ABSTRACT
In recent decades, bacteriocins have received substantial attention as antimicrobial compounds. Although bacteriocins have been predominantly exploited as food preservatives, they are now receiving increased attention as potential clinical antimicrobials and as possible immune-modulating agents. Infections caused by antibiotic-resistant bacteria have been declared as a global threat to public health. Bacteriocins represent a potential solution to this worldwide threat due to their broad- or narrow-spectrum activity against antibiotic-resistant bacteria. Notably, despite their role in food safety as natural alternatives to chemical preservatives, nisin remains the only bacteriocin legally approved by regulatory agencies as a food preservative. Moreover, insufficient data on the safety and toxicity of bacteriocins represent a barrier against the more widespread use of bacteriocins by the food and medical industry. Here, we focus on the most recent trends relating to the application of bacteriocins, their toxicity and impacts.
Keywords: antimicrobials, bacteriocins, gastrointestinal bioavailability, toxicity, safety evaluation, regulations
Antimicrobial activity, gastrointestinal bihaviour and toxicity of bacteriocins.
INTRODUCTION
The widespread emergence of antibiotic-resistant bacteria and slowdown in the discovery of new classes of antibiotics are considered as serious public health issues (Baquero and Moreno 1984; Klaenhammer 1988; May 2014; O'Neill 2016). With regard to the former, the overuse of antibiotics over a long period has allowed infectious organisms to adapt to antibiotics and, in turn, reduce their effectiveness. Moreover, the subsequent spread of resistant bacterial strains from person to person or to humans from non-human sources in the environment, such as from livestock or food animal treated with antibiotics similar to those employed to treat human infection, has been a major factor in the expansion of antibiotic resistance (Ansari 2015; CDC 2018). Due to the loss of efficiency of many antibiotics, researchers are under pressure to identify new types of therapeutic molecules and strategies.
From a food quality and safety perspective, the emergence of new pathogens, the diversification of supply sources and new eating habits of consumers who increasingly opt for fresher and ‘more natural’ food products, with no preservatives or salt, are global challenges (Farber 2016). Therefore, to ensure the quality and safety of food products, maintain the competitiveness of farms, and limit antibiotic residues and the spread of resistance genes, it is necessary to find substitutes for chemical additives, salt and antibiotics.
Advances in the identification of bacteriocins and their characterization have prompted an interest in the use of these molecules as either new food additives or therapeutic agents. These antimicrobial peptides or proteins are produced by Gram-positive and Gram-negative bacteria (Duquesne et al. 2007; Drider and Rebuffat 2011; Hammami et al. 2013), and their effectiveness, and that of their producer strains, with regard to inhibiting numerous food spoilage and pathogenic bacteria has been shown in various food matrices, including cheese, meat and vegetables (Gálvez et al. 2011). Moreover, the efficacy of many bacteriocins with the potential to treat human and animal infections has been described (Kim et al. 2010; Van Staden, Brand and Dicks 2012; Campion et al. 2013)
Several bacteriocins have been shown to be effective against many pathogenic bacteria (Oman and van der Donk 2009; Hanchi et al. 2017); however, the most widely employed bacteriocin in the food (Ramu et al. 2015) and veterinary (Pieterse, Todorov and Dicks 2010) industry is nisin. This is partially due to the lack of detailed scientific information regarding the safety of numerous isolated bacteriocins and their impact on animal and human health when used orally. To address this issue, in this review, we have compiled the most recent data available relating to the safety of different bacteriocins.
GENERAL ASPECTS OF BACTERIOCINS: UPDATED CLASSIFICATION
Bacteriocins were first discovered almost 100 years ago and have been found to be produced by many species of bacteria and archaea (Baquero and Moreno 1984; Klaenhammer 1988; Riley and Wertz 2002; Shand and Leyva 2008; Besse et al. 2015). They comprise a heterogeneous family of small ribosomally synthesized proteinaceous molecules with strong antimicrobial activity at precise concentrations (Chikindas et al. 2018). These molecules are mostly synthesized as non-biologically active precursor peptides containing an N-terminal leader sequence (Kanmani et al. 2013). In some cases, these precursors undergo post-translational modifications (PTMs) before cleavage of the leader region and export outside the cell (Mokoena 2017). These antimicrobial peptides have a bacteriostatic or bactericidal spectrum of activity that is mainly directed against bacteria closely related to the producing strain (Hatakka and Saxelin 2008) and in rarer cases against a broader range of unrelated groups of bacteria (Cotter, Ross and Hill 2013). Bacteriocin-producing cells usually set up mechanisms to prevent them from being killed by their own bacteriocins, either by synthesizing self-immunity proteins or using efflux pumps, or using both systems (de Freire Bastos, Coelho and da Silva Santos 2015; Bountra et al. 2017).
An updated classification of bacteriocins
Bacteriocins are abundant and have a large diversity (Cotter, Hill and Ross 2005; Cotter, Ross and Hill 2013; Yang et al. 2014). To capture this diversity, our group developed an integrated open-access database named BACTIBASE (http://bactibase.hammamilab.org), which contains structural and functional information relating to >200 bacteriocins (Hammami et al. 2010). Specific data include sequence and specific properties, such as antimicrobial, physicochemical and structural properties. Different classification systems have been proposed for bacteriocins over years. Indeed, bacteriocins of lactic acid bacteria, of which there are many, alone have been classified in a variety of ways (de Freire Bastos, Coelho and da Silva Santos 2015), leading to two (Cotter, Ross and Hill 2013) to four (Johnson et al. 2018) subclasses. These classification systems have been modified many times based on new developments regarding structures and modes of action of bacteriocins (Lagha et al. 2017). According to the classification outlined by Cotter, Ross and Hill (2013), which has the advantages of being clear and simple and applying to bacteriocins from both Gram-positive and Gram-negative bacteria, bacteriocins were mainly categorized into two fundamental classes based on the presence or not of post-translationally modified motifs. Moreover, this classification considered only antimicrobial peptides and not larger antimicrobial proteins, such as colicins from Escherichia coli. Based on these rules and taking into account recent developments on ribosomally synthesized and post-translationally modified peptides (RiPPs) (Arnison et al. 2013), we propose here an updated classification of bacteriocins from Gram-positive and Gram-negative bacteria in two large classes assembling the modified (class I) and unmodified (class II) bacteriocins (Table S1, Supporting Information). Class I assembles peptides with molecular masses <5 kDa that all contain PTMs ensured by dedicated enzymes encoded in the bacteriocin gene cluster. These class I bacteriocins thus belong to the family of RiPPs. Class II bacteriocins are essentially unmodified peptides of 6–10 kDa and including or not stabilizing disulfide bridges. The modifications make generally class I bacteriocins more stable to high temperatures, extreme pHs or proteolytic enzymes than class II ones, although disulfide bridges also increase the class II peptide stability. Class I is further subdivided following the RiPP nomenclature (Arnison et al. 2013). It includes lanthipeptides, sactipeptides, circular peptides and glycocins from Gram-positive bacteria (Cotter, Ross and Hill 2013), linear azole(ine)-containing peptides (LAP) and lasso peptides from both Gram-positive and Gram-negative bacteria, and nucleotide peptides and siderophore peptides from Gram-negative ones (Duquesne et al. 2007; Cotter, Ross and Hill 2013; Gabrielsen et al. 2014; Norris and Patchett 2016). In addition, linaridins (Claesen and Bibb 2010) and thiopeptides (Bagley et al. 2005) essentially produced by Actinobacteria, and cyanobactins from diverse cyanobacteria (Jaspars 2014; Martins and Vasconcelos 2015; Martins et al. 2018), belong to this class. Lantibiotics (the term frequently used to qualify antimicrobial lanthipeptides) have been extensively studied for many years. They consist of 19 to >50 amino acids (Jack, Tagg and Ray 1995; McAuliffe, Ross and Hill 2001; Willey and Van Der Donk 2007) and are characterized by the presence of unusual amino acids, named lanthionine and β-methyllanthionine, which result from dehydration of serines and threonines yielding didehydroalanine and didehydrobutyrine residues, respectively. They are stabilized by thioether linkages established between two β-carbons (Willey and Van Der Donk 2007). The stabilization of sactibiotics distinguishes by the presence of intramolecular sulfur to α-carbon linkages involving a cysteine and another residue instead of the β-carbon thioether linkages that are signature of lanthipeptides. For their part, class II bacteriocins are further subdivided into three classes: pediocin-like bacteriocins that contain the YGNGV consensus sequence, non-pediocin-like bacteriocins that are devoid of this characteristic motif and two-peptide bacteriocins.
APPLICATIONS
Since their discovery, bacteriocins have been established as promising antimicrobial compounds with potential applications in the food, health and veterinary sectors. These aspects will be reviewed in the following sections.
Food industry
As natural antimicrobial agents, bacteriocins are an attractive alternative to chemical preservatives when it comes to satisfying the increasing consumer demands for safe and ready-to-eat foods (Gálvez et al. 2007) with minimum processing (Abbasiliasi et al. 2017). Since bacteriocins are colorless, odorless and tasteless (Perez, Zendo and Sonomoto 2014), they can be incorporated in food products without changing their organoleptic properties. Additionally, several bacteriocins are stable at low pH, high temperature (Yang et al. 2018) and across a broad range of salt concentrations (Wilaipun et al. 2004; Fatima and Mebrouk 2013). The use of bacteriocins as food preservatives offers several advantages: They (i) extend shelf life of foods, (ii) provide extra protection during temperature abuse conditions and at other critical control points, (iii) decrease the risk of transmission of foodborne pathogens through the food chain, (iv) reduce the economic losses due to food spoilage, recalls or outbreaks, and (v) allow the application of less severe treatments during food processing without compromising food safety, which results in better preservation of nutrients, vitamins and organoleptic properties of food products (Thomas, Clarkson and Delves-Broughton 2000; Gálvez et al. 2007). The potential applications of bacteriocins in the food products have been extensively reviewed by Gálvez et al. (2011).
Nisin is the only bacteriocin licensed as a biopreservative (Alvarez-Sieiro et al. 2016) and is exploited in different commercial preparations, such as Nisaplin® (Danisco, Copenhagen, Denmark), Chrisin® (Chris Hansen, Horsholm, Denmark) and Delvo®Nis (DSM, Delft, Netherlands). It is widely used in dairy industries to control clostridia (Krivorotova et al. 2016) and post-processing contamination from Listeria strains (Thomas and Delves-Broughton 2001). Furthermore, nisin and other known bacteriocins have been shown to inhibit several pathogens or spoilage bacteria in different food matrices, including dairy products, meat and meat products, fish products and seafoods, juices and beverages, fruits, vegetables and cereals (Delves‐Broughton 1990; Gálvez et al. 2011; Verma et al. 2014; Arqués et al. 2015; Gharsallaoui et al. 2016; Xavier, Gopalan and Ramana 2017). Bacteriocins can also be employed to favor early ripening of cheddar cheese by inducing early lysis of cells in the starter cultures, leading to the release of intracellular enzymes into the cheese matrix (O'sullivan, Ross and Hill 2003).
Bacteriocins can be incorporated in the food products as semi-purified compounds or in the form of bioactive powders, frequently containing a mixture of antimicrobial compounds (bacteriocins, organic acids, etc.). Such powders are usually obtained by cultivation of the producer strain in an appropriate growth medium, followed by bacterial heat inactivation and drying of the medium (Gálvez et al. 2011). Commercial bioactive powders that can be found in the market include MicroGARD® fermentates (DuPont) and DuraFresh products (Kerry), which are effective against yeasts, molds and Gram-positive or Gram-negative bacteria. Bacteriocins can also be incorporated into food packaging films to inhibit the spoilage or growth of pathogenic microorganisms during the storage period of food products (Guo et al. 2014; Damania et al. 2016; Benabbou et al. 2018).
Despite these successes, it should be noted that the merits of adding specific bacteriocins to food products may be limited by a limited, too-narrow spectrum of activity (Gharsallaoui et al. 2016) and/or a hydrophobic nature, which may cause them to be partitioned in the organic fat phase within a food matrix. Moreover, poor solubility or uneven distribution of the bacteriocin molecules can occur in food products, which may significantly affect the antimicrobial activity of these compounds. In order to overcome shortcomings, the use of bacteriocins can be coupled with other preservation approaches to increase their antibacterial activity (Mills et al. 2011).
There are potential strategies that could be developed to overcome such shortcomings, such as encapsulation technology that provides protection and facilitates controlled release of bacteriocins depending on the type and conditions of use. Moreover, there are some reports regarding implementation of certain strategies that have shown to improve efficiency of bacteriocins. For example, plantaricin BM1 applied to the surface of ham has been reported to be more effective in inhibiting Listeria monocytogenes than being incorporated into the cooked ham before homogenization (Zhou et al. 2015). The loss of activity of incorporated bacteriocin was suggested to be due to higher adsorption of bacteriocin molecule to meat components, uneven distribution of bacteriocin in food matrix and slower diffusion (Zhou et al. 2015). Another example is nisin, which can easily lose its activity in food products due to interaction with its components or inactivation by proteolytic enzymes. However, encapsulation of nisin Z in liposomes could effectively improve nisin stability and inhibitory action in the cheddar cheese matrix (Benech et al. 2002). Similarly, in a recent study by Hassan et al. (2019), it was shown that encapsulating nisin with alginate/resistant starch increased its efficiency by protecting it and enabling its gradual release in cheddar cheese without affecting the starter culture. As a result, Clostridium tyrobutyricum count was significantly reduced after 1 week and its growth was completely inhibited after 4 weeks, compared with the control. Another innovation in this regard is the use of bacteriocins in antimicrobial packaging. In this strategy, bacteriocins are embedded in layers of packaging films and coatings (Chandrakasan et al. 2019) and are applied on food surfaces to inhibit the spoilage or growth of pathogenic microorganisms during the storage period. By this approach, bacteriocins improve food safety without interacting with food ingredients or risk of inactivation.
Besides legal approval of bacteriocins as safe additives, their large-scale production, purification and long-term stability during storage hold an important challenge if direct addition of pure bacteriocins to food is considered; thus, further research is required for the cost-effective production of bacteriocins (Bali, Panesar and Bera 2016).
Clinical applications
Bacteriocins can exhibit desirable properties of relevance to medical use, including high activity in the nanomolar range, specific mechanisms of action and high specific activity (van Heel, Montalban-Lopez and Kuipers 2011). The potential medical uses of bacteriocins are discussed in the following subsections. Several studies have demonstrated the efficacy of bacteriocins in vivo, supporting the fact that they can be potentially used in clinical settings. Indeed, some bacteriocins have progressed toward clinical evaluations; however, despite their conceivable potential, there are some limitations such as bioavailability, stability, solubility under physiological conditions, susceptibility to proteolytic enzymes, high production costs and lack of cytotoxic assessment, which restrict the exploitation of bacteriocins for clinical studies and their commercialization for future therapeutic usage. Nevertheless, bioengineering approaches can be implemented to improve physicochemical and biological characteristics of bacteriocins.
Inhibition of bacterial growth
The rapid increase and spread of multidrug-resistant bacterial pathogens has led to the search for alternative methods of fighting infection since almost 20 years (Riley and Wertz 2002). Several bacteriocins have shown distinct mode of action compared with conventional antibiotics, which may decrease the risk of cross-resistance development, allowing them to be considered as promising alternatives to antibiotics. For example, the possibility of resistance development by lanthipeptides such as nisin is decreased, due to their multiple modes of action and the pyrophosphate moiety nature of their target (which is not common in conventional antibiotics), lipid II (Hasper et al. 2006). Moreover, combination of bacteriocins and other antimicrobials or antibiotics with different mechanisms of action may increase their antimicrobial potency while reducing the risk of resistance development (Cavera et al. 2015; de Freire Bastos, Coelho and da Silva Santos 2015). Many studies have shown the inhibitory effects of different bacteriocins against pathogens responsible for hospital-acquired infections, such as methicillin-resistant Staphylococcus aureus (MRSA) (Aunpad and Na-Bangchang 2007; Piper et al. 2009; Hanchi et al. 2017), vancomycin-resistant Enterococcus (Oman and van der Donk 2009), Clostridium difficile (Rea et al. 2007; Le Lay et al. 2016) and many Gram-negative pathogenic bacteria, such as Moraxella catarrhalis, Neisseria spp. and Haemophilus influenza (Castiglione et al. 2008; Jabés et al. 2011). As an example, microcin J25, a Gram-negative bacteriocin, was shown to exhibit a high antimicrobial activity against multidrug-resistant Salmonella and E. coli (Yu et al. 2019). Additionally, bacteriocins can be used as inhibitors of multidrug-resistant Pseudomonas aeruginosa, Klebsiella pneumoniae, Acinetobacter spp. and New Delhi Metallo-beta-lactamase-1 (NDM-1) expressing Enterobacteriaceae (Falagas, Grammatikos and Michalopoulos 2008; Kumarasamy et al. 2010). Bacteriocins have been mainly studied in vitro for their effectiveness against clinically important pathogens; therefore, they can be potential candidates for treatment of infectious diseases, such as those affecting the oral, respiratory, gastrointestinal and urogenital tracts. Purified and microbiologically characterized bacteriocins, such as mutacin 1140 (Ghobrial, Derendorf and Hillman 2009) and salivaricin D (Birri, Brede and Nes 2012), have shown high inhibitory activities against strains responsible for respiratory infections in humans, namely Streptococcus pneumoniae, S. aureus and P. aeruginosa. In addition, nisin F was reported to exhibit in vitro and in vivo (De Kwaadsteniet, Ten Doeschate and Dicks 2008; De Kwaadsteniet, Doeschate and Dicks 2009) inhibitory activities against clinical strains of S. aureus. There are also several reports on the efficiency of bacteriocins against bacteria responsible for gastric ulcers (Kim et al. 2003), and skin infections (Kang et al. 2009). Moreover, topical application of bacteriocins has been reported to be successfully tested for skin infection (Heunis, Smith and Dicks 2013), oral diseases (Tong, Ni and Ling 2014) and mastitis in breastfeeding women (Fernández et al. 2008). In addition, bacteriocin therapy has been efficiently used as an alternative to antibiotics in treating C. difficile infection (Rea et al. 2011).
The narrow spectrum of inhibition of some bacteriocins requires that the strains responsible for an infection need to be identified before a treatment with bacteriocins can commence, i.e. precluding their use to treat infections of unknown aetiology. However, this trait limits the likelihood of side effects on the natural healthy microbiota (Francino 2016). For instance, antibiotic treatment of C. difficile causes antibiotic-induced disruption of gut microbiota, allowing the pathogen to regrow and cause C. difficile-associated diarrhoea (CDAD) (Cotter, Ross and Hill 2013). However, the sactibiotic thuricin CD, which is produced by a strain of Bacillus thuringiensis, is a narrow spectrum bacteriocin that targets C. difficile (Rea et al. 2010). Thuricin CD has shown high antimicrobial activity that is comparable to that of vancomycin and metronidazole, but differs by virtue of having minimal impacts on the commensal microbiota of the gut (Rea et al. 2011). Other examples include pediocin PA-1, which successfully treated L. monocytogenes infection in mice without significantly affecting commensals (Dabour et al. 2009), and subtilosin A, which inhibits Gardnerella vaginalis without affecting Lactobacillus spp. (Sutyak et al. 2008).
The involvement of bacteriocins in the prevention of enteric infections in the GI tract has been demonstrated in only a few studies. Corr et al. (2007) have shown that administration of Lactobacillus salivarius UCC118, a producer of Abp118 bacteriocins, prevented L. monocytogenes infection in mice, while a mutant of Lactobacillus salivarius UCC118, a non-producer of the Abp118 bacteriocin, did not provide protection. This suggests that the anti-Listeria effect of Lactobacillu salivarius UCC118 was directly associated with the production of the bacteriocin. It has also been shown that the bacteriocin-producing Lactobacillus casei strain LAFTI L26 exerts inhibitory effects against L. monocytogenes and enterohemorrhagic E. coli in mouse (Su, Henriksson and Mitchell 2007). Similarly, Millette et al. (2008) have shown that the use of Lactococcus lactis MM19 and Pediococcus acidilactici MM33, two bacteriocin-producing strains from intestinal origin, reduced the intestinal colonization of vancomycin-resistant variants of Enterococcus in mice. Interestingly, administration of five probiotic strains was effective in controlling Salmonella enterica serovar Typhimurium infection in pigs and, notably, the only bacteriocin-producing strain, Lactobacillus salivarus str. DPC 6005, with the most potent anti-Salmonella activity dominated over co-administered strains in ileal digesta and in mucosa. However, the exact contribution of bacteriocins in the activity of these various bacterial strains was not clearly demonstrated. With regard to purified bacteriocins, Dabour et al. (2009) have shown that intragastric administration of purified pediocin PA-1 to L. monocytogenes-infected mice significantly reduced Listeria count in feces and its translocation into the liver and spleen. Ultimately, these combined studies suggest that some bacteriocins may resist gastrointestinal tract (GI) conditions and exert their inhibitory activity in vivo. Similarly, in Gram-negative bacteria, the siderophore peptides microcins M and H47 produced by the probiotic strain E. coli Nissle 1917 commercialized under the name Mutaflor® (Germany) and microcin J25 originating from an E. coli strain isolated from an infant feces were shown to kill the pathogen S. enterica ser. Enteritidis in mouse models (Lopez et al. 2007; Sassone-Corsi et al. 2016).
Despite intensive research during the last decade suggesting bacteriocins as potential agents for clinical applications, companies are not significantly investing in the development of bacteriocins for clinical applications. In fact, large-scale production could be one of the limitations for clinical development and marketability of bacteriocins (Ongey and Neubauer 2016). Hence, optimization of the biological processing for the production of pharma grade bacteriocins in a cost-effective manner requires more research. Microbisporicin NAI-107 is an example of such successfully produced bacteriocin (Sosio et al. 2013). One way to overcome this limitation can be production of synthetic or bioengineered bacteriocins, some variants of which are under clinical development (Ongey and Neubauer 2016). Recent update for clinical studies of bacteriocins may be found in the ‘Global clinical trials data’ (https://globalclinicaltrialdata.com). For example, Novacta Biosystems Ltd has developed a mersacidin analog that has a remarkable activity against clinically important pathogens, such as MRSA, VRE, C. difficile and Streptococcus pyogenes, and is in the pre-clinical stage, to be used in medical applications (Appleyard et al. 2009). Additionally, aerosolized duramycin, commercialized as Moli1901 by AOP Orphan Pharmaceuticals and Lantibio (Vienna, Austria), has completed phase II clinical trial for the treatment of cystic fibrosis (Sandiford 2015). In addition, mutacin 1140 (MU1140, Oragenics, US) and microbisporicin (NAI-107, Naicons SRL and Sentinella Pharmaceuticals) have been developed to control multidrug-resistant Gram-positive infections and are under pre-clinical trials (Sandiford 2015). Moreover, bacteriocin producing strains of Streptococcus salivarius have been commercialized as BLIS K12 and BLIS M18 (New Zealand) and have been progressed into clinical evaluation. BLIS K12 is used in oral hygiene products and has currently completed phase II/III trials for the treatment of throat infection. A potential limitation of bacteriocins in pharmaceutical application is their sensitivity to proteolytic enzymes in case of oral administration; however, encapsulation technologies offer a great solution to protect them from degradation. Ultimately, pharmacokinetic and pharmacodynamic characteristics of bacteriocins are important factors to predict their success as therapeutic agents in clinical studies. Indeed, in vitro potency may not necessarily correlate with clinical efficacy. In particular, bioengineering and semi-synthetic strategies have been implemented to develop novel variants of bacteriocins with optimized stability, biological activity and pharmacokinetic profiles, such as rapid distribution and elimination rates, good bioavailability and fecal excretion (Ongey et al. 2017).
In conclusion, the clinical use of bacteriocins depends on advances in bioengineering technologies, large-scale production of the bacteriocin or different analogs and further studies on their pharmacokinetic and pharmacodynamic properties. Importantly, greater emphasis must be given on researches regarding their toxicity and interaction with the host.
Anticancer properties
Several bacteriocins have shown anticancer activities (Papo and Shai 2005; Hoskin and Ramamoorthy 2008; Kaur and Kaur 2015) by selectively acting against cancer cells (Kaur and Kaur 2015). Bacteriocins produced by Gram-negative bacteria, such as microcin E492 (Lagos et al. 2009) and colicins (A, D, E1, E2, E3) (Lancaster, Wintermeyer and Rodnina 2007), or by Gram-positive bacteria, including nisin (Joo et al. 2012; Kamarajan et al. 2015; Ahmad et al. 2017), have demonstrated cytotoxic effects against malignant human cell lines. The cytotoxicity of bacteriocins against cancer cells is caused by the induction of apoptosis and/or depolarization of the cell membrane leading to permeability changes (Kaur and Kaur 2015). For example, it was shown that, in head and neck squamous cell carcinoma cells, nisin induced increased DNA fragmentation or apoptosis and reduced cell proliferation through induction of cell cycle arrest in the cancerous cells (Joo et al. 2012). In addition, nisin administrations reduced the size of tumors in mice with oral cancer (Joo et al. 2012). In another instance, azurin, a bacteriocin produced by a P. aeruginosa strain, was studied as a potential anticancer drug due to its selective binding to human cancer cells, and resultant cytotoxic and apoptotic effects, without apparently affecting normal cells (Yamada et al. 2004). For further information, Kaur and Kaur (2015) have extensively reviewed the anticancer activity of bacteriocins. It is important to note that the majority of studies relating to the anticancer properties of bacteriocins have been of an in vitro nature and, thus, there is a need for in vivo validation.
Potential birth control
Nisin (Aranha, Gupta and Reddy 2004), subtilosin (Sutyak et al. 2008), fermenticin (Kaur et al. 2013) and lacticin 3147 (Silkin et al. 2008) have been shown to exhibit spermicidal property by causing human spermatozoa motility to be decreased or altered, thus highlighting their potential as contraceptives for birth control (Dicks et al. 2018). In a study conducted by Reddy et al. (2004), intravaginal application of nisin in rabbits for 2 weeks prevented conception without inducing inflammation or damaging the vaginal epithelium. However, if the concentrations of nisin employed in birth control assays using animal models were to be extrapolated for human usage, the levels involved would likely severely impact on the healthy human vaginal microbiota (Dicks et al. 2018) and thus this factor must be considered when further developing bacteriocins, especially broad-spectrum bacteriocins, as potential birth control agents.
Antiviral activity
Bacteriocins also possess value as antiviral substances. Several bacteriocins exhibit antiviral activity against Herpes simplex virus (HSV-1 and HSV-2). According to previous studies, bacteriocins produced by Enterococcus spp. exhibit notable antiviral activity against viruses from Herpesviridae family. As it was shown by Wachsman et al. (2003), enterocins CRL35 and ST4V, which are produced by strains of Enterococcus faecium, exert their antiviral activities against HSV-1 and HSV-2 by affecting intracellular viral multiplication and interfering with the last stage of replication. Also, subtilosin A from Bacillus subtilis and the pediocin-like bacteriocin ST5Ha, produced by E. faecium, have shown anti-HSV activity with a selectivity index (CC50/EC50) of 173 (Todorov et al. 2010; Quintana et al. 2014). In another study, inhibition of PV-1 by Geo9, Ge12 and He17, i.e. bacteriocins from Enterococcus durans, was reported (Cavicchioli et al. 2018). Labyrinthopeptin A1 (LabyA1) (Meindl et al. 2010) produced by Actinomadura namibiensis, the prototype peptide from lantibiotics containing the unique carbacyclic post-translationally modified labionin residue, has also attracted attention due to its dual antiviral activity against HIV and HSV infection and transmission (Férir et al. 2013). LabyA1 was found to inhibit viral cell-to-cell transmission between HIV-infected T cells and uninfected CD4 + T cells and also inhibited transmission of HIV captured by DC-SIGN+-cells to uninfected CD4 + T cells (Férir et al. 2013). However, based on the studies carried out thus far, bacteriocins produced by Lactococcus lactis subsp. lactis, including nisin, were shown as ineffective against HSV-1, murine norovirus, influenza A H1N1, Newcastle disease virus Montana or Feline Herpesvirus KS285 (Lange-Starke et al. 2014).
Despite the information provided above, and in contrast to the killing of bacteria by bacteriocins, little is known about the mode of action of bacteriocins against viruses. As it was suggested by Wachsman et al. (2003), bacteriocins can block receptor sites on host cells and avoid aggregation of viral particles. In addition, they may affect the key reaction in the viral multiplication stage. As bacteriocins identified as antiviral molecules are hydrophobic, they may bind to the lipidic membranes of enveloped viruses, thus interfering with the fusion of cellular and viral membranes, and exert their inhibitory effect in that way. Thus, it is conceivable that the inactivity of many bacteriocins against non-enveloped viruses is due to structural differences from those that have been found active and particularly the lack of hydrophobicity (Badani, Garry and Wimley 2014).
Veterinary applications
Due to the increased incidence of diseases caused by drug-resistant pathogens in the human population, there have been growing debates about the systematic use of antibiotics to protect livestock and enhance growth performance. Indeed, many countries have banned the use of antibiotics as growth promoters as a result. Nonetheless, the use of antibiotics in veterinary medicine is regarded as a contributory factor in the emergence of antibiotic resistance and is regarded by certain authors as an unnecessary risk to human health (Diez-Gonzalez 2007). As a result, the use of bacteriocins and their producer strains in animal feed has been proposed as a safe alternative to antibiotics.
Animal growth performance and pathogen growth inhibition are intimately linked. Bacteriocins can be used to promote animal growth in livestock by controlling or inhibiting pathogens and having a positive impact on animal health (Diez-Gonzalez 2007). For example, a partially purified fraction of pediocin PA-1 has been shown to significantly improve the growth performance of broiler chickens infected with Clostridium perfringens (Grilli et al. 2009). In another study, Hu et al. (2018) have demonstrated that the circular bacteriocin gassericin A produced by Lactobacillus gasseri LA39, representing a species known to be a predominant intestinal Lactobacillus in weaned piglets, binds to the plasma membrane of the intestinal epithelial cells to increase fluid absorption and thus, can be used as antibiotic alternatives for preventing diarrhea in mammals. Divercin AS7 has been shown to be efficient in controlling pathogenic bacterial strains, such as Campylobacter species (Stern et al. 2005), S. enterica Typhimurium (Gillor, Kirkup and Riley 2004) and C. perfringens in chicken or swine (Udompijitkul, Paredes‐Sabja and Sarker 2012). Additionally, several bacteriocins, such as nisin and lacticin 3147, can inhibit the growth of mastitis-causing bacteria, including S. aureus and Streptococcus agalactiae in dairy cattle (Barboza-Corona et al. 2009; Kitazaki et al. 2010; Pieterse, Todorov and Dicks 2010). Besides, clinical studies have shown that intramammary administration of nisin can be used for successful treatment of clinical/subclinical mastitis caused by S. aureus in lactating dairy cows (Cao et al. 2007; Kitching et al. 2019). Some of the nisin-based products for mastitis treatment have been commercialized and available in the market. For example, Mast Out® is an intramammary product developed as an alternative to traditional antibiotics in the treatment of mastitis in lactating dairy cows (Immucell Corporation). Similarly, Teatseal® (Cross Vetpharm Group Ltd) is currently used in a large scale in cow farms. In addition, nisin-based wipe called ‘Wipe Out’ commercialized by Immucell Corporation is FDA (Food and Drug Administration) approved. Furthermore, Lagha et al. (2017) have reviewed the potential uses of bacteriocins as antimicrobial compounds in swine and poultry production.
Gram-negative bacteriocins have also been studied for their potential veterinary applications. Microcin J25 has been used for many years in diverse preparations for the control of Salmonella in poultry (Stavric and D'aoust 1993) and was shown more recently to improve the growth performance of weaned pigs (Yu et al. 2017). Even an antibacterial protein produced by E. coli, colicin E1, has been also reported to significantly improve the growth performance of piglets when incorporated in their diet, by inhibiting the growth of an enterotoxic strain of E. coli (Cutler et al. 2007).
EVALUATION OF BACTERIOCIN SAFETY
As potential antimicrobial agents, few bacteriocins have been commercially applied. The legislation concerning the approval and acceptance of bacteriocins for medical, veterinary and food applications has contributed to this pattern. Another factor relates to the fact that companies have not been willing to invest in the time and effort required to have new bacteriocins approved. Although bacteriocins are generally thought to be non-toxic for mammalian cells, enterococcal cytolysin has shown toxicity at high concentrations (Cox, Coburn and Gilmore 2005). Therefore, various assays must be performed to establish the safety of bacteriocins before their use in food, medicine and veterinary industry. Moreover, the potential development of bacterial resistance to bacteriocins on repeated administration requires evaluation (Behrens et al. 2017).
The assays generally used to establish the safety of bacteriocins are described in the following subsection. Since the mechanism of action of nisin is known, it is often used as a reference compound for toxicity testing (Paiva et al. 2012).
Gastrointestinal stability
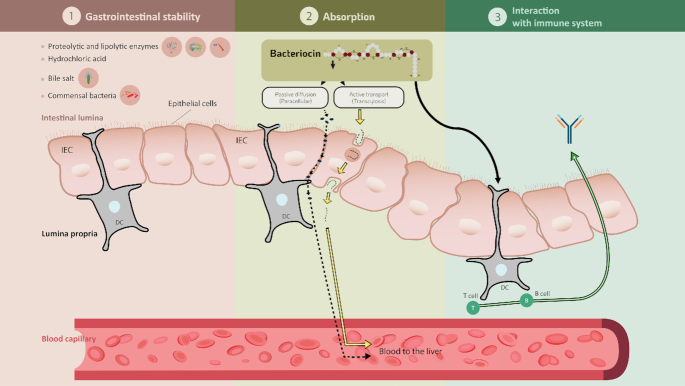
When administered by the oral route, bacteriocins are subject to several barriers that may affect their stability and biological activity. Figure 1 summarizes the different barriers encountered within the GI transit. Numerous in vitro and in vivo studies have demonstrated that several orally ingested bacteriocins or those produced in situ are rapidly inactivated or degraded by proteolytic enzymes in the stomach and the small intestine, such as pepsin, trypsin and chymotrypsin (Cleveland et al. 2001; De Vuyst and Leroy 2007; Fernandez et al. 2013). Class II bacteriocins are known to be very sensitive to intestinal proteases, thus reducing their antimicrobial activity when consumed orally. Kheadr et al. (2010) evaluated the physicochemical and biological stability of pediocin PA-1 in the upper GI conditions using a dynamic in vitro model. They have shown that pediocin was stable in the stomach but completely degraded upon exposure to conditions equivalent to those found in the small intestine. Class I bacteriocins are naturally more resistant to proteases than class II bacteriocins due to undergoing extensive PTMs (Birri, Brede and Nes 2012; Johnson et al. 2018). Nonetheless, nisin A has been shown to be inactivated and digested by intestinal proteases (Heinemann and Williams 1966; Jarvis and Mahoney 1969). These results were confirmed by Gough et al. (2017) who showed complete degradation of nisin following oral, gastric and small intestinal digestion. According to Pomares et al. (2009), microcin J25 was highly resistant to digestion by proteolytic enzymes present in the stomach and the intestinal contents. More recently, Naimi et al. (2018) examined the degradome of microcin J25 using both dynamic and static models of digestion, associated with antibacterial assays, LC-MS/MS and molecular networking analysis. Although microcin J25 is remarkably stable in extreme conditions due to its lasso topology, it was partly degraded in the GI, specifically by the pancreatic protease elastase and it lost its antimicrobial activity.
Figure 1.
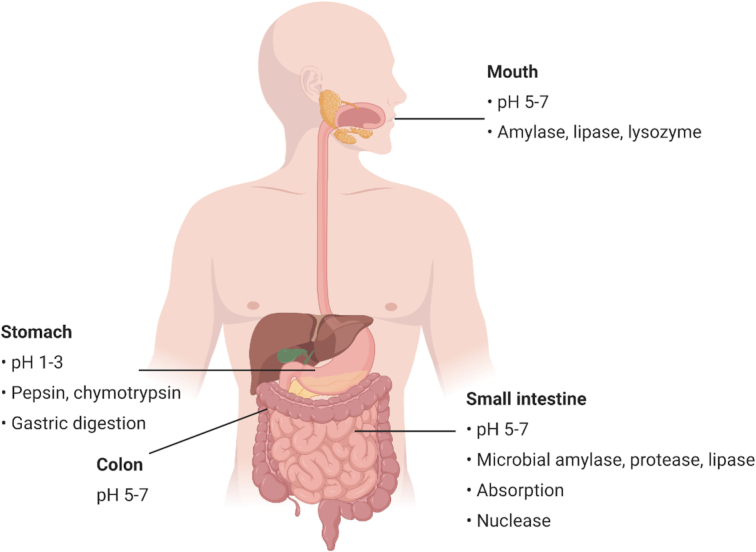
Physiological conditions that may influence the stability and biological activity of bacteriocins during the gastrointestinal transit. Figure created in biorender, https://biorender.com/.
However, for peptides such as nisin or microcin J25, that remain partly stable in such drastic conditions present in the gastrointestinal context, two main methods allow keeping the peptide active at the gastrointestinal level, which are peptide engineering and peptide encapsulation. It was shown by O'Shea et al. (2010) that it is possible to engineer bacteriocins to make derivatives that display resistance to protease action, while retaining high antimicrobial activity. In lantibiotics and particularly nisin, both stability and activity were enhanced by bioengineering the residues that serve as target sites for the digestive enzymes and mutating residues in the specific hinge region of the lantibiotic, respectively (Rollema et al. 1995; Field et al. 2015, 2019). Moreover, nisin bioengineering has been shown to overcome its resistance to proteolytic cleavage of its C-terminal region by the nisin resistance protein NSR, while maintaining also its activity (Rollema et al. 1995; Field et al. 2019). Since the site of action of most bacteriocins is at the end of the small intestine and in the colon, systems have been developed for protection and controlled delivery of these molecules. Encapsulation of bacteriocins (Gomaa et al. 2017) or their incorporation within coated tablets (Habib and Sakr 1999) protects them from the digestive enzymes encountered during passage through the GI. Recently, Gough et al. (2018) have shown that incorporation of nisin into two different starch-based matrices protects it from degradation in the upper GI. The encapsulation strategy should very probably allow developing more efficiently many bacteriocins lacking full stability in all compartments of the GI.
Absorption
The potential toxicity of bacteriocins is dependent on their bioavailability and absorption following oral administration (Fig. 2). Potential systemic effects of bacteriocins can be investigated by estimating the amount absorbed after administration. Nisin has been observed to be absorbed through the vaginal epithelium into the blood circulation following intravaginal administration in rabbits (Reddy et al. 2004). Maximum levels of nisin were detected in blood samples after 1 h of treatment. However, the levels declined to baseline after 12 h, suggesting a rapid systemic turnover of nisin. Oral administration of enterocin E50–52 in chicken has been shown to decrease S. enterica Enteritidis levels in their liver and spleen (Svetoch et al. 2008), which suggests the absorption of this bacteriocin through the intestinal wall (Bogovič-Matijašić and Rogelj 2011). It is interesting to note that Dreyer et al. (2019) reported the migration of bacteriocins across gastrointestinal epithelial and vascular endothelial cells in vitro for the first time. The authors showed that nisin and plantaricin 423 could cross the gut–blood barrier, with activity loss being dependent on the concentration and type of bacteriocins, i.e. class II bacteriocins retained more activity than nisin (Dreyer et al. 2019). However, further analysis is needed to determine the concentration of bacteriocins that exist in blood following gastrointestinal treatment. Similarly, further studies will be required to gain a better understanding of how bacteriocins can be absorbed in order to optimize potential systematic benefits. Figure 2 depicts the bacteriocin interplays in the GI.
Figure 2.
Bacteriocin interplays in the gastrointestinal tract. 1. Stability in gastrointestinal condition including enzymes, pH changes, commensal bacteria; 2. Possible pathways for bacteriocin absorption by epithelial cells; 3. Bacteriocin interaction with immune system.
Acute and subacute toxicity
The evaluation of acute toxicity is the preliminary screening step required for the determination of toxic characteristics of a bioactive compound (Akhila, Shyamjith and Alwar 2007). Acute toxicity assays determine the adverse effects of a product resulting from a single exposure or multiple exposures in a short period of time. In addition, they are designed to determine the lethal dose or LD50 values of a compound. According to OECD guideline 425 for the testing of chemicals (OECD 2008a), the test substance is either administered in a single dose to rodents via oral gavage or given in smaller fractions over a period of no more than 24 h. The general behavior of the rodents, their body weight changes, signs of toxicity and mortality are monitored (Son, Chang and Lee 2015) at least once during the initial 30 min, periodically within the first 24 h of bacteriocin administration and daily thereafter. All rodents are subjected to a gross necropsy at the end of the observation period (OECD 2008a). Subacute oral toxicity assays can be carried out following acute toxicity testing to assess possible health hazards, arising from chronic exposures to a substance, with respect to a wide variety of potential targets of toxicity, including the nervous, immune and endocrine systems. According to the OECD (2008b), for subacute oral toxicity assays, the test substance is administered orally to rodents by gavage or via the diet or drinking water in graduated doses over 28 days. During this period, animals are observed daily to detect clinical signs of toxicity and mortality (OECD 2008b).
In a study by Frazer, Sharratt and Hickman (1962), oral administration of nisin (1000 mg kg−1 body weight) in rats did not show any sign of acute toxicity. However, in another study, signs of possible toxicity, such as histological changes in the spleen, skin and liver, were observed in mice treated with 0.825 mg kg−1 nisin daily for 21 days. These changes were indicative of possible inflammatory processes, which could be associated with the high salt content of commercial nisin (Nisaplin®) used in this study (Vaucher et al. 2011). The LD50 of the bacteriocin TSU4, produced by the L. animalis TSU4 strain, was found to be >200 mg kg−1 when orally administered to mice (Sahoo et al. 2017). Furthermore, mice orally administered with 0.5 mg kg−1 bacteriocin TSU4 daily for 21 days showed no mortality or treatment-induced changes with respect to their physiological conditions, indicating that this bacteriocin was not toxic (Sahoo et al. 2017). In another study by Marlida et al. (2016), oral administration of up to 20 000 mg kg−1 pediocin N6 in mice did not show any sign of acute toxicity. Lactocin 160, which is produced by Lactobacillus rhamnosus 160, is active against the most relevant species associated with bacterial vaginosis; 1.8 mL of a 10 mg mL−1 solution of this bacteriocin was administered to female rabbits intravaginally. Both in vitro and in vivo safety evaluations have shown that lactocin 160 did not cause any severe irritation of vaginal epithelial tissue or toxicity to lactobacilli, and hence can be used for intravaginal application (Dover et al. 2007).
Studies have also been performed involving sites other than the gut or vagina. Enterocin AS-48 produced by Enterococcus faecalis showed no toxicological effects when administered intraperitoneally to mice (5 mg kg−1; 100 g/mouse) in 6 doses (one every 8 h). It is interesting to note that this bacteriocin also did not induce any skin sensitization or allergic contact dermatitis after topical application (Cebrián et al. 2019). Additionally, OG716, a derivative of mutacin 1140, which is considered as a potential candidate for CDAD treatment, has shown no toxicity or side effects upon oral administration in Golden Syrian Hamster; hence, it may progress toward clinical development (Pulse et al. 2019).
Table 1 summarizes the studies regarding in vivo acute and subacute toxicity of bacteriocins. Based on the few studies reported thus far, bacteriocins may be safe for different applications. However, more research is required to determine the safe dose for each bacteriocin. This is essential as insufficient data in this area can be considered as one of the reasons for the underuse of these molecules.
Table 1.
Overview of recent literature and current data on in vivo acute and subacute toxicity of bacteriocins.
| Acute and subacute toxicity | |||
|---|---|---|---|
| Bacteriocin | Administration | Toxicity | References |
| Enterocin AS-48 | 1, 10, 20 μg/dose of 5 μL for 3 days | No skin sensitization | (Cebrián et al. 2019) |
| Lacticin 160 | 1.8 mL of 10 mg mL−1 intravaginally in rat | No severe irritation | (Dover et al. 2007) |
| Nisin | 1000 mg kg−1 BW in rat | No sign of acute toxicity | (Frazer et al. 1962) |
| 20 000 mg kg−1 BW in mice | (Marlida et al. 2016) | ||
| Pediocin N6 | >20 000 mg kg−1 in mice | LD50 | (Marlida et al. 2016) |
| TSU4 | Daily intake dose of 0.5 mg kg−1 for 21 days | No mortality and no changes in physical condition | (Sahoo et al. 2017) |
| 0.825 mg kg−1 daily intake for 21 days | Histological changes in spleen, liver and skin | (Vaucher et al. 2011) | |
Long-term exposure and side effects
It has been estimated that the vast majority of bacterial species produce bacteriocins (Riley and Wertz 2002; Cotter, Hill and Ross 2005). As many fermented foods harbor very high levels of lactic acid bacteria, including bacteriocin-producing strains, these foods also contain bacteriocins that are consumed by humans (Swain et al. 2014). For example, many lactic acid bacteria used in cheese manufacture were shown to produce bacteriocins (Molloy et al. 2011). Moreover, nisin, which is commercially available since 1953 (Collins et al. 2010), is widely used as a food preservative (Cotter, Ross and Hill 2013) without any report of toxicity in humans. However, due to limited reports on the effect of long-term exposure to bacteriocins on human health through the consumption of food products, further studies should be carried out. The aforementioned enterocin AS-48, shown to exhibit no toxicity following acute exposure, also did not cause any significant toxic effect in BALB/c mice fed with 50, 100 or 200 mg kg−1 for 90 days (Baños et al. 2019). Similarly, upon consumption of nisin A by rats at a dietary level of up to 5% for 90 days, no toxicologically significant changes in their clinical signs, body weights or food consumption were observed, confirming the safety of nisin at the concentrations used (Hagiwara et al. 2010). Moreover, long-term incorporation of nisin into the diet of rats did not cause any deleterious effect on their organs (Frazer, Sharratt and Hickman 1962).
Cytotoxicity against eukaryotic cells
As summarized above, bacteriocins are considered to be non-toxic and safe antimicrobial peptides. However, it has been reported that some bacteriocins exert some degree of cytotoxicity when assayed using cell culture-based assays. For instance, it should be noted that the cytotoxicity of some bacteriocins in mammalian cells was shown to be observed at concentrations significantly higher than the minimum inhibitory concentrations (MIC) of these compounds required to avoid food spoilage or the presence of pathogenic bacteria in food products (Lohans and Vederas 2011). Several bacteriocins, such as nisin (Maher and McClean 2006), colicin E1, E3, E7, K (Murinda, Rashid and Roberts 2003), enterocin DD14 (Caly et al. 2017), enterocin S37 (Belguesmia et al. 2011), carnobacteriocins Cbn BM1 and Cbn B2 (Jasniewski et al. 2009), plantaricin DM5 (Das and Goyal 2014), enterocin AS-48 (Abengózar et al. 2017), as well as semi-purified bacteriocins produced by Lactococcus lactis subsp. lactis and E. durans (Cerqueira et al. 2018), showed absence or low levels of cytotoxicity at MIC concentrations against various eukaryotic cell lines. On the other hand, nisin, pediocin PA-1 and colicin E1, E3, E7, K have been shown to be cytotoxic at significantly higher concentrations against Vero cell lines (Murinda, Rashid and Roberts 2003). It is not surprising that some of these phenomena vary in a concentration-dependent manner (Murinda, Rashid and Roberts 2003; Paiva et al. 2012). This was also apparent when a semi-purified bacteriocin produced by Lactobacillus plantarum ST8SH was demonstrated to be highly cytotoxic at the concentration of 25 µg mL−1 but not at 5 µg mL−1 (Favaro and Todorov 2017). Moreover, in wound healing, specific bacteriocins may exhibit strong antimicrobial activity at low concentrations, while at higher concentrations, they might be toxic to eukaryotic cells (Chalekson, Neumeister and Jaynes 2003).
Cox, Coburn and Gilmore (2005) have reported that cytolysin, a two-peptide lytic toxin, produced by some strains of E. faecalis exerts cytotoxicity against a broad spectrum of cells, including human retinal cells, erythrocytes, leucocytes and intestinal epithelial cells. The cytotoxicity of this cationic bacteriocin is proposed to be conferred by binding to anionic membranes or cells through hydrophobic interactions and directly disrupting it (Pessione 2014). Table 2 summarizes the results from in vitro assessments of cytotoxicity for some bacteriocins from different studies.
Table 2.
In vitro assessment of cytotoxicity of bacteriocins using different eukaryotic cells.
| Cytotoxicity | |||||
|---|---|---|---|---|---|
| Bacteriocin | Producer organism | Cell line | Type of assay | Toxicity | References |
| Enterocin DD14 | Enterococcus faecalis 14 | IPEC-1 | CCK-8 assay | No toxicity at MIC and 2× MIC concentrations | (Caly et al. 2017) |
| Enterocin S37 | Enterococcus faecalis S37 | Caco2-TS7 | LDH release assay | No toxicity at 2 and 10 μg mL−1 | (Belguesmia et al. 2011) |
| Enterocin AS-48 | Enterococcus faecalis UGRA 10 | Melanoma cell line A2058 | MTT assay | No toxicity at MIC and higher concentrations (up to 200 μg mL−1) | (Abengózar et al. 2017) |
| Carnobacteriocin Cbn BM1 | |||||
| Cbn B2 | Carnobacterium maltaromaticum CP5 | Caco-2 | MTT assay | No toxicity at 100-fold MIC | (Jasniewski et al. 2009) |
| Plantaricin DM5 | Lactobacillus plantarum DM5 | Hek 293 | MTT assay | No cytotoxic effect on mammalian cells | (Das and Goyal 2014) |
| HeLa | |||||
| Nisin (Nutrition 21/USA) | HT29 | MTT assay | Cytotoxic at 4× MIC value in HT29 | (Maher and McClean 2006) | |
| Caco-2 | Neutral red | Cytotoxic at 2× MIC in Caco-2 | |||
| Nisin (Sigma-Aldrich) | SV40 HC | Trypan blue exclusion | Toxic to both cells at high concentration (<50% viability at >350 AU mL−1) | (Murinda et al. 2003) | |
| Vero cell | |||||
| Nisaplin® (Danisco) | Vero cell | MTT assay | EC50: 0.33 μg mL−1 MTT | (Vaucher et al. 2011) | |
| LDH release assay | 0.79 μg mL−1 LDH | ||||
| Neutral red assay | 0.62 μg mL−1 NUR | ||||
| Nisin Chrisin® | Vero cell | MTT | IC50 | (Paiva et al. 2012) | |
| (Chr. Hansen—Colors & Blends) | MCF-7 | 13.48 μM Vero cell | |||
| HepG2 | 105.46 μM MCF-7 | ||||
| 112.25 μM HepG2 | |||||
| Pediocin PA-1 | Pediococcus acidilactis PA-1/ACH | SV40 HC | Trypan blue exclusion | Toxic to both cells at high concentration (<50% viability at >170 AU mL−1) | (Murinda et al. 2003) |
| Vero cell | |||||
| Col E1 | E. coli 50164 | SV40 HC | Trypan blue exclusion | Col E6 cytotoxic for both cells | (Murinda et al. 2003) |
| Col E3 | Vero cell | Col E1, E3, E7 and Col K little toxic (350 and 700 AU mL−1) | |||
| Col E6 | |||||
| Col E7 | |||||
| Col K | |||||
| Microcin E492 | K. pneumoniae RYC492 | HeLa | LDH release assay (at 14 μg mL−1) | Toxic against Jurkat, HeLa, RJ2.2.5 (different degree) | (Hetz et al. 2002) |
| Jurkat | AMG-3, KG-1 insensitive | ||||
| RJ2.2.5 | Ramos slightly sensitive | ||||
| Ramos | |||||
| KG-1 | |||||
| AMG-3 | |||||
| Bacteriocin-like P40 | Bacillus licheniformis P40 | Vero cell | MTT assay | EC50: | (Vaucher et al. 2011) |
| LDH release assay | 0.30 μg mL−1 MTT | ||||
| Neutral red assay | 0.51 μg mL−1 LDH | ||||
| 0.57 μg mL−1 NUR | |||||
| Bovicin HC5 | Streptococcus bovis HC5 | Vero cell | MTT | IC50: | (Paiva et al. 2012) |
| MCF-7 | 65.42 μM in Vero | ||||
| HepG2 | 279.39 μM MCF-7 | ||||
| 280.30 μM HPG2 | |||||
Since cytotoxic effects of bacteriocins have been evaluated in different types of eukaryotic cell lines, such as monkey kidney epithelial cell line Vero (Murinda, Rashid and Roberts 2003), human intestinal epithelial cell lines HT29 and Caco-2 (Maher and McClean 2006), human embryonic kidney cell line HEK 293 and human cervix epitheloid carcinoma cell line HeLa (Das and Goyal 2014), the comparison of their toxicity levels becomes complicated (Favaro and Todorov 2017). As it was reported, SV40-HC cells could be significantly more sensitive to nisin, pediocin PA-1 and colicin E6 than normal Vero cells (Murinda, Rashid and Roberts 2003). In addition, the IC50 value of commercial nisin (Nisaplin®) was reported to be 105 μM in Vero cell line and nisin A (from Nutrition 21) was shown to exert cytotoxicity against epithelial cell lines HT29 and Caco-2 with IC50 values of 89.9 and 115 μM, respectively (Maher and McClean 2006). However, it should be noted that these products are not pure and contain salts that may affect the results. In a study by Paiva et al. (2012), nisin has been reported to be cytotoxic in Vero, MCF-7 and HepG2 cell lines with IC50 values of 13.48, 105.46 and 112.25 μM, respectively. This difference can be related to different compositions of the cell membranes. Membrane lipid or cholesterol composition can increase the rigidity of lipid bilayers and inhibit its disruption by antimicrobial peptides such as bacteriocins. Hence, higher concentrations of bacteriocins are required to permeabilize the membrane (Wessman et al. 2008; Laverty and Gilmore 2014). Furthermore, differences in hydrophobicity of cell surfaces may affect the interaction and binding of bacteriocins; however, the exact reason for the different cytotoxic activities in different cell types is yet to be known.
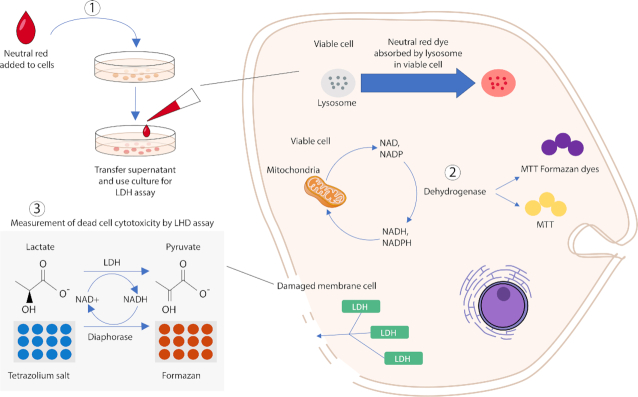
The purity of bacteriocins is another parameter that may affect their toxicity against mammalian cells. The exact purity of bacteriocins has not been stated in some studies, thus making comparisons difficult. Also, different types of assays were used for in vitro cytotoxicity evaluation. Figure 3 illustrates an overview of in vitro assays based on cell functions that may be used for cytotoxicity evaluation of bacteriocins. Altogether, in order to achieve comparative cytotoxicity evaluation of different bacteriocins, the precise tested concentrations, the exact purity of the bacteriocins, the type of eukaryotic cells and their metabolic activity and the type of assay (Paiva et al. 2012) are the factors to be considered.
Figure 3.
In vitro toxicity assays. Three different assays that have been mostly employed for toxicity evaluation of bacteriocins based on various cell functions: 1. Neutral red assay (measures cell viability upon lysosome function), 2. MTT assay (measures cell viability upon mitochondria activity), and 3. LDH release assay (membrane Integrity).
Immunogenicity
The immunogenicity of each bacteriocin should be tested as per safety concerns in order to prevent immune responses in the body of humans or animals following ingestion (De Groot and Scott 2007) or intravenous administration of bacteriocins (Lohans and Vederas 2011). The bacteriocins pediocin AcH (Bhunia et al. 1990) and TSU4 (Sahoo et al. 2017) have been shown to be non-immunogenic. On the other hand, long-term administration to mice of a diet comprising Nisaplin®, which as noted above is a nisin-containing preparation that also contains milk proteins and salt, led to a significant increase in the amount of macrophages/monocytes, isolated from the peripheral blood (Pablo et al. 1999). Moreover, low levels of antibodies were measured in the serum of animals exposed to pyocin S5 (Scholl and Martin 2008; McCaughey et al. 2016).
Impact on cell–cell adhesion or tight junction integrity
Ensuring that the integrity of tight junctions or cell–cell adhesion is not negatively impacted in response to bacteriocin treatment is highly important if these peptides are to be used for the treatment of bacterial infections of epithelial barriers (Belguesmia et al. 2011). To evaluate the impact of different compounds on the integrity of tight junctions, intestinal epithelial cells, such as HCT-8, HT29 and Caco-2, can be used to mimic the GI or the intestinal mucosa (Fujiwara et al. 1997, 2001; Di Cagno et al. 2010; Villarante et al. 2011). Before treatment, cells are allowed to grow and develop the morphological and functional characteristics of enterocytes, such as the formation of intercellular tight junctions. The effect of bacteriocins on the integrity of the tight junctions can be evaluated by measuring the electrical resistance across a cellular monolayer, otherwise known as transepithelial electrical resistance (TEER) (Putaala et al. 2008; Srinivasan et al. 2015).
There are several studies of different bacteriocins that have highlighted the absence of an impact on the integrity of tight junctions. According to Belguesmia et al. (2011), no significant reduction in epithelial integrity was observed in Caco-2/TC7 cells after treatment with 10 µg mL−1 nisin or enterocin S37. Similarly, treating Caco-2 cells with 2 µg mL−1 divercin AS7 for 24 h did not alter TEER (Olejnik-Schmidt et al. 2014). In another study, using the same cell line, plantaricin A was shown to protect the integrity of cellular tight junctions (Di Cagno et al. 2010).
Impact on the intestinal microbiota
The evaluation of the impact of bacteriocins on the intestinal microbiota is an important issue because modifications in the diversity and function of this ecosystem have been associated with different diseases, such as inflammatory immune diseases, functional bowel disorders, insulin resistance and obesity (Arqués et al. 2015). Unlike many classes of antibiotics, bacteriocins are known to often exhibit a narrow-spectrum activity (Cotter, Ross and Hill 2013) and, thus, the use of such bacteriocins should ensure that there are only limited effects on the commensal microbiota.
The effects of bacteriocins have been studied on the intestinal microbiota of the upper GI (Kheadr et al. 2010), the terminal ileum (Le Blay et al. 2012) or the distal colon (Guinane et al. 2006; Le Lay et al. 2015) using different in vitro models. Nisin and pediocin PA-1 did not appear to cause any significant disturbances of the commensal gut microbiota in vitro (Guinane et al. 2006; Le Blay et al. 2012; Le Lay et al. 2015) and in vivo (Bernbom et al. 2006; Dabour et al. 2009). Nevertheless, another in vitro study (Rea et al. 2011) showed that the lantibiotic lacticin 3147 induced a significant shift in the microbiota from Firmicutes to Proteobacteria. Recently, using metagenomic approaches, Umu et al. (2016) demonstrated the effects of five bacteriocins on the gut microbiota at lower taxonomic levels. Through these approaches, some changes were observed in the microbiota that would have remained unnoticed by conventional culture-dependent methods (Gálvez et al. 2012).
Although several studies have demonstrated the role of bacteriocins as a potential mechanism by which certain probiotics exert their beneficial effects, few studies have focused on the direct or indirect impact of bacteriocinogenic probiotics on the colonic microbiota. Padilla, Lobos and Hubert (2004) demonstrated that isolates of E. coli and Bacterioides fragilis from healthy gut were highly sensitive to bacteriocins produced by Shigella flexneri. In a study on rats, Bernbom et al. (2006) have shown that the use of a nisin-producing Lactococcus lactis strain or purified nisin increases the fecal bifidobacteria count but decreases that of enterococci. However, in a study on bacterial cultures representing colic microbiota in humans, nisin A and nisin Z were demonstrated to significantly inhibit Bifidobacterium and Lactobacillus genera, while pediocin did not exert any effects (Blay et al. 2007). These results were confirmed by Dabour et al. (2009) in a study on mice. In addition, Dobson et al. (2011) have shown that oral administration of Lactococcus lactis DPC6520, a lacticin 3147-producing strain, did not alter the microbiota of the distal colon, both in vitro and in vivo. As reported by Riboulet-Bisson et al. (2012), Lactobacillus salivarius UCC118, a producer of the Abp 118 bacteriocin, did not exert significant effect on the proportion of the main communities of mouse gut microbiota, except a significant decrease in Spirochetes and Firmicutes. Murphy et al. (2013) showed that same bacteriocin producing strain with no significant impact on Firmicutes could increase Bacteroidetes and Proteobacteria and decrease Actinobacteria in mice. In addition, it was shown that in a murine model, Lactobacillus salivarius UCC118 could cause an increase in the proportion of Peptococcaceae and a reduction in the proportion of Riknelleaceae and Porphyromonadaceae (Clarke et al. 2013). In the light of the few studies described above, it is evident that the real impact of bacteriocins on the colonic microbiota eubiosis is not yet well understood and that results reported to date are fragmentary and can vary greatly depending on the strain and the model used. To date, there are few such studies on bacteriocins produced by Gram-negative bacteria. However, microcins M and H47, the siderophore microcins responsible for the anti-Salmonella properties of the probiotic E. coli Nissle 1917, were shown to exert their activity without significant impact on the microbiota (Sassone-Corsi et al. 2016). Besides, there are no reports in the literature regarding the real impact of bacteriocinogenic strains on the overall metabolic profile of the colonic microbiota.
More in-depth studies combining metagenomic and metabolomic approaches remain essential in order to study the real effects of bacteriocins on the composition and balance of the colonic microbiota. These studies should include different bacteriocins with different structures and mechanisms.
Bacteriocins may mediate probiotics functionality by different mechanisms. Bacteriocins may facilitate colonization of the host by competitive inhibition of resident microbiota. They may also directly inhibit pathogens. Finally, bacteriocins may act as signaling peptides, either signaling other bacteria through quorum sensing or signaling cells of the host immune system and hence modulating host immune system (Egan, Ross and Hill 2017). In a study by Pablo et al. (1999), nisin was shown to be able to modulate the immune system of mice by increasing CD4 and CD8 T-lymphocytes and fundamentally by increasing macrophages/monocytes population isolated from peripheral blood. Besides, the effect of nisin on innate immune cells, including T cells, is suggested to be due to the modulation of the activity of antigen‐presenting cells. Additionally, nisin was suggested to be used as an immunomodulatory agent in pig breeding (Małaczewska et al. 2019). Bacteriocins may exert immunomodulatory effects in addition to their antimicrobial properties; however, their effect on the immune system has not been yet completely demonstrated.
MECHANISMS OF ACTION AND EMERGENCE OF RESISTANCE TO BACTERIOCINS
General trends of bacteriocin mechanisms of action
Bacteriocins show diverse mechanisms of antimicrobial action depending on their physicochemical properties and the presence or not of PTMs (Cotter et al. 2013) (Fig. 4A). Many bacteriocins, essentially of class II, exhibit a cationic nature. Therefore, interaction with and perturbation of the bacterial cytoplasmic membrane are considered to play an essential role in the killing activity (Héchard and Sahl 2002), similar to what happens with cationic peptides from multicellular organisms (Andres 2012). Electrostatic interactions between cationic bacteriocins and the negatively charged components of the bacterial membrane, i.e. phospholipids and teichoic acids of Gram-positive bacteria or lipopolysaccharide of Gram-negative bacteria, constitute a first mechanistic step initiating their activity. Specific and non-specific binding of bacteriocins to the membrane surface resulting from electrostatic interactions can subsequently lead to insertion of bacteriocins into the cytoplasmic membrane. This first step causes the formation of ion-permeable channels or pores, followed by passive efflux of intracellular metabolites, such as potassium and magnesium ions, amino acids and ATP and further dissipation of vital ion gradients, which leads to leakage of cell content and finally cell death (Todorov 2009). Cell lysis can also occur due to a release and activation of autolytic enzymes linked to teichoic, lipoteichoic and teichuronic acids that result from interactions of bacteriocins with these Gram-positive cell wall components (Karpiński et al. 2016).
Figure 4.
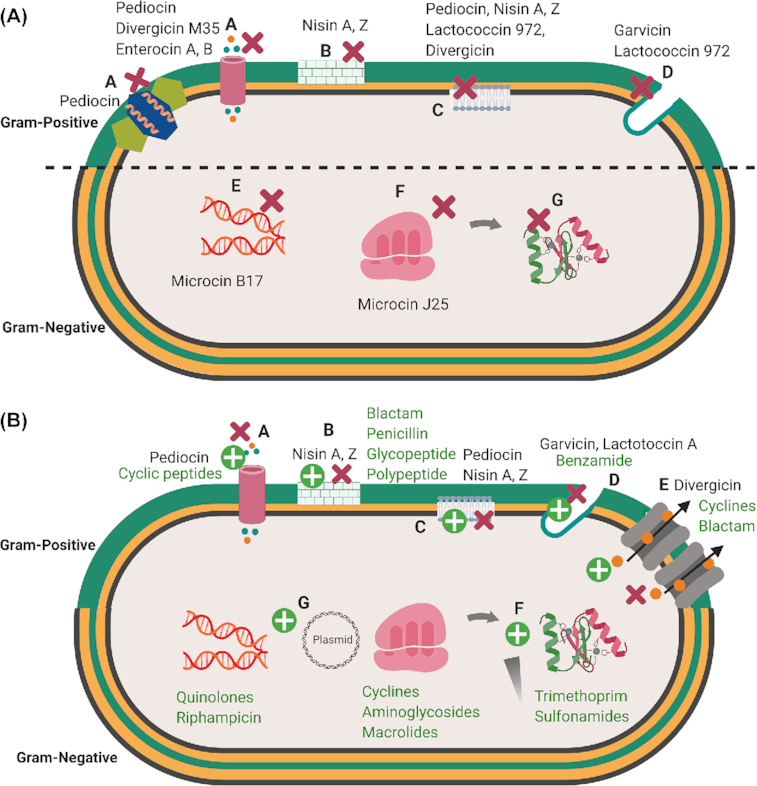
4A. Main targets for bacteriocins in Gram-positive and Gram-negative bacteria. A. Perturbations of the membrane bilayer by pore formation and efflux of ions and metabolites; B. Perturbation of cell wall synthesis; C. Membrane depolarization; D. Perturbation of septum formation; E. Disruption of replication and transcription; F. Inhibition of ribosomal function and perturbation of protein synthesis; G. Blocking of chaperon functions necessary for proper folding of proteins. Bacteriocins ( ) 4B. Mechanism of bacterial resistance to antibiotics and bacteriocins. A. Mutation of a receptor; B and C. Modifications of the membrane composition; D. Septum formation E. Expression of efflux pumps; F. Expression of immunity genes; G. Degradation or inactivation of chaperones. Bacteriocins ( ), Antibiotics ( ) Figures created in biorender https://biorender.com/. (There are symbols for bacteriocins as red cross in bracets and antibiotics as green cross in brackets which can't be added here please consider those.
In addition to the phospholipid bilayer, a large panel of intracytoplasmic targets has been identified. Ruminococcin C, a sactipeptide recently identified from the human microbiota bacterium Ruminococcus gnavus, was shown to be unable to perturb the membrane bilayers of target bacteria but rather would interfere with nucleic acids synthesis (Chiumento et al. 2019). Moreover, a large panel of transporters allowing uptake of essential nutrients or enzymes involved in vital functions has been identified as direct target or as gate involved in the killing mechanisms used by bacteriocins (Cotter 2014). Lipid II, which is an intermediate of the biosynthesis of peptidoglycan, the maltose ABC-transporter, a zinc-dependent metallopeptidase, the undecaprenyl pyrophosphate phosphatase and the large-conductance mechanosensitive channel MscL have been identified as receptors for several Gram-positive bacteriocins, namely nisin and some other lanthipeptides, garvicin ML, the leaderless class II bacteriocin LsbB, lactococcin G and sublancin 168, respectively (Kouwen et al. 2009; Cotter 2014). Sugar-phosphotransferase systems (PTS), such as mannose-PTS or glucose-PTS, have been shown to be targets for both Gram-positive (pediocin-like bacteriocins, sublancin) and Gram-negative (microcin E492) bacteriocins (Bieler et al. 2006) (Cotter, Hill and Ross 2005; Wu et al. 2018). Other Gram-negative bacteriocins, namely microcin B17, microcin J25 and microcin C, bind and inhibit DNA gyrase, RNA polymerase and Asp-tRNA synthetase, respectively, which have been identified as their direct targets (Drider and Rebuffat 2011).
Microbial cell killing may also occur by inhibition of the germination of bacterial spores (Thomas et al. 2002; Karpiński et al. 2016), non-specific degradation of cellular DNA or inhibition of protein synthesis through the specific cleavage of 16s rRNA (Heu et al. 2001; De Vuyst and Vandamme 2012; James, Lazdunski and Pattus 2013).
It should be noted that the outer membrane of Gram-negative bacteria acts as an effective barrier against cationic bacteriocins produced by Gram-positive bacteria, making Gram-negative bacteria more resistant to these bacteriocins (Cao-Hoang et al. 2008). By contrast, bacteriocins produced by Gram-negative bacteria evolved a variety of strategies to overcome this barrier. They use either porins that favor passive diffusion of hydrophilic nutrients, or Trojan horse approaches where receptors required for major functions of bacteria, such as iron uptake, are piratized to allow translocation of the bacteriocins through the outer membrane and enter the periplasmic space. In that respect, iron siderophore receptors of the different types (catechol, hydroxamate) constitute a major target of Gram-negative bacteriocins, similar to the Gram-negative antimicrobial proteins named colicins (Cascales et al. 2007).
Development of resistance
Evaluation of bacteriocin resistance development upon prolonged exposure in target strains is another important consideration before the use of bacteriocins in food products or for treating human and animal diseases. For a complete review of bacterial resistance to bacteriocins, see de Freire Bastos, Coelho and da Silva Santos (2015).
Resistance to bacteriocins is either innate (intrinsically found in particular genera or species) or acquired (developed by a formerly susceptible strain) (Collins et al. 2012). Innate resistance to bacteriocins varies among different bacterial strains (Katla et al. 2003). For example, up to 8% of wild-type strains of L. monocytogenes were shown to be naturally resistant to pediocin-like bacteriocins (Collins et al. 2010). Similarly, in another study evaluating bacteriocin susceptibility, among 381 strains of L. monocytogenes, 20 strains were found to be resistant to pediocin PA-1, but in contrast, there were no naturally nisin-resistant strains (Rasch and Knöchel 1998). Both innate and acquired bacteriocin resistance can be linked to several genetic loci in bacteria. Mutation in genes involved in innate resistance leads to sensitivity to bacteriocins, whereas upon mutation genes associated with sensitivity can contribute to acquired resistance. de Freire Bastos, Coelho and da Silva Santos (2015) have described various mechanisms leading to innate resistance. Another phenomenon observed in non-bacteriocin-producing bacterial strains is immune mimicry, where functional homologs of bacteriocin immunity systems are expressed (Draper et al. 2012). The heterologous expression of genes encoding homologs of bacteriocin immunity confers protection in cells against cognate bacteriocins (Draper et al. 2012). Proteolytic cleavage of bacteriocins is another potential mechanism through which resistance to bacteriocins might occur in non-bacteriocin-producing bacteria (Sun et al. 2009; Nocek et al. 2012). Extracellular proteases or peptidases produced by bacteriocin-resistant strains can degrade bacteriocins, reducing or diminishing their antimicrobial activity (Nes et al. 2015). Similarly, Sedgley, Clewell and Flannagan (2009) demonstrated that gelatinase secreted by E. faecalis is responsible for the degradation and inactivation of pediocin-like bacteriocins.
Exposure to bacteriocins can result in the development of resistance in formerly susceptible bacterial cells (Collins et al. 2012). Acquired resistance may be a result of numerous mechanisms including a spontaneous mutation of associated genes, such as those involved in the expression of specific receptors, cell wall synthesis, transcriptional regulation or energy metabolism and transport (Gravesen et al. 2002; Katla et al. 2003; de Freire Bastos, Coelho and da Silva Santos 2015). The frequencies of these mutations vary according to the producer strain, the bacteriocin and the culture conditions. In general, the frequency of genes undergoing spontaneous mutation, upon cellular exposure to low concentrations of bacteriocins, which results in bacteriocin resistance, is low (Pessione 2014). For example, nisin-resistant L. monocytogenes mutants were observed at frequencies of 10−6 to 10−8 (Harris, Fleming and Klaenhammer 1991), while lacticin 3147-resistant Lactococcus lactis occurred at frequencies of 10−8 to 10−9 (Guinane et al. 2006). The bacteriocin-resistant mutants may arise from changes in bacterial membrane fluidity, lipid composition, electrical potential and load or cell wall thickness, or a combination of all these factors (Fig. 4B).
Other potential mechanisms involving bacterial cell envelope or the cytoplasmic membrane synthesis, reduction of membrane permeability have been identified to explain the acquired resistance to bacteriocins (Cotter, Ross and Hill 2013; de Freire Bastos, Coelho and da Silva Santos 2015). For example, an alteration in the bacterial cell membrane hydrophobicity caused by a change in its fatty acid composition was shown to be involved in the development of nisin resistance in L. monocytogenes cells (Martínez and Rodríguez 2005). As antimicrobial agents, some lantibiotics bind to lipid II, which acts as a receptor or docking molecule to both promote pore formation and inhibit the synthesis of peptidoglycan. Reducing bacteriocin accessibility to their receptors or downregulating the expression of genes involved in receptors may result in an enhanced bacterial resistance (Kramer et al. 2006; Collins et al. 2010; Kjos et al. 2011). So, bacterial resistance can be evaluated by determining the expression of bacterial genes related to receptors alteration, such as lipid II (vanA) and Man-PTS (mPTS), synthesis of peptidoglycans (dal and ddl), proteolytic cleavage [genes for nisinase (NSR protease)] and other proteases (Gravesen et al. 2002, 2004; Cotter, Ross and Hill 2013). Some bacterial strains have developed multiple resistance mechanisms that may operate simultaneously, giving rise to the overall resistance phenotype (Lohans and Vederas 2011). Therefore, an adequate insight into the exact mechanism involved in development of bacteriocin resistance should facilitate their application in clinical and/or food settings.
Co-resistance and cross-resistance between bacteriocins or between antibiotics and bacteriocins
Co-resistance is defined as the resistance of a bacterial strain to the same class bacteriocins as that produced by the strain, while the resistance of a microorganism against bacteriocins from different classes or resistance to both bacteriocins and antibiotics is named cross-resistance. Strains of L. monocytogenes have been reported to be resistant against various class II (Dykes and Hastings 1998; Rasch and Knöchel 1998; Ramnath et al. 2000) and class I bacteriocins (Gravesen et al. 2001), which suggests an identical or similar resistance mechanism (Gravesen et al. 2002). Moreover, sometimes acquired resistance of a bacterial strain to a bacteriocin due to an alteration of the target cell receptor (Kicza et al. 2016) or a change in the composition of the cell membrane (Asaduzzaman and Sonomoto 2011) increases bacterial resistance to other bacteriocins as well (Naghmouchi et al. 2007). Although bacteriocins and antibiotics do not have the same mode of action in most cases (Cleveland et al. 2001), increased resistance to some antibiotics was observed in variants resistant to bacteriocins, such as pediocin, nisin (Kaur et al. 2014) or microcin 24 (Carlson, Frana and Griffith 2001). In addition, Mantovani and Russell (2001) reported a 1000-fold increase in resistance to ampicillin in nisin-resistant mutants of Streptococcus bovis compared with the original nisin-sensitive isolates. On the contrary, Naghmouchi et al. (2007) demonstrated that in L. monocytogenes cells, acquired bacteriocin resistance led to reduced antibiotic sensitivity.
The phenomenon of cross-resistance has been observed among bacteriocins belonging to different classes (de Freire Bastos, Coelho and da Silva Santos 2015). According to Crandall and Montville (1998), nisin-resistant L. monocytogenes showed cross-resistance to pediocin PA-1. Moreover, there are different reports on cross-resistance among nisin, pediocin PA-1, leuconocin S and leucocin A (Bruno and Montville 1993; Gravesen et al. 2004). For instance, Guinane et al. (2006) and Van Schaik, Gahan and Hill (1999) demonstrated the incidence of cross-resistance between nisin and lacticin 3147 or lacticin 481. However, Rasch and Knöchel (1998) found no cross-resistance between nisin and pediocin PA-1 in strains of L. monocytogenes naturally resistant to either of these bacteriocins, while Naghmouchi et al. (2007) demonstrated that acquired resistance to nisin A or Z increased the resistance of L. monocytogenes cells to pediocin and divergicin.
Nevertheless, several studies demonstrated that the use of mixtures of bacteriocins from different classes, especially with different mechanisms of action, increases their antimicrobial activity and broadens their spectrum of activity (Vignolo et al. 2000; Turgis et al. 2016) while reducing the resistance of bacterial strains to these bacteriocins (de Freire Bastos, Coelho and da Silva Santos 2015). For example, the combination of nisin and curvaticin, a bacteriocin produced by Lactobacillus curvatus SB13, significantly decreased the resistance of a L. monocytogenes strain to these bacteriocins used individually (Bouttefroy and Millière 2000). Cross-resistance of bacteriocin-resistant variants to several antimicrobial agents, such as nisin, pediocin 34 and enterocin FH99, has been reported previously (Kaur et al. 2014). In the same study, the combinations of different bacteriocins have been demonstrated more potent against L. monocytogenes ATCC 53135 compared with single compounds. The nisin-resistant variants were more susceptible to most antimicrobial peptides tested than their wild-type counterparts (Kaur et al. 2014). Mathur et al. (2017) have reviewed the effects of different combinations of bacteriocins with other antimicrobials that are effective against clinical and food pathogens. In addition to combination therapy, bioengineering strategies can also be exploited to generate bacteriocins with enhanced potency and reduced incidence of antimicrobial resistance. However, further research is required to understand the precise mechanisms of action of bacteriocins and develop engineered bacteriocins with unique target ranges and modes of action.
BACTERIOCIN APPROVAL BY REGULATORY AGENCIES FOR DIFFERENT APPLICATIONS
Despite the GRAS (Generally recognized as safe) status of a high number of bacteriocins described in the literature, their use for various food, medical or veterinary applications is very limited. It should be noted that most current applications are related to food preservation, while only a few examples are reported in the veterinary and medical sectors, which may be due to the lack of specific guidelines for legal approval of bacteriocin or bacteriocin-producing bacteria. Different guidelines may be used for bacteriocin approval and they vary from one country to another.
Guideline for evaluation and approval of new bacteriocins for food applications
As new active biological compounds, bacteriocins are currently considered by regulatory agencies as ‘technological agents‘ or ‘new’ food additives by regulatory agencies. It should be noted that depending on its intended use, the same bacteriocin might be considered a technological agent or a food additive. A ‘technological agent’ is a substance that is used to produce a technical effect during food manufacturing and processing; however, unlike food additives, it does not alter the intrinsic characteristics of the food. Besides, it inhibits residue generation in the final product or its by-products. A ‘food additive’ refers to any substance that is added to the food during its preparation or before its storage to modify the food in order to achieve the desired technical effect.
In general, using technological agents in foods is not subjected to any regulatory requirements. Food additives can play several roles, including maintaining the nutritive value of the food, increasing its shelf life, or enhancing its appearance and facilitating its processing, packaging or storage. In many countries, as per the demand, regulatory agencies may provide overviews regarding the acceptability of uses as technological agents in food processing. Unlike technological agents, food additives must be previously approved by regulatory agencies and should obtain marketing authorization before their use in the food sector.
According to our knowledge, to be approved as an additive, a new bacteriocin should be subjected to several evaluation steps (Fig. 5). Application for approval of a new bacteriocin should include the following criteria:
Figure 5.
Guideline for evaluation and approval of new bacteriocins for food applications.
The identity and chemical composition of the new bacteriocin. This means that the active molecule should be highly purified and its amino acid sequence must be determined using gold-standard biochemical and molecular techniques.
The method of preparation and stabilization.
A statement indicating the appropriate concentration or the amount of bacteriocin proposed for its proper use and the purpose for which it is proposed, together with all directions, recommendations and suggestions regarding its use.
An acceptable method of analysis, suitable for regulatory purposes that will determine the final concentration of the bacteriocin in the finished food.
Data showing the efficacy of the bacteriocin for its intended use.
Detailed reports on the safety of bacteriocin under the recommended conditions of use. These include acute and subacute toxicity reports and the long-term exposure effects. Bacteriocins with the history of use in foods might be considered as safe. A guideline for the evaluation of the safety of bacteriocin intended to be used as a food additive is presented in Fig. 5.
Data on the acceptable residual concentration in the finished food product when the additive or bacteriocin is used according to the good manufacturing practice.
A proposed maximum concentration of the additive or bacteriocin in the finished food product.
The legislation concerning the use of bacteriocins in food products varies according to their intended use and the laws of each country (Gálvez et al. 2011). Nisin was added to the European food additive list in 1983 (Directive 83/463/EEC; Directive 95/2/EC) and is used as a food preservative (E234). Also, it was approved by the FDA in 1988 (21CFR) and more recently by Health Canada (NOP/ADP-0028) in 2017. Nisin is now used as a food additive in >80 countries, and it may be used in different foods, such as meat products, processed and pasteurized cheeses, fruits, and vegetables. However, the type of food products in which nisin can be incorporated and its maximum levels of an addition vary among different countries (Gálvez et al. 2011; López-Cuellar, Rodríguez-Hernández and Chavarría-Hernández 2016). Moreover, some bacteriocins are in a process to receive legal approval, such as pediocin (Fargo 23, Quest International, B.V., Naarden, The Netherlands and ALTA® 2351, 2341; Kerry Bioscience, Carrigaline, Co. Cork, Ireland), sakacin (Bactoferm FLC®, Chr. Hansen, Hørsholm, Denmark) and micocin (Micocin, CanBiocin, Edmonton, Canada) (López-Cuellar, Rodríguez-Hernández and Chavarría-Hernández 2016).
Bacteriocin-producing protective cultures are also used as food additives. Carnobacterium maltaromaticum CB1 (https://www.canada.ca/en/health-canada/services/food-nutrition/public-involvement-partnerships/use-microbiological-preparation-carnobacterium-maltaromaticum-strain-certain-ready-meat-poultry-products/document.html), Leuconostoc carnosum 4010 (Danisco, HOLDBAC®) or Carnobacterium divergens M35 have been approved in many countries for use in the processing of products, such as vacuum-packed meat products and sliced ready-to-eat cold-smoked salmon or trout (https://www.canada.ca/fr/sante-canada/services/aliments-nutrition/participation-public-partenariats/proposition-permettre-recours-nouvel-additif-alimentaire-carnobacterium-divergens-agent-conservation-antimicrobien-saumon/consultation.html).
Guideline for evaluation and approval of new bacteriocins for human and veterinary applications
Bacteriocins can be used for human and animal applications as well and, therefore, must undergo a rigorous evaluation protocol, aiming to analyze their safety and efficacy by various in vivo tests, including animal and human trials. The intended purpose of the product dictates whether the product is classified as a feed ingredient or a therapeutic drug, although some ingredients may be used in both therapeutic and nutritional applications. For instance, veterinary drugs in Canada are regulated under The Food and Drugs Act and Regulations administered by Health Canada, which ensures the safety and effectiveness of the product (https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/legislation-guidelines/guidance-documents/guidance-document-classification-veterinary-drugs-livestock-feeds/document.html). The identification of the active bacteriocin and evaluation of its safety are essential aspects needed to be investigated. Besides, the efficacy of bacteriocins for the intended uses should be demonstrated using appropriate in vivo tests. The results of the in vitro tests must then be validated by in vivo studies using appropriate animal models, followed by clinical studies. In the animal healthcare sector, a nisin-based udder disinfectant named as Wipe Out® dairy wipes has been approved by the FDA (Dover et al. 2008). Figure 6 shows a proposed guideline that can be implemented for the evaluation of bacteriocins for human and animal uses.
Figure 6.
Guideline for evaluation and approval of new bacteriocins for human and veterinary applications.
Alternative incorporation of any product in animal feed, either directly or indirectly, must be according to food additive regulations, unless it is generally recognized among qualified experts to be safe under the conditions of its intended use (FDA 2018). The FDA regulates the use of bacteriocins and bacteriocinogenic strains under the Federal Food, Drug, and Cosmetic Act (FFDCA) where they are regulated as food ingredients. The approval of a food additive petition is a prerequisite and must meet the criteria listed under Title 21 CFR 570 and 571 of the FFDCA Act, which consists of required data on human and target animal safety, impact on the environment, manufacturing details, proposed labeling and regulations (https://www.fda.gov/animal-veterinary/animal-food-feeds/product-regulation). In Canada, livestock feeds are regulated by the Feeds Act and Regulations, which is managed by the Canadian Food Inspection Agency (CFIA), whose mission is to ensure the safety, effectiveness and correct labeling of livestock feeds (https://www.canada.ca/en/health-canada/services/drugs-health-products/veterinary-drugs/legislation-guidelines/guidance-documents/guidance-document-classification-veterinary-drugs-livestock-feeds/document.html). In addition, feeds in Canada are not allowed to have therapeutic claims or purposes, but they can act as carriers for therapeutic products.
Guideline for safety evaluation of bacteriocins intended for food, human and animal uses
In order to assess the safety of bacteriocins for animal and consumers, much information is required, including technical (production, purity, methods of analysis, etc.) and toxicological data (principal of toxicological assessment, protocols, data report, results and conclusions, etc). It is necessary to identify and characterize the compounds, and they should be identical to that to be used in the commercial product. Figure 7 addresses the main steps for safety evaluation of bacteriocins, which includes identification of compounds, bioavailability, in vitro and in vivo assay, exposure study, ADME study (absorption, distribution, metabolism, excretion) and setting the accepted daily intake (ADI) for determination of safe dose. In case that the highest proposed concentration of bacteriocins in the final product would result in animal or human exposure exceeding the highest safe dose, the measure should be taken to reduce consumers’ exposure.
Figure 7.
Guideline for safety evaluation of bacteriocins intended for food, human, and animal uses.
CONCLUDING REMARKS AND PERSPECTIVES
Bacteriocins have properties due to which they may be used for a broad range of food and medical applications. Thus, the safety of bacteriocins requires much attention. Antimicrobial activity of bacteriocins has been widely studied; however, there is a lack of available in vitro and in vivo data regarding safety and toxicity of bacteriocins. In order to confirm the safety of bacteriocins, investigation of their immunogenicity and both in vitro and in vivo toxicity is necessary. In fact, for this purpose, different assays are needed to be performed to assess their cytotoxicity in eukaryotic cells, ability to induce apoptosis, growth inhibitory effect, hemolytic activity, acute and subchronic toxicity, etc. Approval of health agencies for using bacteriocins in food, livestock or medicine is a time-consuming process involving clinical trials and tests. Regulations proposed by the FDA should be strictly followed to achieve legal approval of bacteriocins as safe and reliable food preservatives or therapeutic agents.
FUNDING
This work is supported by the Natural Sciences and Engineering Research Council of Canada(NSERC) and the Centre de recherches pour le développement international (CRDI).
Supplementary Material
Contributor Information
Samira Soltani, Food Science Department, Faculty of Agriculture and Food Sciences, Université Laval, G1V 0A6 Québec, Canada.
Riadh Hammami, School of Nutrition Sciences, Faculty of Health Sciences, University of Ottawa, 75 Laurier Ave. E, Ottawa, ON K1N 6N5, Canada.
Paul D Cotter, Teagasc Food Research Centre, Moorepark, Fermoy, Co. Cork, P61 C996 Ireland; APC Microbiome Ireland, Institute and school of Microbiology, University College Cork, Western Road, Cork, T12 YN60, Ireland.
Sylvie Rebuffat, Muséum National d'Histoire Naturelle, Centre National de la Recherche Scientifique, Laboratory Molecules of Communication and Adaptation of Microorganisms (MCAM), UMR 7245 CNRS-MNHN, CP 54, 57 rue Cuvier, 75005 Paris, France.
Laila Ben Said, Food Science Department, Faculty of Agriculture and Food Sciences, Université Laval, G1V 0A6 Québec, Canada.
Hélène Gaudreau, Food Science Department, Faculty of Agriculture and Food Sciences, Université Laval, G1V 0A6 Québec, Canada.
François Bédard, Faculty of Pharmacy and Centre de Recherche en Endocrinologie Moléculaire et Oncologique et Génomique Humaine, Université Laval, 2705 Boulevard Laurier, Quebec G1V 4G2, Canada.
Eric Biron, Faculty of Pharmacy and Centre de Recherche en Endocrinologie Moléculaire et Oncologique et Génomique Humaine, Université Laval, 2705 Boulevard Laurier, Quebec G1V 4G2, Canada.
Djamel Drider, Institut Charles Viollette, Université de Lille, EA 7394, 53955 Villeneuve d'Ascq, France.
Ismail Fliss, Food Science Department, Faculty of Agriculture and Food Sciences, Université Laval, G1V 0A6 Québec, Canada; Institute of Nutrition and Functional Foods, Université Laval, 2440 Boulevard Hochelaga, Québec G1V 0A6, Canada.
Conflicts of interest
None declared.
REFERENCES
- Abbasiliasi S, Tan JS, Ibrahim TAT et al. Fermentation factors influencing the production of bacteriocins by lactic acid bacteria: a review. RSC Adv. 2017;7:29395–420. [Google Scholar]
- Abengózar MÁ, Cebrián R, Saugar JM et al. Enterocin AS-48 as evidence for the use of bacteriocins as new leishmanicidal agents. Antimicrob Agents Chemother. 2017;61:e02288–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad V, Khan MS, Jamal QMS et al. Antimicrobial potential of bacteriocins: in therapy, agriculture and food preservation. Int J Antimicrob Agents. 2017;49:1–11. [DOI] [PubMed] [Google Scholar]
- Akhila JS, Shyamjith D, Alwar M. Acute toxicity studies and determination of median lethal dose. Curr Sci. 2007;93;917–20. [Google Scholar]
- Alvarez-Sieiro P, Montalbán-López M, Mu D et al. Bacteriocins of lactic acid bacteria: extending the family. Appl Microbiol Biot. 2016;100:2939–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres E. Cationic antimicrobial peptides in clinical development, with special focus on thanatin and heliomicin. Eur J Clin Microbiol Infect Dis. 2012;31:881–8. [DOI] [PubMed] [Google Scholar]
- Ansari A. Bacteriocin from LAB for medical and health applications. In: Beneficial Microorganisms in Medical and Health Applications, Cham, Switzerland: Springer, 2015, 199–221. [Google Scholar]
- Appleyard AN, Choi S, Read DM et al. Dissecting structural and functional diversity of the lantibiotic mersacidin. Chem Biol. 2009;16:490–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranha C, Gupta S, Reddy K. Contraceptive efficacy of antimicrobial peptide nisin: in vitro and in vivo studies. Contraception. 2004;69:333–8. [DOI] [PubMed] [Google Scholar]
- Arnison PG, Bibb MJ, Bierbaum G et al. Ribosomally synthesized and post-translationally modified peptide natural products: overview and recommendations for a universal nomenclature. Nat Prod Rep. 2013;30:108–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arqués JL, Rodríguez E, Langa S et al. Antimicrobial activity of lactic acid bacteria in dairy products and gut: effect on pathogens. Biomed Res Int. 2015;2015:584183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asaduzzaman SM, Sonomoto K. Responses of lactic acid bacteria to bacteriocins and other antimicrobials. In: Stress Responses of Lactic Acid Bacteria. Boston, MA: Springer, 2011, 439–58. [Google Scholar]
- Aunpad R, Na-Bangchang K. Pumilicin 4, a novel bacteriocin with anti-MRSA and anti-VRE activity produced by newly isolated bacteria Bacillus pumilus strain WAPB4. Curr Microbiol. 2007;55:308–13. [DOI] [PubMed] [Google Scholar]
- Badani H, Garry RF, Wimley WC. Peptide entry inhibitors of enveloped viruses: the importance of interfacial hydrophobicity. BBA Biomembranes. 2014;1838:2180–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagley MC, Dale JW, Merritt EA et al. Thiopeptide antibiotics. Chem Rev. 2005;105:685–714. [DOI] [PubMed] [Google Scholar]
- Bali V, Panesar PS, Bera MB. Trends in utilization of agro-industrial byproducts for production of bacteriocins and their biopreservative applications. Crit Rev Biotechnol. 2016;36:204–14. [DOI] [PubMed] [Google Scholar]
- Baquero F, Moreno F. The microcins. FEMS Microbiol Lett. 1984;23:117–24. [Google Scholar]
- Barboza-Corona JE, de la Fuente-Salcido N, Alva-Murillo N et al. Activity of bacteriocins synthesized by Bacillus thuringiensis against Staphylococcus aureus isolates associated to bovine mastitis. Vet Microbiol. 2009;138:179–83. [DOI] [PubMed] [Google Scholar]
- Baños A, García JD, Núñez C et al. Subchronic toxicity study in BALBc mice of enterocin AS-48, an anti-microbial peptide produced by Enterococcus faecalis UGRA10. Food Chem Toxicol. 2019;132:110667. [DOI] [PubMed] [Google Scholar]
- Behrens HM, Six A, Walker D et al. The therapeutic potential of bacteriocins as protein antibiotics. Emerg Top Life Sci. 2017;1:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belguesmia Y, Madi A, Sperandio D et al. Growing insights into the safety of bacteriocins: the case of enterocin S37. Res Microbiol. 2011;162:159–63. [DOI] [PubMed] [Google Scholar]
- Benabbou R, Subirade M, Desbiens M et al. The impact of chitosan-divergicin film on growth of Listeria monocytogenes in cold-smoked salmon. Front Microbiol. 2018;9:2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech R-O, Kheadr E, Lacroix C et al. Antibacterial activities of nisin Z encapsulated in liposomes or produced in situby mixed culture during cheddar cheese ripening. Appl Environ Microbiol. 2002;68:5607–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernbom N, Licht TR, Brogren C-H et al. Effects of Lactococcus lactis on composition of intestinal microbiota: role of nisin. Appl Environ Microbiol. 2006;72:239–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besse A, Peduzzi J, Rebuffat S et al. Antimicrobial peptides and proteins in the face of extremes: lessons from archaeocins. Biochimie. 2015;118:344–55. [DOI] [PubMed] [Google Scholar]
- Bhunia AK, Johnson M, Ray B et al. Antigenic property of pediocin AcH produced by Pediococcus acidilactici H. J Appl Bacteriol. 1990;69:211–5. [DOI] [PubMed] [Google Scholar]
- Bieler S, Silva F, Soto C et al. Bactericidal activity of both secreted and nonsecreted microcin E492 requires the mannose permease. J Bacteriol. 2006;188:7049–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birri DJ, Brede DA, Nes IF. Salivaricin D, a novel intrinsically trypsin-resistant lantibiotic from Streptococcus salivarius 5M6c isolated from a healthy infant. Appl Environ Microbiol. 2012;78:402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay GL, Lacroix C, Zihler A et al. In vitro inhibition activity of nisin A, nisin Z, pediocin PA‐1 and antibiotics against common intestinal bacteria. Lett Appl Microbiol. 2007;45:252–7. [DOI] [PubMed] [Google Scholar]
- Bogovič-Matijašić B, Rogelj I. Bacteriocins of probiotics and enteric cytoprotection. In: Probiotic Bacteria and Enteric Infections. Dordrecht: Springer, 2011, 313–54. [Google Scholar]
- Bountra K, Hagelueken G, Choudhury HG et al. Structural basis for antibacterial peptide self‐immunity by the bacterial ABC transporter McjD. EMBO J. 2017;36:3062–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouttefroy A, Millière J-B. Nisin–curvaticin 13 combinations for avoiding the regrowth of bacteriocin resistant cells of Listeria monocytogenes ATCC 15313. Int J Food Microbiol. 2000;62:65–75. [DOI] [PubMed] [Google Scholar]
- Bruno ME, Montville TJ. Common mechanistic action of bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1993;59:3003–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly DL, Chevalier M, Flahaut C et al. The safe enterocin DD14 is a leaderless two-peptide bacteriocin with anti-Clostridium perfringens activity. Int J Antimicrob Agents. 2017;49:282–9. [DOI] [PubMed] [Google Scholar]
- Campion A, Casey PG, Field D et al. In vivo activity of Nisin A and Nisin V against Listeria monocytogenes in mice. BMC Microbiol. 2013;13:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao-Hoang L, Marechal P-A, Le-Thanh M et al. Synergistic action of rapid chilling and nisin on the inactivation of Escherichia coli. Appl Microbiol Biotechnol. 2008;79:105–9. [DOI] [PubMed] [Google Scholar]
- Cao L, Wu J, Xie F et al. Efficacy of nisin in treatment of clinical mastitis in lactating dairy cows. J Dairy Sci. 2007;90:3980–5. [DOI] [PubMed] [Google Scholar]
- Carlson SA, Frana TS, Griffith RW. Antibiotic resistance in Salmonella enterica serovar Typhimurium exposed to microcin-producing Escherichia coli. Appl Environ Microbiol. 2001;67:3763–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascales E, Buchanan SK, Duché D et al. Colicin biology. Microbiol Mol Biol Rev. 2007;71:158–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castiglione F, Lazzarini A, Carrano L et al. Determining the structure and mode of action of microbisporicin, a potent lantibiotic active against multiresistant pathogens. Chem Biol. 2008;15:22–31. [DOI] [PubMed] [Google Scholar]
- Cavera VL, Arthur TD, Kashtanov D et al. Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents. 2015;46:494–501. [DOI] [PubMed] [Google Scholar]
- Cavicchioli VQ, de Carvalho OV, de Paiva JC et al. Inhibition of herpes simplex virus 1 (HSV-1) and poliovirus (PV-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int J Antimicrob Agents. 2018;51:33–7. [DOI] [PubMed] [Google Scholar]
- CDC About antimicrobial resistance, Centers for disease control and prevention, 2018. Available at https://www.cdc.gov/drugresistance/about.html (10 March 2020, date last accessed). [Google Scholar]
- Cebrián R, Rodríguez-Cabezas ME, Martín-Escolano R et al. Preclinical studies of toxicity and safety of the AS-48 bacteriocin. J Adv Res. 2019;20:129–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira J, Dimitrov S, Silva A et al. Inhibition of herpes simplex virus 1 and poliovirus (PV-1) by bacteriocins from Lactococcus lactis subsp. lactis and Enterococcus durans strains isolated from goat milk. Int J Antimicrob Agents. 2018;51:33–7. [DOI] [PubMed] [Google Scholar]
- Chalekson CP, Neumeister MW, Jaynes J. Treatment of infected wounds with the antimicrobial peptide D2A21. J Trauma Acute Care Surg. 2003;54:770–4. [DOI] [PubMed] [Google Scholar]
- Chandrakasan G, Rodríguez-Hernández A-I, del Rocío López-Cuellar M et al. Bacteriocin encapsulation for food and pharmaceutical applications: advances in the past 20 years. Biotechnol Lett. 2019;41:453–69. [DOI] [PubMed] [Google Scholar]
- Chikindas ML, Weeks R, Drider D et al. Functions and emerging applications of bacteriocins. Curr Opin Biotech. 2018;49:23–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiumento S, Roblin C, Kieffer-Jaquinod S et al. Ruminococcin C, a promising antibiotic produced by a human gut symbiont. Sci Adv. 2019;5:eaaw9969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claesen J, Bibb M. Genome mining and genetic analysis of cypemycin biosynthesis reveal an unusual class of posttranslationally modified peptides. Proc Natl Acad Sci USA. 2010;107:16297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SF, Murphy EF, O'Sullivan O et al. Targeting the microbiota to address diet-induced obesity: a time dependent challenge. PLoS One. 2013;8:e65790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland J, Montville TJ, Nes IF et al. Bacteriocins: safe, natural antimicrobials for food preservation. Int J Food Microbiol. 2001;71:1–20. [DOI] [PubMed] [Google Scholar]
- Collins B, Curtis N, Cotter PD et al. The ABC transporter AnrAB contributes to the innate resistance of Listeria monocytogenes to nisin, bacitracin, and various β-lactam antibiotics. Antimicrob Agents Chemother. 2010;54:4416–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins B, Guinane CM, Cotter PD et al. Assessing the contributions of the LiaS histidine kinase to the innate resistance of Listeria monocytogenes to nisin, cephalosporins, and disinfectants. Appl Environ Microbiol. 2012;78:2923–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corr SC, Li Y, Riedel CU et al. Bacteriocin production as a mechanism for the antiinfective activity of Lactobacillus salivarius UCC118. Proc Natl Acad Sci USA. 2007;104:7617–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotter PD, Hill C, Ross RP. Food microbiology: bacteriocins: developing innate immunity for food. Nat Rev Microbiol. 2005;3:777. [DOI] [PubMed] [Google Scholar]
- Cotter PD, Ross RP, Hill C. Bacteriocins—a viable alternative to antibiotics? Nat Rev Microbiol. 2013;11:95. [DOI] [PubMed] [Google Scholar]
- Cotter PD. An ‘Upp'‐turn in bacteriocin receptor identification. Mol Microbiol. 2014;92:1159–63. [DOI] [PubMed] [Google Scholar]
- Cox CR, Coburn PS, Gilmore MS. Enterococcal cytolysin: a novel two component peptide system that serves as a bacterial defense against eukaryotic and prokaryotic cells. Curr Protein Pept Sci. 2005;6:77–84. [DOI] [PubMed] [Google Scholar]
- Crandall AD, Montville TJ. Nisin resistance in Listeria monocytogenes ATCC 700302 is a complex phenotype. Appl Environ Microbiol. 1998;64:231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutler SA, Lonergan SM, Cornick N et al. Dietary inclusion of colicin e1 is effective in preventing postweaning diarrhea caused by F18-positive Escherichia coli in pigs. Antimicrob Agents Chemother. 2007;51:3830–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabour N, Zihler A, Kheadr E et al. In vivo study on the effectiveness of pediocin PA-1 and Pediococcus acidilactici UL5 at inhibiting Listeria monocytogenes. Int J Food Microbiol. 2009;133:225–33. [DOI] [PubMed] [Google Scholar]
- Damania P, Patel R, Shaw R et al. Development of antimicrobial packaging materials for food preservation using bacteriocin from Lactobacillus casei. Microbiol Res. 2016;7:6622. [Google Scholar]
- Das D, Goyal A. Characterization of a noncytotoxic bacteriocin from probiotic Lactobacillus plantarum DM5 with potential as a food preservative. Food Funct. 2014;5:2453–62. [DOI] [PubMed] [Google Scholar]
- de Freire Bastos MdC, Coelho MLV, da Silva Santos OC. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiol. 2015;161:683–700. [DOI] [PubMed] [Google Scholar]
- De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. 2007;28:482–90. [DOI] [PubMed] [Google Scholar]
- De Kwaadsteniet M, Doeschate K, Dicks L. Nisin F in the treatment of respiratory tract infections caused by Staphylococcus aureus. Lett Appl Microbiol. 2009;48:65–70. [DOI] [PubMed] [Google Scholar]
- De Kwaadsteniet M, Ten Doeschate K, Dicks L. Characterization of the structural gene encoding nisin F, a new lantibiotic produced by a Lactococcus lactis subsp. lactis isolate from freshwater catfish (Clarias gariepinus). Appl Environ Microbiol. 2008;74:547–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delves‐Broughton J. Nisin and its application as a food preservative. Int J Dairy Technol. 1990;43:73–6. [Google Scholar]
- De Vuyst L, Leroy F. Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microb Biotech. 2007;13:194–9. [DOI] [PubMed] [Google Scholar]
- De Vuyst L, Vandamme EJ. Bacteriocins of Lactic Acid Bacteria: Microbiology, Genetics and Applications. New York NY: Springer, 2012. [Google Scholar]
- Di Cagno R, De Angelis M, Calasso M et al. Quorum sensing in sourdough Lactobacillus plantarum DC400: induction of plantaricin A (PlnA) under co‐cultivation with other lactic acid bacteria and effect of PlnA on bacterial and Caco‐2 cells. Proteomics. 2010;10:2175–90. [DOI] [PubMed] [Google Scholar]
- Dicks LM, Dreyer L, Smith C et al. A review: the fate of bacteriocins in the human gastro-intestinal tract: do they cross the gut–blood barrier? Front Microbiol. 2018;9:2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Gonzalez F. Applications of bacteriocins in livestock. Curr Issues Intest Microbiol. 2007;8:15. [PubMed] [Google Scholar]
- Dobson A, Crispie F, Rea MC et al. Fate and efficacy of lacticin 3147-producing Lactococcus lactis in the mammalian gastrointestinal tract. FEMS Microbiol Ecol. 2011;76:602–14. [DOI] [PubMed] [Google Scholar]
- Dover S, Aroutcheva A, Faro S et al. Natural antimicrobials and their role in vaginal health: a short review. Int J Probiotics Prebiotics. 2008;3:219. [PMC free article] [PubMed] [Google Scholar]
- Dover SE, Aroutcheva AA, Faro S et al. Safety study of an antimicrobial peptide lactocin 160, produced by the vaginal Lactobacillus rhamnosus. Infect Dis Obstet Gynecol. 2007;2007:78248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draper LA, Tagg JR, Hill C et al. The spiFEG locus in Streptococcus infantarius subsp. infantarius BAA-102 confers protection against nisin U. Antimicrob Agents Chemother. 2012;56:573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyer L, Smith C, Deane SM et al. Migration of bacteriocins across gastrointestinal epithelial and vascular endothelial cells, as determined using in vitro simulations. Sci Rep. 2019;9:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drider D, Rebuffat S. Prokaryotic Antimicrobial Peptides: From Genes to Applications. Berlin/Heidelburg, Germany: Springer Science & Business Media, 2011. [Google Scholar]
- Duquesne S, Destoumieux-Garzón D, Peduzzi J et al. Microcins, gene-encoded antibacterial peptides from enterobacteria. Nat Prod Rep. 2007;24:708–34. [DOI] [PubMed] [Google Scholar]
- Dykes G, Hastings J. Fitness costs associated with class IIa bacteriocin resistance in Listeria monocytogenes B73. Lett Appl Microbiol. 1998;26:5–8. [DOI] [PubMed] [Google Scholar]
- Egan K, Ross RP, Hill C. Bacteriocins: antibiotics in the age of the microbiome. Emerg Top Life Sci. 2017;1:55–63. [DOI] [PubMed] [Google Scholar]
- Falagas ME, Grammatikos AP, Michalopoulos A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert Rev Anti Infect Ther. 2008;6:593–600. [DOI] [PubMed] [Google Scholar]
- Farber JM. Food safety advice for the soul. Food Prot. 2016;79 (Suppl. A):2. [Google Scholar]
- Fatima D, Mebrouk K. Characterization and determination of the factors affecting anti-listerial bacteriocins from Lactobacillus plantarum and Pediococcus pentosaceus isolated from dairy milk products. Afr J Food Sci. 2013;7:35–44. [Google Scholar]
- Favaro L, Todorov SD. Bacteriocinogenic LAB strains for fermented meat preservation: Perspectives, challenges, and limitations. Probiotics Antimicrob Proteins. 2017;9:444–58. [DOI] [PubMed] [Google Scholar]
- FDA Ingredients & additives. 2018. Available at https://www.fda.gov/food/food-ingredients-packaging/determining-regulatory-status-food-ingredient (10 March 2020, date last accessed). [Google Scholar]
- Fernandez B, Le Lay C, Jean J et al. Growth, acid production and bacteriocin production by probiotic candidates under simulated colonic conditions. J Appl Microbiol. 2013;114:877–85. [DOI] [PubMed] [Google Scholar]
- Fernández L, Delgado S, Herrero H et al. The bacteriocin nisin, an effective agent for the treatment of staphylococcal mastitis during lactation. J Hum Lact. 2008;24:311–6. [DOI] [PubMed] [Google Scholar]
- Field D, Blake T, Mathur H et al. Bioengineering nisin to overcome the nisin resistance protein. Mol Microbiol. 2019;111:717–31. [DOI] [PubMed] [Google Scholar]
- Field D, Cotter PD, Hill C et al. Bioengineering lantibiotics for therapeutic success. Front Microbiol. 2015;6:1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francino M. Antibiotics and the human gut microbiome: dysbioses and accumulation of resistances. Front Microbiol. 2016;6:1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A, Sharratt M, Hickman J. The biological effects of food additives. I. Nisin. J Sci Food Agr. 1962;13: 32–42. [Google Scholar]
- Fujiwara S, Hashiba H, Hirota T et al. Inhibition of the binding of enterotoxigenic Escherichia coli Pb176 to human intestinal epithelial cell line HCT-8 by an extracellular protein fraction containing BIF of Bifidobacterium longum SBT2928: suggestive evidence of blocking of the binding receptor gangliotetraosylceramide on the cell surface. Int J Food Microbiol. 2001;67:97–106. [DOI] [PubMed] [Google Scholar]
- Fujiwara S, Hashiba H, Hirota T et al. Proteinaceous factor(s) in culture supernatant fluids of bifidobacteria which prevents the binding of enterotoxigenic Escherichia coli to gangliotetraosylceramide. Appl Environ Microbiol. 1997;63:506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Férir G, Petrova MI, Andrei G et al. The lantibiotic peptide labyrinthopeptin A1 demonstrates broad anti-HIV and anti-HSV activity with potential for microbicidal applications. PLoS One. 2013;8:e64010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielsen C, Brede DA, Nes IF et al. Circular bacteriocins: biosynthesis and mode of action. Appl Environ Microbiol. 2014;80:6854–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharsallaoui A, Oulahal N, Joly C et al. Nisin as a food preservative: part 1: physicochemical properties, antimicrobial activity, and main uses. Crit Rev Food Sci Nutr. 2016;56:1262–74. [DOI] [PubMed] [Google Scholar]
- Ghobrial OG, Derendorf H, Hillman JD. Pharmacodynamic activity of the lantibiotic MU1140. Int J Antimicrob Agents. 2009;33: 70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillor O, Kirkup BC, Riley MA. Colicins and microcins: the next generation antimicrobials. Adv Appl Microbiol. 2004;54:129–46. [DOI] [PubMed] [Google Scholar]
- Gomaa AI, Martinent C, Hammami R et al. Dual coating of liposomes as encapsulating matrix of antimicrobial peptides: development and characterization. Front Chem. 2017;5:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough R, Cabrera Rubio R, O'Connor PM et al. Oral delivery of nisin in resistant starch based matrices alter the gut microbiota in mice. Front Microbiol. 2018;9:1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gough R, O'Connor PM, Rea MC et al. Simulated gastrointestinal digestion of nisin and interaction between nisin and bile. LWT Food Sci Technol. 2017;86:530–7. [Google Scholar]
- Gravesen A, Kallipolitis B, Holmstrøm K et al. pbp2229-mediated nisin resistance mechanism in Listeria monocytogenes confers cross-protection to class IIa bacteriocins and affects virulence gene expression. Appl Environ Microbiol. 2004;70:1669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravesen A, Ramnath M, Rechinger KB et al. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiol. 2002;148:2361–9. [DOI] [PubMed] [Google Scholar]
- Gravesen A, Sørensen K, Aarestrup FM et al. Spontaneous nisin-resistant Listeria monocytogenes mutants with increased expression of a putative penicillin-binding protein and their sensitivity to various antibiotics. Microb Drug Resist. 2001;7:127–35. [DOI] [PubMed] [Google Scholar]
- Grilli E, Messina M, Catelli E et al. Pediocin A improves growth performance of broilers challenged with Clostridium perfringens. Poult Sci. 2009;88:2152–8. [DOI] [PubMed] [Google Scholar]
- Guinane CM, Cotter PD, Hill C et al. Spontaneous resistance in Lactococcus lactis IL1403 to the lantibiotic lacticin 3147. FEMS Microbiol Lett. 2006;260:77–83. [DOI] [PubMed] [Google Scholar]
- Guo M, Jin TZ, Wang L et al. Antimicrobial films and coatings for inactivation of Listeria innocua on ready-to-eat deli turkey meat. Food Control. 2014;40:64–70. [Google Scholar]
- Gálvez A, Abriouel H, López RL et al. Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol. 2007;120:51–70. [DOI] [PubMed] [Google Scholar]
- Gálvez A, Abriouel H, Omar NB et al. Food applications and regulation. In: Prokaryotic Antimicrobial Peptides, New York: Springer, 2011, 353–90. [Google Scholar]
- Gálvez A, Lucas R, Abriouel H et al. Bacteriocins. In: Decontamination of Fresh and Minimally Processed Produce. New York: John Wiley & Sons, 2012, 317. [Google Scholar]
- Habib W, Sakr A. Development and human in vivo evaluation of a colonic drug delivery system. Pharm Ind. 1999;61:1145–9. [Google Scholar]
- Hagiwara A, Imai N, Nakashima H et al. A 90-day oral toxicity study of nisin A, an anti-microbial peptide derived from Lactococcus lactis subsp. lactis, in F344 rats. Food Chem Toxicol. 2010;48:2421–8. [DOI] [PubMed] [Google Scholar]
- Hammami R, Fernandez B, Lacroix C et al. Anti-infective properties of bacteriocins: an update. Cell Mol Life Sci. 2013;70:2947–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammami R, Zouhir A, Le Lay C et al. BACTIBASE second release: a database and tool platform for bacteriocin characterization. BMC Microbiol. 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanchi H, Hammami R, Gingras H et al. Inhibition of MRSA and of Clostridium difficile by durancin 61A: synergy with bacteriocins and antibiotics. Future Microbiol. 2017;12:205–12. [DOI] [PubMed] [Google Scholar]
- Harris LJ, Fleming HP, Klaenhammer TR. Sensitivity and resistance of Listeria monocytogenes ATCC 19115, Scott A, and UAL500 to nisin. J Food Prot. 1991;54:836–40. [DOI] [PubMed] [Google Scholar]
- Hasper HE, Kramer NE, Smith JL et al. An alternative bactericidal mechanism of action for lantibiotic peptides that target lipid II. Science. 2006;313:1636–7. [DOI] [PubMed] [Google Scholar]
- Hassan H, Gomaa A, Subirade M et al. Novel design for alginate/resistant starch microcapsules controlling nisin release. Int J Biol Macromol. 2019;153:1186–92. [DOI] [PubMed] [Google Scholar]
- Hatakka K, Saxelin M. Probiotics in intestinal and non-intestinal infectious diseases – clinical evidence. Curr Pharm Design. 2008;14:1351–67. [DOI] [PubMed] [Google Scholar]
- Heinemann B, Williams R. Inactivation of nisin by pancreatin. J Dairy Sci. 1966;49:312–4. [DOI] [PubMed] [Google Scholar]
- Hetz C, Bono MR, Barros LF et al. Microcin E492, a channel-forming bacteriocin from Klebsiella pneumoniae, induces apoptosis in some human cell lines. Proc Natl Acad Sci. 2002;99:2696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heunis TD, Smith C, Dicks LM. Evaluation of a nisin-eluting nanofiber scaffold to treat Staphylococcus aureus-induced skin infections in mice. Antimicrob Agents Chemother. 2013;57:3928–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heu S, Oh J, Kang Y et al. gly Gene cloning and expression and purification of glycinecin A, a bacteriocin produced by Xanthomonas campestris pv. glycines 8ra. Appl Environ Microbiol. 2001;67:4105–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. BBA Biomembranes. 2008;1778:357–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ma L, Nie Y et al. A microbiota-derived bacteriocin targets the host to confer diarrhea resistance in early-weaned piglets. Cell Host Microbe. 2018;24:817–32.e8. [DOI] [PubMed] [Google Scholar]
- Héchard Y, Sahl H-G. Mode of action of modified and unmodified bacteriocins from Gram-positive bacteria. Biochimie. 2002;84:545–57. [DOI] [PubMed] [Google Scholar]
- Jabés D, Brunati C, Candiani G et al. Efficacy of the new lantibiotic NAI-107 in experimental infections induced by multidrug-resistant Gram-positive pathogens. Antimicrob Agents Chemother. 2011;55:1671–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack RW, Tagg JR, Ray B. Bacteriocins of gram-positive bacteria. Microbiol Mol Biol Rev. 1995;59:171–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R, Lazdunski C, Pattus F. Bacteriocins, Microcins and Lantibiotics, Vol. 65, Berlin Heidelberg: Springer Science & Business Media, 2013. [Google Scholar]
- Jarvis B, Mahoney R. Inactivation of nisin by alpha-chymotrypsin. J Dairy Sci. 1969;52:1448–50. [DOI] [PubMed] [Google Scholar]
- Jasniewski J, Cailliez-Grimal C, Chevalot I et al. Interactions between two carnobacteriocins Cbn BM1 and Cbn B2 from Carnobacterium maltaromaticum CP5 on target bacteria and Caco-2 cells. Food Chem Toxicol. 2009;47:893–7. [DOI] [PubMed] [Google Scholar]
- Jaspars M. The origins of cyanobactin chemistry and biology. Chem Comm. 2014;50:10174–6. [DOI] [PubMed] [Google Scholar]
- Johnson EM, Jung DY-G, Jin DY-Y et al. Bacteriocins as food preservatives: challenges and emerging horizons. Crit Rev Food Sci Nutr. 2018;58:2743–67. [DOI] [PubMed] [Google Scholar]
- Joo NE, Ritchie K, Kamarajan P et al. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012;1:295–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan P, Hayami T, Matte B et al. Nisin ZP, a bacteriocin and food preservative, inhibits head and neck cancer tumorigenesis and prolongs survival. PLoS One. 2015;10:e0131008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang BS, Seo J-G, Lee G-S et al. Antimicrobial activity of enterocins from Enterococcus faecalis SL-5 against Propionibacterium acnes, the causative agent in acne vulgaris, and its therapeutic effect. J Microbiol. 2009;47:101–9. [DOI] [PubMed] [Google Scholar]
- Kanmani P, Satish Kumar R, Yuvaraj N et al. Probiotics and its functionally valuable products – a review. Crit Rev Food Sci Nutr. 2013;53:641–58. [DOI] [PubMed] [Google Scholar]
- Karpiński TM, Szkaradkiewicz AK, Caballero B et al. Bacteriocins. In: Encyclopedia of Food and Health. Amsterdam: Elsivier Science, 2016, 312–9. [Google Scholar]
- Katla T, Naterstad K, Vancanneyt M et al. Differences in susceptibility of Listeria monocytogenes strains to sakacin P, sakacin A, pediocin PA-1, and nisin. Appl Environ Microbiol. 2003;69:4431–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur B, Balgir PP, Mittu B et al. Biomedical applications of fermenticin HV6b isolated from Lactobacillus fermentum HV6b MTCC10770. Biomed Res Int. 2013;2013:168438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur G, Singh TP, Malik RK et al. Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against L. monocytogenes, E. faecium and E. faecalis and bacteriocin cross resistance and antibiotic susceptibility of their bacteriocin resistant variants. Int J Food Sci Tech. 2014;51:233–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur S, Kaur S.. Bacteriocins as potential anticancer agents. Front Pharmacol. 2015;6:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheadr E, Zihler A, Dabour N et al. Study of the physicochemical and biological stability of pediocin PA‐1 in the upper gastrointestinal tract conditions using a dynamic in vitro model. J Appl Microbiol. 2010;109:54–64. [DOI] [PubMed] [Google Scholar]
- Kicza A, Pureka C, Proctor D et al. The phenotypic and genotypic landscape of colicin resistance. Bacteriocins. 2016;1:141. [Google Scholar]
- Kim S, Shin S, Koo H et al. In vitro antimicrobial effect and in vivo preventive and therapeutic effects of partially purified lantibiotic lacticin NK34 against infection by Staphylococcus species isolated from bovine mastitis. J Dairy Sci. 2010;93:3610–5. [DOI] [PubMed] [Google Scholar]
- Kim T-S, Hur J-W, Yu M-A et al. Antagonism of Helicobacter pylori by bacteriocins of lactic acid bacteria. J Food Prot. 2003;66:3–12. [DOI] [PubMed] [Google Scholar]
- Kitazaki K, Baba T, Koga Y et al. The use of nisin A in preventing bovine mastitis infection. Food Foods Ingred J JPN. 2010;215:449–56. [Google Scholar]
- Kitching M, Mathur H, Flynn J et al. A live bio-therapeutic for mastitis, containing Lactococcus lactis DPC3147 with comparable efficacy to antibiotic treatment. Front Microbiol. 2019;10:2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjos M, Borrero J, Opsata M et al. Target recognition, resistance, immunity and genome mining of class II bacteriocins from Gram-positive bacteria. Microbiol. 2011;157:3256–67. [DOI] [PubMed] [Google Scholar]
- Klaenhammer TR. Bacteriocins of lactic acid bacteria. Biochimie. 1988;70:337–49. [DOI] [PubMed] [Google Scholar]
- Kouwen TR, Trip EN, Denham EL et al. The large mechanosensitive channel MscL determines bacterial susceptibility to the bacteriocin sublancin 168. Antimicrob Agents Chemother. 2009;53:4702–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer NE, van Hijum SA, Knol J et al. Transcriptome analysis reveals mechanisms by which Lactococcus lactis acquires nisin resistance. Antimicrob Agents Chemother. 2006;50:1753–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivorotova T, Cirkovas A, Maciulyte S et al. Nisin-loaded pectin nanoparticles for food preservation. Food Hydrocoll. 2016;54:49–56. [Google Scholar]
- Kumarasamy KK, Toleman MA, Walsh TR et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10:597–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagha AB, Haas B, Gottschalk M et al. Antimicrobial potential of bacteriocins in poultry and swine production. Vet Res. 2017;48:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos R, Tello M, Mercado G et al. Antibacterial and antitumorigenic properties of microcin E492, a pore-forming bacteriocin. Curr Pharm Biotechnol. 2009;10:74–85. [DOI] [PubMed] [Google Scholar]
- Lancaster LE, Wintermeyer W, Rodnina MV. Colicins and their potential in cancer treatment. Blood Cell Mol Dis. 2007;38:15–8. [DOI] [PubMed] [Google Scholar]
- Lange-Starke A, Petereit A, Truyen U et al. Antiviral potential of selected starter cultures, bacteriocins and d,l-lactic acid. Food Environ Virol. 2014;6:42–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laverty G, Gilmore B. Cationic antimicrobial peptide cytotoxicity. SOJ Microbiol Infect Dis. 2014;2:1–8.29756026 [Google Scholar]
- Le Blay G, Hammami R, Lacroix C et al. Stability and inhibitory activity of pediocin PA-1 against Listeria sp. in simulated physiological conditions of the human terminal ileum. Probiotics Antimicrob Proteins. 2012;4:250–8. [DOI] [PubMed] [Google Scholar]
- Le Lay C, Dridi L, Bergeron MG et al. Nisin is an effective inhibitor of Clostridium difficile vegetative cells and spore germination. J Med Microbiol. 2016;65:169–75. [DOI] [PubMed] [Google Scholar]
- Le Lay C, Fernandez B, Hammami R et al. On Lactococcus lactis UL719 competitivity and nisin (Nisaplin®) capacity to inhibit Clostridium difficile in a model of human colon. Front Microbiol. 2015;6:1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohans CT, Vederas JC. Development of class IIa bacteriocins as therapeutic agents. Int J Microbiol. 2011;2012:386410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez FE, Vincent PA, Zenoff AM et al. Efficacy of microcin J25 in biomatrices and in a mouse model of Salmonella infection. J Antimicrob Chemother. 2007;59:676–80. [DOI] [PubMed] [Google Scholar]
- López-Cuellar MdR, Rodríguez-Hernández A-I, Chavarría-Hernández N. LAB bacteriocin applications in the last decade. Biotechnol Biotechnol Equip. 2016;30:1039–50. [Google Scholar]
- Maher S, McClean S. Investigation of the cytotoxicity of eukaryotic and prokaryotic antimicrobial peptides in intestinal epithelial cells in vitro. Biochem Pharmacol. 2006;71:1289–98. [DOI] [PubMed] [Google Scholar]
- Mantovani HC, Russell JB. Nisin resistance of Streptococcus bovis. Appl Environ Microbiol. 2001;67:808–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlida Y, Arnim A, Yuherman Y et al. Toxicity test pediocin N6 powder produced from isolates Pediococcus Pentosaceus strain N6 on white mice. J Food Pharm Sci. 2016;4:12–6. [Google Scholar]
- Martins J, Leikoski N, Wahlsten M et al. Sphaerocyclamide, a prenylated cyanobactin from the Cyanobacterium Sphaerospermopsis sp. LEGE 00249. Sci Rep. 2018;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins J, Vasconcelos V. Cyanobactins from cyanobacteria: current genetic and chemical state of knowledge. Mar Drugs. 2015;13:6910–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez B, Rodríguez A. Antimicrobial susceptibility of nisin resistant Listeria monocytogenes of dairy origin. FEMS Microbiol Lett. 2005;252:67–72. [DOI] [PubMed] [Google Scholar]
- Mathur H, Field D, Rea MC et al. Bacteriocin-antimicrobial synergy: a medical and food perspective. Front Microbiol. 2017;8:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May M. Time for teamwork. Nature. 2014;509:S4. [DOI] [PubMed] [Google Scholar]
- Małaczewska J, Kaczorek‐Łukowska E, Wójcik R et al. In vitro immunomodulatory effect of nisin on porcine leucocytes. J Anim Physiol Anim Nutr. 2019;103:882–93. [DOI] [PubMed] [Google Scholar]
- McAuliffe O, Ross RP, Hill C. Lantibiotics: structure, biosynthesis and mode of action. FEMS Microbiol Rev. 2001;25:285–308. [DOI] [PubMed] [Google Scholar]
- McCaughey LC, Ritchie ND, Douce GR et al. Efficacy of species-specific protein antibiotics in a murine model of acute Pseudomonas aeruginosa lung infection. Sci Rep. 2016;6:30201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meindl K, Schmiederer T, Schneider K et al. Labyrinthopeptins: a new class of carbacyclic lantibiotics. Angew Chem Int Ed. 2010;49:1151–4. [DOI] [PubMed] [Google Scholar]
- Millette M, Cornut G, Dupont C et al. Capacity of human nisin- and pediocin-producing lactic acid bacteria to reduce intestinal colonization by vancomycin-resistant enterococci. Appl Environ Microbiol. 2008;74:1997–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills S, Stanton C, Hill C et al. New developments and applications of bacteriocins and peptides in foods. Annu Rev Food Sci Technol. 2011;2:299–329. [DOI] [PubMed] [Google Scholar]
- Mokoena MP. Lactic acid bacteria and their bacteriocins: classification, biosynthesis and applications against uropathogens: a mini-review. Molecules. 2017;22:1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy E, Hill C, Cotter P et al. Bacteriocins. : Fuquay JW, Fox PF, McSweeney PLH (). Encyclopedia of Dairy Sciences, 2nd edn Cambridge: Academic Press, 2011, 420–9. [Google Scholar]
- Murinda S, Rashid K, Roberts R. In vitro assessment of the cytotoxicity of nisin, pediocin, and selected colicins on simian Virus 40-transfected human colon and Vero monkey kidney cells with trypan blue staining viability assays. J Food Prot. 2003;66:847–53. [DOI] [PubMed] [Google Scholar]
- Murphy EF, Cotter PD, Hogan A et al. Divergent metabolic outcomes arising from targeted manipulation of the gut microbiota in diet-induced obesity. Gut. 2013;62:220–6. [DOI] [PubMed] [Google Scholar]
- Naghmouchi K, Kheadr E, Lacroix C et al. Class I/Class IIa bacteriocin cross-resistance phenomenon in Listeria monocytogenes. Food Microbiol. 2007;24:718–27. [DOI] [PubMed] [Google Scholar]
- Naimi S, Zirah S, Hammami R et al. Fate and biological activity of the antimicrobial lasso peptide microcin J25 under gastrointestinal tract conditions. Front Microbiol. 2018;9:1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nes IF, Gabrielsen C, Brede DA et al. Novel developments in bacteriocins from lactic acid bacteria. In: Biotechnology of Lactic Acid Bacteria: Novel Applications, 2nd edn New York: John Wiley & Sons, 2015, 80–99. [Google Scholar]
- Nocek B, Tikhonov A, Babnigg G et al. Structural and functional characterization of microcin C resistance peptidase MccF from Bacillus anthracis. J Mol Biol. 2012;420:366–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris GE, Patchett ML. The glycocins: in a class of their own. Curr Opin Struc Biol. 2016;40:112–9. [DOI] [PubMed] [Google Scholar]
- O'Neill L. BVA welcomes report on tackling drug-resistant infections. Vet Rec. 2016;178:590. [DOI] [PubMed] [Google Scholar]
- O'Shea EF, O'Connor PM, Cotter PD et al. Synthesis of trypsin-resistant variants of the Listeria-active bacteriocin salivaricin P. Appl Environ Microbiol. 2010;76:5356–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'sullivan L, Ross R, Hill C. A lacticin 481‐producing adjunct culture increases starter lysis while inhibiting nonstarter lactic acid bacteria proliferation during Cheddar cheese ripening. J Appl Microbiol. 2003;95:1235–41. [DOI] [PubMed] [Google Scholar]
- OECD Guideline for testing of chemicals: acute oral toxicity: up-and-down procedure. 2008a;Section 4, Part 425:27. [Google Scholar]
- OECD Guidline for testing of chemicals: repeated dose 28-day oral toxicity study in rodents. 2008b;Section 4, Part 407:13. [Google Scholar]
- Olejnik-Schmidt AK, Schmidt MT, Sip A et al. Expression of bacteriocin divercin AS7 in Escherichia coli and its functional analysis. Ann Microbiol. 2014;64:1197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oman TJ, van der Donk WA. Insights into the mode of action of the two-peptide lantibiotic haloduracin. ACS Chem Biol. 2009;4:865–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongey EL, Neubauer P. Lanthipeptides: chemical synthesis versus in vivo biosynthesis as tools for pharmaceutical production. Microb Cell Fact. 2016;15:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongey EL, Yassi H, Pflugmacher S et al. Pharmacological and pharmacokinetic properties of lanthipeptides undergoing clinical studies. Biotechnol Lett. 2017;39:473–82. [DOI] [PubMed] [Google Scholar]
- Pablo MA, Gaforio JJ, Gallego AM et al. Evaluation of immunomodulatory effects of nisin-containing diets on mice. FEMS Immunol Med Mic. 1999;24:35–42. [DOI] [PubMed] [Google Scholar]
- Padilla C, Lobos O, Hubert E. Shigella flexneri strains produce bacteriocins active against members of the human microbial intestinal flora. Rev Latinom Microbiol. 2004;46:85–8. [PubMed] [Google Scholar]
- Paiva AD, de Oliveira MD, de Paula SO et al. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology. 2012;158:2851–8. [DOI] [PubMed] [Google Scholar]
- Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez RH, Zendo T, Sonomoto K. Novel bacteriocins from lactic acid bacteria (LAB): various structures and applications. Microb Cell Fact. 2014;13:S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessione E. Fighting off human infections: a new role for bacteriocin molecules. In: Interactive Probiotics. Boca Raton: CRC Press, 2014, 30–59. [Google Scholar]
- Pieterse R, Todorov SD, Dicks LM. Mode of action and in vitro susceptibility of mastitis pathogens to macedocin ST91KM and preparation of a teat seal containing the bacteriocin. Braz J Microbiol. 2010;41:133–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper C, Draper LA, Cotter PD et al. A comparison of the activities of lacticin 3147 and nisin against drug-resistant Staphylococcus aureus and Enterococcus species. J Antimicrob Chemother. 2009;64:546–51. [DOI] [PubMed] [Google Scholar]
- Pomares MF, Salomón RA, Pavlova O et al. Potential applicability of chymotrypsin-susceptible microcin J25 derivatives to food preservation. Appl Environ Microbiol. 2009;75:5734–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulse ME, Weiss WJ, Kers JA et al. Pharmacological, toxicological, and dose range assessment of OG716, a novel lantibiotic for the treatment of Clostridium difficile-associated infection. Antimicrob Agents Chemother. 2019;63:e01904–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putaala H, Salusjärvi T, Nordström M et al. Effect of four probiotic strains and Escherichia coli O157: H7 on tight junction integrity and cyclo-oxygenase expression. Res Microbiol. 2008;159:692–8. [DOI] [PubMed] [Google Scholar]
- Quintana VM, Torres NI, Wachsman MB et al. Antiherpes simplex virus type 2 activity of the antimicrobial peptide subtilosin. J Appl Microbiol. 2014;117:1253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramnath M, Beukes M, Tamura K et al. Absence of a putative mannose-specific phosphotransferase system enzyme IIAB component in a leucocin A-resistant strain of Listeria monocytogenes, as shown by two-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Appl Environ Microbiol. 2000;66:3098–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramu R, Shirahatti PS, Devi AT et al. Bacteriocins and their applications in food preservation. Crit Rev Food Sci Nutr. 2015, 10.1080/10408398.2015.1020918. [DOI] [Google Scholar]
- Rasch M, Knöchel S. Variations in tolerance of Listeria monocytogenes to nisin, pediocin PA‐1 and bavaricin A. Lett Appl Microbiol. 1998;27:275–8. [DOI] [PubMed] [Google Scholar]
- Rea MC, Clayton E, O'connor PM et al. Antimicrobial activity of lacticin 3147 against clinical Clostridium difficile strains. J Med Microbiol. 2007;56:940–6. [DOI] [PubMed] [Google Scholar]
- Rea MC, Dobson A, O'Sullivan O et al. Effect of broad- and narrow-spectrum antimicrobials on Clostridium difficile and microbial diversity in a model of the distal colon. Proc Natl Acad Sci USA. 2011;108:4639–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea MC, Sit CS, Clayton E et al. Thuricin CD, a posttranslationally modified bacteriocin with a narrow spectrum of activity against Clostridium difficile. Proc Natl Acad Sci USA. 2010;107:9352–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy K, Aranha C, Gupta S et al. Evaluation of antimicrobial peptide nisin as a safe vaginal contraceptive agent in rabbits: in vitro and in vivo studies. Reproduction. 2004;128:117–26. [DOI] [PubMed] [Google Scholar]
- Riboulet-Bisson E, Sturme MH, Jeffery IB et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS One. 2012;7:e31113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley MA, Wertz JE. Bacteriocins: evolution, ecology, and application. Annu Rev Microbiol. 2002;56:117–37. [DOI] [PubMed] [Google Scholar]
- Rollema HS, Kuipers OP, Both P et al. Improvement of solubility and stability of the antimicrobial peptide nisin by protein engineering. Appl Environ Microbiol. 1995;61:2873–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo TK, Jena PK, Prajapati B et al. In vivo assessment of immunogenicity and toxicity of the bacteriocin TSU4 in BALB/c mice. Probiotics Antimicrob Proteins. 2017;9:345–54. [DOI] [PubMed] [Google Scholar]
- Sandiford SK. Perspectives on lantibiotic discovery – where have we failed and what improvements are required?, Expert Opin Drug Discov. 2015;10:315–20. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi M, Nuccio S-P, Liu H et al. Microcins mediate competition among Enterobacteriaceae in the inflamed gut. Nature. 2016;540:280–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholl D, Martin DW. Antibacterial efficacy of R-type pyocins towards Pseudomonas aeruginosa in a murine peritonitis model. Antimicrob Agents Chemother. 2008;52:1647–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgley CM, Clewell DB, Flannagan SE. Plasmid pAMS1-encoded, bacteriocin-related “Siblicide” in Enterococcus faecalis. J Bacteriol. 2009;191:3183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shand RF, Leyva KJ. Archaeal antimicrobials: an undiscovered country. In: Archaea: New Models for Prokaryotic Biology. 2008, UK: Caister Academic Press. [Google Scholar]
- Silkin L, Hamza S, Kaufman S et al. Spermicidal bacteriocins: lacticin 3147 and subtilosin A. Bioorg Med Chem Lett. 2008;18:3103–6. [DOI] [PubMed] [Google Scholar]
- Son H-K, Chang H-C, Lee J-J. Acute and subacute oral toxicity evaluation of crude antifungal compounds produced by Lactobacillus plantarum HD1 in Rats. Prev Nutr Food Sci. 2015;20:190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosio M, Donadio S, Maffioli S et al. LAPTOP: lantibiotic production, technology, optimization and improved process. In: 12th International Symposium on the Genetics of Industrial Microorganisms (GIM-2013), 2013. [Google Scholar]
- Srinivasan B, Kolli AR, Esch MB et al. TEER measurement techniques for in vitro barrier model systems. J Lab Autom. 2015;20:107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavric S, D'aoust J-Y. Undefined and defined bacterial preparations for the competitive exclusion of Salmonella in poultry – a review. J Food Prot. 1993;56:173–80. [DOI] [PubMed] [Google Scholar]
- Stern NJ, Svetoch EA, Eruslanov BV et al. Paenibacillus polymyxa purified bacteriocin to control Campylobacter jejuni in chickens. J Food Prot. 2005;68:1450–3. [DOI] [PubMed] [Google Scholar]
- Sun Z, Zhong J, Liang X et al. Novel mechanism for nisin resistance via proteolytic degradation of nisin by the nisin resistance protein NSR. Antimicrob Agents Chemother. 2009;53:1964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su P, Henriksson A, Mitchell H. Survival and retention of the probiotic Lactobacillus casei LAFTI® L26 in the gastrointestinal tract of the mouse. Lett Appl Microbiol. 2007;44:120–5. [DOI] [PubMed] [Google Scholar]
- Sutyak KE, Anderson RA, Dover SE et al. Spermicidal activity of the safe natural antimicrobial peptide subtilosin. Infect Dis Obstet Gynecol. 2008;2008:540758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetoch EA, Eruslanov BV, Perelygin VV et al. Diverse antimicrobial killing by Enterococcus faecium E50–52 bacteriocin. J Agr Food Chem. 2008;56:1942–8. [DOI] [PubMed] [Google Scholar]
- Swain MR, Anandharaj M, Ray RC et al. Fermented fruits and vegetables of Asia: a potential source of probiotics. Biotechnol Res Int. 2014;2014:250424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas L, Clarkson M, Delves-Broughton J. Natural Food Antimicrobial Systems. Washington D.C.: CRC Press, 2000, 265–93. [Google Scholar]
- Thomas LV, Delves-Broughton J. New Advances in the Application of the Food Preservative Nisin. Res Adv Food Sci. 2001;2:11–22. [Google Scholar]
- Thomas LV, Ingram RE, Bevis HE et al. Effective use of nisin to control Bacillus and Clostridium spoilage of a pasteurized mashed potato product. J Food Prot. 2002;65:1580–5. [DOI] [PubMed] [Google Scholar]
- Todorov SD, Wachsman M, Tomé E et al. Characterisation of an antiviral pediocin-like bacteriocin produced by Enterococcus faecium. Food Microbiol. 2010;27:869–79. [DOI] [PubMed] [Google Scholar]
- Todorov SD. Bacteriocins from Lactobacillus plantarum production, genetic organization and mode of action: produção, organização genética e modo de ação. Braz J Microbiol. 2009;40:209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Ni L, Ling J. Antibacterial peptide nisin: a potential role in the inhibition of oral pathogenic bacteria. Peptides. 2014;60:32–40. [DOI] [PubMed] [Google Scholar]
- Turgis M, Khanh D, Majid J et al. Synergistic antimicrobial effect of combined bacteriocins against food pathogens and spoilage bacteria. Microb Res Inter. 2016;4:1–5. [Google Scholar]
- Udompijitkul P, Paredes‐Sabja D, Sarker MR. Inhibitory effects of nisin against Clostridium perfringens food poisoning and nonfood‐borne isolates. J Food Sci. 2012;77:M51–6. [DOI] [PubMed] [Google Scholar]
- Umu ÖC, Bäuerl C, Oostindjer M et al. The potential of class II bacteriocins to modify gut microbiota to improve host health. PLoS One. 2016;11:e0164036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heel AJ, Montalban-Lopez M, Kuipers OP. Evaluating the feasibility of lantibiotics as an alternative therapy against bacterial infections in humans. Expert Opin Drug Metab Toxicol. 2011;7:675–80. [DOI] [PubMed] [Google Scholar]
- Van Schaik W, Gahan CG, Hill C. Acid-adapted Listeria monocytogenes displays enhanced tolerance against the lantibiotics nisin and lacticin 3147. J Food Prot. 1999;62:536–40. [DOI] [PubMed] [Google Scholar]
- Van Staden A, Brand A, Dicks L. Nisin F‐loaded brushite bone cement prevented the growth of Staphylococcus aureus in vivo. J Appl Microbiol. 2012;112:831–40. [DOI] [PubMed] [Google Scholar]
- Vaucher AR, Velho CGC, Correa A et al. Evaluation of the immunogenicity and in vivo toxicity of the antimicrobial peptide P34. Int J Pharm. 2011;421:94–8. [DOI] [PubMed] [Google Scholar]
- Verma A, Banerjee R, Dwivedi H et al. Bacteriocins: potential in food preservation. In: Encyclopedia of Food Microbiology. United States: Academic Press, Elsivier Science, 2014, 180–6. [Google Scholar]
- Vignolo G, Palacios J, Farías ME et al. Combined effect of bacteriocins on the survival of various Listeria species in broth and meat system. Curr Microbiol. 2000;41:410–6. [DOI] [PubMed] [Google Scholar]
- Villarante KI, Elegado FB, Iwatani S et al. Purification, characterization and in vitro cytotoxicity of the bacteriocin from Pediococcus acidilactici K2a2-3 against human colon adenocarcinoma (HT29) and human cervical carcinoma (HeLa) cells. World J Microbiol Biotechnol. 2011;27:975–80. [Google Scholar]
- Wachsman MB, Castilla V, de Ruiz Holgado AP et al. Enterocin CRL35 inhibits late stages of HSV-1 and HSV-2 replication in vitro. Antivir Res. 2003;58:17–24. [DOI] [PubMed] [Google Scholar]
- Wessman P, Strömstedt AA, Malmsten M et al. Melittin-lipid bilayer interactions and the role of cholesterol. Biophys J. 2008;95:4324–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilaipun P, Zendo T, Sangjindavong M et al. The two-synergistic peptide bacteriocin produced by Enterococcus faecium NKR-5-3 isolated from Thai fermented fish (Pla-ra). Sci Asia. 2004;30:115–22. [Google Scholar]
- Willey JM, Van Der Donk WA. Lantibiotics: peptides of diverse structure and function. Annu Rev Microbiol. 2007;61:477–501. [DOI] [PubMed] [Google Scholar]
- Wu C, Biswas S, Garcia De Gonzalo CV et al. Investigations into the mechanism of action of sublancin. ACS Infect Dis. 2018;5:454–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xavier J, Gopalan N, Ramana K. Immobilization of lactic acid bacteria and application of bacteriocin for preservation of fruit juices and bacteriocin production. Defence Life Sci J. 2017;2:231–8. [Google Scholar]
- Yamada T, Hiraoka Y, Ikehata M et al. Apoptosis or growth arrest: modulation of tumor suppressor p53’s specificity by bacterial redox protein azurin. Proc Natl Acad Sci USA. 2004;101:4770–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E, Fan L, Yan J et al. Influence of culture media, pH and temperature on growth and bacteriocin production of bacteriocinogenic lactic acid bacteria. Amb Express. 2018;8:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S-C, Lin C-H, Sung CT et al. Antibacterial activities of bacteriocins: application in foods and pharmaceuticals. Front Microbiol. 2014;5:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Ding X, Li N et al. Dietary supplemented antimicrobial peptide microcin J25 improves the growth performance, apparent total tract digestibility, fecal microbiota, and intestinal barrier function of weaned pigs. J Anim Sci. 2017;95:5064–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Li N, Zeng X et al. A comprehensive antimicrobial activity evaluation of the recombinant microcin J25 against the foodborne pathogens Salmonella and E. coli O157:H7 by using a matrix of conditions. Front Microbiol. 2019;10:1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H, Xie Y, Liu H et al. Effects of two application methods of plantaricin BM-1 on control of Listeria monocytogenes and background spoilage bacteria in sliced vacuum-packaged cooked ham stored at 4°C. J Food Prot. 2015;78:1835–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.