This editorial refers to ‘Cysteine 202 of cyclophilin D is a site of multiple post-translational modifications and plays a role in cardioprotection’ by G. Amanakis et al., pp. 212–223.
Haworth and Hunter first described the characteristics of permeability transition in bovine mitochondria in the late 1970s, establishing it to be a consequence of the reversible opening of a pore within the inner mitochondrial membrane. The role of this pore in myocardial ischaemia/reperfusion (I/R) injury has been the subject of considerable interest in the intervening years. During myocardial ischaemia, this pore, now known as the mitochondrial permeability transition pore (PTP) remains closed because metabolic acidosis prevents its opening. Upon reperfusion, PTP opening in response to biochemical changes [restoration of pHi, generation of reactive oxygen species, and mitochondrial calcium (Ca2+) overload], causes significant cellular injury. In part, this injury happens because PTP opening has deleterious effects on the mitochondria themselves: swelling and rupture, membrane depolarization, and uncoupling of oxidative phosphorylation.1 In part, substances released from the mitochondria through PTP initiate cell death. Together, these events contribute to the overall injury sustained by the heart during myocardial I/R injury. Since the PTP is a key mediator of myocardial I/R injury, much attention is focused on understanding its intrinsic regulatory pathways and how these may be targeted for therapeutic gain. The peptidylprolyl isomerase, cyclophilin D (CypD) is widely recognized as a regulator of the PTP. I/R-induced cell death in vivo is reduced in CypD knockout mice, confirming that CypD and PTP contribute to the tissue damage mediated by such injury.2
Importantly, the CypD inhibitor cyclosporin A (CsA) attenuates infarct size in small and large animal models of I/R. Promisingly, CsA also protected human atrial trabeculae subjected to simulated I/R injury1 and in a small pilot trial, its intravenous administration attenuated infarct size by approximately 40%3 in patients with ST-segment elevation myocardial infarction. However, data generated from the much larger CIRCUS trial proved less promising,4 to the consternation of many. Since CsA affects the mitochondria of all organs it may produce deleterious off-target effects. Gaining a greater insight into CypD regulation and function will therefore allow for the development of alternative and more specific therapies targeted at the cardiac PTP.
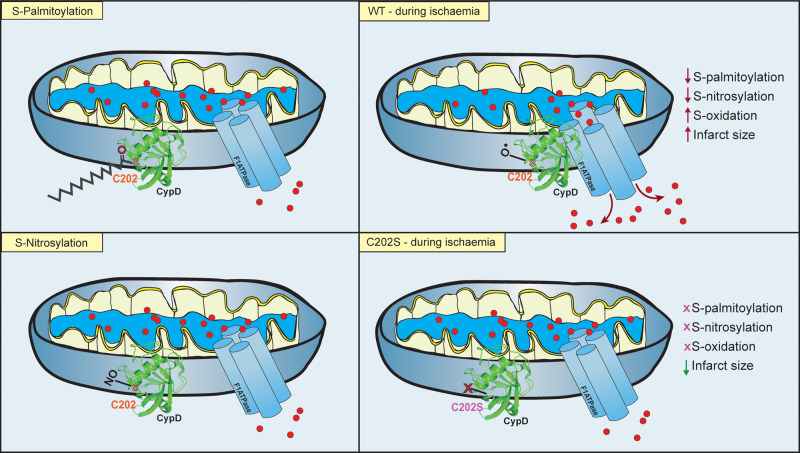
Although CypD knockout mice have significantly improved our understanding of its role in cardiac (patho)physiology, more subtle knock in animals are important to identify the key residues involved in its function. Amanakis et al. have used this knock in approach to evaluate the importance of CypD cysteine 202 in I/R injury.5 This residue had already been established to be oxidized during I/R injury, reversibly nitrosylated during ischaemic preconditioning6 and can also form an intramolecular disulphide.7 Amanakis et al. now demonstrate novel, dynamic S-palmitoylation of this residue. Their study reports substantial protection from I/R injury in C202S hearts compared to wild type, alongside a significantly enhanced resistance of C202S mitochondria to calcium overload-induced permeability transition. Interestingly C202S hearts confer no additional protection following I/R injury from CsA, suggesting PTP assembly requires CypD C202. CypD S-palmitoylation is reduced significantly during ischaemia (in intact hearts) and following calcium overload (in isolated mitochondria). Evidently, the signalling poise of this single amino acid has the potential to swing life and death decisions in the mitochondrial matrix.
To further elucidate the role of CypD palmitoylation in PTP assembly, the mechanisms by which the palmitoylation and de-palmitoylation occur warrant further study. S-palmitoylation is catalyzed by Asp-His-His-Cys (DHHC)-palmitoyl acyltransferase enzymes (zDHHC-PATs) which are integral membrane proteins localized throughout the secretory pathway. The activity of this enzyme family has been implicated in I/R injury by others. The cell surface localized zDHHC5 regulates a number of important cardiac substrates8 and is a key component of the process of massive endocytosis (MEND) following anoxia/reperfusion. The MEND pathway is triggered by PTP opening and acyl-CoA release from mitochondria, which leads to substantial remodelling of the cell surface membrane by zDHHC5.9 The sensitivity of MEND to PTP suggests CypD palmitoylation may itself regulate this pathway. As such, understanding which zDHHC-PAT palmitoylates CypD will be important to determine the impact of palmitoylation on CypD function and PTP assembly. Of the 23 zDHHC-PATs identified in humans, most are localized in the Golgi apparatus, endoplasmic reticulum and on the cell surface. However, proteomic studies have revealed several mitochondrial proteins are palmitoylated,10 so there is indirect evidence to suggest that some zDHHC-PATs localize to mitochondria. In particular, zDHHC13 was identified in a quantitative analysis of the liver S-palmitoylome, where its absence significantly impacted expression of proteins implicated in mitochondrial function,11 and zDHHC8 has been suggested to play an important role in regulating mitochondria in the brain.12 Although these DHHC-PATs have not been studied in the context of cardiac mitochondria, they could provide an insight into the mechanism of CypD palmitoylation. Conversely, it is noteworthy that as a mitochondrial protein, CypD is likely exposed to high local concentrations of acyl-CoA, and it is conceivable that this could cause it to be auto-palmitoylated, as has been demonstrated with other soluble proteins.13
In the work of Amanakis et al., exposing cardiac mitochondria to high calcium triggers CypD de-palmitoylation, potentially increasing availability of C202 for oxidation and subsequent PTP-induced injury at reperfusion (Figure 1). Calcium regulated de-palmitoylation is a previously unreported phenomenon that may have important consequences for palmitoylated cardiac substrates—particularly those involved in calcium handling.14 The mechanism of CypD de-palmitoylation is therefore of greatest interest. Recent work has revealed that APT1, one of seven protein thioesterases identified to date, localizes in mitochondria as well as the cytosol.15 In addition, ABHD10 (recently reported to act as a thioesterase), is exclusively localized in mitochondria where it de-palmitoylates the mitochondrial homeostasis regulator peroxiredoxin-5 (PRXD5).16 Both enzymes warrant investigation as the source of CypD de-palmitoylation during ischaemia.
Figure 1.
CypD C202 Modification in Cardiac Mitochondria. Amanakis et al. demonstrate that under basal conditions, the mitochondrial permeability transition pore (PTP) regulator cyclophillin-D (CypD) is reported to be both S-nitrosylated and S-palmitoylated at position cysteine 202 (C202). Under conditions of ischaemia, CypD S-palmitoylation and S-nitrosylation are reduced as C202 undergoes oxidation. This leads to greater association of CypD with the F1-ATPase subunit of the PTP and enhances pore opening and calcium efflux at reperfusion, thus leading to greater myocardial damage and increased infarct size. Mutation of C202 to a serine (S) results in no possible modification by S-nitrosylation, S-palmitoylation but also S-oxidation and transgenic mice have significantly reduced infarct size and enhanced functional recovery as a result.
While the significance of C202 in CypD function has been clearly demonstrated in this study, an important outstanding question concerns CypD palmitoylation stoichiometry, to understand what population of the protein is being regulated by palmitoylation. It remains a significant challenge to the field of cysteine post-translational modifications that chemically distinct post-translational modifications can compete for the same residue, complicating our understanding of the phenotypes of knock in models. Typically, it is the same solvent-exposed cysteines that form disulphides and are subjected to S-nitrosylation, S-acylation, oxidation, S-glutathiolation, etc. Since protection of C202 from oxidation may generate significant therapeutic benefit, understanding the cross-talk between modifications will be of vital importance.
Acknowledgements
We acknowledge financial support from the British Heart Foundation, SP/16/3/32317 to WF.
Conflict of interest: none declared.
References
- 1. Hausenloy DJ, Yellon DM.. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 2013;123:92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, Robbins J, Molkentin JD.. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005;434:658–662. [DOI] [PubMed] [Google Scholar]
- 3. Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N, Elbelghiti R, Cung TT, Bonnefoy E, Angoulvant D, Macia C, Raczka F, Sportouch C, Gahide G, Finet G, André-Fouët X, Revel D, Kirkorian G, Monassier J-P, Derumeaux G, Ovize M.. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med 2008;359:473–481. [DOI] [PubMed] [Google Scholar]
- 4. Zografos TA, Katritsis DG.. Cyclosporine before PCI in acute myocardial infarction. N Engl J Med 2016;374:88–90. [DOI] [PubMed] [Google Scholar]
- 5. Amanakis G, Sun J, Fergusson MM, Mcginty S, Liu C, Molkentin JD, Murphy E. Cysteine 202 of cyclophilin D is a site of multiple post-translational modifications and plays a role in cardioprotection. Cardiovasc Res 2021;117:212–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kohr MJ, Sun J, Aponte A, Wang G, Gucek M, Murphy E, Steenbergen C.. Simultaneous measurement of protein oxidation and S-nitrosylation during preconditioning and ischemia/reperfusion injury with resin-assisted capture. Circ Res 2011;108:418–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Linard D, Kandlbinder A, Degand H, Morsomme P, Dietz KJ, Knoops B.. Redox characterization of human cyclophilin D: identification of a new mammalian mitochondrial redox sensor? Arch Biochem Biophys 2009;491:39–45. [DOI] [PubMed] [Google Scholar]
- 8. Howie J, Reilly L, Fraser NJ, Vlachaki Walker JM, Wypijewski KJ, Ashford MLJ, Calaghan SC, McClafferty H, Tian L, Shipston MJ, Boguslavskyi A, Shattock MJ, Fuller W.. Substrate recognition by the cell surface palmitoyl transferase DHHC5. Proc Natl Acad Sci USA 2014;111:17534–17539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin M-J, Fine M, Lu J-Y, Hofmann SL, Frazier G, Hilgemann DW.. Massive palmitoylation-dependent endocytosis during reoxygenation of anoxic cardiac muscle. Elife 2013;2:e01295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kostiuk MA, Corvi MM, Keller BO, Plummer G, Prescher JA, Hangauer MJ, Bertozzi CR, Rajaiah G, Falck JR, Berthiaume LG.. Identification of palmitoylated mitochondrial proteins using a bio‐orthogonal azido‐palmitate analogue. FASEB J 2008;22:721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen LF, Chen YJ, Liu KM, Haddad ANS, Song IW, Roan HY, Chen LY, Yen JJY, Chen YJ, Wu JY, Chen YT.. Role of S-palmitoylation by ZDHHC13 in mitochondrial function and metabolism in liver . Sci Rep 2017;7:2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maynard TM, Meechan DW, Dudevoir ML, Gopalakrishna D, Peters AZ, Heindel CC, Sugimoto TJ, Wu Y, Lieberman JA, LaMantia AS.. Mitochondrial localization and function of a subset of 22q11 deletion syndrome candidate genes. Mol Cell Neurosci 2008;39:439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Duncan JA, Gilman AG.. Autoacylation of G protein α subunits. J Biol Chem 1996;271:23594–23600. [DOI] [PubMed] [Google Scholar]
- 14. Reilly L, Howie J, Wypijewski K, Ashford MLJ, Hilgemann DW, Fuller W.. Palmitoylation of the Na/Ca exchanger cytoplasmic loop controls its inactivation and internalization during stress signaling. FASEB J 2015;29:4532–4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kathayat RS, Cao Y, Elvira PD, Sandoz PA, Zaballa ME, Springer MZ, Drake LE, Macleod KF, Van Der Goot FG, Dickinson BC.. Active and dynamic mitochondrial S-depalmitoylation revealed by targeted fluorescent probes. Nat Commun 2018;9:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cao Y, Qiu T, Kathayat RS, Azizi SA, Thorne AK, Ahn D, Fukata Y, Fukata M, Rice PA, Dickinson BC.. ABHD10 is an S-depalmitoylase affecting redox homeostasis through peroxiredoxin-5. Nat Chem Biol 2019;15:1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]