Abstract
In a preregistered, cross-sectional study, we investigated whether olfactory loss is a reliable predictor of COVID-19 using a crowdsourced questionnaire in 23 languages to assess symptoms in individuals self-reporting recent respiratory illness. We quantified changes in chemosensory abilities during the course of the respiratory illness using 0–100 visual analog scales (VAS) for participants reporting a positive (C19+; n = 4148) or negative (C19−; n = 546) COVID-19 laboratory test outcome. Logistic regression models identified univariate and multivariate predictors of COVID-19 status and post-COVID-19 olfactory recovery. Both C19+ and C19− groups exhibited smell loss, but it was significantly larger in C19+ participants (mean ± SD, C19+: −82.5 ± 27.2 points; C19−: −59.8 ± 37.7). Smell loss during illness was the best predictor of COVID-19 in both univariate and multivariate models (ROC AUC = 0.72). Additional variables provide negligible model improvement. VAS ratings of smell loss were more predictive than binary chemosensory yes/no-questions or other cardinal symptoms (e.g., fever). Olfactory recovery within 40 days of respiratory symptom onset was reported for ~50% of participants and was best predicted by time since respiratory symptom onset. We find that quantified smell loss is the best predictor of COVID-19 amongst those with symptoms of respiratory illness. To aid clinicians and contact tracers in identifying individuals with a high likelihood of having COVID-19, we propose a novel 0–10 scale to screen for recent olfactory loss, the ODoR-19. We find that numeric ratings ≤2 indicate high odds of symptomatic COVID-19 (4 < OR < 10). Once independently validated, this tool could be deployed when viral lab tests are impractical or unavailable.
Keywords: anosmia, chemosensory, coronavirus, hyposmia, olfactory, prediction
Introduction
The novel coronavirus SARS-CoV-2 responsible for the global COVID-19 pandemic has left a staggering level of morbidity, mortality, and societal and economic disruption in its wake (CDC 2020). Initial publications indicated that sudden smell and taste loss are cardinal, early, and potentially specific symptoms of COVID-19, including in otherwise asymptomatic individuals (Giacomelli et al. 2020; Hornuss et al. 2020; Kim et al. 2020; Lechien et al. 2020; Menni, Sudre, et al. 2020; Menni, Valdes, et al. 2020; Mizrahi et al. 2020; Moein et al. 2020; Paderno et al. 2020; Parma, Ohla, et al. 2020; Walsh-Messinger et al. 2020; Weiss et al. 2020; Yan, Faraji, Prajapati, Boone, et al. 2020). While fever and cough are common symptoms of diverse viral infections, the potential specificity of chemosensory loss to COVID-19 could make it valuable in screening and diagnosis.
Anosmia and other chemosensory disorders have serious health and quality-of-life consequences for patients. However, the general lack of awareness of anosmia and other chemosensory disorders by clinicians and the public, including their association with upper respiratory infections (Soler et al. 2020), contributed to an underappreciated role of chemosensory symptoms in the diagnosis of COVID-19. Additionally, the impact of smell loss as a clinical consequence of COVID-19 has not been adequately addressed. Thus, there is an urgent need to better define the chemosensory dysfunctions associated with COVID-19 and to determine their relevance as predictors of this disease. Additionally, it is critical to develop rapid clinical tools to efficiently and effectively integrate chemosensory assessments into COVID-19 screening and treatment protocols. Information on the duration and reversibility of post-COVID-19 chemosensory impairment is also lacking.
We used binary, categorical, and continuous self-report measures to determine the chemosensory phenotype, along with other symptoms and characteristics, of COVID-19-positive (C19+) and COVID-19-negative (C19−) individuals who had reported recent symptoms of respiratory illness. Using those results in logistic regression models, we identified predictors of COVID-19 and recovery from smell loss. Finally, we propose the Olfactory Determination Rating scale for COVID-19 (ODoR-19) as a quick, simple-to-use, telemedicine-friendly tool to improve the utility of current COVID-19 screening protocols, particularly when access to rapid testing for SARS-CoV-2 is limited.
Materials and methods
Study design
This preregistered (Parma, Veldhuizen, et al. 2020), cross-sectional online study was approved by the Office of Research Protections of The Pennsylvania State University (STUDY00014904); it is in accordance with the revised Declaration of Helsinki, and compliant with privacy laws in the United States and European Union. Data reported here were collected from the Global Consortium for Chemosensory Research (GCCR) core questionnaire (Appendix 1 and https://gcchemosensr.org; Parma, Ohla, et al. 2020). This online crowdsourced survey is currently deployed in 35 languages among community-dwelling individuals via social and traditional media as well as the GCCR website. It was also presented to clinicians to relay to their patients. The goal of the survey was to determine if changes in chemosensory function distinguish individuals with COVID-19 from those with other respiratory illnesses. The survey included binary response and categorical questions (e.g., Appendix 1, Questions 6, 9) and visual analog scales (VAS) (e.g., Appendix 1, Question 13) to measure self-reported chemosensory ability and other symptoms in adults with current or recent respiratory illness. Data reported here include responses in Arabic, Bengali, Chinese (Simplified and Traditional), Danish, Dutch, English, Farsi, Finnish, French, German, Greek, Hebrew, Hindi, Italian, Japanese, Korean, Norwegian, Portuguese, Russian, Spanish, Swedish, Turkish, and Urdu.
The GCCR survey has been online since 7 April 2020. Data collected between 7 April and 18 April 2020 were previously analyzed with respect to chemosensory function of participants with a positive COVID-19 diagnosis (Parma, Ohla, et al. 2020).
Sample description
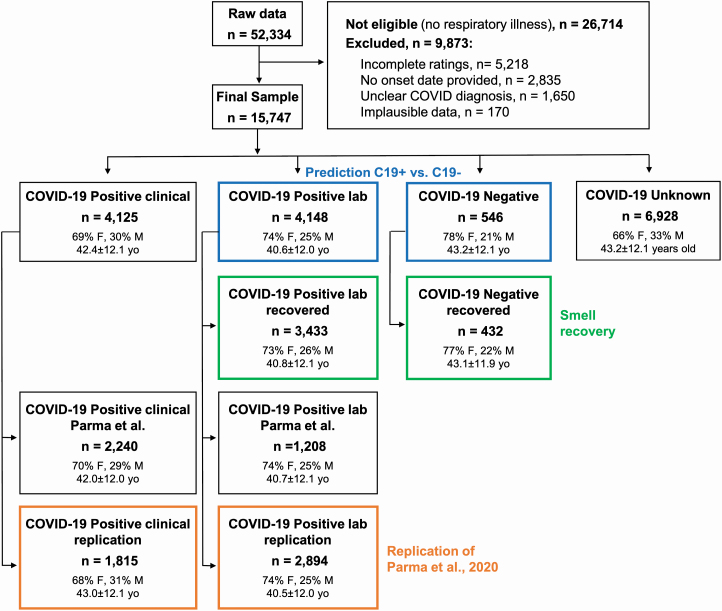
After applying preregistered exclusion criteria (Parma, Veldhuizen, et al. 2020), 15 747 participants were included in reported analyses. Their demographic information is summarized in Figure 1. The inclusion criteria for the present analyses were: recent or current respiratory illness (resolution of symptoms no more than 2 weeks prior to survey completion, if no, excluded), a specific date of onset for respiratory illness symptoms (any date since 1 January 2020), and a reported COVID-19 diagnosis via laboratory test (e.g., viral PCR or antigen test, based on Question 6; see below). The entry criterion for participation in the survey was a recent (symptoms present in the past 2 weeks) or current respiratory illness. Accordingly, only participants who responded “yes” to Question 6—“Within the past 2 weeks, have you been diagnosed with or suspect that you have a respiratory illness?”—were allowed to complete the survey (see Appendix 1 for all survey questions). To investigate the recovery of chemosensory functions, we only included participants who provided the date of onset of respiratory illness symptoms (Question 7: “What date did you first notice symptoms of your recent respiratory illness?”). Based on responses to Question 8—“Have you been diagnosed with COVID-19?”—participants were assigned to either of four groups (Figure 1). The C19+ Lab-tested group (C19+) included those that responded with either option 2 (“Yes—diagnosed with viral swab”) or option 3 (“Yes—diagnosed with another lab test”). The C19− Lab-tested group (C19−) responded with option 5 (“No, I had a negative test, but I have symptoms). The C19+ Clinical group responded with option 1 (“Yes—diagnosed with symptoms only”). The C19 Unknown group responded with option 4 (“No, I was not diagnosed, but I have symptoms”). Participants who responded with option 6 (“No—I do not have any symptoms”), option 7 (“Don’t know”), or option 8 (“Other”) were deemed undefinable and excluded from these analyses. Symptom characteristics in C19+ and C19− groups are reported in Supplementary Table S1.
Figure 1.
Flow diagram showing participant demographics. Participants included in the prediction of COVID-19 status are framed in blue. Participants included in the smell recovery models are framed in green. Participants included in the replication of a previous study (Parma, Ohla, et al. 2020; Parma, Veldhuizen, et al. 2020) are framed in orange. Gender percentages omit <1% of participants who answered “other” or “preferred not to say.” Participants described in the green boxes are a subset of those described in the blue boxes. n = number of participants; yo = age in years; W = women; M = men; unclear COVID diagnosis = responses “No—I do not have any symptoms,” “Don’t know” or “Other” to survey Question 8 (“Have you been diagnosed with COVID-19?”).
The specific collider bias characterizing this sample due to the high fraction of C19+ participants and high prevalence of chemosensory disorders in both groups underestimates the positive correlation between smell loss and COVID-19 (Supplementary Figure S1). Thus, it represents a conservative scenario to test the hypothesis that smell loss reliably predicts COVID-19 status. We also conducted propensity matching to produce equally sized populations of C19+ and C19− subjects (n = 546 each) with matched age and gender distributions, obtaining results similar to those reported in the main text (Supplementary Figure S1). We benchmarked the GCCR data set to the representative samples collected with the Imperial College London YouGov Covid 19 Behaviour Tracker (henceforth, YouGov; countries shared across data sets: Brazil, Canada, Denmark, Finland, France, Germany, Italy, Mexico, the Netherlands, Norway, Spain, Sweden, the United Kingdom, the United States; YouGov: N = 8674, GCCR: N = 3962; data publicly available at https://github.com/YouGov-Data/covid-19-tracker). Benchmarking shows the GCCR sample underestimates the positive association between smell loss and C19+ (Supplementary Figure S1, Supplementary Table S2). The country-wise fraction of C19+ participants is correlated (r ~0.45) when responses from the same calendar week are aligned (Supplementary Figure S2). These findings are in line with other comparisons between crowdsourced versus representative health data (Kraemer et al. 2017), confirming that trends identified in crowdsourced data reasonably approximate population data. Because the GCCR cohort is not demographically balanced, it should not be used to estimate prevalence. However, the representative YouGov cohort indicates globally one-third of C19+ individuals report smell loss (Supplementary Table S1).
Statistical analyses
Statistical analyses were performed in Python 3.7.6 using the pandas (Reback et al. 2020), scikit-learn (Pedregosa et al. 2011), and statsmodels (Seabold and Perktold 2010) packages. The data and annotated code are included as Supplementary material and will be publicly available on GitHub (http://github.com/GCCR/GCCR002) upon publication. The data matrix derived from survey responses had strictly nonnegative values and was normalized (column-wise min = 0, max = 1) to apply regularization in an equitable fashion across variables and give regression coefficients the same interpretation for each feature. Missing values were handled as follows: we only included participants in the recovery group who responded to questions pertinent to recovery (Appendix 1, questions 28–32). Other missing data were limited to: (1) a detailed breakdown of smoking frequency (questions 35 and 37) for self-reported nonsmokers, imputed as zero frequency; (2) an absence of any listed prior conditions in question 38 (including “None”) for 6% of respondents, imputed as no prior conditions; (3) 1.9% of respondents did not indicate whether they had recovered from illness or not, and who were dropped from analysis of recovery; and (4) <0.02% of questions about specific taste qualities, imputed with median values. Prediction targets themselves were never imputed. Responses incompatible with model generalization (e.g., open-ended questions) were excluded. A one-hot encoding was applied to all categorical variables to produce binary indicators of category membership.
L1-regularized logistic regression models using a range of penalty values (α) were assessed using cross-validation. Each model attempted to predict, using the value of the response to a single question (and an additive constant), whether a subject reported a C19+ or C19− status. Coefficients in a logistic regression model can be interpreted as changes in odds or as odds ratios when two values are compared. Each such model included an intercept term and one or more normalized variables. We obtained very similar results for all values of α (results not shown), as expected, because the sample size is much larger than the number of variables. Models with similar AUC values (but with nonzero coefficients for additional, likely spurious variables) were obtained for smaller values of α, and inferior results for larger ones (which contained fewer or no nonzero coefficients). We reported results for α = 1 here, as it consistently produced sparse models with the highest cross-validation accuracy. Quantitatively similar AUC values were obtained for other models predicting COVID-19 status using multiple variables including ridge regression and random forest, but L1-regularized logistic regression consistently produced sparser models with comparable cross-validation accuracy.
Model quality was measured using receiver operating characteristic (ROC) area under the curve (AUC). Each ROC curve—constructed using predictions on holdout test sets and concatenated over these test sets—summarizes the tradeoff between sensitivity (fraction of C19+ cases correctly identified) and specificity (fraction of C19− cases correctly identified) as the threshold value for the predictor is varied. Cross-validation was performed in 100 random splits of 80% training set and 20% test set, and ROC curves were concatenated over each test set. ROC curves were computed on predicted probabilities from each model, circumventing the high-cardinality bias of AUC. For univariate models, ROC curves are invariant to all monotonic transformations of the data, and thus AUC is independent of most modeling details. To correctly compute P values for model coefficients, the normalized data were standardized (mean 0, variance 1) and then coefficients back-transformed to normalized form after fitting. Uncertainty is given as ±standard deviation for descriptive analyses, and ±standard error for model-derived quantities (e.g., AUC). For the replication of Parma, Ohla, et al. (2020), we included Bayes factors (BF) and we used data newly collected from 19 April to 3 July 2020 (see Supplementary Material, Supplementary Figure S3, Supplementary Table S2).
Results
Chemosensory loss associates with COVID-19
In a previous publication using an earlier tranche of data obtained from this survey, we reported that smell, taste, and chemesthesis abilities drop significantly in both lab-tested C19+ participants and those diagnosed by clinical assessment (Parma, Ohla, et al. 2020). In a preregistered replication of that analysis, we confirm those findings using the current data tranche (Supplementary Figure S3, Supplementary Table S3).
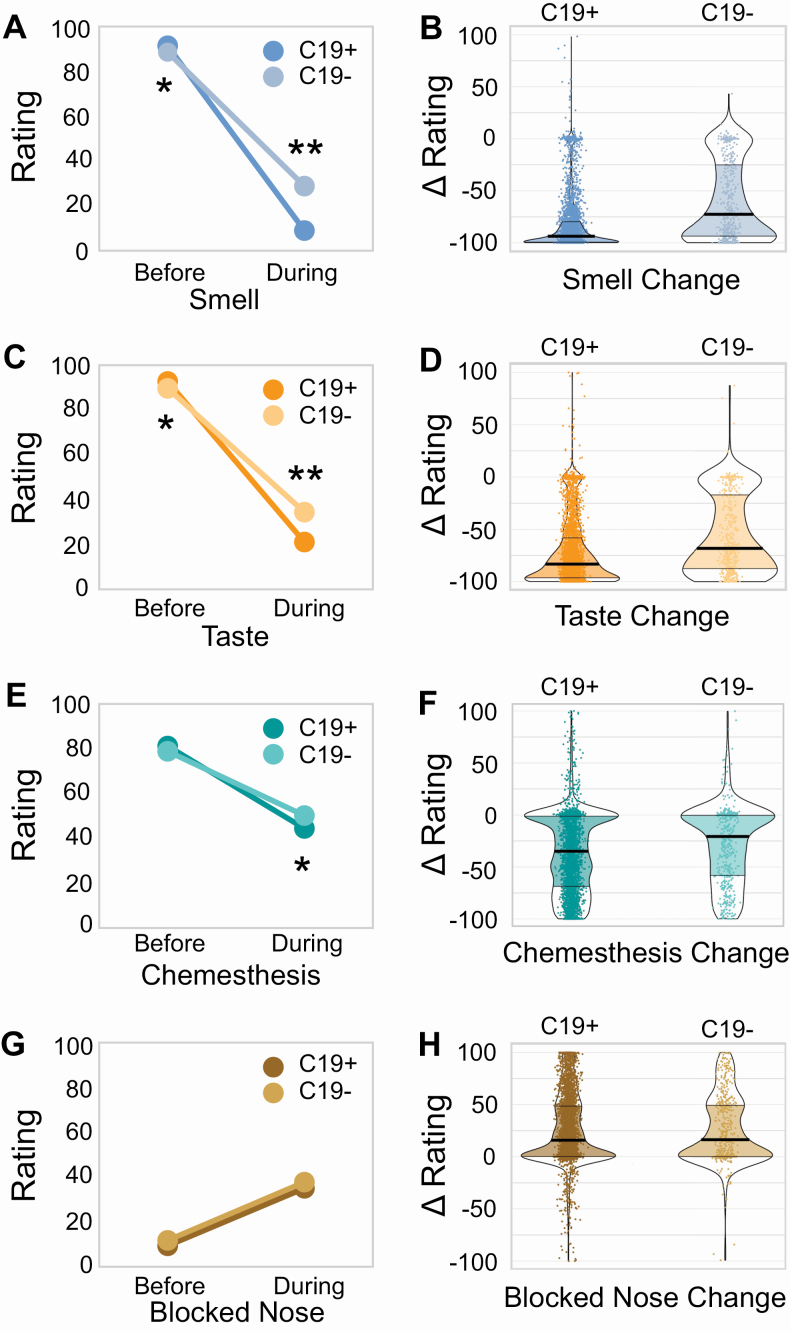
Next, we compared chemosensory abilities and nasal blockage in lab-tested C19+ and C19− participants. Ratings for each of these before the onset of respiratory illness (baseline ratings) show small (2–3 points on a 100-point scale) but significant (smell, P = 3.2 × 10−5; taste, P = 1.8 × 10−6; chemesthesis, P = 0.016; nasal blockage, P = 0.004) differences between C19+ and C19− individuals, as expected with very large sample sizes (Figure 2). However, these differences are much smaller than the chemosensory differences seen between C19+ and C19− individuals during their illness: C19+ participants reported a greater loss of smell (C19+: −82.5 ± 27.2 points; C19−: −59.8 ± 37.7 points; P = 1.1 × 10−59, extreme evidence of difference: BF10 = 8.97 × 10+61; Figure 2A,B, Supplementary Table S4), taste (C19+: −71.6 ± 31.8 points; C19−: −55.2 ± 37.5 points; P = 7 × 10−24, extreme evidence of difference: BF10 = 6.67 × 10+24; Figure 2C,D, Supplementary Table S4) and chemesthesis ability (C19+: −36.8 ± 37.1 points; C19−: −28.7 ± 37.1 points; P = 4.6 × 10−5, extreme evidence of difference: BF10 = 3182; Figure 2E,F, Supplementary Table S4). However, both groups reported a similar degree of nasal obstruction (Figure 2G,H, Supplementary Table S4). Self-reported changes in smell, taste, and chemesthesis were highly correlated within both groups (C19+: 0.71 < r < 0.83; C19−: 0.76 < r < 0.87) and orthogonal to nasal obstruction changes (C19+: r = −0.20; C19−: r = −0.13).
Figure 2.
Chemosensory ability and nasal obstruction in C19+ and C19− participants. Self-reported smell (A, B), taste (C, D), chemesthesis (E, F), and nasal obstruction (G, H: formulated as “How blocked was your nose?”) before and during respiratory illness in C19+ (darker shades) and C19− (lighter shades) participants. Ratings were given on 0–100 visual analog scales. Left panels (A, C, E, G) show mean values. Right panels (B, D, F, H) show distributions of the change scores (during minus before). Thicker sections indicate relatively more subjects (higher density of responses). The thick black horizontal bar indicates the median, the shaded area within each violin indicates the interquartile range. Each dot represents the rating of a single participant. * indicates P < 10–4, ** indicates P < 10–23.
Prediction of COVID-19 status from survey responses
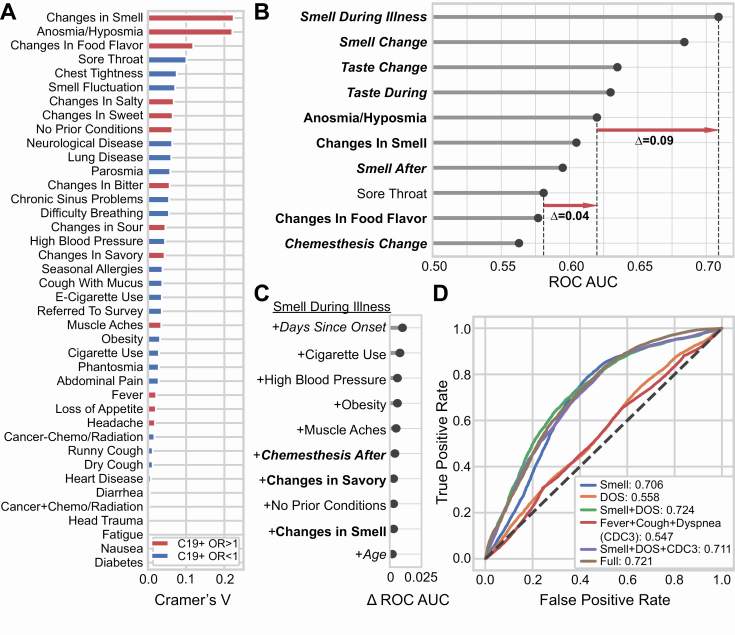
When only binary (yes/no) and categorical responses are analyzed, we found that chemosensory symptoms are more strongly associated with COVID-19 than are fever, cough, or other common non-chemosensory symptoms (Figure 3A). Using AUC to assess prediction quality (Figure 3B), we found that self-reported smell ability during illness, reported on a continuous scale, was the most predictive survey question for COVID-19 status (AUC = 0.71). Changes in smell as a result of illness (i.e., the difference between smell ability during and before illness) were similarly predictive (AUC = 0.69). Changes in taste ability (assessed via rating) were the next most predictive variables (AUC = 0.64–0.65) (Figure 3B). Models fit to the same data but with shuffled COVID-19 status consistently produced AUC ~0.5 for all variables. The most predictive non-chemosensory symptom, sore throat (which was negatively associated with COVID-19) was substantially less predictive (AUC = 0.58) than the top chemosensory symptoms. Nasal obstruction was not predictive (AUC = 0.52). Responses given on a continuous scale were more predictive (AUC = 0.71) than binary responses to parallel questions (e.g., Appendix 1, Question 10 and 15 vs. 14, Supplementary Figure S5) (AUC = 0.60–0.62), likely because a continuous scale contains a greater amount of diagnostic information (Supplementary Figure S4).
Figure 3.
Smell loss is the strongest predictor of COVID-19 status. (A) A normalized measure of association (Cramer’s V) between binary or categorical responses on COVID-19 status. V = 0 reflects no association between the response and COVID-19 status; V = 1 reflects a perfect association; V > 0.1 is considered a meaningful association. Variables in red are positively associated with C19+ (odds ratio > 1); variables in blue are negatively associated with C19+ (odds ratio < 1). (B) Logistic regression is used to predict COVID-19 status from individual variables. Top-10 single variables are ranked by performance (cross-validated area under the ROC curve, AUC). Chemosensory-related variables (bold) show greater predictive accuracy than non-chemosensory variables (non-bold). Responses provided on the numeric scale (italic) were more informative than binary responses (non-italic). Red arrows indicate differences in prediction quality (in AUC) between variables. (C) Adding variables to “Smell During Illness” results in little improvement to the model; only Days Since Onset of Respiratory Symptoms (DOS) relative to survey completion date yields meaningful improvement. (D) ROC curves for several models. A model using “Smell during illness” (Smell Only, abbreviated “Smell” in the figure) is compared against models containing this feature along with DOS, as well as models including the three cardinal CDC variables (fever, dry cough, difficulty breathing). “Full” indicates a regularized model fit using 70 survey variables, which achieves prediction accuracy similar to the parsimonious model “Smell Only + DOS.”
Next, we examined which simple multivariate model would best predict COVID-19 status. As some questions have highly correlated responses, the question most complementary to “Smell during illness” is unlikely to be one that carries redundant information. Adding “Days since Onset of Respiratory Symptoms” (DOS), which was measured relative to the survey completion date, to “Smell during illness” (Smell Only) produced the largest incremental gain in predictive performance (AUC = 0.72, +0.01 vs. the Smell Only model) (Figure 3C).
We directly compared the Smell Only + DOS model to other candidate models. The Smell Only + DOS model (Figure 3D) yielded an equal or higher AUC than the model including the three cardinal symptoms (fever, cough, difficulty breathing) identified by the US Centers for Disease Control and Prevention (CDC) (AUC = 0.55) or the full model using 70 variables (AUC = 0.72). Because the Smell Only + DOS model exhibits the same AUC as the full model it strikes a good balance between model parsimony and predictive accuracy for C19+. However, the Smell Only model also offers reasonable sensitivity of 0.85 (at specificity = 0.51, cutoff = 13 on the 100-point VAS) and/or specificity of 0.75 (at sensitivity = 0.51, cutoff = 1) as desired. By sharp contrast, fever has a sensitivity of only 0.54 with specificity of 0.49, and dry cough has sensitivity of 0.52 and specificity of 0.46. Since some subjects had already fully (N = 1867) or partially (N = 1998) recovered from their respiratory symptoms by the date of completion of the survey, we asked how effectively Smell loss and DOS predict COVID-19 status in the most clinically actionable population: those whose core respiratory symptoms had not resolved. If we exclude those participants who had fully recovered from their respiratory symptoms at the time of survey completion, the ability to predict C19+ status based on Smell Only and Smell Only + DOS increases (Smell Only AUC = 0.73, Smell Only + DOS AUC = 0.76; N = 2827). Further excluding those who had partially recovered from respiratory symptoms produced even larger gains (Smell Only AUC = 0.750 Smell During + DOS AUC = 0.788; N = 829).
Recovery from smell loss
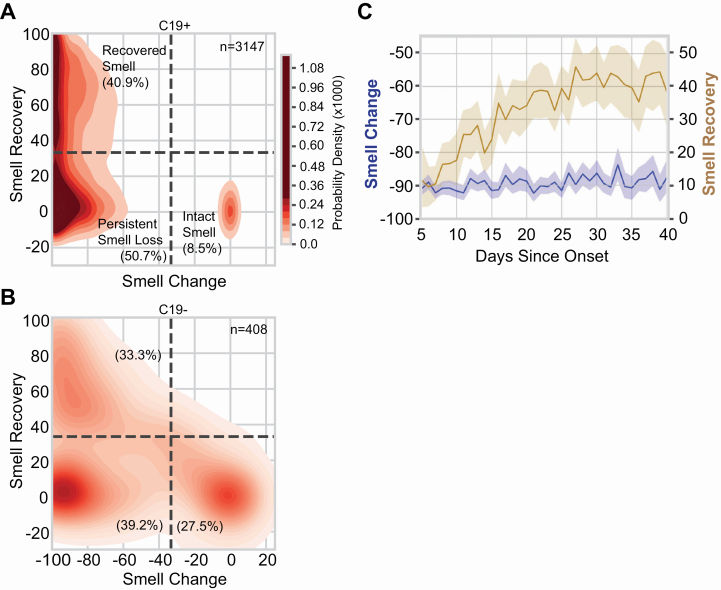
Recovery from smell loss was modest (approximately half the initial average loss) in C19+ participants with full or partial resolution of respiratory symptoms. Overall, self-reported, post-illness olfactory ability was still lower for C19+ (39.9 ± 34.7) than C19− (52.2 ± 35.2, P = 2.8 × 10−11, Supplementary Figure S6A). However, the mean recovery of smell (after illness relative to during illness) was greater for C19+ (30.5 ± 35.7) than C19− (24.6 ± 31.9, P = 0.0002, Supplementary Figure S6B). A similar but smaller effect of COVID-19 status on recovery was observed for taste (Supplementary Figure S6C,D), whereas little to no association with COVID-19 was observed for recovery of chemesthesis (Supplementary Figure S6E,F) or nasal obstruction (Supplementary Figure S6G,H). When illness-induced change in olfactory function (during minus before illness) and recovery of olfactory function (after minus during illness) were evaluated, we identified three respondent clusters: those self-reporting no loss of smell (Intact Smell), those reporting recovery from smell loss (Recovered Smell), and those reporting smell loss without recovery by up to 40 days (Persistent Smell Loss, Figure 4, Supplementary Table S4). Intact smell was reported by only 8.5% of the participants in the C19+ group but by 27.5% in the C19− group (P = 3.8 × 10−31). A greater proportion of C19+ participants were included in both the Recovered Smell group (C19+: 40.9%, C19−: 33.3%; P = 4.9 × 10−10) and the Persistent Smell Loss group (C19+: 50.7%, C19−: 39.2%; P = 5 × 10−5; Figure 4A,B). Using logistic regression, the only variable that could predict the probability of recovery from smell loss was DOS (AUC = 0.62); all other single variables were extremely poor predictors (AUC <= 0.54). DOS is not acting as a proxy for initial smell loss: C19+ participants in both the Recovered Smell and Persistent Smell Loss clusters reported a similar magnitude of olfactory loss, irrespective of time since respiratory symptom onset. By contrast, the degree of self-reported smell recovery increased over time, with a plateau at 30 days (Figure 4C).
Figure 4.
Smell loss, recovery, and time course. (A, B) Joint distribution of smell loss (during minus before illness ratings) and smell recovery (after minus during illness ratings) for C19+ (A) and C19− (B) participants. Darker color indicates a higher probability density; the color map is shared between (A) and (B); dashed lines are placed at a third of the way across the rating scale to aid visualization of the clusters. Severe smell loss that is either persistent (lower left) or recovered (upper left) was more common in C19+ than C19−. n indicates the number of participants in each panel. % indicates the percentage of participants of the given COVID status in each quadrant. (C) In C19+ participants who lost their sense of smell (Recovered Smell + Persistent Smell Loss), the degree of smell recovery (right y axis) increased over ~30 days since onset of respiratory symptoms before plateauing; the degree of reported smell change (left y axis) did not vary in that window of observation. Solid lines indicate the mean of the measure, the shaded region indicates the 95% confidence interval.
Simple screening for COVID-19: the ODoR-19
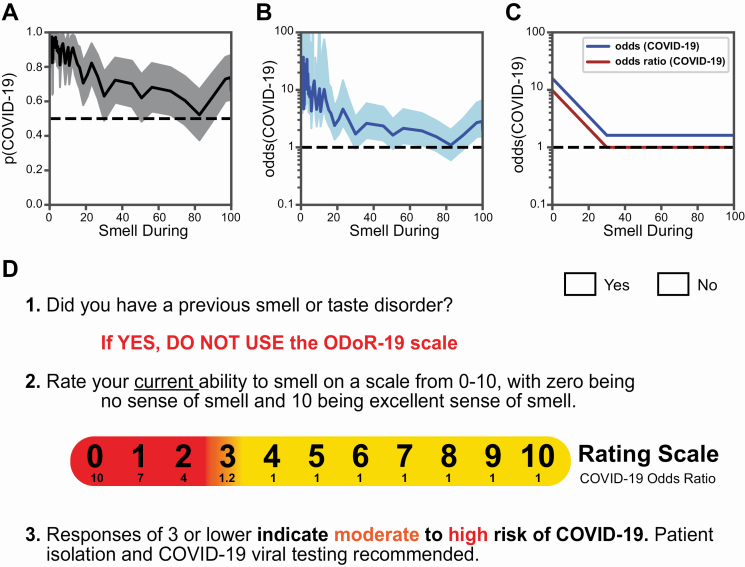
Our results indicate that a continuous rating of current olfactory function is the single best predictor of COVID-19 in the presence of respiratory symptoms and it improves the discrimination between C19+ and C19− compared with a binary question on smell loss. For example, the Smell Only model reached a specificity of 0.83 at the low end of the VAS (sensitivity = 0.36, cutoff = 0). When considering the odds of a COVID-19 diagnosis as a function of current olfactory ability, our data indicate that this probability is greater than 0.8 when current smell ability is rated at 20 or below on a 0–100 scale (Figure 5A). This rating translates into an odds ratio >4 (Figure 5B). The inflection point at which the odds ratio plateaus at 1 is 30/100 (Figure 5C).
Figure 5.
The odds of a COVID-19 diagnosis as a function of olfactory ability in individuals with respiratory symptoms. (A) The solid line indicates the probability of a COVID-19 diagnosis as a function of “Smell during illness” ratings in our sample. The shaded region indicates the 95% confidence interval. (B) The solid line expresses the probability of a COVID-19+ diagnosis as a function of “Smell during illness” in odds (p/[1−p]); it is shown on a logarithmic scale. The shaded region indicates the 95% confidence interval. (C) Stylized depiction of change in the odds of a COVID-19 diagnosis and of the odds ratio. (D) The ODoR-19 tool. After healthcare providers or contact tracers have excluded previous smell and/or taste disorders (such as those resulting from head trauma, chronic rhinosinusitis, or previous viral illness) in patients with respiratory symptoms, the patient can be asked to rate their current ability to smell on a scale from 0 to 10, with 0 being no sense of smell and 10 being excellent sense of smell. If the patient reports a value below or equal to 3, there is a high (red) or moderate (orange) probability that the patient has COVID-19. Values in yellow (ratings above 3) cannot rule out COVID-19.
A 0–10 rating scale, such as the pain scale, is widely used in clinical environments. With the goal of enabling clinicians and other health professionals to quickly and simply assess self-reported smell loss in the context of COVID-19, we transformed the 0–100 rating scale used in this survey to a 0–10 numeric rating scale, the ODoR-19. In our samples, responses to the ODoR-19 scale ≤2 indicate high odds of COVID-19 positivity (4 < OR < 10, Figure 5D). An ODoR-19 response of 3 indicates a borderline risk (OR = 1.2). Upon independent validation, the ODoR-19 could be administered in person or via telemedicine to improve early COVID-19 screening for individuals without preexisting smell and/or taste disorders.
Discussion
Self-reported smell loss was much greater in C19+ than C19− participants, but present in both groups. The use of a VAS to assess olfactory loss better predicted COVID-19 status than using a binary question. We found that the best predictor of COVID-19-associated smell recovery, within the time frame captured by the survey (~40 days), was DOS.
The SARS-CoV-2 pandemic requires healthcare providers and contact tracers to quickly and reliably assess an individual’s COVID-19 risk, often remotely. Thus, reliable screening tools are critical to assess a person’s likelihood of having COVID-19 and to justify self-quarantine and/or testing recommendations. Indeed, some reports suggest that COVID-19-associated smell loss might be an indicator of disease severity (Paderno et al. 2020; Yan, Faraji, Prajapati, Ostrander, et al. 2020). Current symptom criteria (e.g., fever, dry cough) are less specific than severe olfactory loss in distinguishing between COVID-19 and other respiratory illnesses. Indeed, the value of our ODoR-19 tool would lie in the high specificity of values ≤2 for indicating COVID-19 positivity, as seen with our sample, therefore representing a valuable addition to the current repertoire of COVID-19 screening tools. Those who receive a negative outcome from a COVID-19 viral test, yet report significant idiopathic smell loss, should be considered as high-priority candidates for COVID-19 re-testing and self-isolation.
Our online survey and sampling methodology likely selected participants with a heightened interest in smell and taste and/or their disturbances. This self-selection bias could be viewed as a limitation because the C19− group also showed chemosensory loss. However, finding a difference between groups in a sample with a high barrier for discriminating between C19+ and C19− supports the robustness of our findings. A simple model of collider bias suggests that our findings are likely conservative estimates of the association between COVID-19 and smell loss (Supplementary Figure S1). Additionally, this observation is supported by the correlation between the GCCR survey data and that of the representative YouGov data reported here (Supplementary Figure S2, Supplementary Table S2).
Our results suggest that chemosensory impairment has strong COVID-19 predictive value, and is useful when access to viral testing is limited or absent. As with any self-report measure, veracity of self-reports cannot be guaranteed. However, the ability to screen individuals in real-time should outweigh this potential confound (Mermelstein et al. 2007). While objective smell tests are the gold standard for assessing olfactory function (Doty et al. 1984; Hummel et al. 1997), they are costly, time-consuming to administer, and can require in-person interactions with potentially infectious patients (Doty et al. 1984; Oleszkiewicz et al. 2019). By contrast, self-report measures are free, quick, and can be administered in person or remotely. We cannot exclude that our C19− sample contains COVID-19 false negatives (Kucirka et al. 2020). However, self-reported smell during illness distinguishes between C19+ and C19−, but not between randomly shuffled cases, suggesting that the difference between C19+ and C19−, even in a sample with over-represented chemosensory dysfunction, is substantial and can be captured via self-report.
Approximately half of the participants in the C19+ group had recovered their sense of smell by the date that they completed the survey (Figure 4A). The mean recovery from smell loss increased with duration from respiratory symptom onset for ~30 days before reaching a steady-state for at least an additional 10 days, suggesting the presence of at least two subgroups of participants: one group (40.9%) that recovers more quickly (within 30 days of respiratory symptom onset) and another (50.7%) that may take more time to recover. Our survey may overestimate the size of the latter group because some individuals who recover from respiratory symptoms within 2 weeks of their onset may have been missed in our sample due to the recruitment criteria. While we cannot offer a complete picture of recovery from olfactory loss in COVID-19-positive individuals, they do align with other early reports (Chiesa‐Estomba et al. 2020). The COVID-19 pandemic will greatly increase the number of patients suffering from anosmia and other chemosensory disorders (Rawal et al. 2016), conditions that significantly affect quality of life (Smeets et al. 2009; Croy, Nordin, et al. 2014), dietary behavior (Kershaw and Mattes 2018), cardiovascular health (Gallo et al. 2020), and mental health (Malaty and Malaty 2013; Croy, Symmank, et al. 2014). Thus, it is necessary to prepare healthcare providers to address the long-term needs of these patients.
Based on our results, we propose the use of the ODoR-19 tool, a quick, free, and effective smell-based screening method for COVID-19. ODoR-19 combines the utility of a continuous scale with the ease and speed needed for a screening tool for individuals without preexisting smell and taste disorders (e.g., from head trauma, chronic rhinosinusitis; Malaty and Malaty 2013). ODoR-19 is safe for remote administration during an illness with high viral spread and can precede and complement viral testing. This tool has the potential to improve screening for patients with limited or no access to medical care around the globe.
Supplementary Material
Acknowledgments
The authors wish to thank all study participants, patients, and patient advocates that have contributed to this project, including members of the AbScent Facebook group. The authors wish to thank Micaela Hayes, MD for her input on the clinical relevance of this project, Shannon Alshouse and Olivia Christman for their help in implementing the survey, Sara Lipson for her support, and the international online survey research firm YouGov for providing data gathered with the Imperial College London YouGov Covid 19 Behaviour Tracker.
Contributor Information
GCCR Group Author:
Sanne Boesveldt, Jasper H B de Groot, Caterina Dinnella, Jessica Freiherr, Tatiana Laktionova, Sajidxa Marino, Erminio Monteleone, Alexia Nunez-Parra, Olagunju Abdulrahman, Marina Ritchie, Thierry Thomas-Danguin, Julie Walsh-Messinger, Rashid Al Abri, Rafieh Alizadeh, Emmanuelle Bignon, Elena Cantone, Maria Paola Cecchini, Jingguo Chen, Maria Dolors Guàrdia, Kara C Hoover, Noam Karni, Marta Navarro, Alissa A Nolden, Patricia Portillo Mazal, Nicholas R Rowan, Atiye Sarabi-Jamab, Nicholas S Archer, Ben Chen, Elizabeth A Di Valerio, Emma L Feeney, Johannes Frasnelli, Mackenzie E Hannum, Claire Hopkins, Hadar Klein, Coralie Mignot, Carla Mucignat, Yuping Ning, Elif E Ozturk, Mei Peng, Ozlem Saatci, Elizabeth A Sell, Carol H Yan, Raul Alfaro, Cinzia Cecchetto, Gérard Coureaud, Riley D Herriman, Jeb M Justice, Pavan Kumar Kaushik, Sachiko Koyama, Jonathan B Overdevest, Nicola Pirastu, Vicente A Ramirez, S Craig Roberts, Barry C Smith, Hongyuan Cao, Hong Wang, Patrick Balungwe Birindwa, and Marius Baguma
Funding
Deployment of the GCCR survey was supported by an unrestricted gift from James and Helen Zallie to support sensory science research at Penn State. R.C.G. is supported by National Institute of Neurological Disorders and Stroke (NINDS) (U19NS112953) and National Institute on Deafness and Other Communication Disorders (NIDCD) (R01DC018455). P.V.J. is supported by the National Institute of Nursing Research (NINR) under award number 1ZIANR000035-01. P.V.J. is also supported by the Office of Workforce Diversity, National Institutes of Health and The Rockefeller University Heilbrunn Nurse Scholar Award. V.V.V. is supported by Institute of Ecology and Evolution Russian Academy of Sciences (IEE RAS) basic project 0109-2018-0079. M.E.H. is supported by National Institutes of Health (NIH) T32 funding (DC000014). M.Y.N. is supported by Israel Science Foundation (ISF) grant #1129/19.
Conflict of interest
R.C.G. is an advisor for Climax Foods, Equity Compensation (RCG); K.O. consults for for-profit corporations and nonprofit organizations on topics related to food/consumer product perception; J.E.H. has consulted for for-profit food/consumer product corporations in the last 3 years on projects wholly unrelated to this study; also, he is Director of the Sensory Evaluation Center at Penn State, which routinely conducts product tests for industrial clients to facilitate experiential learning for students. Since 2018, T.H. collaborates with and received funding from Sony, Stuttgart, Germany; Smell and Taste Lab, Geneva, Switzerland; Takasago, Paris, France; aspUraclip, Berlin, Germany. C.E.K. is the founder of AbScent, a charity registered in England and Wales, No. 1183468. C.L. has received funding from scent related institutions and corporations, however, for work totally unrelated to the field of the present study. S.D.M. is Editor-in-Chief of Chemical Senses. He played no role in the editorial assessment of this paper.
Author contributions
Author contributions are described according to the following single letter codes and follow the CRediT Contributor Roles taxonomy.
Conceptualization—“C,” Methodology—“M,” Data Curation—“D,” Formal Analysis—“A,” Funding Acquisition—“F,” Investigation—“I,” Project Administration—“A,” Resources—“R,” Software—“S,” Supervision—“U,” Validation—“L,” Visualization—“V,” Writing Original Draft—“W,” Writing Review Editing—“E.”
Author contributions are as follows: V.P.—CMDAIRULVWE; R.C.G.—CMDAIRSLVWE; S.D.M.—CMDAIAULVWE; K.O.—CMIARULVWE; A.J.B.—CMDIARSUE; A.D.P.—CMIARULWE; J.E.H.—CMDFIARUE; M.Y.N.—CMDIARUWE; M.Y.P.—DAIRULVWE; M.G.V.—CMIARUVWE; K.W.C.—DIARSLWE; M.D.—AIARULWE; P.B.S.—CMFIRUWE; V.E.B.—CMDIARLE; D.R.R.—CMIAUWE; I.C.—CMDIRWE; M.K.A.—CMDIARE; M.A.F.—FIARULE; M.A.S.—MIARUWE; P.V.J.—CMARUWE; A.A.—IARUWE; A.W.F.—DIRUWE; A.M.—CMIRWE; C.H.—MIARWE; C.E.K.—CMARUE; D.P.—IRULWE; G.A.—CMIARE; G.M.—CMIRWE; G.M.S.—MILWE; I.K.—IARUWE; J.G.—IARUWE; J.W.H.—CMIUWE; K.L.W.—CMIUWE; L.D.H.—MIARWE; L.Ö.—IARUWE; M.H.O.—CMIRWE; O.C.—CMIRWE; S.B.O.—MIARUE; T.H.—CMAUWE; V.P.L.—MILWE; A.D.E.—CIRWE; A.M.—MIRUL; C.L.—DIARS; E.R.—CIRUE; F.P.S.F.—MIRLE; H.Y.—MIRWE; J.L.—MIRWE; J.N.L.—MFIRE; J.K.O.—MFIRE; L.R.S.—IARUE; M.B.—MIUWE; M.A.B.—DIRUE; C.M.P.—CIUWE; R.P.—MILWE; S.B.—CMIWE; S.S.—MIRWE; V.V.V.—IRUWE; A.A.N.—MIWE; A.N.P.—IRWE; C.D.—IRWE; C.L.—MIRE; D.S.—MDRE; E.C.—CIWE; E.M.—IRWE; E.S.—IAWE; F.F.—MRUE; J.A.—DIRE; J.C.—DIRE; J.F.—IRWE; J.W.M.—CMIE; J.d.G.—CIWE; K.E.S.—CMWE; M.D.G.—IRWE; M.N.—CMIR; O.A.—RLWE; P.M.—MIWE; P.P.M.—IRWE; P.R.D.—IRWE; R.A.A.—CIUE; S.B.—MIRE; S.T.M.—IRWE; T.H.—IUWE; T.L.—IRWE; T.T.D.—ILWE; V.D.C.S.—IUWE; W.F.—IRUE; B.C.—IRE; C.B.—SLE; C.H.—IWE; C.M.—IWE; E.L.F.—IWE; E.E.O.—IRE; J.F.—IRE; M.P.C.—IWE; M.C.F.—IWE; M.E.H.—MIE; M.P.—IUE; N.S.A.—IWE; N.K.—CMD; N.R.R.—IWE; O.S.—IWE; S.M.—RSU; M.R.—IRE; A.S.J.—LE; B.C.S.—ME; C.C.—WE; C.M.—AU; C.H.Y.—IE; E.B.—RE; E.A.D.V.—VE; E.A.S.—IE; G.C.—WE; H.K.—DR; J.M.J.—CE; J.B.O.—CE; K.C.H.—WE; N.P.—DV; P.K.K.—RE; R.A.—WE; R.A.—IR; R.D.H.—IE; S.K.—WE; S.C.R.—IE; V.A.R.—LE; Y.N.—IE; H.C.—IE; H.W.—IE; M.B.—IE; M.v.d.B.—IR; P.B.B.—IE.
References
- CDC. 2020. Coronavirus disease 2019 (COVID-19) – symptoms. Available from: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. [Google Scholar]
- Chiesa‐Estomba CM, Lechien JR, Radulesco T, Michel J, Sowerby LJ, Hopkins C, Saussez S. 2020. Patterns of smell recovery in 751 patients affected by the COVID-19 outbreak. Eur J Neurol. 27:2318–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Nordin S, Hummel T. 2014. Olfactory disorders and quality of life – an updated review. Chem Senses. 39(3):185–194. [DOI] [PubMed] [Google Scholar]
- Croy I, Symmank A, Schellong J, Hummel C, Gerber J, Joraschky P, Hummel T. 2014. Olfaction as a marker for depression in humans. J Affect Disord. 160:80–86. [DOI] [PubMed] [Google Scholar]
- Doty RL, Shaman P, Kimmelman CP, Dann MS. 1984. University of Pennsylvania smell identification test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 94(2 Pt 1):176–178. [DOI] [PubMed] [Google Scholar]
- Gallo S, Byham-Gray L, Duffy VB, Hoffman HJ, Hayes JE, Rawal S. 2020. Associations of olfactory dysfunction with anthropometric and cardiometabolic measures: findings from the 2013–2014 national health and nutrition examination survey (NHANES). Physiol Behav. 215:112702. [DOI] [PubMed] [Google Scholar]
- Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L, Rusconi S, Gervasoni C, Ridolfo AL, Rizzardini G, et al. 2020. Self-reported olfactory and taste disorders in patients with severe acute respiratory Coronavirus 2 infection: a cross-sectional study. Clin Infect Dis. 71(15):889–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornuss D, Lange B, Schröter N, Rieg S, Kern WV, Wagner D. 2020. Anosmia in COVID-19 patients. Clin Microbiol Infect. 26(10):1426–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. 1997. ‘Sniffin’ sticks’: olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 22(1):39–52. [DOI] [PubMed] [Google Scholar]
- Kershaw JC, Mattes RD. 2018. Nutrition and taste and smell dysfunction. World J Otorhinolaryngol Head Neck Surg. 4(1):3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GU, Kim MJ, Ra SH, Lee J, Bae S, Jung J, Kim SH. 2020. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect. 26(7):948.e1–948.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer JD, Strasser AA, Lindblom EN, Niaura RS, Mays D. 2017. Crowdsourced data collection for public health: a comparison with nationally representative, population tobacco use data. Prev Med. 102:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucirka LM, Lauer SA, Laeyendecker O, Boon D, Lessler J. 2020. Variation in false-negative rate of reverse transcriptase polymerase chain reaction-based SARS-CoV-2 tests by time since exposure. Ann Intern Med. 173(4):262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, et al. 2020. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 277(8):2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaty J, Malaty IA. 2013. Smell and taste disorders in primary care. Am Fam Physician. 88(12):852–859. [PubMed] [Google Scholar]
- Menni C, Sudre CH, Steves CJ, Ourselin S, Spector TD. 2020. Quantifying additional COVID-19 symptoms will save lives. Lancet. 395(10241):e107–e108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, et al. 2020. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med. 26(7):1037–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mermelstein R, Hedeker D, Flay B, Shiffman S. 2007. Real-time data capture and adolescent cigarettes moking: moods and smoking. In: Stone A, Shiffman S, Atienza A, Nebeling L, editors. The science of real-time data capture: self-reports in health research. p. 117–135. [Google Scholar]
- Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, Keshet A, Shamir-Stein N, Shalev V, Zohar AE, et al. 2020. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 11:6208. doi: 10.1038/s41467-020-20053-y. [DOI] [PMC free article] [PubMed]
- Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. 2020. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 10(8):944–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oleszkiewicz A, Schriever VA, Croy I, Hähner A, Hummel T. 2019. Updated Sniffin’ Sticks normative data based on an extended sample of 9139 subjects. Eur Arch Otorhinolaryngol. 276(3):719–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, Taboni S, Zorzi S, Lombardi D, Deganello A, et al. 2020. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 10(8):955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Ohla K, Veldhuizen MG, Niv MY, Kelly CE, Bakke AJ, Cooper KW, Bouysset C, Pirastu N, Dibattista M, et al. ; GCCR Group Author . 2020. More than Smell-COVID-19 is associated with severe impairment of smell, taste, and chemesthesis. Chem Senses. 45(7):609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V, Veldhuizen M, Ohla K, Gerkin RC, Reed D, Hayes J. 2020. Is olfactory loss a sensitive symptomatic predictor of COVID-19? A preregistered, crowdsourced study. Available from: https://osf.io/gxu7e.
- Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, Blondel M, Prettenhofer P, Weiss R, Dubourg V, et al. 2011. Scikit-learn: machine learning in Python. J Mach Learn Res. 12:2825–2830. [Google Scholar]
- Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. 2016. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses. 41(1):69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reback J, McKinney W, Jbrockmendel, Bossche JVD, Augspurger T, Cloud P, Gfyoung, Sinhrks, Klein A, Hawkins S, et al. 2020. pandas-dev/pandas: Pandas 1.0.5. Zenodo. Available from: https://zenodo.org/record/3708035#.X_CRz2RKi3I. [Google Scholar]
- Seabold S, Perktold J. 2010. Statsmodels: econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference; 2010 June 28–July 3; Austin, TX. 61 p. [Google Scholar]
- Smeets MAM, Veldhuizen MG, Galle S, Gouweloos J, de Haan AJA, Vernooij J, Visscher F, Kroeze JHA. 2009. Sense of smell disorder and health-related quality of life. Rehabil Psychol. 54(4):404–412. [DOI] [PubMed] [Google Scholar]
- Soler ZM, Patel ZM, Turner JH, Holbrook EH. 2020. A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol. 10(7):814–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh-Messinger J, Kaouk S, Manis H, Kaye R, Cecchi G, Meyer P, Malaspina D. 2020. Standardized testing demonstrates altered odor detection sensitivity and hedonics in asymptomatic college students as SARS-CoV-2 emerged locally. MedRxiv. 2020. doi: 10.1101/2020.06.17.20106302. [DOI]
- Weiss JJ, Attuquayefio TN, White EB, Li F, Herz RS, White TL, Campbell M, Geng B, Datta R, Wyllie AL, et al. 2020. Tracking smell loss to identify healthcare workers with SARS-CoV-2 infection. MedRxiv. 2020. doi: 10.1101/2020.09.07.20188813. [DOI] [PMC free article] [PubMed]
- Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. 2020. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol. 10(7):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. 2020. Self-reported olfactory loss associates with outpatient clinical course in COVID-19. Int Forum Allergy Rhinol. 10(7):821–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.