Abstract
Background
Acute cough in children often causes discomfort to children and parents, reducing their quality of life. Despite the extensive utilization of over-the-counter remedies for cough, the efficacy of most of these treatments in children has not been confirmed.
Methods
We conducted a randomized, double blind, placebo-controlled clinical trial of 106 children with acute cough to evaluate the efficacy and safety of KalobaTUSS®, a paediatric cough syrup based on acacia honey and on Malva sylvestris extract, Inula helenium extract, Plantago major extract, and Helichrysum stoechas extract by using a validated 6 points Likert scale.
Results
Children were orally treated with KalobaTUSS® or placebo for 8 days. Children receiving KalobaTUSS® showed an early and significant reduction in night-time and day-time cough scores measured using a specific scale and a shorter duration of cough than children treated with the placebo.
Conclusions
KalobaTUSS® is well tolerated and produces positive effects by reducing the severity and shortening the duration of cough in children.
Trial registration
Clinicaltrials.gov no. NCT04073251. Retrospectively registered.
Keywords: Syrup, Cough, Children, Honey, Malva sylvestris, Inula helenium, Plantago major, Helichrysum stoechas
Background
Cough is classified as acute or chronic. Acute cough is a very common symptom resolving in the short term, while chronic cough lasts for more than 3 weeks [1]. Acute cough in children is a common reason why parents seek medical treatment and it is a real challenge for paediatricians. Cough is probably the most bothersome symptom associated with upper respiratory tract infections for children and their parents. Cough frequently produces distress and sleep disorders in the whole family [2].
In most cases, acute cough is self-restrained, but its perseverance can be exasperating and can worsen the quality of life and common social participation [3].
The effect of cough experienced by children on the family’s life may create increasing discomfort. Indeed, children with acute cough may experience a transient disability, prompting parents to miss work and children to miss school, thus increasing the global community cost [4]. Generally, parents’ concerns increase when their children’s cough lasts for more than a week. Consequently, parents often seek for a medical consultation with a requirement for drug treatment, although most antitussive drugs lack evidence of effectiveness [5].
In recent years, the inappropriate prescription of antitussive pharmacological treatments in children has decreased; however, paediatricians’ prescriptions currently do not always reflect an accurate treatment of cough [3]. According to epidemiological data, cough occurring in children produces more anxiety than in cough occurring in adults, and many people view this symptom as “a disease”. A recent Italian survey confirms this view, suggesting that some inclinations of paediatricians’ therapeutic practice should be modified to achieve better control of cough in children and to reduce its impact [3]. Moreover, when attempting to treat cough, children are administered over-the-counter products with little or no scientific evidence of proven efficacy [6].
The World Health Organization has identified honey as a possible demulcent treatment for cough [7, 8]. Demulcent is a substance that is usually based on polysaccharides, covers the throat and reduces pain when the mucosa is irritated by increasing saliva production to reduce the cough reflex [9].
Parents often believe that the administration of honey to relieve cough and improve sleep quality at night is more desirable than the administration of drugs such as diphenhydramine or dextromethorphan. Moreover, evidence from some clinical studies support the use of honey to relieve cough. Results from a study comparing a single dose of honey, dextromethorphan and no treatment indicated that parents view honey as the most favourable treatment for symptomatic relief of nocturnal cough due to upper respiratory tract infection in children aged 2–18 years [10].
Another study in which the effects of honey on the nightly cough and sleep quality of children and their parents were compared with dextromethorphan and diphenhydramine showed that the administration of honey before sleep produced a greater effect on alleviating cough induced by an upper respiratory infection in children aged 24–60 months [11].
In 2018, the Cochrane Collaboration reviewed six randomized controlled clinical studies that investigated the use of honey to treat acute cough in children. The authors concluded that honey alleviates cough with a greater size effect than diphenhydramine, an antihistamine drug classified as a cough suppressant in the United States, placebo or no treatment and with little or no difference compared to dextromethorphan [12].
Due to assumptions described above, an evaluation of a different approach to treat cough, instead of synthetic drugs, appears to be interesting. The safeguarding effect of a mechanical barrier in the throat may be considered a well-founded therapeutic approach that indirectly exhibits anti-inflammatory activity and is able to reduce the damage produced by irritant agents or microbes. This protective activity may be acquired from natural substances such as honey and plant extracts.
Based on the considerations listed above and the lack of effective paediatric antitussive products, a cough syrup for children (KalobaTUSS®) based on acacia honey in combination with Malva sylvestris, Inula helenium, Plantago major and Helichrysum stoechas extracts has been developed for acute cough in children. This syrup is a Medical Device class IIa, classified in accordance with Directive 93/42/EC.
Malva sylvestris is a biennial–perennial herbaceous medicinal plant known as “common mallow” that is indigenous to North Africa, Asia and Europe. It has been used in folk medicine for its mucus formed of flavonoids and polysaccharides. Traditionally, M. sylvestris has been used to treat upper respiratory tract infections. The entire plant possesses beneficial properties, and it has been used in folk medicine for its mucus because the leaves and flowers are rich in flavonoids and mucilage. Mucilage is present at a percentage of 6–8% in M. sylvestris leaves and is composed of high-molecular weight acidic polysaccharides of the rhamnogalacturonan type that are also observed in epidermal cells [13]. In 2018, the European Medicinal Agency (EMA) assessed the traditional use of M. sylvestris as a “demulcent preparation for the symptomatic treatment of oral or pharyngeal irritation and associated dry cough” [14, 15]. The extract used in the syrup is a Malva sylvestris leaf aqueous extract, containing no less than 80% mucilage.
Inula helenium (Elecampane) is a perennial herbaceous plant of the Asteraceae family that is native to England and Europe, but it also grows in the northern and eastern United States, Canada Asia, India and Siberia. This plant was used to treat cough based on its medicinal properties [16].
Inula helenium roots are rich in coumarins, flavonoids, polysaccharides (up to 44% inulin and pectic substances), fatty acids and saponins [17]. The sesquiterpene lactones present in the phytocomplex are responsible for the expectorant properties of this plant [18]. Traditionally, extracts of the plant have been used to treat bronchial/tracheal catarrh and dry irritating cough in children [19]. The extract used in the syrup is an Inula helenium roots aqueous extract, containing no less than 30% polysaccharides.
Plantago major is a plant of the Plantaginaceae family that is native to temperate areas of Asia and Europe. Aerial parts contain mucilage (up to 12%). In traditional medicine, the plant was employed as a treatment for cough related to inflammation of the upper respiratory airways [20]. The plant is a quite effective soothing, moistening, expectorant for a dry irritable cough, because the mucous membranes are unable to produce the immune factor-rich mucous that coats, soothes and protects the membrane; thus, it becomes dry, inflamed and easily irritated [21]. Plants belonging to the genus Plantago significantly attenuate cough [22, 23]. The extract used in the syrup is a Plantago major aerial part aqueous extract, containing no less than 30% polysaccharides.
Helichrysum stoechas is an annual herb belonging to the family Asteraceae and is indigenous to the occidental Mediterranean regions [24]. The extract used in the syrup is a Helichrysum stoechas aerial part aqueous extract, containing no less than 30% polysaccharides.
We performed a randomized, controlled double blind clinical trial to investigate the effects of KalobaTUSS®, an innovative syrup containing acacia honey and herbal extracts of Malva sylvestris, Inula helenium, Plantago major and Helichrysum stoechas, on acute cough in children aging 3–6 years. The extract of Malva sylvestris is titrated to 80% based on mucilage, and the extracts of Inula, Plantago and Helichrysum are titrated to 30% based on polysaccharides. The rich composition of the study syrup makes it suitable for use as a treatment for cough in children, because it forms a mechanical barrier on the mucosa. Mucilage is a complex of polysaccharide molecules that is part of different organs of the plant, mainly the aerial parts. When in contact with water, mucilage tends to swell and to create a jelly-like film on the contact surface. When mucilage contacts the respiratory mucosa, it adsorbs moisture present in the mucus, gels and forms bonds with the structure of the mucosa [25]. In the presence of an inflammatory state, this film exerts a soothing and emollient effect on irritated mucosa: this action is defined as “demulcent” activity. Demulcent indicates a soothing and protective effect on the irritated or inflamed mucosal tissue [9].
The aim of this trial was to evaluate the efficacy and safety of KalobaTUSS® compared with the placebo on nocturnal and diurnal acute cough in children.
Methods
Participants were recruited for this study in the paediatric consulting rooms of the Azienda Ospedaliera Provinciale of Messina in collaboration with the Azienda Ospedaliera Universitaria (AOU) Policlinico “G. Martino” of Messina, Italy. The following inclusion criteria were adopted: children aged 3–6 years with acute cough that had persisted for at least three consecutive days, prompting parents to seek a paediatrician consultation. Exclusion criteria were a cough lasting more than 3 weeks, children with a history of obstructive pulmonary diseases, heart diseases, cystic fibrosis, diabetes, neurological diseases, and immunodeficiencies. After the visit to the paediatrician to exclude asthma or other hyperactive airway diseases as the cause of cough, children suitable for the study according to the inclusion criteria were randomly allocated to one of the two treatment groups (KalobaTUSS® or placebo).
Parents signed the informed consent form for children to participate in this trial. Random allocation to the two treatment groups was performed by in a blinded manner by paediatricians who were not aware of the treatment assignment as described below. At admission, subjects were assigned identification numbers and randomly allocated to treatment groups through an external centre for randomization using block randomization with the 1:1 allocation method and blocks of 2 and 4. Allocation concealment was guaranteed by the use of numbered containers. The KalobaTUSS® or placebo assignment was sealed in sequentially numbered identical opaque and sealed containers according to the allocation sequence. Containers were opened sequentially only after the child’s name was written on the envelope. Both participants (their parents) and care providers were double-blinded regarding the interventions, and group membership was disclosed after the analysis of the results. Children belonging to the active group received the KalobaTUSS® syrup and the second group received a placebo as the syrup formulation. Each of two treatment regimens, KalobaTUSS® or placebo, was administered in 4 doses daily in 5 ml per dose for 8 days. The placebo syrup contained 20% fructose and excipients, but it did not contain honey or plant extracts. The appearance, consistency, organoleptic characteristics and viscosity of KalobaTUSS® and the placebo were very similar. Therefore, the effect of the KalobaTUSS® syrup on the verum group might be associated with the honey and plant extracts.
The study was approved by the Ethics Committee of AOU Policlinico “G. Martino” with protocol number 95/18 on 17 December 2018. The trial was conducted according to the ethical principles of the Declaration of Helsinki and Good Clinical Practice principles were adopted. The size of the sample to enrol in the study was calculated based on an average baseline cough score of 3 points in both groups, and an average change in the cough score of 2 points occurring in the group treated with the study product and 1 point for the placebo group (ClinicalTrial.gov identifier: NCT04073251).
The primary outcome of interest was the change in the night-time cough score before treatment (N0) and nocturnal scores obtained after the first (N1), fourth (N4) and eighth nights of treatment, and the change in the diurnal time cough score before treatment (D0) and scores obtained after the first (D1), fourth (D4) and eighth days of treatment. The secondary outcome was the safety of the syrup when administered to children with acute cough for 8 days, which was evaluated by recording potential adverse reactions to treatment.
Cough was clinically diagnosed and evaluated by paediatricians during the first visit and successively monitored by parents, who completed a daily diary after receiving instructions from the paediatricians. The clinical efficacy of KalobaTUSS® was assessed using a validated 6 points Likert scale that was completed by parents and chosen because it is able to establish the severity of cough and to measure the effects of the treatments. The 6 points Likert scale was used to measure the effects of the interventions on all outcomes. The scale ranged from 0 (absence of cough) to 5 (disturbing cough) points corresponding to six increasing degrees of severity for cough at night-time and six other increasing degrees of severity for cough during the day [26]. The syrup was always administered by parents. At the end of the administration period, paediatricians examined the children again and collected the daily diary completed by the parents for each child.
The mean period of coughing before recruitment was calculated to be 3.53 (± SD 0.57) days for the group of children taking KalobaTUSS® syrup and 3.52 (± SD 0.58) days for the group treated with the placebo, and the difference between the two groups was not significant (p = 0.93624). None of the children monitored for the study required antibiotics or any other pharmacological therapy during the treatment period or during the follow-up period of 1 week after the end of the study.
Adverse events (AEs) were monitored throughout the clinical study. AEs were described as inappropriate medical events that were or were not associated with the procedures or the product.
Statistical analysis
The statistical analysis of changes in the cough score between the treatment groups was performed using the Mann-Whitney U test. Demographic data are reported as means ± standard deviations (SD), changes produced by treatments are presented as means ± standard errors (SE). Estimated effect sizes (Cohen d with Hedges bias correction) with 95% CIs are provided for night-time and day-time cough scores. Evaluations of events (cough) between the two groups are reported as the relative risk and relative risk reduction (RR and RRR, respectively), along with 95% confidence intervals (CI) and p-values. A p-value less than 0.05 was considered statistically significant (Table 5).
Table 5.
Evaluation of events (cough) between the two groups is expressed as Relative Risk and Relative Risk reduction (RR, RRR), 95% confidence intervals (CI) and p-values. A p-value smaller than 0.05 was considered statistically significant
| KalobaTUSS® (children with cough) | Placebo (children with cough) | RR (95% CI) | RRR (95% CI) | p | |
|---|---|---|---|---|---|
| Night time cough after 4 days of treatment | 34/54 | 43/52 | 0.76 (0.6–0.9) | 0.24 (0.032–0.40) | 0.0256 |
| Night time cough after 8 days of treatment | 7/54 | 22/52 | 0.306 (0.143–0.65) | 0.69 (0.34–0.85) | 0.0023 |
| Day time cough after 4 days of treatment | 38/54 | 45/52 | 0.81 (0.66–0.99) | 0.18 (0.0032–0.33) | 0.046 |
| Day time cough after 8 days of treatment | 8/54 | 24/52 | 0.32 (0.16–0.65) | 0.68 (0.35–0.84) | 0.0016 |
Results
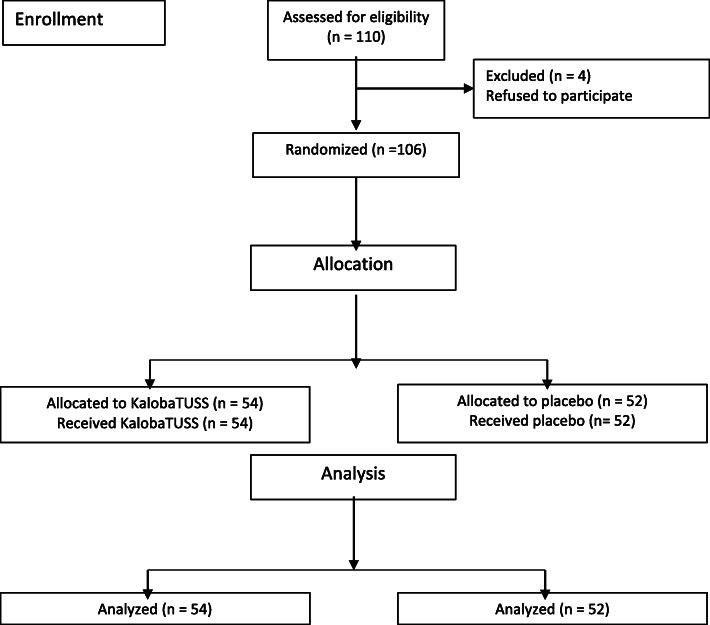
One hundred ten (110) children with acute cough were consecutively enrolled and divided into two groups. Enrolment began on 15 February 2019 and was completed on 10 May 2019. Four children, two from each group, left the study before beginning treatment. One hundred six children began and completed the treatment (Fig. 1). Fifty-four (54) children received the KalobaTUSS® syrup and another group composed of fifty-two (52) children received the placebo syrup. The median age of children completing the study was 53.02 ± a standard deviation (SD) of 12.02 months (range, 38–70 months; median age 4.3 months), with no significant difference in age between the two treatment groups (p = 0.8181). Boys and girls accounted for 50.8 and 49.2%, respectively, of the total sample of enrolled children. Similar percentages of boys and girls were included in the KalobaTUSS® and placebo groups (Table 1).
Fig. 1.
Study Flowchart
Table 1.
Baseline characteristics of children enrolled for the study
| KalobaTUSS® | Placebo | Whole sample | Statistics | |
|---|---|---|---|---|
| Children n. | 54 | 52 | 106 | |
| Mean age (months) | 53.2 ± 11.8 | 52.9 ± 12.3 | 53.0 ± 12.0 | NS |
| Median age (months) | 52 | 51.5 | 52 | __ |
| Sex (male/female) | 28/26 | 26/26 | 54/52 | NS |
| Consecutive days of cough before recruitment | 3.53 ± 0.57 | 3.52 ± 0.58 | 3.59 ± 0.65 | NS |
Data are expressed as means ± standard deviation. NS Not significant
Night-time cough
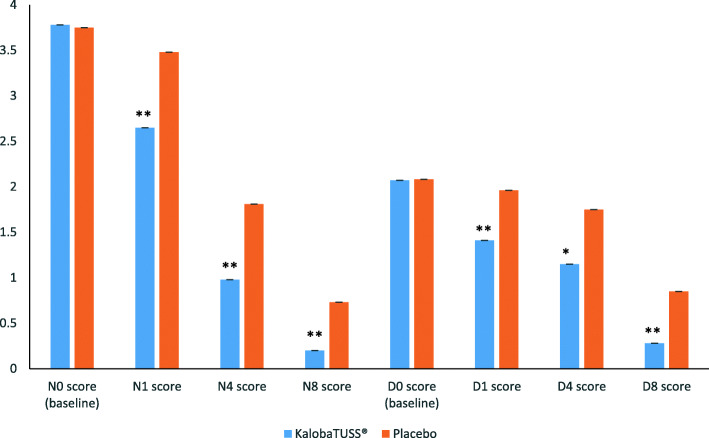
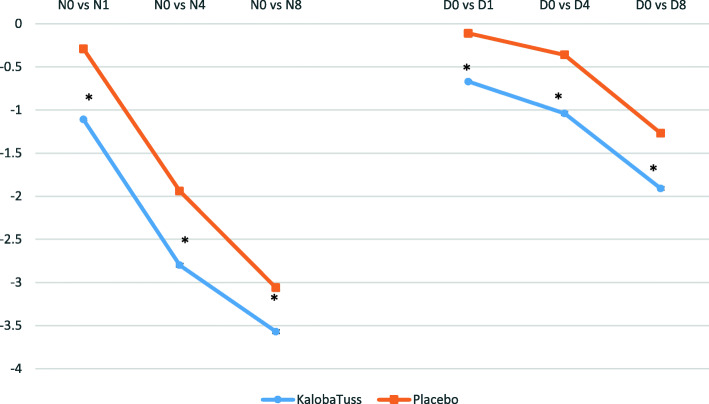
Children who received the KalobaTUSS® syrup already showed a significant reduction in night-time score after the first day of treatment, 1.11 ± 0.01 (mean ± SE) from N0 (last night before treatment) to N1 (first night after treatment), compared to a 0.29 ± 0.01 change in the N0 to N1 score for children receiving the placebo syrup (p = 0.00001), thus indicating a quick improvement in night-time cough (Tables 2 and 4 and Fig. 2). A reduction in the scores compared with N0 was also observed after four or eight nights of treatment. This effect was observed when comparing the scores obtained after four (N4) or eight nights (N8) with the score recorded on N0 or by comparing the total sum of nocturnal scores after four nights or eight nights of treatment with KalobaTUSS® with the night scores of the placebo group. The application of the formulas developed by Hedges and Cohen showed a large effect size after the first night and at the end of treatment compared to the placebo (Table 2). Differences in the reduction of nocturnal cough scores between N0 (baseline) and N1 (night 1), between N0 and night 4 (N4) or between N0 and night 8 (N8), were greater in children treated with KalobaTUSS® than in the placebo group: the N0 vs N1 score was − 1.11 ± 0.01 (SE) for the KalobaTUSS® group and - 0.29 ± 0.01 (SE) for the placebo group; the N0 vs N4 score was − 2.80 ± 0.02 (SE) for the KalobaTUSS® group and − 1.94 ± 0.02 (SE) for the placebo group; and the N0 vs N8 score was − 2.80 ± 0.02 (SE) for the KalobaTUSS® group and − 1.94 ± 0.02 (SE) for the placebo group (Table 4 and Fig. 3). Nocturnal cough was no longer present in a larger group of children treated with KalobaTUSS® compared to the placebo group when assessed either after 4 days or 8 days of treatment (Table 5).
Table 2.
Night-time cough score before and after treatment with KalobaTUSS® or placebo
| Score | KalobaTUSS® | Placebo | Effect size (Hedges’ g) | Effect size (Cohen’s d) | p |
|---|---|---|---|---|---|
| N0 score (baseline) | 3.78 ± 0.01 | 3.75 ± 0.02 | 0.036806 | 0.036776 | 0.8493 |
| N1 score | 2.65 ± 0.02 | 3.48 ± 0.02 | 0.760777 | 0.76118 | 0.0006 |
| N4 score | 0.98 ± 0.02 | 1.81 ± 0.02 | 0.737756 | 0.736129 | 0.0008 |
| N8 score | 0.20 ± 0.01 | 0.73 ± 0.02 | 0.605444 | 0.602798 | 0.0075 |
| Total score of first 4 nights | 6.46 ± 0.07 | 9.36 ± 0.08 | 0.734184 | 0.733648 | 0.0003 |
| Total score of all 8 nights | 8.20 ± 0.11 | 13.40 ± 0.11 | 0.838006 | 0.83697 | 0.00001 |
Data are expressed as means ± standard error. N = night; p was considered significant when < 0.05
Table 4.
Differences in cough scores of the night (N0 score; baseline) and the day (D0 score; baseline) before enrollment compared with night 1 (N1), night 4 (N4), night 8 (N8), and day 1 (D1), day 4 (D4), day 8 (D8), respectively
| KalobaTUSS® | Placebo | p | |
|---|---|---|---|
| N0 vs N1 | - 1.11 ± 0.01 | - 0.29 ± 0.01 | 0.00001 |
| N0 vs N4 | - 2.80 ± 0.02 | −1.94 ± 0.02 | 0.00014 |
| N0 vs N8 | - 3.57 ± 0.02 | - 3.06 ± 0.02 | 0.00758 |
| D0 vs D1 | - 0.67 ± 0.01 | - 0.11 ± 0.01 | 0.00036 |
| D0 vs D4 | - 1.04 ± 0.02 | - 0.36 ± 0.02 | 0.00804 |
| D0 vs D8 | - 1.91 ± 0.02 | - 1.27 ± 0.03 | 0.03940 |
Data are expressed as means ± standard error. p was considered significant when < 0.05
Fig. 2.
Night-time or Day-time cough score before and after treatment with KalobaTUSS® or placebo. * = p < 0.05 vs placebo; * = p < 0.01 vs placebo
Fig. 3.
Reduction in night-time and day-time cough scores in children treated with KalobaTUSS® or Placebo. * = p < 0.01 vs Placebo
Day-time cough
KalobaTUSS® significantly reduced the day-time score after the first day of treatment in the interval from D0 (last day before treatment) to D1 (first day after treatment). The day-time score from D0 to D1 was reduced by 0.67 ± 0.01 (mean ± SE) points in the KalobaTUSS® group compared to 0.11 ± 0.01 in the placebo group (p < 0.00001), thus indicating a quick improvement in the day-time cough as well. The improvement in cough was subsequently revealed by the larger difference in the reduction in the day-time cough score from D0 to D1 (− 1.11 ± 0.01) in the group of children treated with KalobaTUSS® compared to the placebo treatment (− 0.29 ± 0.01) (p = 0.00001). A significant difference in the reduction of cough scores was also observed after 4 days of treatment. This effect was observed when we compared the score recorded on the fourth day (D4) with the score on D0 or by comparing the sum of daily cough scores after 4 days of treatment with KalobaTUSS® with the scores recorded at the same time for the placebo group (Tables 3 and 4 and Fig. 2). The effect size of KalobaTUSS® on daytime cough was larger than the effect of the study syrup on night-time cough after the first night and at the end of treatment (Table 3). Differences in diurnal cough scores between D0 and D1 (day 1), D4 (day 4) or D8 (day 8) were greater in children treated with KalobaTUSS® than in the placebo group (Fig. 2). The D0 vs D1 score was − 0.67 ± 0.01 (SE) for the KalobaTUSS® group and − 0.11 ± 0.01 (SE) for the placebo group, the D0 vs D4 score was − 1.04 ± 0.02 (SE) for the KalobaTUSS® group and − 0.36 ± 0.02 (SE) for the placebo group, and the D0 vs D8 score was − 1.91 ± 0.02 (SE) for the KalobaTUSS® group and − 1.27 ± 0.03 (SE) for the placebo group (Table 4 and Fig. 3). Diurnal cough was no longer present in a larger percentage of children treated with KalobaTUSS® in comparison to the placebo group, either after 4 days or after 8 days of treatment (Table 5). No AEs were observed throughout the whole duration of the clinical study in the two groups of treatment.
Table 3.
Day-time cough score before and after treatment with KalobaTUSS® or placebo
| Score | KalobaTUSS® | Placebo | Effect size (Hedges’ g) |
Effect size (Cohen’s d) |
p |
|---|---|---|---|---|---|
| D0 score (baseline) | 2.07 ± 0.01 | 2.08 ± 0.01 | 0.013795 | 0.013793 | 0.9442 |
| D1 score | 1.41 ± 0.01 | 1.96 ± 0.01 | 0.794567 | 0.793031 | 0.0004 |
| D4 score | 1.15 ± 0.02 | 1.75 ± 0.02 | 0.543784 | 0.542748 | 0.0139 |
| D8 score | 0.28 ± 0.01 | 0.85 ± 0.02 | 0.682739 | 0.676823 | 0.0063 |
| Total score of first 4 nights | 5.09 ± 0.05 | 7.58 ± 0.06 | 1.041877 | 1.031956 | 0.0002 |
| Total score of all 8 nights | 7.33 ± 0.11 | 11.83 ± 0.12 | 0.766493 | 0.764947 | 0.0002 |
Data are expressed as means ± standard error. D = day; p was considered significant when < 0.05
Discussion
Cough is frequent in children and its presence is responsible for numerous visits to paediatricians. It represents a separate clinical entity when it occurs in the absence of an organic origin, and its persistence is generally considered a functional disorder [27].
In the present study, we describe a clinical investigation designed to evaluate the potential beneficial effects of the KalobaTUSS® syrup containing extracts of Malva sylvestris, Inula helenium, Plantago major and Helichrysum stoechas, and acacia honey on acute cough in children. The study product treated cough through a mechanical mode of action: its active substances, honey and herbal extracts, were specifically chosen for their abilities to form a barrier over the irritated mucosa and indirectly reduce the throat inflammation that triggers the cough reflex.
Preclinical data obtained from in vitro experiments (not yet published) showed that the study syrup possesses very good mucoadhesive properties in human oral mucosa cells (static mucoadhesiveness) and is also able to form a mucoadhesive layer (dynamic mucoadhesiveness) that is sufficient to resist the action of the mucosal liquids with which it comes into contact. This peculiarity, namely, the formation of a compact mucosal layer and, in particular a more resistant layer, results in a greater permanence of the product on the mucous membranes and therefore in a greater and prolonged protective effect.
The results of the present clinical trial show a significant reduction in night-time (N) and day-time (D) cough symptoms in children receiving KalobaTUSS® syrup, as measured using a specific evaluation scale. Cough score indicated that parents rated KalobaTUSS® as better than placebo. Reductions in both night- and day-time scores were rapidly observed after 24 h, as evidenced by the evaluation of the differences between basal night (N0 score) and day (D0 score) scores and the scores recorded during the next night (N1 score) and day (D1 score). Moreover, the antitussive effects were maintained after 4 days of treatment, based on the comparison of the scores recorded for children treated with KalobaTUSS® or the placebo with basal values or the comparison of the total sum of daily scores obtained by recording the score for each child after 4 days of treatment with KalobaTUSS® with the scores of the placebo group.
Scores obtained with the questionnaire completed by parents showed a greater reduction in the severity of night-time cough by treatment with the study syrup than the reduction in the severity of the day-time cough: P < 0.01 (= 0.00758) after 8 nights and P < 0.05 (= 0.03940) after 8 days. We did not further investigate this result, although diurnal cough is influenced by diurnal changes in respiratory physiology, and changes in environmental triggers may be confounding factors in the determination of cough [28]. Nevertheless, the reduction in night-time cough scores is a very important topic in cough management as a reduction in night-time cough is difficult to obtain, causes considerable discomfort and produces sleep disturbances both in children and their parents.
Although the analysis was not complete, the relief of symptoms by the study syrup was already significant after the first 24 h, thus suggesting an early response to treatment. The early effect was also associated with a quick relief of cough. The fastest relief was observed in a larger percentage of children without nocturnal and diurnal cough in the group treated with the study syrup than in the group of children treated with placebo after both 4 and 8 days of treatment. The safety of the antitussive product has been assessed, with no reports of adverse events. The palatability, colour, door and density of both the study syrup and placebo syrup were also acceptable.
Despite the inherent limitation associated with the indirect collection of data by the children’s parents, this clinical trial suggests acacia honey together with specific fractions derived from Malva sylvestris, Inula helenium, Plantago major, and Helichrysum stoechas extracts exert beneficial effects on acute cough in children. The significant improvement observed in the cough score may be attributed to the demulcent and expectorant characteristics of the study product. While antitussive drugs act either on the cough centre of central nervous system that controls coughing or on the peripheral cough receptors located in the trachea, pharynx and branch points of large airways and distal smaller airways, the effects of many natural substances on cough are due to the protective and demulcent activities of mucilage [29].
The ingredients of KalobaTUSS® create a protective film on the mucosa, calming cough and protecting the upper respiratory tract. Active components include a large amount of polysaccharides and mucilage that create a film barrier on the oropharyngeal and larynx mucosa through a bioadhesion mechanism of action. The adhesion of these components to the upper respiratory tract mucosa limits the contact with external irritating agents, promotes hydration and limits irritative processes [30].
In addition to the known protective effects of mucilage, and anti-inflammatory and antimicrobial properties of honey, the palatability of honey itself might promote salivation, reducing cough caused by low levels of irritation in the larynx and pharynx [31]. In conclusion, KalobaTUSS® is well tolerated and exerts positive effects by reducing the severity and shortening the duration of cough in children.
Acknowledgements
For their kind collaboration we thank the following pediatricians: Silvana Barone, Mario Fiamingo Adolfo Porto, Maria Lidia Vinciguerra.
The authors also thank Phactory (http://www.phactory.it) for the study design and organization.
Abbreviations
- AOU
Azienda Ospedaliera Universitaria
- AEs
Adverse events
- SD
Standard deviation
- SE
Standard error
- RR
Relative risk
- RRR
Relative risk reduction
- CI
Confidence intervals
- N
Nighttime
- D
Day-time
Authors’ contributions
CI, Contributed to analysis and interpretation and drafted the manuscript; LPR, contributed to conception and design, acquisition and interpretation and critically revised the manuscript; PL, Contributed to analysis and interpretation; RF Contributed to conception and design and contributed to analysis and interpretation; TM, contributed to analysis and interpretation and critically revised the manuscript; MC, contributed to analysis and interpretation, drafted the manuscript and critically revised the manuscript; SEE, contributed to analysis and interpretation; CG, contributed to conception and design, contributed to acquisition, analysis and interpretation, drafted the manuscript. All authors have read and approved the manuscript.
Funding
No funding was obtained for this study.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the Ethic Committee of Azienda Ospedaliera Universitaria (AOU) Policlinico “G. Martino” with protocol number 95/18 on 17 December 2018.
The signed informed consent to partecipate in the study was obtained from all children parents or tutors.
Consent for publication
Not applicable.
Competing interests
Authors Ilaria Carnevali, Lara Pauletto, Floriana Raso, Marco Testa, are employed by the company Schwabe Pharma Italia, Egna (BZ), Italy. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oduwole O, Udoh EE, Oyo-Ita A, Meremikwu MM. Honey for acute cough in children. Cochrane Database Syst Rev 2018, Issue 4. Art. No.: CD007094. doi: 10.1002/14651858.CD007094.pub5. [DOI] [PMC free article] [PubMed]
- 2.De Blasio F, Dicpinigaitis PV, Rubin BK, De Danieli G, Lanata L, Zanasi A. An observational study on cough in children: epidemiology, impact on quality of sleep and treatment outcome. Cough. 2012;8(1). 10.1186/1745-9974-8-1. [DOI] [PMC free article] [PubMed]
- 3.Zanasi A, Morcaldi L, Cazzato S, et al. Survey on attitudes of Italian pediatricians toward cough. Clinicoecon Outcomes Res. 2017;9:189–199. doi: 10.2147/CEOR.S129696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French CT, Irwin RS, Fletcher KE, Adams TM. Evaluation of a cough-specific quality-of-life questionnaire. Chest. 2002;121(4):1123–1131. doi: 10.1378/chest.121.4.1123. [DOI] [PubMed] [Google Scholar]
- 5.Dal Negro RW, Mazzolini M, Turco P, Zanasi A. Cough: impact, beliefs, and expectations from a national survey. Multidiscip Respir Med. 2016;11:34. doi: 10.1186/s40248-016-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith SM, Schroeder K, Fahey T. Over-the-counter (OTC) medications for acute cough in children and adults in ambulatory settings. Cochrane Database Syst Rev. 2014;8:CD001831. doi: 10.1002/14651858.CD001831.pub5. [DOI] [PubMed] [Google Scholar]
- 7.Ahmed N, Sutcliffe A, Tipper C. Feasibility study: honey for treatment of cough in children. Pediatr Rep. 2013;5(2):31–34. doi: 10.4081/pr.2013.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . Cough and cold remedies for the treatment of acute respiratory infections in young children. Geneva: World Health Organization; 2001. [Google Scholar]
- 9.Ziment I. Herbal antitussives. Pulm Pharmacol Ther. 2002;15(3):327–333. doi: 10.1006/pupt.2002.0343. [DOI] [PubMed] [Google Scholar]
- 10.Paul IM, Beiler J, McMonagle A, Shaffer ML, Duda L, Berlin CM. Effect of honey, dextromethorphan, and no treatment on nocturnal cough and sleep quality for coughing children and their parents. Arch Pediatr Adolesc Med. 2007;161:1140–1146. doi: 10.1001/archpedi.161.12.1140. [DOI] [PubMed] [Google Scholar]
- 11.Shadkam MN, Mozaffari-Khosravi H, Mozayan MR. A comparison of the effect of honey, dextromethorphan, and diphenhydramine on nightly cough and sleep quality in children and their parents. J Altern Complement Med. 2010;16(7):787–793. doi: 10.1089/acm.2009.0311. [DOI] [PubMed] [Google Scholar]
- 12.Dicpinigaitis PV, Gayle YE, Solomon G, Gilbert RD. Inhibition of cough-reflex sensitivity by benzonatate and guaifenesin in acute viral cough. Respir Med. 2009;103(6):902–906. doi: 10.1016/j.rmed.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 13.Gasparetto JC, Martins CA, Hayashi SS, Otuky MF, Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. J Pharm Pharmacol. 2012;64:172–189. doi: 10.1111/j.2042-7158.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 14.Assessment report on Malva sylvestris L. and/or Malva neglecta Wallr., folium and Malva sylvestris L., flos,, EMA/HMPC/749518, 2018. https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-malva-sylvestris-l/malva-neglecta-wallr-folium-malva-sylvestris-l-flos-first-version_en.pdf Checked on 31 october 2019.
- 15.Barros L, Carvalho AM, Ferreira IC. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: a comparative study of the nutraceutical potential and composition. Food Chem Toxicol. 2010;48(6):1466–1472. doi: 10.1016/j.fct.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Seca AM, Grigore A, Pinto DC, Silva AM. The genus Inula and their metabolites: from ethnopharmacological to medicinal uses. J Ethnopharmacol. 2014;154(2):286–310. doi: 10.1016/j.jep.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Petkova N, Ivanov I, Vrancheva R, Denev P, Pavlov A. Ultrasound and Microwave-Assisted Extraction of Elecampane (Inula helenium) Roots. Nat Prod Commun. 2017;12(2):171–174. [PubMed] [Google Scholar]
- 18.Zhao YM, Zhang ML, Shi QW, Kiyota H. Chemical constituents of plants from the genus Inula. Chem Biodivers. 2006;3(4):371–384. doi: 10.1002/cbdv.200690041. [DOI] [PubMed] [Google Scholar]
- 19.Kalachaveedu M, Raghavan D, Telapolu S, Kuruvilla S, Kedike B. Phytoestrogenic effect of Inula racemosa Hook f - A cardioprotective root drug in traditional medicine. J Ethnopharmacol. 2018;210:408–416. doi: 10.1016/j.jep.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Najafian Y, Hamedi SS, Farshchi MK, Feyzabadi Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: a narrative review. Electron Physician. 2018;10(2):6390–6399. doi: 10.19082/6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matev M, Angelova I, Koĭchev A, Leseva M, Stefanov G. Clinical trial of a Plantago major preparation in the treatment of chronic bronchitis. Vutr Boles. 1982;21(2):133–137. [PubMed] [Google Scholar]
- 22.Wegener T, Kraft K. Plantain (Plantago lanceolata L.):anti-inflammatory action in upper respiratory tract infections. Wien Med Wochenschr. 1999;149(8–10):211–216. [PubMed] [Google Scholar]
- 23.Beer AM, Loew D. Medicinal plants for infections of the upper and lower respiratory tract: practical recommendations. MMW Fortschr Med. 2008;150(41):29–33. [PubMed] [Google Scholar]
- 24.Memariani Z, Moeini R, Hamedi SS, Gorji N, Mozaffarpur SA. Medicinal plants with antithrombotic property in Persian medicine: a mechanistic review. J Thromb Thrombolysis. 2018;45(1):158–179. doi: 10.1007/s11239-017-1580-3. [DOI] [PubMed] [Google Scholar]
- 25.Bylka W, Witkowska-Banaszczak E, Studzińska-Sroka E, Matławska I. Phytotherapy of respiratory tract diseases. Wiad Lek. 2012;65(2):124–131. [PubMed] [Google Scholar]
- 26.Chung KF. Assessment and measurement of cough: the value of new tools. Pulm Pharmacol Ther. 2002;15(3):267–272. doi: 10.1006/pupt.2002.0360. [DOI] [PubMed] [Google Scholar]
- 27.Weinberger M, Lockshin B. When is cough functional, and how should it be treated? Breathe (Sheff). 2017;13(1):22–30. doi: 10.1183/20734735.015216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kelly EA, Houtman JJ, Jarjour NN. Inflammatory changes associated with circadian variation in pulmonary function in subjects with mild asthma. Clin Exp Allergy. 2004;34(2):227–233. doi: 10.1111/j.1365-2222.2004.01866.x. [DOI] [PubMed] [Google Scholar]
- 29.Puodziūniene G, Janulis V, Milasius A, Budnikas V. Development of cough-relieving herbal teas. Medicina (Kaunas). 2005;41(6):500–505. [PubMed] [Google Scholar]
- 30.Schmidgall J, Schnetz E, Hensel A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000;66(1):48–53. doi: 10.1055/s-2000-11118. [DOI] [PubMed] [Google Scholar]
- 31.Peixoto DM, Rizzo JA, Schor D, et al. Use of honey associated with Ananas comosus (Bromelin) in the treatment of acute irritative cough. Rev Paul Pediatr. 2016;34(4):412–417. doi: 10.1016/j.rpped.2016.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.