Abstract
The lack of reproducibility of published studies is one of the major issues facing the scientific community, and the field of biofilm microbiology has been no exception. One effective strategy against this multifaceted problem is the use of minimum information guidelines. This strategy provides a guide for authors and reviewers on the necessary information that a manuscript should include for the experiments in a study to be clearly interpreted and independently reproduced. As a result of several discussions between international groups working in the area of biofilms, we present a guideline for the spectrophotometric and fluorometric assessment of biofilm formation in microplates. This guideline has been divided into 5 main sections, each presenting a comprehensive set of recommendations. The intention of the minimum information guideline is to improve the quality of scientific communication that will augment interlaboratory reproducibility in biofilm microplate assays.
Keywords: Biofilm, Reproducibility, Guidelines, Microplate, Spectrophotometry, Fluorometry
Introduction
A major challenge facing science today is the lack of reproducibility between published studies [1,2]. Many factors contribute to this phenomenon, including the selective or insufficient reporting of experimental details in the published literature, either in the methodology or data processing, that are essential for conducting the experiment [3]. Furthermore, due to the rapid development of science, new terms are often introduced, or existing terminology is repurposed, which can create confusion when trying to understand a paper or reproduce a study [4].
Minimum information guidelines are an effective strategy for addressing the reproducibility crisis [5]. These guidelines instruct authors and reviewers on the minimum information required for the experiments to be reproducible and the data to be comparable. They also allow the scientific community to standardise terminology leading to the development of ontology databases. However, they do not offer any information on whether a method is appropriate for a certain study nor endorse any specific protocols. The Minimum Information for Biological and Biomedical Investigations (MIBBI) Project is a web based platform (www.mibbi.org) that gathers different minimum information guidelines in the biological and biomedical field, as well as any databases or standard ontologies related to them [4].
Minimum information about a biofilm experiment (MIABiE) (www.miabie.org) is one of the guidelines presented in MIBBI [6]. It offers a broad view of the information necessary when conducting experiments related to biofilms. Biofilms are defined as a community of microorganisms embedded in an extracellular polymeric substance, often attached to a biotic or abiotic surface, which are essential in certain ecosystems but can also have detrimental effects in industry and healthcare [7]. MIABiE includes several modules, each addressing specific parts of a biofilm study, and presents an initiative for a biofilm ontology guide.
The present guideline will expand some of the MIABiE modules by focusing on spectrophotometric and fluorometric methods of biofilm assessment in microplate experiments. These are widely used biofilm assessment methods due to their versatile applications in medical, industrial and environmental biofilm research [8]. They can serve as a generic test, which does not require overly specialised or expensive equipment or training, and can generate high-throughput data because they are microplate compatible.
Although several options for photometric or fluorescence-based methods in microplates exist, this guideline will focus on those methods most frequently used. This includes spectrophotometric methods used to quantify total biofilm mass based on the binding of dyes, such as crystal violet and safranin to cells and negatively-charged molecules (such as polysaccharides) in the biofilm matrix [[9], [10], [11]]. Additionally, the guideline is applicable to fluorometric (or fluorescence-based) methods used to quantify the metabolic activity of cells within a biofilm, including those based on resazurin (also known as alamarBlueTM), fluorescein diacetate (FDA) and various tetrazolium salts like 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT), 2-(4,5-dimethyl-2-thiazolyl)-3,5-diphenyl-2H-tetrazolium bromide (MTT) and 2,3,5-triphenyl-tetrazolium chloride (TTC) [8,[12], [13], [14], [15]]. Furthermore, methods that stain specific biofilm components such as SYTO 9, which stains nucleic acids [8,16], and Wheat Germ Agglutinin (WGA), which stains the extracellular polymeric substances (EPS), are also compatible with the guideline [17].
The guideline
This guideline focuses on spectrophotometric and fluorometric measurement of biofilm grown in microplates and is divided into 5 different sections labelled 01–05 (Fig. 1). Although there may be minor differences between staining reagents and techniques, this outline is designed to follow the chronological order in which the assays are typically performed and described. Section 01 pertains to the experimental design. Here the investigators determine the research question and how they may answer it. Once the experiment is mapped out, the next step is to grow the biofilm (section 02). This step includes inoculum preparation as well as biofilm growth in the microplate. Subsequently the biofilm is typically quantified or assessed using a specific stain and this biofilm assessment method is detailed in section 03. This process allows for variations depending on the target and the stain; however, the main steps are generally the same: washing, drying, staining, elution of stain and/or measuring absorbance or fluorescence (Fig. 1). Once the reading is concluded and the data are collected, the next step is to analyse them (04). Moreover, in the interest of data sharing and communication we propose that data should be submitted to biofilm databases in the future (05).
Fig. 1.
Schematic diagram of the guideline and critical steps for spectrophotometric and fluorometric methods of biofilm assessment. Schematic diagram of the different sections of this guideline, highlighting the various critical steps that can increase variability in biofilm experiments. Different approaches to washing were illustrated to showcase how variable these can be in different protocols. (Illustration courtesy of Jill Story).
While developing the guideline, it became clear that methodological details that may be essential to achieve reproducibility of a biofilm experiment are often lacking critical information or omitted entirely. Therefore, in Table 1 we describe the most common omissions in reporting microplate methods and reflect on the potential impact of these omissions on the outcome of the experiment. At the end of each section of the guideline, we provide an example of a hypothetical simple experiment related to biofilm formation using crystal violet. An example of similar guidance for a more complex analysis, involving the effect of antibiotic exposure on biofilms, can be found as supplementary information (Table S1). Additionally, it was of vital importance to gather a group of international researchers actively working in the area of biofilms in order to provide a balanced view on what can realistically be requested of most groups reporting these methods. All researchers involved in the process are listed as authors in this article.
Table 1.
Common omissions in reporting spectrophotometric and fluorometric methods of biofilm assessment.
| Omission | Impact |
|---|---|
| References | Often papers cited as containing the protocol followed in the study do not describe the full protocol and redirect you to another paper. This can create confusion when trying to understand the protocol that was followed. |
| Replicates | The number of replicates within one experiment is not reported in the published paper. Furthermore, there are inconsistencies in the terminology used when describing replicates. For example, biological and technical replicates vs day-to-day and within experiment replicates. |
| Controls | While controls are mostly mentioned in the published articles, their values and variation are usually not reported. This makes it difficult to understand the variability associated with the method and how the raw data was processed. |
| Inoculum preparation | Different culturing methods can affect the behaviour of microorganisms, their ability to attach to a surface, formation of aggregates, and response to different stimuli, chemicals, or other microorganisms [32]. |
| Environmental factors | In dry conditions, the microplate wells easily dry out, which affects biofilm formation. Hence, investigators take precautions to avoid the problem which are usually not reported in the methodology section. |
| Position of samples in the wells | The layout of plates is often not reported, but the position of samples in the microplate can affect the results. For example, the “edge effect” is a suspected phenomenon which might be due to differences in evaporation between the outer and inner wells, as well as thermal changes in the plate. |
| Orbital shaker settings | Most papers only refer to the rpm settings on their orbital shaker and omit other details such as the orbital diameter which can affect the shear stress exerted in the wells [33]. |
| Washing | Description of this step is often omitted or vague terms such as, “gently rinse” or “slowly tip over plate” are used, which leave it up to the reader to determine how to perform the step [11,34]. |
| Drying | This step is very often omitted altogether from the method description or contains very little detail on how it was performed. |
| Raw data | Most articles do not provide their raw data and omit information on how this was analysed [35]. |
| Outliers | Outliers are very often not included in the paper or, if reported, their exclusion is simply mentioned with little argumentation for it and how the final data analysis was affected by their removal. |
| Data presentation | The most common way of presenting microplate experiment data is through bar charts. However, often they do not provide all the relevant information from a dataset (distribution, outliers, paired data relations). Hence the way data is presented can limit its interpretation. Changing to a scatter plot or a box plot can provide more details for the same dataset [19]. |
01. Experimental design
-
1.
Describe the main question to be addressed in the study. This includes proposed main (and possibly secondary) hypothesis(es).
-
2.
Explain the experimental design for the study, in other words, what type of experiment is being conducted to test the hypothesis (es)? For example, a comparison between different treatments or factors; different microbial bacterial strains (i.e. reference “type” strains, mutant constructs or clinical isolates) or different concentrations and exposure times?
-
3.
State the number of biological replicates, meaning the independent repeats of the same experiment. Ideally, these should be day-to-day replicates to account for changes in humidity and room temperatures, for example. Include the number of technical replicates within the experiment, meaning the number of replicates for each sample group in the experiment. If applicable state whether the technical replicates are within one plate or in separate ones.
-
4.
Include the number of replicates for the controls used in the experiment. Additionally, describe what these controls were and report their data to improve overall understanding of results. Depending on the test hypothesis they could be very straightforward, such as a growth check as a positive control and a sterility check as a negative control. On the other hand, they could be more complex. For example, if an antimicrobial agent is added and then rinsed off, an appropriate control is to use a mock carrier (e.g. saline) which accounts for the removal of microbes resulting from the exchange of fluids. Other appropriate controls for antimicrobial testing include solvent controls (e.g. DMSO) to verify that decrease in biofilm is due to the compound and not the solvent, and pre-treatment controls to verify that the effects observed are due to bactericidal not bacteriostatic activity. Furthermore, when biocide tests are performed it is recommended to perform a neutralizer verification, as well as checking for interactions between the microplate material, biocide (e.g. bleach) and dye. It should be noted that controls are highly dependent on the experimental design, therefore it is important to report all the relevant controls.
-
5.
When applicable, reference published protocols followed, ideally to the original articles containing all the necessary information. Additionally, if any changes were made to these published protocols they need to be described in detail.
-
6.
Provide a link to any supplementary information or data not reported in the main body of the article, such as more detailed method descriptions, a metadata sheet containing raw data and layouts of microplate designs, etc.
“This study investigated the effect of growth media concentration onStaphylococcus aureusbiofilm formation in a microplate. Total biofilm mass formation after 24 hours for four different concentrations of Tryptic Soy Broth (TSB) was compared. Each experiment consisted of one plate that used 6 sample wells per TSB concentration, and 6 negative control wells containing only TSB for all four concentrations tested. Each experiment was repeated in three independent weeks. A more detailed description of the methodology together with a schematic illustration of the sample and negative control positions within the plate can be found in our supplementary data section [Link].”
02. Biofilm formation
-
1.
Describe the microorganisms selected for the experiment. List the species and strain number, and if available the strain numbers assigned in international culture collections, e.g. ATCC, BCCM/LMG bacteria Collection, or DSMZ, or provide a reference in which the relevant details of the strains are reported. Alternatively, if clinical or environmental isolates are used, provide all available and relevant background and ethical information. Describe the stock preservation conditions, and any modifications made to the microorganism (plasmid insertions, gene knockouts, etc) using established genetic nomenclature.
-
2.
Describe the inoculum preparation protocol. Include information on incubation conditions such as concentration, growth phase, temperature, time, shaking (rpm and orbital diameter or static conditions) and growth media (ingredients, concentration, origin). Depending on the microorganism, include other applicable incubation conditions such as light, CO2 concentration, humidity, etc. Additionally, if any washing steps were performed include detailed information on centrifugation conditions (g force, time, equipment) and the washing agent used (water, PBS, etc). Other important factors might be whether a culture was grown up then diluted to a specific concentration, and how this was measured, i.e. optical density is commonly used.
-
3.
Describe the compounds or conditions being tested. In case of antimicrobials, describe their concentration (molarity, g/L or any other appropriate SI units), origin (manufacturers if purchased and catalogue numbers if allowed by the journal of choice), and time point in the experiment when they were added, and whether an agent was used to neutralize the active ingredient. If applicable, describe pH, any solvents used, activity corrections and whether agents were filtered prior to use.
-
4.
Provide information on microplates used. This includes type of plate (clear, white or black), number of wells (6, 9, 24, 96 or 384), shape of the wells (flat, rounded, U-shaped or V-shaped), the material and the manufacturing company, including catalogue numbers if allowed by the journal of choice. Report any modifications made to the manufactured microplate such as pre-coating of the wells or addition of coupons.
-
5.
Describe how the microplate was prepared. Provide information on the inoculum conditions at harvest such as growth phase, optical density (wavelength, zero solution, equipment) and concentration of microorganism (CFU/mL for bacteria or cells/mL for yeast) and growth media (if different from point 2). If a biofilm prevention experiment is being conducted provide information on the antibiofilm agent used (concentration in relevant SI units, preparation and origin).
If possible, provide a short description of the layout of the microplate showing the position of controls and samples. Additionally, if applicable mention any extra steps taken such as adding water to the outer wells to avoid “edge effects”.
-
6.
Provide a description of incubation conditions for the microplate. Include information on temperature, time and shaking (rpm and orbital diameter or stationary). Similar to point 2 include a description of any other relevant conditions such as light, CO2 concentration or humidity. Additionally, if applicable mention any extra steps such as sealing the plate with parafilm or other films or incubating within a humidified container.
“Staphylococcus aureus strain ATCC 25923TMwas used. To prepare the inoculum, -80 °C glycerol stocks were streaked out on Tryptic Soy Agar [Manufacturer] plates. One colony from the plate was transferred into 15 ml TSB and incubated at 37 °C, 125 rpm in a shaker incubator [Model number] with an orbital diameter of 1.9 cm. After 18 hours a 1:100 dilution of the inoculum was incubated at 37 °C, 125 rpm until it reached the exponential growth phase (OD=0.300 [595nm; Model number]). Four 2 ml aliquots of the suspension were made and washed by centrifugation (2000 g for 15 minutes [Model number]) and resuspending the pellets in PBS [pH 7.4; Manufacturer] twice. Subsequently, the pellets were resuspended in 4 different TSB broths (30 g/ml, 3 g/ml, 0.3 g/ml and 0.03 g/ml) and 200 μl per well of each of these suspensions was added to a flat bottom polystyrene 96 well plate [Manufacturer] according to the layout in the supplementary data. The plate was incubated at 37 °C under static conditions in a non-humidified incubator for 24 hours. To prevent excess drying the outer wells were filled with 200 μl/well of sterile water.”
03. Biofilm assessment method
-
1.
Describe the method followed to discard the planktonic suspension, e.g. pipetting, suction manifold.
-
2.
Describe all washing steps in detail. Provide information on the washing agent such as sterility, origin, concentration and pH, if applicable. Additionally, describe the number of washes and method(s) used to add and remove the washing agent (immersion, rinsing or pipetting). When possible, avoid the sole use of vague terms such as “gently” which are subject to interpretation and include more detailed descriptions. For example, describing the angle and depth at which a pipette tip was inserted into the well or stating the number of times the plate was shaken to remove excess liquid when inverted. If automatic liquid handling devices are used provide information of equipment and settings.
-
3.
Describe the staining process. This includes information on the stain: origin (manufacturer), stock and working solution concentrations, solvents used as well as information on the staining: time and incubation conditions (light, temperature, volumes, shaking etc.). If applicable, provide information on any standard curves performed with the experiment.
-
4.
In cases where extra steps such as fixation, drying and elution are required, describe how these were performed and any solvents or chemicals (origin, concentration) used.
-
5.
Describe how the spectrophotometric or fluorometric signal was measured. Provide information on the equipment (model number, company, software) used as well as its settings (excitation, emission and detection wavelengths, end-point or continuous read, shaking). When using fluorometric reading, provide information on the type of readout (top or bottom reading). If bottom reading is performed, provide information on the number and distribution of the points measured across each well.
- Furthermore, if imaging functions of the microplate reader were used, describe the settings (time, shaking, imaging mode, filters, camera).“The planktonic suspension was carefully removed using a multichannel pipette [Model, Manufacturer] fitted with a 300 μL tip inserted slowly at a 45° angle while making sure to avoid touching the sides and bottom of the wells. The plate was washed twice with 250 μl/well of PBS using a multichannel pipette fitted with a 300 μL tip and left to air-dry for 15 min in under laminar flow at room temperature (RT, 20 ± 5 °C). The biofilm was fixed for 15 min with 200 μl/well of 99% v/v ethanol [Manufacturer] and then allowed to air-dry until fully dry, between 5 and 10 minutes. The plate was stained with 200 μL of 0.1% v/v Crystal violet [Manufacturer] for 15 min at RT, under static conditions. After staining the plates were washed twice with 250 μl/well of MilliQ water using a multichannel pipette and left to air-dry for 15 min in laminar flow. The stain was eluted with 200 μl/well of 99% v/v ethanol for 30 min at RT, no shaking. The eluted stain was mixed by pipetting up and down 4 times and 100 μl/well of it were transferred to an empty 96-well plate using a multichannel pipette. The absorbance was measured at 595 nm using a [Company; Model number] plate reader.”
04. Statistical assessment and data presentation
-
1.
Describe how the raw data were processed and/or transformed. If possible, include raw data in the supplementary data section.
-
2.
Present all outliers. Argumentation should be given if they were removed from the analysis in the results and ideally how their removal affected the data.
-
3.
Test the data for normality. Report if the data has been transformed or normalised for example, using a standard curve, log transformation, square root or any other appropriate normalisation method.
-
4.
Describe statistical tests and rationale for use (i.e. parametric, non-parametric, small sample, paired etc.) performed and any post-hoc tests. Provide information on the test parameters, descriptive statistics such as significant differences, standard errors, standard deviation, variance and confidence intervals. Additionally, include descriptive statistics for the controls used in the experiment. If a high-throughput screening assay is being reported, it is recommended to include the calculation of the screening windows coefficient, or Z’ [18].
-
5.
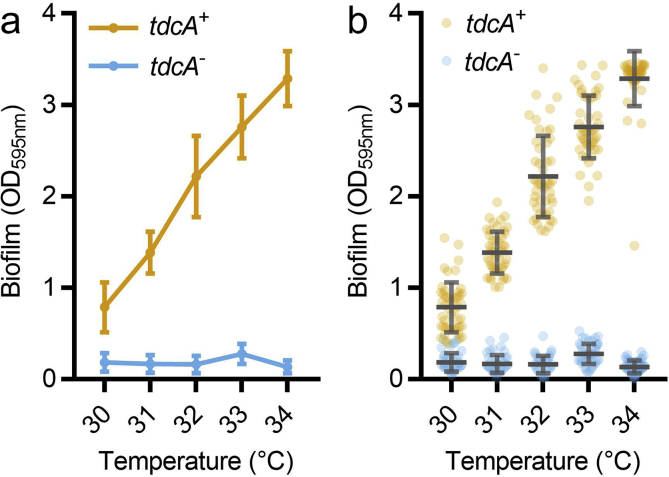
Ensure that the appropriate graph types and data visualizations are used. Figures should provide all the essential and relevant information necessary for a full understanding of the results [19]. We suggest the use of scatter plots or box and whisker plots instead of line graphs or bar charts, which often do not portray all the necessary information in a dataset (Fig. 2). For instance, many different normal, skewed or bimodal data distributions can lead to the same mean and standard deviation values [19]. Summarizing data as a mean with standard deviation can also conceal unequal sample sizes and outliers [19,20]. Plotting all measurements in tandem with means and standard deviations provides transparency and allows readers to evaluate data for themselves (Fig. 2).
-
6.
Provide details of the statistical package used and its version. If more than one was used, they all need to be mentioned. Additionally, if any open source systems such as R packages were used, provide a reference or a link to it.
“Raw absorbance data can be found in our supplementary data section. To evaluate the within plate variability, the mean ± 1 standard deviation (STDEV) of all the technical replicates for each sample were calculated and are summarised in table [1]. The means of all the different samples were corrected by subtracting the corresponding negative control (TSB only) values. The data from all three replicate experiments were analysed using a one-way ANOVA test with a Levene’s post-hoc analysis to compare the absorbance values. These results were represented inFig. 1and a more detailed description can be found in the figure legend. [Statistical Programme; version] was used to perform all tests.”
Fig. 2.
Show the dots on plots: scatter graphs allow readers to evaluate data distributions for themselves. Biofilm formation was measured for Pseudomonas aeruginosa strains CF39S and CF39, which express functional and mutant alleles of the thermosensory diguanylate cyclase (tdcA+ and tdcA−), respectively. Each condition has 48 replicates, representing sixteen technical replicates from each of three independent biological replicates. (a) Line graph. Datum points represent means and standard deviations. (b) Scatter plot. Each point denotes a replicate datum point and lines and bars represent means and standard deviations, respectively. (Data courtesy of Joe J. Harrison).
05. Bioinformatics (optional)
-
1.
Use standard terminology. In the coming years ontology guidelines for biofilm terminology are expected to be developed. A starting guide can be found on the MIABiE website [21].
-
2.
The data should be formatted in a way that makes it easier to submit and extrapolate it to existing databases such as BiofOmics (http://www.biofomics.org/) or other databases currently in development [22].
As illustrated above, the amount of information necessary to fully characterise a complex system such as a biofilm experiment is significant. Therefore, a simplified checklist of the guideline has been included in this paper (Table 2). This checklist can assist authors during their writing process as well as reviewers during the peer-review process. In fact, complementary fields such as ecology and evolution have very recently started to make checklists available in their field of knowledge [23]. Moreover, certain sections of this guideline can be applied to other biofilm assessment methods in microplate experiments, such as when viable plate counts are used to assess biofilm density and treatment efficacy.
Table 2.
Simplified checklist for minimum information guideline spectrophotometric methods of biofilm assessment.
| 01. Experimental design | |
| Aim of the experiment/hypothesis presented | ✓ |
| Type of experiment | |
| Biological and technical replicates | |
| Control replicates and descriptions | |
| Reference to original article containing protocol (If applicable) | |
| Supplementary information (If applicable) |
|
| 02. Biofilm formation | |
| Microorganism description | |
| Inoculum preparation protocol | |
| Treatment description (If applicable) | |
| Microplate description | |
| Plate layout i.e. sample distribution (Optional) | |
| Incubation conditions for microplate |
|
| 03. Biofilm assessment method | |
| Planktonic suspension removal | |
| Washing description | |
| Staining description | |
| Additional steps: fixing, drying, buffer solutions (if applicable) | |
| Absorbance/Fluorescence measurement |
|
| 04. Statistical assessment and data interpretation | |
| Raw data handling | |
| Outliers | |
| Normality testing | |
| Appropriate data presentation | |
| Statistical test with post-hoc and descriptive stats | |
| Statistical programme used |
|
| 05. Bioinformatics (Optional) | |
| Standardised terminology | |
| Data formatting according to data submission guidelines | |
| Submission to online database | |
Discussion
Microplate-based spectrophotometric and fluorometric methods of biofilm assessment have led to the generation of a vast amount of data throughout the years. However, while these data have provided essential information on biofilm biology and experimental therapeutic strategies to tackle biofilms, biofilm experiments have often been difficult to reproduce. Furthermore, most of the time it is not possible to compare data between studies, which means that attempting to draw conclusions by combining data from different studies is not feasible. To minimize this problem, we suggest that a minimum information guideline should be adopted by researchers.
Lack of data comparability can in part be attributed to the high variability of protocols used for these types of methods. Table 3 illustrates this phenomenon of variability in protocols of the crystal violet assay for three common organisms: Staphylococcus aureus, Pseudomonas aeruginosa and Candida albicans. It contains the range of parameters (low to high) for different conditions of inoculum preparation, biofilm growth and biofilm assessment for each microorganism. Major differences in the inoculum preparation and biofilm growth parameters, are expected as the parameters of these steps are largely dictated by the physiology of the microorganism being investigated and the type of experiment being performed. However, Table 3 shows that large differences are also present among the biofilm assessment parameters such as dye concentrations and absorbance wavelengths. Taken together, this information means that comparing different datasets at this stage is not possible for different studies and that the guidelines can only facilitate reproducibility and comparison to a certain degree. On the other hand, it is important to note that the variability in protocols used in the biofilm area is often due to the differences in the subject of the investigations.
Table 3.
Example of the variability in protocol conditions of crystal violet assays for three different example microorganisms.
| Condition | Organism |
||
|---|---|---|---|
| Staphylococcus aureus spp. | Pseudomonas aeruginosa spp. | Candida albicans | |
| Inoculum preparation | |||
| Media | TSB, TSB wS*, LBb*, Water [8,11,18,[36], [37], [38], [39], [40], [41]] | TSB, TSB wS*, LBb*, LB*, BHI*, MHI*, T-broth*, AB* [8,[42], [43], [44], [45], [46], [47], [48], [49]] | YNB*, YPD*, RPMI-1640*, SDB* [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Inoculum incubation temperature (°C) | 35-37 [8,11,18,[36], [37], [38], [39], [40], [41]] | 25-37 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 30-37 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Incubation time (hours) | 0**-24 [8,11,18,[36], [37], [38], [39], [40], [41]] | 0**-24 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 12-24 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Inoculum shaking conditions | 0–200 rpm/min [8,11,18,[36], [37], [38], [39], [40], [41]] | 0–250 rpm/min [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 0–200 rpm/min, Roller drum [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Inoculum concentration/OD/growth phase at harvest | 103–108 CFU/mL, 0.5 McFarland, OD600nm=0.1 [8,11,18,[36], [37], [38], [39], [40], [41]] | 10–108 CFU/mL, OD600nm=0.0025, OD595=1.5 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 104–108 CFU/mL, OD600nm=1 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Biofilm growth | |||
| Media | TSB, LB*, BHI* [8,11,18,[36], [37], [38], [39], [40], [41]] | TSB, T-broth*, AB*, BHI*, MHI* [8,[42], [43], [44], [45], [46], [47], [48], [49]] | YNB*, YPD*, RPMI-1640*, ASM*, SDB*, PBS* [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Incubation temperature (°C) | 35-37 [8,11,18,[36], [37], [38], [39], [40], [41]] | 25-37 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 37 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Incubation time (hours) | 18-48 [8,11,18,[36], [37], [38], [39], [40], [41]] | 2-48 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 2-48 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Shaking conditions | 0–200 rpm/min [8,11,18,[36], [37], [38], [39], [40], [41]] | 0–180 rpm/min [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 0–120 rpm/min [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Biofilm Assessment | |||
| Washing agent | Water, Saline, PBS*, MilliQ water [8,11,18,[36], [37], [38], [39], [40], [41]] | Saline, Water, PBS* [8,[42], [43], [44], [45], [46], [47], [48], [49]] | PBS*, Water, Saline [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Washing (x times) | 1-3 [8,11,18,[36], [37], [38], [39], [40], [41]] | 1-3 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 1-3 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Crystal violet concentration | 0.01–2.3% [8,11,18,[36], [37], [38], [39], [40], [41]] | 0.1–2% [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 0.02–1% [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Staining time | 1–20 min [8,11,18,[36], [37], [38], [39], [40], [41]] | 5–30 min [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 5–45 min [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Solubilisation agent | 33% acetic acid, 95–100% ethanol [8,11,18,[36], [37], [38], [39], [40], [41]] | 30–33% acetic acid, 95–100% ethanol, DMSO* [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 30–33% acetic acid, 95% ethanol, 0.1% Triton-X [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
| Absorbance wavelength (nm) | 540-595 [8,11,18,[36], [37], [38], [39], [40], [41]] | 550-595 [8,[42], [43], [44], [45], [46], [47], [48], [49]] | 540-595 [8,[50], [51], [52], [53], [54], [55], [56], [57]] |
*wS - with Supplement (i.e. added yeast and/or glucose); TSB- Tryptic Soy Broth; LBb – Luria Bertani broth; BHI- Brain Heart Infusion; LB – Lysogeny broth; MHI – Mueller-Hinton broth; T-broth – Terrific broth; AB – minimal growth media; YNB – Yeast Nitrogen Base; YPD – Yeast Peptone Dextrose; SDB – Sabauraund Dextrose Broth; RPMI-1640 - Roswell Park Memorial Institute–1640 medium; ASM – Artificial Saliva Medium; PBS - Phosphate buffered saline; DMSO - Dimethyl sulfoxide.
**0 – Inoculum prepared directly from agar culture.
Hence, a consensus regarding certain aspects of the methodology is necessary to improve reproducibility. On this matter, there are already standardised biofilm methods approved by the American Society for Testing and Materials (ASTM) which could serve as a starting point for this process, such as the E2647-08 Standard Test Method for Quantification of a Pseudomonas aeruginosa Biofilm Grown Using a Drip Flow Biofilm Reactor with Low Shear and Continuous Flow [24], the E2562-17 Standard Test Method for Quantification of Pseudomonas aeruginosa Biofilm Grown with High Shear and Continuous Flow using CDC Biofilm Reactor [25] and the E2799-17 Standard Test Method for Testing Disinfectant Efficacy against Pseudomonas aeruginosa Biofilm using the MBEC Assay [26]. They can also provide an excellent example on how to identify critical steps and describe the methodology in order to ensure reproducibility.
In addition, many investigations aim at optimising and modifying spectrophotometric and fluorometric methods to increase their efficiency, reliability and their applications. For example, Skogman et al. proposed the use of consecutive staining with resazurin, WGA and crystal violet to improve the assessment of antimicrobial effectivity against biofilms [17]. More recently, Junka et al., developed a way to assess wound dressing effectiveness in 24-well plates using crystal violet and TTC analysis [27]. This means that with time, as new steps are introduced or more robust ways of performing certain steps are developed, the methods will evolve. Minimum information guidelines have the advantage of remaining applicable to the methods despite these changes.
As science evolves, we will be able to measure new parameters and conditions which affect reproducibility. For example, even when manufactured from the same base polymer, microplates can have different surface properties depending on the production process, resulting in differences in cell adhesion [28]. As the methods to characterise surface properties become more accessible, parameters such as surface roughness might be used in future. Since guidelines are often part of an online database such as MIBBI, they can be updated when necessary and evolve together with the methods.
As is the case with compliance to MIABiE and other guidelines, compliance to the new guideline presented here will be difficult as it needs to be endorsed by both authors and journals [29,30]. To improve compliance a balance needs to be obtained between the level of detail asked, and the ability of most labs to be able to provide such data. As an example, many studies have shown that oxygen availability influences biofilm formation and can lead to different physiological features being expressed [31]. Therefore, understanding the oxygen availability within a well and across different wells in a microplate might be useful. However, most laboratories lack the kind of system needed to assess this environmental parameter and it would be very difficult to implement this reading routinely. Hence, the oxygen profile within the microplate is not a requirement in the guideline.
We are convinced that the implementation of minimum information guidelines will contribute to solving the reproducibility crisis and thus improve the use that the research community makes of data and ultimately advance science.
Methodology
To create the minimum information guideline, we conducted a literature review using three different databases: Pubmed, Google Scholar and Web of Science. The research was separated into literature related to the methods and literature related to biofilm properties and the various factors affecting them. For the former, very broad search terms such as, “Biofilm AND microtit* plate”, “Biofilm AND Spectro*” and “Biofilm AND Fluor*” were used as a starting point. These resulted in thousands of hits from all three databases, and to further refine this output more specific terms such as “Crystal violet”, “Resazurin Or Alamar Blue”, “XTT”, “TTC”, “MTT”, “FDA”, “Syto9” and “WGA” were used. The results were ordered according to number of citations (most to least) and publishing date (newest to oldest). 180 papers were selected to be used as references to write the guideline. These were categorised into papers evaluating the methods and highlighting critical factors or steps, and papers that used the method in a specific investigation. The latter were used to create an understanding of what is commonly reported in scientific articles. Approximately 30 of the papers in this category were discarded from the literature review, as the only description of the method was a reference to a previously published paper.
When researching the literature on biofilm properties and what affects them, terms such as “impact”, “influence”, “effect or affect”, “changes or differences” were used. These helped in creating an understanding of the different parameters that should be reported for a biofilm experiment. Additionally, other minimum information guidelines were used as templates in the initial drafting process.
The final guidelines are the result of a dialog among biofilm experts familiar with microplate methods. These experts are included in the authors list and contributed throughout the drafting process of the manuscript.
Declaration of competing interest
The authors declare no competing interests.
Acknowledgements
We would like to thank Prof. Dr. Mark E. Shirtliff for his contribution in the development of this guideline and the review of early manuscript drafts prior to his untimely death. We would also like to thank Jill Story (Montana State University, Center for Biofilm Engineering) for designing Fig. 1.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under the Marie Sklodowska – Curie grant agreement No 722467, as part of the Print-Aid consortium. The information and views set out in this article are those of the authors and do not necessarily reflect the official opinion of the European Union. Neither the European Union institutions and bodies nor any person acting on their behalf may be held responsible for the use which may be made of the information contained therein.
This work received additional financial support by: project UID/EQU/00511/2019 - Laboratory for Process Engineering, Environment, Biotechnology and Energy – LEPABE funded by national funds through FCT/MCTES (PIDDAC); Project “LEPABE-2-ECO-INNOVATION” – NORTE-01-0145-FEDER-000005, funded by Norte Portugal Regional Operational Programme (NORTE 2020), under PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioflm.2019.100010.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Baker M. Reproducibility crisis? Nature. 2016;533:26. [Google Scholar]
- 2.Dirnagl U. Rethinking research reproducibility. EMBO J. 2019;38 doi: 10.15252/embj.2018101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grimes D.R., Bauch C.T., Ioannidis J.P. Modelling science trustworthiness under publish or perish pressure. R. Soc. Open Sci. 2018;5:171511. doi: 10.1098/rsos.171511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taylor C.F. Promoting coherent minimum reporting guidelines for biological and biomedical investigations: the MIBBI project. Nat Biotechnol. 2008;26:889–896. doi: 10.1038/nbt.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anon A checklist for our community. Nat. Ecol. Evol. 2018;2:913. doi: 10.1038/s41559-018-0574-7. [DOI] [PubMed] [Google Scholar]
- 6.Lourenço A. Minimum information about a biofilm experiment (MIABiE): standards for reporting experiments and data on sessile microbial communities living at interfaces. Pathog. Dis. 2014;70:250–256. doi: 10.1111/2049-632X.12146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall-Stoodley L., Costerton J.W., Stoodley P. Bacterial biofilms: from the Natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 8.Peeters E., Nelis H.J., Coenye T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods. 2008;72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Lopes S.P., Azevedo N.F., Pereira M.O. Emergent bacteria in cystic fibrosis: in vitro biofilm formation and resilience under variable oxygen conditions. BioMed Res Int. 2014;2014 doi: 10.1155/2014/678301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machado I., Graça J., Sousa A.M., Lopes S.P., Pereira M.O. Effect of antimicrobial residues on early adhesion and biofilm formation by wild-type and benzalkonium chloride-adapted Pseudomonas aeruginosa. Biofouling. 2011;27:1151–1159. doi: 10.1080/08927014.2011.636148. [DOI] [PubMed] [Google Scholar]
- 11.Stepanović S. Quantification of biofilm in microtiter plates: overview of testing conditions and practical recommendations for assessment of biofilm production by staphylococci. APMIS. 2007;115:891–899. doi: 10.1111/j.1600-0463.2007.apm_630.x. [DOI] [PubMed] [Google Scholar]
- 12.Alonso B., Cruces R., Pérez A., Sánchez-Carrillo C., Guembe M. Comparison of the XTT and resazurin assays for quantification of the metabolic activity of Staphylococcus aureus biofilm. J Microbiol Methods. 2017;139:135–137. doi: 10.1016/j.mimet.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 13.van Hengel I.A.J. Selective laser melting porous metallic implants with immobilized silver nanoparticles kill and prevent biofilm formation by methicillin-resistant Staphylococcus aureus. Biomaterials. 2017;140:1–15. doi: 10.1016/j.biomaterials.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 14.Honraet K., Goetghebeur E., Nelis H.J. Comparison of three assays for the quantification of Candida biomass in suspension and CDC reactor grown biofilms. J Microbiol Methods. 2005;63:287–295. doi: 10.1016/j.mimet.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Tunney M.M., Ramage G., Field T.R., Moriarty T.F., Storey D.G. Rapid colorimetric assay for antimicrobial susceptibility testing of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2004;48:1879–1881. doi: 10.1128/AAC.48.5.1879-1881.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stiefel P., Schmidt-Emrich S., Maniura-Weber K., Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015;15:36. doi: 10.1186/s12866-015-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skogman M.E., Vuorela P.M., Fallarero A. Combining biofilm matrix measurements with biomass and viability assays in susceptibility assessments of antimicrobials against Staphylococcus aureus biofilms. J Antibiot (Tokyo) 2012;65:453. doi: 10.1038/ja.2012.49. [DOI] [PubMed] [Google Scholar]
- 18.Sandberg M., Määttänen A., Peltonen J., Vuorela P.M., Fallarero A. Automating a 96-well microtitre plate model for Staphylococcus aureus biofilms: an approach to screening of natural antimicrobial compounds. Int J Antimicrob Agents. 2008;32:233–240. doi: 10.1016/j.ijantimicag.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 19.Weissgerber T.L., Milic N.M., Winham S.J., Garovic V.D. Beyond bar and line graphs: time for a new data presentation paradigm. PLoS Biol. 2015;13 doi: 10.1371/journal.pbio.1002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anon Show the dots in plots. Nat. Biomed. Eng. 2017;1:0079. [Google Scholar]
- 21.Sousa A.M., Pereira M.O., Azevedo N.F., Lourenço A. 8th international Conference on practical Applications of computational biology & bioinformatics (PACBB 2014) Springer; Cham: 2014. Designing an ontology tool for the unification of biofilms data; pp. 41–48. [DOI] [Google Scholar]
- 22.Lourenço A. BiofOmics: a web platform for the systematic and standardized collection of high-throughput biofilm data. PLoS One. 2012;7 doi: 10.1371/journal.pone.0039960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parker T.H. Empowering peer reviewers with a checklist to improve transparency. Nat. Ecol. Evol. 2018;2:929–935. doi: 10.1038/s41559-018-0545-z. [DOI] [PubMed] [Google Scholar]
- 24.ASTM E2647-08 . ASTM Int.; West Conshohocken PA: 2008. Standard test method for quantification of a Pseudomonas aeruginosa biofilm grown using a Drip flow biofilm reactor with low shear and continuous flow. [Google Scholar]
- 25.ASTM E2562-17 . ASTM Int. West Conshohocken PA; 2017. Standard test method for quantification of Pseudomonas aeruginosa biofilm grown with high shear and continuous flow using CDC biofilm reactor. [Google Scholar]
- 26.ASTM E2799-17 . ASTM Int. West Conshohocken PA; 2017. Standard test method for testing disinfectant efficacy against Pseudomonas aeruginosa biofilm using the MBEC assay. [Google Scholar]
- 27.Junka AF, Zywicka A, Szymczk, Dziadas M., Bartoszewicz M, Fijalkowski K. A.D.A.M. test (Antibiofilm Dressing’s Activity Measurement) — simple method for evaluating anti-biofilm activity of drug-saturated dressings against wound pathogens. J Microbiol Methods. 2017;143:6–12. doi: 10.1016/j.mimet.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 28.Zeiger A.S., Hinton B., Van Vliet K.J. Why the dish makes a difference: quantitative comparison of polystyrene culture surfaces. Acta Biomater. 2013;9:7354–7361. doi: 10.1016/j.actbio.2013.02.035. [DOI] [PubMed] [Google Scholar]
- 29.Deutsch E.W. Minimum information specification for in situ hybridization and immunohistochemistry experiments (MISFISHIE) Nat Biotechnol. 2008;26:305–312. doi: 10.1038/nbt1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anon. Time for leadership. Nat Biotechnol. 2007;25:821. doi: 10.1038/nbt0807-821. [DOI] [PubMed] [Google Scholar]
- 31.Xu K.D., Stewart P.S., Xia F., Huang C.-T., Mcfeters G.A. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl Environ Microbiol. 1998;64:5. doi: 10.1128/aem.64.10.4035-4039.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kragh K.N. The inoculation method could impact the outcome of microbiological experiments. Appl Environ Microbiol. 2018;84 doi: 10.1128/AEM.02264-17. e02264–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Azevedo N.F., Pinto A.R., Reis N.M., Vieira M.J., Keevil C.W. Shear stress, temperature, and inoculation concentration influence the adhesion of water-stressed Helicobacter pylori to stainless steel 304 and polypropylene. Appl Environ Microbiol. 2006;72:2936–2941. doi: 10.1128/AEM.72.4.2936-2941.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Erriu M. Microtiter spectrophotometric biofilm production assay analyzed with metrological methods and uncertainty evaluation. Measurement. 2012;45:1083–1088. [Google Scholar]
- 35.Anon Standardizing data. Nat Cell Biol. 2008;10:1123–1124. doi: 10.1038/ncb1008-1123. [DOI] [PubMed] [Google Scholar]
- 36.Stepanović S., Vuković D., Ježek P., Pavlović M., Švabic-Vlahović M. Influence of dynamic conditions on biofilm formation by staphylococci. Eur J Clin Microbiol Infect Dis. 2001;20:0502–0504. doi: 10.1007/s100960100534. [DOI] [PubMed] [Google Scholar]
- 37.Izano E.A., Amarante M.A., Kher W.B., Kaplan J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zmantar T., Kouidhi B., Miladi H., Mahdouani K., Bakhrouf A. A Microtiter plate assay for Staphylococcus aureus biofilm quantification at various pH levels and hydrogen peroxide supplementation. The New Microbiologica. 2010;33(2):137. [PubMed] [Google Scholar]
- 39.Xu Z. Crystal violet and XTT assays on Staphylococcus aureus biofilm quantification. Curr Microbiol. 2016;73:474–482. doi: 10.1007/s00284-016-1081-1. [DOI] [PubMed] [Google Scholar]
- 40.Mohanty S. An investigation on the antibacterial, cytotoxic, and antibiofilm efficacy of starch-stabilized silver nanoparticles. Nanomed Nanotechnol Biol Med. 2012;8:916–924. doi: 10.1016/j.nano.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Kaplan J.B. Low levels of beta-lactam antibiotics induce extracellular DNA release and biofilm formation in Staphylococcus aureus. mBio. 2012;3 doi: 10.1128/mBio.00198-12. e00198-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez C.J. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infect Dis. 2013;13:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim H.-S., Park H.-D. Ginger extract inhibits biofilm formation by Pseudomonas aeruginosa PA14. PLoS One. 2013;8 doi: 10.1371/journal.pone.0076106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palanisamy N.K. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J Nanobiotechnol. 2014;12:2. doi: 10.1186/1477-3155-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Friedman L., Kolter R. Two genetic loci produce distinct carbohydrate-rich structural components of the Pseudomonas aeruginosa biofilm matrix. J Bacteriol. 2004;186:4457–4465. doi: 10.1128/JB.186.14.4457-4465.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabaeifard P., Abdi-Ali A., Soudi M.R., Dinarvand R. Optimization of tetrazolium salt assay for Pseudomonas aeruginosa biofilm using microtiter plate method. J Microbiol Methods. 2014;105:134–140. doi: 10.1016/j.mimet.2014.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Jagani S., Chelikani R., Kim D.-S. Effects of phenol and natural phenolic compounds on biofilm formation by Pseudomonas aeruginosa. Biofouling. 2009;25:321–324. doi: 10.1080/08927010802660854. [DOI] [PubMed] [Google Scholar]
- 48.O’Toole G.A. Microtiter dish biofilm formation assay. J Vis Exp JoVE. 2011;47 doi: 10.3791/2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bauer J., Siala W., Tulkens P.M., Van Bambeke F. A combined pharmacodynamic quantitative and qualitative model reveals the potent activity of daptomycin and delafloxacin against Staphylococcus aureus biofilms. Antimicrob Agents Chemother. 2013;57:2726–2737. doi: 10.1128/AAC.00181-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marcos-Zambrano L.J., Escribano P., Bouza E., Guinea J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: comparison of biomass production and metabolic activity and development of cut-off points. Int J Med Microbiol. 2014;304:1192–1198. doi: 10.1016/j.ijmm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 51.Negri M. Crystal violet staining to quantify Candida adhesion to epithelial cells. Br J Biomed Sci. 2010;67:120–125. doi: 10.1080/09674845.2010.11730308. [DOI] [PubMed] [Google Scholar]
- 52.Monteiro D.R. Silver colloidal nanoparticles: antifungal effect against adhered cells and biofilms of Candida albicans and Candida glabrata. Biofouling. 2011;27:711–719. doi: 10.1080/08927014.2011.599101. [DOI] [PubMed] [Google Scholar]
- 53.Li Z., Sun J., Lan J., Qi Q. Effect of a denture base acrylic resin containing silver nanoparticles on Candida albicans adhesion and biofilm formation. Gerodontology. 2016;33:209–216. doi: 10.1111/ger.12142. [DOI] [PubMed] [Google Scholar]
- 54.Deveau A., Hogan D.A. Linking quorum sensing regulation and biofilm formation by Candida albicans. In: Rumbaugh K.P., editor. vols. 219–233. Humana Press; 2011. (Quorum sensing: methods and protocols). [DOI] [PubMed] [Google Scholar]
- 55.Dovigo L.N. Susceptibility of clinical isolates of Candida to photodynamic effects of curcumin. Lasers Surg Med. 2011;43:927–934. doi: 10.1002/lsm.21110. [DOI] [PubMed] [Google Scholar]
- 56.Khan S., Alam F., Azam A., Khan A.U. Gold nanoparticles enhance methylene blue–induced photodynamic therapy: a novel therapeutic approach to inhibit Candida albicans biofilm. Int J Nanomed. 2012;7:3245–3257. doi: 10.2147/IJN.S31219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li X., Yan Z., Xu J. Quantitative variation of biofilms among strains in natural populations of Candida albicans. Microbiology. 2003;149:353–362. doi: 10.1099/mic.0.25932-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.