Abstract
Acute myeloid leukemia (AML) is a malignant clonal disease derived from hematopoietic stem/progenitor cell. Leukemia blasts cause extensive hypoxia of bone marrow (BM), which lead to disorder and remodeling of BM niche, thereby becoming “leukemic niche” to support the development and drug-resistance of AML as well as the maintenance of normal hematopoietic stem cells. In this study, the biological characteristics (such as self-renewal, apoptosis, migration, autocrine) and function (vascularization) of mesenchymal stem cells (MSCs) and human umbilical artery endothelial cells (HUAECs) that make up BM arteriolar niche in simulated hypoxia AML context were investigated. It was found that moderate hypoxia enhanced the viability of the arteriolar niche cells, but severe hypoxia of AML BM resulted in the damage of arteriolar niche cells and the disorder of vascular cytokines C-X-C motif chemokine ligand 6 (CXCL6). The dynamic changes of CXCL6 in the system as well as its anti-apoptotic and promoting angiogenic effects suggested that CXCL6 played an important role in the remodeling of BM arteriolar niche in AML. Taking advantage of CXCL6 can save the damaged MSCs and HUAECs, which is the hope of rescuing arteriolar niche. It is suggested that CXCL6 may be an assistant strategy for microenvironment targeted therapy of AML.
Keywords: Hypoxia, arteriolar niche, endothelial progenitor cells, mesenchymal stem cells, CXCL6, acute myeloid leukemia
Impact statement
Prevention of hematopoiesis microenvironment collapse is an important strategy to effectively eliminate LSCs and restore bone marrow reconstruction. As an optimal niche of long-term HSCs, the arteriolar niche in bone marrow should be protected as a priority by remodeling its integrity and effectiveness, or otherwise it will become the source of relapsed AML blasts. This study points out that severe hypoxia caused by AML blasts led to fatal damage and depletion of MSCs and EPCs in the arteriolar niche, while excessive consumption of CXCL6, which were helpful for arterioles but affected by hypoxia, caused irreversible atrophy of the arteriolar niche. Therefore, it is suggested that if CXCL6 could be supplemented in time, it will be possible to reverse the malignant remodeling of the arteriolar niche.
Introduction
At present, the cure rate of acute myeloid leukemia (AML) is 35–40% in young adult (<60 years old) patients, while only 5–15% of elderly patients can be cured, and the five-year survival rate of patients over 65 years old is only 7%.1 Therefore, relapse/drug-resistance is an urgent problem which needs to be solved. The main cause of this problem is minimal residual disease, which is recognized as leukemia stem cells (LSCs) escaping chemotherapy. It is generally accepted that the bone marrow microenvironment (BM niche) can provide favorable growth advantages for LSCs. On the other hand, LSCs could also change the niche. LSCs may misinterpret the signals from niches and remodel them, which then provide a shelter for LSCs and protect them from the cytotoxic effects of chemotherapy. Due to the limited number of BM niches, LSCs and normal hematopoietic stem cells (HSCs) compete for the same functional area.2
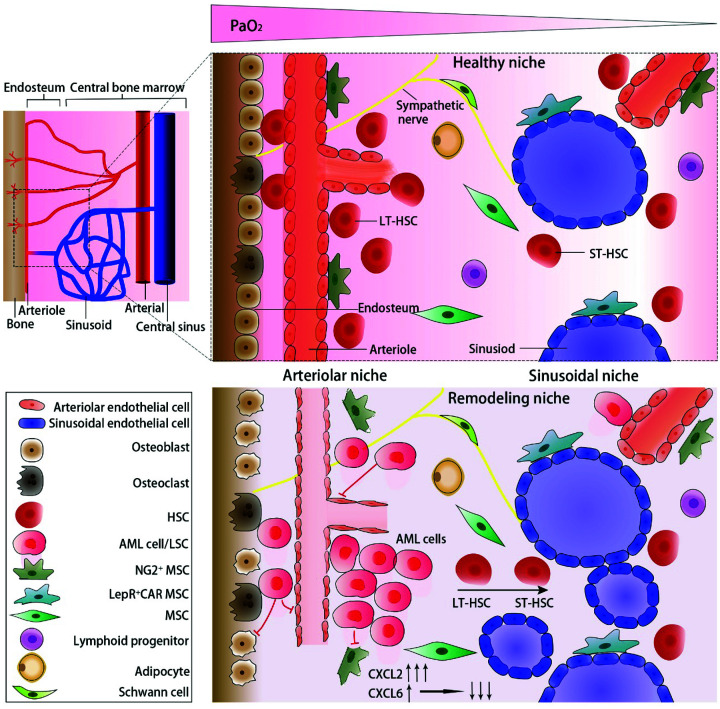
HSCs are mainly located in two specific niches: the endosteal niche (also known as the arteriolar niche3) and the sinusoidal niche. It is generally believed that the marrow cavity is a physiologically hypoxic tissue. In 2014, Spencer’s team used two-photon phosphorescence lifetime microscope technology to directly measure the partial oxygen pressure (PaO2) in the BM of living mice, which showed that the absolute PaO2 in BM was lower than 32 mmHg (4.2%). Compared with the sinusoidal niche in the center of the marrow cavity, the hypoxia level was lower (as low as 10 mmHg or 1.3%) in the endosteal niche, because there were abundant thin-walled arterioles in this area.4 As Kunisaki’s team observed by a 3D imaging method, quiescence HSCs were mainly located in the arteriolar niche.5 Once stimulated by the signals of AML cells, the endothelial cells (ECs) of arterioles and mesenchymal stem cells (MSCs) around would be changed. Then the permeability of arterioles increased, followed by disappearance of the oxygen gradient in BM, resulting in extreme hypoxia of the whole BM niche and circulation of HSCs. The above observations suggest that the damage of the arteriolar niche was mainly responsible for disturbing the quiescence of HSCs. AML cells initially destroyed these arterioles. Conversely, the loss of these arterioles accelerated the development and drug resistance of AML resulting from extreme hypoxia, low perfusion, drug delivery reduction, and LSC transformation. The arteriolar niche was normally protected and supported by peripheral MSCs and pericytes (a kind of NG2+ cell). In the later stage of AML, the differentiation of MSCs into NG2+ cells around the arterioles of AML mice was limited,6 which made the arterioles lose their protection property (Figure 1). HSCs in the arteriolar niche were maintained in a quiescent state by moderate hypoxia rather than extreme hypoxia, as with MSCs. It has been proved that moderate hypoxia enhances the property of MSCs.7 The damage of MSCs and depletion of endothelial progenitor cells (EPCs) are the main events of the niche changes induced by AML, and the subsequent loss of osteoblasts and HSCs aggravates niche destruction. Therefore, the first priority of rescuing the arteriolar niche is to save MSCs and EPCs.
Figure 1.
Normal bone marrow niche and remolding AML niche. (A color version of this figure is available in the online journal.)
We have learnt that AML cells and stromal cells often display crosstalk through cytokines, which results in remodeling of the BM niche. It has been found that while the angiogenesis in the endosteum area was inhibited, there was no prominent change in the central sinusoid area. The transformation is directly related to AML blasts in the endosteal niche promoting the secretion of angiogenesis inhibitor CXCL2 and TNF, which play a reverse role in EC survival.8 The abnormality of the tumor microenvironment vascular cytokines has been given increasing attention in recent decades. Some clinical research has been devoted to the antiangiogenic effect of endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR), but to little effect, which calls researchers to reflect that the improvement of the vascular niche is far more than simply anti-angiogenesis.9 Not only to prevent the invalid angiogenesis but also to promote the effective angiogenesis should be the direction of research for improving the vascular niches. Understanding the dynamic changes of vascular niches in time and space will help to find new treatment strategies. In this study, we have screened out some potential vascular cytokines such C-X-C motif chemokine ligand 6 (CXCL6) and C-X-C motif chemokine ligand 12 (CXCL2; both of them combined with the same receptor). Unfortunately, our knowledge of how these cytokines are related to the arteriolar niche of AML is not clear at present. Therefore, we attempted to investigate more dynamic changes of the hypoxia arteriolar niche of AML in vitro.
Materials and methods
Cell culture and co-culture
The second parcener (P2) of human umbilical cord mesenchymal stem cells (hUC-MSCs, hereinafter referred to as MSCs) was a gift from Professor Hai Bai. The purity was identified by flow cytometry as CD90+CD44+CD34−CD45− cells >94%. MSCs were cultured with a serum-free culture system (BI, 05-200/201, Kibbutz Beit-Haemek, Israel). P3–P8 of MSCs were used for the following experiments. Human umbilical artery endothelial cells (HUAECs, P4) were purchased from Jennio Biotech (Guangdong, China), which passed short tandem repeat authentication. The cell culture system used the endothelial cell medium (ECM, ScienCell, 1001, USA). P5–P9 of HUAECs were used for the experiments. THP-1 cells line was purchased from the Stem Cell Institute of Chinese Academy of Sciences (Shanghai, China), which was cultured with RPMI-1640 culture medium containing 15% fetal bovine serum. Standard cells were cultured at 37°C in a 5% CO2 incubator. Hypoxia cells were cultured in a hypoxia incubator (Billups-rothenberg, mic-101, USA) and filled with N2 and O2 pre-mixed with 5% CO2 to regulate the oxygen content in the culture system. After the adherent cells (MSCs and HUAECs) attached, the suspension cells (THP-1) were inoculated for a proportion of 1:1 for directly contacted co-culture. The culture groups were as follows: (a) single MSC/HUAEC, 21% O2; (b) single MSC/HUAEC, 1% O2; (c) MSC/HUAEC + THP-1, 21% O2; (d) MSC/HUAEC + THP-1, 1% O2. The supernatant and adherent cells of each group were collected at 24, 48, and 72 h respectively for subsequent experiments.
CCK-8 assay
Make growth curves of MSCs and HUAECs in different oxygen conditions
MSCs (P3) or HUAECs (P5) in good growth condition were selected to inoculate into 18 pieces of 96-well plate with a density of 2 × 103/well, respectively. The final system of each well was 100 µL, and each 96-well plate set triple wells. The complete medium without cells was used as the blank control. Nine 96-well plates were cultured in 37°C, 5% CO2, 21% O2 incubators, the others were cultured in 37°C, 5% CO2, 1%O2 (3% O2, 6% O2) incubators. The culture medium was changed every two days. Cell proliferation was measured by the CCK-8 Cell Counting Kit (Meilun, Dalian, China) according to the manufacturer’s instructions. In brief, a 96-well plate was removed every day and given a change of fresh culture medium with 10 µL CCK-8 solution. The plate wall was gently tipped to help mixing. After incubation in the incubator for 2 h, the absorbance (OD) value was measured at 450 nm to represent the cell viability. Mean values and standard deviation (SD) of triple wells were taken to draw the growth curves.
Proliferation analysis
MSCs/HUVECs were inoculated in 96-well plates at a density of 2 × 103/well and treated with a specific concentration of CXCL2 (PeproTech, LA) or CXCL6 (PeproTech) for indicated hours under standard culture conditions. After that, cells were incubated with the CCK-8 solution to measure OD value.
Flow cytometry
Assessment of apoptotic cells was conducted with the FITC Annexin V Apoptosis Detection Kit I (BD Pharmingen, San Jose, CA, USA) after being cultured in 21% O2 or 1% O2. Flow cytometry data were acquired using FACSCantoII6-8 color (BD, San Jose, CA, USA). Analysis of annexin-V and PI expression was performed using FlowJo 7.6 software.
Real-time PCR
Cells of each group, which were stimulated by hypoxia, CXCL6 or BAY87-2243 (MCE, NJ) were used to extract total RNA with Trizol Reagent (TransStart, Beijing, China) following the manufacturer's instructions. A FastKing-RT Kit (Tiangen, Beijing, China) was used for cDNA synthesis. Real-time PCR amplifications were performed using SYBR-Green Ι qPCR SuperMix (TransStart) and detected by RocheLightCycler® 480 (Roche Diagnostics, Basel, Switzerland) according to the manufacturer's recommendations. Real-time PCR amplification was performed with the following parameters: 30 s at 94°C and then 40 cycles of 5 s at 94°C, 15 s at 61°C and 10 s at 72°C. An additional cycle of 1 s at 95°C, 15 s at 61°C, 15 s at 95°C was performed at the end of the reaction to generate the dissociation curve of the amplicon to ensure a single and specific product with the corresponding melting temperature was produced. A housekeeping gene (β-actin) was amplified from the same samples to ensure all samples were used at an equal baseline. The primer sequences for β-actin, CXCL6, CXCL2, and hypoxia-inducible factor 1α (HIF-1α) are listed in Table 1. The relative quantification of genes was performed using the 2−ΔΔCt method.
Table 1.
Sequences of primers used for real-time PCR.
| Gene name | Forward primer (5ʹ–3ʹ) | Reverse primer (5ʹ–3ʹ) |
|---|---|---|
| β-actin | GGAGATTACTGCCCTGGCTCCTA | GACTCATCGTACTCCTGCTTGCTG |
| CXCL6 | AAGTTTGTCTGGACCCGGAAG | AAACTGCTCCGCTGAAGACTG |
| CXCL2 | CGCATCGCCCATGGTTAAG | ACATTAGGCGCAATCCAGGTG |
| HIF-1α | CTCATCAGTTGCCACTTCCACATA | AGCAATTCATCTGTGCTTTCATGTC |
CXCL6: C-X-C motif chemokine ligand 6; CXCL12: C-X-C motif chemokine ligand 12; HIF-1α: hypoxia-inducible factor 1α.
Enzyme-linked immunosorbent assay (ELISA)
The cytokine levels in the supernatant of each group were respectively detected by the human CXCL6 ELISA kit (BOSTER, Wuhan, China) and human CXCL2 ELISA Kit (MULTI SCIENCES, Hangzhou, China) according to the manufacturer’s protocol. The concentration was calculated based on the standard curves.
Transwell migration assay
Migration of MSCs and HUAECs was assessed in transwell permeable 24-well plates with 8.0 µm pores (Costar, WA, USA). The lower compartment contained 500 µL of media with or without indicated concentrations of CXCL6/CXCL2, while 7.5 104 cells were inoculated in the upper compartment. After 16 h, the compartments with migrated cells were fixed with methanol and then dyed with crystal violet. Under the phase-contrast microscope (Olympus, Japan) with magnification of 200×, five random sights were selected in each sample to count migration. The number of migrated cells was calculated by Image J software (National Institutes of Health, USA).
Immunofluorescence analysis
MSCs or HUAECs were inoculated onto cell carrier plates and allowed to attach for 24 h in the incubator. Then cells were cultured at 1% O2 for 48 h, with or without CXCL6. Cells cultured for the same time in standard conditions were considered as control. Then cells were washed in phosphate buffer solution (PBS) twice and fixed with 4% paraformaldehyde for 20 min. Permeabilization was performed by solution with 0.1% Triton X-100 for 5 min. The cells were incubated with 5% goat serum to prevent nonspecific reaction and incubated with cleaved-caspase3 (ab32042, 1:200; abcam, Cambridge, MA, USA) at room temperature for 2 h. Alexa Fluor 488 (Immunoway, 1:500; Plano, TX, USA) was used as the secondary antibody. Nuclei were stained with 4ʹ,6-diamidino-2-phenylindole (DAPI; 1:100, Meilunbio, Dalian, China). Plates were imaged with the automated Operetta High Content Imaging System (Perkin Elmer, MA, USA). Multiple fields of views were imaged with 20× objective magnification at two different excitation wavelengths depending on the fluorophores used: 358 nm (DAPI), 488 nm (cleaved-caspase 3). Mean fluorescence intensity value for all detected cells was calculated for comparison.
Western blot
The cells of each group stimulated were washed with ice-cold PBS, lysed in RIPA buffer with protease inhibitors (Meilunbio) and protein phosphatase inhibitor complex (Meilunbio) for 30 min at 4°C. After centrifugation at 14,000g for 10 min, the supernatant was collected as protein extract. Protein concentration was measured by BCA kit (Meilunbio). Equal concentrations of proteins were fractionated by polyacrylamide gel electrophoresis and were transferred onto a polyvinylidene fluoride membrane (Biosharp, Hefei, China), followed by blotting with antibodies against cleaved-caspase3, HIF-1α (1:1000, CST, Boston, MA, USA), mTOR (1:1000, CST), p-mTOR (1:1000, CST) and β-actin (YM3028, 1:5000; Immunoway) at 4°C overnight. Peroxidase-conjugated secondary antibody (1:5000; Immunoway) was used to incubate the membrane at room temperature for 1 h. Finally, electrochemiluminescence was applied to detect the bands with a chemiluminescence imaging system (Amersham Imager 680, Massachusetts, USA).
Angiogenesis assay
To investigate the influence of hypoxia and CXCL6 on tubule formation by HUAECs, HUAECs were inoculated in 96-well plates coated with growth factor reduced Matrigel (R&D Systems, NJ, USA). A 200 µmol/L CoCl2 was used to simulate hypoxia. CXCL6 was supplied as the concentration of 50 ng/mL. MSCs were cultured for 48 h and collected the supernatant to be conditional medium (CM). Then the CM and ECM were mixed with 1:1 for angiogenesis assay. The same counts of HUVECs were set as positive control. After 15 h of incubation, tubule formation was examined under a phase-contrast microscope. Parameters of angiogenesis were measured quantitatively over three different microscopic fields for each well using Image J software.
Quantificational and statistical analyses
Data were analyzed using GraphPad Prism 8 (GraphPad, USA). Group means were compared using the unpaired Student’s t test. For all data, differences were considered significant whenever P < 0.05, specifically ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Results
Moderate hypoxia was helpful for self-renewal of MSCs and HUAECs, while severe hypoxia reduced the viability and increased the apoptosis
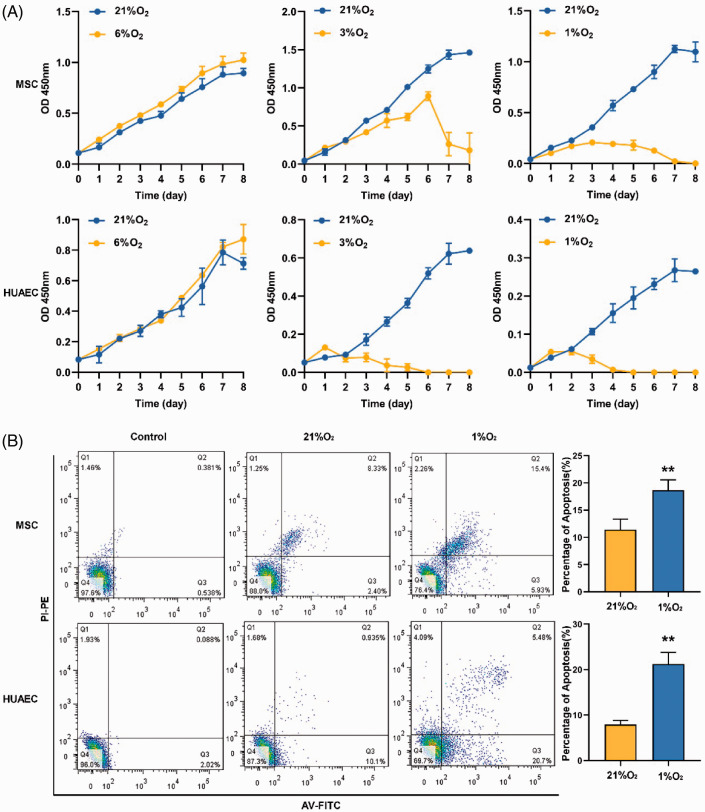
Since BM is a physiologically hypoxic tissue, the optimal growth oxygen level of living cells in the microenvironment may not be 21%. For this reason, we have made the growth curves of MSCs and HUAECs (represent arteriolar EPCs) in different oxygen conditions. The growth curves showed that MSCs and HUAECs had stronger self-renewal ability at 6% O2 than 21% O2. However, when the oxygen level was lower than 3%, the cells (especially HUAECs) demonstrated prominent growth suppression in the long term, in spite of increasing in the early stage (within 24 h) of low oxygen condition (Figure 2(A)). It was sufficiently proved that the optimal oxygen level for the long-term growth of these BM stem/progenitor cells was about 6%, which was consistent with the previous research results.7
Figure 2.
Effects of hypoxia on self-renewal and apoptosis of MSCs and HUAECs. (A) Growth curves of MSCs and HUAECs in 21% O2, 6% O2, 3% O2, and 1% O2 at continuous time points detected by CCK-8 assay. (B) Apoptosis (early apoptosis + late apoptosis) of MSCs and HUAECs increased in 1% O2 conditions compared with 21% O2. (A color version of this figure is available in the online journal.)
Although many studies have shown that a low oxygen level could reduce apoptosis of MSCs,10 our study indicated that severe hypoxia (1% O2) increased apoptosis of MSCs and HUAECs (Figure 2(B)). Therefore, for the subsequent function experiments, we used 1% O2 to represent hypoxia. Twenty-one percent O2 was adopted to represent normoxia of the BM model, because the limitations of experimental conditions did not allow us to carry out two kinds of low oxygen cultural conditions at the same time.
Severe hypoxia up-regulated the expression of vascular cytokines in MSCs and HUAECs
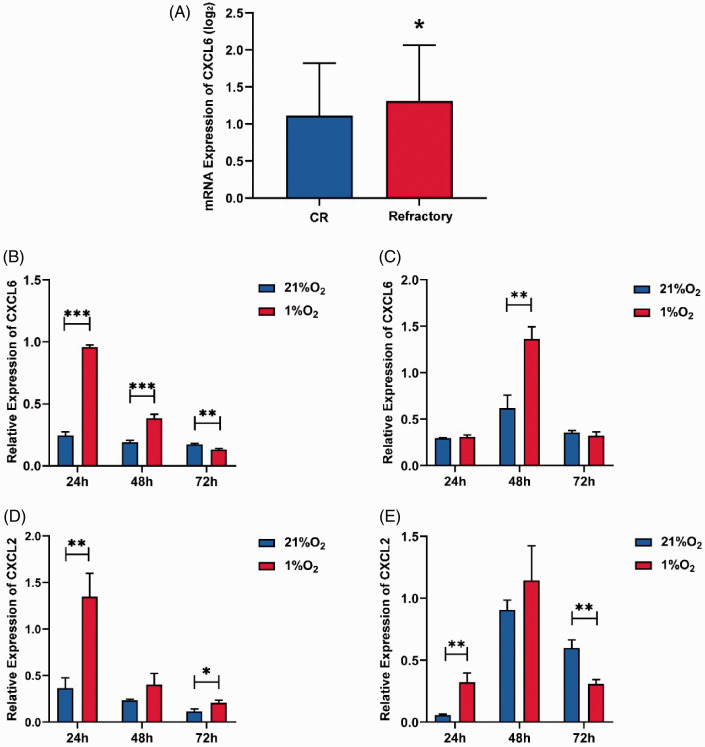
Firstly, we identified that the BM hypoxia level in leukemia was more serious than normal BM based on the oxygen content of BM measure before (AML patients vs. normal people: 4.35 ± 0.76% vs. 5.65 ± 1.53%). Then we used the data of 342 AML patients in the TARGET database (https://ocg.cancer.gov/programs/target/data-matrix) to screen the related cytokines. The results showed that the expression of CXCL6 in relapsed/refractory patients (with high blast load) was significantly higher than that in remission patients. This showed that CXCL6 was up-regulated in the AML BM microenvironment under severe hypoxia. In order to verify the effect of AML hypoxia on CXCL6 expression, we detected the gene expression of CXCL6 in MSCs and HUAECs at three time points in four groups (as described in “Cells culture and co-culture” section). The presence of THP-1 down-regulated the level of CXCL6 expressed in MSCs (statistic MSC + THP1/MSC < 1). In addition, the expression of CXCL6 in MSCs decreased with the time prolonged in the AML microenvironment. While in the same AML cells co-culture condition, hypoxia up-regulated the expression of CXCL6 within 48 h, but after 48 h, the expression of CXCL6 was significantly inhibited in hypoxia. The expression of HUAECs in the co-culture group showed a sharp increase due to compensation in 48 h at hypoxia condition, but it also decreased with time. The above results showed that AML cells generally inhibited the expression of CXCL6 in MSCs and HUAECs. Moreover, hypoxia up-regulated CXCL6 in a short time (within 24–48 h), but the long-term hypoxia resulted in the impairment of their own synthesis ability. CXCL2 has a high homology with CXCL6, and, both of them belonging to CXC-cytokines, were studied together, although we did not find any obvious differences in patient samples (data not shown). We then used the same method to detect the expression of CXCL2 in cells, and found that hypoxia continued to significantly upregulate the expression of CXCL2, especially in MSCs (Figure 3).
Figure 3.
Effects of hypoxia on CXCL6 and CXCL2 gene expression in co-culture systems. (A) The difference of CXCL6 gene expression between censored (n = 95) and relapse/refractory (n = 247) patients in TARGET database. (B to E) The gene expression of CXCL6 and CXCL2 of MSCs and HUAECs in the co-culture systems were measured by the co-culture/mono culture ratio. (B and D) MSC + THP-1/MSC; (C and E) HUAEC + THP-1/HUAEC. *P < 0.05, **P < 0.01, ***P < 0.001, and “ns” for no statistical significance. (A color version of this figure is available in the online journal.)
Severe hypoxia depleted vascular cytokines in the AML microenvironment
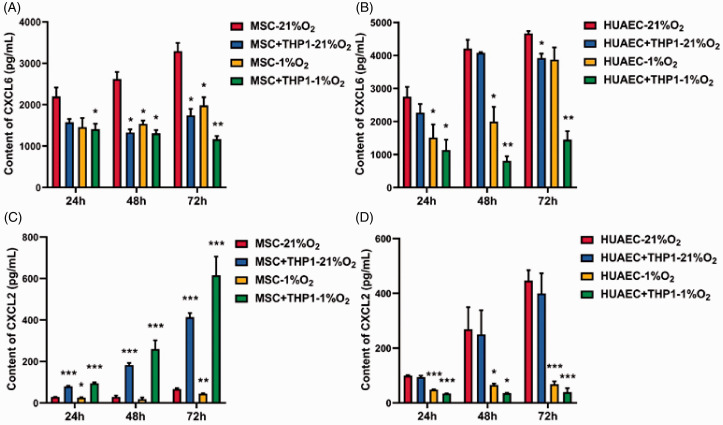
In order to verify the secretion and consumption of CXCL6 and CXCL2 in the microenvironment, we measured the content of two kinds of cytokines under the culture conditions of 21% O2 and 1% O2 at different time points. For the content of CXCL6, both hypoxia and co-culture with THP-1 significantly reduced the content of CXCL6 in MSC culture systems. The reduction trend was enhanced when the two adverse factors were combined, either in MSCs or HUAECs. Furthermore, the inhibition increased with time. Since the autocrine and ego depletion of CXCL6 in the constant system were in dynamic change, the content of CXCL6 in the system decreasing with time sufficiently proved that CXCL6 secreted by MSCs or HUAECs in the malignant microenvironment is gradually in short supply under hypoxia and AML conditions as time progresses. The difference of gene expression supports the result. For the content of CXCL2, in MSC culture systems, THP-1 is obviously a main factor to accelerate the accumulation of CXCL2. Despite the singular hypoxia condition having a certain inhibition effect, the accumulation effect of CXCL2 is significantly enhanced by the superposition of the two factors. In HUAEC culture systems, whether hypoxia or THP-1, both seemed to have a significant inhibiting effect on the accumulation of CXCL2, especially when the two factors are superposed (Figure 4).
Figure 4.
CXCL6 and CXCL2 content in the supernatant of hypoxia and THP-1 co-culture systems. (A and B) CXCL6 content in supernatant. (C and D) CXCL2 content in supernatant. *P < 0.05, **P < 0.01, and ***P < 0.001. (A color version of this figure is available in the online journal.)
Vascular cytokines promoted proliferation and migration of MSCs and HUAECs
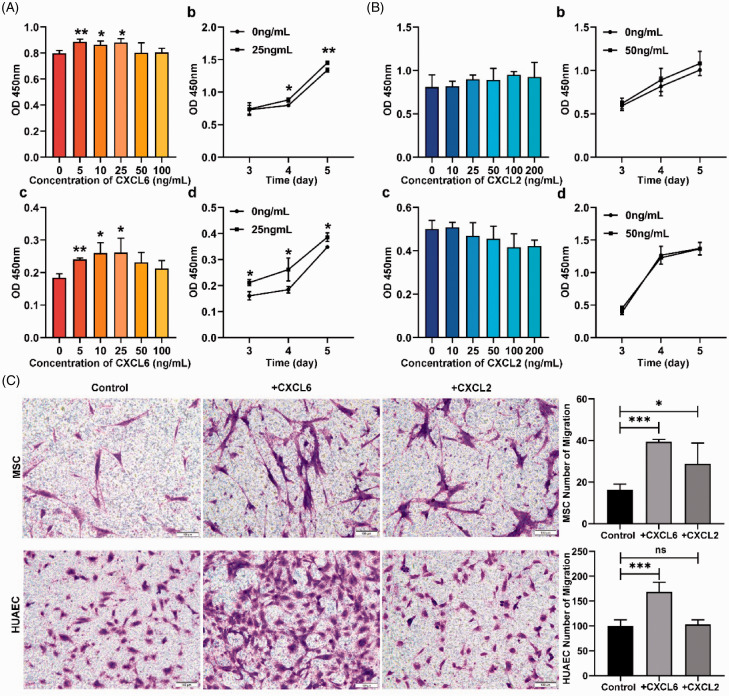
In order to verify the biological effect of the vascular cytokines on MSCs and HUAECs, we treated MSCs and HUAECs with different concentrations of CXCL6 or CXCL2 for different times. The results indicated that CXCL6 could enhance the viability of MSCs and HUAECs in a certain concentration range, which is consistent with the previous study.11 Specifically, the effect of CXCL6 on MSCs was that the cell viability increased significantly between 5 and 25 ng/mL, but decreased when it was greater than 25 ng/mL. The growth trend of HUAECs treated with 0–100 ng/mL CXCL6 was like an “inverted U”, which means at the concentration of 5–25 ng/mL, the cell activity gradually increased (P < 0.05), reaching the maximum value at the concentration of 25 ng/mL, while at the concentration of 25–100 ng/mL, the curve showed a downward trend, indicating that the viability of HUAECs decreased with the increase of concentration, although compared with the untreated group, the viability was still higher (Figure 5(A)). CXCL2 slightly promoted proliferation of MSCs but inhibited HUAECs. However, neither of the differences were of statistical significance (Figure 5(B)).
Figure 5.
The effects of CXCL6 and CXCL2 on the proliferation and migration of MSCs and HUAECs. (A and B) The effects of CXCL6 (A) and CXCL2 (B). Among them, (a and b) described the effects of cytokines on MSCs and (c and d) described the effects of cytokines on HUAECs. (a and c) showed the effects of different concentrations of cytokines on target cells. (b and d) showed the time-dependent effect of cytokines on target cells. (C) The chemotaxis of CXCL6 and CXCL2 to MSCs and HUAECs respectively. The concentration of CXCL6 was 50 ng/mL and of CXCL2 was 100 ng/mL. Scale = 100 µm. (A color version of this figure is available in the online journal.)
Migration experiments by transwell suggested that CXCL6 showed significant chemotaxis on both MSCs and HUAECs. CXCL2 promoted MSCs to migrate, while it had no significant effect on HUAECs. Interestingly, the effect of CXCL6 on MSCs was stronger than that of CXCL2 in the same experiment (Figure 5(C)).
In view of the above research, the effect of CXCL2 on MSCs and HUAECs had not been obvious, which was considered to apply to another research. Thus, the follow-up research was mainly focused on CXCL6.
CXCL6 played a pregnant role in anti-apoptosis and angiogenesis in vitro
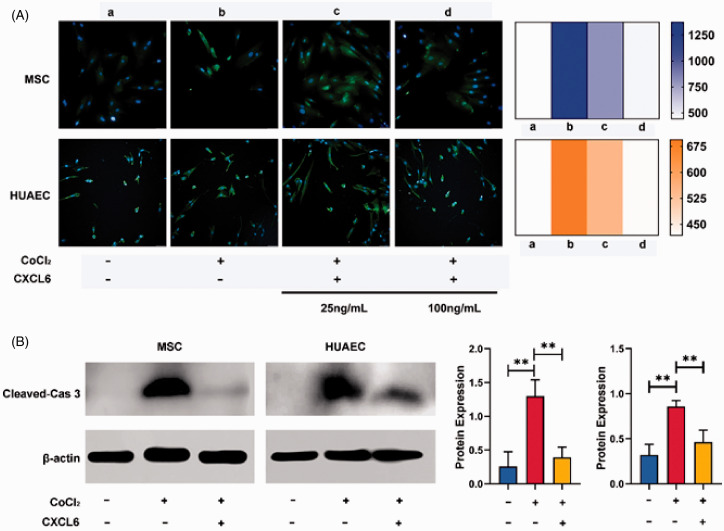
In order to investigate whether CXCL6 has an effect on the cells undergoing apoptosis due to hypoxia, we used cleaved-caspase3 as a marker and detected that hypoxia significantly accelerated apoptosis in MSCs and HUAECs. Furthermore, once CXCL6 was added, the expression of cleaved-caspase3 was shown to be down-regulated. Independent experiments through immunofluorescence and Western blot showed that CXCL6 could reverse the apoptosis induced by hypoxia, and the reversal effect was related to the concentration of CXCL6 (Figure 6).
Figure 6.
Expression of cleaved-caspase3 in MSCs and HUAECs. (A) Expression of cleaved-caspase-3 (green fluorescence) measured by immunofluorescence method increased significantly in hypoxia (1% O2) for 48 h, but decreased after CXCL6 was supplied. The reversion depended on the concentration of CXCL6. MSCs were pictured at 20× water objective and HUAECs were pictured at 20× air objective. The heatmap represented the change of average fluorescence intensity in each group. (B) Western blot method verified the above results. The histograms (MSCs on the left and HUAECs on the right) represented the relative gray value of each group. (A color version of this figure is available in the online journal.)
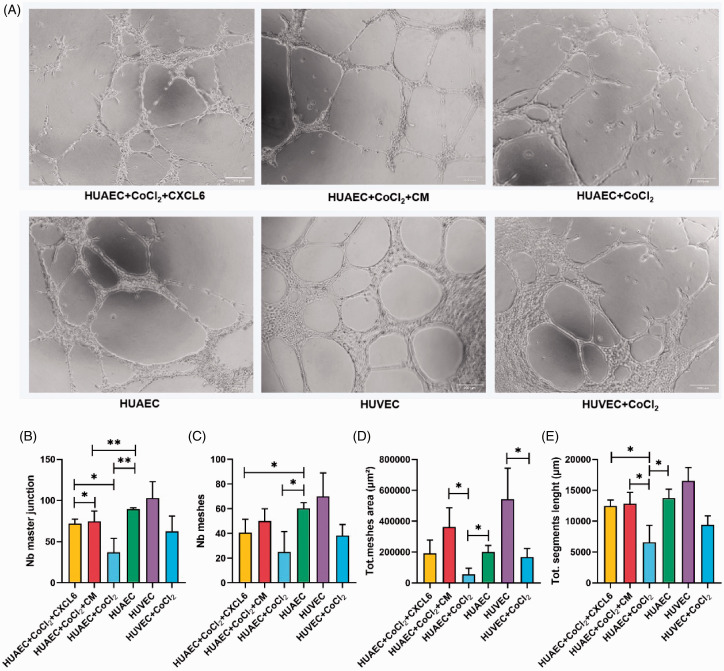
Given the remarkable limitation of HUAECs survival in severe hypoxia conditions and relatively insufficient level of CXCL6, which was beneficial to HUAECs, we speculated that the angiogenic ability of HUAECs might be greatly reduced. To prove the hypothesis, we inoculated HUAECs on Matrigel and simulated hypoxia conditions with 200 µmol/L CoCl2, with or without CXCL6, to observe angiogenesis. We found that in the hypoxia group, evaluation indexes of tubule forming ability such as the number of main nodes and meshes, the total area of meshes as well as the total length of segments were lower than those in the control group. In addition, the integrity of tubule wall was decreased. In fact, researchers even observed that hypoxia led to incomplete or malformed angiogenesis, and the average diameter of blood vessels decreased in vivo,12 which was consistent with the present results. Nevertheless, when 50 ng/mL of CXCL6 was added to the system, or treated with CM, this situation was clearly reversed (Figure 7). In this study, we used HUVECs as the positive control, which were mostly adopted to study angiogenesis in vitro according to published literature. HUVECs were easier to form tubules than HUAECs, and the lumen area was larger, which conformed to the morphological characteristics of the two types of vessel.
Figure 7.
Angiogenesis ability of vascular endothelial cells authenticated in vitro. (A) HUAECs in standard culture conditions, CoCl2 simulate hypoxia and hypoxia plus CXCL6 or CM conditions in vitro were used to simulated angiogenesis. HUVECs tubule forming experiment was taken as positive control. (B to E) Quantitative comparison of the number of master junction and meshes as well as total meshes area and total segments length. Scale = 200 µm. (A color version of this figure is available in the online journal.)
The expression of CXCL6 was regulated by HIF-1α/CXCL6 feedback loop in MSCs and HUAECs under hypoxia condition
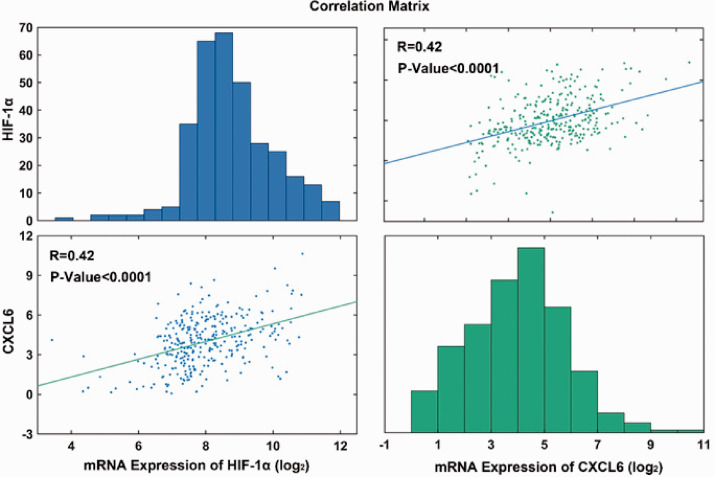
In order to study whether the expression of CXCL6 is related to HIF1-α, we used AML gene data recently published by Oregon Health & Science University (OHSU)13 to analyze the correlation between CXCL6 expression and HIF1-α, and the positive correlation was verified by Person test (Figure 8).
Figure 8.
Correlation between CXCL6 gene expression and HIF-1a gene expression in AML patients. The original data were downloaded from Cbioporta (https://www.cbioportal.org/datasets), n = 323. (A color version of this figure is available in the online journal.)
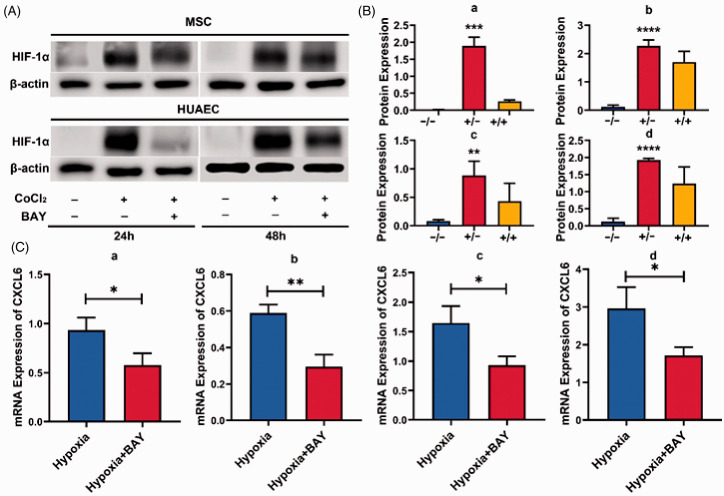
In order to further verify whether CXCL6 is regulated by HIF-1a, we treated MSCs and HUAECs with BAY87-2243 in hypoxia. BAY87-2243 is a selective inhibitor of HIF-1α, which can inhibit the expression of HIF-1α protein and its target genes.14 After HIF-1α was inhibited for 24 or 48 h, CXCL6 gene expression was significantly down-regulated. The expression of HIF-1α protein was detected to verify the effect of BAY87-2243 (Figure 9). These results indicate that the expression of CXCL6 in both cells is regulated by HIF-1a.
Figure 9.
Regulation of CXCL6 expression by HIF-1a. (A) MSCs or HUAECs were treated with CoCl2 (200 µmol/L), with or without BAY87-2243 (1 µmol/L), and the protein expression of HIF-1α was detected by Western blot method after 24 or 48 h. (B) The relative expression of HIF-1a in three independent experiments was statistically analyzed. The x-axis coordinates represented CoCl2(−)/BAY(−), CoCl2(+)/BAY(−), CoCl2(+)/BAY(+), respectively. (a) MSC–24 h; (b) MSC–48 h; (c) HUAEC–24 h; (d) HUAEC–48 h. (C) MSCs and HUAECs were cultured for 24 or 48 h under the condition of 1% O2, with or without BAY87-2243 (1 µmol/L). The relative expression of CXCL6 mRNA was detected by real-time PCR. (a) MSC–24 h; (b) MSC–48 h; (c) HUAEC–24 h; (d) HUAEC–48 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. (A color version of this figure is available in the online journal.)
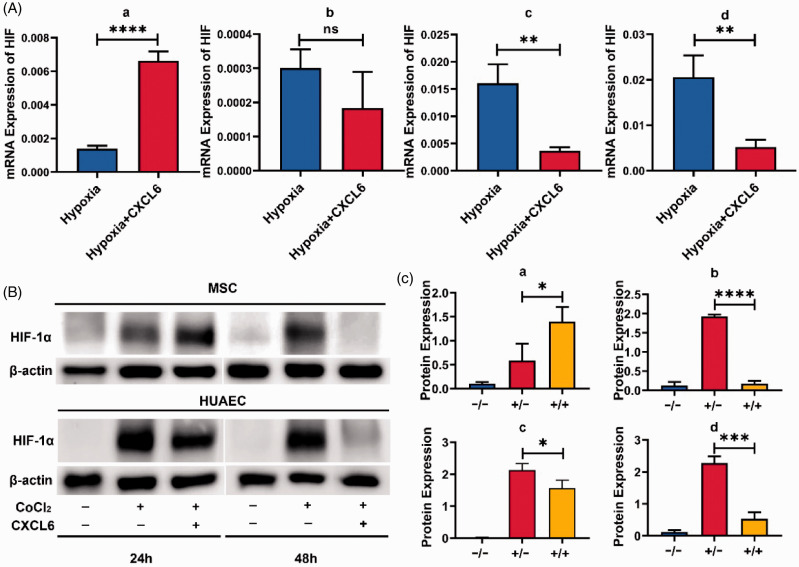
However, whether the transcriptional regulation of HIF-1α will be infinitely positive regulated had aroused our interest. Thus, we treated MSCs and HUAECs with appropriate amount of CXCL6 for 24 or 48 h respectively. The interesting result was that the expression of HIF-1α in MSCs increased within 24 h, but decreased significantly at 48 h. For HUAECs, CXCL6 significantly inhibited the expression of HIF-1α, whether within 24 or 48 h (Figure 10). These results suggest that CXCL6 has a negative feedback effect on HIF-1α.
Figure 10.
Negative feedback regulation of CXCL6 on HIF-1a. (A) MSCs and HUAECs were cultured for 24 or 48 h under 1% O2 condition with or without CXCL6 (50 ng/mL). The relative mRNA expression of HIF-1a was detected by real-time PCR. (a) MSC–24 h; (b) MSC–48 h; (c) HUAEC–24 h; (d) HUAEC–48 h. (B) MSCs or HUAECs were treated with CoCl2, with or without CXCL6 (50 ng/mL). After 24 or 48 h, the expression of HIF-1α protein was detected by Western blot method. (C) The relative expression of HIF-1α protein in three independent experiments was statistically analyzed, and the x-axis coordinates were respectively representative CoCl2(−)/CXCL6(−), CoCl2(+)/CXCL6(−), CoCl2(+)/CXCL6(+), (a) MSC–24 h; (b) MSC–48 h; (c) HUAEC–24 h; (d) HUAEC–48 h. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, and “ns” for no statistical significance. (A color version of this figure is available in the online journal.)
CXCL6 activated mTOR to promote the survival of MSCs and HUAECs under hypoxia condition
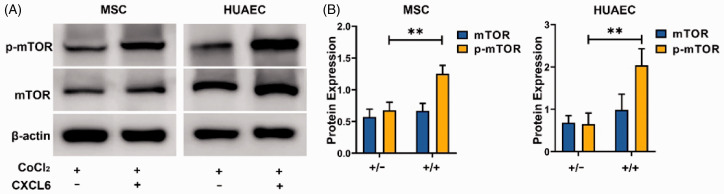
mTOR is reported to be a signal molecule expressed in a variety of cells, which is activated in various cell processes and regulates cell growth, metabolism, and angiogenesis,15 but hypoxia inhibits mTOR activity.15,16 In order to study the mechanism of CXCL6 promoting the growth and survival of MSCs and HUAECs in hypoxic microenvironment, we detected the expression and activation of mTOR under simulated hypoxia condition, with or without CXCL6. The results showed that CXCL6 significantly enhanced the activation of mTOR in MSCs and HUAECs under hypoxia (Figure 11), which indicated that CXCL6 promoted the survival and angiogenesis of MSCs and HUAECs partly through mTOR related pathway.
Figure 11.
CXCL6 activate mTOR in MSCs and HUAECs. (A) CoCl2 (200 µmol/L) was used to treat MSCs or HUAECs for 24 h with or without CXCL6 of 50 ng/Ml. (B) The statistical results of three groups of independent experiments, **P < 0.01. (A color version of this figure is available in the online journal.)
Discussion
This study describes changes of CXCL6 caused by hypoxia in the AML context, which led to the biological properties and functions transforming of MSCs and EPCs, and further resulted in the remodeling of the arteriolar niche. This change caused more severe and continuous hypoxia in the BM niche, bringing out the development and drug-resistance of AML.
Previous studies have found that BM infiltrated by leukemia cells was more hypoxic than healthy BM through leukemic rat model17 and human BM blood gas analysis. The niche in the development of AML is generally out of balance, remodeled, and disordered. MSCs and EPCs can help to restore tissue and support angiogenesis. However, we found that long-term (>48 h) severe hypoxia (1% O2) directly reduced the survival and self-renewal of MSCs and EPCs. What is worse, long-term and severe hypoxia caused high oxidative stress-related damage, and eventually led to apoptosis through the significant increase of HIF1-α and accumulation of DNA damage, although the exposure to moderate hypoxia (5–10% O2) within 24 h could activate the anti-apoptosis mechanism and even promote the proliferation,18 as in our study. Once MSCs or EPCs interacted with AML cells, they obtained intensive functions, such as abnormal MSCs or EPCs secreting a large amount of growth factors, cytokines, and chemokines,19 which promoted EPCs to differentiate into mature ECs.20 In vitro studies have shown that leukemic cells affected ECs through several pathways in the AML microenvironment, which were mainly mediated by angiogenic cytokines (such as members of VEGF family21). The present study involved vascular cytokines CXCL6, which could dramatically promote the proliferation of MSCs and EPCs. Although the gene expression of CXCL6 increased under the stimulation of hypoxia in the early stage, it gradually decreased with long-term hypoxia. Additionally, the protein content measured in the simulated microenvironment decreased significantly, which proved that prolonging the time of the hypoxia and AML cell condition, the malignant microenvironment developed to a situation of large consumption of CXCL6 but a difficulty to supply. When the hypoxia condition lasted for more than 48 h, both MSCs and HUAECs started the apoptotic pathway. This can be proved by the increased expression of cleaved-caspase3. However, the addition of CXCL6 blocked or delayed apoptosis of MSCs and HUAECs. The above results were sufficient to prove that the depletion of MSCs and HUAECs in the later stage of the disease was related to the relative lack of CXCL6. Migration experiments showed that CXCL6 could recruit MSCs and HUAECs to form blood vessels. We found that severe hypoxia made the EPCs state worse, which greatly limited their differentiation into mature ECs and function of angiogenesis. However, when added CM with high-level CXCL6 or recombinant human CXCL6, vascular formation could be saved to a certain extent, which further proved CXCL6 played an important role in arterioles. It has been found that the vascular barrier function was still damaged after chemotherapy, and that BM hypoxia was not normalized after leukemic cells are removed through in vivo imaging on genetically deficient mice model of AML.12 The results emphasized that there was a persistent vascular system with poor function in BM of AML model after chemotherapy. The above data suggested that short-term hypoxia could increase viability of the constituent cells of the arteriolar niche as compensation, but with prolonging time in severe hypoxia, there subsequently occurs serious depletion of those cells, which finally becomes irreversible. Briefly, the primary changes induced by AML blast in the early stage affected the vascular structure and function of the niche,22 followed by the loss of osteoblasts and the inhibition of normal hematopoietic function in the later stage of the disease.8 These events were concentrated and developed continuously, initiated by AML but ended with the irreversible atrophy of the niche. Just as we found that the CXCL6 gene expression and the corresponding protein content in the culture system is contradicted at some time points, this indicated that these events happen in different stages of the dynamic process, which also explains why, for the patients who relapsed or were refractory in large samples, sequencing data expressed CXCL6 higher than those in remission.
In the early stage of hypoxia, CXCL6 was up-regulated in response to HIF-1α transcription regulation. However, the subsequent increase of CXCL6 inhibited the expression of HIF-1α in MSCs or HUAECs through negative feedback loop, so as to avoid excessive accumulation of HIF-1α in nucleus. Studies have shown that HIF-1α regulates the expression of many plasma membrane receptors, thus inducing endocytosis and promoting the release of exosomes. Abnormal exosome secretion is related to the adaptation to different microenvironments, which can promote cancer survival.23 In addition, HIF-1α up-regulates genes involved in many characteristics of cancer, including metabolic reprogramming, cell proliferation, invasion, metastasis, and drug resistance.24 CXCL6 benefits MSCs and EPCs mainly due to the activation of mTOR signaling. Recent studies have shown that mTOR plays an important role in driving the growth and proliferation of stem cells and progenitor cells, promoting the differentiation of MSCs and determining the differentiation process of pluripotent stem cell population.25
Therefore, for the complex and delicate BM microenvironment, simply targeting AML cells is not enough to restore the ecological balance of the BM microenvironment. Only by recovering the components of the microenvironment, can we restore normal hematopoiesis more sufficiently. The highlight is once the vessel wall collapsed, there is no treatment strategy to induce its recovery. As a result, it is suggested that the best treatment strategy is to prevent AML-induced hypoxia leading to arteriole loss in the endosteum, so as to prevent the development of AML due to the hypoxia area (the real “shelter” of LSCs) derived from BM niche.26 Some studies have made efforts to this end. For example, it was found that the ECs of AML mice model produced more nitric oxide, and the recovery of normal vascular function in BM through nitric oxide synthetase (NOS) inhibition improved the treatment response. Thus, they appealed to take the inclusion of NOS inhibitors to target abnormal vascular wall so as to improve the treatment response in clinical trials.12 Equally, we believe that the depletion of CXCL6 caused by severe hypoxia is related to the later atrophy of arterioles. Therefore, regulating CXCL6 may be a promising therapeutic method to normalize the BM vascular system, and eventually to enhance drug delivery and reduce hypoxia in the BM niche.
In conclusion, AML resulted in extensively severe hypoxia of BM. CXCL6 up-regulated as compensation, attempting to promote ECs viability and angiogenesis. However, due to the long-term hypoxia accumulated damage of MSCs and EPCs, it was difficult to supply CXCL6 needed by autocrine, resulting in irreversible EC depletion, which led to the collapse of the arteriolar niche. Therefore, CXCL6 can be considered as an auxiliary strategy to restore abnormal arteriole niches, although its specific mechanism and timing need further research in vivo.
ACKNOWLEDGMENTS
The authors thank professor Hai Bai, 940 Hospital of the people's Liberation Army of China, for providing sorted hUC-MSCs.
AUTHORS' CONTRIBUTIONS: All authors participated in the design, interpretation of the studies and analysis of the data and review of the manuscript; LJL and JCM conducted the experiments, LJL wrote the manuscript, and LZ contributed supervision.
Declaration OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the Natural Science Foundation of Gansu province (grant number 18JR3RA342).
ORCID iD: Li Zhao https://orcid.org/0000-0001-5600-5087
References
- 1.Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, Jemal A, Kramer JL, Siegel RL. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin 2019; 69:363–85 [DOI] [PubMed] [Google Scholar]
- 2.Bernasconi P, Farina M, Boni M, Dambruoso I, Calvello C. Therapeutically targeting SELF-reinforcing leukemic niches in acute myeloid leukemia: a worthy endeavor?. Am J Hematol 2016; 91:507–17 [DOI] [PubMed] [Google Scholar]
- 3.Nombela-Arrieta C, Silberstein LE. The science behind the hypoxic niche of hematopoietic stem and progenitors. Hematol Am Soc Hematol Educ Prog 2014; 2014:542–7 [DOI] [PubMed] [Google Scholar]
- 4.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, Zaher W, Mortensen LJ, Alt C, Turcotte R, Yusuf R, Cote D, Vinogradov SA, Scadden DT, Lin CP. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014; 508:269–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, Mizoguchi T, Wei Q, Lucas D, Ito K, Mar JC, Bergman A, Frenette PS. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature 2013; 502:637–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanoun M, Zhang D, Mizoguchi T, Pinho S, Pierce H, Kunisaki Y, Lacombe J, Armstrong SA, Dührsen U, Frenette PS. Acute myelogenous leukemia-induced sympathetic neuropathy promotes malignancy in an altered hematopoietic stem cell niche. Cell Stem Cell 2014; 15:365–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kwon SY, Chun SY, Ha Y-S, Kim DH, Kim J, Song PH, Kim HT, Yoo ES, Kim BS, Kwon TG. Hypoxia enhances cell properties of human mesenchymal stem cells. Tissue Eng Regen Med 2017; 14:595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte D, Hawkins ED, Akinduro O, Ang H, De Filippo K, Kong IY, Haltalli M, Ruivo N, Straszkowski L, Vervoort SJ, McLean C, Weber TS, Khorshed R, Pirillo C, Wei A, Ramasamy SK, Kusumbe AP, Duffy K, Adams RH, Purton LE, Carlin LM, Lo Celso C. Inhibition of endosteal vascular niche remodeling rescues hematopoietic stem cell loss in AML. Cell Stem Cell 2018; 22:64–77.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Passaro D, Bonnet D. How to say NO to vascular disruption and stem cell mobilization. Expert Opin Ther Targets 2018; 22:563–5 [DOI] [PubMed] [Google Scholar]
- 10.Lavrentieva A, Majore I, Kasper C, Hass R. Effects of hypoxic culture conditions on umbilical cord-derived human mesenchymal stem cells. Cell Commun Signal 2010; 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma J-C, Sun X-W, Su H, Chen Q, Guo T-K, Li Y, Chen X-C, Guo J, Gong Z-Q, Zhao X-D, Qi J-B. Fibroblast-derived CXCL12/SDF-1α promotes CXCL6 secretion and co-operatively enhances metastatic potential through the PI3K/Akt/mTOR pathway in colon cancer. World J Gastroenterol 2017; 23:5167–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Passaro D, Di Tullio A, Abarrategi A, Rouault-Pierre K, Foster K, Ariza-McNaughton L, Montaner B, Chakravarty P, Bhaw L, Diana G, Lassailly F, Gribben J, Bonnet D. Increased vascular permeability in the bone marrow microenvironment contributes to disease progression and drug response in acute myeloid leukemia. Cancer Cell 2017; 32:324–41 e326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyner JW, Tognon CE, Bottomly D, Wilmot B, Kurtz SE, Savage SL, Long N, Schultz AR, Traer E, Abel M, Agarwal A, Blucher A, Borate U, Bryant J, Burke R, Carlos A, Carpenter R, Carroll J, Chang BH, Coblentz C, d’Almeida A, Cook R, Danilov A, Dao K-HT, Degnin M, Devine D, Dibb J, Edwards DK, Eide CA, English I, Glover J, Henson R, Ho H, Jemal A, Johnson K, Johnson R, Junio B, Kaempf A, Leonard J, Lin C, Liu SQ, Lo P, Loriaux MM, Luty S, Macey T, MacManiman J, Martinez J, Mori M, Nelson D, Nichols C, Peters J, Ramsdill J, Rofelty A, Schuff R, Searles R, Segerdell E, Smith RL, Spurgeon SE, Sweeney T, Thapa A, Visser C, Wagner J, Watanabe-Smith K, Werth K, Wolf J, White L, Yates A, Zhang H, Cogle CR, Collins RH, Connolly DC, Deininger MW, Drusbosky L, Hourigan CS, Jordan CT, Kropf P, Lin TL, Martinez ME, Medeiros BC, Pallapati RR, Pollyea DA, Swords RT, Watts JM, Weir SJ, Wiest DL, Winters RM, McWeeney SK, Druker BJ. Functional genomic landscape of acute myeloid leukaemia. Nature 2018; 562:526–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellinghaus P, Heisler I, Unterschemmann K, Haerter M, Beck H, Greschat S, Ehrmann A, Summer H, Flamme I, Oehme F, Thierauch K, Michels M, Hess-Stumpp H, Ziegelbauer K. Bay 87-2243, a highly potent and selective inhibitor of hypoxia-induced gene activation has antitumor activities by inhibition of mitochondrial complex I. Cancer Med 2013; 2:611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxton RA, Sabatini DM. mTOR signaling in growth, metabolism, and disease. Cell 2017; 168:960–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schito L, Rey S. Cell-Autonomous metabolic reprogramming in hypoxia. Trends Cell Biol 2018; 28:128–42 [DOI] [PubMed] [Google Scholar]
- 17.Jensen PO, Mortensen BT, Hodgkiss RJ, Iversen PO, Christensen IJ, Helledie N, Larsen JK. Increased cellular hypoxia and reduced proliferation of both normal and leukaemic cells during progression of acute myeloid leukaemia in rats. Cell Prolif 2000; 33:381–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baldea I, Teacoe I, Olteanu DE, Vaida-Voievod C, Clichici A, Sirbu A, Filip GA, Clichici S. Effects of different hypoxia degrees on endothelial cell cultures – time course study. Mech Ageing Dev 2018; 172:45–50 [DOI] [PubMed] [Google Scholar]
- 19.Melzer C, Yang Y, Hass R. Interaction of MSC with tumor cells. Cell Commun Signal 2016; 14:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge Q, Zhang H, Hou J, Wan L, Cheng W, Wang X, Dong D, Chen C, Xia J, Guo J, Chen X, Wu X. VEGF secreted by mesenchymal stem cells mediates the differentiation of endothelial progenitor cells into endothelial cells via paracrine mechanisms. Mol Med Rep 2018; 17:1667–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Poulos MG, Gars EJ, Gutkin MC, Kloss CC, Ginsberg M, Scandura JM, Rafii S, Butler JM. Activation of the vascular niche supports leukemic progression and resistance to chemotherapy. Exp Hematol 2014; 42:976–86 e973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duarte D, Hawkins ED, Lo Celso C. The interplay of leukemia cells and the bone marrow microenvironment. Blood 2018; 131:1507–11 [DOI] [PubMed] [Google Scholar]
- 23.Choudhry H, Harris AL. Advances in hypoxia-Inducible factor biology. Cell Metab 2018; 27:281–98 [DOI] [PubMed] [Google Scholar]
- 24.Rankin EB, Giaccia AJ. Hypoxic control of metastasis. Science 2016; 352:175–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng D, Frank AR, Jewell JL. mTOR signaling in stem and progenitor cells. Development 2018; 145:dev152595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernasconi P, Borsani O. Targeting leukemia stem cell-niche dynamics: a new challenge in AML treatment. J Oncol 2019; 2019:8323592. [DOI] [PMC free article] [PubMed] [Google Scholar]