Abstract
Initially, the SARS-CoV-2 virus was considered as a pneumonia virus; however, a series of peer reviewed medical papers published in the last eight months suggest that this virus attacks the brain, heart, intestine, nervous and vascular systems, as well the blood stream. Although many facts remain unknown, an objective appraisal of the current scientific literature addressing the latest progress on COVID-19 is required. The aim of the present study was to conduct a critical review of the literature, focusing on the current molecular structure of SARS-CoV-2 and prospective treatment modalities of COVID-19. The main objectives were to collect, scrutinize and objectively evaluate the current scientific evidence-based information, as well to provide an updated overview of the topic that is ongoing. The authors underlined potential prospective therapies, including vaccine and phototherapy, as a monotherapy or combined with current treatment modalities. The authors concluded that this review has produced high quality evidence, which can be utilized by the clinical scientific community for future reference, as the knowledge and understanding of the SARS-CoV-2 virus are evolving, in terms of its epidemiological, pathogenicity, and clinical manifestations, which ultimately map the strategic path, towards an effective and safe treatment and production of a reliable and potent vaccine.
Keywords: SARS-CoV-2, COVID-19, virus pathogenicity, cytokines storm, diagnostic methods, immunotherapy, vaccine, antiviral, photobiomodulation therapy, PBMT, photodynamic therapy, PDT, clinical trials
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Summary
1. The spread of SARS-CoV-2 virus has shown a potential zoonotic origin. Advancements in ongoing research activity is trying to identify further possible animal hosts of COVID-19
2. Classic Kawasaki disease (KD) has been identified in pediatric COVID-19.
3. Newer epidemiological facts, properties of the virus, immune responses against the virus and challenges in vaccine production are surfacing each day.
4. Prospective therapies: cellular therapy, ACE2 (vital component of renin-angiotensin system; RAS) treated with inhibitors of RAS; ACEI and AT1R.
5. Phototherapy can be considered as a potential treatment modality in COVID-19 management subjected to robust clinical trials.
Introduction
Since the World Health Organization (WHO) declared the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral infection a pandemic, researchers and scientists have been making phenomenal efforts to understand the novel coronavirus behavior, clinical presentations, host–virus interaction and its impact on the host immune responses, as well as attempt to establish optimal treatments and produce a safe and effective vaccine. As of October 20th,2020, approximately 40,472,505 cases have been tested positive for coronavirus disease 2019 (COVID-19), including 1,119,283 deaths.1 Initially, SARS-CoV-2 was considered as a respiratory virus but a series of peer reviewed medical papers published in the last eightmonths, suggested that this virus attacks the brain, heart, intestine, vascular system via the blood stream.2–4 The main driving force in violating the blood vessels endothelium lining is the spike protein (S), which binds to the angiotensin-converting enzyme 2 (ACE2), leading to a cytokine storm.5–8 In this context, many cases of multiple-organ dysfunction or failure have been reported in COVID-19 patients with comorbidities.9–11 Nevertheless, many facts regarding the host–virus interaction still remain unknown. On this note, reliable diagnostic methods for an early virus detection and effective and safe treatment modalities to curb the spread of the disease have challenged clinicians and scholars across the globe. The current lines of COVID-19 patients management are palliative and restoring organ functionality. To date, more than 3652 studies are registered in clinical trials.gov and a large number of trials are ongoing, aiming to establish an effective treatment, as well a safe and potent vaccine,12 however this might take a length of time. Therefore, the quest for discovering alternative therapies shall persist until the ultimate cure, which is COVID-19 vaccine is produced.
This aim of this review article was to critically appraise the latest update on disease progression, by assimilating the current knowledge and understanding of COVID-19. The main objectives were to collect, scrutinize and objectively evaluate the available scientific evidence-based information and provide an overview of antiviral treatment modalities.
Epidemiology
Origin
Although origins of SARS-CoV-2 are not entirely understood, the majority of patients in the initial stages of this outbreak reported its link to the Huanan South China Seafood Market, which is a live animal or “wet” market, suggesting a zoonotic origin of the virus.9,10 More recently, several studies that conducted genomic analyses suggesting that SARS-CoV-2 probably evolved from a strain found in horseshoe bats because the whole genome-wide nucleotide sequence of SARS-CoV-2 is 96% identical to SARS-like CoV isolated from an intermediate horseshoe bat (Rhinolophus affinis) CoV, and 89% identical to two SARS-like CoVs isolated from Chinese horseshoe bats (Rhinolophus sinicus).13,14 Some researchers believe that the likelihood for the existence of an amplifying mammalian host intermediate between bats and humans remains questionable, due to the mutation in the original strain, which could have directly triggered virulence towards humans.15 Conflictingly, notable research has proven that the SARS-CoV-2 has been isolated from pangolins and CoV genomes found in the latter have approximately 85.5–92.4% similarity to SARS-CoV-2, suggesting that the pangolin may be a potential intermediate host for SARS-CoV-2.16 There is also a substantial number of ongoing research to discover other probable animal hosts of SARS-CoV-2, which is of a great significance for the prevention and control of COVID-19.17
Physicochemical Properties
Most of the knowledge about the physicochemical properties of CoVs comes from SARS-CoV and MERS-CoV.17 The virus may survive and be detected on different surfaces for hours to days with a half-life ranging from 24 h on cardboard to approximately 72 h on plastic and stainless-steel surfaces, depending on the humidity and temperature.18,19 Like other CoVs, it is sensitive to ultraviolet (UV) rays, heat (56°C for 30 min) and most disinfectants. In order to achieve a 99.99% reduction of the virus, surfaces can be decontaminated by a one minute exposure to products such as, ether (75%), ethanol (95%), isopropyl alcohol (70–100%), sodium hypochlorite (0.21%), hydrogen peroxide (0.5%), povidone-iodine (0.23–7.5%), etc, except chlorhexidine gluconate.18,20 It has been found that soaps and detergents form a lather, which produce bubble-like structures called micelles that can degrade the fatty protective layer on the virus, thus deactivating and separating it from the surface of the skin as well as other surfaces, upon rinsing with water.18 Thus, they can be deemed as effective, if hand-washing procedure is performed correctly.
Clinical Presentation and Disease Progression
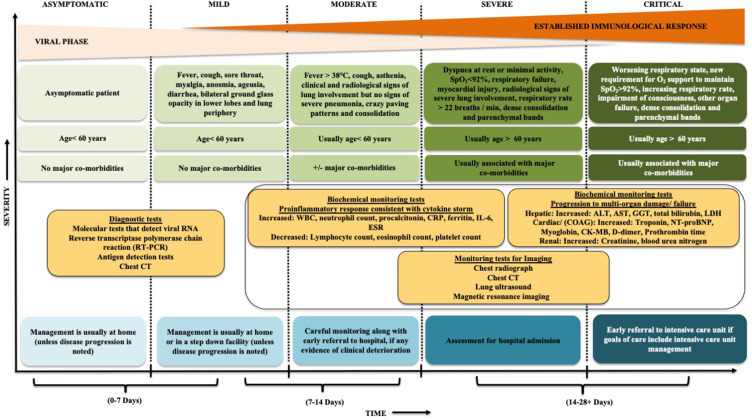
Evidence-based literature of COVID-19 clinical manifestations have shown that the stage of disease and severity of the symptoms and the corresponding care settings vary amongst the infected individuals (Figure 1).2,3,21–23 Most often, a suspected COVID-19 patient can exhibit symptoms such as; fever, cough, myalgia, dyspnea, fatigue, and altered sense of taste/smell.9,10 Approximately 15% of the infected patients have shown signs of fever, cough, and dyspnea,10 whilst the less common symptoms were as follows: sputum production, sore throat, gastrointestinal symptoms, chest pain, confusion, dizziness, headache, rhinorrhoea or nasal congestion, hemoptysis, conjunctivitis, cutaneous manifestations, and anorexia.10 Approximately 90% of the total infected population has exhibited more than one symptom.10 Many studies have been conducted to identify the disease progression roadmap.2,3 The results of the findings were based on the onset of the disease and immediately after contracting the virus, which revealed that every infected individual is in an asymptomatic state.2 This phase is known as viremia, which lasts for approximately 1–2 days.2,3 Although the localized spread of the infection, corresponding with limited innate immune response occurs at this stage and the viral burden may be low, patients are infectious and the virus can be detected via nasal swabs.2,3 Subsequently, over the next few days the virus replicates and spreads through the respiratory tract along the air conduction pathway, initiating a further stronger immune response.2,4 The positive results of nasopharyngeal swabs and early immune markers reveal a presence of SARS-CoV-2, during which the clinical symptoms are predominant at this stage. Alternatively, the acute phase in this context is termed as the pneumonia phase,3 in which patients are home-quarantined and most often are asked to self-isolate and continuously monitored by health officials and or placed on symptomatic therapy. If the host immune response is effective, almost 80% of the infected patients remain mildly infected.4 In this case, the virus is effectively suppressed and the disease is contained in the upper conducting airways and subsequently, the patient enters a recovery phase.3 Unfortunately, the remaining 20% of the population have comorbidities and are considered as immunosuppressed,4 where the disease progresses to an advanced stage. On this note, a severe form of the disease develops with a formation of “ground glass” pulmonary infiltrates detected on chest computed tomography (CT), associated with hypoxia and successively the disease deteriorates to an acute distress respiratory syndrome (ARDS) and multi-organ failure (Figure 1).2,4 Approximately 2.3% of the reported cases resulted in fatal outcomes.4 It has been noted that the severely affected COVID-19 patients are more contagious than the mildly affected counterparts, although asymptomatic infected persons in an incubation period have the capability to spread the infection.2,3 Apart from the abovementioned scenarios, atypical clinical features involving delayed presentation of symptoms have also been reported among older individuals as well as those with medical comorbidities.22 At present, although several cross-sectional studies have reported a high prevalence of asymptomatic infection, as of now, since asymptomatic patients are not routinely tested, the ratio of asymptomatic to symptomatic infection is uncertain and needs to be explored further.22
Figure 1.
Modified schematic diagram illustrates COVID-19 clinical presentation, diagnostic investigations, assessment of disease severity and consideration of care settings accordingly.2,3,21–23
Susceptible Population
Notable research has pointed out that all populations are generally susceptible to SARS-CoV-2. It is factual that elderly patients with or without underlying comorbidities-cardiovascular diseases (CVDs), diabetes mellitus (DM), obesity- have a low immune function and response9–11 and are at a high risk, due to a failure to repair the damaged epithelium and reduced mucociliary clearance, leading to a rapid migration of the virus to the lung gas exchange units and further across multiple organs.24 Reports presented by the Center for Disease Control (CDC) China states that the population, within the age range of 30–79-year-olds, were the most severely affected.25 This data is in accordance to the global reports.11,26 It has also been shown that men have a higher rate of disease severity and mortality rate, independent of age and susceptibility, compared to women.11,26,27 In addition, pregnant women and new-born babies infected with SARS-CoV-2 are also prone to develop a severe pneumonia.28 Thus, the treatment of these vulnerable patients should be given prime importance. Until recently, no severe cases had been reported among young children and it was assumed that a robust innate immune response is a significant factor for disease outcome.9 However, surprising new evidence, linking COVID-19 with Kawasaki disease (KD) has been reported.29 Jones et al, reported a case of a six-month-old infant diagnosed with classic KD who also screened positive for COVID-19 has showed signs of fever and mild respiratory distress.29 The association of KD with COVID-19 is more than a mere coincidence because reports of the former’s correlation with other human CoVs have been established in the past.30 In this context, further evidence is importantly required to achieve a definitive line of action of pediatric COVID-19 management. An association between the number of infected patients with ethnic origin, race, socioeconomic status and illiteracy have also been acknowledge on a global platform.11,26,27 The CDC has stated that people living in rural communities, experiencing homelessness, newly settled refugee populations, people residing in nursing homes/long-term health-care facilities/group homes for the disabled have been shown to be at a high risk of acquiring COVID-19 infection and hence should take extra precautions.31 Recent evidence highlighted that the Bacille Calmette–Guérin (BCG) vaccine could have a potential role in reducing the viremia caused by SARS-CoV-2 and the subsequent illness severity.32 However, the research in this context is ongoing and conclusive results can be expected only in the near future.
Transmission Routes (Disease Spectrum)
The primary route of contagion is through close contact with infected individuals, which is often exhibited via respiratory droplets, arising from coughing, sneezing, or talking.33 However, this may not be the only route of transmission.33 Close contact has also been confirmed to be a potential source for spread of SARS-CoV-2.34 Touching contaminated surfaces and then the face also could increase the likelihood of the disease spread. This occurs through a direct or an indirect contact with the mucous membrane of the eyes, mouth and nose.34 In addition, recent evidence has indicated that the airborne transmission route associated with an aerosol generation is the cause of the disease.34 Aerosol generation in an enclosed environment such as a dental clinic can result in continuous exposure to a high concentration of aerosols containing the virus. It has revealed that SARS-CoV-2 remains viable for approximately three hours in aerosols.24
Incubation Period
The mean incubation period of SARS-CoV-2 is three to seven days (range: 2–14 days), which represents the current official estimated range for the novel coronavirus COVID-19.35 This finding is in accordance to the incubation periods reported by the WHO (two and 10 days),36 China’s National Health Commission (NHC) (10–14 days)37 and the United States’ CDC (two and 14 days).38 According to the pooled analysis of confirmed COVID-19 cases reported between January 4 and February 24, 2020, as an incubation period of five days has been suggested by the research group at the Johns Hopkins University, USA.35 These authors suggested that the latent period of SARS-CoV-2 is consistent with those of SARS-CoV (mean five days, range: 2–14 days), which coincided with the Canadian research group‘s findings39 and with MERS-CoV (mean 5.7 days, range: 2–14 days).39 In spite of the long transmission period of the virus, evidence suggests that asymptomatic COVID-19 patients can effectively transmit infection during the incubation periods.40 Controversially, some discrete cases have been reported with a very longer incubation period of 24 days,41 27 days,42 and 19 days.43 According to the WHO, the long incubation period may suggest a possibility of a double exposure and a potential for relapse, which needs further investigations.36
Histopathological Characteristics
The study of the pathological characteristics of a severe case of COVID-19 can assist in designing a treatment strategy for the acute and severely ill patients, which ultimately reduces the mortality rate.21 Nonetheless, the histopathological examination of the lung tissue specimen obtained from a SARS-CoV-2 infected patient shows a desquamation of pneumocytes along with the formation of hyaline membrane and interstitial mononuclear inflammatory infiltration, indicating ARDS.44 Additionally, the intra-alveolar spaces consisted of multinucleated giant cells, which were indicative of suggesting viral cytopathic-like changes44 Studies have confirmed similarity in the pathological findings of COVID-19 with SARS and MERS.45 Deshmukh et al, and co-workers have recently conducted a systematic review to summarize the histopathological observations in COVID-19. The authors have presented a critical appraisal of 45 studies which have assessed the latest histopathological changes in different organs observed after autopsy of COVID-19 cases. They concluded that although the respiratory and immune systems are the worst affected, other systems such as, cardiovascular, urinary, gastrointestinal tract, reproductive system, nervous system, and integumentary system also show significant histopathological changes which are especially observed in elderly cases and those with comorbidities.46
Diagnostic Tests and Radiographic Imaging Characteristics (Chest CT Presentation)
A COVID-19 patient can show the following changes on chest CT scan: bilateral pulmonary parenchymal ground-glass opacity, pulmonary consolidation and nodules, bilateral diffuse distribution, sometimes with a rounded morphology and a peripheral lung distribution.47 During an early phase of the disease, multiple small patchy shadows and interstitial changes are evident in the lung periphery and appreciated on a chest CT image.16 As the severity of the disease progresses, changes can be observed in the bronchi, which gradually manifest across the entire lung with infrequent interlobar pleural thickening and pleural effusion.21,47 On this note, the moderate lung abnormalities such as; patchy ground-glass opacities’ (normalized during treatment) findings observed in the adults’ CT scan been reported in a study evaluated CT changes in children.48
The rapid and precise detection of the SARS-CoV-2 is quintessential to win this race against time. This has been facilitated through the laboratory diagnosis, using RT-qPCR technique, which is based on detection of nucleic acid sequences.21 RT-qPCR or viral gene sequencing of nasopharyngeal and oropharyngeal swabs (stool, sputum,or blood samples) have been conducted to facilitate SARS-CoV-2 detection.49 However, this testing method has several shortcomings such as, difficulty during sample collection, need for close contact with health professionals, which can increase the risk of contagion, initiation of bleeding, and gag reflex.21,50 Furthermore, evidence has shown that COVID-19 detection varies from different sample sites,51 in which nasopharyngeal swabs are more reliable than oropharyngeal swabs.52 In spite of the controversies growing around this method, there has been a worldwide exponential ramping up of testing procedures over the last eight months. The detection of the virus in saliva has also been proven, suggesting that saliva might serve as, a noninvasive site, to diagnose and monitor the rate of infectivity.53 Nonetheless, coronavirus swab test can only detect an ongoing viral status and provides no information, if the individual was previously infected and recovered. Moreover, the results of the swab tests take a minimum of 24 h for diagnosis.54 In contrast to these findings, the use of a serological test (antibody testing) can identify if an individual was previously infected with COVID-19 and recovered.54 This involves a finger-prick testing method with results obtained at approximately 15–30 mins, at a higher level of diagnostic accuracy has been reported than the swab test.54 Research on the use of synthetically produced RNA fragments of the SARS-CoV-2, as a diagnostic tool has shown notable progress and this could hold a promising role in the future.55 Figure 1 provides an illustration of the diagnostic tests as well as various biochemical and imagining tests that are performed for monitoring COVID-19 patients.
Pathophysiology and Etiopathogenesis
General Characteristic Features and Morphology of SARS-CoV-2
The Coronaviridae family (subfamily: Orthocoronaviridae, order: Nideovirales), are a large family of enveloped, nonsegmented, positive-sense, single-stranded RNA viruses as observed under an electron microscope.15 This family of viruses are genotypically and serotypically further subclassified as; alpha-coronavirus (α-CoV), beta-coronavirus (β-CoV), delta-coronavirus (δ-CoV), and gamma-coronavirus (γ-CoV).15,17 Several systemic ailments such as, respiratory, enteric, hepatic, and neurological diseases in different animal species can be caused by this family of viruses.15 Human CoV infections are specifically caused by α- and β-CoVs.5 SARS-CoV-2 belongs to the subdivision β-CoV, which is similar to its predecessors, severe acute respiratory syndrome-coronavirus (SARS-CoV) and Middle East respiratory syndrome (MERS-CoV).15 SARS-CoV-2 is round or elliptic and often has a pleomorphic form, and a diameter of approximately 60–140 nm.15 The genome that has a size of approximately 30 kilobytes (kb) contains 29,891 nucleotides, encoding for 9860 amino acids.15 Of the several proteins possessed by the virus, there are four structural proteins, which consist of the spike surface glycoprotein, the membrane protein, the envelope protein and the nucleocapsid protein, which are fundamental for the assembly and potential for infectivity, of SARS-CoV-2.21
Structure and Function of the Spike Protein
The CoVs derive their peculiar name from the Latin term “coronam”, which means crown, due to the presence of spike surface glycoproteins on the envelope.15 The host viral interaction takes place in five steps namely, attachment, penetration, biosynthesis, maturation, and release.56 The spike protein plays a crucial role in facilitating the virus–host cell attachment. Upon attachment to the host receptors, the virus penetrates via endocytosis or membrane fusion into the host cells.56 Host proteases have the potential to cleave the spike protein into an N-terminal S1 region for primary attachment and a membrane-bound C-terminal S2 region for viral infusion.57 In order to engage a host receptor, the receptor-binding domain (RBD) of the S1 subunit undergoes hinge-like conformational movements, which transiently hides or exposes the determinants of receptor binding.5,6 These two states of the S1 subunit can be regarded, as the down conformation (inaccessible state of the receptor) and the up conformation (accessible state of the receptor).5,7 Upon binding of the S1 region of the protein to the host receptor cells, the pre-fusion trimer is destabilized, leading to shedding of the S1 subunit.8 This facilitates the transition of the S2 sub-unit into a highly stable postfusion conformation.8 Subsequent to the transfer of all the viral contents into the host cells, the viral RNA enters the nucleus for replication and to biosynthesize the viral proteins. The newly formed viral particles then undergo maturation and release phases.56 Researchers are optimistic that understanding the structure and function of the spike protein can aid in the development of various antiviral drug regimens, as well in a direct development of vaccines in the near future.57
Host Defense Against SARS-CoV-2 Viral Interaction
Initially, the SARS-CoV-2 targets the nasal and bronchial epithelial cells, alveolar epithelial type I and II pneumocytes and capillary endothelial cells through the viral structural spike (S) protein to initiate an inflammatory response, by activating the inflammatory pathways of the immune system.58,59 Scientific evidence of the spike protein binding to angiotensin converting enzyme 2 (ACE2), which is present in the host cell has identified the latter as a functional receptor for SARS-CoV.60 The ACE2 receptor is predominantly expressed in the lung epithelial cells, heart, ileum, kidney and bladder.61 The entry of the SARS-CoV-2 into the host cells is promoted by the cleaving of ACE2 by the type 2 transmembrane serine protease (TMPRSS2), which is present in the host cell, particularly the alveolar epithelial type II pneumocyte cells.61–63 The inflammatory response, consisting both of the innate and the adaptive immune response (comprising of humoral and cell-mediated immunity) can impair lymphopoiesis and increase the T lymphocyte cell apoptosis resulting in profound lymphopenia.64–66
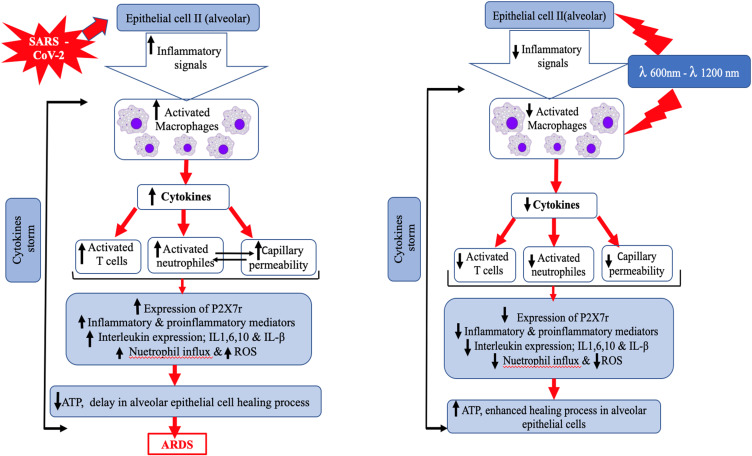
Cytokines are an essential part of the inflammatory process and are produced by several immune cells including the innate macrophages, dendritic cells, natural killer cells and the adaptive T and B lymphocytes.67 As the viral load increases, an exaggerated and unusual hostimmune response comes into play, leading to a continuous activation and expansion of immune cells, lymphocytes, and macrophages, which produce immense amounts of cytokines, resulting in a cytokine storm (CS), which is a serious life-threatening condition.67 The clinical findings associated with CS are linked to the action of the proinflammatory and anti-inflammatory cytokines such as, Interleukin-1 (IL-1), IL-6, IL-18, IL-10, IFN-γ, and tumor necrosis factor-alpha (TNF-α), which are elevated in the serum of COVID-19 patients.68 IL-6 and TNF-α have been recognized to play a major role sequence of events occurring, as a result of the CS and consequent disease progression.67
In later stages of the infection when the viral replication is accelerated, an exponential rise in cytokine levels result in the influx of other immune cells such as; macrophages, monocytes, neutrophils, and T cells from the circulation into the site of infection.67,68 Various destructive events start to occur which are as follows: destabilization of the epithelial-endothelial barrier, damage of vascular barrier, capillary damage, infection spreading to the pulmonary capillary endothelial cells, diffuse alveolar damage, diffuse thickening of the alveolar wall with mononuclear cells and macrophages infiltrating airspaces appearing as ground-glass opacities on CT imaging and pulmonary edema filling the alveolar spaces with formation of hyaline membrane, dysfunctional alveolar-capillary oxygen transmission, and impaired oxygen diffusion capacity (Figure 2).44,64 Lung injury is one consequence of the cytokine storm that can progress into early phase acute respiratory distress syndrome (ARDS) or its more severe form.64,67,68
Figure 2.
Modified schematic diagram illustrates the etiopathogenesis and pathophysiology of COVID-19. Data from Wiersinga et al.64
With a further progression into COVID-19 disease severity, a disproportionate activation of the complement system stimulates the coagulation cascade, endothelial cells and platelets, inhibits fibrinolysis and results in the formation of microthrombi and a high incidence of thrombotic complications in critically ill patients.69,70 The advancement of the disease process causes dysregulated host immune response mediated viral sepsis (critical organ dysfunction) which may further contribute to multi-organ failure.64 The characteristic clinical presentation of CS includes; signs of overwhelming systemic inflammation, hyperferritinemia and hemodynamic instability. Additionally, researchers have successfully linked the excessive production of pro-inflammatory cytokines as one of the major contributing factors in the development of ARDS in COVID-19 patients.9,10,71 ARDS leading to low oxygen saturation levels is a major cause of multi-organ failure and mortality in COVID-19.67 Hence, the early recognition of CS and the prompt treatment can improve the clinical outcome of the COVID-19 infection.67,72 Several antiviral and anti-inflammatory drug therapies have been proposed for treating SARS-CoV-2 mediated CS, in order to decrease both the morbidity and mortality in COVID-19 patients, and a comprehensive review and critical appraisal of these therapies has been made in this review.
Current Scenario for Treatment of COVID-19
General Management
Presently, there are no definitive therapeutic protocols for COVID-19 management.21 For suspected and mild confirmed cases, isolation in designated areas or self-isolation at home has been suggested.21,73 The general line of treatment revolves around establishing symptomatic relief includes: ample bed rest, maintaining a good water-electrolyte balance and monitoring vital signs (temperature, heart rate, oxygen saturation, blood pressure, pulse rate, respiratory rate).21,73 Apart from these, it is important to note that all patients irrespective of the disease severity need to receive a balanced diet full of high nutritive value with a good intake of foods rich in antioxidants.73 An overview of the contemporary trends for the treatment COVID-19 has been essayed in this review.
Antiviral Therapy
Although specific antiviral drugs and drug regimes have not been established to date, a variety of antiviral therapeutic drugs have been tried and tested worldwide with fluctuating results, to obtain COVID-19 symptomatic relief.17 The use of these drugs has been reported through many case series and case reports. On this note, the level of usage of these drugs is low and controversial, due to the limited number of human clinical trials.17,74 The advantages and drawbacks of the potential antiviral therapeutic drugs that have been utilized for COVID-19 management are listed in Table 1 and a detailed review is been presented below:
Table 1.
The Advantages and Drawbacks of the Therapeutic Strategies in COVID-19 Management
| Therapeutic Modality | Category | Generic Name | Advantages | Drawbacks |
|---|---|---|---|---|
| Antiviral therapy | Antiviral cytokines | INFα and INFβ81 | Broad antiviral activity Broad spectrum antivirals, exhibiting both direct inhibitory effects on viral replication and supporting an immune response to clear virus infection INFβ is better tolerated than INFα No signs of adverse birth outcome |
Flu-like symptoms, nausea, fatigue, weight loss, hematological toxicities, elevated transaminases, and psychiatric problems (eg, depression and suicidal ideation). The most serious drug-drug interactions with IFNs are the potential for added toxicity with concomitant use of other immunomodulators and chemotherapeutic agents |
| Antiviral drugs | Ribivarin89 | Existing inventory Reliable supply chain |
Hepatic injury, myopathy, neuropathy, bone marrow suppression, and pancreatitis | |
| Remdesivir93 | Short recovery time Does not alter the QTc interval Shortens the time to recovery in adults with no effect on mortality |
Diarrhea, rash, renal failure, and hypotension, increased liver enzymes. | ||
| Lopinavir Ritonavir99–101 |
Ability to bind to the endopeptidase C30 of SARS-CoV-2 and downgrade the respiratory distress | Hyperlipidemia, systemic hypersensitivity syndromes, and Achilles Tendinopathy | ||
| Nelfinavir103 | Strongly inhibited replication of the SARS-CoV-2 expression of viral antigens | Nausea and diarrhoea | ||
| Arbidol105,106 | Broad-spectrum antiviral drug Blocks the viral replication process of SARS-CoV Well tolerated in children and adults |
Allergic reactions, nausea, diarrhoea, dizziness and elevated serum transaminase | ||
| Antimalarial drug | Chloroquine110,111 | Potent antiviral effect Broad-spectrum antiviral drug Inexpensive drug Good patient safety report and tolerance levels Interference with ACE2 to block virus invasion; increase of endosomal pH required for virus fusion; mild immune suppression |
Retinal or psychiatric symptoms | |
| Corticosteroids | Glucocorticoids | Dexamethasone115 | Potent anti-inflammatory effects against the cytokine storm Anti-allergic |
Increased appetite, irritability, difficulty in sleeping (insomnia), swelling in your ankles and feet (fluid retention), heartburn, muscle weakness, impaired wound healing, increased blood sugar levels |
| Hydrocortisone115,117 | Antishock therapy Can be used as an alternative to dexamethasone to treat patients severely ill with COVID-19 |
Slow wound healing, thinning skin, increased body hair, irregular menstrual periods, changes in sexual function/muscle weakness, tired feeling, depression, anxiety, feeling irritable | ||
| Immunotherapy | CPT120,121 | Safe, clinically effective, and reduces mortality, used in past epidemics | Transfusion related acute lung injury, transfusion associated circulatory overload, and allergic/anaphylactic reactions, transmission of infections, febrile nonhemolytic transfusion reactions, RBC alloimmunization, and hemolytic transfusion reactions | |
| Tocilizumab127–129 Anakinra131 |
Potent anti-inflammatory action | Headache or dizziness, mouth ulcers, high blood pressure, hypercholesterolemia, allergic reactions, weight gain or swollen ankles, stomach irritation or abdominal pain, affects immune system and healing, neutropenia, immunosuppressive therapy may lead to reactivation of latent tuberculosis or other atypical or opportunistic infections |
||
| Antibody cocktail146,147 | Targeted killing, fast symptom-relief, speedy recovery, minimal side effects | None encountered to date | ||
| ACE receptor binding strategy169 | Denoted as novel SARS-CoV-2 receptor and plays an important role in modulating immunity, inflammation, ACE2, and cardiovascular disease | None encountered to date | ||
| Exosomes170,171 | Helps in disease detection and treatment Natural drug delivery vehicles High specificity and efficiency |
Inefficient separation methods, difficulties in characterization, and lack of specific biomarkers | ||
| Cellular therapy | MSCs172,173 | Stem cells are able to suppress the activities of viruses via Chaf1a- mediated and Sumo2-mediated epigenetic regulation (termed pro-viral silencing) Beneficial effects in ARDS |
Lack of clarity with regard to optimal dose and route of MSC delivery, difficulties in large-scale production and cryopreservation, and the potential for substantial variability | |
| RAS blockers174 | Not associated with an increased risk of death, admission to ICU, mechanical ventilation requirement or progression to severe or critical pneumonia in COVID‐19-infected hypertensive patients, can be used safely in children and adults | Insufficient evidence of their potential harm and overwhelming evidence on their benefits | ||
| Traditional Chinese medicines | Used in previous epidemics, remarkable symptomatic relief, antipyretic, faster recovery, reduced stay in hospital | Hypersensitivity pneumonitis, lung injury, fatigue with gastrointestinal discomfort | ||
| Antioxidants | Food products rich in vitamins C and E | Can prevent or reduce the damage caused by oxidation Can reduced the risk of cardiovascular disease |
Bruising under the skin, diarrhea, dizziness, joint pain | |
| Curcumin | Scientifically proven health benefits Potent anti-inflammatory and antioxidant and may also help improve symptoms of depression and arthritis |
None encountered to date | ||
| Natural therapies | Omega 3 fatty acids Eicosapentaenoic acid (EPA) Docosahexaenoic acid (DHA)182 |
Anti-inflammatory | EPA and DHA can make cell membranes more susceptible to non-enzymatic oxidation mediated by reactive oxygen species, leading to the formation of potentially toxic oxidation products and increasing the oxidative stress | |
| Manuka honey183 | Antibacterial Anti-inflammatory Promotes wound healing The higher the concentration of MGO, the stronger the antibiotic effect |
None encountered to date | ||
| Potato starch184 | Prebiotic effect | None encountered to date | ||
| Phototherapy | PDT/a-PDT | There is no an accumulative dose: a lack of microbial specificity and the development of resistance mechanisms212 Good helper in the phagocytosis process, including inactivation of pathogen proliferation213 Clinical PDT protocol against pharyngotonsillitis in reducing more than 90% of the symptoms related to the disease after 24 h were observed. Hence, PDT is significant to inactivation the viral infection and reduce viral load in the respiratory tract221 MB mediated PDT could be a potential treatment modality for an early and advanced bronchopulmonary infection223 The delivery of three photosensitizers (ICG, the chlorine photodithazine, and porphyrin photogem) in a jet nebulizer device has showed to be effective to target the lung directly225 Effective, innovative, accessible, cost-effective treatment modality226 A reduction in mortality rate and severity of the course of disease, a reduction of viral load in COVID-19 patients226 Plasma inactivation of SARS-CoV-2 to ensure safe blood transfusion227 |
None encountered to date | |
| Laser-PBMT | Anti-inflammatory: attenuates cytokine storm at multiple levels and reduces the major inflammatory metabolites189–194 Increases collagen and protein production, cell proliferation, normalized of impaired circulation, decreases edema and swelling, and improves quality and tensile strength of tissue189–194 PBMT reduces pulmonary microvascular leakage, IL-1β, IL-6, and intracellular ROS. On this note, PBMT as a single or adjunct treatment modality can modulate the cytokine storm and ARDS via its anti-inflammatory action196–199 Reduces inflammation and promotes lung healing199,205,206 and minimizes the scarring process189–194 Has an immunomodulator effect in improving immune functional and modulating the cellular and molecular activities of the cytokine storm of SARS-CoV-2200,203,206 It is a safe, effective, low-cost modality without any side effects that may be combined with conventional treatment of ARDS196–199 Minimizes the length of time needed on a ventilator, enhance the healing process, and shorten recovery time in COVID-19 patients199,200 PBMT as a single or adjunct treatment modality can modulate the cytokine storm and ARDS via its anti-inflammatory action196–199 Antioxidant: reduces pain related to inflammation via dose-dependent reduction of prostaglandin E2, prostaglandin-endoperoxide synthase-2, IL-1, IL-6, TNFa, as well as the cellular influx of neutrophils, oxidative stress201–203 Prevents acidosis and hypoxia in COVID-19 patients results in SpO2 improvement and lung healing in COVID-19 pneumonia199,205,206,210 PBMT combined with conventional medical therapy has a potential to prevent COVID-19 progress and improve symptoms200 PBMT combined with conventional treatment in patients with severe COVID-19 and morbid obesity is safe and effective211 and is potential to prevent COVID-19 progress and improve symptoms200 |
None encountered to date | ||
| LEDs-PBMT | It has the same advantages as the laser-PBMT; however, the difference is that LEDs-PBMT delivers noncoherent light via a clustered probe of multiple wavelengths, which provide various penetration depths187,188 Itis safe, efficient and cost-effective treatment modality that can irradiate a large surface area, which can be useful in COVID-19 management.210 |
None encountered to date | ||
Antiviral Cytokines
Antiviral cytokines are proteins, having broad antiviral activity which exhibits both direct inhibitory effects on viral replication and spread through several mechanisms and supporting an immune response to clear viral infection which will enhance the adaptive immunity and improve the host resistance to the viral infection.75 In previous supporting literature, the use of interferons alpha and beta (INFα and INFβ) to mitigate SARS-CoV and MERS-CoV infections have been proven,75,76 as well in combination with other antiviral drugs such as, lopinavir/ritonavir, ribavirin, remdesivir, corticosteroids have been reported.77–80 INFβ is better tolerated than INFα and no signs of adverse birth outcome have been noted with their use in COVID-19 management. Some frequently encountered disadvantages with the utilization of antiviral cytokine therapy include, flu-like symptoms, nausea, fatigue, weight loss, hematological toxicities, elevated transaminases, and psychiatric problems (Table 1).81 The potential for added toxicity with concurrent use of other immunomodulators and chemotherapeutic agents is seen as the most serious drug-drug interactions with this therapy.
In an uncontrolled, exploratory study by Zhou et al, 77 adults who had tested positive for COVID-19 were treated with either nebulized IFN-α2b (five milli international units twice a day), or arbidol (200 mg three times a day; tds) or a combination of IFNα2b and arbidol. The authors concluded that the patients who received IFNα2b with or without arbidol showed a significant reduction in the detectable virus duration in the upper respiratory tract along with a reduction in the duration of elevated blood levels for IL-6 and CRP.81 However, as per the recommendations of the COVID-19 Treatment Guidelines Panel, National Institutes of Health (NIH), the use of interferons for severe of critical COVID-19 illness must be avoided except in a clinical trial. The panel has provided a substantial evidence to highlight the drawbacks of interferons in the previous coronavirus infections, as well underlying toxicities that prevail over the potential benefits of this treatment strategy.82 Concurrently, the NIH are conducting a study called the Adaptive COVID-19 Treatment Trial 3 (ACTT 3), which would take place in the USA and other sites globally. The combination of INF1β and remdesivir for treating COVID-19 has been evaluated in a few small-scale randomized controlled trials (RCTs) and hence the current trial has been undertaken on a larger platform.83 The various clinical trials related to the utilization of this therapy in COVID-19 patients are listed in Table 2.83–85
Table 2.
Shows the Clinical Trials Registered on ClinicalTrials.gov to October 20, 2020, Utilizing Antiviral Therapies for the Treatment of COVID-19
| Study Identifier and Citation (Superscript) | Treatment Protocol | Study Phase; Estimated Enrolment (n) | Primary Outcome Measure(s) | Recruitment Status |
|---|---|---|---|---|
| NCT0449247583 | Drug: IFNβ1a: rebif (R) is a purified 166 amino acid human IFNβ glycoprotein with an amino acid sequence identical to natural fibroblast derived human IFNβ. Each 0.5 mL prefilled syringe contains 44 µg of IFNβ1a, 4 mg human albumin, USP; 27.3 mg mannitol, USP; 0.4 mg sodium acetate; and water for injection, USP. Other: placebo: the IFNβ1a placebo contains either 0.5 mL 0.9% normal saline or 0.5 mL sterile water for injection. Drug: remdesivir: is a single diastereomer monophosphoramidate prodrug designed for the intracellular delivery of a modified adenine nucleoside analog GS-441,524. In addition to the active ingredient, the lyophilized formulation of remdesivir contains the following inactive ingredients: water for injection, sulfobutylether beta-cyclodextrin sodium (SBECD), and hydrochloric acid and/or sodium hydroxide |
Phase III n=1038 |
Time to recovery (Time frame: day 1 through day 29) Day of recovery is defined as the first day on which the subject satisfies one of the following three categories from the ordinal scale: (1) hospitalized, not requiring supplemental oxygen and no longer requires ongoing medical care; (2) not hospitalized, limitation on activities and/or requiring home oxygen; (3) not hospitalized, no limitations on activities. |
Recruiting |
| NCT0434397684 | Drug: pegylated IFNλ 180 µg subcutaneous injection of pegylated IFNλ Other name: lambda |
Phase II n=20 |
Undetectable COVID PCR at day 7 (Time frame: 1 week) Negative COVID PCR testing 7 days after first lambda dose |
Enrolling by invitation |
| NCT0434376885 | Experimental: hydroxychloroquine+lopinavir/ritonavir+IFNβ1a Experimental: hydroxychloroquine+lopinavir/ritonavir+IFNβ1b Active comparator: control group: hydroxychloroquine+lopinavir/ritonavir |
Phase II n=60 |
Time to clinical improvement (Time frame: from date of randomization until 14 days later) Improvement of two points on a seven-category ordinal scale (recommended by the WHO: Coronavirus disease (COVID-2019) R&D. Geneva: WHO) or discharge from the hospital, whichever came first. |
Completed |
| NCT0428070595 | Placebo comparator: placebo: 200 mg of remdesivir placebo administered IV on day 1, followed by a 100 mg once-daily maintenance dose of remdesivir placebo while hospitalized for up to a 10-day total course n=286. Experimental: remdesivir: 200 mg of remdesivir administered IV on day 1, followed by a 100 mg once-daily maintenance dose of remdesivir while hospitalized for up to a 10-day total course n=286. |
Phase III n=1062 |
Percentage of subjects reporting each severity rating on the 7-point ordinal scale (Time frame: day 15) The ordinal scale is an assessment of the clinical status at the first assessment of a given study day. The scale is as follows: (1) death; (2) hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO); (3) hospitalized, on noninvasive ventilation or high flow oxygen devices; (4) hospitalized, requiring supplemental oxygen; (5) hospitalized, not requiring supplemental oxygen; (6) not hospitalized, limitation on activities; (7) not hospitalized, no limitations on activities. |
Completed |
| NCT0429289996 | Drug: remdesivir: administered as an IV infusion (other names: GS 5734TM, Veklury® Drug: standard of care: standard of care treatment for COVID-19 infection |
Phase III n=4891 |
The odds ratio for improvement on a 7-point ordinal scale on day 14 (time frame: day 14) The odds ratio represents the odds of improvement in the ordinal scale between the treatment groups. The ordinal scale is an assessment of the clinical status at a given day. Each day, the worst score from the previous day will be recorded. The scale is as follows: (1) death (2) hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) (3) hospitalized, on noninvasive ventilation or high flow oxygen devices (4) hospitalized, requiring low flow supplemental oxygen (5) hospitalized, not requiring supplemental oxygen—requiring ongoing medical care (coronavirus (COVID-19) related or otherwise) (6) hospitalized, not requiring supplemental oxygen—no longer required ongoing medical care (other than per protocol remdesivir administration (7) not hospitalized |
Completed |
| NCT0429273097 | Drug: remdesivir: administered as an IV infusion (other names- GS 5734TM, veklury® Drug: standard of care: standard of care treatment for COVID-19 infection |
Phase III n=1113 |
The odds ratio for improvement on a 7-point ordinal scale on day 11 (Time frame: day 11) The odds ratio represents the odds of improvement in the ordinal scale between the treatment groups. The ordinal scale is an assessment of the clinical status at a given day. Each day, the worst score from the previous day will be recorded. The scale is as follows: (1) death (2) hospitalized, on invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) (3) hospitalized, on noninvasive ventilation or high flow oxygen devices (4) hospitalized, requiring low flow supplemental oxygen (5) hospitalized, not requiring supplemental oxygen—requiring ongoing medical care (coronavirus (COVID-19) related or otherwise) (6) hospitalized, not requiring supplemental oxygen—no longer required ongoing medical care (other than per protocol remdesivir administration (7) not hospitalized. |
Completed |
| NCT0440926298 | Experimental: remdesivir+tocilizumab (RDV+TCZ): participants assigned to the RDV+TCZ arm will receive an RDV loading dose followed by one infusion of TCZ on day 1, and a once-daily maintenance dose of remdesivir from days 2–10. Interventions: drug: remdesivir; drug: tocilizumab Active comparator: remdesivir+placebo (RDV+placebo): participants assigned to the RDV+placebo arm will receive an RDV loading dose followed by one infusion of TCZ-placebo on day 1, and a once-daily maintenance dose of RDV from days 2–10. Interventions: drug: remdesivir; drug: placebo |
Phase III n=450 |
Clinical status as assessed by the investigator using a 7-category ordinal scale of clinical status on day 28 (Time frame: day 28) | Recruiting |
| NCT04381936104 | Drug: lopinavir-ritonavir: lopinavir 400 mg-ritonavir 100 mg by mouth (or nasogastric tube) every 12 h for 10 days. Drug: corticosteroid: corticosteroid in the form of dexamethasone administered as an oral (liquid or tablets) or IV preparation 6 mg once daily for 10 days. In pregnancy or breastfeeding women, prednisolone 40 mg administered by mouth (or IV hydrocortisone 80 mg twice daily) should be used instead of dexamethasone. Corticosteroid (in children ≤44 weeks gestational age, or >44 weeks gestational age with PIMS-TS only) in the form of hydrocortisone or methylprednisolone sodium succinate (see protocol for timing and dosage) Drug: hydroxychloroquine: hydroxychloroquine by mouth for a total of 10 days (see protocol for timing and dosage). Drug: azithromycin: azithromycin 500 mg by mouth (or nasogastric tube) or IV once daily for 10 days. Biological: convalescent plasma: single unit of ABO compatible convalescent plasma (275mL±75 mL) IV per day on study days 1 (as soon as possible after randomization) and 2 (with a minimum of 12 h interval between first and second units) Drug: tocilizumab: tocilizumab by IV infusion with the dose determined by body weight (see protocol for dosage) Biological: immunoglobulin: IV immunoglobulin (IVIg) for children >44 weeks gestational age and <18 years with PIMS-TS only (see protocol for dosage) Drug: synthetic neutralizing antibodies: for participants ≥12 years only: a single dose of REGN10933+REGN10987 8 g (4 g of each monoclonal antibody) in 250 mL 0.9% saline infused IV over 60in±15 min as soon as possible after randomization (other name: REGN-COV2) |
Phase: II, III n=15,000 |
All-cause mortality (Time frame: within 28 days after randomization) For each pairwise comparison with the “no additional treatment“ arm, the primary objective is to provide reliable estimates of the effect of study treatments on all-cause mortality. |
Recruiting |
| NCT04260594109 | Drug: arbidol Arbidol tablets: take 2 tablets/time, 3 times/day for 14–20 days Other name: the basic treatment used by the investigator was based on the condition of the patient Other: basic treatment basic treatment |
Phase IV n=380 |
Virus negative conversion rate in the first week (Time frame: first week) | Not yet recruiting |
| NCT04347980113 | Drug: dexamethasone and hydroxychloroquine: patients included in the hydroxychloroquine/dexamethasone group will benefit from standardized ventilatory management and administration of hydroxychloroquine in the same manner as the hydroxychloroquine group. They will receive in addition to dexamethasone at a rate of 20 mg IV for 15 min once a day for 5 days (D1 to D5) then at a rate of 10 mg per day from D6 to D10. If the patient is extubated before the tenth day, he will receive his last dose of dexamethasone before. (Other name: standard ventilatory management) Drug: hydroxychloroquine: patients included in the hydroxychloroquine group will benefit from standardized ventilatory management. Patients included in the hydroxychloroquine group will receive 200 mg⨰3/day enterally from J1 of the HCQ for 10 days. If the patient is extubated before the tenth day, he will receive his last dose of HCQ before. (Other name: standard ventilatory management) |
Phase III n=122 |
Day-28 mortality (time frame: 28 days after randomization) Mortality rate evaluated 28 days after randomization |
Recruiting |
| NCT04334928114 | Drug: emtricitabine/tenofovir disoproxil Emtricitabine/tenofovir disoproxil, 200 mg/245 mg tablets. A dose of one tablet once a day will be administered. Drug: hydroxychloroquine Hydroxychloroquine, 200 mg tablets. A dose of one tablet once a day will be administered. Drug: placebo: emtricitabine/tenofovir disoproxil placebo Placebo: tablets similar in appearance to emtricitabine/tenofovir disoproxil Drug: placebo: hydroxychloroquine Placebo: tablets similar in appearance to hydroxychloroquine |
Phase III n=4000 |
Number of confirmed symptomatic infections of SARS-CoV-2 (COVID-19) (time frame: 12 weeks) | Recruiting |
| NCT04513184118 | Drug: IV dexamethasone 6 mg from day 1 to 10 after randomization Other name: ST Drug: nasal dexamethasone 0.12 mg/kg/daily for 3 days from day 1, followed by 0.06 mg/kg/daily from day 4 to 10 after randomization. Other name: nasal DXM |
Phase II n=60 |
Time of clinical improvement (time frame: 10 days after randomization) Evaluation of the clinical status of patients after randomization, defined as a two-point improvement in the WHO 7-point ordinal scale |
Recruiting |
| NCT04509973119 | Experimental: dexamethasone 12 mg IV bolus injection of dexamethasone 12 mg once daily in addition to standard care for up to 10 days. We will allow the use of betamethasone 12 mg at sites, where dexamethasone is not available. Intervention: drug: dexamethasone Active comparator: dexamethasone 6 mg IV bolus injection of dexamethasone 6 mg once daily in addition to standard care for up to 10 days. We will allow the use of betamethasone 6 mg at sites, where dexamethasone is not available. Intervention: Drug: dexamethasone |
Phase III n=1000 |
Days alive without life support at day 28 (time frame: day 28 after randomization) Days alive without life support (ie invasive mechanical ventilation, circulatory support or renal replacement therapy) from randomization to day 28 |
Recruiting |
| NCT04310228133 | Drug: favipiravir combined with tocilizumab Favipiravir: On the first day, 1600 mg each time, twice a day; from the second to the seventh day, 600 mg each time, twice a day. Oral administration, the maximum number of days taken is not more than 7 days. Tocilizumab: the first dose is 4~8 mg/kg and the recommended dose is 400 mg. For fever patients, an additional application (the same dose as before) is given if there is still fever within 24 h after the first dose and the interval between two medications ≥12 h. IV infusion, the maximum of cumulative number is two, and the maximum single dose does not exceed 800 mg. Drug: favipiravir On the first day, 1600 mg each time, twice a day; from the second to the seventh day, 6 00 mg each time, twice a day. Oral adm inistration, the maximum number of days taken is not more than 7 days. Drug: tocilizumab The first dose is 4~8 mg/kg and the recommended dose is 400 mg. For fever patients, an additional application (the same dose as before) is given if there is still fever within 24 h after the first dose and the interval between two medications ≥12 h. IV infusion, The maximum of cumulative number is two, and the maximum single dose does not exceed 800 mg. |
Phase: not applicable n=150 |
Clinical cure rate (time frame: 3 months) Definition of clinical cure: the viral load of the respiratory specimen was negative for two consecutive times (the interval between the two tests was greater than or equal to one day), the lung image improved, and the body temperature returned to normal for more than 3 days, and the clinical manifestation improved. |
Recruiting |
| NCT04320615134 | Drug: tocilizumab (TCZ) Participants will receive one dose of IV TCZ. One additional dose may onee given if clinical symptoms worsen or show no improvement. Drug: placebo Participants will receive one dose of IV placebo matched to TCZ. Up to 1 additional dose may be given if clinical symptoms worsen or show no improvement. |
Phase: II n=450 |
The primary and secondary endpoints of the study include clinical status, mortality, mechanical ventilation, and ICU variables. | Completed |
| NCT04317092135 | Drug: tocilizumab injection Tocilizumab 8 mg/kg (up to a maximum of 800 mg per dose), with an interval of 12 h. |
Phase: II n=400 |
Two primary outcome measures: arrest in deterioration of pulmonary function, and improvement in pulmonary function. | Recruiting |
| NCT04315480136 | Single dose of tocilizumab 8 mg/kg | Phase: II n=38 |
To evaluate its role in the virus-induced cytokine storm, in blocking deterioration of lung function or even promoting a rapid improvement of clinical conditions, preventing nasotracheal intubation and/or death. | Active, not recruiting |
| NCT04322773137 | Drug: roactemra IV single dose treatment with tocilizumab 400 mg IV Other name: tocilizumab 400 mg Drug: roactemra sc single dose treatment with tocilizumab 2⨰162 mg subcutaneously Other name: tocilizumab 2⨰162 mg Drug: kevzara sc single dose treatment with sarilumab 1⨰200 mg subcutaneously Other name: sarilumab 1⨰200 mg Other: standard medical care management as usual |
Phase: II n=200 |
To compare the effect of either one of three IL-6 inhibitor administrations (ie IV tocilizumab, subcutaneous tocilizumab, and subcutaneous sarilumab), relative to the standard of care, in patients with severe SARS-CoV-2 pneumonia | Recruiting |
| NCT04331808138–140 | Drug: tocilizumab Tocilizumab 8 mg/kg D1 and if no response (no decrease of oxygen requirement) a second injection at D3. |
Phase: II n=129 |
A significantly lower proportion of the patients in tocilizumab arm attained the primary outcome of need for ventilation or death at day 14 | Active, not recruiting |
| NCT04315298141,142 | Drug: sarilumab Single or multiple IV doses of sarilumab. Additional doses may be administered if the patient meets protocol defined criteria. Other names: kevzara®/REGN88/SAR153191 Drug: placebo Single or multiple IV doses of placebo to match sarilumab administration |
Phase: II, III n=1912 |
The first part is recruiting patients across multiple sites in the USA and will evaluate the effect of sarilumab on fever and need for supplemental oxygen. The second, larger, part of the trial will evaluate improvement in longer-term outcomes, including prevention of mortality and reduction in need for mechanical ventilation, supplemental oxygen, and/or hospitalization. The early results from this study seem to show that its utility may be reserved for the critically ill patients | Completed |
| NCT04324073143 | Drug: sarilumab (an IV dose of 400 mg of sarilumab in a one-hour infusion at D1 |
Phase: II, III n=239 |
The recruited participants were COVID-19 patients with moderate, severe or critical pneumonia. The trials aim to compare the outcomes of sarilumab-treated patients with those receiving outcomes of standard of care as well as with patients being treated with other immunomodulators. More studies have since been registered or initiated for assessing sarilumab | Active, not recruiting |
| NCT04329650144 | Drug: siltuximab A single-dose of 11 mg/kg of siltuximab will be administered by IV infusion. Drug: methylprednisolone A dose of 250 mg/24 h of methylprednisolone during 3 days followed by 30 mg/24 h during 3 days will be administered by IV infusion. If the patient is taking lopinavir/ritonavir, the dose will be 125 mg/24 h during 3 days followed by 15 mg/24 h during 3 days. |
Phase: II, III n=239 |
Compare efficacy and safety of siltuximab vs corticosteroids in hospitalized patients with COVID19 pneumonia proportion of patients requiring ICU admission at any time within the study period |
Recruiting |
| NCT04443881145 | Drug: anakinra 149 mg/mL prefilled syringe (Kineret) Anakinra (100 mg/6 h) IV infusión during 15 days |
Phase: II, III n=180 |
Treatment success, defined as number of patients not requiring mechanical ventilation to assess the effect of anakinra in addition to standard treatment on the need for mechanical ventilation in patients with severe COVID-19 and CSS pneumonia. (Time frame: day 15) Treatment success, defined as number of patients not requiring mechanical ventilation by day 15. Number of patients not requiring mechanical ventilation to assess the effect of anakinra in addition to standard treatment on the need for mechanical ventilation in patients with severe COVID-19 and CSS pneumonia. (Time frame: day 28) Number of patients not requiring mechanical ventilation Time to mechanical ventilation to assess the effect of anakinra in addition to standard treatment on the need for mechanical ventilation in patients with severe COVID-19 and CSS pneumonia. ((Time frame: up to 28 days) Time to mechanical ventilation Time to oxygen saturation normalization to assess the effect of anakinra in addition to standard treatment on the need for mechanical ventilation in patients with severe COVID-19 and CSS pneumonia. (Time frame: up to 28 days) Time to oxygen saturation normalization Stay in ICU and hospitalization to assess the effect of anakinra in addition to standard treatment on the need for mechanical ventilation in patients with severe COVID-19 and CSS pneumonia. (Time frame: up to 28 days) Stay in ICU and hospitalization |
Recruiting |
Antiviral Drugs
The nucleoside analogs are vital antiviral drugs, which have been used most commonly to treat HIV, hepatitis B virus (HBV), cytomegalovirus (CMV), HSV infections.86 These drugs impair viral replication by competitive inhibition of the viral polymerase or termination of the DNA chain.86 Due to its wide use in the past during the SARS pandemic, ribavirin was one of the first drugs tested as a potential remedy for COVID-19.87 In the past, it has been utilized in combination with steroidal drugs, as well as IFNβ.88 Owing to an existing inventory and a reliable supply chain, it can be used globally for COVID-19 management. However, hepatic injury, myopathy, neuropathy, bone marrow suppression, and pancreatitis are some of the most common adverse effects that have been encountered in the use of this drug (Table 1).86,89 Another important nucleoside analog, remdesivir was utilized on a large-scale for the treatment of SARS-CoV and MERS-CoV.90 The antiviral efficacy of the drug against SARS-CoV-2 has been proven through in vitro analysis.91 In fact, this drug was also utilized in the treatment of COVID-19 in the USA.92 Although, nucleoside analogs have been used extensively to treat viral pneumonia, these drugs have several adverse effects such as, diarrhea, rash, renal failure, and hypotension, increased liver enzymes (Table 1).93 In a recent randomized double-blind, placebo-controlled, multicenter trial at 10 hospitals in Hubei, China, the antiviral efficacy of remdesivir was tested on COVID-19 patients. Over a period of 10 days, the patients were randomly assigned in a 2:1 ratio to intravenous remdesivir. The dosage was 200 mg on day one, followed by 100 mg on days 2–10 in single daily infusion) or the same volume of placebo infusions. Although the findings were not statistically significant, the test group patients showed a faster clinical improvement than the placebo group.94 To date, several clinical trials have been registered to assess the efficacy of these nucleoside analogs in COVID-19 management which are listed in Table 2.83,95–98
The HIV protease inhibitors are a class of antiviral drugs, which play a vital role in the highly active antiretroviral therapy (HAART) utilized in HIV/AIDS management.99 Lopinavir and ritonavir, apart from their virtually identical molecular structures, inhibit the HIV protease by impairing cytochrome P450 activity, which affects the viral replication and synthesis (Table 1).99 Therapeutic efficacy of these drugs has been reported in the management of SARS and MERS.100 It has been suggested that these drugs have the ability to bind to the endopeptidase C30 of SARS-CoV-2 and downgrade the respiratory distress.101 However, higher doses of both lopinavir and ritonavir have been linked to a number of toxicity conditions such as, hyperlipidemia, systemic hypersensitivity syndromes, and Achilles tendinopathy.101 Hence, the safe use of these drugs is of utmost importance and should be performed only in the light of substantial evidence. Nonetheless, a recent review paper by Khalili et al, indicates that there are a number of ongoing clinical trials in which many of them, being conducted in China are to evaluate the role of ribavirin in the COVID-19 outbreak.89 Some of these studies have compared the effectiveness of ribavirin vs many of the antiviral drugs mentioned above, whilst the others have been designed to evaluate whether these drugs are more effective when used in different combinations.61 The results of these studies will indeed show researchers the path forward. Nelfinavir is a selective HIV protease inhibitor, whose antiviral efficacy has been proven against SARS-CoV.102 This drug can strongly inhibit the replication of the SARS-Cov-2 expression of viral antigens (Table 1).103 The potential implications for the use of this drug in COVID-19 patients, is nevertheless, subject to the development of the scientific evidence. The most common side effects of this drug are nausea and diarrhea (Table 1).100 The clinical trials utilizing this class of antiviral drug are listed in Table 2.85,104
Alternatively, the use of arbidol, as a broad-spectrum antiviral compound blocks the viral replication process of SARS-CoV-2.105 It is well-tolerated in children and adults (Table 1).106 As a result, it has been one of the recommended antiviral drugs for COVID-19 treatment.107 Allergic reactions, nausea, diarrhea, dizziness and elevated serum transaminase are some of the adverse effects associated with this drug (Table 1).106 A recent clinical trial was conducted to evaluate the efficacy of using arbidol as a monotherapeutic agent, compared to lopinavir/ritonavir in COVID-19 patients.108 The results in this study were in favor of the patients on arbidol monotherapy who recovered in shorter duration period with no apparent side effects, compared to the lopinavir/ritonavir group.108 Further use of this drug should be based on safe drug regimens standardized through RCTs, one of which is listed in Table 2.109
Chloroquine is a drug extensively used for malarial prophylaxis.110 One of its biochemical properties proposes a potent antiviral effect, which has led to its recognition as a broad-spectrum antiviral drug.111 In the past, chloroquine has shown beneficial effects in SARS-CoV inhibition by impairing ACE2,102 which has also been proven in the in vitro analysis against SARS-CoV-2.111 Furthermore, chloroquine is an inexpensive drug and with a good patient safety report and tolerance levels.63 Its adverse effects such as, retinal or psychiatric symptoms have been reported only with higher doses.110 A systematic review by Cortegiani et al, evaluated the efficacy and safety of chloroquine in COVID-19 treatment. The authors concluded that the drug is effective to reduce SARS-CoV-2 multiplication. Moreover, they suggested the necessity to conduct long-term quality RCTs in the near future to propagate its safe use.112 The clinical trials which have evaluated the efficacy chloroquine in COVID-19 patients are highlighted in Table 2.85,104,113,114
It can be gathered that all antiviral drugs can play a vital role in the treatment of COVID-19. Nonetheless, to date, there are no approved antiviral drugs for the same.17,74 Moreover, the adverse effects of each of them should not be overlooked. Research on the combination of these drugs has shown promising results, and further clinical evaluation of all these drugs is needed through long-term trials.74
Corticosteroids
Corticosteroids have potent anti-inflammatory effects against the COVID-19 cytokine storm (Table 1).115 The National Institute of Health and Care Excellence in line with the WHO guidance have confirmed that the dexamethasone (oral or intravenous; IV) and hydrocortisone (IV) play a role in COVID-19 management.116 Clinical evidence has shown that glucocorticoids may modulate inflammation-mediated lung injury, which is a key feature of COVID-19 infection, thereby reducing progression to respiratory failure and death.116 Dexamethasone (6 mg once a day; od) can lower the 28-day mortality among those who receive either invasive MV or oxygen alone compared to those receive no respiratory support in the treatment of shock and/or ARDS.116 In their a meta-analysis of seven trials, the WHO Rapid Evidence Appraisal for Covid-19 Therapies (REACT) Working Group evaluated corticosteroids (mainly hydrocortisone or dexamethasone) in 1703 critically ill patients in 12 countries and reported that both these drugs reduced the risk of death by about a third, in comparison to the control group.117 The RECOVERY (Randomized Evaluation of COVID-19 Therapy) collaborative group have conducted a controlled, open label trial to assess the role of dexamethasone (6 mg od, oral or IV) for up to 10 days compared to the standard care in 28-day mortality. The primary results of this study demonstrated the beneficial role of dexamethasone in lowering the abovementioned primary outcome compared to the control group (Table 2).104 In addition, antishock therapy using small doses of corticosteroids such as hydrocortisone could help in mitigating of CS, which in turn can minimize the occurrence of respiratory distress, septic shock and multi-organ failure,73 as well an alternative to dexamethasone in some COVID-19 patients. Some disadvantages include, increased appetite, irritability, difficulty in sleeping (insomnia), swelling in your ankles and feet (fluid retention), heartburn, muscle weakness, impaired wound healing, increased blood sugar levels (Table 1).115,117 Other clinical trials based on application of dexamethasone drug therapy in COVID-19 management are listed in Table 2.104,113,118,119
Immunotherapy
Convalescent plasma is plasma rich in antibodies, which is obtained from a patient who has recovered from a virus. Discernibly, the plasma can be taken from patient who has shown a full recovery, and has received ample time to develop a robust antibody response against the virus. The therapy is safe, clinically effective and decreases the mortality rate (Table 1).120,121 Convalescent plasma therapy (CPT) has been widely used for diseases like influenza A,122 Ebola123 and SARS-CoV.124 Recently, this form of passive immunization was utilized in COVID-19 management.125 The results of this study have shown that CPT was well-tolerated with a significant neutralization of the viremia and drastic improvement in the clinical presentation of severely affected COVID-19 patients.125 Nevertheless, the benefit of this therapy needs to be established through further large and well-controlled clinical investigations like the one listed in Table 2.104 Some drawbacks of this therapy include; transfusion related acute lung injury, transfusion associated circulatory overload and allergic/anaphylactic reactions, transmission of infections, febrile nonhemolytic transfusion reactions, RBC alloimmunization, and hemolytic transfusion reactions (Table 1).120,121
Another immunotherapy is a protective monoclonal antibody (mAb) that targets vulnerable sites of viral surface proteins and deactivates the same, and thus prevents the strain propagation. Recently, researchers isolated the human mAb 47D11, which has demonstrated potential benefits in deactivation of SARS-CoV-2 spike protein.126 Although the precise mechanisms of action remain unknown. On this note, the first report on the application of protective mAB for management of COVID-19 launches a gate for further exploration.
Currently, it is well-known that IL-6 is associated with the inflammation seen in COVID-19 patients and hence IL-6 and IL-6 receptor (IL-6R) inhibition appear to be promising targets to mitigate the adverse effects of this viral infection. Tocilizumab also known as atlizumab, is an anti- interleukin-6 receptor humanized mAb.127 It has a potent anti-inflammatory action and is immunosuppressive in nature and has been recommended for the treatment of rheumatoid arthritis and systemic juvenile idiopathic arthritis, a severe form of arthritis in children (Table 1).127–129 Lan et al, conducted a systematic review and meta-analysis of seven retrospective studies to assess the efficacy of tocilizumab (IV infusion: 4–8 mg/kg over 60 min; if needed repeat at 12 h max dose 800 mg), as a potential treatment modality for COVID-19. All-cause mortality of severe COVID-19 patients was lower in the groups receiving tocilizumab, compared to the controls, however, this difference was not statistically significant. Factors such as risk of intensive care unit admission and requirement for mechanical ventilation were similar between the treatment groups. Based on this, the authors concluded that a high quality evidence from future large RCTs data will decide the potential role of this drug in the management of COVID-19.130
The use of a novel human anti-interleukin-1β neutralizing mAb showing in vivo efficacy known as anakinra has surfaced for COVID-19 management in the last few months. Researchers believe that this recombinant IL-1 receptor antagonist can neutralise the hyperinflammatory state, which is considered to be one cause of acute respiratory distress among patients with COVID-19 (Table 1).131 A prospective cohort study from Groupe Hospitalier Paris Saint-Joseph (Paris, France) conducted by Huet et al, to assess the off-label use of subcutaneous anakinra (100 mg twice a day for 72 h, then 100 mg daily for seven days) in severely ill COVID-19 patients having symptoms indicative of worsening respiratory function. The group that received anakinra showed 50% fewer ICU admissions for invasive mechanical ventilation or death, compared to the control group.132 Some drawbacks of both these drugs are as follows, headache or dizziness, mouth ulcers, high blood pressure, hypercholesterolemia, allergic reactions, weight gain or swollen ankles, stomach irritation or abdominal pain. The immunosuppressive nature of anakinra may result in an adverse effect on the immune system (neutropenia) and healing, which may lead to reactivation of latent tuberculosis or other atypical or opportunistic infections (Table 1).127–129,131 The clinical trials that have been registered to evaluate the efficacy of both these drugs are listed in Table 2.133–145
Over the past few months, Regeneron Pharmaceuticals, Inc. have designed an antibody cocktail REGN-COV2, which has been investigated for its potential role in COVID-19 management. Their clinical data have proven that the two antibodies Regeneron selected, REGN10933 and REGN10987, were less likely to generate escape mutants than individual antibodies or other cocktails that have been designed.146 The cocktail has demonstrated the following benefits: targeted killing to reduce viral load and the time to alleviate symptoms in nonhospitalized COVID-19 patients, as well in reducing medical visits (Table 1).146–148 The University of Oxford in collaboration with Regeneron Pharmaceuticals, Inc. have announced that RECOVERY, which is one of the world’s largest RCTs of potential COVID-19 treatments will evaluate Regeneron’s investigational antiviral antibody cocktail, REGN-COV2. This will be a phase three open-label trial in hospitalized COVID-19 patients to assess the effects of adding REGN-COV2 to the usual standard-of-care vs standard-of-care on its own (Table 2).104 This antibody cocktail has been chosen by the university owing to its emerging safety profile in humans, preclinical data showing it could protect against viral escape mutations and reduce the amount of virus and associated damage in the lungs as demonstrated successfully in in vitro and in vivo nonhuman primate treatment studies.104,146 Other antibody cocktail therapies that have been announced are by Eli Lilly with AbCellera-partnered LY-CoV555, AstraZeneca with researchers from Vanderbilt University and a collaboration in between GlaxoSmithKline and Vir Biotechnology and all trials of the above are in difference phases at present.147 Use of systemic anti-inflammatory drugs such as baricitinib to reduce the adverse effects of the cytokine storm has also been assessed, due to its anti-inflammatory and antiviral effects.148
Prospective Therapies for COVID-19 Management
Apart from the various treatment options mentioned above, there are some upcoming modalities that have emerged, as prospective alternatives and the corresponding registered clinical trials are listed in Table 3.149–168 These include:
Table 3.
Shows the Clinical Trials Registered on ClinicalTrials.gov To October 20, 2020, Utilizing Prospective Therapies for the Treatment of COVID-19
| Therapy Modality | Study Identifier and Citation (Superscript) | Treatment Protocol | Study Phase; Estimated Enrolment (n) | Primary Outcome Measure(s) | Recruitment Status |
|---|---|---|---|---|---|
| Recombinant ACE2 receptors | NCT04375046149 | Experimental: Experimental: rbACE2 group 0.4 mg/kg IV BID for 7 days (unblinded) + standard of care Intervention: drug: recombinant bacterial ACE2 receptors—like enzyme of B38-CAP (rbACE2) No intervention: no Intervention: control group Standard of care; no placebo |
Phase I n=24 |
Time course of body temperature (fever) (time frame: 14 days) Compare the time course of body temperature (fever) between two groups over time. Viral load over time (time frame: 14 days) Compare viral load between two groups over time. |
Not yet recruiting |
| NCT04382950150 | Experimental: Experimental: rbACE2 group plus aerosolized isotretinoin rbACE2 0.4 mg/kg IV BID for 7 days (unblinded) plus aerosolized 13 cis retinoic acid in gradual in 2 divided doses increases from 0.2 mg/kg/day to 4 mg/kg/day as inhaled 13 cis retinoic acid therapy for 14 days Intervention: combination product: recombinant bacterial ACE2 receptors—like enzyme of B38-CAP (rbACE2) plus aerosolized 13 cis retinoic acid No intervention: no Intervention: control group Standard of care; no placebo |
Phase I n=24 |
Viral load over time (time frame: 14 days) Compare viral load between two groups over time. P/F ratio over time (time frame: 14 days) PaO2/FiO2 ratio Sequential organ failure assessment score (SOFA score) over time (time frame: 14 days) SOFA, including assessment of respiratory, blood, liver, circulatory, nerve, kidney, from 0 to 4 scores in each systems, the higher scores mean a worse outcome. Pulmonary severity index (PSI) (time frame: 14 days) Image examination of chest over time (time frame: 14 days) Based on radiologist’s assessment of inflammatory exudative disease, category as follows: significant improvement, partial improvement, no improvement, increase of partial exudation, significant increase in exudation, unable to judge. Proportion of subjects who progressed to critical illness or death (time frame: at 14 days) Time from first dose to conversion to normal or mild pneumonia (time frame: 14 days) T-lymphocyte counts over time (time frame: 14 days) C-reactive protein levels over time (time frame: 14 days) Angiotensin II (Ang II) changes over time (time frame: 14 days) Angiotensin 1–7 (Ang 1–7) changes over time (time frame: 14 days) Angiotensin 1–5 (Ang 1–5) changes over time (time frame: 14 days) Renin changes over time (time frame: 14 days) Aldosterone changes over time (time frame: 14 days) Angiotensin-converting enzyme (ACE) changes over time (time frame: 14 days) Interleukin 6 (IL-6) changes over time (time frame: 14 days) Soluble tumor necrosis factor receptor type II (sTNFrII) changes over time (time frame: 14 days) Plasminogen activator inhibitor type-1 (PAI-1) changes over time (time frame: 14 days) Von Willebrand factor (VWF) changes over time (time frame: 14 days) Tumor necrosis factor-α (TNFα) changes over time (time frame: 14 days) Soluble receptor for advanced glycation end products (sRAGE) changes over time (time frame: 14 days) Surfactant protein-D (SP-D) changes over time (time frame: 14 days) Frequency of adverse events and severe adverse events(time frame: 14 days) |
Not yet recruiting | |
| Exosomes | NCT04491240151 | Procedure: EXO 1 inhalation Twice a day during 10 days inhalation of 3 mL special solution contained 0.5–2⨰10^10 of nanoparticles (exosomes) of the first type. Procedure: EXO 2 inhalation Twice a day during 10 days inhalation of 3 mL special solution contained 0.5–2⨰10^10 of nanoparticles (exosomes) of the second type. Procedure: placebo inhalation Twice a day during 10 days inhalation of 3 mL special solution free of nanoparticles (exosomes) |
Phase I PhaseII n=90 |
Number of participants with non-serious and serious adverse events during trial (time frame: 30 days after clinic discharge) Safety assessment such as adverse events will be registered. Adverse events will be monitored during all trial Number of participants with non-serious and serious adverse during inhalation procedure (time frame: after each inhalation during 10 days) Safety assessments such as adverse events during the inhalation procedures will be registered |
Enrolling by invitation |
| NCT04389385152 | Biological: COVID-19 specific T cell derived exosomes (CSTC-Exo) | Phase I n=60 |
Adverse reaction (AE) and severe AE (SAE) (time frame: 28 days) Safety assessment efficacy assessment (time frame: 28 days) Time to clinical recovery (TTCR) The rate of recovery without mechanical ventilator (time frame: 28 days) Efficacy assessment |
Not yet recruiting | |
| MSCs | NCT04384445153 | Zofin is an acellular, minimally manipulated product, derived from human amniotic fluid (HAF). This product contains over 300 growth factors, cytokines, and chemokines as well as other extracellular vesicles/nanoparticles derived from amniotic stem and epithelial cells. Biological: zofin Biological: zofin will be administered intravenously with 1 mL, containing 2–5⨰10^11 particles/mL in addition to the standard care. The zofin dose will be diluted in 100 mL of sterile saline at subject’s bedside. Participants in this group will receive standard of care plus zofin on day 0, day 4 and day 8. Intervention: Biological: zofin Other: placebo Other: placebo Placebo (saline) will be administered intravenously with 1 mL in addition to the standard care. The placebo dose will be diluted in 100 mL of sterile saline at subject’s bedside. Participants in this group will receive standard of care plus placebo (saline) on day 0, day 4 and day 8. |
Phase I Phase II n=20 |
Incidence of any infusion associated adverse events (time frame: 60 days) Safety will be defined by the incidence of any infusion associated adverse events as assessed by treating physician Incidence of severe adverse events (time frame: 60 days) Safety will be defined by the incidence of severe adverse events as assessed by treating physician |
Recruiting |
| NCT04276987154 | Experimental: MSCs-derived exosomes treatment group Conventional treatment and aerosol inhalation of MSCs-derived exosomes treatment participants will receive conventional treatment and 5 times aerosol inhalation of MSCs-derived exosomes (2.0*10E8 nano vesicles/3 mL at days 1, 2, 3, 4, and 5). Intervention: Biological: MSCs-derived exosomes |
Phase I n=24 |
Adverse reaction (AE) and severe adverse reaction (SAE) (time frame: up to 28 days) Safety evaluation within 28 days after first treatment, including frequency of adverse reaction (AE) and severe adverse reaction (SAE) Time to clinical improvement (TTIC) (time frame: up to 28 days) Efficiency evaluation within 28 days, including time to clinical improvement (TTIC) |
Completed | |
| NCT04457609155 | Drug: oseltamivir Current standardized treatment for COVID-19 Drug: azithromycin Current standardized treatment for COVID-19 Biological: umbilical cord MSCs Adjuvant therapy on top of current standardized treatment (oseltamivir+azithromycin) |
Phase I n=40 |
Clinical improvement: presence of dyspnea (time frame: 15 days) Assessing whether the patients still have dyspnea, one of cardinal symptoms of COVID-19, assessed from the respiratory rate Clinical improvement: presence of sputum (time frame: 15 days) Assessing whether the patients still have productive cough, one of cardinal symptoms of COVID-19, assessed from lung auscultation Clinical improvement: fever (time frame: 15 days) Assessing the presence of fever from measurement of body temperature checking, assessed on daily basis Clinical improvement: ventilation status (time frame: 15 days) Assessing whether the patients still require ventilation, one of cardinal symptoms of ARDS in COVID-19, assessed from patients’ ability during ventilation weaning phase Clinical improvement: blood pressure (time frame: 15 days) Assessing the patients’ blood pressure on daily basis Clinical improvement: heart rate (time frame: 15 days) Assessing the patients’ heart rate on daily basis Clinical improvement: respiratory rate (time frame: 15 days) Assessing the patients’ respiratory rate on daily basis Clinical improvement: oxygen saturation (time frame: 15 days) Assessing the patients’ oxygen saturation on daily basis |
Recruiting | |
| RAS blockers | NCT04331574156 | Observational model: Case-only time perspective: cross-sectional Patients with certified diagnosis of COVID-19 recruited in Italian hospitals |
Phase: not applicable n=2000 |
Numbers of COVID-19 patients enrolled that use ACE inhibitors and/or angiotensin receptor blockers (ARB) as antihypertensive agents (time frame: 3 months) Using anamnestic data collected from the health record of the hospital or of the general practitioner, we will count the number of COVID-19 patients enrolled that were treated with ACE Inhibitors or ARB. Numbers of COVID-19 patients enrolled with no symptoms, with moderate symptoms or with severe symptoms of pneumonia based on the WHO specification for ARDS that also used ACE inhibitors and/or angiotensin receptor blockers (ARB) as antihypertensive agents (time frame: 3 months) This study wants to observe whether the assumption of antihypertensive ACE inhibitors or ARB increases the severity of the clinical manifestation of COVID19 |
Recruiting |
| NCT04329195157 | Drug: 1: discontinuation of RAS blocker therapy discontinuation of RAS blocker therapy Drug: 2: continuation of RAS blocker therapy continuation of RAS blocker therapy |
Phase III n=554 |
Time to clinical improvement from day 0 to day 28 (improvement of two points on a seven-category ordinal scale, or live discharge from the hospital, whichever comes first) (time frame: from day 0 to day 28 or hospital discharge) | Recruiting | |
| NCT04353596158 | Drug: ACE inhibitor, angiotensin receptor blocker In patients randomized to stopping/replacing ACEI or ARB, it may be necessary to switch to another drug without direct effect on the RAS system. In patients, randomized to continuation, it may be needed to stop ACEI or ARB (eg hypotension with beginning sepsis) irrespective of the study. |
Phase IV n=208 |
Combination of maximum sequential organ failure assessment (SOFA) score and death (time frame: 30 days) The minimal value of the SOFA score will be 0 and the maximal value 24 points. All-cause death is classified as the maximum score (24 points). In case of a subclinical disease progress without need for hospitalization, the SOFA score will be 0. Composite of admission to an ICU, the use of mechanical ventilation, or all-cause death (time frame: 30 days) |
Recruiting | |
| Traditional Chinese medicines | NCT04306497159 | Drug: TCM prescriptions TCM prescriptions 1: take decocted or granule, one dose a day; TCM prescriptions 2: take decocted or granule, one dose a day. |
Phase—no information n=340 |
The relief of main symptoms/disappearance rate of time (time frame: 9 days) Comparison of the time of relief/disappearance of three main symptoms of fever, cough and shortness of breath chest CT absorption (time frame: 9 days) with reference to the “pneumonia chest X-ray absorption evaluation scale” developed by Renyi Yin et al, the final absorption judgment will be used to evaluate the chest CT absorption of patients with pneumonia, which is divided into four levels according to the degree of absorption: complete absorption, majority absorption, partial absorption and no absorption. |
Completed |
| NCT04251871160 | Drug: conventional medicines (oxygen therapy, alfa interferon via aerosol inhalation, and lopinavir/ritonavir) and TCMs granules Conventional medicines: oxygen therapy, antiviral therapy (alfa interferon via aerosol inhalation, and lopinavir/ritonavir, 400 mg/100 mg, p.o., bid) for 14 days. TCMs granules: 20 g, p.o., bid, for 14 days. Drug: conventional medicines (oxygen therapy, alfa interferon via aerosol inhalation, and lopinavir/ritonavir) Conventional medicines: oxygen therapy, antiviral therapy (alfa interferon via aerosol inhalation, and lopinavir/ritonavir, 400 mg/100 mg, p.o., bid) for 14 days. |
Not applicable n=150 |
The incidents of acute respiratory distress syndrome (ARDS) development (time frame: 14 days) The incidence rate of acute respiratory distress syndrome (ARDS) development |
Recruiting | |
| Antioxidants | NCT04363216161 | Drug: Ascor® ascorbic acid 2-h infusion daily (for 6 days), escalating dose (0.3 g/kg, 0.6 g/kg, 0.9 g/kg). Other name: vitamin C |
Phase II n=66 |
Clinical Improvement (time frame: 72) •Clinical improvement at 72-h of treatment, defined as a 50% reduction in the highest flow rate of oxygen during the 72-h period, a 50% reduction in the most frequent use of bronchodilators within a 12-h window within the 72-h period, or hospital discharge (whichever comes first). |
Not yet recruiting |
| NCT04323514162 | Dietary supplement: vitamin C 10 g of vitamin C IV in addition to conventional therapy. |
Not applicable n=500 |
In-hospital mortality (time frame: 72 h) Change of hospital mortality |
Recruiting | |
| NCT04519034163 | Audit 1 All vitamin D results performed since January 2020 (n= ~15,000) together with age, weight and height if available, ethnicity and other relevant laboratory markers (Ca, adjusted calcium, PTH, Mg, phosphate, liver and renal profile, COVID-19 screening, CRP, hematinics, FBC) Intervention: other: no intervention Audit 2 all COVID-19 screening results together with vitamin D, ethnicity, age, weight, height, length of stay in hospital including ICU (if applicable), type of illness, recovered or not, associated health conditions, CRP, ferritin, hematinics, vitamin A and E, procalcitonin, LDH, INR, fibrinogen, FBC, D-dimers, CK, troponin-T, cytokines, renal function and electrolytes from patients tested at GSTT NHS Trust. Intervention: other: no intervention |
Phase—not applicable n=27,000 |
Collecting vitamin D results in patients from the South-East London area together with age, sex, ethnicity and BMI and other relevant laboratory results. (time frame: January–June 2020) All vitamin D results performed by the Nutristasis Unit at St. Thomas’ since January 2020 (n= ~15,000) together with age, weight and height if available, ethnicity and other relevant laboratory markers (Ca, adjusted calcium, PTH, Mg, phosphate, liver and renal profile, COVID-19 screening, CRP, hematinics, FBC) if they were tested within two weeks of the sample being measured for vitamin D will be acquired. The results of this audit will provide us with a snap shot of vitamin D status in patients from the South-East London area by age, sex, ethnicity and BMI (weight in kg/height2). Correlation analysis will also be undertaken with other laboratory parameters. |
Not yet recruiting | |
| NCT04323228164 | Experimental: intervention—- the intervention groups will receive daily oral antioxidant supplement enriched in vitamins A, C, E, selenium and zinc. The composition of one capsule of the intervention-supplement includes: 1500 µg vitamin A (as β-carotene), 250 mg vitamin C, 90 mg vitamin E, 15 µg selenium, and 7.5 mg zinc. Intervention: dietary Supplement: oral supplement enriched in antioxidants Placebo comparator: placebo Placebo group will receive daily intervention in form of cellulose-containing gelatin capsules with the same color and shape. Intervention: dietary Supplement: cellulose-containing placebo capsules |
Phase II Phase III n=40 |
Change from baseline score of nutrition risk screening-2002 (NRS-2002) at end of the trial (time frame: up to 3 months) Changes in scores of the NRS-2002 for patients with COVID-19 at the end of the study, from 0 to 7 scores, with those scores <3 means no risk of malnutrition and ≥3 means malnutrition. Change from baseline serum ferritin level at end of the trial (time frame: up to 3 months) Change in serum ferritin at the end of the trial as ferritin is considered as a COVID-19 fatality predictor. Change from baseline serum IL-6 concentration at end of the trial (time frame: up to 3 months) Change in IL-6 at the end of the trial as it represents the cytokine storm and it is considered as a COVID-19 fatality predictor Change from baseline serum C-reactive protein concentration at end of the trial (time frame: up to 3 months) Change in C-reactive protein in the serum at the end of the trial which reflects the acute phase Change from baseline serum TNFα concentration at end of the trial (time frame: up to 3 months) Change in the TNF a in the serum at the end of study as it represents severity of the cytokine storm Change from baseline serum monocyte chemoattractant protein 1 (MCP-1) at end of the trial (time frame: up to 3 months) plasma MCP-1 represents severity of the cytokine storm |
Recruiting | |
| Dietary Supplement | NCT04382040165 | ArtemiC is a medical spray comprised of artemisinin (6 mg/mL), curcumin (20 mg/mL), frankincense (=Boswellia) (15 mg/mL) and vitamin C (60 mg/mL) in micellar formulation for spray administration. Drug: ArtemiC: Treatment will be sprayed orally twice a day for the first 2 days in the treatment period Drug: placebo: Treatment will be sprayed orally twice a day for the first 2 days in the treatment period |
Phase II n=50 |
Time to clinical improvement, defined as a national early warning score 2 (NEWS2) of ≤ 2 maintained for 24 h in comparison to routine treatment (time frame: 24 hours) patient will be assessed using a scoring table for changes in clinical signs Percentage of participants with definite or probable drug related adverse events (time frame: 14 days) adverse events caused by the study drug will be assessed |
Recruiting |
| NCT04347382166 | Experimental: Nigella sativa and honey group Drug: Nigella sativa seed Powder 80 mg/kg/day ground in a capsule up to a max of 14 days) Drug: natural honey 1 mg/kg/day orally up to a max of 14 days) along with standard medical care Interventions: Drug: honey; Drug: Nigella sativa/black cumin placebo comparator- standard medical Care: standard supportive medical care prescribed by treating physician, Lahore which includes standard symptomatic care along with use of antibacterial or antiviral (if advised by pulmonologist or infectious disease specialist) Intervention: Drug: placebos: |
Phase III n=313 |
Days required to get a positive COVID-19 PCR to negative (time frame: up to max 14 days) RT-PCR will be done on admission day (0 day) and then after every fourth day for 14 days or till the symptoms resolved and RT-PCR gets negative. RT-PCR will only be shown as positive or negative (as per limitation of Pakistan). HRCT/X-ray findings of disease progression (time frame: up to max 14 days] HRCT will be conducted at admission day (0-day) and a total of maximum four CT-scans will be conducted after every fourth day. The minimum and score at which we label COVID-19 positive will be 5 and 25 respectively using internationally standard nomenclature as described by Fleischner Society glossary and peer-reviewed literature on viral pneumonia. Severity of symptoms progression (time frame: up to max 14 days) Clinically disease progression will be evaluated depending upon the severity of symptoms being classified as mild, moderate and severe. Duration of hospital stay (time frame: up to max 14 days) Duration of hospital stay would be categorized as the number of days the patient stayed in the ward during treatment. The date of admission and date of discharge would give us total duration of stay. 30 day mortality (time frame: 30 days) 30 days mortality rate in each arm |
Completed | |
| NCT04323345167 | Dietary supplement: natural honey: natural honey supplement 1 g/kg/day divided into 2 to 3 doses for 14 days either orally or through nasogastric tube. Other: Standard Care: supportive measures and lopinavir/ritonavir tablets or arbidol or chloroquine phosphate or hydroxychloroquine or oseltamivir with or without azithromycin. |
Phase III n=1000 |
Rate of recovery from positive to negative swaps (time frame: 14 days) Percentage of patients turned from positive to negative swaps at day 14 Fever to normal temperature in days (time frame: 14 days) Number of days till no fever Resolution of lung inflammation in CT or X- ray(time frame: 30 days) Number of days till lungs recovery in chest X-ray or CT |
Recruiting | |
| NCT04342689168 | Drug: dietary supplement containing resistant starch: two tablespoons (~20 g) to be taken twice daily for 14 days (start with 2 tablespoons once daily for three days, followed by twice daily on days 4 through 14) (Other name: resistant starch) Dietary supplement: placebo starch: two tablespoons (~20 g) to be taken twice daily for 14 days (start with 2 tablespoons once daily for three days, followed by twice daily on days 4 through 14) (other name: nonresistant starch) |
Phase III n=1500 |
Rates of hospitalization for a COVID-19 related complication (time frame: One month from the start of treatment) Subject hospitalized while presenting symptoms of fever, shortness of breath, myalgia, cough, or hypoxia with an admission diagnosis of hypoxic respiratory failure, pneumonia, or viral pneumonia on review of electronic health record (EHR). Death prior to hospitalization thought to be secondary to COVID-19 will also be defined as an event. All hospital admissions will be reviewed and adjudicated by a site PI. |
Recruiting |
1. Gheblawi et al, in their review have highlighted the role of ACE2 as the novel SARS-CoV-2 receptor and have provided a critical link between immunity, inflammation, ACE2, and cardiovascular disease.169 The authors have stated that the action of ACE2 is enhanced by recombinant ACE2, gene-delivery of Ace2, Ang 1–7 analogs, and Mas receptor agonists.169 The latter serves as potential therapies for disease conditions associated with an activated RAS (Table 3).149,150
2. Molecular mechanism of exosome-based (30–120 nm extracellular vesicles) strategies are being tested to fight the COVID-19 infection (Table 3).151,152 These vesicles help in intercellular communication via delivering biomolecules like nucleic acids, proteins, and lipids to recipient cells (Table 1).170,171 They contain receptors for viruses (CD9 and ACE2) that make recipient cells susceptible to virus entry. They are released by virus-infected cells and are associated with the transfer of viral components.170 Researchers believe that exosomes have a role in the spread of the SARS-CoV-2 virus and a thorough understanding of their relation to the COVID-19 infection could aid in identifying vital information regarding the entry, replication and spread of the virus in order to combat its adverse effects (Table 1).170,171 However, some drawbacks that have been noted with regards to this therapy are as follows: inefficient separation methods, difficulties in characterization and lack of specific biomarkers. At present, the strategies that are being tested include; inhibition of exosome biogenesis and uptake, exosome-therapy, exosome-based drug delivery system, and exosome-based vaccine (Table 3).151,152
3. Cellular therapy: an intravenous transplantation of ACE2-mesenchymal stem cells (MSCs) or natural killer cells to mediated-antiviral therapy.21,172 MSCs are able to suppress the activities of viruses via Chaf1a- mediated and Sumo2-mediated epigenetic regulation (termed proviral silencing) and have demonstrated beneficial effects in ARDS. However, there is a lack of clarity with regards to optimal dose and route of MSC delivery, difficulties in large-scale production and cryopreservation, and the potential for substantial variability (Table 1).172,173 Table 3 provides data on three clinical trials which have been registered for assessed MSCs in COVID-19 management.153–155
4. As the ACE2 is a vital component of renin-angiotensin system (RAS), it can be a possible treatment by utilizing the RAS inhibitors such as; ACE-inhibitors (ACEI) and angiotensin II receptor type 1 (AT1R).174 These drugs are not associated with an increased risk of death, admission to intensive care unit (ICU), mechanical ventilation requirement or progression to severe or critical pneumonia in COVID‐19–infected hypertensive patients and they can be used safely in children and adults. There is insufficient evidence of their potential harm and only overwhelming evidence shows their benefits, thus favoring their utilization in COVID-19 management in the clinical trials listed in Table 3.156–158
5. Traditional Chinese medicines (TCMs) such as; qingfei paidu decoction (QPD), gancaoganjiang decoction, sheganmahuang decoction, qingfei touxie fuzheng recipe, glycyrrhizin, hesperetin, baicalin and quercetin, are rich in naturally occurring flavonoids.175,176 The advantages of TCMs include, remarkable symptomatic relief, antipyretic, faster recovery, reduced hospital stay results in their utilization in previous epidemics. However, some commonly associated drawbacks include, hypersensitivity pneumonitis, lung injury, fatigue with gastrointestinal discomfort (Table 1).175–178 Table 3 lists two important clinical trials that have been registered to assess TCMs, as a potential treatment strategy in COVID-19 patients.159,160
6. Consumption of food products rich in vitamins A, C, D, and E, which are naturally occurring antioxidants.73 They can prevent or reduce the damage caused by oxidation as well as reduce the risk of cardiovascular disease. Some drawbacks include; bruising under the skin, diarrhea, dizziness, joint pain (Table 1).179,180 Clinical trials which have been registered to evaluate antioxidant therapy in COVID-19 are listed in Table 3.161–164
7. Curcumin may have a potential role against COVID-19, owing to its scientifically proven benefits such as its ability to modulate various molecular targets that contribute to the attachment and internalization of SARS‐CoV‐2 in various human organs, cellular signaling pathways and RNA replication, as well its role in suppression of pulmonary edema and fibrosis‐associated pathways (Table 1).181 It has also demonstrated strong inhibitory effects on NF‐κB and several pro‐inflammatory cytokines for which it can be used, as an adjunct in reversing the fatal CS that occurs in severely-ill COVID-19 patients.181 Owing to no known drawbacks of curcumin it is being assessed in a clinical trial (Table 3).165
8. The role of omega-3 fatty acids, honey and its various components as well as potato starch have been highlighted as novel natural therapies to fight against the symptoms of COVID-19 infection.182 These therapies are known to have a potent antibacterial and anti-inflammatory effects (Table 1).183–185 Methylglyoxal (MGO) is a component of manuka honey which can inhibit enveloped virus growth and inhibit SARS-CoV-2 replication.184,186 In addition, the higher the concentration of MGO, the stronger the antibiotic effect. There are no known drawbacks of honey and starch, however, the omega 3 fatty acids namely, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) can make cell membranes more susceptible to nonenzymatic oxidation mediated by ROS, leading to the formation of potentially toxic oxidation products and increasing the oxidative stress (Table 1).183–185 Researchers believe that these natural products can help boost the host immune system, improve comorbid conditions and antiviral activities in COVID-19 patients and hence some clinical trials have been registered to assesses their outcomes (Table 3)166–168
Vaccine
Presently, the entire world has joined hands and engaged all the available resources and manpower to develop a vaccine against COVID-19. It is believed that vaccination could be acknowledged as the epitome of all existing remedies assigned for controlling the COVID-19 outbreak. Quite a large number of vaccines are undergoing trials across different parts of the world to fight against the present pandemic situation.73,74 Epitopes, mRNA and S protein-receptor binding domain structure-based vaccines are the most coming vaccine proposals, which have been initiated.75 However, scientists have estimated that the process is time consuming and consisting of sequential phase-wise trials with quite a few safety evaluations. Notably, this entire process will take almost 12–18 months in achieving optimal results, as well as mass manufacture.76 Moreover, after the vaccine is available to the general population, human clinical trials would be required to prove the efficacy and safety of the same. The efforts of the entire scientific fraternity in the fight against this brutal fatality have just started materializing. Newer epidemiological facts, properties of the virus, immune responses against the virus and challenges in vaccine production are surfacing each day.50 Evidently, the efforts invested into research have to be amplified with an urgent application in order to combat this disease. Perhaps this is how research will evolve and the vaccine will be developed.
Future Noninvasive Therapy
COVID-19 remains a serious concern, until now, as there are a lack of effective antiviral drugs and the ongoing studies are yet to produce a safe and effective vaccine. Hence, phototherapy could be considered to treat COVID-19, as a therapeutic or preventive treatment modality. The term phototherapy encompasses photobiomodulation therapy (PBMT), including lasers light and laser-emitted diodes (LEDs) and photodynamic therapy (PDT).187,188 It is well-documented in the scientific literature that phototherapy presents a promising noninvasive treatment modality in tissue healing and regeneration, as well in pain management.189–194
The Rationale for Use of PBMT (Laser and LEDs) in COVID-19
The role of PBMT in modulating the molecular and cellular activities plays a significant part in achieving the optimal therapeutic outcomes.190,195 Experimental studies utilizing murine models of acute airway and lung inflammation have shown that PBMT reduces pulmonary microvascular leakage, IL-1β, IL-6, and intracellular ROS. On this note, PBMT as a single or adjunct treatment modality can modulate the cytokine storm and ARDS via its anti-inflammatory action196–199 and this has demonstrated the potential effect of PBMT in reducing the need for ventilators in patients with COVID-19.199,200
Several in vivo animal studies have utilized various wavelengths such as; λ 650 nm, λ 660±20 nm201 and λ 780 nm lasers for PBM irradiation in which positive results reported as follows: downregulation of inflammatory mediators, reduction in the activation of neutrophils influx, improvement in the endothelial damage, reduction in TNFα, and IL-1β in the lung, as well lung edema.201–203 On this note, ARDS symptoms, as a result of SARS-CoV-2 infection can be improved with PBMT.204 Additionally, in vivo experimental animal model studies of respiratory disease suggested that PBMT reduces inflammation and promotes lung healing.199,205,206 These in vivo animal studies have shown PBMT effectiveness in minimize the length of time needed on a ventilator, enhance the healing process, and shorten recovery time. As a result, this could significantly reduce severely overwhelmed health-care systems. Hence, there is an urgency of proposing this therapy for COVID-19 management.207 This statement is supported by the evidence-based clinical results, which showed a significant improvement in patients with chronic obstructive pulmonary syndrome (COPD) and asthma following PBMT.208,209
Figure 3 illustrates the mechanism in which PBM can reverse the cellular and molecular activities of the SARS-CoV-2 virus.200,203,206 On this note, the first severe case of COVID-19 pneumonia treated with PBMT, as an adjunct to standard conventional protocol was recently treated by Sigman et al, which aimed to reduce inflammation and promote lung healing. The presentation of this case is related to a 57-year-old African American man with severe COVID-19 who, once-daily for four consecutive days, received PBMT sessions by a laser scanner with pulsed λ 808 nm (peak power of 3 W, frequency 1500 Hz, 330 microseconds, 50% duty cycle) and super-pulsed λ 905 nm (peak power 75 W⨰3, frequency 1500 Hz, 10 nanoseconds) for 28 min (14 mins for each lung).210 The fluence was 7.2 J/cm2 and the total energy 3600 J. The laser device utilized scanner, which positioned 20 cm above the target tissue and scanned 250 cm2 over each lung. The patient was evaluated before and after treatment via radiological assessment of lung edema (RALE) by CXR, pulmonary severity indices, blood tests, oxygen requirements and patient questionnaires. The results have shown that oxygen saturation (SpO2) has increased from 93–94% to 97–100%, whilst the oxygen requirement has decreased from 2–4 L/min to 1 L/min. The RALE score improved from eight to five. Furthermore, the pneumonia severity index (PSI) improved from Class V (142) to Class II (67). Furthermore, both pulmonary indices (Brescia-COVID and SMART-COP) were decreased from four to zero. The CRP normalized from 15.1 to 1.23. The patient has reported a substantial improvement on the community-acquired pneumonia assessment (CAPA) tool. Importantly it wasreported that the respiratory indices, radiological findings, oxygen requirements and patient outcomes have improved over several days of treatment without the need for a ventilator. This report has presented the significance of supportive PBMT in a patient with severe COVID-19 pneumonia. Future randomized controlled clinical trials with large data are warranted to evaluate PBMT effects on clinical outcomes in patients with COVID-19 pneumonia.
Figure 3.
Modified schematic diagram illustrates the cellular and molecular cascade of PBMT that reserve SARS-CoV-2 cellular and molecular activities to prevent ARDS.
Notes: Adapted with permission from Mokmeli S, Vetrici M. Low level laser therapy as a modality to attenuate cytokine storm at multiple levels, enhance recovery, and reduce the use of ventilators in COVID–19. Can J Respir Ther. 2020;56:1–7.200
In the present review, we highlighted that patients with comorbidities can be subjected to a higher risk of complications when infected with SARS-CoV-2 virus.9–11 Obesity is one them. This is due to a low respiratory system compliance, increased inflammatory cytokines and an activated immune system secondary to excess adiposity. Another recent case report by Sigman et al, evaluated the effect of PBMT in treating a morbidly obese 32-year-old Asian female with severe COVID-19211 who was irradiated with the same protocol of the previous case report.102 The utilized evaluation methods of PBMT outcomes are as follows: on CXR, pneumonia severity indices (SMART-COP and Brescia-COVID) and pneumonia severity indices blood inflammatory markers (IL-6, ferritin, and CRP), and oxygen requirements and saturation in a patient with severe COVID-19 and morbid obesity. The results have shown that SpO2 via pulse oximetry increased to 97–99% on 1–3 L oxygen after PBMT, compared to baseline where SpO2 via pulse oximetry was 88–93% on 5–6 L oxygen. Additionally, a reduction in the following values noted after PBMT: RALE score from eight to three, Brescia-COVID from four to zero, and SMART-COP from five to zero, IL-6 from 45.89 to 11.7 pg/mL, ferritin from 359 to 175 ng/mL and CRP from 3.04 to 1.43 mg/dL. This report suggests that adjunct PBMT can be safely combined with conventional treatment in patients with severe COVID-19 and morbid obesity. This coincides with a recent review by Mokmeli et al, who showed that PBMT combined with conventional medical therapy has the potential to prevent COVID-19 progress and improve symptoms.200
Given the abovementioned two cases, there is a relatively recent trend towards utilization of PBMT in minimizing complications with patients who are more vulnerable to COVID-19 than others. Hence, there is an urgent need for RCTs with large data to validate this promising noninvasive therapy.
The Rationale for Use of PDT in COVID-19
The most appropriate photosensitizer (PS) for PDT in the airway tract seems to be methylene blue (MB, phenothiazine derivative) for its good performance for safety in various clinical therapeutic uses. MB has two absorption peaks at λ 635 and λ 670 nm and its complete absorption spectrum ranges from of λ 609 nm to λ 690 nm, with a low slope trend of the ascending part of the absorption curve and a sudden drop of the descending part.212 The scientific literature clearly shows that both MB and riboflavin can be used to inactivate coronaviruses, using a photodynamic process, although the cellular mechanisms are not completely understood. It is assumed that the COVID-19 viruses accumulate the photosensitive molecules due to their energetic potential. Photodynamic excitation by an appropriately adapted light source (lasers or LEDs) leads to the formation of ROS and singlet O2 (1O2), which destroys the cell membranes of the viruses.213,214 Knowing that most of the viruses have either DNA or RNA (single or double stranded) core and an outer protein cover or lipid, thereby, the basic structure of viruses contains three principal molecular targets for viral protein disulfide isomerase (PDI) and for the reaction with the generated ROS: nucleic acids, virus proteins, and if present, viral lipids.215 Those with viral lipids and/or protein envelope, in general seem to be more sensitive to using PDT/PDI (photodynamic inactivation) than those without.216 It is noteworthy that photodynamic damage is likely to occur very close to the intracellular location of the PS, which plays a crucial part in the apoptosis process, in conjunction with other factors such as the overall PDT dose (PS concentration ⨰ light fluence).217
Interestingly, SARS-CoV-2 has a number of distinguishing features including a protein envelope and lipids that would make it susceptible to treatment with PDT,218 as well as positive stranded RNA virus and belongs to the beta CoVs category. It has a round/elliptic and often pleomorphic form, and a diameter of approximately 60–140 nm. Like other CoVs, it is sensitive to ultraviolet rays and heat.218 As a result, safe and potent vaccine production faces many challenges, which might take a longer time to be on the market. Importantly, at the onset of the symptoms such as, fever, cough, and headaches, a significant number of viruses are bound to ACE2 receptors in the mucosa of the oral cavity, throat and nasal cavity,219 which allow easy access to PDT. Reduced viral load via PDT process can stimulate an immune reaction and the formation of protective antibodies, while favoring a mild or moderate course of disease without severe lung dysfunction or damage.220 It is noteworthy that the upper respiratory tract is the main harbor for opportunistic pathogen propagation. This is due to the natural colonization in the oropharynx. On this note the pharyngotonsillitis PDT can facilitate a reduction in these pathogens and act as a safeguard to prevent their penetration into the mucosal barriers. Hence, it is a good helper in the phagocytosis process, including inactivation of pathogen proliferation.213 This has been demonstrated by Blanco et al, reporting a clinical PDT protocol against pharyngotonsillitis in reducing more than 90% of the symptoms related to the disease after 24 h were observed. Hence, PDT is significant to inactivation the viral infection and reduce viral load in the respiratory tract.221 Interestingly, many studies investigated the methods of delivery of the PS to the respiratory tract.
The most common vehicle for PS delivery for PDT of pulmonary diseases is via intravenous administration. This, however, is subjected to an intrinsic difficulty in targeting respiratory tract pathogen.222 As a result, nebulization can assist to overcome the lack of equal distribution of the PS and reduce adverse effects by delivering the PS directly to the lung. Fine catheter is another vehicle to deliver the light through the cricothyroid membrane. This technique could be useful in COVIS-19 patients with tracheostomy, thereby, MB-PDT in a topical setting is safe without expected morbidity.223 Given the current treatment protocols for COVID-19 management, MB-mediated PDT could be a potential treatment modality for an early and advanced bronchopulmonary infection.
An in vivo animal study by Geralde et al, 224 has shown the efficiency of PDT protocol in eradicating bacterial pneumonia by using an extracorporeal illumination via a custom-made laser device contained 18 clusters of laser diodes, emitting monochromatic light of λ 780 nm at irradiance of 60 mW/cm2 and a total dose of 120 J/cm2 to activate nebulized indocyanine green (ICG) as PS. The ICG concentration of 100 μmol/L and 15 μL was instilled in each nostril of the mouse. The number of recruited infected mice with Streptococcus pneumoniae cells was ten. The authors observed no deaths in the PDT group, compared to 60% in the control group. In addition, the colony forming unit recovery ranged from 103–104/mouse in the control group, whilst no bacteria recovered in 80% of the animals in the PDT group. Therefore, the authors concluded that clinical implementation of this protocol (extracorporeally‐illuminated photodynamic inactivation of pneumonia) is a significant steping-stone toward PDT potential as a single therapy or as an adjunct to antibiotics treatment modality. Another in vivo animal study by Kassab et al, evaluated the viability of three photosensitizers (ICG, the chlorine photodithazine, and porphyrin photogem) in a jet nebulizer device, which was shown to be effective to target the lung directly.225
A randomized controlled trial/placebo study by Schikora et al, evaluated the effects of antimicrobial PDT (a-PDT) on following variables: SARS-CoV-2 viral load at early stages of the infection, viral load in the lung, inflammation and severe damage on the lung, clinical course of treatment and mortality rate.226 The following a-PDT protocol was utilized and applied on 300 participants who tested positive for COVID-19, whereas the placebo group (n=330) received sham. MB (1% solution of methylthioninium-chloride dissolved in a 5% glucose solution) was applied to flush the oral cavity and throat and it was sprayed in the nostrils: λ 660 nm photonic energy irradiation applied at power output of 240 mW for five minutes, at a fluence of 72 J/cm2 (total fluence 360 J/cm2). The PCR real time testing employed to determine the viral load pretreatment (baseline) and immediately after a-PDT. In addition, four weeks post a-PDT the patients were tested, using ELISA test to evaluate the formation of antibodies to SARS-CoV-2 virus. The results identified the following findings: (1) a reduction in mortality rate (0.7 in PDT and placebo group vs 3.3% in placebo group), (2) a significant reduction in the severity of the course of disease (2.6% vs 19%) and attenuation of COVID-19 progression (97% vs 81%) in PDT group. As a result, a reduction of viral load in throat, oral and nasal cavities revealed by PCR test immediately after each five-stage treatment cycle. Ultimately, no adverse effects were reported. In is important to mention that MB alone has no impact in reducing the viral load without photodynamic excitation with a light source. The authors concluded that a-PDT is an effective, innovative, accessible, cost-effective treatment modality in COVID-19 management without adverse effects. It is noteworthy that another significant application of PDT in COVID-19 pandemic is plasma inactivation of the SARS-CoV–2 to ensure safe blood transfusion.227
Several studies have reported successful treatments of coronaviruses by PDT, using riboflavin as PS.228,229 Riboflavin and UV light effectively reduced the titer of MERS-CoV in human plasma products, including the platelets to below the limit of detection, suggesting that the treatment process may reduce the risk of transfusion transmission of MERS-CoV.228,229 It is Important to note that a study employed λ 405 nm light can have antimicrobial properties to eliminate the infection risk and can be propagated despite commonly known approaches in PDT and PDI. The design of personal mobile devices for the purpose of biological war can be proposed and used in such a simple form as mobile application, which program LED to irradiate specific wavelengths. Moreover, in terms of viruses inactivation, light therapy with a range of blue wavelengths between λ 420 nm and λ 430 nm can inactivate the leukemia virus.200 In this context, the visible light of the electromagnetic spectrum contributes into the PDT of the plasma to inactivate many viruses such as herpes simplex (HSV-1) and HIV.213,230,231 It is noteworthy that the recent reports have shown that blue light in a pulsed emission mode was 20–100 times more potent than in continuous emission mode to inactivate the opportunistic bacterial infections.232,233 This light source could be of great potential, as an antiviral agent to inactivate SARS-CoV-2, which requires further investigations.
Based on the abovementioned science and evidenced-based practice, phototherapy can be utilized as a single treatment modality or as an adjunct to the standard treatment protocol/s to inactivate SARS-CoV-2 virus, decrease its viral load, and reverse the cytokine storm, which can assist in modulating the host immune system.
The Current Ongoing Phototherapy Registered RCTs for COVID-19 Management
For all the abovementioned, phototherapy deserves great attention from researchers and clinicians for future long-term RCTs in the management of COVID-19. This coincides with a recent critical review by Hanna et al, showed that phototherapy is a promising treatment modality in COVID-19 pandemic that demands to be validated by robust and rigorous randomized, double blind, placebo-controlled, clinical trials to evaluate its impartial outcomes and safety.234
Taking into consideration the abovementioned potential of phototherapy in COVID-19 management, at this stage only four RCTs have been registered at clinicaltrials.gov, which are either in the recruiting phase or not recruiting yet or completed phase without reported results (Table 4). These trials are highlighted below.
Table 4.
Shows the Clinical Trials Registered on ClinicalTrials.gov To October 20, 2020, Utilizing Phototherapy for the Treatment of COVID-19
| Photo-Therapy | Study Identifier and Citation (Superscript) | Laser Treatment Protocol | Study Phase; Estimated Enrolment (n) | Primary Outcome Measure(s) | Recruitment Status |
|---|---|---|---|---|---|
| PDT + PBMT | NCT04416113 | The patients will be divided into 3 equal groups. Group I will receive low level laser (diode laser 980 nm) from laser watch for 30 min, 20 J for 3 to 5 days and laser acupuncture. Group 2 will be treated with photodynamic therapy by injecting the methylene blue as a photosensitizer and irradiated with laser watch (diode laser 670 nm). Group 3 will serve as a control. Evaluation methods will include laboratory investigations and CT chest. | Phase: Not applicable n=60 |
Participants achieving either a major clinical response or partial clinical response (PCR) defined by WHO All patients will be subjected to thorough examination with complete personal and clinical history and will do the following before and after treatment: PCR, CBC, CRP, ESR, D dimers, liver enzymes, and ferritin, CXR. Endpoint treatment will be stopped if no improvement occurred after one week |
Active— Not recruiting yet |
| PBMT | NCT04386694 | PBMT combined with static magnetic field (sMF) (PBMT/sMF) on respiratory muscles of patients admitted to the Intensive Care Unit (ICU) with COVID-19 using invasive mechanical ventilation. The device has 4 diodes of 905 nm (1.25 mW each diode, 0.32 cm2 each), 8 diodes of 633 nm (25 mW each diode, 0.85 cm2 each), and 8 diodes of 850 nm (40 mW each diode, 0.56 cm2 each). The static magnetic field is 110 mT. PBMT/sMF application time will be 60 seconds per site. The dose used in the lower thorax will be 31.50 J per site, a total of 6 sites will be irradiated, totaling a dose of 189 J. In addition, the dose used in the neck area (bilaterally) will be 31.50 J per site, a total of 1 site (bilaterally) will be irradiated, totaling a dose of 31.50 J (bilaterally). PBMT/sMF will be applied using the direct contact method with light pressure on the skin. |
Phase: not applicable n=30 |
Time until discharge (time frame: from date of randomization until the date of discharge or date death from any cause, whichever came first, assessed up to 20 days)Number of days hospitalized in the ICU until discharge or death Survival rate, diaphragm muscle function Platelet count leukogram and erythrogram. C-reactive protein D-dimer Levels of immunoglobulin G and M, TNFα, and vitamin D Levels of positive end-expiratory pressure (PEEP), PO2, FiO2 |
Recruiting |
| NCT04524715 | PBMT: to reduce pneumonia inflammation by applying red light PBMT in respiratory system. | Phase: Not applicable n=64 |
Inflammation of lungs-O2 Inflammation of lungs-CRP Inflammation of lungs-IL6 |
Recruiting | |
| NCT04391712 | Multi-wave locked system (MLS) laser therapy for COVID-19 positive patients with pulmonary disease. Laser treatment was delivered when patient at a prone position, using a 10 by 25 cm laser field across each lung with 7 J/cm2 at 1500 Hz. The treatment frequency and duration: one daily for 4 days. |
Phase: II n=10 |
Patient disposition post treatment [time frame: 7 days]. ICU on vent, ICU not requiring ventilation, discharge to rehab requiring assistance, discharge home unable to perform ADLs, discharge to home able to perform ADLs. Oxygenation (time frame; daily for 4 days). Patients O2 requirements pulse oximetry evaluation for change from pre- and post-treatment and end of protocol IL-6 levels (time frame: first four days of trial). The change in pre-treatment and 24 h post final treatment. CXR results (time frame: 7 days). Pre-treatment CXR, compared to post treatment, using RALE CXR evaluation scale. -Brescia-COVID Respiratory Severity Scale (BCRSS) (time frame: 7 days). Change in the pre- and post-treatment evaluated with BCRSS. SMART-COP score (time frame: 7 days). Change between pre- and post-treatment. PSI score: change between pre- and post-treatment. CRP levels (time frame: 7 days). |
Completed (No study results posted on ClinicalTrials. gov for this study yet). |
An ongoing RCT clinical trial (not recruiting yet stage)235 aims to evaluate the effects of PBMT and PDT, compared to control in treating COVID-19 patients, in order to achieve a major clinical response or partial respond defined by WHO in a time-frame of two weeks (Table 4). This trial is including hospitalized patients with positive COVID-19 with one or a combination of the following symptoms; fever, cough, shortness of breath, chills, muscle pain, a new loss of taste or smell, vomiting or diarrhea and/or sore throat, whilst it is excluding critical cases admitted to ICU, respiratory distress grade III and IV, as well patients on mechanical ventilator. Laboratory investigations (molecular-based assays; serology and antigen) and CT chest utilized to evaluate the trial clinical outcomes. The 60 recruited patients would be randomized as follows: group I irradiates with λ 980 nm PBM from laser watch, which is applied on wrist on the radial artery for 30 min, 20 J for 3–5 days and laser acupuncture trigger points; group 2 receives PDT according to the following protocol, 670 nm laser watch, methylene blue (MB) as a photosensitizer (PS) of 0.1 to 0.2 mL (1% solution per kilogram of body weight) injected intravenously very slowly over a period of several minutes, after one hour, an application of light photonic dose: 100–200 J/cm2, 50–100 mW/cm2 (50 mW/cm2 increased the phototoxic response as well as the fractionated light application) twice per week; whilst group 3 serves as a control (conventional treatment protocol).
In terms of PBMT, there are two ongoing RCTs that are in the recruiting stage (Table 4).236,237 One of these RCTs aims to evaluate the effects of LED PBMT on lung inflammation in COVID-19 patients.237 Sixty-four participants above 18-years-old diagnosed with COVID-19 and exhibiting moderate-acute respiratory distress were recruited, whilst participants with photosensitive condition or medication, active chemotherapy treatment or other cancer treatment, autoimmune disorders, as well pregnant or possibly pregnant or planning pregnancy prior to the end of study participation would be excluded. In this trial, LED light source in the red and IR range of PBMT is utilized. All participants continue receiving standard treatments for COVID-19 symptoms. This trial aims to determine if a reduction of pneumonic inflammation occurs after PBMT, applying red-light LED source on the respiratory system of COVID-19 patients, suffering from acute viral pneumonia. The protocol of PBMT was not recorded. The following methods of assessment of outcomes are SpO2, CRP, and IL-6 levels. Alternatively, a randomized, triple-blind, placebo-controlled trial is registered (not recruiting yet)237 to investigate the effects LEDs PBMT combined with static magnetic field (sMF) (LEDs PBMT/sMF) on respiratory muscles of patients admitted to the ICU with COVID-19, using invasive mechanical ventilation. The laser protocol described in Table 1 shows that a clustered probe of eight diodes of λ 850 nm, eight diodes of λ 633 nm and four diodes of λ 905 nm. This indicates that the light would interact with tissue at various depth of penetration to achieve optimal outcome. Various variables would be assessed (Table 4) to determine whether this combined therapy has an influence in minimizing the impact of COVID-19 on the respiratory muscles, accelerating the ventilatory weaning process and optimizing the functional capacity of the involved muscles.
One completed RCT238 evaluated the effectiveness of multiwave locked system (MLS) laser therapy, as a treatment for pulmonary complications due to COVID-19 infection. Laser treatment was delivered with patients in a prone position, using a 10 by 25 cm laser field across each lung with 7 J/cm2 at 1500 Hz. The treatment frequency and duration: once daily for four days (Table 4). However, no study results are posted on clinicaltrials.gov yet.
It is noteworthy that despite the scientific evidence-based research consistently suggesting the potential of PDT in COVID-19 which is intensively emphasized in our review, only one RCT to date is registered to evaluate the effects of PDT combined with PBMT in COVID-19 management. Further studies are warranted to confirm the study design of an ideal photodynamic protocol. Moreover, the authors of the present review observed that phototherapy protocols for each stage (early and medium-severe) of COVID-19 are warranted to justify therapeutic outcomes and ensure safety.
Conclusion
This review has produced a high quality evidence, which can be utilized by the clinical and the scientific community for future reference, as the knowledge and understanding the virus SARS-CoV-2 are evolving, in terms of its epidemiological and clinical manifestations, which ultimately map the strategic path toward an effective and safe treatment and production of potent and safe vaccine. To date, there is neither a reliable safe vaccine nor an effective safe treatment modality, specifically designed to treat COVID-19. There are novel therapeutic innovations such as immunotherapy and cellular therapy, which require further investigation. Phototherapy is a well-documented treatment modality, which can play a crucial role in COVID-19 management. However, further clinical research is mandatory in this regard.
Funding Statement
This work was funded by National Research Development Projects to finance excellence (PFE)-37/2018- 2020 granted by the Romanian Ministry of Research and Innovation.
Disclosure
The authors have declared no conflicts of interest in this work.
References
- 1.Available from: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed October20, 2020.
- 2.Lin L, Lu LF, Cao W, et al. Hypothesis for potential pathogenesis of SARS-CoV-2 infection–a review of immune changes in patients with viral pneumonia. Emerg Microb Infect. 2020;9(1):727–732. doi: 10.1080/22221751.2020.1746199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mason RJ. Pathogenesis of COVID-19 from a cell biology perspective. Eur Respir J. 2020;55(4):2000607. doi: 10.1183/13993003.00607-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention [published online ahead of print, 2020 Feb 24]. JAMA. 2020;10.1001/jama.2020.2648. doi: 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 5.Wrapp D, Wang N, Corbett KS, et al. Cryo- EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3(1):237–261. doi: 10.1146/annurev-virology-110615-042301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gui M, Song W, Zhou H, et al. Cryo-electron microscopy structures of the SARS-CoV spike glycoprotein reveal a prerequisite conformational state for receptor binding. Cell Res. 2017;27(1):119–129. doi: 10.1038/cr.2016.152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walls AC, Xiong X, Park YJ, et al. Unexpected receptor functional mimicry elucidates activation of coronavirus fusion. Cell. 2019;176(5):1026–39.e15. doi: 10.1016/j.cell.2018.12.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Available from: https://coronavirus.jhu.edu/data/racial-data-transparency. Accessed October20, 2020.
- 12.Available from: https://clinicaltrials.gov/ct2/results?cond=COVID-19. Accessed October20, 2020.
- 13.Mackenzie JS, Smith DW. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol Aust. 2020;MA20013. doi: 10.1071/MA20013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascella M, Rajnik M, Cuomo A, et al. Features, evaluation, and treatment of coronavirus (COVID-19) In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 16.Lam TT, Shum MH, Zhu HC, et al. Identifying SARS-CoV-2 related coronaviruses in Malayan pangolins. Nature. 2020;583(7815):282–285. doi: 10.1038/s41586-020-2169-0 [DOI] [PubMed] [Google Scholar]
- 17.Jin Y, Yang H, Ji W, et al. Virology, epidemiology, pathogenesis, and control of COVID-19. Viruses. 2020;12(4):372. doi: 10.3390/v12040372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Doremalen N, Bushmaker T, Morris DH. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382(16):1564–1567. doi: 10.1056/NEJMc2004973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salehi S, Abedi A, Balakrishnan S, et al. Coronavirus disease 2019 (COVID- 19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol. 2020:1–7. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 20.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Liu SM, Yu XH, et al. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrobial Agents. 2020;55(5):105951. doi: 10.1016/j.ijantimicag.2020.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html. Accessed October20, 2020.
- 23.Thevarajan I, Buising KL, Cowie BC. Clinical presentation and management of COVID-19. Med J Aust. 2020;213(3):134–139. doi: 10.5694/mja2.50698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ho JC, Chan KN, Hu WH, et al. The effect of aging on nasal mucociliary clearance, beat frequency, and ultrastructure of respiratory cilia. Am J Respir Crit Care Med. 2001;163:983–988. doi: 10.1164/ajrccm.163.4.9909121 [DOI] [PubMed] [Google Scholar]
- 25.Epidemiology Working Group for NCIP Epidemic Response. Chinese center for disease control and prevention. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145–151. doi: 10.3760/cma.j.issn.0254-6450.2020.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Di Stadio A, Ricci G, Greco A, et al. Mortality rate and gender differences in COVID-19 patients dying in Italy: a comparison with other countries. Eur Rev Med Pharmacol Sci. 2020;24(8):4066–4067. doi: 10.26355/eurrev-202004-20980 [DOI] [PubMed] [Google Scholar]
- 27.Jin JM, Bai P, He W, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen H, Guo J, Wang C, et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: a retrospective review of medical records. Lancet. 2020;395(10226):809–815. doi: 10.1016/S0140-6736(20)30360-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones VG, Mills M, Suarez D, et al. COVID-19 and Kawasaki disease: novel virus and novel case. Hosp Pediatr. 2020;10(6):537–540. doi: 10.1542/hpeds.2020-0123 [DOI] [PubMed] [Google Scholar]
- 30.Esper FED, Shapiro C, Weibel D, et al. Association between a novel human coronavirus and Kawasaki disease. J Infect Dis. 2005;191(4):499–502. doi: 10.1086/428291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Available from: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/index.html. Accessed October20, 2020.
- 32.Curtis N, Sparrow A, Ghebreyesus TA, et al. Considering BCG vaccination to reduce the impact of COVID-19. Lancet. 2020;395(10236):1545–1546. doi: 10.1016/S0140-6736(20)31025-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu CW, Liu XF, Jia ZF. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395(10224):e39. doi: 10.1016/S0140-6736(20)30313-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia J, Tong J, Liu M, et al. Evaluation of coronavirus in tears and conjunctival secretions of patients with SARS-CoV-2 infection. J Med Virol. 2020;92(6):589–594. doi: 10.1002/jmv.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lauer SA, Grantz KH, Bi Q, et al. The incubation period of coronavirus disease 2019 (COVID-19) from publicly reported confirmed cases: estimation and application. Ann Intern Med. 2020;172(9):577–582. doi: 10.7326/M20-0504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Novel coronavirus (2019-ncov) situation report-7. World Health Organization (WHO) 2020. January 27 Accessed October20, 2020. [Google Scholar]
- 37.China’s national health commission news conference on coronavirus. Al Jazeera. 2020. January 26 Accessed October20, 2020.
- 38.Symptoms of novel coronavirus (2019-nCoV)-CDC. Accessed October20, 2020.
- 39.Varia M, Wilson S, Sarwal S, et al. Investigation of a nosocomial outbreak of severe acute respiratory syndrome (SARS) in Toronto, Canada. CMAJ. 2003;169:285–292. [PMC free article] [PubMed] [Google Scholar]
- 40.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quilty BJ, Clifford S, Flasche S, et al. Effectiveness of airport screening at detecting travellers infected with novel coronavirus (2019-nCoV). Euro Surveill. 2020;25(5):2000080. doi: 10.2807/1560-7917.ES.2020.25.5.2000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coronavirus incubation could be as long as 27 days, Chinese provincial government says. Reuters. 2020. February 22 Accessed October20, 2020.
- 44.Xu Z, Shi L, Wang Y, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200(3):282–289. doi: 10.1002/path.1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deshmukh V, Motwani R, Kumar A, et al. Histopathological observations in COVID-19: a systematic review. J Clin Pathol. 2020:jclinpath-2020-206995. doi: 10.1136/jclinpath-2020-206995. [DOI] [PubMed] [Google Scholar]
- 47.Chung M, Bernheim A, Mei X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 2020;295(1):202–207. doi: 10.1148/radiol.2020200230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li W, Cui H, Li K, et al. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50(6):796–799. doi: 10.1007/s00247-020-04656-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25(3):2000045. doi: 10.2807/1560-7917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carver C, Jones N. Comparative accuracy of oropharyngeal and nasopharyngeal swabs for diagnosis of COVID-19. Oxford COVID-19 evidence service team centre for evidence-based medicine; 2020. Available from: https://www.cebm.net/covid-19/comparative-accuracy-of-oropharyngeal-and-nasopharyngeal-swabs-for-diagnosis-of-covid-19/. Accessed October20, 2020.
- 52.Wang W, Xu Y, Gao R, et al. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.To KK, Tsang OT, Yip CC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020;71(15):841–843. doi: 10.1093/cid/ciaa149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lipsitch M, Kahn R, Mina MJ. Antibody testing will enhance the power and accuracy of COVID-19 prevention trials. Nat Med. 2020;26(6):818–819. doi: 10.1038/s41591-020-0887-3 [DOI] [PubMed] [Google Scholar]
- 55.Zhang F, Abudayyeh OO, Gootenberg JS. A Protocol for Detection of COVID-19 Using CRISPR Diagnostics. Cambridge, MA: Broad Institute, MIT; 2020. [Google Scholar]
- 56.Bosch BJ, van der Zee R, de Haan CA, et al. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J Virol. 2003;77(16):8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yuan Y, Cao D, Zhang Y, et al. Cryo-EM structures of MERS-CoV and SARS-CoV spike glycoproteins reveal the dynamic receptor binding domains. Nat Commun. 2017;8(1):15092. doi: 10.1038/ncomms15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoffmann M, Kleine-Weber H, Schroeder S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Braciale TJ, Hahn YS. Immunity to viruses. Immunol Rev. 2013;255(1):5–12. doi: 10.1111/imr.12109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li W, Moore MJ, Vasilieva N, et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426(6965):450–454. doi: 10.1038/nature02145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zou X, Chen K, Zou J, et al. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front Med. 2020;14(2):185–192. doi: 10.1007/s11684-020-0754-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sungnak W, Huang N, Bécavin C, et al. HCA lung biological network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das SK. The pathophysiology, diagnosis and treatment of corona virus disease 2019 (COVID-19). Indian J Clin Biochem. 2020;35(4):1–12. doi: 10.1007/s12291-020-00919-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wiersinga WJ, Rhodes A, Cheng AC, et al. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324(8):782–793. doi: 10.1001/jama.2020.12839 [DOI] [PubMed] [Google Scholar]
- 65.Mancia G, Rea F, Ludergnani M, et al. Renin-angiotensin-aldosterone system blockers and the risk of COVID-19. N Engl J Med. 2020;382(25):2431–2440. doi: 10.1056/NEJMoa2006923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fosbøl EL, Butt JH, Østergaard L, et al. Association of angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use with COVID-19 diagnosis and mortality. JAMA. 2020;324(2):168–77. doi: 10.1001/jama.2020.11301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 cytokine storm; what we know so far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shimizu M. Clinical features of cytokine storm syndrome In: Cron R, Behrens E, editors. Cytokine Storm Syndrome. Cham: Springer; 2019:31–42. doi: 10.1007/978-3-030-22094-5_3 [DOI] [Google Scholar]
- 69.Fletcher-Sandersjöö A, Bellander BM. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review [published online ahead of print, 2020 Jun 18]. Thromb Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lai C, Shih T, Ko W, Tang H, Hsueh P-R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ishikawa T. Clinical preparedness for cytokine storm induced by the highly pathogenic H5N1 influenza virus. J Pharmacogenom Pharmacoproteomics. 2012;3(6):1000e131. doi: 10.4172/2153-0645.1000e131 [DOI] [Google Scholar]
- 73.Task Force BM, BASE Medicine Task Force. COVID-19: facts and recommendations from A to Z. Sci Insight. 2020;33(1):138–158. doi: 10.15354/si.20.re061 [DOI] [Google Scholar]
- 74.Yousefifard M, Zali A, Mohamed Ali K, et al. Antiviral therapy in management of COVID-19: a systematic review on current evidence. Arch Acad Emerg Med. 2020;8(1):e45. [PMC free article] [PubMed] [Google Scholar]
- 75.Scagnolari C, Vicenzi E, Bellomi F, et al. Increased sensitivity of SARS-coronavirus to a combination of human type I and type II interferons. Antivir Ther. 2004;9:1003–1011. [PubMed] [Google Scholar]
- 76.Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med. 2006;3(9):e343. doi: 10.1371/journal.pmed.0030343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chan JF, Yao Y, Yeung ML, et al. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913. doi: 10.1093/infdis/jiv392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chen F, Chan KH, Jiang Y, et al. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol. 2004;31(1):69–75. doi: 10.1016/j.jcv.2004.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290(24):3222–3228. doi: 10.1001/jama.290.24.3222 [DOI] [PubMed] [Google Scholar]
- 80.Jr SB, Mossel EC, Peters CJ, et al. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology. 2004;329(1):11–17. doi: 10.1016/j.virol.2004.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhou Q, Chen V, Shannon CP, et al. Interferon-α2b treatment for COVID-19. Front Immunol. 2020;11:1061. doi: 10.3389/fimmu.2020.01061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Available from: https://www.covid19treatmentguidelines.nih.gov/immune-basedtherapy/immunomodulators/interferons/. Accessed October20, 2020.
- 83.Adaptive COVID-19 treatment trial 3 (ACTT-3). Available from: https://clinicaltrials.gov/ct2/show/NCT04492475?term=NCT04492475&draw=2&rank=1. Accessed October20, 2020.
- 84.Pegylated interferon lambda treatment for COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04343976?term=NCT04343976&draw=2&rank=1. Accessed October20, 2020.
- 85.An investigation into beneficial effects of interferon beta 1a, compared to interferon beta 1b and the base therapeutic regiment in moderate to severe COVID-19: a randomized clinical trial (COVIFERON). Available from: https://clinicaltrials.gov/ct2/show/NCT04343768?term=NCT04343768&draw=2&rank=1. Accessed October20, 2020.
- 86.LiverTox. Clinical and Research Information on Drug-Induced Liver Injury. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases; 2012. [PubMed] [Google Scholar]
- 87.Wenzel RP, Edmond MB. Managing SARS amidst uncertainty. N Engl J Med. 2003;348(20):1947–1948. doi: 10.1056/NEJMp030072 [DOI] [PubMed] [Google Scholar]
- 88.Morgenstern B, Michaelis M, Baer PC, et al. Ribavirin and interferon-beta synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun. 2005;326(4):905–908. doi: 10.1016/j.bbrc.2004.11.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Khalili JS, Zhu H, Mak NSA, et al. Novel coronavirus treatment with ribavirin: groundwork for evaluation concerning COVID-19. J Med Virol. 2020;92(7):740–746. doi: 10.1002/jmv.25798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Wit E, Feldmann F, Cronin J, et al. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci. 2020;117(12):6771–6776. doi: 10.1073/pnas.1922083117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269–271. doi: 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929–936. doi: 10.1056/NEJMoa2001191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of covid-19 - final report [published online ahead of print, 2020 Oct 8]. N Engl J Med. 2020:NEJMoa2007764. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395(10236):1569–1578. doi: 10.1016/S0140-6736(20)31022-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Adaptive COVID-19 treatment trial (ACTT). Available from: https://clinicaltrials.gov/ct2/show/NCT04280705?term=NCT04280705&draw=2&rank=1. Accessed October20, 2020.
- 96.Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with severe coronavirus disease (COVID-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04292899?term=NCT04292899&draw=2&rank=1. Accessed October20, 2020.
- 97.Study to evaluate the safety and antiviral activity of remdesivir (GS-5734™) in participants with moderate coronavirus disease (COVID-19) compared to standard of care treatment. Available from: https://clinicaltrials.gov/ct2/show/NCT04292730?term=NCT04292730&draw=2&rank=1. Accessed October20, 2020.
- 98.A study to evaluate the efficacy and safety of remdesivir plus tocilizumab compared with remdesivir plus placebo in hospitalized participants with severe COVID-19 pneumonia (REMDACTA). Available from: https://clinicaltrials.gov/ct2/show/NCT04409262?term=NCT04409262&draw=2&rank=1. Accessed October20, 2020.
- 99.Lv Z, Chu Y, Wang Y. HIV protease inhibitors: a review of molecular selectivity and toxicity. HIV AIDS (Auckl). 2015;7:95–104. doi: 10.2147/HIV.S79956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim UJ, Won EJ, Kee SJ, et al. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-alpha for Middle East respiratory syndrome. Antivir Ther. 2016;21(5):455–459. doi: 10.3851/IMP3002 [DOI] [PubMed] [Google Scholar]
- 101.Lin S, Shen R, Guo X. Molecular modeling evaluation of the binding abilities of ritonavir and lopinavir to Wuhan pneumonia coronavirus proteases. bioRxiv. 2020. doi: 10.1101/2020.01.31.929695 [DOI] [Google Scholar]
- 102.Yamamoto N, Yang R, Yoshinaka Y, et al. HIV protease inhibitor nelfinavir inhibits replication of SARS-associated coronavirus. Biochem Biophys Res Commun. 2004;318(3):719–725. doi: 10.1016/j.bbrc.2004.04.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yamamoto N, Matsuyama S, Hoshino T, et al. Nelfinavir inhibits replication of severe acute respiratory syndrome coronavirus 2 in vitro. bioRxiv. 2020. doi: 10.1101/2020.04.06.026476 [DOI] [Google Scholar]
- 104.Randomised evaluation of COVID-19 therapy (recovery). Available from: https://clinicaltrials.gov/ct2/show/NCT04381936?term=NCT04381936&draw=2&rank=1. Accessed October20, 2020.
- 105.Khamitov RA, Loginova S, Shchukina VN, et al. Antiviral activity of arbidol and its derivatives against the pathogen of severe acute respiratory syndrome in the cell cultures. Vopr Virusol. 2008;53:9–13. [PubMed] [Google Scholar]
- 106.Huang L, Zhang L, Liu Y, et al. Arbidol for preventing and treating influenza in adults and children. Cochrane Database Syst Rev. 2017;2017(2):CD011489. doi: 10.1002/14651858.CD011489.pub2 [DOI] [Google Scholar]
- 107.Zhang J, Zhou L, Yang Y, et al. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir Med. 2020;8(3):e11–e12. doi: 10.1016/S2213-2600(20)30071-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhu Z, Lu Z, Xu T, et al. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clinical study of arbidol hydrochloride tablets in the treatment of pneumonia caused by novel coronavirus. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04260594?term=arbidol&cond=covid+19&draw=2&rank=3. Accessed October20, 2020.
- 110.Savarino A, Boelaert JR, Cassone A, et al. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3(11):722–727. doi: 10.1016/s1473-3099(03)00806-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yan Y, Zou Z, Sun Y, et al. Anti-malaria drug chloroquine is highly effective in treating avian influenza A H5N1 virus infection in an animal model. Cell Res. 2013;23(2):300–302. doi: 10.1038/cr.2012.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cortegiani A, Ingoglia G, Ippolito M, et al. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J Crit Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dexamethasone treatment for severe acute respiratory distress syndrome induced by COVID-19 (DHYSCO). Available from: https://clinicaltrials.gov/ct2/show/NCT04347980?term=NCT04347980&draw=2&rank=1. Accessed October20, 2020.
- 114.Randomized clinical trial for the prevention of sars-cov-2 infection (COVID-19) in healthcare personnel (EPICOS). Available from: https://clinicaltrials.gov/ct2/show/NCT04334928?term=NCT04334928&draw=2&rank=1. Accessed October20, 2020.
- 115.Available from: http://chemocare.com/chemotherapy/drug-info/dexamethasone.aspx. Accessed October20, 2020.
- 116.Available from: https://www.nice.org.uk/guidance/ng159/resources/covid19-prescribing-briefing-corticosteroids-pdf-8839913581. Accessed October20, 2020.
- 117.Mahase E. Covid-19: hydrocortisone can be used as alternative to dexamethasone, review finds. BMJ. 2020;370:m3472. doi: 10.1136/bmj.m3472 [DOI] [PubMed] [Google Scholar]
- 118.Randomized clinical trial of intranasal dexamethasone as an adjuvant in patients with COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04513184?term=NCT04513184&draw=2&rank=1. Accessed October20, 2020.
- 119.Higher vs. lower doses of dexamethasone for COVID-19 and severe hypoxia (COVIDSTEROID2). Available from: https://clinicaltrials.gov/ct2/show/NCT04509973?term=NCT04509973&draw=2&rank=1. Accessed October20, 2020.
- 120.Available from: https://www.popsci.com/story/health/convalescent-plasma-covid-19-coronavirus/. Accessed October20, 2020.
- 121.Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. 2012;52(Suppl 1):65S–79S. doi: 10.1111/j.1537-2995.2012.03663.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357(14):1450–1451. doi: 10.1056/NEJMc070359 [DOI] [PubMed] [Google Scholar]
- 123.van Griensven J, Edwards T, de Lamballerie X, et al. Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med. 2016;374(1):33–42. doi: 10.1056/NEJMoa1511812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cheng Y, Wong R, Soo YO, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24(1):44–46. doi: 10.1007/s10096-004-1271-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Duan K, Liu B, Li C, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. 2020;117(17):9490. doi: 10.1073/pnas.2004168117:202004168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang C, Li W, Drabek D, et al. A human monoclonal antibody blocking SARS-CoV-2 infection. Nat Commun. 2020;11(2251):1–6. doi: 10.1038/s41467-020-16256-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Guaraldi G, Meschiari M, Cozzi-Lepri A, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study [published correction appears in lancet rheumatol. 2020;2(10):e591]. Lancet Rheumatol. 2020;2(8):e474–e484. doi: 10.1016/S2665-9913(20)30173-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Available from: https://www.versusarthritis.org/about-arthritis/treatments/drugs/tocilizumab/. Accessed October20, 2020.
- 129.Sheppard M, Laskou F, Stapleton PP, et al. Tocilizumab (Actemra). Hum Vaccin Immunother. 2017;13(9):1972–1988. doi: 10.1080/21645515.2017.1316909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lan SH, Lai CC, Huang HT, et al. Tocilizumab for severe COVID-19: a systematic review and meta-analysis. Int J Antimicrob Agents. 2020;56(3):106103. doi: 10.1016/j.ijantimicag.2020.106103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Available from: https://www.drugs.com/kineret.html. Accessed October20, 2020.
- 132.Huet T, Beaussier H, Voisin O, et al. Anakinra for severe forms of COVID-19: a cohort study. Lancet Rheumatol. 2020;2(7):e393–e400. doi: 10.1016/S2665-9913(20)30164-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Favipiravir combined with tocilizumab in the treatment of corona virus disease; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT04310228. Accessed October20, 2020.
- 134.A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA). Available from: https://clinicaltrials.gov/ct2/show/NCT04320615. Accessed October20, 2020.
- 135.Tocilizumab in COVID-19 pneumonia (TOCIVID-19) (TOCIVID-19). Available from: https://clinicaltrials.gov/ct2/show/NCT04317092. Accessed October20, 2020.
- 136.Tocilizumab for SARS-CoV2 (COVID-19) severe pneumonitis. Available from: https://clinicaltrials.gov/ct2/show/NCT04315480. Accessed October20, 2020.
- 137.Anti-il6 treatment of serious COVID-19 disease with threatening respiratory failure (TOCIVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04322773. Accessed October20, 2020.
- 138.CORIMUNO-19-tocilizumab trial-TOCI (CORIMUNO-TOCI) (CORIMUNO-TOC). Available from: https://clinicaltrials.gov/ct2/show/NCT04331808. Accessed October20, 2020.
- 139.Tocilizumab improves significantly clinical outcomes of patients with moderate or severe COVID-19 pneumonia. Available from: https://pipelinereview.com/index.php/2020042874458/Antibodies/Tocilizumab-improves-significantly-clinical-outcomes-of-patients-with-moderate-or-severe-COVID-19-pneumonia.html. Accessed October20, 2020.
- 140.Press release. First randomized study favorable to tocilizumab in Covid-19, in France. Available from: https://www.apmnews.com/depeche/0/350489/premiere-etude-randomisee-favorable-au-tocilizumab-dans-le-covid-19%2C-en-france. Accessed October20, 2020.
- 141.Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04315298. Accessed October20, 2020.
- 142.Erman M, Joseph S. Regeneron, Sanofi arthritis drug may only help critical coronavirus patients: study. Available from: https://www.reuters.com/article/us-health-coronavirus-regeneron-pharms/regeneron-sanofi-to-treat-only-critical-covid-19-patients-with-arthritis-drug-idUSKCN2291OD. Accessed October20, 2020.
- 143.Cohort multiple randomized controlled trials open-label of immune modulatory drugs and other treatments in COVID-19 patients—sarilumab trial—CORIMUNO-19—SARI (CORIMUNO-SARI). Available from: https://clinicaltrials.gov/ct2/show/study/NCT04324073. Accessed October20, 2020.
- 144.Efficacy and safety of siltuximab vs. corticosteroids in hospitalized patients with COVID-19 pneumonia. Available from: https://clinicaltrials.gov/ct2/show/record/NCT04329650?cond=siltuximab+covid+19&draw=2&rank=1. Accessed October20, 2020.
- 145.Clinical trial of the use of anakinra in cytokine storm syndrome secondary to Covid-19 (ANA-COVID-GEAS) (ANA-COVID-GEAS). Available from: https://clinicaltrials.gov/ct2/show/NCT04443881?term=NCT04443881&draw=2&rank=1. Accessed October20, 2020.
- 146.Available from: https://www.recoverytrial.net/news/recovery-covid-19-phase-3-trial-to-evaluate-regeneron2019s-regn-cov2-investigational-antibody-cocktail-in-the-uk. Accessed October20, 2020.
- 147.Available from: https://investor.regeneron.com/news-releases/news-release-details/regenerons-regn-cov2-antibody-cocktail-reduced-viral-levels-and. Accessed October20, 2020.
- 148.Lo Caputo S, Corso G, Clerici M, et al. Baricitinib: a chance to treat COVID-19? [published online ahead of print, 2020 May 21]. J Med Virol. 2020:10.1002/jmv.26033. doi: 10.1002/jmv.26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Recombinant bacterial ACE2 receptors -like enzyme of B38-CAP could be promising COVID-19 infection- and lung injury preventing drug better than recombinant human ACE2 (bacterial ACE2). Available from: https://clinicaltrials.gov/ct2/show/NCT04375046?term=NCT04375046&draw=2&rank=1. Accessed October20, 2020.
- 150.Combination of recombinant bacterial ACE2 receptors -like enzyme of B38-CAP and isotretinoin could be promising COVID-19 infection- and lung injury preventing drug better than recombinant human ACE2. Available from: https://clinicaltrials.gov/ct2/show/NCT04382950?term=NCT04382950&draw=2&rank=1. Accessed November23, 2020.
- 151.Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated pneumonia. (COVID-19EXO). Available from: https://clinicaltrials.gov/ct2/show/NCT04491240?term=NCT04491240&draw=2&rank=1. Accessed October20, 2020.
- 152.COVID-19 specific T cell derived exosomes (CSTC-Exo). Available from: https://clinicaltrials.gov/ct2/show/NCT04389385?term=NCT04389385&draw=2&rank=1. Accessed October20, 2020.
- 153.Zofin (organicell flow) for patients with COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04384445?term=NCT04384445&draw=2&rank=1. Accessed October20, 2020.
- 154.A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia. Available from: https://clinicaltrials.gov/ct2/show/NCT04276987?term=NCT04276987&draw=2&rank=1. Accessed October20, 2020.
- 155.Administration of allogenic UC-MSCs as adjuvant therapy for critically-ill COVID-19 patients. Available from: https://clinicaltrials.gov/ct2/show/NCT04457609?term=NCT04457609&draw=1&rank=1. Accessed October20, 2020.
- 156.Renin-angiotensin system inhibitors and COVID-19 (SARS-RAS). Available from: https://clinicaltrials.gov/ct2/show/NCT04331574?term=NCT04331574&draw=2&rank=1. Accessed October20, 2020.
- 157.ACE inhibitors or ARBs discontinuation in context of SARS-CoV-2 pandemic (ACORES-2). Available from: https://clinicaltrials.gov/ct2/show/NCT04329195?term=NCT04329195&draw=1&rank=1. Accessed October20, 2020.
- 158.Stopping ACE-inhibitors in COVID-19 (ACEI-COVID). Available from: https://clinicaltrials.gov/ct2/show/NCT04353596?term=NCT04353596&draw=2&rank=1. Accessed October20, 2020.
- 159.TCM differentiation and treatment protocol of COVID-19 (TDATPOC). Available from: https://clinicaltrials.gov/ct2/show/NCT04306497?term=NCT04306497&draw=2&rank=1. Accessed October20, 2020.
- 160.Treatment and prevention of traditional Chinese medicines (TCMs) on COVID-19 infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04251871?term=NCT04251871&draw=2&rank=1. Accessed October20, 2020.
- 161.Pharmacologic ascorbic acid as an activator of lymphocyte signaling for COVID-19 treatment. Available from: https://clinicaltrials.gov/ct2/show/NCT04363216?term=NCT04363216&draw=2&rank=1. Accessed October20, 2020.
- 162.Use of ascorbic acid in patients with COVID 19. Available from: https://clinicaltrials.gov/ct2/show/NCT04323514?term=NCT04323514&draw=2&rank=1. Accessed October20, 2020.
- 163.Vitamin D status and immune-inflammatory status in different UK populations with COVID-19 infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04519034?term=NCT04519034&draw=2&rank=1. Accessed October20, 2020.
- 164.Anti-inflammatory/antioxidant oral nutrition supplementation in COVID-19 (ONSCOVID19). Available from: https://clinicaltrials.gov/ct2/show/NCT04323228?term=NCT04323228&draw=2&rank=1.Accessed October20, 2020.
- 165.A phase II, controlled clinical study designed to evaluate the effect of artemiC in patients diagnosed with COVID-19. Available from: https://clinicaltrials.gov/ct2/show/NCT04382040?term=NCT04382040&draw=2&rank=1. Accessed October20, 2020.
- 166.Honey & nigella sativa trial against COVID-19 (HNS-COVID-PK). Available from: https://clinicaltrials.gov/ct2/show/NCT04347382?term=NCT04347382&draw=2&rank=1. Accessed October20, 2020.
- 167.Efficacy of natural honey treatment in patients with novel coronavirus. Available from: https://clinicaltrials.gov/ct2/show/NCT04323345?term=NCT04323345&draw=2&rank=1. Accessed October20, 2020.
- 168.The role of resistant starch in COVID-19 infection. Available from: https://clinicaltrials.gov/ct2/show/NCT04342689?term=NCT04342689&draw=2&rank=1. Accessed October20, 2020.
- 169.Gheblawi M, Wang K, Viveiros A, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hassanpour M, Rezaie J, Nouri M, et al. The role of extracellular vesicles in COVID-19 virus infection [published online ahead of print, 2020 Jun 13]. Infect Genet Evol. 2020;85:104422. doi: 10.1016/j.meegid.2020.104422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li X, Corbett AL, Taatizadeh E, et al. Challenges and opportunities in exosome research-perspectives from biology, engineering, and cancer therapy. APL Bioeng. 2019;3(1):011503. doi: 10.1063/1.5087122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: from mechanisms to potential therapeutic tools. Virol Sin. 2020;35(3):266. doi: 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhong J, Tang J, Ye C, et al. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2(7):e428–e436. doi: 10.1016/S2665-9913(20)30120-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Barochiner J, Martínez R. Use of inhibitors of the renin-angiotensin system in hypertensive patients and COVID-19 severity: a systematic review and meta-analysis [published online ahead of print, 2020 Aug 7]. J Clin Pharm Ther. 2020;10.1111/jcpt.13246. doi: 10.1111/jcpt.13246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chen H, Du Q. Potential natural compounds for preventing 2019-nCoV infection. Preprints. 2020;2020010358. doi: 10.20944/preprints202001.0358.v3 [DOI] [Google Scholar]
- 176.Ren JL, Zhang AH, Wang XJ. Traditional Chinese medicine for COVID-19 treatment [published correction appears in pharmacol res. 2020 Mar 25;:104768]. Pharmacol Res. 2020;155:104743. doi: 10.1016/j.phrs.2020.104743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang Y, Zeng X, Zhao Y, et al. The pros and cons of traditional Chinese medicines in the treatment of COVID-19. Pharmacol Res. 2020;157:104873. doi: 10.1016/j.phrs.2020.104873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gray PE, Belessis Y. The use of traditional Chinese medicines to treat SARS-CoV-2 may cause more harm than good. Pharmacol Res. 2020;156:104776. doi: 10.1016/j.phrs.2020.104776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yoshihara D, Fujiwara N, Suzuki K. Antioxidants: benefits and risks for long-term health. Maturitas. 2010;67(2):103–107. doi: 10.1016/j.maturitas.2010.05.001 [DOI] [PubMed] [Google Scholar]
- 180.Available from: https://www.webmd.com/drugs/2/drug-15583/antioxidant-vitamins-oral/details/list-sideeffects. Accessed October20, 2020.
- 181.Zahedipour F, Hosseini SA, Sathyapalan T, et al. Potential effects of curcumin in the treatment of COVID-19 infection [published online ahead of print, 2020 May 19]. Phytother Res. 2020:10.1002/ptr.6738. doi: 10.1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Available from: https://www.nutraingredients-asia.com/Article/2020/04/20/Can-honey-omega-3-resistant-potato-starch-help-fight-COVID-19-Researchers-to-examine-via-clinical-trials. Accessed October20, 2020.
- 183.Rogero MM, Leão MC, Santana TM, et al. Potential benefits and risks of omega-3 fatty acids supplementation to patients with COVID-19. Free Radic Biol Med. 2020;156:190–199. doi: 10.1016/j.freeradbiomed.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Available from: https://www.webmd.com/a-to-z-guides/manuka-honey-medicinal-uses#1. Accessed October20, 2020.
- 185.Available from: https://atlasbiomed.com/blog/potato-resistant-starch/. Accessed October20, 2020.
- 186.Hossain K, Hossain M, Moni A, et al. Prospects of honey in fighting against COVID-19: pharmacological insights and therapeutic promises. OSF Preprints. 2020. doi: 10.31219/osf.io/w3hqu [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Heiskanen V, Hamblin MR. Photobiomodulation: lasers vs. light emitting diodes? Photochem Photobiol Sci. 2018;17(8):1003–1017. doi: 10.1039/c8pp90049c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Solmaz H, Ulgen Y, Gulsoy M. Photobiomodulation of wound healing via visible and infrared laser irradiation. Lasers Med Sci. 2017;32(4):903–910. doi: 10.1007/s10103-017-2191-0 [DOI] [PubMed] [Google Scholar]
- 189.Gavish L, Houreld NN. Therapeutic efficacy of home-use photobiomodulation devices: a systematic literature review. Photomed Laser Surg. 2019;37(1):4–16. doi: 10.1089/photob.2018.4512 [DOI] [PubMed] [Google Scholar]
- 190.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. doi: 10.1109/JSTQE.2016.2561201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Amaroli A, Agas D, Lus F, et al. The effects of photobiomodulation of 808 nm diode laser therapy at higher fluence on the in vitro osteogenic differentiation of bone marrow stromal cell. Front Physiol. 2018;9(123):1–11. doi: 10.3389/fphys.2018.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Amaroli A, Ferrando S, Hanna R, et al. The photobiomodulation effect of higher-fluence 808-nm laser therapy with a flat-top handpiece on the wound healing of the earthworm Dendrobaena veneta: a brief report. Lasers Med Sci. 2018;33:221–225. doi: 10.1007/s10103-016-2132-3 [DOI] [PubMed] [Google Scholar]
- 193.Hamblin MR. Mechanisms and applications of the anti-inflammatory effects of photobiomodulation. AIMS Biophys. 2017;4(3):337–361. doi: 10.3934/biophy.2017.3.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Hanna R, Dalvi S, Amaroli A, et al. Effects of photobiomodulation on bone defects grafted with bone substitutes: a systematic review of in vivo animal studies. J Biophotonics. 2020;e202000267:1–41. doi: 10.1002/jbio.202000267 [DOI] [PubMed] [Google Scholar]
- 195.Hanna R, Agas D, Benedicenti S, et al. A comparative study between the effectiveness of 980 nm photobiomodulation delivered by hand-piece with Gaussian vs. flat-top profiles on osteoblasts maturation. Front Endocrinol. 2019;10:92. doi: 10.3389/fendo.2019.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Aimbire F, Albertine R, de Magalhães RG, et al. Effect of LLLT Ga-Al-As (685 nm) on LPS-induced inflammation of the airway and lung in the rat. Lasers Med Sci. 2005;20(1):11–20. doi: 10.1007/s10103-005-0339-9 [DOI] [PubMed] [Google Scholar]
- 197.Aimbire F, Lopes-Martins RA, Albertini R, et al. Effect of low-level laser therapy on hemorrhagic lesions induced by immune complexes in rat lungs. Photomed Laser Surg. 2007;25(2):112–117. doi: 10.1089/pho.2006.1041 [DOI] [PubMed] [Google Scholar]
- 198.Aimbire F, Ligeiro de Oliveira AP, Albertini R, et al. Low level laser therapy (LLLT) decreases pulmonary microvascular leakage, neutrophil influx and IL-1beta levels in airway and lung from rat subjected to LPS- induced inflammation. Inflammation. 2008;31(3):189–197. doi: 10.1007/s10753-008-9064-4 [DOI] [PubMed] [Google Scholar]
- 199.Oliveira MC, Greiffo FR, Rigonato-Oliveira NC, et al. Low level laser therapy reduces acute lung inflammation in a model of pulmonary and extrapulmonary LPS-induced ARDS. J Photochem Photobiol B. 2014;134:57–63. doi: 10.1016/j.jphotobiol.2014.03.021 [DOI] [PubMed] [Google Scholar]
- 200.Mokmeli S, Vetrici M. Low level laser therapy as a modality to attenuate cytokine storm at multiple levels, enhance recovery, and reduce the use of ventilators in COVID-19. Can J Respir Ther. 2020;56:1–7. doi: 10.29390/cjrt-2020-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mafra de Lima F, Villaverde AB, Salgado MA, et al. Low intensity laser therapy (LILT) in vivo acts on the neutrophils recruitment and chemokines/cytokines levels in a model of acute pulmonary inflammation induced by aerosol of lipopolysaccharide from Escherichia coli in rat. J Photochem Photobiol B. 2010;101:271–278. doi: 10.1016/j.jphotobiol.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 202.Brochetti A, Leal MP, Rodrigues R, et al. Photobiomodulation therapy improves both inflammatory and fibrotic parameters in experimental model of lung fibrosis in mice. Lasers Med Sci. 2017;32(8):1825–1834. doi: 10.1007/s10103-017-2281-z [DOI] [PubMed] [Google Scholar]
- 203.de Brito AA, da Silveira EC, Rigonato-Liveira NC, et al. Low-level laser therapy attenuates lung inflammation and airway remodeling in a murine model of idiopathic pulmonary fibrosis, relevance to cytokines secretion from lung structural cells. J Photochem Photobiol B. 2020;203:111731. doi: 10.1016/j.jphotobiol.2019.111731 [DOI] [PubMed] [Google Scholar]
- 204.Lai CC, Shih TP, Ko WC, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19), The epidemic and the challenges. Int J Antimicrob Agents. 2020;55(3):105924. doi: 10.1016/j.ijantimicag.2020.105924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Miranda da Silva C, Peres Leal M, Brochetti RA, et al. Low level laser therapy reduces the development of lung inflammation induced by formaldehyde exposure. PLoS One. 2015;10(11):e0142816. doi: 10.1371/journal.pone.0142816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.da Cunha Moraes G, Vitoretti LB, de Brito AA, et al. Low-level laser therapy reduces lung inflammation in an experimental model of chronic obstructive pulmonary disease involving p2x7 receptor. Oxid Med Cell Longev. 2018;2018:6798238. doi: 10.1155/2018/6798238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Enwemeka CS, Bumah VV, Masson-Meyers DS. Light as a potential treatment for pandemic coronavirus infections: a perspective. J Photochem Photobiol B. 2020;207:111891. doi: 10.1016/j.jphotobiol.2020.111891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Yusupalieva MM, Savtchenko VM. The effectiveness of combined laser therapy for the treatment of the patients presenting with bronchial asthma and concomitant allergic rhinitis. Vopr Kurortol Fizioter Lech Fiz Kult. 2017;94(4):14–18. doi: 10.17116/kurort201794414-18 [DOI] [PubMed] [Google Scholar]
- 209.Askenova IZ, Burduli NM. Pathogenetic effects of low-intensity laser therapy for chronic obstructive pulmonary disease. Ter Arkh. 2015;88:32–35. doi: 10.17116/terarkh201688332-35 [DOI] [PubMed] [Google Scholar]
- 210.Sigman SA, Mokmeli S, Monici M, et al. A 57-year-old african american man with severe COVID-19 pneumonia who responded to supportive photobiomodulation therapy (PBMT): first use of PBMT in COVID-19. Am J Case Rep. 2020;21:e926779. doi: 10.12659/AJCR.926779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sigman SA, Mokmeli S, Vetrici MA. Adjunct low level laser therapy (LLLT) in a morbidly obese patient with severe COVID-19 pneumonia: a case report. Can J Respir Ther. 2020;56:52–56. doi: 10.29390/cjrt-2020-022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Giannelli M, Bani D. Appropriate laser wavelengths for photodynamic therapy with methylene blue. Lasers Med Sci. 2018;33(8):1837–1838. doi: 10.1007/s10103-018-2566-x [DOI] [PubMed] [Google Scholar]
- 213.Dias LD, Bagnato VS. An update on clinical photodynamic therapy for fighting respiratory tract infections: a promising tool against COVID-19 and its co-infections. Laser Phys Lett. 2020;17(8):1–9. doi: 10.1088/1612-202X/ab95a9 [DOI] [Google Scholar]
- 214.Wainright M, Crossley KB. Photosensitizing agents- circumventing resistance and breaking down biofilms: a review. Int Biodeter Biodegr. 2004;53(2):119–126. doi: 10.1016/j.ibiod.2003.11.006 [DOI] [Google Scholar]
- 215.Hu XQ, Huang YY, Wang YG. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front Microbiol. 2019;9(1299):1–24. doi: 10.3389/fmicb.2018.01299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Wiehe A, O’Brien JM, Senge MO. Trends and targets in antiviral phototherapy. Photochem Photobiol Sci. 2019;18(11):2565–2612. doi: 10.1039/c9pp00211a [DOI] [PubMed] [Google Scholar]
- 217.Moan J, Berg K, Kvam E, et al. Intracellular localization of photosensitizers. Ciba Found Symp. 1989;146:95–107. doi: 10.1002/9780470513842.ch7 [DOI] [PubMed] [Google Scholar]
- 218.Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome -coronavirus-2 (SARS-CoV-2; coronavirus disease-19). Clin Exp Pediatr. 2020;63(4):119–124. doi: 10.3345/cep.2020.00493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Woelfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized cases of coronavirus disease 2019. medRxiv. 2020;03:05.20030502. doi: 10.1101/2020.03.05.20030502 [DOI] [Google Scholar]
- 220.Oh MD, Park WB, Choe PG, et al. Viral load kinetics of MERS coronavirus infection. N Engl J Med. 2016;375(13):1303–1305. doi: 10.1056/NEJMc1511695 [DOI] [PubMed] [Google Scholar]
- 221.Blanco KC, Inada NM, Carbinatto FM, et al. Treatment of recurrent pharyngotonsillitis by photodynamic therapy. Photodiagnosis Photodyn Ther. 2017;18:138–139. doi: 10.1016/j.pdpdt.2017.01.187 [DOI] [PubMed] [Google Scholar]
- 222.Sécher T, Alexie Mayor A, Heuzé-Vourc’h N. Inhalation of immuno-therapeutics/- prophylactics to fight respiratory tract infections: an appropriate drug at the right place! Front Immunol. 2019;10(2760):1–6. doi: 10.3389/fimmu.2019.02760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Moghissi K, Dixon K, Gibbins S. Does PDT have potential in the treatment of COVID-19 patients? Photodiagnosis Photodyn Ther. 2020;31(101889):1–2. doi: 10.1016/j.pdpdt.2020.101889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Geralde MC, Leite IS, Inada NM, et al. Pneumonia treatment by photodynamic therapy with extracorporeal illumination - an experimental model. Physiol Rep. 2017;5(5):e13190. doi: 10.14814/phy2.13190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kassab G, Geralde MC, Inada NM, Achiles AE, Guerra VG, Bagnato VS. Nebulization as a tool for photosensitizer delivery to the respiratory tract. J Biophotonics. 2019;12(4):e201800189. doi: 10.1002/jbio.201800189 [DOI] [PubMed] [Google Scholar]
- 226.Schikora D, Hepburn J, Plavin SR. Reduction of the viral load by non-invasive photodynamic therapy in early stages of COVID-19 infection. Am J Virol Dis. 2020;2(1):01–05. [Google Scholar]
- 227.Jin C, Yu B, Zhang J. Methylene blue photochemical treatment as a reliable SARS-CoV-2 plasma virus inactivation method for blood safety and convalescent plasma therapy for the COVID-19 outbreak. Res Square. 2020;1–15. doi: 10.21203/rs.3.rs-17718/v1 [DOI] [Google Scholar]
- 228.Keil SD, Bowen R, Marschner S. Inactivation of Middle East respiratory syndrome coronavirus (MERS-CoV) in plasma products using a riboflavin-based and ultraviolet light-based photochemical treatment. Transfusion. 2016;56(12):2948–2952. doi: 10.1111/trf.13860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ruane PH, Edrich R, Gampp D, et al. Photochemical inactivation of selected viruses and bacteria in platelet concentrates using riboflavin and light. Transfusion. 2014;44(6):877. doi: 10.1111/j.1537-2995.2004.03355.x [DOI] [PubMed] [Google Scholar]
- 230.Wagner SJ. Virus inactivation in blood components by photoactive phenothiazine dyes. Transfus Med Rev. 2002;16(1):61–66. doi: 10.1053/tmrv.2002.29405 [DOI] [PubMed] [Google Scholar]
- 231.Bachmann B, Knüver-Hopf J, Lambrecht B, et al. Target structures for HIV-1 inactivation by methylene blue and light. J Med Virol. 1995;47(2):172–178. doi: 10.1002/jmv.1890470211 [DOI] [PubMed] [Google Scholar]
- 232.Bumah VV, Masson-Meyers DS, Enwemeka CS. Pulsed 450 nm blue light suppresses MRSA and propionibacterium acnes in planktonic cultures and bacterial biofilms. J Photochem Photobiol B. 2020;202:111702. doi: 10.1016/j.jphotobiol.2019.111702 [DOI] [PubMed] [Google Scholar]
- 233.Masson-Meyers DS, Bumah VV, Castel C, et al. Pulsed 450 nm blue light significantly inactivates Propionibacterium acnes more than continuous wave blue light. J Photochem Photobiol B. 2020;202:111719. doi: 10.1016/j.jphotobiol.2019.111719 [DOI] [PubMed] [Google Scholar]
- 234.Hanna R, Dalvi S, Sălăgean T, et al. Phototherapy as a rational antioxidant treatment modality in COVID-19 management; new concept and strategic approach: critical review. Antioxidants. 2020;9(875):1–23. doi: 10.3390/antiox9090875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Photodynamic therapy for the treatment of COVID-19. Available from: https://clinicaltrials.gov/show/NCT04416113. Accessed October20, 2020.
- 236.Photobiomodulation therapy combined with static magnetic field in patients with COVID-19. Available from: https://clinicalTrials.govshow/NCT04386694. Accessed October20, 2020.
- 237.Lung treatment of lung inflammation in COVID-19. Available from: https://clinicalTrials.govshow/NCT04524715. Accessed October20, 2020.
- 238.Photobiomodulation laser therapy for COVID-19 positive patients with pulmonary disease. Available from: https://clinicalTrials.govshow/NCT04391712. Accessed October20, 2020.