Abstract
Currently, the world is challenged by the coronavirus disease 2019 (COVID-19) pandemic. Epidemiologists and researchers worldwide are invariably trying to understand and combat this precarious new disease. Scrutinizing available drug options and developing potential new drugs are urgent needs to subdue this pandemic. Several intervention strategies are being considered and handled worldwide with limited success, and many drug candidates are yet in the trial phase. Despite these limitations, the development of COVID-19 treatment strategies has been accelerated to improve the clinical outcome of patients with COVID-19, and some countries have efficiently kept it under control. Recently, the use of natural and traditional medicine has also set the trend in coronavirus treatment. This review aimed to discuss the prevailing COVID-19 treatment strategies available globally by examining their efficacy, potential mechanisms, limitations, and challenges in predicting a future potential treatment candidate and bridging them with the effective traditional Chinese medicine (TCM). The findings might enrich the knowledge on traditional alternative medication and its complementary role with Western medicine in managing the COVID-19 epidemic.
Keywords: COVID-19, treatment, Chinese Medicine, strategies
1. Introduction
Since December 2019, the new coronavirus disease (COVID-19) has become a major global epidemic threat. On January 30, 2020, the World Health Organization (WHO) announced COVID-19 as a public health emergency of international concern (PHEIC) [1]. Up to November 23, 2020, 86,464 confirmed cases, 81,508 (94.27%) cured cases, and 4634 (5.36%) deaths were reported in mainland China (www.nhc.gov.cn/xcs/yqtb/list_gzbd.shtml). China has efficiently controlled this epidemic crisis. However, the global scenario seems out of control with the death toll more than one million, and more than 57 million people have been diagnosed with the viral infection (www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports).
COVID-19 is caused by a new coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which belongs to the β-coronavirus group and is highly pathogenic to humans. SARS-CoV-2 is the third coronavirus strain discovered so far that can cause zoonotic diseases. The other two strains are SARS-CoV and Middle East respiratory syndrome (MERS) coronavirus (MERS-CoV). COVID-19 is the third global plague caused by coronavirus in the last 20 years. During 2002-2003, bat-originated SARS-CoV, which could be transmitted to humans through the intermediary host palm civet cat, infected 8422 people in China and Hong Kong. SARS-CoV resulted in 916 deaths and increased the cumulative mortality rate to 11% [2]. Ten years later, another coronavirus named MERS-CoV swept Saudi Arabia, which also originated from bats, but was transmitted to humans through its intermediate host camel. MERS-CoV infected 2494 people, caused 858 deaths, and increased the cumulative mortality rate to 34% [3].
Phylogenetic analysis has shown that SARS-CoV-2 belongs to the Sarbecovirus subtype of β-coronavirus, which is similar to the other two bat-derived severe acute respiratory syndrome (SARS)-like coronaviruses collected in 2018, named bat-SL-CoVZC45 and bat-SL-CoVZXC21 (88% similarity), but far away from SARS-CoV (about 79% similarity) and MERS-CoV (about 50% similarity) [4]. A recent report has revealed that the β-coronavirus found in Malayan pangolins (Manis javanica) is a close relative of SARS-CoV-2 with a high degree of identity (97%) in the receptor-binding domain (RBD) sequence [5]. The homology modeling has revealed that SARS-CoV-2 shares a similar RBD structure to SARS-CoV, indicating similar pathogenesis between these two diseases [4]. S protein of coronavirus can bind to angiotensin-converting enzyme 2 (ACE2) of the host cell to promote the entry of the virus into the target cell [6], while the tissue distribution of the host receptor is usually consistent with the tropisms of the virus [7]. ACE2 is mainly distributed in human respiratory epithelial cells, lung parenchyma, vascular endothelium, kidney cells, and small intestine cells [8, 9]. Mutation and recombination are the most important mechanisms in the evolution of RNA viruses and also the main reason for the genetic diversity of SARS-CoV-2. Nine putative recombination patterns have been identified in the SARS-CoV-2 genome, including six key recombination regions in the S gene, RNA-dependent RNA polymerase (RdRp), nsp13, and ORF3a. [10] The number and incidence of mutations are much higher in Europe and North America compared with Asia, which also indicates different mutation patterns. [11] A high-throughput and high-coverage SARS-CoV-2 complete genome sequence study has observed that distinct time-course evolution patterns are divided into four main mutation groups. [12] Two nonsynonymous mutations A23403G and C14408T are mainly from Europe and North America, one nonsynonymous mutation T28144C is mainly distributed in Asia and Spain, two nonsynonymous mutations G11083T and G26144T are mainly distributed in Asia and some European countries, while two other nonsynonymous mutations G1440A and G2891A are mainly found in Europe. However, some virus strains may be eliminated with the control of the disease and the passage of time. At present, the S protein D614G mutant strain (A23403G) has become the most common form of a global pandemic. G614 mutation is related to higher infectivity. The quantitative analysis has revealed that the infectivity titer of virus particles with D614G mutation increases by 2.6-9.3 times [13]. Drug screening should pay attention to the efficiency on different SARS-CoV-2 lineages or clades or subtypes to speed up drug development.
SARS-CoV-2- and SARS-CoV-infected patients have similar symptoms of lower respiratory tract infection and gastrointestinal manifestations; four amino acid variations of S protein exist between SARS-CoV-2 and SARS-CoV [14, 15]. Animal experiments have proved that SARS-CoV-2 can effectively replicate in respiratory epithelial cells of the entire respiratory tract tissues (including nasal cavity, bronchi, bronchioles, and alveoli) [16]. The symptoms of SARS-CoV-2 infection are mainly lower respiratory tract infections, such as fever and dry cough. Meanwhile, some patients have nasal congestion and runny nose; some patients might suffer from fatigue and myalgia; while a small number of patients also show digestive tract reactions, such as vomiting and diarrhea [17-20]. In severe cases, dyspnea and/or hypoxemia usually occur within a week of onset. More severe cases quickly progress to acute respiratory distress syndrome, septic shock, diffuse coagulation, and multiple-organ failure. SARS-CoV-2 has three main routes of person-to-person transmission: respiratory droplets, such as coughing, sneezing, and droplet inhalation; contact transmission, such as contact with the oral cavity, nasal cavity, and eye mucosa [21]; and aerosol transmission, for which experiments have shown that SARS-CoV-2 can remain viable in aerosols for 3 h [22], and virus aerosolization in a confined space may be contagious [23]. Patients with COVID-19 are most infectious before the onset of symptoms, while SARS-CoV-2 begins to shed 2-3 days before the first wave of symptoms appears. After symptoms appear, the viral load in the patient's body decreases faster [24]. A report of a SARS-CoV-2 infection in Germany indicates that the spread of the virus may also occur through contact with asymptomatic patients [25]. In a new study of an asymptomatic cynomolgus monkey model, SARS-CoV-2 virus shedding peaked in the early stage (4 days) of the infection process, similar to the situation of asymptomatic patients [16]. However, no reliable evidence has shown the efficiency of fecal-oral transmission route and vertical transmission risk yet, which seems relatively low temporarily, but still needs further research and confirmation [26, 27].
Data on pathology, computerized tomography scan (CT) imaging, clinical characteristics, or complications have shown that both MERS and SARS are more severe than COVID-19 [28-49] (Table 1). The mortality rate of MERS-CoV infection is more than three and six times of SARS-CoV and SARS-CoV-2, respectively. However, MERS-CoV is not easy to be transmitted from person to person through close contact with infected patients; it is primarily through droplets of infected persons [50]. Despite the low case mortality rate, the number of deaths caused by COVID-19 has already exceeded the sum of SARS and MERS [51]. Taken together, it indicates that the differences in the epidemiological characteristics of these viruses can be caused by other factors, including high viral load of the upper respiratory tract, possibility of asymptomatic distribution and transmission of the virus in people infected with SARS-CoV-2, as well as other related social aspects. It has been found that the SARS-CoV RNA viral load reaches its peak 7-10 days after the onset of SARS symptoms, while the peak of SARS-CoV-2 RNA is within 5 days after the onset of symptoms [52]. The Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8) states that the incubation period is infectious, and highly infectious within 5 days after the onset [49]. In addition, SARS-CoV-2 begins to shed 2-3 days before the onset of symptoms [24]. When the patient’s symptoms are still mild and the symptoms of upper respiratory tract infection have just appeared, the new coronavirus can spread more effectively than the SARS virus through active pharynx virus shedding activities. The intensive care unit (ICU) air samples and low-altitude samples of Wuhan Fangcang Hospital have been tested positive for the virus, indicating that the deposition and resuspension of virus aerosols on protective clothing or floor surfaces are potential transmission routes [53]. Meanwhile, exposure to virus-contaminated objects can also cause infection [49]. The airborne SARS-CoV-2 concentration increases significantly with the increase in the flow of people. The gathering of asymptomatic carriers may be a potential source of airborne SARS-CoV-2 [53]. Experiments have also shown that the stability of SARS-CoV-2 in aerosols and on metal surface is similar to that of SARS-CoV [22]. Currently, no specific and effective clinical treatment exists for these three diseases. The official clinical management strategies of SARS [46, 47], MERS [48], and COVID-19 [49] are summarized in Table 1, which are mainly based on supportive treatments focusing on relieving symptoms and preventing or treating complications. The specific treatment should depend on clinical manifestations and patient factors (for example, age and whether they have comorbidities). Meanwhile, many drug candidates have shown good therapeutic prospects and may be valuable in the future.
Table 1.
Pathogenetic, epidemiological and clinical characteristics of SARS-CoV-2, SARS-CoV and MERS-CoV.
| SARS-Cov | MERS-Cov | SARS-Cov-2 | |
|---|---|---|---|
| Genus | Beta-CoVs lineage B | Beta-CoVs lineage C | Beta-CoVs lineage B |
| Date/Place first detected | November 2002, Guangdong China | June 2012, Jeddah, Saudi Arabla | December 2020, Wuhan China |
| Possible nature reservoir | Bat | Bat | Bat |
| Possible intermediate host | Palm civets | Camel | Pangolin |
| Virus transmission | 1. Respiratory droplets 2. Contact 3. Aerosol [28] |
1. Respiratory droplets 2. Contact [29] |
1. Respiratory droplets 2. Contact 3. Aerosol [30] |
| Predominant cellular receptor [31] | ACE2 | DDP4 | ACE2 |
| Receptor distribution [31] | Arterial and venous endothelium; arterial smooth muscle; small intestine, respiratory tract epithelium; alveolar monocytes and macrophages | Respiratory tract epithelium; kidney, small intestine; liver and prostate; activated leukocytes | Arterial and venous endothelium; arterial smooth muscle; small intestine, respiratory tract epithelium; alveolar monocytes and macrophages |
| Number of affected countrie and area | 29 | 27 | 183 |
| Confirmed cases | 8096 | 2494 | 571678 |
| Death cases | 744 | 858 | 26494 |
| Mortality rate | 9.19% | 34.40% | 4.63% |
| Severity Rate | 10-20% [28] | -- | 7-21% [17, 18, 32] |
| Incubation Period | 4d (2-10d)[33] | 5.2d (2-14d)[34] | 5.2d (2-14d)[35] |
| Epidemic doubling time[36] | 4.6-14.2 | 90 | 6.4 |
| Reproductive number, R0[36, 37] | 1.4-5.5 | <1 | 2.2-3.6 |
| Ventilation support | 13-26% [38] | 85.2% [39] | 4.2% [32] |
| ICU admission | 19-34% [38] | 53-89% [39] | 10% [40] |
| invasive mechanical ventilation | 17% [31] | 37% [31] | 7-9.6% [17, 18] |
| Symptom | Fever (99%); Headache (39%); Myalgia (59%); Cough(58%); Shortness of breath (27%); Sore throat (17%); Nausea/vomiting (15%); Diarrhoea (17%)[31] |
Fever (84%); Headache (19%); Myalgia (98%); Cough(63%); Shortness of breath 35%); Sore throat (13%); Nausea/vomiting (15%); Diarrhoea (20%)[31] |
Fever (83%) Cough (82%) Shortness of breath (31%) Muscle ache (11%) Confusion (9%) Headache (8%) Sore throat (5%) Rthinorrhoea (4%) [18] |
| Pathology | Edematous lung, bronchial epithelial denudation, loss of cilia, squamous metaplasia fibrosis [41]. | Exudative diffuse alveolar damage with hyaline membranes, pulmonary edema, type II pneumocyte hyperplasia, interstitial pneumonia [41]. | Inflammation, mucus and fibrosis [18]. |
| CT imaging | 1. Air-space opacities; 2. ~50% unilateral multifocal or bilateral involvement [31]. |
1. Ground glass opacities and consolidation; 2. Higher rate of Pleural effusion and pneumothorax [31]. |
1. Small patches and interstitial changes; 2. Ground glass opacity; 3. Rare Pleural effusion [17]. |
| Clinical characters | 1. Hypoalbuminemia; 2. Thrombocytopenia; 3. Leukopenia; 4. Lymphopenia.[28, 42] |
1. Increase White Blood Cells count; 2. Decrease lymphocytes count; 3. Decrease platelets count; 4. Decrease Red Blood Cells count [43]. |
1. The total number of peripheral blood leukocytes was normal or decreased; 2. Decreased lymphocyte count; 3. Increased CRP and erythrocyte sedimentation rate [44]. |
| Complication | 1. Acute kidney injury (AKI) is a significant characteristic of SARS patients [38]. | 1. Acute kidney injury (AKI) is a significant characteristic of MERS patients; 2. Vasopressor therapy was much more common in MERS, (81%)[31]. |
1. Acute respiratory distress syndrome; 2. RNA aemia, acute cardiac injury; 3. Secondary (super-)infections [41]. |
| Affected organ | Respiratory tract; kidney; liver [38] | Respiratory tract; intestinal tract; genitourinary tract; liver, kidney, neurons; monocyte; T lymphocyte; Cardiovascular [31]. | Respiratory tract; intestinal tract; liver; kidney [32]. |
| Prognostic factor | 1. Age 2. Underlying condition 3. Male 4. LDH level 5. Neutrophil count 6. CD4 7. CD8 [38] |
1. Age 2. Underlying condition 3. Male [45] |
1. Age 2. Underlying condition 3. Male 4. Lymphocyte count 5. Lactic acid 6. IL-6 and CRP [18] |
| Clinical management | -Continuous nasal cannula oxygen is given early (the oxygen concentration is generally 1~3 L/min); -Given oseltamivir within 48 hours of onset can help reduce the symptoms. -Fever>38.5, or obvious body aches, can use antipyretic analgesics; Those with high fever should be given physical cooling measures; Salicylic acid antipyretic analgesics are forbidden for children. -Cough and expectorants can be given antitussive and expectorant drugs; -With damage to organs such as heart, liver, kidney, etc. should be treated accordingly. -With diarrhea should be paid attention to rehydration and correct water and electrolyte imbalances; -Use of glucocorticoids, the recommended adult dose is equivalent to 80-320 mg/d of methylprednisolone, reduced or stopped when the clinical manifestations have been improved or the chest radiograph shown the absorbed shadows in lung; -Antiviral therapy has not yet found specific drugs; Possible to try protease inhibitors such as lopinavir and ritonavir; -When the diagnosis is unclear, new quinolones or β-lactams combined with macrolides can be used for trial treatment; -The pathogens of secondary infections include gram-negative bacilli, drug-resistant gram-positive cocci, fungi, and Mycobacterium tuberculosis, and appropriate antibacterial drugs should be selected accordingly; -Chinese medicine as an alternative strategy. [46, 47] |
For patients with pneumonia or comorbidities: -With signs of severe respiratory distress, shock or hypoxemia, oxygen therapy should be started immediately. -It is recommended that fluid management in patients is necessary, while provided that there is no sign of shock. -Empirical antibacterial treatment (including antibiotics and antiviral drugs) should be initiated for hospitalized patients with suspected MERS pneumonia; If sepsis is suspected, it should be started within one hour; -Antipyretic/analgesic is recommended to control fever and pain. -Corticosteroids are generally not recommended; however, stress doses can be given when needed. -Patients who are about to develop or have developed respiratory failure should be admitted to the ICU ward. For patients without pneumonia or comorbidities: -Supportive treatment is recommended, including the use of antipyretics and analgesics (such as acetaminophen, ibuprofen) to relieve pain and fever; -Patients should stay hydrated, but should not consume too much fluid, as this may worsen oxygenation [48]. |
-General treatment: strengthen supportive treatment, ensure adequate energy intake; pay attention to water and electrolyte balance, maintain internal environment stability; give effective oxygen therapy measures in time, including nasal cannula, mask oxygen and nasal high flow oxygen therapy; avoid blind or inappropriate use of antibacterial drugs, especially the combined use of broad-spectrum antibacterial drugs. -Antiviral treatment: drugs with potential antiviral effects (such as α-interferon, ribavirin, chloroquine, and arbidol) should be used early, and recommended to be applied to patients with severe high-risk factors and severely ill tendencies. -Immunotherapy: convalescent plasma from recovered patients, intravenous injection of COVID-19 human immunoglobulin, tocilizumab; -Glucocorticoid treatment can be applied for patients with progressive deterioration of oxygenation indicators, rapid imaging progress, and excessive activation of the body's inflammatory response for a short period of time (equivalent to methylprednisolone 0.5~1mg/kg/day, 3 to 5 days); -For severe and critical cases: On the basis of the above treatment, actively prevent and treat complications, treat basic diseases, prevent secondary infections, and provide organ function support in time; -Chinese medicine as an alternative strategy [49]. |
Accumulating recent pieces of evidence have unraveled the understanding of the development and direction of the epidemic. The National Health Commission of China has issued and implemented a series of guidelines based on the epidemiological evidence of pathogens responsible for COVID-19 infection, as well as epidemiological characteristics, clinical characteristics, prevention, diagnosis, staging, treatment, traditional Chinese medicine (TCM) syndrome differentiation treatment, and rehabilitation management [49, 54, 55]. Since the beginning of the pandemic, clinicians and epidemiologists around the world have been exploring the most effective treatment strategy to greatly benefit those who are affected by potentially life-threatening complications of COVID 19, as well as to further subdue this pandemic.
2. Current Therapeutics and Drug Development
According to clinical syndromes, patients with COVID-19 can be divided into four categories: (1) mild cases, presenting mild fever and mild fatigue, but no symptom of pneumonia on imaging; (2) moderate cases, with fever, respiratory tract and other symptoms, and manifestations of pneumonia on imaging; (3) severe cases, presenting shortness of breath, respiratory rate ≥30 times/min, resting oxygen saturation ≤93%, arterial oxygen partial pressure (PaO2)/oxygen inhalation concentration (FiO2) ≤300 mmHg (1 mm Hg = 0.133 kPa); and (4) critical cases, with shortness of breath and the need for mechanical ventilation, or shock, or combined with other organ failure, which requires ICU care [49]. For mild and moderate cases, the main clinical measures are to implement close observation of vital signs, blood oxygen saturation, and electrolyte balance; ensure adequate sleep; as well as strengthen nutrition. Oxygen therapy and antiviral therapy should be given in time according to real-time conditions such as blood routine, biochemical indicators, blood gas analysis, and chest imaging. For severe and critical cases, the number of peripheral blood lymphocytes progressively decreases, along with the progressive increase in the expression of peripheral blood inflammatory factors such as interleukin 6 (IL-6) and C-reactive protein (CRP), as well as the progressive elevation of lactic acid. A rapid progression of intrapulmonary lesions in the short term is a clinical warning indicator for severe and critical cases. Besides symptomatic treatment, these patients should also be paid great attention to prevent complications and secondary infections and provide organ function support promptly. These patients usually require noninvasive ventilation or high-flow nasal catheter oxygen therapy. When the airway platform pressure is less than 30 cm H2O, mechanical ventilation should be provided. Closed sputum aspiration and bronchoscopy are performed when necessary along with the other appropriate treatments to act on the airway secretions. Extracorporeal membrane oxygenation (ECMO) is also considered in severe cases without contraindications [49]. However, for severe and critically affected patients, circulatory support, renal replacement therapy, and solutions to reverse cytokine storms (CS) should also be considered and administered.
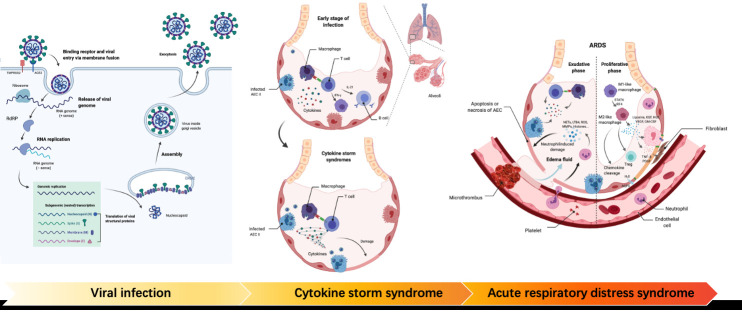
As mentioned earlier, SARS-CoV-2 can infect the human body by binding to host cell receptors, thereby causing body damage. As shown in Figure 1, in severe cases, it may be associated with the induction of excessive immune response, which is related to "self-attack" followed by multiple-organ damage and CS [56]. At present, the research and development of drugs mainly focus on three aspects: antiviral drugs, suppression of excessive immune response, and vaccination.
Figure 1.
The development of COVID19. Viral infection: TMPRSS2 cleaves the S protein of SARS-CoV-2; The RBD on the S1 subunit binds to ACE2 on the cell surface. Following entry into the cell, viral RNA is released and combined with RdRp to synthesize a full-length negative-strand RNA as an RNA replication template. After translation, structural proteins are localized to the inner membrane of Golgi for assembly. Cytokine storm syndrome: The immune system is over-activated, followed by the overproduction of multiple inflammatory factors. Multiple immune cells are recruited. As a result, healthy cells are damaged by overactive immune response. Acute respiratory distress syndrome: In the exudative phase, macrophages are activated and release pro-inflammatory mediators, which leads to the aggregation of neutrophils and monocytes. Activated neutrophils induce further damage. The injury leads to loss of barrier function and fluid accumulation in the interstitium and alveoli. In the proliferative period, the tissue homeostasis is recovered.
2.1 Antiviral treatment
Currently, the Food and Drug Administration (FDA) has authorized the emergency use of remdesivir to treat COVID-19. China has gained a lot of experience in the treatment process and developed “Diagnosis and Treatment Protocol for COVID-19 (Trial Version 8).” The guideline has recommended the use of α-interferon, ribavirin combined with α-interferon or lopinavir/ ritonavir, chloroquine phosphate, and arbidol. However, no specific antiviral treatment for COVID-19 still exists. Therefore, the development of antiviral drugs is imminent. The main antigen component S protein (spike protein) on SARS-CoV-2 plays an important role during the viral infection process. The S protein comprises two subunits, S1 and S2, which can be cleaved by the transmembrane protease Serine 2 (TMPRSS2). The RBD on the S1 subunit binds to ACE2 on the host cell surface [57] to promote the virus's entry into the host cell through endocytosis [58]. After entering the cell, the viral RNA is released. Then, two open reading frames and protein transcriptase [RNA-dependent RNA polymerase (RdRp)] form a viral transcriptase complex [59]. Subsequently, a full-length negative-strand RNA is synthesized from the viral RNA (positive strand) as a full-length genomic RNA replication template [60]. After translation, structural proteins are localized to the inner membrane of the Golgi for assembly [61]. According to the mechanism of viral infection, blocking virus entry, inhibiting the process of viral replication and assembly, and removing the virus are the key strategies to the development of antiviral drugs.
2.1.1 Blocking the virus from entering the host cell
ACE2 is the host cell binding site for viral infection. At the beginning of the disease outbreak, it is believed that the use of inhibitors of the renin-angiotensin-aldosterone system (RAAS) may increase the expression level of ACE2, thereby making individuals susceptible to severe COVID-19 [62]. A large case study by Francisco et al. [63] has confirmed that the application of RAAS inhibitors does not increase the proportion of COVID-19 admissions, nor does it increase ICU admissions and mortality after adjusting for age, sex, cardiovascular comorbidities, and risk factors, suggesting that these inhibitors need not be banned and can still be used as appropriate.
TMPRSS2-cracked virus S protein plays an important role in initiating the SARS-CoV-2 virus infection process. Camostat mesilate is an effective TMPRSS2 inhibitor. Preclinical evidence has shown that camostat mesilate can prevent the SARS-CoV-2 virus from infecting 293T cell lines [57] and reduce the mortality of SARS-CoV-infected mice [64]. Compared with camostat mesilate, its analogue nafamostat mesilate has a stronger activity to block the entry of the SARS-CoV-2 virus in in vitro tests [65]. Theoretically, imatinib can also have a type II transmembrane serine protease inhibitory effect [66, 67], while α-1antitrypsin, as a TMPRSS2 inhibitor, can also exhibit the ability to block the virus from entering host cells [68]. These TMPRSS2 inhibitors have all entered the clinical phase. Interestingly, androgens can mediate the upregulation of TMPRSS2 mRNA [69], which may be related to the difference in the proportion of men and women infected with the SARS-CoV-2 virus. A large observational study has revealed that patients with prostate cancer receiving androgen-deprivation therapies (ADTs) are partially protected from infection [70]. ADT therapeutic drugs such as bicalutamide and enzalutamide, which are androgen receptor blockers, can reduce the expression of TMPRSS2 or the entry of SARS-CoV-2 virus into host cells. This hypothesis has entered the clinical verification stage. In addition, CD147 expressed by host cells can bind to the spike protein of SARS-CoV-2 and participate in host cell invasion [71], indicating that the anti-CD147 antibody meplazumab may prevent SARS-CoV-2 infection [72].
Since the sixth edition of “Diagnosis and Treatment Protocol for COVID-19,” arbidol (200 mg tid) and chloroquine phosphate (500 mg bid) have been included in the plan. Arbidol has the characteristic core of indole, which can inhibit the fusion between the viral envelope and the host cell, thereby preventing the virus from entering the target cell [73]. A comparative analysis of protein sequences has revealed that the trimerization domain (S2) of the SARS-CoV-2 spike protein is similar to the hemagglutinin (HA) protein in influenza virus H3N2, which may be the binding site of arbidol [74]. Arbidol can also stimulate the humoral immune response and induce the production of interferon, thereby exhibiting a regulatory effect on the immune system [75]. However, the results of a retrospective analysis have shown that arbidol treatment cannot improve the symptoms of the disease or shorten the negative turning time of respiratory specimen virus nucleic acid [76]. However, chloroquine and hydroxychloroquine have been regarded as drug candidates with great therapeutic potential because they can block viral infection by increasing the endosomal pH value required for viral cell fusion [77] and inhibit viral replication through the suppression of p38 mitogen-activated protein kinase (MAPK) activation [78]. On March 28, 2020, the FDA issued an Emergency Use Authorization (EUA), allowing the distribution of hydroxychloroquine sulfate and chloroquine phosphate products donated to the Strategic National Stockpile to certain hospitalized patients with COVID-19 (www.fda.gov/media/138945/download). However, further clinical studies have revealed that chloroquine and hydroxychloroquine are not beneficial to hospitalized patients with COVID-19 and even have potential cardiac side effects. [79, 80] Therefore, on June 15, the FDA officially announced the withdrawal of the EUA of chloroquine and hydroxychloroquine for treating COVID-19 [81]. Recently, analogue mefloquine is under clinical trial.
2.1.2 Blocking virus replication
In vitro studies have revealed that SARS-CoV-2 can infect human lung tissue more effectively and replicate more efficiently compared with SARS-CoV. The number of viral particles in lung tissues infected by SARS-CoV-2 is more than 3.2 times the number of SARS-CoV within 48 h. [82] Blocking viral replication is particularly important in anti-SARS-CoV-2 virus therapy. RNA-dependent RNA polymerase (RdRP) is a key enzyme in the life cycle of RNA viruses and one of the most promising drug targets in anti-coronavirus treatment. Compared with several other types of positive-sense RNA viruses (hepatitis C virus, Zika virus) [83], SARS-CoV-2 polymerase and several key amino acid residues conserved in the active site show the structural similarity with SARS-CoV (97%) [84, 85]. Therefore, the inhibition of RdRP for SARS-CoV drug development may have therapeutic potential for SARS-CoV-2. Chloroquine, hydroxychloroquine, and mefloquine can block membrane fusion, while also exhibiting an RdRP inhibitory effect [86].
Nucleoside analogue antiviral drugs, mainly including adenosine analogues, guanosine analogues, and pyrimidine analogues, also target RdRP. Among these, remdesivir is an adenosine analogue considered to be one of the most promising therapeutic drugs in the early stage of the virus outbreak. The first patient with COVID-19 in the United States [87] was given an intravenous infusion of remdesivir on the seventh day of admission, and the symptoms improved significantly ever since the next day. In the clinical trial of compassionate drug use [88], remdesivir has shown a certain clinical improvement effect, but the experiment lacks a control group and the median observation time is short, which needs further clinical verification. Currently, a Phase III clinical trial, including mild, moderate, and severe cases, of remdesivir is also underway. In the “Handbook of COVID-19 Prevention and Treatment” [89], darunavir/cobicistat is also recommended as an antiviral treatment. Taken together, it suggests that the drugs that can reduce viral replication may also be promising for treating COVID-19.
Ribavirin, also known as "tribavirin," is a nucleoside antiviral drug and a guanosine analogue, which has been approved by the FDA mainly for treating respiratory syncytial virus infections in adults and children (inhalation only). In Trial Version 8, it is recommended to use ribavirin in combination with α-interferon or lopinavir/ritonavir. α-Interferon is an important cytokine in innate immunity, which can enhance the killing activity of the immune system against virus-infected cells. In a clinical trial of α-interferon/ribavirin combined treatment of patients with severe MERS, the 28-day mortality rate was not improved, although the 14-day mortality rate was significantly reduced [90]. α-Interferon/Ribavirin combined treatment was also applied in SARS, but no significant improvement was observed in prognosis. [91] Other adenosine analogue investigational drugs used in treating SARS-CoV [85], such as galidesivi, favipiravir, atazanavir, daclatasvir, and oseltamivir, are currently in clinical trials. Similarly, nucleoside analogues, such as triazavirin, clevudine, and clofazimine, have also been included in clinical trials. In addition, computer matching analysis has found that the inhibitors of nucleoside transport, reverse transcriptase, and nucleic acid synthesis may have therapeutic potential, which have entered the clinical validation stage.
The coronavirus genome contains two open reading frames (ORF1a and ORF1b), which encode polyproteins. These polyproteins are processed by 3C-like protease (3CL) and a papain-like protease (PLpro), which can produce 16 kinds of nonstructural proteins, including RdRP [92]. Therefore, the addition of 3CL inhibitors can affect the production of RdRP, thereby reducing viral replication. In addition, the level of viral replication is regulated by the cellular de novo pyrimidine nucleotide biosynthesis pathway. Dihydroorotate dehydrogenase (DHODH) is a restriction enzyme on this pathway and can be a therapeutic target for antiviral drugs. By inhibiting sphingosine kinase 2, lipid metabolism can be changed, while phospholipid aggregation can be reduced, thereby limiting virus assembly. Even by inhibiting heat shock protein 90 (Hsp90), the virus hijacks infected cells through the process of autophagy, thus suppressing virus replication. These therapeutic regimens are currently in clinical trials. Other promising drugs such as azithromycin, carrimycin, and DAS181 are also in clinical trials. For example, in combination with antibiotics and proton pump inhibitors, the bismuth salt for treating Helicobacter pylori was found to inhibit both NTPase and SARS-CoV-2 nsp13 helicase, thereby reducing viral replication [93]
2.1.3 Virus removal
Clinically, the immune response induced by SARS-CoV-2 infection is divided into two stages [94]. In the early and nonsevere stage, a specific adaptive immune response is required to eliminate the virus and prevent the disease from progressing to the severe stage. In the severe stage, a large number of damaged cells induce an excessive immune response, mainly an innate immune response. Therefore, in the first stage, strategies to enhance the immune response (antiserum, hyperreactive immunoglobulin, or interferon (IFN)) are essential. In this process, IFN, interleukin 7 (IL-7), and thymosin are used to stimulate the production of T cells and activate T-cell functions to clear the virus. The Trial Version 8 has mentioned α-interferon (5 million U, bid, nebulized inhalation) or its combination with ribavirin (500 mg, bid or tid, intravenous infusion) for antiviral treatment. Other drugs such as Toll-like receptor agonist (PUL-042 and polyinosinic:polycytidylic acid), Bacillus Calmette-Guérin, and bacterial lysate, which can enhance lung epithelial mucosa or innate immunity of the body, are also in clinical trials [95-97].
Members of the coronavirus family, including SARS-CoV-2, are associated with strong interferon suppression, including blood lymphopenia-related abnormality of NK cells [98]. Natural killer (NK) cells are important lymphocytes in the innate immune system to fight infection. The differentiation of NK cells from stem cells and cord blood cells is currently under clinical investigation (Clinical trial identifier: NCT04299152, NCT04324996). NK cells expressing ACE2 and natural killer group 2D (NKG2D) constructed by chimeric antigen receptor T cell (CART) technology can enhance the antiviral effect by directly targeting the S proteins of SARS-CoV-2 and NKG2DL on the surface of infected cells (Clinical trial identifier: NCT04324996). The S protein-binding antibody or virus-neutralizing antibody developed for SARS-CoV-2 is also a research hotspot of antiviral drugs.
2.1.4 Combined use of different antiviral treatments
New drug development and research are time-consuming and cannot cope with the current virus outbreak. Drug repurposing is a popular exploratory method, including exploring new indications from old drugs [99]. Currently, most of the drugs clinically used to fight the virus are for influenza, viral hepatitis, and HIV, as well as drugs developed for MERS and SARS [100-170] (Supplementary Table 1). However, since the viral outbreak, many exploratory clinical trials of antiviral treatments have been published. Unfortunately, no specific drug has been developed to fight the virus. Another method of rapid development strategy is based on the mechanism for the combination therapy of different antiviral drugs.
Trial Version 8 also mentions the antiviral drug lopinavir/ritonavir (lopinavir: 400 mg, bid; ritonavir: 100 mg, bid, oral), with the commercial name of Aluvia. Lopinavir is an anti-retroviral of the protease inhibitor, while ritonavir is often used as a booster of other protease inhibitors. It has been considered as a promising therapeutic drug in the early stage of the epidemic, recommended from the first version of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia to the seventh version. However, in a clinical study including 199 patients with severe COVID-19, lopinavir/ritonavir did not significantly improve the 28-day mortality rate compared with standard treatment [171]. The updated Trial Version 8 recommended a combination with ribavirin. An open-label, randomized, Phase II clinical trial showed that the early use of INF-β1, lopinavir/ritonavir, and ribavirin triple therapy could significantly shorten the time for nasopharyngeal swab virus to turn negative, reduce the hospital stay, and relieve symptoms for patients with mild-to-moderate COVID-19, compared with the application of lopinavir/ritonavir. A large number of clinical trials of hydroxychloroquine, azithromycin, chloroquine, lopinavir/ritonavir, or nucleoside analogue antiviral drugs in combination with drugs of different antiviral mechanisms (Table 2) have been performed to observe the effectiveness of the combined treatments for antiviral therapy. This drug development strategy saves a lot of preclinical research and can also avoid the embarrassing situation of poor efficacy of a single drug.
Table 2.
Combined use of different treatments in COVID19.
| Treatment | Drug | Drug target | Dosage | Source |
|---|---|---|---|---|
| Arbidol + Bromhexine | Arbidol | Membrane fusion inhibitor | PO | NCT04273763 (CN) |
| Bromhexine | Expectorant | PO | ||
| Arbidol + IFN-β1α | Arbidol | Membrane fusion inhibitor | PO, 200 mg, tid, 14 d | NCT04254874 (CN) |
| IFN-β1α | Immunomodulator | INH, 14 d | ||
| Chloroquine + Ivermectin | Chloroquine | Membrane fusion inhibitor and immunomodulator | PO | NCT04382846 (EG) |
| Ivermectin | Importin (IMP) α/β receptor | PO | ||
| Chloroquine + Ivermectin + Vitamin D | Chloroquine | Membrane fusion inhibitor and immunomodulator | PO, 500 mg, 4 d, 30 d | NCT04399746 (MX) |
| Ivermectin | IMP α/β receptor | PO, 6 mg, qd, Day 1, 7 and 8 | ||
| Vitamin D | Vitamins | PO, 400 UI, bid | ||
| Chloroquine + Losartan | Chloroquine | Membrane fusion inhibitor and immunomodulator | PO, 450 mg, bid | NCT04428268 (MX) |
| Losartan | Angiotensin II receptor (type AT1) antagonis | PO, 25 mg, bid | ||
| Chloroquine + Zinc | Chloroquine | Membrane fusion inhibitor and immunomodulator | PO | NCT04447534 (EG) |
| Zinc | PO | |||
| Hydroxychloroquine + Azithromycin | Hydroxychloroquine | --/ Receptor binding and membrane fusion inhibitor | PO, 400 mg, bid, 7 d |
NCT04321278 (IL) NCT04322123 (BR) NCT04329832 (US) |
| Azithromycin | Tetrcycline | PO, 500 mg, qd | ||
| Hydroxychloroquine + Azithromycin + / - tocilizumab | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 800 mg, qd | NCT04347031 (RU) |
| Azithromycin | Tetrcycline | PO, 250 mg, bid | ||
| Tocilizumab | Anti-IL-6R antibody | IV | ||
| Hydroxychloroquine + Azithromycin + Convalescent plasma | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 400 mg, bid, 5 d | NCT04441424 (IQ) |
| Azithromycin | Tetrcycline | PO, 500 mg, qd, 5 d | ||
| Convalescent plasma | Immunomodulator | IV, 400 mL, 5 d | ||
| Hydroxychloroquine + Azithromycin + Oseltamivir | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 5 d |
NCT04338698 (BR) NCT04338698 (PK) |
| Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | ||
| Oseltamivir | Nucleoside analog | PO, 75 mg, bid, 5 d | ||
| Hydroxychloroquine + Azithromycin + Sarilumab | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid | NCT04341870 (FR) |
| Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | ||
| Sarilumab | Anti-IL-6R antibody | IV, 400 mg, Day 1 | ||
| Hydroxychloroquine + Azithromycin + zinc | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 600 mg, Day 1, 200 mg, Day 2-9 | NCT04528927 (TN) |
| Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | ||
| Zinc | PO, 220 mg, 10 d | |||
| Hydroxychloroquine + Baricitinib | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 14 d | NCT04373044 (US) |
| Baricitinib | JAK inhibitor | PO, 2 mg, qd, 14 d | ||
| Hydroxychloroquine + Bromhexine | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid |
NCT04273763 (CN) NCT04340349 (MX) |
| Bromhexine | Expectorant | PO, 8 mg, tid | ||
| Hydroxychloroquine + Camostat mesylate | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 400 mg, bid, 10 d | NCT04355052 (IL) |
| Camostat mesylate | TMPRSS2 inhibitor | PO, 200 mg, qd, 10 d | ||
| Hydroxychloroquine + Clindamycin | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 7 d | NCT04349410 (US) |
| Clindamycin | Lincomycin antibiotics | IV, 4800 mg | ||
| Hydroxychloroquine + Clindamycin + Primaquine | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 7 d | NCT04349410 (US) |
| Clindamycin | Lincomycin antibiotics | IV, 4800 mg, 7 d | ||
| Primaquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, qd, 7 d | ||
| Hydroxychloroquine + Daclatasvir + Sofosbuvir | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 400 mg, bid, 14 d | NCT04443725 (EG) |
| Daclatasvir | Nucleoside analog | PO, 90 mg, qd, 14 d | ||
| Sofosbuvir | Nucleoside analog | PO, 400 mg, qd, 14 d | ||
| Hydroxychloroquine + Doxycycline | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 10 d | NCT04349410 (US) |
| Doxycycline | Tetrcycline | IV, 100 mg, bid, 10 d | ||
| Hydroxychloroquine + Favipiravir | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, bid, 7 d |
NCT04359615 (IR) NCT04376814 (IR) |
| Favipiravir | Nucleoside analog | PO, 1600 mg, Day 1, 600 mg, tid, 7 d | ||
| Hydroxychloroquine + IFN-α2β | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO | NCT04273763 (CN) |
| IFN-α2β | Immunomodulator | INH | ||
| Hydroxychloroquine + Imatinib | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, bid | NCT04346147 (ES) |
| Imatinib | TMPRSS2 inhibitor | PO, 400 mg, qd | ||
| Hydroxychloroquine + Indomethacin +Azithromycin | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, bid, 7d | NCT04344457 (US) |
| Indomethacin | Nonsteroidal anti-inflammatory drug | PO, 50 mg, tid, 14 d | ||
| Zithromax | Tetrcycline | PO, 500 mg, qd, 3d | ||
| Hydroxychloroquine + Lopinavir + Ritonavir + IFN-β1α | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO |
NCT04350684 (IR) NCT04343768 (IR) NCT04350671 (IR) |
| Lopinavir | Nucleoside analog | PO | ||
| Ritonavir | Nucleoside analog | PO | ||
| IFN-β 1a | Immunomodulator | IV | ||
| Hydroxychloroquine + Lopinavir + Ritonavir | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, bid |
NCT04390152 (CO) NCT04346147 (ES) |
| Lopinavir | Nucleoside analog | PO, 200 mg, qd | ||
| Ritonavir | Nucleoside analog | PO, 50 mg, qd | ||
| Hydroxychloroquine + Lopinavir + Ritonavir + IFN-β1β | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO | NCT04343768 (IR) |
| Lopinavir | Nucleoside analog | PO | ||
| Ritonavir | Nucleoside analog | PO | ||
| IFN-β1β | Immunomodulator | PO | ||
| Hydroxychloroquine + Oseltamivir | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, bid, 5 d |
NCT04338698 (PK) NCT04303299 (TH) |
| Oseltamivir | Nucleoside analog | PO, 75 mg, bid, 5 d | ||
| Hydroxychloroquine + Sirolimus f | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 600 mg, 10 d | NCT04374903 (JO) |
| Sirolimus | Immunosuppressant | PO, 250 mg, 10 d | ||
| Hydroxychloroquine + Tofacitinib | Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 200 mg, tid, 14 d | NCT04390061(US) |
| Tofacitinib | JAK inhibitor | PO, 10 mg, bid, 14 d | ||
| Azithromycin + Amoxicillin | Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5, 2 d | NCT04363060 (FR) |
| Amoxicillin/Clavulanate | Antibacterial drugs | PO, 250 mg, tid, 2 d | ||
| Azithromycin + Atovaquone | Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | NCT04339426 (US) |
| Atovaquone | PO, 750 mg, bid, 10 d | |||
| Azithromycin + Clavulanate | Azithromycin | Tetrcycline | PO, 250 mg, tid, 7 d | NCT04363060 (FR) |
| Clavulanate | ||||
| Azithromycin + Doxycycline | Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | NCT04528927 (TN) |
| Doxycycline | Tetrcycline | PO, 200 mg, qd, 10 d | ||
| Azithromycin + Ivermectin + Nitazoxanide | Azithromycin | Tetrcycline | PO | NCT04382846 (EG) |
| Ivermectin | IMP α/β receptor | PO | ||
| Nitazoxanide | Immunomodulator | PO | ||
| Azithromycin + Mefloquine + / - Tocilizumab | Azithromycin | Tetrcycline | PO, 250 mg, tid, 7 d | NCT04347031 (RU) |
| Mefloquine | Membrane fusion inhibitor and immunomodulator | PO, 500 mg, bid, 7 d | ||
| Tocilizumab | Anti-IL-6R antibody | IV | ||
| Azithromycin + Nitazoxanide | Azithromycin | Tetrcycline | PO | NCT04382846 (EG) |
| Nitazoxanide | Immunomodulator | PO | ||
| Azithromycin + Oseltamivir | Azithromycin | Tetrcycline | PO, 500 mg, Day 1; 250 mg, Day 2-5 | NCT04338698 (PK) |
| Oseltamivir | Nucleoside analog | PO, 75 mg, bid | ||
| Azythromycin + Ivermectin + Dutasteride | Azythromycin | Tetrcycline | PO, 500 mg, qd | NCT04446429 (BR) |
| Ivermectin | IMP α/β receptor | PO, 200 mcg/kg, qd | ||
| Dutasteride | TMPRSS2 inhibitor | PO, 0.5 mg | ||
| Azythromycin + Ivermectin+ Proxalutamide | Azythromycin | Tetrcycline | PO, 500 mg, qd | NCT04446429 (BR) |
| Ivermectin | IMP α/β receptor | PO, 200 mcg/kg, qd | ||
| Proxalutamide | TMPRSS2 inhibitor | PO, 200 mg | ||
| Ivermectin + Doxycycline | Ivermectin | IMP α/β receptor | PO, 200 mcg/kg, qd, 5 d |
NCT04407130 (BD) NCT04523831 (BD) NCT04403555 (EG) |
| Doxycycline | Tetrcycline | PO, 200 mg, 5 d | ||
| Ivermectin + Dutasteride + | Ivermectin | IMP α/β receptor | PO, 200 mcg/kg, qd | NCT04446429 (BR) |
| Dutasteride | TMPRSS2 inhibitor | PO, 0.5 mg | ||
| Ivermectin + Losartan | Ivermectin | IMP α/β receptor | PO, 12 mg, qd, 15 d | NCT04447235 (BR) |
| Losartan | Angiotensin II receptor (type AT1) antagonis | PO, 50 mg, qd, 15 d | ||
| Ivermectin + Nitazoxanide | Ivermectin | IMP α/β receptor | PO | NCT04382846 (EG) |
| Nitazoxanide | Immunomodulator | PO | ||
| Ivermectin + Nitazoxanide + Ribavirin | Ivermectin | IMP α/β receptor | PO, 7 d | NCT04392427 (EG) |
| Nitazoxanide | Immunomodulator | PO, 7 d | ||
| Ribavirin | Nucleoside analog | PO, 7 d | ||
| Ledipasvir + Sofosbuvir | Ledipasvir | Nucleoside analog | PO, 90 mg, qd, 14 d | NCT04498936 (EG) |
| Sofosbuvir | Nucleoside analog | PO, 400 mg, qd, 14 d | ||
| Daclatasvir + Sofusbuvir | Daclatasvir | Nucleoside analog | PO, 120 mg, Day 1; 60 mg, Day 2-9 |
NCT04468087 (BR) NCT04460443 (EG) |
| Sofusbuvir | Nucleoside analog | PO, 800 mg, Day 1; 400 mg, Day 2-9 | ||
| Danoprevir + Ritonavir | Danoprevir | Nucleoside analog | PO, 100 mg, bid, 10 d |
NCT04345276 (CN) NCT04291729 (CN) |
| Ritonavir | Nucleoside analog | PO, 100 mg, bid | ||
| Darunavir + Cobicistat | Darunavir | Viral RNA-dependent RNA polymerase inhibitor/ CYP3A protein inhitor | PO, 800 mg, qd, 5 d |
NCT04252274 (CN) NCT04425382 (QA) |
| Cobicistat | Protease inhibitor | PO, 150 mg, qd, 5 d | ||
| Darunavir + Ritonavir + Favipiravir+ Hydroxychloroquine | Darunavir | Nucleoside analog | PO, 400 mg, tid | NCT04303299 (TH) |
| Ritonavir | Nucleoside analog | PO, 200 mg, qd | ||
| Favipiravir | Nucleoside analog | PO, 2400 mg, qd | ||
| Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | PO, 800 mg, qd | ||
| Darunavir + Ritonavir + Oseltamivir + Hydroxychloroquine | Darunavir | Nucleoside analog | PO, 400 mg, tid | NCT04303299 (TH) |
| Ritonavir | Nucleoside analog | PO, 200 mg, qd | ||
| Oseltamivir | Nucleoside analog | PO, 300 mg, qd | ||
| Hydroxychloroquine | Membrane fusion inhibitor and immunomodulator | 8 PO, 00 mg, qd | ||
| Darunavir + Ritonavir + Oseltamivir | Darunavir | Nucleoside analog | PO, 400 mg, tid | NCT04303299 (TH) |
| Ritonavir | Nucleoside analog | PO, 200 mg, qd | ||
| Oseltamivir | Nucleoside analog | PO, 300 mg, qd | ||
| Emtricitabine + Tenofovir | Emtricitabine | Protease inhibitor | PO, 300 mg, qd, 8 d | NCT04519125 (CO) |
| Tenofovir | Nucleoside analog | PO, 200 mg, qd | ||
| Emtricitabine + Tenofovir Alafenamide | Emtricitabine | Protease inhibitor | PO, 200 mg, qd | NCT04405271 (AR) |
| Tenofovir alafenamide | Nucleoside analog | PO, 25 mg, qd | ||
| Favipiravir + Maraviroc | Favipiravir | Nucleoside analog | PO, 200 mg, qd, 10 d | NCT04475991 (MX) |
| Maraviroc | Chemokine receptor antagonist | PO, 300 mg, qd, 10 d | ||
| Favipiravir + Tocilizumab | Favipiravir | Nucleoside analog | PO, 1600 mg, Day 1, 600 mg, tid, 6 d | NCT04310228 (CN) |
| Tocilizumab | Anti-IL-6R antibody | IV, 4-8 mg/kg, 7 d | ||
| Oseltamivir + ASC09F | Oseltamivir | RdRP inhibitor | PO, 75 mg, qd, 14 d | NCT04261270 (CN) |
| ASC09F | CYP3A4 inhibitor | PO, 400 mg, bid, 14 d | ||
| Oseltamivir + Mesenchymal stem cells | Oseltamivir | Nucleoside analog | PO, 4 w | NCT04371601 (CN) |
| Mesenchymal stem cells | MSC therapy | IV, 1 ×10^6 cell/kg/w, 4 w | ||
| Oseltamivir + Ritonavir | Oseltamivir | Viral RNA-dependent RNA/ Booster of other protease polymerase inhibitor | PO, 75 mg, qd, 7 d |
NCT04315896 (MX) NCT04318444 (US) NCT04328285 (FR) |
| Ritonavir | Nucleoside analog | PO, 300 mg, bid, 7 d | ||
| Lopinavir + Ritonavir | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 400 mg, bid, 5 d | Guidelines (version 7) for treatment of COVID-19 NCT04328285 (FR) NCT04328012 (US) |
| Ritonavir | Nucleoside analog | PO, 100 mg, bid, 5 d | ||
| Lopinavir + Ritonavir + Arbidol | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 400 mg, bid, 5-21 d |
NCT04350671 (IR) NCT04403100 (BR) NCT04376814 (IR) |
| Ritonavir | Nucleoside analog | PO, 100 mg, bid, 5-21 d | ||
| Arbidol | Membrane fusion inhibitor and immunomodulator/Anti-retroviral of the protease inhibitor/booster of other protease | PO, 200 mg, tid, 5-21 d | ||
| Lopinavir + Ritonavir + Atorvastatin | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 200 mg, qd, 10 d | NCT04466241 (FR) |
| Ritonavir | Nucleoside analog | PO, 50 mg, qd, 10 d | ||
| Atorvastatin | Statin medication | PO, 20 mg, qd, 10 d | ||
| Lopinavir + Ritonavir + Favipiravir | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 400 mg, bid, 7 d |
NCT04499677 (GB) NCT04303299 (TH) |
| Ritonavir | Nucleoside analog | PO, 100 mg, bid, 7 d | ||
| Favipiravir | Nucleoside analog | PO, 1800 mg, bid, Day 1; 400 mg, 4 times, 7 d | ||
| Lopinavir + Ritonavir + IFN-β1α | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 200 mg, qd |
NCT04315948 (FR) NCT04276688 (CN) |
| Ritonavir | Nucleoside analog | PO, 50 mg, qd | ||
| IFN-β1α | Immunomodulator | INH, 44 μg/ 0.5 mL | ||
| Lopinavir + Ritonavir + Oseltamivir | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 800 mg, qd | NCT04303299 (TH) |
| Ritonavir | Nucleoside analog | PO, 200 mg, qd | ||
| Oseltamivir | Nucleoside analog | PO, 300 mg, qd | ||
| Lopinavir + Ritonavir + Telmisartan | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 200 mg, qd, 10 d | NCT04466241 (FR) |
| Ritonavir | Nucleoside analog | PO, 50 mg, qd, 10 d | ||
| Telmisartan | Angiotensin II receptor (type AT1) antagonis | PO, 40 mg, qd, 10 d | ||
| Lopinavir + Ritonavir +Ribavirin + IFN-β1α | Lopinavir | Anti-retroviral of the protease inhibitor/booster of other protease inhibitors | PO, 400 mg, bid, 14 d |
NCT04276688 (CN) NCT04343768 (IR) |
| Ritonavir | Nucleoside analog | PO, 100 mg, bid, 14 d | ||
| Ribavirin | Nucleoside analog | PO, 400 mg, bid, 14 d | ||
| IFN-β1α | Immunomodulator | SC, 0.25 mg | ||
| Remdesivir + Apremilast | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04488081 (US) |
| Apremilast | Antiemetic | PO, 30 mg, bid, 9 d | ||
| Remdesivir + Cenicriviroc | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04488081 (US) |
| Cenicriviroc | CCR5 inhibitor | PO, 150 mg, bid, 28 d | ||
| Remdesivir + Baricitinib | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04401579 (US) |
| Baricitinib | JAK inhibitor | PO, 4 mg, qd, 14 d | ||
| Remdesivir + Icatibant | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04488081 (US) |
| Icatibant | Peptide-based hormone | SC, 30 mg, 9 d | ||
| Remdesivir + IFN-β1α | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04492475 (US) |
| IFN-β1α | Immunomodulator | SC, 44 μg/ 0.5 mL | ||
| Remdesivir + Merimepodib | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04410354 (US) |
| Merimepodib | Inosine monophosphate dehydrogenase (IMPDH) inhibitor | IV, 400 mg, tid, 10 d | ||
| Remdesivir + NA-831 | Remdesivir | Nucleoside analog | PO, 1 mg/kg | NCT04480333 (US) |
| NA-831 | Endogenous small molecule | INH, 0.2 mg/kg | ||
| Remdesivir + Razuprotafib | Remdesivir | Nucleoside analog | PO, 200 mg, qd, Day 1; 100 mg, qd, Day 2-9 | NCT04488081 (US) |
| Razuprotafib | VE-PTP inhibitor | SC, 10 mg, tid, 7d | ||
| Remdesivir + Tocilizumab | Remdesivir | Nucleoside analog | PO, 10 d | NCT04409262 (US) |
| Tocilizumab | Anti-IL-6R antibody | IV, 10 d | ||
| Ribavirin + Sofosbuvir | Ribavirin | Nucleoside analog | PO | NCT04460443 (EG) |
| Sofosbuvir | Nucleoside analog | PO | ||
| Ritonavir + ASC09 | Ritonavir | Nucleoside analog | PO, 100 mg, bid, 14 d | NCT04261907 (CN) |
| ASC09 | Protease inhibitors | PO, 300 mg, bid, 14 d | ||
| Ritonavir + Ganovo + IFN-Nebulization | Ritonavir | Nucleoside analog | PO, 100 mg, bid, 14 d | NCT04291729 (CN) |
| Danoprevir | Nucleoside analog | PO, 100 mg, bid, 14 d | ||
| IFN-Nebulization | Immunomodulator | INH, 50 μg, bid, 14 d | ||
| IFN-α1β + Thymosin α1 | IFN-α1β | Immunomodulator | ISIN, 2-3 drops, 4 times | NCT04320238 (CN) |
| Thymosin α1 | Immunomodulator | SC, 1 time per week | ||
| IFN-β1β + clofazimine | IFN-β1β | Immunomodulator | SC or IV, 16 million UI, 3 d | NCT04465695 (CN) |
| Clofazimine | Nucleoside analog | PO, 100 mg, bid | ||
| Adalimumab + Tocilizumab | Adalimumab | Humanized monoclonal antibody against the TNF-alpha | Adalimumab: SC, 40 mg, every 2 wekks; Tocilizumab: IV, 8 mg/kg, 6 times in 4 weeks |
ChiCTR2000030580 |
| Tocilizumab | Anti-IL-6R antibody | IV | ||
| C486 | - | SC or IV | ||
| REGN10933 + REGN10987 | REGN10933 | Anti-Spike (S) SARS-CoV-2 antibody | SC or IV |
NCT04426695 (US) NCT04519437 (US) NCT04452318 (US) |
| REGN10987 | ||||
| Tocilizumab + Dexamethasone | Tocilizumab | Anti-IL-6R antibody | IV, 8 mg/kg, Day 1 and 3 | NCT04476979 (GF) |
| Dexamethasone | Corticosteroids | IV, 10 mg for 5 d, 2.5 mg for 4 d | ||
| Tocilizumab + Methylprednisolone | Tocilizumab | Anti-IL-6R antibody | IV, 8 mg/kg, Day 1 and 3 | NCT04377503 (ES) |
| Methylprednisolone | Corticosteroids | IV, 1.5 mg/kg/d, 21 d | ||
| Tocilizumab + Pembrolizumab | Tocilizumab | Anti-IL-6R antibody | IV, 8 mg/kg | NCT04335305 (ES) |
| Pembrolizumab | PD-1 antibody | IV, 200 mg | ||
| Toremifene + Melatonin | Toremifene | Hormone | PO, 60 mg, qd | NCT04531748 (US) |
| Melatonin | Hormone | PO, 40 mg, morning; 60 mg, evening | ||
| Anakinra + Ruxolitinib | Anakinra | IL antagonists | IV, 300 mg/d, 5 d | NCT04366232 (FR) |
| Ruxolitinib | JAK inhibitor | PO, 5 mg, bid, 14-28 d | ||
| Anakinra + Siltuximab | Anakinra | IL antagonists | SC, 100 mg, qd | NCT04330638 (BE) |
| Siltuximab | Anti-IL-6R antibody | IV, 11 mg/kg | ||
| Anakinra + Tocilizumab | Anakinra | IL antagonists | SC, 100 mg, qd | NCT04330638 (BE) |
| Tocilizumab | Anti-IL-6R antibody | IV, 8 mg/kg | ||
| Colchicine + Edoxaban | Colchicine | NLRP Inflammasome inhibitor | PO, 0.5 mg, qd | NCT04516941 (CH) |
| Edoxaban | Thrombolytic medication | PO, 60 mg, qd | ||
| Colchicine + Methylprednisolone | Colchicine | NLRP Inflammasome inhibitor | PO, 0.5 mg, qd, 14 d | NCT04492358 (ES) |
| Methylprednisolone | Corticosteroids | PO, 60 mg, qd, 3d | ||
| Colchicine + Rosuvastatin | Colchicine | NLRP Inflammasome inhibitor | PO, 0.6 mg, qd, 3 d | NCT04472611 (US) |
| Rosuvastatin | HMG-CoA reductase inhibitors | PO, 40 mg, qd, 3 d | ||
| Dexamethasone + Placenta-Derived MMSCs | Dexamethasone | Corticosteroids | IV | NCT04461925 (UA) |
| Placenta-Derived MMSCs | MSC therapy | IV, 1 ×10^6 cell/kg, Day 1, 4 and 7 | ||
| Diltiazem + Niclosamide | Diltiazem | Calcium-channel blocker | PO, 500 mg × 4 times, 10 d | NCT04372082 (FR) |
| Niclosamide | - | PO, 60 mg, tid, 10 d | ||
| Dipyridamole + Aspirin | Dipyridamole | Thrombolytic medication | PO, 200 mg, qd, 14 d | NCT04410328 (US) |
| Aspirin | Thrombolytic medication | PO, 25 mg, qd, 14 d | ||
| Enoxaparin + Methylprednisolone | Enoxaparin | Thrombolytic medication | SC, 4000-6000 UI | NCT04528888 (IT) |
| Methylprednisolone | Corticosteroids | IV, 0.5 mg/kg | ||
| Heparin + Methylprednisolone | Heparin | Thrombolytic medication | IV, 18 UI/kg/h |
NCT04485429 (BR) NCT04528888 (IT) |
| Methylprednisolone | Corticosteroids | IV, 0.5 mg/kg | ||
| Heparin + Umbilical Cord Mesenchymal Stem Cells | Heparin | Thrombolytic medication | IV | NCT04355728 (US) |
| Umbilical Cord Mesenchymal Stem Cells | MSC therapy | IV, 100 ×10^6 cell | ||
| Levamisole + Isoprinosine | Levamisole | - | PO, 50 mg, tid, 14 d |
NCT04383717 (EG) NCT04360122 (EG) |
| Isoprinosine | - | PO, 1 g, 4 times | ||
| Metenkefalin + Tridecactide | Metenkefalin | Opioid delta receptor agonists | IV, 5 mg | NCT04374032 (YU) |
| Tridecactide | Th1 cell modulators | IV, 1 mg | ||
| Nitazoxanide + atazanavir+ ritonavir | Nitazoxanide | Immunomodulator | PO, 1000 mg, bid | NCT04459286 (NG) |
| Atazanavir | Nucleoside analog | PO, 300 mg, qd | ||
| Ritonavir | Nucleoside analog | PO, 100 mg, qd | ||
| Paracetamol + ChAdOx1 nCoV-19 | Paracetamol | Non-steroidal anti-inflammatory drugs | PO, | NCT04324606 (GB) |
| ChAdOx1 nCoV-19 | Vaccine | IV, 5 ×10^10 vp | ||
| Paracetamol + MenACWY | Paracetamol | Non-steroidal anti-inflammatory drugs | IV | NCT04324606 (GB) |
| MenACWY | Vaccine | IV | ||
| Ruxolitinib + Simvastatin | Ruxolitinib | JAK inhibitor | PO, 5 mg, bid, 14 d | NCT04348695 (ES) |
| Simvastatin | Statin medication | PO, 40 mg, qd, 14 d | ||
| SnPP Protoporphyrin + Sunlight exposure | SnPP Protoporphyrin | Photodynamic therapy | IV, 5, 7 and 9 mg, 14 d | NCT04371822 (EG) |
| Sunlight exposure | 1 h, 14 d | |||
| Sulfonatoporphyrin(TPPS) + Sunlight exposure | Sulfonatoporphyrin(TPPS) | Photodynamic therapy | IV, 5 mg, 14 d | NCT04371822 (EG) |
| Sunlight exposure | 1 h, 14 d | |||
| Tacrolimus + Methylprednisolone | Tacrolimus | Immunosuppressant | PO, 8-10 ng/mL blood level | NCT04341038 (ES) |
| Methylprednisolone | Corticosteroids | PO, 120 mg, 3 d | ||
| Tirofiban + Clopidogrel + Acetylsalicylic acid + Fondaparinux | Tirofiban | Thrombolytic medication | IV, 0.15 μg/kg/min | NCT04368377 |
| Clopidogrel | Thrombolytic medication | PO, initial dose of 300 mg, then 75 mg/d | ||
| Acetylsalicylic acid | Thrombolytic medication | IV, initial dose of 75 mg, then 30 mg/d | ||
| Fondaparinux | Thrombolytic medication | IV, 2.5 mg |
PO: Oral administration; IV: Intravenous administration; INH: Inhalation; IM: intramuscular administration; SC: Subcutaneous administration; ISIN: Intrasinal administration; ID: intradermal injection; SL: Sublingual administration; TOPp: patch applied on the skin; ET: Endotracheal instillation
Although antiviral drugs seem to be promising for treating COVID-19, their potential toxicities and side effects still need attention. In addition, the dosage and starting time point of antiviral drugs require clinical investigation. The large-scale administration can make the virus mutate under selection pressure and produce drug resistance because the new coronavirus has different variants [172] or different mutations [173, 174].
2.2 Immunomodulatory therapy
In the severe stage of COVID-19, when the immune system is overactivated to combat the virus, multiple inflammatory factors are produced. It results in a severe cytokine storm through cascade amplification, continuous activation, and expansion of immune cells (even some T cells and B cells) (Fig. 1), leading to organ damage, edema, subsequent air exchange dysfunction, acute respiratory distress syndrome (ARDS), acute heart injury, multiple-organ failure, and death eventually [175]. These injuries persist if only antiviral therapy is given, and the excessive immune response is ignored. From this point, immune regulation may be the key to the treatment of patients with severe COVID-19.
Corticosteroids can antagonize pathophysiological processes of ARDS, mainly to inhibit excessive inflammation, excessive cell proliferation, and abnormal collagen deposition. At present, the application of corticosteroids in SARS-CoV-2 infection is inconclusive [176]; however, they are widely used for treating SARS-CoV [177] and MERS-CoV infections [178]. A retrospective study of 401 patients with SARS found that the administration of corticosteroids at lower doses reduced the mortality of patients with severe SARS and shortened their hospital stay, which was not observed in patients with severe secondary lower respiratory infections or other complications [177]. Corticosteroids and immunomodulators were usually prescribed empirically in the early stages of the COVID-19 outbreak. The application of corticosteroids in COVID-19 is still controversial due to the methodological limitations of the available evidence [176]. In the WHO-issued interim guidelines on “Clinical management of severe acute respiratory infection when COVID-19 is suspected” (published on March 13, 2020) [179], corticosteroids are not recommended unless otherwise indicated [180]. Nevertheless, some scholars think that the application of pulsed dose or long-term high dose, or early administration of corticosteroids to treat serious viral pneumonia can affect virus clearance rate [178, 181] or survival [182]. On the contrary, some researchers found that adjuvant therapy with low-dose corticosteroids could reduce mortality in critical cases [183]. A retrospective analysis showed that the rational use of low-dose corticosteroids could suppress the cytokine storm and prevent secondary multiple-organ damage in patients with severe COVID-19, thus gaining time for intensive treatment to bring survival advantages [184]. A systematic review and meta-analysis found that the application of corticosteroids in SARS-CoV-2, SARS-CoV, and MERS-CoV infections was associated with the delay in virus clearance, with a mean difference (MD) = 3.78 days (95% CI = 1.16), while the duration of hospital stay was prolonged and the use of mechanical ventilation increased. The Diagnosis and Treatment Protocol for COVID-19 (Trial Version 8) [49] has pointed out that a low dose of corticosteroids 3-5 days, not exceeding 1-2 mg/(kg.d) of methylprednisolone equivalent is appropriate for patients with a worsening oxygenation index, rapid imaging progression, and overactivation of an inflammatory response, whereas a high dose of corticosteroids can result in a strong immunosuppressive effect, thereby delaying the clearance of the virus. In short, corticosteroids should be used with caution in patients with COVID-19, and the indications and dosage should be strictly followed.
Intravenous immunoglobulin (IVIG) has been used to treat patients with autoimmunity and chronic inflammatory diseases; it targets viral, bacterial, and fungal infections [185]. IVIG can fight against infection by regulating a variety of mechanisms, such as downregulation of pro-inflammatory cytokines, upregulation of anti-inflammatory cytokines [186, 187], inhibition of Th1-involved delayed hypersensitivity inflammation, and inhibition of Th1-involved autoimmunity and chronic inflammation [187], as well as neutralizing pathogens. The guideline has pointed out that IVIG may be considered for critical and severe cases [102]. IVIG is derived from nonspecific gamma globulin (IgG type) extracted from the plasma of healthy people. The antibodies in the plasma of the recovered patients can inhibit viremia. According to recent clinical experiences and literature reports [188], the IgM type usually appears 3-4 days after the onset of COVID-19 symptoms, while the titer of IgG antibody can be higher than four times in the recovery stage compared with the acute stage. Therefore, convalescent plasma has been suggested as a therapeutic approach [189]. A clinical study revealed that four patients who received mechanical ventilation and ECMO no longer needed respiratory support, while the CT features recovered within 1-12 days with the negative viral load 9 days after receiving convalescence plasma from patients with severe COVID-19 [190]. The National Health Commission of China has also released the "Convalescent plasma for treating severe and critical COVID-19 (Trial Version 2)" [191], which incorporates the plasma of the rehabilitated patients into comprehensive treatment.
Pneumonia and lung injury associated with COVID-19 are accompanied by lymphocytic decline and interferon suppression, which is part of virus-induced immunosuppression. As mentioned earlier, the progressive decrease in the number of lymphocytes, the progressive increase in plasma IL-6 and CRP levels, the increase in the lactate level, as well as the rapid development of medical image processing are the predictors of severe cases. The levels of IL-1β, IL-6, IL-12, IL-8, tumor necrosis factor (TNF)-α, and CCL2 are usually elevated in infected people [192-194]. SARS-CoV can induce the production of more IL-6 in human epithelial cells, compared with influenza A virus and human parainfluenza virus Type II (HPIV II), which can be the basis for the expansion of IL-6 response [98, 195]. Trial Version 7 has recommended that severe cases with an elevated IL-6 level can be treated with tocilizumab [102]. A retrospective observational study found that the application of tocilizumab could reduce the risk of death by 24% [196]. Meanwhile, humanized monoclonal antibodies that antagonize IL-6R, such as clazakizumab, sarilumab, and siltuximab, are also in clinical trials. In addition, monoclonal antibodies infliximab, emapalumab, BMS-986253, canakinumab, and anakinra, which can antagonize TNF-α, IFN-γ, IL-8, IL-1, and IL-1R, are also in clinical exploration to prove their efficacy in treating COVID-19-related CS. Granulocyte-macrophage colony-stimulating factor (GM-CSF) is an important myelopoietic factor and pro-inflammatory cytokine. Myeloid cells activated by GM-CSF can secrete reactive oxygen species and express high levels of pro-inflammatory cytokines (IL-1, IL-6, and TNF) and various chemokines (CCL2, IL-8, and CCL17) [197]. GM-CSF can also enhance CD4+ T helper cells (TH) [198]. These TH cells can further enhance the immune response generated by pro-inflammatory myeloid cells [199]. The iInhibition of GM-CSF has shown benefits in conditions of high inflammation [200]. Triggering receptor expressed on myeloid cells 1 (TREM1) can also promote inflammation during this process. [201] Pattern recognition receptors [Toll or Toll-like receptors (TLR)] can recognize pathogens (pathogen-related molecular patterns or pathogen-associated molecular pattern (PAMPs) or components of injured cells (risk-related molecular patterns or damage-associated molecular patterns (DAMPs)). The CD24Fc can bind to DAMP to capture inflammatory stimulation, prevent its interaction with TLR receptors, and ameliorate excessive inflammation caused by tissue damage. Also, CD24Fc can also bind to another pattern recognition receptor Siglec G/10, which negatively regulates the activity of NF-κB, thereby reducing tissue damage (Clinical trials identifier: NCT04317040). Besides, the activation of many receptors of cytokines can activate downstream Janus kinase (JAK) family kinases. In a case report, three of four critically ill patients using JAK inhibitor ruxolitinib were out of danger, while one patient died [202]. Lymphocyte depletion is a sign of severe exacerbation of COVID-19. Thus, immunomodulators that can reverse the loss of lymphocytes are considered to have therapeutic potential in critical cases. The complement protein C5 inhibitor eculizumab can effectively block the cleavage of C5, thereby inhibiting the production of pro-inflammatory complement components C5a and C5b-9 [203] and preventing neutrophil recruitment and the formation of lytic membrane attack complex (C5b-9), which promotes inflammation and tissue damage [204], thus reversing the loss of T lymphocytes. In addition, BDB-001 is also a monoclonal antibody that antagonizes C5a complement and is currently in clinical trials. The failure of CD8 + T cells expressing inhibitory receptor programmed cell death 1 (PD-1), promotes the persistence of some viruses [205]. Also, the blockade of PD-1 signaling improves the proliferation of specific T lymphocytes in vitro. PD-1 monoclonal antibody treatment of COVID-19 is also in clinical trials. The activation of T lymphocytes also depends on the C3 receptor [206] and C-C chemokine receptor type 5 (CCR5) on the cell surface. Therefore, C3 antagonists also have therapeutic potential in CS.
Sphingosine-1-phosphate receptor 1 (S1P receptor) is widely distributed on the surface of vascular endothelial cells and lymphocytes, and is related to CS. Evidence shows that S1P receptor inhibitors are still effective against CS in lymphocyte-deficient Rag2-/- mice [207]. This is mainly related to S1P1 receptor inhibitors that can reduce the capillary leakage caused by CS. Artificial intelligence predicts that drugs related to AP2-related protein kinase 1 (AAK1) can destroy these proteins, thereby inhibiting the virus from entering target cells, indicating that AAK1 inhibitor baricitinib is also expected to be a potential drug [132]. In theory, anti-inflammatory drugs aiming at targets such as dihydroorotate dehydrogenase (DHODH), nucleotide-binding domain leucine-rich repeat and pyrin domain containing receptor 3 (NLRP3), Janus kinase (JAK), and Bruton's tyrosine kinase (BTK) [208] can also be used to regulate tissue damage and excessive inflammation caused by COVID-19. mTOR inhibitors can selectively inhibit the activation of memory B cells, preventing antibody-dependent enhancement, thereby slowing down CS to reduce the severity of COVID-19 [209]. At present, these therapeutic options have entered the clinical trial stage. When the disease is progressively worse, the CS can generate an uncontrolled systemic inflammatory response, which further aggravates the severity of the disease and becomes the main cause of patient death, together with ARDS [130]. Therefore, the application of inflammatory cytokine antagonists in the early stages of progressive diseases may benefit in prognosis.
2.3 Treatment of ARDS
Cytokine storm is an important cause of ARDS and the main cause of death of patients with COVID-19. Diffuse alveolar injury is the histological feature of ARDS, with rapid development of capillary congestion, atelectasis, intra-alveolar hemorrhage, and alveolar edema, followed by hyaline membrane formation, epithelial cell proliferation, and interstitial edema in the following days [210]. The pathogenesis of ARDS is divided into three stages, corresponding to histological characteristics. The first stage is ARDS exudative stage characterized by innate immune cell-mediated alveolar endothelial and epithelial barrier damage. In this stage, a large number of pro-inflammatory cytokines are produced, leading to the recruitment of neutrophils and monocytes or macrophages and the activation of effector T cells, thereby maintaining and promoting inflammation to continuous tissue damage [211]. In this process, endothelial activation and microvascular damage further promote and worsen tissue damage [212]. The second stage is the proliferative stage. In this stage, the tissue damage repair begins, the integrity of the epithelium is restored, alveolar edema is absorbed, and alveolar structure and function are restored [213]. This stage is critical to the survival of the host. The last stage is fibrosis, which is not present in all patients and is closely related to the duration of mechanical ventilation.
For the first stage of ARDS exudation, it is particularly important to control and prevent CS and excessive immune response. In addition, reducing endothelial cell damage and improving alveolar edema are also the treatment directions during this period. Angiotensin-converting enzyme (ACE) and ACE2 exist in the epithelium of the respiratory tract and have antagonistic physiological functions [214]. ACE cleaves angiotensin I into angiotensin II, which in turn binds to and activates type 1 angiotensin II receptor (angiotensin type 1 receptor, AT1), and hence exhibits vasoconstriction, pro-inflammatory, and pro-oxidant effects. On the contrary, ACE2 also degrades angiotensin II into angiotensin 1-7 (angiotensin peptide 1-7) and degrades angiotensin I into angiotensin 1-9. When angiotensin 1-9 binds to the Mas receptor, it exerts anti-inflammatory, antioxidant, and vasodilator effects. When the virus binds to ACE2, it reduces the activity of ACE2 so that the balance between ACE/ACE2 is biased toward the angiotensin II enhancement state [17], thus intensifying tissue damage. Therefore, the application of ACE inhibitors and angiotensin peptide 1-7 may benefit; currently, captopril, ramipril, losartan, and angiotensin peptide 1-7 have entered the clinical practice. The inhalation of nitric oxide can inhibit neutrophil migration and oxidative activity, thereby reducing endothelial damage [215]. Vascular endothelial growth factor (VEGF) is the most effective inducing factor to increase vascular permeability. Bevacizumab can antagonize VEGF, thereby reducing the increase in vascular permeability, which has also been exploratorily applied in clinical practice [216]. Drugs that achieve the function of reducing edema through different targets are also in clinical trials. In the exudative phase of ARDS, TNF-mediated tissue factor expression promotes platelet aggregation and microthrombosis, as well as coagulation and hyaline membrane formation in the alveoli [217]. Blocking the internal coagulation process and preventing the formation of microthrombus can also become therapeutic targets in this period. Activated FXIIa catalyzes FXI to produce coagulation factor XII (FXII), which initiates the activation of the internal coagulation pathway. Targeting FXII can prevent thrombosis and does not interfere with hemostasis [218]. Garadacimab is a drug candidate for treatment during this period. In addition, iloprost as prostacyclin protects endothelial damage and has the function of regulating blood clotting.
The second stage of ARDS proliferation is the key recovery period for the host. This stage is characterized by the temporary expansion of resident fibroblasts and the formation of a temporary matrix [217]. Promoting endothelial repair and preventing fibrosis are the key strategies during this period. Oxidative stress caused by harmful reactive oxygen species (ROS) may directly or indirectly participate in human lung fibrosis [219]. NF-E2-related factor 2 (NRF2) is a transcriptional activator of antioxidant/defense genes mediated by antioxidant response elements. The activation of Nrf2 can increase the level of glutathione-S-transferase (GST), NADP(H): quinone oxidoreductase 1 (NQO1), thioredoxin, glutathione peroxidase (GPx), and other antioxidant enzymes, thereby decreasing ROS and reducing pulmonary fibrosis [220]. A variety of drugs in the research and development for anti-fibrosis treatment have entered clinical trials.
The treatment with mesenchymal stem cells (MSCs) has been the research hotspot of ARDS immunotherapy in recent years [221]. MSCs exert anti-inflammatory protective effects on host tissues through the potential anti-inflammatory paracrine factors IL-1ra, TNF-α-stimulated gene 6 protein (TSG-6), insulin-like-growth factor 1 (IGF-1), and prostaglandin E2 (PEG 2) pathway [222]. Meanwhile, MSCs can maintain epithelial cell function [223] and increase the clearance rate of alveolar fluid [224]. MCSs have currently been regarded as a treatment option for patients with severe COVID-19 and are practiced in compassionate use [175, 225, 226] or in clinical trials. Thymosin has shown dual immunoregulatory functions; it is widely used in infectious diseases such as hepatitis B, hepatitis C, sepsis, and aspergillosis [227], as well as in severe COVID-19 [228]. Pulmonary hypertension is a characteristic of ARDS. The dynamic observation of patients with ARDS has revealed that sildenafil can improve pulmonary hypertension [229] but may also lead to the deterioration of oxygenation [229, 230]. However, the application of sildenafil in treating ARDS caused by COVID-19 remains controversial. In addition, immunomodulators such as thalidomide, fingolimod, and aviptadil; immune-stimulants such as GM-CSF; amniotic fluid; as well as anti-inflammatory agents such as tradipitant, colchicine, and losartan are already in clinical trials for treating COVID-19.
2.4 Coagulation disorders in COVID-19
Disseminated intravascular coagulation (DIC) is a secondary syndrome characterized by intravascular coagulation caused by other causes of local damage. It manifests as coagulation failure and the intermediate link to develop into multiple-organ failure (MOF) [231]. The initial coagulopathy of COVID-19 is manifested by a significant increase in the levels of D-dimer and fibrin/fibrinogen degradation products [232]. CS can activate the coagulation pathway, leading to the excessive consumption of coagulation factors and platelets [192]. The excessive consumption of coagulation substrates causes coagulation dysfunction, forming a vicious circle and hence leading to DIC. Therefore, D-dimer antagonists and plasminogen activators may be used to prevent the deterioration of coagulopathy and the development of DIC. At present, these anticoagulant drugs have entered the clinical trial stage. China has issued the “Expert consensus for diagnosis and treatment of coagulation dysfunction in COVID-19,” recommending that patients with severe COVID-19 combined with coagulation dysfunction undergo anticoagulation treatment with unfractionated heparin or low-molecular-weight heparin [231]. If patients with severe COVID-19 develop heparin-induced thrombocytopenia, the anticoagulant argatroban/bivalirudin must be replaced. If continuous renal replacement therapy (CRRT) is required for severe COVID-19 with active bleeding, local citrate anticoagulation is recommended.
2.5 Vaccines
Vaccines need to be urgently developed as the epidemic spreads and the situation continues to escalate. From the long discovery process, traditional vaccine development may take 15 years or more. Vaccines will be designed, and preclinical experiments will be conducted in this stage. The development of the SARS-CoV-2 vaccine has shortened the discovery process due to the knowledge and experience gained from the development of SARS-CoV and MERS-CoV vaccines, as well as the emergency situation based on the global pandemic. The process is simplified as the interim analysis of Phase I/II results, and then the Phase III trial is directly started. At the same time, vaccine manufacturers have begun the mass production of several risky vaccine candidates. According to the information released by the WHO, research teams from companies and universities around the world are developing more than 100 vaccines against SARS-CoV-2. These vaccines are in different stages of development: some are being tested in animals, while some have reached the clinical stage. The mainstream promising vaccines are listed in Supplementary Table 1. To date, seven main types of vaccine candidates are in the clinical stage: inactivated vaccines, subunit vaccines, adenovirus vector vaccines, lentiviral vector vaccines, measles virus vector vaccines, DNA vaccines, and mRNA vaccines. Inactivated and subunit vaccines are virus-based vaccines made from the killed virus or the fragment of the virus. In China, two inactivated vaccines BBIBP-CorV [161] and CoronaVac [160] developed by Sinopharm and Sinovac Biotech, respectively, are already in clinical Phase III. Adenovirus vector, lentiviral vector, and measles virus vector vaccines are viral vector-based vaccines divided into two types according to the technology: replicating viral vector and nonreplicating viral vector vaccines. ChAdOx1 nCov-2019 (Clinical Trial Identifier: ISRCTN89951424) is a recombinant adenovirus vaccine produced by the cooperation between Oxford University and AstraZeneca. The preliminary results showed an average efficacy of 70% in preventing COVID-19. The vaccine can be stored at normal refrigerated conditions (2-8 °C) (www.astrazeneca.com/media-centre/press-releases/2020/azd1222hlr.html). Ad5-nCoV is another adenovirus vector vaccine developed by CanSino Biologics Inc./Academy of Military Medical Sciences from China. Johnson & Johnson’s JNJ-78436735 vaccine [233] is based on nonreplicating viral vector vaccine technology. DNA- and RNA-based vaccines are nucleic acid vaccines that only need genetic materials to produce the vaccines. Technically, this is the easiest vaccine production strategy. MRT5500 [167] is an mRNA vaccine produced by Sanofi and Translate Bio. Recently, the US pharmaceutical company Pfizer and the German pharmaceutical company BioNTech announced their initial research data on mRNA-based vaccine candidate BNT162b2 [168], claiming that the vaccine jointly developed by the two companies is as efficient as 90%, which is much higher than the minimum requirement of the FDA (50%). Shortly afterward, the first interim analysis of Moderna’s COVID-19 vaccine candidate (mRNA-1273) was published [234]. The result met the primary efficacy endpoint with an efficacy of 94.5% (P < 0.0001) (https://investors.modernatx.com/node/10316/pdf). All current vaccines in the clinical stage can induce neutralizing antibodies against viral spike (S) proteins, thereby preventing the virus entry into host cells through the human ACE2 receptor. Once the safety and effectiveness of vaccine candidates are confirmed, COVAX (led by the WHO, Gavi, and Coalition for Epidemic Preparedness Innovations (CEPI)) will promote the fair access and distribution of these vaccines to protect people in all countries.
3. Understanding COVID-19 From the Perspective of TCM
Currently, numerous drug candidates for COVID-19 are in clinical trials; finding a specific drug may still take time. On May 1, the FDA issued an EUA that authorized the use of remdesivir to treat suspected or laboratory-confirmed adult and pediatric patients with severe COVID-19 [235]. So far, the information on the safety and efficacy of remdesivir in treating COVID-19 remains incomplete. Gilead Sciences Inc. (GILD) announced the results of the NCT04280705 trial, which recruited 1062 patients, and showed a significant reduction in the clinical recovery time of 31% (11 days vs 15 days, P < 0.001) in the remdesivir group compared with the placebo group, with no significant reduction in mortality (8% vs 11%, P = 0.059) [236]. The efficacy of remdesivir against COVID-19 was not demonstrated in a previous clinical trial involving 53 patients [88]. In a randomized, double-blind, placebo-controlled trial with severe COVID-19, the administration of remdesivir was not associated with the duration of clinical improvement. However, among patients with symptoms lasting 10 days or less, the duration of clinical improvement was significantly shorter in the remdesivir group than in the placebo group [237]. In the early stage of the epidemic, lopinavir/ritonavir, which was a promising drug combination for treating COVID-19, showed no clinical benefit in 99 severe cases [171]. Still, no effective antiviral drugs are available for severe cases, while clinical treatments mainly focus on the prevention and treatment of symptoms and complications. On the contrary, some traditional medicine practices, such as TCM, have shown certain group efficacy and advantages in this large-scale epidemic.
In the TCM theory, COVID-19 belongs to the category of blight (yì bìng), caused by plague, which refers to dampness (shī dú zhī xié). The characteristics of the disease are damp-heat and turbidity (shī rè zhuó dú), located in the lung, often involving the spleen, stomach, and large intestine [238]. "The Plague Theory (wēnyì lùn)" by Wu Youke (1582-1652 C.E.) in Ming Dynasty pointed out that the infection mode of external dampness was "the evil of the epidemic comes from the mouth and nose." The basic pathogenesis is exogenous dampness, hurting the lung guard; transforming into heat to enter deeper in the body, thereby consuming qi and hurting yin; and invading the viscera. The blood is transformed into sediment and transmitted directly to the pericardium quickly with the poisonous heat intertwining. This dampness eventually develops into a critical illness. In addition, the pathological characteristics of COVID-19 are "wet, blood stasis, poison, and deficiency" [239].
Ever since ancient times, TCM has been successful in treating plague. The treatment of plague is not only against the virus but also for the overall adjustment of the relationship between the upright (zhèng) and the evil ( xié) after the disease has invaded the body. "Book of Jin (jìn shū)" in the Tang Dynasty (completed in 648 AD) also has a record of "the upright stays inside, and the evil cannot interfere" [240]. According to "The Plague Theory," whether the dampness develops into disease after invading the human body depends on the ups and downs of the upright qi (zhèng qì) and the severity of the evil qi. The dampness is one type of evil qi. Upright qi is the opposite of evil qi (xié qì), which refers to a class of fine substances in the body that have the functions of disease resistance, evil elimination, regulation, repair, and so forth [241]. The theory of upright qi and evil qi has provided a reasonable explanation for asymptomatic infected persons and the virus transmission caused by asymptomatic infected persons [25]. "Those with strong upright qi can't be infected even though they face strong evil qi." That is, the body's strong immunity is not easy to be attacked by external evil qi, thus equating upright qi to a certain extent with nonspecific immunity [242]. As mentioned earlier, "The upright stays inside, and the evil cannot interfere." However, "when upright qi coincides with the loss, external evil qi would take advantage of it between breathing." And "if the plague prevails in this year, and the patients will be severe, the disease will be most contagious " [240], indicating that the epidemic goes into a vicious circle without a deep understanding of the etiology. As TCM emphasizes that the treatment of epidemic diseases needs to not only get rid of evil qi but also support upright qi, it is evident that some TCM-based treatment concepts for acute infectious diseases are very advanced.
TCM adopts the diagnosis and treatment method combining disease identification and syndrome differentiation, making a more accurate judgment on the condition of the disease. Modern science and technology have promoted the rapid development of modern medicine. In the face of the need to diagnose diseases and improve objective indicators, diseases diagnosed by modern medicine are renamed, classified, and staged according to the TCM theory in this epidemic [102, 238, 244] (Table 3). According to TCM, COVID-19 is caused by dampness involving different geographical locations, climates, customs, habits, physique differences, and so forth. This dampness has changed from cold to heat during the development of the disease. A person with a white face and yang (yáng) deficiency easily becomes cold after experiencing dampness or other evil qi, thereby developing cold dampness and presenting the syndrome of "cold-damp depressing the lung (hán shī yù fèi)." On the contrary, it is easy to heat up and develop heat dampness for a person with a red face and yin (yīn) deficiency, thus reflecting the syndrome of "damp-heat accumulating in the lung (shī rè yùn fèi)." These are the two main syndromes in the early stage of infection with dampness, which are more common in mild and moderate patients. At this time, the treatment should focus on squandering excessive qi and removing evil qi. This is also how antiviral drugs (recognized as qu xie) have shown a certain effect in the early stage of COVID-19. The cold-damp can further develop into a syndrome that not only obstructs the lung but also traps the spleen, showing clinical signs of chest tightness, vomiting, anorexia, and loose stools. In treating diseases in this stage, warm energy is also needed to disperse cold besides demonstrating lung qi. If symptoms of toxic dampness appear, it is necessary to strengthen the spleen and clean the lung. At this time, the damp-heat syndrome may also form the syndrome of phlegm-heat obstructing the lungs during further development; it is necessary to clear the heat and detoxify, as well as expel evil qi from the lungs.
Table 3.
Prescription for COVID-19 recommended by Chinese medicine.
| Clinical stage | Syndrome | Symptom | Formula | Ref. | |
|---|---|---|---|---|---|
| Observation period | Fatigue with gastrointestinal discomfort | Huoxiang Zhengqi (HXZQ) capsule (pill, liquid or oral solution) | [102, 238] | ||
| Fatigue with fever |
Jinhua Qinggan granules; Lianhua Qingwen capsule (or granules); Shufeng Jiedu capsule (or granules); Fang Feng Tong Sheng pill (or granules) |
[102, 238] | |||
| Treatment period | Mild cases | Cold-damp invading the lung | Aversion to cold with fever or no fever, muscle aches or headaches, dry throat with cough, tiredness and fatigue, stuffy feeling in chest and stomach, or vomiting, loose stools, light or red tongue, white and greasy fur, tight, wiry or moisten pulse i |
Rhizoma Atractylodes 15g, dried Pericarpium Citri Reticulatae 10g, Cortex Magnoliae Officinalis 10g, Chinese Patchouli 10g, Fructus Amomi 6g, raw Herba Ephedrae 6g, Rhizoma et Radix Notopterygii 10g, Rhizoma Zingiberis Recens 10g, Semen Arecae 10g | [238] |
| Raw Herba Ephedrae 6g, Gypsum Fibrosum 15g, Semen Armeniacae Amarum 9g, Rhizoma et Radix Notopterygii 15g, Semen Lepidii 15g, Rhizoma Cyrtomii 9g, earthworm Pheretima 15g, Radix Cynanchi Paniculati 15g, Chinese Patchouli 15g, Herba Eupatorii 9g, Rhizome Atratylodes 15g, Poria Cocos 45g, raw white Atractylodes Rhizome 30g, charred triplet (charred malt, charred hawthorn, charred medicated leaven) 9g for each, Cortex Magnoliae Officinalis 15g, charred Semen Arecae 9g, roasted Fructus Amomi 9g, Rhizoma Zingiberis Recens 15g | [102] | ||||
| Damp-heat obstructing the lung | Low or no fever, slight aversion to cold, fatigue, heavy head and body feeling, muscular soreness, dry cough with less sputum, sore throat, dry mouth without desire of drinking, or accompanied by stuffy feeling in chest and stomach, no sweat or sweating is not smooth, with a disgusted and dull expression, laxness or loose stools, reddish tongue with thick and greasy or light yellow fur, rolling, rapid or moisten pulse | Semen Arecae 10g, Fructus Amomi 10g, Cortex Magnoliae Officinalis 10g, Rhizoma Anemarrhenae 10g, Radix Scutellariae 10g, Radix Bupleuri 10g, Radix Paeoniae Rubra 10g, Fructus Forsythiae 15g, Herba Artemisiae Annuae 10g (decocted later), Rhizoma Atractylodes 10g, Folium Isatidis 10g, raw Radix Glycyrrhizae 5g | [102] | ||
| Chinese Patchouli 10g (decocted later), Cortex Magnoliae Officinalis 10g, Rhizoma Pinellinae Praeparata 10g, Poria Cocos 15g, Radix Bupleuri 15g, Radix Scutellariae 10g, Radix Codonopsis 10g, Semen Armeniacae Amarum 10g, Semen Coicis 20g, Agar 10g, Rhizoma Alismatis 10g, Fructus Amomi Rotundu 10g (decocted later), Semen Sojae Preparatum 10g, Medulla Tetrapanacis 10g, Rhizoma Zingiberis Recens 5g, Fructus Jujubae 5gg | [243] | ||||
| Moderate cases | Toxic damp depressing the lung | Low to medium fever, which is obvious in the afternoon, slight aversion to cold, dry cough with less sputum, dry and sore throat, light tongue with yellow greasy fur, rolling and rapid pulse | Folium Isatidis 15g, Radix Scrophulariae 20g, Radix Bupleuri 15g, Radix Scutellariae 15g, Rhizoma Pinellinae Praeparata 10g, Fructus Arctii 10g, Fructus Forsythiae 10g, Herba Taraxaci 20g, Rhizoma Cyrtomii 10g, Herba Schizonepetae 10g, raw Radix Glycyrrhizae 10g, Radix Isatidis 10g, Poria Cocos 15g, Semen Armeniacae Amarum 12g | [238] | |
| Raw Herba Ephedrae 6g, Semen Armeniacae Amarae 15g, Gypsum Fibrosum 30g, raw Semen Coicis 30g, Rhizoma Atractylodes 10g, Chinese Patchouli 15g, Herba Artemisiae Annuae 12g, Rhizoma Polygontum Cuspidatum 20g, Herba Verbenae 30g, dried Rhizoma Phragmitis 30g, Semen Lepidii 15g, Exocarpium Citri Grandis 15g, raw Radix Glycyrrhizae 10g | [102] | ||||
| Cold-damp obstructing the lung | Hidden low fever, dry cough with less sputum, tiredness and fatigue, stuffy feeling in chest and stomach, or vomiting, and loose stools, pale or light red tongue with white or greasy fur, moisten pulse | Rhizoma Atractylodes 15g, dried Pericarpium Citri Reticulatae 10g, Cortex Magnoliae Officinalis 10g, Chinese Patchouli 10g, Fructus Amomi 6g, raw Ephedrae 6g, Rhizoma et Radix Notopterygii 10g, Rhizoma Zingiberis Recens 10g, Semen Arecae 10g | [102] | ||
| Toxic damp accumulated in interior, involving lung and spleen | Low fever, which is obvious in the afternoon, dry cough with less sputum, dry and sore throat, stuffy feeling in chest and stomach, vomiting and anorexia, laxness and fatigue, pale tongue with greasy fur, rolling and rapid pulse | Folium Isatidis 15g, Radix Scrophulariae 20g, Radix Bupleuri 15g, Radix Scutellariae 15g, Rhizoma Pinellinae Praeparata 10g, Fructus Arctii 10g, Fructus Forsythiae 10g, Herba Taraxaci 20g, Rhizoma Cyrtomii 10g, Herba Schizonepetae 10g, raw Radix Glycyrrhizae 10g, Poria Cocos 15g, Semen Armeniacae Amarum12g, Radix Angelica Dahurica 10g, Chinese Patchouli 10g, Herba Eupatorii 10g | [238] | ||
| Toxic damp obstructing the spleen | Mental depression, fatigue, weakness, anorexia, loose stools, borborygmus, abdominal fullness, chest tightness and shortness of breath, pale tongue with greasy fur, soft and moisten pulse | Chinese Patchouli 15g, Radix Angelica Dahurica 10g, Folium Perillae 6g, Poria Cocos 30g, Rhizoma Pinelliae processed by ginger juice 12g, Rhizoma Atractylodes 10g, white Atractylodes Rhizome 15g, dried Pericarpium Citri Reticulatae 10g, Cortex Magnoliae Officinalis 10g, Radix Glycyrrhiza Preparata 6g, Fructus Amomi Rotundu 10g, Radix Platycodonis 10g | [238] | ||
| Evil heat obstructing the lung | Fever or high fever, cough, yellow or thick phlegm, fatigue, headache, muscular stiffness, dry and bitter mouth, anxiety, red urinary and constipation, red tongue with yellow or greasy fur, rolling and rapid pulse | Fried Herba Ephedrae 8g, Semen Armeniacae Amarum 10g, Gypsum Fibrosum 30g, Radix Glycyrrhizae 10g, Semen Arecae 10g, Cortex Magnoliae Officinalis 10g, Fructus Amomi 10g, Rhizoma Anemarrhenae 10g, Radix Paeoniae Alba 10g, Radix Scutellariae 15g | [243] | ||
| Severe cases | Toxic plague blocking the lung | Chest tightness, shortness of breath, coughing and panting, extreme fatigue, asthmatic, cough with less sputum or hemoptysis or yellow sputum, thirsty and irritable, high fever does not retreat, bloating and constipation, dark red tongue with yellow greasy fur, slippery or heavy pulse | Semen Armeniacae Amarum 10g, Gypsum Fibrosum 30g, Pericarpium Trichosanthis or Semen Trichosanthis 30g, raw Radix et Rhizoma Rhei 6g (decocted later), fried Herba Ephedrae 6g, Semen Lepidii 10g, Semen Persicae 10g, Fructus Amomi 6g, Semen Arecae 10g, Rhizoma Atractylodes 10g | [238] | |
| Semen Armeniacae Amarum 15g, Gypsum Fibrosum 30g (chopped), Fructus Trichosanthis 30g, Fructus Aurantii Immaturus 15g, raw Radix et Rhizoma Rhei 15g (decocted later), raw Herba Ephedrae 6~10g, Semen Lepidii 30g, Semen Persicae 10g, Radix Paeoniae Rubra 15g, raw Radix Glycyrrhizae 6g, Rhizoma Phragmitis 30g | [238] | ||||
| Raw Herba Ephedrae 6g, Semen Armeniacae Amarum 9g, Gypsum Fibrosum 15g, Radix Glycyrrhizae 3g, Chinese Patchouli 10g (decocted later), Cortex Magnoliae Officinalis 10g, Rhizoma Atractylodes 15g, Fructus Amomi 10g, Rhizoma Pinellinae Praeparata 9g, Poria Cocos 15g, raw Radix et Rhizoma Rhei 5g (decocted later), raw Radix Astragali 10g, Semen Lepidii 10g, Radix Paeoniae Rubra 10g | [102] | ||||
| Fever, cough, thick yellow phlegm, chest tightness, wheezing, thirst, stinking tone, bloating and constipation, dark red tongue with thick and yellow fur, slippery or tight pulse | Raw Herba Ephedrae 8g, Semen Armeniacae Amarum 12g, Gypsum Fibrosum 30g, raw Radix et Rhizoma Rhei 10g, Semen Trichosanthis 30g, Semen Persicae 10g, Radix Paeoniae Rubra 15g, Semen Lepidii 20g, Rhizoma Coptidis 3g, Radix Scutellariae 10g, Cortex Mori 10g, Rhizoma Paridis 10g, Cortex Moutan 15g, Radix Curcumae 15g, Rhizoma Acori Graminei 15g, raw Radix Rehmanniae 15g, Radix Scrophulariae 15g | [243] | |||
| Hidden fever, disturbed hidrosis, panting, dry coughing or bucking, or with sore throat, stuffy feeling in chest and stomach, dry mouth, bitter or sticky in the mouth, sticky stools, dark red tongue with yellow and greasy fur, slippery pulse |
Raw Herba Ephedrae 8g, Semen Armeniacae Amarum 12g, Gypsum Fibrosum 30g, raw Radix Glycyrrhizae 10g, Talcum powder 30g, Herba Artemisiae Scopariae 20g, Radix Scutellariae 15g, Fructus Amomi Rotundu 10g (decocted later), Chinese Patchouli 15g, Rhizoma Pinellinae Praeparata 15g, Rhizoma Atractylodes 15g, Semen Lepidii 20g, Fructus Forsythiae 15g, Bombyx Batryticatus 5g, Periostracum Cicadae 5g, Rhizoma Curcumae Longae 10g, raw Radix et Rhizoma Rhei 5g, Rhizoma Paridis 10g, Cortex Moutan 15g, Radix Paeoniae Rubra 15g, Radix Curcumae 15g, Rhizoma Acori Graminei 15g, raw Radix Rehmanniae 15g, Radix Scrophulariae 15g | [243] | |||
| raw Herba Ephedrae 8g, Semen Armeniacae Amarum 12g, Gypsum Fibrosum 30g, raw Radix Glycyrrhizae 10g, Talcum powder 30g, Herba Artemisiae Scopariae 20g, Radix Scutellariae 15g, Fructus Amomi Rotundu 10g (decocted later), Chinese Patchouli 15g, Rhizoma Pinellinae Praeparata 15g, Rhizoma Atractylodes 15g, Semen Lepidii 20g, Fructus Forsythiae 15g, Bombyx Batryticatus 5g, Periostracum Cicadae 5g, Rhizoma Curcumae Longae 10g, raw Radix et Rhizoma Rhei 5g, Rhizoma Paridis 10g, Cortex Moutan 15g, Radix Paeoniae Rubra 15g, Radix Curcumae 15g, Rhizoma Acori Graminei 15g, raw Radix Rehmanniae 15g, Radix Scrophulariae 15g | [243] | ||||
| Blazing heat in qi and ying fen | Hot and thirsty, panting, dizzy delirium, blurred vision with macula, hematemesis and epistaxis, convulsions in the limbs, crimson tongue with little or no fur, deep, thin and rapid pulse, or floating and rapid pulse | Gypsum Fibrosum 30~60g (decocted first), Rhizoma Anemarrhenae 30g, Radix Rehmanniae Preparata 30~60g, Cornu Bubali 30g (decocted first), Radix Paeoniae Rubra 30g, Radix Scrophulariae 30g, Fructus Forsythiae 15g, Cortex Moutan 15g, Rhizoma Coptidis 6g, Folium Phyllostachys 12g, Semen Lepidii 15g, raw Radix Glycyrrhizae 6g | [102] | ||
| Critical cases | Inner blocking causing collapse | Dyspnea, asthmatic, or assisted ventilation needed, with dizziness and irritability, sweating, cold limbs, dark purple tongue with thick, greasy or dry fur, large floating and weak pulse | Radix Ginseng 15g, Radix Aconiti Lateralis Preparata 10g (decocted first), Fructus Corni 15g; with Suhexiang pill or Angong Niuhuang pill; high fever plus Zixue powder; severe breathing plus Semen Lepidii and Herba Ephedrae | [238] | |
| Radix Panacis Quinquefolii 15g, Radix Aconiti Lateralis Preparata 10g, Fructus Corni 30g, crude Os Draconis 30g, Concha Ostreae 30g, Magnetitum 30g; with Angong Niuhuang pill | [238] | ||||
| Radix Ginseng 15g, Radix Aconiti Lateralis Preparata 10g (decocted first), Fructus Corni 15g; with Suhexiang pill or Angong Niuhuang pill | [102] | ||||
| High fever and irritability, cough and shortness of breath, the nose wings incite, phlegm in the throat, holding breath in embarrassment, intermittent voice, spots of rash, or even dizzy coma, sweating, cold limbs, dark purple lips, dark red tongue with yellow and greasy fur, deep, thin and delicate pulse | Radix Ginseng Rubra 10g, Radix Aconiti Lateralis Preparata 10g (decocted first), Fructus Corni 30g, Radix Ophiopogonis 20g, Radix Notoginseng 10g | [243] | |||
| Recovery period | Qi deficiency of lung and spleen | Shortness of breath, fatigue, anorexia, vomiting, fullness, weak and loose stools, pale fat tongue with white and greasy fur. | Rhizoma Pinellinae Praeparata 9g, dried Pericarpium Citri Reticulatae 10g, Radix Codonopsis 15g, Radix Astragali Preparata 30g, Poria Cocos 15g, Chinese Patchouli 10g, Fructus Amomi 6g (decocted later) | [102, 238] | |
| Dried Radix Ginseng 10g (boiled separately), fried Rhizoma Atractylodis Macrocephalae 15g, Poria Cocos 15g, Semen Lablab Album 30g, Fructus Amomi 6g (chopped, decocted later), Semen Nelumbinis 30g, Radix Glycyrrhiza Preparata 6g, Radix Platycodonis 10g, Rhizoma Dioscoreae 15g, Semen Coicis 30g, fried Fructus Hordei Germinatus 30g, medicated leaven 10g | [243] | ||||
| Deficiency of both qi and yin | Shortness of breath, fatigue, coughing, less phlegm, low or no fever, thirst, anorexia, dry and dark tongue with dry and white fur, thin and weak pulse | Radix Adenophorae 10g, Radix Glehniae 10g, Radix Ophiopogonis 6g, Fructus Schisandrae 10g, Gypsum Fibrosum 15g, Herba Lophatheri 10g, Folium Mori 6g, Rhizoma Phragmitis 15g, Radix Trichosanthis 15g, Poria Cocos 10g, Radix Salviae Miltiorrhizae 15g, Semen Persicae 10g, Radix Glycyrrhizae 6g | [102, 238] | ||
|
Buzhong Yiqi pill; Shengmai drink; Xiangsha Liujunzi pill; Shen Ling Baizhu powder |
[238] | ||||
| Radix Panacis Quinquefolii 20g, Herba Dendrobii 10g, Radix Ophiopogonis 10g, Rhizoma Anemarrhenae 10g, Herba Lophatheri 10g, Rhizoma Coptidis 3g, Radix Glycyrrhizae 6g, Poria Cocos 15g, Rhizoma Pinellinae Praeparata 10g, Exocarpium Citri Rubrum 10g, dried Pericarpium Citri Reticulatae 10g, fried Fructus Hordei Germinatus 30g | [243] | ||||
| Prevention | High-risk group | Radix Astragali 15g, fried Rhizoma Atractylodes 10g, Radix Sileris 10g, Flos Lonicerae 10g, Rhizoma Dryopteris Crassirhizomatis 10g, dried Pericarpium Citri Reticulatae 10g, Semen Lablab Album 15g, Poria Cocos 10g | [238] | ||
| Raw Radix Astragali 10g, fried white Atractylodes Rhizome 10g, Radix Sileris 6g, Flos Lonicerae 10g, Herba Eupatorii 10g, dried Pericarpium Citri Reticulatae 10g, Chinese Patchouli 10g, Poria Cocos 10g, raw Radix Glycyrrhizae 6g | [238] | ||||
| Healthy group | Roasted Rhizoma Atractylodes 3g, Flos Lonicerae 3g, dried Pericarpium Citri Reticulatae 3g, Rhizoma Phragmitis 6g, Folium Mori 3g, Folium Perillae 3g, raw Radix Astragali 6g | [238] | |||
| Radix Codonopsis 20g, Folium Perillae 10g, Herba Schizonepetae 10g, Radix Sileris 10g | [243] | ||||
| Rhizoma Zingiberis Recens 10g, Fructus Jujubae 15g, Radix Sileris 10g | [243] | ||||
In COVID-19 disease, cold dampness changes rapidly, with the main event of yang injury, combined with heat, dryness, yin injury, blood stasis, and atrophy. This means that cold-damp can also turn into damp-heat during disease development. In late mild cases or early severe cases, common symptoms of toxic plague blocking the lung (yì dú bì fèi) are always observed. Damp-heat causes dryness, which damages yin. When the disease develops into middle and later stages, many yin injuries occur. In severe cases, both qi and yin are depleted, presenting a dark red tongue with less or peeling fur. Most of the patients who are about to recover from the disease have qi deficiency of the lung and spleen, thereby presenting the symptoms of blazing heat in both qi and blood. In critical cases, the yin and yang are almost departed and separated. Due to qi desertion, patients are more willing to lie down in low spirits. As the upright qi is to take off, the yin fluid loses astringency, presenting shortness of breath and sweating. When the yin fluid is deficient, it causes sudden yang collapse, presenting with cold limbs, severe shortness of breath, dripping cold sweat, and delicate and desperate pulse. In this case, the treatment strategy should be tonifying qi, recuperating depleted yang, and rescuing the patient from collapse. During the recovery period, the qi deficiency of the lung and spleen are observed, which are more common in patients who have been discharged from the hospital after treatment.
Currently, some commercial Chinese medicine preparations are also used in this epidemic. A randomized clinical trial evaluated the clinical efficacy of Huoxiang Zhengqi drop pills and/or Lianhua Qingwen granules combined with Western medicine in treating COVID-19 [245]. The results showed that Huoxiang Zhengqi drop pills combined with Lianhua Qingwen granules and Western medicine had advantages in improving nausea, vomiting, and sore limbs, as well as reducing the use of antibiotics. The efficacy evaluation of Xiyanping injection (NCT04275388) and Huai’er granules for COVID-19 has also entered clinical trials. Herbal extracts, such as licorice, acai palm berry, black locust, and black cumin; star compounds of herbal origin, such as silymarin, artesunate, pyrrolidine-artesunate, tetrandrine, resveratrol, quercetin, vitamins, and folic acid; and even Ayurveda also play a role in adjuvant therapy as supplementary or alternative therapies during treatment. Recently, several observational studies have been carried out worldwide (Table 4).
Table 4.
Commercial Chinese medicine preparations and herb supplement in treatment of COVID19.
| Treatment | Administration and Dosage | Outcome Measures | Source |
|---|---|---|---|
| Lianhua Qingwen | PO, 4 capsules, tid, 7 d | Virological clearance | NCT04433013 (SG) |
| Xiyanping injection | IV, 10-20 mL, qd, Combination medication, 14 d | Clinical recovery time |
NCT04275388 (CN) NCT04295551 (CN) |
| Huaier keli | PO, 20 g, 14 d | Mortality | NCT04291053 (CN) |
| T89 (Danshen Diwan) | PO, 30 pills, bid | Improve oxygen saturation | NCT04285190 (CN) |
| Fuzheng Huayu Tablet | PO, 1.6 g, tid | Evaluation of Pulmonary fibrosis Improvement. | NCT04279197 (CN) |
| Conventional medicines (Oxygen therapy, alfa interferon via aerosol inhalation, and lopinavir/ritonavir) and Traditional Chinese Medicines (TCMs) granules | PO, 20 g, bid, 14 d | The incidence rate of ARDS development | NCT04251871 (CN) |
| Chinese Herbal Medicine | PO | Patient reported main complaint | NCT04380870 (US) |
| Reginmune | PO, 1000 mg, tid, 10 d | Time to clinical improvement | NCT04494204 (IN) |
| Licorice extract | PO, 250 mg standardized extract, 10 d | Increased number of recovery from COVID19 | NCT04487964 (EG) |
| Iota-Carrageenan | ISIN, 4 times, 21 d | Change in SARS-CoV-2 antibody titers | NCT04521322 (AR) |
| çaí palm berry extract | PO, 520 mg, tid, 30 d | 7-point ordinal symptom scale | NCT04404218 (CA) |
| P2Et (Caesalpinia spinosa extract) | PO, 250 mg, bid, 14 d | Proportion of patients who reduce the time in the hospital | NCT04410510 (CO) |
| Nigella sativa | PO, 500 mg, Black seed oil | Proportion of patients who are clinically recovered | NCT04401202 (SA) |
| Nigella Sativa / Black Cumin | PO, 80 mg/kg/d, 14 d | Time needed to turn positive COVID-19 PCR to negative |
NCT04472585 (PK) NCT04347382 |
| Natural Honey | PO, 30 mL, bid, 14 d | Time needed to turn positive COVID-19 PCR to negative |
NCT04323345 (EG) NCT04347382 (PK) |
| Antioxidation Therapy | Two proprietary formulations composed of reduced glutathione, N-acetylcysteine, supero×ide dismutase, and bovine lactoferrin and immunoglobulins. PO, 28 d | Time to clinical improvement | NCT04466657 (NG) |
| Aromatherapy | INH, 15 min | Change from baseline in state anxiety on the State portion of the State Trait Anxiety Scale (STAI-S) at 15 minutes | NCT04495842 (US) |
| Ayurveda | PO | Time to achieve afebrile |
NCT04395976 (GB) NCT04345549 (GB) NCT04351542 |
| Ayurvedic Kadha | PO, 30 d | Prevention | NCT04387643 (IN) |
| Natural Honey | PO, 1 g/kg/d, 14 d | Rate of recovery |
NCT04323345 (EG) NCT04347382 (PK) |
| QuadraMune(TM) | PO, 2 pills, 84 d | Prevention | NCT04421391 (US) |
| Resveratrol | PO, 15 d | Length of stay in hospital | NCT04400890 (US) |
| Tetrandrine | PO, 60 mg, qd, 7 d | Survival rate | NCT04308317 (CN) |
| Quercetin | PO, 500 mg, 90 d | Prevalence of COVID-19 | NCT04377789 (TR) |
| Silymarin | PO, 140 mg, tid, 7-28 d | Time to clinical improvement | NCT04394208 (EG) |
| Escin | PO, 40 mg, tid, 12 d | Mortality | NCT04322344 (IT) |
| Pyronaridine-Artesunate | PO, Pyronaridine 180mg/ Artesunate 60mg, 7 d | Virological clearance | NCT04475107 (KR) |
| Artemisinin / Artesunate | PO, 100 mg, 5 d | Length of stay in hospital | NCT04387240 (SA) |
| ArtemiC | PO | Time to clinical improvement | NCT04382040 (IL) |
| Fisetin | PO, 20 mg/kg/d | Serious adverse events and change in oxygenation status | NCT04476953 (US) |
| LEAF-4L6715 | IV, initial dose of 5 mg, 2.5 mg, qd | Improve oxygenation status | NCT04378920 (FR) |
| LEAF-4L7520 | IV, 0.25 mg/kg per 3h | Improve oxygenation status | NCT04378920 (FR) |
| Manremyc | PO, 1×10^5 heat-inactivated Mycobacterium s. 14 d | Documented cumulative incidence of SARS-CoV-2 infection | NCT04452773 (ES) |
| Glycine | PO, 0.5 g/kg | Mortality | NCT04443673 (US) |
| EPA-FFA | PO, 1 g, bid | Rate of treatment failure | NCT04335032 (CH) |
| omega3-FA | PO, 300 mg, 60 d | Inflammatory factor levels | NCT04483271 (JO) |
| Vitamins | AZINC forme etvitalité®, 2 tablets, PO, 9 d | Occurrence of hospitalization and mortality | NCT04356495 (FR) |
| Vitamin D | PO, 1000-500000 UI, 30 d | Mortality |
NCT04334005 (ES) NCT04335084 (US) NCT04344041 (FR) |
| Vitamin D3 | PO, 2000-100000 UI, 10-30 d | Severity, mortality and inflammatory factor levels |
NCT04351490 (FR) NCT04395768 (AU) NCT04400890 (US) |
| Vitamin C | IV, 10-12 g or 50-60 mg/kg, 9 d | Mortality and dependency on mechanical ventilation, renal replacement, or vasopressors |
NCT03680274 (CN) NCT04323514 (IT) NCT04328961 (US) |
| PO, 500-1000 mg | Symptom Severity |
NCT04354428 (US) NCT04530539 (US) |
|
| Vitamin C +Vitamin D +zinc | PO | Prevention |
NCT04335084 (US) NCT04334512 (US) |
| Vitamin C + Folic Acid | VC: PO, 500 mg, 9 d Folic acid: PO, 400 mcg, 9 d |
Incidence of hospitalization or mortality and change in upper respiratory viral shedding | NCT04354428 (US) |
| Vitamin B | Super B-Comple×, bid, PO, 42 d | Prevention | NCT04343248 (US) |
| Vitamin B12 | Adjuvant therapy: PO, 14 d | Symptoms, length of hospital stay and mortality | NCT04395768 (AU) |
| Vitamin B3 | IV, 20000 UI, 3 d | Change in SARS-CoV-2 antibody titers | NCT04482673 (US) |
| Zinc gluconate | PO, 15-20 mg, bid, 60 d | Survival rate in asymptomatic subjects at inclusion |
NCT04351490 (FR) NCT04447534 (EG) NCT04472585 (PK) |
| L-citrulline | PO, 7 d | SOFA score for organ failures on D7 or last known SOFA score if the patient has died or been resuscitated | NCT04404426 (FR) |
| Nutritional support system | Diet based on the Basal Energy E×penditure plus the stress factor using the Harris Benedict equation. | Oxygen saturation | NCT04507867 (MX) |
| SivoMixx | Composition of SivoMi××: Streptococcus thermophilus DSM322245, Bifidobacterium lactis DSM 32246, Bifidobacterium lactis DSM 32247, Lactobacillus acidophilus DSM 32241, Lactobacillus helveticus DSM 32242, Lactobacillus paracasei DSM 32243, Lactobacillus plantarum DSM 32244, Lactobacillus brevis DSM 27961 (NB: DSM n°... : bacterial strain identification code), 21 d | Delta of time of disappearance of acute diarrhea | NCT04368351 (IT) |
| Omnibiotic AAD | Bacterial strains in Omni-Biotic® 10 AAD are Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W37, Lactobacillus acidophilus W55, Lactobacillus paracasei W20, Lactobacillus plantarum W1, Lactobacillus plantarum W62, Lactobacillus rhamnosus W71 and Lactobacillus salivarius W24 which are embedded in a matri× containing maize starch, maltode×trin, inulin, potassium chloride, hydrolysed rice protein, magnesium sulphate, fructooligosaccharide (FOS), enzymes (amylases), vanilla flavour and manganese sulphate, 30 d | Delta of time of disappearance of acute diarrhea | NCT04420676 (AT) |
| MRx-4DP0004 | Lyophilised formulation of a proprietary strain of bacteria: 4 × 10^9 to 4 ×10^10 colony forming units, PO, 14 d | Change in mean clinical status score in each treatment arm | NCT04363372 (GB) |
| Probiotic | PO, 1×10^9 cfu of the probiotic, 30 d | Cases with discharge to ICU |
NCT04390477 (ES) NCT04517422 |
| bacTRL-Spike | Single dose of bacTRL-Spike, equivalent to 1-3 billion colony forming units (cfu) of Bifidiobacterium longum; PO | Genetically modified probiotic bacteria colonize the gut, bind directly to intestinal epithelial cells and constitutively replicate, secrete and deliver plasmid DNA molecules encoding antigenic transgenes and neutralizing nanobodies. | NCT04334980 (US, CA) |
| Previfenon® | PO, 250 mg, tid | Improve symptoms | NCT04446065 (AU) |
Although Western medicine-based treatment proposes to release the isolation and discharge standards, it lacks follow-up treatment for these patients. Establishing this period of TCM-based treatment plan can benefit patients in terms of their further recovery. Also, adding corresponding notes can fill the gap in the follow-up treatment of these patients by Western medicine, which has potential significance and unique advantages. So far, TCM-based treatment of COVID-19 can significantly improve the clinical symptoms of patients. Mild and moderate patients are easily cured after treatment with TCM. Meanwhile, the conversion of moderate into severe cases has been significantly reduced. Also, patients in the recovery period benefit from TCM.
4. Discussion
The present review outlined the drug development and treatment strategies since the outbreak of SARS-CoV-2, as well as the theory and practice of TCM treatment of COVID-19. The development of Western medicine for COVID-19 is based on the pathogenesis of the disease, involving viral infection, immune suppression induced during the infection process, and CS, ARDS, and DIC caused by excessive inflammation. During TCM treatment, the cause of the disease is examined, the pathogenesis is analyzed, and the measures are adapted to individual conditions. The treatment options of the two medical systems are very different, but similarities exist in the treatment concept. Western medicine has several contradictory drugs, such as the controversial application of glucocorticoids, interferon and interferon antagonist monoclonal antibodies, nonsteroidal anti-inflammatory drugs, anticoagulation and hemostatic therapy, as well as antagonistic GM-CSF monoclonal antibodies and GM-CSF. In the initial stage of infection, blocking the infection and removing the virus are the primary tasks. Besides directly targeting viral invasion and replication, improving innate immunity contributes to clearing the virus in patients with mild COVID-19. The application of interferon is beneficial during this period. In this stage, preventive glucocorticoids can suppress the immune response and delay viral clearance and prolong the course of the disease. Patients with severe and critical COVID-19 also experience CS at the same time. Inhibiting the inflammatory response and preventing the further development of CS are the core tasks of treatment during this period. Therefore, the application of glucocorticoids and interferon antagonism is required.
Taken together, treatment for COVID-19 should be based on the severity of the disease, period of infection, and individual differences, which coincides with the idea of "treatment based on an overall diagnosis" (stages and types of diseases), "prescribing the right medicine," and "appropriate measures to the individual" of TCM theory. From a social perspective, drug development often takes a long time and is costly, making it difficult to respond to the current global outbreak on time. In this case, traditional medicine, as empirical medicine, can make up for the shortcomings of drug treatment as supplementary treatment, adjuvant treatment, and preventive means. Moreover, the feasibility of the combined use of Chinese and Western medicine has been verified in clinical practice. A 58-year-old severely ill female patient received nonselective antiviral Western medicine treatment (ropinavir/ritonavir, arbidol, and methyl-prednisolone) and also a formula including 16 Chinese medicines based on the patient’s symptoms, which showed the therapeutic effect of TCM on the critical COVID-19 [246]. The Shanghai Public Health Clinical Center reported four mild or severe cases given antiviral lopinavir/ritonavir and arbidol treatment combined with a Chinese patent medicine Shufeng Jiedu Capsule. Two out of four patients recovered after the treatment, while the other two patients showed significant improvement. [247] In a retrospective clinical study, 63 patients were treated with Qingfei Paidu Decoction combined with antiviral drugs such as interferon, lopinavir, or arbidol, leading to obvious anti-inflammatory benefits. Although the mortality rate and duration of hospital stay were not affected, combination therapy tended to reduce multiple-organ damage. [248] In addition, several meta-analyses showed that the combination of TCM, such as Lianhua Qingwen decoction [249], Qingfei Paidu Decoction [250], and Reduning injection [251], with Western medicine achieved certain clinical benefits.
Supplementary Materials
The Supplemenantry data can be found online at: www.aginganddisease.org/EN/10.14336/AD.2020.1124.
Acknowledgments
This work was supported by Macau Science and Technology Development Fund (0049/2019/A1 and 039/2017/AFJ), and multi-year research grants, University of Macau (MYRG2018-00242-ICMS and MYRG2019-00160-ICMS).
Footnotes
Conflicts of interest
The authors declared no conflicts of interest.
References
- [1].World Health Organization (2020). Statement on the second meeting of the International Health Regulations (2005) Emergency Committee regarding the outbreak of novel coronavirus (2019-nCoV). [Google Scholar]
- [2].Chan-Yeung M, Xu RH (2003). SARS: epidemiology. Respirology, 8 Suppl:S9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Al-Omari A, Rabaan AA, Salih S, Al-Tawfiq JA, Memish ZA (2019). MERS coronavirus outbreak: Implications for emerging viral infections. Diagn Microbiol Infect Dis, 93:265-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, et al. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 395:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jaimes JA, André NM, Chappie JS, Millet JK, Whittaker GR (2020). Phylogenetic Analysis and Structural Modeling of SARS-CoV-2 Spike Protein Reveals an Evolutionary Distinct and Proteolytically Sensitive Activation Loop. J Mol Biol, 432:3309-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, et al. (2003). Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature, 426:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tang JW, To KF, Lo AW, Sung JJ, Ng HK, Chan PK (2007). Quantitative temporal-spatial distribution of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) in post-mortem tissues. J Med Virol, 79:1245-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004). Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol, 203:631-637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].To KF, Lo AW (2004). Exploring the pathogenesis of severe acute respiratory syndrome (SARS): the tissue distribution of the coronavirus (SARS-CoV) and its putative receptor, angiotensin-converting enzyme 2 (ACE2). J Pathol, 203:740-743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Shafique L, Ihsan A, Liu Q (2020). Evolutionary trajectory for the emergence of novel coronavirus SARS-CoV-2. Pathogens, 9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Rahimi A, Mirzazadeh A, Tavakolpour S (2020). Genetics and genomics of SARS-CoV-2: A review of the literature with the special focus on genetic diversity and SARS-CoV-2 genome detection. Genomics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Liu S, Shen J, Fang S, Li K, Liu J, Yang L, et al. (2020). Genetic spectrum and distinct evolution patterns of SARS-CoV-2. Front Microbiol, 11:2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Korber B, Fischer WM, Gnanakaran S, Yoon H, Theiler J, Abfalterer W, et al. (2020). Tracking changes in SARS-CoV-2 Spike: evidence that D614G increases infectivity of the COVID-19 virus. Cell, 182:812-827.e819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Peng X, Xu X, Li Y, Cheng L, Zhou X, Ren B (2020). Transmission routes of 2019-nCoV and controls in dental practice. Int J Oral Sci, 12:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wan Y, Shang J, Graham R, Baric RS, Li F (2020). Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J Virol, 94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, et al. (2020). Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science, 368:1012-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. (2020). Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet, 395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. (2020). Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet, 395:507-513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu XW, Wu XX, Jiang XG, Xu KJ, Ying LJ, Ma CL, et al. (2020). Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ, 368:m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Team C-NIRS (2020). COVID-19, Australia: Epidemiology Report 2 (Reporting week ending 19:00 AEDT 8 February 2020). Commun Dis Intell (2018), 44. [DOI] [PubMed] [Google Scholar]
- [21].Lu CW, Liu XF, Jia ZF (2020). 2019-nCoV transmission through the ocular surface must not be ignored. Lancet, 395:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med, 382:1564-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cai J, Sun W, Huang J, Gamber M, Wu J, He G (2020). Indirect Virus Transmission in Cluster of COVID-19 Cases, Wenzhou, China, 2020. Emerg Infect Dis, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. (2020). Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine, 26:672-675. [DOI] [PubMed] [Google Scholar]
- [25].Rothe C, Schunk M, Sothmann P, Bretzel G, Froeschl G, Wallrauch C, et al. (2020). Transmission of 2019-nCoV Infection from an Asymptomatic Contact in Germany. N Engl J Med, 382:970-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gu J, Han B, Wang J (2020). COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology, 158:1518-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Qiao J (2020). What are the risks of COVID-19 infection in pregnant women? Lancet, 395:760-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sampathkumar P, Temesgen Z, Smith TF, Thompson RL (2003). SARS: epidemiology, clinical presentation, management, and infection control measures. Mayo Clin Proc, 78:882-890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Rajakaruna SJ, Liu WB, Ding YB, Cao GW (2017). Strategy and technology to prevent hospital-acquired infections: Lessons from SARS, Ebola, and MERS in Asia and West Africa. Mil Med Res, 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].van Doremalen N, Bushmaker T, Morris DH, Holbrook MG, Gamble A, Williamson BN, et al. (2020). Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N Engl J Med, 382:1564-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yin Y, Wunderink RG (2018). MERS, SARS and other coronaviruses as causes of pneumonia. Respirology, 23:130-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. (2020). Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med, 8:475-481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ashour HM, Elkhatib WF, Rahman MM, Elshabrawy HA (2020). Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens, 9:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Azhar EI, Hui DSC, Memish ZA, Drosten C, Zumla A (2019). The Middle East Respiratory Syndrome (MERS). Infect Dis Clin North Am, 33:891-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y, et al. (2020). Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med, 382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Prompetchara E, Ketloy C, Palaga T (2020). Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol, 38:1-9. [DOI] [PubMed] [Google Scholar]
- [37].Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W, et al. (2020). Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: A data-driven analysis in the early phase of the outbreak. Int J Infect Dis, 92:214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chan KS, Zheng JP, Mok YW, Li YM, Liu YN, Chu CM, et al. (2003). SARS: prognosis, outcome and sequelae. Respirology, 8 Suppl:S36-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Arabi YM, Al-Omari A, Mandourah Y, Al-Hameed F, Sindi AA, Alraddadi B, et al. (2017). Critically Ill Patients With the Middle East Respiratory Syndrome: A Multicenter Retrospective Cohort Study. Crit Care Med, 45:1683-1695. [DOI] [PubMed] [Google Scholar]
- [40].Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. (2020). Clinical progression of patients with COVID-19 in Shanghai. China J Infect, 80:e1-e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Liu J, Zheng X, Tong Q, Li W, Wang B, Sutter K, et al. (2020). Overlapping and discrete aspects of the pathology and pathogenesis of the emerging human pathogenic coronaviruses SARS-CoV, MERS-CoV, and 2019-nCoV. J Med Virol, 92:491-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Su YJ, Lai YC (2020). Comparison of clinical characteristics of coronavirus disease (COVID-19) and severe acute respiratory syndrome (SARS) as experienced in Taiwan. Travel Med Infect Dis, 36:101625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Nassar MS, Bakhrebah MA, Meo SA, Alsuabeyl MS, Zaher WA (2018). Middle East Respiratory Syndrome Coronavirus (MERS-CoV) infection: epidemiology, pathogenesis and clinical characteristics. Eur Rev Med Pharmacol Sci, 22:4956-4961. [DOI] [PubMed] [Google Scholar]
- [44].Mo P, Xing Y, Xiao Y, Deng L, Zhao Q, Wang H, et al. (2020). Clinical characteristics of refractory COVID-19 pneumonia in Wuhan, China Clin Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Matsuyama R, Nishiura H, Kutsuna S, Hayakawa K, Ohmagari N (2016). Clinical determinants of the severity of Middle East respiratory syndrome (MERS): a systematic review and meta-analysis. BMC Public Health, 16:1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ho W (2003). Guideline on management of severe acute respiratory syndrome (SARS). Lancet, 361:1313-1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Zhong NS (2003). Infectious severe acute respiratory syndrome (SARS) diagnosis and treatment protocol. Zhonghua Yi Xue Za Zhi, 83:1731-1752. [PubMed] [Google Scholar]
- [48].Zumla A, Hui DS, Perlman S (2015). Middle East respiratory syndrome. Lancet, 386:995-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].National Health Commission of the PRC (2020). Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 8). [Google Scholar]
- [50].Killerby ME, Biggs HM, Midgley CM, Gerber SI, Watson JT (2020). Middle East Respiratory Syndrome Coronavirus Transmission. Emerg Infect Dis, 26:191-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mahase E (2020). Coronavirus covid-19 has killed more people than SARS and MERS combined, despite lower case fatality rate. BMJ, 368:m641. [DOI] [PubMed] [Google Scholar]
- [52].Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. (2020). Virological assessment of hospitalized patients with COVID-2019. Nature, 581:465-469. [DOI] [PubMed] [Google Scholar]
- [53].Liu Y, Ning Z, Chen Y, Guo M, Liu Y, Gali NK, et al. (2020). Aerodynamic Characteristics and RNA Concentration of SARS-CoV-2 Aerosol in Wuhan Hospitals during COVID-19 Outbreak. bioRxiv. [Google Scholar]
- [54].National Health Commission of the PRC. 2020. Health Management Protocol for Discharged COVID-19 patient. [Google Scholar]
- [55].National Health Commission of the PRC. 2020. Prevention and Control (IPC) for Novel Coronavirus (COVID-19)(Trial Version 6). [Google Scholar]
- [56].Zumla A, Hui DS, Azhar EI, Memish ZA, Maeurer M (2020). Reducing mortality from 2019-nCoV: host-directed therapies should be an option. Lancet, 395:e35-e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. (2020). SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell, 181:271-280.e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, et al. (2020). Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun, 11:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang Y, Anirudhan V, Du R, Cui Q, Rong L (2020). RNA-dependent RNA polymerase of SARS-CoV-2 as a therapeutic target. J Med Virol. [DOI] [PubMed] [Google Scholar]
- [60].Azkur AK, Akdis M, Azkur D, Sokolowska M, Veen W, Brüggen MC, et al. (2020). Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy, 75:1564-1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Schaecher SR, Diamond MS, Pekosz A (2008). The Transmembrane Domain of the Severe Acute Respiratory Syndrome Coronavirus ORF7b Protein Is Necessary and Sufficient for Its Retention in the Golgi Complex. J Virol, 82:9477-9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].South AM, Tomlinson L, Edmonston D, Hiremath S, Sparks MA (2020). Controversies of renin-angiotensin system inhibition during the COVID-19 pandemic. Nat Rev Nephrol, 16:305-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].de Abajo FJ, Rodriguez-Martin S, Lerma V, Mejia-Abril G, Aguilar M, Garcia-Luque A, et al. (2020). Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet, 395:1705-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zhou Y, Vedantham P, Lu K, Agudelo J, Carrion R, Nunneley JW, et al. (2015). Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res, 116:76-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Hoffmann M, Schroeder S, Kleine-Weber H, Müller MA, Drosten C, Pöhlmann S (2020). Nafamostat Mesylate Blocks Activation of SARS-CoV-2: New Treatment Option for COVID-19. Antimicrob Agents Chemother, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dong L, Hu S, Gao J (2020). Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther, 14:58-60. [DOI] [PubMed] [Google Scholar]
- [67].Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A, et al. (2020). Coronavirus Disease 2019 (COVID-19) and Cardiovascular Disease. Circulation, 141:1648-1655. [DOI] [PubMed] [Google Scholar]
- [68].Azouz NP, Klingler AM, Rothenberg ME (2020). Alpha 1 Antitrypsin is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2. Preprint from bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Lin B, Ferguson C, White JT, Wang S, Vessella R, True LD, et al. (1999). Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res, 59:4180-4184. [PubMed] [Google Scholar]
- [70].Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. (2020). Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol, 31:1040-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, et al. (2020). SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Bian H, Zheng Z-H, Wei D, Zhang Z, Kang W-Z, Hao C-Q, et al. (2020). Meplazumab treats COVID-19 pneumonia: an open-labelled, concurrent controlled add-on clinical trial. medRxiv. [Google Scholar]
- [73].Boriskin YS, Pecheur EI, Polyak SJ (2006). Arbidol: a broad-spectrum antiviral that inhibits acute and chronic HCV infection. Virol J, 3:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Vankadari N (2020). Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking trimerization of the spike glycoprotein. Int J Antimicrob Agents, 56:105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Glushkov RG, Gus'kova TA, Krylova L, Nikolaeva IS (1999). [Mechanisms of arbidole's immunomodulating action]. Vestn Ross Akad Med Nauk: 36-40. [PubMed] [Google Scholar]
- [76].Li J, Fan JG (2020). Characteristics and Mechanism of Liver Injury in 2019 Coronavirus Disease. J Clin Transl Hepatol, 8:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res, 30:269-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Kono M, Tatsumi K, Imai AM, Saito K, Kuriyama T, Shirasawa H (2008). Inhibition of human coronavirus 229E infection in human epithelial lung cells (L132) by chloroquine: involvement of p38 MAPK and ERK. Antiviral Res, 77:150-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Self WH, Semler MW, Leither LM, Casey JD, Angus DC, Brower RG, et al. (2020). Effect of Hydroxychloroquine on Clinical Status at 14 Days in Hospitalized Patients With COVID-19: A Randomized Clinical Trial. JAMA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Torjesen I (2020). Covid-19: Hydroxychloroquine does not benefit hospitalised patients, UK trial finds. BMJ, 369. [DOI] [PubMed] [Google Scholar]
- [81].Gupta A, Malviya A (2020). Chloroquine and hydroxychloroquine for COVID-19: time to close the chapter. Postgrad Med J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Yuen K-Y, Au W-K, Sit K-Y, Zhang AJ, Chan IH-Y, To KK-W, et al. (2020). Comparative Replication and Immune Activation Profiles of SARS-CoV-2 and SARS-CoV in Human Lungs: An Ex Vivo Study With Implications for the Pathogenesis of COVID-19. Clin Infect Dis, 71:1400-1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Wang Q, Wu J, Wang H, Gao Y, Liu Q, Mu A, et al. (2020). Structural Basis for RNA Replication by the SARS-CoV-2 Polymerase. Cell, 182:417-428.e413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Gao Y, Yan L, Huang Y, Liu F, Zhao Y, Cao L, et al. (2020). Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science, 368:779-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Elfiky AA (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci, 258:118350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Nimgampalle M, Devanathan V, Saxena A (2020). Screening of Chloroquine, Hydroxychloroquine and its derivatives for their binding affinity to multiple SARS-CoV-2 protein drug targets. J Biomol Struct Dyn, 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, et al. (2020). First Case of 2019 Novel Coronavirus in the United States. N Engl J Med, 382:929-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, et al. (2020). Compassionate Use of Remdesivir for Patients with Severe Covid-19. N Engl J Med, 382:2327-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].The First Affiliated Hospital, Zhejiang University School of Medicine. 2020. Handbook of COVID-19 Prevention and Treatment. [Google Scholar]
- [90].Omrani AS, Saad MM, Baig K, Bahloul A, Abdul-Matin M, Alaidaroos AY, et al. (2014). Ribavirin and interferon alfa-2a for severe Middle East respiratory syndrome coronavirus infection: a retrospective cohort study. Lancet Infect Dis, 14:1090-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Stockman LJ, Bellamy R, Garner P (2006). SARS: systematic review of treatment effects. PLoS Med, 3:e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Rathnayake AD, Zheng J, Kim Y, Perera KD, Mackin S, Meyerholz DK, et al. (2020). 3C-like protease inhibitors block coronavirus replication in vitro and improve survival in MERS-CoV-infected mice. Sci Transl Med, 12:eabc5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Shu T, Huang M, Wu D, Ren Y, Zhang X, Han Y, et al. (2020). SARS-Coronavirus-2 Nsp13 Possesses NTPase and RNA Helicase Activities That Can Be Inhibited by Bismuth Salts. Virol Sin, 35:321-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Shi Y, Wang Y, Shao C, Huang J, Gan J, Huang X, et al. (2020). COVID-19 infection: the perspectives on immune responses. Cell Death Differ, 27:1451-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Lythgoe MP, Middleton P (2020). Ongoing Clinical Trials for the Management of the COVID-19 Pandemic. Trends Pharmacol Sci, 41:363-382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Shahabi nezhad F, Mosaddeghi P, Negahdaripour M, Dehghani Z, Farahmandnejad M, Moghadami M, et al. (2020). Therapeutic Approaches for COVID-19 Based on the Dynamics of Interferon-mediated Immune Responses. Preprints. [Google Scholar]
- [97].Ayoub B (2020). COVID-19 vaccination clinical trials should consider multiple doses of BCG. Pharmazie, 75:159-159. [DOI] [PubMed] [Google Scholar]
- [98].McGonagle D, Sharif K, O'Regan A, Bridgewood C (2020). The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev, 19:102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Jones LH, Bunnage ME (2017). Applications of chemogenomic library screening in drug discovery. Nat Rev Drug Discov, 16:285-296. [DOI] [PubMed] [Google Scholar]
- [100].Dong L, Hu S, Gao J (2020). Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther, 14:58-60. [DOI] [PubMed] [Google Scholar]
- [101].Mollica V, Rizzo A, Massari F (2020). The pivotal role of TMPRSS2 in coronavirus disease 2019 and prostate cancer. Future Oncol, 16:2029-2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].National Health Commission of the PRC. 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Prajapat M, Sarma P, Shekhar N, Avti P, Sinha S, Kaur H, et al. (2020). Drug targets for corona virus: A systematic review. Indian J Pharmacol, 52:56-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Zhou D, Dai SM, Tong Q (2020). COVID-19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents, 55:105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zou Quanming LH, Zeng Hao (2020). Current status and counter measures for development of drugs to treat coronavirus disease 2019. J Third Mil Med Univ, 42:5. [Google Scholar]
- [107].Elfiky AA (2020). Ribavirin, Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir against SARS-CoV-2 RNA dependent RNA polymerase (RdRp): A molecular docking study. Life Sci, 253:117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ekins S, Lane TR, Madrid PB (2020). Tilorone: a Broad-Spectrum Antiviral Invented in the USA and Commercialized in Russia and beyond. Pharm Res, 37:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Liu X, Li Z, Liu S, Chen Z, Zhao Z, Huang Y-y, et al. (2020). Therapeutic effects of dipyridamole on COVID-19 patients with coagulation dysfunction. medRxiv. [Google Scholar]
- [110].Johns Hopkins ABX Guide. 2020. Coronavirus COVID-19 (SARS-CoV-2). [Google Scholar]
- [111].Huang A, Xue Tang, Huimin Wu, Jun Zhang, Wanqi Wang, Zhiwei Wang, Li Song, Min-an Zhai, Lihui Zhao, Hailong Yang, Xiaohui Ma, Shuiping Zhou, Jinyong Cai (2020). Virtual Screening and Molecular Dynamics on Blockage of Key Drug Targets as Treatment for COVID-19 Caused by SARS-CoV-2. Preprints. [Google Scholar]
- [112].Vautrin A, Manchon L, Garcel A, Campos N, Lapasset L, Laaref AM, et al. (2019). Both anti-inflammatory and antiviral properties of novel drug candidate ABX464 are mediated by modulation of RNA splicing. Sci Rep, 9:1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Izzedine H, Jhaveri KD, Perazella MA (2020). COVID-19 therapeutic options for patients with kidney disease. Kidney Int, 97:1297-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Wu C-J, Jan J-T, Chen C-M, Hsieh H-P, Hwang D-R, Liu H-W, et al. (2004). Inhibition of Severe Acute Respiratory Syndrome Coronavirus Replication by Niclosamide. Antimicrob Agents Chemother, 48:2693-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Sheahan TP, Sims AC, Zhou S, Graham RL, Pruijssers AJ, Agostini ML, et al. (2020). An orally bioavailable broad-spectrum antiviral inhibits SARS-CoV-2 in human airway epithelial cell cultures and multiple coronaviruses in mice. Sci Transl Med, 12:eabb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S (2020). A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care, 57:279-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Pan X, Zhou P, Fan T, Wu Y, Zhang J, Shi X, et al. (2020). Immunoglobulin fragment F(ab’)2 against RBD potently neutralizes SARS-CoV-2 in vitro. Antiviral Res, 182:104868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Chen L, Xiong J, Bao L, Shi Y (2020). Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis, 20:398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Haodong C (2020). Safety application of novel coronavirus pneumonia antiviral drugs. Adverse Drug React, 22:8. [Google Scholar]
- [120].Luo SH, Liu W, Liu ZJ, Zheng XY, Hong CX, Liu ZR, et al. (2020). A confirmed asymptomatic carrier of 2019 novel coronavirus. Chin Med J (Engl), 133:1123-1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Qiu R, Wei X, Zhao M, Zhong C, Zhao C, Hu J, et al. (2020). Outcome reporting from protocols of clinical trials of Coronavirus Disease 2019 (COVID-19): a review. medRxiv. [Google Scholar]
- [122].Monneret G, de Marignan D, Coudereau R, Bernet C, Ader F, Frobert E, et al. (2020). Immune monitoring of interleukin-7 compassionate use in a critically ill COVID-19 patient. Cell Mol Immunol, 17:1001-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y (2020). The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents, 55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Little P (2020). Non-steroidal anti-inflammatory drugs and covid-19. BMJ, 368:m1185. [DOI] [PubMed] [Google Scholar]
- [125].Matsuyama S, Kawase M, Nao N, Shirato K, Ujike M, Kamitani W, et al. (2020). The inhaled corticosteroid ciclesonide blocks coronavirus RNA replication by targeting viral NSP15. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang Y, Jiang W, He Q, Wang C, Wang B, Zhou P, et al. (2020). Early, low-dose and short-term application of corticosteroid treatment in patients with severe COVID-19 pneumonia: single-center experience from Wuhan, China. medRxiv. [Google Scholar]
- [127].Cron RQ, Chatham WW (2020). The Rheumatologist’s Role in COVID-19. J Rheumatol, 47:639-642. [DOI] [PubMed] [Google Scholar]
- [128].Deng Xiaobing YX, Jianfeng Pei (2020). Regulation of interferon production as a potential strategy for COVID-19 treatment. ArXiv. [Google Scholar]
- [129].Li X, Yu J, Zhang Z, Ren J, Peluffo AE, Zhang W, et al. (2020). Network Bioinformatics Analysis Provides Insight into Drug Repurposing for COVID-2019. Preprints. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. (2020). COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet, 395:1033-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Martin TR, Wurfel MM, Zanoni I, Ulevitch R (2020). Targeting innate immunity by blocking CD14: Novel approach to control inflammation and organ dysfunction in COVID-19 illness. EBioMedicine, 57:102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Richardson P, Griffin I, Tucker C, Smith D, Oechsle O, Phelan A, et al. (2020). Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet, 395:e30-e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. (2020). COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis, 20:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, et al. (2020). The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol, 214:108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Singh AK, Singh A, Shaikh A, Singh R, Misra A (2020). Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: A systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr, 14:241-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].CytoDyn Inc. (2020). CytoDyn Files FDA-Suggested Modifications to IND and Protocol for Phase 2 Clinical Trial for COVID-19 Patients with Mild to Moderate Indications and a Second Randomized Protocol for All COVID-19 Patients in Severe Condition Will be Filed Next Week per FDA Recommendation. [Google Scholar]
- [137].Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC, et al. (2020). The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol, 215:108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Zheng M, Gao Y, Wang G, Song G, Liu S, Sun D, et al. (2020). Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol Immunol, 17:533-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Rasmussen SA, Jamieson DJ (2020). Coronavirus Disease 2019 (COVID-19) and Pregnancy. Obstet Gynecol, 135:999-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Yang D, Yang Y, Zhao Y (2020). Ibudilast, a Phosphodiesterase-4 Inhibitor, Ameliorates Acute Respiratory Distress Syndrome in Neonatal Mice by Alleviating Inflammation and Apoptosis. Med Sci Monit, 26:e922281-1-e922281-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Grimes JM, Grimes KV (2020). p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J Mol Cell Cardiol 144:63-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Mauvais-Jarvis F, Klein SL, Levin ER (2020). Estradiol, Progesterone, Immunomodulation, and COVID-19 Outcomes. Endocrinology, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Zhang X, Zhu Z, Jiao W, Liu W, Liu F, Zhu X (2019). Ulinastatin treatment for acute respiratory distress syndrome in China: a meta-analysis of randomized controlled trials. BMC Pulm Med, 19:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Wu C, Hu X, Song J, Du C, Xu J, Yang D, et al. (2020). Heart injury signs are associated with higher and earlier mortality in coronavirus disease 2019 (COVID-19). medRxiv. [Google Scholar]
- [145].Golchin A, Seyedjafari E, Ardeshirylajimi A (2020). Mesenchymal Stem Cell Therapy for COVID-19: Present or Future. Stem Cell Rev Rep, 16:427-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Mevorach D (2020). Allocetra-Ots: Early Apoptotic Cells for Immune Homeostasis in Human Stem Cell Transplantation (HSCT) and for the Prevention of Graft Versus Host Disease (GvHD). Biol Blood Marrow Transplant, 26:S313-S314. [Google Scholar]
- [147].Amat-Santos IJ, Santos-Martinez S, López-Otero D, Nombela-Franco L, Gutiérrez-Ibanes E, Del Valle R, et al. (2020). Ramipril in High-Risk Patients With COVID-19. J Am Coll Cardiol, 76:268-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Sallari Jazzi AMK; Hossein Hejazi S; Damavandi MS; Sadeghi P; Zeinalian M; Tabesh F; Mirbod SM; Khanahmad H. (2020). Inhibition of Viral Macrodomain of COVID-19 and Human TRPM2 by losartan. Preprints. [Google Scholar]
- [149].Rothlin RP, Vetulli HM, Duarte M, Pelorosso FG (2020). Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev Res, 81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Kintscher U, Slagman A, Domenig O, Röhle R, Konietschke F, Poglitsch M, et al. (2020). Plasma Angiotensin Peptide Profiling and ACE2-Activity in COVID-19 Patients treated with Pharmacological Blockers of the Renin Angiotensin System. Hypertension, 76:e34-e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Berra L, Lei C, Su B, Dong H, Safaee Fakhr B, Grassi LG, et al. (2020). Protocol for a randomized controlled trial testing inhaled nitric oxide therapy in spontaneously breathing patients with COVID-19. medRxiv. [Google Scholar]
- [152].Marchetti M (2020). COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol, 99:1701-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Chen CS, Qi F, Shi KQ, Li YP, Li J, Chen YP, et al. (2020). Thalidomide Combined with Low-dose Glucocorticoid in the Treatment of COVID-19 Pneumonia. Preprints. [Google Scholar]
- [154].Rosa SGV, Santos WC (2020). Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica, 44:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Solaimanzadeh I (2020). Acetazolamide, Nifedipine and Phosphodiesterase Inhibitors: Rationale for Their Utilization as Adjunctive Countermeasures in the Treatment of Coronavirus Disease 2019 (COVID-19). Cureus, 12:e7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Robertson CE (2020). Could CGRP Antagonists Be Helpful in the Fight Against COVID-19? Headache, 60:1450-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Shetty AK (2020). Mesenchymal Stem Cell Infusion Shows Promise for Combating Coronavirus (COVID-19)- Induced Pneumonia. Aging Dis, 11:462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Munera-Campos M, Carrascosa JM (2020). Innovation in Atopic Dermatitis: From Pathogenesis to Treatment. Actas Dermosifiliogr, 111:205-221. [DOI] [PubMed] [Google Scholar]
- [159].Seelhammer TG, Rowse P, Yalamuri S (2020). Bivalirudin for Maintenance Anticoagulation During Venovenous Extracorporeal Membrane Oxygenation for COVID-19. J Cardiothorac Vasc Anesth. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Shervani Z, Khan I, Khan T, Qazi UY (2020). COVID-19 Vaccine. Adv Infect Dis, 10:195-210. [Google Scholar]
- [161].Xia S, Zhang Y, Wang Y, Wang H, Yang Y, Gao GF, et al. (2020). Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Gupta T, Gupta SK (2020). Potential adjuvants for the development of a SARS-CoV-2 vaccine based on experimental results from similar coronaviruses. Int Immunopharmacol, 86:106717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Yang J, Wang W, Chen Z, Lu S, Yang F, Bi Z, et al. (2020). A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature, 586:572-577. [DOI] [PubMed] [Google Scholar]
- [164].Thanh Le T, Andreadakis Z, Kumar A, Gomez Roman R, Tollefsen S, Saville M, et al. (2020). The COVID-19 vaccine development landscape. Nat Rev Drug Discov, 19:305-306. [DOI] [PubMed] [Google Scholar]
- [165].Mercado NB, Zahn R, Wegmann F, Loos C, Chandrashekar A, Yu J, et al. (2020). Single-shot Ad26 vaccine protects against SARS-CoV-2 in rhesus macaques. Nature, 586:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Agrawal S, Goel AD, Gupta N (2020). Emerging prophylaxis strategies against COVID-19. Monaldi Arch Chest Dis, 90. [DOI] [PubMed] [Google Scholar]
- [167].Kalnin KV, Plitnik T, Kishko M, Zhang J, Zhang D, Beauvais A, et al. (2020). Immunogenicity of novel mRNA COVID-19 vaccine MRT5500 in mice and non-human primates. Preprint from bioRxiv. [Google Scholar]
- [168].Walsh EE, Frenck RW, Falsey AR, Kitchin N, Absalon J, Gurtman A, et al. (2020). Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N Engl J Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Liu C, Zhou Q, Li Y, Garner LV, Watkins SP, Carter LJ, et al. (2020). Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent Sci, 6:315-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Sanchis-Gomar F, Lavie CJ, Morin DP, Perez-Quilis C, Laukkanen JA, Perez MV (2020). Amiodarone in the COVID-19 Era: Treatment for Symptomatic Patients Only, or Drug to Prevent Infection? Am J Cardiovasc Drugs, 20:413-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. (2020). A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N Engl J Med, 382:1787-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Xiong C, Jiang L, Chen Y, Jiang Q (2020). Evolution and variation of 2019-novel coronavirus. bioRxiv. [Google Scholar]
- [173].Ceraolo C, Giorgi FM (2020). Genomic variance of the 2019-nCoV coronavirus. J Med Virol, 92:522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Forster P, Forster L, Renfrew C, Forster M (2020). Phylogenetic network analysis of SARS-CoV-2 genomes. Proc Natl Acad Sci U S A, 117:9241-9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Atluri S, Manchikanti L, Hirsch JA (2020). Expanded Umbilical Cord Mesenchymal Stem Cells (UC-MSCs) as a Therapeutic Strategy in Managing Critically Ill COVID-19 Patients: The Case for Compassionate Use. Pain Physician, 23:E71-E83. [PubMed] [Google Scholar]
- [176].Shang L, Zhao J, Hu Y, Du R, Cao B (2020). On the use of corticosteroids for 2019-nCoV pneumonia. Lancet, 395:683-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Chen RC, Tang XP, Tan SY, Liang BL, Wan ZY, Fang JQ, et al. (2006). Treatment of severe acute respiratory syndrome with glucosteroids: the Guangzhou experience. Chest, 129:1441-1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Arabi YM, Mandourah Y, Al-Hameed F, Sindi AA, Almekhlafi GA, Hussein MA, et al. (2018). Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am J Respir Crit Care Med, 197:757-767. [DOI] [PubMed] [Google Scholar]
- [179].World Health Organization (2020). Clinical management of severe acute respiratory infection when COVID-19 is suspected. [Google Scholar]
- [180].Russell CD, Millar JE, Baillie JK (2020). Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet, 395:473-475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Wang W, Rong P, Ma X, Liu J, Zeng Q, Mei J, et al. (2020). Clinical Factors Associated with Progression and Prolonged Viral Shedding in COVID-19 Patients: A Multicenter Study. Aging Dis, 11:1069-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Moreno G, Rodriguez A, Reyes LF, Gomez J, Sole-Violan J, Diaz E, et al. (2018). Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med, 44:1470-1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Li H, Yang SG, Gu L, Zhang Y, Yan XX, Liang ZA, et al. (2017). Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza Other Respir Viruses, 11:345-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zhou W, Liu Y, Tian D, Wang C, Wang S, Cheng J, et al. (2020). Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther, 5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Jawhara S (2020). Could Intravenous Immunoglobulin Collected from Recovered Coronavirus Patients Protect against COVID-19 and Strengthen the Immune System of New Patients? Int J Mol Sci, 21:2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Seite JF, Shoenfeld Y, Youinou P, Hillion S (2008). What is the contents of the magic draft IVIg? Autoimmun Rev, 7:435-439. [DOI] [PubMed] [Google Scholar]
- [187].Maddur MS, Trinath J, Rabin M, Bolgert F, Guy M, Vallat JM, et al. (2015). Intravenous immunoglobulin-mediated expansion of regulatory T cells in autoimmune patients is associated with increased prostaglandin E2 levels in the circulation. Cell Mol Immunol, 12:650-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Haveri A, Smura T, Kuivanen S, Osterlund P, Hepojoki J, Ikonen N, et al. (2020). Serological and molecular findings during SARS-CoV-2 infection: the first case study in Finland, January to February 2020. Eurosurveillance, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Chen L, Xiong J, Bao L, Shi Y (2020). Convalescent plasma as a potential therapy for COVID-19. Lancet Infect Dis, 20:398-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. (2020). Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA, 323:1582-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].National Health Commission of the PRC (2020). Convalescent plasma for the treatment of severe and critical COVID-19. [Google Scholar]
- [192].Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. (2020). Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA, 323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Chen L, Liu HG, Liu W, Liu J, Liu K, Shang J, et al. (2020). Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi, 43:E005. [DOI] [PubMed] [Google Scholar]
- [194].Wan S, Yi Q, Fan S, Lv J, Zhang X, Guo L, et al. (2020). Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP). medRxiv. [Google Scholar]
- [195].Okabayashi T, Kariwa H, Yokota S, Iki S, Indoh T, Yokosawa N, et al. (2006). Cytokine regulation in SARS coronavirus infection compared to other respiratory virus infections. J Med Virol, 78:417-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Lazzeri C, Ip A, Berry DA, Hansen E, Goy AH, Pecora AL, et al. (2020). Hydroxychloroquine and tocilizumab therapy in COVID-19 patients-An observational study. Plos One, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Hamilton JA (2020). GM-CSF in inflammation. J Exp Med, 217:e20190945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Becher B, Tugues S, Greter M (2016). GM-CSF: From Growth Factor to Central Mediator of Tissue Inflammation. Immunity, 45:963-973. [DOI] [PubMed] [Google Scholar]
- [199].Zhang J, Roberts AI, Liu C, Ren G, Xu G, Zhang L, et al. (2013). A novel subset of helper T cells promotes immune responses by secreting GM-CSF. Cell Death Differ, 20:1731-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Lang FM, Lee KMC, Teijaro JR, Becher B, Hamilton JA (2020). GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol, 20:507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Bouchon A, Dietrich J, Colonna M (2000). Cutting Edge: Inflammatory Responses Can Be Triggered by TREM-1, a Novel Receptor Expressed on Neutrophils and Monocytes. J Immunol, 164:4991-4995. [DOI] [PubMed] [Google Scholar]
- [202].Won C, Damsky W, Singh I, Joseph P, Chichra A, Oakland H, et al. (2020). Hijaking the SARS-CoV-2 Cytokinopathy: Janus Kinase Inhibitors for Moderate to Severe COVID-19. SSRN. [Google Scholar]
- [203].Thomas TC, Rollins SA, Rother RP, Giannoni MA, Hartman SL, Elliott EA, et al. (1996). Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol, 33:1389-1401. [DOI] [PubMed] [Google Scholar]
- [204].Barilla-Labarca ML, Toder K, Furie R (2013). Targeting the complement system in systemic lupus erythematosus and other diseases. Clin Immunol, 148:313-321. [DOI] [PubMed] [Google Scholar]
- [205].Fuller MJ, Callendret B, Zhu B, Freeman GJ, Hasselschwert DL, Satterfield W, et al. (2013). Immunotherapy of chronic hepatitis C virus infection with antibodies against programmed cell death-1 (PD-1). Proc Natl Acad Sci U S A, 110:15001-15006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Wilson AB, Prichard-Thomas S, Coombs RR (1979). Receptors for activated C3 on thymus-dependent (T) lymphocytes of normal guinea-pigs. Immunology, 37:377-384. [PMC free article] [PubMed] [Google Scholar]
- [207].Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, et al. (2011). Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell, 146:980-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Stebbing J, Phelan A, Griffin I, Tucker C, Oechsle O, Smith D, et al. (2020). COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis, 20:400-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Zheng Y, Li R, Liu S (2020). Immunoregulation with mTOR inhibitors to prevent COVID-19 severity: A novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol, 92:1495-1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Katzenstein AL, Bloor CM, Leibow AA (1976). Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am J Pathol, 85:209-228. [PMC free article] [PubMed] [Google Scholar]
- [211].Aggarwal NR, King LS, D'Alessio FR (2014). Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol, 306:L709-L725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Guice KS, Oldham KT, Caty MG, Johnson KJ, Ward PA (1989). Neutrophil-dependent, oxygen-radical mediated lung injury associated with acute pancreatitis. Ann Surg, 210:740-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Vaughan AE, Brumwell AN, Xi Y, Gotts JE, Brownfield DG, Treutlein B, et al. (2014). Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature, 517:621-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [214].Bavishi C, Maddox TM, Messerli FH (2020). Coronavirus Disease 2019 (COVID-19) Infection and Renin Angiotensin System Blockers. JAMA Cardiol, 5:745-747. [DOI] [PubMed] [Google Scholar]
- [215].Bloomfield GL, Holloway S, Ridings PC, Fisher BJ, Blocher CR, Sholley M, et al. (1997). Pretreatment with inhaled nitric oxide inhibits neutrophil migration and oxidative activity resulting in attenuated sepsis-induced acute lung injury. Crit Care Med, 25:584-593. [DOI] [PubMed] [Google Scholar]
- [216].Rosa SGV, Santos WC (2020). Clinical trials on drug repositioning for COVID-19 treatment. Rev Panam Salud Publica, 44:e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Thompson BT, Drazen JM, Chambers RC, Liu KD (2017). Acute Respiratory Distress Syndrome. N Engl J Med, 377:562-572. [DOI] [PubMed] [Google Scholar]
- [218].Renné T, Nieswandt B, Gailani D, Renné C, Burfeind P, Pauer H-U, et al. (2006). Targeting coagulation factor XII provides protection from pathological thrombosis in cerebral ischemia without interfering with hemostasis. J Exp Med, 203:513-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [219].Cantin AM, North SL, Fells GA, Hubbard RC, Crystal RG (1987). Oxidant-mediated epithelial cell injury in idiopathic pulmonary fibrosis. J Clin Invest, 79:1665-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Cho H-Y, Reddy SPM, Yamamoto M, Kleeberger SR (2004). The transcription factor NRF2 protects against pulmonary fibrosis. FASEB, 18:1258-1260. [DOI] [PubMed] [Google Scholar]
- [221].Garcovich S, Calabrese C, Pizzicannella J, Sterodimas A, Gentile P (2020). Research progress on Mesenchymal Stem Cells (MSCs), Adipose-Derived Mesenchymal Stem Cells (AD-MSCs), Drugs, and Vaccines in Inhibiting COVID-19 Disease. Aging Dis, 11:1191-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Walter J, Ware LB, Matthay MA (2014). Mesenchymal stem cells: mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir Med, 2:1016-1026. [DOI] [PubMed] [Google Scholar]
- [223].Eklund L, Saharinen P (2013). Angiopoietin signaling in the vasculature. Exp Cell Res, 319:1271-1280. [DOI] [PubMed] [Google Scholar]
- [224].Lee JW, Fang X, Gupta N, Serikov V, Matthay MA (2009). Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc Natl Acad Sci U S A, 106:16357-16362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ (2020). Current Status of Cell-Based Therapies for Respiratory Virus Infections: Applicability to COVID-19. Eur Respir J, 55:2000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Zhao RC, Jin K, Caruso C, Ellison-Hughes G, Min K-J, Chakrabarti S, et al. (2020). Transplantation of ACE2- Mesenchymal Stem Cells Improves the Outcome of Patients with COVID-19 Pneumonia. Aging Dis, 11:216-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Camerini R, Garaci E (2015). Historical review of thymosin alpha 1 in infectious diseases. Expert Opin Biol Ther, 15 Suppl 1:S117-127. [DOI] [PubMed] [Google Scholar]
- [228].Zhai P, Ding Y, Wu X, Long J, Zhong Y, Li Y (2020). The epidemiology, diagnosis and treatment of COVID-19. Int J Antimicrob Agents, 55:105955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Cornet AD, Hofstra JJ, Swart EL, Girbes AR, Juffermans NP (2010). Sildenafil attenuates pulmonary arterial pressure but does not improve oxygenation during ARDS. Intensive Care Med, 36:758-764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Blanch L, Albaiceta GM (2010). Sildenafil for pulmonary hypertension in ARDS: a new pleasant effect? Intensive Care Med, 36:729-731. [DOI] [PubMed] [Google Scholar]
- [231].Song J-C, Wang G, Zhang W, Zhang Y, Li W-Q, Zhou Z (2020). Chinese expert consensus on diagnosis and treatment of coagulation dysfunction in COVID-19. Mil Med Res, 7:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Levy JH, Connors JM (2020). COVID-19 and its implications for thrombosis and anticoagulation. Blood, 135:2033-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Doshi P (2020). Covid-19 vaccine trial protocols released. BMJ, 371. [DOI] [PubMed] [Google Scholar]
- [234].Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, Makhene M, Coler RN, et al. (2020). An mRNA Vaccine against SARS-CoV-2 - Preliminary Report. N Engl J Medicine, 383:1920-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].U.S. Food and Drug Administration. 2020. Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. [Google Scholar]
- [236].Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. (2020). Remdesivir for the Treatment of Covid-19 - Final Report. N Engl J Med, 383:1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Wang Y, Zhang D, Du G, Du R, Zhao J, Jin Y, et al. (2020). Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet, 395:1569-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Shandong Provincial Health Committee. (2020). Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia with Traditional Chinese Medicine. [Google Scholar]
- [239].Wang Guangyu QW, Ma Jiaju, Luan Lianguo, Lu Youran, Li XuCheng, Zhao Xin, Zhang Zhongde, Liu Qingquan (2020). Preliminary study on clinical features and syndrome differentiation treatment of new coronavirus (2019-nCoV) pneumonia. J Tradit Chin Med, 61:6. [Google Scholar]
- [240].Yude W (2020). The epidemic situation and countermeasures in ancient China. Jianghan Tribune, 9:5. [Google Scholar]
- [241].Fuchun S. 2005. Basic Theory of Traditional Chinese Medicine: People's Military Medical Press. [Google Scholar]
- [242].Lu Jianwu DY, Yin Shipeng (2016). Discussion on the function correlation between spleen and stomach and non-specific immunity with the theory of "positive qi". J Gansu Univ Tradit Chin Med, 6:3. [Google Scholar]
- [243].Yu Feng LJ, Ma Han (2020). Talking about new coronavirus pneumonia. Acta Chin Med: 8. [Google Scholar]
- [244].Zhang Wei WY, Zhang Huiyong, Chen Wei, Shi Kehua, Wang Zhenwei (2020). Interpretation of the“TCM Diagnosis and Treatment Program for Novel Coronavirus Pneumonia in Shanghai (Trial)”. Shanghai J Tradit Chin Med, 54:4. [Google Scholar]
- [245].Xiao M, Tian J, Zhou Y, Xu X, Min X, Lv Y, et al. (2020). Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: A randomized controlled trial. Pharmacol Res, 161:105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Zhang K, Tian M, Zeng Y, Wang L, Luo S, Xia W, et al. (2020). The combined therapy of a traditional Chinese medicine formula and Western medicine for a critically ill case infected with COVID-19. Complement Ther Med, 52:102473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [247].Wang Z, Chen X, Lu Y, Chen F, Zhang W (2020). Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends, 14:64-68. [DOI] [PubMed] [Google Scholar]
- [248].Xin S, Cheng X, Zhu B, Liao X, Yang F, Song L, et al. (2020). Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed Pharmacother, 129:110500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [249].Zhang X, Cao D, Liu J, Zhang Q, Liu M (2020). Efficacy and safety of Lianhua Qingwen combined with conventional antiviral Western Medicine in the treatment of coronavirus disease (covid-19) in 2019: Protocol for a systematic review and meta-analysis. Medicine, 99:e21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [250].Gao K, Song YP, Chen H, Zhao LT, Ma L (2020). Therapeutic efficacy of Qingfei Paidu decoction combined with antiviral drugs in the treatment of corona virus disease 2019: A protocol for systematic review and meta analysis. Medicine, 99:e20489. [DOI] [PubMed] [Google Scholar]
- [251].Cao C, Zhen Z, Kuang S, Xu T (2020). Reduning injection combined with western medicine for pneumonia: A protocol for systematic review and meta-analysis. Medicine, 99:e22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.