Abstract
Simple Summary
The BRAFV600E mutation accounts for 8–10% of metastatic colorectal cancer (mCRC) patients and it is an established prognostic factor. Median overall survival of this subset of patients is indeed so poor that it is similar to first line PFS of patients without this molecular alteration. An exception is represented by patients displaying concomitant MSI-H status who can benefit from immunotherapy with checkpoint inhibitors (CPIs). Recently, a targeted therapy with the combination of encorafenib and cetuximab provided for the first time a survival gain and thus translation in the clinic, even though acquired resistance limits the possibility of more than an incremental benefit. Many studies exploiting other different strategies are ongoing. In this review we present current therapies specifically headed to BRAFV600E mutant mCRC and systematically review ongoing clinical trials identifying different approaches under investigations: targeting MAPK pathway (monotherapy or combinations), targeting MAPK pathway combined with cytotoxic agents, intensive cytotoxic regimen combinations, targeted agents combined with CPIs, oxidative stress induction, and cytotoxic agents combined with antiangiogenic drugs and CPIs.
Abstract
The BRAFV600E mutation is found in 8–10% of metastatic colorectal cancer (mCRC) patients and it is recognized as a poor prognostic factor with a median overall survival inferior to 20 months. At present, besides immune checkpoint inhibitors (CPIs) for those tumors with concomitant MSI-H status, recommended treatment options include cytotoxic chemotherapy + anti-VEGF in the first line setting, and a combination of EGFR and a BRAF inhibitor (cetuximab plus encorafenib) in second line. However, even with the latter targeted approach, acquired resistance limits the possibility of more than an incremental benefit and survival is still dismal. In this review, we discuss current treatment options for this subset of patients and perform a systematic review of ongoing clinical trials. Overall, we identified six emerging strategies: targeting MAPK pathway (monotherapy or combinations), targeting MAPK pathway combined with cytotoxic agents, intensive cytotoxic regimen combinations, targeted agents combined with CPIs, oxidative stress induction, and cytotoxic agents combined with antiangiogenic drugs and CPIs. In the future, the integration of new therapeutic strategies targeting key players in the BRAFV600E oncogenic pathways with current treatment approach based on cytotoxic chemotherapy and surgery is likely to redefine the treatment landscape of these CRC patients.
Keywords: BRAF, colon cancer, immune checkpoint inhibitors, targeted agents, FOLFOXIRI
1. Introduction
Colorectal cancer (CRC) is the third most common diagnosed type of cancer and the third cause of cancer related death worldwide in both women and men [1]. Despite recent improvements in CRC treatment, only 12% of patients diagnosed with metastatic colorectal cancer (mCRC) are still alive after five years [2]. As per clinical guidelines, pan-RAS, BRAF, HER2, and mismatch repair (MMR) status assessments are recommended to define patients prognosis and treatment strategy [3,4,5]. Particularly, BRAF mutations account for 8–10% of mCRCs and more than 90% are missense mutations occurring in codon 600, leading to an aminoacidic substitution of a valine for a glutamic acid (V600E) [6]. Furthermore, BRAF mutations different from V600E (BRAFnon-V600E) account for about 2% of mCRCs and they have been associated with specific clinicopathological features and a better clinical outcome [7,8,9,10,11]. Considering that BRAF-V600E mutation in mCRC is still a clinical unmet need, we focused our manuscript on treatment of BRAFV600E mutant mCRC.
In mCRC BRAFV600E mutation represents a poor prognostic factor and median overall survival (OS) of patients diagnosed with advanced disease harboring this mutation ranges between 10 to 20 months [7,12]. Biologically, BRAFV600E mutant mCRCs are frequently characterized by hypermethylation, microsatellite instability (MSI) and consensus molecular subtype 1 (CMS1) [13]. Particularly, MSI features and BRAFV600E mutations frequently overlap and up to 50% of BRAFV600E mutant mCRCs are also MSI [14,15,16]. Notably, those MSI mCRCs harboring BRAFV600E mutation are always sporadic and do not arise in the context of Lynch Syndrome [14,15,16]. This is relevant since MSI and microsatellite stable (MSS) mCRCs are well-known to represent two distinct diseases with specific etiology, prognosis and different treatment implications [13,17].
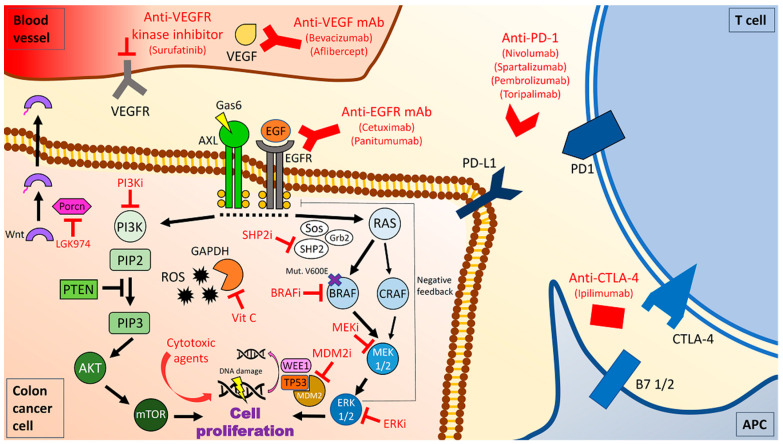
BRAF is a serine-threonine kinase playing a key role as downstream RAS effector in the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) signal transduction cascade. BRAFV600E mutation causes an inappropriate activation of this pathway leading to uncontrolled cell proliferation, migration, angiogenesis, and escape from apoptosis [18] (Figure 1). BRAFV600E mutation is a target of treatment in various types of malignancies such as melanoma, non-small cell lung cancer (NSCLC), and hairy-cell leukemia [3,19,20,21].
Figure 1.
Schematic representation of pathways currently under investigations as actionable therapeutic targets harnessing BRAFV600E mutant metastatic colorectal cancer (mCRC). Legend: mAb = monoclonal antibodies; i = inhibitor. Vit = vitamin. Mut = mutation.
In mCRC, initial studies targeting BRAFV600E mutant disease were disappointing, still demonstrating signs of activity [18,22,23]. However, initial exploitation of BRAF inhibitors as monotherapy in mCRC paved the way for the understanding of molecular mechanisms which led to rational combinations of MAPK targeting agents against BRAFV600E mutant disease [24,25,26,27]. Progressively, subsequent clinical trials reshaped the therapeutic landscape toward specific targeted or cytotoxic treatment regimens for this subset of patients [25,28]. However, prognosis of BRAFV600E mutant mCRC patients remains poor [12,18,25]. Further treatment improvements are needed to tackle this clinical still unmet need.
In this review, we first discuss current treatments options for BRAFV600E mutant mCRC patients and then we systematically review ongoing clinical trials focusing on novel strategies under investigation in this subset of patients.
2. Current Treatment Strategies
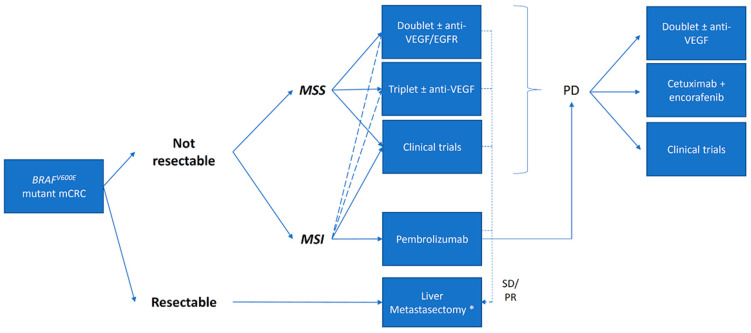
Treatment strategies for BRAFV600E mutant mCRC have been the same of all mCRCs up to recent times. However, given the poor prognosis of these subset of patients, specific treatment regimens have been recently investigated with successful results [25,28]. These studies led to National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) recommendations of focused treatments improving outcomes [3,4,5]. Current options of treatment for BRAFV600E mutant mCRC are summarized in Figure 2. Although rarely BRAFV600E mutant mCRC patients present with liver or lung limited disease, international clinical guidelines recommend evaluating feasibility of surgical resection with curative intent in oligometastatic disease given its long-term survival implication [29,30]. However, it should be taken into account that shorter (OS) and relapse-free survival after metastasectomy have been reported [30,31,32]. In this regard, BRAFV600E mutation has been indicated as an exclusion criteria for most ongoing experimental trials of liver transplantation for mCRC, such as in the ongoing COLT Study (NCT03803436).
Figure 2.
Current treatment options for BRAFV600E mutant metastatic colorectal cancer (mCRC). According to most recent studies, treatment opportunities for BRAFV600E mutant mCRC are fast developing if compared to only a decade ago. The panorama of treatment now includes the following options: surgery, combinations of cytotoxic drugs, targeted and immunological agents. All these approaches should be carefully evaluated when discussing the treatment approach to BRAFV600E mCRCs in multidisciplinary teams (MDT). Given the peculiarity of this subset of mCRCs, clinical trial enrolment should always be considered also in the upfront setting. Based on current evidence, MSI BRAFV600E mutant mCRC progressing to first line treatment with pembrolizumab should be managed as microsatellite stable (MSS) BRAFV600E mutant mCRC. Keys: * = Metastasectomy should be -considered in liver limited disease in case of response or prolonged disease control obtained with medical treatments even if relapse-free and overall survival is poorer than BRAF wild-type mCRCs. Legend: mCRC = metastatic colorectal cancer. SD = stable disease; PD = progressive disease; PR = partial response. “Dashed line” means consider. “Continuous line” means recommended.
2.1. Immune Checkpoint Inhibitors in BRAFV600E Mutant MSI-H mCRC
A fundamental step to identify the best treatment for a BRAFV600E mutant mCRC patient, given the increasing number of evidences showing a dramatic impact of treating MSI mCRC with checkpoint inhibitors (CPIs) [33,34,35,36], is the assessment of tumor’s MMR status. In the CheckMate 142 trial, 12 out of 74 MSI mCRC patients treated with nivolumab had BRAFV600E mutant disease [36]. Overall response rate (ORR) and disease control rate (DCR) were 31 and 69% in BRAF wild-type mCRCs and 25 and 75% in BRAFV600E mutant mCRCs [36]. In the nivolumab and ipilimumab cohort 29 out of 119 patients had BRAFV600E mutant MSI mCRC [33]. In this cohort response rates were higher in both BRAFV600E mutant and wild-type mCRC with a remarkable 55% ORR and 80% DCR in the former group [33]. In addition, the recent phase III trial KEYNOTE-177 demonstrated the superiority of pembrolizumab in first-line setting over standard regimens in MSI mCRC, independently from BRAF status (Table S1) [35]. According to these trials, CPIs seem to perform better than standard therapies in BRAFV600E mutant MSI mCRC [25,33,35]. The ongoing phase III trial CheckMate 8HW (NCT04008030) is evaluating the combination of nivolumab and ipilimumab in the same setting and it is expected to provide further data for this subset of patients. Summarizing, these studies support the administration of a CPI as upfront treatment in BRAFV600E mutant MSI mCRC patients. Indeed, following KEYNOTE-177 data, both Food and Drug Administration (FDA) and European Medicines Agency (EMA) recently approved pembrolizumab in the first line setting for MSI mCRC, including those BRAFV600E mutant [37,38]. Today, pembrolizumab is the new standard of care for MSI mCRC harboring BRAFV600E mutation. If immunotherapy is contraindicated or not available, standard cytotoxic treatments remain an option (Figure 2).
2.2. Doublet Cytotoxic Combination Plus Biological Agents
Standard doublet chemotherapy leads to poor outcome in terms of progression-free survival (PFS) in advanced mCRC harboring BRAFV600E mutation in first-, second- and third-line treatment [39]. Furthermore, the use of oxaliplatin or irinotecan does not modify PFS to first-line treatment [39].
In addition to standard cytotoxic agents, the added value of an anti-VEGF drug has never been shown through a dedicated trial in mCRCs harboring BRAFV600E mutation. However, AVF2107g and AGITG MAX trials showed a numerical improvement in survival outcomes for patients with BRAFV600E mutant mCRC with the addiction of bevacizumab to cytotoxic agents [40,41]. Also, a subgroup analysis of the second-line study VELOUR described a greater benefit in terms of OS from the addition of aflibercept to FOLFIRI in BRAFV600E mutant mCRC patients than in the wild-type ones, even though by its nature it is not powered to drive conclusions for this subset of patients [42].
As far as anti-EGFR treatment, initial data generated retrospectively in the advanced lines with cetuximab or panitumumab used as monotherapy, and supported by in vitro data, clearly showed that BRAFV600E mutation is a mechanism of resistance to this treatment [43]. This hypothesis has then been tested in subgroup analyses of prospective trials with conflicting results. In second line treatment, the addition of anti-EGFR to FOLFIRI did not confer any clinical benefit in BRAFV600E mutant mCRC patients and it is reported as potentially deleterious [44,45]. In contrast, in a first line setting, BRAFV600E mutation was not identified as a negative predictive biomarker of response to cetuximab or panitumumab added on top to FOLFOX or FOLFIRI, but rather a poor prognostic biomarker [46,47]. To assess the real impact of BRAFV600E mutation as predictive biomarker to anti-EGFR treatment, two meta-analyses were published showing conflicting results [48,49]. Furthermore, methodological limitations hampered definitive conclusions from these two publications [18]. Interestingly, the FIRE-3 trial is a first-line setting study which compared FOLFIRI plus bevacizumab versus FOLFIRI plus cetuximab [50]. Among BRAFV600E mutant mCRC enrolled in this trial (N = 48; 14%), cetuximab led to a higher ORR but no difference in terms of PFS and OS were captured between the two arms [50]. In conclusion, latest NCCN guidelines recommend the use of anti-EGFR agents only in BRAF wild-type tumor, while ESMO guidelines are less restrictive [3,4]. Overall, anti-EGFR drugs represent a weak option for treatment of BRAFV600E mutant mCRC patients in the first line and even more in further lines of therapy (Figure 2).
2.3. Triplet Cytotoxic Combination Plus Biological Agent
In all mCRC patients, an intensive chemotherapy regimen of FOLFOXIRI plus a bevacizumab can be considered in the first-line setting [18]. In particular, this regimen is currently recommended by clinical guidelines for BRAFV600E mutant mCRC fit (meaning ECOG performance status 0 or 1) patients [3,4]. Initially, a phase II trial with FOLFOXIRI plus bevacizumab specifically designed for BRAFV600E mutant mCRC showed promising results in terms of median PFS and OS [51]. Following, in a subgroup analysis of the phase III TRIBE study, BRAFV600E mutant mCRC patients appeared to benefit more from triplet combination plus bevacizumab if compared to FOLFIRI plus bevacizumab, even if statistical significance was not reached (Table S1) [28]. In contrast, the TRIBE 2 study did not confirmed the advantage of FOLFOXIRI plus bevacizumab versus the doublet regimens plus bevacizumab in the BRAFV600E mutant mCRC patients [52]. This has been recently confirmed by a meta-analysis from the same group, demonstrating no benefit from FOLFOXIRI plus bevacizumab if compared to standard doublet cytotoxic combinations [53]. These data relight the debate on current clinical guidelines recommendation, making FOLFOXIRI plus bevacizumab no longer the treatment of choice in first line for BRAFV600E mutant mCRC patients [3,4,53]. In addition to FOLFOXIRI plus bevacizumab, in the randomized phase II VOLFI trial FOLFOXIRI plus panitumumab has also been studied in first-line, showing a high ORR improvement (85% versus 22%) in BRAFV600E mutant mCRC [54], requiring confirmation in larger studies and possibly leading to reconsider the role anti-EGFR in this setting.
2.4. BRAF-Targeted Combinations
In BRAFV600E mutant melanoma BRAF-inhibition led to dramatic results both in the metastatic and adjuvant settings [55,56,57]. In mCRC, initial studies of monotherapy with BRAF-inhibitors provided poor outcomes, with fewer than 10% responders and poor PFS [22,23]. Subsequently, preclinical studies allowed to shed light on mechanisms of primary resistance to BRAF blockade in this tumor: differently from melanoma, in CRC anti-BRAF monotherapy induces a feedback activation of EGFR that re-activate the oncogenic pathway providing pharmacological escape [27] (Figure 1). As a consequence, multiple studies combined EGFR and BRAF inhibitors in BRAFV600E mutant mCRC, demonstrating improved results [24,58,59]. Recently, the BEACON phase III trial compared the combination of the newer anti-BRAF agent encorafenib plus the MEK inhibitor binimetinib and cetuximab versus encorafenib and cetuximab versus FOLFIRI or irinotecan plus cetuximab after failure of first-line therapy [25]. In this study, both the triplet and the doublet combinations were superior to control arm obtaining a median OS of nine and 8.4 months respectively, compared to 5.4 months in the control arm [25]. Objective responses were 29% with the triplet, 23% with the doublet and 2% with the control arm [25]. Severe toxicities of grade 3 and higher were reported in 58% of patients in the triplet arm, 50% in the doublet and 61% in control arm [25]. Based on these data, FDA and EMA recently approved the doublet combination of cetuximab plus encorafenib after failure of a first-line treatment for BRAFV600E mutant mCRC patients (Table S1) [60,61]. This study has been criticized in two aspects. First, the percentage of MSI CRC patients enrolled was lower than 10% which appears lower than expected among BRAFV600E mutant mCRC [15,16,62]. Second, the control arm has been questioned since the use of a regimen including an anti-EGFR in the second line setting is of very limited efficacy [44,45]. Based on the BEACON results, the active but not recruiting phase II ANCHOR-CRC trial is going to explore the role of the triplet combination in first-line setting [63]. Initial results of the triplet combination in the first-line setting were recently presented and demonstrated a 50% ORR and 85% DCR with a mPFS 4.9 months and a safety profile similar to the BEACON study [63]. Based on that, a comparison with standard chemotherapy in the upfront setting is awaited with great interest. In addition to these combinations, preclinical data described an increased PI3K/AKT pathway activation as a possible mechanism of resistance to BRAF-targeted monotherapy (Figure 1), thus cetuximab and encorafenib has been compared to the triplet cetuximab, encorafenib plus alpelisib [64]. ORR was 19% and 18% while median PFS was 3.7 and 4.2 months for the doublet and triplet, respectively [64]. Further studies are warranted to clarify the potential role of adding alpelisib to the combination of cetuximab and encorafenib. Finally, it should be considered that acquired resistance eventually takes place also in face of multiple layers of BRAF-blockade, being associated with the expansion of pre-existing minor RAS mutant clones [24]. In this regard, in vitro data suggest considering an upfront convergent targeting with also an ERK inhibitor to prevent resistance [24].
Overall, even if the recent approval of the combination of cetuximab and encorafenib represents a step forward for treatment of BRAFV600E mutant mCRC, it is estimated that only 60% of these patients actually reach second-line treatment due to the aggressiveness of this disease [11,12]. Because of this prognostic impact, it is therefore crucial to consider for all BRAFV600E mCRC patients early enrollment in clinical trials right from the first-line setting.
3. Ongoing Clinical Trials
3.1. Material and Methods
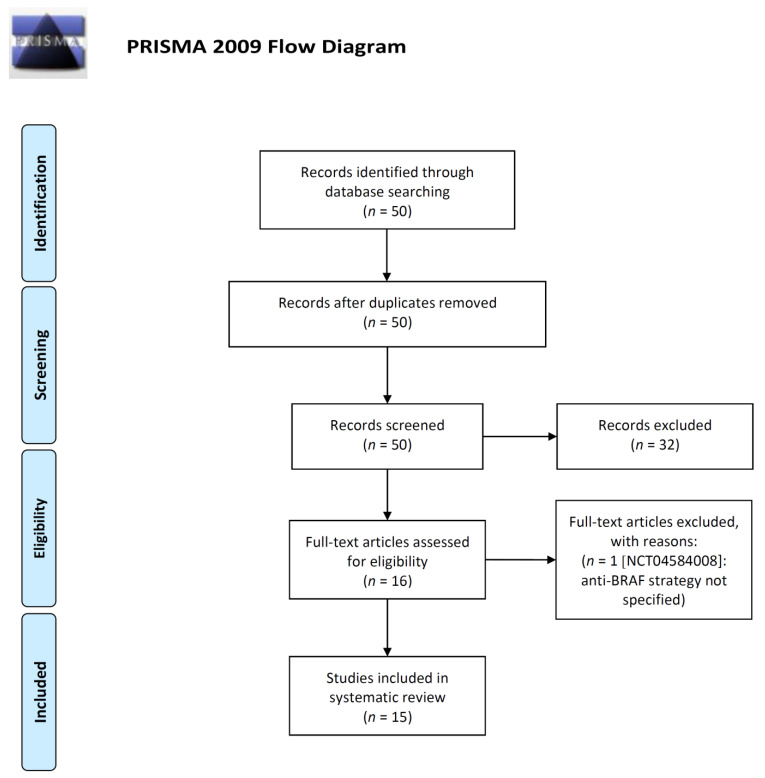
An initial systematic review process was performed on 23 November and then updated throughout the revision process on 23 December 2020 with the aim to guarantee a more comprehensive and timely assessing of the panorama of strategies currently under investigations harnessing BRAFV600E mutant mCRC. We performed a systematic review of ongoing clinical trials on Clinicaltrial.gov according to PRISMA guidelines (Figure 3) [65]. The Medical Subject Headings terms used for the search in ClinicalTrials.gov were (“Recruiting or not yet recruiting” as status), (“colo-rectal cancer” as condition/disease) and (“BRAF” as other terms). The systematic review process was performed independently by two authors (G.M. and V.G). and checked by other two authors (E.B. and A.S-B.). All ongoing studies not detailing the anti-BRAF regimen under investigation were excluded.
Figure 3.
PRISMA 2009 Flow diagram representing the systematic review performed on ClinicaTrial.gov on 23 December 2020 [65]. For more information, visit www.prisma-statement.org.
3.2. Results
The treatment panorama of BRAFV600E mutant mCRC is evolving according to the results of focused clinical studies. Besides the above-mentioned trials (Table S1) [25,28,35], many others are currently ongoing to further improve prognosis of these patients. To capture the whole picture of clinical strategies specifically directed to BRAFV600E mutant mCRC, we collected data of currently ongoing clinical trials in this subset of patients. Throughout a systematic review process, we gathered 50 studies of whom 16 were assessed for eligibility and 15 included in this review (Table 1 and Figure 3). One trial (NCT04584008) was excluded since the anti-BRAF treatment strategy is not detailed. Indeed, the clinical studies identified were classified according to the treatment strategy adopted: targeting MAPK pathway (monotherapy or combinations), targeting MAPK pathway combined with cytotoxic agents, intensive cytotoxic regimens plus standard biological agents, targeted agents combined with CPIs, oxidative stress induction and cytotoxic agents combined with antiangiogenic drugs and CPIs (Table 1).
Table 1.
Interventional ongoing clinical trials targeting specifically BRAFV600 mutant metastatic colorectal cancer (mCRC) retrieved through a systematic review process performed on 23 December 2020.
| Strategy | Study ID Status Main Location |
Ph. | Drug Schedule | Main Inclusion Criteria |
|---|---|---|---|---|
| Targeting MAPK pathway (monotherapy or combinations) |
NCT04294160 Recruiting Germany |
Ib | -Dabrafenib + LTT462 (ERKi) -Dabrafenib + Trametinib + LTT462 (ERKi) -Dabrafenib + LTT462 (ERKi) + LXH254 (pan-RAFi) -Dabrafenib + LTT462 (ERKi) + TNO155 (SHP2i) |
-BRAFV600 mutation -Site for biopsy at baseline and on treatment |
|
NCT03087071 Recruiting USA |
II | -Panitumumab + Trametinib (Cohort 2) | -KRAS, NRAS, or BRAF mutation -Prior treatment with MEKi, ERKi or anti-EGFR not allowed |
|
|
NCT03714958 Recruiting France |
I | -Trametinib + HDM201 (Mdm2i) | -RAS or BRAF mutation and TP53 wild-type (also BRAF translocation are eligible) | |
|
NCT02465060 (MATCH) Recruiting USA |
II | -Dabrafenib + Trametinib | -Solid tumor with BRAFV600E/R/K/D mutation | |
|
NCT04190628 Recruiting USA |
I | -ABM-1310 (BRAFi) | -Solid tumor BRAFV600 mutation -Patients with active brain metastases are eligible |
|
| Targeting MAPK pathway combined with cytotoxic agents |
NCT03727763 (IMPROVEMENT) Recruiting China |
II | -FOLFIRI + vemurafenib + cetuximab | -BRAFV600E mutation and extended RAS wild-type |
|
NCT02857270 Recruiting USA |
Ib | -LY3214996 (ERK1/2i) ± other agents (Part E) | -BRAFV600E mutation | |
|
NCT04607421 (BREAKWATER) Not yet recruiting |
III | -Encorafenib + Cetuximab ± FOLFOX/FOLFIRI vs. FOLFOX/FOLFIRI/FOLFOXIRI/CAPOX ± bevacizumab | -BRAFV600E mutation -1st line treatment -MSI is an exclusion criteria unless the patient is not eligible to CPIs |
|
| Intensive cytotoxic regimens plus standard biological agents |
NCT04034459 (AIO-KRK-0116) Recruiting Germany |
II | -FOLFOXIRI + cetuximab -FOLFOXIRI + bevacizumab |
-BRAFV600E mutant and pan-RAS wild-type -1st line treatment |
| Targeted agents combined with checkpoint inhibitors |
NCT03668431 Recruiting USA |
II | -Dabrafenib + Trametinib + Spartalizumab (PDR001) | -BRAFV600E mutation and pan-RAS wild-type -Any line -prior anti-EGFR, BRAFi or MEKi, or prior CPIs allowed |
|
NCT04294160 Recruiting Germany |
Ib | -Dabrafenib + LTT462 (ERKi) + Spartalizumab (PDR001) | -BRAFV600E mutation -Site for biopsy at baseline and on treatment |
|
|
NCT01351103 Recruiting USA |
I | -LGK974 (porcupine inhibitor) ± Spartalizumab (PDR001) | -BRAF mutant colorectal cancer ± RNF43 mutation and/or RSPO fusion | |
|
NCT04017650 Recruiting USA |
I/II | -Cetuximab + Encorafenib + Nivolumab | -BRAFV600E mutation MSS -Prior BRAFi, MEKi, ERKi, anti-EGFR and CPIs not allowed |
|
|
NCT04044430 Recruiting USA |
I/II | -Encorafenib + Binimetinib + Nivolumab | -BRAFV600E mutation MSS -prior anti-EGFR, BRAFi or MEKi, or prior CPIs not allowed |
|
| Oxidative stress induction |
NCT03146962 Recruiting USA |
II | -Vitamin C | -RAS (e.g. KRAS or NRAS) or BRAF mutation |
| Cytotoxic agents combined with antiangiogenic drugs and checkpoint inhibitors |
NCT04653480 Recruiting China |
II | -Oxaliplatin or irinotecan cytotoxic regimens + Surufatinib (anti-VEGFR and FGFR) + Toripalimab (anti-PD-1) | -RAS or BRAF mutation MSS -less than 2 previous systemic line of treatment |
Legend: Ph. = phase; i = inhibitor; CPI = checkpoint inhibitors.
The first strategy currently under investigation to target BRAFV600E mutant mCRC includes the use of agents targeting the MAPK pathway, alone or in combination, and it is currently one of the more represented with 5 ongoing clinical trials. Three of them are phase I while two are phase II trials. One trial (NCT04294160) is evaluating multiple targeted combinations of BRAF inhibitors, ERK inhibitors, SHIP2 inhibitors or pan-RAF inhibitor (Figure 1) [66,67]. A further study (NCT03714958) is testing the option of targeting the P53 inhibitor MDM2 and MEK [68,69].
The second strategy being tested is to combine MAPK targeting agents with cytotoxic agents. We retrieved three studies currently pursuing this option. One of them is a phase II trial (NCT03727763) combines FOLFIRI with cetuximab and vemurafenib based on previous encouraging data combining irinotecan with anti-BRAF molecules [58,59]. Among them, the BREAKWATER study (NCT04607421) is the only phase III trial currently ongoing specifically designed for BRAFV600E mutant mCRC patients. Based on promising results from BEACON and ANCHOR-CRC trials [25,63], this trial is evaluating the efficacy of the combination cetuximab plus encorafenib compared to the same combination plus FOLFOX or FOLFIRI compared to physician choice. Interestingly, MSI patients are excluded unless they are ineligible to receive CPIs. Furthermore, the intensive regimen FOLFOXIRI plus bevacizumab is allowed among physician choices in the control arm.
The third strategy is represented by the upfront administration of intensive cytotoxic regimens combined with standard biological agents. Considering the high number of BRAFV600E mutant mCRC patients who will never receive a second-line treatment, the rational of this strategy is to maximize treatment outcome within the first-line setting [11,12]. TRIBE and VOLFI trials do support a potential benefit of this approach [28,54]. The AIO-KRK-0116 trial is a randomized phase II trial (NCT04034459) comparing FOLFORIXI plus cetuximab versus FOLFOXIRI plus bevacizumab. This trial is expected to provide data on intensive regimens efficacy and tolerability and to define the role of anti-EGFR compared to anti-VEGF agents on top of FOLFOXIRI.
The fourth strategy combines targeted agents with CPIs such as nivolumab or spartalizumab (PDR001). Five studies have been retrieved. Two of them are evaluating BRAF and MEK inhibitors combined with a CPI (NCT03668431 and NCT04044430) and one is testing a combination of BRAF and ERK inhibitors with spartalizumab (NCT04294160). Another one is investigating cetuximab plus encorafenib combined with nivolumab (NCT04017650), while the last one is testing a porcupine inhibitor with spartalizumab (NCT01351103). Similarly to early trials of BRAF targeting in mCRC, this strategy has been derived from melanoma [70,71]. In CRC, a positive correlation between the expression of programmed death ligand-1 (PD-L1) and the presence of BRAFV600E mutation has been shown, with also higher levels of CD8+ tumor-infiltrating lymphocytes [72]. This led to reason that BRAFV600E mutant MSS mCRC patients might benefit from a combination of targeted agents and a CPI. Interestingly, initial results obtained with the combination of dabrafenib, trametinib and spartalizumab (NCT03668431) were recently presented and demonstrated a promising 35% ORR and 75% DCR [73]. Of note, patients pretreated with CPIs or BRAF inhibitors were allowed to enter the trial but efficacy was reported lower [73]. Translational analysis of circulating tumor DNA (ctDNA) and patients-derived organoids (PDO) carried out in this trial are expected to clarify mechanisms of resistance and efficacy of this approach [73]. Further results from these studies are awaited with great interest.
The fifth strategy currently under investigation is the exploitation of the oxidative stress induced by high-dose vitamin C administration. Vitamin C has been preclinically demonstrated able to selectively kill RAS and BRAF mutant mCRC cells [74]. This killing activity is mediated by the stalling of glyceraldehyde 3-phosphate dehydrogenase, (GAPDH) which causes an energetic crisis in highly glycolytic KRAS and BRAF mutant but not in wild-type CRC cells (Figure 1) [74]. Following this study, a currently ongoing trial is investigating this strategy in RAS and BRAF mutant mCRC (NCT03146962). Recently, an enhanced activity of CPIs induced by concomitant administration of vitamin C has been reported [75]. Further clinical studies are warranted to test this combination in this subset of patients.
Finally, the sixth strategy under investigation harnessing BRAFV600E mutant mCRC is an intensive approach combining cytotoxic agents plus an antiangiogenic drug and a CPI in patients receiving first- or second-line treatment (NCT04653480). Similarly to intensive cytotoxic regimens combined with standard biological agents, this last approach aims to sooner maximize treatment outcome [11,12].
4. Discussion
BRAFV600E mutant mCRC is a currently an unmet medical need requiring both preclinical and clinical research. Even if dedicated treatment options have been included in the latest clinical guidelines, prognosis of BRAFV600E mutant mCRC patients is still dismal [3,25,28]. Accordingly, many clinical trials are currently ongoing (Table 1) and given the amount of research targeting this subset of CRCs, clinical recommendations are likely to change in the future. Differently from BRAFV600E mutant mCRC, BRAFnon-V600E mutant mCRC are usually left-sided, non-mucinous, MSS, without peritoneal involvement leading to a better OS, and not requiring the same treatment approach [7,8,11].
Differently from recent publications on this topic [11,18,76], in this review we focused on ongoing clinical trials with the aim to define future developments of treatment for this subset of patients [11,18,76] (Figure 3). Our search led to identification of six different treatment strategies directed against BRAFV600E mutant mCRC. Among these, the exploitation of agents targeting the MAPK pathway and intensive chemotherapy regimens appear as the most promising based on previous results derived from published clinical trials [25,28]. However, a recent meta-analysis, showing no benefit from FOLFOXIRI plus bevacizumab if compared to standard cytotoxic doublets plus bevacizumab in BRAFV600E mutant mCRC, will lead to reconsider current clinical guidelines recommendations [3,4,53]. Moreover, the combination of targeted agents plus CPIs is of great interest, particularly for MSS tumors, even if there are only initial data in mCRC [73]. Among other avenues, a provocative opportunity is represented by high-dose vitamin C, even though several issues are still to be addressed such as the right dosages and infusion scheduling.
BRAFV600E mutation is commonly recognized as a poor prognostic factor in mCRC with a median OS of less than 20 months for metastatic disease [12]. However, around 20% of patients with BRAFV600E mCRC patients survives beyond 24 months from the initial diagnosis [12,28,51,77,78]. The reason for this prognostic heterogeneity has not been identified yet. According to molecular consensus subtypes (CMS), BRAFV600E mutant mCRC are identified for the vast majority in the CMS1 subgroup while the few remaining are scattered across the other CMS subtypes [13]. However, CMS classification does not explain this prognostic heterogeneity. Barras and coworkers from a cohort of 218 BRAFV600E mutant CRC identified two subtypes of disease with different prognosis: BM1 (BRAF mutant 1) and BM2 (BRAF mutant 2) [79]. These two subgroups were characterized by substantial differences both at transcriptomic and proteomic level and they are independent from patients’ gender, sidedness, MMR status and PI3K status [79]. BM1 is less common (1/3 of cases) and is characterized by strong activation of AKT/mTOR, KRAS, 4EBP1 and epithelial-mesenchymal transition features [79]. On the other hand, BM2 represent most of cases and it is characterized by cell cycle deregulation, high level of CDK1 and low level of cyclin D1 [79]. Despite prognostic subdivision, this classification has no direct implication for the BRAFV600E treatment decision algorithm. In addition to molecular characterization, a retrospective platform of 395 BRAFV600E mutant mCRC led to the identification of three different prognostic subgroups based on the use of clinical data [80]. Even if this classification might have potential implication for treatment decision and for guiding translational research, its integration with molecular classification such as BM1/BM2 or CMS is warranted [80]. It should be noted that neither any molecular sub-grouping nor clinical classification has been used to date to design ongoing clinical trials against BRAFV600E mutant mCRC. A closer interaction between preclinical and clinical researchers is needed therefore to design future trials.
5. Conclusions
The treatment of BRAFV600E mutant mCRC has been relentlessly improving over the last decade thanks to the parallel evolution of preclinical and clinical knowledge. The advent of cancer immune therapy with CPIs has clearly changed the scenario providing striking results also in this subset of MSI tumors, although the true challenge is represented by patients harboring BRAFV600E MSS cancer. Results of currently ongoing clinical trials exploiting new strategies, such as the combination of different targeted agents and with CPIs, are awaited to further expand the spectrum of treatment for this peculiar subtype of CRC under the paradigm of precision oncology.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/13/1/137/s1, Table S1: Main completed clinical trials which demonstrated to improve clinical outcome in BRAFV600E mutant metastatic colorectal cancer (mCRC) if compared to standard doublet plus anti-VEGF or anti-EGFR agents and supporting the current recommendation by NCCN and ESMO clinical guidelines.
Funding
The work of the authors is supported, in part, by Fondazione Regionale Ricerca Biomedica Regione Lombardia, Project CP 12/2018 IANG CRC (S.S., A.S.-B.); H2020 grant agreement no. 635342-2 MoTriColor (S.S.); AIRC IG no. 20685 (S.S.); Terapia Molecolare Tumori by Fondazione Oncologia Niguarda Onlus (A.S.-B. and S.S.); FONDAZIONE AIRC under 5 per Mille 2018-ID. 21091 program–G.L. Siena Salvatore.
Conflicts of Interest
Salvatore Siena is advisory board member for Amgen, Bayer, BMS, CheckmAb, Clovis, Daiichi-Sankyo, Merck, Roche-Genentech, and Seattle Genetics. Andrea Sartore-Bianchi is advisory board member for Amgen, Bayer, Sanofi and Servier. The other authors declare no competing interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. CA Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C.E., Lin C.C., Mariotto A.B., Siegel R.L., Stein K.D., Kramer J.L., Alteri R., Robbins A.S., Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J. Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology. [(accessed on 21 March 2020)]; Available online: https://www.nccn.org/professionals/physician_gls/default.aspx.
- 4.Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 5.Yoshino T., Arnold D., Taniguchi H., Pentheroudakis G., Yamazaki K., Xu R.-H., Kim T.W., Ismail F., Tan I.B., Yeh K.-H., et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: A JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann. Oncol. 2018;29:44–70. doi: 10.1093/annonc/mdx738. [DOI] [PubMed] [Google Scholar]
- 6.Davies H., Bignell G.R., Cox C., Stephens P., Edkins S., Clegg S., Teague J., Woffendin H., Garnett M.J., Bottomley W., et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 7.Schirripa M., Biason P., Lonardi S., Pella N., Pino M.S., Urbano F., Antoniotti C., Cremolini C., Corallo S., Pietrantonio F., et al. Class 1, 2, and 3 BRAF-Mutated Metastatic Colorectal Cancer: A Detailed Clinical, Pathologic, and Molecular Characterization. Clin. Cancer Res. 2019;25:3954–3961. doi: 10.1158/1078-0432.CCR-19-0311. [DOI] [PubMed] [Google Scholar]
- 8.Cremolini C., Di Bartolomeo M., Amatu A., Antoniotti C., Moretto R., Berenato R., Perrone F., Tamborini E., Aprile G., Lonardi S., et al. BRAF codons 594 and 596 mutations identify a new molecular subtype of metastatic colorectal cancer at favorable prognosis. Ann. Oncol. 2015;26:2092–2097. doi: 10.1093/annonc/mdv290. [DOI] [PubMed] [Google Scholar]
- 9.Jones J.C., Renfro L.A., Al-Shamsi H.O., Schrock A.B., Rankin A., Zhang B.Y., Kasi P.M., Voss J.S., Leal A.D., Sun J., et al. Non-V600 BRAF Mutations Define a Clinically Distinct Molecular Subtype of Metastatic Colorectal Cancer. J. Clin. Oncol. 2017;35:2624–2630. doi: 10.1200/JCO.2016.71.4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao Z., Yaeger R., Rodrik-Outmezguine V.S., Tao A., Torres N.M., Chang M.T., Drosten M., Zhao H., Cecchi F., Hembrough T., et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548:234–238. doi: 10.1038/nature23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fanelli G.N., Dal Pozzo C.A., Depetris I., Schirripa M., Brignola S., Biason P., Balistreri M., Dal Santo L., Lonardi S., Munari G., et al. The heterogeneous clinical and pathological landscapes of metastatic Braf-mutated colorectal cancer. Cancer Cell Int. 2020;20:30. doi: 10.1186/s12935-020-1117-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Seligmann J.F., Fisher D., Smith C.G., Richman S.D., Elliott F., Brown S., Adams R., Maughan T., Quirke P., Cheadle J., et al. Investigating the poor outcomes of BRAF-mutant advanced colorectal cancer: Analysis from 2530 patients in randomised clinical trials. Ann. Oncol. 2017;28:562–568. doi: 10.1093/annonc/mdw645. [DOI] [PubMed] [Google Scholar]
- 13.Dienstmann R., Vermeulen L., Guinney J., Kopetz S., Tejpar S., Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 14.Capper D., Voigt A., Bozukova G., Ahadova A., Kickingereder P., von Deimling A., von Knebel Doeberitz M., Kloor M. BRAF V600E-specific immunohistochemistry for the exclusion of Lynch syndrome in MSI-H colorectal cancer. Int. J. Cancer. 2013;133:1624–1630. doi: 10.1002/ijc.28183. [DOI] [PubMed] [Google Scholar]
- 15.Venderbosch S., Nagtegaal I.D., Maughan T.S., Smith C.G., Cheadle J.P., Fisher D., Kaplan R., Quirke P., Seymour M.T., Richman S.D., et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: A pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin. Cancer Res. 2014;20:5322–5330. doi: 10.1158/1078-0432.CCR-14-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lochhead P., Kuchiba A., Imamura Y., Liao X., Yamauchi M., Nishihara R., Qian Z.R., Morikawa T., Shen J., Meyerhardt J.A., et al. Microsatellite instability and BRAF mutation testing in colorectal cancer prognostication. J. Natl. Cancer Inst. 2013;105:1151–1156. doi: 10.1093/jnci/djt173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Müller M.F., Ibrahim A.E.K., Arends M.J. Molecular pathological classification of colorectal cancer. Virchows Arch. 2016;469:125–134. doi: 10.1007/s00428-016-1956-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taieb J., Lapeyre-Prost A., Laurent Puig P., Zaanan A. Exploring the best treatment options for BRAF-mutant metastatic colon cancer. Br. J. Cancer. 2019;121:434–442. doi: 10.1038/s41416-019-0526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michielin O., van Akkooi A., Ascierto P., Dummer R., Keilholz U., ESMO Guidelines Committee Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019;30:1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 20.Wu Y.-L., Planchard D., Lu S., Sun H., Yamamoto N., Kim D.-W., Tan D.S.W., Yang J.C.-H., Azrif M., Mitsudomi T., et al. Pan-Asian adapted Clinical Practice Guidelines for the management of patients with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 2019;30:171–210. doi: 10.1093/annonc/mdy554. [DOI] [PubMed] [Google Scholar]
- 21.Tiacci E., Trifonov V., Schiavoni G., Holmes A., Kern W., Martelli M.P., Pucciarini A., Bigerna B., Pacini R., Wells V.A., et al. BRAF mutations in hairy-cell leukemia. N. Engl. J. Med. 2011;364:2305–2315. doi: 10.1056/NEJMoa1014209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hyman D.M., Puzanov I., Subbiah V., Faris J.E., Chau I., Blay J.-Y., Wolf J., Raje N.S., Diamond E.L., Hollebecque A., et al. Vemurafenib in Multiple Nonmelanoma Cancers with BRAF V600 Mutations. N. Engl. J. Med. 2015;373:726–736. doi: 10.1056/NEJMoa1502309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kopetz S., Desai J., Chan E., Hecht J.R., O’Dwyer P.J., Maru D., Morris V., Janku F., Dasari A., Chung W., et al. Phase II Pilot Study of Vemurafenib in Patients With Metastatic BRAF-Mutated Colorectal Cancer. J. Clin. Oncol. 2015;33:4032–4038. doi: 10.1200/JCO.2015.63.2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corcoran R.B., André T., Atreya C.E., Schellens J.H.M., Yoshino T., Bendell J.C., Hollebecque A., McRee A.J., Siena S., Middleton G., et al. Combined BRAF, EGFR, and MEK Inhibition in Patients with BRAFV600E-Mutant Colorectal Cancer. Cancer Discov. 2018;8:428–443. doi: 10.1158/2159-8290.CD-17-1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kopetz S., Grothey A., Yaeger R., Van Cutsem E., Desai J., Yoshino T., Wasan H., Ciardiello F., Loupakis F., Hong Y.S., et al. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2019;381:1632–1643. doi: 10.1056/NEJMoa1908075. [DOI] [PubMed] [Google Scholar]
- 26.Oddo D., Sennott E.M., Barault L., Valtorta E., Arena S., Cassingena A., Filiciotto G., Marzolla G., Elez E., van Geel R.M.J.M., et al. Molecular Landscape of Acquired Resistance to Targeted Therapy Combinations in BRAF-Mutant Colorectal Cancer. Cancer Res. 2016;76:4504–4515. doi: 10.1158/0008-5472.CAN-16-0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prahallad A., Sun C., Huang S., Di Nicolantonio F., Salazar R., Zecchin D., Beijersbergen R.L., Bardelli A., Bernards R. Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature. 2012;483:100–103. doi: 10.1038/nature10868. [DOI] [PubMed] [Google Scholar]
- 28.Cremolini C., Loupakis F., Antoniotti C., Lupi C., Sensi E., Lonardi S., Mezi S., Tomasello G., Ronzoni M., Zaniboni A., et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16:1306–1315. doi: 10.1016/S1470-2045(15)00122-9. [DOI] [PubMed] [Google Scholar]
- 29.Johnson B., Jin Z., Truty M.J., Smoot R.L., Nagorney D.M., Kendrick M.L., Kipp B.R., Grothey A. Impact of Metastasectomy in the Multimodality Approach for BRAF V600E Metastatic Colorectal Cancer: The Mayo Clinic Experience. Oncologist. 2018;23:128–134. doi: 10.1634/theoncologist.2017-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tosi F., Magni E., Amatu A., Mauri G., Bencardino K., Truini M., Veronese S., De Carlis L., Ferrari G., Nichelatti M., et al. Effect of KRAS and BRAF Mutations on Survival of Metastatic Colorectal Cancer After Liver Resection: A Systematic Review and Meta-Analysis. Clin. Colorectal. Cancer. 2017;16:e153–e163. doi: 10.1016/j.clcc.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 31.Schirripa M., Bergamo F., Cremolini C., Casagrande M., Lonardi S., Aprile G., Yang D., Marmorino F., Pasquini G., Sensi E., et al. BRAF and RAS mutations as prognostic factors in metastatic colorectal cancer patients undergoing liver resection. Br. J. Cancer. 2015;112:1921–1928. doi: 10.1038/bjc.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Renaud S., Romain B., Falcoz P.-E., Olland A., Santelmo N., Brigand C., Rohr S., Guenot D., Massard G. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br. J. Cancer. 2015;112:720–728. doi: 10.1038/bjc.2014.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Overman M.J., Lonardi S., Wong K.Y.M., Lenz H.-J., Gelsomino F., Aglietta M., Morse M.A., Van Cutsem E., McDermott R., Hill A., et al. Durable Clinical Benefit With Nivolumab Plus Ipilimumab in DNA Mismatch Repair-Deficient/Microsatellite Instability-High Metastatic Colorectal Cancer. J. Clin. Oncol. 2018;36:773–779. doi: 10.1200/JCO.2017.76.9901. [DOI] [PubMed] [Google Scholar]
- 34.Le D.T., Uram J.N., Wang H., Bartlett B.R., Kemberling H., Eyring A.D., Skora A.D., Luber B.S., Azad N.S., Laheru D., et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.André T., Shiu K.-K., Kim T.W., Jensen B.V., Jensen L.H., Punt C., Smith D., Garcia-Carbonero R., Benavides M., Gibbs P., et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020;383:2207–2218. doi: 10.1056/NEJMoa2017699. [DOI] [PubMed] [Google Scholar]
- 36.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.-J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.FDA Approves Pembrolizumab for First-Line Treatment of MSI-H/dMMR Colorectal Cancer. [(accessed on 23 December 2020)]; Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-first-line-treatment-msi-hdmmr-colorectal-cancer.
- 38.New Indication Concerns the First-Line Treatment of Metastatic MSI-H or dMMR Colorectal Cancer. [(accessed on 23 December 2020)]; Available online: https://www.esmo.org/oncology-news/ema-recommends-extension-of-indications-for-pembrolizumab4.
- 39.Morris V., Overman M.J., Jiang Z.-Q., Garrett C., Agarwal S., Eng C., Kee B., Fogelman D., Dasari A., Wolff R., et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin. Colorectal. Cancer. 2014;13:164–171. doi: 10.1016/j.clcc.2014.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ince W.L., Jubb A.M., Holden S.N., Holmgren E.B., Tobin P., Sridhar M., Hurwitz H.I., Kabbinavar F., Novotny W.F., Hillan K.J., et al. Association of k-ras, b-raf, and p53 status with the treatment effect of bevacizumab. J. Natl. Cancer Inst. 2005;97:981–989. doi: 10.1093/jnci/dji174. [DOI] [PubMed] [Google Scholar]
- 41.Price T.J., Hardingham J.E., Lee C.K., Weickhardt A., Townsend A.R., Wrin J.W., Chua A., Shivasami A., Cummins M.M., Murone C., et al. Impact of KRAS and BRAF Gene Mutation Status on Outcomes From the Phase III AGITG MAX Trial of Capecitabine Alone or in Combination With Bevacizumab and Mitomycin in Advanced Colorectal Cancer. J. Clin. Oncol. 2011;29:2675–2682. doi: 10.1200/JCO.2010.34.5520. [DOI] [PubMed] [Google Scholar]
- 42.Wirapati P., Pomella V., Vandenbosch B., Kerr P., Maiello E., Jeffery G.M., Curca R.-O.D., Karthaus M., Bridgewater J.A., Mihailov A.C., et al. Velour trial biomarkers update: Impact of RAS, BRAF, and sidedness on aflibercept activity. J. Clin. Oncol. 2017;35:3538. doi: 10.1200/JCO.2017.35.15_suppl.3538. [DOI] [Google Scholar]
- 43.Di Nicolantonio F., Martini M., Molinari F., Sartore-Bianchi A., Arena S., Saletti P., De Dosso S., Mazzucchelli L., Frattini M., Siena S., et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J. Clin. Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 44.Seymour M.T., Brown S.R., Middleton G., Maughan T., Richman S., Gwyther S., Lowe C., Seligmann J.F., Wadsley J., Maisey N., et al. Panitumumab and irinotecan versus irinotecan alone for patients with KRAS wild-type, fluorouracil-resistant advanced colorectal cancer (PICCOLO): A prospectively stratified randomised trial. Lancet Oncol. 2013;14:749–759. doi: 10.1016/S1470-2045(13)70163-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peeters M., Oliner K.S., Price T.J., Cervantes A., Sobrero A.F., Ducreux M., Hotko Y., André T., Chan E., Lordick F., et al. Analysis of KRAS/NRAS Mutations in a Phase III Study of Panitumumab with FOLFIRI Compared with FOLFIRI Alone as Second-line Treatment for Metastatic Colorectal Cancer. Clin. Cancer Res. 2015;21:5469–5479. doi: 10.1158/1078-0432.CCR-15-0526. [DOI] [PubMed] [Google Scholar]
- 46.Douillard J.-Y., Oliner K.S., Siena S., Tabernero J., Burkes R., Barugel M., Humblet Y., Bodoky G., Cunningham D., Jassem J., et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 47.Bokemeyer C., Van Cutsem E., Rougier P., Ciardiello F., Heeger S., Schlichting M., Celik I., Köhne C.-H. Addition of cetuximab to chemotherapy as first-line treatment for KRAS wild-type metastatic colorectal cancer: Pooled analysis of the CRYSTAL and OPUS randomised clinical trials. Eur. J. Cancer. 2012;48:1466–1475. doi: 10.1016/j.ejca.2012.02.057. [DOI] [PubMed] [Google Scholar]
- 48.Pietrantonio F., Petrelli F., Coinu A., Di Bartolomeo M., Borgonovo K., Maggi C., Cabiddu M., Iacovelli R., Bossi I., Lonati V., et al. Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab: A meta-analysis. Eur. J. Cancer. 2015;51:587–594. doi: 10.1016/j.ejca.2015.01.054. [DOI] [PubMed] [Google Scholar]
- 49.Rowland A., Dias M.M., Wiese M.D., Kichenadasse G., McKinnon R.A., Karapetis C.S., Sorich M.J. Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer. Br. J. Cancer. 2015;112:1888–1894. doi: 10.1038/bjc.2015.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stintzing S., Miller-Phillips L., Modest D.P., Fischer von Weikersthal L., Decker T., Kiani A., Vehling-Kaiser U., Al-Batran S.-E., Heintges T., Kahl C., et al. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: Analysis of the FIRE-3 (AIO KRK-0306) study. Eur. J. Cancer. 2017;79:50–60. doi: 10.1016/j.ejca.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 51.Loupakis F., Cremolini C., Salvatore L., Masi G., Sensi E., Schirripa M., Michelucci A., Pfanner E., Brunetti I., Lupi C., et al. FOLFOXIRI plus bevacizumab as first-line treatment in BRAF mutant metastatic colorectal cancer. Eur. J. Cancer. 2014;50:57–63. doi: 10.1016/j.ejca.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 52.Cremolini C., Antoniotti C., Rossini D., Lonardi S., Loupakis F., Pietrantonio F., Bordonaro R., Latiano T.P., Tamburini E., Santini D., et al. Upfront FOLFOXIRI plus bevacizumab and reintroduction after progression versus mFOLFOX6 plus bevacizumab followed by FOLFIRI plus bevacizumab in the treatment of patients with metastatic colorectal cancer (TRIBE2): A multicentre, open-label, phase 3, randomised, controlled trial. Lancet Oncol. 2020;21:497–507. doi: 10.1016/S1470-2045(19)30862-9. [DOI] [PubMed] [Google Scholar]
- 53.Cremolini C., Antoniotti C., Stein A., Bendell J., Gruenberger T., Rossini D., Masi G., Ongaro E., Hurwitz H., Falcone A., et al. Individual Patient Data Meta-Analysis of FOLFOXIRI Plus Bevacizumab Versus Doublets Plus Bevacizumab as Initial Therapy of Unresectable Metastatic Colorectal Cancer. J. Clin. Oncol. 2020;38:JCO2001225. doi: 10.1200/JCO.20.01225. [DOI] [PubMed] [Google Scholar]
- 54.Geissler M., Klingler T., Knorrenschild J.R., Tannapfel A., Greeve J., Seufferlein T., Kanzler S., Held S., Heinemann V., Reinacher-Schick A., et al. 1st-line mFOLFOXIRI + panitumumab vs FOLFOXIRI treatment of RAS wt mCRC: A randomized phase II VOLFI trial of the AIO (KRK-0109) Ann. Oncol. 2018;29:viii150. doi: 10.1093/annonc/mdy281.001. [DOI] [Google Scholar]
- 55.Chapman P.B., Hauschild A., Robert C., Haanen J.B., Ascierto P., Larkin J., Dummer R., Garbe C., Testori A., Maio M., et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Robert C., Grob J.J., Stroyakovskiy D., Karaszewska B., Hauschild A., Levchenko E., Chiarion Sileni V., Schachter J., Garbe C., Bondarenko I., et al. Five-Year Outcomes with Dabrafenib plus Trametinib in Metastatic Melanoma. N. Engl. J. Med. 2019;381:626–636. doi: 10.1056/NEJMoa1904059. [DOI] [PubMed] [Google Scholar]
- 57.Long G.V., Hauschild A., Santinami M., Atkinson V., Mandalà M., Chiarion-Sileni V., Larkin J., Nyakas M., Dutriaux C., Haydon A., et al. Adjuvant Dabrafenib plus Trametinib in Stage III BRAF-Mutated Melanoma. N. Engl. J. Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 58.Yaeger R., Cercek A., O’Reilly E.M., Reidy D.L., Kemeny N., Wolinsky T., Capanu M., Gollub M.J., Rosen N., Berger M.F., et al. Pilot trial of combined BRAF and EGFR inhibition in BRAF-mutant metastatic colorectal cancer patients. Clin. Cancer Res. 2015;21:1313–1320. doi: 10.1158/1078-0432.CCR-14-2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hong D.S., Morris V.K., El Osta B., Sorokin A.V., Janku F., Fu S., Overman M.J., Piha-Paul S., Subbiah V., Kee B., et al. Phase IB Study of Vemurafenib in Combination with Irinotecan and Cetuximab in Patients with Metastatic Colorectal Cancer with BRAFV600E Mutation. Cancer Discov. 2016;6:1352–1365. doi: 10.1158/2159-8290.CD-16-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.FDA Approves Encorafenib in Combination with Cetuximab for Metastatic Colorectal Cancer with a BRAF V600E Mutation. [(accessed on 23 December 2020)]; Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-encorafenib-combination-cetuximab-metastatic-colorectal-cancer-braf-v600e-mutation.
- 61.Braftovi. [(accessed on 7 June 2020)]; Available online: https://www.ema.europa.eu/en/medicines/human/summaries-opinion/braftovi-0.
- 62.Pietrantonio F. Encorafenib, Binimetinib, and Cetuximab in BRAF V600E-Mutated Colorectal Cancer. N. Engl. J. Med. 2020;382:876–877. doi: 10.1056/NEJMc1915676. [DOI] [PubMed] [Google Scholar]
- 63.Grothey A., Tabernero J., Taieb J., Yaeger R., Yoshino T., Maiello E., Fernandez E.E., Casado A.R., Ross P., André T., et al. LBA-5 ANCHOR CRC: A single-arm, phase 2 study of encorafenib, binimetinib plus cetuximab in previously untreated BRAF V600E-mutant metastatic colorectal cancer. Ann. Oncol. 2020;31:S242–S243. doi: 10.1016/j.annonc.2020.04.080. [DOI] [Google Scholar]
- 64.van Geel R.M.J.M., Tabernero J., Elez E., Bendell J.C., Spreafico A., Schuler M., Yoshino T., Delord J.-P., Yamada Y., Lolkema M.P., et al. A Phase Ib Dose-Escalation Study of Encorafenib and Cetuximab with or without Alpelisib in Metastatic BRAF-Mutant Colorectal Cancer. Cancer Discov. 2017;7:610–619. doi: 10.1158/2159-8290.CD-16-0795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;62:1006–1012. [PMC free article] [PubMed] [Google Scholar]
- 66.Morris E.J., Jha S., Restaino C.R., Dayananth P., Zhu H., Cooper A., Carr D., Deng Y., Jin W., Black S., et al. Discovery of a novel ERK inhibitor with activity in models of acquired resistance to BRAF and MEK inhibitors. Cancer Discov. 2013;3:742–750. doi: 10.1158/2159-8290.CD-13-0070. [DOI] [PubMed] [Google Scholar]
- 67.Nichols R.J., Haderk F., Stahlhut C., Schulze C.J., Hemmati G., Wildes D., Tzitzilonis C., Mordec K., Marquez A., Romero J., et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 2018;20:1064–1073. doi: 10.1038/s41556-018-0169-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shattuck-Brandt R.L., Chen S.-C., Murray E., Johnson C.A., Crandall H., O’Neal J.F., Al-Rohil R.N., Nebhan C.A., Bharti V., Dahlman K.B., et al. Metastatic Melanoma Patient-Derived Xenografts Respond to MDM2 Inhibition as a Single Agent or in Combination with BRAF/MEK Inhibition. Clin. Cancer Res. 2020;26:3803–3818. doi: 10.1158/1078-0432.CCR-19-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hata A.N., Rowley S., Archibald H.L., Gomez-Caraballo M., Siddiqui F.M., Ji F., Jung J., Light M., Lee J.S., Debussche L., et al. Synergistic activity and heterogeneous acquired resistance of combined MDM2 and MEK inhibition in KRAS mutant cancers. Oncogene. 2017;36:6581–6591. doi: 10.1038/onc.2017.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ribas A., Lawrence D., Atkinson V., Agarwal S., Miller W.H., Carlino M.S., Fisher R., Long G.V., Hodi F.S., Tsoi J., et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat. Med. 2019;25:936–940. doi: 10.1038/s41591-019-0476-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Luke J.J., Flaherty K.T., Ribas A., Long G.V. Targeted agents and immunotherapies: Optimizing outcomes in melanoma. Nat. Rev. Clin. Oncol. 2017;14:463–482. doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 72.Rosenbaum M.W., Bledsoe J.R., Morales-Oyarvide V., Huynh T.G., Mino-Kenudson M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016;29:1104–1112. doi: 10.1038/modpathol.2016.95. [DOI] [PubMed] [Google Scholar]
- 73.Corcoran R., Giannakis M., Allen J., Chen J., Pelka K., Chao S., Meyerhardt J., Enzinger A., Enzinger P., McCleary N., et al. SO-26 Clinical efficacy of combined BRAF, MEK, and PD-1 inhibition in BRAFV600E colorectal cancer patients. Ann. Oncol. 2020;31:S226–S227. doi: 10.1016/j.annonc.2020.04.041. [DOI] [Google Scholar]
- 74.Yun J., Mullarky E., Lu C., Bosch K.N., Kavalier A., Rivera K., Roper J., Chio I.I.C., Giannopoulou E.G., Rago C., et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350:1391–1396. doi: 10.1126/science.aaa5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magrì A., Germano G., Lorenzato A., Lamba S., Chilà R., Montone M., Amodio V., Ceruti T., Sassi F., Arena S., et al. High-dose vitamin C enhances cancer immunotherapy. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.aay8707. [DOI] [PubMed] [Google Scholar]
- 76.Nakayama I., Hirota T., Shinozaki E. BRAF Mutation in Colorectal Cancers: From Prognostic Marker to Targetable Mutation. Cancers. 2020;12:3236. doi: 10.3390/cancers12113236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ducreux M., Chamseddine A., Laurent-Puig P., Smolenschi C., Hollebecque A., Dartigues P., Samallin E., Boige V., Malka D., Gelli M. Molecular targeted therapy of BRAF-mutant colorectal cancer. Ther. Adv. Med. Oncol. 2019;11 doi: 10.1177/1758835919856494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kayhanian H., Goode E., Sclafani F., Ang J.E., Gerlinger M., Gonzalez de Castro D., Shepherd S., Peckitt C., Rao S., Watkins D., et al. Treatment and Survival Outcome of BRAF-Mutated Metastatic Colorectal Cancer: A Retrospective Matched Case-Control Study. Clin. Colorectal. Cancer. 2018;17:e69–e76. doi: 10.1016/j.clcc.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 79.Barras D., Missiaglia E., Wirapati P., Sieber O.M., Jorissen R.N., Love C., Molloy P.L., Jones I.T., McLaughlin S., Gibbs P., et al. BRAF V600E Mutant Colorectal Cancer Subtypes Based on Gene Expression. Clin. Cancer Res. 2017;23:104–115. doi: 10.1158/1078-0432.CCR-16-0140. [DOI] [PubMed] [Google Scholar]
- 80.Loupakis F., Intini R., Cremolini C., Orlandi A., Sartore-Bianchi A., Pietrantonio F., Pella N., Spallanzani A., Dell’Aquila E., Scartozzi M., et al. A validated prognostic classifier for V600EBRAF-mutated metastatic colorectal cancer: The “BRAF BeCool” study. Eur. J. Cancer. 2019;118:121–130. doi: 10.1016/j.ejca.2019.06.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.