Abstract
Alzheimer's disease (AD) is caused by several risk factors leading to dementia. It’s diagnosis usually depends on clinical presentation and certain biomarkers in the cerebrospinal fluid (CSF). The brain has a high content of cholesterol and the metabolism of cholesterol in the brain can be associated with beta-amyloid plaques formation, which is seen in Alzheimer’s disease. Given these implications, we studied if plasma lipid levels can vary in Alzheimer's disease and if these can be used as biomarkers to diagnose and predict the progression of Alzheimer's disease. Certain mutations in the brain cholesterol transport receptors and proteins and their association with Alzheimer's were also studied. This systematic review abides by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched multiple databases, such as Pubmed, Google Scholar, Pubmed central, ScienceDirect, Web of Science, and Medline with the help of keywords like Alzheimer's disease, cognitive impairment, plasma lipid biomarkers, cholesterol, brain cholesterol metabolism separately and in combination with each other. We collected 49 quality appraised articles on the association between plasma lipids and Alzheimer's disease and the genetic mutations in alleles related to cholesterol metabolism and Alzheimer's disease by applying the inclusion and exclusion criteria. Based on the finding of the studies reviewed, we found an association between plasma lipids, polymorphisms in genes associated with cholesterol transport, and Alzheimer's disease. Increased serum low-density lipoprotein (LDL-C), triglycerides (TG), total cholesterol (TC), sphingolipids, 24S hydroxycholesterol (24S-HC), 27O hydroxycholesterol (27O-HC) was associated with Alzheimer's. Decreased high-density lipoprotein (HDL-C) and phospholipids were noticed. Genetic mutations in apolipoprotein E (ApoE), apolipoprotein B (ApoB), apolipoprotein A (ApoA), ATP binding cassette transporter 1 (ABCA1), ATP binding cassette transporter 7 (ABCA7), amyloid precursor protein (APP), cytochrome P450 family 46 subfamilies A member 1 (CYP46A1), presenilin 1 (PSEN1), presenilin 2 (PSEN2) are also associated with increased risk of Alzheimer's disease. This study found an association between plasma lipids and Alzheimer's, proving that plasma lipids can be used as biomarkers for early diagnosis of Alzheimer's disease. It may also help predict the prognosis and stage the disease severity. Further studies are needed to find out the exact mechanism behind these changes.
Keywords: alzheimers disease, cognitive impairment, plasma lipid biomarkers, cholesterol, brain cholesterol metabolism
Introduction and background
Alzheimer's disease and other causes of dementia constitute an increasing challenge in the health care system worldwide. There are approximately 50 million people currently living with dementia [1]. Alzheimer's disease is associated with a high mortality rate; it is the seventh leading cause of death in older people [2]. Alzheimer's disease (AD) was discovered based on the findings of an autopsy, which suggested atrophy of the brain cortex [3].
Alzheimer's disease is characterized by memory loss and cognitive impairment due to degeneration in the brain. It's the most common type of dementia. It's a progressive disease beginning with mild cognitive impairment resulting in memory loss, language, and thinking ability. Mild cognitive impairment is the phase between the cognitive impairment normally seen in the elderly and the cognitive decline due to complex conditions causing dementia. Pathologically, Alzheimer's disease is due to the building up of beta amyloid-forming plaques in the brain cortex and deposition of phosphorylated tau protein in the neurofibrillary tangles [3].
Both genetic and non-genetic risk factors can cause Alzheimer's disease. Ageing is the most important risk factor. Other causes include cerebrovascular disease, increased blood pressure, increase insulin resistance in type two diabetes mellitus, bodyweight, metabolic syndrome, smoking, traumatic brain injury, plasma lipid levels, diet, intellectual activity, and decreased physical activity [4]. Research focusing on the genetic component of Alzheimer's disease shows that Individuals with the apolipoprotein E4 (ApoE4) allele are at a higher chance of the disease [5-7]. Early-onset Alzheimer's disease is usually caused due to genetic variants in genes coding for amyloid precursor protein (APP) or presenilin (PSEN 1 and PSEN 2) [8-10]. Late-onset Alzheimer's is not known to be due to mutations in these genes.
Currently, the diagnosis of Alzheimer's disease is made based on the clinical presentation of the patients and the laboratory diagnosis of three biomarkers in the cerebrospinal fluid (CSF): amyloid-beta 42, total tau, and phospho-tau, out of which beta-amyloid 42 is the most sensitive biomarker [11]. Biomarkers of cognitive decline help to detect the biochemical and pathological changes of AD in the cerebrospinal fluid. Cerebrospinal fluid is present in the brain, and hence a sample of the cerebrospinal fluid would help us evaluate the onset and progression of the disease. But a collection of a cerebrospinal fluid sample is an inconvenient process requiring a lumbar puncture, which is not routinely done in primary care, geriatric care, or psychiatry. This has led to the discovery of other biomarker molecules found in the plasma, which can help us evaluate Alzheimer's disease without the need for invasive, expensive tests and can also be done as large-scale screening tests. These biomarkers are plasma lipids: high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C), Total serum cholesterol, total cholesterol/HDL ratio, triglycerides, 24S-hydroxycholesterol (24S-HC), lipoprotein A, phospholipids, and sphingolipids. It is better to test this entire panel of biomarkers, as a single biomarker will not help detect a complex condition, such as Alzheimer's disease. Many recent research pieces have found a link between plasma lipids and Alzheimer's disease [12-15]. The brain has very high lipid content; hence changes in the brain phospholipids and cholesterol levels can easily lead to pathology in the brain [16,17].
The blood-brain barrier (BBB) prevents the entry of circulating cholesterol into the brain. The brain produces cholesterol from the astrocytes, which is later converted to 24S-hydroxycholesterol. 24S-Hydroxycholesterol can cross the blood-brain barrier and is excreted via the bile from the plasma [5]. In the case of hyperlipidemia, increased plasma cholesterol leads to the formation of free radicals, which disrupt the blood-brain barrier and lead to increased cholesterol in the brain. Cholesterol plays a key role in amyloidogenesis in the brain, causing more beta-amyloid plaques formation and leading to neurodegeneration. ApoE4 is a carrier for cholesterol transport; hence an individual carrying an allele of ApoE4 is prone to developing Alzheimer's [5,6]. Many other genetic mutations of the receptors and transporters involved in cholesterol metabolism and transport, such as ATP binding cassette transporter 1 and 7 (ABCA1, ABCA7), APP have caused Alzheimer's disease [8].
This study aims to show the relationship between plasma lipids and Alzheimer's disease, to demonstrate if increased plasma cholesterol increases the risk for Alzheimer's disease. It will help us understand if these plasma lipid biomarkers can be used as (1) diagnostic biomarker - to evaluate the risk of the onset of AD and (2) monitoring biomarkers - to estimate the progression of the condition. We will also discuss the various genetic variants associated with the onset of Alzheimer's disease.
Review
Method
This systematic review strictly follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [18]. For data collection, we searched multiple electronic databases, such as PubMed, Google Scholar, Medline, ScienceDirect, PubMed Central, and Web of Science and the website of Neuropathology. Data were collected in September 2020. We used keywords such as "Alzheimer's disease," "cognitive impairment," "plasma lipid biomarkers," "cholesterol," "brain cholesterol metabolism" separately and in combination with each other. We found 3,417 articles with the help of these keywords. The articles' screening was done by going through the topics and abstracts and keeping the ones relevant to our research question. Inclusion and exclusion criteria were applied, and articles were further narrowed down to relevant ones. We did the quality appraisal of all the reference articles by following guidelines, and good quality forty-nine articles were kept.
Inclusion Criteria
Study selection included the following criteria: studies conducted in English, on humans over 40 years of age, in the last 20 years, that were relevant to our topic and research question, peer-reviewed, full texts, including these study types - clinical trials, observation studies (case-control, cohort, and cross-sectional studies), systematic review, meta-analysis and literature review.
Exclusion Criteria
Grey literature, books, letter to editor, editorials, duplicate and overlapping studies, in vitro or animal studies.
Result
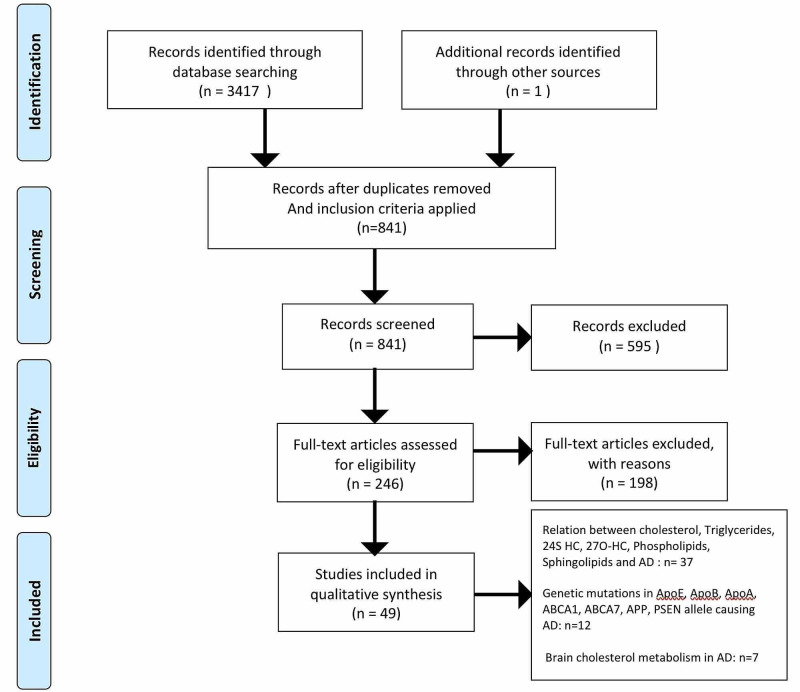
A total number of 3,417 studies were identified from the databases. Filters applied based on inclusion criteria (full-text studies in English, last 20 years, on humans, clinical trials, all types of reviews, observational studies), and studies were filtered and reduced to 246. Screening of the articles was done, and relevant studies kept - 54. Quality appraisal was done for all studies, and the number of studies included reduced to 48. These included 11 systematic reviews/meta-analyses, 12 literature reviews, two randomized control trials, 13 case-control studies, seven cohort studies, and three cross-sectional studies. One article from the website of Neuropathology was included. A total of 49 articles were studied [1-49]. This study includes 37 studies that proved the relationship between increased plasma cholesterol, triglycerides, 24S hydroxycholesterol, 27O hydroxycholesterol, sphingolipids and phospholipids, and Alzheimer’s disease. Twelve studies prove that genetic mutations in ApoE, ApoB, ApoA, ABCA1, ABCA7, APP, and PSEN 1 and PSEN2 alleles are associated with AD. Seven studies explain the metabolism of cholesterol in the brain and the pathology associated with AD (Figure 1).
Figure 1. PRISMA flow chart .
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; AD: Alzheimer's disease, APO: apolipoprotein, APP: amyloid precursor protein; 24S HC: 24S hydroxycholesterol; 27O-HC: 27O hydroxycholesterol; PSEN: presenilin; ABCA1 and ABCA7: ATP binding cassette transporter 1 and 7
Discussion
We studied 49 previously published articles, some stressing the association between Alzheimer’s disease and plasma lipids and some about the genetic variants of various cholesterol transporters causing AD. In this study, we found that a dysregulation of brain lipid homeostasis can lead to cognitive disorders such as Alzheimer's disease. The exact pathway by which certain mutations happen is still not clear and more research is needed to confirm that.
Cholesterol Metabolism in the Brain and Pathology Related to AD
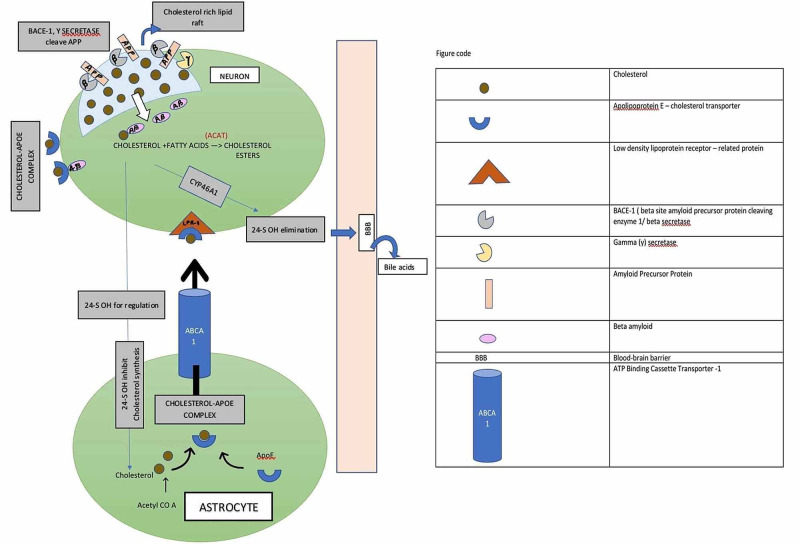
The brain has a rich lipid content. The majority of cholesterol in the central nervous system (CNS) is present in two places; one is the oligodendrocytes of the myelin sheath, and the other is the plasma membrane of astrocytes and neurons. Myelin comprises 70% lipids containing cholesterol, sphingolipids, and phospholipids [19]. Cholesterol, sphingolipids are essential components of the neuronal plasma membrane present in the form of lipid rafts. It participates in signal transduction, neurotransmitter release, synaptogenesis, and membrane trafficking [11,20]. It is believed that the developing brain produces a high level of cholesterol, but this gradually reduced in the adult brain. The adult brain synthesizes cholesterol by the astrocytes, then transported to the neurons to carry out its functions.
Cholesterol metabolism in the brain depends on brain cells' synthesis, transport across the cells, BBB, and catabolism [5]. Neurons depend on glial cells (astrocytes) for cholesterol. Astrocytes synthesize cholesterol from acetyl CoA by the HMG-CoA reductase enzyme. The transport of cholesterol to neural cells takes place with the help of ApoE which is also synthesized by astrocytes. The ApoE-cholesterol complex is transported with ABCA-1 and is taken up by the neurons via endocytosis through the LDL receptor-related protein (LRP-1) [5]. The endocytosed cholesterol in the neuron is further hydrolyzed to form free cholesterol. Free cholesterol undergoes esterification with acyl-coenzyme A cholesterol acyltransferase to form cholesterol esters stored in the neuron's cytoplasm. Some of the free cholesterol also controls the expression of cholesterol synthesizing enzymes and lipoprotein receptors such as liver X receptors (LXRs). These LXRs lead to increased expression of the ABCA1, thus mediating the transport of cholesterol from cells to apolipoproteins [5,6]. There is no degradation mechanism for any excess cholesterol in the brain. The excess cholesterol is converted to 24S-HC by the cytochrome P450 family 46 subfamilies A member 1 (CYP46A1) enzyme and is transported out of the brain to the blood-brain barrier [21].
The pathognomonic feature of AD is the building up of beta-amyloid plaques in the brain. In a healthy brain, the beta-secretase/beta-site amyloid precursor protein cleavage enzyme -1 (BACE-1) and gamma-secretase are present in the lipid rafts of the neuronal plasma membrane and cause cleavage of APP forming beta-amyloid. However, in AD, increased cholesterol lipid rafts induce raft clustering and enhance BACE-1 and APP interaction leading to increased beta-amyloid production [5,20,22].
The BBB maintains CNS homeostasis by regulating the transport of solutes between blood and brain. The BBB allows diffusion of oxygen and carbon dioxide freely, although lipophilic molecules such as cholesterol enter through receptors or channels. The brain lipid nature's homeostatic balance helps in controlled beta-amyloid production by APP cleavage, maintains the receptor channels, vesicle formation, secretion, signaling, inflammation, oxidation, membrane biosynthesis, and remodeling. Dysregulation in the brain lipid environment attributes to disturbed BBB, abnormal APP processing, abnormal cytosis, signaling, increased inflammation, oxidation. Long term, these can result in neuronal death, leading to AD (Figure 2) [22].
Figure 2. Demonstrating the process of synthesis, metabolism, and transport of brain cholesterol.
The Relation Between Plasma Lipids and AD
Many researchers have found a link between cholesterol homeostasis and AD; however, the exact pathogenesis remains unclear. We know that brain is rich in cholesterol content. The cholesterol in the brain and periphery are two separate units as they are well separated by a blood barrier, which restricts the entry of peripheral cholesterol. The cholesterol present in the lipid rafts of plasma neuronal membrane causes cleavage of APP. In the case of increased cholesterol in the lipid rafts, cholesterol enhances the activity of BACE-1 and Gamma-secretase and causes cleavage of APP leading to increased beta-amyloid production. These beta-amyloid plaques are characteristic hallmarks of AD. Increased plasma LDL-C, TC, triglycerides (TG), and decreased HDL-C was associated with increased beta-amyloid plaques causing AD. A cohort study by Pappolla et al. emphasized the association between high plasma cholesterol and patients with AD [23]. They found that increased cholesterol in the plasma was leading to increased beta-amyloid production. Many other studies found the same association between cholesterol and beta-amyloid plaques in the brain [4,24]. Some studied proved that beta-amyloid isoforms 1-40, 1-42 are associated explicitly with plasma cholesterol [7,13]. The study by Iqbal et al. was a systematic review showing that increased LDL-C, TC, TG were increasing beta-amyloid production. In contrast, decreased HDL-C was associated with AD [24].
Apart from amyloid plaques, AD is also associated with atrophy of the left/right hippocampal and entorhinal cortex. These changes are seen in the early stage of AD even before symptoms could arise. A case-control study by Proitsi et al., including 300 patients, also found that increased plasma lipids led to amyloid plaques in the brain and hippocampal and entorhinal cortex atrophy patients with AD [25]. Another study by Wolf et al. also studied the hippocampal atrophy of the brain and found its link with cholesterol; however, it was only associated with HDL-C [26]. The role of HDL-C in the disease process of AD is controversial. HDL is found to reduce the build-up of beta-amyloid plaques, thus reducing inflammation. Many researchers studied this protective role of HDL-C. Formiga et al. included 321 patients in a cohort study and proved the association between decreased HDL and AD [27]. Few other studies also proved the same objective [26,28]. Physical activity can decrease HDL levels and has proven to improve symptoms and progression of AD by a randomized control trial of 170 patients [28].
Cholesterol and triglycerides are also known to be a predictive marker for cardiovascular diseases. These lipids build up and obstruct the arteries supplying the heart. A similar mechanism was assumed for AD. Scientists believed that AD happens due to the brain's poor oxygenation due to the lipids' clogged vessels. Some studies determined the relation between these serum lipids and AD and if it is similar to the cardiac risk profile. A cohort study of nearly 4000 subjects by Helzner et al. shows that plasma lipids are associated with AD and other vascular diseases [29]. However, the mini-mental state exam (MMSE) was not affected, and hence it is still unclear how the cardiac risk profile affects cognition [15,30]. The relation between cholesterol and low MMSE was seen in a study by Hall et al. [31].
Unlike the studies already discussed, some studies say that only TC has an association with AD. The theory remains unclear, though. A study by He et al. proved that LDL, HDL, and triglycerides levels remain normal and only increased TC was seen in AD [32]. He included 130 patients in his case-control study. Similar studies by other authors also proved the same concept [33,34]. These studies by Solomon et al. and Anstey et al. suggest that total cholesterol has a bidirectional association with AD. It was seen to rise to midlife, suggesting cognitive impairment, and later declined with age in patients with AD. Solomon et al. proved this by conducting a large-scale case-control study, including 1321 patients with AD and 1203 controls [34]. Some other studies also found that only a single lipoprotein- low-density lipoprotein was elevated in AD, serum levels of rest were normal.
LDL-C is seen to cause vascular and neurotoxic effects in the brain [35,36]. Another study by Zhon, a systematic review including nearly 6500 patients, found the same association; however, LDL-C was high in patients with AD mostly around 60-70 years of age, gradually reduced with ageing [37]. Some studies found an association between AD and only LDL-C, TC. Many hypotheses are present. Some say LDL-C, TC is associated with increased tau concentration, according to some LDL-C, TC cause increased amyloid build-up, and some found that LDL and TC disrupt the cell cycle [38]. A study by Liu et al., including around 2333 AD patients and 3615 healthy controls, also suggested the association between LDL-C, TC, and AD [39]. Two studies also found that cholesterol remains normal, and only the serum triglyceride level is increased in the case of AD [22,40].
It is known that de novo synthesis of cholesterol occurs in the brain, and any disruption in this mechanism can lead to AD. 24S Hydroxycholesterol is the elimination product of neuronal cholesterol that leaves the brain and enters the periphery by crossing the BBB. 24S-HC and 27O hydroxycholesterol in the plasma indicates the degree of beta-amyloid production, loss of active grey matter, phosphorylated tau accumulation, and brain atrophy, thus indicating AD [11]. A case-control study by Popp et al., including 200 patients, found an association between increased plasma 24S hydroxycholesterol, 27O hydroxycholesterol, and AD [41]. Another study also showed a similar association [30].
Another group of lipids associated with AD is the sphingolipids and phospholipids. Sphingolipids such as sphingomyelin, ceramide, sulfatide, and sphingosine are major constituents of the neuron's plasma membrane. They are present in the lipid rafts, hence have a role in enhancing the activity of BACE-1 and gamma-secretase that causes cleavage of APP, forming beta-amyloid [20]. Phospholipids such as phosphatidylcholine, plasmogens, phosphatidylinositol, too, are a part of the membrane-forming lipid [20]. An association between serum and CSF levels of sphingolipids and phospholipids and AD has been studied. A study by Wong et al. showed that CSF sphingomyelin levels increase in the prodromal stage in AD, CSF ceramide increases in AD, CSF sulfatide levels decrease in AD, CSF phospholipids levels also increase. Whereas in the blood, ceramide level increases, sphingomyelin levels decrease, and phospholipids decrease in AD [20]. Another study by Kosicek et al. also found similar results [17]. A case-control study by Costa et al. studied ten membrane phospholipids and their association with AD. These were found to decrease in AD (Table 1) [42].
Table 1. Summarizing the association between plasma lipids and AD.
LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides; AD: Alzheimer's disease; APO: apolipoprotein; Aβ: beta-amyloid; MCI: mild cognitive impairment; BBB: blood-brain barrier; 24S HC: 24S hydroxycholesterol; 27O-HC: 27O hydroxycholesterol; PC: phosphatidylcholine; PLA2: phospholipase 2
| Author | Year of Publication | Type of Study | Purpose of Study | Intervention Studied | Result/Conclusion | |
| 1. | Saiz-Vazquez et al. [35] | 2020 | Meta-analysis | To determine the association of serum cholesterol and AD. | Total serum cholesterol, HDL-C, LDL-C, and serum triglycerides | The relation between increased LDL-C and AD was found. No association between TC, TG, HDL-C, and AD were found. |
| 2. | Zhou et al. [37] | 2020 | A systematic review and meta-analysis | To determine the association of serum cholesterol and AD. | LDL-C was measured | Elevated LDL-C leads to AD. This association is more in patients 60–70 years of age and gradually declines with age. |
| 3. | Iqbal et al. [24] | 2020 | Systematic review | To determine the association of serum lipids and AD | HDL-C, LDL-C, TG, TC | HDL-c was found to be low, whereas LDL-C, TG, TC were high in cases of AD. |
| 4. | Bernath et al. [40] | 2020 | Cross-sectional study | To determine the association of triglycerides with AD | Serum TG | Serum TG was found to be high in cases of AD |
| 5. | Jensen et al. [28] | 2020 | Randomized control trial | To determine if physical exercise influences the lipid profile and AD | HDL-C, TC, LDL-C, triglycerides | With an increase in physical activity, cholesterol was lower, thus decreasing the risk of AD. |
| 6. | Chew et al. [22] | 2020 | Literature review | To determine the factors affecting lipid metabolism and the association between plasma lipids and AD. | Age, sex, race, diet, plasma lipids level | Decreased plasma HDL-C and increased triglyceride is associated with AD. |
| 7. | Liu et al. [39] | 2019 | Meta-analysis | To determine the association between plasma cholesterol and AD | Total serum cholesterol, HDL-C, LDL-C, and serum triglycerides | Serum LDL-C and total cholesterol were found to be elevated in AD. No association between HDL-C and triglycerides was found. |
| 8. | Wu et al. [38] | 2019 | Meta-analysis | To determine the association between plasma lipids and AD | HDL-C, TC, LDL-C | LDL-C, TC are increased in AD |
| 9. | Costa et al. [42] | 2019 | Case-control study | To determine the association between phospholipids and AD, and the activity of PLA2 in the brain | Serum phospholipids, PLA2 by radio enzymatic assay | Ten serum phospholipids were assessed and found to decrease in AD; PLA2 activity was also decreased in the neuronal membrane in AD. |
| 10. | Anstey et al. [34] | 2017 | A systematic review and meta-analysis | To determine the association between plasma lipids and AD in midlife | HDL-C, LDL-C, TC, TG | Increased TC in midlife is found to be associated with late-onset AD. No association between HDL and TG with AD was found. |
| 11. | Proitsi et al. [25] | 2017 | Case-control study | To determine the association between plasma cholesterol and AD and the associated brain atrophy | Plasma lipids. Brain – left/right hippocampal area, entorhinal cortex | Increased plasma lipids were associated with AD, causing brain atrophy. |
| 12. | Wong et al. [20] | 2017 | Literature review | To determine the progress in lipidomics research in AD with the help of mass spectrometry | Phospholipids, sphingolipids, and cholesterol | Increase total cholesterol increase the risk of AD. Sphingolipids: in CSF – sphingomyelin levels increase in prodromal AD, ceramide increases in AD, sulfatide decreases in AD. As blood – ceramide level increases, sphingomyelin decreases in AD. Phospholipids: increases in CSF and decreases in blood in AD. |
| 13. | Wang et al. [11] | 2016 | Meta-analysis | To determine if 24S-HC and 27 O HC are biomarkers for AD | 24S-HC, 27S-HC | 24S-HC and 27O-HC are sensitive biomarkers for AD diagnosis |
| 14. | He et al. [32] | 2016 | Case-control study | To determine the association of plasma lipids with MCI in the elderly | Total cholesterol, HDL-C, LDL-C, serum triglycerides were measured | TC was elevated in the elderly with AD. No association between LDL-C and AD was found. HDL-C and TG were negatively related to AD. |
| 15. | Hall et al. [31] | 2014 | Systematic review | To determine if increased cholesterol in AD is gender-specific | Total serum cholesterol | Males were found to have higher total Cholesterol in AD |
| 16. | Toro et al. [44] | 2014 | Cohort study | To determine the association of total cholesterol in AD and MCI and the ApoE genotype | Total cholesterol | Increased TC is seen in patients with MCI and AD even before symptom scan arise. This is independent of the APOE genotype. |
| 17. | Lukiw [49] | 2014 | Literature review | To determine if cholesterol and 24 hydroxycholesterol trafficking in the brain causes AD and CYP46A1 gene effects on AD | Cholesterol and 24 hydroxycholesterol, beta-amyloid plaques, CYP46A1 genotyping | Beta-amyloid plaques, which is characteristic of AD, were found with increased CSF 24 hydroxycholesterol, a mutation in the CYP46A1 gene causes dysfunctional cholesterol metabolism |
| 18. | Reitz and Mayeux [4] | 2014 | Literature review | To determine the genetic and non-genetic risk factor of AD | Plasma and CSF lipid markers, genetic mutations, age, physical activity, BMI, cerebrovascular disease. | Cholesterol was found to be increased in cases of AD. |
| 19. | Popp et al. [41] | 2013 | Case-control study | To determine the association between cerebral and extracerebral cholesterol and its relation with AD. | Plasma and CSF cholesterol, cholesterol precursors, 24 hydroxycholesterols, and 27 hydroxycholesterols were measured. | Cholesterol synthesis was found to be de novo in the brain. Twenty-four hydroxycholesterols were found to increase in the case of AD. |
| 20. | Kosicek and Hecimovic [17] | 2013 | Literature review | To determine the association between phospholipids and AD | Sulfatide, ceramide, sphingomyelin, PC | Sphingomyelin increases in the prodromal stage of AD, ceramide increases, Sulfatide decreases, and PC metabolites decreased in AD. |
| 21. | Formiga et al. [27] | 2012 | Cohort study | To determine the association of HDL-C levels with mental, physical activity, and cognition | HDL-C | Decreased HDL-C was only found to improve functional aspect but not cognitive performance |
| 22. | Helzner et al. [29] | 2009 | Cohort study | To determine the role of vascular risk factors in AD. | Total cholesterol, HDL-C, LDL-C | High total cholesterol and LDL-C levels and diabetes cause an increased incidence of AD, thus confirming that vascular risk factors play a role in AD. |
| 23. | Mamo et al. [13] | 2008 | Case-control study | To investigate if lipoproteins are bound to Aβ isoforms | Fasting state lipoproteins | The majority of plasma triglycerides, VLDL, and IDL, was bound to Aβ1–40 isoform |
| 24. | Solomon et al. [33] | 2007 | Case-control study | To determine the association between TC and AD | TC | Serum TC increases in midlife in AD and later decreases with age. |
| 25. | Raygani et al. [ 46] | 2006 | Case-control study | To determine the association between plasma lipids, ApoE polymorphism, and with AD | HDL-c, LDL-C, TC, APOB, APOE4, APOA1 | Apolipoprotein e4 is associated with AD. Also, decreased apoA1, HDL-C, increased Apo B, increased LDL-C, TC is seen in AD. |
| 26. | Solfrizzi et al. [30] | 2006 | Literature review | To determine the association between biomarkers such as HDL-C, TC, LDL-C, homocysteine, lipoprotein A, inflammatory cytokines, and AD | HDL-C, LDL-C, TC, triglycerides, homocysteine, LP(A), cytokines | All these biomarkers were found to increase in association with AD |
| 27. | Sabbagh et al. [15] | 2005 | Case-control study | To determine the association between plasma lipids and AD | HCL-C, LDL-C, TC, TG, TOTAL/HDL RATIO | Increased TC, TG, LDL-C was associated with AD. HDL-C and total/HDL ratio remains normal in AD. |
| 28. | Wolf et al. [26] | 2004 | Randomized control trial | To determine the association between hippocampal volume (presumptive index of AD) and plasma lipids | Plasma HDL-C, LDL-C, TC were studied | HDL-C was found to be associated with hippocampal volume in the brain. |
| 29. | Pappolla et al. [23] | 2003 | Cohort study | To determine the association between amyloid plaques and hypercholesterolemia | Amyloid deposits using imaging and immunohistochemistry and serum cholesterol | Association was seen between hypercholesterolemia and beta-amyloid plaques deposited in the human brain |
| 30. | Kuo et al. [7] | 1998 | Case-control study | To determine if elevated LDL-C in AD is related to beta-amyloid 1-42 | TC, HDL-C, LDL-C, lipoproteins, ApoB | A correlation was found between the levels of serum total cholesterol, LDL-C, and ApoB with only Aβ N-42 in AD, not Aβ N-40. |
| 31. | Moroney et al. [36] | 1999 | Cohort study | To determine the effect of lipids on dementia with stroke | HDL-C, LDL-C, triglycerides, total cholesterol, lipoprotein A, APOE4. | Increased plasma LDL-C was found in patients with dementia with stroke, but its relationship with the ApoE4 allele was not established. |
Genetic Mutations in Cholesterol-Related Genes and AD
Apolipoprotein E is produced by the astrocytes and is a carrier transport protein, shifting cholesterol from astrocytes to neurons. Apolipoprotein E also has an affinity for beta-amyloid in the presence of cholesterol. Some studies found that ApoE is required for the clearance of beta-amyloid. Thus, any mutation in this could lead to a decrease in the clearance and building up of plaques [6]. There are three isoforms of ApoE: ApoE2, ApoE3, ApoE4. ApoE3 is the commonest isoform present in the majority of the population; however, ApoE4 has the strongest established association with AD. Any individual homozygous for this allele and old can develop AD [6]. Since it has such a strong association, any mutations in ApoE4 can result in AD. According to studies, ApoE4 is an independent risk factor for AD [10]. A Cohort study by Kivipelto et al., including 1449 AD patients, suggests that ApoE 4 is an independent risk factor for AD [43]. Another cohort study by Toro et al. also found the same association [44]. Other isoforms of ApoE are not as closely related to causing AD as ApoE4. ApoE2 does not have any role in AD, and ApoE3 has protective effects against AD. A systematic review by Agarwal et al. suggests the above and found that all alleles of ApoE4 - ApoE 2/4,3/4,4/4 are associated with AD [45].
Some other types of apolipoproteins are also related to AD. ApoB is another lipoprotein associated with AD; the exact reason remains unclear. In the brain, most of the cholesterol is present in density similar to that of HDL-C and transported via ApoE or ApoA [5]. Any mutation in the ApoA gene can also cause AD. A study conducted by Raygani et al. found that apart from ApoE 4, increased ApoB and decreased ApoA1 are associated with Alzheimer's disease [46]. Early-onset AD has also been associated with mutations in the genes coded for enzymes and transporters involved in beta-amyloid metabolisms, such as APP, PSEN1, and PSEN2. APP cleaves to form beta-amyloid, and presenilin is a protein present in the gamma-secretase complex [8]. Studies have shown the association between AD and mutation in the gene of APP, PSEN1, PSEN2, and ApoB [47].
Some studies have found the association between another cholesterol transporter - ABCA1 and AD. ABCA1 is essential for the cholesterol efflux from the CSF to the serum. ABCA1 is also found to reduce beta-amyloid accumulation. Lack of gene/defect in the gene of ABCA1 is associated with building up of amyloid plaques, causing AD [16]. A study by Li et al. studied the association between ABCA1 and Cholesterol efflux causing AD [48]. The same study also found the association between another genotype, ABCA7, and AD. By the metabolism of cholesterol, we have studied that cholesterol does not cross BBB; hence it is metabolized to 24S hydroxycholesterol with the help of the enzyme CYP46A1 and further eliminated via the BBB. Any mutation in the gene coding for CYP46A1 can lead to defects in elimination, causing increased cholesterol and more amyloid plaques leading to AD. A study by Lukiw et al. found the association between 24S-HC, cholesterol synthesizing enzyme, and AD (Table 2, Figure 3) [49].
Table 2. Summarizing association between mutations in cholesterol metabolism protein and Alzheimer's disease .
LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides; AD: Alzheimer's disease; Apo: apolipoprotein; Aβ: beta-amyloid; APP: amyloid precursor protein; MCI: mild cognitive impairment; BBB: blood-brain barrier; 24S HC: 24S hydroxycholesterol; 27O-HC: 27O hydroxycholesterol; PSEN: presenilin; ABCA1 and ABCA7: ATP binding cassette transporter 1 and 7; CYP46A1: cytochrome P450 family 46 subfamilies A member 1
| Author | Year of Publication | Type of Study | Purpose of Study | Intervention Studied | Result/Conclusion |
| Wings et al. [8] | 2019 | Case-control study | To determine if plasma cholesterol and genetic variants of various cholesterol transport proteins are associated with AD. | Total cholesterol, LDL-C, HDL-C, TG, and genetic variants in ApoB, ApoE, APP, PSEN1 and PSEN2. | Primary outcome: the relationship between plasma cholesterol and AD was seen. Secondary outcome: AD was associated with mutations in APOE, APP, PSEN1, PSEN2, and APOB. |
| Jeong et al. [6] | 2019 | Literature review | To determine if APOE induced cholesterol dysfunction affects the various brain cells and causes AD. | APOE4, APOE3, APOE2 alleles | APOE ɛ4 one or more alleles increase the risk of AD |
| Li et al. [48] | 2017 | Case-control study | To determine if the ABCA7 genotype of cholesterol transport protein it's associated with sporadic AD | ABCA7 genotyping | ABCA7 genotype was associated with lipid homeostasis and AD. |
| Yassine et al. [16] | 2016 | Cross-sectional study | To determine if ABCA-1 mediated cholesterol efflux is affected in patients with AD and MCI | CSF's role in cholesterol transport was assessed using a BHK cell line that expressed the ABCA1 transporter. | In case of MCI and AD, the role of CSF was impaired. ABCA1‐mediated cholesterol efflux does not take place as normal, leading to AD. |
| Agarwal et al. [45] | 2014 | Meta-analysis | To determine the association between ApoE alleles and the risk for AD | APOE genotyping | All genotypes of the ApoE e4 allele, increase the risk of AD, although the ApoE e2, e3 alleles protect from AD. |
| Toro et al. [44] | 2014 | Cohort study | To determine the association of total cholesterol in AD and MCI and the ApoE genotype | Total cholesterol | High TC levels are associated with AD but are independent of the APOE genotype. |
| Lukiw [49] | 2014 | Literature review | To determine if cholesterol and 24 hydroxycholesterol trafficking in the brain causes AD and CYP46A1 gene effects on AD | Cholesterol and 24 hydroxycholesterol, beta-amyloid plaques, CYP46A1 genotyping | Beta-amyloid plaques, which is characteristic of AD, were found with increased CSF 24 hydroxycholesterol, a mutation in the CYP46A1 gene causes dysfunctional cholesterol metabolism |
| Caramelli et al. [47] | 1999 | Case-control study | To determine the relationship between plasma lipids and AD. | VLDL, HDL-C, LDL-C, triglycerides, ApoB, lipoprotein (a) | Significantly higher Apolipoprotein B levels were found in AD patients, whereas the concentration of lipoprotein (a) and plasma lipids was not statistically different. This shows that APOE may not be the only transporter associated with AD. |
| Raygani et al. [46] | 2006 | Case-control study | To determine the association between plasma lipids, ApoE polymorphism, and with AD | HDL-C, LDL-C, TC, APOB, APOE4, APOA1 | Apolipoprotein e4 is associated with AD. Also, decreased ApoA1, HDL-C, increased ApoB, increased LDL-C, TC is seen in AD |
| Panza et al. [10] | 2006 | Literature review | To determine the association between TC, 24 S hydroxycholesterol, LDL-C, Lp(A), ApoE levels with AD | TC, 24S HC, LDL-C, Lp(A), APOE | TC, LDL-C, Lp(A) was found to be elevated in patients with AD. No consistent association between ApoE and AD have been found in this study |
| Martins et al. [5] | 2006 | Literature review | To understand cholesterol mechanism, the relation between APOE allele and AD, determine convergence risk factors for AD and CAD | APOE ɛ4 allele | APOE ɛ4 one or more alleles increase the risk of AD. A decrease in APO A1 is associated with AD. |
| Kivipelto et al. [43] | 2002 | Cohort study | To determine the association between APOE ɛ4 allele and AD | APOE genotype | APOE ɛ4 alleles are associated with AD |
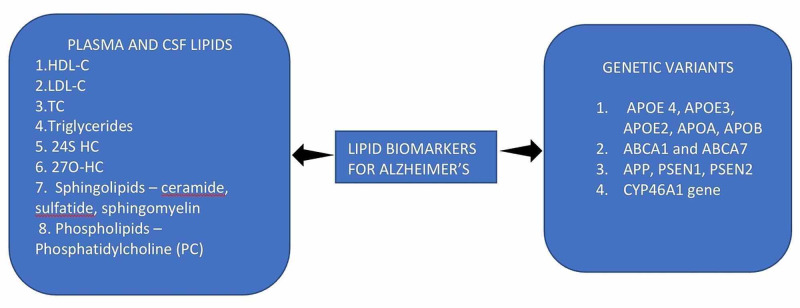
Figure 3. Plasma biomarkers of Alzheimer's disease and their genetic variants .
LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; TC: total cholesterol; TG: triglycerides; Apo: apolipoprotein; APP: amyloid precursor protein; 24S HC: 24S hydroxycholesterol; 27O-HC: 27 O hydroxycholesterol; PSEN: presenilin; ABCA1 and ABCA7: ATP binding cassette transporter 1 and 7; CYP46A1: cytochrome P450 family 46 subfamilies A member 1
Limitations
Even though the study clearly shows the relation between AD and plasma cholesterol, there are many aspects of cholesterol metabolism in the brain that are still not clearly understood. Different studies included in this study have different theories about AD pathology due to high plasma cholesterol. The exact reasons for polymorphism in many genes and associated AD also remain unclear. We were also unable to find if these biomarkers are altered by gender and physical activity. Only studies published in English were included; we did not include articles that were in other languages. Case reports, case series, letter to the editor, editorials were not included. Similarly, studies that did not find any association between AD and cholesterol were also excluded.
Conclusions
We studied many articles on the association between AD and plasma lipids. Based on those, we found that levels of plasma cholesterol, triglycerides, sphingolipids, phospholipids are altered in AD, and genetic variants of the various cholesterol metabolism-related proteins also lead to AD. The brain is made up of high lipid content, and thus any alteration in the cholesterol metabolism in the brain can cause dysregulation of the brain lipid homeostasis. Cholesterol is associated with the beta-amyloid build-up in the brain, thus increased plasma cholesterol results in increased beta-amyloid plaques, which is pathognomonic of AD. We also found out that ApoE4 and mutations in many other transporters of cholesterol in the brain are linked with increasing the chances of AD. This article thus points out all the lipid-associated risk factors causing AD. This will help us improve our knowledge and scope of the disease. Plasma lipids biomarkers can also be studied by a simple blood test making diagnosis and prediction of AD much easier. However, more advanced studies and research must understand the exact pathology behind this, as brain and peripheral cholesterol are two different entities and separated by a strict BBB.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
Footnotes
The authors have declared that no competing interests exist.
References
- 1.Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. GBD 2016 Dementia Collaborators. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.2009 Alzheimer's disease facts and figures. Alzheimer's Association. Alzheimers Dement. 2009;5:234–270. doi: 10.1016/j.jalz.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Degenerative diseases: Neuropathology. https://neuropathology-web.org 1998
- 4.Alzheimer disease: epidemiology, diagnostic criteria, risk factors and biomarkers. Reitz C, Mayeux R. Biochem Pharmacol. 2014;88:640–651. doi: 10.1016/j.bcp.2013.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Apolipoprotein E, cholesterol metabolism, diabetes, and the convergence of risk factors for Alzheimer's disease and cardiovascular disease. Martins IJ, Hone E, Foster JK, et al. Mol Psychiatry. 2006;11:721–736. doi: 10.1038/sj.mp.4001854. [DOI] [PubMed] [Google Scholar]
- 6.ApoE4-induced cholesterol dysregulation and its brain cell type-specific implications in the pathogenesis of Alzheimer's disease. Jeong W, Lee H, Cho S, Seo J. Mol Cells. 2019;42:739–746. doi: 10.14348/molcells.2019.0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elevated low-density lipoprotein in Alzheimer's disease correlates with brain abeta 1-42 levels. Kuo YM, Emmerling MR, Bisgaier CL, Essenburg AD, Lampert HC, Drumm D, Roher AE. Biochem Biophys Res Commun. 1998;252:711–715. doi: 10.1006/bbrc.1998.9652. [DOI] [PubMed] [Google Scholar]
- 8.Association of early-onset Alzheimer disease with elevated low-density lipoprotein cholesterol levels and rare genetic coding variants of APOB. Wings TS, Cutler DJ, Wingo AP, et al. JAMA Neurol. 2019;76:809–817. doi: 10.1001/jamaneurol.2019.0648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genetic predisposition to increased blood cholesterol and triglyceride lipid levels and risk of Alzheimer disease: a Mendelian randomization analysis. Proitsi P, Lupton MK, Velayudhan L, et al. PLoS Med. 2014;11:1001713. doi: 10.1371/journal.pmed.1001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipid metabolism in cognitive decline and dementia. Panza F, D'Introno A, Colacicco AM, et al. Brain Res Rev. 2006;51:275–292. doi: 10.1016/j.brainresrev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Cholesterol, 24-hydroxycholesterol, and 27-hydroxycholesterol as surrogate biomarkers in cerebrospinal fluid in mild cognitive impairment and Alzheimer's disease: a meta-analysis. Wang H, Wang Y, Liu X, Kuo SH, Liu N, Song QY, Wang MW. J Alzheimers Dis. 2016;51:45–55. doi: 10.3233/JAD-150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Association between serum cholesterol levels and Alzheimer's disease in China: a case-control study. Chen H, Du Y, Liu S, Ge B, Ji Y, Huang G. Int J Food Sci Nutr. 2019;70:405–411. doi: 10.1080/09637486.2018.1508426. [DOI] [PubMed] [Google Scholar]
- 13.Plasma lipoprotein β-amyloid in subjects with Alzheimer's disease or mild cognitive impairment. Mamo JCL, Jian L, James AP, Flicker L, Esselmann H, Wiltfang J. Ann Clin Biochem. 2008;45:395–403. doi: 10.1258/acb.2008.007214. [DOI] [PubMed] [Google Scholar]
- 14.Lipids and adipokines as risk factors for Alzheimer's disease. Warren M, Hynan L, Weiner M. J Alzheimers Dis. 2012;29:151–157. doi: 10.3233/JAD-2012-111385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Is there a characteristic lipid profile in Alzheimer's disease? Sabbagh M, Zahiri R, Ceimo J, Cooper K, Gaul W, Connor D, Sparks DL. J Alzheimers Dis. 2004;6:585–589. doi: 10.3233/jad-2004-6602. [DOI] [PubMed] [Google Scholar]
- 16.ABCA1-mediated cholesterol efflux capacity to cerebrospinal fluid is reduced in patients with mild cognitive impairment and Alzheimer's disease. Yassine HN, Feng Q, Chiang J, Petrosspour LM, Fonteh AN, Chui HC, Harrington MG. J Am Heart Assoc. 2016;5:2886. doi: 10.1161/JAHA.115.002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phospholipids and Alzheimer's disease: alterations, mechanisms and potential biomarkers. Kosicek M, Hecimovic S. Int J Mol Sci. 2013;14:1310–1322. doi: 10.3390/ijms14011310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. https://doi.org/10.1371/journal.pmed.1000097. PLoS Med. 2009;6:1000097. [PMC free article] [PubMed] [Google Scholar]
- 19.Brain cholesterol: long secret life behind a barrier. Björkhem I, Meaney S. Arterioscler Thromb Vasc Biol. 2004;24:806–815. doi: 10.1161/01.ATV.0000120374.59826.1b. [DOI] [PubMed] [Google Scholar]
- 20.Dysregulation of lipids in Alzheimer's disease and their role as potential biomarkers. Wong MW, Braidy N, PoljakA PoljakA, Pickford R, Thambisetty M, Sachdev PS. Alzheimers Dement. 2017;13:810–827. doi: 10.1016/j.jalz.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 21.Cholesterol in brain disease: sometimes determinant and frequently implicated. Martín MG, Pfrieger F, Dotti CG. EMBO Rep. 2014;15:1036–1052. doi: 10.15252/embr.201439225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Involvement of lipids in Alzheimer's disease pathology and potential therapies. Chew H, Solomon VA, Fonteh AN. Front Physiol. 2020;11:598. doi: 10.3389/fphys.2020.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mild hypercholesterolemia is an early risk factor for the development of Alzheimer amyloid pathology. Pappolla MA, Bryant-Thomas TK, Herbert D, et al. Neurology. 2003;61:199–205. doi: 10.1212/01.wnl.0000070182.02537.84. [DOI] [PubMed] [Google Scholar]
- 24.Blood-based biomarkers for predictive diagnosis of cognitive impairment in a Pakistani population. Iqbal G, Braidy N, Ahmed T. Front Aging Neurosci. 2020;12:223. doi: 10.3389/fnagi.2020.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Association of blood lipids with Alzheimer's disease: a comprehensive lipidomics analysis. Proitsi P, Kim M, Whiley L, et al. Alzheimers Dement. 2017;13:140–151. doi: 10.1016/j.jalz.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 26.Serum lipids and hippocampal volume: the link to Alzheimer's disease? Wolf H, Hensel A, Arendt T, Kivipelto M, Winblad B, Gertz HJ. Ann Neurol. 2004;56:745–748. doi: 10.1002/ana.20289. [DOI] [PubMed] [Google Scholar]
- 27.Serum high-density lipoprotein cholesterol levels correlate well with functional but not with cognitive status in 85-year-old subjects. Formiga F, Ferrer A, Chivite D, Pinto X, Badia T, Padrós G, Pujol R. J Nutr Health Aging. 2012;16:449–453. doi: 10.1007/s12603-012-0018-z. [DOI] [PubMed] [Google Scholar]
- 28.Physical exercise may increase plasma concentration of high-density lipoprotein-cholesterol in patients with Alzheimer's disease. Jensen CS, Musaeus CS, Frikke-Schmidt R, et al. Front Neurosci. 2020;14:532. doi: 10.3389/fnins.2020.00532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Contribution of vascular risk factors to the progression in Alzheimer disease. Helzner EP, Luchsinger JA, Scarmeas N, Cosentino S, Brickman AM, Glymour MM, Stern Y. Arch Neurol. 2009;66:343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Circulating biomarkers of cognitive decline and dementia. Solfrizzi V, D'Introno A, Colacicco AM, et al. Clin Chim Acta. 2006;364:91–112. doi: 10.1016/j.cca.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 31.Total cholesterol and neuropsychiatric symptoms in Alzheimer's disease: the impact of total cholesterol level and gender. Hall JR, Wiechmann AR, Johnson LA, et al. Dement Geriatr Cogn Disord. 2014;38:300–309. doi: 10.1159/000361043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Relationship between plasma lipids and mild cognitive impairment in the elderly Chinese: a case-control study. He Q, Li Q, Zhao J, Wu T, Ji L, Huang G, Ma F. Lipids Health Dis. 2016;15:146. doi: 10.1186/s12944-016-0320-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serum cholesterol changes after midlife and late-life cognition, Twenty-one-year follow-up study. Solomon A, Kåreholt I, Ngandu T, et al. Neurology. 2007;68:751–756. doi: 10.1212/01.wnl.0000256368.57375.b7. [DOI] [PubMed] [Google Scholar]
- 34.Updating the evidence on the association between serum cholesterol and risk of late-life dementia: review and meta-analysis. Anstey KJ, Ashby-Mitchell K, Peters R. J Alzheimers Dis. 2017;56:215–228. doi: 10.3233/JAD-160826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cholesterol and Alzheimer's disease risk: a meta-meta-analysis. Sáiz-Vazquez O, Puente-Martínez A, Ubillos-Landa S, Pacheco-Bonrostro J, Santabárbara J. Brain Sci. 2020;10:386. doi: 10.3390/brainsci10060386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Low-density lipoprotein cholesterol and the risk of dementia with stroke. Moroney JT, Tang M, Berglund L, et al. JAMA. 1999;282:254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 37.Low-density lipoprotein cholesterol and Alzheimer's disease: a systematic review and meta-analysis. Zhou Z, Liang Y, Zhang X, et al. Front Aging Neurosci. 2020;12:5. doi: 10.3389/fnagi.2020.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Prediction of Alzheimer's disease with serum lipid levels in Asian individuals: a meta-analysis. Wu Y, Wang Z, Jia X, et al. Biomarkers. 2019;24:341–351. doi: 10.1080/1354750X.2019.1571633. [DOI] [PubMed] [Google Scholar]
- 39.Elevated serum TC and LDL-C levels in Alzheimer's disease and mild cognitive impairment: a meta-analysis study. Liu Y, Zhong X, Shen J, et al. Brain Res. 2020;1727:146554. doi: 10.1016/j.brainres.2019.146554. [DOI] [PubMed] [Google Scholar]
- 40.Serum triglycerides in Alzheimer disease: relation to neuroimaging and CSF biomarkers. Bernath M, Bhattacharyya S, Nho K, et al. Neurology. 2020;94:2088–2098. doi: 10.1212/WNL.0000000000009436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer's disease. Popp J, Meichsner S, Kölsch H, et al. Biochem Pharmacol. 2013;86:37–42. doi: 10.1016/j.bcp.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Plasma lipids metabolism in mild cognitive impairment and Alzheimer's disease. Costa AC, Joaquim HPG, Forlenza O, Talib LL, Gattaz WF. World J Biol Psychiatry. 2019;20:190–196. doi: 10.1080/15622975.2017.1369566. [DOI] [PubMed] [Google Scholar]
- 43.Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Kivipelto M, Helkala EL, Laakso MP, et al. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- 44.Cholesterol in mild cognitive impairment and Alzheimer's disease in a birth cohort over 14 years. Toro P, Degen C, Pierer M, Gustafson D, Schröder J, Schönknecht P. Eur Arch Psychiatry Clin Neurosci. 2014;264:485–492. doi: 10.1007/s00406-013-0468-2. [DOI] [PubMed] [Google Scholar]
- 45.Association of apolipoprotein E genetic variation in Alzheimer's disease in Indian population: a meta-analysis. Agarwal R, Tripathi CB. Am J Alzheimers Dis Other Demen. 2014;29:575–582. doi: 10.1177/1533317514531443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Association between apolipoprotein E polymorphism and serum lipid and apolipoprotein levels with Alzheimer's disease. Raygani AV, Rahimi Z, Kharazi H, Tavilani H, Pourmotabbed T. Neurosci Lett. 2006;408:68–72. doi: 10.1016/j.neulet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 47.Increased apolipoprotein B serum concentration in Alzheimer's disease. Caramelli P, Nitrini R, Maranhão R, Lourenço AC, Damasceno MC, Vinagre C, Caramelli B. Acta Neurol Scand. 1999;100:61–63. doi: 10.1111/j.1600-0404.1999.tb00724.x. [DOI] [PubMed] [Google Scholar]
- 48.A complex association between ABCA7 genotypes and blood lipid levels in Southern Chinese Han patients of sporadic Alzheimer's disease. Li H, Zhou J, Yue Z, et al. J Neurol Sci. 2017;382:13–17. doi: 10.1016/j.jns.2017.09.016. [DOI] [PubMed] [Google Scholar]
- 49.Cholesterol and 24S-hydroxycholesterol trafficking in Alzheimer's disease. Lukiw WJ. Expert Rev Neurother. 2014;6:683–693. doi: 10.1586/14737175.6.5.683. [DOI] [PubMed] [Google Scholar]