Abstract
Background
Neuregulin 1 (NRG1) fusions, which activate ErbB signaling, are rare oncogenic drivers in multiple tumor types. Afatinib is a pan‐ErbB family inhibitor that may be an effective treatment for NRG1 fusion‐driven tumors.
Patients and Methods
This report summarizes pertinent details, including best tumor response to treatment, for six patients with metastatic NRG1 fusion‐positive tumors treated with afatinib.
Results
The six cases include four female and two male patients who ranged in age from 34 to 69 years. Five of the cases are patients with lung cancer, including two patients with invasive mucinous adenocarcinoma and three patients with nonmucinous adenocarcinoma. The sixth case is a patient with colorectal cancer. NRG1 fusion partners for the patients with lung cancer were either CD74 or SDC4. The patient with colorectal cancer harbored a novel POMK‐NRG1 fusion and a KRAS mutation. Two patients received afatinib as first‐ or second‐line therapy, three patients received the drug as third‐ to fifth‐line therapy, and one patient received afatinib as fifteenth‐line therapy. Best response with afatinib was stable disease in two patients (duration up to 16 months when combined with local therapies) and partial response (PR) of >18 months in three patients, including one with ongoing PR after 27 months. The remaining patient had a PR of 5 months with afatinib 40 mg/day, then another 6 months after an increase to 50 mg/day.
Conclusion
This report reviews previously published metastatic NRG1 fusion‐positive tumors treated with afatinib and summarizes six previously unpublished cases. The latter include several with a prolonged response to treatment (>18 months), as well as the first report of efficacy in NRG1 fusion‐positive colorectal cancer. This adds to the growing body of evidence suggesting that afatinib can be effective in patients with NRG1 fusion‐positive tumors.
Key Points
NRG1 fusions activate ErbB signaling and have been identified as oncogenic drivers in multiple solid tumor types. Afatinib is a pan‐ErbB family inhibitor authorized for the treatment of advanced non‐small cell lung cancer that may be effective in NRG1 fusion‐driven tumors.
This report summarizes six previously unpublished cases of NRG1 fusion‐driven cancers treated with afatinib, including five with metastatic lung cancer and one with metastatic colorectal cancer.
Several patients showed a prolonged response of >18 months with afatinib treatment. This case series adds to the evidence suggesting a potential role for afatinib in this area of unmet medical need.
Keywords: Gene fusion, NRG1, Afatinib, ErbB‐targeted treatment, Case series
Short abstract
This case series describes six patients with NRG1 fusion‐driven tumors who received treatment with afatinib and reviews relevant published data.
Introduction
Oncogenic gene fusions, such as those involving ALK, ROS1, RET, and NTRK1/2/3, can lead to deregulated activity and have been detected across a wide range of solid tumors, many of which are clinically actionable [1, 2]. NRG1 encodes the growth factor neuregulin 1 (NRG1). NRG1 contains an epidermal growth factor (EGF)‐like domain, which binds to human tyrosine kinases of the ErbB/HER receptor family, particularly ErbB3 and ErbB4, leading to heterodimerization (ErbB3/HER2 or ErbB3/ErbB4, or ErbB4/HER2) and activation of ErbB‐mediated downstream signaling pathways [3]. NRG1 gene fusions have been identified in multiple tumor types, including non‐small cell lung cancer (NSCLC), pancreatic ductal adenocarcinoma (PDAC), colorectal cancer (CRC), and cholangiocarcinoma [4, 5], and are clinically actionable oncogenic drivers. The overall estimated frequency of NRG1 fusions is 0.2% across solid tumors [4], with a reported prevalence of up to 31% in invasive mucinous lung adenocarcinomas [6, 7]. Numerous NRG1 fusion partners have been identified, with CD74 and SDC4 being reported as the most common 5' fusion partners in lung cancer [4]. Many other gene fusion partners have also been detected both in lung and other solid tumors [4].
NRG1 fusions and their downstream signaling pathways represent rational targets for therapeutic intervention. Afatinib, a first‐line treatment option for patients with metastatic epidermal growth factor receptor (EGFR)–mutated NSCLC [8, 9], is an irreversible pan‐ErbB family inhibitor, which inhibits ErbB signaling. Afatinib irreversibly binds to EGFR (ErbB1), HER2 (ErbB2), and ErbB4 and blocks transphosphorylation of ErbB3 [10], which makes it a treatment option for patients with NRG1 fusion‐driven tumors. Afatinib has demonstrated activity in preclinical tumor models harboring NRG1 fusions [11, 12]. In addition, crizotinib‐resistant ALK + NSCLC cells harboring a NRG1 fusion were sensitive to afatinib in vitro [13], in accordance with in vitro data showing sensitivity to afatinib of ALK + or ROS1 + NSCLC cell lines resistant to crizotinib via inhibition of NRG1 signaling [14].
A number of published case reports have described responses to afatinib in patients with solid tumors harboring NRG1 fusions (Table 1) [11, 12, 15, 16, 17, 18, 19, 20]. In this article, we present a case series of six additional patients with NRG1 fusion‐driven tumors who received treatment with afatinib, adding to the body of available data regarding the potential of afatinib as targeted therapy in this setting. To identify relevant published data on NRG1 and NRG1 gene fusions, we conducted literature searches with PubMed using the medical subject headings “Neuregulin‐1” and “afatinib” and “gene fusion.” We also searched Google Scholar, as well as conference proceedings from recent major congresses, including the American Society of Clinical Oncology, European Society for Medical Oncology, American Association for Cancer Research, and World Conference on Lung Cancer. Searches were updated on March 12, 2020.
Table 1.
Published cases of patients with metastatic NRG1 fusion‐driven solid tumors who were treated with afatinib
| Age, gender, ethnicity, a authors’ country of origin | Tumor type | NRG1 fusion partner | NRG1 fusion detection method | Best response, physician assessed (duration in months) | Reference |
|---|---|---|---|---|---|
| Cases of lung cancer | |||||
| 42 yr, male, white, U.S. | Nonmucinous ADC | SLC3A2 | RNA sequencing | PR (12) | [15] |
| 62 yr, male, white, U.S. | Mucinous ADC | CD74 | RNA sequencing | Durable response (10) | [15] |
| 43 yr, female, Canada | Nonmucinous ADC | SDC4 | WGTA | PR (12) | [16] |
| 62 yr, female, Asian, Canada | Invasive mucinous ADC | CD74 | NGS solid fusion assay | PR (6.5) | [17] |
| 86 yr, male, U.S. | Invasive mucinous ADC | CD74 | MSK‐IMPACT | PD (19) | [12] |
| 81 yr, male, U.S. | Invasive mucinous ADC | CD74 | MSK‐Solid Fusion Assay, Archer FusionPlex | SD (1.4) | [12] |
| 56 yr, female, U.S. | Invasive mucinous ADC | SDC4 | MSK‐Solid Fusion Assay | PD (at 1.2) | [12] |
| 51 yr, male, U.S. | Invasive mucinous ADC | CD74 | MSK‐IMPACT | PD (at 1.8) | [12] |
| Cases of gastrointestinal cancer | |||||
| 59 yr, male, Canada | PDAC | ATP1B1 | WGTA | PR (5.5) | [18, 20] |
| 54 yr, male, Canada | PDAC | APP | WGTA | PR (9) | [18, 20] |
| 30 yr, female, Germany | PDAC | ATP1B1 | WGTA | PR (3) | [11] |
| 38 yr, female, Canada | Cholangiocarcinoma | ATP1B1 | WGTA and FISH | Response (8) | [16] |
| Cases of genitourinary cancers | |||||
| NA, female, Finland | LGS ovarian cancer | CLU | RNA sequencing | Disease control (>36) b | [19] |
If reported.
Treatment included afatinib monotherapy followed by a combination of trastuzumab and pertuzumab.
Abbreviations: ADC, adenocarcinoma; FISH, fluorescence in situ hybridization; LGS, low‐grade serous; NA, not available; NRG1, neuregulin 1; NGS, next‐generation sequencing; PD, progressive disease; PDAC, pancreatic ductal adenocarcinoma; PR, partial response; SD, stable disease; WGTA, whole‐genome and transcriptome analysis.
Case Series
Previously unpublished data from six new cases are described in detail below, and findings are summarized in Table 2. The cutoff for data collection was February 2020.
Table 2.
New cases of patients with metastatic NRG1 fusion‐driven solid tumors who were treated with afatinib
| Patient | Age, gender, ethnicity | Tumor type | NRG1 fusion partner | NRG1 fusion detection method | Initial afatinib regimen | Line of treatment | Best response, physician assessed (duration in months) | Previous presentations |
|---|---|---|---|---|---|---|---|---|
| Cases of lung cancer | ||||||||
| 1 | 56 yr, female, white | Nonmucinous ADC | NR | NanoString™ | 40 mg/day | 15th | PR (24) a | [20, 21, 22, 23] |
| 2 | 62 yr, female, Asian | Nonmucinous ADC | CD74 | Oncomine™ | 40 mg/day | Fifth | PR (ongoing after 27) | [20, 21, 22, 23, 24] |
| 3 | 68 yr, male, white | Invasive nonmucinous ADC | SDC4 | RNA sequencing | 30 mg/day | Third | SD (4) | [20, 21, 22, 23] |
| 4 | 43 yr, female, white | Invasive mucinous ADC | CD74 | RNA sequencing | 40 mg/day | Third | PR (>18) | [20, 21, 22, 23, 24] |
| 5 | 34 yr, female, African | Invasive mucinous ADC | SDC4 | RNA sequencing | 40 mg/day | First (metastatic) | PR (5, then 6) | [26] |
| Cases of gastrointestinal cancer | ||||||||
| 6 | 69 yr, male, white | Colorectal cancer | POMK | Caris® profiling | 30 mg/day | Second | SD (16) | [20, 21, 22, 24, 27] |
NRG1 fusion was identified after afatinib treatment. The patient was rechallenged with afatinib, and best response was PR (4 months).
Abbreviations: ADC, adenocarcinoma; NR, not reported; NRG1, neuregulin 1; PR, partial response; SD, stable disease.
Cases of Lung Cancer
Case 1
A 56‐year‐old, white, female, never‐smoker was diagnosed in 2004 with nonmucinous lung adenocarcinoma [20, 21, 22, 23]. After lobectomy in 2004, the patient relapsed in 2006 with metastases in the left lung. Between December 2006 and February 2015, she received 14 lines of systemic therapy including chemotherapy and EGFR tyrosine kinase inhibitors, with progressive disease (PD) or short durations of response to each treatment. She received carboplatin plus gemcitabine (twice), erlotinib, pemetrexed (twice), docetaxel (twice), gefitinib, vinorelbine, and weekly paclitaxel. The patient's tumor did not harbor alterations in KRAS, EGFR, ALK, ROS1, or ERRB2.
Left lung metastases close to the chest wall were detected in December 2011, and the patient received palliative radiation therapy (30 Gy). From April 2012 to August 2013, she received six cycles of gemcitabine with stable disease (SD), followed by 10 cycles of pemetrexed, with a partial response (PR) in January 2013. Between November 2013 and July 2014, the patient received 10 cycles of gemcitabine with SD, and from August 2014 to November 2014, 11 weekly paclitaxel infusions. Paclitaxel was stopped because of fever, cough, hypoxia, and diffuse pulmonary infiltrates on computed tomography (CT) scan leading to suspicion of intolerability to paclitaxel. She was also treated with antibiotics and corticosteroids.
Afatinib (30 mg/day) was initiated in February 2015, escalating to 40 mg/day after 2 weeks; the patient rapidly achieved a PR shown on CT scan after 2.5 months (Fig. 1) that was maintained for a total of 24 months before discontinuation in March 2017 because of PD (Fig. 2). Rebiopsy was performed to screen for a new treatment target, but nothing actionable was found. Four cycles of pemetrexed followed by three cycles of gemcitabine were administered, but the disease continued to progress. A rebiopsy and an extended molecular analysis were performed, given her previous response to afatinib. An NRG1 fusion was identified in September 2017 by NanoString™ analysis (NanoString Technologies, Seattle, WA).
Figure 1.
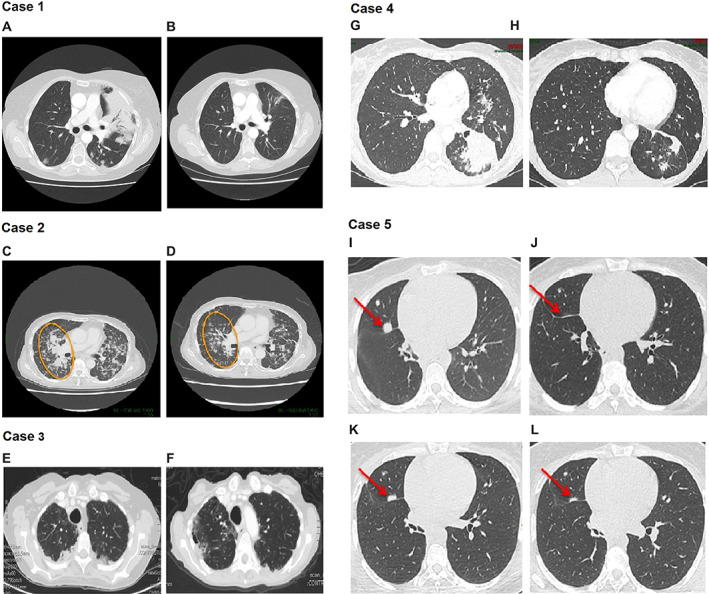
Computed tomography images of patients with metastatic lung cancer showing response to afatinib treatment. Case 1: Nonmucinous lung adenocarcinoma. (A): Prior to treatment in February 2015. (B): Partial response (PR) in April 2015 after 2.5 months on afatinib treatment. Case 2: Nonmucinous lung adenocarcinoma. (C): Prior to treatment in December 2017. (D): PR in November 2018 after 11 months on afatinib treatment. Case 3: Invasive nonmucinous lung adenocarcinoma. (E): Prior to treatment in July 2018. (F): Stable disease for 4 months in December 2018 after 4 months of afatinib treatment in December 2018. (Scans taken early December 2018 before PD.) Case 4: Invasive mucinous lung adenocarcinoma. (G): Prior to treatment in July 2017. (H): PR in March 2019 after 18 months of afatinib treatment. Case 5: Invasive mucinous lung adenocarcinoma. (I): Prior to treatment in Jan 2018. (J): PR in March 2018 after afatinib treatment. (K): Progressive disease (PD) in June 2018 while on afatinib. (L): PR in August 2018 after an afatinib dose increase to 50 mg/day.
Figure 2.
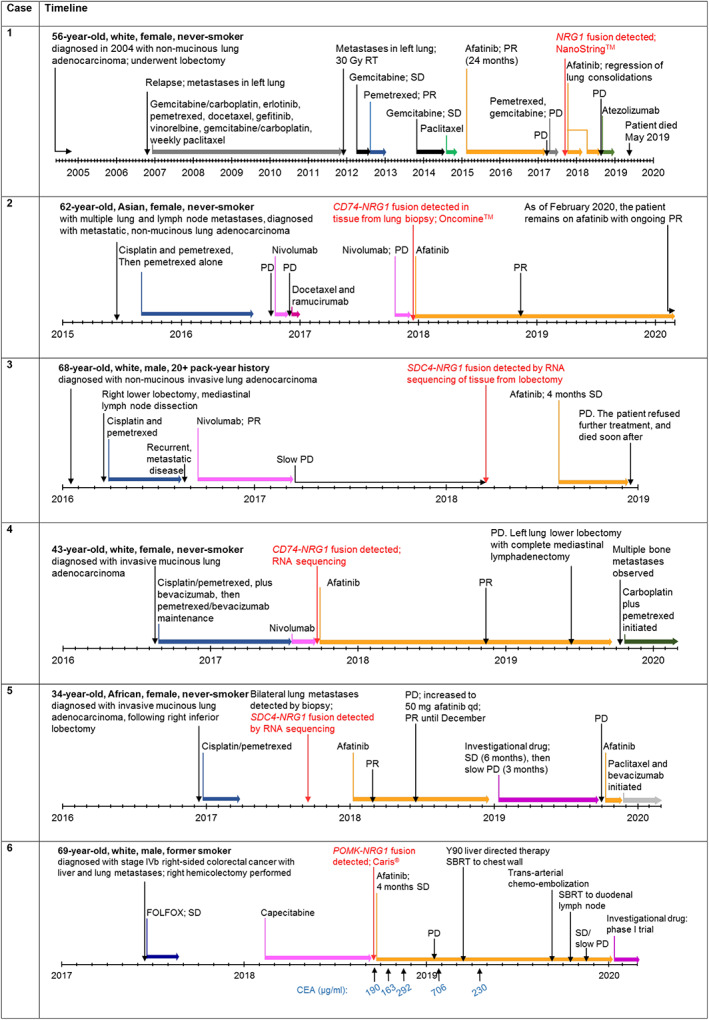
Timelines for new cases of patients with metastatic NRG1 fusion‐driven solid tumors who were treated with afatinib.
Abbreviations: CEA, carcinoembryonic antigen; FOLFOX, 5‐fluorouracil, oxaliplatin, and folinic acid; NRG1, neuregulin 1; PD, progressive disease; PR, partial response; qd, every day; RT, radiotherapy; SBRT, stereotactic body radiation; SD, stable disease.
Afatinib treatment (30 mg/day) was reinitiated in October 2017, giving cough relief, and the dose was increased to 40 mg/day after 2 weeks. Treatment led to regression in lung consolidations 1 month after initiation; however, the patient discontinued treatment after 4 months because of cough/fever and pulmonary infiltrates, which were thought to be afatinib‐related. She was treated with corticosteroids and antibiotics.
Afatinib 30 mg/day was reintroduced in April 2018, giving cough relief, and the dose was increased to 40 mg/day after 1 month. In August 2018, a CT scan showed PD, and afatinib treatment was discontinued. The patient was then given six cycles of atezolizumab between August and December 2018 but had PD during therapy and died in May 2019.
Case 2
A 62‐year‐old, Asian, female, never‐smoker with multiple lung and lymph node metastases was diagnosed with metastatic nonmucinous lung adenocarcinoma in June 2015 [20, 21, 22, 23, 24]. The patient received four lines of treatment prior to afatinib, and best response to any therapy was SD (Fig. 2). Between September 2015 and August 2016, she received four cycles of cisplatin (75 mg/m2) and pemetrexed (500 mg/m2) followed by 11 cycles of pemetrexed alone. After PD, between October and November 2016, she received three cycles of nivolumab. After further PD, she received two cycles of docetaxel and ramucirumab in December 2016 but discontinued because of grade 3 malaise. Nivolumab was reinitiated for two cycles between October and December 2017 but discontinued after progression.
In December 2017, a CD74‐NRG1 fusion was identified using the Oncomine™ Comprehensive Assay (Thermo Fisher Scientific, Waltham, MA) in material taken from a lung biopsy. Afatinib treatment (40 mg/day) was initiated in the same month. The patient had several afatinib dose adjustments to a minimum of 20 mg/day because of diarrhea and malaise symptoms. The dose was increased from 20 mg to 30 mg after an elevation of carcinoembryonic antigen (CEA) was detected, and she tolerated the higher dose. The patient achieved a PR in November 2018, after 11 months on afatinib treatment (Fig. 1). As of February 2020, after 27 months, the patient remains on afatinib (30 mg/day) with ongoing PR.
Case 3
A 68‐year‐old, white man with a 20+ pack‐year smoking history, was diagnosed in January 2016 with invasive nonmucinous lung adenocarcinoma [20, 21, 22, 23]. In March 2016, he underwent right lower lobectomy with mediastinal lymph node dissection and received two cycles of cisplatin and pemetrexed until August 2016. A CT scan of the chest showed signs of recurrent metastatic disease, confirmed by biopsy from the right lower lobe of the lung, leading to discontinuation of chemotherapy. Nivolumab was then administered at 3 mg/kg every 2 weeks from September until November 2016. An initial CT scan in December 2016 showed a good response, but nivolumab was discontinued in March 2017 because of immune‐mediated hepatitis and PD. Slow PD was further observed on CT and positron emission tomography (PET)–CT scans from March 2017 to March 2018.
An SDC4‐NRG1 fusion was identified by RNA sequencing in March 2018 using material from the right lower lobectomy performed in March 2016. Afatinib (30 mg/day) was initiated in August 2018 (Fig. 2). The patient had SD for 4 months (Fig. 1), but rapid PD in December 2018 led to discontinuation of treatment. The patient opted to receive no further treatment and died shortly after in a hospice.
Case 4
A 43‐year‐old, white, female, never‐smoker was diagnosed with metastatic invasive mucinous lung adenocarcinoma in August 2016 [20, 21, 22, 23, 24] after biopsy of the left lower lung. Initial testing revealed wild‐type ALK and ROS1 (by immunohistochemistry [IHC] and fluorescence in situ hybridization), wild‐type EGFR, BRAF, KRAS, MET, and HER2 by next‐generation sequencing (NGS), and no expression of programmed death ligand‐1 (PD‐L1) by IHC. Staging studied confirmed a 6.4 cm primary tumor with bilateral lung nodules (cT3N0M1a, stage IV). Prior to afatinib, she received pemetrexed, cisplatin, and bevacizumab, achieving a PR before beginning bevacizumab/pemetrexed maintenance therapy until July 2017. Subsequently, she received nivolumab until September 2017, with best response of PD, when a CD74‐NRG1 fusion was detected after exome and RNA sequencing (ArcherDx, Boulder, CO) during a secondary evaluation of the original biopsy specimen. Afatinib 40 mg/day was initiated (Fig. 2); the patient achieved a PR after 13 months of afatinib treatment in November 2018 with further response noted after 18 months of afatinib treatment (March 2019) (Figs. 1 and 3).
Figure 3.
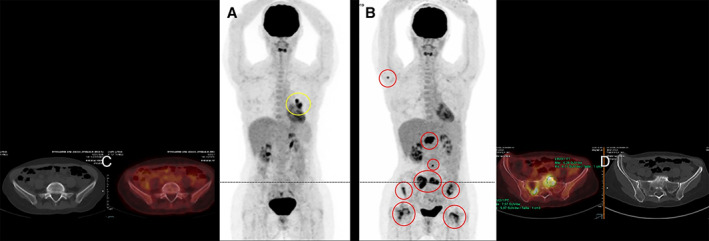
Case 4: Total body positron emission tomography‐computed tomography (PET‐CT) scan with fusion imaging. (A): May 2019, preoperative PET‐CT staging showed a non‐RECIST progression of the left lower lobe only residual target lesion (yellow circle) while the patient received afatinib (40 mg/day). (B): October 2019, the patient was admitted for spine and sacrum pains. PET‐CT showed numerous osteolytic and osteocondensant bone metastatic lesions (red circles). Comparison between May (C) and October (D) PET‐CT fusion centered on the sacrum.
In June 2019, the left lower lobe residual tumor target increased in size. Brain magnetic resonance imaging (MRI) and PET‐CT were normal. The afatinib plasma concentration was in the normal range (expected 26 ± 22 ng/mL) [25]. A left lung lower lobe lobectomy with complete mediastinal lymphadenectomy was performed. Sixteen lymph nodes were negative, and there was a 3.5 cm diameter residual tumor (30% tumor, 70% fibrosis). PD‐L1 expression was between 0% and 20%. NGS revealed no comutations, and ArcherDx sequencing confirmed the known NRG1 fusion. Afatinib was continued. In October 2019, bone metastases in the spine, femoral head, and sacrum were discovered on PET‐CT (Fig. 3). Afatinib was discontinued after a total of 24 months. The patient was rechallenged with carboplatin plus pemetrexed and received palliative radiotherapy on the spine and sacrum. A biopsy of the sacrum during cementoplasty detected the previously confirmed NRG1 fusion (ArcherDx). PET‐CT and MRI showed a dissociated response with improvement on bone lesion standardized uptake values; however, a right adrenal gland metastasis was also detected. The patient is alive and on treatment.
Case 5
A 34‐year‐old, African, female, never‐smoker was diagnosed with invasive mucinous lung adenocarcinoma after a right inferior lobectomy in December 2016 [26]. The tumor was pT4N2M0, and profiling showed no expression of PD‐L1 with a low tumor mutation burden (1.26 mutations per megabase). She was treated with four cycles of adjuvant cisplatin plus pemetrexed.
In September 2017, 9 months after surgery, she relapsed with bilateral lung metastases, confirmed by CT‐guided biopsy. Whole‐transcriptome sequencing revealed an SDC4‐NRG1 fusion. Afatinib 40 mg/day was initiated in January 2018 as first‐line metastatic treatment (Fig. 2). After 6 weeks, PR was observed on the CT scan, which was confirmed by PET‐CT scan at 3 months. She experienced grade 2 skin toxicity. In June 2018, after 5 months of treatment, lung progression was observed while skin toxicity disappeared. The afatinib plasma level was found to be low, and the dose was increased to 50 mg/day. A PR was again observed that persisted until December 2018.
To understand the mechanism of afatinib resistance, a repeat CT‐guided thoracic biopsy was performed, which again showed invasive mucinous adenocarcinoma without any histological transformation. Unfortunately, RNA sequencing was unsuccessful. Using the AmpliSeq Colon and Lung Research Panel (ThermoFisher Scientific Inc., Les Ullis, France) and Lung NGS panels, some differences between the surgical resection (before treatment) and the biopsy at progression while on afatinib were observed. Initially, a SMAD4 p.G386V c. 1157G > T mutation appeared in a 15% allelic ratio, whereas at progression on afatinib, the allelic ratio was 1%, suggesting that the SMAD4‐mutated clone had become the minor clone.
In January 2019, she received an investigational drug in a phase I clinical trial as second‐line treatment and experienced SD for 6 months, followed by slow progression over 3 months. Once treatment was stopped, she experienced rapid progression in the lungs and was rechallenged with afatinib at 50 mg/day over 6 weeks without benefit. Paclitaxel and bevacizumab were initiated in November 2019, and treatment is ongoing as of February 2020.
Case of Gastrointestinal Cancer
Case 6
A 69‐year‐old, white, male, former smoker presented with gastrointestinal bleeding in June 2017 [20, 21, 22, 24, 27]. He was diagnosed with a KRAS‐mutant (G12D), stage IVb, right‐sided CRC with liver and lung metastases. A right hemicolectomy was performed, and first‐line 5‐fluorouracil (5‐FU), oxaliplatin, and folinic acid (FOLFOX) was initiated. He received four cycles of FOLFOX, but a full 5‐FU infusion was never tolerated, and oxaliplatin was not used in all cycles. Irinotecan was not tolerated after a single dose. The lung lesion was stable (0.8–1.0 cm), and there was no hepatic response. In November 2017, the patient underwent a liver metastasectomy. A CT scan showed an increase in lung nodule size. In February 2018, he began capecitabine (500 mg/day Monday to Friday, titrated to 500 mg b.i.d. Monday to Friday). In May 2018, the patient underwent lung metastasectomies. Caris® (Irving, TX) NGS profiling identified a novel POMK‐NRG1 fusion in both lung and liver metastases.
Afatinib 30 mg/day was initiated in September 2018, with a reduction in CEA from 190 μg/mL to 163 μg/mL observed in October 2018 (Fig. 2). In November 2018, a CT scan showed SD, and the CEA was 292 μg/mL. In January 2019, PET‐CT revealed an increase in metastatic lesion size (CEA 706 μg/mL), giving approximately 4 months of SD on afatinib. In March 2019, he received Y90 liver‐directed therapy plus stereotactic body radiation (SBRT) to the chest wall and afatinib (30 mg/day). In April 2019, his CEA level was 230 μg/mL. In September 2019, the patient received transarterial chemoembolization using irinotecan beads to the liver and SBRT to a duodenal lymph node in October 2019. The patient remained on afatinib for 16 months with stable to slightly increasing disease (last CT November 2019; Fig. 4) until he entered a phase I trial of an investigational drug in January 2020.
Figure 4.
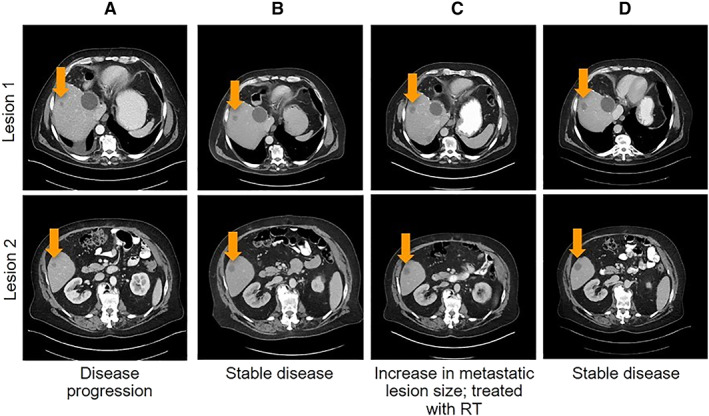
Computed tomography imaging from Case 6 of a patient with metastatic colorectal cancer showing stable disease while on afatinib treatment. (A): Pretreatment in August 2018. (B): After 2 months of treatment with afatinib in November 2018. (C): After 4 months of treatment with afatinib in January 2019. (D): After 7 months of treatment with afatinib in April 2019.
Abbreviation: RT, radiotherapy.
Discussion
NRG1 fusions are uncommon genomic events but are enriched in invasive mucinous lung adenocarcinoma [4]. At this time, there are no approved targeted treatments for NRG1 fusion‐driven tumors. Encouraging responses with afatinib were reported across six new cases, which included five patients with NSCLC (mucinous and nonmucinous) and the first clinical report in a patient with metastatic CRC (mCRC).
An encouraging response was reported in Case 4 with invasive mucinous disease (PR ongoing for >18 months) harboring a CD74‐NRG1 fusion, similar to durable responses (PR for up to 10 months) previously reported in patients with CD74‐NRG1–positive invasive mucinous adenocarcinoma [15, 17]. Durable responses of ≥24 months were also observed in Cases 1 and 2, heavily pretreated patients with nonmucinous disease harboring NRG1 fusions. Similar findings were reported in other published case reports, with PR lasting up to 12 months [15, 18]. It is also noteworthy that Case 1 had previously received treatment with erlotinib and gefitinib, with PD as best response, before experiencing a 24‐month PR on afatinib. Also of interest is that Case 5 had a PR lasting 5 months with afatinib 40 mg/day, after which lung progression was observed. However, afatinib plasma concentrations were found to be low at that time, and the patient had a subsequent PR of 6 months after an increase in afatinib dose to 50 mg/day.
NRG1 fusions may also serve as a mechanism of resistance to other targeted therapies, such as ALK inhibitors in patients with NSCLC harboring an ALK gene rearrangement (ALK +) [13]. In a recent study, a RALGAPA1‐NRG1 fusion was detected in the primary tumor of a patient with stage IV ALK + NSCLC progressing after treatment with crizotinib and alectinib [13, 28]. The NRG1 fusion was also detected in samples taken prior to crizotinib treatment, suggesting that this alteration may coexist with the ALK fusion and may represent an intrinsic mechanism of resistance to ALK inhibitors [13]. Another example of concurrent ALK and NRG1 fusions has been observed in a patient with metastatic lung adenocarcinoma, suggesting that multiple fusions of this type may not be that uncommon [29].
NRG1 gene fusions have previously been reported in 0.1%–0.5% of gastrointestinal tumors, including PDAC, CRC, and cholangiocarcinoma [4]. Although NRG1 fusions are relatively uncommon, there remains a need for new targeted therapies. In Case 6 of the present series, SD (16 months) was reported for the first time (to our knowledge) in a patient with mCRC treated with second‐line afatinib. Notably, this patient also had KRAS‐mutated disease. This is in contrast to some studies that report that NRG1 gene fusions are mutually exclusive with other oncogenic driver mutations, including coexistent KRAS mutations in tumors [4, 11, 16, 30, 31]. Nevertheless, exceptions to these findings have been described in other studies [4, 6, 7, 13].
We have not discussed the finer molecular details of the NRG1 fusions in the cases in this report, for example, whether they were in‐frame or retained the EGF‐like domain. These details may prove to be clinically relevant as our knowledge of the biology of NRG1 fusions grows. Our cases were from different institutions and were detected using a variety of techniques, which precludes comparisons between the cases. Although liquid biopsy– and plasma‐based NGS have emerged as appealing complements or alternatives to tissue‐based genotyping, the NRG1 fusions in these and previously reported cases were detected from tissue [11, 15, 16, 18] or serous effusion [19] by a variety of detection methods. A wider, prospective study of NRG1 fusions, with centralized testing, would be useful to provide more information on the response of each fusion to various treatments.
Analysis of large data sets suggests that targeted RNA sequencing protocols may be more robust than hybrid capture techniques for detecting NRG1 fusions in solid tumors [4, 12]. Increased screening of patients and new detection techniques may identify additional niches for potential targeted treatments.
Approaches for repurposing drugs for use in cancers with a high unmet clinical need may prove to be a fruitful strategy. Murumägi et al. have described the clinical implementation of precision systems oncology in the treatment of ovarian cancer based on ex vivo drug sensitivity testing, with a panel of 528 approved and investigational oncology drugs, and molecular profiling [19]. A CLU‐NRG1 fusion was detected in a metastatic low serous grade ovarian cancer sample, which conferred sensitivity of the tumor to afatinib. Based on these data, the patient received afatinib monotherapy, followed by trastuzumab in combination with pertuzumab, resulting in disease control for over 3 years.
Recent developments in bispecific antibody technology may be important to patients with NRG1 fusion‐driven tumors. Zenocutuzumab (MCLA‐128) is a bispecific antibody targeting HER2 and ErbB3 [32]; it may, therefore, be effective in patients with NRG1 fusions, which preferentially activate ErbB signaling via ErbB3‐containing heterodimers. Three patients with NRG1 fusions treated with zenocutuzumab have been reported, including a patient with PDAC who had a PR for 7 months (ongoing), another patient with PDAC who experienced SD for 7 months (ongoing), and a patient with NSCLC who had a PR for 4.5 months (ongoing) [33]. Zenocutuzumab is currently being investigated in a phase I/II study in patients with solid tumors harboring an NRG1 fusion (NCT02912949), as well as an Early Access Program (NCT04100694).
Other small molecules are also under investigation. Tarloxotinib, a prodrug that releases a hypoxia‐activated, potent, irreversible pan‐ErB inhibitor, showed greater, more durable antitumor activity in CLU‐NRG1 patient‐derived xenografts than afatinib [34] and is currently undergoing phase II testing in patients with NSCLC harboring HER2‐activating mutations and patients with solid tumors harboring NRG1 or ERBB fusions (NCT03805841).
Although findings from the current case series and previous case reports are encouraging, such reports may be subject to publication bias, where only cases with a favorable response are published. Prospective clinical trials are needed to assess the efficacy of afatinib in larger numbers of patients with NRG1 fusion‐driven tumors. Of note, studies of afatinib in patients with cancer harboring NRG1 rearrangements in the Drug Rediscovery Protocol trial (DRUP; NCT02925234) are ongoing, and results are awaited with interest. In addition, the TAPUR (Targeted Agent and Profiling Utilization Registration; NCT02693535) trial is currently recruiting patients. This is a nonrandomized, single group assignment study, the aim of which is to learn from the real‐world practice of prescribing targeted therapies to patients with advanced cancer whose tumor harbors a genomic variant known to be a drug target, or to predict sensitivity to a drug. In this study, afatinib will be administered to patients whose tumors harbor an NRG1 rearrangement.
In conclusion, data from this case series add to the growing body of evidence suggesting that afatinib may be a potential treatment option for patients with NRG1 fusion‐positive tumors. This evidence has to be confirmed by results of ongoing trials and put in the perspective of other therapeutic approaches.
Author Contributions
Conception/design: Jacques Cadranel, Stephen V. Liu, Michaël Duruisseaux, Richard F. Schlenk, Khaled Tolba, Agnieszka Cseh, Flavio Solca, Janessa J. Laskin
Provision of study material or patients: Jacques Cadranel, Stephen V. Liu, Eva Branden, Yasushi Goto, Benjamin A. Weinberg, Alexander Drilon, Khaled Tolba, Valerie Gounant, Janessa J. Laskin, Daniel J. Renouf
Collection and/or assembly of data: Jacques Cadranel, Stephen V. Liu, Yasushi Goto, Benjamin A. Weinberg, Khaled Tolba
Data analysis and interpretation: Jacques Cadranel, Stephen V. Liu, Michaël Duruisseaux, Yasushi Goto, Benjamin A. Weinberg, Richard F. Schlenk, Parneet Cheema, Alexander Drilon, Agnieszka Cseh, Daniel J. Renouf
Manuscript writing: Jacques Cadranel, Stephen V. Liu, Michaël Duruisseaux, Eva Branden, Yasushi Goto, Benjamin A. Weinberg, Christoph Heining, Richard F. Schlenk, Parneet Cheema, Martin R. Jones, Alexander Drilon, Domenico Trombetta, Lucia Anna Muscarella, Agnieszka Cseh, Flavio Solca, Janessa J. Laskin, Daniel J. Renouf
Final approval of manuscript: Jacques Cadranel, Stephen V. Liu, Michaël Duruisseaux, Eva Branden, Yasushi Goto, Benjamin A. Weinberg, Christoph Heining, Richard F. Schlenk, Parneet Cheema, Martin R. Jones, Alexander Drilon, Domenico Trombetta, Lucia Anna Muscarella, Khaled Tolba, Valerie Gounant, Agnieszka Cseh, Flavio Solca, Janessa J. Laskin, Daniel J. Renouf
Disclosures
Jacques Cadranel: AstraZeneca, Boehringer Ingelheim, Bristol‐Myers Squibb, Eli Lilly & Co., Novartis, Merck Sharp & Dohme, Pfizer, Roche, Takeda (C/A), AstraZeneca, Boehringer Ingelheim, Pfizer, Novartis (RF); Stephen V. Liu: AstraZeneca, Boehringer‐Ingelheim, Bristol‐Myers Squibb, Catalyst, Celgene, G1 Therapeutics, Genentech/Roche, Guardant Health, Inivata, Janssen, Eli Lilly & Co., Loxo, Merck Sharp & Dohme, Pfizer, PharmaMar, Regeneron, Takeda (C/A), Alkermes, AstraZeneca, Bayer, Blueprint, Bristol‐Myers Squibb, Corvus, Genentech, Eli Lilly & Co., Lycera, Merck, Merus, Molecular Partners, Pfizer, Rain, RAPT, Spectrum, Turning Point Therapeutics (RF); Michaël Duruisseaux: Nanostring, Blueprint (RF), Roche, AstraZeneca, Boehringer Ingelheim, Merck Sharp & Dohme, Bristol‐Myers Squibb, Novartis, Takeda, AbbVie, Pfizer (SAB); Yasushi Goto: Eli Lilly & Co., Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Pfizer, Novartis, AstraZeneca, Glaxo Smith Kline, Merck Sharp & Dohme, Guardant Health, Dai‐ichi Sankyo, Kyorin, Chugai, Illumina (C/A), AstraZeneca, Eli Lilly & Co., Chugai, Taiho Pharmaceutical, Boehringer Ingelheim, Ono Pharmaceutical, Bristol Myers Squibb, Pfizer, Merck Sharp & Dohme, Shionogi Pharma, Novartis (H); Benjamin A. Weinberg: Bayer, Taiho, Sirtex, Eli Lilly & Co. (other—speaker's bureau), Bayer (C/A); Richard F. Schlenk: AstraZeneca, Pfizer, Daiichi Sankyo, Boehringer Ingelheim, PharmaMar (RF), Pfizer, Novartis, Astellas (SAB); Parneet Cheema: AstraZeneca, Bristol‐Myers Squibb, Novartis, Pfizer, Amgen, Roche, Merck, Takeda (C/A), Merck, AstraZeneca, Novartis, Takeda, Pfizer (H); Martin R. Jones: Qiagen (E); Alexander Drilon: Ignyta/Genentech/Roche, Loxo/Bayer/Eli Lilly & Co., Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Axis, Peerview Institute, OncLive, Paradigm Medical Communications LLC, Remedica Ltd., ArcherDx, Foundation Medicine, PeerVoice, Research to Practice, Medscape, WebMD (H, SAB), Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho, PharmaMar (RF—to institution), Foundation Medicine (RF), Wolters Kluwer (other—royalties), Merck, Puma (other—food/beverage), Merus, Boehringer Ingelheim (Other), Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice (H–CME); Domenico Trombetta: Boehringer Ingelheim, Roche (H); Lucia Anna Muscarella: AstraZeneca (RF), Boehringer Ingelheim, Astra Zeneca (H); Khaled Tolba: Merck, Bristol‐Myers Squibb (C/A), Foundation Medicine (E); Valerie Gounant: Chugai, Merck Sharp & Dohme, Boehringer Ingelheim (H), Bristol‐Myers Squibb, Takeda, AstraZeneca, Roche (SAB); Agnieszka Cseh: Boehringer Ingelheim International GmbH (E); Flavio Solca: Boehringer Ingelheim RCV GmbH & Co KG (E); Janessa J. Laskin: Roche Canada, Pfizer, Takeda (C/A), Roche, AstraZeneca (RF), AstraZeneca (H); Daniel J. Renouf: Celgene, Servier, Taiho, Ipsen, AstraZeneca, Bayer, Roche (C/A), Bayer, Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (CME) Continuing Medical Education; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
Medical writing assistance, supported financially by Boehringer Ingelheim, was provided by Caroline Perry of GeoMed, an Ashfield company, part of UDG Healthcare plc, and Toby Allinson, contracted on behalf of GeoMed, during the preparation of this article. The authors acknowledge Dr. Martine Antoine, Pathologist (Hôpital Tenon, Paris, France), Pr. Roger Lacave (Hôpital Tenon), and Pr. Pierre Laurent Puig (Hôpital Européen Georges Pompidou, Paris, France), Molecular Biologists, for their contribution in the diagnosis of the NRG1 fusion‐driven patient of Case 4. This work was funded by Boehringer Ingelheim.
Disclosures of potential conflicts of interest may be found at the end of this article.
No part of this article may be reproduced, stored, or transmitted in any form or for any means without the prior permission in writing from the copyright holder. For information on purchasing reprints contact Commercialreprints@wiley.com. For permission information contact permissions@wiley.com.
References
- 1. Schram AM, Chang MT, Jonsson P et al. Fusions in solid tumours: Diagnostic strategies, targeted therapy, and acquired resistance. Nat Rev Clin Oncol 2017;14:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Drilon A, Laetsch TW, Kummar S et al. Efficacy of larotrectinib in TRK fusion‐positive cancers in adults and children. N Engl J Med 2018;378:731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fernandez‐Cuesta L, Thomas RK. Molecular pathways: Targeting NRG1 fusions in lung cancer. Clin Cancer Res 2015;21:1989–1994. [DOI] [PubMed] [Google Scholar]
- 4. Jonna S, Feldman RA, Swensen J et al. Detection of NRG1 gene fusions in solid tumors. Clin Cancer Res 2019;25:4966–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fernandez‐Cuesta L, Plenker D, Osada H et al. CD74‐NRG1 fusions in lung adenocarcinoma. Cancer Discov 2014;4:415–422. [DOI] [PubMed] [Google Scholar]
- 6. Shin DH, Lee D, Hong DW et al. Oncogenic function and clinical implications of SLC3A2‐NRG1 fusion in invasive mucinous adenocarcinoma of the lung. Oncotarget 2016;7:69450–69465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trombetta D, Graziano P, Scarpa A et al. Frequent NRG1 fusions in Caucasian pulmonary mucinous adenocarcinoma predicted by phospho‐ErbB3 expression. Oncotarget 2018;9:9661–9671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. U.S. Food and Drug Administration; Afatinib (Gilotrif) U.S. Prescribing Information. 2018. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/201292s014lbl.pdf. Accessed December 15, 2019. [Google Scholar]
- 9. European Medicines Agency . Afatinib (Giotrif) EU Summary of Product Characteristics. 2019. Available at https://www.ema.europa.eu/en/medicines/human/EPAR/giotrif#product‐information‐section. Accessed December 15, 2019. [Google Scholar]
- 10. Solca F, Dahl G, Zoephel A et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342–350. [DOI] [PubMed] [Google Scholar]
- 11. Heining C, Horak P, Uhrig S et al. NRG1 fusions in KRAS wild‐type pancreatic cancer. Cancer Discov 2018;8:1087–1095. [DOI] [PubMed] [Google Scholar]
- 12. Drilon A, Somwar R, Mangatt BP et al. Response to ERBB3‐directed targeted therapy in NRG1‐rearranged cancers. Cancer Discov 2018;8:686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McCoach CE, Le AT, Gowan K et al. Resistance mechanisms to targeted therapies in ROS1(+) and ALK(+) non‐small cell lung cancer. Clin Cancer Res 2018;24:3334–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen H, Zhang Q, Zhang Y et al. Afatinib reverses ceritinib resistance (CR) in ALK/ROS1‐positive non‐small‐cell lung cancer cell (NSCLC) via suppression of NRG1 pathway. Onco Targets Ther 2018;11:8201–8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gay ND, Wang Y, Beadling C et al. Durable response to afatinib in lung adenocarcinoma harboring NRG1 gene fusions. J Thorac Oncol 2017;12:e107–e110. [DOI] [PubMed] [Google Scholar]
- 16. Jones MR, Lim H, Shen Y et al. Successful targeting of the NRG1 pathway indicates novel treatment strategy for metastatic cancer. Ann Oncol 2017;28:3092–3097. [DOI] [PubMed] [Google Scholar]
- 17. Cheema PK, Doherty M, Tsao MS. A case of invasive mucinous pulmonary adenocarcinoma with a CD74‐NRG1 fusion protein targeted with afatinib. J Thorac Oncol 2017;12:e200–e202. [DOI] [PubMed] [Google Scholar]
- 18. Jones MR, Williamson LM, Topham JT et al. NRG1 gene fusions are recurrent, clinically actionable gene rearrangements in KRAS wild‐type pancreatic ductal adenocarcinoma. Clin Cancer Res 2019;25:4674–4681. [DOI] [PubMed] [Google Scholar]
- 19. Murumägi A, Ungureanu D, Khan S et al. Clinical implementation of precision systems in oncology in the treatment of ovarian cancer based on ex‐vivo drug testing and molecular profiling. Cancer Res 2019;79(suppl 13):2945a. [Google Scholar]
- 20. Liu SV, Duruisseaux M, Tolba K et al. Targeting NRG1‐fusions in multiple tumour types: Afatinib as a novel potential treatment option. Ann Oncol 2019;30(suppl 5):v791–v792. [Google Scholar]
- 21. Goto Y, Cadranel J, Weinberg BA et al. NRG1 fusion‐driven solid tumors: A case series indicating the therapeutic potential of afatinib. Ann Oncol 2019;30(suppl 9):ix23–ix24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu SV, Duruisseaux M, Tolba K et al. NRG1 fusion‐positive (NRG1+) tumors: Afatinib as a novel potential treatment option. Presented at the Chinese Society of Clinical Oncology Annual Meeting; Xiamen, China; September 18–22, 2019. Available at https://www.inoncology.com/sites/default/files/poster/csco_2019_nrg1_smartposter_final_version.pdf. Accessed December 15, 2019.
- 23. Duruisseaux M, Laskin JJ, Tolba K et al. Targeting NRG1‐fusions in lung adenocarcinoma: Afatinib as a novel potential treatment strategy. J Thorac Oncol 2019;10:S563. [Google Scholar]
- 24. Laskin JJ, Cadranel J, Renouf DJ et al. Afatinib as a novel potential treatment option for NRG1 fusion‐positive tumors. J Global Oncol 2019;5:110–110. [Google Scholar]
- 25. Wind S, Schmid M, Erhardt J et al. Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet 2013;52:1101–1109. [DOI] [PubMed] [Google Scholar]
- 26. Duruisseaux M, Liu SV, Han JY et al. NRG1 fusion‐positive lung cancers: Clinicopathologic profile and treatment outcomes from a global multicenter registry. J Clin Oncol 2019;37(suppl 15):9081a. [Google Scholar]
- 27. Weinberg BA, Renouf DJ, Lim H et al. NRG1 fusion‐positive gastrointestinal tumours: Afatinib as a novel potential treatment option. Ann Oncol 2019;30(suppl 4):iv80. [Google Scholar]
- 28. Doebele RC. Addressing drug resistance beyond kinase domain mutations. Presented at the International Association for the Study of Lung Cancer (IASLC) 19th World Conference on Lung Cancer; Toronto, Canada; September 23–26, 2018. Available at https://wclc2018.iaslc.org/wp‐content/uploads/2018/11/Online‐WCLC2018‐CME‐Overview‐2018.11.01.pdf. Accessed December 15, 2019.
- 29. Muscarella LA, Trombetta D, Fabrizio FP et al. ALK and NRG1 fusions coexist in a patient with signet ring cell lung adenocarcinoma. J Thorac Oncol 2017;12:e161–e163. [DOI] [PubMed] [Google Scholar]
- 30. Nakaoku T, Tsuta K, Ichikawa H et al. Druggable oncogene fusions in invasive mucinous lung adenocarcinoma. Clin Cancer Res 2014;20:3087–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Shim HS, Kenudson M, Zheng Z et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol 2015;10:1156–1162. [DOI] [PubMed] [Google Scholar]
- 32. Geuijen CAW, De Nardis C, Maussang D et al. Unbiased combinatorial screening identifies a bispecific IgG1 that potently inhibits HER3 signaling via HER2‐guided ligand blockade. Cancer Cell 2018;33:922–936. [DOI] [PubMed] [Google Scholar]
- 33. Schram AM, O'Reilly EM, Somwar R et al. Clinical proof of concept for MCLA‐128, a bispecific HER2/3 antibody therapy, in NRG1 fusion‐positive cancers. Mol Targets Cancer Ther 2019;18(suppl 12):PR02a. [Google Scholar]
- 34. Tirunagaru VG, Estrada‐Bernal A, Yu H et al. Tarloxotinib exhibits potent activity in NRG1 fusion and rearranged cancers. Cancer Res 2019;79(suppl 13):2202a. [Google Scholar]


