Abstract
The lipid raft hypothesis postulates that lipid-lipid interactions can laterally organize biological membranes into domains of distinct structures, lipid/protein compositions, and functions. This proposal has in equal measure exhilarated and frustrated membrane research for decades. While the physicochemical principles underlying lipid-driven domains has been explored and is well understood, the existence and relevance of such domains in cells remains elusive, despite decades of research. Here, we review the conceptual underpinnings of the raft hypothesis and critically discuss the supporting and contradicting evidence in cells, focusing on why controversies about the composition, properties, and even the very existence of lipid rafts remain unresolved. Finally, we highlight several recent breakthroughs that may resolve existing controversies and suggest general approaches for moving beyond questions of rafts existence and towards understanding of their physiological significance.
Introduction
Few topics in cell biology have spawned quite the level of spirited debate as have lipid rafts. The introduction of the raft concept [1] launched a new field and recruited to membrane biology a generation of physical chemists, cell biologists, biophysicists, optical engineers, and computational scientists [2], all to explore the seemingly trivial notion that living membranes are not laterally homogeneous. From this perspective, the raft concept has been a tremendous success, yielding insights and development across numerous disciplines. On the other hand, the exciting promise of a comprehensive mechanism for regulating signaling at the plasma membrane (PM) has so far not been definitively substantiated. Worse still, despite decades of research, thousands of papers, and countless debates, the pressing question in the field remains: “…but do rafts exist?”
These competing notions of success for the raft hypothesis are perhaps not incompatible: it is exactly the enduring mystery of the nature and function of rafts that keeps the field vital and progressive. In this review, we will discuss (1) the physicochemical principles underlying the raft concept, (2) how cooperative regulation between proteins and lipids establish lateral structure in biomembranes, and (3) recent developments in understanding of the raft concept and why it has proven so difficult to definitively establish the existence and relevance of lipid driven membrane subdomains. In the final section, we highlight several recent breakthroughs across multiple fields that have overcome these complications to provide some of the most compelling evidence supporting the functional roles for raft domains in cell biology.
Order out of Chaos
Compartmentalization is a ubiquitous theme in biology: membranes originally evolved as semi-permeable barriers to envelop cells and delimit compartments within the cytoplasm. Perhaps it is not surprising that compartmentalization is also possible within the plane of the membrane itself. One possibility for such compartmentalization is known as the “raft hypothesis”, which posits that preferential interactions between sterols and certain phospholipids can induce the formation of tightly packed membrane domains with distinct protein and lipid compositions from their surrounding membranes. The physiological implications of such organization are self-evident: with minimal energy expenditure, cells could concentrate specific reactants, exclude negative regulators, induce conformational changes, or regulate local membrane properties. Thus, rafts have been extensively implicated in signal transduction through the PM in a variety of physiological contexts [3]. A separate functionality powered by lateral membrane partitioning, and the physiological role for which they were originally invoked, is in the sorting and trafficking of membrane components between subcellular organelles [4–6]. All cellular organelles have unique membrane compositions and the mechanisms by which proteins and lipids traffic between them remain largely unresolved. In particular, the secretory pathway is believed to present a gradient of lipid compositions and physical properties, with endoplasmic reticulum (ER) membranes being poor in raft-forming lipids like cholesterol and sphingolipids, while plasma membranes (PMs) are highly enriched in these components (30–40 mol% cholesterol / 10–30% sphingolipids) [7]. The Golgi stacks are likely intermediate, possibly with progressive accumulations from cis to trans [8]. Laterally segregated raft lipid-enriched domains could provide a self-organizing explanation for such lipid distributions by formation of selective compartments that are preferentially moved from earlier to later steps in secretory pathway. A similar principle may be functional in the endosomal pathway, with the lysosome standing in for the cholesterol-poor ER and various endosomes as intermediate compartments.
Despite the long-standing debates about raft existence and confusion about their very definition, the basic physical chemistry underlying lipid-driven domains is relatively straightforward. All lipids interact with one another very favorably compared to their interactions with water; this is the basis of membrane self-assembly. However, certain lipids interact more favorably with each other than with other lipids because of various chemical and geometric features [9]. With hundreds of different lipid subtypes in a given mammalian membrane [10, 11], a plethora of differential interactions are a certainty and therefore ideal mixing should not be expected. Put another way, lateral heterogeneity is the rule rather than the exception. However, the time- and length-scale of this heterogeneity, which ultimately dictate their biological relevance, are highly dependent on the molecular details. Furthermore, the physical models that best describe how differential binary lipid interactions lead to lateral structures remain under active debate; some of these are discussed in the following section.
For lipid rafts, the most relevant interactions are between saturated lipids, sphingolipids, and sterols, which are preferred relative to their interactions with highly unsaturated lipids (Fig. 1A). Individually, the magnitudes of these relative preferences are small. However, membranes are formed by a multitude of lipids, whose inherent collectivity can multiply these small differences into macroscopic behaviors (Fig. 1B). The physicochemical basis for the raft hypothesis grew out of extensive model membrane studies that established that collective interactions between sterols and saturated lipids can form a unique state of matter called the liquid ordered phase [12–14]. This Lo phase is fluid, like the more common disordered lipid (Ld) phase, allowing molecular motion and flexibility. However, the molecular arrangement and various physical properties (lipid packing, rigidity, permeability) are distinct from the Ld phase, allowing Lo and Ld phases to coexist throughout a broad space of compositional and physical parameters [12]. It is this coexistence of ordered and disordered lipid phases that is believed to underlie raft assembly in living cells.
Figure 1 -. Differential lipid interactions drive lateral organization.
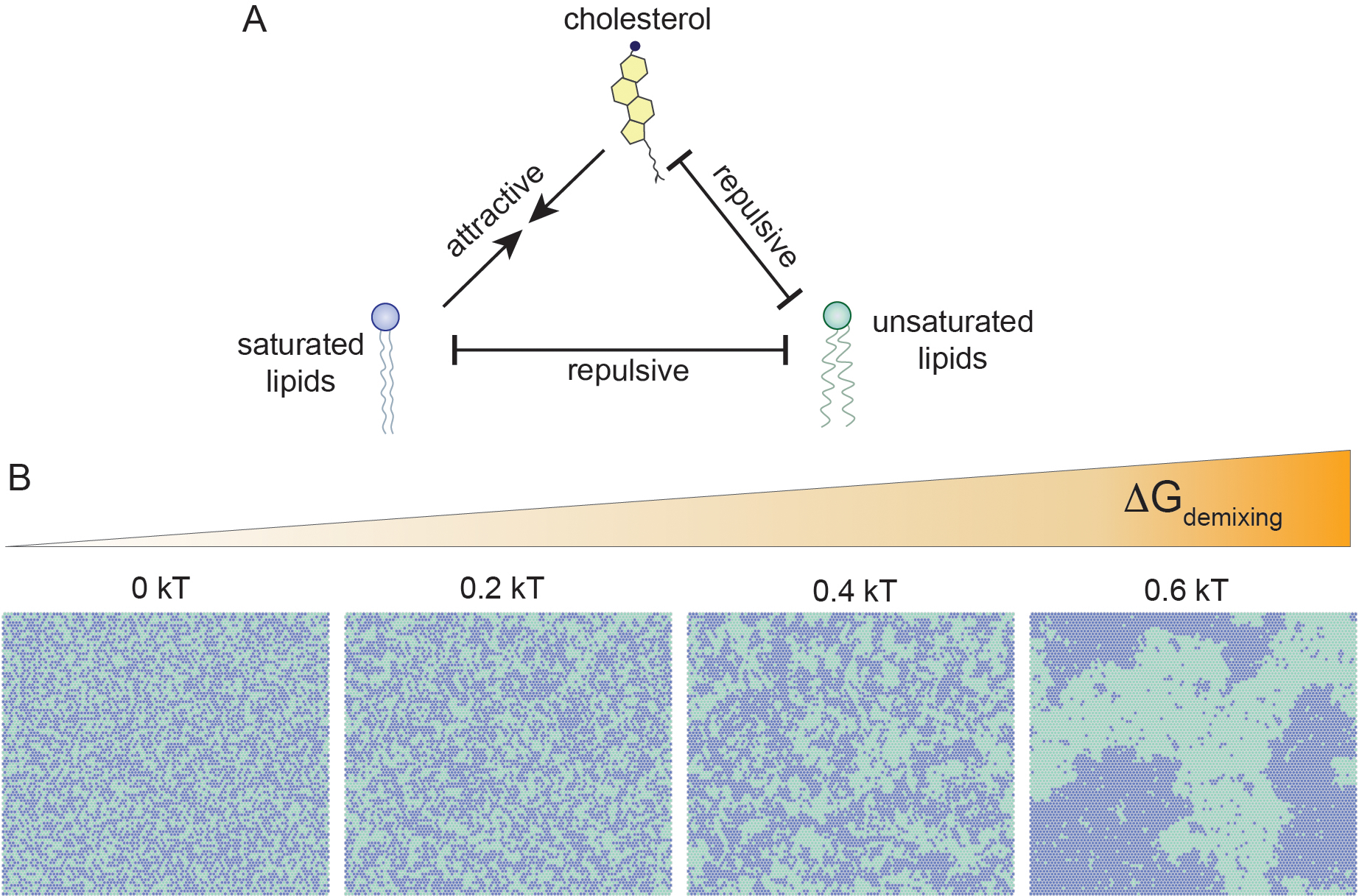
(A) Saturated lipids and cholesterol interact more favorably with each other than with unsaturated lipids. (B) Collectively, these differential interactions oppose the entropic tendency towards random mixing. When the various interactions sufficiently outweigh the demixing cost (dGdemixing ~ −kT), stable lateral domains are formed.
In the biological world, this simplistic version of phase coexistence is complicated by the inherent complexity of living systems. In contrast to model membranes, biomembranes contain hundreds of different lipids and a vast array of transmembrane proteins which comprise up to 25% of the cross-sectional area of the PM [15]. However, strong evidence supporting the biological relevance of Lo/Ld phase coexistence comes from studies in Giant Plasma Membrane Vesicles (GPMVs) [16, 17], which are intact, isolated PM blebs that retain the lipid diversity and protein content of living membranes [16–18]. These vesicles macroscopically phase separate into coexisting ordered and disordered phases [19] that sort both lipid and protein components in concordance with the precepts of the raft hypothesis. Namely, saturated lipids, sterols, glycolipids, and certain proteins are co-recruited to more ordered phases, away from unsaturated lipids and most other proteins [16, 20]. This spontaneous formation of coexisting fluid domains demonstrates the self-organizing capacity of mammalian plasma membranes and the potential for such organization to laterally sort membrane components. Further research has illuminated the structural determinants of protein partitioning to the more ordered or domains, suggesting that post-translational lipid modifications and transmembrane domain features enable raft affinity [21, 22]. A persistent observation from these studies, and analogous ones on synthetic membranes, is that ordered domains tend to exclude most transmembrane proteins [19, 23], and even those proteins that are included are rarely enriched. This finding suggests the possibility that perhaps rafts are relatively lipid-rich / protein-poor membrane regions whose major role is exclusion of most components, rather than concentration of some. However, it should be noted that such measurements have only been made in isolated or synthetic systems, which may not faithfully represent some essential aspects of living cell membranes. On the other hand, protein-depleted membrane domains in cells have been directly observed by super-resolved microscopy [24], though their underlying structure and mechanisms have not been resolved.
Let’s Work Together: cooperative regulations between membrane proteins and lipid domains
Recent studies utilizing computations, synthetic membranes of increasing complexity, and cell-derived GPMVs have moved beyond simple models to begin to understand the determinants of lateral structure in living cells. The major insights can be summarized into three broad themes: (a) lipid context is essential for lateral structure; (b) proteins play an essential role in regulating (clustering, localizing, templating) the lipid template (Fig. 2); and (c) the principles of macroscopic phase separation can be co-opted to produce nanodomains.
Figure 2 -. Cooperative regulation between membrane proteins and lateral domains.
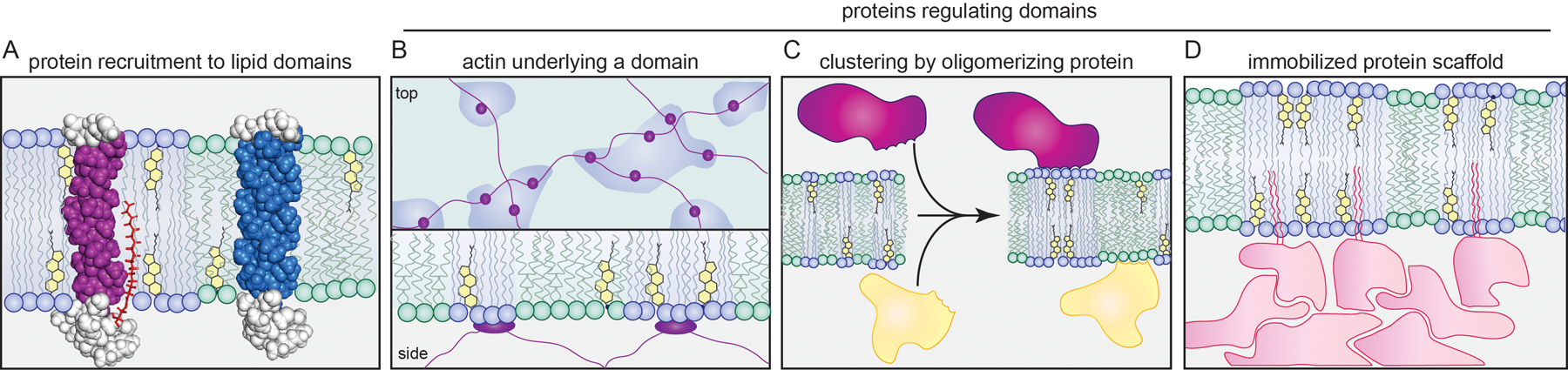
(A) Freely-diffusing proteins can be recruited to membrane domains by post-translational lipid modifications (e.g. palmitoylations) and features such as long and thin transmembrane domains. (B-D) Proteins can also template and regulate membrane domains. (B) Cytoskeletal protein networks can template membrane domains by tethering specific lipids or proteins. (C) Oligomerization of raft-associated proteins can cluster and stabilize raft domains. (D) Immobilized, membrane-bound scaffolds (e.g. post-synaptic density) may recruit specific membrane domains based on the raft affinity of their membrane-anchoring proteins.
Lipid context in lateral structure.
The original model membrane studies that provided the crucial physical foundation of the raft hypothesis typically relied on a composition inspired by the apical PM of epithelial cells [13, 25]: an approximately equimolar mixture of cholesterol, a saturated lipid like sphingomyelin (SM), and an unsaturated phosphatidylcholine usually bearing two unsaturated acyl chains (e.g. dioleoyl phosphatidylcholine; DOPC). Since then, it has become clear that this mixture is a rather poor model of most mammalian PMs. First, this combination of headgroups is a reasonable mimic of the PM outer leaflet, whereas the inner leaflet contains abundant charged and polyunsaturated lipids [26, 27]. The possible consequences of asymmetry on membrane structure are discussed below. More subtly, lipids with two unsaturated acyl chains are relatively scarce in mammalian membranes, which are composed largely of mixed chain (“hybrid”) lipids with one saturated and one unsaturated acyl chain (e.g. palmitoyl-oleoyl PC; POPC). This subtle distinction turns out to be of fundamental significance, as membranes with hybrid lipids as the major unsaturated components typically do not macroscopically phase separate, but rather form nanoscopic ordered domains (discussed in detail below). These studies highlight an important theme: the composition, structure, and stability of ordered domains depends not only on the abundance of order-preferring lipids (sterols and sphingolipids) but also on the type and abundance of disordered lipids. A potentially generalizable framework is that the “contrast” between order- and disorder-preferring lipids determines the ultimate structure: disordered phases with highly polyunsaturated or very short lipids will enhance the contrast to a thick, tightly packed ordered phase and thereby stabilize phase separation. This principle has been demonstrated in simulations [28], model membranes [29–35], and isolated plasma membranes [10, 36–39]. An important conclusion from this line of evidence is that the detailed properties (size, lifetime, stability) and compositions of domains are intrinsically context dependent and reflect the specifics of the membrane in which they arise. Thus, investigations aimed at defining the size or lifetime of such structures in cells may be doomed by fundamental heterogeneity.
Co-operative protein and lipid organization.
Much of the discussion of rafts in biophysics and cell biology presents a paradox: while the central purpose of membrane domains is to sort and organize membrane proteins (Fig. 2A), little attention is paid to the structural roles of proteins in raft organization, which are often treated as passengers to lipid-driven organization. This is somewhat surprising, as some of earliest support for the raft hypothesis came from experiments that used antibodies or toxins to cluster raft-associated molecules [40, 41] (Fig. 2C). In cells, such clustering produces stable, microscopic structures that sort PM components. Similarly, in synthetic and cell-derived membranes, clustering of the glycolipid GM1 by cholera toxin is sufficient to induce macroscopic domains in previously uniform membranes [40], demonstrating that even subtle perturbations may have profound consequences on lipids with the capacity for phase separation. Similar behavior on the nanoscopic scale was recently reported by super-resolution microscopy in B-cells, wherein clustering by B-cell receptor (or cholera toxin) crosslinking produced selective ordered nanodomains [42].
Recently, several notable examples have expanded on the principle that membrane organization involves a cooperative clustering of proteins and lipids, wherein lipid self-assembly provides the tunable template regulated by proteins (Fig. 2). A striking example is the implication of the mammalian cell’s predominant structural element, the actomyosin cytoskeletal network, in membrane organization. Simulations demonstrated that filamentous supports coupled to lipids have the potential to segregate membranes into corrals and stabilize domain formation, even at relatively low connectivity to the membrane [43, 44] (Fig. 2B). At conditions where domains are not preferred, templating by filaments can supplement lipid connectivity to stabilize domain formation. Alternatively, when domains are present, an overlying filamentous meshwork may fragment domains into smaller sub-regions. Some of these predictions have been directly demonstrated in model membranes, by reconstituting an actin network linked to a bilayer capable of phase separation [43, 45–47]. Actin was also demonstrated to be an essential determinant of diffusion in cell-derived membranes [48]. Importantly, a similar mechanism was shown to drive lipid domains in cells, with specific phospholipids mediating connection to an active, dynamically rearranging actomyosin cortex to effect transbilayer registration of raft-associated lipid-anchored proteins [49–51]. A conceptually similar mechanism was proposed to organize the post-synaptic PM in neurons, wherein the post-synaptic density may recruit raft-like domains via immobilization of palmitoylated proteins [52] (Fig. 2D). This hypothesis presents a potentially generalizable paradigm that inverts a common conception of rafts: rather than lipid-driven domains recruiting freely-diffusing proteins, immobilized order-preferring proteins could recruit a dynamic assembly of raft-forming lipids [52]. However, both models are limited, as membrane organization must inherently involve the interplay between protein and lipid components.
Raft nanodomains.
Lipid phase separation in model membranes tends to yield macroscopic domains on the orders of many micrometers, which are generally not observed in live cells, where domains remain nanoscopic [53, 54]. However, clear and compelling evidence across a variety of experimental contexts has demonstrated that the principles of liquid-liquid phase separation can be co-opted to produce nanoscopic domains (Fig. 1). As discussed above, when raft-mimicking membranes are constructed using hybrid lipids like POPC (with one saturated and one unsaturated acyl chain) as the unsaturated component, they appear microscopically uniform. However, FRET, electron spin resonance spectroscopy, and neutron scattering have definitively demonstrated that microscopic uniformity disguises the presence of nanoscopic ordered and disordered domains [33, 55–57]. While it is still not completely clear what sets the size scale of such domains or why they transition to macroscopic upon addition of di-unsaturated lipids, a critical factor appears to be the contrast between hydrophobic thickness of the domains [33], in analogy with the “contrast” arguments discussed above.
A distinct, though not necessarily exclusive, possibility for producing nanodomains using liquid-liquid phase separation principles is critical fluctuations. It has been convincingly demonstrated that some synthetic membrane compositions [58] and most isolated plasma membranes [59–61] are poised near high specific regions in compositional space that allow access to so-called critical phenomena. At these compositions, domains do not simply melt away under unfavorable conditions (e.g. high temperatures). Rather they begin to fragment into smaller and ever more dynamic assemblies called critical fluctuations. Such criticality has been extensively explored in many non-biological phase separated systems [62] and shown to possess some universal characteristics that are independent of the molecular details of a given system [63]. This universality thus allows translation of insights between systems. One such insight is how fluctuation size and lifetime should scale with temperature, which predicts that domains on the order of tens of nanometers should persist on millisecond time scales in plasma membranes under physiological temperatures. Such sub-microscopic domains have been previously reported [64]. Another universal property is the high susceptibility of critical systems to external perturbations, which has been directly demonstrated in Lo/Ld membranes [65] and may account for the susceptibility of lipid raft assembly to protein-mediated crosslinking described above.
Regardless of the mechanistic specifics, if rafts exist in cells, they are almost always nanoscopic (on the order of tens of nanometers) and dynamic, both in the sense of lateral diffusion and possibly also formation/dissolution. One obvious practical consequence of such small, dynamic domains is that they would be very difficult to detect in live cells. Despite the dramatic advances in super-resolution microscopy, there is no current technology with the combined temporal and spatial resolution to directly observe such objects, were they to exist. However, the presence of nanoscopic lipid domains has been extensively reported using techniques like FRET [66–70], fluorescence quenching [71], super-resolution diffusion [69, 72, 73], electron microscopy [20, 74, 75], and single-molecule tracking [76, 77]. Moreover, lipid nanodomains have been extensively observed in fixed cell membranes and shown to sort critical signaling proteins in the PM [37, 68, 74, 78].
Hard to Find a Raft, or Looking for Rafts in All the Wrong Places
It is essential to emphasize that although coexisting domains have been inferred through a variety of indirect measurements [66–73, 76, 77], they have not been directly, microscopically observed. Additionally, there have been several independent lines of evidence that have failed to find clear evidence of lipid-driven domains [79, 80] or thermotropic phase transitions [81] in live cells. Thus, a healthy skepticism persists about the nature and function of lipid-driven lateral domains in vivo.
Seeing is believing.
Lack of direct observation persists as the most common reason for skepticism of the raft hypothesis, raising an obvious question: if ordered domains are so easy to observe in biomimetic and isolated membranes, why are they so elusive (or illusive [82]) in live cells? One possibility is discussed above: there are several plausible mechanisms for generating Lo/Ld nanodomains that would be inaccessible to even modern methodology. Therefore, it may be necessary to manipulate membranes, for example by clustering raft components or releasing them from their cytoskeletal constraints – to directly observe domains in living cells.
However, the scales involved are far from the only methodological concern. Historically, a major issue was the lack of validated, domain-specific probes. This problem is conceptual as much as methodological: the liquid ordered phase is defined by unique structural arrangements of tight, specific lipid packing. Almost by definition, tagging any raft lipid with a bulky, often hydrophilic label reduces their affinity for raft domains. Consistently, most fluorescent lipids are excluded from ordered phases [39]. Recently, this problem has been overcome by the development of novel probes that use the clever strategy of tagging lipid headgroups with the fluorophore far removed from the membrane by an inert spacer (e.g. polyethylene glycol) [83–85]. Another important tool in the arsenal for tagging rafts in cells are minimal proteins, which retain only the membrane-interacting portions of native proteins and therefore represent expressible probes for raft domains in vivo. Examples of these include GPI-anchored fluorophores [20], isolated protein transmembrane domains [21, 86], and lipidated intracellular anchors [20, 74, 75]. An exciting development in this field is the use of such selective anchors to target various reporters or effectors to specific membrane domains [10, 87, 88]. These have been used to selectively read out Akt activity and cAMP concentration in raft domains, or to sequester second messengers in specific membrane sub-compartments [87, 88].
You say yes, I say no.
Negative evidence, i.e. lack of direct visualization, is not the only reason for skepticism of the raft hypothesis. Widely used methods for raft “isolation” have relied on cold-temperature detergent solubilization to generate detergent-resistant membranes (DRM), which have been sometimes construed as proxies for rafts in cells. These DRMs are enriched in sterols, sphingolipids, and lipidated proteins, the same components that partition to Lo phases in model and isolated PMs [89, 90]. Further, Lo phases in model membranes are detergent resistant, lending credence to the assumption that these DRMs represent living Lo phases [12, 91]. On the other hand, it is clear that residue arising from detergent dissolution of cells are very unlikely to directly represent any native structure present in live cells for numerous reasons, including loss of native asymmetry, temperature-dependent lipid properties, and complex membrane-detergent interactions [82, 92–96]. Thus, DRMs should not be equated with rafts and cannot provide conclusive evidence of raft composition or properties.
Finally, several methods have failed to detect rafts, despite cleverly designed approaches. One such attempt relied on mass spectrometry to scan the surface of fixed cells for chemical signatures of cholesterol and sphingolipid enrichment. While domains rich in SM [80] or putative raft proteins [97] were detected, these were not enriched in cholesterol. A separate approach attempted to immobilize GPI-anchored proteins (GPI-APs) to recruit ordered domains and did not detect enrichment of other putatively raft-preferring molecules or changes in diffusion in GPI-rich zones [79]. These are consistent with older studies of GPI-AP clustering [98]. On the contrary, a study combining total internal reflection fluorescence (TIRF) microscopy with correlation analysis detected clear signatures of GPI-APs associating with membrane domains and stabilization of domains upon GPI-APs crosslinking [99]. It remains to be determined whether the above methods have the appropriate spatial and molecular resolution to definitively rule out the presence of compositionally distinct subdomains. An important concern is how different domains would need to be from each other and their surrounding bulk, in order for these differences to be detectable. For example, model membrane experiments have reported relatively small differences in cholesterol concentration between Lo and Ld phases [100]. Moreover, lipid packing differences are much smaller in natural membrane (i.e. GPMVs) than in the synthetic models often used to calibrate analytical methods [36, 101]. Similarly, proteins and lipids rarely enrich by more than 2-fold in the ordered phase of GPMVs.
Looking for rafts in all the wrong places.
As discussed [5], lipid rafts may be used to organize subcellular traffic in the cell, segregating components in sorting organelles (Golgi and endosomes) to facilitate their trafficking to the PM. An extreme prediction from this hypothesis is that the PM is a “post-sorting” compartment so distilled with raft lipids that stable domains may be hard to observe. Indeed, there is evidence that ordered domains comprise a major portion of the PM in some cells [54, 66, 69, 102–104]. If this is the case, perhaps the search for lipid domains must be expanded to internal membranes that may have compositions more amenable to phase separation. Supporting evidence for this possibility is the direct observations of raft-like domains in endosomal compartments (vacuoles) of yeast [105]. These domains show the essential hallmarks of lipid-lipid phase separation [106] and may be the first direct demonstrations of raft domains in vivo. More indirect data (also discussed below) suggests that sorting of raft proteins occurs in endosomes of mammalian cells, suggesting the possibility of stable domains therein [86]. Unfortunately, the organization of internal membranes is difficult to access because they cannot be labeled form the outside and because they are generally small and not flat. However, a recent demonstration suggests that hypotonic swelling of cells allows inflation of organelles and perhaps direct visualization of membrane domains therein [107].
Break on Through to the Other Raft
The ultimate burden of proof for whether rafts are physiologically meaningful remains on proponents of the raft hypothesis. While a wealth of biophysical and cell biological data is supportive of selective, functional membrane domains in cells, definitive demonstrations have remained elusive. However, several recent advances have combined cross-validated probes with biophysical and cellular insights to provide some of the most compelling evidence to date supporting relevance of liquid-liquid phase separation in mammalian membranes.
Correlations light the way.
A pair of studies used similar approaches to explore the detergent resistance, ordered phase preference, and in vivo diffusion of fluorescent analogs of raft lipids [76, 77]. One group focused on SM analogs, while the other focused on a large array of analogs of glycosphingolipids. Both cross-validated their probes, showing that only specific designs (fluorophore separated from the membrane by an inert linker) were enriched in raft domains in GPMVs and that those same analogs were found in DRMs. These validated analogs were then evaluated via single-molecular tracking, revealing distinct behaviors for raft probes, which transiently associated with GPI-anchored proteins in nanoscopic raft-like clusters in living cells. These remarkably convergent observations from independent labs show that Lo/DRM partitioning is directly related to diffusion in live cells, providing strong evidence for the presence of sub-microscopic ordered domains in live cells [76, 77] (Fig. 3A).
Figure 3 -. Accumulating evidence for raft domains in real life.
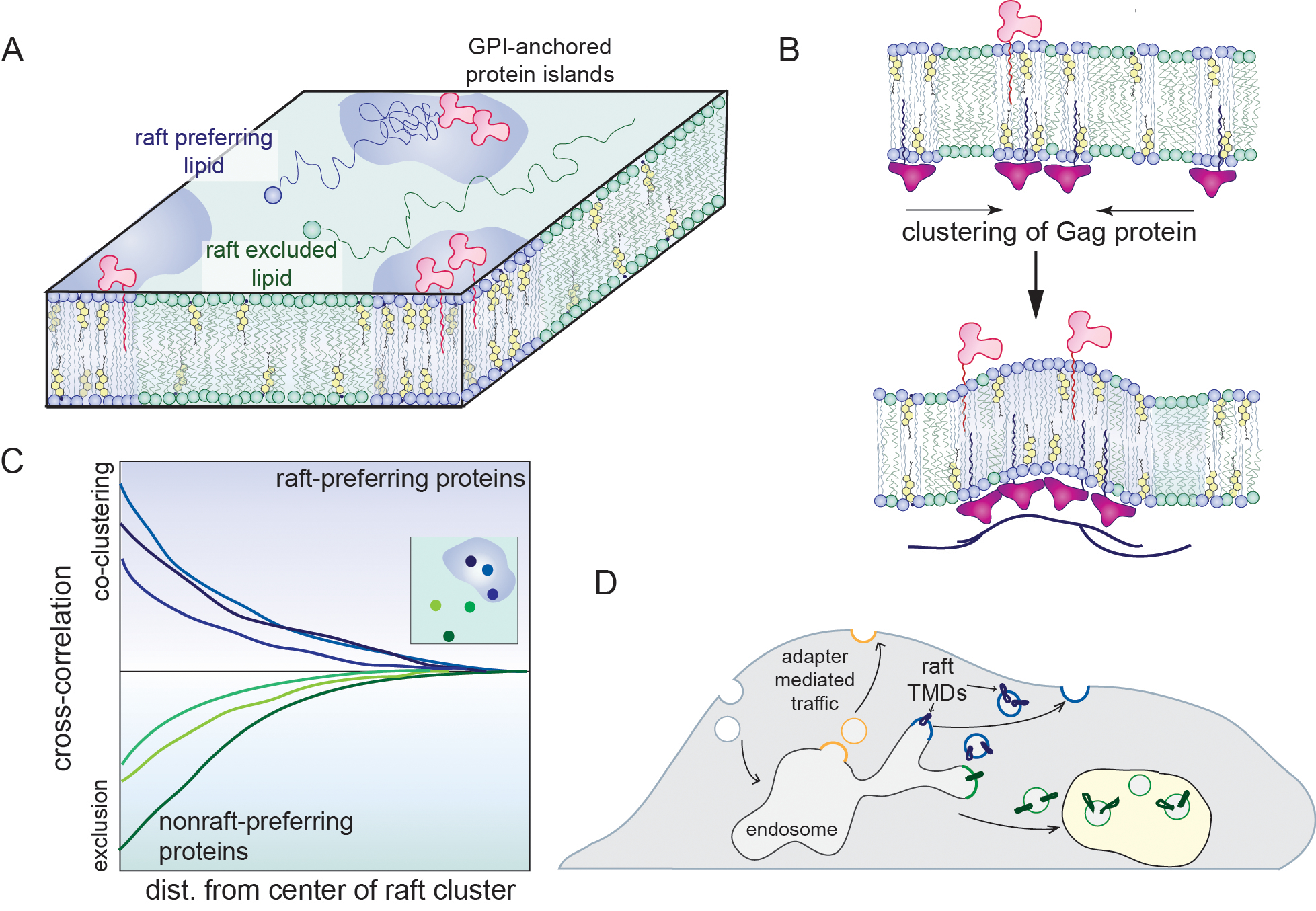
(A) A variety of cross-validated probes have recently been developed, allowing comparison of single-molecule diffusion behaviors between raft-enriched and raft-excluded probes. Fluorescence lipid analogs that prefer ordered phases in model and bio-derived membranes also tend to be detergent-resistant and show distinct diffusive behaviors, statistically enriching in GPI-anchored protein-rich zones. (B) Clustering of viral Gag by matrix proteins leads to microscopically observable membrane domains that sort host proteins based on their affinity for ordered lipid phases. (C) Super-resolution microscopy in live cells reveals statistical enrichment of raft preferring proteins near activated B-cell receptors. (D) Some transmembrane proteins rely on raft affinity for their subcellular localization. For these, the TMDs are fully sufficient to recapitulate the trafficking fates of the full-length protein, and their raft affinity is essential, as non-raft mutants are mis-sorted and ultimately degraded.
Super-resolving domain assembly in vivo.
A technical tour-de-force applied super-resolution microscopy to living B-cells [108] and showed that proteins partitioning to ordered phases in GPMVs form raft-like nanoscopic clusters in activated cells [42] (Fig. 3C). This behavior was shown to affect immune signaling, as clustering of the B-cell receptor led to its recruitment to ordered nanodomains that sorted key regulators of cell activation. These observations were rationalized with a simple model wherein receptor clustering stabilizes pre-existing ordered fluctuations to form a signaling-competent, “extended ordered domain”. These results are fully consistent with classical literature in mast cells, which relied on DRMs to draw similar conclusions about the role of rafts in immune cell activation [109–113].
Viruses organize domains.
raft domains have long been implicated in the function of viruses and other pathogens [114]. Specifically, it has been proposed that certain viral proteins can recruit a raft domain at the host PM, allowing passive recruitment of other viral proteins for ultimate assembly of the viral envelope. This process was recently directly observed by imaging of clustered matrix protein (Gag) from the HIV virus [115] (Fig. 3B). These clusters recruited host raft proteins (raft affinity directly validated by GPMVs) as well as cholesterol and sphingolipids, all consistent with raft assembly during viral budding. A separate study with the same virus revealed that the receptor for HIV (CD4) is localized in raft domains in GPMVs, whereas the co-receptor (CCR5) enriches at the ordered-disordered boundary [116]. This configuration appears to facilitate the fusion of virus with host cell membranes, providing mechanistic insight into previous implications of rafts in viral entry [114].
Rafting through traffic:
as discussed above, raft domains are well suited to provide a platform for accurate and robust sorting of membrane components between organelles [4, 6]. Isolation of post-Golgi vesicles from yeast appears to confirm the potential for lipid-mediated sorting of proteins in the secretory pathway [117, 118]. The involvement of rafts in subcellular sorting in mammalian cells was recently confirmed by analysis of the trafficking and localization of single-pass protein transmembrane domains (TMDs) (Fig. 3D). Such TMDs have been extensively engineered to evaluate the determinants of transmembrane protein affinity for raft domains in GPMVs [21, 86], revealing structural features like TMD length and surface area as key drivers of raft affinity. Because these raft-partitioning TMDs have no residues that can interact with known cytosolic trafficking machineries, they act as genetically encoded probes of trafficking of ordered domains in cells. Remarkably, for a broad panel of TMDs, raft association was fully sufficient for their recycling to the PM after endocytosis, establishing a quantitative and functional relationship between raft association and subcellular protein localization [86]. These observations support the conclusion that ordered membrane domains mediate recycling of specific membrane components from the endosomal compartments to the PM. Subsequent bioinformatic analyses suggest that PM resident proteins tend to have higher raft affinity than those of secretory organelles [21], in line with the putative role of rafts in secretory sorting. Finally, sorting of SM into specific post-Golgi vesicles destined for the PM has been recently directly observed [119, 120].
An over-arching conclusion of the studies discussed in this section is that Lo-like domains are present in live cell membranes as nanoscopic entities that influence cellular physiology, signaling, and trafficking. Importantly, the features of live cell rafts inferred from these observations – nanometric, highly dynamic, potentially heterogeneous – make them inherently difficult to observe in their native context. Studies like those discussed above, which rely on natural membrane models (i.e. GPMVs) to directly quantify raft-associated behaviors and relate them to cellular effects, are enabling rapid progress towards resolving the raft controversy.
Concluding Remarks: Rafts keep on slipping, slipping, slipping… into the future
The studies described in the previous section have provided compelling evidence that strongly supports the existence of rafts in vivo. These advances have some common themes: they rely on bio-orthogonal probes for ordered membrane environments (e.g. synthetic lipids or non-natural TMD sequences) and validate those probes using several independent methods. The behavior of these probes is then evaluated in living cells relative to analogous probes with distinct preferences for disordered membrane regions. Finally, the methodology for detection must either be extremely sensitive to nanometer/millisecond scales or rely on cellular machineries to amplify differences between probes. This general set of approaches may provide a useful guide for coherent characterization of the physiological roles of membrane domains.
One of the broadest and most important open questions in membrane biology concerns the biophysical consequences of lipid asymmetry (see “Outstanding Questions” box). The asymmetric distribution of lipids between the two bilayer leaflets is a fundamental feature of nearly all PMs [27], but the effect of this asymmetry on various physical properties (fluidity, permeability, packing, stiffness) is largely unexplored. Part of the reason is the historical lack of well-controlled experimental model systems with stable, robust lipid asymmetry. This limitation has been recently overcome by several clever approaches [121–123], paving the way towards exploration of the coupling between lateral domains and transverse lipid organization. A fundamental open question is, what is lateral organization of a membrane wherein one leaflet is expected to separate into coexisting Lo/Ld phases whereas the other leaflet would not. Such is the most likely configuration of the mammalian PM, with a SM- and chol-rich outer leaflet and an inner leaflet generally lacking in saturated lipids. In most experiments (both in model membranes [124, 125] and in cells [67, 115]), information about lateral domains appears to be transmitted across the bilayer midplane; however, the mechanism of this transbilayer coupling remains mysterious. An exciting possibility is that asymmetric membranes represent an entirely unexplored state of matter, wherein large-scale domain formation is suppressed due to a strong mismatch penalty between leaflets, which can be rapidly released by modulation of lipid asymmetry.
Finally, continuing advances across microscopic modalities suggest that “seeing” rafts in real life may be just a matter of time. Expansion microscopy and other forms of increasing contrast combined with continuing extension of the boundaries of temporal and spatial resolution may eventually allow direct observations of domains in vivo. As noted above, these searches should not be limited to the PM, as internal membranes may be no less likely to have observable domains. Finally, the recent developments in cryogenic electron microscopy in intact cells [126] may open doors towards directly imaging membrane properties in situ.
Supplementary Material
Acknowledgements:
We gratefully acknowledge funding for IL and KRL provided by the NIH/National Institute of General Medical Sciences (GM114282, GM124072, GM120351, GM134949), the Volkswagen Foundation (grant 93091), and the Human Frontiers Science Program (RGP0059/2019). FAH was supported by National Science Foundation (NSF) Grant No. MCB-1817929 (to FAH). All authors have no competing interests.
References
- 1.Simons K and Ikonen E (1997) Functional rafts in cell membranes. Nature 387 (6633), 569–72. [DOI] [PubMed] [Google Scholar]
- 2.Jacobson K et al. (2007) Lipid rafts: at a crossroad between cell biology and physics. Nat Cell Biol 9 (1), 7–14. [DOI] [PubMed] [Google Scholar]
- 3.Simons K and Toomre D (2000) Lipid rafts and signal transduction. Nat Rev Mol Cell Biol 1 (1), 31–9. [DOI] [PubMed] [Google Scholar]
- 4.Schuck S and Simons K (2004) Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci 117 (Pt 25), 5955–64. [DOI] [PubMed] [Google Scholar]
- 5.Diaz-Rohrer B et al. (2014) Rafting through traffic: Membrane domains in cellular logistics. Biochim. Biophys. Acta-Biomembr 1838 (12), 3003–3013. [DOI] [PubMed] [Google Scholar]
- 6.Smart EJ et al. (1996) A role for caveolin in transport of cholesterol from endoplasmic reticulum to plasma membrane. J Biol Chem 271 (46), 29427–35. [DOI] [PubMed] [Google Scholar]
- 7.van Meer G et al. (2008) Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9 (2), 112–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orci L et al. (1981) Heterogeneous distribution of filipin--cholesterol complexes across the cisternae of the Golgi apparatus. Proc Natl Acad Sci U S A 78 (1), 293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almeida PF (2009) Thermodynamics of lipid interactions in complex bilayers. Biochim Biophys Acta 1788 (1), 72–85. [DOI] [PubMed] [Google Scholar]
- 10.Levental KR et al. (2017) omega-3 polyunsaturated fatty acids direct differentiation of the membrane phenotype in mesenchymal stem cells to potentiate osteogenesis. Sci Adv 3 (11), eaao1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerl MJ et al. (2012) Quantitative analysis of the lipidomes of the influenza virus envelope and MDCK cell apical membrane. J Cell Biol 196 (2), 213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown DA and London E (1998) Structure and origin of ordered lipid domains in biological membranes. J Membr Biol 164 (2), 103–14. [DOI] [PubMed] [Google Scholar]
- 13.Simons K and Vaz WL (2004) Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct 33, 269–95. [DOI] [PubMed] [Google Scholar]
- 14.Ipsen JH et al. (1987) Phase equilibria in the phosphatidylcholine-cholesterol system. Biochim Biophys Acta 905 (1), 162–72. [DOI] [PubMed] [Google Scholar]
- 15.Dupuy AD and Engelman DM (2008) Protein area occupancy at the center of the red blood cell membrane. Proc Natl Acad Sci U S A 105 (8), 2848–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumgart T et al. (2007) Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci U S A 104 (9), 3165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lingwood D et al. (2008) Plasma membranes are poised for activation of raft phase coalescence at physiological temperature. Proc Natl Acad Sci U S A 105 (29), 10005–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sezgin E et al. (2012) Elucidating membrane structure and protein behavior using giant plasma membrane vesicles. Nat Protoc 7 (6), 1042–51. [DOI] [PubMed] [Google Scholar]
- 19.Levental I et al. (2011) Raft domains of variable properties and compositions in plasma membrane vesicles. Proc Natl Acad Sci U S A 108 (28), 11411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levental I et al. (2010) Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc Natl Acad Sci U S A 107 (51), 22050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lorent JH et al. (2017) Structural determinants and functional consequences of protein affinity for membrane rafts. Nat Commun 8 (1), 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin X et al. (2018) Protein partitioning into ordered membrane domains: insights from simulations. Biophys J 114 (8), 1936–1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levental KR and Levental I (2015) Giant plasma membrane vesicles: models for understanding membrane organization. Curr Top Membr 75, 25–57. [DOI] [PubMed] [Google Scholar]
- 24.Saka SK et al. (2014) Multi-protein assemblies underlie the mesoscale organization of the plasma membrane. Nat Commun 5, 4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simons K and van Meer G (1988) Lipid sorting in epithelial cells. Biochemistry 27 (17), 6197–202. [DOI] [PubMed] [Google Scholar]
- 26.Lorent JH et al. (2019) The mammalian plasma membrane is defined by transmembrane asymmetries in lipid unsaturation, leaflet packing, and protein shape. bioRxiv, 10.1101/698837. [DOI] [Google Scholar]
- 27.Devaux PF (1991) Static and dynamic lipid asymmetry in cell membranes. Biochemistry 30 (5), 1163–73. [DOI] [PubMed] [Google Scholar]
- 28.Lin X et al. (2016) Domain stability in biomimetic membranes driven by lipid polyunsaturation. J Phys Chem B 120 (46), 11930–11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang C et al. (2015) Push and pull forces in lipid raft formation: the push can be as important as the pull. J Am Chem Soc 137 (2), 664–6. [DOI] [PubMed] [Google Scholar]
- 30.Krause MR et al. (2014) Push-pull mechanism for lipid raft formation. Langmuir 30 (12), 3285–9. [DOI] [PubMed] [Google Scholar]
- 31.Heberle FA (2019) With Lipid Rafts, Context Is Everything. Biophys J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nyholm TKM et al. (2019) Impact of Acyl Chain Mismatch on the Formation and Properties of Sphingomyelin-Cholesterol Domains. Biophys J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heberle FA et al. (2013) Bilayer thickness mismatch controls domain size in model membranes. J Am Chem Soc 135 (18), 6853–9. [DOI] [PubMed] [Google Scholar]
- 34.Georgieva R et al. (2015) Docosahexaenoic acid promotes micron scale liquid-ordered domains. A comparison study of docosahexaenoic versus oleic acid containing phosphatidylcholine in raft-like mixtures. Biochim Biophys Acta 1848 (6), 1424–35. [DOI] [PubMed] [Google Scholar]
- 35.Shaikh SR et al. (2004) Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: a comparative NMR, DSC, AFM, and detergent extraction study. Biophys J 87 (3), 1752–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sezgin E et al. (2015) Adaptive lipid packing and bioactivity in membrane domains. PLoS One 10 (4), e0123930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Y et al. (2013) Bile acids modulate signaling by functional perturbation of plasma membrane domains. J. Biol. Chem 288 (50), 35660–35670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levental KR et al. (2016) Polyunsaturated lipids regulate membrane domain stability by tuning membrane order. Biophys J 110(8), 1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sezgin E et al. (2012) Partitioning, diffusion, and ligand binding of raft lipid analogs in model and cellular plasma membranes. Biochim Biophys Acta 1818 (7), 1777–84. [DOI] [PubMed] [Google Scholar]
- 40.Hammond AT et al. (2005) Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci U S A 102 (18), 6320–6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harder T et al. (1998) Lipid domain structure of the plasma membrane revealed by patching of membrane components. J Cell Biol 141 (4), 929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stone MB et al. (2017) Protein sorting by lipid phase-like domains supports emergent signaling function in B lymphocyte plasma membranes. Elife 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arumugam S et al. (2015) Cytoskeletal pinning controls phase separation in multicomponent lipid membranes. Biophys J 108 (5), 1104–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Machta BB et al. (2011) Minimal model of plasma membrane heterogeneity requires coupling cortical actin to criticality. Biophys J 100 (7), 1668–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Honigmann A et al. (2014) A lipid bound actin meshwork organizes liquid phase separation in model membranes. Elife 3, e01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vogel SK et al. (2017) Control of lipid domain organization by a biomimetic contractile actomyosin cortex. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu AP and Fletcher DA (2006) Actin polymerization serves as a membrane domain switch in model lipid bilayers. Biophys J 91 (11), 4064–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider F et al. (2017) Diffusion of lipids and GPI-anchored proteins in actin-free plasma membrane vesicles measured by STED-FCS. Mol Biol Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gowrishankar K et al. (2012) Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell 149 (6), 1353–67. [DOI] [PubMed] [Google Scholar]
- 50.Koster DV and Mayor S (2016) Cortical actin and the plasma membrane: inextricably intertwined. Curr Opin Cell Biol 38, 81–9. [DOI] [PubMed] [Google Scholar]
- 51.Goswami D et al. (2008) Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell 135 (6), 1085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tulodziecka K et al. (2016) Remodeling of the postsynaptic plasma membrane during neural development. Mol Biol Cell 27 (22), 3480–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pralle A et al. (2000) Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol 148 (5), 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meder D et al. (2006) Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci U S A 103 (2), 329–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ackerman DG and Feigenson GW (2015) Multiscale Modeling of Four-Component Lipid Mixtures: Domain Composition, Size, Alignment, and Properties of the Phase Interface. J. Phys. Chem. B 119 (11), 4240–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Usery RD et al. (2017) Line Tension Controls Liquid-Disordered + Liquid-Ordered Domain Size Transition in Lipid Bilayers. Biophys J 112 (7), 1431–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heberle FA et al. (2010) Comparison of three ternary lipid bilayer mixtures: FRET and ESR reveal nanodomains. Biophys J 99 (10), 3309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Veatch SL et al. (2007) Critical fluctuations in domain-forming lipid mixtures. PNAS 104 (45), 17650–17655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veatch SL et al. (2008) Critical fluctuations in plasma membrane vesicles. ACS Chem Biol 3 (5), 287–93. [DOI] [PubMed] [Google Scholar]
- 60.Gray E et al. (2013) Liquid general anesthetics lower critical temperatures in plasma membrane vesicles. Biophys J 105 (12), 2751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gray EM et al. (2015) Growth Conditions and Cell Cycle Phase Modulate Phase Transition Temperatures in RBL-2H3 Derived Plasma Membrane Vesicles. PLoS One 10 (9), e0137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Honerkamp-Smith AR et al. (2009) An introduction to critical points for biophysicists; observations of compositional heterogeneity in lipid membranes. Biochim Biophys Acta 1788 (1), 53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Levental I and Veatch SL (2016) The continuing mystery of lipid rafts. J. Mol. Biol 428 (24), 4749–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ge M et al. (2003) Ordered and disordered phases coexist in plasma membrane vesicles of RBL-2H3 mast cells. An ESR study. Biophys J 85 (2), 1278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao J et al. (2013) Adhesion stabilizes robust lipid heterogeneity in supercritical membranes at physiological temperature. Biophys J 104 (4), 825–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez SA et al. (2012) Laurdan generalized polarization fluctuations measures membrane packing micro-heterogeneity in vivo. Proc Natl Acad Sci U S A 109 (19), 7314–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghupathy R et al. (2015) Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell 161 (3), 581–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y et al. (2015) SIGNAL TRANSDUCTION. Membrane potential modulates plasma membrane phospholipid dynamics and K-Ras signaling. Science 349 (6250), 873–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Owen DM et al. (2012) Sub-resolution lipid domains exist in the plasma membrane and regulate protein diffusion and distribution. Nat Commun 3, 1256. [DOI] [PubMed] [Google Scholar]
- 70.Sengupta P et al. (2007) Fluorescence resonance energy transfer between lipid probes detects nanoscopic heterogeneity in the plasma membrane of live cells. Biophys J 92 (10), 3564–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kreder R et al. (2015) Solvatochromic Nile Red probes with FRET quencher reveal lipid order heterogeneity in living and apoptotic cells. ACS Chem Biol 10 (6), 1435–42. [DOI] [PubMed] [Google Scholar]
- 72.Eggeling C et al. (2009) Direct observation of the nanoscale dynamics of membrane lipids in a living cell. Nature 457 (7233), 1159–62. [DOI] [PubMed] [Google Scholar]
- 73.Sezgin E et al. (2019) Measuring nanoscale diffusion dynamics in cellular membranes with super-resolution STED-FCS. Nat Protoc 14 (4), 1054–1083. [DOI] [PubMed] [Google Scholar]
- 74.Prior IA et al. (2001) GTP-dependent segregation of H-ras from lipid rafts is required for biological activity. Nat Cell Biol 3 (4), 368–75. [DOI] [PubMed] [Google Scholar]
- 75.Prior IA et al. (2003) Direct visualization of Ras proteins in spatially distinct cell surface microdomains. J Cell Biol 160 (2), 165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komura N et al. (2016) Raft-based interactions of gangliosides with a GPI-anchored receptor. Nat Chem Biol 12 (6), 402–10. [DOI] [PubMed] [Google Scholar]
- 77.Kinoshita M et al. (2017) Raft-based sphingomyelin interactions revealed by new fluorescent sphingomyelin analogs. J Cell Biol 216 (4), 1183–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Janosi L et al. (2012) Organization, dynamics, and segregation of Ras nanoclusters in membrane domains. Proc Natl Acad Sci U S A 109 (21), 8097–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sevcsik E et al. (2015) GPI-anchored proteins do not reside in ordered domains in the live cell plasma membrane. Nat Commun 6, 6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Frisz JF et al. (2013) Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc Natl Acad Sci U S A 110 (8), E613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee IH et al. (2015) Live cell plasma membranes do not exhibit a miscibility phase transition over a wide range of temperatures. J Phys Chem B 119 (12), 4450–9. [DOI] [PubMed] [Google Scholar]
- 82.Munro S (2003) Lipid rafts: elusive or illusive? Cell 115 (4), 377–88. [DOI] [PubMed] [Google Scholar]
- 83.Momin N et al. (2015) Designing lipids for selective partitioning into liquid ordered membrane domains. Soft Matter 11 (16), 3241–50. [DOI] [PubMed] [Google Scholar]
- 84.Bordovsky SS et al. (2016) Engineering Lipid Structure for Recognition of the Liquid Ordered Membrane Phase. Langmuir 32 (47), 12527–12533. [DOI] [PubMed] [Google Scholar]
- 85.Mobarak E et al. (2018) How to minimize dye-induced perturbations while studying biomembrane structure and dynamics: PEG linkers as a rational alternative. Biochim Biophys Acta Biomembr 1860 (11), 2436–2445. [DOI] [PubMed] [Google Scholar]
- 86.Diaz-Rohrer BB et al. (2014) Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A 111 (23), 8500–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Averaimo S et al. (2016) A plasma membrane microdomain compartmentalizes ephrin-generated cAMP signals to prune developing retinal axon arbors. Nat Commun 7, 12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gao X et al. (2011) PI3K/Akt signaling requires spatial compartmentalization in plasma membrane microdomains. Proc Natl Acad Sci U S A 108 (35), 14509–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brown DA and Rose JK (1992) Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68 (3), 533–44. [DOI] [PubMed] [Google Scholar]
- 90.Ahmed SN et al. (1997) On the origin of sphingolipid/cholesterol-rich detergent-insoluble cell membranes: physiological concentrations of cholesterol and sphingolipid induce formation of a detergent-insoluble, liquid-ordered lipid phase in model membranes. Biochemistry 36 (36), 10944–10953. [DOI] [PubMed] [Google Scholar]
- 91.Brown DA and London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14, 111–36. [DOI] [PubMed] [Google Scholar]
- 92.Kenworthy AK (2008) Have we become overly reliant on lipid rafts? Talking Point on the involvement of lipid rafts in T-cell activation. EMBO Rep 9 (6), 531–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lichtenberg D et al. (2005) Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci 30 (8), 430. [DOI] [PubMed] [Google Scholar]
- 94.Brown DA (2006) Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology (Bethesda) 21, 430–9. [DOI] [PubMed] [Google Scholar]
- 95.Schuck S et al. (2003) Resistance of cell membranes to different detergents. Proc Natl Acad Sci U S A 100 (10), 5795–5800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Heerklotz H (2002) Triton promotes domain formation in lipid raft mixtures. Biophys J 83 (5), 2693–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wilson RL et al. (2015) Hemagglutinin clusters in the plasma membrane are not enriched with cholesterol and sphingolipids. Biophys J 108 (7), 1652–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kenworthy AK and Edidin M (1998) Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J Cell Biol 142 (1), 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang H et al. (2015) Effect of receptor dimerization on membrane lipid raft structure continuously quantified on single cells by camera based fluorescence correlation spectroscopy. PLoS One 10 (3), e0121777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Feigenson GW and Buboltz JT (2001) Ternary phase diagram of dipalmitoyl-PC/dilauroyl-PC/cholesterol: nanoscopic domain formation driven by cholesterol. Biophys J 80 (6), 2775–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kaiser HJ et al. (2009) Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci U S A 106 (39), 16645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levental I et al. (2009) Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem J 424 (2), 163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kilin V et al. (2015) Fluorescence lifetime imaging of membrane lipid order with a ratiometric fluorescent probe. Biophys J 108 (10), 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Swamy MJ et al. (2006) Coexisting domains in the plasma membranes of live cells characterized by spin-label ESR spectroscopy. Biophys J 90 (12), 4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Toulmay A and Prinz WA (2013) Direct imaging reveals stable, micrometer-scale lipid domains that segregate proteins in live cells. J Cell Biol 202 (1), 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rayermann SP et al. (2017) Hallmarks of Reversible Separation of Living, Unperturbed Cell Membranes into Two Liquid Phases. Biophys J 113 (11), 2425–2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.King C et al. (2019) ER membranes exhibit phase behavior at sites of organelle contact. biorXiv 707505; doi: 10.1101/707505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stone MB and Veatch SL (2015) Steady-state cross-correlations for live two-colour super-resolution localization data sets. Nat Commun 6, 7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gosse JA et al. (2005) Transmembrane sequences are determinants of immunoreceptor signaling. J Immunol 175 (4), 2123–31. [DOI] [PubMed] [Google Scholar]
- 110.Young RM et al. (2005) Reconstitution of regulated phosphorylation of FcepsilonRI by a lipid raft-excluded protein-tyrosine phosphatase. J Biol Chem 280 (2), 1230–5. [DOI] [PubMed] [Google Scholar]
- 111.Young RM et al. (2003) A lipid raft environment enhances Lyn kinase activity by protecting the active site tyrosine from dephosphorylation. J Biol Chem 278 (23), 20746–52. [DOI] [PubMed] [Google Scholar]
- 112.Cheng PC et al. (2001) Translocation of the B cell antigen receptor into lipid rafts reveals a novel step in signaling. J Immunol 166 (6), 3693–701. [DOI] [PubMed] [Google Scholar]
- 113.Sohn HW et al. (2006) Fluorescence resonance energy transfer in living cells reveals dynamic membrane changes in the initiation of B cell signaling. Proc Natl Acad Sci U S A 103 (21), 8143–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manes S et al. (2003) Pathogens: raft hijackers. Nat Rev Immunol 3 (7), 557–68. [DOI] [PubMed] [Google Scholar]
- 115.Sengupta P et al. (2019) A lipid-based partitioning mechanism for selective incorporation of proteins into membranes of HIV particles. Nat Cell Biol 21 (4), 452–461. [DOI] [PubMed] [Google Scholar]
- 116.Yang ST et al. (2017) HIV virions sense plasma membrane heterogeneity for cell entry. Sci Adv 3 (e1700338). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Surma MA et al. (2012) Lipid-dependent protein sorting at the trans-Golgi network. Biochim Biophys Acta 1821 (8), 1059–67. [DOI] [PubMed] [Google Scholar]
- 118.Klemm RW et al. (2009) Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. J Cell Biol 185 (4), 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Deng Y et al. (2016) Sphingomyelin is sorted at the trans Golgi network into a distinct class of secretory vesicle. Proc Natl Acad Sci U S A 113 (24), 6677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sundberg EL et al. (2019) Syndecan-1 Mediates Sorting of Soluble Lipoprotein Lipase with Sphingomyelin-Rich Membrane in the Golgi Apparatus. Dev Cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lin Q and London E (2014) Preparation of artificial plasma membrane mimicking vesicles with lipid asymmetry. PLoS One 9 (1), e87903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Doktorova M et al. (2018) Preparation of asymmetric phospholipid vesicles for use as cell membrane models. Nat Protoc 13 (9), 2086–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Enoki TA and Feigenson GW (2019) Asymmetric bilayers by hemifusion: method and leaflet behaviors. Biophys J 117 (6), 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Collins MD and Keller SL (2008) Tuning lipid mixtures to induce or suppress domain formation across leaflets of unsupported asymmetric bilayers. Proc Natl Acad Sci U S A 105 (1), 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Veatch SL and Keller SL (2005) Seeing spots: complex phase behavior in simple membranes. Biochim Biophys Acta 1746 (3), 172–85. [DOI] [PubMed] [Google Scholar]
- 126.Fischer TD et al. (2018) Morphology of mitochondria in spatially restricted axons revealed by cryo-electron tomography. PLoS Biol 16 (9), e2006169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


