Abstract
Background
Abemaciclib demonstrated efficacy in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer. Here we provide a comprehensive summary of the most common adverse events (AEs), their management, and whether AEs or dose reductions influenced progression‐free survival (PFS), in the MONARCH 2 and 3 trials.
Materials and Methods
Incidence of the most clinically relevant AEs, management, and outcomes were summarized. Time‐dependent covariate analyses examined the impact of dose reductions on PFS. PFS was estimated for patients with and without early onset of diarrhea or neutropenia.
Results
The most frequently reported AE was diarrhea, with clinically significant diarrhea (grade ≥2) reported for 42.8% of patients taking abemaciclib. Median time to onset was 1 week, and duration ranged from 6 to 12 days, depending on grade and study. Diarrhea was adequately managed by antidiarrheal medication (72.8%), dose omissions (17.3%), and reductions (16.7%). The highest rates of grade ≥2 diarrhea were observed in the first cycles and decreased in subsequent cycles. Neutropenia (grade ≥3) occurred in 25.4% of abemaciclib‐treated patients. Neutropenia resolved with dose omissions (16.8%) and/or dose reductions (11.2%). Incidence of febrile neutropenia (0.7%) or other relevant grade ≥3 hematological events (<9%) was low. Venous thromboembolic events (5.3%) were primarily treated with anticoagulants. Interstitial lung disease/pneumonitis (3.4%) was treated with corticosteroids and/or antibiotics. PFS benefit of abemaciclib was not impacted by dose reductions or early onset of toxicities.
Conclusion
Abemaciclib was generally well tolerated. The most common AEs were effectively managed by supportive medications, and/or dose adjustments, with no detriment to PFS.
Implications for Practice
Treatment with abemaciclib plus fulvestrant or nonsteroidal aromatase inhibitors is generally well tolerated in patients with hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer. In MONARCH 2 and MONARCH 3, any‐grade diarrhea and grade ≥3 neutropenia were effectively managed with supportive medication and/or dose adjustment. Venous thromboembolic events were treated with anticoagulants and did not often require treatment discontinuation. Interstitial lung disease/pneumonitis was infrequent and treated with corticosteroids and/or antibiotics. Clinicians should be aware of and implement management strategies, including dose adjustments according to local labels, for commonly occurring and serious adverse events to ensure continued treatment and optimize clinical benefit/risk ratio.
Keywords: Abemaciclib, Safety, Advanced breast cancer, Diarrhea, Neutropenia
Short abstract
This article presents the results of a comprehensive safety analysis of abemaciclib‐associated adverse events, focusing on management of serious adverse events and whether adverse events and dose adjustments affect progression‐free survival.
Introduction
Hormone receptor‐positive (HR+), human epidermal growth factor receptor 2‐negative (HER2−) advanced breast cancer (ABC) is routinely treated with sequential endocrine therapies (ETs) [1, 2]; however, nearly all of these tumors have intrinsically or acquired ET resistance [3]. Recent efforts have focused on identifying new therapeutic strategies to overcome ET resistance. With the advent of highly specific cyclin‐dependent kinase 4 and 6 (CDK4 and CDK6) inhibitors, new treatment options are available [4].
Abemaciclib is an orally available CDK4 and CDK6 inhibitor, approved for continuous dosing [5]. In the first‐line setting (MONARCH 3) [6, 7], there was significantly longer progression‐free survival (PFS) in the abemaciclib arm compared with the placebo arm [7]. In the second‐line setting (MONARCH 2), there was statistically significant improvement in the primary outcomes of investigator‐assessed median PFS [5] and median overall survival (OS) [8].
The safety profile of abemaciclib was consistent across MONARCH 2 and 3 [5, 6, 7, 8]. The most common adverse event (AE) across both trials was early‐onset diarrhea, most commonly grades 1 and 2 [5, 6]. The most common grade ≥3 AE was neutropenia, typically occurring in the first two cycles [5, 6, 9]. Although detailed MONARCH 2 and 3 safety analyses were published [5, 6, 7, 8], additional information may be useful for clinical management. Here, we present the results of a comprehensive safety analysis of abemaciclib‐associated AEs and outcomes of the most frequently occurring AEs. Moreover, we determined if AEs and dose adjustments affected PFS. Finally, management of less frequent but potentially serious AEs was examined, including increased aminotransferases, venous thromboembolic events (VTEs), and interstitial lung disease (ILD)/pneumonitis [5, 6].
Materials and Methods
Study Design and Treatment
MONARCH 2 (NCT02107703) and MONARCH 3 (NCT02246621) were randomized, double blind, phase III trials in women with HR+, HER2− ABC [5, 6]. The eligibility criteria, study design, and primary outcomes for both studies have been previously described [5, 6, 7, 8]. In brief, MONARCH 2 enrolled women of any menopausal status, with disease progression on ET, who were chemotherapy naïve in the metastatic setting [5]. Patients were randomly assigned 2:1 to receive abemaciclib plus fulvestrant or placebo plus fulvestrant per label [5]. Abemaciclib or placebo were administered on a continuous schedule, initially at 200 mg (n = 121). After a blinded review of safety data and dose reduction rates, which revealed a high number of dose adjustments for abemaciclib or placebo principally due to diarrhea occurrence in the first treatment cycle, the protocol was amended; the abemaciclib starting dose was reduced to 150 mg b.i.d. for subsequent patients (n = 320), and all patients receiving 200 mg underwent a mandatory dose reduction to 150 mg. MONARCH 3 enrolled postmenopausal women with no prior therapy in the advanced setting [6]. Patients were randomly assigned 2:1 to abemaciclib (150 mg b.i.d.) plus a nonsteroidal aromatase inhibitor (NSAI; 1 mg anastrozole or 2.5 mg letrozole, daily, per physician's choice), or placebo plus NSAI [6].
Dose Adjustments
Protocol guidelines permitted abemaciclib dose omission, and/or dose reduction by up to two dose levels (i.e., 150 mg to 100 mg to 50 mg, all b.i.d.), as needed, and based on the nature of toxicity (hematologic or nonhematologic), severity, persistence, and recurrence (supplemental online Table 1; Fig. 1). The need for dose reduction beyond 50 mg b.i.d. required abemaciclib discontinuation. Fulvestrant dose modifications were permitted according to label. NSAI dose modifications were not permitted. Appropriate supportive care was allowed per investigator's discretion, including but not limited to over‐the‐counter antidiarrheal medication, such as loperamide, anticoagulants, antibiotics, and/or corticosteroids. In MONARCH 2 and 3 protocols, abemaciclib could be suspended and/or reduced per investigators’ discretion for persistent or recurrent grade 2 abnormal liver tests not resolving within 7 days (supplemental online Table 1). For grade ≥3 abnormal liver tests, per local labels and protocol, dose suspension was required until resolution of liver tests to baseline or grade 1; if abemaciclib was resumed, dose reduction was required (supplemental online Table 1).
Figure 1.
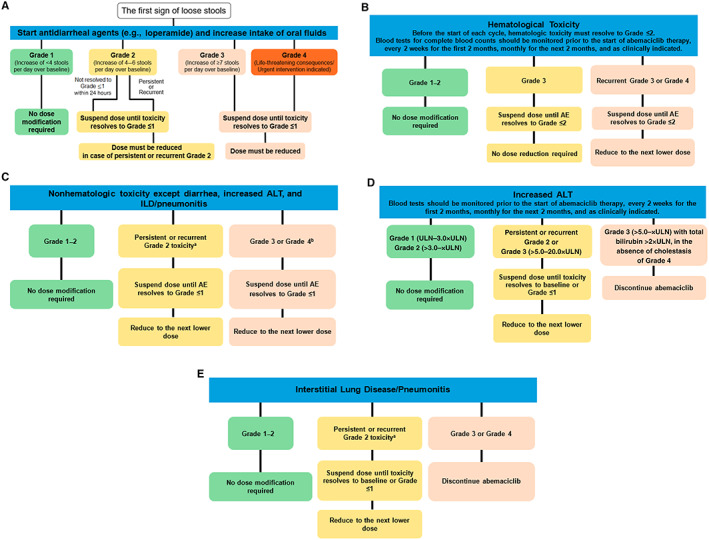
Recommendation for management of adverse events. (A), hematological toxicities (B), nonhematologic toxicities except diarrhea, increased ALT, and ILD/pneumonitis (C), increased ALT (D), and interstitial lung disease/pneumonitis (E) management. A dose reduction corresponds to a reduction of 50 mg of abemaciclib at a time. Discontinue abemaciclib for patients unable to tolerate 50 mg twice daily. For neutropenia evaluation, blood counts should be performed before starting abemaciclib treatment, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. If blood cell growth factors are administered, abemaciclib treatment must be suspended for at least 48 hours after the last administration of cell growth factors and until toxicity resolves to grade ≤2. Reduce the abemaciclib dose, unless already performed, for the toxicity that led to the use of growth factor. aGrade 2 toxicity that does not resolve with maximal supportive measures within 7 days to grade ≤1. bFor grade 4 increased aminotransferases, discontinue abemaciclib. Abbreviations: AE, adverse event; ALT, alanine aminotransferase; ILD, interstitial lung disease; ULN, upper limit of normal.
Safety Measures
Type, severity, incidence, seriousness, timing, and relatedness to study drug of AEs were all assessed. AE severity was graded using the National Cancer Institute Common Terminology Criteria, version 4.0. Central laboratory assessments were conducted at baseline, the first day of each cycle, and approximately 30 days after discontinuation of study therapy. MONARCH 2 had an additional laboratory assessment on day 15 of cycle 1.
Statistical Analyses
Safety was assessed in all patients who received ≥1 dose of the study drug at the time of the final PFS analysis (cutoff dates: MONARCH 2, February 14, 2017; MONARCH 3, November 3, 2017). AEs were summarized by maximum toxicity regardless of causality. Clinically synonymous terms were grouped together under a consolidated preferred term. For the most common AE overall (diarrhea) and the most common grade ≥3 AE (neutropenia), the incidence of clinically relevant events was derived relative to total drug exposure for each cycle. Time‐dependent covariate analysis of dose level versus PFS examined the impact of dose levels over time on PFS.
To investigate any potential association of early toxicities, defined as within time of median onset, and outcome, PFS was estimated for patients with and without any‐grade diarrhea within 7 days of abemaciclib initiation, corresponding to the median time to onset of diarrhea. A similar analysis compared PFS for patients with and without grade ≥2 neutropenia within 56 days of abemaciclib initiation, corresponding to the median time to onset of grade ≥3 neutropenia.
Results
Overall Safety Profile
Baseline patient and disease characteristics were previously described [5, 6, 7, 8]. The MONARCH 2 safety population included 441 patients in the abemaciclib arm and 223 in the placebo arm. The MONARCH 3 safety population included 327 and 161 patients in the abemaciclib and placebo arms, respectively.
In MONARCH 2 and 3, 99% of all patients experienced any‐grade AE; 58% (MONARCH 3) and 62% (MONARCH 2) experienced a grade ≥3 AE. In MONARCH 2 and MONARCH 3, 44.4% and 47.4%, respectively, of patients experienced a grade ≥3 abemaciclib‐related AE. Table 1 summarizes the most common AEs, pooled across trials and occurring in ≥20% of either study arm.
Table 1.
Safety summary: pooled data for MONARCH 2 and MONARCH 3 showing any‐grade, grade 3, and grade 4 adverse events occurring in ≥10% of patients in the abemaciclib or placebo arms
| Preferred term | Abemaciclib plus fulvestrant or NSAI (n = 768) | Placebo plus fulvestrant or NSAI (n = 384) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | |
| Diarrhea | 650 (84.6) | 90 (11.7) | 0 (0) | 107 (27.9) | 3 (0.8) | 0 (0) |
| Neutropenia | 346 (45.1) | 176 (22.9) | 19 (2.5) | 12 (3.1) | 4 (1.0) | 2 (0.5) |
| Nausea a | 334 (43.5) | 16 (2.1) | — | 84 (21.9) | 4 (1.0) | — |
| Fatigue a | 311 (40.5) | 18 (2.3) | — | 114 (29.7) | 1 (0.3) | — |
| Abdominal pain a | 258 (33.6) | 17 (2.2) | — | 56 (14.6) | 4 (1.0) | — |
| Anemia | 231 (30.1) | 54 (7.0) | 1 (0.1) | 21 (5.5) | 4 (1.0) | 0 (0) |
| Vomiting | 213 (27.7) | 9 (1.2) | 0 (0) | 44 (11.5) | 8 (2.1) | 0 (0) |
| Decreased appetite | 203 (26.4) | 10 (1.3) | 0 (0) | 44 (11.5) | 2 (0.5) | 0 (0) |
| Leukopenia | 197 (25.7) | 65 (8.5) | 2 (0.3) | 8 (2.1) | 0 (0) | 1 (0.3) |
| Alopecia b | 159 (20.7) | — | — | 22 (5.7) | — | — |
| Headache | 154 (20.1) | 6 (0.8) | — | 60 (15.6) | 1 (0.3) | — |
| Blood creatinine increased | 119 (15.5) | 10 (1.3) | 1 (0.1) | 8 (2.1) | 0 (0) | 0 (0) |
| Constipation | 117 (15.2) | 5 (0.7) | 0 (0) | 53 (13.8) | 1 (0.3) | 0 (0) |
| Alanine aminotransferase increased | 116 (15.1) | 37 (4.8) | 2 (0.3) | 24 (6.3) | 7 (1.8) | 0 (0) |
| Dysgeusia b | 110 (14.3) | — | — | 11 (2.9) | — | — |
| Thrombocytopenia | 110 (14.3) | 17 (2.2) | 8 (1.0) | 11 (2.9) | 1 (0.3) | 1 (0.3) |
| Aspartate aminotransferase increased | 109 (14.2) | 22 (2.9) | 0 (0) | 27 (7.0) | 8 (2.1) | 0 (0) |
| Arthralgia a | 108 (14.1) | 1 (0.1) | — | 65 (16.9) | 1 (0.3) | — |
| Stomatitis | 108 (14.1) | 2 (0.3) | 0 (0) | 40 (10.4) | 0 (0) | 0 (0) |
| Cough a | 107 (13.9) | 0 (0) | — | 45 (11.7) | 0 (0) | — |
| Pruritus a | 104 (13.5) | 0 (0) | — | 28 (7.3) | 0 (0) | — |
| Dizziness a | 99 (12.9) | 4 (0.5) | — | 31 (8.1) | 0 (0) | — |
| Rash | 99 (12.9) | 8 (1.0) | 0 (0) | 18 (4.7) | 0 (0) | 0 (0) |
| Back pain a | 94 (12.2) | 6 (0.8) | — | 54 (14.1) | 3 (0.8) | — |
| Dyspnea | 88 (11.5) | 13 (1.7) | 2 (0.3) | 36 (9.4) | 4 (1.0) | 0 (0) |
| Edema peripheral a | 84 (10.9) | 0 (0) | — | 25 (6.5) | 0 (0) | — |
| Pyrexia | 82 (10.7) | 3 (0.4) | 1 (0.1) | 30 (7.8) | 1 (0.3) | 0 (0) |
| Upper respiratory tract infection | 82 (10.7) | 0 (0) | 0 (0) | 26 (6.8) | 2 (0.5) | 0 (0) |
| Weight decreased a | 82 (10.7) | 4 (0.5) | — | 10 (2.6) | 2 (0.5) | — |
| Hot flush a | 79 (10.3) | 0 (0) | — | 50 (13.0) | 0 (0) | — |
Data are presented as n (%).
Additional adverse events of clinical importance but with incidence <10% are described here: (a) Interstitial lung disease/pneumonitis [abemaciclib arm: any grade, 26 (3.4); grade ≥3, 7 (0.9%); placebo arm: any grade, 2 (0.5%); grade ≥3, 0 (0%)] and (b) venous thromboembolic events: [abemaciclib arm: any grade, 41 (5.3%); grade ≥3, 19 (2.5%); placebo arm: any grade, 3 (0.8%); grade ≥3, 2 (0.5%)].
Maximum grade 3, so grade 4 is not applicable.
Maximum grade 2, so grades 3 or 4 are not applicable.
Abbreviations: —, no data; NSAI, nonsteroidal aromatase inhibitor.
Clinically important AEs included increased alanine aminotransferase (ALT; 13%–17%), increased aspartate aminotransferase (AST; 12%–17%), any‐grade VTEs, (5%–6%), and ILD/pneumonitis (2%–5%; Table 1).
Diarrhea Management and Occurrence
The first step in diarrhea management was antidiarrheal medication. At study onset, patients were prescribed and counseled to take antidiarrheal medication at the first sign of loose stools (Fig. 1). If diarrhea did not resolve to grade ≤1 within 24 hours of antidiarrheal therapy, dose adjustments were made, per study protocol. Specific recommendations included suspending abemaciclib at the first occurrence of grade ≥2 diarrhea. Abemaciclib could be resumed when diarrhea resolved to grade ≤1, with a dose reduction for persistent or recurrent grade 2, or grade ≥3 diarrhea (Fig. 1A).
The most frequently reported AE was diarrhea, occurring in 85% of patients across MONARCH 2 and 3 (Table 1). Diarrhea was typically low‐grade; 10%–13% of MONARCH 2 and 3 patients taking abemaciclib reported grade 3, and no patients reported grade ≥4 diarrhea (Table 2). In MONARCH 2, incidence of any‐grade, grade 2, and grade 3 diarrhea was higher for patients starting at abemaciclib 200 mg b.i.d. than for patients starting at 150 mg b.i.d. (any grade: 94.2% vs. 83.4%; grade 2: 43.8% vs. 27.2%; grade 3: 19.0% vs. 11.3%).
Table 2.
Diarrhea a characteristics in MONARCH 2 and MONARCH 3
| MONARCH 2 | MONARCH 3 | |||
|---|---|---|---|---|
| Characteristics | Abemaciclib + fulvestrant (n = 441) | Placebo + fulvestrant (n = 223) | Abemaciclib + NSAI (n = 327) | Placebo + NSAI (n = 161) |
| Diarrhea a | 381 (86.4) | 55 (24.7) | 269 (82.3) | 52 (32.3) |
| Grade 1 | 182 (41.3) | 43 (19.3) | 139 (42.5) | 36 (22.4) |
| Grade 2 | 140 (31.7) | 11 (4.9) | 99 (30.3) | 14 (8.7) |
| Grade 3 | 59 (13.4) | 1 (0.4) | 31 (9.5) | 2 (1.2) |
| Diarrhea SAEs | 7 (1.6) | 0 (0) | 5 (1.5) | 0 (0) |
| Time to onset, median, days | 6.0 | 57.0 | 8.0 | 59.5 |
| Duration of grade 2, median, days | 9.0 | 2.5 | 12.0 | 6.0 |
| Duration of grade 3, median, days | 6.0 | 7.0 | 8.0 | 2.0 |
| Dose reduction | 83 (18.8) | 1 (0.4) | 45 (13.8) | 3 (1.9) |
| Dose omission | 83 (18.8) | 0 (0) | 50 (15.3) | 3 (1.9) |
| Treatment discontinuation | 13 (2.9) b | 0 (0) | 6 (1.8) | 0 (0) |
| Antidiarrheal medication | 333 (75.5) | 40 (17.9) | 226 (69.1) | 15 (31.3) |
Data are presented as n (%) unless otherwise specified.
No grade ≥4 events were reported.
Eight out of 13 patients who discontinued treatment because of diarrhea initiated treatment at 200 mg.
Abbreviations: NSAI, nonsteroidal aromatase inhibitor; SAEs, serious adverse events.
Clinically significant diarrhea (defined as grade 2 or 3) occurred early in abemaciclib treatment, with median time to onset for any‐grade diarrhea of 6 days in MONARCH 2 and 8 days in MONARCH 3 (Table 2). The median duration of diarrhea was 9 to 12 days for grade 2 events and ranged from 6 to 8 days for grade 3 events (Table 2). The highest rates of clinically significant diarrhea (32% in MONARCH 2 and 20.8% in MONARCH 3) were observed in the first cycles of MONARCH 2 and 3, with decreasing incidence in all subsequent cycles (Fig. 2).
Figure 2.
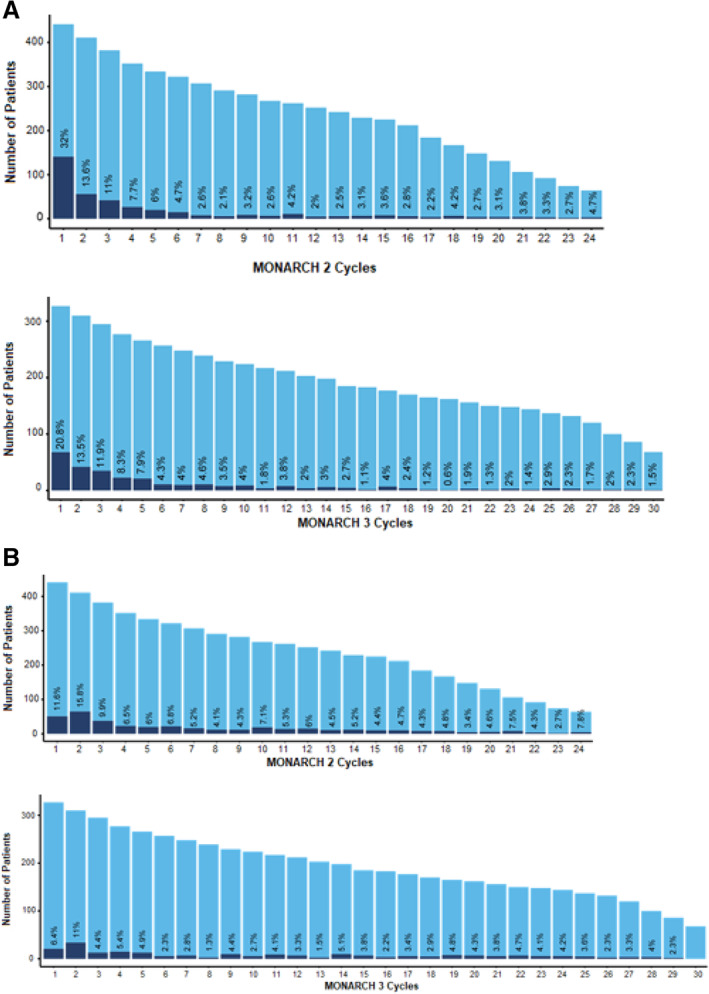
Percentage of patients with clinically significant diarrhea or neutropenia, relative to exposure by cycle for MONARCH 1 and MONARCH 2. aAbemaciclib at 150 mg after amendment twice a day, plus fulvestrant. bAbemaciclib at 150 mg twice a day plus nonsteroidal aromatase inhibitor. Each light blue bar corresponds to the number of patients who received a cycle of treatment, representing total exposure by cycle. Each dark blue bar represents those with grade ≥2 diarrhea or grade ≥3 neutropenia. The number displayed above each dark blue bar is the percentage of patients with clinically significant diarrhea or neutropenia within each cycle. No grade ≥4 diarrhea was observed.
Antidiarrheal medication, most commonly loperamide, was taken by 69%–76% of patients receiving abemaciclib in both trials (Table 2). Abemaciclib dose reduction due to diarrhea occurred in 13%–19% of patients across both studies (Table 2). Abemaciclib dose omission due to diarrhea occurred in 15%–19% of patients, with a median duration of 1.7 days and 3.1 days in MONARCH 2 and 3, respectively (Table 2). Abemaciclib discontinuation due to diarrhea occurred in 2.3%–2.9% of patients (Table 2).
Neutropenia Management and Occurrence
Neutropenia was primarily managed with dose adjustment. Recommendations included abemaciclib suspension for grade ≥3 neutropenia until resolution to grade ≤2. Abemaciclib could be resumed with a dose reduction required for recurrent grade 3 or grade 4 neutropenia (Fig. 1B). Granulocyte colony‐stimulating factor (GCSF) use was permitted per protocol and consistent with American Society of Clinical Oncology guidelines [10].
In the abemaciclib arms, the most frequently reported grade ≥3 AE was neutropenia, occurring in 25% of patients across both trials (Table 3) [5, 6]. Median time to onset for clinically significant (defined as grade ≥3) neutropenia ranged from 29 to 36.5 days, and median time to resolution from onset ranged from 11.5 to 15 days in MONARCH 2 and 3 (Table 3). In MONARCH 2, the highest rates of clinically significant neutropenia occurred during the first two cycles (11.6% and 15.8%, respectively), with <10% in all subsequent cycles (Fig. 2). In MONARCH 3, the trend was similar, with clinically significant neutropenia in 6.4% and 11.0% in cycles 1 and 2, and ≤10% in all subsequent cycles (Fig. 2). Owing to neutropenia, dose reduction occurred in 10%–13% of all abemaciclib patients, omission in 16%–17%, and discontinuation in 1%–3% of patients (Table 3).
Table 3.
Neutropenia characteristics (any grade) in MONARCH 2 and MONARCH 3
| MONARCH 2 | MONARCH 3 | |||
|---|---|---|---|---|
| Characteristics | Abemaciclib + fulvestrant (n = 441) | Placebo + fulvestrant (n = 223) | Abemaciclib + NSAI (n = 327) | Placebo + NSAI (n = 161) |
| Neutropenia | 203 (46.0) | 9 (4.0) | 143 (43.7) | 3 (1.9) |
| Grade 1 | 23 (5.2) | 4 (1.8) | 12 (3.7) | 0 (0) |
| Grade 2 | 63 (14.3) | 1 (0.4) | 53 (16.2) | 1 (0.6) |
| Grade 3 a | 104 (23.6) | 3 (1.3) | 72 (22.0) | 1 (0.6) |
| Grade 4 a | 13 (2.9) | 1 (0.4) | 6 (1.8) | 1 (0.6) |
| Neutropenia SAEs | 1 (0.2) | 1 (0.4) | 2 (0.6) | 2 (1.2) |
| Time to onset (grade ≥3), median, days | 29.0 | 223.0 | 36.5 | 176.5 |
| Duration b (grade ≥3), median, days | 15.0 | 8.0 | 11.5 | 7.5 |
| Dose reduction | 44 (10.0) | 0 (0) | 42 (12.8) | 1 (0.6) |
| Dose omission | 72 (16.3) | 0 (0) | 57 (17.4) | 1 (0.6) |
| Treatment discontinuation | 7 (1.6) | 0 (0) | 9 (2.8) | 0 (0) |
| GCSF/GM‐CSF | 31 (7.0) | 2 (0.9) | 15 (4.6) | 2 (1.2) |
Data are presented as n (%) unless otherwise specified.
Grade ≥3 toxicities reported as treatment‐emergent adverse events are consistent with central laboratory abnormalities.
Duration is calculated based on duration for total number of cases for each respective subgroup.
Abbreviations: GCSF, granulocyte colony‐stimulating factor; GM‐CSF, granulocyte‐macrophage colony‐stimulating factor; NSAI, nonsteroidal aromatase inhibitor; SAEs, serious adverse events.
Infection that was temporally related to grade ≥3 neutropenia (concurrent or within 1 week) ranged from 1.5% to 4.0% in both trials. Febrile neutropenia occurred in <1% of patients and was not associated with severe infection (grade ≥3) [5, 6]. GCSF use was low in MONARCH 2 (7.0%) and 3 (4.6%). Incidence of other hematological grade ≥3 toxicities was <10% in both trials and included leukopenia (9%), anemia (7%), thrombocytopenia, and lymphopenia (3% each).
Other Relevant Toxicities
In the abemaciclib arms, any‐grade increased ALT occurred in 13%–17%; any‐grade increased AST occurred in 12%–17% of patients (Table 4). Rates of grade ≥3 increased ALT and AST and of increased ALT and AST ≥3 times the upper limit of normal (ULN) with total bilirubin ≥2 times the ULN were low in both studies (Table 4). The median time to onset for grade ≥3 increased ALT in the abemaciclib arms was approximately 60 days in both studies. The median times to onset for grade ≥3 increased AST, in the abemaciclib arms of MONARCH 2 and 3, were 185 days and 71 days, respectively. Effects were reversible in both studies, as demonstrated by short duration of grade ≥3 increased ALT and AST, with a median time to resolution (for all patients regardless of dose adjustments) from onset of approximately 2 weeks. Dose reduction or discontinuation due to increased ALT or AST was infrequent (<1%; Table 4).
Table 4.
Hepatic events, venous thromboembolic events, interstitial lung disease, and change in creatinine in MONARCH 2 and MONARCH 3
| MONARCH 2 | MONARCH 3 | |||
|---|---|---|---|---|
| Characteristics | Abemaciclib + fulvestrant (n = 441) | Placebo + fulvestrant (n = 223) | Abemaciclib + NSAI (n = 327) | Placebo + NSAI (n = 161) |
| Hepatic events | ||||
| ALT increased, any grade | 59 (13.4) | 12 (5.4) | 57 (17.4) | 12 (7.5) |
| Grade ≥3 | 18 (4.1) | 4 (1.8) | 21 (6.4) | 3 (1.9) |
| ≥3×ULN and TBILI ≥2×ULN | 1 (0.2) | 1 (0.5) | 1 (0.3) | 0 (0) |
| Dose reduction | 7 (1.6) | 0 (0) | 8 (2.4) | 1 (0.6) |
| Discontinuation | 3 (0.7) | 1 (0.4) | 7 (2.1) | 0 (0) |
| AST increased, any grade | 54 (12.2) | 15 (6.7) | 55 (16.8) | 12 (7.5) |
| Grade ≥3 | 10 (2.3) | 6 (2.7) | 12 (3.7) | 2 (1.2) |
| ≥3×ULN and TBILI ≥2×ULN | 2 (0.5) | 2 (0.9) | 0 (0) | 0 (0) |
| Dose reduction | 2 (0.5) | 0 (0) | 0 (0) | 0 (0) |
| Discontinuation | 2 (0.5) | 1 (0.4) | 2 (0.6) | 0 (0) |
| Venous thromboembolic events | ||||
| Any grade | 21 (4.8) | 2 (0.9) | 20 (6.1) | 1 (0.6) |
| Grade ≥3 | 9 (2.0) | 1 (0.4) | 10 (3.1) | 1 (0.6) |
| Type of VTE | ||||
| PE | 11 (2.5) | 0 (0) | 11 (3.4) a | 1 (0.6) |
| DVT | 10 (2.3) | 2 (0.9) | 9 (2.8) | 0 (0) |
| Death | 0 (0) | 0 (0) | 3 (0.9) | 0 (0) |
| SAE | 8 (1.8) | 1 (0.4) | 9 (2.8) | 1 (0.6) |
| PE | 4 (0.9) | 0 (0) | 7 (2.1) b | 1 (0.6) |
| DVT | 4 (0.9) | 1 (0.4) | 4 (1.2) b | 0 (0) |
| Dose reduction | 2 (0.5) | 0 (0) | 0 (0) | 1 (0.6) |
| Discontinuation | 2 (0.5) | 0 (0) | 4 (1.2) c | 0 (0) |
| Anticoagulant treatment | 19 (4.3) | 0 (0) | 17 (5.3) | 0 (0) |
| VTE risk factors | ||||
| History of blood clots | 1 (0.2) | 0 (0) | 4 (1.2) | 0 (0) |
| Recent surgery | 3 (0.7) | 0 (0) | 2 (0.6) | 0 (0) |
| Lung metastases | 2 (0.5) | 1 (0.4) | 7 (2.1) | 0 (0) |
| Body mass index >30 | 3 (0.7) | 1 (0.4) | 3 (0.9) | 1 (0.6) |
| Age >65 | 10 (2.3) | 2 (0.9) | 12 (3.7) | 0 (0) |
| Interstitial lung disease/pneumonitis events | ||||
| Any grade | 9 (2.0) | 1 (0.4) | 17 (5.2) | 1 (0.6) |
| Grade ≥3 | 3 (0.7) | 0 (0) | 4 (1.2) | 0 (0) |
| Preferred term | ||||
| Pneumonitis | 8 (1.8) | 1 (0.4) | 9 (2.8) | 1 (0.6) |
| ILD | 0 (0) | 0 (0) | 4 (1.2) | 0 (0) |
| Pulmonary fibrosis | 0 (0) | 0 (0) | 4 (1.2) | 0 (0) |
| Organizing pneumonia | 1 (0.2) | 0 (0) | 0 (0) | 0 (0) |
| Death | 2 (0.5) | 0 (0) | 1 (0.3) | 0 (0) |
| SAE | 4 (0.9) | 0 (0) | 6 (1.8) | 0 (0) |
| Dose reduction | 0 (0) | 0 (0) | 2 (0.6) | 0 (0) |
| Discontinuation | 2 (0.4) | 0 (0) | 4 (1.2) | 0 (0) |
| Antibiotic treatment d | 6 (1.4) | 0 (0) | 4 (1.2) | 0 (0) |
| Corticosteroid treatment d | 2 (0.5) | 0 (0) | 7 (2.1) | 0 (0) |
| Creatinine increased, by laboratory assessment e | ||||
| Any grade | 427 (98.4) | 161 (73.5) | 308 (98.1) | 131 (84.0) |
| Grade 1 | 231 (53.2) | 154 (70.3) | 135 (43.0) | 124 (79.5) |
| Grade 2 | 191 (44.0) | 7 (3.2) | 166 (52.9) | 7 (4.5) |
| Grade 3 | 5 (1.2) | 0 (0) | 7 (2.2) | 0 (0) |
| Grade 4 | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Dose reduction | 2 (0.5) | 0 (0) | 8 (2.4) | 0 (0) |
| Dose omission | 6 (1.4) | 0 (0) | 6 (1.6) | 0 (0) |
| Discontinuation | 0 (0) | 0 (0) | 4 (1.2) | 0 (0) |
Data are presented as n (%).
Three patients experienced both PE and DVT.
Two patients with an SAE experienced both a PE and a DVT.
Includes three patients who died.
In MONARCH 2, two patients had both antibiotics and corticosteroids; in MONARCH 3, one patient had antibiotics and corticosteroids.
Percentages here are calculated based on denominator of number of patients who had creatinine laboratory assessments completed: 434 patients in the MONARCH 2 abemaciclib arm; 219 patients in the MONARCH 2 placebo arm; 314 patients in the MONARCH 3 abemaciclib arm; and 156 patients in the MONARCH 3 placebo arm.
Abbreviations: DVT, deep vein thrombosis; NSAI, nonsteroidal aromatase inhibitor; PE, pulmonary embolism; SAE, serious adverse event; TBILI, total bilirubin; ULN, upper limit of normal; VTE, venous thromboembolic event.
VTEs are a known AE of special interest for abemaciclib. Any‐grade VTEs, including pulmonary embolism or deep vein thrombosis, occurred in 4.8% and 6.1% of abemaciclib‐treated patients in MONARCH 2 and 3 (Table 4). The incidence of VTE was statistically significantly higher in the abemaciclib arm compared with the placebo arm. VTEs were managed with anticoagulant treatment (Table 4), most commonly low‐molecular‐weight heparin, with continued anticoagulant treatment for the duration of the study. VTEs infrequently resulted in abemaciclib dose reduction or discontinuation (≤1%; Table 4). Most patients (≥95%) who experienced VTEs had pre‐existing risk factors for VTE in addition to ABC, which were balanced between study arms (Table 4).
ILD/pneumonitis was reported for 9 (2.0%) and 17 (5.2%) abemaciclib‐treated patients in MONARCH 2 and 3, respectively, with ≤1% of cases grade ≥3 (Table 4). Patients experiencing ILD/pneumonitis had lung metastases (n = 8) and prior radiotherapy (n = 6) in MONARCH 2 and lung metastases (n = 6) and prior radiotherapy (n = 9) in MONARCH 3. Breakdown by how ILD/pneumonitis was reported (preferred term) is in Table 4; treatment (per physician discretion) included steroids (2 of 9 patients in MONARCH 2 and 4 of 17 in MONARCH 3) and/or antibiotics (6 of 9 patients in MONARCH 2 and 7 of 17 in MONARCH 3; Table 4). Dose reductions (0.0% in MONARCH 2 and 0.6% in MONARCH 3) and/or discontinuation (0.4% in MONARCH 2 and 1.2% in MONARCH 3) were infrequent (Table 4).
Creatinine Increased
Laboratory‐based abnormalities of increased creatinine were observed in 98.3% of abemaciclib‐treated patients in MONARCH 2 and 3. Creatinine elevation is known to occur with abemaciclib owing to inhibition of renal tubular transporters, organic cation transporter 2, and multidrug and toxin extrusion (MATE) 1 and MATE2‐K transporters in vitro and is not associated with reduced renal function [11]. In MONARCH 2, most cases of creatinine elevations were grade 1 (53.2%) or grade 2 (44.0%); similar incidence was reported in MONARCH 3 (grade 1, 43.0%; grade 2, 52.9%; Table 4). In both studies, ≤2.5% of patients required dose reduction, omission, or discontinuation because of creatinine elevations (Table 4). Of note, a majority of patients with laboratory‐based abnormalities of creatinine elevation, regardless of grade, had postbaseline creatinine values less than or equal to the ULN (abemaciclib: 61%–68%). Overall, creatinine rises typically occurred after the first cycle, remained elevated but stable for treatment duration, and were reversible upon abemaciclib treatment discontinuation.
Impact of Adverse Events on Progression‐Free Survival
In the abemaciclib arms of MONARCH 2 and 3, 189 (42.9%) and 142 (43.4%) patients had dose reductions owing to AEs. The most frequent AEs accounting for ≥10% of dose reductions were grade 2 or 3 diarrhea (14%–19%) and grade ≥3 neutropenia (10%–13%). In both studies, there was no difference in PFS when the dose was reduced to 100 mg, or to 50 mg at any point in the treatment, compared with being treated at the 150‐mg dose (Fig. 3).
Figure 3.
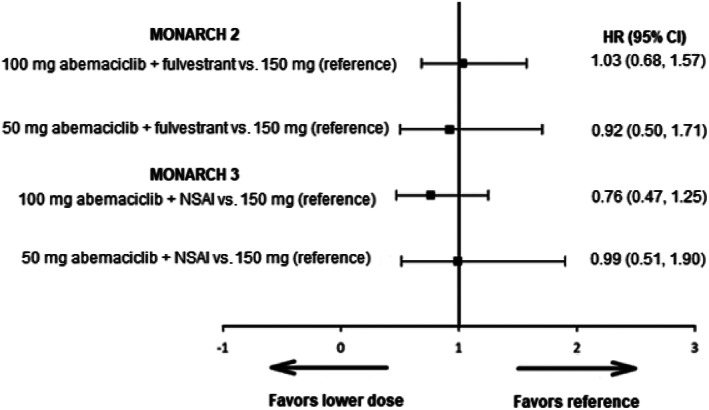
Time‐dependent covariate analysis of progression‐free survival among patients with reduced dose compared with those without. Abbreviations: CI, confidence interval; HR, hazard ratio; NSAI, nonsteroidal romatase inhibitor.
Clinical efficacy, as measured by median PFS, favored the abemaciclib arm, regardless of experiencing diarrhea within the first 7 days of abemaciclib initiation (supplemental online Fig. 1). In MONARCH 2, relative to the placebo arm, PFS was improved in abemaciclib‐treated patients who experienced any‐grade diarrhea within the first 7 days (hazard ratio [HR], 0.50; 95% confidence interval [CI], 0.39–0.64) and in abemaciclib‐treated patients who did not experience any‐grade diarrhea within the first 7 days (HR, 0.61; 95% CI, 0.48–0.77). For patients who received abemaciclib in combination with fulvestrant, the median PFS was 17.5 months among patients with an early onset of diarrhea (n = 215) and 14.8 months among patients without an early onset of diarrhea (n = 212). Similarly, in MONARCH 3, relative to the placebo arm, PFS was improved in abemaciclib‐treated patients who experienced any‐grade diarrhea within the first 7 days (HR, 0.49; 95% CI, 0.35–0.67) and in abemaciclib‐treated patients who did not experience diarrhea within the first 7 days (HR, 0.58; 95% CI, 0.43–0.78). For patients who received abemaciclib in combination with an NSAI, the median PFS was 28.2 months among patients with an early onset of diarrhea (n = 134) and 29.1 months among patients without an early onset of diarrhea (n = 183; supplemental online Fig. 1).
Clinical efficacy, as measured by PFS, favored the abemaciclib arm, regardless of experiencing grade ≥3 neutropenia within the first 56 days of abemaciclib initiation, corresponding to the median time of onset of grade ≥3 neutropenia (supplemental online Fig. 2). In MONARCH 2, relative to the placebo arm, PFS was improved in abemaciclib‐treated patients who experienced grade ≥3 neutropenia within the first 56 days of abemaciclib initiation (HR, 0.58; 95% CI, 0.45–0.74) and in abemaciclib‐treated patients who did not experience grade ≥3 neutropenia within the first 56 days (HR, 0.56; 95% CI, 0.43–0.73). For patients who received abemaciclib in combination with fulvestrant, median PFS was 16.6 months among those with (n = 226) and 17.6 months among those without (n = 186) grade ≥3 neutropenia (supplemental online Fig. 2). Similarly, in MONARCH 3, relative to the placebo arm, PFS was improved in abemaciclib‐treated patients who experienced grade ≥3 neutropenia within the first 56 days (HR, 0.54; 95% CI, 0.39–0.75) and who did not experience grade ≥3 neutropenia within the first 56 days (HR, 0.52; 95% CI, 0.38–0.70). For patients who received abemaciclib in combination with an NSAI, the median PFS was 28.2 and 31.1 months among those with (n = 122) and without (n = 178) grade ≥3 neutropenia, respectively (supplemental online Fig. 2).
Discussion
In MONARCH 2 and 3, abemaciclib in combination with ET demonstrated a significant and clinically meaningful PFS benefit, OS advantage (MONARCH 2), and a generally tolerable safety profile [5, 6, 7, 8]. This report expands on existing knowledge by providing a more in‐depth investigation of AEs associated with abemaciclib treatment.
The abemaciclib safety profile differs from other CDK4 and CDK6 inhibitors and is characterized by an increased rate of diarrhea [5, 6, 12, 13, 14]. Diarrhea, the most commonly occurring AE, typically occurred within the first 7 days of abemaciclib therapy and resolved within 2 weeks with antidiarrheal medication and/or dose‐adjustments. Importantly, no patients experienced grade 4 diarrhea. Decreasing incidence of clinically relevant diarrhea in subsequent cycles indicated low recurrence. In both MONARCH 2 and 3, the PFS benefit achieved with the addition of abemaciclib was observed regardless of early onset of diarrhea. Patients should be counseled regarding the early onset of diarrhea and to begin antidiarrheal medication at the first sign of loose stools. Dose adjustments should be considered after initiation of antidiarrheal medication if symptoms do not resolve within 24 hours of antidiarrheal therapy. It should be noted that patients may vary in the acceptance or tolerance of different grades of diarrhea, and there may be regional or ethnic differences. Although the exact mechanism for the lower rate of diarrhea with abemaciclib compared with other CDK4 and CDK6 inhibitors is unknown, these results demonstrate that early and proactive management of diarrhea with antidiarrheal medications, in combination with early dose adjustments, provides an effective strategy to manage this side effect.
The most common grade ≥3 AE in MONARCH 2 and 3 was neutropenia, typically occurring within the first two cycles of treatment and resolving with dose adjustments. The decreasing incidence in subsequent cycles following abemaciclib dose adjustment indicated low recurrence. Post hoc exploratory analyses indicated that patients taking abemaciclib received PFS benefit regardless of whether they experienced grade ≥3 neutropenia during the first 2 months of treatment. Dose reductions due to neutropenia occurred in approximately 43% of patients across both studies; these dose reductions did not affect the PFS benefit observed. Infection temporally related to grade ≥3 neutropenia was not common (1.5%–4%), nor was febrile neutropenia (<1%). Per label recommendations, neutropenia monitoring includes blood counts assessed at baseline, every 2 weeks for the first 2 months, monthly for the subsequent 2 months, and as clinically indicated [15].
In contrast to other CDK4 and CDK6 inhibitors, which require intermittent dosing, neutropenia observed with abemaciclib was not dose limiting in a phase I study and did not limit the ability of continuous abemaciclib dosing [16]. Lower rates of grade ≥3 neutropenia were seen with abemaciclib compared with other CDK4 and CDK6 inhibitors, which ranged from 59% to 66% in phase III trials of other CDK4 and CDK6 inhibitors [12, 13, 17]. Abemaciclib is a more potent inhibitor of cyclin D1/CDK4 than cyclin D3/CDK6 [7, 18] and exhibits greater selectivity for CDK4 compared with CDK6 [16]. CDK6 has a relatively larger role in hematopoietic stem cell differentiation compared with CDK4 [19], which may explain the lower frequency of neutropenia with abemaciclib compared with other drugs in this class. Of note, the effects of neutropenia caused by CDK4 and CDK6 inhibitors versus chemotherapy are thought to differ; neutropenia caused by CDK4 and CDK6 inhibitors, purely cytostatic drugs, is reversible upon discontinuation, but neutropenia due to chemotherapy can result in prolonged bone marrow suppression and the possibility for cumulative bone marrow aplasia [20].
Abnormal liver tests, including increased ALT and AST, occurred at low rates, with short median times from onset to resolution. No cases met the Food and Drug Administration's (FDA) definition of Hy's Law, characterized by elevation of ALT or AST ≥3 × ULN; elevation of total bilirubin ≥2 × ULN, without initial findings of cholestasis; and with no other reason to explain the combination of increased ALT/AST and bilirubin (i.e., viral hepatitis, pre‐existing or acute liver disease, or another drug) [21]. Following analysis of MONARCH 2 and 3 data, increased ALT and AST were identified as adverse drug reactions for abemaciclib in combination with ET. Thus, there is now a more conservative recommendation that ALT and AST should be monitored prior to the start of abemaciclib therapy, every 2 weeks for the first 2 months, monthly for the next 2 months, and as clinically indicated. Increased ALT should be managed with dose modifications, omissions, or discontinuations, as appropriate (Fig. 1D). For persistent or recurrent grade 2 or grade 3 increased ALT, we do not recommend rechallenging at the same dose, but rather dose omit until resolution to baseline or grade 1, followed by a dose reduction.
In both studies, VTEs occurred in approximately 5%–6% of abemaciclib‐treated patients, which was higher than the rate in the placebo arms (0.8%). Most patients were treated with anticoagulants, primarily low‐molecular‐weight heparin, and continued treatment with abemaciclib; dose adjustments due to VTEs were infrequent. VTEs are not specific to abemaciclib but have been reported for CDK inhibitors as a class effect [22, 23]. A recent retrospective analysis in 424 patients treated with CDK4 and CDK6 inhibitors (palbociclib in 92% of cases) showed a 1‐year cumulative incidence of thromboembolic events of 6.3% [24]. Recommended VTE management includes investigating and treating per usual clinical practice, with clinicians’ discretion for anticoagulant choice; abemaciclib dose reduction is not recommended.
Rates of ILD/pneumonitis reported here are similar to those observed in studies of other CDK4 and CDK6 inhibitors [12, 25], and a recent review by the FDA suggests that the occurrence of ILD/pneumonitis may be a class effect [26]. Patients who develop new or worsening symptoms, such as dyspnea, cough, and fever, should be evaluated and treated per local clinical practice and/or guidelines, including corticosteroids as appropriate. Confirming an ILD/pneumonitis diagnosis can be challenging, given nonspecific respiratory symptoms and numerous differential diagnoses including pneumonia, lung metastases, and pulmonary edema [27]. Investigations should include imaging, such as high‐resolution computed tomography, bronchoalveolar lavage, and/or biopsy, as clinically indicated. ILD/pneumonitis management is described in Figure 1E. Clinicians should refer to the local abemaciclib label for dose adjustment guidelines. The benefit of resuming abemaciclib treatment must be carefully evaluated.
Abemaciclib has been shown to increase serum creatinine owing to inhibition of renal tubular transporters without affecting glomerular function, and these increases were not accompanied by changes in markers of renal function, such as blood urea nitrogen, cystatin C, or calculated glomerular filtration rate based on cystatin C [11]. Other drugs such as trimethoprim and cimetidine have a similar pharmacodynamic effect. Although creatinine levels typically remained elevated during abemaciclib treatment, they returned to normal upon treatment discontinuation. In both trials, there was no evidence of renal failure/injury identified for patients who experienced increased blood creatinine. Therefore, dose adjustments of abemaciclib should not solely be based on interpretation of serum creatinine values, because these may not reflect renal function. If a clinician suspects deterioration of renal function, alternative measurements, including those listed above (i.e., blood urea nitrogen, cystatin C, or calculated glomerular filtration rate), should be evaluated to accurately assess renal function, and dose alteration should follow protocol or local label guidelines for nonhematological toxicities (Fig. 1C).
Some considerations should be made when interpreting results presented here. In MONARCH 2, patients enrolled at the 200‐mg (n = 121) dose received a median of 34 days of treatment prior to the mandatory dose reduction, compared with an entire treatment duration of 54.7 weeks among those starting at 150 mg (n = 320); given the similar dose intensity, no separate analysis was performed. Given that dose reductions and treatment duration are positively correlated (i.e., those whose dose reduced were more likely to remain on treatment and those who remained on treatment longer were more likely to have a dose adjustment), a time‐dependent covariate analysis was selected to assess the impact of dose reductions on efficacy. In both MONARCH 2 and 3, patients received PFS benefit regardless of whether they experienced dose reductions.
Conclusion
The addition of abemaciclib to ET resulted in a clinically and statistically significant improvement in PFS for patients with HR+, HER2− ABC. Based on these data, clinicians are currently incorporating abemaciclib along with ET as a new standard‐of‐care treatment for these patients. A thorough understanding of the abemaciclib safety profile and management of its associated toxicities can help ensure patients continue treatment with optimal clinical benefit. In general, abemaciclib plus ET exhibits a generally tolerable safety profile, and side effects in MONARCH 2 and MONARCH 3 were predictable, manageable, and reversible upon treatment discontinuation. The most common AE, low‐grade diarrhea, was properly managed with antidiarrheal medication and dose adjustments. Other toxicities, including hematological toxicities, were well managed with dose adjustments. Given the potential severity of ILD/pneumonitis, patients should be made aware of symptoms and report any to their physician. In both MONARCH 2 and 3, patients received PFS benefit regardless of dose reductions and regardless of early onset of diarrhea and/or neutropenia. These findings demonstrate that concomitant medications and/or dose adjustments are an effective way to manage toxicity without compromising efficacy.
Author Contributions
Conception/design: Hope S. Rugo, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Provision of study material or patients: Hope S. Rugo, Norikazu Masuda, Joo Hyuk Sohn, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Collection and/or assembly of data: Hope S. Rugo, Norikazu Masuda, Joo Hyuk Sohn, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Data analysis and interpretation: Hope S. Rugo, Jens Huober, José A. García‐Sáenz, Norikazu Masuda, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Manuscript writing: Hope S. Rugo, Jens Huober, José A. García‐Sáenz, Norikazu Masuda, Joo Hyuk Sohn, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Final approval of manuscript: Hope S. Rugo, Jens Huober, José A. García‐Sáenz, Norikazu Masuda, Joo Hyuk Sohn, Valerie A.M. Andre, Susana Barriga, Joanne Cox, Matthew Goetz
Disclosures
Hope S. Rugo: Pfizer, Novartis, Eli Lilly and Company, Genentech/Roche, MacroGenics, Merck, Eisai, Odonate Therapeutics, Daiichi Sankyo, Seattle Genetics, Immunomedics (RF), PUMA Biotechnology, Mylan, Amgen, AstraZeneca, Daiichi Sankyo, Merck, MacroGenics, Pfizer (other); Jens Huober: Eli Lilly and Company, Novartis, Roche, Pfizer, AstraZeneca, Merck Sharp & Dohme, Celgene, Eisai, Abbvie (H), Eli Lilly and Company, Novartis, Roche, Pfizer, Hexal, AstraZeneca, Merck Sharp & Dohme, Celgene, Abbvie (C/A), Roche, Pfizer, Novartis, Celgene, Daiichi (travel expenses), Celgene, Novartis, Hexal (RF); José A. García‐Sáenz: Eli Lilly and Company, Novartis, Pfizer, Celgene, Daiichi Sankyo, Eisai, AstraZeneca (C/A), AstraZeneca (RF), Roche‐Genentech (other); Norikazu Masuda: Chugai, AstraZeneca, Pfizer, Eli Lilly & Company, Eisai, Takeda (H), Chugai, AstraZeneca, Kyowa‐Kirin, Merck Sharp & Dohme, Novartis, Pfizer, Eli Lilly and Company, Eisai, Daiichi Sankyo (RF [to institution]); Valerie A.M. Andre: Eli Lilly and Company (E, OI); Susana Barriga: Eli Lilly and Company (E, OI); Joanne Cox: Eli Lilly and Company (E); Matthew Goetz: Eli Lilly and Company, Pfizer, Sermonix (RF), Eli Lilly and Company, Pfizer, Novartis, Sermonix, Biovica, Context Therapeutics (C/A). Joo Hyuk Sohn indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Protocol guidelines for dose adjustments and delays by toxicity type
Supplementary Figure 1 PFS among those with and without diarrhea (any grade) within 7 days in MONARCH 2 (Panel A) and MONARCH 3 (Panel B). Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
Supplementary Figure 2. PFS among those with and without laboratory Grade ≥3 neutropenia within 56 days in MONARCH 2 (Panel A) and MONARCH 3 (Panel B). Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
Acknowledgments
We thank the MONARCH study steering committee, as well as the patients and their caregivers, for participating in the MONARCH trials. We also thank the investigators and their support staff who generously participated in these trials. Eli Lilly and Company contracted with Syneos Health for writing and editorial support from Andrea Metti, Ph.D., M.P.H.
Disclosures of potential conflicts of interest may be found at the end of this article.
Endnotes
Interstitial lung disease/pneumonitis. Abemaciclib arm: any grade, 26 (3.4); grade ≥3, 7 (0.9%). Placebo arm: any grade, 2 (0.5%); grade ≥3, 0 (0%).
Venous thromboembolic events. Abemaciclib arm: any grade, 41 (5.3%); grade ≥3, 19 (2.5%). Placebo arm: any grade, 3 (0.8%); grade ≥3, 2 (0.5%).
References
- 1. Castrellon AB. Novel strategies to improve the endocrine therapy of breast cancer. Oncol Rev 2017;11:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lumachi F, Luisetto G, Basso SM et al. Endocrine therapy of breast cancer. Curr Med Chem 2011;18:513–522. [DOI] [PubMed] [Google Scholar]
- 3. Alves CL, Elias D, Lyng MB et al. SNAI2 upregulation is associated with an aggressive phenotype in fulvestrant‐resistant breast cancer cells and is an indicator of poor response to endocrine therapy in estrogen receptor‐positive metastatic breast cancer. Breast Cancer Res 2018;20:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. AlFakeeh A, Brezden‐Masley C. Overcoming endocrine resistance in hormone receptor‐positive breast cancer. Curr Oncol 2018;25(suppl 1):S18–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sledge GW Jr, Toi M, Neven P et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2‐ advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017;35:2875–2884. [DOI] [PubMed] [Google Scholar]
- 6. Goetz MP, Toi M, Campone M et al. MONARCH 3: Abemaciclib as initial therapy for advanced breast cancer. J Clin Oncol 2017;35:3638–3646. [DOI] [PubMed] [Google Scholar]
- 7. Johnston S, Martin M, Di Leo A et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sledge GW Jr, Toi M, Neven P et al. The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor‐positive, ERBB2‐negative breast cancer that progressed on endocrine therapy ‐ MONARCH 2: A randomized clinical trial. JAMA Oncol 2020;6:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rugo HS, Sledge GW, Johnston SRD et al. The association of early toxicity and outcomes for patients treated with abemaciclib. J Clin Oncol 2018;36(suppl 15):1053. [Google Scholar]
- 10. Smith TJ, Bohlke K, Lyman GH et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol 2015;33:3199–3212. [DOI] [PubMed] [Google Scholar]
- 11. Chappell JC, Turner PK, Pak YA et al. Abemaciclib inhibits renal tubular secretion without changing glomerular filtration rate. Clin Pharmacol Ther 2019;105:1187–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cristofanilli M, Turner NC, Bondarenko I et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone‐receptor‐positive, HER2‐negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA‐3): Final analysis of the multicentre, double‐blind, phase 3 randomised controlled trial. Lancet Oncol 2016;17:425–439. [DOI] [PubMed] [Google Scholar]
- 13. Hortobagyi GN, Stemmer SM, Burris HA et al. Ribociclib as first‐line therapy for HR‐positive, advanced breast cancer. N Engl J Med 2016;375:1738–1748. [DOI] [PubMed] [Google Scholar]
- 14. O'Shaughnessy J, Petrakova K, Sonke GS et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2‐ advanced breast cancer in the randomized MONALEESA‐2 trial. Breast Cancer Res Treat 2018;168:127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Eli Lilly and Company . Verzenio drug label. Availabile at http://uspl.lilly.com/verzenio/verzenio.html#pi. Accessed September 16, 2019.
- 16. Patnaik A, Rosen LS, Tolaney SM et al. Efficacy and safety of abemaciclib, an inhibitor of CDK4 and CDK6, for patients with breast cancer, non‐small cell lung cancer, and other solid tumors. Cancer Discov 2016;6:740–753. [DOI] [PubMed] [Google Scholar]
- 17. Finn RS, Martin M, Rugo HS et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016;375:1925–1936. [DOI] [PubMed] [Google Scholar]
- 18. Dickler MN, Tolaney SM, Rugo HS et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2‐ metastatic breast cancer. Clin Cancer Res 2017;23:5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tigan AS, Bellutti F, Kollmann K et al. CDK6‐a review of the past and a glimpse into the future: From cell‐cycle control to transcriptional regulation. Oncogene 2016;35:3083–3091. [DOI] [PubMed] [Google Scholar]
- 20. Kassem L, Shohdy KS, Lasheen S et al. Hematological adverse effects in breast cancer patients treated with cyclin‐dependent kinase 4 and 6 inhibitors: A systematic review and meta‐analysis. Breast Cancer 2018;25:17–27. [DOI] [PubMed] [Google Scholar]
- 21. Food and Drug Administration . Guidance for industry drug‐induced liver injury: Premarketing clinical evaluation. 2009. Available at https://www.fda.gov/media/116737/download. Accessed September 16, 2019.
- 22. Thein KZ, Ball S, Zaw MH et al. Risk of venous thromboembolism with abemaciclib based regimen versus other CDK 4/6 inhibitor containing regimens in patients with hormone receptor‐positive HER2‐negative metastatic breast cancer. Cancer Res 2019;79(suppl 4):P1‐16‐04a. [Google Scholar]
- 23. Thein KZ, Zaw MH, Tun AM et al. Incidence of venous thromboembolism in patients with hormone receptor‐positive HER2‐negative metastatic breast cancer treated with CDK 4/6 inhibitors: A systematic review and meta‐analysis of randomized controlled trials. Cancer Res 2018;78(suppl 4):p3‐14‐02a. [DOI] [PubMed] [Google Scholar]
- 24. Gervaso L, Montero AJ, Jia X et al. Venous thromboembolism in breast cancer patients receiving cyclin‐dependent kinase inhibitors. J Thromb Haemost 2020;18:162–168. [DOI] [PubMed] [Google Scholar]
- 25. Slamon DJ, Neven P, Chia S et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor‐positive, human epidermal growth factor receptor 2‐negative advanced breast cancer: MONALEESA‐3. J Clin Oncol 2018;36:2465–2472. [DOI] [PubMed] [Google Scholar]
- 26. Food and Drug Administration . FDA warns about rare but severe lung inflammation with ibrance, kisqali, and verzenio for breast cancer. Posted September 13, 2019. Available at https://www.fda.gov/drugs/drug‐safety‐and‐availability/fda‐warns‐about‐rare‐severe‐lung‐inflammation‐ibrance‐kisqali‐and‐verzenio‐breast‐cancer. Accessed September 16, 2019.
- 27. Omarini C, Thanopoulou E, Johnston SRD. Pneumonitis and pulmonary fibrosis associated with breast cancer treatments. Breast Cancer Res Treat 2014;146:245–258. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Table 1 Protocol guidelines for dose adjustments and delays by toxicity type
Supplementary Figure 1 PFS among those with and without diarrhea (any grade) within 7 days in MONARCH 2 (Panel A) and MONARCH 3 (Panel B). Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.
Supplementary Figure 2. PFS among those with and without laboratory Grade ≥3 neutropenia within 56 days in MONARCH 2 (Panel A) and MONARCH 3 (Panel B). Abbreviations: CI, confidence interval; HR, hazard ratio; PFS, progression‐free survival.


