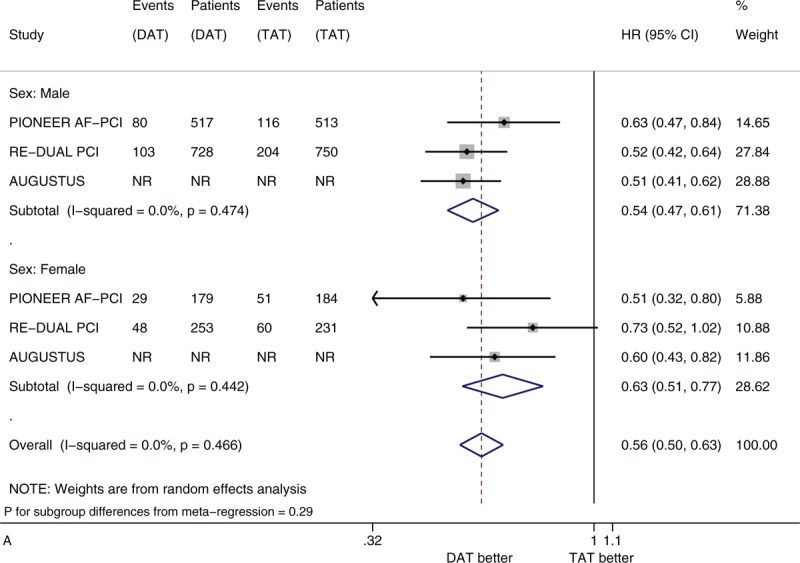
(A) Forest plot of meta-analysis on clinically significant bleeding, stratified by sex. (B) Forest plot of meta-analysis on clinically significant bleeding, stratified by age. (C) Forest plot of meta-analysis on clinically significant bleeding, stratified by race. (D) Forest plot of meta-analysis on clinically significant bleeding, stratified by history of diabetes. (E) Forest plot of meta-analysis on clinically significant bleeding, stratified by type of P2Y12 inhibitor.
Figure 2 (Continued).
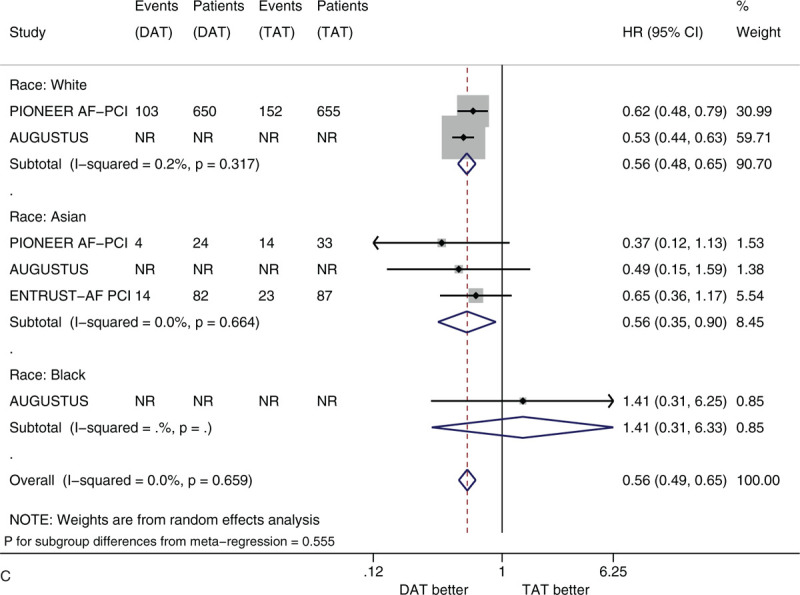
(A) Forest plot of meta-analysis on clinically significant bleeding, stratified by sex. (B) Forest plot of meta-analysis on clinically significant bleeding, stratified by age. (C) Forest plot of meta-analysis on clinically significant bleeding, stratified by race. (D) Forest plot of meta-analysis on clinically significant bleeding, stratified by history of diabetes. (E) Forest plot of meta-analysis on clinically significant bleeding, stratified by type of P2Y12 inhibitor.
Figure 2 (Continued).
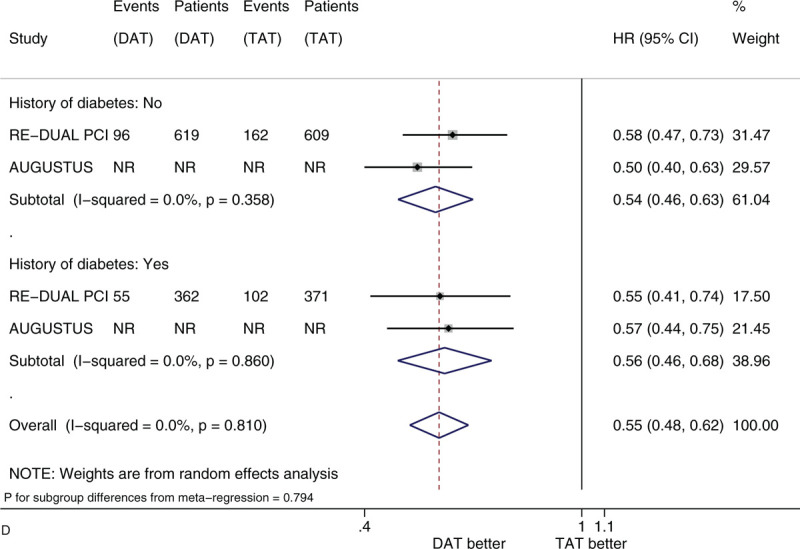
(A) Forest plot of meta-analysis on clinically significant bleeding, stratified by sex. (B) Forest plot of meta-analysis on clinically significant bleeding, stratified by age. (C) Forest plot of meta-analysis on clinically significant bleeding, stratified by race. (D) Forest plot of meta-analysis on clinically significant bleeding, stratified by history of diabetes. (E) Forest plot of meta-analysis on clinically significant bleeding, stratified by type of P2Y12 inhibitor.
Figure 2 (Continued).
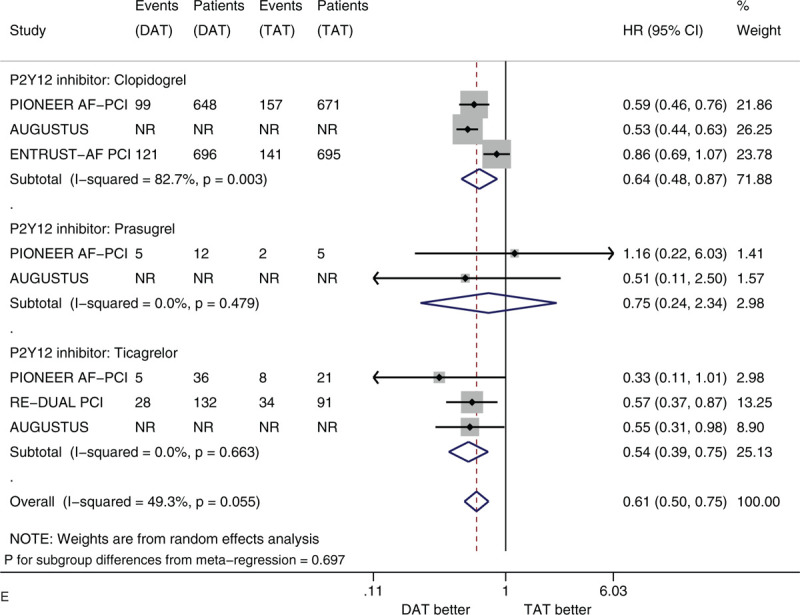
(A) Forest plot of meta-analysis on clinically significant bleeding, stratified by sex. (B) Forest plot of meta-analysis on clinically significant bleeding, stratified by age. (C) Forest plot of meta-analysis on clinically significant bleeding, stratified by race. (D) Forest plot of meta-analysis on clinically significant bleeding, stratified by history of diabetes. (E) Forest plot of meta-analysis on clinically significant bleeding, stratified by type of P2Y12 inhibitor.
Figure 3 shows the forest plots of meta-analysis on MACE stratified by the 5 factors of interest. Compared with TAT, DAT led to the similar risk of MACE (HR 0.98, 95% CI 0.89–1.08, I2 0%). DAT vs. TAT led to the similar risk in the subgroups of male patients (HR 1.01, 95% CI 0.81–1.26) and of female patients (HR 1.04, 95% CI 0.87–1.25) (Fig. 3 A). DAT vs TAT led to the similar risk in the subgroups of patients with age <75 years (HR 0.96, 95% CI 0.86–1.07) and of patients with age ≥75 years (HR 1.05, 95% CI 0.82–1.34) (Fig. 3 B). DAT vs. TAT led to the similar risk in the subgroups of White patients (HR 0.90, 95% CI 0.79–1.01), of Black patients (HR 2.33 95% CI 0.93–5.83) and of Asian patients (HR 1.11, 95% CI 0.50–2.45) (Fig. 3 C). DAT vs TAT led to the similar risk in the subgroups of patients with no diabetes (HR 1.06, 95% CI 0.89–1.25) and of patients with diabetes (HR 0.88, 95% CI 0.71–1.10) (Fig. 3 D). DAT vs TAT led to the similar risk in the subgroups of patients with baseline use of clopidogrel (HR 0.93, 95% CI 0.83–1.04), of patients with baseline use of ticagrelor (HR 1.01, 95% CI 0.74–1.39) and of patients with baseline use of prasugrel (HR 1.79, 95% CI 0.64–5.04) (Fig. 3 E). All of these subgroup effects were not statistically significant (P value for subgroup differences ranged from .308 to .828).
Figure 2 (Continued).
(A) Forest plot of meta-analysis on clinically significant bleeding, stratified by sex. (B) Forest plot of meta-analysis on clinically significant bleeding, stratified by age. (C) Forest plot of meta-analysis on clinically significant bleeding, stratified by race. (D) Forest plot of meta-analysis on clinically significant bleeding, stratified by history of diabetes. (E) Forest plot of meta-analysis on clinically significant bleeding, stratified by type of P2Y12 inhibitor.
Figure 3.
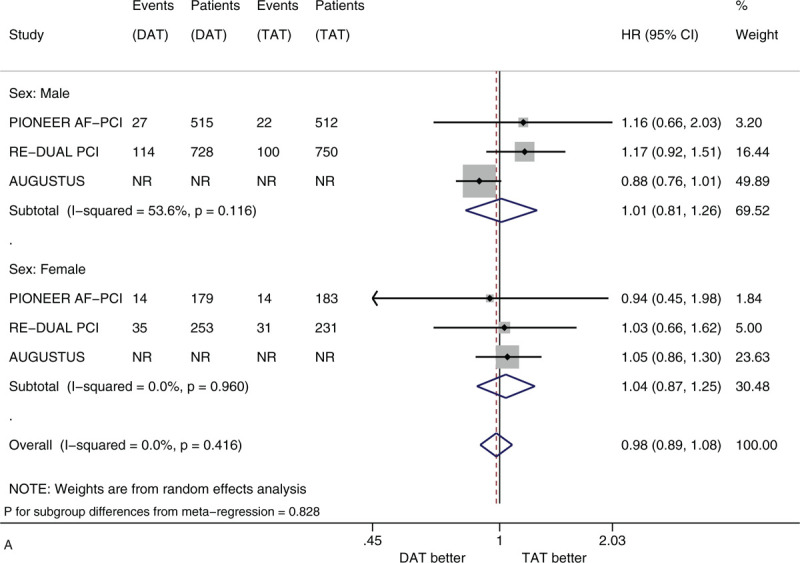
(A) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by sex. (B) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by age. (C) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by race. (D) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by history of diabetes. (E) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by type of P2Y12 inhibitor.
Figure 3 (Continued).
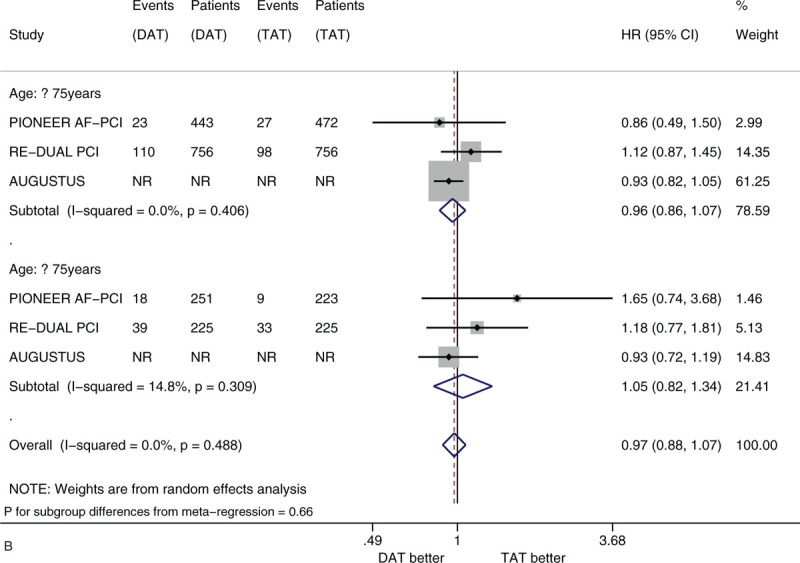
(A) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by sex. (B) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by age. (C) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by race. (D) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by history of diabetes. (E) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by type of P2Y12 inhibitor.
Figure 3 (Continued).
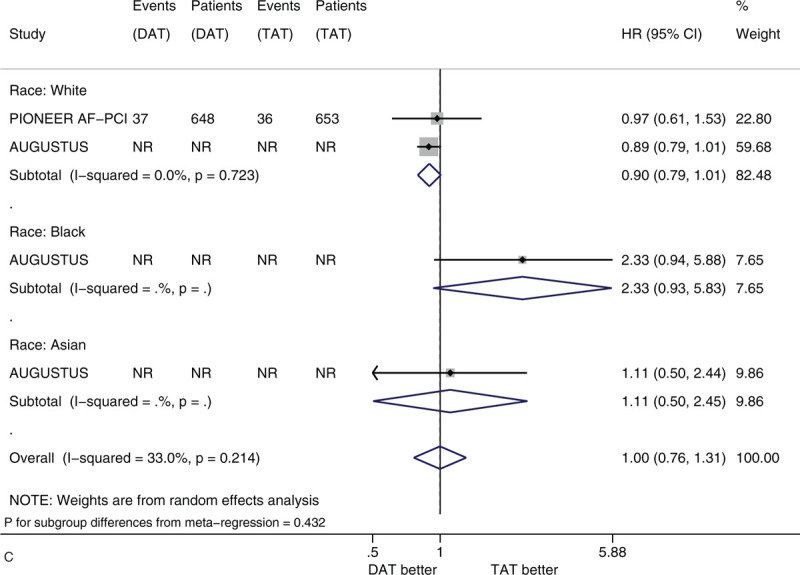
(A) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by sex. (B) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by age. (C) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by race. (D) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by history of diabetes. (E) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by type of P2Y12 inhibitor.
Figure 3 (Continued).
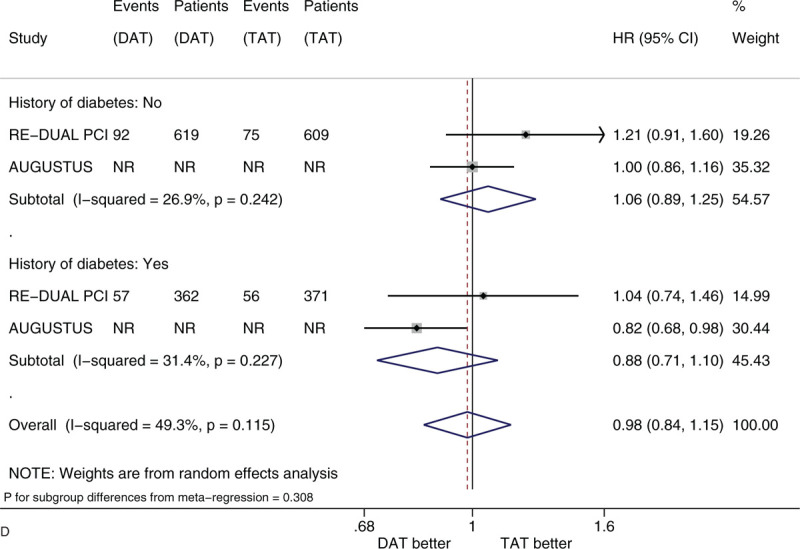
(A) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by sex. (B) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by age. (C) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by race. (D) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by history of diabetes. (E) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by type of P2Y12 inhibitor.
Figure 3 (Continued).
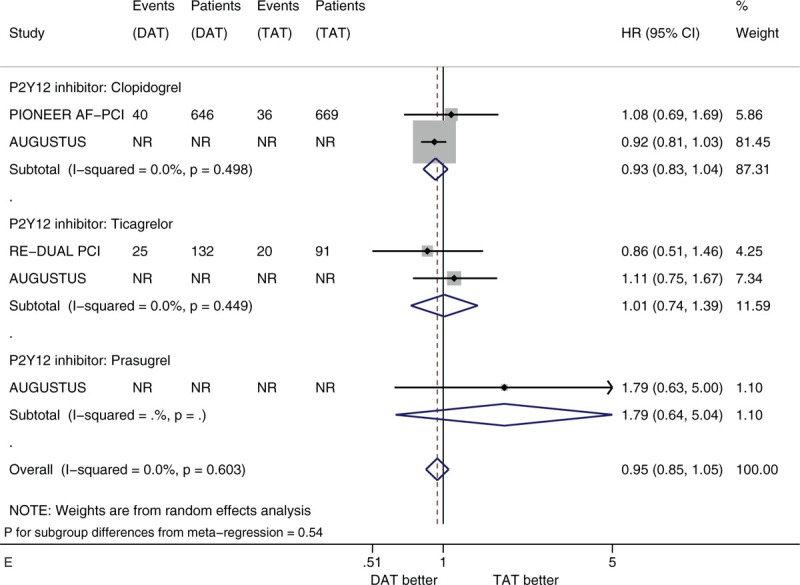
(A) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by sex. (B) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by age. (C) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by race. (D) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by history of diabetes. (E) Forest plot of meta-analysis on major adverse cardiovascular events, stratified by type of P2Y12 inhibitor.
We did not find substantial heterogeneity in most subgroups of interest, whereas we found substantial heterogeneity in terms of meta-analysis on clinically significant bleeding in the subgroups of patients with age <75 years (I2 = 64.1%) and patients with baseline use of clopidogrel (I2 = 82.7%) and meta-analysis on MACE in the subgroup of male patients (I2 = 53.6%). We did not observe publication bias in most subgroups of interest, whereas we observed publication bias as for clinically significant bleeding in the subgroup of male patients (Figs. S1-S24 in Supplementary data 3).
4. Discussion
We assessed the efficacy of DAT vs. TAT for prevention of bleeding and ischemic events in various subgroups among patients with atrial fibrillation following PCI by conducting subgroup meta-analysis stratified by 5 factors of interest (i.e., sex, age, race, history of diabetes, and type of P2Y12 inhibitor). Thus, we summarized the findings in this study.
First, DAT vs. TAT significantly reduced the risk of clinically significant bleeding (HR 0.56, 95% CI 0.50–0.63). The significant reduction in the risk of clinically significant bleeding shown by DAT was observed in most subgroups of interest (HR ranged from 0.54 to 0.69), and was consistent across various subgroups defined by each of the 5 factors of interest (Psubgroup ranged from 0.290 to 0.794). Second, DAT vs TAT led to the similar risk of MACE (HR 0.98, 95% CI 0.89–1.08). The similar risk of MACE shown by DAT was observed in all subgroups of interest (all 95% CIs of HRs were across 1.0), and was consistent across various subgroups defined by each of the 5 factors of interest (Psubgroup ranged from 0.308 to 0.828).
Previously published meta-analysis studies[5–8] in the same field assessed the efficacy of DAT vs TAT in patients with atrial fibrillation and coronary artery disease, whereas this study[5–8] failed to assess the efficacy of DAT vs TAT in various subgroups of patients with different baseline characteristics. For the first time, our study evaluated the efficacy of DAT vs TAT in various subgroups of patients with different sex, age, race, history of diabetes, and type of P2Y12 inhibitor used at baseline. Three meta-analyses[5–7] demonstrated that DAT had a lower rate of bleeding compared with TAT. Similarly, our study further revealed that DAT vs TAT significantly reduced the risk of clinically significant bleeding irrespective of sex, age, race, history of diabetes, and type of P2Y12 inhibitor used at baseline. Furthermore, our study revealed that DAT led to the similar risk of MACE compared with TAT irrespective of sex, age, race, history of diabetes, and type of P2Y12 inhibitor used at baseline. The findings in this study will inform heart specialists and specific patients with atrial fibrillation who underwent PCI to make informed clinical decisions about the selection of DAT and TAT.
The study has 2 main weaknesses. First, in a few subgroups substantial heterogeneity was found, and we failed to explore the sources of heterogeneity due to the limited subgroup analysis data provided in original studies. Second, publication bias was observed as for clinically significant bleeding in the subgroup of male patients, and therefore the result from meta-analysis on this outcome in this subgroup needs to be interpreted with caution.
In conclusion, compared with TAT, DAT significantly reduces the risk of clinically significant bleeding and leads to the similar risk of MACE in patients with atrial fibrillation following PCI, irrespective of sex, age, race, history of diabetes, and type of P2Y12 inhibitor used at baseline.
Author contributions
Conceptualization: Mei Qiu.
Data curation: Mei Qiu, Shu-Yan Liu, Hai-Rong Zhou.
Formal analysis: Shu-Yan Liu.
Methodology: Shu-Yan Liu.
Validation: Hai-Rong Zhou.
Writing – original draft: Mei Qiu.
Writing – review & editing: Hai-Rong Zhou.
Supplementary Material
Supplementary Material
Supplementary Material
Footnotes
Abbreviations: ACS = acute coronary syndrome, CI = confidence interval, DAT = double antithrombotic therapy, HR = hazard ratio, MACE = major adverse cardiac events, PCI = percutaneous coronary intervention, RCTs = randomized controlled trials, TAT = triple antithrombotic therapy, VKA = vitamin K antagonist.
How to cite this article: Qiu M, Liu SY, Zhou HR. Double antithrombotic therapy for prevention of bleeding and ischemic events after percutaneous coronary intervention in patients with atrial fibrillation: a meta-analysis. Medicine. 2021;100:1(e24188).
All authors disclose that they have no conflicts of interest.
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Supplemental digital content is available for this article.
References
- [1].Gibson CM, Mehran R, Bode C, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med 2016;375:2423–34. [DOI] [PubMed] [Google Scholar]
- [2].Cannon CP, Bhatt DL, Oldgren J, et al. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med 2017;377:1513–24. [DOI] [PubMed] [Google Scholar]
- [3].Lopes RD, Heizer G, Aronson R, et al. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med 2019;380:1509–24. [DOI] [PubMed] [Google Scholar]
- [4].Vranckx P, Valgimigli M, Eckardt L, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet 2019;394:1335–43. [DOI] [PubMed] [Google Scholar]
- [5].Gargiulo G, Goette A, Tijssen J, et al. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J 2019;40:3757–67. [DOI] [PubMed] [Google Scholar]
- [6].Ravi V, Pulipati P, Vij A, et al. Meta-analysis comparing double versus triple antithrombotic therapy in patients with atrial fibrillation and coronary artery disease. Am J Cardiol 2020;125:19–28. [DOI] [PubMed] [Google Scholar]
- [7].Cavallari I, Patti G. Meta-analysis comparing the safety and efficacy of dual versus triple antithrombotic therapy in patients with atrial fibrillation undergoing percutaneous coronary intervention. Am J Cardiol 2018;121:718–24. [DOI] [PubMed] [Google Scholar]
- [8].Andò G, Costa F. Double or triple antithrombotic therapy after coronary stenting and atrial fibrillation: a systematic review and meta-analysis of randomized clinical trials. Int J Cardiol 2020;302:95–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


