Abstract
Probiotics are beneficial bacteria that exist within the human gut, and which are also present in different food products and supplements. They have been investigated for some decades, due to their potential beneficial impact on human health. Probiotics compete with pathogenic microorganisms for adhesion sites within the gut, to antagonize them or to regulate the host immune response resulting in preventive and therapeutic effects. Therefore, dysbiosis, defined as an impairment in the gut microbiota, could play a role in various pathological conditions, such as lactose intolerance, gastrointestinal and urogenital infections, various cancers, cystic fibrosis, allergies, inflammatory bowel disease, and can also be caused by antibiotic side effects. MicroRNAs (miRNAs) are short non-coding RNAs that can regulate gene expression in a post-transcriptional manner. miRNAs are biochemical biomarkers that play an important role in almost all cellular signaling pathways in many healthy and disease states. For the first time, the present review summarizes current evidence suggesting that the beneficial properties of probiotics could be explained based on the pivotal role of miRNAs.
Video Abstract
Keywords: MicroRNAs, Probiotics, Biomarkers, Cancer, Inflammatory bowel disease, Supplements
Background
The gastrointestinal tract (GIT) is an active ecosystem within the human body, and normally contains beneficial bacteria essential for maintaining metabolism and immune cell maturation. In the gut, healthy bacteria are part of the normal microbiota that arrive in the intestine via food intake, and naturally coexist with each other. These microorganisms are in a balanced relationship with immune cells associated with the lamina propria in the gut, and lead to the stimulation and maturation of these immune cells [1].
Probiotics are defined as a group of beneficial living bacteria, which are naturally members of the intestinal microbiota, and some of them have been incorporated into food products and supplements to improve GIT health through maintaining a good microbial balance. To improve host health or to treat various infectious and non-infectious diseases, the potential properties of probiotics have been widely explored in experimental models. The mentioned beneficial effects include: a decrease in GIT inflammatory responses [2], preventive activity against malignancy [3–5], protective role against infections [6–8], allergy prevention [9, 10], inhibition of Helicobacter pylori growth [11], and relief of irritable bowel syndrome [12]. In addition, the antioxidant, anti-inflammatory and anticancer activity of probiotics may be explained by saturated fatty acid production in the gut [13, 14]. Although probiotics have shown encouraging results in several human diseases, such as irritable bowel syndrome, multi-drug resistant pathogens, and diabetes [15–17], more extensive studies are still needed to confirm their preliminary effectiveness on nutrition, human health, and modulation of various diseases.
MicroRNAs (miRNAs) are members of the family of non-coding RNAs, and are 19–25, nucleotides in length [18]. miRNAs are generated from endogenous primary miRNA precursors [19]. Recently, interest in miRNAs has taken off due to their roles in the treatment and development of a wide variety of diseases. Additionally, miRNAs play key roles in numerous normal physiological networks. Deregulation of miRNAs has been implicated in the pathogenesis of several disorders, including cancer and infections [20, 21].
This review summarizes the available data on the modulatory effects of probiotics on miRNA expression and function in pathological conditions,
Probiotics in health and disease
Microbial populations found within the GIT, have numerous roles in different aspects of human health [22]. Any change in the normal microbiome can be the cause of various diseases, including gastrointestinal cancers and obesity [23, 24]. Probiotics are externally administered living microbes that are beneficial for human health via their regulatory function on the host GI microbiome, host immune system, and systemic inflammation [25–27]. Probiotics, if administered in a sufficient amount, can improve diseases, or the lessen the complications of various disorders, including GI cancers, inflammatory bowel disease (IBD), rheumatoid arthritis, obesity, and diabetes (Table 1) [106, 107]. Lactobacillus and Bifidobacterium species are the major bacterial strains that are generally consumed as probiotics [107].
Table 1.
Selected probiotic preparations that have been investigated to treat various diseases
| Physiology or pathological condition | Probiotic agent | Probiotic concentration | Duration of study | Effect (s) | Model | Sample (n) | Ref |
|---|---|---|---|---|---|---|---|
| Colorectal cancer | Lactobacillus acidophilus, L. rhamnosus | 2 × 109 CFU (colony-forming units) | 12 weeks | Enhance bowel signs and QOL | Human | 28 | [28] |
| Gastric cancer | Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, Bacillus cereus | > 106 CFU/tablet | 6–7 days | Improve immune response and decrease severity of inflammation | Human | 50 | [29] |
| Colorectal cancer |
Lactobacillus acidophilus, L. casei, L. lactis, Bifidobacterium bifidum, B. longum, B. infantis |
3 × 1010 CFU | 8 weeks | Improve QOL, decrease inflammatory biomarkers, reduce side effects of chemotherapy | Human | 70 | [30] |
| Colorectal cancer | Lactobacillus acidophilus, L. casei. L. lactis, Bifidobacterium bifidum, B. longum, B. infantis | 3 × 1010 CFU | 1 week | Accelerate return of normal gut function | Human | 20 | [31] |
| Asthma |
Lactobacillus rhamnosus GG (LGG) |
1 × 1010 CFU | 6 months | Enhance T-regulatory induction | Human | 10 | [32] |
| Eczema and Asthma | Lactobacillus rhamnosus GG (LGG) | 1 × 1010 CFU | 6 months | Prevent the eczema or asthma development | Human | 92 | [33] |
| Seasonal allergic rhinitis and Intermittent asthma |
Bifidobacterium spp (B. longum BB536, B. infantis M-63, B. breve M-16V) |
3 × 109 CFU, 1 × 109 CFU and 1 × 109 CFU |
4 weeks | Enhance AR and QOL | Human | 40 | [34] |
| Atopic | Bifidobacterium bifidumW23, Bifi-dobacterium lactisW52 and Lactococcus Lactis W58 | 3 × 109 CFU | 6 years | Minor impact on gut microbiota composition | Human | 99 | [35] |
| Necrotizing enterocolitis (NEC) | Bifidobacterium and Lactobacillus | 3 × 109 CFU | At least 10 days | Decreased frequency of necrotizing enterocolitis in preterm neonates | Human | 52 | [36] |
| Necrotizing enterocolitis (NEC) | Bifidobacterium lactis and Lactobacillus rhamnosus | 1 × 108 CFU and 1 × 109 CFU | 3 years | No significant effects | Human | 332 | [37] |
| Necrotizing enterocolitis (NEC) | Lactobacillus acidophilus and Bifidobacterium bifidum | 1 × 109CFU and 1 × 109 CFU | 8 month | No significant effects | Human | 31 | [38] |
| Inflammatory bowel disease (IBD) | Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 |
1 × 103 CFU and 2 × 107 CFU |
30 days | Anti-inflammatory effects | Human | 8 | [39] |
| Inflammatory bowel disease (IBD) | Lactobacillus acidophilus La-5 and Bifidobacterium BB-12 | 1 × 106 CFU | 8 weeks | Enhanced intestinal function | Human | 105 | [40] |
| Rheumatoid arthritis (RA) |
Lactobacillus rhamnosus GR-1 and Lactobacillus reuteri RC-14 |
2 × 109 CFU | 4 month | No significant effects | Human | 14 | [41] |
| Rheumatoid arthritis | Lactobacillus acidophilus, L. casei and Bifidobacterium bifidum | 6 × 109 CFU | 8 weeks | Therapeutic effects | Human | 30 | [42] |
| Rheumatoid arthritis | Lactobacillus casei 01 | 1 × 108 CFU | 8 weeks | Reduced disease activity and inflammatory status | Human | 22 | [43] |
| Atopic dermatitis | Lactobacillus rhamnosus (LR) | NA | 8 weeks | Reduce signs of atopic dermatitis | Human | 30 | [44] |
| Atopic dermatitis | Lactobacillus plantarum IS-10506 | 1 × 1010 CFU | 12 weeks | Therapeutic effects | Human | 12 | [45] |
| Atopic dermatitis (AD) | Lactobacillus rhamnosus GG (LGG), Bifidobacterium animalis subsp. lactis Bb-12 (Bb-12), d L. acidophilus La-5 (La-5) | 5 × 1010 CFU,5 × 1010 CFU and 5 × 109 CFU | 2 years | Decreased proportion of Th22 cells. | Human | 68 | [46] |
| Irritable bowel syndrome (IBS) | Bacillus subtilis, Bifidobacterium spp., Lactobacillus spp., L. lactis, and Streptococcus thermophilus | 8 × 109 CFU | 16 weeks | Significantly improvement in IBS symptoms well tolerance | Human | 181 | [47] |
| Irritable bowel syndrome (IBS) | Lactobacillus acidophilus CL1285, L. casei LBC80R, L. rhamnosus CLR2 | 5 × 1010 CFU | 12 weeks | Improved stool consistency and frequency, QOL, and IBS symptoms | Human | 76 | [48] |
| Irritable bowel syndrome (IBS) | Bifidobacterium longum (BL) | 1 × 1010 CFU | 6 weeks | Decreased depression | Human | 18 | [49] |
| Irritable bowel syndrome (IBS) | Lactobacillus brevis KB290 | 1 × 109 CFU | 12 weeks | Improved symptoms and inflammatory status | Human | 20 | [50] |
| Gastroenteritis | Lactobacillus rhamnosus GG | 1 × 1010 CFU | 5 days | No significant effects | Human | 468 | [51] |
| Acute gastroenteritis (AGE) | Lactobacillus rhamnosus (LGG) | 1 × 1010 CFU (twice a day) | 5 days | - | Human | - | [52] |
| Gastroenteritis | Lactobacillus rhamnosus GG (LGG) | 1 × 1010 CFU | 4 weeks | Immuno-modulatory effects | Human | 65 | [53] |
| Eczema |
Lactobacillus salivarius CUL61, L. paracasei CUL08, Bifidobacterium animalis subspecies lactis CUL34 and B. bifidum CUL20 |
1 × 1010 CFU | 2 years | Prevent eczema | Human | 187 | [54] |
| Eczema | Bifidobacterium longum (BL999) and Lactobacillus rhamnosus (LPR) | NA | 5 years | No significant effects | Human | 124 | [55] |
| Allergic rhinitis | Bifidobacterium lactis NCC 2818 | 4 × 109 CFU | 8 weeks | Improved immune parameters and allergic symptoms | Human | 10 | [56] |
| Allergic rhinitis | Lactobacillus paracasei LP-33 | 2 × 109 CFU | 5 weeks | Enhanced QOL | Human | 179 | [57] |
| Allergic rhinitis | Lactobacillus paracasei HF.A00232 (LP) | 5 × 109 CFU | 8 weeks | No significant effects | Human | 32 | [58] |
| Celiac disease | Bifidobacterium longum CECT 7347 | 1 × 109 CFU | 3 months | Enhanced health status | Human | 17 | [59] |
| Celiac disease | Bifidobacterium infantis | 2 × 109 CFU | 3 weeks | - | Human | 12 | [60] |
| Celiac disease | Bifidobacterium breve BR03 and B632 | 1 × 109 CFU | 3 month | Reduced TNF-α levels | Human | 22 | [61] |
| Obesity | Lactobacillus casei strain Shirota (LcS) | ≥ 4 × 1010 CFU | 6 months | Decreased body weight and increased high density lipoprotein cholesterol concentration | Human | 12 | [62] |
| Obesity | Lactobacillus rhamnosus (LPR) | 1.6 × 108 CFU | 12 weeks | Improve fasting fullness and cognitive restraint in men | Human | 62 | [63] |
| Obesity | Lactobacillus curvatus HY7601, L. plantarum KY1032 | 5 × 109 CFU | 12 weeks | Induced weight loss and decreased adiposity . | Human | 32 | [64] |
| Type I diabetes and Type II diabetes |
Lactobacillus acidophilus ZT-L1, Bifidobacterium bifidum ZT-B1, L. reuteri ZT-Lre, L. fermentum ZT-L3 |
8 × 109 CFU | 12 weeks | Beneficial effects on glycemic control and markers of cardio-metabolic risk. | Human | 30 | [65] |
| Type II diabetes |
Bifidobacterium bifidum W23, B. lactis W52, L. acidophilus W37, L. brevis W63, L. casei W56, L. salivarius W24, Lactococcus lactis W19 and L.s lactis W58 |
2.5 × 109 CFU | 12 weeks | Enhanced HOMA-IR | Human | 39 | [66] |
| Type II diabetes | Lactobacillus reuteri DSM 17938 | 1 × 108 CFU | 12 weeks | No significant effects | Human | 30 | [67] |
| Type II diabetes |
concentrated biomass of 14 probiotic bacteria genera Bifidobacterium, Lactobacillus, Lactococcus, Propionibacterium |
Lactobacillus + Lactococcus (6 × 1010 CFU/g), Bifidobacterium (1 × 1010/g), Propionibacterium (3 × 1010/g) and Acetobacter (1 × 106/g) |
8 weeks | Reversed insulin resistance | Human | 31 | [68] |
| Type II diabetes | Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp lactis BB-12 | 1 × 109 CFU | 6 weeks | Enhanced glycemic control in T2D patients, however, the intake of fermented milk seems to be involved with other metabolic changes, such as reduced inflammatory cytokines (TNF-α and resistin) . | Human | 23 | [69] |
| HIV | Lactobacillus casei Shirota (LcS) | 6.5 × 108 CFU | 8 weeks | Immunological and virological effects | Human | 20 | [70] |
| HIV |
Lactobacillus plantarum, Streptococcus thermophilus, Bifidobacterium breve, L. paracasei, L. delbrueckii subsp. bulgaricus, L. acidophilus, B. longum, B. infantis |
1.8 × 1012 CFU | 6 months | Beneficial effects on the reconstitution of physical and immunological integrity of the mucosal intestinal barrier | Human | 10 | [71] |
| HIV | Saccharomyces boulardii | NA | 12 weeks | Therapeutic effects | Human | 22 | [72] |
| Non-alcoholic fatty liver disease | a mixture of eight probiotic strains (Streptococcus thermophilus, bifidobacteria SPP., Lactobacillus acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. bulgaricus) | NA | 4 month | Reduced body mass index (BMI) in probiotic supplemented children | Human | 22 | [73] |
| Non-alcoholic fatty liver disease |
Lactobacillus acidophilus La5 and Bifidobacterium lactis Bb12 |
6.46 × 106 and 4.97 × 106 CFU/g | 8 weeks |
Improved hepatic enzymes, serum total cholesterol, and low density lipoprotein cholesterol levels |
Human | 36 | [74] |
| Non-alcoholic fatty liver disease |
Lactobacillus casei, L. acidophilus, L. rhamnosus, L. bulgaricus, Bifidobacterium breve, B. longum, and Streptococcus thermophilus. |
Lactobacillus casei (3 × 109 CFU/g), Lactobacillus acidophilus (3 × 1010 CFU/ g), Lactobacillus rhamnosus (7 × 109 CFU/g), Lactobacillus bulgaricus (5 × 108 CFU/g), Bifidobacterium breve (2 × 1010 CFU/g), Bifidobacterium longum (1 × 109 CFU/g), and Streptococcus thermophilus (3 × 108 CFU/g). |
8 weeks | Reduced insulin requirement, reversed insulin resistance, TNF-α, and IL-6 | Human | 21 | [75] |
| Non-alcoholic steatohepatitis |
Lactobacillus casei, L. rhamnosus, L. bulgaris, Bifidobacterium longum, and Streptococcus thermophilus |
1 × 108 CFU | 12 weeks | Reduced body mass index (BMI) and serum cholesterol | Human | 38 | [76] |
| Non-alcoholic steatohepatitis | Lactobacillus plantarum, L. deslbrueckii, L. acidophilus, L. rhamnosus and Bifidobacterium bifidum | 2 × 108 CFU | 6 months | No changes in body mass index, waist circumference, glucose or lipid levels but decreased liver fat and aspartate transaminase (AST) level | Human | 10 | [77] |
| Urinary Tract Infections | Lactobacillus crispatus GAI 98322 | 1 × 108 CFU | 1 year | Prevent recurrence of UTI | Human | 9 | [78] |
| Urinary Tract Infections | Lactobacillus crispatus | 1 × 108 CFU | 10 weeks | Less recurrence of UTI | Human | 43 | [79] |
| Urinary Tract Infections | Lactobacillus GG | 6 × 109 CFU | 1 week | Decreased incidence of UTIs, necrotizing enterocolitis (NEC) and sepsis | Human | 295 | [80] |
| Urinary Tract Infections | Lactobacillus crispatus CTV-05 | 5 × 108 CFU | 5 days | Minimal side effects | Human | 15 | [81] |
| Vaccine Adjuvants | Bifidobacterium lactis, Lactobacillus acidophilus, L. plantarum, L. paracasei and L. salivarius | 2 × 1010 CFU | 3 weeks | Faster immune response. Specific probiotics may be adjuvants to humoral immune response following oral vaccination | Human | 63 | [82] |
| Vaccine Adjuvants | Lactobacillus fermentum CECT5716 | 1 × 1010 CFU | 4 weeks | Potentiated the immunologic response and improved systemic protection from infection | Human | 25 | [83] |
| Vaccine Adjuvants | Lactobacillus GG | 1 × 1010 CFU | 4 weeks | Improved influenza vaccine immunogenicity | Human | 19 | [84] |
| Plaque and carries | Lactobacillus reuteri | 1 × 108 CFU | 1 years | Decreased caries prevalence and gingivitis score | Human | 60 | [85] |
| Plaque and carries | Streptococcus uberis KJ2™, Streptococcus. oralis KJ3™, Streptococcus. rattus JH145 | 1 × 108 CFU | 1 year | Reduced early childhood caries development | Human | 54 | [86] |
| Plaque and carries | Lactobacillus brevis CD2 | NA | 6 weeks | Beneficial effects on variables associated with oral health | Human | 91 | [87] |
| Plaque and carries | Bifidobacterium animalis subsp. lactis BB-12 | 1 × 1010 CFU | 2 years | No significant effects | Human | 32 | [88] |
| Plaque and carries | Bifidobacterium animalis subsp. lactis DN-173010 | ≥ 108 CFU | 4 weeks | Effect on plaque accumulation and gingival inflammatory parameters | Human | 26 | [89] |
| Oral wounds |
A mixture of two probiotic strains, Lactobacilli reuteri DSM 17938 and ATCC PTA 5289 |
2 × 108 CFU/mL | 1 week | No significant effects | Human | - | [90] |
| oral lichen planus (OLP) | Lactobacilli reuteri (DSM 17938 and ATCC PTA 5289) | NA | 16 weeks | No significant effects | Human | 9 | [91] |
| Periodontitis |
L. rhamnosus SP1 |
2 × 107 CFU | 3 month | Clinical and microbiological improvements. | Human | 16 | [92] |
| Periodontitis | Lactobacillus reuteri | 1 × 108 CFU | 3 weeks |
Decreased pro-inflammatory cytokine response and improved clinical parameters |
Human | 24 | [93] |
| Periodontitis | Lactobacillus reuteri | 1 × 108 CFU | 12 weeks | Useful adjunct to scaling and root planing (SRP) in chronic periodontitis. | Human | 15 | [94] |
| chronic periodontitis (CP) | Lactobacillus Reuteri | NA | 3 weeks | Reduced inflammatory markers | Human | 15 | [95] |
| Periodontitis | L. rhamnosus SP1 | 2 × 107 CFU | 3 months | Clinical improvement | Human | 12 | [96] |
| Gingivitis | Bifidobacterium animalis | ≥ 108 CFU | 4 weeks | Effects on plaque accumulation and gingival inflammation | Human | 26 | [89] |
| Gingivitis | Lactobacillus rhamnosus PB01, DSM 14869 and Lactobacillus curvatus EB10, DSM 32307 | ≤ 108 CFU/tablet | 4 weeks | Enhanced gingival health | Human | 23 | [97] |
| Gingivitis |
Bacillus subtilis, Bacillus megaterium and Bacillus pumulus as a toothpaste |
5 × 107 CFU | 8 weeks | No significant effects | Human | 20 | [98] |
| Gingivitis | Lactobacillus plantarum, Lactobacillus brevis and Pediococcus acidilactici | NA | 6 weeks | Significant changes in mean gingival index | Human | 29 | [99] |
|
Pregnancy Gingivitis |
Lactobacillus reuteri DSM 17938 | ≥ 108 CFU | 7 weeks | Useful adjunct in the control of pregnancy gingivitis | Human | 24 | [100] |
| Halitosis | Lactobacillus salivarius WB21 | 2 × 109 CFU | 4 weeks | Improved halitosis | Human | 20 | [101] |
| Halitosis | Lactobacillus brevis CD2 | NA | 2 weeks | No significant effects | Human | 10 | [102] |
| Candida infection | Lactobacillus reuteri | 1 × 108 CFU | 1 month | Decreased incidence of sepsis in addition to improving symptoms and feeding | Human | 150 | [103] |
| Candida infection | Lactobacillus fermentum LF10 and L. acidophilus LA02 | ≥ 4 × 108 CFU | 11 weeks | - | Human | 57 | [104] |
| Candida infection |
Lactobacillus acidophilus, L. rhamnosus, Streptococcus thermophilus, and L. delbrueckii subsp. bulgaricus |
NA | Prevented relapse | Human | 201 | [105] |
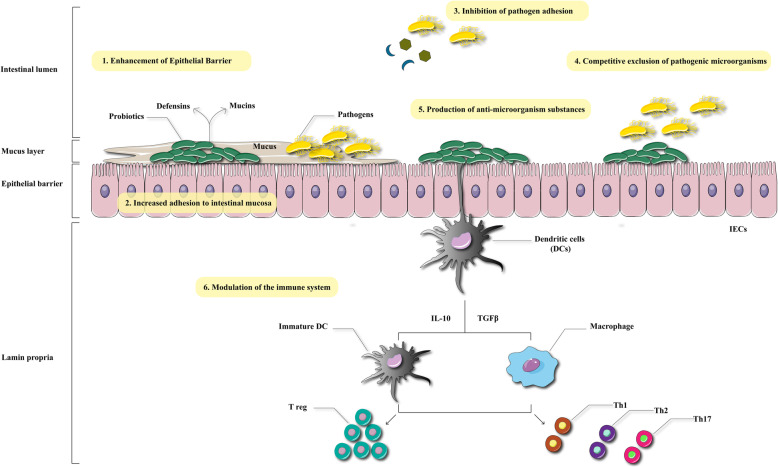
The major mechanisms of action of probiotics, include enhancement of the gut epithelial barrier, adhesion to intestinal mucosa, and concomitant inhibition of pathogen adhesion, competitive exclusion of pathogenic microorganisms, production of anti-microbial substances, and modulation of the immune system (Fig. 1).
Fig 1.
Major mechanisms of action of probiotics. Probiotics exert its therapeutic effects via different mechanisms including enhancement of epithelial barrier, inhibition of pathogen adhesion, and modulation of immune system. This figure adapted from Bermudez-Brito et al (Ann Nutr Metab 2012;61:160–174)
Epidemiological studies clearly show that, despite all the advances made in the field of diagnosis, prevention and treatment of cancer, the prevalence of cancer is still increasing. About 80% of all types of cancer are due to environmental and lifestyle factors [108]. Due to the high burden of cancer around the world, effective treatment cancer is very important [109]. There are many treatment methods such as chemotherapy, radiotherapy, targeted therapy and immunotherapy, but their overall efficacy is still not satisfactory [110, 111]. Probiotics have recently been used to improve cancer treatment, relieve symptoms and increase the quality of life [112]. One of the reasons for the occurrence of cancers, in particular GIT cancers, may be changes in the normal GI microbial flora, and therefore the use probiotics is attractive to modulate these changes, reduce complications, or even treat cancer [113]. In one study by Gao et al., probiotic therapy reduced the number of mucosal-associated pathogens in patients with colorectal cancer (CRC) by altering the profile of microbial flora in the mucosa. A total of 22 CRC patients completed the trial. Patients were randomized into a PGT group (n = 11) taking probiotics or CGT group (n = 11) taking preoperative placebo. It should be noted that the participants in the PGT group took an encapsulated probiotic combination consisting of living Bifidobacterium longum, Enterococcus faecalis, and Lactobacillus acidophilus (1:1:1) with at least 1×107 CFU/g viable cells, 3 times a day, with a total daily dosage of 6×107 CFU for 5 days. The participants in the CGT group took only encapsulated maltodextrin 3 times a day. The results showed that the probiotic supplement regimen could efficiently modify the composition and diversity of the gut microflora. It could also suppress specific potential pathogens such as Peptostreptococcus and Fusobacterium strains. In addition, probiotics enhanced the numbers of specific beneficial microorganisms [114]. Abnormal blood vessels and hypoxic and necrotic regions are common features of solid tumors and related to the malignant phenotype and therapy resistance. Certain obligate or facultative anaerobic bacteria exhibit inherent ability to colonize and proliferate within solid tumors in vivo. Escherichia coli Nissle 1917, a non-pathogenic probiotic in European markets, has been known to proliferate selectively in the interface between the viable and necrotic regions of solid tumors. Li et al. established a tumor-targeting therapy system using the genetically engineered E. coli Nissle 1917 for targeted delivery of cytotoxic compounds, including glidobactin, colibactin, and luminmide. Biosynthetic gene clusters of these cytotoxic compounds were introduced into E. coli Nissle 1917 and the corresponding compounds were detected in the resultant recombinant strains. The recombinant E. coli Nissle 1917 showed cytotoxic activity in vitro and in vivo as well, and suppressed the tumor growth [115]. Another study was conducted to evaluate the effect of probiotic supplementation in patients with laryngeal cancer. After the intervention, biochemical markers of stress were reduced. A total of 20 healthy controls and 30 patients with laryngeal cancer were included. Then, for two weeks prior to the surgical operation, 20 patients were randomly assigned to take a placebo or probiotic supplement (Clostridium butyricum; 420 mg/capsule) two times per day. In addition, the degree of anxiety and the heart rate were assessed. It was found that the level of serum corticotropin-releasing factor (CRF) in patients with laryngeal cancer was increased as they approached the time of surgery, but no corresponding increase in CRF, anxiety or heart rate was seen after probiotic use. Probiotics reduced the level of the patient anxiety on the Hamilton Anxiety Scale (HAMA) from 19.8 to 10.2. Consequently, clinical anxiety and biochemical features of stress were decreased by probiotics administration in the participants assigned for laryngectomy [116]. In another study carried out on patients with gastric cancer, the results showed that the combination of dietary fiber and probiotics was effective in treating post-operative diarrhea. The study included 120 patients suffering from GC. Patients were assigned to one of 3 groups as follows: (1) Fiber-enriched nutrition formula (FE group, n = 40), (2) Fiber-free nutrition formula (FF group, n = 40), and (3) Fiber and probiotic-enriched nutrition formula, a combination of live bifidobacterium and lactobacillus in tablets , (FEP group, n = 40). Then, each patient received enteral nutrition (EN) formulae for seven successive days after the surgical operation [117]. Earlier investigations had shown that addition of the fiber or probiotics may preserve the intestinal microecology, and diminish diarrhea related to EN [118]. Dietary fiber is a kind of carbohydrate polymer, which cannot be digested by humans. Some researchers have shown that the effects of combined probiotics and fiber on the treatment of diarrhea were indecisive [119–121]. According to the present RCT investigation, the combination of probiotics and fiber may reduce diarrhea, augment intestinal motility, and diminish intestinal dysfunction in the post-operative GC patients receiving EN. Additionally, probiotics may shorten the length of hospital stay (LOHS) as part of enhanced recovery after surgery (ERAS) protocols. Therefore, the combination of fiber and probiotics when beginning EN, may avoid diarrhea related to EN, improving comfort, and enhancing recovery after surgical operation [117].
Ventilator-associated pneumonia (VAP) is often caused by aspiration of pathogenic bacteria from the oropharynx. Oral decontamination by using antiseptics, such as antibiotics or chlorhexidine (CHX), can be used as prophylaxis. Klarin et al. examined the probiotic effect of bacteria Lactobacillus plantarum 299 (Lp299) as CHX in reducing the pathogenic bacteria in the oropharynx. Fifty tracheally intubated, mechanically ventilated, critically ill patients critically ill patients administrated to either oral cleansing by 0.1% CHX solution or to the same washing procedure and oral using of an emulsion of Lp299. Oropharynx samples showed that pathogenic bacteria that were not present at inclusion were detected in the patients treated with Lp299 was less than those in control group [122].
Inflammatory bowel disease (IBD) consists of two disorders, Crohn's disease and ulcerative colitis [123]. In the pathogenesis of IBD, it is thought that pathogenic or resident luminal bacteria continuously activate the mucosal and systemic immune systems, and ultimately cause an inflammatory cascade [124]. IBD is a chronic immunological disease that is related to lack of dietary fiber, saturated fatty acids, poor sleep, and low levels of vitamin D in the body [123]. For medical therapy, drugs such as immunomodulators and 5-aminosalicylic acid (5-ASA) can be used [125]. Antibiotics and probiotics are also used to treat IBD [126]. So far, several studies have been performed to evaluate the efficacy of probiotics in IBD. In one study carried out by Shadnoush et al., supplementation with probiotics improved intestinal function in patients with IBD. A total of 305 participants were classified into 3 groups. Group A (IBD patients taking probiotic yogurt (contained Lactobacillus acidophilus La-5 and Bifidobacterium BB-12): n = 105), group B (IBD patients taking a placebo: n = 105), or control group (healthy persons taking probiotic yogurt: n = 95). Stool samples were obtained before and after eight weeks of intervention. Afterwards, the numbers of Bifidobacterium, Lactobacillus, and Bacteroides species in the stool samples were measured. It was found that the mean number of Bifidobacterium, Lactobacillus, and Bacteroides CFU in group A was increased compared to group B. Moreover, the mean number of all 3 bacteria was significantly different between groups A and B compared to healthy control group C. The differences between the two groups were seen both at the base-line and the completion of the study. It has been found that consuming probiotic yogurt by IBD patients can contribute to the improved intestinal function via enhancing the numbers of the beneficial bacteria in the gut. Nonetheless, it is still necessary to do more studies to confirm this concept [40]. In one study that used a Lactobacillus reuteri rectal infusion in children with chronic ulcerative colitis (UC), mucosal inflammation and the expression of some pro-inflammatory cytokines was decreased. Oliva et al. investigated the effects of a Lactobacillus (L) reuteri ATCC 55730 enema on children with active distal UC, and measured inflammation and cytokine expression in the rectal mucosa. In a prospective, randomized, placebo-controlled trial, in addition to taking oral mesalazine, the patients (n=40) received an enema solution containing 1010 CFU of L. reuteri or placebo for eight weeks. The Mayo score (endoscopic and clinical characteristics) was considerably reduced in the L. reuteri group in comparison to the placebo. Moreover, histological scores showed a considerable decline in the L. reuteri group. In addition, at the post-trial assessment of the level of mucosal cytokine expression, the anti-inflammatory IL-10 was significantly increased, while the pro-inflammatory TNFα, IL-8 and IL-1β were reduced in the L. reuteri group [127].
Kekkonen eta l. investigated production of cytokine in human peripheral blood mononuclear cells (PBMC) in response to stimulation with probiotic bacteria including Streptococcus thermophilus THS, Lactobacillus rhamnosus GG (ATCC 53103), Lactobacillus rhamnosus Lc705 (DSM 7061), Lactobacillus helveticus 1129, Lactobacillus helveticus Lb 161, Bifidobacterium longum 1/10, Bifidobacterium animalis ssp. lactis Bb12, Bifidobacterium breve Bb99 (DSM 13692), Lactococcus lactis ssp. cremoris ARH74 (DSM 18891), Leuconostoc mesenteroides ssp. cremoris PIA2 (DSM 18892) and Propionibacterium freudenreichii ssp. shermanii JS (DSM 7067). All of examined bacteria could induce TNF-α production. Streptococcus and Leuconostoc induced Th1 type cytokines IL-12 and IFN-γ more than other. All Propionibacterium and Bifidobacterium strains induced higher IL-10 production. They showed that Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus are more potent inducers of Th1 type cytokines IL-12 and IFN-γ than the probiotic Lactobacillus strains [128].
Atopic dermatitis (AD) is an inflammatory skin disease can be due to the imbalance between Tcell-related immune responses. Sheikhi et al. investigated the effects of lactobacillus Bulgaricus in the yogurt culture on the secretion of Th1/Th2/Treg type cytokines by PBMCsin 20 children with AD. Results showed that L. Delbrueckii subsp. Bulgaricus significantly up-regulated the secretion of IL-10, IL-12 and IFN-γ, while secretion of IL-4 was decreased by PBMCs compared to control [129].
Some studies have suggested that arthralgia is a common extra-intestinal manifestation of IBD. It is possible that disturbing the immune profile within the gut plays a role in the pathogenesis of arthralgia. A study by Karimi showed that administration of probiotics (VSL#3) could improve pouchitis in IBD patients. The safety and efficacy of VSL#3 administration for 2 weeks in patients with quiescent IBD also suffering from arthralgia, was assessed in an open-label trial. The pre-treatment and post-treatment intensity of joint pain was measured using a visual analog scale and the Ritchie Articular Index. Moreover the Truelove-Witts and the Harvey-Bradshaw scores were used to assess severity of IBD symptoms. 16 of 29 patients completed the trial. 10 of these 16 patients showed a remarkable improvement in joint pain using the Ritchie Articular Index. No patients suffered a relapse of intestinal disease while on probiotics. The above results indicated that the probiotic supplement VSL#3 could be a good therapeutic option for arthralgia inpatients suffering from IBD. Since probiotics can also serve as an IBD treatment, patients suffering from comcomitant arthralgia could take advantage of a dual treatment modality [130]. Another study carried out in laboratory dogs suggested that the anti-inflammatory effects of probiotic administration could be due to reduced mucosal immune cell infiltration, accompanied by increased levels of putrescine (PUT), ornithine decarboxylase (ODC) and diamino-oxidase (DAO), which play an anti-inflammatory role [131].
Irritable bowel syndrome (IBS) is a common chronic disease of the GIT with an incidence of 3 - 20 % in the US [132, 133]. The exact mechanisms of IBS pathogenesis are still not fully understood, but immunological disturbances and low levels of inflammation contribute to the symptoms of the disease [134]. IBS is a complex of different symptoms, such as abdominal pain, diarrhea, constipation, and general bodily weakness [132]. The main treatments are drug therapy with anti-spasmodic and anti-diarrheal drugs, fiber-rich diet for constipation, and supportive treatment with low dose antidepressants [132]. In addition to these treatments, probiotics can also be used to treat IBS; Nevertheless the role of probiotic microorganisms in the treatment of IBS has not yet been fully confirmed [135]. Several studies have shown the reduction of IBS symptoms by probiotic administration; however, there is still not enough evidence about their impact on psychiatric comorbidity. For example, Pinto-Sanchez et al. carried out a prospective study to evaluate the effects of Bifidobacterium longum NCC3001 (BL) on depression and anxiety symptoms in patients suffering from IBS. The researchers randomly selected 44 IBS patients suffering from diarrhea for the trial. The patients took either BL (n=22) or placebo (n=22) for 6 weeks. Afterwards, the levels of depression and anxiety, IBS symptoms, quality of life (QOL), and somatization were measured at weeks 0, 6, and 10. This study demonstrated a reduction in depression by probiotic BL; however, they observed no reduction in the anxiety scores. Moreover, the probiotic BL increased QOL in IBS patients. They found a correlation between the psychological improvements and changes in the brain activation pattern, indicating a reduction in limbic reactivity by probiotics [49]. Mezzasalma et al. investigated the efficacy of a supplementary regimen containing multi-species probiotics to alleviate patient IBS symptoms, such as constipation (IBS-C), and also measured their gut microbiota. They conducted their study in 150 IBS-C participants who received orally administered probiotic mixtures F_1 or F_2 or else placebo F_3 for 60 days. The results showed that the improvement in symptoms was greater in the probiotic group in comparison with the placebo group. The symptoms also remained in remission during the follow-up period. Moreover, fecal analyses showed that the probiotics enhanced fecal bacterial DNA in participants who received F_1 and F_2, but not with F_3. A similar level continued during the follow-up course [136]. In another study, Choi et al. explored the effectiveness of a combined treatment with mosapride and probiotics in IBS patients without diarrhea. They randomly assigned 285 IBS patients to receive over 4 weeks, a combined treatment with probiotics (Streptococcus faecium & Bacillus subtilis) plus mosapride at 4 different dosages (groups 1-4), or a placebo. The proportion gaining AR at the fourth week was greater in all treatment groups in comparison with the placebo group. Moreover, the proportion of the patients who improved on the SGA was also greater in the treatment groups compared to the placebo group. In addition, abdominal discomfort and pain scores in treatment group 4 showed the best improvement in comparison to the placebo group. In patients suffering from constipation-predominant IBS, greater improvement was observed in stool frequency and consistency in the treatment groups 4 and 1 as compared to the placebo group [137].
Celiac disease (CD) is a chronic immune-mediated disease caused by the consumption of foods containing gluten, especially wheat, and is most often seen in genetically-predisposed individuals [138]. The part of the intestines involved in CD is the proximal section of the small intestine [139]. The prevalence of celiac disease is different worldwide, but its incidence has increased in the last few decades [140]. Currently, the only effective treatment for patients with CD is a gluten-free diet (GFD) [141]. Of course, the effectiveness of this treatment depends on the strict avoidance of gluten, which may sometimes be difficult [141]. In patients with celiac disease, as compared to healthy people, the beneficial gut microbes have been shown to be decreased, and the potentially pathogenic microbes are increased [142]. This change in the microbiome increases the inflammatory response in the intestine and worsens celiac disease [142]. Considering the role of probiotics in modulating microbial populations, probiotics could be used to reduce inflammatory response and to improve the symptoms of celiac disease. In a study evaluating the effects of Bifidobacterium infantis Natren Life Start (NLS) strain super strain in patients with CD, it was concluded that this strain could relieve the symptoms of untreated individuals. 22 patients who were positive for two different CD-specific tests were enrolled in this study. The patients were randomly assigned to take two capsules before meals for three weeks, containing either placebo or Bifidobacterium infantis super strain (Lifestart 2). It was found that B. infantis alleviated the symptoms in the untreated CD patients. Moreover, probiotics produced a number of immunologic alterations, but the abnormal intestinal permeability was not affected [60]. Another study conducted by Olivares et al. used Bifidobacterium longum CECT 7347 in children with newly diagnosed CD, showed that this probiotic could improve the QOL in subjects. They assessed the effects of oral administration of the B. longum CECT 7347 in 33 children who were diagnosed with CD, after they had been on a gluten-free diet (GFD) for a three-month period. It was concluded that oral administration of B. longum CECT 7347 in combination with a GFD decreased the potentially pro-inflammatory bacteria in the gut (B. fragilis group) which have been associated with CD in earlier studies, as well as fecal sIgA (soluble immunoglobulin A). In addition, B. longum CECT 7347 decreased activated T-lymphocytes and inflammatory markers (TNF-a), possibly showing improved immune homeostasis in CD patients [59]. Another study that investigated the effects of a probiotic consisting of two strains Bifidobacterium breve BR03 and B. breve B632 in children with CD combined with a GFD, showed that the TNF-α inflammatory cytokine was decreased in these children after the intervention [143]. In vitro investigations also showed that several strains of Bifidobacteria could lower levels of pro-inflammatory cytokines (TNF-α, IFN-γ, & IL-2) and increase levels of the anti-inflammatory cytokine (IL-10). Bifidobacteria may be able to reverse the pro-inflammatory milieu caused by the microbiota of patients with CD [144–146]. Moreover, many researchers have shown that B. breve strains can exert immuno-modulatory effects both in vitro and in vivo [147, 148]. It has been established that these probiotics bacteria possess the Qualified Presumption of Safety status [149]. Two recent studies have shown that intestinal inflammation can be prevented by B. breve strains via induction of a population of T-regulatory 1 (Tr1) cells that secrete IL-10 [150, 151]. It is possible that gliadin-specific Tr1 cell clones could suppress the proliferation of pathogenic T-cells in CD patients [152]. Autoimmune-type dysfunction in known to be increased in CD patients [153]. Supplementation with B. breve probiotics plus a GFD can ameliorate the pro-inflammatory environment in the CD gut, and thus reduce the recurrence of the disease. In another study, Klemenak et al. determined the effects of a combined treatment with B. breve strains B632 and BR03 plus a GFD on the immunological function of CD children by measuring serum levels of TNF-a and IL-10. They randomly assigned 49 CD children on a GFD into treatment and placebo groups, plus 18 healthy children in a control group. The first group (24 CD children) took B. breve strains BR03 and B632 (2×109 colony-forming units) per day, while the second group (25 CD children) took a placebo for three months. The results showed a significant reduction in the level of TNF-α in the first group after the administration of B. breve for three months. Follow-up three months after the end of the study showed that level of TNF-α increased once again. The levels of IL-10 were below the detection level in each group. They concluded that probiotic B. breve strains could decrease the proinflammatory cytokine TNF-a in CD children on a GFD [61]. Quagliariello et al. also found that a supplement of Bifidobacterium breve (B632 and BR03) in CD children treated with GFD could increase the amount of beneficial microbial compounds in the gut [154].
In a study the response of intestinal epithelial cells to Enterococcus faecium NCIMB 10415 (E. faecium) and two pathogenic E. coli strains was examined, with focus on the probiotic modulation of the response to the pathogenic challenge. Intestinal cells (IPEC-J2 and Caco-2) were incubated without bacteria (control), with E. faecium, with enteropathogenic (EPEC) or enterotoxigenic E. coli (ETEC) each alone or in combination with E. faecium. Results showed that the ETEC strain decreased transepithelial resistance (TER) and increased IL-8 mRNA and protein expression in both cell lines compared with control cells, an effect that could be prevented by pre- and coincubation with E. faecium. Similar effects were observed for the increased expression of heat shock protein 70 in Caco-2 cells. When the cells were challenged by the EPEC strain, no such pattern of changes could be observed. The reduced decrease in TER and the reduction of the proinflammatory and stress response of enterocytes following pathogenic challenge indicate the protective effect of the probiotic [155].
Rheumatoid arthritis (RA) is the most common type of inflammatory arthritis, and is a main cause of disability [156]. Inflammatory cytokines play an important role in the pathogenesis of RA. An imbalance between pro-inflammatory and anti-inflammatory cytokines causes induction of autoimmunity and chronic inflammation resulting in joint damage [157]. Epidemiological studies have estimated a 0.5-1% prevalence worldwide, and an annual average incidence of 0.02-0.05% in the Northern European and North American regions [158]. There are several drugs given to patients with RA, one of which is methotrexate (MTX) [159]. However all of these drugs have side effects that can adversely affect the QOL of RA patients. For example, MTX commonly causes GI symptoms and elevation of hepatic enzymes, while severe hemocytopenia and MTX pneumonitis occur less frequently [160]. Given the role that probiotics play in down-regulating inflammatory cytokines [161], they could be substituted for drugs in the treatment of RA. In a study by Mandel, supplementation with Bacillus coagulans GBI-30 was shown to be effective in patients with RA [162]. A probiotic mixture of three strains of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium bifidum was given for 8 weeks to patients with RA. Probiotics showed positive effects on serum insulin levels, assessment of B cell function (HOMA-B), and serum high-sensitivity C-reactive protein (hs-CRP) concentrations in a study by Zamani et al [42]. Another study was conducted to investigate the effects of Lactobacillus casei 01 in RA patients. At the end of the intervention it was found that this strain could reduce the symptoms of this disease. The researchers randomly assigned women with established RA to receive either a placebo or one capsule containing 108 colony-forming units (CFU) of L. casei 01 for eight weeks. Serum levels of cytokines IL-1β, IL-10, IL-6, TNF-α and IL-12 were measured. They concluded that L. casei 01 could decrease the serum level of hs-CRP, improve swollen joints and tenderness, and increase the global health score and disease activity score-28 (P < 0.05). They showed a significant difference between both groups for IL-12, IL-10, and TNF-α levels over the study (P<0.05), in favor of the probiotic group. There were no adverse effects in the intervention group. They concluded that probiotics could be a useful supplement for patients with RA, to alleviate symptoms and reduce inflammatory cytokines [163]. In a study by Hatakka et al. supplementation with Lactobacillus rhamnosus GG (LGG) reduced the severity of RA. However, probiotic therapy with Lactobacillus GG showed no effects on RA severity. Therefore, it is necessary to do more research into probiotic bacteria for RA patients [164].
Modulation of microRNA by probiotics
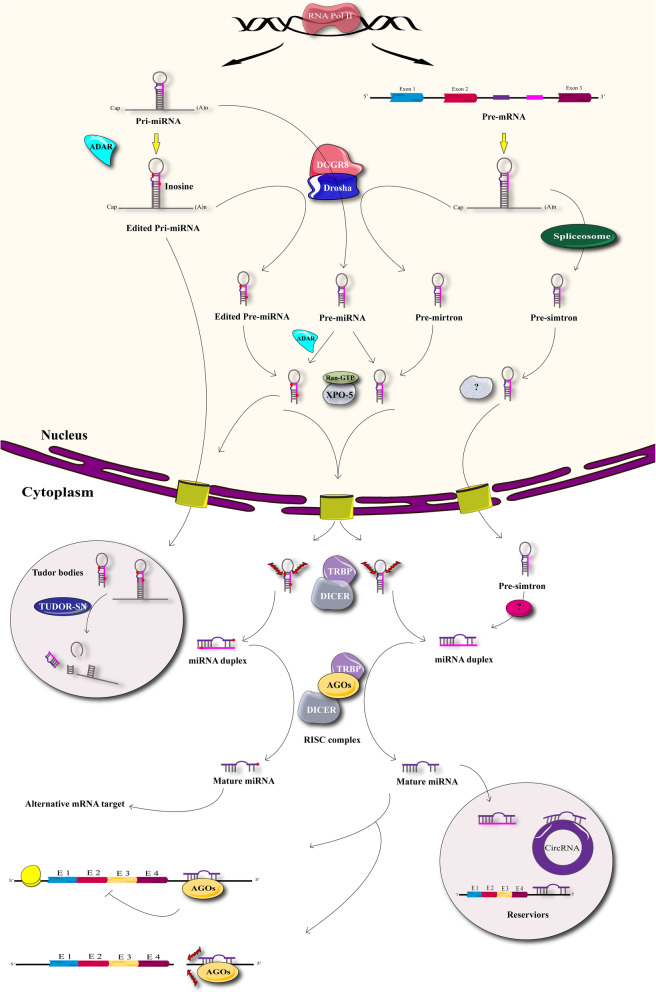
MicroRNAs (miRNAs) are short non-coding RNAs comprising 20 to 24 nucleotide bases, which play important roles in all the biological and physiological pathways in multicellular organisms [165]. Figure 2 shows the scheme of miRNA biogenesis. Dysregulation of microRNAs plays an important role in the pathogenesis of many different diseases [165]. These diseases include, central nervous system disorders [166], autoimmune diseases [167], cancers [168] and many other common diseases worldwide. Some treatments that are used to cure these diseases act by influencing gene expression and affecting miRNA regulation [169–171].
Fig 2.
Biogenesis of miRNA. Starts in the nucleus when miRNA genes are transcribed by RNA polymerase II as large polyadenylated RNA molecules named primary miRNAs (pri-miRNAs). Pri-miRNAs are processed in the nucleus by RNase III Drosha and microprocessor complex subunit DGCR8. As a result, pri-miRNAs are cleaved into smaller double-stranded RNA (dsRNA) molecules known as pre-miRNAs and then are exported to the cytoplasm by exportin 5 (XPO5). Pre-miRNAs in the cytoplasm are cleaved and despoiled of their loops by the RNase III enzyme Dicer in association with TRBP into mature miRNAs consisting of a ∼22-nucleotide duplex. The last processing step is carried out by a ribonucleoprotein complex known as RNA-induced silencing complex (RISC), which can unwind both strands. Although either strand of the miRNA duplex could potentially act as a mature miRNA, usually only one of the strands is incorporated into the RISC complex to induce mRNA silencing. RISC-loading complex (RLC), consisted of Dicermm, Argonaute 2 (Ago2), and TRBP. Once loaded, the RISC complex finds a complementary strand, activates RNase and cleaves the RNA
Probiotics are among other biological factors that have recently been discussed with regard to whether they have any effects on miRNAs. Many laboratory studies have so far been done to investigate the effects of probiotics on miRNAs (Table 2).
Table 2.
Modulation of microRNA by probiotics in pathological conditions
| MicroRNA | Probiotics | Probiotic concentrations | Expression | Target gene | Effects | Model | Sample (n) | Ref |
|---|---|---|---|---|---|---|---|---|
|
miR-215-5p, miR-10b-5p, miR-21-5p, miR-26a-5p, miR-22-3p, miR-10a-5p, miR-148a-3p, miR-194, miR-92-3p, miR-30d, miR-181a-5p, miR-429-3p, let-7f-5p, miR-30a-5p, miR-133a-3p, miR-199-3p, miR-30c-5p, miR-200a-3p, miR-126-5p, miR-27b-3p |
Lactobacillus plantarum Z01 (LPZ01) | 1 × 108 CFU/mL | Down-regulation of miR-215-5p, miR-3525, miR-122-5p and up regulation of miR-193a-5p, miR-375 and miR-215-5p | cAMP-dependent protein kinase activity, stress-activated MAPK cascade, MAPK and Wnt signaling pathways. | Decrease inflammation in S. typhimurium infection in neonatal broiler chicks |
In vivo (Newly hatched chicks) |
- | [172] |
|
miR-135b, miR-155 miR-26b and miR-18a |
Lactobacillus acidophilus and Bifidobacterium bifidum (Bla/016P/M) | 1 × 109 CFU/g and 1 × 109 CFU/g | Up regulation of miR-135b, miR-155 and down regulation of miR-26b, miR-18a | APC, PTEN, KRAS, and PU.1 | -. | In vivo (Mice) | - | [173] |
| miR-423-5p | Enterococcus faecium NCIMB 10415 | 3.6 × 106 CFU/g | Up regulation of miR-423-5p | Immunoglobulin lambda light C region (IGLC) and immunoglobulin kappa constant (IGKC) | - | In vivo (Landrace pigs) | - | [174] |
|
Lactobacillus rhamnosus GG, Bifidobacterium animalis subsp. lactis Bb-12 and L. acidophilus La-5 |
5 × 1010 CFU, 5 × 1010 CFU and 5 × 109 CFU | Human | 54 | [175] | ||||
| miR-122a | Lactobacillus rhamnosus GG | 1 × 109 CFU | Down-regulation of miR-122a | ? | Decrease ethanol-elevated miR122a levels and attenuate ethanol-induced liver injury |
In vivo (Mice) |
- | [176] |
| miR-146a | Escherichia coli Nissle 1917 (EcN) (O6:K5:H1) | NA | Up-regulation of miR-146a in both EPEC and ECN | IRAK1 and TRAF6 | Reduce IL-8 as well as CXCL1 in T84 cells |
In vitro (Human epithelial and THP-1 cells) |
- | [177] |
|
miR-21, miR-92a, miR-155, miR-663 |
Lactobacillus acidophilus (La) ATCC strain 4356 |
109 – 1010 CFU/ml |
Up-regulation of miR-21 and down-regulation of miR-155 |
MiR-92a targets integrin a5, klf-2 and klf-4/ MiR-155 targets AtR1 and Ets-1/ MiR-663 targets VEGF |
Reduce apoptosis, necrosis and inflammatory | In vitro(HUVEC) | - | [178] |
|
miR-155, miR-223, miR-150, miR-375 and miR-143 |
Lactobacillus fermentum CECT5716 and L. salivarius CECT5713 | 5 × 108 CFU | Down-regulation of miR-155, miR-223 and miR-150 and up-regulation of miR-143 | c-Myb (miR-150) | Reduce expression of pro-inflammatory cytokine IL-1β | In vivo (Mice) | - | [179] |
|
miR-203, miR-483-3p and miR-595 |
Escherichia coli Nissle 1917 (EcN) | NA | Up-regulation of miR-483-3p and down-regulation of miR-203 and miR-595 |
miR-203 targets tight junction protein ZO-2/ miR-595 targets Mucin-4-precursor/ miR-483-3p targets beta-defensin 111 precursor |
Improve effects of EcN. | In vitro (T84 epithelial cells) | - | [180] |
| miR-21 and miR-200b | Leuconostoc mesenteroides | NA | Down-regulation of miRNA-21 and miRNA-200b | NF-kB inhibitory subunit (IKB) and RelA | Induce apoptosis in colon cancer cell line by up-regulation of MAPK1, Bax, and caspase 3, and down-regulation of AKT, NF-kB, Bcl-XL |
In vitro (HT-29 cells) |
- | [181] |
|
miRNA-29a miRNA-29b miRNA-29c |
A mixture of: Lactobacillus plantarum DSM 24730, Streptococcus thermophiles DSM 24731, Bifidobacterium breve DSM 24732, L. paracasei DSM 24733, L. delbrueckii subsp. Bulgaricus DSM 24734, L. acidophilus DSM 24735, B. longum DSM 24736, and B. infantis DSM 24737 |
1.8 × 109 CFU | Unchanged | - | -. | Human | 10 | [182] |
| miR-146a and miR-155 |
Lactobacillus rhamnosus GG (LGG) and L. delbrueckii subsp. Bulgaricus (L.del) |
NA | Up-regulation of miR-155 and down regulation of miR-146a. | - | Down-regulation of p38 while IκB expression was significantly decreased in L. del-treated DCs. |
In vitro (Human monocyte-derived dendritic cells) |
- | [183] |
|
miR-143, miR-150, miR-155, miR-223, and miR-375 |
Escherichia coli Nissle 1917 (EcN) |
5 × 108 CFU | miR-150, miR-155, and miR-223 were up-regulated. miR-375 was down-regulated. | Housekeeping gene (SNORD95) | The intestinal anti-inflammatory effects of EcN were associated with altered gut microbiome in mouse experimental colitis. | In vivo (Mice) | - | [184] |
| miR-148a | Bifidobacterium bifidum MIMBb75 B. bifidum NCC390 or B. longum NCC2705 | 1 × 108 CFU |
Up-regulation after 1 and 4 hours, but not after 24 h (in vitro) Up-regulation after 2 but not 14 days (in vivo) |
EPAS1 | Reduce EPAS1 expression in Caco-2 cells and mouse cecum. | In vitro (Caco-2 cells) and In vivo (Mice) | - | [185] |
| miR-744 | Lactobacillus crispatus 2743 and L. gasseri 3396 | 5 × 107 CFU/ml | Up-regulation | ARHGAP5 | Trigger lactic acid-induced migration and invasion in SiHa cells. | In vitro (SiHa cells) | - | [186] |
| Let-7b | Encapsulated mixture of Lactobacillus plantarum, L. acidophilus -11 and Bifidobacterium longum-88 or encapsulated mixture of Enterococcus faecium and Bacillus subtilis | 2.6×1014 CFU in first mixture and 5.0×108 CFU in second mixture | Down-regulation |
HMGA2 FIGN MAPK6 ARID3B |
- | Human and In vitro (NCM460 cells) | 79 | [187] |
In one study by Heydari et al. supplementation with Lactobacillus acidophilus and Bifidobacterium bifidum probiotics in a mouse model of azoxymethane (AOM)-induced colon cancer was investigated. The results showed that the expression levels of miR-135b, miR-155, and KRAS (one of the target genes of these miRNAs) increased after azoxymethane cancer induction, and administration of a probiotic preparation containing Lactobacillus acidophilus and Bifidobacterium bifidum decreased the above-mentioned factors. Conversely, cancer induction with azoxymethane reduced the expression of miR-26b, miR-18a, APC, PU.1, and PTEN in mice, and probiotic supplementation increased them again. It seems that Lactobacillus acidophilus and Bifidobacterium bifidum though increasing the expression of the tumor suppressor miRNAs and their target genes and decreasing the oncogenes can improve colon cancer treatment [173].
Enterococcus faecium NCIMB 10415 is a probiotic species that has been shown to affect the intestinal microbial flora, and improve the immune system response in numerous human and animal studies [188, 189]. In one in vitro study using next-generation sequencing, Kreuzer-Redmer and colleagues analyzed the differential expression of the miRNAs and potential target genes in the ileal and jejunal lymphatic tissue isolated from piglets which had been fed with E. faecium NCIMB 10415 versus control animals. They found that feeding E. faecium affected the expression of miR-423-5p as well as regulating its target gene IGLC. Therefore, E. faecium benefits the immune cells in the small intestine probably by affecting the expression of miR-423-5p [174].
Escherichia coli Nissle 1917 (EcN) is a non-pathogenic Gram-negative bacterium of the Enterobacteriaceae family, which when used as a probiotic, has beneficial effects on human health [190]. In one in vitro study, E. coli (EPEC) pathogenic strain E2348/69 and E. coli Nissle 1917 (EcN) were tested in human T84 and THP-1 cells to compare the effects of the two strains on cytokine and miRNA expression. EcN increased the expression of CXCL1 and IL-8 in human T84 epithelial cells infected from the basolateral side. miR-146a is a molecular adaptor in the Toll-like receptor (TLR)/NF-κB signaling pathway. In this study, miR-146a was increased in T84 and THP-1 cells treated with EPEC, but this increase was less pronounced when these cells were incubated with EcN. Two miR-146a target genes were also identified, including IRAK1 and TRAF6. So, the probiotic EcN induced the expression of miR-146a in epithelial and immune cells, though this induction was reduced by incubation with pathogenic strain EcN [177].
miR-122 is the most frequent miRNA found in the liver, and plays an important role in liver biology and the pathogenesis of liver diseasess [191]. MiR-122 can down-regulate the proliferation and transactivation of hepatic stellate cells (HSCs) which play a role in liver fibrosis [192]. It is well known that alcoholic liver disease (ALD) causes a great burden of morbidity and death. In fact, persistent use of alcohol affects the homeostasis of the intestinal microflora, increases endotoxemia, disrupts the intestinal cell tight junction barrier, and causes liver steatosis or steatohepatitis. It was shown that a bacteria-free LGG culture supernatant (LGGs) and probiotic Lactobacillus rhamnosus GG (LGG) both promoted intestinal epithelial integrity and protected intestinal barrier function in ALD. Nonetheless, there is insufficient information on the mechanism of action of LGGs for increasing intestinal tight junction proteins. Another study conducted by Zhao et al. found that chronic ethanol exposure could increase the expression of the intestinal miR122a and decrease the expression of occludin, causing increased intestinal permeability. Supplementation with LGGs decreased the levels of miR122a caused by ethanol administration, and lessened ethanol-induced liver damage in mice. Over-expression of miR122a in a Caco-2 cell mono-layer caused a remarkable reduction in the level of occludin protein, similar to ethanol exposure. On the contrary, inhibiting miR122a increased the expression of occludin. It was suggested that a LGG supplementary regimen could affect intestinal integrity via inhibiting miR122a, thereby restoring levels occludin in the mice subjected to chronic ethanol intake [176].
Infection with high-risk human papillomavirus (HPV), and subsequent genomic integration leads to cervical cancer. Notably, E6 and E7 oncogenes are expressed leading to immortalizing the cells, and the effects on cell migration and invasion predispose to tumor metastasis [193]. Nonetheless, there is insufficient data on the fundamental mechanisms underlying this process, including the movement of the cervical cancer cells into the tumor micro-environment. In addition, lactic acid has been commonly viewed as an important constituent of the tumor microenvironment, as it is a by-product of glycolysis. In fact, researchers have suggested that the lactic acid content in primary malignant lesions could be a prognostic marker for the patient overall survival and risk of metastasis [194]. Based on some studies, there was a correlation between higher amounts of lactic acid and the risk of metastasis and poor prognosis in head and neck squamous cell carcinoma, rectal adenocarcinoma and cervical cancer [194, 195]. However, little attention has been paid to the biology of lactic acid in cervical cancer. For instance, Li et al. assessed the effects of lactic acid on the invasion and migration of the HPV16 positive SiHa cells. It was found that the addition of extra-cellular lactic acid reduced the expression of E7 and 16 HPV-16 E6 oncogenes, and increased the migration and invasion of SiHa cells by up-regulating miRNA-744 levels. This research added further knowledge about the relationship between lactic acid, cell migration and invasion, and implicated the miR-774 in the mechanism [186].
In a study by Liu et al., a study population of 186 patients was recruited who underwent surgery for colorectal cancer. The intervention group (n=93) received either an encapsulated mixture of Lactobacillus plantarum (CGMCC No.1258), L. acidophilus-11 and Bifidobacterium longum-88 or an encapsulated mixture of Enterococcus faecium and Bacillus subtilis, while the placebo group (n=93) received maltodextrin capsules. The intervention group showed reduced levels of complications and infection, accompanied by increased serum and tissue levels of the miRNA let-7b. In this study, P38 MAPK was identified as a target gene for let-7b using NCM460 cells. Therefore, it could be suggested that mixture of probiotics used in this study reduced the complications and infection after surgery for colorectal cancer probably through increasing the level of the miRNA let-7b [187].
The mir-148/mir-152 family is composed of three highly conserved mature miRNAs with similar sequences, structures and the same core region (i.e., UCAGUGCA). This family includes mir-148a, mir-148b and mir-152. The precursor mir-148/mir-152 has a stem-loop structure which is cleaved by a series of enzymes in the nucleus and cytoplasm to form mir-148a, mir-148b and mir-152 sequences [196]. In one study, the miRNAs in Caco-2 cells and cecal tissue from mice were evaluated after treatment with Bifidobacterium bifidum MIMBb75, B. bifidum NCC390 or B. longum NCC2705 probiotics. After 1 and 4 hours miR-148a was increased in response to B. bifidum MIMBb75 in Caco-2 cells. In the animal study, Taibi et al. explored whether Bifidobacterium strains (including Bifidobacterium bifidum MIMBb75, B. bifidum NCC390 or B. longum NCC2705) could change the expression of the miR-148a and its target gene in the intestines. The expression of miR-148a and its respective validated target EPAS1 in Caco-2 cells and mouse cecum in response to B. bifidum NCC390, B. bifidum MIMBb75, and B. bifidum NCC2705 was evaluated. It was concluded that in vitro exposure to B. bifidum MIMBb75 (but not B. bifidum NCC390 or B. longum NCC2705) increased the expression of miR-148a after 1-4 hours (p<0.01) but not after 24 hours. Administration of B. bifidum MIMBb75 to C57BL/6J mice enhanced the expression of miR-148a in the cecum after 2 but not 14 days (p<0.05). It was found that increased miR-148a expression was followed by reduced EPAS1 expression in the Caco-2 cells and cecal tissue (p<0.05). Finally, it was shown that silencing miR-148a could reverse the B. bifidum MIMBb75-dependent down-regulation of EPAS1 [185].
Another study used E. coli Nissle 1917 as a probiotic, and investigated the effects of this strain on the expression of inflammatory cytokines and miRNAs in mice with dextran sodium sulfate (DSS)-induced colitis. Probiotic treatment reduced the secretion of inflammatory cytokines and restored epithelial integrity. Probiotic treatment also restored to normal levels the various types of miRNAs involved in the inflammation process (miR-143, miR-150, miR-155, miR-223, and miR-375). They concluded that probiotics could modulate the expression of miRNAs in the colitis affected mice to ameliorate inflammation and restore gut homeostasis [184].
Toll like receptor 4 (TLR4) is an pathogen recognition receptor (PRR) expressed on many immune cells [197]. The TLR4 signaling pathway plays an important role in triggering the innate immune response in many inflammatory disorders [198]. P38 mitogen-activated protein kinases (MAPKs) are activated in response to many factors, such as inflammatory cytokines. There are four members of the p38MAPK family (p38α, p38β, p38γ and p38δ). The p38MAPK signaling pathway plays a major role in the biosynthesis of inflammatory cytokines, and can be used as a target to treat various disorders of the immune system [199]. One in vitro study investigated the effects of probiotics on immune responses using Lactobacillus rhamnosus GG (LGG) and L. delbrueckii subsp. Bulgaricus (L. del) as probiotics added to human monocyte-derived dendritic cells (DCs). The use of LGG reduced the expression of p38 MAP kinase during the intervention. Also, the expression of IκB was significantly reduced in the L. del. group. The probiotic LGG could regulate immune system responses through increasing the level of miR-155 as well as reduction the expression of miR-146a which targets NFκB [183].
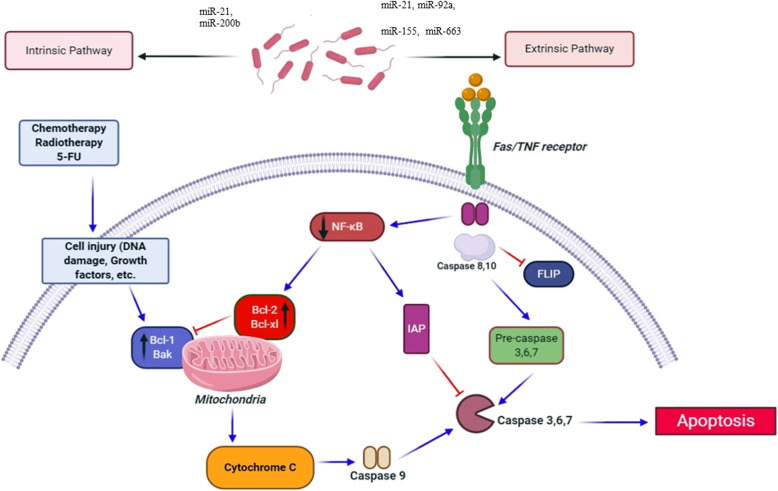
Leuconostoc species are lactic acid bacteria used as fermentation agents in numerous foods, and in the food and beverage industry. Leuconostoc spp. naturally produce chemical compounds that have inhibitory properties against other bacteria. The most important of these compounds are called bacteriocins (antibacterial peptides) [200]. One study performed by Vahed et al. investigated the anticancer effects of Leuconostoc mesenteroides in colon cancer cells (HT-29). Researchers isolated the L. mesenteroides strain from conventional dairy products. HT-29 cells were treated with the conditioned-medium of L. mesenteroides bacteria, and apoptosis was examined. This study showed that L. mesenteroides medium as a potential adjunctive treatment for cancer could promote apoptosis in the colon cancer cells via up-regulating Bax, MAPK1 and caspase 3, and down-regulating NF-κB, AKT, Bcl-XL, probably by reducing the expression of onco-miRNAs, including miR-200b and miR-21 (Fig. 3 )[181].
Fig 3.
A schema of anti- apoptotic effects of probiotics. Various microRNAs i.e., miR-21, miR-200b and miR-21 can indirectly affect on apoptosis pathways
Conclusions
The investigation of the interaction between human health and the gut microbiota, is essential for understanding many diseases of the modern world. As the human diet has become steadily more processed, it is thought that the gut is no longer exposed to many bacteria as it has been for most of human evolution. Accumulating data suggests that the administration of probiotics, living microorganisms typical of the healthy human gut, could have a crucial role in the prevention and treatment of many pathological conditions. Probiotics may function through several signaling pathways and by regulating biochemical biomarkers, such as miRNAs. Therefore, evaluation of the possible relationship between probiotics and miRNAs is important to discover the underlying role of gut microbiota in human health. Regarding the concept of the application of probiotics in different foods, such as yoghurt, milk and cheese, and their use as supplements to the human diet, future investigations should focus on the role of miRNAs, and further animal studies and clinical trials will be required before clear guidelines can be laid down.
Acknowledgements
Not applicable.
Abbreviations
- 5-ASA
5-aminosalicylic acid
- AD
Atopic dermatitis
- AGE
Acute gastroenteritis
- Ago2
Argonaute 2
- ALD
Alcoholic liver disease
- AOM
Azoxymethane
- APC
Adenomatous polyposis coli
- AR
Adequate relief
- AST
Aspartate transaminase
- B
Bifidobacterium
- Bcl-XL
B-cell lymphoma-extra large
- BL
Bifidobacterium longum NCC3001
- BL
Bifidobacterium longum
- BMI
Body mass index
- CD
Celiac disease
- CFU
Colony-forming unit
- CP
Chronic periodontitis
- CRC
Colorectal cancer
- CRF
Corticotropin-releasing factor
- CXCL1
Chemokine (C-X-C motif) ligand 1
- DAO
Diamino-oxidase
- DC
Dendritic cells
- dsRNA
Double-stranded RNA
- DSS
Dextran sodium sulfate
- E. faecium
Enterococcus faecium
- ECN; EcN
Escherichia coli Nissle
- EN
Enteral nutrition
- EPAS1
Endothelial PAS domain-containing protein 1
- EPEC
Escherichia coli
- ERAS
Enhanced recovery after surgery
- FE
Fiber-enriched
- FEP
Fiber- and probiotic-enriched
- FF
Fiber-free
- GC
Gastric cancer
- GFD
Gluten-Free Diet
- GIT
Gastrointestinal tract
- HAMA
Hamilton Anxiety Scale
- HOMA-B
Homeostatomy model assessment-B cell function
- HPV
Human papillomaviruses
- hs-CRP
High-sensitivity C -reactive protein
- HSC
Hepatic stellate cells
- IBD
Inflammatory bowel disease
- IBS-C
IBS symptoms related to constipation
- IBS
Irritable bowel syndrome
- IFN-γ
Interferon gamma
- IGKC
Immunoglobulin kappa constant
- IGLC
Immunoglobulin Lambda Constant 1
- IGLC
Immunoglobulin lambda light C region
- IL-1β
Interleukin-1beta
- IL
Interleukin
- KRAS
Kirsten rat sarcoma viral oncogene
- L
Lactobacillus
- L.del
Lactobacillus delbrueckii
- La
Lactobacillus acidophilus
- LcS
Lactobacillus casei strain Shirota
- LGG
Lactobacillus rhamnosus GG
- LOHS
length of hospital stay
- LP
Lactobacillus paracasei
- LPR
Lactobacillus rhamnosus
- LPZ01
Lactobacillus plantarum Z01
- LR
Lactobacillus rhamnosus
- MAPK
Mitogen-activated protein kinase
- miRNAs
MicroRNAs
- MTX
Methotrexate
- MYB
Myeloblastosis
- NEC
Necrotizing enterocolitis
- NF-κB
Nuclear factor kappa-light-chain-enhancer of activated B cells
- ODC
Ornithine decarboxylase
- PCR
Polymerase chain reaction
- Pri-miRNAs
Primary miRNAs
- PRR
Pathogen recognition receptor
- PTEN
Phosphatase and tensin homolog
- PUT
Putrescine
- RA
Rheumatoid arthritis
- RCT
Randomized controlled trial
- RISC
RNA-induced silencing complex
- RLC
RISC-loading complex
- SGA
Subjects' global assessment
- SRP
Scaling and root planning
- TLR
Toll-like receptor
- TNFα
Tumor necrosis factor alpha
- Tr1
T-regulatory 1
- TRBP
Transactivation response element RNA-binding protein
- UC
Ulcerative colon
- UTI
Urinary tract infection
- XPO5
Exportin 5
Authors’ contributions
HM and MRH contributed in conception, design, statistical analysis and drafting of the manuscript. AD, HM, PG, AS, MM-T, SR, and HT contributed in data collection and manuscript drafting. All authors approved the final version for submission.
Funding
The present study was founded by a grant from the Vice Chancellor for Research, Kashan University of Medical Sciences, in Iran. MRH was supported by US NIH Grants R01AI050875 and R21AI121700.
Availability of data and materials
The primary data for this study is available from the authors on request.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
MRH declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc, Cleveland, OH; BeWell Global Inc, Wan Chai, Hong Kong; Hologenix Inc. Santa Monica, CA; LumiTheraInc, Poulsbo, WA; Vielight, Toronto, Canada; Bright Photomedicine, Sao Paulo, Brazil; Quantum Dynamics LLC, Cambridge, MA; Global Photon Inc, Bee Cave, TX; Medical Coherence, Boston MA; NeuroThera, Newark DE; JOOVV Inc, Minneapolis-St. Paul MN; AIRx Medical, Pleasanton CA; FIR Industries, Inc. Ramsey, NJ; UVLRx Therapeutics, Oldsmar, FL; Ultralux UV Inc, Lansing MI; Illumiheal&Petthera, Shoreline, WA; MB Lasertherapy, Houston, TX; ARRC LED, San Clemente, CA; Varuna Biomedical Corp. Incline Village, NV; Niraxx Light Therapeutics, Inc, Boston, MA. Consulting; Lexington Int, Boca Raton, FL; USHIO Corp, Japan; Merck KGaA, Darmstadt, Germany; Philips Electronics Nederland B.V. Eindhoven, Netherlands; Johnson & Johnson Inc, Philadelphia, PA; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholdings: Global Photon Inc, Bee Cave, TX; Mitonix, Newark, DE. The other authors declare no conflicts of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Amirhossein Davoodvandi, Email: Davoudvandi.am87u@gmail.com.
Havva Marzban, Email: Marzban.ha65t5k@gmail.com.
Pouya Goleij, Email: Goleji.po09o9@gmail.com.
Amirhossein Sahebkar, Email: sahebkaraa@mums.ac.ir.
Korosh Morshedi, Email: morshedik@gmail.com.
Samaneh Rezaei, Email: smnrz83@gmail.com.
Maryam Mahjoubin-Tehran, Email: mmahjoubin@gmail.com.
Hossein Tarrahimofrad, Email: h_tarrahimofrad@yahoo.com.
Michael R. Hamblin, Email: HAMBLIN1@helix.mgh.harvard.edu
Hamed Mirzaei, Email: mirzaei-h@kaums.ac.ir, Email: h.mirzaei2002@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-020-00668-w.
References
- 1.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336(6086):1268–1273. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fábrega M-J, Rodríguez-Nogales A, Garrido-Mesa J, Algieri F, Badía J, Giménez R, Gálvez J, Baldomà L. Intestinal anti-inflammatory effects of outer membrane vesicles from Escherichia coli Nissle 1917 in DSS-experimental colitis in mice. Front Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aragon F, Carino S, Perdigon G, de Moreno de LeBlanc A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with Lactobacillus casei CRL 431. J Immunother. 2015;38(5):185–196. doi: 10.1097/CJI.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 4.So SS, Wan ML, El-Nezami H. Probiotics-mediated suppression of cancer. Curr Opin Oncol. 2017;29(1):62–72. doi: 10.1097/CCO.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 5.Kumar M, Verma V, Nagpal R, Kumar A, Behare P, Singh B, Aggarwal P. Anticarcinogenic effect of probiotic fermented milk and chlorophyllin on aflatoxin-B 1-induced liver carcinogenesis in rats. Br J Nutr. 2012;107(7):1006–1016. doi: 10.1017/S0007114511003953. [DOI] [PubMed] [Google Scholar]
- 6.Park M, Kwon B, Ku S, Ji G. The efficacy of bifidobacterium longum BORI and lactobacillus acidophilus AD031 probiotic treatment in infants with rotavirus infection. Nutrients. 2017;9(8):887. doi: 10.3390/nu9080887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Acurcio L, Sandes S, Bastos R, Sant’Anna F, Pedroso S, Reis D, Nunes Á, Cassali G, Souza M, Nicoli J. Milk fermented by Lactobacillus species from Brazilian artisanal cheese protect germ-free-mice against Salmonella Typhimurium infection. Beneficial Microb. 2017;8(4):579–588. doi: 10.3920/BM2016.0163. [DOI] [PubMed] [Google Scholar]
- 8.Mallina R, Craik J, Briffa N, Ahluwalia V, Clarke J, Cobb A. Probiotic containing Lactobacillus casei, Lactobacillus bulgaricus, and Streptococcus thermophiles (ACTIMEL) for the prevention of Clostridium difficile associated diarrhoea in the elderly with proximal femur fractures. J Infect Public Health. 2018;11(1):85–88. doi: 10.1016/j.jiph.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 9.Velez EM, Galdeano CM, Carmuega E, Weill R, Bonet MEB, Perdigón G. Probiotic fermented milk consumption modulates the allergic process induced by ovoalbumin in mice. Br J Nutr. 2015;114(4):566–576. doi: 10.1017/S0007114515001981. [DOI] [PubMed] [Google Scholar]
- 10.Nelson HS: Allergen immunotherapy now and in the future. In: Allergy & Asthma Proceedings: 2016; 2016. [DOI] [PubMed]
- 11.Fujimura T, Kinoshita J, Makino I, Nakamural K, Oyama K, Fujita H, Tajima H, Takamura H, Ninomiya I, Kitagawa H. Gastric cancer-state of the art in Japan. Rozhledy v chirurgii: mesicnik Ceskoslovenske chirurgicke spolecnosti. 2012;91(6):346. [PubMed] [Google Scholar]
- 12.Hungin A, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J. Philippart de Foy JM, Rubin G: Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice–an evidence-based international guide. Aliment Pharmacol Ther. 2013;38(8):864–886. doi: 10.1111/apt.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sadrzadeh-Yeganeh H, Elmadfa I, Djazayery A, Jalali M, Heshmat R, Chamary M. The effects of probiotic and conventional yoghurt on lipid profile in women. Br J Nutr. 2010;103(12):1778–1783. doi: 10.1017/S0007114509993801. [DOI] [PubMed] [Google Scholar]
- 14.Kich DM, Vincenzi A, Majolo F. Volken de Souza CF, Goettert MI: Probiotic: effectiveness nutrition in cancer treatment and prevention. Nutr Hosp. 2016;33(6):1430–1437. doi: 10.20960/nh.806. [DOI] [PubMed] [Google Scholar]
- 15.He J, Zhang F, Han Y. Effect of probiotics on lipid profiles and blood pressure in patients with type 2 diabetes: A meta-analysis of RCTs. Medicine. 2017;96(51):e9166. doi: 10.1097/MD.0000000000009166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abdelhamid AG, Esaam A, Hazaa MM. Cell free preparations of probiotics exerted antibacterial and antibiofilm activities against multidrug resistant E. coli. Saudi pharmaceutical journal : SPJ : the official publication of the Saudi Pharmaceutical Society. 2018;26(5):603–607. doi: 10.1016/j.jsps.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Majeed M, Nagabhushanam K, Arumugam S, Majeed S, Ali F. Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study. Food Nutr Res. 2018;62. [DOI] [PMC free article] [PubMed]
- 18.Hashemian SM, Pourhanifeh MH, Fadaei S, Velayati AA, Mirzaei H, Hamblin MR. Non-coding RNAs and Exosomes: Their Role in the Pathogenesis of Sepsis. Mol Ther Nucleic Acids. 2020;21:51–74. doi: 10.1016/j.omtn.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma M. Apoptosis-antagonizing transcription factor (AATF) gene silencing: role in induction of apoptosis and down-regulation of estrogen receptor in breast cancer cells. Biotechnol Lett. 2013;35(10):1561–1570. doi: 10.1007/s10529-013-1257-8. [DOI] [PubMed] [Google Scholar]
- 20.Chen JQ, Papp G, Szodoray P, Zeher M. The role of microRNAs in the pathogenesis of autoimmune diseases. Autoimmun Rev. 2016;15(12):1171–1180. doi: 10.1016/j.autrev.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Chen XM, Huang QC, Yang SL, Chu YL, Yan YH, Han L, Huang Y, Huang RY. Role of Micro RNAs in the Pathogenesis of Rheumatoid Arthritis: Novel Perspectives Based on Review of the Literature. Medicine. 2015;94(31):e1326. doi: 10.1097/MD.0000000000001326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shreiner AB, Kao JY, Young VB. The gut microbiome in health and in disease. Curr Opin Gastroenterol. 2015;31(1):69. doi: 10.1097/MOG.0000000000000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rauch M, Lynch S. The potential for probiotic manipulation of the gastrointestinal microbiome. Curr Opin Biotechnol. 2012;23(2):192–201. doi: 10.1016/j.copbio.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Hirayama K, Rafter J. The role of probiotic bacteria in cancer prevention. Microb Infect. 2000;2(6):681–686. doi: 10.1016/S1286-4579(00)00357-9. [DOI] [PubMed] [Google Scholar]
- 25.Guarner F. Prebiotics, probiotics and helminths: the ‘natural’solution? Dig Dis. 2009;27(3):412–417. doi: 10.1159/000228582. [DOI] [PubMed] [Google Scholar]
- 26.Oelschlaeger TA. Mechanisms of probiotic actions–a review. Int J Med Microbiol. 2010;300(1):57–62. doi: 10.1016/j.ijmm.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Plaza-Diaz J, Gomez-Llorente C, Fontana L, Gil A. Modulation of immunity and inflammatory gene expression in the gut, in inflammatory diseases of the gut and in the liver by probiotics. World J Gastroenterol. 2014;20(42):15632. doi: 10.3748/wjg.v20.i42.15632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee J-Y, Chu S-H, Jeon JY, Lee M-K, Park J-H, Lee D-C, Lee J-W, Kim N-K. Effects of 12 weeks of probiotic supplementation on quality of life in colorectal cancer survivors: a double-blind, randomized, placebo-controlled trial. Dig Liver Dis. 2014;46(12):1126–1132. doi: 10.1016/j.dld.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 29.Zheng C, Chen T, Wang Y, Gao Y, Kong Y, Liu Z, Deng X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer. 2019;10(3):568–576. doi: 10.7150/jca.29072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Golkhalkhali B, Rajandram R, Paliany AS, Ho GF, Wan Ishak WZ, Johari CS, Chin KF. Strain-specific probiotic (microbial cell preparation) and omega-3 fatty acid in modulating quality of life and inflammatory markers in colorectal cancer patients: a randomized controlled trial. Asia Pac J Clin Oncol. 2018;14(3):179–191. doi: 10.1111/ajco.12758. [DOI] [PubMed] [Google Scholar]
- 31.Tan CK, Said S, Rajandram R, Wang Z, Roslani AC, Chin KF. Pre-surgical administration of microbial cell preparation in colorectal cancer patients: a randomized controlled trial. World J Surg. 2016;40(8):1985–1992. doi: 10.1007/s00268-016-3499-9. [DOI] [PubMed] [Google Scholar]
- 32.Durack J, Kimes NE, Lin DL, Rauch M, McKean M, McCauley K, Panzer AR, Mar JS, Cabana MD, Lynch SV. Delayed gut microbiota development in high-risk for asthma infants is temporarily modifiable by Lactobacillus supplementation. Nat Commun. 2018;9(1):707. doi: 10.1038/s41467-018-03157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cabana MD, McKean M, Caughey AB, Fong L, Lynch S, Wong A, Leong R, Boushey HA, Hilton JF. Early probiotic supplementation for eczema and asthma prevention: a randomized controlled trial. Pediatrics. 2017:e20163000. [DOI] [PMC free article] [PubMed]
- 34.Del Giudice MM, Indolfi C, Capasso M, Maiello N, Decimo F, Ciprandi G. Bifidobacterium mixture (B longum BB536, B infantis M-63, B breve M-16V) treatment in children with seasonal allergic rhinitis and intermittent asthma. Ital J Pediatr. 2017;43(1):25. doi: 10.1186/s13052-017-0340-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rutten N, Gorissen D, Eck A, Niers L, Vlieger A. Besseling-Van der Vaart I, Budding A, Savelkoul P, Van der Ent C, Rijkers G: Long term development of gut microbiota composition in atopic children: impact of probiotics. Plos One. 2015;10(9):e0137681. doi: 10.1371/journal.pone.0137681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chowdhury T, Ali MM, Hossain MM, Singh J, Yousuf A, Yasmin F, Chowdhury FR. Efficacy of probiotics versus placebo in the prevention of necrotizing enterocolitis in preterm very low birth weight infants: a double-blind randomized controlled trial. J Coll Physicians Surg Pak. 2016;26:770–774. [PubMed] [Google Scholar]
- 37.Lambæk ID, Fonnest G, Gormsen M, Brok J, Greisen G. Probiotics to prevent necrotising enterocolitis in very preterm infants. Dan Med J. 2016;63(3):A5203. [PubMed] [Google Scholar]
- 38.Saengtawesin V, Tangpolkaiwalsak R, Kanjanapattankul W. Effect of oral probiotics supplementation in the prevention of necrotizing enterocolitis among very low birth weight preterm infants. J Med Assoc Thai. 2014;97(Suppl 6):S20–S25. [PubMed] [Google Scholar]
- 39.Lorea Baroja M, Kirjavainen P, Hekmat S, Reid G. Anti-inflammatory effects of probiotic yogurt in inflammatory bowel disease patients. Clin Exp Immunol. 2007;149(3):470–479. doi: 10.1111/j.1365-2249.2007.03434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shadnoush M, Hosseini RS, Khalilnezhad A, Navai L, Goudarzi H, Vaezjalali M. Effects of probiotics on gut microbiota in patients with inflammatory bowel disease: a double-blind, placebo-controlled clinical trial. Korean J Gastroenterol. 2015;65(4):215–221. doi: 10.4166/kjg.2015.65.4.215. [DOI] [PubMed] [Google Scholar]
- 41.de los Angeles Pineda M, Thompson SF, Summers K, de Leon F, Pope J, Reid G. A randomized, double-blinded, placebo-controlled pilot study of probiotics in active rheumatoid arthritis. Med Sci Monit. 2011;17(6):CR347. doi: 10.12659/MSM.881808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zamani B, Golkar HR, Farshbaf S, Emadi-Baygi M, Tajabadi-Ebrahimi M, Jafari P, Akhavan R, Taghizadeh M, Memarzadeh MR, Asemi Z. Clinical and metabolic response to probiotic supplementation in patients with rheumatoid arthritis: a randomized, double-blind, placebo-controlled trial. In J Rheum Dis. 2016;19(9):869–879. doi: 10.1111/1756-185X.12888. [DOI] [PubMed] [Google Scholar]
- 43.Vaghef-Mehrabany E, Alipour B, Homayouni-Rad A, Sharif S-K, Asghari-Jafarabadi M, Zavvari S. Probiotic supplementation improves inflammatory status in patients with rheumatoid arthritis. Nutrition. 2014;30(4):430–435. doi: 10.1016/j.nut.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y-J, Wu W-F, Hung C-W, Ku M-S, Liao P-F, Sun H-L, Lu K-H, Sheu J-N, Lue K-H. Evaluation of efficacy and safety of Lactobacillus rhamnosus in children aged 4–48 months with atopic dermatitis: An 8-week, double-blind, randomized, placebo-controlled study. J Microbiol Immunol Infect. 2017;50(5):684–692. doi: 10.1016/j.jmii.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 45.Prakoeswa C, Herwanto N, Prameswari R, Astari L, Sawitri S, Hidayati A, Indramaya D, Kusumowidagdo E, Surono I. Lactobacillus plantarum IS-10506 supplementation reduced SCORAD in children with atopic dermatitis. Beneficial Microb. 2017;8(5):833–840. doi: 10.3920/BM2017.0011. [DOI] [PubMed] [Google Scholar]
- 46.Rø A, Simpson MR, Rø TB, Storrø O, Johnsen R, Videm V, Øien T. Reduced Th22 cell proportion and prevention of atopic dermatitis in infants following maternal probiotic supplementation. Clin Exp Allergy. 2017;47(8):1014–1021. doi: 10.1111/cea.12930. [DOI] [PubMed] [Google Scholar]
- 47.Ishaque SM, Khosruzzaman S, Ahmed DS, Sah MP. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol. 2018;18(1):71. doi: 10.1186/s12876-018-0788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Preston K, Krumian R, Hattner J, de Montigny D, Stewart M, Gaddam S. Lactobacillus acidophilus CL1285, Lactobacillus casei LBC80R and Lactobacillus rhamnosus CLR2 improve quality-of-life and IBS symptoms: a double-blind, randomised, placebo-controlled study. Beneficial Microb. 2018:1–10. [DOI] [PubMed]
- 49.Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, Martin F-P, Cominetti O, Welsh C, Rieder A. Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153(2):448–459. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 50.Fuke N, Aizawa K, Suganuma H, Takagi T, Naito Y. Effect of combined consumption of Lactobacillus brevis KB290 and β-carotene on minor diarrhoea-predominant irritable bowel syndrome-like symptoms in healthy subjects: a randomised, double-blind, placebo-controlled, parallel-group trial. Int J Food Sci Nutr. 2017;68(8):973–986. doi: 10.1080/09637486.2017.1311843. [DOI] [PubMed] [Google Scholar]
- 51.Schnadower D, Tarr PI, Casper TC, Gorelick MH, Dean JM, O’connell KJ, Mahajan P, Levine AC, Bhatt SR, Roskind CG. Lactobacillus rhamnosus GG versus placebo for acute gastroenteritis in children. N Engl J Med. 2018;379(21):2002–2014. doi: 10.1056/NEJMoa1802598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schnadower D, Tarr PI, Casper TC, Gorelick MH, Dean MJ, O’Connell KJ, Mahajan P, Chun TH, Bhatt SR, Roskind CG. Randomised controlled trial of Lactobacillus rhamnosus (LGG) versus placebo in children presenting to the emergency department with acute gastroenteritis: the PECARN probiotic study protocol. BMJ Open. 2017;7(9):e018115. doi: 10.1136/bmjopen-2017-018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sindhu KN, Sowmyanarayanan TV, Paul A, Babji S, Ajjampur SS, Priyadarshini S, Sarkar R, Balasubramanian K, Wanke CA, Ward HD. Immune response and intestinal permeability in children with acute gastroenteritis treated with Lactobacillus rhamnosus GG: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 2014;58(8):1107–1115. doi: 10.1093/cid/ciu065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Allen SJ, Jordan S, Storey M, Thornton CA, Gravenor MB, Garaiova I, Plummer SF, Wang D, Morgan G: Probiotics in the prevention of eczema: a randomised controlled trial. Archives of disease in childhood 2014:archdischild-2013-305799. [DOI] [PMC free article] [PubMed]
- 55.Loo EX, Llanora GV, Lu Q, Aw MM, Lee BW, Shek LP. Supplementation with probiotics in the first 6 months of life did not protect against eczema and allergy in at-risk Asian infants: a 5-year follow-up. Int Arch allergy Immunol. 2014;163(1):25–28. doi: 10.1159/000356338. [DOI] [PubMed] [Google Scholar]
- 56.Singh A, Hacini-Rachinel F, Gosoniu M, Bourdeau T, Holvoet S, Doucet-Ladeveze R, Beaumont M, Mercenier A, Nutten S. Immune-modulatory effect of probiotic Bifidobacterium lactis NCC2818 in individuals suffering from seasonal allergic rhinitis to grass pollen: an exploratory, randomized, placebo-controlled clinical trial. Eur J Clin Nutr. 2013;67(2):161. doi: 10.1038/ejcn.2012.197. [DOI] [PubMed] [Google Scholar]
- 57.Costa D, Marteau P, Amouyal M, Poulsen LK, Hamelmann E, Cazaubiel M, Housez B, Leuillet S, Stavnsbjerg M, Molimard P. Efficacy and safety of the probiotic Lactobacillus paracasei LP-33 in allergic rhinitis: a double-blind, randomized, placebo-controlled trial (GA2LEN Study) Eur J Clin Nutr. 2014;68(5):602. doi: 10.1038/ejcn.2014.13. [DOI] [PubMed] [Google Scholar]
- 58.Lin W-Y, Fu L-S, Lin H-K, Shen C-Y, Chen Y-J. Evaluation of the effect of Lactobacillus paracasei (HF. A00232) in children (6–13 years old) with perennial allergic rhinitis: a 12-week, double-blind, randomized, placebo-controlled study. Pediatr Neonatol. 2014;55(3):181–188. doi: 10.1016/j.pedneo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 59.Olivares M, Castillejo G, Varea V, Sanz Y. Double-blind, randomised, placebo-controlled intervention trial to evaluate the effects of Bifidobacterium longum CECT 7347 in children with newly diagnosed coeliac disease. Br J Nutr. 2014;112(1):30–40. doi: 10.1017/S0007114514000609. [DOI] [PubMed] [Google Scholar]
- 60.Smecuol E, Hwang HJ, Sugai E, Corso L, Chernavsky AC, Bellavite FP, Gonzalez A, Vodanovich F, Moreno ML, Vazquez H. Exploratory, randomized, double-blind, placebo-controlled study on the effects of Bifidobacterium infantis natren life start strain super strain in active celiac disease. J Clin Gastroenterol. 2013;47(2):139–147. doi: 10.1097/MCG.0b013e31827759ac. [DOI] [PubMed] [Google Scholar]
- 61.Klemenak M, Dolinšek J, Langerholc T, Di Gioia D, Mičetić-Turk D. Administration of Bifidobacterium breve Decreases the Production of TNF-α in Children with Celiac Disease. Dig Dis Sci. 2015;60(11):3386–3392. doi: 10.1007/s10620-015-3769-7. [DOI] [PubMed] [Google Scholar]
- 62.Nagata S, Chiba Y, Wang C, Yamashiro Y. The effects of the Lactobacillus casei strain on obesity in children: a pilot study. Beneficial Microb. 2017;8(4):535–543. doi: 10.3920/BM2016.0170. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez M, Darimont C, Panahi S, Drapeau V, Marette A, Taylor VH, Doré J, Tremblay A. Effects of a diet-based weight-reducing program with probiotic supplementation on satiety efficiency, eating behaviour traits, and psychosocial behaviours in obese individuals. Nutrients. 2017;9(3):284. doi: 10.3390/nu9030284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim M, Kim M, Kang M, Yoo HJ, Kim MS, Ahn Y-T, Sim J-H, Jee SH, Lee JH. Effects of weight loss using supplementation with Lactobacillus strains on body fat and medium-chain acylcarnitines in overweight individuals. Food Funct. 2017;8(1):250–261. doi: 10.1039/C6FO00993J. [DOI] [PubMed] [Google Scholar]
- 65.Mafi A, Namazi G, Soleimani A, Bahmani F, Aghadavod E, Asemi Z. Metabolic and genetic response to probiotics supplementation in patients with diabetic nephropathy: a randomized, double-blind, placebo-controlled trial. Food Funct. 2018;9(9):4763–4770. doi: 10.1039/C8FO00888D. [DOI] [PubMed] [Google Scholar]
- 66.Sabico S, Al-Mashharawi A, Al-Daghri NM, Yakout S, Alnaami AM, Alokail MS, McTernan PG. Effects of a multi-strain probiotic supplement for 12 weeks in circulating endotoxin levels and cardiometabolic profiles of medication naïve T2DM patients: a randomized clinical trial. J Transl Med. 2017;15(1):249. doi: 10.1186/s12967-017-1354-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mobini R, Tremaroli V, Ståhlman M, Karlsson F, Levin M, Ljungberg M, Sohlin M, Bertéus Forslund H, Perkins R, Bäckhed F. Metabolic effects of L actobacillus reuteri DSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diab Obes Metab. 2017;19(4):579–589. doi: 10.1111/dom.12861. [DOI] [PubMed] [Google Scholar]
- 68.Kobyliak N, Falalyeyeva T, Mykhalchyshyn G, Kyriienko D, Komissarenko I: Effect of alive probiotic on insulin resistance in type 2 diabetes patients: randomized clinical trial. Diabetes & Metabolic Syndrome: Clinical Research & Reviews 2018. [DOI] [PubMed]
- 69.Tonucci LB, dos Santos KMO, de Oliveira LL, Ribeiro SMR, Martino HSD. Clinical application of probiotics in type 2 diabetes mellitus: A randomized, double-blind, placebo-controlled study. Clin Nutr. 2017;36(1):85–92. doi: 10.1016/j.clnu.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 70.Ishizaki A, Bi X, Nguyen LV, Matsuda K, Pham HV, Phan CTT, Khu DTK, Ichimura H. Effects of Short-Term Probiotic Ingestion on Immune Profiles and Microbial Translocation among HIV-1-Infected Vietnamese Children. Int J Mol Sci. 2017;18(10):2185. doi: 10.3390/ijms18102185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.d'Ettorre G, Rossi G, Scagnolari C, Andreotti M, Giustini N, Serafino S, Schietroma I, Scheri GC, Fard SN, Trinchieri V. Probiotic supplementation promotes a reduction in T-cell activation, an increase in Th17 frequencies, and a recovery of intestinal epithelium integrity and mitochondrial morphology in ART-treated HIV-1-positive patients. Immun Inflamm Dis. 2017;5(3):244–260. doi: 10.1002/iid3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Villar-García J, Güerri-Fernández R, Moya A, González A, Hernández JJ, Lerma E, Guelar A, Sorli L, Horcajada JP, Artacho A. Impact of probiotic Saccharomyces boulardii on the gut microbiome composition in HIV-treated patients: a double-blind, randomised, placebo-controlled trial. PloS One. 2017;12(4):e0173802. doi: 10.1371/journal.pone.0173802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C, Giammaria P, Reali L, Anania F, Nobili V. Randomised clinical trial: the beneficial effects of VSL# 3 in obese children with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2014;39(11):1276–1285. doi: 10.1111/apt.12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nabavi S, Rafraf M, Somi M, Homayouni-Rad A, Asghari-Jafarabadi M. Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci. 2014;97(12):7386–7393. doi: 10.3168/jds.2014-8500. [DOI] [PubMed] [Google Scholar]
- 75.Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG, Mojtaba S, Mohammad S, Ghader G, Seyed Moayed A. Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr. 2016;35(6):500–505. doi: 10.1080/07315724.2015.1031355. [DOI] [PubMed] [Google Scholar]
- 76.Manzhalii E, Virchenko O, Falalyeyeva T, Beregova T, Stremmel W. Treatment efficacy of a probiotic preparation for non-alcoholic steatohepatitis: A pilot trial. J Dig Dis. 2017;18(12):698–703. doi: 10.1111/1751-2980.12561. [DOI] [PubMed] [Google Scholar]
- 77.Wai-Sun Wong V, Wong GL-H, Chim AM-L, Chu WC-W, Yeung DK-W, Li KC-T, Chan HL-Y. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2015;12(2):256–262. doi: 10.1016/S1665-2681(19)31364-X. [DOI] [PubMed] [Google Scholar]
- 78.Uehara S, Monden K, Nomoto K, Seno Y, Kariyama R, Kumon H. A pilot study evaluating the safety and effectiveness of Lactobacillus vaginal suppositories in patients with recurrent urinary tract infection. Int J Antimicrob Agents. 2006;28:30–34. doi: 10.1016/j.ijantimicag.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Stapleton AE, Au-Yeung M, Hooton TM, Fredricks DN, Roberts PL, Czaja CA, Yarova-Yarovaya Y, Fiedler T, Cox M, Stamm WE. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52(10):1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dani C, Biadaioli R, Bertini G, Martelli E, Rubaltelli FF. Probiotics feeding in prevention of urinary tract infection, bacterial sepsis and necrotizing enterocolitis in preterm infants. Neonatology. 2002;82(2):103–108. doi: 10.1159/000063096. [DOI] [PubMed] [Google Scholar]
- 81.Czaja CA, Stapleton AE, Yarova-Yarovaya Y, Stamm WE: Phase I trial of a Lactobacillus crispatus vaginal suppository for prevention of recurrent urinary tract infection in women. Infectious diseases in obstetrics and gynecology 2007, 2007. [DOI] [PMC free article] [PubMed]
- 82.Paineau D, Carcano D, Leyer G, Darquy S, Alyanakian M-A, Simoneau G, Bergmann J-F, Brassart D, Bornet F, Ouwehand AC. Effects of seven potential probiotic strains on specific immune responses in healthy adults: a double-blind, randomized, controlled trial. FEMS Immunol Med Microbiol. 2008;53(1):107–113. doi: 10.1111/j.1574-695X.2008.00413.x. [DOI] [PubMed] [Google Scholar]
- 83.Olivares M, Díaz-Ropero MP, Sierra S, Lara-Villoslada F, Fonollá J, Navas M, Rodríguez JM, Xaus J. Oral intake of Lactobacillus fermentum CECT5716 enhances the effects of influenza vaccination. Nutrition. 2007;23(3):254–260. doi: 10.1016/j.nut.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 84.Davidson LE, Fiorino A-M, Snydman DR, Hibberd PL. Lactobacillus GG as an immune adjuvant for live-attenuated influenza vaccine in healthy adults: a randomized double-blind placebo-controlled trial. Eur J Clin Nutr. 2011;65(4):501. doi: 10.1038/ejcn.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Stensson M, Koch G, Coric S, Abrahamsson T, Jenmalm M, Birkhed D, Wendt L-K. Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. 2014;48(2):111–117. doi: 10.1159/000354412. [DOI] [PubMed] [Google Scholar]
- 86.Hedayati-Hajikand T, Lundberg U, Eldh C, Twetman S. Effect of probiotic chewing tablets on early childhood caries–a randomized controlled trial. BMC Oral Health. 2015;15(1):112. doi: 10.1186/s12903-015-0096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Campus G, Cocco F, Carta G, Cagetti MG, Simark-Mattson C, Strohmenger L, Lingström P. Effect of a daily dose of Lactobacillus brevis CD2 lozenges in high caries risk schoolchildren. Clin Oral Investig. 2014;18(2):555–561. doi: 10.1007/s00784-013-0980-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Administration of Bifidobacteriumanimalis subsp. lactis BB-12 in early childhood: a post-trial effect on caries occurrence at four years of age. Caries Res. 2013;47(5):364–372. doi: 10.1159/000348424. [DOI] [PubMed] [Google Scholar]
- 89.Kuru BE, Laleman I, Yalnızoğlu T, Kuru L, Teughels W. The Influence of a Bifidobacterium animalis Probiotic on Gingival Health: A Randomized Controlled Clinical Trial. J Periodontol. 2017;88(11):1115–1123. doi: 10.1902/jop.2017.170213. [DOI] [PubMed] [Google Scholar]
- 90.Twetman S, Pedersen AML, Yucel-Lindberg T. Probiotic supplements containing Lactobacillus reuteri does not affect the levels of matrix metalloproteinases and interferons in oral wound healing. BMC Res Notes. 2018;11(1):759. doi: 10.1186/s13104-018-3873-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Keller MK, Kragelund C. Randomized pilot study on probiotic effects on recurrent candidiasis in oral lichen planus patients. Oral Dis. 2018;24(6):1107–1114. doi: 10.1111/odi.12858. [DOI] [PubMed] [Google Scholar]
- 92.Morales A, Gandolfo A, Bravo J, Carvajal P, Silva N, Godoy C, Garcia-Sesnich J, Hoare A, Diaz P, Gamonal J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: a randomized placebo-controlled trial with 9-month follow-up. J Appl Oral Sci. 2018;26. [DOI] [PMC free article] [PubMed]
- 93.Szkaradkiewicz AK, Stopa J, Karpiński TM. Effect of oral administration involving a probiotic strain of Lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic periodontitis. Arch Immunol Ther Exp. 2014;62(6):495–500. doi: 10.1007/s00005-014-0277-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Teughels W, Durukan A, Ozcelik O, Pauwels M, Quirynen M, Haytac MC. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: a randomized placebo-controlled study. J Clin Periodontol. 2013;40(11):1025–1035. doi: 10.1111/jcpe.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ince G, Gursoy H, Ipci DS, Cakar G, Alturfan EE. Ylmaz S: clinical and biochemical evaluation of Lactobacillus reuteri containing lozenges as an adjunct to nonsurgical periodontal therapy in chronic periodontitis: p0199. J Clin Periodontol. 2015;42:126. [Google Scholar]
- 96.Morales A, Carvajal P, Silva N, Hernandez M, Godoy C, Rodriguez G, Cabello R, Garcia-Sesnich J, Hoare A, Diaz PI. Clinical Effects of Lactobacillus rhamnosus in Non-Surgical Treatment of Chronic Periodontitis: A Randomized Placebo-Controlled Trial With 1-Year Follow-Up. J Periodontol. 2016;87(8):944–952. doi: 10.1902/jop.2016.150665. [DOI] [PubMed] [Google Scholar]
- 97.Keller M, Brandsborg E, Holmstrøm K, Twetman S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: a randomised controlled trial. Beneficial Microb. 2018;9(3):487–494. doi: 10.3920/BM2017.0104. [DOI] [PubMed] [Google Scholar]
- 98.Alkaya B, Laleman I, Keceli S, Ozcelik O, Cenk Haytac M, Teughels W. Clinical effects of probiotics containing Bacillus species on gingivitis: a pilot randomized controlled trial. J Periodontal Res. 2017;52(3):497–504. doi: 10.1111/jre.12415. [DOI] [PubMed] [Google Scholar]
- 99.Montero E, Iniesta M, Rodrigo M, Marín MJ, Figuero E, Herrera D, Sanz M. Clinical and microbiological effects of the adjunctive use of probiotics in the treatment of gingivitis: A randomized controlled clinical trial. J Clin PeriodontolS. 2017;44(7):708–716. doi: 10.1111/jcpe.12752. [DOI] [PubMed] [Google Scholar]
- 100.Schlagenhauf U, Jakob L, Eigenthaler M, Segerer S, Jockel-Schneider Y, Rehn M. Regular consumption of Lactobacillus reuteri-containing lozenges reduces pregnancy gingivitis: an RCT. J Clin Periodontol. 2016;43(11):948–954. doi: 10.1111/jcpe.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Iwamoto T, Suzuki N, Tanabe K, Takeshita T, Hirofuji T. Effects of probiotic Lactobacillus salivarius WB21 on halitosis and oral health: an open-label pilot trial. Oral Surg Oral Med Oral Pathol Oral Radiol Endodontol. 2010;110(2):201–208. doi: 10.1016/j.tripleo.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 102.Marchetti E, Tecco S, Santonico M, Vernile C, Ciciarelli D, Tarantino E, Marzo G, Pennazza G. Multi-sensor approach for the monitoring of halitosis treatment via Lactobacillus brevis (CD2)—containing lozenges—a randomized, double-blind placebo-controlled clinical trial. Sensors. 2015;15(8):19583–19596. doi: 10.3390/s150819583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Oncel MY, Arayici S, Sari FN, Simsek GK, Yurttutan S, Erdeve O, Saygan S, Uras N, Oguz SS, Dilmen U. Comparison of Lactobacillus reuteri and nystatin prophylaxis on Candida colonization and infection in very low birth weight infants. J Maternal-Fetal Neonatal Med. 2015;28(15):1790–1794. doi: 10.3109/14767058.2014.968842. [DOI] [PubMed] [Google Scholar]
- 104.Murina F, Graziottin A, Vicariotto F, De Seta F. Can Lactobacillus fermentum LF10 and Lactobacillus acidophilus LA02 in a slow-release vaginal product be useful for prevention of recurrent vulvovaginal candidiasis?: a clinical study. J Clin Gastroenterol. 2014;48:S102–S105. doi: 10.1097/MCG.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 105.Kovachev SM, Vatcheva-Dobrevska RS. Local probiotic therapy for vaginal Candida albicans infections. Probiotics Antimicrobial Proteins. 2015;7(1):38–44. doi: 10.1007/s12602-014-9176-0. [DOI] [PubMed] [Google Scholar]
- 106.Daliri EB-M, Lee BH. New perspectives on probiotics in health and disease. Food Sci Hum Wellness. 2015;4(2):56–65. doi: 10.1016/j.fshw.2015.06.002. [DOI] [Google Scholar]
- 107.Soccol CR, de Souza Vandenberghe LP, Spier MR, Medeiros AP, Yamaguishi CT, De Dea LJ, Pandey A, Thomaz-Soccol V. The potential of probiotics: a review. Food Technol Biotechnol. 2010;48(4):413–434. [Google Scholar]
- 108.Organization WH . Report of a WHO consultation on xenotransplantation, Geneva, Switzerland, 28-30 October 1997. Geneva: World Health Organization; 1998. [Google Scholar]
- 109.Murthy N, Mathew A. Cancer epidemiology, prevention and control. Curr Sci. 2004;86(4):518–527. [Google Scholar]
- 110.Lopes C, Dourado A, Oliveira R. Phytotherapy and nutritional supplements on breast cancer. BioMed Res Int. 2017;2017. [DOI] [PMC free article] [PubMed]
- 111.Xue R, Cai X, Xu H, Wu S, Huang H. The efficacy of concurrent weekly carboplatin with radiotherapy in the treatment of cervical cancer: A meta-analysis. Gynecol Oncol. 2018;150(3):412–419. doi: 10.1016/j.ygyno.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 112.Bai J, Behera M, Bruner DW. The gut microbiome, symptoms, and targeted interventions in children with cancer: a systematic review. Support Care Cancer. 2018;26(2):427–439. doi: 10.1007/s00520-017-3982-3. [DOI] [PubMed] [Google Scholar]
- 113.Vétizou M, Daillère R, Zitvogel L. Gut microbiota and efficacy of cancer therapies. Biologie aujourd'hui. 2017;211(1):51–67. doi: 10.1051/jbio/2017009. [DOI] [PubMed] [Google Scholar]
- 114.Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. 2015;12(4):6119–6127. doi: 10.3892/mmr.2015.4124. [DOI] [PubMed] [Google Scholar]
- 115.Li R, Helbig L, Fu J, Bian X, Herrmann J, Baumann M, Stewart AF, Müller R, Li A, Zips D, et al. Expressing cytotoxic compounds in Escherichia coli Nissle 1917 for tumor-targeting therapy. Res Microbiol. 2019;170(2):74–79. doi: 10.1016/j.resmic.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Yang H, Zhao X, Tang S, Huang H, Zhao X, Ning Z, Fu X, Zhang C. Probiotics reduce psychological stress in patients before laryngeal cancer surgery. Asia-Pacific J Clin Oncol. 2016;12(1):e92–e96. doi: 10.1111/ajco.12120. [DOI] [PubMed] [Google Scholar]
- 117.Zhao R, Wang Y, Huang Y, Cui Y, Xia L, Rao Z, Zhou Y, Wu X: Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine 2017, 96(43). [DOI] [PMC free article] [PubMed]
- 118.Zaman MK, Chin K-F, Rai V, Majid HA. Fiber and prebiotic supplementation in enteral nutrition: a systematic review and meta-analysis. World J Gastroenterol. 2015;21(17):5372. doi: 10.3748/wjg.v21.i17.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.McRorie JW, Jr, McKeown NM. Understanding the physics of functional fibers in the gastrointestinal tract: an evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J Acad Nutr Diet. 2017;117(2):251–264. doi: 10.1016/j.jand.2016.09.021. [DOI] [PubMed] [Google Scholar]
- 120.Rayes N, Hansen S, Seehofer D, Müller AR, Serke S, Bengmark S, Neuhaus P. Early enteral supply of fiber and Lactobacilli versus conventional nutrition: a controlled trial in patients with major abdominal surgery. Nutrition. 2002;18(7-8):609–615. doi: 10.1016/S0899-9007(02)00811-0. [DOI] [PubMed] [Google Scholar]
- 121.Williams NT. Probiotics. Am J Health-Syst Pharm. 2010;67(6):449–458. doi: 10.2146/ajhp090168. [DOI] [PubMed] [Google Scholar]
- 122.Klarin B, Molin G, Jeppsson B, Larsson A. Use of the probiotic Lactobacillus plantarum 299 to reduce pathogenic bacteria in the oropharynx of intubated patients: a randomised controlled open pilot study. Critical Care. 2008;12(6):R136. doi: 10.1186/cc7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015;12(4):205. doi: 10.1038/nrgastro.2015.34. [DOI] [PubMed] [Google Scholar]
- 124.Sartor RB. Pathogenesis and immune mechanisms of chronic inflammatory bowel diseases. Am J Gastroenterol. 1997;92. [PubMed]
- 125.Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD) Pharmacol Rep. 2011;63(3):629–642. doi: 10.1016/S1734-1140(11)70575-8. [DOI] [PubMed] [Google Scholar]
- 126.Gionchetti P, Rizzello F, Lammers KM, Morselli C, Sollazzi L, Davies S, Tambasco R, Calabrese C, Campieri M. Antibiotics and probiotics in treatment of inflammatory bowel disease. World J Gastroenterol. 2006;12(21):3306. doi: 10.3748/wjg.v12.i21.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S, Stronati L. Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther. 2012;35(3):327–334. doi: 10.1111/j.1365-2036.2011.04939.x. [DOI] [PubMed] [Google Scholar]
- 128.Kekkonen R-A, Kajasto E, Miettinen M, Veckman V, Korpela R, Julkunen I. Probiotic Leuconostoc mesenteroides ssp. cremoris and Streptococcus thermophilus induce IL-12 and IFN-gamma production. World J Gastroenterol. 2008;14(8):1192–1203. doi: 10.3748/wjg.14.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheikhi A, Giti H, Heibor MR, Jafarzadeh A, Shakerian M, Baharifar N, Niruzad F, Moghaddam AS, Kokhaei P, Baghaeifar M. Lactobacilus delbrueckii subsp. bulgaricus modulates the secretion of Th1/Th2 and Treg cell-related cytokines by PBMCs from patients with atopic dermatitis. Drug Res. 2017;67(12):724–729. doi: 10.1055/s-0043-117612. [DOI] [PubMed] [Google Scholar]
- 130.Karimi O, Peña AS. Bodegraven AAv: Probiotics (VSL# 3) in arthralgia in patients with ulcerative colitis and Crohn's disease: a pilot study. Drugs Today. 2005;41(7):453–460. doi: 10.1358/dot.2005.41.7.917341. [DOI] [PubMed] [Google Scholar]
- 131.Rossi G, Cerquetella M, Scarpona S, Pengo G, Fettucciari K, Bassotti G, Jergens A, Suchodolski J. Effects of probiotic bacteria on mucosal polyamines levels in dogs with IBD and colonic polyps: a preliminary study. Beneficial Microb. 2018;9(2):247–255. doi: 10.3920/BM2017.0024. [DOI] [PubMed] [Google Scholar]
- 132.Grundmann O, Yoon SL. Irritable bowel syndrome: epidemiology, diagnosis and treatment: an update for health-care practitioners. J Gastroenterol Hepatol. 2010;25(4):691–699. doi: 10.1111/j.1440-1746.2009.06120.x. [DOI] [PubMed] [Google Scholar]
- 133.Choung RS. Epidemiology of IBS. Gastroenterol Clin North Am. 2011;40(1):1–10. doi: 10.1016/j.gtc.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 134.Öhman L, Simrén M. Pathogenesis of IBS: role of inflammation, immunity and neuroimmune interactions. Nat Rev Gastroenterol Hepatol. 2010;7(3):163. doi: 10.1038/nrgastro.2010.4. [DOI] [PubMed] [Google Scholar]
- 135.Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59(3):325–332. doi: 10.1136/gut.2008.167270. [DOI] [PubMed] [Google Scholar]
- 136.Mezzasalma V, Manfrini E, Ferri E, Sandionigi A, La Ferla B, Schiano I, Michelotti A, Nobile V, Labra M, Di Gennaro P. A randomized, double-blind, placebo-controlled trial: the efficacy of multispecies probiotic supplementation in alleviating symptoms of irritable bowel syndrome associated with constipation. BioMed Res Int. 2016;2016. [DOI] [PMC free article] [PubMed]
- 137.Choi C, Kwon J, Kim S, Myung SJ, Park K, Sohn CI, Rhee PL, Lee K, Lee O, Jung HK. Efficacy of combination therapy with probiotics and mosapride in patients with IBS without diarrhea: a randomized, double-blind, placebo-controlled, multicenter, phase II trial. Neurogastroenterol Motil. 2015;27(5):705–716. doi: 10.1111/nmo.12544. [DOI] [PubMed] [Google Scholar]
- 138.Di Sabatino A, Corazza GR. Coeliac disease. Lancet. 2009;373(9673):1480–1493. doi: 10.1016/S0140-6736(09)60254-3. [DOI] [PubMed] [Google Scholar]
- 139.Brown I, Mino-Kenudson M, Deshpande V, Lauwers GY. Intraepithelial lymphocytosis in architecturally preserved proximal small intestinal mucosa: an increasing diagnostic problem with a wide differential diagnosis. Arch Pathol Lab Med. 2006;130(7):1020–1025. doi: 10.5858/2006-130-1020-ILIAPP. [DOI] [PubMed] [Google Scholar]
- 140.Catassi C, Gatti S, Fasano A. The new epidemiology of celiac disease. J Pediatr Gastroenterol Nutr. 2014;59:S7–S9. doi: 10.1097/01.mpg.0000450393.23156.59. [DOI] [PubMed] [Google Scholar]
- 141.Gujral N, Freeman HJ, Thomson AB. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18(42):6036. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Marasco G, Di Biase AR, Schiumerini R, Eusebi LH, Iughetti L, Ravaioli F, Scaioli E, Colecchia A, Festi D. Gut microbiota and celiac disease. Dig Dis Sci. 2016;61(6):1461–1472. doi: 10.1007/s10620-015-4020-2. [DOI] [PubMed] [Google Scholar]
- 143.Kverka M, Tlaskalova-Hogenova H. Two faces of microbiota in inflammatory and autoimmune diseases: triggers and drugs. Apmis. 2013;121(5):403–421. doi: 10.1111/apm.12007. [DOI] [PubMed] [Google Scholar]
- 144.De Palma G, Cinova J, Stepankova R, Tuckova L, Sanz Y. Pivotal Advance: Bifidobacteria and Gram-negative bacteria differentially influence immune responses in the proinflammatory milieu of celiac disease. J Leukoc Biol. 2010;87(5):765–778. doi: 10.1189/jlb.0709471. [DOI] [PubMed] [Google Scholar]
- 145.López P, Gueimonde M, Margolles A, Suárez A. Distinct Bifidobacterium strains drive different immune responses in vitro. Int J Food Microbiol. 2010;138(1-2):157–165. doi: 10.1016/j.ijfoodmicro.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 146.He F, Morita H, Ouwehand AC, Hosoda M, Hiramatsu M. Kurisaki Ji, Isolauri E, Benno Y, Salminen S: Stimulation of the secretion of pro-inflammatory cytokines by Bifidobacterium strains. Microbiol Immunol. 2002;46(11):781–785. doi: 10.1111/j.1348-0421.2002.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 147.Aloisio I, Santini C, Biavati B, Dinelli G, Cencič A, Chingwaru W, Mogna L, Di Gioia D. Characterization of Bifidobacterium spp. strains for the treatment of enteric disorders in newborns. Appl Microbiol Biotechnol. 2012;96(6):1561–1576. doi: 10.1007/s00253-012-4138-5. [DOI] [PubMed] [Google Scholar]
- 148.Mogna L, Del Piano M, Mogna G. Capability of the two microorganisms Bifidobacterium breve B632 and Bifidobacterium breve BR03 to colonize the intestinal microbiota of children. J Clin Gastroenterol. 2014;48:S37–S39. doi: 10.1097/MCG.0000000000000234. [DOI] [PubMed] [Google Scholar]
- 149.Hazards EPoB: Scientific Opinion on the maintenance of the list of QPS biological agents intentionally added to food and feed (2013 update). EFSA J 2013, 11(11):3449.
- 150.Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H, Tsuji NM, Kiyono H, Ma JS, Kusu T. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLos Pathog. 2012;8(5):e1002714. doi: 10.1371/journal.ppat.1002714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Zheng B, van Bergenhenegouwen J, Overbeek S, van de Kant HJ, Garssen J, Folkerts G, Vos P, Morgan ME, Kraneveld AD. Bifidobacterium breve attenuates murine dextran sodium sulfate-induced colitis and increases regulatory T cell responses. Plos One. 2014;9(5):e95441. doi: 10.1371/journal.pone.0095441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T. Reduced expression of the regulatory CD4+ T cell subset is related to Th1/Th2 balance and disease severity in rheumatoid arthritis. Arthritis Rheum. 2000;43(3):617–627. doi: 10.1002/1529-0131(200003)43:3<617::AID-ANR19>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 153.Lauret E, Rodrigo L. Celiac disease and autoimmune-associated conditions. BioMed Res Int. 2013;2013. [DOI] [PMC free article] [PubMed]
- 154.Quagliariello A, Aloisio I, Bozzi Cionci N, Luiselli D, D’Auria G, Martinez-Priego L, Pérez-Villarroya D, Langerholc T, Primec M, Mičetić-Turk D. Effect of Bifidobacterium breve on the intestinal microbiota of coeliac children on a gluten free diet: a pilot study. Nutrients. 2016;8(10):660. doi: 10.3390/nu8100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Klingspor S, Bondzio A, Martens H, Aschenbach JR, Bratz K, Tedin K, Einspanier R. Lodemann U: <i>Enterococcus faecium</i> NCIMB 10415 Modulates Epithelial Integrity, Heat Shock Protein, and Proinflammatory Cytokine Response in Intestinal Cells. Mediators Inflamm. 2015;2015:304149. doi: 10.1155/2015/304149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423(6937):356. doi: 10.1038/nature01661. [DOI] [PubMed] [Google Scholar]
- 157.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7(6):429. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 158.Alamanos Y, Drosos AA. Epidemiology of adult rheumatoid arthritis. Autoimmun Rev. 2005;4(3):130–136. doi: 10.1016/j.autrev.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 159.Lipsky PE, van der Heijde DM, St. Clair EW, Furst DE, Breedveld FC, Kalden JR, Smolen JS, Weisman M, Emery P, Feldmann M. Infliximab and methotrexate in the treatment of rheumatoid arthritis. N Engl J Med. 2000;343(22):1594–1602. doi: 10.1056/NEJM200011303432202. [DOI] [PubMed] [Google Scholar]
- 160.Schnabel A, Gross WL: Low-dose methotrexate in rheumatic diseases—efficacy, side effects, and risk factors for side effects. In: Seminars in arthritis and rheumatism: 1994: Elsevier; 1994: 310-327. [DOI] [PubMed]
- 161.Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME. Probiotics and immunity. J Gastroenterol. 2009;44(1):26–46. doi: 10.1007/s00535-008-2296-0. [DOI] [PubMed] [Google Scholar]
- 162.Mandel DR, Eichas K, Holmes J. Bacillus coagulans: a viable adjunct therapy for relieving symptoms of rheumatoid arthritis according to a randomized, controlled trial. BMC Complement Altern Med. 2010;10(1):1. doi: 10.1186/1472-6882-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Alipour B, Homayouni-Rad A, Vaghef-Mehrabany E, Sharif SK, Vaghef-Mehrabany L, Asghari-Jafarabadi M, Nakhjavani MR, Mohtadi-Nia J. Effects of L actobacillus casei supplementation on disease activity and inflammatory cytokines in rheumatoid arthritis patients: a randomized double-blind clinical trial. Int J Rheum Dis. 2014;17(5):519–527. doi: 10.1111/1756-185X.12333. [DOI] [PubMed] [Google Scholar]
- 164.Hatakka K, Martio J, Korpela M, Herranen M, Poussa T, Laasanen T, Saxelin M, Vapaatalo H, Moilanen E, Korpela R. Effects of probiotic therapy on the activity and activation of mild rheumatoid arthritis–a pilot study. Scand J Rheumatol. 2003;32(4):211–215. doi: 10.1080/03009740310003695. [DOI] [PubMed] [Google Scholar]
- 165.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Maes OC, Chertkow HM, Wang E, Schipper HM. MicroRNA: implications for Alzheimer disease and other human CNS disorders. Curr Genomics. 2009;10(3):154–168. doi: 10.2174/138920209788185252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jinnin M. microRNA in autoimmune disorders. Nihon Rinsho Men'eki Gakkai kaishi=Japanese journal of clinical immunology. 2011;34(6):439–446. doi: 10.2177/jsci.34.439. [DOI] [PubMed] [Google Scholar]
- 168.Croce CM. Causes and consequences of microRNA dysregulation in cancer. Nat Rev Genet. 2009;10(10):704. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hecker M, Thamilarasan M, Koczan D, Schröder I, Flechtner K, Freiesleben S, Füllen G, Thiesen H-J, Zettl U. MicroRNA expression changes during interferon-beta treatment in the peripheral blood of multiple sclerosis patients. Int J Mol Sci. 2013;14(8):16087–16110. doi: 10.3390/ijms140816087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wang J, Huang W, Wu Y, Hou J, Nie Y, Gu H, Li J, Hu S, Zhang H. MicroRNA-193 pro-proliferation effects for bone mesenchymal stem cells after low-level laser irradiation treatment through inhibitor of growth family, member 5. Stem Cells Dev. 2012;21(13):2508–2519. doi: 10.1089/scd.2011.0695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368(18):1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 172.Chen Q, Tong C, Ma S, Zhou L, Zhao L, Zhao X. Involvement of microRNAs in probiotics-induced reduction of the cecal inflammation by Salmonella typhimurium. Front Immunol. 2017;8:704. doi: 10.3389/fimmu.2017.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Heydari Z, Rahaie M, Alizadeh AM, Agah S, Khalighfard S, Bahmani S. Effects of Lactobacillus acidophilus and Bifidobacterium bifidum Probiotics on the Expression of MicroRNAs 135b, 26b, 18a and 155, and Their Involving Genes in Mice Colon Cancer. Probiotics Antimicrob Proteins. 2018:1–8. [DOI] [PubMed]
- 174.Kreuzer-Redmer S, Bekurtz JC, Arends D, Bortfeldt R, Kutz-Lohroff B, Sharbati S, Einspanier R, Brockmann GA. Feeding of Enterococcus faecium NCIMB 10415 leads to intestinal miRNA-423-5p-induced regulation of immune-relevant genes. Appl Environ Microbiol. 2016;82(8):2263–2269. doi: 10.1128/AEM.04044-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Simpson MR, Brede G, Johansen J, Johnsen R, Storrø O, Sætrom P, Øien T. Human breast milk miRNA, maternal probiotic supplementation and atopic dermatitis in offspring. Plos One. 2015;10(12):e0143496. doi: 10.1371/journal.pone.0143496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhao H, Zhao C, Dong Y, Zhang M, Wang Y, Li F, Li X, McClain C, Yang S, Feng W. Inhibition of miR122a by Lactobacillus rhamnosus GG culture supernatant increases intestinal occludin expression and protects mice from alcoholic liver disease. Toxicol Lett. 2015;234(3):194–200. doi: 10.1016/j.toxlet.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 177.Sabharwal H, Cichon C, Ölschläger TA, Sonnenborn U, Schmidt MA: Interleukin-8, CXCL1, and MicroRNA miR-146a responses to probiotic Escherichia coli nissle 1917 and enteropathogenic E. coli in human intestinal epithelial T84 and Monocytic THP-1 cells after apical or basolateral infection. Infect Immun 2016, 84(9):2482-2492. [DOI] [PMC free article] [PubMed]
- 178.Kalani M, Hodjati H, Khanian MS, Doroudchi M. Lactobacillus acidophilus increases the anti-apoptotic micro RNA-21 and decreases the pro-inflammatory micro RNA-155 in the LPS-treated human endothelial cells. Probiotics Antimicrobial Proteins. 2016;8(2):61–72. doi: 10.1007/s12602-016-9214-1. [DOI] [PubMed] [Google Scholar]
- 179.Rodríguez-Nogales A, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, Garcia F, Olivares M, Rodríguez-Cabezas ME, Gálvez J. Differential intestinal anti-inflammatory effects of Lactobacillus fermentum and Lactobacillus salivarius in DSS mouse colitis: impact on microRNAs expression and microbiota composition. Mol Nutr Food Res. 2017;61(11):1700144. doi: 10.1002/mnfr.201700144. [DOI] [PubMed] [Google Scholar]
- 180.Veltman K, Hummel S, Cichon C, Sonnenborn U, Schmidt MA. Identification of specific miRNAs targeting proteins of the apical junctional complex that simulate the probiotic effect of E. coli Nissle 1917 on T84 epithelial cells. int J Biochem Cell Biol. 2012;44(2):341–349. doi: 10.1016/j.biocel.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 181.Vahed SZ, Barzegari A, Saadat YR, Goreyshi A, Omidi Y. Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed Pharmacother. 2017;94:1094–1100. doi: 10.1016/j.biopha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 182.Ceccarelli G, Fratino M, Selvaggi C, Giustini N, Serafino S, Schietroma I, Corano Scheri G, Pavone P, Passavanti G, Alunni Fegatelli D. A pilot study on the effects of probiotic supplementation on neuropsychological performance and micro RNA-29a-c levels in antiretroviral-treated HIV-1-infected patients. Brain Behav. 2017;7(8):e00756. doi: 10.1002/brb3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Giahi L, Aumueller E, Elmadfa I, Haslberger A. Regulation of TLR4, p38 MAPkinase, IκB and miRNAs by inactivated strains of lactobacilli in human dendritic cells. Beneficial Microb. 2012;3(2):91–98. doi: 10.3920/BM2011.0052. [DOI] [PubMed] [Google Scholar]
- 184.Nogales AR, Algieri F, Garrido-Mesa J, Vezza T, Utrilla MP, Chueca N, Fernandez-Caballero JA, García F, Rodriguez-Cabezas ME, Gálvez J. The administration of Escherichia coli Nissle 1917 ameliorates development of DSS-induced colitis in mice. Front Pharmacol. 2018;9:468. doi: 10.3389/fphar.2018.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Taibi A, Singh N, Chen J, Arioli S, Guglielmetti S, Comelli EM. Time-and strain-specific downregulation of intestinal EPAS1 via miR-148a by Bifidobacterium bifidum. Mol Nutr Food Res. 2017;61(5):1600596. doi: 10.1002/mnfr.201600596. [DOI] [PubMed] [Google Scholar]
- 186.Li C, Jia L, Yu Y, Jin L. Lactic acid induced microRNA-744 enhances motility of SiHa cervical cancer cells through targeting ARHGAP5. Chem Biol Interact. 2019;298:86–95. doi: 10.1016/j.cbi.2018.10.027. [DOI] [PubMed] [Google Scholar]
- 187.Liu Z, Tian Y, Jiang Y, Chen S, Liu T, Moyer MP, Qin H, Zhou X. Protective Effects of Let-7b on the Expression of Occludin by Targeting P38 MAPK in Preventing Intestinal Barrier Dysfunction. Cell Physiol Biochem. 2018;45(1):343–355. doi: 10.1159/000486815. [DOI] [PubMed] [Google Scholar]
- 188.Starke I, Zentek J, Vahjen W. Effects of the probiotic Enterococcus faecium NCIMB 10415 on selected lactic acid bacteria and enterobacteria in co-culture. Beneficial Microb. 2014;6(3):345–352. doi: 10.3920/BM2014.0052. [DOI] [PubMed] [Google Scholar]
- 189.Siepert B, Reinhardt N, Kreuzer S, Bondzio A, Twardziok S, Brockmann G, Nöckler K, Szabó I, Janczyk P, Pieper R. Enterococcus faecium NCIMB 10415 supplementation affects intestinal immune-associated gene expression in post-weaning piglets. Vet Immunol Immunopathol. 2014;157(1-2):65–77. doi: 10.1016/j.vetimm.2013.10.013. [DOI] [PubMed] [Google Scholar]
- 190.Schultz M. Clinical use of E. coli Nissle 1917 in inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(7):1012–1018. doi: 10.1002/ibd.20377. [DOI] [PubMed] [Google Scholar]
- 191.Bandiera S, Pfeffer S, Baumert TF. Zeisel MB: miR-122–a key factor and therapeutic target in liver disease. J Hepatol. 2015;62(2):448–457. doi: 10.1016/j.jhep.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 192.Lou G, Yang Y, Liu F, Ye B, Chen Z, Zheng M, Liu Y. MiR-122 modification enhances the therapeutic efficacy of adipose tissue-derived mesenchymal stem cells against liver fibrosis. J cell Mol Med. 2017;21(11):2963–2973. doi: 10.1111/jcmm.13208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Sadri NJ, Moghoofei M, Salmaninejad A, Bahmanpour Z, Karimzadeh M, Nasiri M, Mirzaei H, Pourhanifeh M, Bokharaei-Salim F, Mirzaei H. Pathogenic role of exosomes and microRNAs in HPV-mediated inflammation and cervical cancer: A review. Int J Cancer. 2019. [DOI] [PMC free article] [PubMed]
- 194.Goetze K, Walenta S, Ksiazkiewicz M, Kunz-Schughart LA, Mueller-Klieser W. Lactate enhances motility of tumor cells and inhibits monocyte migration and cytokine release. Int J Oncol. 2011;39(2):453–463. doi: 10.3892/ijo.2011.1055. [DOI] [PubMed] [Google Scholar]
- 195.Walenta S, Schroeder T, Mueller-Klieser W. Lactate in solid malignant tumors: potential basis of a metabolic classification in clinical oncology. Curr Med Chem. 2004;11(16):2195–2204. doi: 10.2174/0929867043364711. [DOI] [PubMed] [Google Scholar]
- 196.Li Y, Deng X, Zeng X, Peng X. The role of mir-148a in cancer. J Cancer. 2016;7(10):1233. doi: 10.7150/jca.14616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Sepehri Z, Kiani Z, Kohan F, Alavian SM, Ghavami S. Toll like receptor 4 and hepatocellular carcinoma; A systematic review. Life Sci. 2017;179:80–87. doi: 10.1016/j.lfs.2017.04.025. [DOI] [PubMed] [Google Scholar]
- 198.Kuzmich N, Sivak K, Chubarev V, Porozov Y, Savateeva-Lyubimova T, Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines. 2017;5(4):34. doi: 10.3390/vaccines5040034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochimica et Biophysica Acta (BBA)-Molecular. Cell Res. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 200.Stiles ME. Bacteriocins produced by Leuconostoc species. J Dairy Sci. 1994;77(9):2718–2724. doi: 10.3168/jds.S0022-0302(94)77214-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The primary data for this study is available from the authors on request.