Abstract
Background:
Approximately half of depressed adolescents fail to respond to cognitive behavioral therapy (CBT). Given the variability in response, it is important to identify pre-treatment characteristics that predict prognosis. Knowledge of which depressed adolescents are likely to exhibit a positive vs. poor outcome to CBT may have important clinical implications (e.g., informing treatment recommendations). Emerging evidence suggests that neural reward responsiveness represents one promising predictor.
Method:
Adolescents with major depressive disorder (n = 36) received CBT and completed a reward task at three timepoints (pre-treatment, mid-treatment and post-treatment) while 128-channel electroencephalogram (EEG) data were acquired. Healthy control participants (n = 29) completed the same task at three corresponding timepoints. Analyses focused on event-related potentials (ERPs) linked to two stages of neural processing: initial response to rewards (reward-related positivity [RewP]) and later, elaborative processing (late positive potential [LPP]). Moreover, time-frequency analyses decomposed the RewP into two constituent components: reward-related delta and loss-related theta activity.
Results:
Multilevel modeling revealed that greater pre-treatment reward responsiveness, as measured by the LPP to rewards, predicted greater depressive symptom change. In addition, a Group x Condition x Time interaction emerged for theta activity to losses, reflecting normalization of theta power in the MDD group from baseline to post-treatment.
Conclusions:
An ERP measure of sustained (LPP)—but not initial (RewP)—reward responsiveness predicted symptom improvement, which may help inform which depressed adolescents are most likely to benefit from CBT. In addition to alleviating depression, successful CBT may attenuate underlying neural (theta) hypersensitivity to negative outcomes in depressed youth.
Keywords: Cognitive Behavioral Therapy, Depression, Reward Positivity, Time Frequency Decomposition, Late Positive Potential, Adolescence
Introduction
Depression rates increase substantially during adolescence, and by age 18, an estimated 15% of teens will have experienced at least one episode of major depressive disorder (MDD), with females twice as likely as males to have developed MDD (1). Despite these alarming statistics, approximately 40-66% of adolescents do not receive treatment for their depression (1,2). A range of psychotherapeutic and pharmacological treatment options are available for depressed adolescents, and cognitive behavioral therapy (CBT) is among the most empirically supported intervention (3). However, approximately 40-50% of depressed youth fail to respond to CBT (3,4). Given the variability in response, it is important to identify pre-treatment patient characteristics that predict treatment prognosis, as this may have important clinical implications regarding treatment recommendations (e.g., suggesting a more intensive, alternative or combination treatment for those individuals predicted to have a poor response to CBT)(5,6).
Reward-Related Predictors of Treatment Outcome
Several studies have identified pre-treatment neural response to rewards as a predictor of treatment outcome among adults (7,8) and youth (9,10) receiving CBT or SSRI. To assess neural reward responsiveness, researchers have utilized the reward positivity (RewP), an event-related potential (ERP) most commonly examined within monetary reward tasks (11). The RewP, also known as the feedback-related negativity (FRN), is a frontocentral ERP component occurring approximately 250-350ms following rewarding feedback (relative to losses or the omission of rewards). Studies combining ERPs and functional magnetic resonance imaging (fMRI) reveal that the RewP is associated with activation of the mesocorticolimbic reward circuit, including the ventral striatum and medial prefrontal cortex (12,13). Two initial studies in adults with anxiety and/or depression indicated that a reduced pre-treatment RewP (i.e., reflecting blunted reward responsiveness) predicted greater depressive symptom improvement to CBT (8) and SSRI (7, but see 14). Similarly, a more recent study (9) in a sample of children and adolescents with generalized anxiety disorder (GAD) or social anxiety disorder (SAD) receiving CBT or SSRI reported that a reduced RewP to monetary rewards predicted greater depressive—but not anxiety—symptom improvement. Although sample size was small (n = 16 for CBT; n = 11 for SSRI), exploratory analyses suggested that the pattern of reduced RewP predicting depressive symptom change was specific to CBT, and not SSRI. Taken together, these findings are consistent with a “compensatory” model, such that CBT may be well-suited to those with blunted—rather than intact or enhanced—reward responsiveness. However, the first two studies (7,8) focused on adults, whereas the latter study (9) included children and adolescents with GAD or SAD, none of whom had current MDD. The extent to which a blunted RewP to rewards predicts better outcome in CBT for depressed adolescents is unknown. In addition, it may be that depression-related abnormalities in the RewP (11) improve or normalize following successful CBT. CBT may exert its beneficial effects at least in part through ameliorating depression-related deficits in the neural processing of rewards (e.g., via behavioral activation skills aimed at systematically increasing exposure to and engagement with rewarding activities and experiences)(15) and/or attenuating neural hyperreactivity to negative outcomes (e.g., via cognitive reappraisal skills). Of relevance, recent research using time-frequency decomposition approaches reveals that the RewP consists of both delta (< 3 Hz) and theta (4-7 Hz) activity (16–18). Critically, these studies indicate that, whereas delta activity is more sensitive to rewards than losses, theta activity displays the opposite pattern. As a result, time-frequency decomposition may isolate “purer” and more distinguishable measures of neural responsiveness to rewards (delta) vs. losses (theta) than traditional time-domain ERPs. The extent to which CBT modulates these two time-frequency measures of sensitivity to rewards vs. losses is unknown.
Late Positive Potential
In contrast to the RewP, the LPP is a later ERP component (beginning approximately 300 ms post-stimulus and lasting several hundred ms or seconds) linked to the elaborative processing of emotional or motivationally salient stimuli (including–but not specific to–rewards). The LPP is initially observed over parietal regions and then propagates to frontal electrodes later in its time course (19). Previous research has shown that the LPP is enhanced to emotional words, images and rewards, which is consistent with the notion that this ERP reflects sustained cognitive processing of motivationally salient stimuli. The LPP has been shown to be enhanced to monetary rewards in adolescent (16,20) and young adult samples (21). For example, Webb et al.(16) found potentiated LPPs to monetary rewards relative to losses in healthy adolescent girls, and the opposite pattern in depressed teens. Notably, a recent study indicated that a blunted RewP (to monetary rewards) and LPP (to pleasant pictures) are independent predictors of MDD status (i.e., account for unique variance in depression)(22). The extent to which the RewP and LPP account for significant and unique variance in predicting treatment outcome among depressed youth has yet to be examined. Interestingly, and of relevance to CBT, previous research has shown that the LPP can be modulated via cognitive reappraisal (23–26). Accordingly, given its emphasis on the development of cognitive reappraisal skills, successful CBT may modulate the LPP. In addition, pre-treatment LPP may predict depression treatment outcome. For example, Barch et al.(14) recently found that a larger pre-treatment LPP to pleasant pictures predicted better outcomes for young depressed children (4-7 years of age) who received Parent Child Interaction Therapy (PCIT). The latter finding suggests that relatively enhanced elaborative processing of rewarding or positive stimuli among depressed youth may signal an increased likelihood of benefiting from psychotherapy.
The Present Study
The present study tested (1) whether the RewP and/or LPP, assessed at pre-treatment, predict symptom change among depressed adolescents receiving CBT; and (2) the extent to which a course of CBT modulates the RewP and LPP, while addressing several limitations in the literature. First, none of the abovementioned studies (7–9,14) testing the RewP as a predictor of treatment outcome examined whether these effects were attributable to reward-related delta and/or loss-related theta activity. As described above, the latter two components of the RewP can be disaggregated via time-frequency decomposition. Second, with the exception of one study (14), prior research testing neural predictors of treatment response in depression focused on either initial (RewP)(7–9) or later (LPP)(27) neural stages of processing. To test whether early or later neural responsiveness to rewards predicts outcome, we simultaneously examined an ERP probing initial neural responsiveness to rewards (RewP) and later, elaborative processing of rewards (LPP). Given their excellent temporal resolution, ERPs can distinguish between initial vs. later stages of reward responsiveness (28). Finally, with the exception of one recent study of PCIT in young children which included three EEG timepoints (29), prior studies have relied on a single pre-treatment neural assessment (8,9) or pre- and post-treatment measures (7,14). These designs do not allow for the examination of the time course of change in neural abnormalities. For example, similar to the commonly observed curvilinear pattern of depressive symptom change (i.e., greater change early in treatment) in psychotherapy and pharmacotherapy (30–33), neural changes may not be linear. To address a gap in the treatment literature, in the present study we included pre-, mid- and post-treatment EEG assessments.
In summary, based on prior literature (e.g., 7, 8, 9, 14), we hypothesized that blunted delta power to rewards during the timeframe of the RewP and potentiated LPP to rewards will predict greater depressive symptom improvement in CBT for depressed adolescents. In addition, we expected pre- to post-treatment increases in neural sensitivity to rewards (i.e., reflected by increased delta power) and decreased reactivity to losses (i.e., decreased theta power).
Methods and Materials
Participants
Female adolescents (MDD = 36; Healthy Controls [HC] = 33) ages 13-18 years were recruited from the local greater Boston area via community and internet advertisements. All participants were fluent in English and right-handed. Participants in the MDD group were required to meet DSM-IV criteria for a current major depressive episode according to the K-SADS-PL (34). Exclusion criteria for HC participants included a history of MDD, bipolar disorder, psychosis (including mood disorder with psychotic features), anxiety disorders, eating disorders, substance use disorders, attention-deficit/hyperactivity disorder, mental retardation, organic brain syndrome, and head injury with loss of consciousness for ≥5 min or seizures. Similarly, MDD participants could not meet current criteria for any of the above diagnoses (other than MDD [without psychotic features]), with the exception of a secondary diagnosis of generalized anxiety disorder (GAD; n=12). With regards to medications, four participants were prescribed a selective serotonin reuptake inhibitor (SSRI). See Supplement for additional details.
Procedure
Study approval was provided by the Partners Health Care Institutional Review Board. The baseline assessment was conducted over 2 days. On Day 1, adolescents were administered the K-SADS-PL to assess lifetime mental disorders and completed self-report measures of depressive and anxiety symptoms. On Day 2, adolescents completed a monetary reward gambling task while 128-channel EEG data were recorded. Following Day 2, the MDD group were offered 12 weekly sessions of CBT (one 50-minute session per week) based on the following manual (35)(for additional details, see (16)). The EEG assessment and monetary reward task were re-administered 5 weeks after the initial assessment and at post-treatment. The HC participants, who did not receive treatment, completed EEG assessments and the reward task at three corresponding timepoints (n=4 were excluded due to poor EEG quality). For simplicity and consistency of terms across groups, we henceforth refer to these EEG assessments as “initial”, “mid” and “final”. Baseline (i.e., pre-treatment) clinical and EEG data have previously been published on a subset (51/65) of these participants (16,36).
Measures
Depressive symptoms were assessed via the Beck Depression Inventory-II (BDI-II)(37). Both the MDD and HC participants completed the BDI-II at each assessment. The MDD group completed additional BDI-II assessments at the start of each therapy session. Anxiety symptoms were assessed via the Multidimensional Anxiety Scale for Children (MASC)(38), and administered every other session in the MDD group and at each assessment in the HC group.
Experimental Task.
Participants completed a 180-trial monetary reward gambling task while EEG data were recorded (39–41,16). On each trial, participants were presented with three black boxes and instructed to guess which box contained a green ball (the other boxes contained red balls) using a button box. If participants identified the correct box, the green ball was presented for 2,500 ms along with a rising tone (500 ms), which indicated a monetary gain of 30 cents. If a participant selected a box with a red ball, the red ball would appear for 2,500 ms alongside a falling tone (500 ms) and a monetary loss of 15 cents. There were 90 win and 90 loss trials. For additional details, see Supplement and (16).
EEG Recording and Data Reduction
EEG data were recorded using a 128-channel HydroCel Geodesic Sensor Net (Electrical Geodesics, Inc., Eugene, OR) in an electrically and acoustically shielded room. BrainVision Analyzer 2.1.1 (Brain Products, Munich, Germany) was used for EEG data processing. For time-domain analyses, EEG data were segmented from 200 ms before stimulus onset (win or loss feedback) up to 1,000 ms after stimulus onset. A baseline correction was applied using the average amplitude over 200 ms prior to stimulus onset. Consistent with prior work (16), RewP values were computed as the mean amplitude from 250-350 ms post-stimulus at electrode FCz (see Figure 1, Panel A), and the LPP was assessed using the average of frontocentral midline electrode sites (Fz, FCz, and Cz) between 600-1,000 ms post-stimulus (16,36,42,43)(Figure 2). For time-frequency analyses, and consistent with prior work isolating RewP-linked theta and delta power (16), a complex Morlet wavelet transformation was applied (Morlet parameter c = 3.5) from 0.5 to 20 Hz using 30 frequency steps distributed on a logarithmic scale (44)(Figure 1, Panel B). See Supplement for additional details.
Figure 1.
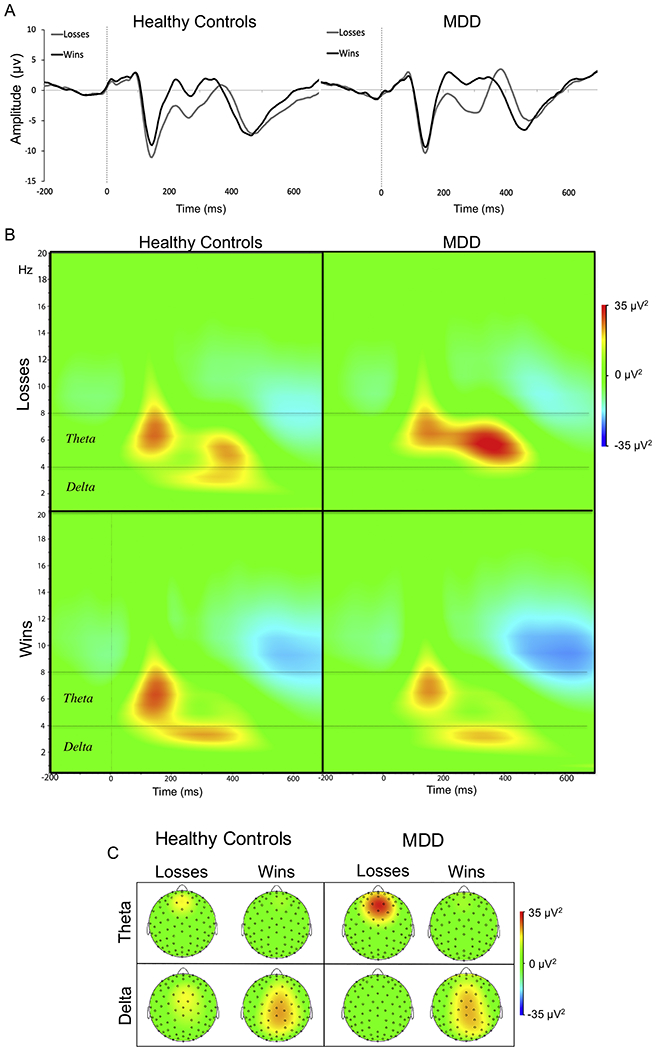
(A) Event-related potentials (Reward Positivity; RewP) elicited by monetary rewards (black) and losses (gray) for healthy controls (left panel) and adolescents with major depressive disorder (MDD) (right panel) shown in the time-domain at electrode FCz at baseline. (B) Time-frequency plots for monetary losses (top panel) vs. rewards (bottom panel) for both groups highlighting theta and delta power. (C). Scalp distribution for theta power (top panel) and delta power (bottom panel) at 300ms for both groups and conditions (wins and losses).
Figure 2.
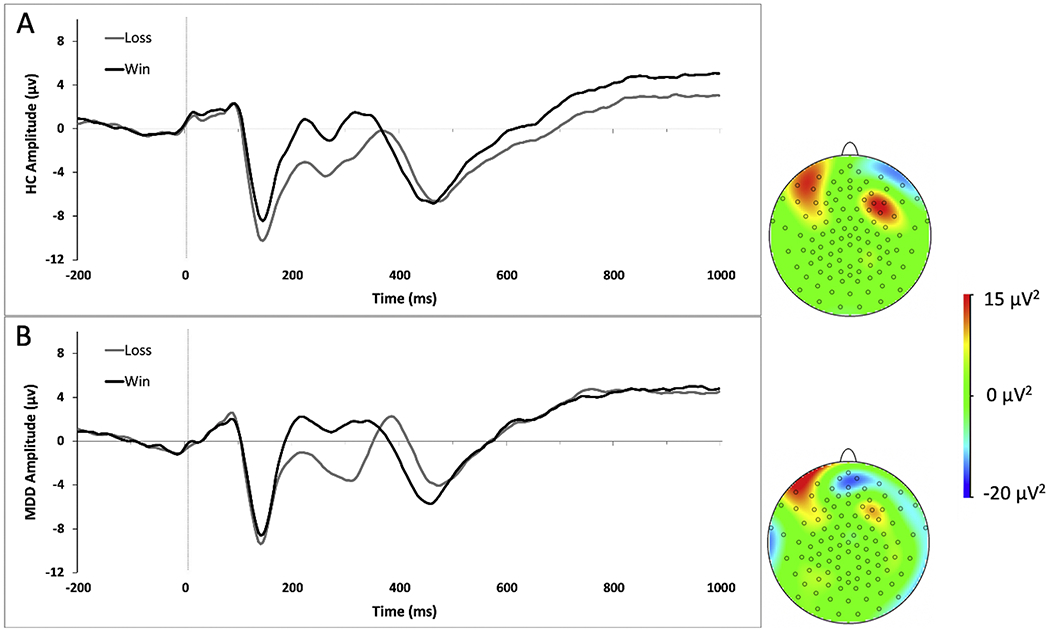
Plots of Late Positive Potential (LPP) for healthy controls (A) and adolescents with major depressive disorder (MDD) at baseline (B) in response to monetary wins (black) and losses (gray). The LPP was averaged across electrodes Fz, FCz and Cz from 600-1,000ms. Scalp distribution of the difference wave from 600-1,000ms are displayed.
Analytic approach
Given the longitudinal, multilevel data structure (i.e., repeated depressive symptom assessments nested within patients), we used a multilevel modeling (MLM; via lme4 (45) and lmerTest (46) packages in R) approach to test whether pre-treatment time-domain (RewP & LPP) and time-frequency (theta and delta power) variables predict depressive symptom improvement. Specifically, to test whether the RewP to wins and/or losses predict symptom change, an MLM simultaneously including RewPWins x Time and RewPLosses x Time interactions was modeled (Time centered to represent estimated post-treatment BDI-II scores, while adjusting for pre-treatment BDI-II scores).1 Corresponding models were run for the LPP, theta power, and delta power (i.e., similar to the above RewP model, including the win and loss interactions in the same model). As stated above, our primary hypotheses focused on whether (1) the delta power to rewards (during the timeframe of the RewP) and (2) the LPP to rewards predicted depression outcome (BDI-II total score). In each model, intercepts and slopes were treated as randomly varying across patients. To adjust for the effect of age, antidepressant medication (on SSRI vs not), and task version (versions A, B or C), Age x Time, Medication x Time, and Task Version x Time interactions were included in all models. All available data were used, including from dropouts, rendering these intent-to-treat analyses. However, patients missing baseline EEG/ERP data or who dropped out prior to completing at least 3 weeks of CBT were excluded (n=4). To examine change in time-domain or time-frequency variables over the course of treatment, we tested Group (MDD/HC) x Time (Initial/Mid/Final) x Condition (Wins/Losses) interactions, separately for the RewP, LPP, theta, and delta (adjusting for age and medication). (In contrast, Group was not included as a factor in the analyses presented in the below CBT Outcomes and Prediction of CBT Outcomes sections given that these analyses pertained only to the MDD group). As described in our hypotheses, we expected significant pre- to post-treatment increased delta power to rewards and decreased theta power to losses in the MDD group (relative to the HC group). All analyses were conducted in R with the exception of the latter Group x Time x Condition interactions which were conducted in SPSS Version 24.
Results
Internal (split-half) reliability and test-retest reliability for time-domain and time-frequency measures, as well as their intercorrelations, are reported in the Supplement.
CBT Outcomes
Intent-to-treat MLM analyses revealed that depressive (BDI-II) symptoms improved significantly over the course of treatment for the MDD group, Time: b = 1.08, t(28.2) = 4.52, p < .001. Among treatment completers, mean pre-treatment BDI-II scores were in the severe range (M = 30.35; SD = 11.57), whereas post-treatment scores were in the mild range (M = 16.93; SD = 14.24). This pre- to post-treatment change represents a large effect (Cohen’s d = 1.00)(Figure 3).
Figure 3.
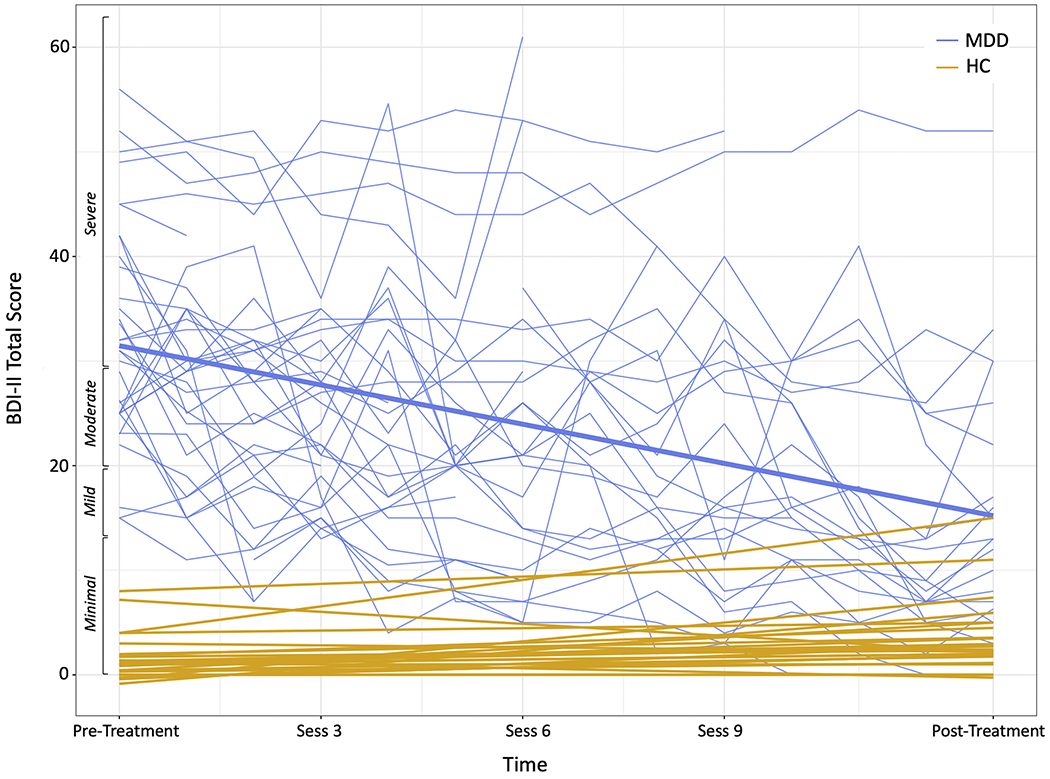
Session-by-session Beck Depression Inventory-II (BDI-II) scores for MDD participants (blue). Thicker blue line represents the regression line. HC participants’ BDI-II scores (gold) are also plotted for comparison (at 2 timepoints corresponding to pre- and post-treatment)
Prediction of CBT Outcomes
The pre-treatment RewP did not predict depressive symptom change (i.e., RewPWins x Time and RewPLoss x Time interactions were not significant; ps >.61). When using the conventional subtraction-based difference score approach (see Footnote 1), the RewP x Time interaction was not significant, p = 0.62). However, a pre-treatment LPPWins x Time interaction emerged, b = 0.81, t(27.6) = 2.38, p = .024, indicating that adolescents with a larger LPP response to wins had greater depressive symptoms improvement (Table 1 & Figure 4). A pre-treatment deltaLosses x Time interaction emerged, b = 0.53, t(27.1) = 2.49, p =.019, indicating that adolescents with a larger delta response to losses had greater depressive symptoms improvement (see Table 2, Figure 5). Corresponding pre-treatment theta x Time interactions were not significant (ps >.86). When both the significant LPPWins x Time and deltaLosses x Time interactions are included in the same model (residualized to adjust for LPPLoss and deltawins, respectively) both remained significant: (b = 0.40, t(27.0) = 2.15, p =.041; b = 0.41, t(25.6) = 2.06, p =.049, respectively).
Table 1.
LPP by time interactions predicting BDI-II symptom change
| Variable | B | SE | p value |
|---|---|---|---|
| (Intercept) | 14.48 | 4.86 | 0.01** |
| Baseline BDI | 10.24 | 0.85 | 0.00** |
| Time | 1.12 | 0.38 | 0.01** |
| Medication | −10.81 | 5.41 | 0.06+ |
| Age | 6.49 | 2.48 | 0.01* |
| Task Version | 9.01 | 6.13 | 0.15 |
| LPPWins | −9.14 | 4.32 | 0.04* |
| LPPLosses | 6.05 | 4.44 | 0.18 |
| Time x Medication | 0.92 | 0.42 | 0.04* |
| Time x Age | −0.46 | 0.19 | 0.02* |
| Time x Task Version | −0.53 | 0.48 | 0.45 |
| Time x LPPWins | 0.81 | 0.34 | 0.02* |
| Time x LPPLosses | −0.53 | 0.35 | 0.14 |
p < 0.10.
p < 0.05.
p < 0.01.
Figure 4.
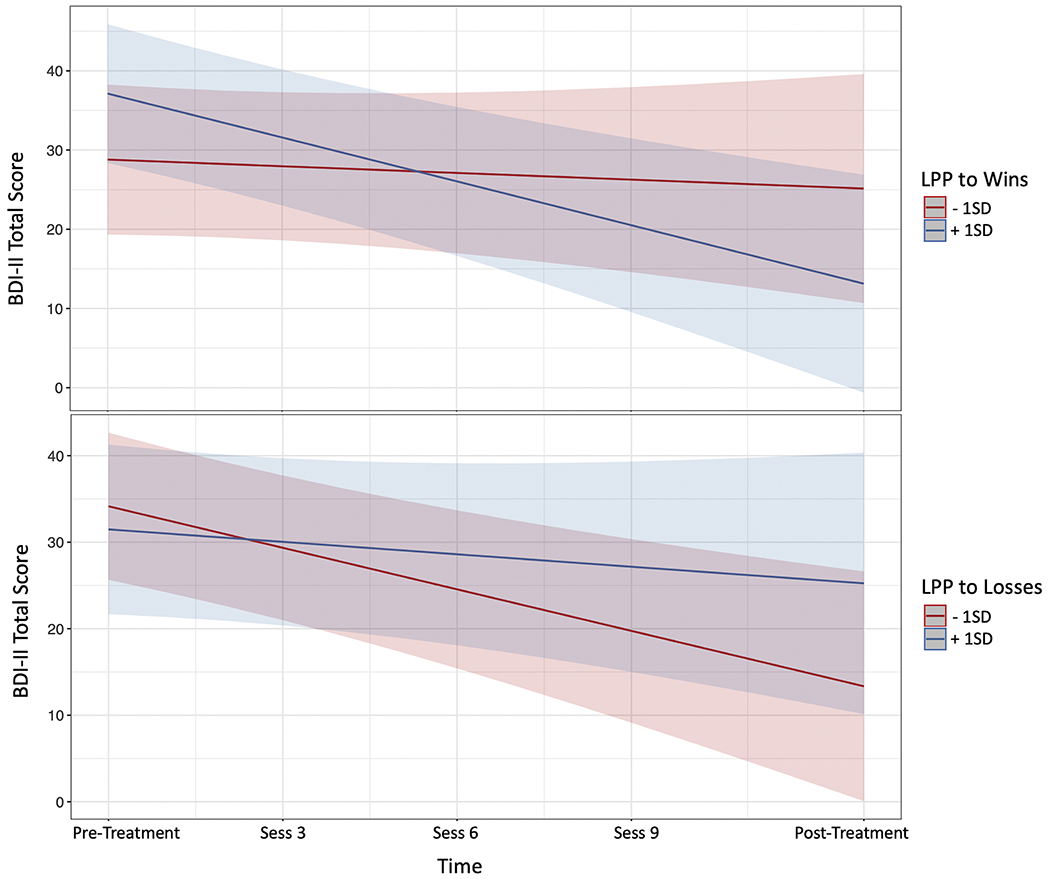
Plot of pre-treatment LPP by time interactions from the model. LPP to wins by time interaction is shown in the top panel, and LPP to losses by time interaction in the bottom panel.
Table 2.
Delta by time interactions predicting BDI-II symptom change
| Variable | B | SE | p value |
|---|---|---|---|
| (Intercept) | 10.69 | 5.17 | 0.04* |
| Baseline BDI | 9.90 | 0.89 | 0.00** |
| Time | 1.48 | 0.40 | 0.00** |
| Medication | −6.19 | 5.35 | 0.26 |
| Age | 8.35 | 2.66 | 0.00** |
| Task Version | 12.04 | 6.35 | 0.09+ |
| DeltaWins | 2.52 | 2.58 | 0.34 |
| DeltaLosses | −5.45 | 2.76 | 0.06+ |
| Time x Medication | 0.48 | 0.41 | 0.25 |
| Time x Age | −0.66 | 0.21 | 0.00** |
| Time x Task Version | −0.83 | 0.49 | 0.13 |
| Time x DeltaWins | −0.27 | 0.20 | 0.18 |
| Time x DeltaLosses | 0.53 | 0.21 | 0.02* |
p < 0.10.
p < 0.05.
p < 0.01.
Figure 5.
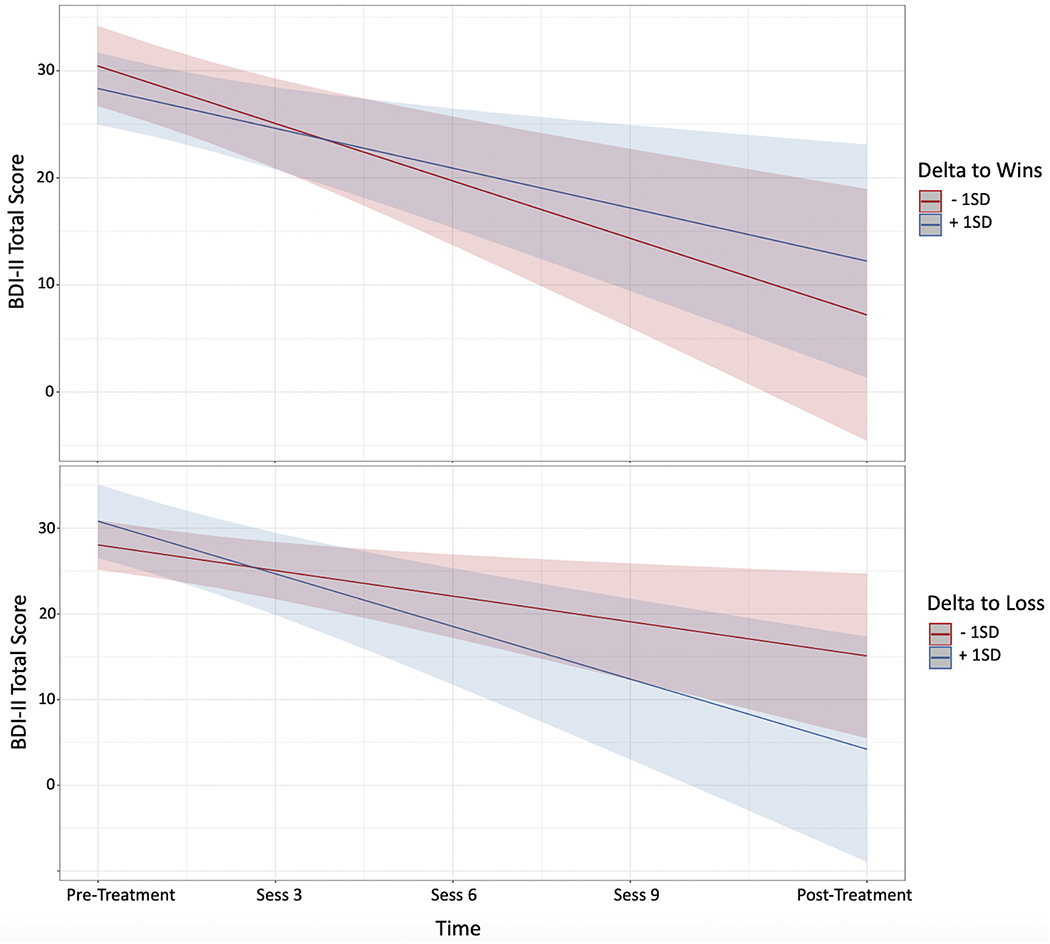
Plot of pre-treatment delta power by time interactions from the model. Delta to wins by time interaction is shown in the top panel, and delta to losses by time interaction in the bottom panel.
Changes in Neural Response Following CBT
No significant Group x Time x Condition interactions emerged for the RewP, LPP or delta power (all ps > .08). A Group x Time x Condition interaction emerged for theta power, F(2,37) = 4.00, p =.027, η2 = 0.18, such that the MDD group exhibited greater pre- to post-treatment reductions in theta response to losses relative to the HC participants (Figure 6). Greater pre- to post-treatment reductions in theta to losses were non-significantly associated with greater anxiety symptom improvement over the course of treatment, r = .44, p = 0.052 (depressive symptoms: r = .01; p = 0.971). Similarly, early reductions in theta to losses (i.e., from pre- to mid-treatment) were non-significantly associated with greater pre- to post-treatment anxiety symptom improvement, r = .43, p = 0.060, (depressive symptoms, r = .05; p = 0.828). Sensitivity analyses excluding the mid EEG assessment (i.e., only including data from initial and final EEG assessments), including number of days between EEG assessments as a covariate, and with imputed missing values yielded the same pattern of findings (see Supplemental Results).
Figure 6.
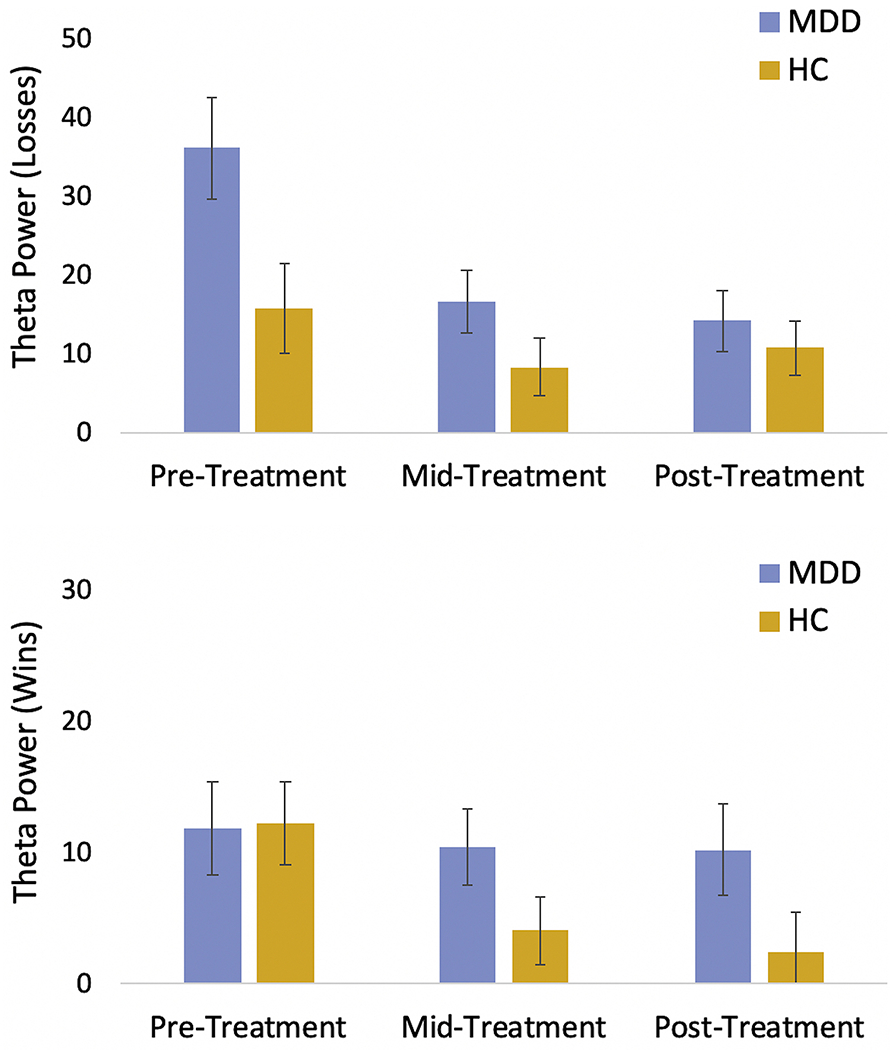
Change in theta power to losses (top panel) and wins (bottom panel) in the MDD participants (blue) vs. HC participants (gold) over time (model-derived estimated marginal means). Error bars represent standard error
Discussion
The present study evaluated whether the RewP and/or LPP, assessed prior to the start of treatment, predicted symptom change among depressed adolescent girls receiving CBT. In addition, we tested whether CBT modulated the RewP and LPP. Strengths of the study include (1) the use of time-frequency decomposition to isolate reward-related (delta power) and loss-related (theta power) neural signals, (2) simultaneous examination of ERPs linked to initial response to rewards (RewP) vs. later, elaborative processing (LPP), and (3) incorporation of pre-, mid- and post-treatment ERP assessments. Multilevel modeling revealed that the pre-treatment LPP, but not RewP, to rewards predicted symptom improvement during CBT. Similarly, Barch et al.(14) showed that larger pre-treatment LPP to pleasant pictures, but not the RewP to rewards, predicted better outcomes for young depressed children receiving PCIT. Although our findings are generally consistent with the latter study, they diverge from two initial studies in adults with depression and/or anxiety indicating that a reduced pre-treatment RewP to monetary rewards predicted greater depressive symptom improvement to CBT (8) and SSRI (7). In other words, in contrast to the latter two studies, our results do not support a “compensatory” model whereby individuals with more blunted—as opposed to intact or enhanced—neural reward responsiveness exhibit greater depressive symptom improvement. Additional research is needed to determine whether these inconsistencies may be due, at least in part, to differences in sample (adolescent girls vs. adults of both genders), diagnosis (MDD vs. depressive or anxiety disorders) and the variant of monetary reward task. It is also important to note that the average adolescent in our sample had severe levels of depression (mean pre-treatment BDI-II = 33), which may have influenced findings.
A consideration of the distinct neural generators of the RewP and LPP may help account for their differential pattern of prediction. Specifically, the RewP has been linked to activity within the mesocorticolimbic reward circuit (e.g., ventral striatum and medial prefrontal cortex) (12,13) and dACC (17); conversely, the LPP has been associated with a more distributed set of cortical and subcortical regions linked with visual, attentional and emotion processing, including occipital, parietal, inferotemporal and lateral prefrontal regions, as well as the amygdala and insula (50–54). In addition, in contrast to the RewP which reflects initial reactivity to the receipt of rewards (but see studies linking the RewP/FRN to unexpected outcomes or feedback indicating safety)(e.g.,56), the LPP reflects more sustained attention towards and engagement with emotional or motivationally salient content (and not specific to only rewards). Although speculative, depressed adolescents exhibiting more sustained neural engagement to rewarding or motivationally salient feedback may be relatively more likely to successfully engage in and benefit from cognitive and behavioral activities prescribed in CBT. Subsequent research including active comparison conditions (e.g., an SSRI or a different psychotherapy modality) are needed to test whether an enhanced LPP to rewards is a prescriptive (i.e., treatment-specific) or prognostic (i.e., treatment non-specific) predictor of outcome among depressed adolescents.
With regards to neural changes in treatment, only theta activity exhibited a significant Group x Time x Condition interaction. As displayed in Figure 6, the elevated pre-treatment theta activity to losses in the MDD group (relative to HC) is attenuated over the course of CBT. Importantly, the inclusion of a mid-treatment EEG assessment revealed that the majority (88.9%) of this pre- to post-treatment reduction occurred early in CBT (i.e., by the time of the mid-treatment EEG assessment). These findings suggest that CBT may attenuate neural hypersensitivity to negative feedback among depressed adolescents (16). In addition, both overall (pre- to post-treatment) and early (pre- to mid-treatment) reductions in theta activity to losses correlated moderately (rs =.43-.44) with pre- to post-treatment improvement in anxiety symptoms, but exhibited weak associations (rs =.01-.05) with depressive symptom improvement. Previous studies indicate that frontal midline theta power is more strongly associated with anxiety than depressive symptoms (56,57,57–59). Frontal midline theta activity is elicited not only by tasks involving negative or loss feedback, as in the present study, but by a range of paradigms requiring the deployment of cognitive control (e.g., tasks involving the commission of errors, stimulus-response conflict and novelty)(56,60). As others have argued, frontal midline theta elicited during these tasks is most likely generated from frontocingulate regions, in particular the ACC, which may be signaling the need to increase cognitive control in the service of adjusting behavior adaptively (56,60). In addition to being correlated with anxiety symptoms, enhanced theta response to aversive/incorrect feedback has been linked to heightened avoidance learning (59,61), suggesting one mechanism through which neural (theta) hypersensitivity to negative feedback may contribute to maladaptive behavior (e.g., anxiety-related avoidance)(56). Research is needed to test whether CBT-related reductions in theta power to negative outcomes are associated with normalization of avoidance learning.
In contrast, we did not observe increases in neural markers of reward sensitivity (RewP and delta power) over the course of CBT. These findings may reflect the fact that anhedonia and associated reward-related deficits in depression are among the most common residual symptoms following psychotherapy or pharmacotherapy and are particularly challenging to successfully target (62–64). Treatments that more directly target anhedonia, such as Behavioral Activation (BA) (15) and positive affect-focused treatments (64), may be more likely to modulate reward related-circuitry (e.g., for a relevant BA example, see (65)). Although CBT includes BA interventions, a substantial proportion of treatment is devoted to teaching patients cognitive skills to identify and modify maladaptive thinking patterns. In contrast, BA may be more likely to target reward circuitry function given its greater focus on teaching depressed individuals an array of behavioral strategies aimed at gradually and systematically increasing their exposure to and engagement with rewarding experiences and activities. Ultimately, a comparative trial is needed in which depressed adolescents are randomly assigned to BA vs. CBT to test for treatment group differences in “target engagement” of reward circuitry function. Finally, the fact that neural markers predicting treatment outcome (LPP to rewards) did not exhibit significant pre- to post-treatment change (relative to HCs), and vice versa (i.e., theta to losses did not predict outcome but did demonstrate significant change from pre- to post-treatment) suggests a dissociation between neural markers predicting symptom improvement vs. neural mechanisms of change.
Several limitations should be noted. First, sample size was small, in particular for detecting interactions, and thus replication in a larger cohort is required. Second, the inclusion of a HC group who completed ERP tasks at three timepoints corresponding to the MDD group controlled for the effect of repeated EEG assessments and task practice effects. However, an active control condition is needed to test the specificity of findings to CBT vs. relevant alternative interventions (e.g., BA or SSRIs) for the treatment of MDD in adolescents. Third, although EEG is a relatively low-cost imaging approach (i.e., compared to fMRI) and has excellent temporal resolution (e.g., allowing us to isolate ERPs linked to initial vs. later, elaborative stages of neural processing), it suffers from poor spatial resolution (e.g., cannot isolate neural activity within relevant subcortical reward-related and emotion-related regions). Fourth, a relatively large number of statistical tests were conducted. Fifth, CBT fidelity was not measured. These limitations notwithstanding, the present study provides initial evidence that an ERP measure of sustained responsiveness to rewards predicts depressive symptom change in CBT. In addition, findings indicate that neural (theta) hypersensitivity to negative outcomes among depressed youth may be attenuated within the first few weeks of CBT. Ultimately, such research may help inform which depressed adolescents are better suited to CBT and may clarify the neural mechanisms underlying depressive symptom improvement.
Supplementary Material
Acknowledgments
This project was partially supported by an F32 MH099801(CAW), K23 MH097786 (RPA), R37 MH068376 (DAP) from the National Institute of Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided through the Tommy Fuss Fund (RPA).
Financial Disclosures
Over the past 3 years, Dr. Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Compass Pathway, Otsuka Pharmaceuticals, and Takeda Pharmaceuticals; one honorarium from Alkermes, and research funding from NIMH, Dana Foundation, Brain and Behavior Research Foundation, Millennium Pharmaceuticals. In addition, he has received stock options from BlackThorn Therapeutics. No funding from these entities was used to support the current work, and all views expressed are solely those of the authors. Dr. Webb, Dr. Auerbach, Ms. Bondy, Mr. Stanton, and Ms. Appleman report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A subtraction-based difference score approach (i.e., RewP to wins minus losses) was not used given recent evidence of its relatively poor psychometric properties (47–49). Instead, and similar to recent treatment outcome prediction efforts using the RewP (9), we included the RewP to wins and losses as separate variables, entered simultaneously in the same model. In other words, the resulting parameter estimate for the RewP to wins x Time interaction adjusts for the RewP to losses x Time interaction (and vice-versa) (see 47–49).
References
- 1.Avenevoli S, Swendsen J, He J-P, Burstein M, Merikangas KR (2015): Major Depression in the National Comorbidity Survey–Adolescent Supplement: Prevalence, Correlates, and Treatment. J Am Acad Child Adolesc Psychiatry 54: 37–44.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.NW 1615 L. St, Suite 800Washington, Inquiries D 20036USA202-419-4300 | M-857-8562 | F-419-4372 | M (n.d.): A growing number of American teenagers – particularly girls – are facing depression. Pew Research Center; Retrieved January 20, 2020, from https://www.pewresearch.org/fact-tank/2019/07/12/a-growing-number-of-american-teenagers-particularly-girls-are-facing-depression/ [Google Scholar]
- 3.Weersing VR, Jeffreys M, Do M-CT, Schwartz KT, Bolano C (2017): Evidence base update of psychosocial treatments for child and adolescent depression. J Clin Child Adolesc Psychol 46: 11–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckshtain D, Kuppens S, Ugueto A, Ng MY, Vaughn-Coaxum R, Corteselli K, Weisz JR (2019): Meta-Analysis: 13-Year Follow-Up of Psychotherapy Effects on Youth Depression. J Am Acad Child Adolesc Psychiatry. [DOI] [PubMed] [Google Scholar]
- 5.Cohen ZD, DeRubeis RJ (2018): Treatment Selection in Depression. Annu Rev Clin Psychol 14: 209–236. [DOI] [PubMed] [Google Scholar]
- 6.Webb CA, Cohen ZD, Beard C, Forgeard M, Peckham AD, Bjorgvinsson T (2020): Personalized prognostic prediction of treatment outcome for depressed patients in a naturalistic psychiatric hospital setting: A comparison of machine learning approaches. J Consult Clin Psychol 88: 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhouse KL, Gorka SM, Klumpp H, Kennedy AE, Karich S, Francis J, et al. (2018): Neural Responsiveness to Reward as an Index of Depressive Symptom Change following Cognitive-Behavioral Therapy and Selective Serotonin Reuptake Inhibitor Treatment. J Clin Psychiatry 79 10.4088/JCP.17m11836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burkhouse KL, Kujawa A, Kennedy AE, Shankman SA, Langenecker SA, Phan KL, Klumpp H (2016): Neural Reactivity to Reward as a Predictor of Cognitive Behavioral Therapy Response in Anxiety and Depression. Depress Anxiety 33: 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kujawa A, Burkhouse KL, Karich SR, Fitzgerald KD, Monk CS, Phan KL (2019): Reduced Reward Responsiveness Predicts Change in Depressive Symptoms in Anxious Children and Adolescents Following Treatment. J Child Adolesc Psychopharmacol 29: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL, Dahl RE (2010): Reward-related brain function as a predictor of treatment response in adolescents with major depressive disorder. Cogn Affect Behav Neurosci 10: 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Proudfit GH (2015): The reward positivity: From basic research on reward to a biomarker for depression. Psychophysiology 52: 449–459. [DOI] [PubMed] [Google Scholar]
- 12.Becker MPI, Nitsch AM, Miltner WHR, Straube T (2014): A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. J Neurosci Off J Soc Neurosci 34: 3005–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson JM, Foti D, Mujica-Parodi LR, Harmon-Jones E, Hajcak G (2011): Ventral striatal and medial prefrontal BOLD activation is correlated with reward-related electrocortical activity: a combined ERP and fMRI study. NeuroImage 57: 1608–1616. [DOI] [PubMed] [Google Scholar]
- 14.Barch DM, Whalen D, Gilbert K, Kelly D, Kappenman ES, Hajcak G, Luby JL (2019): Neural Indicators of Anhedonia: Predictors and Mechanisms of Treatment Change in a Randomized Clinical Trial in Early Childhood Depression. Biol Psychiatry 85: 863–871. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Nagy GA, Cernasov P, Pisoni A, Walsh E, Dichter GS, Smoski MJ (2020): Reward Network Modulation as a Mechanism of Change in Behavioral Activation. Behav Modif 44: 186–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webb CA, Auerbach RP, Bondy E, Stanton CH, Foti D, Pizzagalli DA (2017): Abnormal neural responses to feedback in depressed adolescents. J Abnorm Psychol 126: 19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foti D, Weinberg A, Bernat EM, Proudfit GH (2015): Anterior cingulate activity to monetary loss and basal ganglia activity to monetary gain uniquely contribute to the feedback negativity. Clin Neurophysiol 126: 1338–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernat EM, Nelson LD, Baskin-Sommers AR (2015): Time-frequency theta and delta measures index separable components of feedback processing in a gambling task. Psychophysiology 52: 626–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foti D, Hajcak G, Dien J (2009): Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology 46: 521–530. [DOI] [PubMed] [Google Scholar]
- 20.Broyd SJ, Richards HJ, Helps SK, Chronaki G, Bamford S, Sonuga-Barke EJ (2012): An electrophysiological monetary incentive delay (e-MID) task: a way to decompose the different components of neural response to positive and negative monetary reinforcement. J Neurosci Methods 209: 40–49. [DOI] [PubMed] [Google Scholar]
- 21.Pornpattananangkul N, Nusslock R (2015): Motivated to win: Relationship between anticipatory and outcome reward-related neural activity. Brain Cogn 100: 21–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Klawohn J, Burani K, Bruchnak A, Santopetro N, Hajcak G (2020): Reduced neural response to reward and pleasant pictures independently relate to depression. Psychol Med 1–9. [DOI] [PubMed] [Google Scholar]
- 23.Hajcak G, Nieuwenhuis S (2006): Reappraisal modulates the electrocortical response to unpleasant pictures. Cogn Affect Behav Neurosci 6: 291–297. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Liu F, Chen L, Jiang Z, Shang J (2019): Cognitive reappraisal in children: neuropsychological evidence of up-regulating positive emotion from an ERP study. Front Psychol 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser JS, Hartwig R, Moran TP, Jendrusina AA, Kross E (2014): Neural markers of positive reappraisal and their associations with trait reappraisal and worry. J Abnorm Psychol 123: 91–105. [DOI] [PubMed] [Google Scholar]
- 26.Harrison NR, Chassy P (2017): Habitual Use of Cognitive Reappraisal Is Associated With Decreased Amplitude of the Late Positive Potential (LPP) Elicited by Threatening Pictures. J Psychophysiol. [Google Scholar]
- 27.Stange JP, MacNamara A, Barnas O, Kennedy AE, Hajcak G, Phan KL, Klumpp H (2017):Neural Markers of Attention to Aversive Pictures Predict Response to Cognitive Behavioral Therapy in Anxiety and Depression. Biol Psychol 123: 269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khazanov GK, Ruscio AM, Forbes CN (2019): The Positive Valence Systems Scale:Development and Validation. Assessment 1073191119869836. [DOI] [PubMed] [Google Scholar]
- 29.Luby JL, Gilbert K, Whalen D, Tillman R, Barch DM (in press): The Differential Contribution of the Components of Parent-Child Interaction Therapy Emotion Development for Treatment of Preschool Depression. J Am Acad Child Adolesc Psychiatry. 10.1016/j.jaac.2019.07.937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forand NR, DeRubeis RJ (2013): Pre-treatment Anxiety Predicts Patterns of Change in Cognitive Behavioral Therapy and Medications for Depression. J Consult Clin Psychol 81: 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vittengl JR, Clark LA, Thase ME, Jarrett RB (2013): Nomothetic and Idiographic Symptom Change Trajectories in Acute-Phase Cognitive Therapy for Recurrent Depression. J Consult Clin Psychol 81 10.1037/a0032879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ilardi SS, Craighead WE (1994): The Role of Nonspecific Factors in Cognitive-Behavior Therapy for Depression. Clin Psychol Sci Pract 1: 138–155. [Google Scholar]
- 33.Stassen HH, Angst J, Hell D, Scharfetter C, Szegedi A (2007): Is there a common resilience mechanism underlying antidepressant drug response? Evidence from 2848 patients. J Clin Psychiatry 68: 1195–1205. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. (1997): Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. J Am Acad Child Adolesc Psychiatry 36: 980–988. [DOI] [PubMed] [Google Scholar]
- 35.Auerbach RP, Webb CA, Stewart JG (2016): Cognitive Behavior Therapy for Depressed Adolescents: A Practical Guide to Management and Treatment, 1 edition New York: Routledge. [Google Scholar]
- 36.Auerbach RP, Stanton CH, Proudfit GH, Pizzagalli DA (2015): Self-Referential Processing in Depressed Adolescents: A High-Density ERP Study. J Abnorm Psychol 124: 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beck AT, steer RA, Brown GK (1996): Beck Depression Inventory Manual. [Google Scholar]
- 38.March JS, Parker JD, Sullivan K, Stallings P, Conners CK (1997): The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry 36: 554–565. [DOI] [PubMed] [Google Scholar]
- 39.Johnsrude IS, Owen AM, Zhao WV, White NM (1999): Conditioned Preference in Humans:A Novel Experimental Approach. Learn Motiv 30: 250–264. [Google Scholar]
- 40.Johnsrude IS, Owen AM, White NM, Zhao WV, Bohbot V (2000): Impaired Preference Conditioning after Anterior Temporal Lobe Resection in Humans. J Neurosci 20: 2649–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox SML, Andrade A, Johnsrude IS (2005): Learning to Like: A Role for Human Orbitofrontal Cortex in Conditioned Reward. J Neurosci 25: 2733–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dennis TA, Hajcak G (2009): The late positive potential: a neurophysiological marker for emotion regulation in children. J Child Psychol Psychiatry 50: 1373–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auerbach RP, Bondy E, Stanton CH, Webb CA, Shankman SA, Pizzagalli DA (2016): Self-referential processing in adolescents: Stability of behavioral and ERP markers. Psychophysiology 53: 1398–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen MX (2014): Analyzing Neural Time Series Data: Theory and Practice, 1 edition Cambridge, Massachusetts: The MIT Press. [Google Scholar]
- 45.Bates D, Mächler M, Bolker B, Walker S (2014): Fitting linear mixed-effects models using lme4. ArXiv Prepr ArXiv14065823. [Google Scholar]
- 46.Kuznetsova A, Brockhoff PB, Christensen RHB (2017): lmerTest package: tests in linear mixed effects models. J Stat Softw 82. [Google Scholar]
- 47.Ethridge P, Weinberg A (2018): Psychometric properties of neural responses to monetary and social rewards across development. Int J Psychophysiol 132: 311–322. [DOI] [PubMed] [Google Scholar]
- 48.Luking KR, Nelson BD, Infantolino ZP, Sauder CL, Hajcak G (2017): Internal consistency of functional magnetic resonance imaging and electroencephalography measures of reward in late childhood and early adolescence. Biol Psychiatry Cogn Neurosci Neuroimaging 2: 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meyer A, Lerner MD, De Los Reyes A, Laird RD, Hajcak G (2017): Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology 54: 114–122. [DOI] [PubMed] [Google Scholar]
- 50.Liu Y, Huang H, McGinnis-Deweese M, Keil A, Ding M (2012): Neural Substrate of the Late Positive Potential in Emotional Processing. J Neurosci 32: 14563–14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nieuwenhuis S, Aston-Jones G, Cohen JD (2005): Decision making, the P3, and the locus coeruleus-norepinephrine system. Psychol Bull 131: 510–532. [DOI] [PubMed] [Google Scholar]
- 52.Sabatinelli D, Lang PJ, Keil A, Bradley MM (2007): Emotional Perception: Correlation of Functional MRI and Event-Related Potentials. Cereb Cortex 17: 1085–1091. [DOI] [PubMed] [Google Scholar]
- 53.Sabatinelli D, Keil A, Frank DW, Lang PJ (2013): Emotional perception: Correspondence of early and late event-related potentials with cortical and subcortical functional MRI. Biol Psychol 92: 513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kayser J, Tenke CE, Abraham KS, Alschuler DM, Alvarenga JE, Skipper J, et al. (2016): Neuronal generator patterns at scalp elicited by lateralized aversive pictures reveal consecutive stages of motivated attention. NeuroImage 142: 337–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mulligan EM, Hajcak G (2018): The electrocortical response to rewarding and aversive feedback: The reward positivity does not reflect salience in simple gambling tasks. Int J Psychophysiol 132: 262–267. [DOI] [PubMed] [Google Scholar]
- 56.Cavanagh JF, Shackman AJ (2014): Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J Physiol-Paris. 10.1016/jjphysparis.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cavanagh JF, Meyer A, Hajcak G (2017): Error-Specific Cognitive Control Alterations in Generalized Anxiety Disorder. Biol Psychiatry Cogn Neurosci Neuroimaging 2: 413— 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmidt B, Kanis H, Holroyd CB, Miltner WHR, Hewig J (2018): Anxious gambling: Anxiety is associated with higher frontal midline theta predicting less risky decisions. Psychophysiology 55: e13210. [DOI] [PubMed] [Google Scholar]
- 59.Cavanagh JF, Bismark AW, Frank MJ, Allen JJB (2018): Multiple Dissociations Between Comorbid Depression and Anxiety on Reward and Punishment Processing: Evidence From Computationally Informed EEG. Comput Psychiatry 3: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cavanagh JF, Frank MJ (2014): Frontal theta as a mechanism for cognitive control. Trends Cogn Sci 18: 414–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cavanagh JF, Bismark A, Frank MJ, Allen JJB (2011): Larger Error Signals in Major Depression are Associated with Better Avoidance Learning. Front Psychol 2 10.3389/fpsyg.2011.00331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nierenberg AA (2015): Residual symptoms in depression: prevalence and impact. J Clin Psychiatry 76: e1480–e1480. [DOI] [PubMed] [Google Scholar]
- 63.Dunn BD, German RE, Khazanov G, Xu C, Hollon SD, DeRubeis RJ (2020): Changes in Positive and Negative Affect During Pharmacological Treatment and Cognitive Therapy for Major Depressive Disorder: A Secondary Analysis of Two Randomized Controlled Trials. Clin Psychol Sci 8: 36–51. [Google Scholar]
- 64.Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, Rosenfield D (2019): Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. J Consult Clin Psychol 87: 457–471. [DOI] [PubMed] [Google Scholar]
- 65.Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ (2009): The Effects of Psychotherapy on Neural Responses to Rewards in Major Depression. Biol Psychiatry 66: 886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


