Abstract
Objective
We aimed to provide a meta-analysis of previously published papers on the COVID-19-related clinical features and laboratory findings in children.
Method
This meta-analysis was conducted by using Medline/PubMed, Scopus, Web of Sciences and Google Scholar. Finally, 32 articles were selected for full-text assessment.
Results
The most frequent symptoms were fever, cough, vomiting, diarrhea, sore throat, and dyspnea. Regarding the combined results of the meta-analysis, fever (46%, 95% CI 40–53%), cough (37%, 95% CI 29–46%), diarrhea (19%, 95% CI 9–28%), and pharyngalgia (13%, 95% CI 5–20%) were the most widely reported symptom. Besides, positive RT-PCR test results (43%, 95% CI 33–53%), low oxygen saturation (38%, 95% CI 25–51%), and elevated D-dimer levels (36%, 95% CI 16–56%) were the most common laboratory findings.
Conclusion
This review found that clinical presentations were milder, the prognosis was better, and the mortality rate was lower in children with COVID-19 compared with adult patients; however, children are potential carriers, like adults, and can transmit the infection among the population. Therefore, early identification and intervention in pediatric patients with COVID-19 are essential in order to control the pandemic. Moreover, gastrointestinal symptoms were more common symptoms among children.
Keywords: Coronavirus disease 2019, Children, Clinical symptom, Laboratory features, Meta-analysis
1. Introduction
On December 31, 2019, an “unknown viral pneumonia” was first introduced in Wuhan, China. It was originally referred to as the “2019 coronavirus novel” [1], [2]. Children have always played a significant role in disease outbreaks and epidemics as they are potential carriers and spreaders [3].
On January 20, 2020, the first pediatric case of the novel coronavirus infection was confirmed in Shenzhen, China. [4].
About 20 days later, 398 confirmed pediatric cases and 10,924 adult cases of COVID-19 were reported in China; however, the number of infected children was not accurate because primary screening was not completely performed for them. In a study of 44,672 laboratory-confirmed cases in China, only 416 (0.9%) patients were younger than 10 years and 549 (1.2%) of them were between 10 and 20 years old [3], [5].
The first case of an infant with COVID-19 reported was a 3-month-old female infant, from Xiaogan, Hubei province, with fever, who was diagnosed on January 26 [6]. With the growing number of adult patients, the number of infected children increased as well. It was found that the virus was highly contagious as “second-generation” infections were reported [6].
According to a study of nine hospitalized infected infants, at least one of their family members had been infected [7]. Another study by Zhang et al. of an infected infant raised the question of whether the infection had a shorter incubation time in the infant or whether her parents acquired the infection from the baby [6]. However, all these children belonged to family cluster circles, and thus the onset of aggregation is an important feature in the children's cases, and this was a strong indication that the virus is highly contagious.
The study by Cai et al. conducted with the first pediatric case outside Hubei province was the first study that suggested children as a source of infection in adults [8].
Children with COVID-19 can be asymptomatic or present with fever, dry cough, fatigue, and sometimes gastrointestinal symptoms, including abdominal discomfort, nausea, vomiting, abdominal pain, and diarrhea. They also showed mild symptoms with a good prognosis most of the time and recovered after 1–2 weeks [6], [9]; however, there were severe pediatric cases that were also detected, such as the 1-year-old infant with severe COVID-19 ported by Chen et al. in Wuhan Children's Hospital [10].
A normal white blood cell count and absolute lymphocyte count in most pediatric cases suggests less immune dysfunction after the SARS-CoV-2 infection [6], [9]. It is suggested that mild infection in children is the result of trained immunity. This immunity is related to the use of particular vaccines such as the bacille Calmette–Guerin (BCG) vaccine, which train innate immunity to generate immune memory [5]. It has been proven that BCG provided non-specific protection of mice against the influenza virus [11]. This vaccine has been given to most Asian children, and it is known that the influenza infection causes more severe respiratory symptoms in adults compared with children in these countries [11].
The clinical manifestations of COVID-19 have been unclear because the clinical and laboratory features of infected children were limited. This study is a meta-analysis of SARS-CoV-2 infection in children focusing on the clinical features and laboratory findings.
1.1. Objective
This study aims to analyze the clinical characteristics of COVID-19 in children by summarizing the clinical and laboratory data reported in recent observational studies.
2. Materials and methods
2.1. Identification and study selection
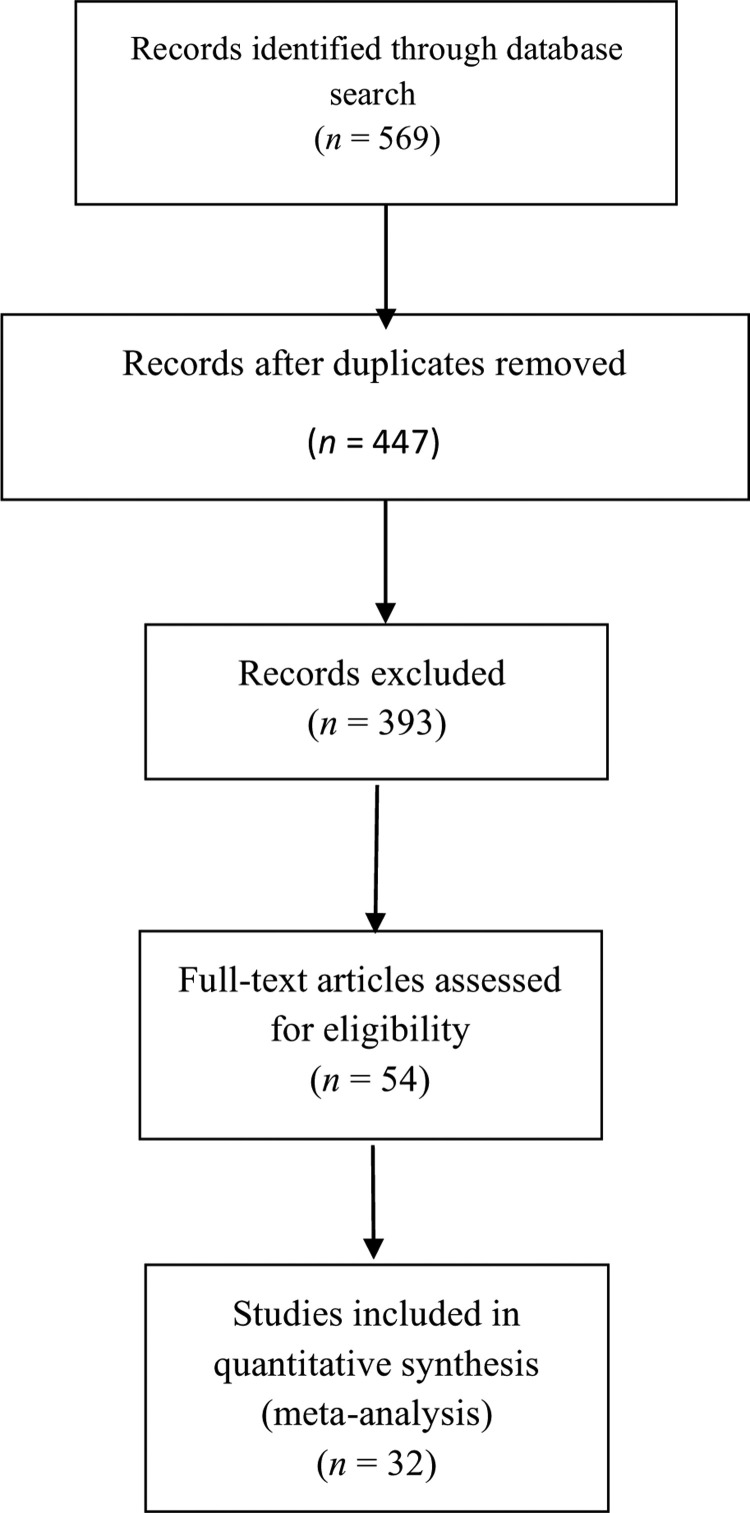
This systematic review was conducted, using Medline/PubMed, Scopus, Web of Sciences, and Google Scholar, to identify studies published on COVID-19 following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The keywords used to search the studies were: “Novel coronavirus”, “Novel coronavirus 2019”, “2019 nCoV”, “COVID-19”, “Wuhan coronavirus”, “Wuhan pneumonia”, and “SARS-CoV-2”, up to April 14, 2020. The results were reviewed by two authors (conventional double-screening), the abstracts were screened, and related studies were selected. Finally, the full texts of the selected studies were reviewed and 32 publications were selected for the meta-analysis (Fig. 1 ). Studies with incomplete information, review articles, opinions, and letters were excluded.
Fig. 1.
Flowchart representing the selection process.
3. Meta-analysis approach
The meta-analysis was performed using Stata software version 14 (StataCorp. 2015, Stata Statistical Software: Release 14, College Station, TX). Due to the nature of the studies, substantial heterogeneity was expected. Heterogeneity was assessed with the Q test and quantified numerically using the I2 index [13]. For I2 < 50%, i.e., non-heterogeneity, a fixed-effects model (DerSimonian–Laird method) was applied; otherwise, sensitivity analysis was used to find out the causes of heterogeneity, and if there was no clinical heterogeneity, a random-effects model was used with the estimate of heterogeneity being taken from an inverse-variance model (DerSimonian and Laird, 1986) [14]. Publication bias was evaluated by Egger's and Begg's tests at the 5% significant level [15]. Forest plots were used to visualize the prevalence in each study and the combined estimated prevalence with 95% confidence intervals (95% CI), with the size of each box indicating the weight of the study and each crossed line referring to the 95% CI.
4. Results
4.1. Literature review
A total of 569 articles were initially retrieved and 32 articles were finally selected for full-text assessment. The main characteristics of the studies included are shown in Table 1 [7], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46]. Out of the 32 publications, three were performed in Iran, the United States, and Spain and the other 29 studies were conducted by Chinese researchers. The total sample size consisted of 759 children, of whom 399 were male.
Table 1.
Characteristics of the included studies on COVID-19, 2020 [7], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46].
| Authors | Country | Publication year | Sample size | Sex (male) | Age |
|---|---|---|---|---|---|
| Feng et al. [16] | China | 2020 | 15 | 5 | 4–14 years |
| Wang et al. [17] | China | 2020 | 31 | 15 | 1 month to 7 years |
| Zhou et al. [18] | China | 2020 | 9 | - | 0–3 years |
| Tan et al. [19] | China | 2020 | 10 | 3 | 1–12 years |
| Qiu et al. [20] | China | 2020 | 36 | 23 | 0–16 years |
| Tagarro et al. [21] | Spain | 2020 | 41 | 18 | < 18 years |
| Tongqiang et al. [22] | China | 2020 | 3 | 3 | 6–9 years |
| Anjue et al. [23] | China | 2020 | 26 | 17 | 1–13 years |
| Qin et al. [24] | China | 2020 | 68 | 40 | 0–10 years |
| Han et al. [25] | China | 2020 | 7 | 4 | < 18 years |
| Liu et al. [26] | China | 2020 | 6 | 2 | 1–7 years |
| Cai et al. [27] | China | 2020 | 10 | 4 | 3 months to 131 months |
| Wei et al. [7] | China | 2020 | 9 | 2 | 3 months to 11 months |
| Xia et al. [28] | China | 2020 | 20 | 13 | < 18 years |
| Zhang et al. [29] | China | 2020 | 4 | 3 | 30 hours to 17 days |
| Liu et al. [30] | China | 2020 | 5 | 4 | 7 months to 13 years |
| Zheng et al. [31] | China | 2020 | 3 | 3 | Newborns |
| Rahimzadeh et al. [32] | Iran | 2020 | 9 | 6 | 2–10 years |
| Li et al. [33] | China | 2020 | 5 | 4 | 1–10 years |
| Bialek et al. [34] | United States | 2020 | 291 | 165 | < 18 years |
| Huanhuan et al. [35] | China | 2020 | 4 | 2 | 2 months to 9 years |
| Li et al. [36] | China | 2020 | 22 | - | Pediatric patients |
| Chen et al. [37] | China | 2020 | 31 | 13 | 0–17 years |
| Sun et al. [38] | China | 2020 | 8 | 6 | 2 months to 15 years |
| Su et al. [39] | China | 2020 | 9 | 3 | 11 months to 9 years |
| Zhu et al. [40] | China | 2020 | 10 | 8 | Neonates |
| Zheng et al. [41] | China | 2020 | 25 | 14 | 1 month to 16 years |
| Shen et al. [42] | China | 2020 | 9 | 3 | 1–12 years |
| Zhu et al. [43] | China | 2020 | 10 | 5 | 1–17 years |
| Du et al. [44] | China | 2020 | 14 | 6 | Children |
| Xing et al. [45] | China | 2020 | 3 | 2 | 1.5–6 years |
| Hu et al. [46] | China | 2020 | 6 | 3 | < 15 years |
4.2. Clinical features
Table 2 presents the clinical symptoms of COVID-19 in children. However, several clinical symptoms were reported in different studies; the most frequent symptoms were fever, cough, vomiting, diarrhea, sore throat, and dyspnea.
Table 2.
Summary of the study characteristics and results of meta-analysis of clinical and laboratory variables.
| Clinical and laboratory variables | Studies in which each variable was evaluated (n) | Number of patients | Percentage of patients | P-value for test (ES = 0) | I2 (%) | P-value for heterogeneity test | P-value for Egger test | Trim and fill results (95% CI) |
|---|---|---|---|---|---|---|---|---|
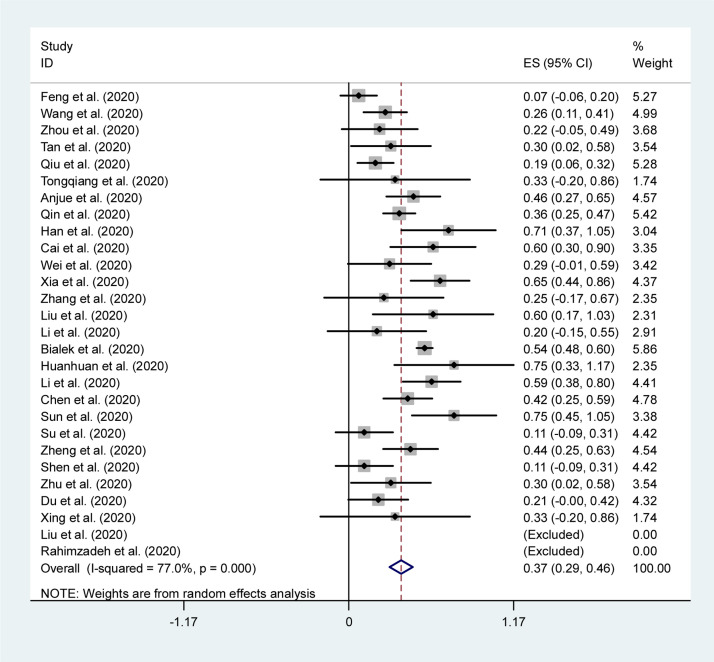
| Dry cough | 28 | 317 | 0.37 (0.29, 0.46) | < 0.001 | 77 | < 0.001 | 0.353 | – |
| Sputum production | 5 | 10 | 0.06 (0.01, 0.11) | 0.011 | 23.2 | 0.267 | 0.132 | 0.04 (−0.03, 0.11) |
| Diarrhea | 11 | 63 | 0.19 (0.09, 0.28) | < 0.001 | 85.1 | < 0.001 | 0.378 | 0.12 (0.01, 0.23) |
| Rhinorrhea | 8 | 45 | 0.11 (0.08, 0.15) | < 0.001 | 9 | 0.360 | 0.116 | – |
| Pharyngalgia | 10 | 86 | 0.13 (0.05, 0.20) | 0.001 | 72.6 | < 0.001 | 0.767 | 0.11 (0.04, 0.19) |
| Vomiting | 14 | 60 | 0.11 (0.06, 0.16) | < 0.001 | 56.2 | 0.005 | 0.023 | – |
| Dyspnea | 10 | 66 | 0.11 (0.04, 0.18) | 0.001 | 74.7 | < 0.001 | 0.147 | – |
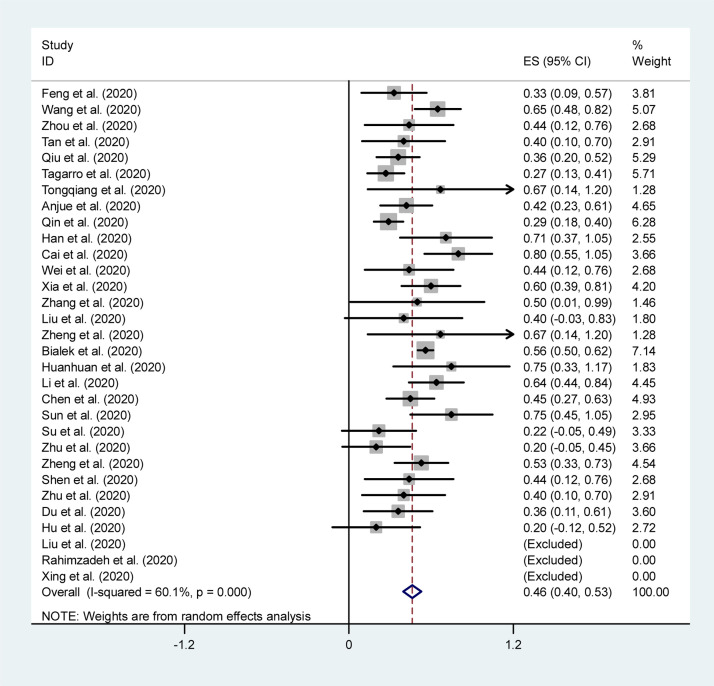
| Fever | 31 | 375 | 0.46 (0.40, 0.53) | < 0.001 | 60.1 | < 0.001 | 0.655 | – |
| Nasal congestion | 3 | 7 | 0.12 (−0.01, 0.26) | 0.071 | 56 | 0.103 | 0.082 | – |
| Abdominal pain | 4 | 21 | 0.06 (0.04, 0.09) | < 0.001 | 0 | 0.738 | 0.065 | – |
| Fatigue | 9 | 89 | 0.11 (0.04, 0.18) | 0.002 | 73 | 0.001 | 0.393 | – |
| Headache | 9 | 95 | 0.11 (0.03, 0.20) | 0.011 | 87.7 | < 0.001 | 0.755 | – |
| Runny nose | 7 | 11 | 0.07 (0.05, 0.10) | < 0.001 | 7.7 | 0.370 | 0.053 | – |
| Laboratory findings | ||||||||
| RT-PCR test+ | 10 | 117 | 0.43 (0.33, 0.53) | < 0.001 | 0 | 0.588 | 0.297 | – |
| Leukopenia | 11 | 66 | 0.22 (0.12, 0.32) | < 0.001 | 70 | < 0.001 | 0.294 | – |
| Leukocytosis | 15 | 49 | 0.23 (0.13, 0.33) | < 0.001 | 62.6 | 0.001 | 0.024 | – |
| Lymphopenia | 14 | 57 | 0.21 (0.13, 0.28) | < 0.001 | 51.5 | 0.016 | 0.012 | – |
| Neutropenia | 8 | 19 | 0.10 (0.03, 0.17) | 0.005 | 21.7 | 0.270 | 0.017 | – |
| Platelet decrease | 4 | 12 | 0.15 (0.02, 0.29) | 0.029 | 56.5 | 0.075 | 0.315 | – |
| Procalcitonin | 9 | 41 | 0.24 (0.09, 0.39) | < 0.001 | 91.8 | < 0.001 | 0.040 | – |
| D-dimer | 8 | 25 | 0.35 (0.15, 0.55) | 0.001 | 80.8 | < 0.001 | 0.883 | – |
| Creatine kinase | 8 | 16 | 0.12 (0.07, 0.17) | < 0.001 | 43.7 | 0.087 | 0.204 | 0.07 (0.03, 0.12) |
| Creatine kinase MB | 8 | 40 | 0.30 (0.17, 0.43) | < 0.001 | 70.8 | 0.001 | 0.002 | 0.16 (0.02, 0.29) |
| Oxygen saturation | 3 | 20 | 0.38 (0.25, 0.51) | < 0.001 | 0 | 0.468 | 0.768 | |
| CRP | 15 | 51 | 0.15 (0.09, 0.22) | < 0.001 | 60.9 | 0.002 | 0.001 | – |
| LDH | 8 | 45 | 0.30 (0.16, 0.43) | < 0.001 | 70.8 | 0.001 | 0.081 | 0.14 (−0.01, 0.29) |
| ESR | 8 | 90 | 0.29 (0.23, 0.35) | < 0.001 | 20.7 | 0.271 | 0.847 | – |
| AST | 8 | 23 | 0.20 (0.13, 0.26) | < 0.001 | 21.7 | 0.257 | 0.365 | 0.15 (0.06, 0.25) |
| ALT | 11 | 38 | 0.21 (0.13, 0.29) | < 0.001 | 50.6 | 0.027 | 0.017 | 0.10 (0.02, 0.19) |
LDH: lactate dehydrogenase; CRP: C-reactive protein; ESR: erythrocyte sedimentation rate; AST: aspartate aminotransferase; ALT: alanine aminotransferase: RT-PCR: reverse transcription polymerase chain reaction; CI: confidence interval.
The combined results of the meta-analysis based on clinical data are presented in Table 2. The forest plots of the most common clinical symptoms are depicted in Fig. 2, Fig. 3 . Regarding the clinical manifestations, fever (46%, 95% CI 40–53%), cough (37%, 95% CI 29–46%), diarrhea (19%, 95% CI 9–28%), and pharyngalgia (13%, 95% CI 5–20%) were the most commonly reported symptom in children.
Fig. 2.
Forest plot of the overall proportion of dry cough in children with COVID-19.
Fig. 3.
Forest plot of the overall proportion of fever in children with COVID-19.
4.3. Laboratory findings
The laboratory findings and the combined estimated prevalence with 95% confidence intervals (95% CI) are shown in Table 2. Our results showed that positive RT-PCR test results (43%, 95% CI 33–53%), low oxygen saturation (38%, 95% CI 25–51%), and elevated D-dimer levels (36%, 95% CI 16–56%) were the most common laboratory findings in children.
4.4. Publication bias
Since the use of funnel plots for assessment of potential publication bias is inaccurate in the meta-analysis of proportion studies [47], we utilized significance tests to identify publication bias. For this purpose, Egger's and Begg's tests were used to assess publication bias. Since Begg's test is less powerful than Egger's test, we report the results of Egger's test. The adjusted trim and fill statistics are also demonstrated for studies with a high risk of publication bias in Table 2.
4.5. Grading the quality of evidence
To assess and evaluate the quality of evidence, we used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) guidelines [48]. The quality of evidence was classified into four levels: “very low”, “low”, “moderate”, or “high” judgment. If there was a dispute, it would be resolved by consensus or consultation. All studies were judged to be of high quality.
5. Discussion
The contagious disease COVID-19 can be transmitted from an asymptomatic infected person or an asymptomatic carrier by contact and respiratory droplets. The incubation period can be up to 24 days. Most of the patients experience mild symptoms, but the elderly or those with underlying conditions are more likely to develop severe symptoms [28], [49]. The clinical manifestations in children as the same as in adults; however, children present with more gastrointestinal symptoms.
When a new infectious disease becomes an epidemic, it can spread to new regions and cause a pandemic. This situation demands epidemiological, diagnostic, therapeutic, and preventive infrastructures and may have devastating effects on the global economy. Many questions about the clinical manifestations, laboratory and imaging findings, morbidity, the mortality rate, and the severity of disease have been raised.
In this systematic review and random-effects meta-analysis, we aimed to summarize the recently published clinical data of COVID-19 confirmed cases. We analyzed data on pediatric patients for major clinical manifestations and their associated significant laboratory findings.
Our findings are robust in that we used a random-effects meta-analysis model. This involves an assumption that the effects being estimated in the different studies are not identical, but follow some distribution. For random-effects analyses, the pooled estimate and 95% CIs refer to the center of the distribution of pooled prevalence but do not describe the width of the distribution. Often the pooled estimate and its 95% CI are quoted in isolation as an alternative estimate of the quantity evaluated in a fixed-effect meta-analysis, which is inappropriate.
Based on initial observation studies in China, patients – particularly adult patients – prevalently presented with fever and cough, as well as dyspnea, and myalgia. Based on the analysis of all the included publications in this study, the same results were also found elsewhere.
This study investigated clinical manifestations, laboratory results, and findings from 32 studies on COVID-19 in children. The total sample size was 759. Out of 759 children, 399 were male. This finding suggested that males are more prone to this disease. Fever, cough, vomiting, diarrhea, sore throat, and dyspnea were the most frequent symptoms in children; however, fever and cough were the main symptoms in both children and adults. While gastrointestinal symptoms were rare in adult patients, approximately 20% of infected children had diarrhea. Most of the articles did not discuss the diagnostic modality in most of the studies (systematic screening around cases or symptomatic patients), and this could explain the sex ratio favoring males.
Analysis of the laboratory findings in children showed that positive RT-PCR test results, low oxygen saturation, and elevated D-dimer levels were the most abnormal findings in children with COVID-19. It should be considered that not all clinical manifestations and laboratory findings were investigated in the studies reviewed.
Han et al. reported that diarrhea and/or vomiting (gastrointestinal involvement) were the more common symptoms in infected children, and elevated creatine kinase isoenzyme levels (57.1%) and leukocyte counts (28.6%) were common laboratory findings too [49]. By contrast, this analysis indicated that fever and cough were the most frequent clinical manifestations of COVID-19 in children, and that leukopenia (22%) and leukocytosis (23%) were the most prevalent laboratory findings.
In the study by Han et al., abnormal coagulation function, hypoalbuminemia, and hyperuricemias were reported in infected pediatric patients [49]. Moreover, elevated creatine kinase isoenzyme levels were detected by myocardial zymography in children. This may be explained by intense chills associated with high fever or by the higher incidence of myocardial damage in children. They also found elevated C-reactive protein (CRP) and D-dimer levels in 28.6% and 28.6% of the pediatric patients, respectively. Based on this analysis, positive CRP and positive D-dimer results were detected in 15% and 35% of the total sample, respectively.
In a study by Su et al., elevated CK-MB levels were detected in six children with COVID-19, which suggests that SARS-CoV-2 may cause heart injury [39] since CK-MB is an indicator of myocardial injury. It is reported that the main mechanisms of SARS-CoV-2-induced myocardial injury may be the direct injury of the virus, of a cytokine storm, and the distribution of the ACE2 receptor. In the present study, the CK-MB level was found to be 30%.
Oualha et al. conducted a retrospective study of 27 pediatric cases of COVID-19 to describe severe forms of pediatric SARS-CoV-2 infection. According to their results, the disease had a wide range of clinical presentation and progression in children. They also found a higher rate of comorbidities in life-threatening cases of COVID-19, since 70% of their cases had sickle cell disease as well as neurological and respiratory comorbidities [50].
Ludvigsson reviewed 45 scientific papers on COVID-19 in children and reported that the infection has milder symptoms, better prognosis, and lower mortality rate in children compared with adult patients [51].
In a systemic review by Chang et al. on clinical characteristics and diagnostic challenges of pediatric COVID-19, it was reported that the higher rates of asymptomatic and milder cases of COVID-19 in children make it difficult to diagnose and control the infection among the pediatric population. Most of the diagnoses were based on epidemiological data and a history of contact with infected patients [52].
Zhang et al. conducted a case series to detect clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China. Based on the results, pediatric patients admitted to hospital presented with higher rates of fever, vomiting, and diarrhea compared with adult patients. An increase in serum amyloid A, high-sensitivity CRP, lactate dehydrogenase, and α-hydroxybutyrate dehydrogenase as well as a decrease in pre-albumin were detected in the majority of the cases [53].
Ultimately, being aware of the differences between the clinical presentations in children and adults is helpful for the clinical diagnosis and treatment approach of COVID-19 in children. It also helps to discuss the age-specific pattern of coronavirus infection more accurately.
Our results show that more comprehensive clinical studies, including short- and long-term follow-up cohort assessments, are still required. It would also be beneficial to have more studies from other countries, as most available studies are currently from one country, i.e., China.
To clarify the clinical spectrum of the disease, further clinical data are required. Currently, more regions, including the Americas and Europe, are struggling with the increasing number of infected cases and more studies from these areas would be extremely helpful. To date, regardless of the types of reports, the clinical findings were very similar.
In the current pandemic, available data and evidence should constantly be evaluated to help manage the situation and decrease the transmission of the virus, to have a better diagnosis and clinical suspicion, and to protect the population and healthcare staff.
This systematic review focused on the clinical manifestation and laboratory findings of COVID-19, which should assist clinicians around the world, particularly those who are practicing in regions with new onset of infection. Physicians would be able to monitor patients, implement control measures, and prevent further transmission if they could recognize the infection in earlier stages.
5.1. Limitations
This review has several limitations. Few studies were available and most of them were from China. It would be better to include studies with a broad geographic scope for a more comprehensive understanding of COVID-19. More detailed patient information, particularly regarding clinical outcomes, was unavailable in most studies at the time of the analyses; however, the data in this review offer an initial summary of the clinical and laboratory characteristics of COVID-19.
6. Conclusion
This review study showed that clinical presentations were milder, the prognosis was better, and the mortality rate was lower in children with COVID-19 compared with adult patients; however, children are potential carriers, like adults, and can transmit the infection among the population. Therefore, early identification and intervention in pediatric patients with COVID-19 are essential to control the pandemic. Moreover, gastrointestinal symptoms were more common symptoms among children.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure of interest
The authors declare that they have no competing interest.
References
- 1.World Health Organization. Coronavirus disease 2019, Outbreak, World Health Organization Coronavirus Dis. (n.d.). https://www.who.int/emergencies/diseases/novel-coronavirus-2019.(accessed April 18, 2020).
- 2.World Health Organization. Coronavirus disease 2019, World Health Organisation Nov. Coronavirus — China. (n.d.). https://www.who.int/csr/don/12-january-2020-novel-coronavirus- china/en (accessed February 15, 2020).
- 3.Cao Q., Chen Y.C., Chen C.L. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan J.F.W., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) – China. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41:145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Y.H., Lin D.J., Xiao M.F. 2019-novel coronavirus infection in a three-month-old baby. Zhonghua Er Ke Za Zhi. 2020;58:E006. doi: 10.3760/cma.j.issn.0578-1310.2020.0006. [DOI] [PubMed] [Google Scholar]
- 7.Wei M., Yuan J., Liu Y. Novel coronavirus infection in hospitalized infants under 1 year of age in China. JAMA. 2020;323:1313–1314. doi: 10.1001/jama.2020.2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai J.H., Wang X.S., Ge Y.L. First case of 2019 novel coronavirus infection in children in Shanghai. Zhonghua Er Ke Za Zhi. 2020;58:E002. doi: 10.3760/cma.j.issn.0578-1310.2020.0002. [DOI] [PubMed] [Google Scholar]
- 9.Zeng L.K., Tao X.W., Yuan W.H. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:E009. doi: 10.3760/cma.j.issn.0578-1310.2020.0009. [DOI] [PubMed] [Google Scholar]
- 10.Chen F., Liu Z.S., Zhang F.R. Frist case of severe childhood novel coronavirus pneumonia in China. Zhonghua Er Ke Za Zhi. 2020;58:E005. doi: 10.3760/cma.j.issn.0578-1310.2020.0005. [DOI] [PubMed] [Google Scholar]
- 11.Netea M.G., Schlitze A., Placek K. Innate and adaptive immune memory: an evolutionary continuum in the host's response to pathogens. Cell Host Microbe. 2019;25:13–26. doi: 10.1016/j.chom.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 12.Moher D., Liberati A., Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Rücker G., Schwarzer G., Carpenter J.R. Undue reliance on I 2 in assessing heterogeneity may mislead. BMC Med Res Methodol. 2008;8:79. doi: 10.1186/1471-2288-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Y., Ding Z., Han C. Efficacy of mesenchymal stromal cells for fistula treatment of Crohn's disease: a systematic review and meta-analysis. Dig Dis Sci. 2017;62:851–860. doi: 10.1007/s10620-017-4453-x. [DOI] [PubMed] [Google Scholar]
- 15.Borenstein M., Hedges L.V., Higgins J.P.T. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 16.Feng K., Yun Y.X., Wang X.F. Analysis of CT features of 15 children with 2019 novel coronavirus infection. Zhonghua Er Ke Za Zhi. 2020;58:E007. doi: 10.3760/cma.j.issn.0578-1310.2020.0007. [DOI] [PubMed] [Google Scholar]
- 17.Wang D., Ju X.L., Xie F. Clinical analysis of 31 cases of 2019 novel coronavirus infection in children from six provinces (autonomous region) of northern China. Zhonghua Er Ke Za Zhi. 2020;58:E011. doi: 10.3760/cma.j.cn112140-20200225-00138. [DOI] [PubMed] [Google Scholar]
- 18.Zhou Y., Yang G.D., Feng K. Clinical features and chest CT findings of coronavirus disease 2019 in infants and young children. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22:215–220. doi: 10.7499/j.issn.1008-8830.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tan Y., Tan B., Pan J. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2020;127:104353. doi: 10.1016/j.jcv.2020.104353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qiu H., Wu J., Hong L. Clinical and epidemiological features of 36 children with coronavirus disease 2019 (COVID-19) in Zhejiang, China: an observational cohort study. Lancet Infect Dis. 2020;20:689–696. doi: 10.1016/S1473-3099(20)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tagarro A., Epalz C., Santos M. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020:e201346. doi: 10.1001/jamapediatrics.2020.1346. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang T., Cui X., Zhao X. Detectable SARS-CoV-2 viral RNA in feces of three children during recovery period of COVID-19 pneumonia. J Med Virol. 2020;92:909–914. doi: 10.1002/jmv.25795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang A., Xu W., Chen P. A retrospective study of the clinical characteristics of COVID-19 infection in 26 children. MedRxiv. 2020 doi: 10.1101/2020.03.08.20029710. [03.08.20029710] [DOI] [Google Scholar]
- 24.Wu Q., Xing Y., Shi L. Epidemiological and clinical characteristics of children with coronavirus disease 2019. MedRxiv. 2020 doi: 10.1101/2020.03.19.20027078. [DOI] [Google Scholar]
- 25.Han Y.N., Feng Z.W., Sun L.N. A comparative-descriptive analysis of clinical characteristics in 2019-Coronavirus-infected children and adults. J Med Virol. 2020;92:1596–1602. doi: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 26.Liu W., Zhang Q., Chen J. Detection of COVID-19 in children in early January 2020 in Wuhan, China. N Engl J Med. 2020;382:1370–1371. doi: 10.1056/NEJMc2003717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai J., Xu J., Lin D. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia W., Shao J., Guo Y. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Z.J., Yu X.J., Fu T. Novel coronavirus infection in newborn babies aged < 28 days in China. Eur Respir J. 2020;55:2000697. doi: 10.1183/13993003.00697-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu M., Song Z., Xiao K. High-resolution computed tomography manifestations of 5 pediatric patients with 2019 novel coronavirus. J Comput Assist Tomogr. 2020;44:311–313. doi: 10.1097/RCT.0000000000001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zeng L., Xia S., Yuan W. Neonatal early-onset infection with SARS-CoV-2 in 33 neonates born to mothers with COVID-19 in Wuhan, China. JAMA Pediatr. 2020;174:722–725. doi: 10.1001/jamapediatrics.2020.0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahimzadeh G., Ekrami Noghabi M., Kadkhodaei Elyaderani F. COVID-19 infection in Iranian children: a case series of 9 patients. J Pediatr Rev. 2020;8:139–144. [Google Scholar]
- 33.Li W., Cui H., Li K. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50:796–799. doi: 10.1007/s00247-020-04656-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bialek S., Gierke R., Hughes M. Coronavirus disease 2019 in children – United States, February 12–April 2. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu H., Liu F., Li J. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–e13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B., Shen J., Li L. Radiographic and clinical features of children with coronavirus disease (COVID-19) pneumonia. Indian Pediatr. 2020;57:423–426. doi: 10.1007/s13312-020-1816-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen C., Cao M., Peng L. 2020. Coronavirus disease-19 among children outside Wuhan, China, China (2/25/2020) [Google Scholar]
- 38.Sun D., Li H., Lu X.X. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. 2020;16:251–259. doi: 10.1007/s12519-020-00354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Su L., Ma X., Yu H. The different clinical characteristics of corona virus disease cases between children and their families in China – the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhu H., Wang L., Fang C. Clinical analysis of 10 neonates born to mothers with 2019-nCoV pneumonia. Transl Pediatr. 2020;9:51–60. doi: 10.21037/tp.2020.02.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng F., Liao C., Fan Q.H. Clinical characteristics of children with coronavirus disease 2019 in Hubei, China. Curr Med Sci. 2020;40:275–280. doi: 10.1007/s11596-020-2172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen Q., Guo W., Guo T. Novel coronavirus infection in children outside of Wuhan, China. Pediatr Pulmonol. 2020;55:1424–1429. doi: 10.1002/ppul.24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu L., Wang J., Huang R. Clinical characteristics of a case series of children with coronavirus disease 2019. Pediatr Pulmonol. 2020;55:1430–1432. doi: 10.1002/ppul.24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Du W., Yu J., Wang H. Clinical characteristics of COVID-19 in children compared with adults in Shandong Province, China. Infection. 2020;48:445–452. doi: 10.1007/s15010-020-01427-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xing Y.H., Ni W., Wu Q. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu Z., Song C., Xu C. Clinical characteristics of 24 asymptomatic infections with COVID-19 screened among close contacts in Nanjing, China. Sci China Life Sci. 2020;63:706–711. doi: 10.1007/s11427-020-1661-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hunter J.P., Saratzis A., Sutton A.J. In meta-analyses of proportion studies, funnel plots were found to be an inaccurate method of assessing publication bias. J Clin Epidemiol. 2014;67:897–903. doi: 10.1016/j.jclinepi.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 48.Guyatt G.H., Oxman A.D., Vist G.E. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–926. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han Y., Feng Z., Sun L. A comparative-descriptive analysis of clinical characteristics in 2019-Coronavirus-infected children and adults. J Med Virol. 2020 doi: 10.1002/jmv.25835. [DOI] [PubMed] [Google Scholar]
- 50.Oualha M., Bendavid M., Berteloot L. Severe and fatal forms of COVID-19 in children. Arch Pediatr. 2020;27:235–238. doi: 10.1016/j.arcped.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludvigsson J.F. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109:1088–1095. doi: 10.1111/apa.15270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang T.H., Wu J.L., Chang L.Y. Clinical characteristics and diagnostic challenges of pediatric COVID-19: a systematic review and meta-analysis. J Formos Med Assoc. 2020;119:982–989. doi: 10.1016/j.jfma.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang C., Gu J., Chen Q. Clinical and epidemiological characteristics of pediatric SARS-CoV-2 infections in China: a multicenter case series. PLoS Med. 2020;17:e1003130. doi: 10.1371/journal.pmed.1003130. [DOI] [PMC free article] [PubMed] [Google Scholar]