Abstract
The complete mitochondrial genome of Isonychia kiangsinensis is a circular molecule of 15,456 bp in length, containing 2 rRNA genes, 13 protein-coding genes, 22 tRNA genes, and a control region. The AT content of the overall base composition is 62.9%. The length of the control region for I. kiangsinensis is 745 bp with 68.6% AT content. In BI and ML phylogenetic trees, Isonychia kiangsinensis was a sister clade to I. ignota and Isonychiidae was shown to be the basal clade of Ephemeroptera excluding Siphluriscidae. The monophyly of the families Isonychiidae, Heptageniidae, Viemamellidae, and Baetidae and the genus Isonychia were well supported.
Keywords: Ephemeroptera, mitochondrial genome, Isonychia kiangsinensis, phylogeny
The family Isonychiidae is composed of one genus (Isonychia) and two subgenera (Isonychia and Prinoides) (Tiunova et al. 2004; Tungpairojwong and Boonsoong 2011). The phylogenetic relationship of Isonychiidae is controversial both in morphological and molecular aspects (Demoulin 1961; McCafferty and Edmunds 1979; Hebert et al. 2003; Ogden and Whiting 2005; Sun et al. 2006; O’Donnell and Jockusch 2008; Ogden et al. 2009; Webb et al. 2012; Saito et al. 2016). More molecular evidence needs to be discovered to clarify the status of this system. Thus, we sequenced the mitochondrial genome of Isonychia kiangsinensis and discussed its phylogenetic relationship within Ephemeroptera.
Samples of I. kiangsinensis were collected in Jingning (27°58′22′′ N, 119°38′10′′ E), Zhejiang province, China and identified by Dr. Zhang. The total genomic DNA was extracted from the hindleg of I. kiangsinensis using an Ezup Column Animal Genomic DNA Purification Kit (Sangon Biotech Company, Shanghai, China). All mayflies samples and DNA samples were stored in the lab of Dr. Zhang, College of Chemistry and Life Science, Zhejiang Normal University. The universal primers and specific primers for polymerase chain reaction (PCR) amplification were designed as in Zhang et al. (2008).
The mitochondrial genome of I. kiangsinensis showed the typical insect arrangement and is a circular molecule of 15,456 bp length. The AT content of the overall base composition is 62.9%, and the length of the control region is 745 bp with 68.6% AT content. Most of the protein-coding genes (PCGs) used ATN (N represents A, T, C, G) as the initiation codon whereas ND2 and ND5 were initiated by GTG. The COX1, COX2, ND4, ND5, and Cyt b genes used T as the termination codon and the other PCGs ended with TAA or TAG.
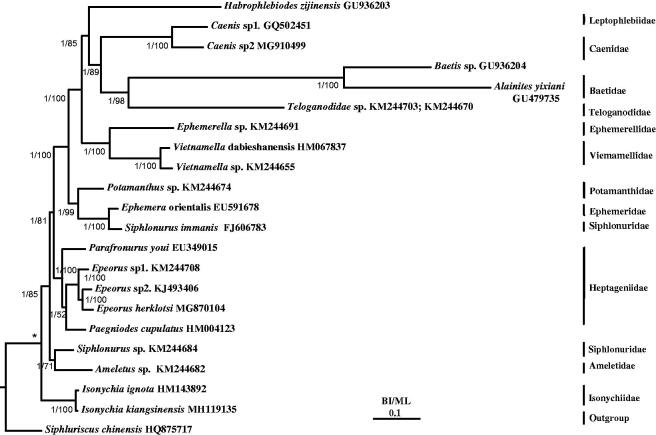
Bayesian inference (BI) and maximum likelihood (ML) trees were constructed using the 13 PCGs from 22 species (Zhang et al. 2008; Li et al. 2014; Tang et al. 2014; Zhou et al. 2016; Gao et al. 2018) including Siphluriscus chinensis (Li et al. 2014) as the outgroup (Figure 1). To select conserved regions of the nucleotides, each alignment was performed by Gblocks 0.91b (Castresana, 2000). BI and ML analyses were performed by MrBayes 3.1.2 (Huelsenbeck & Ronquist, 2001) and RAx ML 8.2.0 (Stamatakis 2014), respectively.
Figure 1.
Phylogenetic tree of the relationships among 22 species of Ephemeroptera, including Isonychia kiangsinensis based on the nucleotide dataset of the 13 mitochondrial protein-coding genes. Numbers above branches specify posterior probabilities as determined from BI (left) and bootstrap percentages from ML (right). The GenBank accession numbers of all species are also shown.
Isonychia kiangsinensis was shown to be a sister clade to I. ignota (HM143892). Siphluriscus chinensis (Siphluriscidae) is the basal clade to Ephemeroptera and Isonychiidae is the basal clade to Ephemeroptera excluding Siphluriscidae. The monophyly of the families Isonychiidae, Heptageniidae, Viemamellidae, and Baetidae and the genus Isonychia were well supported in both BI and ML analyses (Figure 1). The monophyly of Siphlonuridae failed to be supported in BI and ML analyses as also reported by Gao et al. (2018). Long branch attraction was found in Baetidae which may affect the phylogenetic relationship between Teloganodidae and Baetidae. In this study, Teloganodidae is a sister clade to Baetidae (Baetis sp. + Alainites yixiani) as also shown in Gao et al. (2018) but differs from the results of Ogden and Whiting (2005).
Nucleotide sequence accession number
The complete mitochondrial genome of Isonychia kiangsinensis has been assigned the GenBank accession number MH119135.
Acknowledgments
The authors would like to thank Ya-Jie Gao for aid in taxon sampling.
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the article.
References
- Castresana J. 2000. Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol. 17:540–552. [DOI] [PubMed] [Google Scholar]
- Demoulin G. 1961. A propos des donnees recentes sur la caenis maxima Joly (Ephemeroptera). Bull Ann Soc Roy Ent Belg. 97:63–68. [Google Scholar]
- Gao XY, Zhang SS, Zhang LP, Yu DN, Zhang JY, Chen HY.. 2018. The complete mitochondrial genome of Epeorus herklotsi (Ephemeroptera: Heptageniidae) and its phylogeny. Mitochondrial DNA B. 3:303–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert PDN, Cywinska A, Ball SL, Dewaard JR.. 2003. Biological identification through DNA barcodes. Proc Royal Soc Lond B. 270:313–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsenbeck JP, Ronquist F.. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17:754–755. [DOI] [PubMed] [Google Scholar]
- Li D, Qin JC, Zhou CF.. 2014. The phylogeny of Ephemeroptera in Pterygota revealed by the mitochondrial genome of Siphluriscus chinensis (Hexapoda: Insecta). Gene. 545:132. [DOI] [PubMed] [Google Scholar]
- McCafferty WP, Edmunds GF.. 1979. The higher classification of the Ephemeroptera and its evolutionary basis. Ann Entomol Soc Am. 72:5–12. [Google Scholar]
- O’Donnell BC, Jockusch EL. 2008. Phylogenetic relationships of leptophlebiid mayflies as inferred by histone H3 and 28S ribosomal DNA. Syst Entomol. 33:651–667. [Google Scholar]
- Ogden TH, Whiting MF.. 2005. Phylogeny of Ephemeroptera (mayflies) based on molecular evidence. Mol Phylogenet Evol. 37:625–643. [DOI] [PubMed] [Google Scholar]
- Ogden TH, Gattolliat JL, Sartori M, Taniczek AH, Soldán T, Whiting MF.. 2009. Towards a new paradigm in mayfly phylogeny (Ephemeroptera): combined analysis of morphological and molecular data. Syst Entomol. 34:616–634. [Google Scholar]
- Saito R, Jo J, Sekiné K, Bae YJ, Tojo K.. 2016. Phylogenetic analyses of the isonychiid mayflies (Ephemeroptera: Isonychiidae) in the northeast palearctic region. Entomol Res. 46:246–259. [Google Scholar]
- Stamatakis A. 2014. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 30:1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Sabo A, Meyer MD, Randolph RP, Jacobus LM, McCafferty WP, Ferris VR.. 2006. Tests of current hypotheses of Mayfly (Ephemeroptera) phylogeny using molecular (18s rDNA) data. Ann Entomol Soc Am. 99:241–252. [Google Scholar]
- Tang M, Tan MH, Meng GL, Yang SZ, Su X, Liu SL, Song WH, Li YY, Wu Q, Zhang A, Zhou X.. 2014. Multiplex sequencing of pooled mitochondrial genomes – a crucial step toward biodiversity analysis using mito-metagenomics. Nucleic Acids Res. 42:e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiunova TM, Kluge NJ, Ishiwata S.. 2004. Revision of the East Palaearctic genus Isonychia (Ephemeroptera: Isonychiidae). Can Entomol. 136:1–41. [Google Scholar]
- Tungpairojwong N, Boonsoong B.. 2011. New records of Isonychia formosana, Prosopistoma annamense and Prosopistoma sinense (Ephemeroptera) from Thailand. Entomol Res. 41:66–69. [Google Scholar]
- Webb JM, Jacobus LM, Funk DH, Zhou X, Kondratieff B, Geraci CJ, DeWalt RE, Baird DJ, Richard B, Phillips I, Hebert PD.. 2012. A DNA barcode library for North American Ephemeroptera: progress and prospects. PLoS One. 7:e38063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Zhou CF, Gai YH, Song DX, Zhou KY.. 2008. The complete mitochondrial genome of Parafronurus youi (Insecta: Ephemeroptera) and phylogenetic position of the Ephemeroptera. Gene. 424:18–24. [DOI] [PubMed] [Google Scholar]
- Zhou D, Wang YY, Sun JZ, Han YK, Zhou CF.. 2016. The complete mitochondrial genome of Paegniodes cupulatus (Ephemeroptera: Heptageniidae). Mitochondrial DNA A. 27:925–926. [DOI] [PubMed] [Google Scholar]