Abstract
The rapid spread of the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has brought into focus the key role of angiotensin-converting enzyme 2 (ACE2), which serves as a cell surface receptor required for the virus to enter cells. SARS-CoV-2 can decrease cell surface ACE2 directly by internalization of ACE2 bound to the virus and indirectly by increased ADAM17 (a disintegrin and metalloproteinase 17)-mediated shedding of ACE2. ACE2 is widely expressed in the heart, lungs, vasculature, kidney and the gastrointestinal (GI) tract, where it counteracts the deleterious effects of angiotensin II (AngII) by catalyzing the conversion of AngII into the vasodilator peptide angiotensin-(1-7) (Ang-(1-7)). The down-regulation of ACE2 by SARS-CoV-2 can be detrimental to the cardiovascular system and kidneys. Further, decreased ACE2 can cause gut dysbiosis, inflammation and potentially worsen the systemic inflammatory response and coagulopathy associated with SARS-CoV-2. This review aims to elucidate the crucial role of ACE2 both as a regulator of the renin–angiotensin system and a receptor for SARS-CoV-2 as well as the implications for Coronavirus disease 19 and its associated cardiovascular and renal complications.
Keywords: ACE2, COVID-19, Heart Failure, Hypertension, Kidney Diseases, SARS-CoV-2
Introduction
Since its discovery in late 2000, angiotensin-converting enzyme 2 (ACE2) has garnered widespread attention for its multiple physiological roles: a negative regulator of renin–angiotensin system (RAS), a mediator of amino-acid transport and more recently a receptor for severe acute respiratory syndrome coronavirus (SARS-CoV) and the novel SARS-CoV-2, that has caused the Coronavirus disease 2019 (COVID-19). Over the past 20 years, numerous clinical and experimental interventions including recombinant ACE2, delivery of ACE2 gene, analogs of angiotensin-(1-7) (Ang-(1-7)), and Mas receptor agonists, have signified the vital role of ACE2 in ameliorating various cardiovascular diseases (CVDs) [1–10], and validated multiple promising drug targets for the treatment of various disorders associated with alterations in RAS [1,11,12].
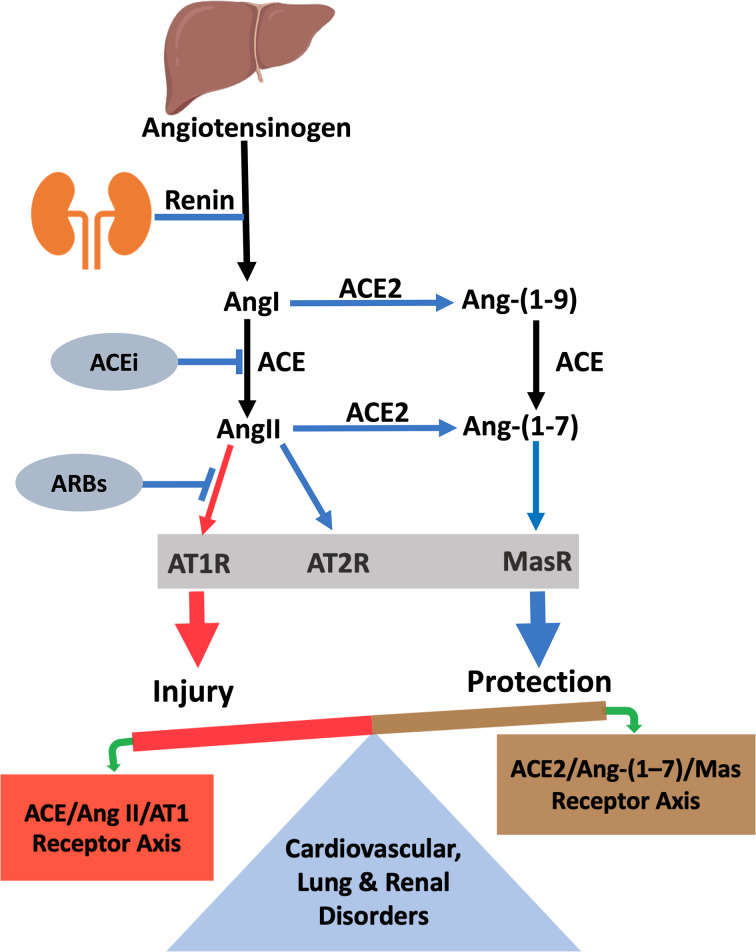
ACE2 is a member of the angiotensin-converting enzyme (ACE) family. It is an ectoenzyme that cleaves the carboxyl-terminal amino acid phenylalanine from angiotensin II (AngII) and leucine from angiotensin I (AngI), hydrolyzing them into vasodilators Ang-(1-7) and angiotensin-(1-9) (Ang-(1-9)), respectively (Figure 1). Thus, ACE2 regulates RAS directly by down-regulating AngII/AT1R signaling and indirectly by activating the counterregulatory Ang-(1-7)/Mas receptor axis. The vasodeleterious AngII/AT1R signaling induces vasoconstriction, profibrotic and proinflammatory effects, whereas Ang-(1-7)/Mas receptor signaling elicits vasodilatory, antiproliferative and antiapoptotic effects that are beneficial in various CVDs [1,5,13,14]. Imbalance between vasodeleterious AngII/AT1R signaling and beneficial Ang (1-7)/Mas receptor signaling has been shown to underlie multiple CVDs as well as pathophysiological states that predispose to CVD such as obesity, diabetes and kidney disease. This view is further supported by the evidence that catalytically active ACE2 treatment ameliorates CVDs in various experimental models of human diseases [1,2,12,15].
Figure 1. The renin–angiotensin system (RAS) with vasodeleterious axis (ACE/Ang II/AT1 receptor) and vasoprotective ACE2/Ang-(1-7)/Mas receptor axis.
ACE2 is a major component of vasoprotective RAS which counterbalances the vasodeleterious effects of AngII activation. Abbreviations: ACEi, ACE inhibitor; ARB, angiotensin receptor blocker.
Recent studies on SARS-CoV-2 infections indicate that individuals with pre-existing conditions such as obesity, diabetes, hypertension (HTN), lung and kidney disease, are at greater risk of severe manifestations of COVID-19. Since these pre-existing conditions are associated with abnormal RAS signaling, it is worth considering the influence of RAS on SARS-CoV-2 infection and COVID-19 disease. In a preliminary study of 12 patients with COVID-19, circulating AngII levels tended to correlate positively with lung injury and viral load [16]. In SARS-CoV infection, ACE2 expression is reduced in the heart and other tissues [1,17]. Many aspects of SARS-CoV and SARS-CoV-2 diseases are similar; this likely occurs in COVID-19, as well. Ongoing global efforts have focused on manipulating the ACE2/Ang-(1-7) axis to mitigate this deadly infection and attenuate lung and cardiovascular system damage from COVID-19. In this review, we summarize recent developments on ACE2 and its role as a receptor for SARS-CoV-2. We also discuss the implications of ACE2 on the pathophysiology of CVDs and potential therapeutic approaches for the treatment of COVID-19.
ACE2 structure and functions
In 2000, a gene encoding a protein with 42% homology in the catalytic domain to ACE, was discovered in a cDNA library prepared from the ventricle of a human heart failure (HF) patient and named ACE homolog or ACE2 [18]. Sequence comparison of ACE2 and ACE indicates that ACE2, like ACE, is an integral transmembrane protein tasked to metabolize circulating peptides with its extracellularly facing catalytic site. ACE has two such catalytic sites but ACE2 only one [2,19]. The ACE2 gene contains 18 exons located on chromosome X (band: Xp22.2) [1,20]. The translated protein comprised an extracellular N-terminal domain with 740 amino acids, a short single transmembrane domain and a short cytosolic C-terminal tail with 44 amino acids [1,20]. ACE2 is widely expressed throughout the body with highest expression in the gastrointestinal (GI) and oral epithelium and substantial levels in lungs, kidney and heart [1,2,18].
ACE2 as a receptor for SARS-CoV-2
Soon after the first outbreak of SARS in 2003, ACE2 was identified as a cell-surface receptor responsible for SARS-CoV entry into cells. ACE2 functions as a primary entry point for most coronaviruses including HCoV-NL63, SARS-CoV and SARS-CoV-2. However, the ACE2-binding domain has higher affinity for SARS-CoV-2 which may partly explain the markedly larger global impact of SARS-CoV-2 than the initial SARS outbreak [21].
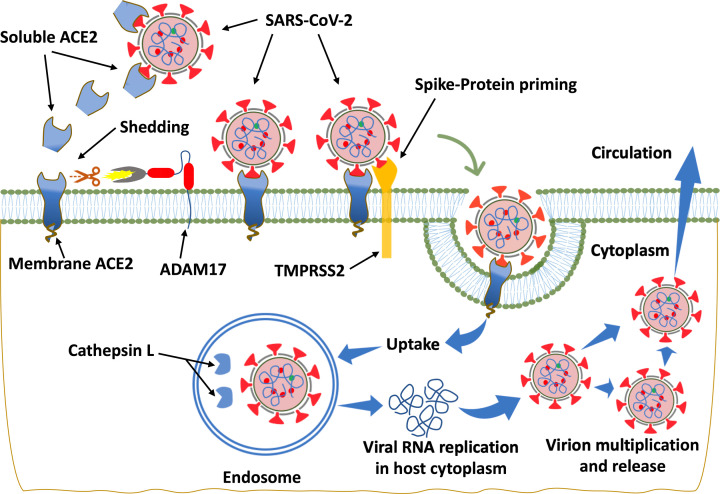
SARS-CoV-2 is an enveloped, RNA virus with virions ranging in size from 55 to 180 nm [1,22]. Coronaviruses invade cells through their spike protein (S-protein), a glycoprotein that binds to the extracellular enzymatic domain of ACE2 located on the surface of the host cells, resulting in subsequent down-regulation of cell surface ACE2 expression [1,2,23]. Following entry into the cell, the virus replicates and induces cytotoxicity that may result in organ failure (Figure 2). The size of S-proteins has been reported to be ∼15.5 nm [1] and it may be found in either open or closed conformations [24]. In a recent study, Hoffmann et al., showed that entry of the SARS-CoV-2 depends on the priming of S-protein by the host cell serine protease TMPRSS2 (transmembrane protease serine 2) and cathepsin L/B [23]. Blocking of this priming by inhibitors of TMPRSS2, camostat mesilate, in combination with cathepsin L/B inhibitor E-64d [23] arrests SARS-CoV-2 infection. However, SARS-CoV-2 also utilizes multiple other host proteases including trypsin, elastase, factor X and furin to prime S-protein and facilitate cell entry following binding to the ACE2 receptor [1,2]. ACE2 and S-protein are both proteolytically modified during their interaction [1,2].
Figure 2. Diagrammatic representation of ACE2-mediated SARS-CoV-2 entry into the cell.
Binding of SARS-CoV-2 with its cellular receptor, ACE2, results in loss of ACE2 and its cardioprotective effects. The entry of SARS-CoV-2 into the cell is facilitated by TMPRSS2 that primes S-protein. Virus may also enter through endocytosis and become activated by cathepsin L, although other proteases such as furin may also activate SARS-CoV-2. Following virus entry into host cells, SARS-CoV-2 undergoes RNA replication within endosomes. ADAM17 (a disintegrin and metalloproteinase 17) activity is up-regulated by the endocytosed S-proteins of SARS-CoV, and though not proven, likely to be by SARS-CoV-2 given the similarity of viral interactions with ACE2. ADAM17 mediates the proteolytic cleavage of ACE2, releasing soluble ACE2 (sACE2) into the circulation.
ADAM17 (a disintegrin and metalloproteinase domain‐containing protein 17), a type I transmembrane protein, is also known to mediate the proteolysis and ectoderm shedding of ACE2 [2] (Figure 2) as well as other proteins including the precursor for TNF-α. Studies have shown that ACE2 shedding mediated by ADAM17 is enhanced upon binding of SARS-CoV S-protein to ACE2 while knocking down ADAM17 with siRNA prevents SARS-CoV entry into the cell [25,26]. Thus, it is reasonable to suggest that enhancement of ADAM17-mediated ACE2 shedding resulted in impaired ACE2-mediated conversion of AngII into Ang-(1-7) by the cells [2], while simultaneously catalyzing the production of TNF-α from its precursor, which damages tissue. However, this remains to be investigated in SARS-CoV-2. Additionally, inhibition of ADAM17 attenuates fibrosis and inflammation indicating its potential as a therapeutic agent for treatment of fibrotic kidney disease [27,28]. Overactivity of the vasodeleterious axis of the RAS predisposes to CVDs including HF, arterial fibrillation, HTN and coronary artery disease [2]. Moreover, loss of cellular ACE2 promotes accumulation of AngII and enhances ADAM17 activity leading to inflammation and other consequences of overactivation of the vasodeleterious RAS axis which may be relevant in SARS-CoV-2 infection.
GI ACE2 plays dual roles: the traditional RAS-dependent role and an RAS-independent role in the transport of amino acids [29]. ACE2 functions as a chaperone for B0AT1 (neutral amino acid transporter), a protein that is highly expressed in the brush border of the small intestine and kidney and tasked with absorbing neutral amino acids [30] such as leucine, tryptophan, glutamine and their metabolites [1]. The transporter activity of B0AT1 in the gut is dramatically augmented by ACE2 co-expression [1,31]. ACE2 in the gut also functions as a key regulator of innate immunity, gut microbial ecology and expression of antimicrobial peptides [29,32,33]. High expression of ACE2 throughout the luminal surface of the GI tract, particularly in enterocytes, suggests that the gut could serve as a secondary site for SARS-CoV-2 infection. Indeed, SARS-CoV particles were found within the surface microvilli of apical enterocytes of the ileum and colon [34]. In support of this concept, patients with COVID-19 show prominent GI manifestations, sometimes prior to the development of classic pulmonary symptoms [35]; Further, SARS-CoV-2 has been detected in the feces of COVID-19 patients, suggesting the possibility of fecal–oral transmission [36]. Importantly, decreased ACE2 activity after binding of SARS-CoV-2 with the intestinal apical membrane ACE2–B0AT1 complex disrupts intestinal RAS, with consequent gut wall inflammation, compromised gut barrier integrity and microbial dysbiosis [2,37]. The impairment of the gut–blood barrier leads to systemic spread of bacteria, microbial metabolites and endotoxins [2,38]. These incidents alone or in combination could potentially affect the host’s response to SARS-CoV-2 infection and contribute to multiorgan system dysfunction and septic shock [39–41].
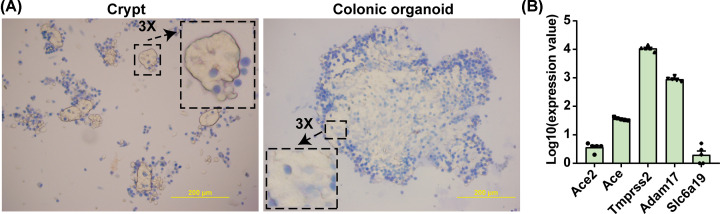
The mechanism by which SARS-COV-2 interacts with ACE2 and its partners such as TMPRSS2 and B0AT1 remains under extensive investigation. We have established an ex vivo 3D organoid system from rat and mouse colon for this purpose. These organoids have characteristics of colonic epithelium and express ACE2, ACE, TMPRSS2 and B0AT1. These are essential components for SARS-CoV-2 infection (Figure 3). These rodent organoids and organoids from human subjects will be key in elucidating interactions among SARS-CoV-2, ACE2 and its partners.
Figure 3. 3D colonic organoid cultures: an ex vivo model to study the interactions of ACE2, its partners and SARS-CoV-2.
(A) Phase microscopic images documenting 7-day growth of organoids from isolated colonic crypts of WKY rats stained with Trypan Blue; dead cells are blue. Scale bar: 200 µM. (B) mRNA levels (by RNA-seq) of ACE2 and its partners in colonic organoids from WKY rats (n=5 per group). The detailed method for 3D colonic organoid cultures was published elsewhere [62]. Abbreviation: Slc6a19, solute carrier family 6 member 19, also known as B0AT1.
ACE2 in cardiovascular system, implications in COVID-19
HTN
HTN is the one of the most prevalent risk factors for CVDs. The most recent American Heart Association report indicates that ∼49% of American adults have high blood pressure (BP) [42] and ∼20% of these patients have drug-resistant HTN (R-HTN) [43] because they do not have adequate BP control even when treated with three (or more) antihypertensive medications. Overactivity of the vasodeleterious axis (ACE/AngII/AT1R) and a decrease in the vasoprotective axis (ACE2/Ang-(1-7)/Mas receptor) of the RAS is a major contributor to HTN. There is ample evidence, particularly from animal studies, in support of this concept. This includes: (i) BP is elevated in ACE2 null mice on a C57BL/6 background [10]; AngII-induced HTN is more severe in these mice [10]. (ii) Lentiviral-mediated overexpression of ACE2 ameliorates high BP and HTN-associated pathophysiology in the spontaneously hypertensive rat (SHR), a rodent model of essential HTN [44,45]. Similarly, vascular ACE2 overexpression increased Ang-(1-7), restored endothelial function and decreased BP [46]. (iii) Renal ACE2 is inversely related to BP and is decreased in the SHR and the stroke-prone SHR [47]. On the other hand, a few studies have failed to find beneficial effects of ACE2 in BP control. For example, Crackower et al. found no change in BP in 3-month-old ACE2 null mice and unanticipated lower BP in the same mice at 6 months of age [48]. These ACE2 null mice do exhibit abnormal cardiac contractility that might have confounded effects on BP. Mas receptor null mice also had normal BP [49]. Administration of diminazene aceturate (DIZE), a putative ACE2 activator, caused an acute lowering of BP in the SHR [50], but it had no effect on BP in rats with kidney disease [51]. Importantly, in wild-type mice, rhACE2 treatment did not affect the baseline plasma AngII, Ang-(1-7) or BP suggesting that substrate availability is a key limiting factor in ACE2’s enzymatic activity [52]. Finally, in a Phase I safety trial, rhACE2 administration to a small cohort of healthy human subjects had no effect on BP and heart rate [53]. These observations, taken together, suggest ambivalence about the effects of ACE2 on high BP in HTN and further studies are warranted to resolve this. It would also be prudent to consider alternate mechanisms for the role of ACE2/Ang-(1-7) in BP control. An attractive option is that this vasoprotective axis exerts direct local beneficial effects on cardiac, vascular, renal and neural signaling mechanisms that indirectly affect BP. The brain RAS and neurogenic HTN may be an example of such organ-specific effects of ACE2/Ang-(1-7).
The brain RAS is a major player in the regulation of autonomic function and has a role in control of BP. Components of the RAS, including ACE2, are widely expressed in many brain cells including neurons [54], microglia and astrocytes [55,56]. ACE2 is generally decreased in brains of animals with HTN and HF [2,48,52,57]. The decrease is suggested to be due to shedding of ACE2 into the CSF by ADAM17 [57]. This is consistent with an increased ACE2 in the CSF of hypertensive patients and a significant correlation of CSF ACE2 with systolic BP [58]. ACE2 gene deletion impairs autonomic and baroreceptor functions and disrupts the central regulation of BP [59,60]. Conversely, ACE2 overexpression is protective against neurogenic HTN [45,61]. Its beneficial effects involve increasing neuronal nitric oxide (NO), reducing proinflammatory cytokines and reactive oxygen species (ROS), and restoring cerebrovascular endothelial function [62]. Additional evidence of ACE2’s involvement is derived from elegant studies with ADAM17 deletion [15]. Selective deletion of ACE2 and ADAM17 from neurons relevant to autonomic function decreases inhibitory inputs to pre-sympathetic neurons controlling BP [15]. Consistent with these observations, intracerebroventricular infusion of Ang-(1-7) into the nucleus tractus solitarius (NTS) [63], caudal ventrolateral medulla (CVLM) [64]), paraventricular nucleus (PVN) of hypothalamus [65] or anterior hypothalamus results in decreases in BP. This effect on BP appears to be specific to certain brain regions since administration of Ang-(1-7) into the rostral ventrolateral medulla (RVLM) increased BP [66,67]. Together, these studies strengthen the concept of a protective role for brain ACE2 in neurogenic HTN.
More than half the patients who are hospitalized with COVID-19 are hypertensive [68]. However, whether this high prevalence is due to more frequent or severe SARS-CoV-2 infection in hypertensive patients or confounding by age, obesity, kidney disease or other comorbidities remains unclear. Antihypertensive treatment with ACE inhibitors (ACEi) or Ang II receptor blockers (ARBs) is associated with increased ACE2, which could potentially increase the chances of SARS-CoV-2 infection or the severity of COVID-19. Lisinopril and losartan treatment increased ACE2 gene expression in the left ventricle of the normotensive Lewis rats [39] and ACE2 protein and activity are increased in the heart of rats treated with enalapril [40]. Similarly, ACE2 protein and mRNA increased following treatment with the ARB, Telmisartan [41]. However, studies from human subjects treated with ACEi or ARB demonstrate contradictory results on whether these medications increase ACE2, with some showing increased ACE2, while others showed no effect [2,44,45,69]. Studies in humans relied mostly on plasma levels of ACE2 to assess ACE2 activity and could account for some of these discrepancies. Retrospective studies examining the use of ACEi/ARB in hypertensive patients have either found improvement in mortality risk [70] or no effect on the incidence or severity of COVID-19 [71–73]. The preliminary results from the randomized controlled BRACE-CORONA trial suggests that continuing ACEi/ARB as opposed to withholding ACEi/ARB therapy does not change clinical outcomes in patients with COVID-19 (NCT04364893). Multiple randomized controlled trials are underway in the United States and elsewhere (Table 1) which should clarify this question.
Table 1. Overview of current clinical trials evaluating various therapeutic agents on COVID-19.
| Clinical trial Identifier | Title of the clinical trial | Setting, severity | Active arm(s) | Status |
|---|---|---|---|---|
| NCT04364893 | Angiotensin Receptor Blockers and Angiotensin-converting Enzyme Inhibitors and Adverse Outcomes in Patients With COVID-19 (BRACE-CORONA) | Hospitalized, COVID-19 (∼700 subjects) | ACEi and ARBs | Recruiting |
| NCT04335136 | Recombinant Human Angiotensin-converting Enzyme 2 (rhACE2) as a Treatment for Patients With COVID-19 (APN01-COVID-19) | Hospitalized, COVID-19 (200 subjects) | RhACE2, APN01 | Recruiting |
| NCT04321096 | The Impact of Camostat Mesilate on COVID-19 Infection (CamoCO-19) | Hospitalized, COVID-19 (580 subjects) | Camostat Mesilate | Recruiting |
| NCT04338009 | Elimination or Prolongation of ACE Inhibitors and ARB in Coronavirus Disease 2019 (REPLACECOVID) | Hospitalized, COVID-19 (152 subjects) | ARB/ACEi | Recruiting |
| NCT04312009 | Losartan for Patients With COVID-19 Requiring Hospitalization | Hospitalized, COVID-19 (200 subjects) | Losartan | Recruiting |
| NCT04311177 | Losartan for Patients With COVID-19 Not Requiring Hospitalization | Patients with COVID-19 (580 subjects) | Losartan | Active, not Recruiting |
| NCT04318418 | ACE Inhibitors, Angiotension II Type-I Receptor Blockers and Severity of COVID-19 (CODIV-ACE) | Hospitalized, COVID-19 (3400 subjects) | ACEi and ARBs | Completed |
| NCT04315298 | Evaluation of the Efficacy and Safety of Sarilumab in Hospitalized Patients With COVID-19 | Hospitalized, COVID-19 (1912 subjects) | Sarilumab | Completed |
| NCT04320615 | A Study to Evaluate the Safety and Efficacy of Tocilizumab in Patients With Severe COVID-19 Pneumonia (COVACTA) | Hospitalized, severe COVID-19 Pneumonia (450 subjects) | Tocilizumab (TCZ) | Completed |
| NCT04330638 | Treatment of COVID-19 Patients With Anti-interleukin Drugs (COV-AID) | Hospitalized, CIVID-19 with systemic cytokine release syndrome (342 subjects) | Anakinra Siltuximab Tocilizumab | Recruiting |
| NCT04322188 | An Observational study of the Use of Siltuximab (SYLVANT) in Patients Diagnosed With COVID-19 Infection Who Have Developed Serious Respiratory Complications (SISCO) | Hospitalized, ARDS, secondary to SARS-COV-2 Infection (220 subjects) | Siltuximab (SYLVANT) | Completed |
HF
HF is a leading cause of deaths and a major public health problem. The prevalence of HF is 6.5 million in the U.S.A., over 25 million worldwide, and increasing. It is a complex, multifactorial syndrome consisting of many overlapping phenotypes. High BP, coronary artery diseases, cardiomyopathy and diabetes are major risk factors involved in the pathophysiology of HF. ACE2 is widely expressed in cardiac fibroblasts, cardiomyocytes and coronary endothelial cells where it negates the deleterious effects of AngII and activates Ang-(1-7)/Mas signaling [74]. Ang-(1-7) produced by ACE2 promotes endothelial NO production via Akt-dependent mechanisms [75], and ameliorates cardiac hypertrophy and fibrosis in a mouse model of insulin resistance [76]. Evidence indicates that many CVDs including HF are associated with relative ACE2 deficiency, and this loss of ACE2 reduces the homeostatic defensive mechanisms [19,77]. ACE2 overexpression prevents or even reverses the HF phenotype [76,78–80], whereas ACE2 deficiency may promote the development of HF [81]. Interestingly, HF patients show increased expression of ACE2 early in the disease, perhaps in an attempt to counter the deleterious effects of elevated AngII levels, but with progressing cardiac disease there is relative deficiency of ACE2 and a concomitant increase in AngII [5,81]. Further, the activity of circulating ACE2 is markedly higher in patients with decompensated HF compared with ambulatory HF patients, associated with poor prognosis [12,82].
Evidence indicates that COVID-19 is strongly associated with HF. In an early study from China involving 799 COVID-19 patients, HF was one of the major complications observed with a reported incidence of 24% in all patients and 49% in patients who died [83]. Of note, the median age of deceased patients (68 years) was significantly older than those who recovered (51 years) [83]. In a separate study from China involving 191 COVID-19 patients, HF was reported in 23% of all patients and 52% of patients who died [84]. Studies using cardiac magnetic resonance imaging, even in younger patients without comorbidities, have shown significant cardiac involvement in patients who had presumably recovered from COVID-19 [85]. Lending credence to direct myocardial involvement in COVID-19, the SARS-CoV genome was detected in the heart tissues in postmortem autopsy samples from SARS patients [17]. Tissue ACE2 is down-regulated while there is a concomitant increase in AngII in patients with COVID-19. The AngII levels correlate linearly with SARS-CoV-2 viral load [16]. Similarly, myocardial ACE2 expression is markedly reduced in postmortem autopsy samples from SARS-CoV-infected patients and was associated with concomitant increases in myocardial inflammation and fibrosis [17]. This decrease in ACE2 is thought to be due to increased endocytosis and proteolytic processing of membrane-bound ACE2 [2]. Importantly, cardiac pericytes with high expression of ACE2 have been shown to be a primary target in SARS-CoV-2 infection [86]. Injured pericytes due to virus infection may impair endothelial function and induce microvascular dysfunction. These adverse effects on endothelial and myocardial function might be compounded by circulating proinflammatory cytokines.
Since ACE2 is required for SARS-CoV-2 entry into cells, it has been suggested that ACE2 up-regulation may increase susceptibility to SARS-CoV-2 infection. On the other hand, increased ACE2 expression may also protect against AngII-induced vasoconstriction and inflammation. However, an association between ACE2 expression and susceptibility or severity of SARS-CoV-2 infection, is not evident [74]. In animal models of HF, use of ACEi/ARB increases ACE2 expression. For example, Losartan and Olmesartan increase ACE2 mRNA expression in the heart of rats after MI [87]. Similarly, Enalapril can normalize ACE2 expression in the left ventricle of rats with HF [88]. However, protective or ameliorating effects of ACEi/ARB use on myocardial function in patients with COVID-19 remain to be proven. In the absence of compelling clinical data, most professional organizations recommend continuing ACEi or ARB therapy in HF patients with or at risk of SARS-CoV-2 infection.
Vasculature/endothelium dysfunction/atherosclerosis
Recent evidence from clinical trials have confirmed the important role of inflammation in atherosclerosis. In healthy vessels the endothelium is important in modulating vascular tone by synthesizing and releasing a variety of endothelium-derived relaxing factors, vasodilator prostaglandins and most importantly NO [89]. Endothelial dysfunction precedes atherosclerosis and is thought to be an important pathogenic mechanism that contributes to the development of atherosclerosis and its complications [90,91]. Thus, endothelial dysfunction is often considered a marker for inherent atherosclerotic risk in an individual [92].
ACE2 expression is crucial for the healthy functioning of endothelial cells. Animal studies demonstrate that ACE2 deletion impairs endothelium-dependent vasodilatation [93] by decreasing bioavailability of NO [93]. ACE2 deficiency leads to a pro-oxidative state with increased reactive oxygen species (ROS) and concurrent decrease in NO bioavailability through formation of peroxynitrile (ONOO–) free radicals [93–95]. ACE2 overexpression, on the other hand, promotes normal endothelial cell migration and tube formation favoring angiogenesis whereas silencing the ACE2 gene reverses these effects [93]. Importantly, ACE2 attenuates the formation of atherosclerotic plaques by regulating monocyte–endothelial cell interactions by decreasing endothelial expression of adhesion molecules (MCP-1, VCAM-1 and E-selectin) through production of Ang-(1-7), which curbs macrophage infiltration into the vessel wall [96]. Finally, ACE2 inhibits vascular smooth muscle cell proliferation and migration [97–99]. Pro-atherosclerotic effects of ACE2 deficiency are amplified in the presence of HTN or diabetes or both [100].
COVID-19 is emerging as a thrombotic and vascular disease affecting endothelial cells and is particularly noticeable in patients with cardiometabolic comorbidities such as HTN, diabetes and kidney disease [101]. Recent evidence indicates that SARS-CoV-2 directly infects blood vessels and initiates endothelial signaling associated with proinflammatory and pro-apoptotic mediators and cytokine dysfunction precipitating the thrombotic cascade [102]. Additionally, viral consumption of membrane ACE2 can also disrupt AngII metabolism leading to increased AngII and concomitant Ang-(1-7) deficiency, further augmenting inflammation, endothelium activation and recruitment of leukocytes and platelets [103]. All these events alone or in combination could induce a localized microvascular inflammation, prothrombotic conditions and tissue damage [103]. Pulmonary embolism and deep vein thrombosis have been reported in one third of hospitalized COVID-19 patients [104] often associated with elevated D-dimer and fibrinogen levels [105].
Very recently in New York, a pediatric multi-inflammatory syndrome mimicking Kawasaki disease was described in children with COVID-19 [106]. Kawasaki disease is a rare acute pediatric vasculitis with coronary artery aneurysms as its main complication. These children have milder vasculitis than adults and better prognosis as well as survival. In Bergamo, Italy, several cases of pediatric vasculitis were also reported in the midst of the pandemic [107]. Vasculitis is suggested to be a direct consequence of SARS-CoV-2 infection [108]. The incidence of multiple vascular diseases point toward vascular complications as a major contributing factor to the pathogenesis of COVID-19. This emphasizes the notion that endothelial injury and inflammation occur due to either direct viral infection, through disordered cytokine release, or both, and might contribute to the systemic microcirculatory impairment in COVID -19 [101].
Renal diseases
Kidney works synchronously with the cardiovascular system to maintain BP and fluid balance [109,110]. These functions of kidney are vital for maintenance of normal cardiac ejection fraction [110,111] and tissue oxygen delivery [112]. In addition, kidney has important roles in timely clearance of uremic toxins [113], maintaining electrolyte and acid–base balance [114], erythropoiesis [115] and bone-mineral metabolism [116]. Progressive decline in renal function increases the risk of CVDs [117]. It is estimated that 37 million (one in seven) adults have chronic kidney disease (CKD), costing Medicare more than 84 billion dollars in the U.S.A. (CDC 2019).
Renal tissue is a major source of Ang-(1-7), a beneficial peptide derived by ACE2-mediated hydrolysis of AngII suggesting the presence of functional ACE2 in the kidney [PMIDs:19578709,14,146]. ACE2 is profoundly expressed in the brush border of proximal tubules and moderately in podocytes and parietal epithelial cells, whereas ACE2 expression is weak in glomerular endothelial cells and mesangial cells [118–120]. Growing evidence suggests that imbalance in the counterbalancing arms of the RAS is a fundamental driver of renal and systemic vascular dysfunction in various pathological states. Renal ACE2 activity and Ang-(1-7) were decreased after 4 h of reperfusion after ischemic injury and were negatively correlated with AngII [121]. Interestingly, the renal expression of Mas receptor was greatly increased after 4 h of reperfusion suggesting a critical role for the RAS components in acute kidney injury (AKI) [121]. Changes in kidney RAS expression has been validated in other animal models of kidney disease including subtotal nephrectomy and sepsis [121–123]. Further, patients with end stage renal disease on hemodialysis had lower plasma ACE2 activity compared with their pre-dialysis counterparts with CKD or renal transplant recipients [124]. In animal models of diabetes, studies have shown decreased glomerular ACE2 whereas intraglomerular ACE expression is increased [125,126]. Deficiency of ACE2 by either pharmacological inhibition or genetic knocking out of ACE2 exacerbates albuminuria, mesangial matrix expansion, glomerular basement membrane thickening and glomerulosclerosis, all of which are characteristic features of diabetic nephropathy [127,128]. Conversely, kidney-specific overexpression of ACE2 in diabetic animals (Akita mice) ameliorates glomerular injury and sclerosis, improves podocyte function and development of overt albuminuria [129,130]. The ratio of ACE to ACE2 positively correlates with mean BP, fasting blood glucose, proteinuria, as well as serum creatinine and inversely with glomerular filtration rate (GFR) [129]. Interestingly, endothelial ACE2 was found to be increased in the glomerular and interstitial capillaries in kidney diseases indicating ACE2 might be a marker of endothelial injury [119]. ACE2 activity is increased early in CKD but as renal function progressively deteriorates relative ACE2 deficiency follows [131]. Is the initial increase in ACE2 seen in earlier stages of CKD a compensatory mechanism to counterbalance the elevated circulating AngII? Understanding the temporal dynamics of these interactions might help identify the subset of patients who are vulnerable to develop progressive decline in renal functions, culminating in end stage renal disease. It could also help to develop novel strategies and the appropriate time of intervention to prevent AKI to CKD progression, which is currently a huge challenge. Therefore, the underlying change in ACE2, regulation of enzymes involved in ACE2 metabolism and its receptors as well as the consequences of these changes is still an area of active research.
Kidney is frequently involved in SARS-CoV infection. During the 2003 SARS outbreak in Hong Kong, 36 out of 536 patients showed elevated plasma creatinine levels during their clinical course [132]. Those who had renal injury tended to be older with high BP. In another study ∼6% of SARS-CoV patients developed AKI, and kidney injury was identified as a fatal complication of SARS-CoV as almost 92% of SARS-infected patients with AKI died [132]. COVID-19 shows a similar high incidence of AKI and poor prognosis. For example, more than 40% of patients have proteinuria at hospital admission, 20–40% of critically ill patients have AKI in Europe and U.S.A., which serves as a negative prognostic marker for the recovery [133]. An early report with 3235 patients indicated that 43% of all hospitalized patients and 68% of those admitted to the intensive care unit developed AKI [134]. In a separate study of critically ill patients with COVID-19 in Wuhan, China, 29% of those who were admitted developed AKI [135]. Further, out of 113 patients who died from COVID-19, 28 of them had AKI during their hospitalization [83]. These data strongly suggest a significantly higher mortality rate in critically ill COVID-19 patients with AKI.
COVID-19-associated pathological changes in the kidney include characteristics of acute tubular necrosis such as diffuse tubular injury with loss of brush border integrity and necrotic debris composed of necrotic epithelium in tubular lumina. In some cases, presence of multiple inflammatory infiltrates is observed in the tubules [118]. In addition, capillary endothelial injury, vacuolar degeneration and erythrocyte aggregates occluding the capillary lumina are frequently seen [118]. The cause of AKI in SARS-CoV-2 infection appears to be multifactorial. Sepsis (or septic shock) is considered the major contributing factor of kidney injury in these patients. Increasing viral infection in alveolar cells (pneumonia) could lead to massive recruitment of immune cells to the kidney which produce high levels of cytokines escalating the systemic inflammatory response called cytokine storm [136]. This could also result in endothelial dysfunction, activation of coagulation cascade and microthrombi eventually resulting in multiple organ failure. This process is not specific to COVID-19 as cytokine-mediated inflammatory AKI has been observed in several clinical conditions [137]. SARS-CoV-2 viral load was quantified in autopsy tissue samples collected from 22 patients who died from COVID-19. Seventeen (77%) of these patients showed SARS-CoV-2 in the kidney [138]. Presence of more than two coexisting conditions is associated with SARS‐CoV‐2 tropism for the kidneys, even in patients without a history of CKD [134,138,139]. Although these findings have been disputed, electron microscopic ultrastructural studies showed viral particles in the renal tubular epithelium morphologically identical with SARS‐CoV‐2, and presence of coronavirus-like particles with distinctive spikes in the tubular epithelium and podocytes of infected individuals indicating a direct infection (or circulating viremia) of the kidney in setting of AKI in COVID‐19 [140]. Examination of single cell databases from 15 human subjects showed that ACE2 and TMPRSS2 are highly co-expressed in podocytes and proximal convoluted tubules of the kidney samples [141]. Since viral entry depends on the activity of protease TMPRSS2 with ACE2, these observations would suggest that kidneys with high co-expression of ACE2 and TMPRSS2 could amplify SARS‐CoV‐2 infection. Presence of high numbers of SARS‐CoV‐2 copies per cell in the kidney supports this view [138]. Finally, the loss of ACE2 via ADAM17-mediated proteolytic cleavage, which is activated in COVID-19 [26,142], might promote further injury to the kidney and cardiovascular system, particularly in patients with diabetes [2,142,143]. This loss of ACE2 leads to accumulation of renal AngII resulting in activation of vasodeleterious axis of RAS. Systemic and glomerular capillary HTN ensue from the increased intrarenal AngII, eventually causing endothelial injury and kidney dysfunction. Further, in a healthy kidney, ACE2 degrades AngII to Ang-(1-7) activating the vasoprotective axis. Ang-(1-7) dilates pre-constricted renal afferent arterioles and increases renal blood flow [144]. Ang-(1-7) also increases the production of renal atrial natriuretic peptide which reduces oxidative stress and fibrotic, proliferative and inflammatory effects of AngII in the kidney [145,146]. Thus Ang-(1-7) is contemplated to be an important physiological regulator opposing the harmful effects of disproportionate AngII production [146].
ACE2 targeted therapeutic strategies against SARS-CoV-2 infection
While research on developing a vaccine/treatment is being pursued aggressively, currently there are a few ACE2 based therapeutic approaches that could be effective during SARS-COV-2 infection that could be available sooner. For instance, up-regulating the function and expression of ACE2 by increasing gene transcription, translation or its catalytic activity has been shown to have beneficial outcomes in various cardiopulmonary disorders characterized by dysregulated RAS. In pulmonary arterial hypertension (PAH), rhACE2 ameliorates vascular damage, lung injury and fibrosis, arterial remodeling and improves right ventricular performance [1,2]. In an experimental model of HTN, rhACE2 treatment produced decreased left ventricular hypertrophy, myocardial fibrosis and improved cardiac function [5]. Recently, Penninger et al. showed that clinical-grade human recombinant soluble (rhACE2) can reduce viral load and block entry of SARS-CoV-2 infection into Vero E6 cells [147]. Further, they showed that SARS-CoV-2 could directly infect human organoids such as kidney and this infection can be inhibited by rhACE2 in earlier stages of SARS-CoV-2 infection [147]. Excessive soluble ACE2 (sACE2) binding with SARS-CoV-2 decreases infectivity up to 5000-fold in blood vessels and kidney organoids [147]. In two Phase II clinical trials, rhACE2 administration was well tolerated in patients with PAH and acute respiratory distress syndrome (ARDS) with no apparent side effects [148,149]. More definitive clinical trials are underway to determine the effectiveness of rhACE2 to block viral entry and decrease replication by regulating systemic RAS (NCT04335136). However, there might be a few inherent barriers that challenge the therapeutic use of ACE2 including its restricted penetrance, lack of stability in vivo (short half-life ∼3.5 h) and high cost associated with the manufacturing of recombinant protein. However, an effort has been made to change the stability of ACE2 via modulating Ser680 phosphorylation which could have significant translational relevance of ACE2 in treating lung disorders [150]. Oral delivery is the preferred route of drug administration. Oral administration of rhACE2 protein has limitations because it is degraded by the acidic environment of the GI tract. To circumvent this problem, our group has developed an oral delivery system which bioencapsulates ACE2 utilizing transplastomic technology. Oral delivery of bioencapsulated ACE2 ameliorated monocrotaline-induced PAH and improved right heart functions in rodents [11]. This was accompanied by increased pulmonary ACE2 and angiotensin type 2 receptors (AT2Rs) and decreased proinflammatory cytokines [11]. Our group also developed an oral delivery system for Ang-(1-7). Moreover, the oral delivery of ACE2 therapeutics is more cost-effective, convenient and likely to be accepted by patients.
Decreased plasma membrane ACE2 due to internalization of SARS-CoV-2 shifts the RAS toward the vasodeleterious axis potentiating the inflammatory, hypertrophic and fibrotic actions of AngII and AT1R that are often observed in SARS-CoV-2 patients. Therefore, increasing membrane expression of ACE2 could be beneficial, as it might reduce the systemic inflammation and shift the RAS balance toward the beneficial side. There could be many potential mechanisms for the beneficial effects of increased ACE2. Increased sACE2 can bind with SARS-CoV-2 and may remove it from the circulation that may be helpful in reducing the infectious load [1]. Finally, increased ACE2 may foster self-dimerization in the cell membrane thereby diminishing affinity for virus, internalization and spread. Proteases such as ADAM17 and TMPRSS2 have been implicated in regulation of plasma membrane ACE2 and therefore may serve as potential therapeutic targets. ADAM17 inhibitors such as paricalcitol and synthetic vitamin D analogs, might be beneficial in COVID-19 and need to be tested for clinical efficacy. A clinical trial is underway to determine the efficacy in SARS-CoV-2 positive patients (NCT04321096). On the other hand, ADAM17 increases sACE2 by cleaving the extracellular domain of ACE2. The resultant sACE2 has enzymatic activity and the ability to bind SARS-CoV [26]. Thus, the final effect of ADAM17 on SARS-CoV2 infection and complications remains to be determined.
Several small molecule activators have been synthesized to enhance the activity of endogenous ACE2 by utilizing structure-based drug design. DIZE, the most potent activator, increases ACE2 activity in plasma, heart, lung, kidney and retina [151–154] and has been shown to have protective effects in several animal models of heart and lung disease [151,153,154]. DIZE was effective in attenuating ischemia-induced pathology in a murine model of myocardial infarction [151], as well as pulmonary HTN and fibrosis [154]. Additionally, Ang-(1-7) receptor agonist AVE0991 has been shown to have beneficial effects in lung and CVD, by stimulating ACE2/Ang-(1-7)/Mas receptor signaling [155]. Therefore, the ACE2/Ang-(1-7) axis together with its widely expressed Mas receptor, may play a dynamic role in maintaining ACE2 or Ang-(1-7) levels and could be a target to decrease severity or prevent complications in patients infected with SARS-CoV-2.
Non-ACE2-targeted RAS inhibition-based therapeutic approaches against SARS-CoV-2 infection
Present therapies aim to inhibit RAS in numerous ways. ARBs and ACEi, in addition to their primary pharmacological function to block AT1 receptor activity or inhibit ACE respectively, up-regulate ACE2 expression and activity in experimental models. The direct implications of increased ACE2 in COVID-19 patients with HTN is not clear yet. Theoretically, an increase in ACE2 expression by ARBs and ACEi could increase the risk for or severity of the SARS-CoV-2 infection. This has led to some controversy around the world whether these inhibitors should be discontinued. Some recent studies have brought some clarity to this issue. For instance, a retrospective study in New York city of 12594 patients, 46.8% of whom were COVID-19 positive, found no association between the use of ARBs or ACEi and COVID-19 infection, severity or mortality [72]. A Chinese study of 362 COVID-19 patients found no difference in severity or mortality compared with the use of ACEi/ARB [156]. Finally, an Italian population-based cohort of 6272 case patients found no evidence ARBs or ACEi increased the risk of COVID-19 [71]. However, a retrospective multicenter study of 1128 patients showed significantly lower mortality with the use of ACEi/ARB [157]. Although increased ACE2 could facilitate cellular entry of the virus, ACE2 could increase the conversion of vasodeleterious AngII into Ang-(1-7), which has known vasodilatory and anti-inflammatory effects. The Randomized Elimination or Prolongation of ARB and ACEi in COVID-19 (REPLACE) trial is a prospective randomized controlled trial underway to examine whether discontinuation of these drugs would ameliorate the severity of the disease (NCT04338009). Several other clinical trials (ClinicalTrial.gov numbers NCT04312009, NCT04311177, and NCT04318418) (Table 1) are underway addressing viral-mediated RAS imbalance and the risks and benefits associated with the use of ARBs and ACEi in COVID-19 patients.
Sepsis and inflammation are characteristic features of SARS-CoV-2 infection. In addition, neurological abnormalities were evident in COVID-19 patients suggesting neuroinflammation could be a key factor in the pathogenesis of COVID-19 [1,158,159]. However, the mechanisms that mediate neuroinflammation and any long-term consequences and management of these neurological symptoms remain to be clarified. Studies have examined the effect of non-steroidal anti-inflammatory drugs in COVID-19, but a clear benefit on outcomes was not discernable. It might be worthwhile to examine if anti-inflammatory drugs like minocycline, an anti-inflammatory antibiotic that crosses the blood–brain barrier and ameliorates neuroinflammation-driven HTN, pulmonary HTN and lung inflammation in animal models [160–162] could be beneficial in COVID-19.
Recent investigations found increased IL-6 in patients with confirmed COVID-19 pneumonia that positively correlated with severity of disease [163]. Elevated levels of some other inflammatory cytokines (IFN-y, TNF-α etc.) are also found in COVID-19 patients [40] indicative of cytokine storm/sepsis. This overproduction of cytokines might cause more damage to the host cell than the SARS-CoV-2. Interestingly, in a pilot study, rhACE2 infusion decreased IL-6 in patients with ARDS [149]. Therefore, considering the critical role of cytokines in severe COVID-19, rhACE2 targeted to decrease IL-6 could be considered as a potential therapeutic approach. Finally, FDA-approved IL-6 inhibitors and anti-IL-6 monoclonal antibodies are under clinical trial currently for their use in COVID-19 patients (Sarilumab: NCT04315298; Tocilizumab: NCT04320615, Siltuximab: NCT04330638, NCT04322188) (Table 1).
Concluding remarks
SARS-CoV-2 requires membrane-bound ACE2 for entry into cells. ACE2 can be down-regulated directly by binding and internalization of SARS-CoV-2 or indirectly via proteolytic processing, autophagy and increased shedding. Decreased ACE2 or increased circulating sACE2 can bind SARS-CoV-2 and potentially decrease SARS-CoV-2 infection or disease severity. On the other hand, decreased ACE2 can cause microbial dysbiosis and inflammation, that can worsen the systemic response to SARS-CoV-2. Studies are ongoing to clarify the effects of modulating ACE2 to mitigate COVID-19. Apart from the effects on SARS-CoV-2 infection, decreased ACE2 can have profound effects on the cardiovascular and pulmonary systems. Multiple lines of incontrovertible evidence have proven the pivotal role of ACE2 in down-regulating detrimental AngII signaling and up-regulating the beneficial Ang-(1-7)/Mas receptor signaling. Thus, altered ACE2 signaling can have detrimental effects on BP, cardiac function, vasculature, kidney and the brain. The long-term consequences of these effects are yet to be determined and could inform us on the relevance of ACE2 signaling to human health and disease.
Abbreviations
- ACE2
angiotensin-converting enzyme 2
- ACEi
ACE inhibitor
- ADAM17
a disintegrin and metalloproteinase 17
- AKI
acute kidney injury
- AngII
angiotensin II
- Ang-(1-7)
angiotensin-(1-7)
- Ang-(1-9)
angiotensin-(1-9)
- ARB
angiotensin receptor blocker
- ARDS
acute respiratory distress syndrome
- BP
blood pressure
- CKD
chronic kidney disease
- COVID-19
coronavirus disease 2019
- CVD
cardiovascular disease
- DIZE
diminazene aceturate
- GI
gastrointestinal
- HF
heart failure
- HTN
hypertension
- NO
nitric oxide
- PAH
pulmonary arterial hypertension
- sACE2
soluble ACE2
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SHR
spontaneously hypertensive rat
- S-protein
spike protein
- TMPRSS2
transmembrane protease serine 2
Contributor Information
Mohan K. Raizada, Email: mraizada@ufl.edu.
Rajesh Mohandas, Email: rajesh.mohandas@medicine.ufl.edu.
Competing Interests
The authors declare that there are no competing interests associated with the manuscript.
Funding
This work was supported by National Institutes of Health [grant numbers HL102033 (to Mohan K. Raizada), HL130945 (to Rajesh Mohandas)].
References
- 1.Sharma R.K., Stevens B.R., Obukhov A.G., Grant M.B., Oudit G.Y., Li Q. et al. (2020) ACE2 (Angiotensin-Converting Enzyme 2) in cardiopulmonary diseases: ramifications for the control of SARS-CoV-2. Hypertension 76, 651–661 10.1161/HYPERTENSIONAHA.120.15595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J. et al. (2020) Angiotensin converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system. Circ. Res. 126, 1456–1474 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamazato Y., Ferreira A.J., Hong K.H., Sriramula S., Francis J., Yamazato M. et al. (2009) Prevention of pulmonary hypertension by Angiotensin-converting enzyme 2 gene transfer. Hypertension 54, 365–371 10.1161/HYPERTENSIONAHA.108.125468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Treml B., Neu N., Kleinsasser A., Gritsch C., Finsterwalder T., Geiger R. et al. (2010) Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit. Care Med. 38, 596–601 10.1097/CCM.0b013e3181c03009 [DOI] [PubMed] [Google Scholar]
- 5.Zhong J., Basu R., Guo D., Chow F.L., Byrns S., Schuster M. et al. (2010) Angiotensin-converting enzyme 2 suppresses pathological hypertrophy, myocardial fibrosis, and cardiac dysfunction. Circulation 122, 717–728, 718 p following 728 10.1161/CIRCULATIONAHA.110.955369 [DOI] [PubMed] [Google Scholar]
- 6.Ferreira A.J., Shenoy V., Yamazato Y., Sriramula S., Francis J., Yuan L. et al. (2009) Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am. J. Respir. Crit. Care Med. 179, 1048–1054 10.1164/rccm.200811-1678OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferreira A.J., Jacoby B.A., Araújo C.A., Macedo F.A., Silva G.A., Almeida A.P. et al. (2007) The nonpeptide angiotensin-(1-7) receptor Mas agonist AVE-0991 attenuates heart failure induced by myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 292, H1113–H1119 10.1152/ajpheart.00828.2006 [DOI] [PubMed] [Google Scholar]
- 8.Patel V.B., Bodiga S., Basu R., Das S.K., Wang W., Wang Z. et al. (2012) Loss of angiotensin-converting enzyme-2 exacerbates diabetic cardiovascular complications and leads to systolic and vascular dysfunction: a critical role of the angiotensin II/AT1 receptor axis. Circ. Res. 110, 1322–1335 10.1161/CIRCRESAHA.112.268029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tikellis C., Pickering R., Tsorotes D., Du X.J., Kiriazis H., Nguyen-Huu T.P. et al. (2012) Interaction of diabetes and ACE2 in the pathogenesis of cardiovascular disease in experimental diabetes. Clin. Sci. (Lond.) 123, 519–529 10.1042/CS20110668 [DOI] [PubMed] [Google Scholar]
- 10.Gurley S.B., Allred A., Le T.H., Griffiths R., Mao L., Philip N. et al. (2006) Altered blood pressure responses and normal cardiac phenotype in ACE2-null mice. J. Clin. Invest. 116, 2218–2225 10.1172/JCI16980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shenoy V., Kwon K.C., Rathinasabapathy A., Lin S., Jin G., Song C. et al. (2014) Oral delivery of Angiotensin-converting enzyme 2 and Angiotensin-(1-7) bioencapsulated in plant cells attenuates pulmonary hypertension. Hypertension 64, 1248–1259 10.1161/HYPERTENSIONAHA.114.03871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basu R., Poglitsch M., Yogasundaram H., Thomas J., Rowe B.H. and Oudit G.Y. (2017) Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J. Am. Coll. Cardiol. 69, 805–819 10.1016/j.jacc.2016.11.064 [DOI] [PubMed] [Google Scholar]
- 13.Vickers C., Hales P., Kaushik V., Dick L., Gavin J., Tang J. et al. (2002) Hydrolysis of biological peptides by human angiotensin-converting enzyme-related carboxypeptidase. J. Biol. Chem. 277, 14838–14843 10.1074/jbc.M200581200 [DOI] [PubMed] [Google Scholar]
- 14.Simões e Silva A.C., Silveira K.D., Ferreira A.J. and Teixeira M.M. (2013) ACE2, angiotensin-(1-7) and Mas receptor axis in inflammation and fibrosis. Br. J. Pharmacol. 169, 477–492 10.1111/bph.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mukerjee S., Gao H., Xu J., Sato R., Zsombok A. and Lazartigues E. (2019) ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension 74, 1181–1191 10.1161/HYPERTENSIONAHA.119.13133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J. et al. (2020) Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci. China Life Sci. 63, 364–374 10.1007/s11427-020-1643-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M. et al. (2009) SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Invest. 39, 618–625 10.1111/j.1365-2362.2009.02153.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N. et al. (2000) A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 87, E1–E9 10.1161/01.RES.87.5.e1 [DOI] [PubMed] [Google Scholar]
- 19.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J. et al. (2020) Response by Gheblawi et al to Letter Regarding Article, “Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2”. Circ. Res. 127, e46–e47 10.1161/CIRCRESAHA.120.317332 [DOI] [PubMed] [Google Scholar]
- 20.Tipnis S.R., Hooper N.M., Hyde R., Karran E., Christie G. and Turner A.J. (2000) A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 275, 33238–33243 10.1074/jbc.M002615200 [DOI] [PubMed] [Google Scholar]
- 21.Shang J., Ye G., Shi K., Wan Y., Luo C., Aihara H. et al. (2020) Structural basis of receptor recognition by SARS-CoV-2. Nature 581, 221–224 10.1038/s41586-020-2179-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bar-On Y.M., Flamholz A., Phillips R. and Milo R. (2020) SARS-CoV-2 (COVID-19) by the numbers. Elife 9, e57309 10.7554/eLife.57309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S. et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271.e278–280.e278 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Walls A.C., Park Y.J., Tortorici M.A., Wall A., McGuire A.T. and Veesler D. (2020) Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 181, 281.e286–292.e286 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haga S., Yamamoto N., Nakai-Murakami C., Osawa Y., Tokunaga K., Sata T. et al. (2008) Modulation of TNF-alpha-converting enzyme by the spike protein of SARS-CoV and ACE2 induces TNF-alpha production and facilitates viral entry. Proc. Natl. Acad. Sci. U.S.A. 105, 7809–7814 10.1073/pnas.0711241105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert D.W., Yarski M., Warner F.J., Thornhill P., Parkin E.T., Smith A.I. et al. (2005) Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J. Biol. Chem. 280, 30113–30119 10.1074/jbc.M505111200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kefaloyianni E., Muthu M.L., Kaeppler J., Sun X., Sabbisetti V., Chalaris A. et al. (2016) ADAM17 substrate release in proximal tubule drives kidney fibrosis. JCI Insight 1, e87023 10.1172/jci.insight.87023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T., Hagiyama M. and Ito A. (2018) Renal ADAM10 and 17: their physiological and medical meanings. Front. Cell Dev. Biol. 6, 153 10.3389/fcell.2018.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashimoto T., Perlot T., Rehman A., Trichereau J., Ishiguro H., Paolino M. et al. (2012) ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 487, 477–481 10.1038/nature11228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Camargo S.M., Singer D., Makrides V., Huggel K., Pos K.M., Wagner C.A. et al. (2009) Tissue-specific amino acid transporter partners ACE2 and collectrin differentially interact with hartnup mutations. Gastroenterology 136, 872–882 10.1053/j.gastro.2008.10.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jando J., Camargo S.M.R., Herzog B. and Verrey F. (2017) Expression and regulation of the neutral amino acid transporter B0AT1 in rat small intestine. PLoS ONE 12, e0184845 10.1371/journal.pone.0184845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sharma R.K., Oliveira A.C., Yang T., Karas M.M., Li J., Lobaton G.O. et al. (2020) Gut pathology and its rescue by ACE2 (Angiotensin-Converting Enzyme 2) in hypoxia-induced pulmonary hypertension. Hypertension 76, 206–216 10.1161/HYPERTENSIONAHA.120.14931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma R.K., Oliveira A.C., Yang T., Kim S., Zubcevic J., Aquino V. et al. (2020) Pulmonary arterial hypertension-associated changes in gut pathology and microbiota. ERJ Open Res. 6, 00253–02019 10.1183/23120541.00253-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leung W.K., To K.F., Chan P.K., Chan H.L., Wu A.K., Lee N. et al. (2003) Enteric involvement of severe acute respiratory syndrome-associated coronavirus infection. Gastroenterology 125, 1011–1017 10.1016/j.gastro.2003.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian Y., Rong L., Nian W. and He Y. (2020) Review article: gastrointestinal features in COVID-19 and the possibility of faecal transmission. Aliment. Pharmacol. Ther. 51, 843–851 10.1111/apt.15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y., Guo C., Tang L., Hong Z., Zhou J., Dong X. et al. (2020) Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol. Hepatol. 5, 434–435 10.1016/S2468-1253(20)30083-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zuo T., Zhang F., Lui G.C.Y., Yeoh Y.K., Li A.Y.L., Zhan H. et al. (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology 159, 944–955 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logsdon A.F., Erickson M.A., Rhea E.M., Salameh T.S. and Banks W.A. (2018) Gut reactions: How the blood-brain barrier connects the microbiome and the brain. Exp. Biol. Med. (Maywood) 243, 159–165 10.1177/1535370217743766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. et al. (2020) Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. et al. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. et al. (2020) Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whelton P.K., Carey R.M., Aronow W.S., Casey D.E., Collins K.J., Dennison Himmelfarb C. et al. (2018) 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension 71, 1269–1324 [DOI] [PubMed] [Google Scholar]
- 43.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P. et al. (2019) Heart Disease and Stroke Statistics-2019 Update: a report from the American Heart Association. Circulation 139, e56–e528 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 44.Díez-Freire C., Vázquez J., Correa de Adjounian M.F., Ferrari M.F., Yuan L., Silver X. et al. (2006) ACE2 gene transfer attenuates hypertension-linked pathophysiological changes in the SHR. Physiol. Genomics 27, 12–19 10.1152/physiolgenomics.00312.2005 [DOI] [PubMed] [Google Scholar]
- 45.Yamazato M., Yamazato Y., Sun C., Diez-Freire C. and Raizada M.K. (2007) Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 49, 926–931 10.1161/01.HYP.0000259942.38108.20 [DOI] [PubMed] [Google Scholar]
- 46.Rentzsch B., Todiras M., Iliescu R., Popova E., Campos L.A., Oliveira M.L. et al. (2008) Transgenic angiotensin-converting enzyme 2 overexpression in vessels of SHRSP rats reduces blood pressure and improves endothelial function. Hypertension 52, 967–973 10.1161/HYPERTENSIONAHA.108.114322 [DOI] [PubMed] [Google Scholar]
- 47.Tikellis C., Johnston C.I., Forbes J.M., Burns W.C., Burrell L.M., Risvanis J. et al. (2003) Characterization of renal angiotensin-converting enzyme 2 in diabetic nephropathy. Hypertension 41, 392–397 10.1161/01.HYP.0000060689.38912.CB [DOI] [PubMed] [Google Scholar]
- 48.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E. et al. (2002) Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature 417, 822–828 10.1038/nature00786 [DOI] [PubMed] [Google Scholar]
- 49.Walther T., Wessel N., Kang N., Sander A., Tschöpe C., Malberg H. et al. (2000) Altered heart rate and blood pressure variability in mice lacking the Mas protooncogene. Braz. J. Med. Biol. Res. 33, 1–9 10.1590/S0100-879X2000000100001 [DOI] [PubMed] [Google Scholar]
- 50.Gjymishka A., Kulemina L.V., Shenoy V., Katovich M.J., Ostrov D.A. and Raizada M.K. (2010) Diminazene aceturate is an ACE2 activator and a novel antihypertensive drug. FASEB J. 24, 1032.3–1032.3 [Google Scholar]
- 51.Velkoska E., Patel S.K., Griggs K., Pickering R.J., Tikellis C. and Burrell L.M. (2015) Short-term treatment with diminazene aceturate ameliorates the reduction in kidney ACE2 activity in rats with subtotal nephrectomy. PLoS ONE 10, e0118758 10.1371/journal.pone.0118758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel V.B., Zhong J.C., Grant M.B. and Oudit G.Y. (2016) Role of the ACE2/Angiotensin 1-7 Axis of the renin-angiotensin system in heart failure. Circ. Res. 118, 1313–1326 10.1161/CIRCRESAHA.116.307708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haschke M., Schuster M., Poglitsch M., Loibner H., Salzberg M., Bruggisser M. et al. (2013) Pharmacokinetics and pharmacodynamics of recombinant human angiotensin-converting enzyme 2 in healthy human subjects. Clin. Pharmacokinet. 52, 783–792 10.1007/s40262-013-0072-7 [DOI] [PubMed] [Google Scholar]
- 54.Doobay M.F., Talman L.S., Obr T.D., Tian X., Davisson R.L. and Lazartigues E. (2007) Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R373–R381 10.1152/ajpregu.00292.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gowrisankar Y.V. and Clark M.A. (2016) Angiotensin II regulation of angiotensin-converting enzymes in spontaneously hypertensive rat primary astrocyte cultures. J. Neurochem. 138, 74–85 10.1111/jnc.13641 [DOI] [PubMed] [Google Scholar]
- 56.Zhu Y., Jiang M., Gao L. and Huang X. (2020) Single cell analysis of ACE2 expression reveals the potential targets for 2019-nCoV. Preprints, 2020020221 10.20944/preprints202002.0221.v1 [DOI] [Google Scholar]
- 57.Xia H., Sriramula S., Chhabra K.H. and Lazartigues E. (2013) Brain angiotensin-converting enzyme type 2 shedding contributes to the development of neurogenic hypertension. Circ. Res. 113, 1087–1096 10.1161/CIRCRESAHA.113.301811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu J., Sriramula S., Xia H., Moreno-Walton L., Culicchia F., Domenig O. et al. (2017) Clinical relevance and role of neuronal AT. Circ. Res. 121, 43–55 10.1161/CIRCRESAHA.116.310509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia H., Feng Y., Obr T.D., Hickman P.J. and Lazartigues E. (2009) Angiotensin II type 1 receptor-mediated reduction of angiotensin-converting enzyme 2 activity in the brain impairs baroreflex function in hypertensive mice. Hypertension 53, 210–216 10.1161/HYPERTENSIONAHA.108.123844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xia H. and Lazartigues E. (2010) Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr. Hypertens. Rep. 12, 170–175 10.1007/s11906-010-0105-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sriramula S., Cardinale J.P., Lazartigues E. and Francis J. (2011) ACE2 overexpression in the paraventricular nucleus attenuates angiotensin II-induced hypertension. Cardiovasc. Res. 92, 401–408 10.1093/cvr/cvr242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sriramula S., Xia H., Xu P. and Lazartigues E. (2015) Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension 65, 577–586 10.1161/HYPERTENSIONAHA.114.04691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chaves G.Z., Caligiorne S.M., Santos R.A., Khosla M.C. and Campagnole-Santos M.J. (2000) Modulation of the baroreflex control of heart rate by angiotensin-(1-7) at the nucleus tractus solitarii of normotensive and spontaneously hypertensive rats. J. Hypertens. 18, 1841–1848 10.1097/00004872-200018120-00019 [DOI] [PubMed] [Google Scholar]
- 64.Alzamora A.C., Santos R.A. and Campagnole-Santos M.J. (2002) Hypotensive effect of ANG II and ANG-(1-7) at the caudal ventrolateral medulla involves different mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 283, R1187–R1195 10.1152/ajpregu.00580.2001 [DOI] [PubMed] [Google Scholar]
- 65.Whitaker A.M. and Molina P.E. (2013) Angiotensin (1-7) contributes to nitric oxide tonic inhibition of vasopressin release during hemorrhagic shock in acute ethanol intoxicated rodents. Life Sci. 93, 623–629 10.1016/j.lfs.2013.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakagaki T., Hirooka Y., Ito K., Kishi T., Hoka S. and Sunagawa K. (2011) Role of angiotensin-(1-7) in rostral ventrolateral medulla in blood pressure regulation via sympathetic nerve activity in Wistar-Kyoto and spontaneous hypertensive rats. Clin. Exp. Hypertens. 33, 223–230 10.3109/10641963.2011.583967 [DOI] [PubMed] [Google Scholar]
- 67.Fontes M.A., Baltatu O., Caligiorne S.M., Campagnole-Santos M.J., Ganten D., Bader M. et al. (2000) Angiotensin peptides acting at rostral ventrolateral medulla contribute to hypertension of TGR(mREN2)27 rats. Physiol. Genomics 2, 137–142 10.1152/physiolgenomics.2000.2.3.137 [DOI] [PubMed] [Google Scholar]
- 68.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. et al. (2020) Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA 323, 2052–2059 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., Diz D.I. and Gallagher P.E. et al. (2005) Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2.. Circulation 111, 2605–10 10.1161/CIRCULATIONAHA.104.510461 [DOI] [PubMed] [Google Scholar]
- 70.Zhang P., Zhu L., Cai J., Lei F., Qin J.-J. and Xie J. et al. (2020) Correction to: Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 127, e147, 10.1161/CIRCRESAHA.120.317134 [DOI] [PubMed] [Google Scholar]
- 71.Mancia G., Rea F., Ludergnani M., Apolone G. and Corrao G. (2020) Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N. Engl. J. Med. 382, 2431–2440 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B. et al. (2020) Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 382, 2441–2448 10.1056/NEJMoa2008975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fosbøl E.L., Butt J.H., Østergaard L., Andersson C., Selmer C., Kragholm K. et al. (2020) Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA 324, 168–177 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo J., Huang Z., Lin L. and Lv J. (2020) Coronavirus Disease 2019 (COVID-19) and cardiovascular disease: a viewpoint on the potential influence of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers on onset and severity of severe acute respiratory syndrome coronavirus 2 infection. J. Am. Heart Assoc. 9, e016219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sampaio W.O., Souza dos Santos R.A., Faria-Silva R., da Mata Machado L.T., Schiffrin E.L. and Touyz R.M. (2007) Angiotensin-(1-7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension 49, 185–192 10.1161/01.HYP.0000251865.35728.2f [DOI] [PubMed] [Google Scholar]
- 76.Mori J., Patel V.B., Abo Alrob O., Basu R., Altamimi T., Desaulniers J. et al. (2014) Angiotensin 1-7 ameliorates diabetic cardiomyopathy and diastolic dysfunction in db/db mice by reducing lipotoxicity and inflammation. Circ. Heart Fail. 7, 327–339 10.1161/CIRCHEARTFAILURE.113.000672 [DOI] [PubMed] [Google Scholar]
- 77.Wang W., Bodiga S., Das S.K., Lo J., Patel V. and Oudit G.Y. (2012) Role of ACE2 in diastolic and systolic heart failure. Heart Fail. Rev. 17, 683–691 10.1007/s10741-011-9259-x [DOI] [PubMed] [Google Scholar]
- 78.Jia G., Hill M.A. and Sowers J.R. (2018) Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circ. Res. 122, 624–638 10.1161/CIRCRESAHA.117.311586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong C. and Marwick T.H. (2007) Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat. Clin. Pract. Cardiovasc. Med. 4, 436–443 10.1038/ncpcardio0943 [DOI] [PubMed] [Google Scholar]
- 80.Patel V.B., Mori J., McLean B.A., Basu R., Das S.K., Ramprasath T. et al. (2016) ACE2 deficiency worsens epicardial adipose tissue inflammation and cardiac dysfunction in response to diet-induced obesity. Diabetes 65, 85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Oudit G.Y., Kassiri Z., Patel M.P., Chappell M., Butany J., Backx P.H. et al. (2007) Angiotensin II-mediated oxidative stress and inflammation mediate the age-dependent cardiomyopathy in ACE2 null mice. Cardiovasc. Res. 75, 29–39 10.1016/j.cardiores.2007.04.007 [DOI] [PubMed] [Google Scholar]
- 82.Epelman S., Tang W.H., Chen S.Y., Van Lente F., Francis G.S. and Sen S. (2008) Detection of soluble angiotensin-converting enzyme 2 in heart failure: insights into the endogenous counter-regulatory pathway of the renin-angiotensin-aldosterone system. J. Am. Coll. Cardiol. 52, 750–754 10.1016/j.jacc.2008.02.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chen T., Wu D., Chen H., Yan W., Yang D., Chen G. et al. (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368, m1091 10.1136/bmj.m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. et al. (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395, 1054–1062 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Puntmann V.O., Carerj M.L., Wieters I., Fahim M., Arendt C., Hoffmann J. et al. (2020) Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 10.1001/jamacardio.2020.3557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chen L., Li X., Chen M., Feng Y. and Xiong C. (2020) The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc. Res. 116, 1097–1100 10.1093/cvr/cvaa078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B. and Ferrario C.M. (2004) Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension 43, 970–976 10.1161/01.HYP.0000124667.34652.1a [DOI] [PubMed] [Google Scholar]
- 88.Ocaranza M.P., Godoy I., Jalil J.E., Varas M., Collantes P., Pinto M. et al. (2006) Enalapril attenuates downregulation of Angiotensin-converting enzyme 2 in the late phase of ventricular dysfunction in myocardial infarcted rat. Hypertension 48, 572–578 10.1161/01.HYP.0000237862.94083.45 [DOI] [PubMed] [Google Scholar]
- 89.Godo S. and Shimokawa H. (2017) Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 37, e108–e114 10.1161/ATVBAHA.117.309813 [DOI] [PubMed] [Google Scholar]
- 90.Ross R. (1993) The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature 362, 801–809 10.1038/362801a0 [DOI] [PubMed] [Google Scholar]
- 91.Deanfield J.E., Halcox J.P. and Rabelink T.J. (2007) Endothelial function and dysfunction: testing and clinical relevance. Circulation 115, 1285–1295 10.1161/CIRCULATIONAHA.106.652859 [DOI] [PubMed] [Google Scholar]
- 92.Bonetti P.O., Lerman L.O. and Lerman A. (2003) Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler. Thromb. Vasc. Biol. 23, 168–175 10.1161/01.ATV.0000051384.43104.FC [DOI] [PubMed] [Google Scholar]
- 93.Lovren F., Pan Y., Quan A., Teoh H., Wang G., Shukla P.C. et al. (2008) Angiotensin converting enzyme-2 confers endothelial protection and attenuates atherosclerosis. Am. J. Physiol. Heart Circ. Physiol. 295, H1377–H1384 10.1152/ajpheart.00331.2008 [DOI] [PubMed] [Google Scholar]
- 94.Rabelo L.A., Todiras M., Nunes-Souza V., Qadri F., Szijártó I.A., Gollasch M. et al. (2016) Genetic deletion of ACE2 induces vascular dysfunction in C57BL/6 mice: role of nitric oxide imbalance and oxidative stress. PLoS ONE 11, e0150255 10.1371/journal.pone.0150255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rabelo L.A., Alenina N. and Bader M. (2011) ACE2-angiotensin-(1-7)-Mas axis and oxidative stress in cardiovascular disease. Hypertens. Res. 34, 154–160 10.1038/hr.2010.235 [DOI] [PubMed] [Google Scholar]
- 96.Zhang Y.H., Dong X.F., Hao Q.Q., Zhou X.M., Yu Q.T., Li S.Y. et al. (2015) ACE2 and Ang-(1-7) protect endothelial cell function and prevent early atherosclerosis by inhibiting inflammatory response. Inflamm. Res. 64, 253–260 10.1007/s00011-015-0805-1 [DOI] [PubMed] [Google Scholar]
- 97.Zhang C., Zhao Y.X., Zhang Y.H., Zhu L., Deng B.P., Zhou Z.L. et al. (2010) Angiotensin-converting enzyme 2 attenuates atherosclerotic lesions by targeting vascular cells. Proc. Natl. Acad. Sci. U.S.A. 107, 15886–15891 10.1073/pnas.1001253107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hayashi N., Yamamoto K., Ohishi M., Tatara Y., Takeya Y., Shiota A. et al. (2010) The counterregulating role of ACE2 and ACE2-mediated angiotensin 1-7 signaling against angiotensin II stimulation in vascular cells. Hypertens. Res. 33, 1182–1185 10.1038/hr.2010.147 [DOI] [PubMed] [Google Scholar]
- 99.Koka V., Huang X.R., Chung A.C., Wang W., Truong L.D. and Lan H.Y. (2008) Angiotensin II up-regulates angiotensin I-converting enzyme (ACE), but down-regulates ACE2 via the AT1-ERK/p38 MAP kinase pathway. Am. J. Pathol. 172, 1174–1183 10.2353/ajpath.2008.070762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Srivastava P., Badhwar S., Chandran D.S., Jaryal A.K., Jyotsna V.P. and Deepak K.K. (2019) Imbalance between Angiotensin II - Angiotensin (1-7) system is associated with vascular endothelial dysfunction and inflammation in type 2 diabetes with newly diagnosed hypertension. Diabetes Metab. Syndr. 13, 2061–2068 10.1016/j.dsx.2019.04.042 [DOI] [PubMed] [Google Scholar]
- 101.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. et al. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418 10.1016/S0140-6736(20)30937-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Labò N., Ohnuki H. and Tosato G. (2020) Vasculopathy and coagulopathy associated with SARS-CoV-2 infection. Cells 9, 1583 10.3390/cells9071583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Leisman D.E., Deutschman C.S. and Legrand M. (2020) Facing COVID-19 in the ICU: vascular dysfunction, thrombosis, and dysregulated inflammation. Intensive Care Med. 46, 1105–1108 10.1007/s00134-020-06059-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bilaloglu S., Aphinyanaphongs Y., Jones S., Iturrate E., Hochman J. and Berger J.S. (2020) Thrombosis in hospitalized patients with COVID-19 in a New York City health system. JAMA 324, 799–801 10.1001/jama.2020.13372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fogarty H., Townsend L., Ni Cheallaigh C., Bergin C., Martin-Loeches I., Browne P. et al. (2020) COVID19 coagulopathy in Caucasian patients. Br. J. Haematol. 189, 1044–1049 10.1111/bjh.16749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Team, C. C.-R. (2020) Coronavirus disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 422–426 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Verdoni L., Mazza A., Gervasoni A., Martelli L., Ruggeri M., Ciuffreda M. et al. (2020) An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet 395, 1771–1778 10.1016/S0140-6736(20)31103-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Becker R.C. (2020) COVID-19-associated vasculitis and vasculopathy. J. Thromb. Thrombolysis 50, 499–511 10.1007/s11239-020-02230-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Roumelioti M.E., Glew R.H., Khitan Z.J., Rondon-Berrios H., Argyropoulos C.P., Malhotra D. et al. (2018) Fluid balance concepts in medicine: principles and practice. World J. Nephrol. 7, 1–28 10.5527/wjn.v7.i1.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Coffman T.M. (2014) The inextricable role of the kidney in hypertension. J. Clin. Invest. 124, 2341–2347 10.1172/JCI72274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mascarenhas J., Laszczynska O., Severo M., Friões F., Alvelos M., Bettencourt P. et al. (2018) Prognostic effect of renal function in ambulatory patients with heart failure and reduced ejection fraction: the kidney is a marker of cardiac function. Can. J. Cardiol. 34, 1325–1332 10.1016/j.cjca.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 112.Haase V.H. (2013) Mechanisms of hypoxia responses in renal tissue. J. Am. Soc. Nephrol. 24, 537–541 10.1681/ASN.2012080855 [DOI] [PubMed] [Google Scholar]
- 113.Masereeuw R., Mutsaers H.A., Toyohara T., Abe T., Jhawar S., Sweet D.H. et al. (2014) The kidney and uremic toxin removal: glomerulus or tubule? Semin. Nephrol. 34, 191–208 10.1016/j.semnephrol.2014.02.010 [DOI] [PubMed] [Google Scholar]
- 114.Nagami G.T. and Hamm L.L. (2017) Regulation of acid-base balance in chronic kidney disease. Adv. Chronic Kidney Dis. 24, 274–279 10.1053/j.ackd.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 115.Donnelly S. (2003) Why is erythropoietin made in the kidney? The kidney functions as a ‘critmeter’ to regulate the hematocrit. Adv. Exp. Med. Biol. 543, 73–87 10.1007/978-1-4419-8997-0_6 [DOI] [PubMed] [Google Scholar]
- 116.Williams M.E. (2009) Chronic kidney disease/bone and mineral metabolism: the imperfect storm. Semin. Nephrol. 29, 97–104 10.1016/j.semnephrol.2009.01.002 [DOI] [PubMed] [Google Scholar]
- 117.Go A.S., Chertow G.M., Fan D., McCulloch C.E. and Hsu C.Y. (2004) Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 351, 1296–1305 10.1056/NEJMoa041031 [DOI] [PubMed] [Google Scholar]
- 118.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J. et al. (2020) Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 251, 228–248 10.1002/path.5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lely A.T., Hamming I., van Goor H. and Navis G.J. (2004) Renal ACE2 expression in human kidney disease. J. Pathol. 204, 587–593 10.1002/path.1670 [DOI] [PubMed] [Google Scholar]
- 120.Ye M., Wysocki J., William J., Soler M.J., Cokic I. and Batlle D. (2006) Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. J. Am. Soc. Nephrol. 17, 3067–3075 10.1681/ASN.2006050423 [DOI] [PubMed] [Google Scholar]
- 121.da Silveira K.D., Pompermayer Bosco K.S., Diniz L.R., Carmona A.K., Cassali G.D., Bruna-Romero O. et al. (2010) ACE2-angiotensin-(1-7)-Mas axis in renal ischaemia/reperfusion injury in rats. Clin. Sci. (Lond.) 119, 385–394 10.1042/CS20090554 [DOI] [PubMed] [Google Scholar]
- 122.Velkoska E., Dean R.G., Burchill L., Levidiotis V. and Burrell L.M. (2010) Reduction in renal ACE2 expression in subtotal nephrectomy in rats is ameliorated with ACE inhibition. Clin. Sci. (Lond.) 118, 269–279 10.1042/CS20090318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gupta A., Rhodes G.J., Berg D.T., Gerlitz B., Molitoris B.A. and Grinnell B.W. (2007) Activated protein C ameliorates LPS-induced acute kidney injury and downregulates renal INOS and angiotensin 2. Am. J. Physiol. Renal Physiol. 293, F245–F254 10.1152/ajprenal.00477.2006 [DOI] [PubMed] [Google Scholar]
- 124.Roberts M.A., Velkoska E., Ierino F.L. and Burrell L.M. (2013) Angiotensin-converting enzyme 2 activity in patients with chronic kidney disease. Nephrol. Dial. Transplant. 28, 2287–2294 10.1093/ndt/gft038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chodavarapu H., Grobe N., Somineni H.K., Salem E.S., Madhu M. and Elased K.M. (2013) Rosiglitazone treatment of type 2 diabetic db/db mice attenuates urinary albumin and angiotensin converting enzyme 2 excretion. PLoS ONE 8, e62833 10.1371/journal.pone.0062833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Reich H.N., Oudit G.Y., Penninger J.M., Scholey J.W. and Herzenberg A.M. (2008) Decreased glomerular and tubular expression of ACE2 in patients with type 2 diabetes and kidney disease. Kidney Int. 74, 1610–1616 10.1038/ki.2008.497 [DOI] [PubMed] [Google Scholar]
- 127.Soler M.J., Wysocki J., Ye M., Lloveras J., Kanwar Y. and Batlle D. (2007) ACE2 inhibition worsens glomerular injury in association with increased ACE expression in streptozotocin-induced diabetic mice. Kidney Int. 72, 614–623 10.1038/sj.ki.5002373 [DOI] [PubMed] [Google Scholar]
- 128.Wong D.W., Oudit G.Y., Reich H., Kassiri Z., Zhou J., Liu Q.C. et al. (2007) Loss of angiotensin-converting enzyme-2 (Ace2) accelerates diabetic kidney injury. Am. J. Pathol. 171, 438–451 10.2353/ajpath.2007.060977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nadarajah R., Milagres R., Dilauro M., Gutsol A., Xiao F., Zimpelmann J. et al. (2012) Podocyte-specific overexpression of human angiotensin-converting enzyme 2 attenuates diabetic nephropathy in mice. Kidney Int. 82, 292–303 10.1038/ki.2012.83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Liu C.X., Hu Q., Wang Y., Zhang W., Ma Z.Y., Feng J.B. et al. (2011) Angiotensin-converting enzyme (ACE) 2 overexpression ameliorates glomerular injury in a rat model of diabetic nephropathy: a comparison with ACE inhibition. Mol. Med. 17, 59–69 10.2119/molmed.2010.00111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wysocki J. and Batlle D. (2013) Reduced plasma ACE2 activity in dialysis patients: another piece in the conundrum of factors involved in hypertension and cardiovascular morbidity? Nephrol. Dial. Transplant. 28, 2200–2202 10.1093/ndt/gft240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chu K.H., Tsang W.K., Tang C.S., Lam M.F., Lai F.M., To K.F. et al. (2005) Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int. 67, 698–705 10.1111/j.1523-1755.2005.67130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ronco C., Reis T. and Husain-Syed F. (2020) Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 8, 738–742 10.1016/S2213-2600(20)30229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hardenberg J.B. and Luft F.C. (2020) Covid-19, ACE2 and the kidney. Acta Physiol. (Oxf.) 230, e13539 10.1111/apha.13539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H. et al. (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. et al. (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395, 1033–1034 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Cantaluppi V., Quercia A.D., Dellepiane S., Ferrario S., Camussi G. and Biancone L. (2014) Interaction between systemic inflammation and renal tubular epithelial cells. Nephrol. Dial. Transplant. 29, 2004–2011 10.1093/ndt/gfu046 [DOI] [PubMed] [Google Scholar]
- 138.Puelles V.G., Lütgehetmann M., Lindenmeyer M.T., Sperhake J.P., Wong M.N., Allweiss L. et al. (2020) Multiorgan and renal tropism of SARS-CoV-2. N. Engl. J. Med. 383, 590–592 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Braun F., Lütgehetmann M., Pfefferle S., Wong M.N., Carsten A., Lindenmeyer M.T. et al. (2020) SARS-CoV-2 renal tropism associates with acute kidney injury. Lancet 396, 597–598 10.1016/S0140-6736(20)31759-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Farkash E.A., Wilson A.M. and Jentzen J.M. (2020) Ultrastructural Evidence for Direct Renal Infection with SARS-CoV-2. J. Am. Soc. Nephrol. 31, 1683–1687 10.1681/ASN.2020040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pan X.W., Xu D., Zhang H., Zhou W., Wang L.H. and Cui X.G. (2020) Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med. 46, 1114–1116 10.1007/s00134-020-06026-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Obukhov A.G., Stevens B.R., Prasad R., Li Calzi S., Boulton M.E., Raizada M.K. et al. (2020) SARS-CoV-2 infections and ACE2: clinical outcomes linked with increased morbidity and mortality in individuals with diabetes. Diabetes 69, 1875–1886 10.2337/dbi20-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wang K., Gheblawi M. and Oudit G.Y. (2020) Angiotensin converting enzyme 2: a double-edged sword. Circulation 142, 426–428 10.1161/CIRCULATIONAHA.120.047049 [DOI] [PubMed] [Google Scholar]
- 144.Sampaio W.O., Nascimento A.A. and Santos R.A. (2003) Systemic and regional hemodynamic effects of angiotensin-(1-7) in rats. Am. J. Physiol. Heart Circ. Physiol. 284, H1985–H1994 10.1152/ajpheart.01145.2002 [DOI] [PubMed] [Google Scholar]
- 145.Bernardi S., Burns W.C., Toffoli B., Pickering R., Sakoda M., Tsorotes D. et al. (2012) Angiotensin-converting enzyme 2 regulates renal atrial natriuretic peptide through angiotensin-(1-7). Clin. Sci. (Lond.) 123, 29–37 10.1042/CS20110403 [DOI] [PubMed] [Google Scholar]
- 146.Pinheiro S.V. and Simões E Silva A.C. (2012) Angiotensin converting enzyme 2, Angiotensin-(1-7), and receptor MAS axis in the kidney. Int. J. Hypertens. 2012, 414128 10.1155/2012/414128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M. et al. (2020) Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 181, 905.e907–913.e907 10.1016/j.cell.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hemnes A.R., Rathinasabapathy A., Austin E.A., Brittain E.L., Carrier E.J., Chen X. et al. (2018) A potential therapeutic role for angiotensin-converting enzyme 2 in human pulmonary arterial hypertension. Eur. Respir. J. 51, 1702638 10.1183/13993003.02638-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Khan A., Benthin C., Zeno B., Albertson T.E., Boyd J., Christie J.D. et al. (2017) A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit. Care 21, 234 10.1186/s13054-017-1823-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang J., Dong J., Martin M., He M., Gongol B., Marin T.L. et al. (2018) AMP-activated protein kinase phosphorylation of angiotensin-converting enzyme 2 in endothelium mitigates pulmonary hypertension. Am. J. Respir. Crit. Care Med. 198, 509–520 10.1164/rccm.201712-2570OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Qi Y., Zhang J., Cole-Jeffrey C.T., Shenoy V., Espejo A., Hanna M. et al. (2013) Diminazene aceturate enhances angiotensin-converting enzyme 2 activity and attenuates ischemia-induced cardiac pathophysiology. Hypertension 62, 746–752 10.1161/HYPERTENSIONAHA.113.01337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Qiu Y., Shil P.K., Zhu P., Yang H., Verma A., Lei B. et al. (2014) Angiotensin-converting enzyme 2 (ACE2) activator diminazene aceturate ameliorates endotoxin-induced uveitis in mice. Invest. Ophthalmol. Vis. Sci. 55, 3809–3818 10.1167/iovs.14-13883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thatcher S.E., Zhang X., Howatt D.A., Yiannikouris F., Gurley S.B., Ennis T. et al. (2014) Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler. Thromb. Vasc. Biol. 34, 2617–2623 10.1161/ATVBAHA.114.304613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shenoy V., Gjymishka A., Jarajapu Y.P., Qi Y., Afzal A., Rigatto K. et al. (2013) Diminazene attenuates pulmonary hypertension and improves angiogenic progenitor cell functions in experimental models. Am. J. Respir. Crit. Care Med. 187, 648–657 10.1164/rccm.201205-0880OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferreira A.J., Murça T.M., Fraga-Silva R.A., Castro C.H., Raizada M.K. and Santos R.A. (2012) New cardiovascular and pulmonary therapeutic strategies based on the Angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor axis. Int. J. Hypertens. 2012, 147825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li J., Wang X., Chen J., Zhang H. and Deng A. (2020) Association of renin-angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID-19) infection in Wuhan, China. JAMA Cardiol. 5, 825–830 10.1001/jamacardio.2020.1624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J. et al. (2020) Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin ii receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ. Res. 2020, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Montalvan V., Lee J., Bueso T., De Toledo J. and Rivas K. (2020) Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin. Neurol. Neurosurg. 194, 105921 10.1016/j.clineuro.2020.105921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A. et al. (2020) Neurological associations of COVID-19. Lancet Neurol. 19, 767–783 10.1016/S1474-4422(20)30221-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma R.K., Oliveira A.C., Kim S., Rigatto K., Zubcevic J., Rathinasabapathy A. et al. (2018) Involvement of neuroinflammation in the pathogenesis of monocrotaline-induced pulmonary hypertension. Hypertension 71, 1156–1163 10.1161/HYPERTENSIONAHA.118.10934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Santisteban M.M., Ahmari N., Carvajal J.M., Zingler M.B., Qi Y., Kim S. et al. (2015) Involvement of bone marrow cells and neuroinflammation in hypertension. Circ. Res. 117, 178–191 10.1161/CIRCRESAHA.117.305853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shi P., Diez-Freire C., Jun J.Y., Qi Y., Katovich M.J., Li Q. et al. (2010) Brain microglial cytokines in neurogenic hypertension. Hypertension 56, 297–303 10.1161/HYPERTENSIONAHA.110.150409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Chen L.D., Zhang Z.Y., Wei X.J., Cai Y.Q., Yao W.Z., Wang M.H. et al. (2020) Association between cytokine profiles and lung injury in COVID-19 pneumonia. Respir. Res. 21, 201 10.1186/s12931-020-01465-2 [DOI] [PMC free article] [PubMed] [Google Scholar]