Abstract
Pituitary apoplexy is a medical and surgical emergency caused by hemorrhage or infarction of the hypophysis, which typically occurs within a pituitary adenoma. It is usually characterized by severe (often thunderclap) headache, visual disturbances, cranial nerve impairments, and hormonal deficiencies. We herein report a case of a previously healthy woman with severe acute respiratory syndrome coronavirus 2 infection associated with pituitary apoplexy. The plausible pathophysiological mechanisms of pituitary apoplexy in infectious coronavirus disease 2019 are discussed.
Keywords: COVID-19, SARS-CoV-2, pituitary apoplexy
In approximately 2% to 12% of pituitary adenomas (mostly nonfunctional), sudden hemorrhagic or infarction may occur, giving rise to a life-endangering medical and surgical emergency known as pituitary apoplexy [1]. The clinical spectrum encompasses severe (often thunderclap) headache, visual disturbances, cranial nerve impairments, and hormonal deficiencies [1]. Although it is a recognized entity, its pathophysiology is poorly understood.
Hemorrhagic viral fevers have long been associated with hypopituitarism, the majority being orthohantavirus and dengue infection [2, 3]. In the case of orthohantavirus infection, ischemic damage to pituitary could be caused by hypotension and/or vasospasms during the acute phase, while thrombocytopenia, thrombopathy, and other coagulation disorders can cause hemorrhage [2]. Further, hypophysitis due to the presence of autoantibodies has been suggested [2]. Leow et al [4] reported postinfectious hypophysitis with related transient hypocortisolism in patients who survived the previous severe acute respiratory syndrome (SARS) epidemic. Chan et al [5] have recently reported a case of pituitary apoplexy associated with a third-trimester pregnancy complicated by infectious coronavirus disease 2019 (COVID-19). A few other cases [6-8] have also been reported thus far (Table 1). However, in none of these cases [5-8] have the authors suggested a causal relationship between pituitary apoplexy and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Interestingly, one of the cases had a spontaneous involution of the sellar mass [8].
Table 1.
Reported cases of pituitary apoplexy associated with severe acute respiratory syndrome coronavirus 2 infection
| Authors | Patient information | Chief complaints | Diagnosis | Treatment/Procedures | Outcome |
|---|---|---|---|---|---|
| Chan et al [5] | Female, pregnant, 28 y | Blurry vision in left eye, mild headache and left ear pain | Pituitary adenoma with apoplexy | Dexamethasone, endoscopic transsphenoidal surgery | Complete recovery of pupillary defect and visual disturbances |
| Solorio-Pineda et al [6] | Male, 27 y | Progressive decrease in visual acuity in both eyes and left exotropia | Pituitary macroadenoma apoplexy and atypical pneumonia | Anticoagulants, dopamine agonist, cardiac surgery, and pituitary dynamic testing | Death 12 h after admission |
| Santos et al [7] | Male, 47 y | Left frontal headache, diplopia, left eye ptosis, and left visual acuity loss | Pituitary macroadenoma with central hemosiderin deposition | Ibuprofen and transsphenoidal pituitary tumor resection | Recovery |
| Bray et al [8] | Female, 28 y | Severe headache | Pituitary adenoma with possible antecedent apoplexy | Cortisol replacement | Spontaneous resolution of the adenoma |
We herein report a case of a previously asymptomatic woman with SARS-CoV-2 infection associated with pituitary apoplexy. Plausible pathophysiological mechanisms of pituitary apoplexy in COVID-19 infection are discussed.
Case Report
A 44-year-old previously healthy woman from rural India was admitted to the emergency department with complaints of a sudden-onset severe headache along with projectile vomiting for 3 days and progressive asymmetric visual blurriness for the last 2 days. These symptoms were preceded by abrupt-onset intermittent fever (temperature 100.7 °F [38.2 °C] on an oral thermometer) 6 days prior, which subsided on its own within the next 2 days. She did not have a cough or a change in sensation in taste or smell. On physical examination, she was conscious and cooperative, but less attentive and was in tremendous pain. Cognitive and cranial nerve functions were intact except for subtly asymmetric bitemporal hemianopic visual field defects. Pupils were equal and reacted normally to light and accommodation. All other neurological and systemic examinations were normal. The differential diagnoses considered were a) subarachnoid hemorrhage due to an aneurysmal rupture of the anterior communicating artery; b) sudden vascular catastrophe within a preexisting tumor, located adjacent to the midoptic chiasm (craniopharyngioma, macroadenoma, or meningioma); c) cerebral venous sinus thrombosis, especially involving the cavernous sinuses; d) pituitary apoplexy; e) infective meningoencephalitis or abscess adjacent to the chiasm; f) inflammatory granuloma in the vicinity of the chiasm; g) chiasmal neuritis following primary demyelinating disorders (neuromyelitis optica spectrum disorder or multiple sclerosis); and h) autoimmune vasculitis involving the chiasm or adjacent structures.
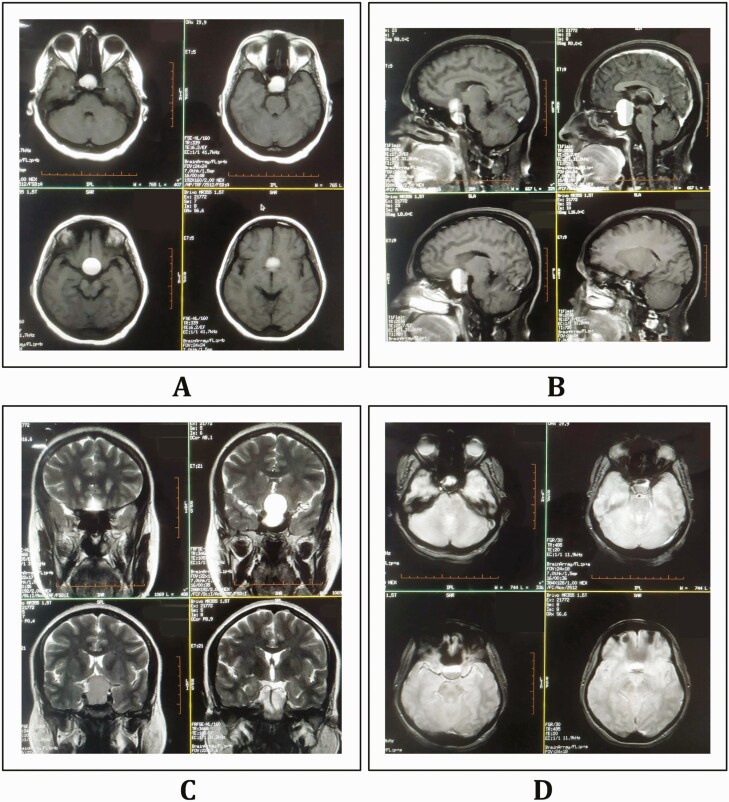
Because a pandemic is ongoing and the patient had a history of febrile illness before presentation, a nasopharyngeal swab test for SARS-CoV-2 by quantitative real-time reverse transcriptase–polymerase chain reaction (RT-PCR) assay was performed and confirmed positive. Complete hemogram revealed thrombocytopenia (85 000/µL, normal 150 000-400 000/µL), raised erythrocyte sedimentation rate (53 mm/hour), and a normal neutrophil to lymphocyte ratio. Renal, liver, and thyroid functions were normal except mild hyponatremia (serum Na+ 126 mEq/L). Glycated hemoglobin A1c was normal and capillary blood glucose was 69 mg/dL. C-reactive protein was 86 mg/L (normal, 3.0-10.0 mg/L); meanwhile, ferritin and D-dimer were minimally elevated. Contrast-enhanced magnetic resonance imaging of the brain revealed a well-defined “snowman-shaped” large heterogeneous solid-cystic lesion in the suprasellar region (measuring 24 × 25 × 31 mm) with fluid-fluid levels and a predominantly cystic component (Fig. 1). The cystic component showed various signal intensities on T1-weighted, T2-weighted, and T2–fluid-attenuated inversion recovery (FLAIR)-weighted images. Foci of blooming on gradient-echo images were observed within the lesion (more on the anterior aspect of the fluid-fluid level) without any diffusion restriction. The optic chiasma was compressed by the superior extension of the lesion. Neuroimaging features were suggestive of pituitary macroadenoma with hemorrhage. The endocrine workup demonstrated a low baseline serum cortisol at 8 am of 2.0 µg/dL (normal, 4.3-22.4 µg/dL) and reduced corresponding plasma adrenocorticotropin (3.6 pg/mL; normal, 6-50 pg/mL). A cosyntropin stimulation test was performed with the utmost care and strict monitoring (because any dynamic pituitary test may further precipitate or aggravate apoplexy). It showed a significant increment in serum levels of cortisol (20.0 µg/dL and 25.2 µg/dL, after 30 and 60 minutes, respectively). Serum thyrotropin (2.43 mIU/L; normal, 0.35-5.50 mIU/L), free 3,5,3′-triiodothyronine (3.0 pg/mL; normal 2.3-4.2 pg/mL) and thyroxine (1.50 ng/dL; normal, 0.89-1.76 ng/dL) levels, follicle-stimulating hormone (2.7 mIU/mL; normal, 1.5-9.1 mIU/mL), luteinizing hormone (7.8 IU/L; normal, 0.5-16.9 IU/L), prolactin (22 ng/mL; normal, 2.8-29.2 ng/mL), and insulin-like growth factor 1 (84 ng/mL; normal, 69-253 ng/mL) and insulin-like growth factor binding protein levels (4.60 µg/mL; normal, 3.30-6.60 µg/mL) were otherwise normal. Plasma and urine osmolarity throughout the hospital stay was normal. Arterial blood gas analysis was within the normal range. A high-resolution computed tomography scan of the thorax revealed no abnormalities. The presence of low blood glucose, hyponatremia in a backdrop of pituitary hemorrhage, hypocortisolism, and a positive cosyntropin test led to a working diagnosis of pituitary apoplexy with pituitary insufficiency. The patient was put on intravenous dexamethasone (24 mg/day in 3 divided doses), clonazepam (2 mg/day), intravenous normal saline (0.9%), acetaminophen infusion for analgesia, and broad-spectrum β-lactam antibiotics. The patient and her caregivers refused surgical intervention during this period. Fortunately, her blood pressure, fluid and electrolyte status, plasma glucose, and plasma and urine osmolarity levels remained stable throughout the hospital stay with conservative management. Headache, vomiting, and visual blurriness decreased subjectively, but bitemporal hemianopia remained. On day 4, oral hydrocortisone was initiated (20 mg at 7 am and 10 mg at 3-4 pm) along with discontinuation of dexamethasone. Because the chiasmal compression persisted, she was offered neurosurgical consultation once more, but she denied, and hence she was being periodically followed up every month with strict monitoring of vital parameters, plasma and urine osmolarity, and electrolytes and plasma glucose levels. At the second month of follow-up, her vital parameters and laboratory parameters were stable and the hemianopia improved. However, she was diagnosed with secondary hypothyroidism. At present, she is on hydrocortisone (15 mg/day in 2 divided doses) and levothyroxine (75 µg/day) supplementation and kept under close follow-up with regular endocrine workup to detect any evidence of late-onset panhypopituitarism.
Figure 1.
Contrast-enhanced magnetic resonance imaging of the brain revealing a well-defined “snowman-shaped” large heterogeneous solid-cystic lesion in the suprasellar region (measuring 24 × 25 × 31 mm) with fluid-fluid levels and predominantly cystic component. The cystic component showed various signal intensities both on T1-weighted (A, axial and B, sagittal views) and T2-weighted (C, coronal view) images. Foci of blooming on gradient echo images were observed within the lesion (more on the anterior aspect of the fluid-fluid level) (D, axial view).
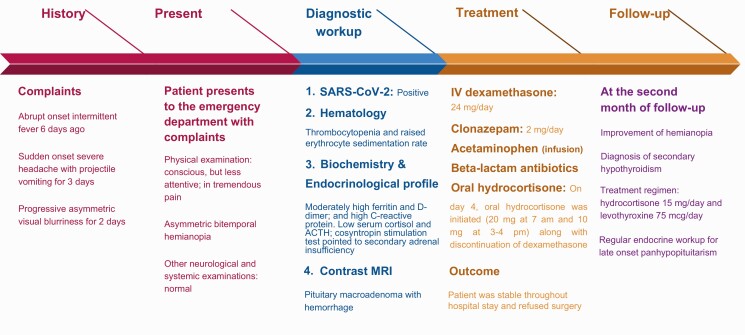
A chronological summary of events, starting from the patient’s hospital visit to her follow-up, is given in Fig. 2.
Figure 2.
A brief timeline of the clinical course of the patient from symptom onset.
Discussion
The patient presented with a history of febrile illness, followed by thunderclap headache, bitemporal hemianopia, decreased visual acuity, and projectile vomiting due to either vascular, infective, demyelinating, or inflammatory etiology. However, with the aid of magnetic resonance imaging and ancillary tests, a diagnosis of pituitary apoplexy following a hemorrhage within a preexisting pituitary macroadenoma was made. Major surgical interventions, arterial hypertension, head injury, anticoagulant therapy, pregnancy, increase in intracranial pressure, stress drugs, and pituitary dynamic tests, among others, might be precipitating factors for pituitary apoplexy [1]. This patient did not have any premorbid conditions. Further, the macroadenoma inside her sella did not produce symptoms needing consultation. This occurs often as the majority of nonfunctional macroadenomas are diagnosed when they present with pituitary apoplexy [9-11]. Infection-induced thrombocytopenia, platelet dysfunction, coagulopathy, and immune-mediated hypophysitis have been documented as precipitants for pituitary apoplexy [2, 3, 12, 13]. Wildemberg et al [13] claimed dengue infection to be a novel condition for pituitary apoplexy. Although SARS-CoV-2 infection is associated with thrombosis and coagulopathies at the same time and is often treated with unfractionated or low-molecular-weight heparins [14], a risk factor for pituitary apoplexy [2], they were not administered in our patient because the serial D-dimer levels were only minimally elevated. Among the numerous hematological complications induced by SARS-CoV-2 infection, thrombocytopenia, coagulopathy, and platelet dysfunction have been reported [14-18]. Our patient had thrombocytopenia, D-dimer elevation, and acute SARS-CoV-2 infection; we postulate that these factors contributed jointly to the acute hemorrhagic picture. In addition, the hypophysial vasculature is well known for its fragility [19], and SARS-CoV-2 infection may cause endotheliitis and endothelial dysfunction [20]. Hence, damage to fragile vessels in the setting of COVID-19 is also a plausible pathomechanism. However, in our patient, we did not have evidence of systemic endotheliitis.
Regarding management, urgent surgical decompression seemed appropriate in our case initially [21]. However, because the patient did not consent to neurosurgical intervention, and rapid and adequate response was achieved with steroids, surgery was deferred. We recognize that our case has some limitations. First, visual perimetry could not be carried out. The reason for this was the extreme circumstances in our hospital at the peak of this pandemic. Further, because surgery was deferred, a biopsy for confirmation of SARS-CoV-2–associated viral hypophysitis could not be performed. Regardless, during the ongoing pandemic, nonoperative conservative management of selected pituitary apoplexy cases has been recommended [21, 22]. Finally, RT-PCR was not performed in the cerebrospinal fluid, which could have shed light on whether there was a direct central nervous system invasion of the virus.
In closing, COVID-19 is a rapidly evolving and emerging pandemic. Its effect on endocrinological disorders is enormous [22]. This case stands out as a genuinely rare manifestation of COVID-19 and adds to the tally of cases reporting SARS-CoV-2 infection and pituitary apoplexy.
Patient Perspective
“I had fever associated with headache and malaise for a few days and I was worried whether I had COVID-19. I did not want to go to the hospital. But when my field of vision was at stake and headache worsened, I had no choice left. I got COVID-19, my treating doctors confirmed. They took me for a brain scan and told me that I had a pituitary tumor which bursted inside my head. I thought I would not survive. The doctors wanted to transfer me to a setting with the provision for neurosurgery. But I refused surgical intervention and requested they treated me with medicines instead. I left any hope of survival. My doctors took great care, and with some injectable drugs, I got somewhat better. They said I will need a hormonal replacement for life. Right now, I am on daily steroid tablets. My eyesight has normalized. I thank the Almighty, my doctors, and my family for constant support.”
Acknowledgments
Financial Support: This study has been supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422) and the European Commission (grant ICT-2011-287739, NeuroTREMOR).
Glossary
Abbreviations
- COVID-19
coronavirus disease 2019
- RT-PCR
reverse transcriptase–polymerase chain reaction
- SARS
severe acute respiratory syndrome
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
Additional Information
Disclosures: The authors have nothing to disclose.
Data Availability
The authors confirm that the data supporting the findings of this case report are available within the article. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.
References
- 1. Briet C, Salenave S, Bonneville JF, Laws ER, Chanson P. Pituitary apoplexy. Endocr Rev. 2015;36(6):622-645. [DOI] [PubMed] [Google Scholar]
- 2. Bhoelan S, Langerak T, Noack D, et al. Hypopituitarism after orthohantavirus infection: what is currently known? Viruses. 2019; 11(4):340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan SK, Seow CJ, Tan E, Chau YP, Dalan R. Pituitary apoplexy secondary to thrombocytopenia due to dengue hemorrhagic fever: a case report and review of the literature. Endocr Pract. 2014;20(4):e58-e64. [DOI] [PubMed] [Google Scholar]
- 4. Leow MK, Kwek DS, Ng AW, Ong KC, Kaw GJ, Lee LS. Hypocortisolism in survivors of severe acute respiratory syndrome (SARS). Clin Endocrinol (Oxf). 2005;63(2):197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chan JL, Gregory KD, Smithson SS, Naqvi M, Mamelak AN. Pituitary apoplexy associated with acute COVID-19 infection and pregnancy. Pituitary. 2020;23(6):716-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Solorio-Pineda S, Almendárez-Sánchez CA, Tafur-Grandett AA, et al. Pituitary macroadenoma apoplexy in a severe acute respiratory syndrome-coronavirus-2-positive testing: causal or casual? Surg Neurol Int. 2020;11:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Santos CDSE, Filho LMDCL, Santos CAT, Neill JS, Vale HF, Kurnutala LN. Pituitary tumor resection in a patient with SARS-CoV-2 (COVID-19) infection. A case report and suggested airway management guidelines [article in Portuguese]. Braz J Anesthesiol. 2020;70(2):165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bray DP, Solares CA, Oyesiku NM. Rare case of a disappearing pituitary adenoma during the COVID-19 pandemic. World Neurosurg. 2020;146:148-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Leyer C, Castinetti F, Morange I, et al. A conservative management is preferable in milder forms of pituitary tumor apoplexy. J Endocrinol Invest. 2011;34(7):502-509. [DOI] [PubMed] [Google Scholar]
- 10. Abbara A, Clarke S, Eng PC, et al. Clinical and biochemical characteristics of patients presenting with pituitary apoplexy. Endocr Connect. 2018;7(10):1058-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wichlińska-Lubińska M, Kozera G. Pituitary apoplexy. Neurol Neurochir Pol. 2019;53(6):413-420. [DOI] [PubMed] [Google Scholar]
- 12. Thomas M, Robert A, Rajole P, Robert P. A rare case of pituitary apoplexy secondary to dengue fever-induced thrombocytopenia. Cureus. 2019;11(8):e5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wildemberg LE, Neto LV, Niemeyer P, Gasparetto EL, Chimelli L, Gadelha MR. Association of dengue hemorrhagic fever with multiple risk factors for pituitary apoplexy. Endocr Pract. 2012;18(5):e97-e101. [DOI] [PubMed] [Google Scholar]
- 14. Bikdeli B, Madhavan MV, Jimenez D, et al. ; Global COVID-19 Thrombosis Collaborative Group, endorsed by the ISTH, NATF, ESVM, and the IUA, supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function . COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(23):2950-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lippi G, Plebani M, Henry BM. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis. Clin Chim Acta. 2020;506:145-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang X, Yang Q, Wang Y, et al. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18(6):1469-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Manne BK, Denorme F, Middleton EA, et al. Platelet gene expression and function in patients with COVID-19. Blood. 2020;136(11):1317-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Le Tissier P, Campos P, Lafont C, Romanò N, Hodson DJ, Mollard P. An updated view of hypothalamic-vascular-pituitary unit function and plasticity. Nat Rev Endocrinol. 2017;13(5):257-267. [DOI] [PubMed] [Google Scholar]
- 20. Mosleh W, Chen K, Pfau SE, Vashist A. Endotheliitis and endothelial dysfunction in patients with COVID-19: its role in thrombosis and adverse outcomes. J Clin Med. 2020;9(6):1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fleseriu M, Buchfelder M, Cetas JS, et al. Pituitary society guidance: pituitary disease management and patient care recommendations during the COVID-19 pandemic—an international perspective. Pituitary. 2020;23(4):327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chatterjee S, Ghosh R, Biswas P, et al. COVID-19: the endocrine opportunity in a pandemic. Minerva Endocrinol. 2020;45(3):204-227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this case report are available within the article. Data sharing is not applicable to this article because no data sets were generated or analyzed during the present study.