Abstract
Background
Feeding dogs with raw meat-based diets (RMBD) has increased in popularity in recent years. Proponents claim that RMBD is more natural for dogs, because it is what their ancestors (wolves) eat. Opponents claim that RMBD is a health hazard to both humans and animals, with a risk of spreading zoonotic bacteria and resistant bacterial strains.
Methods
This cross-sectional study investigated differences in bacteria shedding in faeces between dogs fed RMBD and dogs fed dry kibble. Faeces samples from 50 dogs from the same municipality were analysed for the presence of extended-spectrum beta-lactamases (ESBL)-producing Escherichia coli, Campylobacter and Salmonella.
Results
For the 25 dogs fed RMBD, ESBL E coli was isolated from 13 faeces samples, Campylobacter from 12 and Salmonella from 1. For the 25 dogs fed dry kibble, ESBL-producing E coli was isolated from one faeces sample and Campylobacter from four, while Salmonella was not detected.
Conclusion
There was thus a significant difference in excretion of zoonotic and resistant bacteria in faeces between dogs fed RMBD and dogs fed dry kibble. These results confirm that RMBD can pose a microbiological risk not only for dogs, but also for people handling RMBD and faeces from dogs.
Keywords: Campylobacter, dogs, Escherichia coli, intestinal disease, nutrition, Salmonella
Introduction
In recent years, it has become increasingly popular to feed dogs a raw meat-based diet (RMBD) instead of the more conventional dry kibble or canned pet foods. Raw meat-based diets, also called bones and raw food or biologically appropriate raw food (BARF) or raw animal products (RAP), do not undergo any form of heat treatment before chilling or freezing, and can be homemade or commercially produced. Feeds produced specifically for dogs have been available for the last 150 years. In the past, the diet of dogs usually consisted of household leftovers, food scraps/debris, garbage the dogs foraged themselves on the street or small animals they killed. The first dog biscuit was produced in the 1860s and the first commercial dog feed in 1922. It was not until the late 1950s that dry dog food was introduced in the market.1
There are different opinions about the advantages and disadvantages of RMBD. Proponents of feeding RMBD claim that raw diets featuring fresh, natural ingredients are unequivocally the best nutritional choice. In a survey, owners feeding RMBD to their pets reported important health benefits such as improvements in the immune system, skin and coat, a reduction in dental diseases and a lower incidence of food allergies.2 3 Opponents of RMBD consider it to pose a risk of infection in pet animals and also a risk of transmission of zoonotic and resistant bacteria to humans. Many previous studies have detected zoonotic bacteria in RMBD, sometimes at high levels.4–7 A Finnish study reported the presence of intestinal pathogens in RMBD and identified a risk of the diet becoming imbalanced and of bones perforating the gastrointestinal tract.4
The gastrointestinal microbiota is a highly complex ecosystem in dogs and cats, and also in humans. The gastrointestinal microbiota is significantly influenced by diet type and is estimated to consist of 1010–1014 bacteria per gram.8 9 Dogs fed a natural diet consisting of bones, raw meat and vegetables have been found to host a significantly more variable and abundant gastrointestinal flora than dogs fed a commercial diet.8 In a previous Swedish study of RMBD, Escherichia coli was isolated from all samples of RMBD, and antimicrobial resistance to extended-spectrum cephalosporins (ESC-resistant E coli) was detected in 23 per cent of the samples.10 This means that close contact between dogs and humans provides the opportunity for transmission of antimicrobial-resistant bacteria belonging to the Enterobacteriaceae, posing a risk to human health.11 12 Extended-spectrum beta-lactamases (ESBL)-producing bacteria that are resistant to multiple antibiotics are likely to be a major future challenge in both human and veterinary medicine.
Campylobacter can easily colonise the intestine of dogs and is frequently isolated from the faeces of healthy dogs.13 14 The Campylobacter species most commonly isolated from dog faeces is C upsaliensis, followed by C jejuni.14–17 Campylobacter species is common in the normal intestinal microbiota of both healthy food-producing animals (eg, poultry, pigs, cattle and sheep) and wild animals.18–21 These reservoirs continuously contaminate the environment and food products, and are a source of pathogens for campylobacteriosis in humans.
Salmonella species have been found in RMBD in several studies, at an incidence varying between 2 per cent and 20 per cent of samples tested.4 6 7 22 23 There are various transmission routes for Salmonella to RMBD, for example, the meat can be contaminated with Salmonella originating from the intestinal tract of the various animal species from which the offal derives.24 Salmonella can also originate from the spices, herbs or vegetables used in the RMBD formulation.25 As RMBD does not undergo any form of heat treatment before chilling or freezing, there are possibilities for dogs fed RMBD to be colonised with Salmonella and excrete it into the local environment. Transmission of Salmonella to humans in the same household has been reported, indicating that pet animals are a potential source of infection.26 27
The aim of this study was to investigate differences in the occurrence of ESBL E coli, Campylobacter species and Salmonella species in faeces samples from dogs fed RMBD and faeces samples from dogs receiving a strict dry food diet, that is, kibble.
Materials and methods
Sampling
Dog owners were recruited to participate in the study and submit faeces samples from their dogs for microbiological analysis, through ‘advertising’ in the Facebook groups ‘Veterinärstudenter’ (Veterinary students’) and ‘Vi som bor på Gälbo och Kronåsen’ (Residents in Gälbo and Kronåsen), and through personal requests to students and staff at the Swedish University of Agriculture Sciences (SLU). All dogs included in the study lived in the municipality of Uppsala, mainly on Campus Ultuna around SLU. All analyses were initiated within 24 hours after sampling. The faeces samples were taken from the ground immediately after defecation and stored in clean bags or storage cans until analysis. All sampling was performed in September and October, in most cases in the morning, and the analyses started on the afternoon of the same day.
The dogs had to meet the following criteria in order to be included in the study: at least six months old, clinically healthy (no symptoms of disease) and not treated with antibiotics in the previous two weeks. A total of 50 dogs were recruited and divided into two equal groups: those only fed dry kibble in the previous two weeks (dry feed group) and those fed some type of RMBD at least once a week, or preferably more, in the week before sampling (RMBD group).
In total, 50 faeces samples from 50 different dogs were analysed for presence of ESBL E coli, Campylobacter species and Salmonella species. The age of participating dogs varied from 6 months to 14 years. A range of different breeds were represented, such as Cocker Spaniel, pug, poodle, Nova Scotia duck tolling retriever, labrador retriever, flatcoated retriever, Akita, Norwich terrier, Siberian husky, Weimaraner, Eurasier, Finnish Laphund, collie, Australian shepherd and a number of mixed breeds. Many dogs in the RMBD group ate other feedstuffs in addition to RMBD, such as dry kibble. Of the 25 dogs in the RMBD group, 11 were also fed some dry kibble. The RMBD and the dry kibble originated from several different producers.
ESBL E coli
The presence of ESBL E coli was analysed both by direct culture and by culture by enrichment. Faeces were direct-cultured on CHROMagar Orientation (Chromagar, Paris, France) containing 1 mg cefotaxime (cephalosporin) per litre and incubated at 37°C for 24+24 hours. For enrichment, 10 g of faeces were mixed with 90 ml of buffered peptone water (BPW), dilution 1/10, incubated at 37°C for 18–24 hours, and then cultured on CHROMagar Orientation and incubated at 37°C for 18–24 hours. Suspected E coli colonies on CHROMagar Orientation were recultured on blood agar and incubated at 37°C±1°C for 24±3 hours, and their identity was confirmed by matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF MS).
Susceptibility testing was performed on 13 of the E coli isolated on CHROMagar Orientation as one of the isolates could not be recultured. Susceptibility to selected antimicrobial substances was assessed with VetMIC GN-mo (SVA, Uppsala, Sweden), determining the antimicrobial minimum inhibitory concentrations (MIC). The E coli reference strain CCUG 17620 was used as quality control. Epidemiological cut-off (ECOFF) values for determining susceptibility were obtained from the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (www.eucast.org, retrieved 12 July, 2019; table 1). ESBL is a group of enzymes, which are resistant to penicillins and many cephalosporins and borne on transmissible plasmids common among E coli, and other Enterobacteriaceae, whereas ESBLCARBA is the term for a mechanism of resistance to most penicillins, cephalosporins and carbapenem. Multidrug resistance is defined as resistance against beta-lactams and at least two other antibiotic classes.28 For example, resistance to ciprofloxacin and nalidixic acid was considered resistance to one antibiotic class (quinolones).
Table 1.
Antibiogram of 13 Escherichia coli strains isolated from faeces cultured on CHROMagar orientation with cefotaxime
| Antibiotic | Cut-off | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| Beta-lactam antibiotics | ||||||||||||||
| Ampicillin | >8 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| Cefotaxime | >0.25 | 2 | 2 | >4 | 4 | 2 | 2 | 2 | 2 | >4 | >4 | >4 | >4 | >4 |
| Ceftazidime | >0.5 | 2 | 8 | 2 | 8 | 4 | 4 | 4 | 2 | 2 | 2 | 2 | 2 | 2 |
| Meropenem | >0.125 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 | <0.03 |
| Quinolones | ||||||||||||||
| Ciprofloxacin | >0.06 | <0.015 | 0.25 | <0.015 | <0.015 | 0.03 | <0.015 | <0.015 | <0.015 | 8 | <0.015 | 0.5 | <0.015 | <0.015 |
| Nalidixic acid | >16 | <4 | <4 | 8 | <4 | <4 | <4 | <4 | <4 | >128 | <4 | 16 | <4 | |
| Amphenicols | ||||||||||||||
| Chloramphenicol | >16 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | <8 | >128 | <8 | 128 | <8 | <8 |
| Polymyxins | ||||||||||||||
| Colistin | >2 | <1 | <1 | <1 | 2 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 |
| Aminoglycosides | ||||||||||||||
| Gentamicin | >2 | 1 | 1 | <0.5 | <0.5 | <0.5 | 1 | <0.5 | 1 | <0.5 | <0.5 | <0.5 | <0.5 | 1 |
| Macrolides | ||||||||||||||
| Azithromycin | >16 | 8 | 32 | 8 | 8 | 8 | 8 | 16 | 8 | 16 | 4 | 8 | 4 | 8 |
| Tetracyclines | ||||||||||||||
| Tetracycline | >8 | <2 | <2 | <2 | <2 | 4 | <2 | >64 | <2 | >64 | <2 | 64 | <2 | <2 |
| Tigecycline | >0.5 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 | <0.25 |
| Sulfonamides and trimethoprim | ||||||||||||||
| Sulphamethoxazole | >64 | 16 | 16 | >1024 | 32 | 16 | 16 | 16 | 32 | >1024 | >1024 | <8 | >1024 | >1024 |
| Trimethoprim | >2 | 0.5 | >32 | 0.5 | 0.5 | 1 | 1 | 1 | 1 | >32 | 0.5 | >32 | 0.5 | 1 |
Strain no. 5 originated from a dog fed dry kibble, the others from dogs that received RMBD.
EUCAST, European Committee on Antimicrobial Susceptibility Testing; MIC, minimum inhibitory concentration; RMBD, raw meat-based diet.
Campylobacter species
The occurrence of Campylobacter was analysed according to ISO 10272 part 1 (2017), with some modifications. In brief, faeces were direct-cultured on modified charcoal-cefoperazone deoxycholate agar (mCCDA) (Oxoid, Basingstoke, UK) and the plates were incubated at 37.0°C±1°C for five days in a microaerophilic atmosphere generated by use of CampyGen (Oxoid, Basingstoke). Identification of Campylobacter species was based on typical morphological aspects, white to grey colonies with a metallic sheen according to ISO 10272 part 1 (2017). Suspected Campylobacter colonies were cultured on blood agar and incubated at 37.0°C±1°C for 44±4 hours, and their identity was confirmed by MALDI-TOF MS.
Salmonella species
The presence of Salmonella species was analysed according to method NMKL 187 (2nd edition, 2016). In brief, up to 25 g of faeces were homogenised and preincubated in 225 ml BPW at 37°C for 18±2 hours. If 25 g faeces were not available, one part faeces to nine parts BPW was used. Of all 50 samples analysed, five weighed less than 10 g, 33 weighed more than 20 g and the weight of the remaining 12 ranged from 10 to 20 g. The smallest amount of faeces analysed was 6.5 g. After incubation, three drops with a total volume of 100 µl of the enriched culture with BPW and faeces were added to selective Modified Semi-solid Rappaport-Vassiliadis (MSRV) with 10 mg/l novobiocin (Oxoid), and incubated at 41.5°C for 24±3 hours. If no suspected Salmonella were found on MSRV, the plate was incubated for a further 24±3 hours. Putative Salmonella colonies were subcultured on Brilliant Green agar (BG) (Oxoid) and Xylose Lysine Deoxycholate agar (XLD) (Oxoid), according to NMKL standards. Up to five suspected Salmonella colonies on BG and XLD were re-cultured on purple lactose agar, incubated at 37°C for 24±3 hours, and further analysed by MALDI-TOF MS. Colonies identified as Salmonella by MALDI-TOF MS were sent to the Swedish reference laboratory for Salmonella at the National Veterinary Institute (SVA) for confirmation and species identification. The results are expressed as Salmonella detected or not detected.
Species identification
Bacteria considered interesting and relevant to the study were further analysed by MALDI-TOF MS using a Microflex LT MALDI-TOF MS (Bruker Daltonics, Billerica, Massachusetts, USA). In brief, single colonies were picked from fresh agar plates and smeared/spotted on the MALDI-TOF MS target plate, followed by addition of 1 µl α-cyano-4-hydroxycinnamic acid matrix solution (Bruker Daltonics). After drying, the mass spectral fingerprint was generated with the MALDI-TOF MS instrument and the spectra obtained were compared against a reference database spectrum. Genus and species identification was then performed using a Bruker Maldi Biotyper system.
Statistical analysis
The results from the bacterial cultures were analysed by Fisher’s exact test performed using a statistical program on the internet website ‘Social Science Statistics’ (https://www.socscistatistics.com). The tests verified the association between bacterial secretion and diet. A probability level of P<0.05 was considered to be statistically significant.
Results
In the faeces samples from dogs in the RMBD group, Salmonella species, ESBL E coli and/or Campylobacter species were detected in 18 of the 25 samples (72 per cent). In the faeces samples from dogs in the dry feed group, Salmonella species, ESBL E coli and/or Campylobacter species were detected in 5 of the 25 samples (20 per cent). This difference in incidence was strongly statistically significant (P=0.0005) (figure 1).
Figure 1.
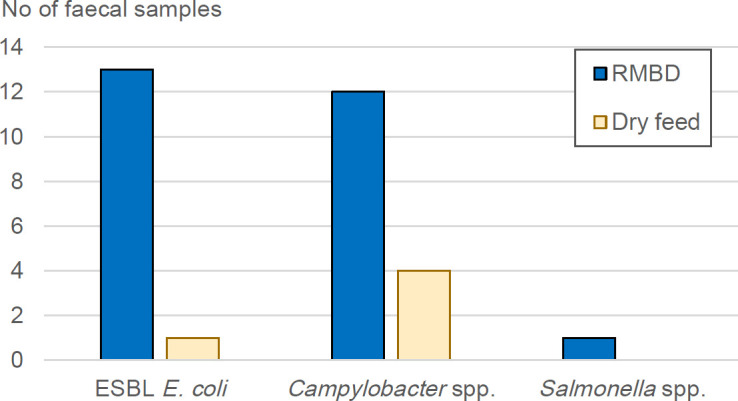
Number of faeces samples from the 25 dogs fed a raw meat-based diet (RMBD) and the 25 dogs fed only dry kibble in which extended-spectrum beta-lactamases Escherichia coli, Campylobacter species and/or Salmonella species were detected.
E coli with ESC resistance was isolated from 14 of the 50 faeces samples analysed. For the RMBD group, ESBL E coli was detected in 13 (52 per cent) of the 25 faeces samples. For the dry feed group, ESBL E coli was detected in only 1 (4 per cent) of the 25 faeces samples (figure 1). This difference was statistically significant (P=0.0003). All 13 tested E coli strains were resistant to ampicillin, cefotaxime and ceftazidime and 3 (isolate 2, 9 and 11) of them were resistant to three or more antibiotic classes, that is, multidrug resistant (table 1). Carbapenem-resistant E coli was not isolated from any of samples.
Campylobacter species were detected in 16 of the 50 faeces samples analysed. For the group of dogs fed RMBD, Campylobacter species were isolated from 12 (48 per cent) of the 25 faeces samples. For the group of dogs fed dry kibble, Campylobacter species were detected in 4 (16 per cent) of the 25 faeces samples (figure 1). This difference was statistically significant (P=0.03). One of the isolates was identified as C jejuni, which was found in a faeces sample from a dog in the RMBD group. The remaining Campylobacter species were identified as either C upsaliensis or C helveticus. Both may have been present in the positive faeces samples, or MALDI-TOF MS may not have been able to distinguish them because they are so closely related.
Salmonella species was only detected in one faeces sample, from a dog in the RMBD group (figure 1). The Salmonella species isolated was identified as S enterica subspecies enterica, serovar Typhimurium. Campylobacter species and ESBL E coli were not detected in the faeces sample that Salmonella species was detected.
Discussion
ESBL E coli was isolated more frequently in faeces samples from dogs fed RMBD than in samples from dogs fed dry kibble. This result was expected, as a previous Swedish study isolated E coli from all (n=39) feed samples of RMBD tested and found that nine (23 per cent) of the RMBD samples contained ESC-resistant E coli.10 ESC-resistant bacteria belonging to family Enterobacteriaceae have also been isolated from livestock and in RMBD in studies performed in other countries.7 29–33 It can be argued that RMBD should be completely abandoned for this reason, partly to avoid the risk to the dogs themselves, but also because there is a risk of spreading resistant bacterial genes to others in the household and in the community. It has been confirmed that ESBL E coli can be spread between animals and humans in the same household.34
In this study, there was a significant difference in the incidence of Campylobacter species in the faeces from dogs fed RMBD and dogs fed dry kibble. Similar results have been found in a study in New Zealand, where dogs consuming RMBD were 12.3 times more likely (P=0.03) to be carriers of C upsaliensis than dogs consuming dry feed.5 In that study, it was also shown that, when poultry was the main ingredient, RMBD was more likely (P=0.006) to be contaminated with Campylobacter species than when the main ingredient was some other type of meat.5 Unfortunately, the authors could not test for differences in the prevalence of Campylobacter species in faeces of dogs fed RMBD based on poultry compared with cattle or pig meat, since the authors did not have access to such data. The detection of Campylobacter species in 48 per cent of the faeces samples was not surprising, as several other studies have isolated Campylobacter species from the faeces of clinically healthy dogs, with a detection rate of 37 per cent–76 per cent.5 13 14 16 Campylobacteriosis is the most common reported zoonotic disease in Europe.24 Although C upsaliensis is not the most common cause of campylobacteriosis in humans, several cases have been described.35
Salmonella was only found in one of the faeces samples. This low level of Salmonella in dog faeces was expected, based on the low levels of Salmonella in livestock in Sweden and other north European countries,24 where most of the RMBD fed for dogs in Sweden is produced. It should be noted that, in 19 of the 50 samples, the amount of faeces analysed was below the amount recommended in the standard (25 g) (NMKL 187, 2nd edition, 2016), which may have influenced the possibility of detection of Salmonella species. Furthermore, based on the literature,24 there is a possibility that Salmonella species could have been isolated in more samples in this study if a faeces sample per day for five consecutive days had been analysed. Increasing the sampling frequency would probably increase the chances of isolating Salmonella species in more samples. In a Canadian study, faeces from dogs were sampled at two-month intervals for a full year and it was found that consumption of RMBD during the two-month period was strongly associated (P<0.001) with presence of Salmonella species in the faeces.33 In another study that investigated potential risk factors for dogs becoming carriers of Salmonella species, feeding RMBD was identified as a statistically significant (P<0.05) risk factor positively associated with colonisation by Salmonella.36 A study in Canada investigated whether dogs that eat Salmonella-contaminated RMBD shed Salmonella in their faeces.37 The feed and dogs were sampled before the study to ensure freedom from Salmonella, and then one group was fed a Salmonella-contaminated RMBD and a control group was fed a Salmonella-free RMBD. Of the 16 dogs receiving Salmonella-contaminated RMBD, Salmonella was isolated from the faeces from seven dogs (44 per cent). Salmonella was not isolated from any of the control dogs and the difference between the groups was statistically significant (P=0.01). The total number of days for which the dogs excreted Salmonella species varied between 1 and 11.37 An interesting finding in that study was that none of the dogs showed any clinical symptoms of gastroenteritis, which means that dogs could be silent carriers and spread Salmonella species to an unknown environment. Salmonella species can persist in food bowls despite cleaning and disinfection, for example, in a study where raw food inoculated with Salmonella was served in stainless steel and plastic bowls, Salmonella was detected in 33 per cent–100 per cent of the bowls after various cleaning procedures.38 This confirms that unconsumed raw meat should not be left in feed bowls, and that bowls should be removed and disinfected shortly after feeding. It also indicates the importance of avoiding inadvertent contact with RMBD and feed bowls by humans with a compromised immune system, for example, children or the elderly.
The present cross-sectional study mainly provides a snapshot of bacterial pathogens present in faeces samples from dogs fed different diets. One of the criteria for participation in the study was that dogs in the dry kibble group were fed only dry kibble during the previous two weeks, while dogs in the RMBD group were fed RMBD at least once, or preferably more, the last weeks before sampling. Eating RMBD at least once in the week before sampling was estimated to be sufficient to affect the gastrointestinal microbiota. Although the threshold for the RMBD group was set fairly low, most dogs included were given RMBD regularly and generally more than the threshold. However, the results may have been influenced by other factors, for example, participating dogs may have eaten carrion that they found outdoors, and thus it is not entirely certain that the feed was the only source of any bacteria detected. As there was no previous sampling of the dog faeces or a parallel sampling of food, the authors could not be sure that pathogens and resistant E coli in the faeces were a result from the RMBD. Moreover, the selection of participants was not entirely random, as recruitment took place via closed groups on Facebook and most participating dogs lived on Campus Ultuna in Uppsala. The advantage of this is that the dogs in the study lived in the same community, which means that their external environment was similar regardless of the feed they received. Despite the above, the results revealed a statistically significant difference (P=0.0005) in bacterial occurrence between dogs fed RMBD and those fed a strict dry food diet. The probability of this difference occurring by chance is thus very small.
Overall, the results obtained in this study show that it is highly important to handle RMBD, and faeces from dogs fed RMBD, with great care and to maintain good hygiene, due to the potential risks these biomaterials pose to human and animal health. Dog owners that fed their dogs with RMBD should (i) keep the RMDB frozen until used, (ii) separate RMBD from human food and handle RMBD with separate kitchen equipment, (iii) pick up the faeces from the dogs and sort it as combustible waste and not bio or organic waste, (iv) avoid feeding dogs RMBD at animal assisted activity/therapy, (vi) avoid feeding dogs with close contact to young and/or immunocompromised individuals. In 2011, the American Animal Hospital Association (AAHA) issued a statement warning that RMBD may pose a risk to the animal that eats it, to other animals that come into contact with that animal or its faeces, to people in the animal’s household and to the general public. The statement goes on to point out that this could not only be a potential health problem for animals, but could become a major problem in a public health perspective.
Conclusions
This study examined whether excretion of certain specific zoonotic bacteria and ESBL-producing E coli in dog faeces differs depending on whether the dogs are fed RMBD or not. The results indicated a high microbiological risk with feeding RMBD. Excretion of zoonotic and resistant bacteria in faeces was significantly higher for dogs fed RMBD than for dogs fed dry kibble. The bacteria in dog faeces pose a risk of being excreted in the local environment and transmitted to other animals and humans. Thus if RMBD is used, it is necessary to have careful handling and sound hygiene procedures to avoid spread of zoonotic and/or resistant bacteria. In view of the resistance problem, dogs should not be fed RMBD while they are being treated with antimicrobials, as this could increase the risk of resistant strains being selected and multiplying. Dogs in families with infants, elderly people or immunocompromised individuals should also not be fed RMBD, as these groups are more susceptible to infections.
Acknowledgments
The authors gratefully acknowledge the dogs and owners participating in the study, for making this work possible and Oskar Nilsson at SVA for fruitful discussions regarding extended-spectrum beta-lactamases Escherichia coli.
Footnotes
Contributors: All authors contributed substantially to interpretation of data, drafting the final manuscript and critical revision for important intellectual content.
Funding: This research was funded by the C August Carlsson Foundation at the Faculty of Veterinary Medicine and Animal Sciences, Swedish University of Agricultural Sciences.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Corresponding author have all data but it is not publicly available
References
- 1. Case LP, Daristotle L, Hayek MG, et al. History and regulation of PET foods. I: canine and feline nutrition Elsevier, 2011: 121–9. https://linkinghub.elsevier.com/retrieve/pii/B9780323066198100143 [Google Scholar]
- 2. Morgan SK, Willis S, Shepherd ML. Survey of owner motivations and veterinary input of owners feeding diets containing RAW animal products. PeerJ 2017;5:e3031 10.7717/peerj.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morelli G, Bastianello S, Catellani P, et al. Raw meat-based diets for dogs: survey of owners’ motivations, attitudes and practices. BMC Vet Res 2019;15:1 10.1186/s12917-019-1824-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fredriksson-Ahomaa M, Heikkilä T, Pernu N, et al. Raw meat-based diets in dogs and cats. Vet Sci 2017;4:33 10.3390/vetsci4030033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bojanić K, Midwinter AC, Marshall JC, et al. Isolation of Campylobacter spp. from Client-Owned Dogs and Cats, and Retail Raw Meat Pet Food in the Manawatu, New Zealand. Zoonoses Public Health 2017;64:438–49. 10.1111/zph.12323 [DOI] [PubMed] [Google Scholar]
- 6. Hellgren J, Hästö LS, Wikström C, et al. Occurrence of Salmonella, Campylobacter, Clostridium and Enterobacteriaceae in raw meat-based diets for dogs. Vet Rec 2019;184:442 10.1136/vr.105199 [DOI] [PubMed] [Google Scholar]
- 7. van Bree FPJ, Bokken GCAM, Mineur R, et al. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet Rec 2018;182:50 10.1136/vr.104535 [DOI] [PubMed] [Google Scholar]
- 8. Kim J, An J-U, Kim W, et al. Differences in the gut microbiota of dogs (Canis lupus familiaris) fed a natural diet or a commercial feed revealed by the Illumina MiSeq platform. Gut Pathog 2017;9:68 10.1186/s13099-017-0218-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sender R, Fuchs S, Milo R. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533 10.1371/journal.pbio.1002533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nilsson O. Hygiene quality and presence of ESBL-producing Escherichia coli in raw food diets for dogs. Infect Ecol Epidemiol 2015;5:1–4. 10.3402/iee.v5.28758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Guardabassi L, Schwarz S, Lloyd DH. Pet animals as reservoirs of antimicrobial-resistant bacteria: review. J Antimicrob Chemother 2004;54:321–32. 10.1093/jac/dkh332 [DOI] [PubMed] [Google Scholar]
- 12. Pomba C, Rantala M, Greko C, et al. Public health risk of antimicrobial resistance transfer from companion animals. J Antimicrob Chemother 2017;72:957–68. 10.1093/jac/dkw481 [DOI] [PubMed] [Google Scholar]
- 13. Hald B, Pedersen K, Wainø M, et al. Longitudinal study of the excretion patterns of thermophilic Campylobacter spp. in young pet dogs in Denmark. J Clin Microbiol 2004;42:2003–12. 10.1128/JCM.42.5.2003-2012.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holmberg M, Rosendal T, Engvall EO, et al. Prevalence of thermophilic Campylobacter species in Swedish dogs and characterization of C. jejuni isolates. Acta Vet Scand 2015;57:1 10.1186/s13028-015-0108-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Parsons BN, Porter CJ, Ryvar R, et al. Prevalence of Campylobacter spp. in a cross-sectional study of dogs attending veterinary practices in the UK and risk indicators associated with shedding. Vet J 2010;184:66–70. 10.1016/j.tvjl.2009.01.009 [DOI] [PubMed] [Google Scholar]
- 16. Acke E, McGill K, Golden O, et al. Prevalence of thermophilic Campylobacter species in household cats and dogs in Ireland. Vet Rec 2009;164:44–7. 10.1136/vr.164.2.44 [DOI] [PubMed] [Google Scholar]
- 17. Leonard EK, Pearl DL, Janecko N, et al. Factors related to Campylobacter spp. carriage in client-owned dogs visiting veterinary clinics in a region of Ontario, Canada. Epidemiol Infect 2011;139:1531–41. 10.1017/S0950268810002906 [DOI] [PubMed] [Google Scholar]
- 18. Jore S, Viljugrein H, Brun E, et al. Trends in Campylobacter incidence in broilers and humans in six European countries, 1997–2007. Prev Vet Med 2010;93:33–41. 10.1016/j.prevetmed.2009.09.015 [DOI] [PubMed] [Google Scholar]
- 19. Stanley KN, Wallace JS, Currie JE, et al. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J Appl Microbiol 1998;85:472–80. 10.1046/j.1365-2672.1998.853511.x [DOI] [PubMed] [Google Scholar]
- 20. Engvall EO, Brändström B, Gunnarsson A, et al. Validation of a polymerase chain reaction/restriction enzyme analysis method for species identification of thermophilic campylobacters isolated from domestic and wild animals. J Appl Microbiol 2002;92:47–54. 10.1046/j.1365-2672.2002.01491.x [DOI] [PubMed] [Google Scholar]
- 21. Tegner C, Sunil-Chandra NP, Wijesooriya WRPLI, et al. Detection, Identification, and Antimicrobial Susceptibility of Campylobacter spp. and Salmonella spp. from Free-ranging Nonhuman Primates in Sri Lanka. J Wildl Dis 2019;55:879 10.7589/2018-08-199 [DOI] [PubMed] [Google Scholar]
- 22. Mehlenbacher S, Churchill J, Olsen KE, et al. Availability, brands, labelling and Salmonella contamination of raw PET food in the Minneapolis/St. Paul area. Zoonoses Public Health 2012;59:513–20. 10.1111/j.1863-2378.2012.01491.x [DOI] [PubMed] [Google Scholar]
- 23. Nemser SM, Doran T, Grabenstein M, et al. Investigation of Listeria, Salmonella, and Toxigenic Escherichia coli in Various Pet Foods. Foodborne Pathog Dis 2014;11:706–9. 10.1089/fpd.2014.1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. EFSA European food safety authority) and ECDC (European centre for disease prevention and control). The European Union summary report on trends and sources of zoonoses, zoonotic agents and food‐borne outbreaks in 2017. EFSA J 2018;16:5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zweifel C, Stephan R. Spices and herbs as source of Salmonella-related foodborne diseases. Food Res Int 2012;45:765–9. 10.1016/j.foodres.2011.02.024 [DOI] [Google Scholar]
- 26. Morse EV, Duncan MA, Estep DA, et al. Canine salmonellosis: A review and report of dog to child transmission of Salmonella enteritidis. Am J Public Health 1976;66:82–3. 10.2105/AJPH.66.1.82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sato Y, Mori T, Koyama T, et al. Salmonella Virchow infection in an infant transmitted by household dogs. J Vet Med Sci 2000;62:767–9. 10.1292/jvms.62.767 [DOI] [PubMed] [Google Scholar]
- 28. Swedres-Svarm Consumption of antibiotics and occurrence of resistance in Sweden. Solna/Uppsala, 2018. [Google Scholar]
- 29. Founou LL, Founou RC, Essack SY. Antibiotic resistance in the food chain: a developing country-perspective. Front Microbiol 1881;2016:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Leonard EK, Pearl DL, Janecko N, et al. Risk factors for carriage of antimicrobial-resistant Salmonella spp and Escherichia coli in pet dogs from volunteer households in Ontario, Canada, in 2005 and 2006. Am J Vet Res 2015;76:959–68. 10.2460/ajvr.76.11.959 [DOI] [PubMed] [Google Scholar]
- 31. Finley R, Reid-Smith R, Ribble C, et al. The occurrence and antimicrobial susceptibility of Salmonellae isolated from commercially available canine raw food diets in three Canadian cities. Zoonoses Public Health 2008;55:462–9. 10.1111/j.1863-2378.2008.01147.x [DOI] [PubMed] [Google Scholar]
- 32. Seiffert SN, Hilty M, Perreten V, et al. Extended-spectrum cephalosporin-resistant gram-negative organisms in livestock: an emerging problem for human health? Drug Resist Updat 2013;16:22–45. 10.1016/j.drup.2012.12.001 [DOI] [PubMed] [Google Scholar]
- 33. Lefebvre SL, Reid-Smith R, Boerlin P, et al. Evaluation of the risks of shedding Salmonellae and other potential pathogens by therapy dogs fed raw diets in Ontario and Alberta. Zoonoses Public Health 2008;55:470–80. 10.1111/j.1863-2378.2008.01145.x [DOI] [PubMed] [Google Scholar]
- 34. Johnson JR, Owens K, Gajewski A, et al. Escherichia coli Colonization Patterns among Human Household Members and Pets, with Attention to Acute Urinary Tract Infection. J Infect Dis 2008;197:218–24. 10.1086/524844 [DOI] [PubMed] [Google Scholar]
- 35. Patrick ME, Henao OL, Robinson T, et al. Features of illnesses caused by five species of Campylobacter, Foodborne Diseases Active Surveillance Network (FoodNet) – 2010–2015. Epidemiol Infect 2018;146:1–10. 10.1017/S0950268817002370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Leonard EK, Pearl DL, Finley RL, et al. Evaluation of pet-related management factors and the risk of Salmonella spp. carriage in pet dogs from volunteer households in Ontario (2005-2006). Zoonoses Public Health 2011;58:140–9. 10.1111/j.1863-2378.2009.01320.x [DOI] [PubMed] [Google Scholar]
- 37. Finley R, Ribble C, Aramini J, et al. The risk of salmonellae shedding by dogs fed Salmonella-contaminated commercial RAW food diets. Can Vet J 2007;48:69–75. [PMC free article] [PubMed] [Google Scholar]
- 38. Weese JS, Rousseau J. Survival of Salmonella Copenhagen in food bowls following contamination with experimentally inoculated raw meat: effects of time, cleaning, and disinfection. Can Vet J 2006;47:887–9. [PMC free article] [PubMed] [Google Scholar]


