Abstract
This randomized controlled trial aimed to evaluate the effects of different whole body vibration (WBV) frequencies on concentric and eccentric leg muscle strength, bone turnover and walking endurance after stroke. The study involved eighty-four individuals with chronic stroke (mean age = 59.7 years, SD = 6.5) with mild to moderate motor impairment (Fugl-Meyer Assessment lower limb motor score: mean = 24.0, SD = 3.5) randomly assigned to either a 20 Hz or 30 Hz WBV intervention program. Both programs involved 3 training sessions per week for 8 weeks. Isokinetic knee concentric and eccentric extension strength, serum level of cross-linked N-telopeptides of type I collagen (NTx), and walking endurance (6-min walk test; 6MWT) were assessed at baseline and post-intervention. An intention-to-treat analysis revealed a significant time effect for all muscle strength outcomes and NTx, but not for 6MWT. The time-by-group interaction was only significant for the paretic eccentric knee extensor work, with a medium effect size (0.44; 95% CI: 0.01, 0.87). Both WBV protocols were effective in improving leg muscle strength and reducing bone resorption. Comparatively greater improvement in paretic eccentric leg strength was observed for the 30 Hz protocol.
Subject terms: Neuroscience, Medical research, Biomarkers
Introduction
Muscle weakness is a major impairment after stroke1 and is associated with various aspects of physical function2 and bone tissue integrity3. According to a recent systematic review4, previous studies involving the use of bone imaging techniques such as peripheral quantitative computed tomography (pQCT)3,5,6 and dual-energy X-ray absorptiometry (DXA)7 to investigate the impact of stroke on lower limb bone outcomes reported strong associations of muscle strength and mass with bone mineral density and indices of bone strength. Previous work has also demonstrated an increased rate of bone resorption in people with stroke, which was correlated with lower hip bone density8,9. Therefore, effective interventions that target muscle strength and bone health are important for stroke rehabilitation.
Whole-body vibration (WBV) augments muscle activation during exercise10,11. The mechanical vibration induces reflex muscle activation and increases motor cortex excitability12,13. WBV has also been shown to increase peak muscle torque in lower limb muscles14, presumably through the recruitment of higher threshold motor units. Improved muscle contractility and force generating capacity have implications for bone health15 as muscle contractions provide an important source of dynamic mechanical loading for maintaining bone tissue15,16. There is evidence that WBV can reduce the rate of bone resorption in different populations (e.g., post-menopausal women, children with severe motor disabilities, and people with metabolic acidosis)17–19.
WBV training has been identified as a potentially viable treatment modality in various patient groups with muscle weakness and consequent bone loss19–22, such as people after stroke3,23–25. However, research on bone metabolism and muscle strength post-stroke after WBV intervention is scarce and the results are inconclusive23,24,26. Thus far, only one study has examined the effects of WBV on bone turnover in people with stroke, and found no significant change in both bone formation and resorption markers following an 8-week WBV intervention (9–15 min, 20–30 Hz)24. More research is needed before the use of WBV for modifying bone turnover rate in people with stroke can be considered conclusive. A meta-analyses by Yang et al. demonstrated that the effects of WBV on maximal isometric knee extension strength (5 studies, SMD = 0.23, 95%CI = − 0.27 to 0.74, p = 0.36), and maximal eccentric knee extension strength (2 studies, SMD = 0.09, 95%CI = -0.38 to 0.56, p = 0.71) yielded wide confidence intervals, indicating that the therapeutic value of WBV on improving knee muscle strength post-stroke requires further investigation26.
Many factors may account for the discrepancies in results across previous studies in stroke (e.g., sample characteristics, WBV type, WBV frequency, treatment duration, etc.). As various studies differed on multiple factors, it was not feasible to delineate the effects of each factor by comparing the results of different studies. Nevertheless, among these factors, vibration frequency may be a particularly important parameter, as revealed by both animal and human studies. Animal studies have shown that higher frequency WBV can enhance osteogenesis more effectively than relatively lower frequency WBV27. In people with stroke, a greater level of leg muscle activation, as indicated by electromyography (EMG) findings, was found during exposure to higher WBV frequency (30 Hz) than lower frequency (20 Hz)11,28. Therefore, repeated exposure to WBV of higher frequencies may lead to a greater strengthening effect of the muscles being stimulated. In a randomized controlled trial, Wei et al. showed that when controlling for the total number of vibrations, a 40 Hz frequency WBV protocol led to the best outcomes in terms of muscle size, strength and physical performance (i.e., 10-m walk test, timed-up-and-go, and sit-to-stand) in patients with sarcopenia29,30. However, these findings are not necessarily generalizable to individuals with chronic stroke. Stroke-related impairments are heterogeneous in presentation, etiologically complex (compensatory movement patterns, learned disuse, etc.) and are often inconsistent with typical muscle changes and performance deficits associated with atrophy or aging alone31,32. Only one study has compared the effects of two different WBV protocols in the same sample of people with stroke (20 Hz vs 30 Hz) and found no difference in knee muscle strength after 10 weeks of intervention. However, the number of vibrations was not controlled and bone turnover was not measured33.
To address these identified gaps in knowledge, we aimed to evaluate the effects of different WBV frequencies in stroke patients. In addition to leg muscle strength and bone turnover, the 6-min walk test (6MWT), an indicator of walking endurance, was also used as an outcome. Leg muscle strength has demonstrated a strong association with 6MWT distance in individuals with stroke34,35. Therefore, any WBV-induced improvement in leg muscle strength was also thought to result in better walking endurance. We hypothesized that a higher WBV frequency (30 Hz) would induce larger improvements in muscle strength and walking endurance, and greater reduction in the level of bone resorption marker compared with a lower WBV frequency (20 Hz).
Methods
Study design
A single-blinded, randomized controlled trial was conducted.
Ethical approval
This study was registered on 06/11/2019 in clinicaltrials.gov (identifier: NCT03982251). Ethical approval for the study was granted by the Human Research Ethics Subcommittee of the Hong Kong Polytechnic University (reference number: HSEARS 20140226001-03), and all of the experiments were conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant prior to data collection.
Participants
This study was conducted in a research laboratory at the University. Participants were recruited from a stroke patient organization in the community via convenience sampling. The screening and enrolment of potential participants were performed by an independent researcher.
The inclusion criteria were as follows: (1) patient aged ≥ 50 years, (2) medically stable, (3) able to stand for at least 1 min with hand support, and (4) able to understand simple verbal commands. Only individuals aged 50 years or more were recruited. It was because stroke is more prevalent in older adults36. Setting an age limit would make the sample more homogeneous in terms of age thereby reducing the potential confounding effect of age on primary outcomes (i.e., muscle strength, bone turnover). The exclusion criteria were as follows: (1) additional neurological conditions, (2) musculoskeletal conditions affecting leg muscle performance (e.g., rheumatoid arthritis), (3) presence of metal implants in the lower extremity, (4) recent fracture in the lower extremity (within 1-year post-onset), (5) receiving medications to treat osteoporosis, (6) vestibular disorders, (7) peripheral vascular disease, and (8) other serious illnesses or contraindications to exercise.
Participant allocation
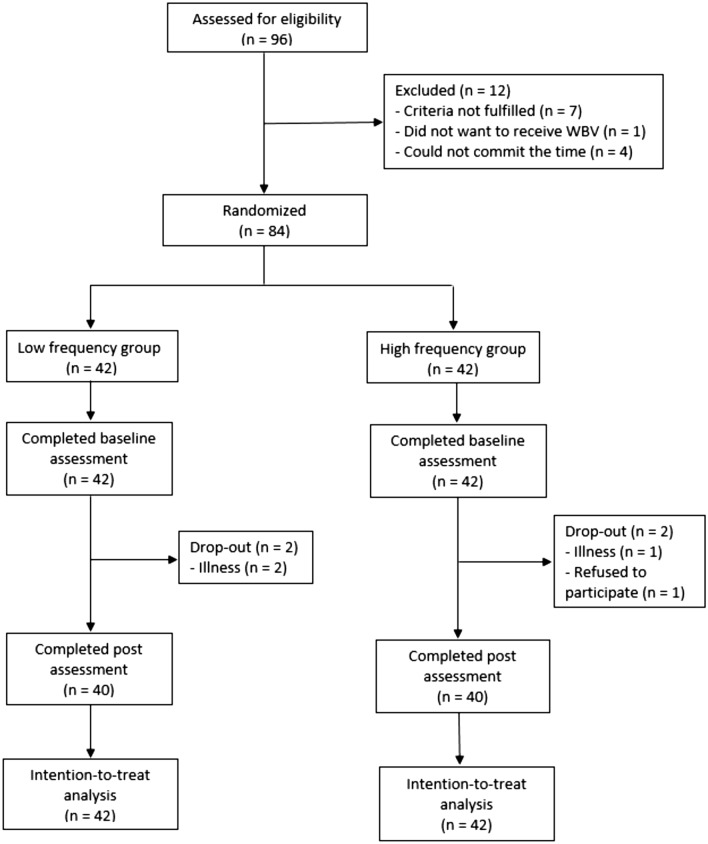
The participants were randomly allocated to one of two groups: a low frequency WBV group (frequency: 20 Hz; amplitude: 0.60 mm) or a relatively higher frequency WBV group (frequency: 30 Hz; amplitude: 0.60 mm). The allocation (with a 1:1 ratio) was completed by an off-site researcher who was not involved in other aspects of the trial, using an online randomization program (http://rct.mui.ac.ir/q/). Prior to randomization, participants were stratified into three clinically meaningful groups according to walking speed (household ambulators: < 0.4 m/s; limited community ambulators: 0.4–0.8 m/s; community ambulators: > 0.8 m/s) and sex37. These variables were used for stratification because they were shown to be associated with muscle strength and bone status3,34,38–41. The stratified random allocation would ensure that the 20 Hz and 30 Hz groups were similar in terms of walking function and proportion of men/women. The reporting of results and procedures was done in accordance with the Consolidated Standards of Reporting Trials (CONSORT) guidelines. A diagram outlining the flow of participant screening, randomization and allocation is provided in Fig. 1.
Figure 1.
CONSORT flow diagram.
Intervention protocol
The two groups of participants completed training sessions 3 days a week for 8 weeks. A make-up session was provided for any missed appointments so that all participants eventually completed 24 training sessions. The duration of the intervention was based on a previously published study that reported a positive effect of a similar training dosage on bone turnover in post-menopausal women19. There is no established WBV protocol for enhancing muscle strength and bone health in people with stroke, and no stroke study has specifically examined the effect of WBV interventions involving different frequencies. Stroke patients also share similar bone health problems to those associated with post-menopausal women (i.e., increased rate of bone resorption and compromised bone density). Therefore, it is reasonable to take reference from this study on post-menopausal women when we developed our WBV protocol. WBV-induced changes in muscle strength and bone turnover marker levels were expected to be evident within the 8-week time frame21,42–45.
In each training session, the participants first performed warm-up exercises for ~ 10 min, which included general mobilization and upper limb stretching exercises performed in a sitting position. A Jet-Vibe System (Danil SMC Co. Ltd., Seoul, Korea) was then used to deliver the WBV. This device delivers synchronous vertical vibrations over a range of frequencies (20–55 Hz), which were adjusted by the researchers. Synchronous vertical WBV was used because it was thought to provide better stability during WBV exercise as it produces only vertical perturbations in comparison to both vertical and horizontal displacements associated with side-alternating or oscillating WBV46,47. Frequencies > 30 Hz were not used. The pilot data showed that high frequencies caused discomfort in this population. Frequencies < 20 Hz were not used due to potential resonance48 and sensorimotor coordination effects49.
During the WBV treatment, the participants were instructed to remove their shoes and stand on the vibration platform with their feet placed a shoulder-width distance apart. Participants were instructed to flex the knee to 60° while standing on the vibration platform. This specified joint angle was chosen to reduce undesirable transmission of vibration to the head50,51. Based on the results of a previous study, knee extensor muscle EMG activity during WBV exercise was shown to be greatest at 60° of knee joint flexion compared to 10° and 30°11. This angle was also determined to be safe and feasible during pilot testing. Participants were also asked to report any symptoms of pain or abnormal discomfort during WBV sessions. To facilitate a meaningful comparison and to delineate the effects of the WBV frequency, the number of loading cycles was matched between the 20 Hz and 30 Hz frequency groups. For both groups, exposure to vibration was provided in 1-min bouts, with a 1-min rest period between bouts. Twelve WBV bouts were delivered per training session to the 20 Hz frequency group, whereas 8 WBV bouts were delivered per training session to the 30 Hz frequency group (i.e., 14,400 loading cycles) so that the total WBV dosage for each session was equivalent between groups. For standardization, all participants gently held onto the handrail of the WBV device only to maintain balance.
Outcome measures
Researchers who were blinded to the intervention groups conducted all of the outcome assessments. Relevant demographic information and clinical history were obtained from all participants through interviews conducted at baseline. During the baseline assessment session, the level of motor impairment of the leg and foot was evaluated using the Fugl-Meyer Motor Assessment (FMA)52. The Physical Activity Scale for the Elderly (PASE)53 was used to measure participant physical activity level. The spasticity of the paretic ankle joint was examined using the Modified Ashworth Scale (MAS)54. All of the following outcomes were assessed at baseline and also the end of the eight-week intervention period.
Isokinetic knee muscle strength: Participants underwent knee muscle strength testing on both sides using an isokinetic dynamometer (HUMAC NORM Testing & Rehabilitation System, Computer Sports Medicine Inc., U.S.A.), which provided good reliability of strength measurements (ICC = 0.89–0.96)55. In brief, participants maintained an upright sitting position while the knee joint was aligned with the mechanical axis of the dynamometer. Straps were used to stabilize the untested limb. Each participant was then instructed to perform maximal concentric/eccentric knee extension throughout a range of 10°–70° knee flexion on each side at a constant angular speed of 120°/s. This range of motion was chosen based on the experience gained in our pilot testing. Some individuals with stroke patients had limited hamstrings flexibility, and were not able to reach the 0° knee flexion (i.e., full knee extension) in a sitting position. Some individuals experienced some discomfort if the knee (particularly on the paretic side) was flexed to more than 80°, potentially indicative of degenerative joint changes. Therefore, to ensure safety, we used a range of motion between 10° and 70° of knee flexion. The relatively high angular speed of 120°/s was chosen for several reasons. First, it was a speed commonly used in previous stroke studies56,57. Second, individuals with stroke typically demonstrated severe muscle weakness at higher contraction speeds1. Also, knee movements at high speeds are involved in daily activities. Previous work showed that during walking over a wide range of speeds (0.4–1.39 m/s), the angular velocity during knee flexion in the swing phase, and that of knee extension during terminal swing, exceeded 120°/s58. As walking speed approached 1.0 m/s, the angular velocity of knee flexion during the loading response also approximated 120°/s. During the sit-to-stand movement, the knee joint angular velocity has also been shown to be roughly 120°/s during the extension phase59. The sequence of testing (i.e., paretic side versus non-paretic side, or type of contraction) was randomized to minimize the order effect. Three trials were recorded for each test condition and the total work (in Joules; J) value was obtained using customized software. Three trials were conducted to obtain the mean value for statistical analysis. The total work represents the accumulated torque output produced as the joint moves through a specified range of motion60. Therefore, the measurement of total work takes into account the ability of the muscle to maintain contraction at a certain strength level through the range of motion. The measure was thus considered by some researchers to be more reflective of muscle function and strength during movement than peak torque61–63. The percent standard error of measurement (%SEM) established for this outcome was 19.2%, which is an appropriate index for detecting change in a group of people64.
Bone resorption analysis: Serum cross-linked N-telopeptides of type I collagen (NTx) was chosen as a surrogate marker of bone resorption to evaluate the dynamic process of bone turnover. In brief, a 5 ml fasting blood sample was collected from all participants in the morning (between 0900 and 1100 h) at defined investigational time points. All of the blood samples were then promptly centrifuged. The serum was separated and then immediately frozen at − 80 °C until further analysis. Serum levels of NTx were assessed using the Osteomark NTx Serum assay (Alere Scarborough, Inc., Scarborough, U.S.A.) according to the protocol provided by the manufacturer. Essentially, appropriately diluted serum samples, together with NTx epitope-containing molecules that are conjugated with horseradish peroxidase, were added to the microplate wells that had been previously coated with antibodies against NTx. NTx in the patient sample thus competed with the conjugated NTx epitopes in the microplate well for antibody binding sites. Following a wash step, a chromogenic substrate solution was added for color development. Absorbance was determined on a spectrophotometer and the NTx concentration was calculated against a standard calibration curve. The assay values were recorded in nanomoles Bone Collagen Equivalents per liter (nM BCE). The reference range was between 3.2 and 40.0 nM BCE. In each assay, three duplicate samples were used to determine the intra-assay coefficient of variation (% CV) (intra-assay %CV = 4.6%). The inter-assay %CV was established between two assays of a total of 18 samples (inter-assay %CV = 6.9%). An intra-assay and inter-assay %CV value of less than 10% is considered to be acceptable65.
6MWT: This test was used to assess endurance66. Participants were asked to walk along a 15-m walkway and cover as much distance as possible within 6 min, using walking aids if necessary. The total distance (meters) walked was recorded. The 6MWT has demonstrated excellent reliability (ICC = 0.97–0.99) in assessing walking endurance among individuals with stroke66.
Compliance and adverse events
Participant attendance of the training sessions was recorded by the researcher who supervised the WBV training sessions. Any adverse events reported by the participants or observed by the researcher were also documented. The total time period taken to complete 24 training sessions (number of days) and the maximum time lapse between any two training sessions (number of days) for each participant were used for subsequent analysis.
Statistical analyses
The sample size was estimated using G*Power 3.1.9.2 software (Heinrich-Heine-Universität Düsseldorf, Germany). Tihanyi et al. found that WBV induced a significant increase in paretic knee muscle strength (Cohen’s d) of 0.46–0.51 (i.e., medium) in people with stroke67. Another study by Wei et al. showed that medium-frequency WBV generated better knee muscle strength outcomes than low-frequency outcomes in patients with sarcopenia (d = 0.24)30. Another study by Turner et al. showed that a WBV protocol similar to that used in the 30 Hz frequency group induced a significant change in bone resorption marker levels, with a large effect size of 0.9619. Overall, a small to medium effect size was assumed (f = 0.2) for a 2 × 2 analysis of variance (ANOVA) with repeated measures. With an alpha of 0.05, a power of 90%, and considering an attrition rate of 15%, the minimum sample size required to detect a significant group × time interaction effect was 80 participants (40 per group).
The Statistical Package for Social Sciences version 23.0 (SPSS; IB, Armonk, NY) was used for all analyses. The normality of the data was checked using the Kolmogorov–Smirnov test. Between-group differences in baseline characteristics were evaluated by an independent t test, a Mann–Whitney U test or a chi-square test, as appropriate. To compare the treatment effect between the two groups, a mixed-design, analysis of variance was used (within-subject factor: time; between-subject factor: group). An intention-to-treat analysis was conducted, in which the last observation carried forward method was used to substitute the missing data for participants who were lost to follow-up (i.e. dropout). This approach was considered to be more conservative but was less susceptible to bias arising from attrition68. Post-hoc analyses were conducted to examine the pre-test and post-test within-group scores (paired t-tests), and also between-group differences in change scores (independent t-tests). The above analyses were repeated after eliminating drop-outs (on-protocol analysis). If both the intention-to-treat and on-protocol analysis approaches yielded similar findings, there would be strong confidence in the study results68.
Results
Study group characteristics
Of the 96 individuals with stroke who were screened for eligibility, 84 fulfilled all of the selection criteria. This was greater than the estimated minimum number of participants required from our sample size calculation (n = 80). As having a greater sample size could further increase statistical power, 84 individuals with stroke were ultimately enrolled in the study rather than 80. They were randomly allocated to either the 20 Hz frequency (n = 42) or 30 Hz frequency (n = 42) groups. Four participants dropped out during the course of the study (2 in each of the treatment groups). By the end of the study, 80 participants had completed the training program and all outcome assessments (Fig. 1). We found no significant between-group differences in terms of demographic variables or stroke characteristics at baseline (Table 1). Therefore, none of the variables shown in Table 1 were considered important confounding factors.
Table 1.
Participant characteristics at baseline*.
| Characteristic | All (n = 84) | 20 Hz WBV (n = 42) | 30 Hz WBV (n = 42) | p† |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 59.7 ± 6.5 | 60.4 ± 5.9 | 59.0 ± 7.0 | 0.299 |
| Sex (men/women) | 54/30 | 29/13 | 25/17 | 0.362 |
| Body mass index h(kg/m2) | 23.5 ± 3.3 | 23.0 ± 3.1 | 24.0 ± 3.4 | 0.203 |
| Walking aid: none/cane/quad/frame | 68/13/3/0 | 35/6/1/0 | 33/7/2/0 | 0.791 |
| PASE score | 95.1 ± 50.3 | 97.2 ± 57.0 | 93.1 ± 43.2 | 0.715 |
| Stroke characteristics | ||||
| Hemiparesis side, n (right/left) | 37/47 | 17/25 | 20/22 | 0.510 |
| Post-stroke duration (years) | 4.6 ± 3.5 | 4.6 ± 3.7 | 4.5 ± 3.4 | 0.951 |
| Type of stroke, n (hemorrhagic/ischemic) | 40/44 | 17/25 | 23/19 | 0.190 |
| Fugl-Meyer lower limb score | 24.0 ± 3.5 | 24.6 ± 2.8 | 23.2 ± 4.1 | 0.060 |
| Paretic ankle MAS score (0–4) | 1.0 (0–4) ‡ | 1.0 (0–4) ‡ | 1.0 (0–4) ‡ | 0.075 |
| Comorbidities, n | ||||
| Hypertension | 54 | 28 | 26 | 0.649 |
| Diabetes mellitus | 15 | 8 | 7 | 0.776 |
| Hyperlipidemia | 32 | 17 | 15 | 0.653 |
| Number of comorbidities | 1.7 ± 0.8 | 1.6 ± 0.9 | 1.8 ± 1.0 | 0.495 |
| Medications, n | ||||
| Antihypertensives | 54 | 28 | 26 | 0.649 |
| Antidiabetic medications | 15 | 8 | 7 | 0.776 |
| Anticonvulsants | 35 | 18 | 17 | 0.825 |
| Anticoagulants | 31 | 16 | 15 | 0.821 |
| Number of medications | 3.0 ± 1.8 | 3.1 ± 1.7 | 2.9 ± 1.8 | 0.462 |
| Compliance | ||||
| Time taken to complete 24 training sessions (d) | 65.4 ± 3.1 | 65.3 ± 3.2 | 65.5 ± 3.1 | 0.784 |
| Maximum time lapse between training sessions (d) | 8.0 ± 2.2 | 7.8 ± 2.3 | 8.1 ± 2.1 | 0.527 |
*Mean ± standard deviation presented unless indicated otherwise.
†Between-group comparison.
‡Median (interquartile range).
Abbreviations: 20 Hz WBV: 20 Hz whole-body-vibration group, 30 Hz WBV: 30 Hz whole-body-vibration group, PASE: Physical Activity Scale for the Elderly, MAS: Modified Ashworth Scale.
Effect on outcome measures
The outcome measurements collected at baseline did not differ between the two groups (Table 2). We identified a significant main effect of time for all muscle strength and bone turnover outcomes (p < 0.001), but not for the 6MWT (p = 0.533) (Table 2). We also identified a significant effect of time × group interaction for the paretic eccentric knee extensor work, with a medium effect size (0.44; 95% CI: 0.01, 0.87). The change of eccentric extensor work in the non-paretic leg also showed a similar trend, but the confidence intervals suggested a small chance that the 20 Hz frequency protocol might be superior (95% CI: -0.08, 0.78). The on-protocol analysis generated similar results (Supplementary Table).
Table 2.
Outcome measurements (Intention-to-treat analysis).
| Variable | 20 Hz WBV (N = 42) | 30 Hz WBV (N = 42) | Between-group difference in change scores | Comparisons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change score | Pre | Post | Change score | Mean (95% CI) | pa | pb | pc | pd | pe | pf | |
| Knee extensor work (J) | |||||||||||||
| Nonparetic concentric*‡§ | 28.1 ± 11.1 | 35.8 ± 17.4 | 7.8 ± 12.4 | 23.5 ± 11.1 | 36.3 ± 23.4 | 12.8 ± 17.6 | 5.0 (− 1.6, 11.7) | 0.061 | 0.000 | 0.135 | 0.000 | 0.000 | 0.135 |
| Paretic concentric*‡§ | 17.4 ± 8.0 | 22.4 ± 9.0 | 5.0 ± 5.1 | 15.3 ± 8.2 | 21.0 ± 10.0 | 5.7 ± 4.8 | 0.7 (− 1.4, 2.9) | 0.242 | 0.000 | 0.496 | 0.000 | 0.000 | 0.496 |
| Nonparetic eccentric*‡§ | 80.3 ± 20.4 | 94.5 ± 22.9 | 14.2 ± 13.4 | 75.1 ± 19.9 | 95.5 ± 30.2 | 20.4 ± 21.0 | 6.2 (− 1.5, 13.8) | 0.243 | 0.000 | 0.113 | 0.000 | 0.000 | 0.113 |
| Paretic eccentric*†‡§¶ | 65.2 ± 21.2 | 73.0 ± 18.7 | 7.8 ± 11.2 | 60.2 ± 23.3 | 74.0 ± 21.6 | 13.8 ± 15.5 | 5.9 (0.05, 11.8) | 0.311 | 0.000 | 0.048 | 0.000 | 0.000 | 0.048 |
| Other outcomes | |||||||||||||
| NTx (nM BCE)*‡§ | 6.1 ± 3.7 | 3.6 ± 2.3 | − 2.2 ± 3.4 | 6.3 ± 4.2 | 3.6 ± 1.8 | − 2.7 ± 4.0 | − 0.5 (− 2.1, 1.1) | 0.792 | 0.000 | 0.540 | 0.000 | 0.000 | 0.540 |
| 6MWT distance (m) | 248.7 ± 89.8 | 250.6 ± 90.3 | 1.9 ± 11.9 | 257.2 ± 111.4 | 256.8 ± 112.5 | − 0.4 ± 11.0 | − 2.3 (− 7.3, 2.7) | 0.701 | 0.533 | 0.365 | 0.302 | 0.835 | 0.365 |
*Significant time effect (p < 0.05).
†Significant group × time interaction effect (p < 0.05).
‡Significant within-group comparison (20 Hz WBV group) (p < 0.05).
§Significant within-group comparison (30 Hz WBV group) (p < 0.05).
¶Between-group comparison of change score (p < 0.05).
aBaseline comparisons (independent t-test).
bTime effect (ANOVA).
cGroup × time interaction effect (ANOVA).
dWithin-group comparison (20 Hz WBV group) (paired t-test).
eWithin-group comparison (30 Hz WBV group) (paired t-test).
fBetween-group comparison of change score (independent t-test).
Abbreviations: 20 Hz WBV: 20 Hz whole-body-vibration group, 30 Hz WBV: 30 Hz whole-body-vibration group, CI: confidence interval, NTx: serum cross-linked N-telopeptides of type I collagen, 6MWT: 6-min walk test.
Compliance and adverse events
The time taken to complete 24 training sessions and the maximum time lapse between any two training sessions were similar between the two groups (p > 0.05). No adverse events occurred during the intervention trial.
Discussion
The key finding is that both the 20 Hz and 30 Hz WBV protocols are effective in increasing knee muscle strength and reducing bone resorption, but the former is better at improving the paretic eccentric knee extensor strength than the latter.
Leg muscle activity measured by EMG can be augmented during WBV exposure69–71. Through regular WBV intervention, the stimulated muscles are repetitively “exercised”. Over time, this may contribute to greater muscle strength15. Our study data confirm that leg muscle strength can be increased following regular WBV intervention over an 8-week period. Improved muscle coordination67, and enhancement of intramuscular blood perfusion72 are some of the proposed mechanisms underlying improved muscle strength following WBV reported in studies involving neurological populations. Other mechanisms associated with improved strength following WBV reported in healthy subjects include increased cortical excitability12, reduced recruitment threshold, increased activation of fast-twitch muscle motor units73, and motor unit reflex activation47. Overall, our findings are largely in line with previous studies showing increased muscle strength in elderly populations, both with and without sarcopenia, following WBV exercise29,74. However, the positive improvement in muscle strength reported in this study cannot be attributed to the WBV stimulation alone. The participants assumed a static, semi-squatting posture (i.e., 60° of knee flexion) during WBV exposure which may have also contributed to the observed increase in muscle strength.
An interesting finding of our study is that the 30 Hz frequency protocol induced a greater gain in eccentric knee extension strength in the paretic leg. The mean increase in eccentric knee strength attained by the 30 Hz frequency group was 22.9%, which exceeded the %SEM value (i.e. 19.2%). Our results thus suggest the 30 Hz frequency protocol produced a clinically meaningful change in muscle strength. These results are accordant with a previous study that found a 30 Hz WBV frequency to be optimal for improving muscle strength among healthy adults75. Compared to relatively lower (20 Hz) and higher WBV frequencies (60 Hz), Wei et al. found that a 40 Hz frequency produced the largest improvement in isokinetic knee extension strength among older adults with sarcopenia30. Therefore, it seems that specific frequencies for producing optimal outcomes are different for various patient populations.
In our study, the eccentric strength results for the non-paretic leg showed a similar trend to the paretic leg. However, the confidence intervals (-0.08, 0.78) indicate a slight probability that the 20 Hz frequency protocol might be superior to the 30 Hz frequency protocol. Baseline paretic muscle strength was also substantially lower than the non-paretic side (Table 2), suggesting there was more room for improvement in the former.
Only one randomized controlled study by Liao et al. has attempted to compare WBV protocols for stroke patients33. However, the addition of low-intensity (peak acceleration: 1.6 g) high-intensity (3.6 g) WBV in the exercise protocol used in their trial did not improve concentric or eccentric muscle strength outcomes33. Differences in the WBV protocol [i.e., smaller (10°) knee flexion angle during WBV exercise, lower number of total bouts per session (2 for 20 Hz, 3 for 30 Hz), longer bout duration (1.5 min), and higher vibration amplitude (1 mm) used in the study by Liao et al.] may provide an explanation for this finding. The authors also compared WBV intensities but were unable to delineate the effect of frequency because of differences in the number of loading cycles involved in different treatment arms33.
Both groups improved in concentric muscle strength on both sides after the intervention period but no significant between-group differences were found. Concentric muscle strength is typically more compromised than eccentric muscle strength after stroke1, and may require more intensive training to elicit a more pronounced difference in improvements between the two WBV protocols. This theory now requires further research.
Our results show that both the 20 Hz and 30 Hz frequency protocols promoted a significant reduction in the expression of NTx indicating that WBV exercise was beneficial in reducing the rate of bone resorption among chronic stroke patients. The amount of reduction in NTx was similar between the two groups (20 Hz frequency: 36%, 30 Hz frequency: 43%) and was not statistically significant. Perhaps a larger differential in WBV frequency and a larger sample size would be required to detect a significant between-group difference in reduction of NTx levels. Only one previous study has investigated the effects of WBV on bone turnover (indicated by C-telopeptide of type I collagen cross linking and bone-specific alkaline phosphatase levels) in people with stroke24. The level of these bone turnover markers showed no significant change after the 8-week intervention period (24 sessions) for both the WBV and control groups. The disparity in results may be related to the difference in WBV protocols24. In the previous study, the number of loading cycles was gradually increased as the training program progressed, and did not reach a level similar to the present study until week 5. The less intensive WBV stimulation in the initial period of the intervention program may partly explain why no significant change in bone resorption marker level was reported in their study24. As there was no sham WBV or no-intervention control group in this study, the difference in the change of NTx levels after WBV intervention versus a sham intervention is unknown. Previous work has shown that individuals with stroke have a higher level of bone resorption than their counterparts without a history of stroke8. Longitudinal studies are required to examine the temporal changes in bone resorption marker levels.
The two treatment protocols did not induce any significant change in 6MWT distance. Apart from muscle strength, aerobic capacity has also been identified as a limiting factor related to 6MWT performance (i.e., walking endurance) among people with stoke34,35,76,77. Previous results indicated that exposure to WBV produced only modest changes in cardiovascular parameters, such as heart rate and blood pressure78. This may explain, in part, why our intervention protocols did not lead to any significant change in 6MWT distance.
This study has several limitations that should be noted. First, the findings cannot be generalized to those who are in the acute or sub-acute stages of stroke recovery, or to those who are wheelchair-bound or have severe motor impairments. Second, while it is unlikely that age is an important confounding factor in this study due to the lack of significant between-group difference (Table 1), how younger stroke patients might respond to the two WBV frequencies remains to be investigated. Third, we did not measure the level of bone formation markers. Incorporating a bone formation maker in our study would have provided a more comprehensive evaluation of the effect of our WBV protocols on bone turnover. Fourth, only synchronous vertical vibrations were used in our study. It may not be meaningful to make a direct comparison between the results of this study with others using oscillating (side-alternating) vibrations as other factors also differed across studies (i.e., participant demographics, body position, external load)46. Whether using side-alternating vibrations may result in greater muscle strength improvement in stroke patients will require further investigation. Finally, whether the beneficial effects can be sustained after the cessation of treatment also remains to be determined.
The results showed that while both the 20 Hz and 30 Hz WBV frequency protocols increased concentric and eccentric knee muscle strength and reduced bone resorption rate, the 30 Hz frequency protocol was more effective than the 20 Hz frequency protocol in improving eccentric knee extension strength on the paretic side after treatment cessation. Therefore, a frequency of 30 Hz may be more appropriate for enhancing leg muscle strength, with possible implications for maintaining bone health among individuals with chronic stroke.
Supplementary Information
Acknowledgements
Zhenhui Yang and Tiev Miller were granted full-time research studentships by the Hong Kong Polytechnic University (funding code RUBE and funding code RL27). This study was supported by the Hong Kong Research Grants Council (General Research Fund no. 151025/14M).
Author contributions
Z.Y. and M.Y.C.P. conceived, designed and managed the project. M.Y.C.P. procured the funding, T.M. conducted the intervention and Z.Y. conducted the baseline and post-intervention assessments. Z.Y., T.M., Z.X. and M.Y.C.P. contributed to the data analysis and interpretation of the results. Z.Y. wrote the initial manuscript. Z.Y., T.M., Z.X. and M.Y.C.P. provided feedback and subsequent revision of the manuscript. All authors have read and approved the final manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-020-80526-4.
References
- 1.Lomaglio MJ, Eng JJ. Nonuniform weakness in the paretic knee and compensatory strength gains in the nonparetic knee occurs after stroke. Cerebrovasc. Dis. (Basel, Switzerland) 2008;26:584–591. doi: 10.1159/000165111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lomaglio MJ, Eng JJ. Muscle strength and weight-bearing symmetry relate to sit-to-stand performance in individuals with stroke. Gait Posture. 2005;22:126–131. doi: 10.1016/j.gaitpost.2004.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pang MY, Ashe MC, Eng JJ. Compromised bone strength index in the hemiparetic distal tibia epiphysis among chronic stroke patients: the association with cardiovascular function, muscle atrophy, mobility, and spasticity. Osteoporos. Int. 2010;21:997–1007. doi: 10.1007/s00198-009-1038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang FZ, Jehu DAM, Ouyang H, Lam FMH, Pang MYC. The impact of stroke on bone properties and muscle–bone relationship: a systematic review and meta-analysis. Osteoporos. Int. 2020;31:211–224. doi: 10.1007/s00198-019-05175-4. [DOI] [PubMed] [Google Scholar]
- 5.Yang FZ, Pang MY. Influence of chronic stroke impairments on bone strength index of the tibial distal epiphysis and diaphysis. Osteoporos. Int. 2015;26:469–480. doi: 10.1007/s00198-014-2864-5. [DOI] [PubMed] [Google Scholar]
- 6.Lam FM, Bui M, Yang FZ, Pang MY. Chronic effects of stroke on hip bone density and tibial morphology: a longitudinal study. Osteoporos. Int. 2016;27:591–603. doi: 10.1007/s00198-015-3307-7. [DOI] [PubMed] [Google Scholar]
- 7.Pang MY, Eng JJ, McKay HA, Dawson AS. Reduced hip bone mineral density is related to physical fitness and leg lean mass in ambulatory individuals with chronic stroke. Osteoporos. Int. 2005;16:1769–1779. doi: 10.1007/s00198-005-1925-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haddaway MJ, Bainbridge NJ, Powell DE, Davie MWJ. Bone Resorption in stroke and institutionalized subjects. Calcif. Tissue Int. 2009;84:118–125. doi: 10.1007/s00223-008-9203-9. [DOI] [PubMed] [Google Scholar]
- 9.Paker N, Bugdayci D, Tekdos D, Dere C, Kaya B. Relationship between bone turnover and bone density at the proximal femur in stroke patients. J. Stroke Cerebrovasc. Dis. 2009;18:139–143. doi: 10.1016/j.jstrokecerebrovasdis.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 10.Roelants MPM, Verschueren MPS, Delecluse MPC, Levin MPO, Stijnen MPV. Whole-body-vibration-induced increase in leg muscle activity during different squat exercises. J. Strength Cond. Res. 2006;20:124–129. doi: 10.1519/00124278-200602000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Liao L-R, Lam FMH, Pang MYC, Jones AYM, Ng GYF. Leg muscle activity during whole-body vibration in individuals with chronic stroke. Med. Sci. Sports Exerc. 2014;46:537–545. doi: 10.1249/mss.0b013e3182a6a006. [DOI] [PubMed] [Google Scholar]
- 12.Mileva KN, Bowtell JL, Kossev AR. Effects of low-frequency whole-body vibration on motor-evoked potentials in healthy men. Exp. Physiol. 2009;94:103–116. doi: 10.1113/expphysiol.2008.042689. [DOI] [PubMed] [Google Scholar]
- 13.Karacan I, Cidem M, Cidem M, Türker KS. Whole-body vibration induces distinct reflex patterns in human soleus muscle. J. Electromyogr. Kinesiol. 2017;34:93–101. doi: 10.1016/j.jelekin.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs PL, Burns P. Acute enhancement of lower-extremity dynamic strength and flexibility with whole-body vibration. J. Strength Cond. Res. 2009;23:51–57. doi: 10.1519/JSC.0b013e3181839f19. [DOI] [PubMed] [Google Scholar]
- 15.Turner CH, Owan I, Takano Y. Mechanotransduction in bone: role of strain rate. Am. J. Physiol. Endocrinol. Metab. 1995;269:E438–E442. doi: 10.1152/ajpendo.1995.269.3.E438. [DOI] [PubMed] [Google Scholar]
- 16.Turner CH, Robling AG. Designing exercise regimens to increase bone strength. Exerc. Sport Sci. Rev. 2003;31:45–50. doi: 10.1097/00003677-200301000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Cardinale M, Leiper J, Farajian P, Heer M. Whole-body vibration can reduce calciuria induced by high protein intakes and may counteract bone resorption: a preliminary study. J. Sports Sci. 2007;25:111–119. doi: 10.1080/02640410600717816. [DOI] [PubMed] [Google Scholar]
- 18.Kilebrant S, et al. Whole-body vibration therapy in children with severe motor disabilities. J. Rehabil. Med. 2015;47:223–228. doi: 10.2340/16501977-1921. [DOI] [PubMed] [Google Scholar]
- 19.Turner S, et al. A randomized controlled trial of whole body vibration exposure on markers of bone turnover in postmenopausal women. J Osteoporos. 2011;2011:710387. doi: 10.4061/2011/710387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belavý DL, et al. Evidence for an additional effect of whole-body vibration above resistive exercise alone in preventing bone loss during prolonged bed rest. Osteoporos. Int. 2011;22:1581–1591. doi: 10.1007/s00198-010-1371-6. [DOI] [PubMed] [Google Scholar]
- 21.Gusso S, et al. Effects of whole-body vibration training on physical function, bone and muscle mass in adolescents and young adults with cerebral palsy. Sci. Rep. 2016;6:22518. doi: 10.1038/srep22518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau RW, et al. The effects of whole body vibration therapy on bone mineral density and leg muscle strength in older adults: a systematic review and meta-analysis. Clin. Rehabil. 2011;25:975–988. doi: 10.1177/0269215511405078. [DOI] [PubMed] [Google Scholar]
- 23.Marín PJ, Ferrero CM, Menéndez H, Martín J, Herrero AJ. Effects of whole-body vibration on muscle architecture, muscle strength, and balance in stroke patients: a randomized controlled trial. Am. J. Phys. Med. Rehabil. 2013;92:881–888. doi: 10.1097/PHM.0b013e318292336c. [DOI] [PubMed] [Google Scholar]
- 24.Pang MYC, Lau RWK, Yip SP. The effects of whole-body vibration therapy on bone turnover, muscle strength, motor function, and spasticity in chronic stroke: a randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2013;49:439–450. [PubMed] [Google Scholar]
- 25.Poole KES, Reeve J, Warburton EA. Falls, fractures, and osteoporosis after stroke. Time to think about protection? Stroke. 2002;33:1432–1436. doi: 10.1161/01.STR.0000014510.48897.7D. [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Wang P, Liu C, He C, Reinhardt JD. The effect of whole body vibration on balance, gait performance and mobility in people with stroke: a systematic review and meta-analysis. Clin. Rehabil. 2015;29:627–638. doi: 10.1177/0269215514552829. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa T, et al. The effect of whole-body vibration on peri-implant bone healing in rats. Clin. Oral Implants Res. 2011;22:302–307. doi: 10.1111/j.1600-0501.2010.02020.x. [DOI] [PubMed] [Google Scholar]
- 28.Liao L-R, Ng GYF, Jones AYM, Chung RCK, Pang MYC. Effects of vibration intensity, exercise, and motor impairment on leg muscle activity induced by whole-body vibration in people with stroke. Phys. Ther. 2015;95:1617–1627. doi: 10.2522/ptj.20140507. [DOI] [PubMed] [Google Scholar]
- 29.Wei N, Pang MY, Ng SS, Ng GY. Optimal frequency/time combination of whole body vibration training for developing physical performance of people with Sarcopenia: a randomized controlled trial. Clin. Rehabil. 2017;31:1313–1321. doi: 10.1177/0269215517698835. [DOI] [PubMed] [Google Scholar]
- 30.Wei N, Pang MY, Ng SS, Ng GY. Optimal frequency/time combination of whole-body vibration training for improving muscle size and strength of people with age-related muscle loss (Sarcopenia): a randomized controlled trial. Geriatr. Gerontol. Int. 2017;17:1412–1420. doi: 10.1111/ggi.12878. [DOI] [PubMed] [Google Scholar]
- 31.Foran JR, Steinman S, Barash I, Chambers HG, Lieber RL. Structural and mechanical alterations in spastic skeletal muscle. Dev. Med. Child Neurol. 2007;47:713–717. doi: 10.1111/j.1469-8749.2005.tb01063.x. [DOI] [PubMed] [Google Scholar]
- 32.Sions JM, Tyrell CM, Knarr BA, Jancosko A, Binder-Macleod SA. Age- and stroke-related skeletal muscle changes: a review for the geriatric clinician. J. Geriatr. Phys. Ther. 2012;35:155–161. doi: 10.1519/JPT.0b013e318236db92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liao FL-R, Ng MGY, Jones CAY, Huang CM-Z, Pang CMY. Whole-body vibration intensities in chronic stroke: a randomized controlled trial. Med. Sci. Sports Exerc. 2016;48:1227–1238. doi: 10.1249/MSS.0000000000000909. [DOI] [PubMed] [Google Scholar]
- 34.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn KE. Functional walk tests in individuals with stroke. Stroke. 2002;33:756–761. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 35.Pang MYC, Eng JJ, Dawson AS. Relationship between ambulatory capacity and cardiorespiratory fitness in chronic stroke: influence of stroke-specific impairments. Chest. 2005;127:495–501. doi: 10.1378/chest.127.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mozaffarian D, et al. Executive summary: heart disease and stroke statistics—2016 update. Circulation. 2016;133:447–454. doi: 10.1161/CIR.0000000000000366. [DOI] [PubMed] [Google Scholar]
- 37.Perry J, Garrett M, Gronley JK, Mulroy SJ. Classification of walking handicap in the stroke population. Stroke. 1995;26:982–989. doi: 10.1161/01.STR.26.6.982. [DOI] [PubMed] [Google Scholar]
- 38.Jørgensen L, Jacobsen BK, Wilsgaard T, Magnus JH. Walking after stroke: does it matter? Changes in bone mineral density within the first 12 months after stroke. A longitudinal study. Osteoporos. Int. 2000;11:381–387. doi: 10.1007/s001980070103. [DOI] [PubMed] [Google Scholar]
- 39.Lynn HS, Lau EMC, Au B, Leung PC. Bone mineral density reference norms for Hong Kong Chinese. Osteoporos. Int. 2005;16:1663–1668. doi: 10.1007/s00198-005-1899-z. [DOI] [PubMed] [Google Scholar]
- 40.Miller AEJ, MacDougall JD, Tarnopolsky MA, Sale DG. Gender differences in strength and muscle fiber characteristics. Eur. J. Appl. Physiol. Occup. Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- 41.Pang MY, Ashe MC, Eng JJ. Tibial bone geometry in chronic stroke patients: influence of sex, cardiovascular health, and muscle mass. J. Bone Miner. Res. 2008;23:1023–1030. doi: 10.1359/jbmr.080224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seibel MJ. Biochemical markers of bone turnover part II: clinical applications in the management of osteoporosis. Clin. Biochem. Rev. 2006;27:123–138. [PMC free article] [PubMed] [Google Scholar]
- 43.Furness TP, Maschette WE. Influence of whole body vibration platform frequency on neuromuscular performance of community-dwelling older adults. J. Strength Cond. Res. 2009;23:1508–1513. doi: 10.1519/JSC.0b013e3181a4e8f9. [DOI] [PubMed] [Google Scholar]
- 44.Rees S, Murphy A, Watsford M. Effects of vibration exercise on muscle performance and mobility in an older population. J. Aging Phys. Act. 2007;15:367. doi: 10.1123/japa.15.4.367. [DOI] [PubMed] [Google Scholar]
- 45.Rees SS, Murphy AJ, Watsford ML. Effects of whole-body vibration exercise on lower-extremity muscle strength and power in an older population: a randomized clinical trial. Phys. Ther. 2008;88:462–470. doi: 10.2522/ptj.20070027. [DOI] [PubMed] [Google Scholar]
- 46.Ritzmann R, Gollhofer A, Kramer A. The influence of vibration type, frequency, body position and additional load on the neuromuscular activity during whole body vibration. Eur. J. Appl. Physiol. 2013;113:1–11. doi: 10.1007/s00421-012-2402-0. [DOI] [PubMed] [Google Scholar]
- 47.Rittweger J. Vibration as an exercise modality: how it may work, and what its potential might be. Eur. J. Appl. Physiol. 2010;108:877–904. doi: 10.1007/s00421-009-1303-3. [DOI] [PubMed] [Google Scholar]
- 48.Kiiski J, Heinonen A, Järvinen TL, Kannus P, Sievänen H. Transmission of vertical whole body vibration to the human body. J. Bone Miner. Res. 2008;23:1318–1325. doi: 10.1359/jbmr.080315. [DOI] [PubMed] [Google Scholar]
- 49.Oullier O, et al. Countering postural posteffects following prolonged exposure to whole-body vibration: a sensorimotor treatment. Eur. J. Appl. Physiol. 2009;105:235–245. doi: 10.1007/s00421-008-0894-4. [DOI] [PubMed] [Google Scholar]
- 50.Tankisheva E, et al. Transmission of whole-body vibration and its effect on muscle activation. J. Strength Cond. Res. 2013;27:2533–2541. doi: 10.1519/JSC.0b013e31827f1225. [DOI] [PubMed] [Google Scholar]
- 51.Huang M, Tang C-Y, Pang MYC. Use of whole body vibration in individuals with chronic stroke: transmissibility and signal purity. J. Biomech. 2018;73:80–91. doi: 10.1016/j.jbiomech.2018.03.022. [DOI] [PubMed] [Google Scholar]
- 52.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 53.Washburn RA, Zhu W, McAuley E, Frogley M, Figoni SF. The physical activity scale for individuals with physical disabilities: development and evaluation. Arch. Phys. Med. Rehabil. 2002;83:193–200. doi: 10.1053/apmr.2002.27467. [DOI] [PubMed] [Google Scholar]
- 54.Li F, Wu Y, Li X. Test-retest reliability and inter-rater reliability of the Modified Tardieu Scale and the Modified Ashworth Scale in hemiplegic patients with stroke. Eur. J. Phys. Rehabil. Med. 2014;50:9–15. [PubMed] [Google Scholar]
- 55.Flansbjer U-B, Holmbäck AM, Downham D, Lexell J. What change in isokinetic knee muscle strength can be detected in men and women with hemiparesis after stroke? Clin. Rehabil. 2005;19:514–522. doi: 10.1191/0269215505cr854oa. [DOI] [PubMed] [Google Scholar]
- 56.Kristensen OH, Stenager E, Dalgas U. Muscle strength and poststroke hemiplegia: a systematic review of muscle strength assessment and muscle strength impairment. Arch. Phys. Med. Rehabil. 2017;98:368–380. doi: 10.1016/j.apmr.2016.05.023. [DOI] [PubMed] [Google Scholar]
- 57.Rabelo M, Nunes GS, da Costa Amante NM, de Noronha M, Fachin-Martins E. Reliability of muscle strength assessment in chronic post-stroke hemiparesis: a systematic review and meta-analysis. Top. Stroke Rehabil. 2016;23:26–35. doi: 10.1179/1945511915Y.0000000008. [DOI] [PubMed] [Google Scholar]
- 58.Mentiplay BF, Banky M, Clark RA, Kahn MB, Williams G. Lower limb angular velocity during walking at various speeds. Gait Posture. 2018;65:190–196. doi: 10.1016/j.gaitpost.2018.06.162. [DOI] [PubMed] [Google Scholar]
- 59.Kotake T, et al. An analysis of sit-to-stand movements. Arch. Phys. Med. Rehabil. 1993;74:1095–1099. doi: 10.1016/0003-9993(93)90068-L. [DOI] [PubMed] [Google Scholar]
- 60.Perrin D. Reliability of isokinetic measures. Athl. Train. 1986;21:319–321. [Google Scholar]
- 61.Rothstein JM, Delitto A, Sinacore DR, Rose SJ. Electromyographic, peak torque, and power relationships during isokinetic movement. Phys. Ther. 1983;63:926–933. doi: 10.1093/ptj/63.6.926. [DOI] [PubMed] [Google Scholar]
- 62.Suomi R, Surburg PR, Lecius P. Reliability of isokinetic and isometric measurement of leg strength on men with mental retardation. Arch. Phys. Med. Rehabil. 1993;74:848–852. doi: 10.1016/0003-9993(93)90012-Y. [DOI] [PubMed] [Google Scholar]
- 63.Hsu A-L, Tang P-F, Jan M-H. Test-retest reliability of isokinetic muscle strength of the lower extremities in patients with stroke. Arch. Phys. Med. Rehabil. 2002;83:1130–1137. doi: 10.1053/apmr.2002.33652. [DOI] [PubMed] [Google Scholar]
- 64.Clark DJ, Condliffe EG, Patten C. Reliability of concentric and eccentric torque during isokinetic knee extension in post-stroke hemiparesis. Clin. Biomech. 2006;21:395–404. doi: 10.1016/j.clinbiomech.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 65.Plested JS, Coull PA, Gidney MAJ. XXXX. In: Herbert MA, Hood DW, Richard Moxon E, editors. Haemophilus Influenzae Protocols. Totowa: Humana Press; 2003. pp. 243–261. [Google Scholar]
- 66.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J. Rehabil. Med. 2005;37:75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 67.Tihanyi J, et al. Low resonance frequency vibration affects strength of paretic and non-paretic leg differently in patients with stroke. Acta Physiol. Hung. 2010;97:172–182. doi: 10.1556/aphysiol.97.2010.2.3. [DOI] [PubMed] [Google Scholar]
- 68.Portney LG. Foundations of Clinical Research: Applications to Practice. 3. Philadelphia: F.A. Davis Company; 2015. [Google Scholar]
- 69.Hazell TJ, Kenno KA, Jakobi JM. Evaluation of muscle activity for loaded and unloaded dynamic squats during vertical whole-body vibration. J. Strength Cond. Res. 2010;24:1860–1865. doi: 10.1519/JSC.0b013e3181ddf6c8. [DOI] [PubMed] [Google Scholar]
- 70.Pollock RD, Woledge RC, Mills KR, Martin FC, Newham DJ. Muscle activity and acceleration during whole body vibration: effect of frequency and amplitude. Clin. Biomech. 2010;25:840–846. doi: 10.1016/j.clinbiomech.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 71.Madou KH. Leg muscle activity level and rate of perceived exertion with different whole-body vibration frequencies in multiple sclerosis patients: an exploratory approach. Hong Kong Physiother. J. 2011;29:12–19. doi: 10.1016/j.hkpj.2011.02.002. [DOI] [Google Scholar]
- 72.Huang M, Miller T, Ying M, Pang MYC. Whole-body vibration modulates leg muscle reflex and blood perfusion among people with chronic stroke: a randomized controlled crossover trial. Sci. Rep. 2020;10:1473. doi: 10.1038/s41598-020-58479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pollock RD, Woledge RC, Martin FC, Newham DJ. Effects of whole body vibration on motor unit recruitment and threshold. J. Appl. Physiol. 2012;112:388–395. doi: 10.1152/japplphysiol.01223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Machado A, García-López D, González-Gallego J, Garatachea N. Whole-body vibration training increases muscle strength and mass in older women: a randomized-controlled trial. Scand. J. Med. Sci. Sports. 2010;20:200–207. doi: 10.1111/j.1600-0838.2009.00919.x. [DOI] [PubMed] [Google Scholar]
- 75.Da Silva ME, et al. Effects of different frequencies of whole body vibration on muscular performance. Biol. Sport. 2006;23:267. [Google Scholar]
- 76.Severinsen K, Jakobsen JK, Overgaard K, Andersen H. Normalized muscle strength, aerobic capacity, and walking performance in chronic stroke: a population-based study on the potential for endurance and resistance training. Arch. Phys. Med. Rehabil. 2011;92:1663–1668. doi: 10.1016/j.apmr.2011.04.022. [DOI] [PubMed] [Google Scholar]
- 77.Patterson SL, et al. Determinants of walking function after stroke: differences by deficit severity. Arch. Phys. Med. Rehabil. 2007;88:115–119. doi: 10.1016/j.apmr.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 78.Liao L-R, Ng GYF, Jones AYM, Pang MYC. Cardiovascular stress induced by whole-body vibration exercise in individuals with chronic stroke. Phys. Ther. 2015;95:966–977. doi: 10.2522/ptj.20140295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.