Thermal stress reduces pocilloporid coral resilience to ocean acidification by impairing control over calcifying fluid chemistry.
Abstract
The combination of thermal stress and ocean acidification (OA) can more negatively affect coral calcification than an individual stressors, but the mechanism behind this interaction is unknown. We used two independent methods (microelectrode and boron geochemistry) to measure calcifying fluid pH (pHcf) and carbonate chemistry of the corals Pocillopora damicornis and Stylophora pistillata grown under various temperature and pCO2 conditions. Although these approaches demonstrate that they record pHcf over different time scales, they reveal that both species can cope with OA under optimal temperatures (28°C) by elevating pHcf and aragonite saturation state (Ωcf) in support of calcification. At 31°C, neither species elevated these parameters as they did at 28°C and, likewise, could not maintain substantially positive calcification rates under any pH treatment. These results reveal a previously uncharacterized influence of temperature on coral pHcf regulation—the apparent mechanism behind the negative interaction between thermal stress and OA on coral calcification.
INTRODUCTION
Coral reefs are some of the most diverse and productive ecosystems on Earth. Increasing anthropogenic atmospheric CO2 causes warming and acidification of the oceans, thereby threatening these precious ecosystems (1, 2). Thermal stress causes coral bleaching, as evidenced by the increasing frequency and magnitude of mass bleaching events over the last several decades (1) and reduced calcification rates of corals. Ocean acidification (OA) can also impair coral calcification. Although it is well established that the combination of thermal stress and OA can more negatively affect coral calcification than either stressor alone (3–5), the mechanism behind this interaction—essential for predicting the impacts of future global change on corals—is not fully understood.
The ability of different coral species to calcify under a range of environmental conditions is partially attributed to the apparently strong control that they exert on their internal calcifying fluid (also known as the “calcifying medium”) (6). In this study, we exposed corals to a range of pCO2 and thermal treatments and estimated their calcifying fluid pH (pHcf) from the boron isotope (δ11Bc) composition of their skeletal aragonite and compared it to direct pH microelectrode measurements of their calcifying fluids (7). We also estimated the carbonate ion concentration of the calcifying fluid ([CO32−]cf) from skeletal B/Ca measurements (8). We then used these two key measurements (pHcf and [CO32−]cf) to calculate other carbonate system parameters of the coral calcifying fluid (DICcf and ΩA) (8–10). Our results provide insight into the impacts of future global oceanic change (11) on the calcifying fluid dynamics of tropical pocilloporid corals and relate these impacts to corals’ ability to produce their skeletons under future high-CO2 and high-temperature conditions.
Background on scleractinian coral biomineralization
In a stable climate, the persistence of coral reefs relies on the capacity of corals to produce new skeleton fast enough to offset processes of skeletal degradation (e.g., dissolution and bioerosion). Many species of tropical corals maintain a symbiosis with photosynthetic algae (i.e., zooxanthellae), which yields additional energy for calcification, thereby allowing them to maintain net positive rates of skeletal accretion and, in some cases, build large reef structures. This symbiosis enables the coral host to access up to 90% of the fixed carbon they require while providing the zooxanthellae with protection and access to nutrients (nitrogen and phosphorus) and dissolved inorganic carbon (DIC).
The precipitation of aragonite is thought to occur in the subcalicoblastic space (between the base of the calicoblastic tissue and the skeleton), from a partially isolated extracellular calcifying fluid (6, 12), with components of the calcifying fluid sourced from seawater (11) and precipitation of aragonite influenced by organic matrices (13).
Corals elevate the pH of their calcifying fluid under both normal and acidified conditions [e.g., (14)], potentially via enzymes that exchange Ca2+ and H+ between seawater and the coral calcifying fluid (e.g., Ca-ATPase) (15). CO2 dissolved in the coral calcifying fluid, which is derived from seawater, coral respiration (16), direct transport (17), and/or passive CO2 diffusion (12, 18, 19), is converted into HCO3− via the enzyme carbonic anhydrase (CA) and increasing pHcf (Eq. 1) (18). Increasing pHcf also causes HCO3− to dissociate into H+ and CO32− (Eq. 1), thereby increasing the aragonite saturation state (Ωar, Eq. 2) and driving calcification via Eq. 3 (6).
| (1) |
| (2) |
| (3) |
Evidence has also emerged that coral skeletal growth is influenced by organic molecules, such as coral acid rich proteins (CARPs) proteins (20, 21). It has also been reported that the precipitation of coral skeletal aragonite may be preceded by a transient phase of amorphous calcium carbonate (ACC) (22, 23). Although the ACC-to-aragonite transformation could introduce complexity into the interpretation of geochemical proxies recorded in coral skeletons, one would still expect the geochemistry of the aragonite skeleton to reflect conditions at the site of calcification and, to some extent, seawater, since the transformation of ACC to aragonite may occur within the high-pH and high-Ω extracellular coral calcifying fluid where nucleating aragonite crystals are observed [e.g., (24)] and involve dissolution and reprecipitation of the ACC in that fluid (25). However, the role of ACC in coral calcification is an ongoing debate (23, 26–28) and, like the potential role of organic molecules in coral calcification, does not materially affect interpretation of the present study.
Boron incorporation and isotope fractionation in coral aragonite
Previous studies support the assertion that B(OH)4− is the primary form of boron incorporated into coral aragonite (29). Inorganic CaCO3 precipitation experiments suggest that B(OH)4− is substituted for CO32− in the aragonite lattice (30, 31). Prior studies also suggest that [CO32−]cf can be estimated from coral skeletal B/Ca [see (8) for a review]. However, several substitution equations have been proposed to maintain charge balance when substituting B(OH)4− for CO32− in aragonite, resulting in divergent definitions for the B/Ca partition coefficient KD (8, 10). The KD defined below (Eq. 4) (10) was used to calculate carbonate system parameters in the present study pursuant to the rationale provided in (8) and because our reconstructed [CO32−]cf following the rationale in (10) was consistent with independent electrode [CO32−]cf (fig. S1) (32).
| (4) |
Nevertheless, carbonate system parameters calculated from a range of published KD equations are also presented (table S1) to acknowledge the ongoing research in this area (8, 10, 33, 34). The main outcomes of this study are preserved whatever KD formulation is used.
Controlled laboratory experiments on corals have revealed that δ11B of the coral skeleton, relative to borate δ11B of the organism’s surrounding seawater, is higher than skeletal δ11B of most other species of marine calcifiers (35, 36). Thus, the δ11B composition of coral aragonite likely reflects pH of the calcifying fluid, which, in corals, is known from independent measurements [microelectrode and pH-sensitive dye; e.g., (14, 37)] to be substantially elevated relative to seawater pH (pHsw). Numerous studies have used this apparent relationship to estimate coral’s pHcf from their skeletal δ11B to explore the ability of corals to regulate their pHcf in response to environmental stress, as summarized below.
Modification of coral calcifying fluid chemistry under future global change scenarios
Response of calcifying fluid to OA
Various experimental studies have used coral skeletal δ11B to examine the ability of scleractinian corals to modify pH of their calcifying fluid under normal and acidified conditions [see (38) for a review]. These studies reveal that corals increase their internal pHcf relative to pHsw by 0.2 to 1.1 units, with the offset generally increasing with the degree of acidification. These observations are consistent with other observations of elevated pHcf based on pH microsensors and pH-sensitive dyes (37, 39–42). The δ11B approach reveals that the sensitivity of pHcf to pHsw (ΔpHcf/ΔpHsw) in culture experiments on zooxanthellae-bearing corals ranges across species from 0.23 to 0.51 (38). However, corals in their natural environment appear to exhibit greater sensitivity in δ11B-based estimates of pHcf to pHsw than corals cultured in laboratory experiments (10).
A study on Stylophora pistillata (42) used controlled laboratory experiments at a single temperature of 25°C to show that changes in DICsw affected pHcf and DICcf. Such findings have also been observed in natural environments, where changes in pHcf and DICcf modulate Ωcf in support of coral calcification (9, 10). In one of the few coral-δ11B studies conducted on a natural reef system, a Porites species was observed to elevate pHcf by 0.4 relative to pHsw (10), with seasonal variations of ±0.25. It was also observed that pHcf and DICcf were strongly inversely correlated (10, 43), with maximum pHcf/minimum DICcf occurring in winter and minimum pHcf/maximum DICcf occurring in summer—effectively dampening thermally induced seasonal variability in Ωsw.
Response of calcifying fluid to thermal stress
Increasing atmospheric pCO2 causes ocean warming (11), which negatively affects zooxanthellate corals by breaking down the coral-algal symbiosis [“bleaching”; e.g., (1, 44), among many others]. Depending on the magnitude and duration of thermal stress, recovery from reduced rates of growth and reproduction accompanying bleaching events can take days to years (45), which may ultimately lead to the collapse of the coral reef ecosystem.
Despite the established impacts of ocean warming on coral bleaching, the impact of thermal stress on the calcifying fluid of corals is not well understood. There is some evidence from field experiments that seasonal changes in water temperature (up to 6°C) are associated with changes in calcifying fluid chemistry (DICcf, pHcf, and Ωcf) (10), as summarized above. Furthermore, the calcifying fluid of a Porites coral located in the central Great Barrier Reef (GBR) exhibited decoupling of pHcf from DICcf (i.e., both pHcf and DICcf decreased), along with decreased growth during the severe bleaching event of 1998 that caused localized bleaching of ca. 60% of zooxanthellate corals (46). D’Olivo et al. (47) analyzed a core obtained from a Porites colony from the GBR and attempted to decouple the long-term impacts of OA and warming on coral pHcf. They concluded that both processes caused pHcf to decline but found that the decline due to temperature was much less than the decline due to OA—which they attributed to the species’ annual exposure to large seasonal temperature changes rendering it more tolerant of thermal stress. However, it is challenging to unequivocally attribute changes in calcifying fluid chemistry to discrete oceanographic parameters owing to covariation of these parameters in the field.
RESULTS AND DISCUSSION
The skeletons of two species of pocilloporid tropical corals (Pocillopora damicornis and S. pistillata) grown under a suite of controlled temperature and pCO2 treatments [28°C/462 parts per million (ppm), 28°C/931 ppm, 28°C/2884 ppm, 31°C/483 ppm, 31°C/908 ppm, and 31°C/3303 ppm; Table 1] were analyzed for δ11B and B/Ca to gain insight into the impacts of temperature and pHsw stress on coral calcifying fluid chemistry. These observations are compared to pHcf microelectrode data reported elsewhere (7) obtained from the same individuals under the same experimental treatments. Although thermal stress is known to be the main cause of coral bleaching, no prior study has examined the combined impacts of temperature and pHsw on the carbonate chemistry of coral calcifying fluid under controlled laboratory conditions.
Table 1. Measured and calculated seawater parameters (±SE) from the culturing experiments (S, salinity; T, temperature; pH (NBS scale); DIC, dissolved inorganic carbon; Alkalinity, total alkalinity; pH on total scale; [CO32−], carbonate ion; [HCO3−], bicarbonate ion; [CO2], partial pressure of CO2; ΩA, saturation state of seawater with respect to aragonite).
| Experiment | Seawater measured parameters | |||||
|
δ11Bsw* (‰) |
S (psu) |
T (°C) |
pH (NBS scale) |
DIC (μmol/kg-seawater) |
Alkalinity (μmol/kg-seawater) |
|
| Exp1 (400 ppm, 28°C) | 41.02 ± 0.49 | 34.9 ± 0.1 | 28.29 ± 0.01 | 8.27 ± 0.01 | 2480 ± 40 | 2915 ± 49 |
| Exp2 (400 ppm, 31°C) | 40.17 ± 0.33 | 35.6 ± 0.1 | 31.72 ± 0.06 | 8.24 ± 0.01 | 2350 ± 44 | 2774 ± 54 |
| Exp3 (1000 ppm, 28°C) | 41.36 ± 0.13 | 35.4 ± 0.1 | 27.88 ± 0.02 | 8.04 ± 0.01 | 2645 ± 44 | 2939 ± 46 |
| Exp4 (1000 ppm, 31°C) | 41.93 ± 0.20 | 36.0 ± 0.1 | 30.83 ± 0.02 | 8.12 ± 0.01 | 2755 ± 37 | 3083 ± 40 |
| Exp5 (2800 ppm, 28°C) | 40.97 ± 0.15 | 35.8 ± 0.1 | 28.17 ± 0.02 | 7.62 ± 0.01 | 2861 ± 82 | 2907 ± 81 |
| Exp6 (2800 ppm, 31°C) | 41.35 ± 0.12 | 35.7 ± 0.1 | 30.93 ± 0.02 | 7.69 ± 0.01 | 3223 ± 57 | 3290 ± 56 |
| Experiment | Seawater calculated parameters | |||||
|
pCO2 (ppmv) |
pH** (total scale) |
[CO32−] (μmol/kg-seawater) |
[HCO3−] (μmol/kg-seawater) |
[CO2] (μmol/kg-seawater) |
ΩA | |
| Exp1 (400 ppm, 28°C) | 466 ± 8 | 8.14 ± 0.01 | 334 ± 9 | 2133 ± 33 | 12.3 ± 0.2 | 5.38 ± 0.14 |
| Exp2 (400 ppm, 31°C) | 499 ± 9 | 8.09 ± 0.01 | 320 ± 11 | 2017 ± 36 | 12.3 ± 0.3 | 5.20 ± 0.18 |
| Exp3 (1000 ppm, 28°C) | 925 ± 15 | 7.90 ± 0.01 | 217 ± 6 | 2424 ± 35 | 24.6 ± 0.4 | 3.48 ± 0.10 |
| Exp4 (1000 ppm, 31°C) | 885 ± 12 | 7.97 ± 0.01 | 265 ± 4 | 2468 ± 35 | 22.0 ± 0.4 | 4.28 ± 0.07 |
| Exp5 (2800 ppm, 28°C) | 2807 ± 120 | 7.49 ± 0.01 | 90 ± 3 | 2697 ± 77 | 74.3 ± 3.2 | 1.44 ± 0.04 |
| Exp6 (2800 ppm, 31°C) | 3194 ± 136 | 7.55 ± 0.01 | 113 ± 4 | 3030 ± 54 | 79.5 ± 3.4 | 1.83 ± 0.06 |
*δ11Bsw-based average of δ11Bsw1 and δ11Bsw2 (see table S5) for each condition.
**pH on total scale recalculated on the basis of pH measured on NBS scale during experiment.
Coral calcification response to pHsw and thermal stress
Net calcification, defined here as the difference between gross calcification and gross dissolution of the coral skeleton, was estimated from the buoyant weight technique (see Materials and Methods). The corals investigated in this study generally exhibited an increase in net calcification in response to OA under both temperature conditions—with the exception of S. pistillata exhibiting stable (but very low) calcification rates across pH treatments under the high-temperature treatment. Both species also exhibited a decrease in net calcification with increasing temperature under each of the three pCO2 treatments (Fig. 1, A and B) (4).
Fig. 1. Calcifying fluid pHcf-based boron isotopes.
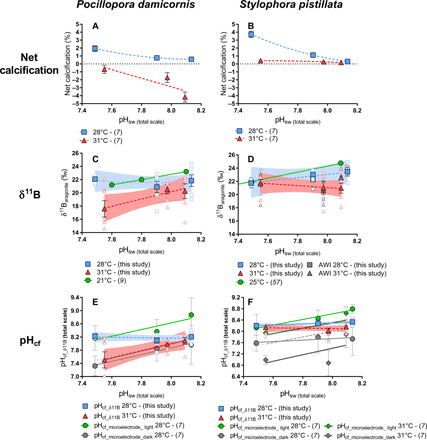
Panels show measured parameters as a function of treatment pHsw. Blue squares correspond to 28°C, red triangles correspond to 31°C, solid symbols represent treatment averages, and open symbols represent data for individual coral specimens. (A) and (B) show net calcification rate as percent change in skeletal mass throughout the experiment (7). (C) and (D) show δ11B measured for P. damicornis and S. pistillata, respectively, and independent measurements of δ11B of S. pistillata by Alfred Wegener Institute for Polar and Marine Research (AWI), with individuals shown in open dark gray and treatment averages shown in solid gray. Dotted horizontal lines represent zero calcification. (E) and (F) show calculated pHcf (see the main text) for P. damicornis and S. pistillata, respectively. Best-fit model (linear or nonlinear regressions) was determined using GraphPad based on lowest akaike information criteria (AIC); solid lines indicate significant regressions. Dashed gray lines represent 1:1 ratio of pHcf:pHsw for comparison to observed trends (28°C, dark; 31°C, light). Error bars are given in SEM and envelopes for the regressions represent 95% confidence interval.
Corals typically exhibit parabolic, rather than linear, growth responses to temperature (48), referred to as thermal performance curves. Coral thermal performance curves vary across species (7, 49–51) and may be influenced by various factors, including the environment (52), factors influencing photosynthesis activity (e.g., symbiont density), and symbiont clade (53). Prolonged thermal stress also causes bleaching, leading to reduction or cessation of calcification. Thermal performance curves previously established for the species investigated here, but collected from different areas, suggest optimal temperatures of 28° to 30°C (48) for S. pistillata and 27°C (54) for P. damicornis.
Although thermal stress reduced net calcification rates of both species (Fig. 1, A and B) (4), S. pistillata exhibited greater resilience than P. damicornis to thermal stress to the extent that S. pistillata maintained mildly positive net rates of calcification rates under the high-temperature treatments, while P. damicornis exhibited net dissolution.
Controlled laboratory experiments have shown that tropical scleractinian corals can exhibit a range of calcification response to OA, including linear negative, threshold negative, and parabolic (49, 55, 56). Previous OA experiments (each conducted at only a single temperature) on the two species investigated here identified different trends than observed in the present study—S. pistillata exhibited declining calcification with increasing pCO2 (57), while P. damicornis exhibited no significant calcification response to pCO2 (9). In the present study, both S. pistillata and P. damicornis exhibited increasing calcification rates with increasing pCO2, except for S. pistillata that exhibited stable calcification rates at 31°C.
It is difficult to deconvolve the parameters responsible for the different outcomes of these experiments, as coral genetics (i.e., population), laboratory environment, feeding, light, and temperature inevitably differed among studies (58). For example, specimens used in these three studies were collected from different places, including from Eilat, Israel [S. pistillata; (57)], Salmon Bay, Western Australia [P. damicornis; (9)], and Fiji Islands (present study)—raising the possibility that local, population-level adaptations to temperature and pHsw contributed to these differing results. Temperatures were substantially different among the experiments, suggesting that they were at least partly responsible for the different responses to OA observed in the various studies. Specifically, Comeau et al. (9) used a temperature of 21°C for P. damicornis, while Krief et al. (57) used a temperature of 25°C for S. pistillata, both below the thermal optimum for these species, compared with the substantially higher temperatures of 28°C (i.e., thermal optimum) and 31°C (above thermal optimum). Thermal optimum might also vary depending of the environment and local conditions (52). However, collectively, these results raise the possibility that these corals’ response to OA is most resilient under their thermal optimum (28°C) and becomes more negative as temperature departs, positively (31°C) or negatively (21°C, 25°C), from this thermal optimum—although this hypothesis requires further investigation.
Response of calcifying fluid chemistry to thermal stress
pHcf
The δ11B measurements for S. pistillata were performed independently in two laboratories for the purpose of interlaboratory validation and to ensure quality control of the data (fig. S2; see also Materials and Methods and Supplemental Information). For all treatments, the mean δ11B values obtained by the two laboratories for the same coral specimens were not significantly different from each other (P > 0.05). Likewise, slopes of the linear regressions (LRs) relating treatment condition to coral skeletal δ11B obtained by the two laboratories were also not statistically different (P > 0.05). Last, the slope of the LR relating skeletal δ11B obtained by the two laboratories for all coral specimens is not significantly different from 1 (P = 0.39), and the intercept is not significantly different from 0 (P = 0.83). It should be noted that measurements were performed on different subsamples of the same coral specimens, meaning that the small, nonsignificant differences in δ11B obtained by the two laboratories may be partly attributable to small variations in δ11B within the coral skeleton. These results cross-validate the δ11B analytical procedures of both laboratories and show that the reported δ11B values are reproducible for both laboratories at the 95% confidence level.
Both species exhibit a significant decrease in δ11B with increasing temperature (P < 0.05) (Fig. 1, C and D), indicating that the ability of both species to elevate pHcf in support of calcification is impaired under thermal stress (Fig. 1, E and F). Furthermore, both species also exhibited greater interspecimen variability in δ11B within the same pCO2 treatment at 31°C than at 28°C (Fig. 1, fig. S3, and Tables 2 and 3), with the greatest δ11B variability exhibited by P. damicornis at 31°C. Higher interspecimen variability in δ11B at 31°C provides further support for the assertion that both species exert less control over pHcf under conditions of thermal stress.
Table 2. Measured values of δ11B and elemental ratios (B/Ca) within the skeletons of P. damicornis and corresponding reconstructed parameters of the calcifying fluid (pHcf, [CO32−]cf, and DICcf).
| Culture conditions | Analytical results | Calculated parameters | ||||||||
| pCO2sw | T | pHsw | Tank | δ11Bc1 (±2 SD) | δ11Bc2 (±2 SD) | δ11Baverage (±2 SD) | B/Ca (±2 SD) | pHcf | [CO32−]cf (±1 SE) | DICcf (±1 SE) |
| (ppm) | (°C) | (total scale) | (‰) | (‰) | (‰) | (μmol/mol) | (total scale) | (μmol/kg) | (μmol/kg) | |
| Pocillopora damicornis | ||||||||||
| 466 | 28 | 8.14 | 1 | 19.70 ± 0.10 | 19.65 ± 0.10 | 19.68 ± 0.07 | 472 ± 11 | 8.04 | 522 ± 66 | 4458 ± 199 |
| 466 | 28 | 8.14 | 2 | 21.52 ± 0.10 | 21.63 ± 0.10 | 21.58 ± 0.07 | 545 ± 11 | 8.19 | 621 ± 57 | 3891 ± 162 |
| 466 | 28 | 8.14 | 3 | 22.76 ± 0.10 | 22.65 ± 0.10 | 22.71 ± 0.07 | 449 ± 11 | 8.28 | 877 ± 70 | 4694 ± 206 |
| 466 | 28 | 8.14 | 4 | 23.67 ± 0.11 | 23.68 ± 0.11 | 23.68 ± 0.08 | 492 ± 11 | 8.34 | 894 ± 65 | 4240 ± 175 |
| 466 | 28 | 8.14 | 1 | 24.42 ± 0.39 | 24.42 ± 0.39 | 489 ± 11 | 8.39 | 975 ± 76 | 4231 ± 178 | |
| 466 | 28 | 8.14 | 2 | 19.01 ± 0.39 | 19.01 ± 0.39 | 460 ± 11 | 7.97 | 463 ± 81 | 4513 ± 222 | |
| 499 | 31 | 8.09 | 4 | 22.02 ± 0.21 | 22.09 ± 0.21 | 22.06 ± 0.29 | 537 ± 11 | 8.25 | 759 ± 61 | 3840 ± 157 |
| 499 | 31 | 8.09 | 4 | 22.38 ± 0.09 | 22.38 ± 0.09 | 537 ± 11 | 8.27 | 789 ± 55 | 3834 ± 162 | |
| 499 | 31 | 8.09 | 1 | 21.02 ± 0.09 | 21.02 ± 0.09 | 501 ± 11 | 8.17 | 707 ± 57 | 4130 ± 176 | |
| 499 | 31 | 8.09 | 22.01 ± 0.11 | 22.02 ± 0.11 | 22.01 ± 0.08 | 500 ± 11 | 8.24 | 809 ± 57 | 4126 ± 174 | |
| 499 | 31 | 8.09 | 3 | 18.53 ± 0.11 | 18.50 ± 0.11 | 18.51 ± 0.08 | 504 ± 11 | 7.96 | 455 ± 58 | 4000 ± 188 |
| 499 | 31 | 8.09 | 4 | 15.56 ± 0.11 | 15.53 ± 0.11 | 15.55 ± 0.08 | 470 ± 11 | 7.60 | 184 ± 60 | 3459 ± 461 |
| 925 | 28 | 7.90 | 2 | 17.22 ± 0.11 | 17.23 ± 0.11 | 17.23 ± 0.08 | 435 ± 11 | 7.73 | 263 ± 58 | 4329 ± 339 |
| 925 | 28 | 7.90 | 1 | 19.78 ± 0.11 | 19.63 ± 0.11 | 19.71 ± 0.08 | 558 ± 11 | 8.01 | 422 ± 45 | 3807 ± 162 |
| 925 | 28 | 7.90 | 3 | 22.98 ± 0.26 | 22.82 ± 0.26 | 22.90 ± 0.18 | 492 ± 11 | 8.27 | 800 ± 55 | 4361 ± 178 |
| 925 | 28 | 7.90 | 2 | 22.25 ± 0.09 | 22.25 ± 0.09 | 491 ± 11 | 8.22 | 735 ± 54 | 4383 ± 185 | |
| 925 | 28 | 7.90 | 4 | 21.60 ± 0.09 | 21.60 ± 0.09 | 503 ± 11 | 8.17 | 652 ± 54 | 4280 ± 178 | |
| 925 | 28 | 7.90 | 1 | 21.69 ± 0.09 | 21.69 ± 0.09 | 619 ± 11 | 8.18 | 538 ± 42 | 3482 ± 136 | |
| 885 | 31 | 7.97 | 4 | 21.32 ± 0.09 | 21.32 ± 0.09 | 743 ± 11 | 8.07 | 387 ± 38 | 2812 ± 114 | |
| 885 | 31 | 7.97 | 3 | 18.94 ± 0.09 | 18.94 ± 0.09 | 474 ± 11 | 7.84 | 356 ± 56 | 4141 ± 230 | |
| 885 | 31 | 7.97 | 1 | 21.89 ± 0.21 | 21.85 ± 0.21 | 21.87 ± 0.15 | 8.11 | |||
| 885 | 31 | 7.97 | 3 | 21.88 ± 0.09 | 21.88 ± 0.09 | 592 ± 11 | 8.11 | 533 ± 46 | 3550 ± 146 | |
| 885 | 31 | 7.97 | 3 | 17.84 ± 0.11 | 17.80 ± 0.11 | 17.82 ± 0.08 | 524 ± 11 | 7.70 | 217 ± 50 | 3422 ± 286 |
| 885 | 31 | 7.97 | 1 | 21.47 ± 0.11 | 21.46 ± 0.11 | 21.46 ± 0.08 | 441 ± 11 | 8.08 | 668 ± 61 | 4751 ± 208 |
| 2807 | 28 | 7.49 | 1 | 22.59 ± 0.26 | 22.53 ± 0.26 | 22.56 ± 0.18 | 460 ± 11 | 8.27 | 865 ± 60 | 4673 ± 192 |
| 2807 | 28 | 7.49 | 4 | 22.60 ± 0.11 | 22.35 ± 0.11 | 22.48 ± 0.08 | 466 ± 11 | 8.26 | 845 ± 57 | 4613 ± 187 |
| 2807 | 28 | 7.49 | 3 | 21.19 ± 0.11 | 21.24 ± 0.11 | 21.21 ± 0.08 | 422 ± 11 | 8.17 | 782 ± 64 | 5118 ± 224 |
| 2807 | 28 | 7.49 | 2 | 22.69 ± 0.11 | 22.57 ± 0.11 | 22.63 ± 0.08 | 496 ± 11 | 8.27 | 811 ± 54 | 4339 ± 175 |
| 2807 | 28 | 7.49 | 2 | 21.48 ± 0.21 | 21.48 ± 0.21 | 8.19 | ||||
| 3194 | 31 | 7.55 | 4 | 22.17 ± 0.39 | 22.17 ± 0.39 | 485 ± 11 | 8.18 | 734 ± 69 | 4300 ± 178 | |
| 3194 | 31 | 7.55 | 2 | 20.47 ± 0.09 | 20.47 ± 0.09 | 486 ± 11 | 8.04 | 557 ± 53 | 4266 ± 184 | |
| 3194 | 31 | 7.55 | 3 | 15.61 ± 0.09 | 15.61 ± 0.09 | 538 ± 11 | 7.39 | 74 ± 7x | 2276 ± 108 | |
| 3194 | 31 | 7.55 | 4 | 16.06 ± 0.09 | 16.06 ± 0.09 | 518 ± 11 | 7.49 | 113 ± 46 | 2774 ± 94 | |
| 3194 | 31 | 7.55 | 1 | 14.60 ± 0.11 | 14.59 ± 0.11 | 14.60 ± 0.08 | 449 ± 11 | 7.02 | 14 ± 4x | 950 ± 175 |
| 3194 | 31 | 7.55 | 4 | 20.24 ± 0.11 | 20.29 ± 0.11 | 20.26 ± 0.08 | 509 ± 11 | 8.02 | 512 ± 50 | 4061 ± 180 |
| 3194 | 31 | 7.55 | 2 | 14.00 ± 0.11 | 13.94 ± 0.11 | 13.97 ± 0.08 | 523 ± 11 | 6.38 | ||
pCO2sw, seawater pCO2 of the culture treatment.
T, seawater temperature of the culture treatment.
pHsw, seawater pH of the culture treatment.
Tank, tank number where the sample was cultured.
δ11Bc1, first measurement of the boron isotopic composition of the sample, uncertainty determined on reproducibility of the AE121 standard (2 SD).
δ11Bc2, duplicate analysis of the boron isotopic composition of the sample, uncertainty determined on reproducibility of the AE121 standard (2 SD).
δ11Baverage, average of δ11Bc1 and δ11Bc2 or δ11Bc1 if only one measurement was performed. When two measurements were carried out, uncertainty was calculated based on uncertainties of δ11Bc1 and δ11Bc2 (Δa = √ (Σi(Δai)2)).
B/Ca measured for the sample. Uncertainty is calculated as 2 SD of repeated measurements of the CamWuellestorfi standard (table S4).
pHcf calculated from δ11B using (8) based on Monte Carlo simulation.
[CO32−]cf calculated from B/Ca using (8).
DICcf calculated from δ11B and B/Ca using (8).
Uncertainties for [CO32−]cf and DICcf calculated using (8), Δ (1 SE) = √ (Σi(Δsysi+Δnonsysi)2), xuncertainty = Δnonsys (1 SE).Additional data not used in this study (Li/Ca, Mg/Ca and Sr/Ca) are presented in table S7 in the Supplementary Materials.
Table 3. Measured values of δ11B and elemental ratios (B/Ca) within the skeletons of S. pistillata and corresponding reconstructed parameters of the calcifying fluid (pHcf, [CO32−]cf, and DICcf).
| Culture conditions | Analytical results | |||||||||
| pCO2sw | T | pHsw | Tank | δ11Bc1 (±2 SD) | δ11Bc2 (±2 SD) | δ11Baverage (±2 SD) | B/Ca (±2 SD) | pHcf | [CO32−]cf (±1 SE) | DICcf (±1 SE) |
| (ppm) | (°C) | (total scale) | (‰) | (‰) | (‰) | (μmol/mol) | (total scale) | (μmol/kg) | (μmol/kg) | |
| Stylophora pistillata | ||||||||||
| 466 | 28 | 8.14 | 2 | 23.20 ± 0.30 | 23.21 ± 0.30 | 23.20 ± 0.21 | 470 ± 11 | 8.31 | 888 ± 70 | 4464 ± 191 |
| 466 | 28 | 8.14 | 2 | 455 ± 11 | 918 ± 71 | 4614 ± 194 | ||||
| 466 | 28 | 8.14 | 2 | 459 ± 11 | 909 ± 70 | 4584 ± 190 | ||||
| 466 | 28 | 8.14 | 4 | 21.85 ± 0.30 | 21.63 ± 0.30 | 21.74 ± 0.21 | 527 ± 11 | 8.21 | 659 ± 62 | 4021 ± 166 |
| 466 | 28 | 8.14 | 1 | 23.95 ± 0.26 | 23.95 ± 0.26 | 432 ± 11 | 8.36 | 1050 ± 78 | 4815 ± 216 | |
| 466 | 28 | 8.14 | 2 | 24.94 ± 0.26 | 24.94 ± 0.26 | 480 ± 11 | 8.43 | 1048 ± 70 | 4291 ± 181 | |
| 499 | 31 | 8.09 | 2 | 18.09 ± 0.26 | 18.09 ± 0.26 | 378 ± 11 | 7.92 | 553 ± 84 | 5283 ± 284 | |
| 499 | 31 | 8.09 | 4 | 21.62 ± 0.09 | 21.62 ± 0.09 | 536 ± 11 | 8.21 | 718 ± 54 | 3850 ± 163 | |
| 499 | 31 | 8.09 | 1 | 23.48 ± 0.09 | 23.48 ± 0.09 | 513 ± 11 | 8.34 | 934 ± 59 | 3968 ± 163 | |
| 499 | 31 | 8.09 | 1 | 526 ± 11 | 983 ± 63 | 4179 ± 184 | ||||
| 499 | 31 | 8.09 | 4 | 20.93 ± 0.26 | 20.93 ± 0.26 | 411 ± 11 | 8.16 | 851 ± 79 | 5046 ± 231 | |
| 499 | 31 | 8.09 | 3 | 19.62 ± 0.09 | 19.62 ± 0.09 | 462 ± 11 | 8.06 | 616 ± 63 | 4451 ± 203 | |
| 499 | 31 | 8.09 | 1 | 22.04 ± 0.09 | 22.04 ± 0.09 | 557 ± 11 | 8.24 | 728 ± 53 | 3692 ± 160 | |
| 499 | 31 | 8.09 | 1 | 473 ± 11 | 859 ± 62 | 4351 ± 187 | ||||
| 925 | 28 | 7.90 | 2 | 23.26 ± 0.36 | 23.26 ± 0.36 | 419 ± 11 | 8.29 | 980 ± 77 | 5097 ± 217 | |
| 925 | 28 | 7.90 | 4 | 22.70 ± 0.26 | 22.70 ± 0.26 | 440 ± 11 | 8.25 | 870 ± 67 | 4885 ± 207 | |
| 925 | 28 | 7.90 | 1 | 22.25 ± 0.26 | 22.25 ± 0.26 | 451 ± 11 | 8.22 | 800 ± 64 | 4770 ± 204 | |
| 925 | 28 | 7.90 | 3 | 23.63 ± 0.26 | 23.63 ± 0.26 | 449 ± 11 | 8.32 | 958 ± 67 | 4747 ± 200 | |
| 925 | 28 | 7.90 | 2 | 23.32 ± 0.26 | 23.32 ± 0.26 | 485 ± 11 | 8.30 | 855 ± 62 | 4403 ± 184 | |
| 885 | 31 | 7.97 | 3 | 23.01 ± 0.21 | 22.79 ± 0.21 | 22.90 ± 0.15 | 8.19 | |||
| 885 | 31 | 7.97 | 1 | 23.21 ± 0.39 | 23.21 ± 0.39 | 417 ± 11 | 8.21 | 920 ± 82 | 5038 ± 220 | |
| 885 | 31 | 7.97 | 3 | 19.64 ± 0.26 | 19.64 ± 0.26 | 378 ± 11 | 7.91 | 541 ± 79 | 5346 ± 286 | |
| 885 | 31 | 7.97 | 4 | 19.76 ± 0.26 | 19.76 ± 0.26 | 595 ± 11 | 7.92 | 353 ± 50 | 3408 ± 161 | |
| 885 | 31 | 7.97 | 1 | 17.49 ± 0.26 | 17.49 ± 0.26 | 394 ± 11 | 7.65 | 251 ± 73 | 4347 ± 474 | |
| 885 | 31 | 7.97 | 2 | 23.27 ± 0.39 | 23.27 ± 0.39 | 417 ± 11 | 8.22 | 929 ± 84 | 5036 ± 219 | |
| 2807 | 28 | 7.49 | 3 | 22.11 ± 0.39 | 22.11 ± 0.39 | 384 ± 11 | 8.23 | 983 ± 86 | 5622 ± 251 | |
| 2807 | 28 | 7.49 | 3 | 21.90 ± 0.39 | 21.90 ± 0.39 | 377 ± 11 | 8.22 | 968 ± 86 | 5726 ± 257 | |
| 2807 | 28 | 7.49 | 4 | 21.51 ± 0.39 | 21.51 ± 0.39 | 357 ± 11 | 8.19 | 969 ± 94 | 6046 ± 287 | |
| 2807 | 28 | 7.49 | 4 | 21.40 ± 0.39 | 21.40 ± 0.39 | 355 ± 11 | 8.18 | 958 ± 95 | 6095 ± 286 | |
| 3194 | 31 | 7.55 | 4 | 21.74 ± 0.39 | 21.74 ± 0.39 | 410 ± 11 | 8.15 | 819 ± 81 | 5101 ± 231 | |
| 3194 | 31 | 7.55 | 2 | 22.75 ± 0.39 | 22.75 ± 0.39 | 425 ± 11 | 8.22 | 903 ± 79 | 4895 ± 214 | |
| 3194 | 31 | 7.55 | 3 | 22.62 ± 0.36 | 22.62 ± 0.36 | 408 ± 11 | 8.21 | 928 ± 78 | 5106 ± 226 | |
| 3194 | 31 | 7.55 | 3 | 22.05 ± 0.36 | 22.05 ± 0.36 | 419 ± 11 | 8.17 | 836 ± 78 | 4984 ± 218 | |
| 3194 | 31 | 7.55 | 1 | 19.15 ± 0.39 | 19.15 ± 0.39 | 342 ± 11 | 7.92 | 605 ± 93 | 5888 ± 330 | |
| 3194 | 31 | 7.55 | 2 | 22.07 ± 0.28 | 22.14 ± 0.28 | 22.10 ± 0.20 | 455 ± 11 | 8.17 | 773 ± 61 | 4585 ± 202 |
pCO2sw, seawater pCO2 of the culture treatment.
T, seawater temperature of the culture treatment.
pHsw, seawater pH of the culture treatment.
Tank, tank number where the sample was cultured.
δ11Bc1, first measurement of the boron isotopic composition of the sample, uncertainty determined on reproducibility of the AE121 standard (2 SD).
δ11Bc2, duplicate analysis of the boron isotopic composition of the sample, uncertainty determined on reproducibility of the AE121 standard (2 SD).
δ11Baverage, average of δ11Bc1 and δ11Bc2 or δ11Bc1 if only one measurement was performed. When two measurements were carried out uncertainty was calculated based on uncertainties of δ11Bc1: and δ11Bc2 (Δa = √ (Σi(Δai)2)).
B/Ca measured for the sample. Uncertainty is based 2 SD on the reproducibility of the CamWuellestorfi (table S4).
pHcf calculated from δ11B using (8) based on Monte Carlo simulation.
[CO32−]cf calculated from B/Ca using (8).
DICcf calculated from δ11B and B/Ca using (8).
Uncertainties for [CO32−]cf and DICcf calculated using (8), Δ (1 SE) = √ (Σi(Δsysi+Δnonsysi)2), xuncertainty = Δnonsys (1 SE).Additional data not used in this study (Li/Ca, Mg/Ca and Sr/Ca) are presented in table S7 in the Supplementary Materials.
The pHcf was also measured with pH microelectrodes both in the light (pHcf_light) and in the dark (pHcf_dark) (Fig. 1, E and F). Both pHcf_light and pHcf_dark yielded an inverse correlation between temperature and pHcf. However, microelectrode measurements of pHcf_light and pHcf_dark were respectively significantly (P < 0.05) greater and less than pHcf measured via δ11B. In addition, despite the offset in the δ11B-based and microlectrode-based measurements of pHcf, temperature-driven decreases in pHcf (e.g., ΔpHcf/ΔT) detected by the two approaches did not significantly differ from each other under any of the pCO2 treatments [S. pistillata at 400 ppm (P = 0.86), at 1000 ppm (P = 0.35), and at 2800 ppm (P = 0.54)] (Figs. 1F and 2).
Fig. 2. Carbonate chemistry of the calcifying fluid–based boron proxies.
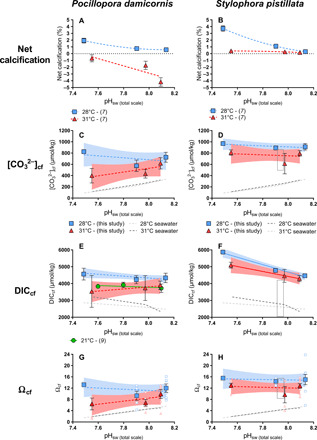
Panels show measured parameters as a function of treatment pHsw. Blue squares correspond to 28°C, red triangles correspond to 31°C, solid symbols represent treatment averages, and open symbols represent data for individual coral specimens. (A) and (B) show net calcification rate as percent change in skeletal mass throughout the experiment (7). (C) and (D) show recalculated [CO32−]cf. (E) and (F) show calculated DICcf. (G) and (H) show calculated saturation state of aragonite in the calcifying fluid (Ωcf). Gray rectangles in (D), (F), and (H) represent the range (2 SD) of LIX data (32) for S. pistillata [pH 7.94 (total scale), T = 25°C, S = 38]. Best-fit model (linear or nonlinear regressions) was determined using GraphPad based on lowest AIC. Solid lines indicate significant regressions (P < 0.05). Dashed gray lines represent 1:1 ratio of [CO32−]cf:[CO32−]sw, DICcf:DICsw, or Ωcf:Ωsw for comparison to observed trends (28°C, dark; 31°C, light). Error bars are given in SEM and envelopes for the regressions represent 95% confidence interval.
The impact of OA on pHcf was more complex than the impact of temperature discussed above. Both species under 28°C and S. pistillata under 31°C exhibited homeostatic pHcf responses to seawater acidification—i.e., pHcf remained relatively constant with declining pHsw. In contrast, P. damicornis under 31°C exhibited pHcf that tracks pHsw, meaning that the coral is not able to maintain pHcf homeostasis. Under pHcf-homeostatic conditions (both species under 28°C and S. pistillata under 31°C), the corals elevate pHcf by up to 0.7 units relative to pHsw, with the greatest increase in pHcf (relative to pHsw) exhibited under the lowest pHsw treatment. Thus, under more acidic conditions, the corals elevate pHcf more, relative to pHsw, than under less acidic conditions—at potentially increased energetic cost to the organism [e.g., (14)]. P. damicornis seems to lose this compensatory ability under thermal stress (31°C).
The sensitivity of pHcf to temperature is also seen in the pH microelectrode data. Again, despite the difference in absolute measured pHcf between the δ11B and microlectrode approaches, pHsw-driven changes in pHcf (e.g., ΔpHcf/ΔpHsw) detected by the two approaches did not significantly differ from each other for either species under any of the temperature treatments (P. damicornis at 28°C, P = 0.14; S. pistillata at 28°C, P = 0.06; S. pistillata at 31°C, P = 0.72; Fig. 1, E and F). Absolute differences in measured pHcf between the two approaches are discussed further in the “Fidelity of coral skeletal δ11B in recording coral pHcf” section.
The observations that S. pistillata is able to maintain pHcf homeostasis under acidified conditions under both temperatures and that P. damicornis can only maintain pHcf homeostasis under the control (optimal) temperature suggest that P. damicornis is more susceptible to this apparent negative interaction between thermal stress and OA. Despite the inability of P. damicornis at 31°C to elevate pHcf above pHsw in support of calcification, gross calcification (but not net calcification) was still occurring, as indicated by the discrete calcein dye layer that was recorded in the coral skeleton to identify new skeletal material for boron isotopic and elemental analysis. This supports previous assertions that the saturation state of the coral calcifying fluid can be increased in support of calcification by increasing DICcf while maintaining pHcf that is elevated relative to the equilibrium pHcf expected from the elevated DICcf (even if pHcf tracks pHsw) (9) and also that organic templates and organic molecules within the calcifying fluid may support the coral calcification process [e.g., (59)].
[CO32−]cf and DICcf
Our results also show that both coral species elevate [CO32−]cf and DICcf relative to the corals’ surrounding seawater under all treatment conditions (Fig. 2 and Tables 2 and 3). Furthermore, both species maintain a relatively constant [CO32−]cf across pCO2 treatments (P = 0.53 for S. pistillata at 28°C, P = 0.27 for S. pistillata at 31°C, P = 0.52 for P. damicornis at 28°C, and P = 0.30 for P. damicornis at 31°C), resulting in a large increase in [CO32−]cf relative to [CO32−]sw in the lowest pHsw treatment (Fig. 2, C and D). Although P. damicornis at 31°C exhibits an apparent decline in [CO32−]cf with decreasing pHsw, this trend was not statistically significant (P = 0.2). In contrast, DICcf trends differed between the two species, with S. pistillata exhibiting increasing DICcf with decreasing pHsw at 28°C [LR: R2 = 0.84, P = 0.09; analysis of variance (ANOVA): P < 0.0001] and 31°C (LR: R2 = 0.31, P = 0.01; ANOVA: P = 0.05), and with P. damicornis exhibiting constant DICcf with decreasing pHsw at 28°C (LR: R2 = 0.22, P = 0.53; ANOVA: P = 0.08) and 31°C (LR: R2 = 0.15, P = 0.12; ANOVA: P = 0.69). However, both species exhibited DICcf that was significantly (P < 0.05) elevated relative to DICsw in each treatment, except for P. damicornis at 31°C that exhibited DICcf that was statistically indistinguishable from DICsw at 1000 and 2800 ppm (P = 0.16). Furthermore, both species exhibited lower [CO32−]cf and DICcf under conditions of thermal stress but only under the highest pCO2 treatment (Figs. 2 and 3; [CO32−]cf: P = 0.02 for both species; DICcf: P = 0.05 for P. damicornis; P = 0.01 for S. pistillata).
Fig. 3. Effect of temperature on the carbonate chemistry of the calcifying fluid in this study.
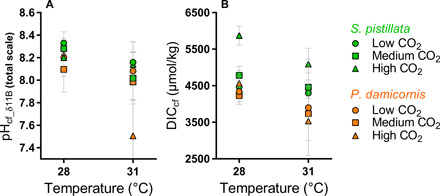
Effect of temperature on pHcf_δ11B (A) and DICcf (B) of S. pistillata (green) and P. damicornis (orange). Error bars are given as SEM.
The observed increases in DICcf cannot be solely explained by the prescribed increases in DICsw because the change in DICcf did not consistently track the change in DICsw (ΔDICcf/ΔDICsw). Neither can the hypothesis that DICcf increases under conditions of OA owing to decreased calcification rate [i.e., via accumulation of DIC in the calcifying fluid; (6)] explain the observed DICcf trends, since both DICcf and calcification rate increased with acidification. Instead, it appears that the changes in DICcf were the cause, rather than the result, of the changes in calcification rate resulting from the prescribed OA.
Ωcf
The capacity of an organism to elevate aragonite saturation state at the site of calcification supports precipitation of its skeleton (6, 38, 46). Prior work has shown that [Ca2+] of the coral calcifying fluid ([Ca2+]cf) can differ from [Ca2+] of seawater ([Ca2+]sw) (Fig. 2, G and H), with [Ca2+]cf/[Ca2+]sw of P. damicornis ranging from 0.85 to 1.41 (32, 60) and S. pistillata exhibiting [Ca2+]cf/[Ca2+]sw of 1.18 (32). Owing to the absence of direct measurements of [Ca2+]cf in the present study, we conservatively used [Ca2+] of seawater but acknowledge that more accurate estimates of Ωcf could be obtained from direct measurements of [Ca2+]cf under the difference treatments. Although using a constant value of [Ca2+]cf yields responses in [CO32−]cf and Ωcf that are of identical shape, estimation of Ωcf is useful because it presents the carbonate chemistry of the calcifying fluid relative to the nonarbitrary chemical division between the precipitation and dissolution of inorganic aragonite (i.e., Ω = 1).
Ωcf is significantly greater at 28°C than at 31°C for S. pistillata under all pCO2 conditions (400 ppm, P = 0.0002; 1000 ppm, P < 0.0001; 2800 ppm, P < 0.0001) and for P. damicornis under a pCO2 of 2800 ppm (P = 0.03). Both species maintained Ωcf ≥ 5 under all treatments, well above the level favoring dissolution of inorganic aragonite (Ωcf < 1). Under the control (optimal) temperature treatments, Ωcf was elevated as high as 16—a level that supports spontaneous precipitation of aragonite from seawater (61). Yet, despite maintaining Ωcf that is elevated relative to Ωsw by 2 to 4 units under thermal stress (31°C), the rate of gross calcification for P. damicornis was less than its rate of gross dissolution, which caused the corals to exhibit net dissolution under all pCO2 conditions at 31°C.
These estimates of Ωcf are consistent with the results of recent studies that used microsensors and fluorescent dyes (32), boron geochemistry (9), and Raman spectroscopy (60) to characterize calcifying fluid chemistry in the same species. Our observations of thermally induced declines in Ωcf are also in line with recent studies on the calcifying fluid dynamics of Porites corals in nature settings (10, 43, 46, 47). However, our results are not consistent with the finding of Comeau et al. (9) that Ωcf decreased with pHcf by ca. 3 units of Ωcf over 0.2 pHsw units. Instead, we found that Ωcf of both species remained constant with decreasing pHsw (for P. damicornis at 28°C: ANOVA, P = 0.19 and at 31°C: ANOVA, P = 0.28; for S. pistillata at 28°C: ANOVA, P = 0.90 and at 31°C: ANOVA, P = 0.51).
Fidelity of coral skeletal δ11B in recording coral pHcf
The skeletal δ11B composition of corals has been widely used as a proxy for pHcf [e.g., (9, 10, 57), among others]. However, few studies have independently measured pHcf to assess the reliability of the δ11B proxy of pHcf in corals (39), and none have done this with the pH microelectrode approach (Fig. 4). Holcomb et al. (62) found that the pH-sensitive dye (SNARF) and skeletal boron isotope approaches yielded different estimates of pHcf for S. pistillata, which they attributed to the two approaches analyzing different portions of the coral skeleton. Sevilgen et al. (32) found that the microelectrode and SNARF approaches yielded statistically indistinguishable estimates of pHcf, although they reported that the microelectrode approach yielded greater variability—possibly due to polyp retraction, stress response, and/or fluid mixing during microelectrode insertion.
Fig. 4. Compilation of pHcf under OA from direct and indirect measurements for S. pistillata and P. damicornis.
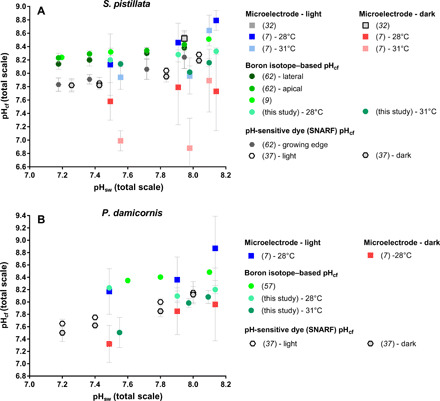
Compilation of existing pHcf data from microelectrode, pH-sensitive-dye (SNARF), and boron isotope data. (A) S. pistillata. (B) P. damicornis. Error bars are given in SD.
In this study, pHcf was independently measured with proton-sensitive microelectrodes (7) in the same coral specimens that were measured for skeletal δ11B, allowing direct comparison of δ11B-derived pHcf and microelectrode-derived pHcf. The microelectrode pHcf measurements were performed in both the light (pHcf_light) and dark (pHcf_dark) (Figs. 1, E and F, 5, and 6; Table 4; and table S2). The δ11B-based estimates of pHcf are significantly correlated with the microelectrode-based pHcf measurements for both species (Fig. 5, A and B). As discussed in the “pHcf” section, pHcf derived from both the δ11B and the microelectrode approaches show the same response to temperature (i.e., decreasing pHcf under thermal stress) and to pHsw. However, the microelectrode estimate of pHcf_light and pHcf_dark are, in some cases, significantly different from the δ11B estimates of pHcf in absolute magnitude. For S. pistillata, the difference between microelectrode pHcf_light and δ11B pHcf is greatest and statistically significant at pHsw 8.1 (28°C, P = 0.01; 31°C, P = 0.02). At lower pHsw, pHcf_light and δ11B estimates of pHcf were not significantly different (P > 0.05) (Figs. 1 and 6). In contrast, pHcf_dark was significantly different from δ11B estimates of pHcf under the lowest pH treatment for both species and at both temperatures (P. damicornis at 28°C, P = 0.03; S. pistillata at 28°C, P = 0.02; S. pistillata at 31°C, P < 0.0001).
Fig. 5. Comparison between pHcf from skeletal δ11B and direct LIX measurements.
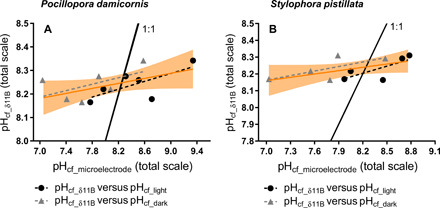
Comparison of pHcf from skeletal δ11B (pHcf_δ11B) and pHcf from microelectrode measurements (7) in the light (pHcf_light, black dots) and in the dark (pHcf_dark, gray triangles) for individual coral specimens of P. damicornis (A) and for S. pistillata (B) measured in the same samples. LRs of pHcf_light (dashed black line), pHcf_dark (dashed gray line), and both pHcf-based microelectrode (orange line) versus pHcf_δ11B. The black line represents 1:1 ratio of pHcf_δ11B:pHcf_microelectrode for comparison to observed trends. The orange line is the LR between pHcf from δ11B and pHcf derived from microelectrode (both light and dark), and the orange envelop represents the 95% confidence interval for LR.
Fig. 6. pHcf and DICcf from boron proxies and pHcf from LIX measurements.
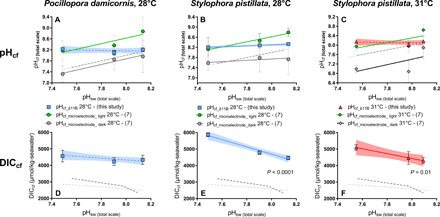
(A to C) Comparison between averages of boron-based pHcf (blue or red symbols) and microelectrode-derived pHcf in the light (open symbols) and in the dark (solid gray symbols) at the two temperature treatments for P. damicornis [(A) 28°C; no microelectrode pHcf data at 31°C] and S. pistillata [(B) 28°C and (C) 31°C]. LRs of pHcf_light (orange, 28°C; green, 31°C), pHcf_dark (black, 28°C; gray, 31°C), and pHcf_δ11B (blue, 28°C; red, 31°C) versus pH treatment. (D to F) DICcf data calculated from B/Ca and δ11B-derived pHcf for P. damicornis [(D) 28°C; no microelectrode pH data at 31°C] and S. pistillata [(E) 28°C and (F) 31°C]. Dashed gray lines represent 1:1 ratio of pHcf:pHsw for comparison to observed trends (28°C, dark; 31°C, light). Error bars are given in SEM and envelopes for the regressions represent 95% confidence interval.
Table 4. Measurements of pHcf from microelectrode in the light (pHcf_light) and dark (pHcf_dark), pHcf_δ11B calculated from skeletal δ11B, and estimated light:dark calcification ratios.
| pHcf_light (±SE) | pHcf_dark (±SE) | ΔpHlight-dark (±SE) | pHcf_δ11B (±SE) | Light:dark calcification | |
| (total scale) | (total scale) | (total scale) | (total scale) | ||
| P. damicornis | |||||
| pCO2 466 ppm, 28°C | 8.93 ± 0.30 | 8.03 ± 0.34 | 0.9 ± 0.5 | 8.20 ± 0.07 | 19:81 |
| pCO2 925 ppm, 28°C | 8.43 ± 0.22 | 7.92 ± 0.22 | 0.5 ± 0.3 | 8.10 ± 0.08 | 35:65 |
| pCO2 2807 ppm, 28°C | 8.24 ± 0.22 | 7.39 ± 0.17 | 0.9 ± 0.3 | 8.23 ± 0.02 | 99:01 |
| S. pistillata | |||||
| pCO2 466 ppm, 28°C | 8.86 ± 0.07 | 7.80 ± 0.30 | 1.1 ± 0.6 | 8.29 ± 0.12 | 50:50 |
| pCO2 925 ppm, 28°C | 8.53 ± 0.15 | 7.86 ± 0.28 | 0.6 ± 0.3 | 8.28 ± 0.02 | 63:37 |
| pCO2 2807 ppm, 28°C | 8.20 ± 0.23 | 7.65 ± 0.14 | 0.6 ± 0.3 | 8.20 ± 0.01 | 100:00* |
| pCO2 499 ppm, 31°C | 8.71 ± 0.12 | 7.96 ± 0.24 | 0.9 ± 0.3 | 8.16 ± 0.05 | 27:73 |
| pCO2 885 ppm, 31°C | 8.03 ± 0.14 | 6.95 ± 0.23 | 0.9 ± 0.3 | 8.04 ± 0.10 | 99:01 |
| pCO2 3194 ppm, 31°C | 8.01 ± 0.09 | 7.06 ± 0.08 | 0.9 ± 0.1 | 8.14 ± 0.11 | 100:00* |
pHcf_light, pHcf measured from microelectrode when the coral was in the light; uncertainty is reported as 1 SE of the replicates.
pHcf_dark, pHcf measured from microelectrode when the coral was in the dark; uncertainty is reported as 1 SE of the replicates.
ΔpHlight-dark, difference in light and dark pHcf microelectrode measurements pHcf_diurnal: pHcf calculated from δ11B; uncertainty is reported as 1 SE of the replicates.
Light:dark calcification, light:dark calcification ratios obtained from the weighting of pHcf_light and pHcf_dark measurements required to generate the δ11B-based estimates of pHcf (pHcf_δ11B) calculated from average of measured specimens within a treatment.
*When pHcf_diurnal average was > or = pHcf_light but still within errors of each other, we attributed a light:dark calcification of 100:00.
Both species exhibited an increase in pHcf in the light relative to the dark under all treatments, most likely owing to photosynthetic drawdown of DIC leading to elevated solution pHsw—a phenomenon previously observed in zooxanthellate corals by Al-Horani et al. (40). Average pHcf derived from δ11B consistently fell between the averages of pHcf_light (e.g., higher than δ11B estimates of pHcf) and pHcf_dark (e.g., lower than δ11B estimates of pHcf). These findings support the assertion that δ11B is reflecting time-averaged pHcf (possibly diurnal), while microelectrode pHcf captures instantaneous pHcf under whatever irradiance conditions exist at the time of measurement (light or dark). Thus, the discrepancy between these two approaches to estimating pHcf may arise from the fact that the microelectrode approach measures instantaneous pHcf at the particular time that the microelectrode is inserted, while δ11B reflects a time-averaged pHcf.
It is also possible that the δ11B and microelectrode measurements are slightly offset because of differing analytical errors and/or the possibility that the two approaches are sampling slightly different regions of the calcifying fluid. However, the fact that both techniques capture the same general trends in pHcf in response to both pHsw and thermal stress suggests that the observed trends are reliable.
The comparison of both approaches to measuring pHcf yields unique insight into the mechanism of coral biomineralization. One hypothesis arising from the observation in both species is that δ11B-based estimates of pHcf at 28°C are more similar to pHcf_dark than to pHcf_light at the lowest pCO2 treatment and that symbiont photosynthesis is DIC-limited under low CO2/high pHsw conditions, potentially shifting the timing of rapid calcification away from the time of peak photosynthesis when DICcf is lowest. Conversely, δ11B pHcf may be more similar to pHcf_light under higher pCO2 treatments because DIC may no longer be limiting for symbiont photosynthesis under these conditions, meaning that maximum calcification can proceed coevally with maximum photosynthesis without competing for DIC.
CO2-induced fertilization of symbiont photosynthesis links OA to observed trends in coral calcification rate and calcifying fluid chemistry
The mechanism linking the prescribed OA to the observed trends in DICcf, pHcf, Ωcf, and calcification rate may be the CO2-induced fertilization of photosynthesis by the coral’s algal symbionts [e.g., (55, 56)]. Under conditions of OA, the photosynthetic activity of the coral’s algal symbionts increases [e.g., (3, 57, 63, 64)] up to the point that CO2 is no longer limiting for the algae. This should increase the energy available to the coral host for physiological processes, including maintenance of the elevated pHcf with respect to acidified seawater via proton pumping [through increased production of adenosine 5′-triphosphate (ATP)], while simultaneously increasing the coral’s metabolic supply of DIC to its calcifying fluid (via increased respiration of translocated photosynthate)—which are both supportive of calcification by increasing Ωcf. Increased DIC in the external seawater under high CO2 may also propagate directly into the coral calcifying fluid, contributing to the higher DICcf observed in the present study.
The CO2-induced fertilization of symbiont photosynthesis seems to be the most parsimonious mechanism to explain the observed link between the prescribed CO2-induced acidification and the resulting increase in DICcf, stable pHcf, and Ωcf, and positive net calcification rate. The one treatment for which these relationships were not observed was P. damicornis at 31°C, for which pHcf declined and DICcf remained constant with decreasing pHsw, and net calcification rate was negative (i.e., net dissolution) under all pCO2 treatments. P. damicornis specimens exhibited severe bleaching under these conditions—up to 60% more than S. pistillata at 31°C. This increased bleaching (i.e., reduction in symbiont density) would have severely limited the benefits arising from the CO2-induced fertilization of symbiont photosynthesis, which may be at least partly responsible for the decline in pHcf with decreasing pHsw (i.e., in the absence of symbionts, the corals lacked the energy to control calcifying fluid chemistry) and the net dissolution exhibited by the P. damicornis specimens under all pCO2 treatments at 31°C. Thus, when the symbionts were removed from the P. damicornis host via thermally induced bleaching, this species was no longer able to maintain constant pHcf and Ωcf across pCO2 treatments, causing calcification rates to become negative (i.e., net dissolution). The impacts of the increased bleaching of P. damicornis relative to S. pistillata are also consistent with the increased offset in pHcf, DICcf, and net calcification rate between the two temperature treatments (i.e., because of increased bleaching in the higher temperature treatments) at equivalent pCO2 for P. damicornis, compared to S. pistillata (Figs. 1 to 3).
However, our hypothesis that CO2-induced fertilization of photosynthesis is the cause of the trends observed in the present study is challenged by the lack of direct measurements of symbiont photosynthesis by reports of recent studies that elevated pCO2 can have a null or even negative effects on symbiont photosynthesis (4, 65). An additional and potentially complementary explanation for the observed trends is that environmental stress impairs coral calcification and control over calcifying chemistry by increasing paracellular permeability (leakiness), as recently observed by Venn et al. (66) for S. pistillata exposed to OA. Although increased paracellular permeability in corals has not yet been observed in response to thermal stress, the results of the present study suggest that thermal stress may exacerbate any increased paracellular permeability associated with OA.
The results of the present study suggest that some species of zooxanthellate corals are able to cope with the deleterious impacts of moderate OA by way of the increased energy acquired through CO2-induced fertilization of their algal symbionts. However, once the coral-symbiont relationship breaks down as a result of thermally induced bleaching, P. damicornis appears to lose this built-in resilience to OA. These results are consistent with the assertion that thermally induced bleaching is the mechanism behind the strongly negative interaction between the impacts of thermal stress and OA on coral calcification. Although it is possible that these trends are driven by thermal impairment of enzymes used in calcification and/or photosynthesis, such as CA, the observation that P. damicornis exhibited greater bleaching than S. pistillata at 31°C and also exhibited calcifying fluid chemistry and calcification rates that were more negatively affected than those of S. pistillata (Figs. 1 and 3) suggests that thermally induced bleaching is the primary driver behind the negative interaction between thermal stress and OA on coral calcification.
Implications for models of scleractinian coral calcification
Mechanisms of coral biomineralization have been interrogated with a wide array of approaches, including isotopic and elemental geochemistry of the coral skeleton (9), cellular biological approaches, histology, ion-sensitive microelectrodes and dyes, and physical/mathematical modeling (7, 24, 39, 40). Many of these studies address the role that pHsw and carbonate chemistry of the calcifying fluid play in coral biomineralization, while others address the roles that organic molecules and tissues play in the nucleation and growth of the aragonite crystals that comprise the coral skeleton (20, 22, 67). Compensatory responses to OA involve not only pHcf regulation but also differential expression of genes and proteins involved in biomineralization, including enzymes such as CA [e.g., (21)].
Studies differ in the emphasis placed on the roles that regulation of calcification site pHcf and carbonate chemistry play in the coral calcification response to environmental change. We use two independent recorders of pHcf: the microelectrode approach that records instantaneous pHcf in the bulk calcifying fluid and skeletal boron geochemistry (δ11B and B/Ca) that records a time-integrated signal of pHcf and carbonate chemistry at the site of crystal formation. These two techniques both reveal a loss of control over coral pHcf under thermal stress and yield estimates of pHcf that are elevated relative to pHsw and intracellular pH. Differences in absolute pHcf estimates between the microelectrode and δ11B techniques under controlled experimental conditions may be most easily explained as reflecting differences in the time scales that these techniques record pHcf, but these differences may also reflect processes of coral biomineralization that are not yet fully constrained, such as maintenance of pH gradients within the calcifying fluid. The boron geochemical and microelectrode data agree that the coral species studied exert less control over pHcf under thermal stress and that this loss of control over calcifying fluid chemistry is strongly associated with decreased rate calcification. These results identify a previously unreported mechanism by which thermal and acidification stressors interact to impair scleractinian coral calcification (i.e., thermal stress reduces coral control over calcifying fluid carbonate chemistry, compromising coral ability to mitigate impacts of acidification) and confirm the role that coral control over calcifying fluid chemistry plays in the coral calcification response to OA.
The following conclusions can be drawn from the present study:
1) Specimens of P. damicornis and S. pistillata exhibit stable or increasing pHcf, DICcf, Ωcf, and net calcification with decreasing pHsw under the corals’ optimal temperature conditions (28°C). However, under conditions of thermal stress (31°C), P. damicornis (but not S. pistillata) exhibits an inversion of these trends—i.e., decreasing pHcf and DICcf with decreasing pHsw combined with net skeletal dissolution under all pHsw treatments. The observation that P. damicornis also exhibited more intense bleaching under the high-temperature treatment than S. pistillata suggests that the coral’s loss of symbionts caused the observed inversion of these trends—lowering its resilience to OA.
2) Specimens of P. damicornis and S. pistillata exhibited significant decreases in pHcf, DICcf, and Ωcf in response to thermal stress, when pCO2 was held constant. These are the first controlled laboratory experiments to reveal that thermal stress, in addition to OA, renders the coral calcifying fluid less supportive of calcification.
3) Collectively, these results suggest that corals that retain their symbionts are able to cope with moderate OA via CO2-induced fertilization of photosynthesis, which should increase the energy available to the coral host for pHcf elevation via proton pumping (via increased production of ATP), while simultaneously increasing the coral’s metabolic supply of DIC to its calcifying fluid (via increased respiration of translocated photosynthate), which leads to stable or increased Ωcf and positive rates of calcification. These results are consistent with the assertion that thermally induced bleaching is the mechanism behind the strongly negative interaction between the impacts of thermal stress and OA on coral calcification.
4) A novel comparison of the δ11B-based and microelectrode-based approaches to measuring pHcf reveal that these measurements are correlated but that δ11B estimates are consistently offset from the microelectrode estimates of pHcf. This may be explained by the fact that the microelectrode approach measures pHcf at an instant in time—i.e., under whatever light condition was deployed at the time of microelectrode deployment—while the δ11B approach records a time-integrated estimate of pHcf that may represent an average of light and dark pHcf. Nevertheless, both techniques support the assertions that corals elevate pHcf relative to pHsw under ambient and elevated pCO2 and exhibit less control over pHcf under thermal stress.
5) Correlation between coral control over calcifying fluid chemistry and net calcification responses to warming and acidification reaffirms the importance of calcifying fluid chemistry in the response of scleractinian corals to global change stressors.
MATERIALS AND METHODS
Experimental design
P. damicornis and S. pistillata are pocilloporid corals that host zooxanthellae dinoflagellates of the family Symbiodiniaceae (68). Specimens of P. damicornis and S. pistillata were obtained from De Jong Marinelife and originally collected from multiple colonies in the Fiji Islands. Seasonal temperatures in the Fiji Islands range from 25° to 29°C, with an annual average temperature of 27°C, a pCO2 of 360 ppmv (parts per million volume), and a pHsw of 8.08 (69). Experiments used three pCO2 regimes (466/499 ppm, 925/885 ppm, and 2807/3194 ppm) crossed with two temperatures (28° and 31°C), yielding six treatments in total that were each replicated in four independent tanks. Although it is unlikely that coral reefs will experience pCO2 levels as high as the highest pCO2 treatments used in the present study, these treatments were used to elicit a strong response in the corals to elucidate the physiological mechanism(s) underlying corals’ response to OA.
Natural seawater, originally collected from Spitsbergen, Norway, was continuously added to the experimental flow-through aquarium system at a rate of 0.6 liters/hour to prevent material depletion of alkalinity, [Ca2+], or other seawater constituents throughout the duration of the study. Aquaria were illuminated at 150 lux with actinic blue and white lights on a 12-hour on/off cycle. Fragments of both species (S. pistillata = 65 and P. damicornis = 63) were mounted onto eggcrate stands labeled with a unique identifier and split evenly among experimental treatments. Corals were fed 1-day-old Artemia salina nauplii hatched from ~40 mg of eggs. Ten milliliters of concentrated live nauplii was introduced to each replicate tank every second day. Ten-liter replicate acrylic tanks were fed by 244-liter recirculating sumps containing protein skimmers, mechanical filters, and activated charcoal filters.
The experiment was conducted over an 8-week interval, which was preceded by 3 weeks of acclimation to laboratory and experimental conditions. The corals were maintained at control conditions during the first week of the acclimation, were exposed to gradually increasing pCO2 and temperature until reaching target conditions during the second week, and were maintained at target conditions during the final week. Coral skeletons were marked with 3.2 g/liter-seawater calcein dye for 5 days before initial buoyant weighing to facilitate identification and extraction of new skeletal material that was produced exclusively under the experimental conditions. The calcein marker was imaged with a Nikon AZ100 stereomicroscope with a Nikon 96320 fluorescence filter cube. Measurements of pHcf were performed with pH-sensitive liquid-ion-exchanger (LIX) microsensors after 30 days of exposure to experimental conditions. As with any controlled laboratory experiment on corals, it is possible that the duration of exposure influenced the results, as corals may function normally over short time frames, but exhibit impaired function over longer time frames, or vice versa.
Seawater chemistry manipulation and measurement
The pCO2 of the experimental tanks was maintained by vigorously bubbling mixtures of CO2-free air and CO2 into the 244-liter treatment sumps with microporous sparging tubes. The pCO2 of the bubbled gases was achieved by mixing compressed CO2-free air and compressed CO2 with solenoid-valve mass flow controllers at flow rates proportional to the target pCO2 conditions. Temperature (±SE) was maintained at 28 (±0.02)°C and 31 (±0.06)°C using 125-W aquarium heaters (EHEIM) controlled with a programmable thermostat.
Temperature, pH, and salinity of all replicate tanks were measured three times per week using a multielectrode probe (WTW Multi 3430 Set K). The pH electrode was calibrated before use at each treatment temperature with pH 4.00 and 7.00 National Bureau of Standards (NBS) buffers. The conductivity (salinity) probe was calibrated at each treatment temperature with certified seawater reference material of 33.347 salinity (Dickson CRM batch #154). Samples for the analysis of DIC and total alkalinity (TA) were collected weekly at midday. Samples for the analysis of DIC and TA were collected in 25-ml borosilicate glass vials sealed with rubber septae and in 50-ml polypropylene centrifuge tubes, respectively. After collection, DIC and TA samples were poisoned with 10 and 20 μM, respectively, of saturated HgCl2 solution and refrigerated until analysis. TA was determined by open-cell potentiometric Gran titration (precision, 10 μmol/kg) and DIC was determined by coulometry using a Shimadzu DIC analyzer (precision, 10 μmol/kg), with both analytical systems calibrated with certified seawater reference material (Dickson CRM batch #154). pHsw (total scale) was calculated on the basis of direct measurements of pH (NBS); results fell within 0.1 pH unit of pHsw calculated from TA and DIC. Seawater pCO2, carbonate ion concentration ([CO32−]), bicarbonate ion concentration ([HCO3−]), aqueous CO2, and aragonite saturation state (Ω) were calculated from measured TA and DIC with the program CO2SYS, using Roy et al. (70) values for the K1 and K2 carbonic acid constants, the Mucci (71) value for the stoichiometric aragonite solubility product, and an atmospheric pressure of 1.015 atm (Table 1).
Seawater chemistry data are summarized in Tables 1 to 3, table S5, Figs. 1 and 2, and figs. S4 and S5. All data presented in those plots were tested for outliers using an extreme studentized deviate method in GraphPad software (72).
Microelectrode pHcf measurements and calcification rate
pHcf of the corals was measured with proton-sensitive LIX microelectrodes using the technique described in (7, 73) (see the Supplementary Materials).
Measurement of coral calcification rate
Calcification rates were obtained using the buoyant weight technique (74) (see the Supplementary Materials).
Geochemical analyses
Sample cleaning was performed following Guillermic et al. (75). A double oxidative step was performed using H2O2 because of high organic matter of the coral skeletal samples. A weak acid leach was also performed using 0.001 N HCl. Boron was purified via microdistillation (75). Boron isotope analyses were carried out on a Thermo Fisher Scientific Neptune Plus multicollector inductively coupled plasma mass spectrometry (MC-ICPMS) at the University of Cambridge maintained at 1013 ohm resistance. Seawater samples were prepared using column chemistry and analyzed at the Pôle Spectrométrie Océan (PSO), Plouzané.
The B concentration of skeletal samples analyzed for δ11B ranged from 10 parts per billion (ppb) B (~5 ng B) to 20 ppb B (~10 ng B) samples. Sensitivity was 8 mV/ppb B (e.g., 80 mV for 10 ppb B) in wet plasma at a sample aspiration rate of 50 μl/min. Procedural boron blanks ranged from 15 to 65 pg B (contributing less than 1% of the sample signal). The 11B of the acid blank was measured at 1 mV, contributing less than 1% of the sample signal. No memory effect was observed within or across sessions. External reproducibility was ensured by repeated measurements of carbonate standard [JCP-1 (76) and NEP, see the Supplementary Materials] microdistilled at the same time as the samples. The δ11B composition of the NEP standard (δ11B NEP) was measured at 25.71 ± 0.79 per mil (‰) (2 SD, n = 22) over eight analytical sessions, with each number representing an ab initio processed sample from the present study, which are within error of the published value for the standard of 25.80 ± 0.89‰ (35). The δ11B composition of the JCP-1 standard was measured at 24.06 ± 0.19‰ (2 SD, n = 6) over six analytical sessions, with each number representing an ab initio processed sample from the present study, which was within error of the published values of 24.37 ± 0.32‰ (77) and 24.42 ± 0.28‰ (table S3) (35).
The δ11B analyses for S. pistillata were also measured independently at the Alfred Wegener Institute for Polar Studies (AWI) in Bremerhaven, Germany. Boron in the dissolved samples was separated from the matrix using the microdistillation technique and analyzed at a B concentration of ~3 to 4 ppb with a Nu Plasma II MC-ICPMS using secondary electron multipliers (SEMs), where high-mass ion counter 5 (IC5) was used for 11B and IC0 for 10B. Sample measurements were bracketed by NBS951 measurements and accompanied by regular analyses of the control standard AE121 (δ11B = 19.82 ± 0.3‰).
Elemental ratio measurements
Elemental ratios were measured on a Thermo Fisher Scientific Element XR HR-ICP-MS at the PSO, Ifremer (Plouzané, France) after Ca analyses on an ICP-AES Ultima 2 HORIBA at the PSO (Plouzané, France) (75). Data quality and external reproducibility were maintained by the repeated measurement of an internal consistency standard “CamWuellestorfi” [courtesy of the University of Cambridge (78)].
Typical measured concentrations of procedural blanks for the trace element analyses for sessions in which samples were diluted to 30 ppm Ca were 7Li < 3%, 11B < 4%, 25Mg < 0.1%, 87Sr < 0.1%, and 43Ca < 0.1%. Typical measured concentrations of blanks for the trace element analyses for sessions in which samples were diluted to 10 ppm Ca were 7Li < 5%, 11B < 6%, 25Mg < 0.3%, 87Sr < 2%, and 43Ca < 0.1%. External reproducibility was quantified via repeated measurement of the consistency standard CamWuellestorfi (table S4). The X/Ca elemental ratio measurements of the external standard CamWuellestorfi were always within error of published values (table S4) (78). Analytical uncertainty of a single measurement was calculated from the repeated measurement of the CamWuellestorfi standard within a given analytical session. The analytical uncertainties on the X/Ca elemental ratios are 0.6 μmol/mol for Li/Ca, 11 μmol/mol for B/Ca, 0.09 mmol/mol for Mg/Ca, and 0.01 mmol/mol for Sr/Ca (2 SD, n = 39).
Calculations of pHcf, [CO32−]cf, and DICcf
The pHcf was calculated from measurements of coral skeletal δ11B following Hemming and Hanson (29)
| (5) |
with pKB*(T, S) representing the dissociation constant, δ11Bsw representing the boron isotopic composition of the culture seawater (measured), δ11Bc representing the boron isotopic composition of the coral skeleton, and ε representing the boron isotopic fractionation between boric acid and borate ion [27.2‰ (79)].
The [CO32−]cf was calculated from coral skeletal B/Ca and a partition coefficient (KD) of 0.00297*exp(−0.0202*[H+]) following McCulloch et al. (10); other partition coefficients were used and are presented in table S1. The DIC and [CO32−] were calculated using the MATLAB code provided in DeCarlo et al. (8), modified for various values of δ11Bsw. The aragonite saturation state from the calcifying fluid (Ωcf, Eq. 3) was calculated using [CO32−]cf, [Ca2+] = 10.5 mmol at 28°C, and 10.3 mmol at 31°C [based on results from (46) on a Porites coral]. K*sp is the stoichiometric solubility product for aragonite, which varies with temperature and salinity.
Supplementary Material
Acknowledgments
Funding: R.A.E. and J.B.R. acknowledge support from National Science Foundation grants OCE-1437166 and OCE-1437371. The work was also supported by the “Laboratoire d’Excellence” LabexMER (ANR-10-LABX-19), cofunded by a grant from the French government under the program “Investissements d’Avenir,” and an IAGC student grant 2017. R.A.E. acknowledges financial and logistical support from the Pritzker Endowment to UCLA IoES, and J.B.R. acknowledges support from the ZMT and the Hanse-Wissenschaftskolleg Fellowship Program and the NSF OCE award #1437371. We thank C. Bouniol for assistance with sample preparation and Y. Germain and C. Liorzou for technical assistance. We thank the staff of the ZMT experimental aquaria facility for their support, without which this study would not have been possible. We thank J. Sutton and J. Drake for providing comments on a draft of this manuscript. Author contributions: R.A.E. and J.B.R. conceived the project and directed the research. L.P.C. and J.B.R. performed culturing experiments, specimen characterization, and pH microsensor experiments with input from D.d.B., C.E.R., H.W., and R.A.E. M.G., I.D.C., and S.M. performed isotope and trace element analyses at the University of Cambridge and the University of Brest, and J.B. oversaw isotope analyses at Bremerhaven. M.G., S.M., R.A.E., J.B., and J.B.R. analyzed and interpreted the geochemical data. M.G. wrote the manuscript with input from R.A.E. and J.B.R. All authors read and edited the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/2/eaba9958/DC1
REFERENCES AND NOTES
- 1.Hughes T. P., Anderson K. D., Connolly S. R., Heron S. F., Kerry J. T., Lough J. M., Baird A. H., Baum J. K., Berumen M. L., Bridge T. C., Claar D. C., Eakin C. M., Gilmour J. P., Graham N. A. J., Harrison H., Hobbs J. P. A., Hoey A. S., Hoogenboom M., Lowe R. J., McCulloch M. T., Pandolfi J. M., Pratchett M., Schoepf V. G., Torda, Wilson S. K., Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–83 (2018). [DOI] [PubMed] [Google Scholar]
- 2.Baker A. C., Glynn P. W., Riegl B., Climate change and coral reef bleaching: An ecological assessment of long-term impacts, recovery trends and future outlook. Estuar. Coast. Shelf Sci. 80, 435–471 (2008). [Google Scholar]
- 3.Reynaud S., Leclercq N., Romaine-Lioud S., Ferrier-Pagés C., Jaubert J., Gattuso J. P., Interacting effects of CO2 partial pressure and temperature on photosynthesis and calcification in a scleractinian coral. Glob. Chang. Biol. 9, 1660–1668 (2003). [Google Scholar]
- 4.Anthony K. R. N., Kline D. I., Diaz-Pulido G., Dove S., Hoegh-Guldberg O., Ocean acidification causes bleaching and productivity loss in coral reef builders. Proc. Natl. Acad. Sci. U.S.A. 105, 17442–17446 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodolfo-Metalpa R., Houlbrèque F., Tambutté É., Boisson F., Baggini C., Patti F. P., Jeffree R., Fine M., Foggo A., Gattuso J.-P., Hall-Spencer J. M., Coral and mollusc resistance to ocean acidification adversely affected by warming. Nat. Clim. Change 1, 308–312 (2011). [Google Scholar]
- 6.Cohen A. L., McConnaughey T. A., Geochemical perspectives on coral mineralization. Rev. Mineral. Geochem. 54, 151–187 (2003). [Google Scholar]
- 7.Cameron L. P., Reymond C., Bijma J., Büscher J., De Beer D., Eagle R. T., Symbiont-assisted calcifying fluid pH elevation aids coral resilience to ocean acidification. In review (2020). [Google Scholar]
- 8.DeCarlo T. M., Holcomb M., McCulloch M. T., Reviews and syntheses: Revisiting the boron systematics of aragonite and their application to coral calcification. Biogeosciences 15, 2819–2834 (2018b). [Google Scholar]
- 9.Comeau S., Cornwall C. E., McCulloch M. T., Decoupling between the response of coral calcifying fluid pH and calcification to ocean acidification. Sci. Rep. 7, 7573 (2017a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McCulloch M. T., D’Olivo J. P., Falter J., Holcomb M., Trotter J. A., Coral calcification in a changing world and the interactive dynamics of pH and DIC upregulation. Nat. Commun. 8, 15686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.IPCC: Climate Change 2014—The Physical Science Basis, Intergovernmental Panel on Climate Change, Ed., (Cambridge Univ. Press, 2014). [Google Scholar]
- 12.Allemand D., Ferrier-Pagès C., Furla P., Houlbrèque F., Puverel S., Reynaud S., Tambutté É., Tambutté S., Zoccola D., Biomineralisation in reef-building corals: From molecular mechanisms to environmental control. Comptes Rendus Palevol 3, 453–467 (2004). [Google Scholar]
- 13.Erez J., The source of ions for biomineralization in foraminifera and their implications for paleoceanographic proxies. Rev. Mineral. Geochemistry 54, 115–149 (2003). [Google Scholar]
- 14.Ries J. B., A physicochemical framework for interpreting the biological calcification response to CO2-induced ocean acidification. Geochim. Cosmochim. Acta 75, 4053–4064 (2011). [Google Scholar]
- 15.McConnaughey T. A., Falk R. H., Calcium-proton exchange during algal calcification. Biol. Bull. 180, 185–195 (1991). [DOI] [PubMed] [Google Scholar]
- 16.Furla P., Galgani I., Durand I., Allemand D., Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445–3457 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Zoccola D., Ganot P., Bertucci A., Caminiti-Segonds N., Techer N., Voolstra C. R., Aranda M., Tambutté E., Allemand D., Casey J. R., Tambutté S., Bicarbonate transporters in corals point towards a key step in the evolution of cnidarian calcification. Sci. Rep. 5, 9983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moya A., Tambutté S., Bertucci A., Tambutté E., Lotto S., Vullo D., Supuran C. T., Allemand D., Zoccola D., Carbonic anhydrase in the scleractinian coral Stylophora pistillata: Characterization, localization, and role in biomineralization. J. Biol. Chem. 283, 25475–25484 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Allison N., Cohen I., Finch A. A., Erez J., Tudhope A. W., Corals concentrate dissolved inorganic carbon to facilitate calcification. Nat. Commun. 5, 5741 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Von Euw S., Zhang Q., Manichev V., Murali N., Gross J., Feldman L. C., Gustafsson T., Flach C., Mendelsohn R., Falkowski P. G., Biological control of aragonite formation in stony corals. Science 356, 933–938 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Drake J. L., Schaller M. F., Mass T., Godfrey L., Fu A., Sherrell R. M., Rosenthal Y., Falkowski P. G., Molecular and geochemical perspectives on the influence of CO2 on calcification in coral cell cultures. Limnol. Oceanogr. 63, 107–121 (2018). [Google Scholar]
- 22.Mass T., Giuffre A. J., Sun C.-Y., Stifler C. A., Frazier M. J., Neder M., Tamura N., Stad C. V., Marcus M. A., Gilbert P. U. P. A., Amorphous calcium carbonate particles form coral skeletons. Proc. Natl. Acad. Sci. U.S.A. 114, E7670–E7678 (2017b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akiva A., Neder M., Kahil K., Gavriel R., Pinkas I., Goobes G., Mass T., Minerals in the pre-settled coral Stylophora pistillata crystallize via protein and ion changes. Nat. Commun. 9, 1880 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venn A., Tambutté E., Holcomb M., Allemand D., Tambutté S., Live tissue imaging shows reef corals elevate pH under their calcifying tissue relative to seawater. PLOS ONE 6, e20013 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giuffre A. J., Gagnon A. C., De Yoreo J. J., Dove P. M., Isotopic tracer evidence for the amorphous calcium carbonate to calcite transformation by dissolution–reprecipitation. Geochim. Cosmochim. Acta 165, 407–417 (2015). [Google Scholar]
- 26.DeCarlo T. M., Characterizing coral skeleton mineralogy with Raman spectroscopy. Nat. Commun. 9, 5325 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiva A., Neder M., Kahil K., Gavriel R., Pinkas I., Goobes G., Mass T., Reply to: Characterizing coral skeleton mineralogy with Raman spectroscopy. Nat. Commun. 9, 5324 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans D., Webb P. B., Penkman K., Kröger R., Allison N., The characteristics and biological relevance of inorganic amorphous calcium carbonate (ACC) precipitated from seawater. Cryst. Growth Des. 19, 4300–4313 (2019). [Google Scholar]
- 29.Hemming N. G., Hanson G. N., Boron isotopic composition and concentration in modern marine carbonates. Geochim. Cosmochim. Acta 56, 537–543 (1992). [Google Scholar]
- 30.Mavromatis V., Montouillout V., Noireaux J., Gaillardet J., Schott J., Characterization of boron incorporation and speciation in calcite and aragonite from co-precipitation experiments under controlled pH, temperature and precipitation rate. Geochim. Cosmochim. Acta 150, 299–313 (2015). [Google Scholar]
- 31.Noireaux J., Mavromatis V., Gaillardet J., Schott J., Montouillout V., Louvat P., Rollion-Bard C., Neuville D. R., Crystallographic control on the boron isotope paleo-pH proxy. Earth Planet. Sci. Lett. 430, 398–407 (2015). [Google Scholar]
- 32.Sevilgen D. S., Venn A. A., Hu M. Y., Tambutté E., de Beer D., Planas-Bielsa V., Tambutté S., Full in vivo characterization of carbonate chemistry at the site of calcification in corals. Sci. Adv. 5, eaau7447 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Holcomb M., DeCarlo T. M., Gaetani G. A., McCulloch M., Factors affecting B/Ca ratios in synthetic aragonite. Chem. Geol. 437, 67–76 (2016). [Google Scholar]
- 34.Allison N., Reconstructing coral calcification fluid dissolved inorganic carbon chemistry from skeletal boron: An exploration of potential controls on coral aragonite B/Ca. Heliyon 3, e00387 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutton J. N., Liu Y.-W., Ries J. B., Guillermic M., Ponzevera E., Eagle R. A., δ11B as monitor of calcification site pH in divergent marine calcifying organisms. Biogeosciences 15, 1447–1467 (2018). [Google Scholar]
- 36.Liu Y. W., Sutton J. N., Ries J. B., Eagle R. A., Regulation of calcification site pH is a polyphyletic but not always governing response to ocean acidification. Sci. Adv. 6, eaax1314 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Venn A. A., Tambutté E., Caminiti-Segonds N., Techer N., Allemand D., Tambutté S., Effects of light and darkness on pH regulation in three coral species exposed to seawater acidification. Sci. Rep. 9, 2201 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCulloch M. T., D’Olivo J. P., Falter J., Georgiou L., Holcomb M., Montagna P., Trotter J. A., Boron isotopic systematics in scleractinian corals and the role of pH up-regulation. Boron Isotopes , 145–162 (2018). [Google Scholar]
- 39.Venn A. A., Tambutte E., Holcomb M., Laurent J., Allemand D., Tambutte S., Impact of seawater acidification on pH at the tissue–skeleton interface and calcification in reef corals. Proc. Natl. Acad. Sci. U.S.A. 110, 1634–1639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Al-Horani F. A., Al-Moghrabi S. M., de Beer D., The mechanism of calcification and its relation to photosynthesis and respiration in the scleractinian coral Galaxea fascicularis. Mar. Biol. 142, 419–426 (2003). [Google Scholar]
- 41.Cai W.-J., Ma Y., Hopkinson B. M., Grottoli A. G., Warner M. E., Ding Q., Hu X., Yuan X., Schoepf V., Xu H., Han C., Melman T. F., Hoadley K. D., Pettay D. T., Matsui Y., Baumann J. H., Levas S., Ying Y., Wang Y., Microelectrode characterization of coral daytime interior pH and carbonate chemistry. Nat. Commun. 7, 11144 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Comeau S., Tambutté E., Carpenter R. C., Edmunds P. J., Evensen N. R., Allemand D., Ferrier-Pagès C., Tambutté S., Venn A. A., Coral calcifying fluid pH is modulated by seawater carbonate chemistry not solely seawater pH. Proc. R. Soc. B 284, 20161669 (2017b). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ross C. L., Falter J. L., McCulloch M. T., Active modulation of the calcifying fluid carbonate chemistry (δ11B, B/Ca) and seasonally invariant coral calcification at sub-tropical limits. Sci. Rep. 7, 13830 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Glynn P. W., Coral reef bleaching: Ecological perspectives. Coral Reefs 12, 1–17 (1993). [Google Scholar]
- 45.Berkelmans R., Oliver J. K., Large-scale bleaching of corals on the Great Barrier Reef. Coral reefs 18, 55–60 (1999). [Google Scholar]
- 46.D’Olivo J. P., McCulloch M. T., Response of coral calcification and calcifying fluid composition to thermally induced bleaching stress. Sci. Rep. 7, 2207 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.D’Olivo J. P., Ellwood G., DeCarlo T. M., McCulloch M. T., Deconvolving the long-term impacts of ocean acidification and warming on coral biomineralisation. Earth Planet. Sci. Lett. 526, 115785 (2019). [Google Scholar]
- 48.Hoegh-Guldberg O., Smith G. J., The effect of sudden changes in temperature, light and salinity on the population density and export of zooxanthellae from the reef corals Stylophora pistillata Esper and Seriatopora hystrix Dana. J. Exp. Mar. Biol. Ecol. 129, 279–303 (1989). [Google Scholar]
- 49.Bove C. B., Ries J. B., Davies S. W., Westfield I. T., Umbanhowar J., Castillo K. D., Common Caribbean corals exhibit highly variable responses to future acidification and warming. Proc. R. Soc. B. 286, 20182840 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loya Y., Sakai K., Yamazato K., Nakano Y., Sambali H., van Woesik R., Coral bleaching: The winners and the losers. Ecol. Lett. 4, 122–131 (2001). [Google Scholar]
- 51.Gattuso J. P., Allemand D., Frankignoulle M., Photosynthesis and calcification at cellular, organismal and community levels in coral reefs: A review on interactions and control by carbonate chemistry. Am. Zool. 39, 160–183 (1999). [Google Scholar]
- 52.Silbiger N. J., Goodbody-Gringley G., Bruno J. F., Putnam H. M., Comparative thermal performance of the reef-building coral Orbicella franksi at its latitudinal range limits. Mar. Biol. 166, 126 (2019). [Google Scholar]
- 53.Rowan R., Thermal adaptation in reef coral symbionts. Nature 430, 742–742 (2004). [DOI] [PubMed] [Google Scholar]
- 54.Clausen C. D., Roth A. A., Effect of temperature and temperature adaptation on calcification rate in the hermatypic coral Pocillopora damicornis. Mar. Biol. 33, 93–100 (1975). [Google Scholar]
- 55.Ries J. B., Cohen A. L., McCorkle D. C., Marine calcifiers exhibit mixed responses to CO2-induced ocean acidification. Geology 37, 1131–1134 (2009). [Google Scholar]
- 56.Castillo K. D., Ries J. B., Bruno J. F., Westfield I. T., The reef-building coral Siderastrea siderea exhibits parabolic responses to ocean acidification and warming. Proc. R. Soc. B 281, 20141856 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krief S., Hendy E. J., Fine M., Yam R., Meibom A., Foster G. L., Shemesh A., Physiological and isotopic responses of scleractinian corals to ocean acidification. Geochim. Cosmochim. Acta 74, 4988–5001 (2010). [Google Scholar]
- 58.McLachlan R. H., Price J. T., Solomon S. L., Grottoli A. G., Thirty years of coral heat-stress experiments: A review of methods. Coral Reefs 39, 885–902 (2020). [Google Scholar]
- 59.S. Tambutté, E. Tambutté, D. Zoccola, D. Allemand, Organic matrix and biomineralization of scleractinian corals, in Handbook of Biomineralization: Biological Aspects and Structure Formation (Wiley, 2007), pp. 243–259. [Google Scholar]
- 60.DeCarlo T. M., Comeau S., Cornwall C. E., McCulloch M. T., Coral resistance to ocean acidification linked to increased calcium at the site of calcification. Proc. R. Soc. B Biol. Sci. 285, 20180564 (2018a). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sun W., Jayaraman S., Chen W., Persson K. A., Ceder G., Nucleation of metastable aragonite CaCO3 in seawater. Proc. Natl. Acad. Sci. U.S.A. 112, 3199–3204 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holcomb M., Venn A. A., Tambutté E., Tambutté S., Allemand D., Trotter J., McCulloch M., Coral calcifying fluid pH dictates response to ocean acidification. Sci. Rep. 4, 5207 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langdon C., Atkinson M. J., Effect of elevated pCO2 on photosynthesis and calcification of corals and interactions with seasonal change in temperature/ irradiance and nutrient enrichment. J. Geophys. Res. 110, C09S07 (2005). [Google Scholar]
- 64.Noonan S. H. C., Fabricius K. E., Ocean acidification affects productivity but not the severity of thermal bleaching in some tropical corals. ICES J. Mar. Sci. 73, 715–726 (2016). [Google Scholar]
- 65.Wall C. B., Fan T.-Y., Edmunds P. J., Ocean acidification has no effect on thermal bleaching in the coral Seriatopora caliendrum. Coral Reefs 33, 119–130 (2013). [Google Scholar]
- 66.Venn A. A., Bernardet C., Chabenat A., Tambutté E., Tambutté S., Paracellular transport to the coral calcifying medium: Effects of environmental parameters. J. Exp. Biol. 223, jeb227074 (2020). [DOI] [PubMed] [Google Scholar]
- 67.Hohn S., Reymond C., Coral calcification, mucus, and the origin of skeletal organic molecules. Coral Reefs 38, 973–984 (2019). [Google Scholar]
- 68.LaJeunesse T. C., Parkinson J. E., Gabrielson P. W., Jeong H. J., Reimer J. D., Voolstra C. R., Santos S. R., Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 28, 2570–2580.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 69.Key R. M., Kozyr A., Sabine C. L., Lee K., Wanninkhof R., Bullister J. L., Feely R. A., Millero F. J., Mordy C., Peng T.-H., A global ocean carbon climatology: Results from Global Data Analysis Project (GLODAP). Global Biogeochem. Cycles 18, GB4031 (2004). [Google Scholar]
- 70.Roy R. N., Roy L. N., Vogel K. M., Porter-Moore C., Pearson T., Good C. E., Millero F. J., Campbell D. M., The dissociation constants of carbonic acid in seawater at salinities 5 to 45 and temperatures 0 to 45°C. Mar. Chem. 44, 249–267 (1993). [Google Scholar]
- 71.Mucci A., The solubility of calcite and aragonite in seawater at various salinities, temperatures, and one atmosphere total pressure. Am. J. Sci. 283, 780–799 (1983). [Google Scholar]
- 72.Grubbs F. E., Procedures for detecting outlying observations in samples. Dent. Tech. 11, 1–21 (1969). [Google Scholar]
- 73.de Beer D., Glud A., Epping E., Kühl M., A fast-responding CO2 microelectrode for profiling sediments, microbial mats, and biofilms. Limnol. Ocean. 42, 1590–1600 (1997). [Google Scholar]
- 74.Davies P. S., Short-term growth measurements of corals using an accurate buoyant weighing technique. Mar. Biol. 101, 389–395 (1989). [Google Scholar]
- 75.Guillermic M., Misra S., Eagle R., Villa A., Chang F., Tripati A., Seawater pH reconstruction using boron isotopes in multiple planktonic foraminifera species with different depth habitats and their potential to constrain pH and pCO2 gradients. Biogeosciences 17, 3487–3510 (2020). [Google Scholar]
- 76.M., Gutjahr, L., Bordier, E., Douville, J., Farmer, G. L., Foster, E., Hathorne, B. Hönisch, D. Lemarchand, P. Louvat, M. M. Culloch, J. Noireaux, N. Pallavicini, I. Rodushkin, P. Roux, J. Stewart, F. Thil, C.-F. You, Boron Isotope Intercomparison Project (BIIP): Development of a new carbonate standard for stable isotopic analyses, in EGU General Assembly Conference Abstracts (Vol. 16) (2014)
- 77.Holcomb M., DeCarlo T. M., Schoepf V., Dissard D., Tanaka K., McCulloch M., Cleaning and pre-treatment procedures for biogenic and synthetic calcium carbonate powders for determination of elemental and boron isotopic compositions. Chem. Geol. 398, 11–21 (2015). [Google Scholar]
- 78.Misra S., Greaves M., Owen R., Kerr J., Elmore A. C., Elderfield H., Determination of B/Ca of natural carbonates by HR-ICP-MS. Geochem. Geophys. Geosyst. 15, 1617–1628 (2014). [Google Scholar]
- 79.Klochko K., Kaufman A. J., Yao W., Byrne R. H., Tossell J. A., Experimental measurement of boron isotope fractionation in seawater. Earth Planet. Sci. Lett. 248, 261–270 (2006). [Google Scholar]
- 80.Barker S., Greaves M., Elderfield H., A study of cleaning procedures used for foraminiferal Mg/Ca paleothermometry. Geochem. Geophys. Geosyst. 4, 8407 (2003). [Google Scholar]
- 81.E. J. Catanzaro, C. E. Champion, A. L. Garner, G. Marinenko, K. M. Sappenfield, W. R. Shields, Boric Acid; Isotopic and Assay Standard Reference Materials (U.S. Natl. Bur. Stand. Spec., Publ., 1970), pp. 260–17, 70p.
- 82.Nir O., Vengosh A., Harkness J. S., Dwyer G. S., Lahav O., Direct measurement of the boron isotope fractionation factor: Reducing the uncertainty in reconstructing ocean paleo-pH, Earth Planet. Sci. Lett. 414, 1–5 (2015). [Google Scholar]
- 83.Gaillardet J., Lemarchand D., Göpel C., Manhès G., Evaporation and sublimation of boric acid: Application for boron purification from organic rich solutions. Geostand. Newsl. 25, 67–75 (2001). [Google Scholar]
- 84.Wang B.-S., You C.-F., Huang K.-F., Wu S.-F., Aggarwal S. K., Chung C.-H., Lin P.-Y., Direct separation of boron from Na- and Ca-rich matrices by sublimation for stable isotope measurement by MC-ICP-MS. Talanta 82, 1378–1384 (2010). [DOI] [PubMed] [Google Scholar]
- 85.Misra S., Owen R., Kerr J., Greaves M., Elderfield H., Determination of δ11B by HR-ICP-MS from mass limited samples: Application to natural carbonates and water samples. Geochim. Cosmochim. Acta 140, 531–552 (2014b). [Google Scholar]
- 86.J. W. B. Rae, Boron isotopes in Foraminifera: Systematics, biomineralisation, and CO2 reconstruction, in Boron Isotopes. Advances in Isotope Geochemistry, H. Marschall, G. Foster, Eds. (Springer, 2018). [Google Scholar]
- 87.Raitzsch M., Bijma J., Benthien A., Richter K.-U., Steinhoefel G., Kučera M., Boron isotope-based seasonal paleo-pH reconstruction for the Southeast Atlantic – A multispecies approach using habitat preference of planktonic foraminifera. Earth Planet. Sci. Lett. 487, 138–150 (2018). [Google Scholar]
- 88.Lloyd N. S., Sadekov A. Y., Misra S., Application of 1013 ohm Faraday cup current amplifiers for boron isotopic analyses by solution mode and laser ablation multicollector inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 32, 9–18 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/2/eaba9958/DC1


