Abstract
Ischaemic colitis (IC) is a common condition with rising incidence, and in severe cases a high mortality rate. Its presentation, severity and disease behaviour can vary widely, and there exists significant heterogeneity in treatment strategies and resultant outcomes. In this article we explore practical challenges in the management of IC, and where available make evidence-based recommendations for its management based on a comprehensive review of available literature. An optimal approach to initial management requires early recognition of the diagnosis followed by prompt and appropriate investigation. Ideally, this should involve the input of both gastroenterology and surgery. CT with intravenous contrast is the imaging modality of choice. It can support clinical diagnosis, define the severity and distribution of ischaemia, and has prognostic value. In all but fulminant cases, this should be followed (within 48 hours) by lower gastrointestinal endoscopy to reach the distal-most extent of the disease, providing endoscopic (and histological) confirmation. The mainstay of medical management is conservative/supportive treatment, with bowel rest, fluid resuscitation and antibiotics. Specific laboratory, radiological and endoscopic features are recognised to correlate with more severe disease, higher rates of surgical intervention and ultimately worse outcomes. These factors should be carefully considered when deciding on the need for and timing of surgical intervention.
Keywords: ischaemia
Key points.
Ischaemic colitis is common, rising in incidence and associated with a high mortality rate, especially in cases where surgical intervention is required.
CT with intravenous contrast is the imaging modality of choice and should be followed by lower gastrointestinal endoscopy (within 48 hours), aiming to reach the distal-most extent of the disease to achieve an endoscopic (and histological) diagnosis.
Isolated right colonic involvement is predictive of poorer outcomes, including higher rates of surgical intervention and death.
High-quality supportive/conservative treatment remains the backbone of medical therapy.
Antibiotics are recommended, but there is little evidence on the benefit of other pharmacological interventions.
Surgical intervention should be considered in the setting of circulatory compromise, abdominal pain without rectal bleeding, pancolonic or isolated right-sided distribution, and in patients with peritoneal signs.
Introduction
Ischaemic colitis (IC) represents the manifestations of compromised blood supply to the colon (ischaemia, derived from the Greek word iskhaimos meaning ‘stopping blood’). When blood supply (however transiently) becomes insufficient to meet the metabolic demands of the colon, mucosal inflammation occurs, giving rise to ulceration and haemorrhage. Inflammation stems from both direct ischaemic insult and reperfusion injury; the latter is caused by the release of reactive oxygen species and inflammatory cytokines during restoration of normal tissue perfusion,1 damage from which may exceed the direct effects of ischaemia. Bacterial translocation, intestinal vasospasm and intestinal dysbiosis (from disruption of the gut microbiome) also contribute to tissue damage.2 Colonic ischaemia occurs in a top-down distribution. The mucosa, as the most metabolically active layer of the colon, is the first layer to be affected. Sloughing of villous tips and mucosal oedema are followed by submucosal haemorrhage and (eventually) transmural necrosis. Clinical manifestations are dependent on the site and extent of ischaemic insult, but include abdominal pain, diarrhoea, melaena and rectal bleeding. The spectrum of severity ranges from self-limiting within days to requiring emergency surgical resection.
IC should be differentiated from mesenteric ischaemia. Acute mesenteric ischaemia (AMI) represents complete loss of blood supply to a segment of bowel, leading to rapid necrosis and necessitating emergency laparotomy. It is usually due to acute thromboembolic arterial occlusion (often of the superior mesenteric artery (SMA)). However, non-occlusive arterial AMI can also occur (generally in the setting of critical illness and haemodynamic compromise); less commonly, it may stem from mesenteric venous thrombosis (which may coexist with chronic pancreatitis or portal hypertension).3 Chronic mesenteric ischaemia (‘mesenteric angina’) involves intermittent, crampy, postprandial abdominal pain, typically within an hour of oral intake, over a period of at least 3 months.
IC is a relatively commonly encountered clinical syndrome (incidence of 22.9/100 000 person-years4), with substantial heterogeneity in clinical approach. There also exists variation in the specialty of clinicians responsible for managing patients with IC. Some cases are managed by surgeons, others by physicians (gastroenterologists). We therefore carried out a literature review in order to make evidence-based recommendations for the practical management of patients with IC.
Methods
Our literature review search strategy and selection criteria can be found in the online supplementary material, along with our Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram. Two reviewers (AH and TC) independently screened citations and abstracts before retrieving full-text publications of all potentially eligible articles.
flgastro-2019-101204supp001.pdf (61.1KB, pdf)
Aetiology
Old age (ie, over 60), atherosclerosis, smoking, chronic kidney disease (CKD) and atrial fibrillation have been consistently demonstrated to increase the risk of developing IC.5 6 Certain medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and oestrogen therapy7 also increase the risk. There is interplay, of course, between these risk factors. Patients with CKD have higher rates of diabetes mellitus, anaemia and hypertension.6 They also have changes in vascular elasticity, and haemodialysis can lead to microthrombus generation.5 The causes of IC can be categorised into thromboembolic, haemodynamic insufficiency (often in the setting of a predisposing factor), iatrogenic and drug-induced. Thromboembolic causes include atrial fibrillation, prothrombotic conditions such as antiphospholipid syndrome (leading to both arterial and venous thromboemboli), and concurrent malignancy. Haemodynamic insufficiency (a ‘supply and demand’ problem) occurs with cardiac failure, severe anaemia, hypovolaemia and septic shock; atherosclerosis (causing vascular narrowing) can be thought of as a predisposing factor. Iatrogenic IC can occur postoperatively during open abdominal aortic aneurysm repair, either through cross-clamping of the aorta or due to sacrifice of the inferior mesenteric artery (IMA) due to its location in the aneurysmal sac. It can also arise through microemboli generated by disruption of aortic plaques during endovascular repair.8 Potential drugs that cause IC are wide-ranging and include chemotherapeutic agents, vasopressors, oestrogen therapy, cocaine, amphetamines, ergotamine, antipsychotics and NSAIDs, among others.9 10 These agents should be specifically excluded when taking history from patients with suspected IC.
Clinical features
Symptoms
IC is a clinical spectrum. The constellation of symptoms varies in relation to the anatomical distribution and severity of the colitis. The most common symptoms (with approximate prevalence) are abdominal pain (87%), rectal bleeding (84%) and diarrhoea (56%).11 PR bleeding is more common in left-sided colitis and often absent in isolated right-sided colitis, where pain predominates. Bleeding usually manifests as fresh red blood PR, particularly when associated with distal colitis. Melaena may occur with more proximal colonic involvement. The left colon is affected in around 75% of cases of IC, with approximately 25% involving the splenic flexure. Isolated right colon ischaemia (IRCI) occurs in around 10%.1 On examination, mild to moderate tenderness may be elicited, but usually without generalised peritonism. Fever is uncommon, but if present may suggest infarction.1 12
Anatomical distribution
Typically, the ‘watershed’ areas of the colon are the most frequently affected. These zones are at the junctions of vascular territories and have the least robust collateral blood supply.13 The splenic flexure is particularly susceptible to ischaemia.14 Griffith’s point describes the point between the territories formed by the middle colic branch of the SMA and the right colic branch of the IMA. Similarly vulnerable is Sudeck’s point, the junction between the last sigmoid branch and the superior rectal branch of the IMA in the rectosigmoid (figure 1).12 Collateral supply to the colon comes largely from the marginal artery of Drummond, which forms a vascular arcade connecting the SMA and the IMA, and is subject to anatomical variants. In up to 5% of cases, blood supply from the marginal artery is absent at the splenic flexure.1 Furthermore, in up to 50% of cases the marginal artery is underdeveloped in the right colon,12 which may explain why the right colon may be vulnerable in low flow states and why some patients are more predisposed to right-sided involvement. The caecum is also felt to be a relative watershed area, and rarely isolated caecal involvement may occur. The rectum is usually spared in IC as it receives collateral blood supply from the middle and inferior rectal arteries, which are branches of the internal iliac vessels rather than the IMA.
Figure 1.
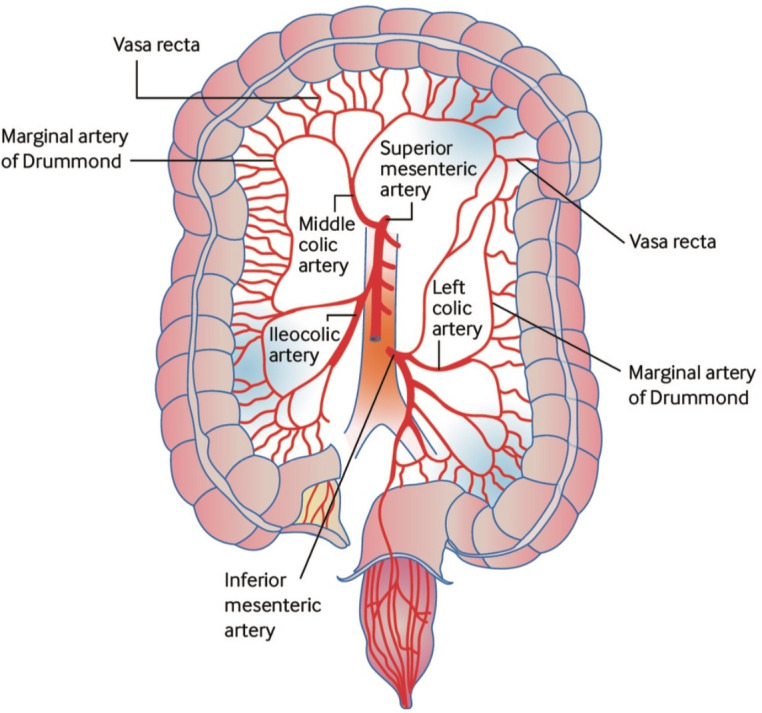
Arterial supply of the colon and the most common sites for ischaemic colitis. The colon receives blood from both the superior and inferior mesenteric arteries. However, there are weak points, or ‘watershed’ areas, at the borders of the territory supplied by each of these arteries, such as the splenic flexure and the transverse colon. These watershed areas are most vulnerable to ischaemia when blood flow decreases as they have the fewest vascular collaterals. Reproduced with permission from Trotter et al.32
Prognostic factors
The most frequently cited factors to confer an unfavourable outcome in IC are the absence of rectal bleeding15–17 and right-sided colonic involvement.4 5 15–17 Given other areas of the colon are more susceptible to ischaemic insult, right-sided involvement can be considered a marker of severity.18 Furthermore, IRCI can be the harbinger of incipient AMI due to large vessel occlusion (given the area supplied by the SMA includes both the distal small bowel and the right colon).19 20
Coexistent atrial fibrillation21 and atherosclerotic disease4 promotes less favourable outcomes. Older patients and those with chronic obstructive pulmonary disease (COPD) also tend to have poorer outcomes,4 the latter likely owing in part to the cardiovascular sequelae of smoking. Other negative prognostic factors include CKD,5 11 thrombocytopaenia,5 high C-reactive protein (CRP)5 and high white cell count (WCC).21 Unsurprisingly, examination findings of guarding or peritonism are linked to a poorer prognosis.15 16
Gastrointestinal investigation
Laboratory tests
All patients with suspected IC should have basic work-up including full blood count, renal profile, liver profile, CRP, serum lactate, coagulation studies, and group and save. Based on limited evidence, there does not currently appear to be any role for faecal markers of inflammation (eg, calprotectin) in IC.22 Table 1 outlines the laboratory findings indicative of a more severe disease.19 Initial investigations should also include faecal culture, Clostridium difficile toxin assay, and studies for ova, cysts and parasites.23
Table 1.
Features associated with severe ischaemic colitis and failure of conservative management
| Patient factors | Clinical features | Laboratory tests | Cross-sectional imaging |
| Male gender. | Peritoneal signs evident. | Anaemia. | Free intraperitoneal fluid. |
| Pre-existing renal dysfunction. | Absence of rectal bleeding. | Leucocytosis. | Disease localised to or involving the right colon. |
| History of atrial fibrillation. | Tachycardia. | Hyponatraemia. | |
| Thrombocytopaenia. | |||
| Elevated CRP. | |||
| Elevated serum lactate. |
CRP, C-reactive protein.
Imaging
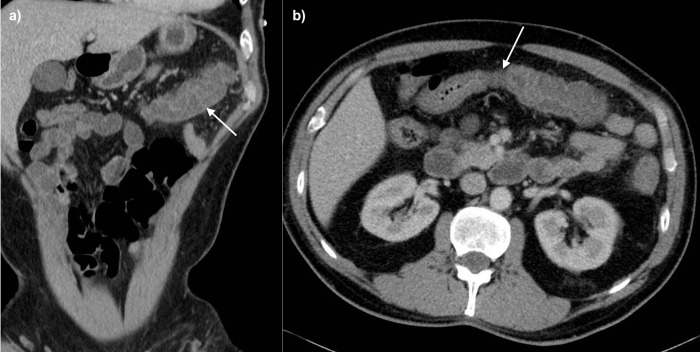
There is currently no standardised pathway for imaging in patients with IC. As a frequently misdiagnosed condition, it is often picked up as part of a work-up for the ‘acute abdomen’, which usually includes CT. Where renal function allows, CT should be performed with intravenous contrast. Formal CT angiography is not necessarily required unless AMI is suspected or IRCI is found. Oral contrast is not necessary and usually unhelpful as it hinders assessment of bowel wall enhancement. Patients with IC demonstrate imaging features of colitis, such as bowel wall thickening and pericolic fat stranding. These are often seen in a segmental distribution, with the left colon most frequently involved.24 However, it must be noted that these imaging findings are non-specific. Only approximately 15%–39% of patients with bowel wall thickening on CT have been found to have endoscopic features of ischaemia.25 Figures 2 and 3 demonstrate common CT findings.
Figure 2.
CT images showing (A) a coronal view with mural thickening and submucosal oedema (arrow) with mild surrounding pericolic oedema, and (B) an axial image demonstrating a sharp cut-off between the normal proximal transverse colon and the abnormal mid/distal transverse colon (arrow).
Figure 3.
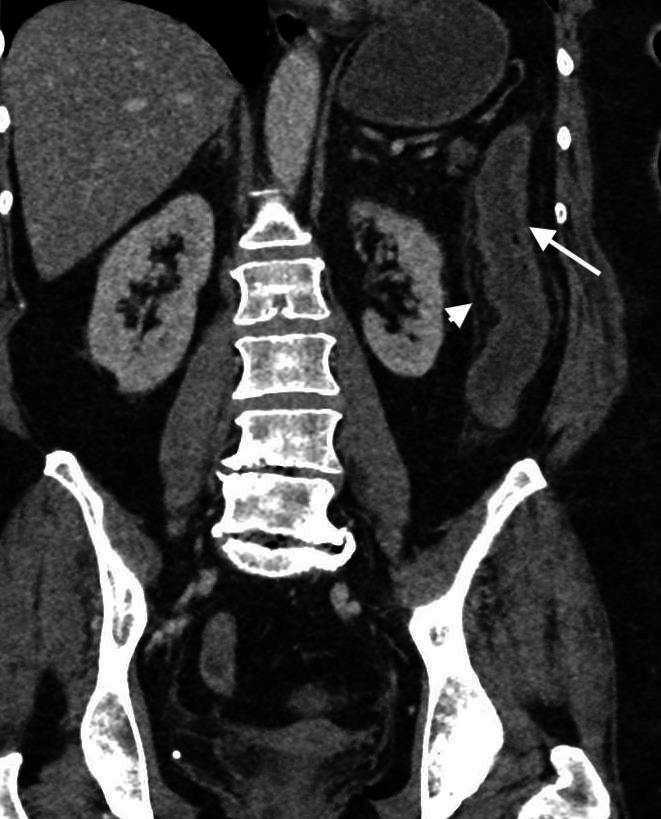
CT image demonstrating acute ischaemia on a background of chronic change due to chronic ischaemia. The colon appears relatively featureless with loss of haustration and reduction in luminal calibre, with superadded mural thickening (arrow) and pericolic oedema (arrowhead) due to acute insult.
Concerning features on CT include right-sided involvement, colonic dilatation, pneumatosis and free abdominal fluid.26 Patients with severe disease necessitating surgical intervention and/or leading to death are five times as likely to have right-sided colonic involvement.18 Factors suggesting an alternative diagnosis include the absence of a target sign (ie, ring of submucosal oedema between enhancing mucosa and serosa), presence of a stricture on presentation and mucosal hyperenhancement. Such findings might raise suspicion for Crohn’s disease.27 However, it is recognised that patients with established IC may develop strictures after the acute phase of the disease. In a case series of eight such patients, a typical CT appearance was a single area of concentric wall thickening, with greater enhancement in the portal phase than arterial phase, and vasa recta prominence.28
Ultrasound of the bowel can (with adequate expertise) also elicit the diagnosis, can differentiate between left-sided and right-sided disease,29 and represents a valid alternative for patients unable to tolerate contrast media for CT. Absence of flow on colour Doppler denotes more severe disease and confers poorer outcomes,30 as does lack of enhancement with microbubble ultrasound contrast medium. However, the inherent user dependency of ultrasound (combined with its lack of out-of-hours availability) renders CT the imaging modality of choice.
Endoscopy
Common endoscopic findings include scattered erythematous mucosa and petechial haemorrhages, with or without erosion and ulceration.31 Figure 4 demonstrates the single-stripe sign (a single longitudinal strip of ulcerated or inflamed colon).32 Features suggesting gangrenous transformation include dark and dusky mucosa, with blue-black mucosal nodules. There is a paucity of robust data regarding the correlation between endoscopic findings and clinical severity of disease. However, previous attempts have been made to risk-stratify patients based on endoscopic findings, with longitudinal recesses or erosions being considered lower risk and longitudinal or circumferential ulcers considered high risk. On this basis, patients with endoscopically severe IC have been found to have longer hospital stays, as well as higher background rates of ischaemic heart disease and connective tissue disorders.33
Figure 4.
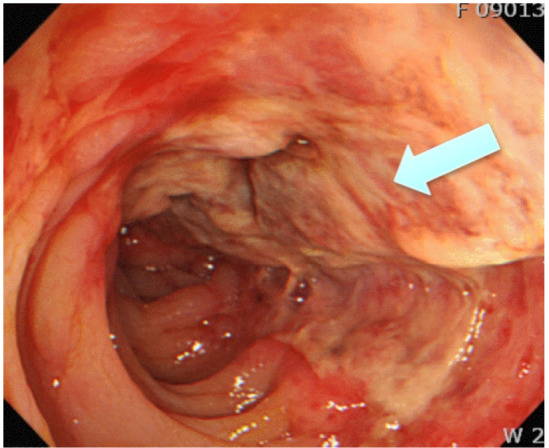
Endoscopic findings of inflamed mucosa and single-stripe sign (a single longitudinal strip of ulcerated or inflamed colon (arrow)) in the segment of ischaemic colitis. Reproduced with permission from Trotter et al.32
The planned extent of lower gastrointestinal endoscopy should be based on the distribution of inflammation seen on CT. The American College of Gastroenterology (ACG) recommends termination of the examination on reaching the distal-most extent of disease.23 As most cases of IC involve the left colon, flexible sigmoidoscopy is usually sufficient to achieve an endoscopic and histopathological diagnosis, but where complete colonic evaluation is necessary minimal insufflation using carbon dioxide (as opposed to air) is recommended.23 As there is evidence to suggest that the diagnostic yield reduces over time, early endoscopic examination is advocated within the first 48 hours.23
As IC may mimic other conditions (such as colorectal malignancy) at endoscopy, histology is important to confirm the diagnosis and exclude alternative pathologies. The most commonly observed histological features are mucosal atrophy, hyperaemia, oedema and features of acute inflammation. Unsurprisingly, patients with gangrenous disease demonstrate both endoscopic and microscopic features of necrosis. Traditionally, ‘ghost cells’ have been viewed as pathognomonic of colonic ischaemia; however, data suggest these to be an inconsistent and therefore unreliable finding.34
Cardiovascular investigation
Patients with IC often have cardiovascular risk factors, including atrial fibrillation, hypertension and CKD. As such, they are more likely to have a potential cardiac precipitant of thromboembolic IC (eg, arrhythmia or valvular abnormality), detectable by a combination of electrocardiography (ECG) and echocardiography. The most commonly observed cardiac abnormality is atrial fibrillation (either paroxysmal or sustained), which in one study occurred in 20% of patients with IC (n=60). In the same cohort, 25% required antiarrhythmic medication and 32% needed anticoagulation.35 All patients with suspected IC should have an ECG, and all patients with confirmed IC should receive an echocardiogram. A Holter monitor should be considered to exclude paroxysmal arrhythmias.
Management
Treatment of IC comprises both medical and surgical components, and so patients should receive joint gastroenterology and surgical input. In mild cases and in the absence of factors predictive of a need for operative intervention (table 1), emphasis lies on medical therapy. However, in borderline cases or where operative intervention appears necessary, patients require frequent surgical review to determine the optimal time for intervention. Such patients may be best placed on a surgical ward with additional gastroenterology input. Our suggested algorithm for management can be seen in figure 5.
Figure 5.
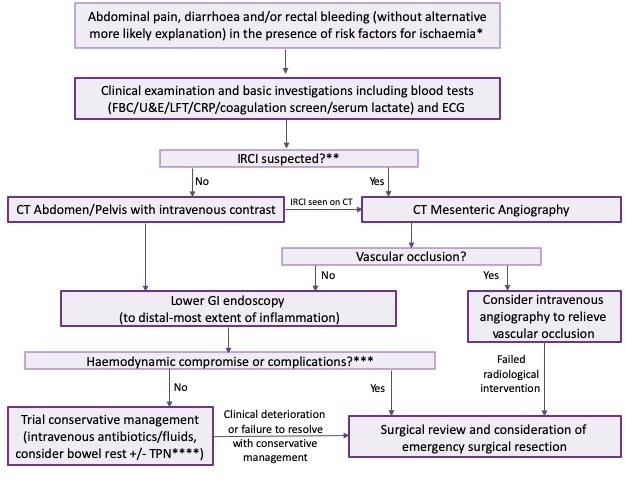
Algorithm for investigation and management of ischaemic colitis. *Risk factors for ischaemia: AF, smoking history, CKD, atherosclerosis, age >60, medications predisposing to IC. **Absence of rectal bleeding/right-sided pain/symptoms of chronic mesenteric ischaemia. ***Either systemic compromise (eg, haemodynamic instability) or complications such as perforation. ****Choice to commence TPN will be influenced by factors suggesting more severe disease and protracted course, such as absence of rectal bleeding, peritonitis, IRCI or presence of biochemical markers of severity (anaemia, leucocytosis, thrombocytopaenia, hyponatraemia, elevated CRP/lactate). AF, atrial fibrillation; CKD, chronic kidney disease; CRP, C-reactive protein; ECG, electrocardiography; FBC, full blood count; GI, gastrointestinal; IRCI, isolated right colon ischaemia; LFT, liver function tests; TPN, total parenteral nutrition; U&E, urea and electrolytes.
Medical management
The mainstay of medical management is careful supportive treatment, with correction of any precipitating factors. In addition to intravenous fluid resuscitation and blood glucose control (in patients with diabetes), this generally consists of bowel rest and intravenous antibiotics.18 Bowel rest is achieved through fasting, and in the presence of ileus a nasogastric tube placement. The duration of bowel rest will depend on severity and clinical response, but in general most improve within 2–3 days (although it is thought to take 1–2 weeks for the colon to heal).23 As noted in the ACG clinical guideline, there is little robust evidence regarding antibiotic choice. Consensus suggests combining anaerobic cover with a third-generation cephalosporin or fluoroquinolone.23 There is also a paucity of data regarding duration of antibiotic treatment, but expert consensus has suggested a pragmatic approach that involves review after 72 hours. If no clinical improvement is seen by this point, then consultation with a microbiologist is recommended to help refine the antibiotic regimen. Where clinical improvement is seen, completion of a 7-day course has been advocated.23 In more severe cases where bowel rest is indicated and the course expected to be protracted, parenteral nutrition is indicated.23 Beyond antimicrobial cover, there are no comparative studies of medical therapies in IC, and there is no evidence to support the use of aminosalicylates, corticosteroids or immunomodulators. Prophylactic low molecular weight heparin is generally recommended, but there is no established role for formal anticoagulation in the acute setting. Secondary prevention with antiplatelets and anticoagulants should, however, be considered at time of discharge. As there is a lack of evidence in support of specific risk-reducing medical therapies following an episode of IC, secondary prophylaxis should be tailored to individual thromboembolic risk factors. Aspirin, then, is appropriate for those with ischaemic heart disease, whereas clopidogrel should be considered for patients with peripheral vascular disease or previous cerebrovascular disease. Oral anticoagulants are recommended for patients with atrial fibrillation.
The thrust of monitoring for response to treatment should be through frequent clinical review (including abdominal examination) and careful monitoring of vital signs. In addition to worsening (or non-resolution) of symptoms, signs such as persistent fever and/or deterioration in biochemical markers (CRP, white cell count or lactate) should prompt reinvestigation. This should include consideration of repeat CT scanning and endoscopic re-evaluation.
Surgical management
Certain factors have been identified that can indicate more severe disease, predict failure of conservative management and a need for surgery (table 1). The presence or absence of these can inform consideration of semielective surgical intervention in the face of probable non-resolution.5 19 20 26 33
The depth of mural involvement can also be used to classify severity.36 Type 1 IC describes inflammation limited to the mucosa, type 2 denoting muscularis layer involvement and type 3 transmural inflammation. The depth of inflammation is best judged using cross-sectional CT imaging as endoscopy alone cannot reliably confirm or exclude transmural involvement.37 For patients with type 1 or 2 IC and no evidence of systemic compromise, conservative management is an appropriate initial approach. However, evidence suggests that in patients with type 2 IC and systemic compromise (ie, circulatory collapse and/or organ failure), operative intervention should be considered. Type 3 IC is generally accompanied by systemic compromise and necessitates surgery.38 Other factors that should prompt consideration of operative intervention include persistent abdominal pain without rectal bleeding, pancolonic or isolated right-sided distribution, and the presence/development of peritoneal signs.16 34 39
Surgical intervention in IC is associated with higher morbidity and mortality than patients managed conservatively (box 1). However, clearly selection bias exists here, as comorbid patients with more severe disease are more likely to require surgery. Surgical intervention usually involves segmental resection and colostomy formation, with the average postoperative hospital stay typically lasting several weeks. Many require intensive care admission. Table 2 demonstrates risk factors for postoperative mortality and such risk factors are understood to be additive.38 40 Based on a study of 177 patients, the Ischemic Colitis Mortality Risk score was proposed (box 1, factors in italics). The number of factors present results in a score ranging from 0 to 5, with mortality rate estimates of 10.5%, 28.9%, 37.1%, 50.0%, 76.7% and 100.0% for each ascending stratification.40
Box 1. Risk factors associated with increased postoperative mortality (factors in italics are included in the Ischemic Colitis Mortality Risk score40).
Age >75.
Multiple organ failure.
American Society of Anesthesiologists (ASA) status ≥4.
Intraoperative blood loss >500 mL.
Preoperative lactate >2.5.
Acute kidney injury.
Preoperative or intraoperative catecholamine use.
Low output heart failure.
Subtotal or total colectomy.
Table 2.
Outcomes in ischaemic colitis
| Year | Patients (n) | Management | Inpatient mortality | |||
| Non-operative | Operative | Non-operative (%) | Operative (%) | |||
| Reissfelder et al 40 | 2011 | 177 | 0 | 177 | – | 85 (48) |
| Moszkowicz et al 38 | 2014 | 191 | 17 | 174 | 0 (0) | 84 (48) |
| Medina et al 18 | 2004 | 53 | 35 | 18 | 0 (0) | 5 (28) |
| Paterno et al 44 | 2010 | 253 | 205 | 48 | 10 (5) | 16 (33) |
| Sadot et al 21 | 2014 | 117 | 96 | 21 | 3 (3) | 2 (10) |
| Genstorfer et al 42 | 2014 | 100 | – | 100 | – | 54 (54) |
| Glauser et al 45 | 2011 | 49 | 45 | 4 | 0 (0) | 1 (25) |
| Flobert et al 46 | 2000 | 60 | 39 | 21 | 3 (7) | 4 (20) |
| Gilshtein et al 47 | 2018 | 63 | 50 | 13 | 12 (24) | 6 (50) |
| Castleberry et al 48 | 2013 | 115 | – | 115 | – | 43 (37) |
| Anon et al 16 | 2006 | 69 | 54 | 15 | 1 (0.02) | 7 (46) |
| Cosme et al 43 | 2013 | 135 | 123 | 12 | 4 (0.03) | 4 (33) |
Complications
Complications include perforation, abscess formation and strictures. Perforation, which occurs in the context of transmural inflammation and sometimes gangrenous ischaemia, may be accompanied by sepsis and requires laparotomy. Abscess formation generally occurs secondary to a (sometimes sealed, localised) perforation. Percutaneous drainage may be necessary. Fulminant ischaemic pancolitis is rare,3 but is potentially life-threatening and may also necessitate colectomy.
One key ‘complication’ of IC is the persistence of symptoms (in the absence of a fulminant decline) to the point where surgical resection is felt to be beneficial. This may be failure of diarrhoea or rectal bleeding to resolve within 1–2 weeks, persistent postprandial pain, or the development of a protein-losing colopathy. The latter describes a constellation of ongoing weight loss, hypoalbuminaemia, inability to sustain oral intake and failure to thrive with conservative management. In most cases this will be a clinical diagnosis, but elevated faecal clearance of alpha-1-antitrypsin is supportive.
Postinflammatory strictures can form following conservative management and may occur in up to 10% of cases.41 As inflammation tends to be segmental (due to its vascular aetiology), strictures tend to be relatively long. As such, they are more likely to require surgical intervention with either stricturoplasty or resection.
Postoperative complications of surgical intervention include anastomotic leak, rectal stump leak, stoma-related issues, malabsorption syndromes and short gut syndrome. Around 16% of patients will experience surgical complications, and these patients are often found to have ischaemic changes at the resection margins.42 Between 20% and 29% of patients will require second-look laparotomies due to clinical deterioration or based on findings during their initial laparotomy.40
As noted, parenteral nutrition may be required. Associated complications include line sepsis, deranged liver function tests and refeeding syndrome.
Long-term outcomes
Recurrence of IC is uncommon, with 5-year recurrence rates reported to be 10.5%. These patients appear to have a similar clinical presentation to index presentation.43 No clear data exist on long-term dysplastic risk following IC. Most cases run an acute course, with relatively few resulting in a state of chronic inflammation which might predispose to dysplastic transformation (as observed in ulcerative colitis, for example).
Conclusions
The management of IC depends on severity at presentation and the presence or absence of poor prognostic features. It is best delivered by a multidisciplinary team including both gastroenterologists and surgeons. Prompt recognition and appropriate investigation, initially with CT and then with lower gastrointestinal endoscopy, are key to making the diagnosis and risk-stratifying patients. Although the majority of cases will settle with conservative management, a minority will require operative intervention and the mortality among this group is high. An understanding of factors which predict surgical outcome is necessary in order to make crucial management decisions and counsel patients appropriately.
Footnotes
AH and TC contributed equally.
Correction notice: This article has been corrected since it published Online First. Figure 3 had been duplicated and has now been replaced and the abstract has been amended.
Contributors: AH, TC, MAS and GW were responsible for planning the content and structure of the article. AH and TC drafted the manuscript, which MAS, AAP and GW critically reviewed and revised.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: MAS: advisory fees: Takeda, Janssen, Sandoz; lecture fees: Takeda, MSD, Janssen, Falk.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; internally peer reviewed.
References
- 1. Theodoropoulou Αngeliki, Κoutroubakis IE. Ischemic colitis: clinical practice in diagnosis and treatment. World J Gastroenterol 2008;14:7302–8. 10.3748/wjg.14.7302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nadatani Y, Watanabe T, Shimada S, et al. Microbiome and intestinal ischemia/reperfusion injury. J Clin Biochem Nutr 2018;63:26–32. 10.3164/jcbn.17-137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Schoots IG, Koffeman GI, Legemate DA, et al. Systematic review of survival after acute mesenteric ischaemia according to disease aetiology. Br J Surg 2004;91:17–27. 10.1002/bjs.4459 [DOI] [PubMed] [Google Scholar]
- 4. Yadav S, Dave M, Edakkanambeth Varayil J, et al. A population-based study of incidence, risk factors, clinical spectrum, and outcomes of ischemic colitis. Clin Gastroenterol Hepatol 2015;13:731–8. quiz e41 10.1016/j.cgh.2014.07.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi SR, Jee SR, Song GA, et al. Predictive factors for severe outcomes in ischemic colitis. Gut Liver 2015;9:761–6. 10.5009/gnl15167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lee TC, Wang HP, Chiu HM, et al. Male gender and renal dysfunction are predictors of adverse outcome in nonpostoperative ischemic colitis patients. J Clin Gastroenterol 2010;44:e96–100. [DOI] [PubMed] [Google Scholar]
- 7. Newman JR, Cooper MA. Lower gastrointestinal bleeding and ischemic colitis. Can J Gastroenterol 2002;16:597–600. 10.1155/2002/374682 [DOI] [PubMed] [Google Scholar]
- 8. Lee MJ, Daniels SL, Drake TM, et al. Risk factors for ischaemic colitis after surgery for abdominal aortic aneurysm: a systematic review and observational meta-analysis. Int J Colorectal Dis 2016;31:1273–81. 10.1007/s00384-016-2606-6 [DOI] [PubMed] [Google Scholar]
- 9. Hass DJ, Kozuch P, Brandt LJ. Pharmacologically mediated colon ischemia. Am J Gastroenterol 2007;102:1765–80. 10.1111/j.1572-0241.2007.01260.x [DOI] [PubMed] [Google Scholar]
- 10. Longstreth GF, Yao JF. Diseases and drugs that increase risk of acute large bowel ischemia. Clin Gastroenterol Hepatol 2010;8:49–54. 10.1016/j.cgh.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 11. Longstreth GF, Yao JF. Epidemiology, clinical features, high-risk factors, and outcome of acute large bowel ischemia. Clin Gastroenterol Hepatol 2009;7:1075–80. quiz 23 10.1016/j.cgh.2009.05.026 [DOI] [PubMed] [Google Scholar]
- 12. Baixauli J, Kiran RP, Delaney CP. Investigation and management of ischemic colitis. Cleve Clin J Med 2003;70:920–1. 10.3949/ccjm.70.11.920 [DOI] [PubMed] [Google Scholar]
- 13. Yamazaki T, Shirai Y, Tada T, et al. Ischemic colitis arising in watershed areas of the colonic blood supply: a report of two cases. Surg Today 1997;27:460–2. 10.1007/BF02385714 [DOI] [PubMed] [Google Scholar]
- 14. Robert JH, Mentha G, Rohner A. Ischaemic colitis: two distinct patterns of severity. Gut 1993;34:4–6. 10.1136/gut.34.1.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O’Neill S, Elder K, Harrison SJ, et al. Predictors of severity in ischaemic colitis. Int J Colorectal Dis 2012;27:187–91. 10.1007/s00384-011-1301-x [DOI] [PubMed] [Google Scholar]
- 16. Añón R, Boscá MM, Sanchiz V, et al. Factors predicting poor prognosis in ischemic colitis. World J Gastroenterol 2006;12:4875–8. 10.3748/wjg.v12.i30.4875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. ten Heggeler LB, van Dam LJH, Bijlsma A, et al. Colon ischemia: right-sided colon involvement has a different presentation, etiology and worse outcome. A large retrospective cohort study in histology proven patients. Best Pract Res Clin Gastroenterol 2017;31:111–7. 10.1016/j.bpg.2016.12.004 [DOI] [PubMed] [Google Scholar]
- 18. Medina C, Vilaseca J, Videla S, et al. Outcome of patients with ischemic colitis: review of fifty-three cases. Dis Colon Rectum 2004;47:180–4. 10.1007/s10350-003-0033-6 [DOI] [PubMed] [Google Scholar]
- 19. Longstreth GF, Hye RJ. Right-Side colon ischemia: clinical features, large visceral artery occlusion, and long-term follow-up. Perm J 2015;19:11–16. 10.7812/TPP/15-024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sotiriadis J, Brandt LJ, Behin DS, et al. Ischemic colitis has a worse prognosis when isolated to the right side of the colon. Am J Gastroenterol 2007;102:2247–52. 10.1111/j.1572-0241.2007.01341.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sadot E, Telem DA, Cohen L, et al. Nonocclusive ischemic colitis: analysis of risk factors for severity. Am Surg 2014;80:454–60. [PubMed] [Google Scholar]
- 22. Hsieh J, Brandt L. Fecal calprotectin in ischemic colitis (IC). Am J Gastroenterol 2009;104 10.14309/00000434-200910003-00438 [DOI] [Google Scholar]
- 23. Brandt LJ, Feuerstadt P, Longstreth GF, et al. Acg clinical guideline: epidemiology, risk factors, patterns of presentation, diagnosis, and management of colon ischemia (CI). Am J Gastroenterol 2015;110:18–44. quiz 5 10.1038/ajg.2014.395 [DOI] [PubMed] [Google Scholar]
- 24. Wiesner W, Mortelé KJ, Glickman JN, et al. Ct findings in isolated ischemic proctosigmoiditis. Eur Radiol 2002;12:1762–7. 10.1007/s00330-001-1288-8 [DOI] [PubMed] [Google Scholar]
- 25. Al-Khowaiter SS, Brahmania M, Kim E, et al. Clinical and Endoscopic Significance of Bowel-Wall Thickening Reported on Abdominal Computed Tomographies in Symptomatic Patients With No History of Gastrointestinal Disease. Can Assoc Radiol J 2014;65:67–70. 10.1016/j.carj.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 26. Cruz C, Abujudeh HH, Nazarian RM, et al. Ischemic colitis: spectrum of CT findings, sites of involvement and severity. Emerg Radiol 2015;22:357–65. 10.1007/s10140-015-1304-y [DOI] [PubMed] [Google Scholar]
- 27. Chen M, Remer EM, Liu X, et al. Identification of the distinguishing features of Crohn's disease and ischemic colitis using computed tomographic enterography. Gastroenterol Rep 2017;5:219–25. 10.1093/gastro/gow037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim JS, Kim HJ, Hong S-M, et al. Post-Ischemic bowel stricture: CT features in eight cases. Korean J Radiol 2017;18:936–45. 10.3348/kjr.2017.18.6.936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pastor-Juan MdelR, Ripollés T, Martí-Bonmatí L, et al. Predictors of severity in ischemic colitis: usefulness of early ultrasonography. Eur J Radiol 2017;96:21–6. 10.1016/j.ejrad.2017.09.003 [DOI] [PubMed] [Google Scholar]
- 30. Danse EM, Van Beers BE, Jamart J, et al. Prognosis of ischemic colitis: comparison of color doppler sonography with early clinical and laboratory findings. AJR Am J Roentgenol 2000;175:1151–4. [DOI] [PubMed] [Google Scholar]
- 31. Zou X, Cao J, Yao Y, et al. Endoscopic findings and clinicopathologic characteristics of ischemic colitis: a report of 85 cases. Dig Dis Sci 2009;54:2009–15. 10.1007/s10620-008-0579-1 [DOI] [PubMed] [Google Scholar]
- 32. Trotter JM, Hunt L, Peter MB. Ischaemic colitis. BMJ 2016;355 10.1136/bmj.i6600 [DOI] [PubMed] [Google Scholar]
- 33. Beppu K, Osada T, Nagahara A, et al. Relationship between endoscopic findings and clinical severity in ischemic colitis. Intern. Med. 2011;50:2263–7. 10.2169/internalmedicine.50.5349 [DOI] [PubMed] [Google Scholar]
- 34. Montoro MA, Brandt LJ, Santolaria S, et al. Clinical patterns and outcomes of ischaemic colitis: results of the Working group for the study of ischaemic colitis in Spain (CIE study). Scand J Gastroenterol 2011;46:236–46. 10.3109/00365521.2010.525794 [DOI] [PubMed] [Google Scholar]
- 35. Hourmand-Ollivier I, Bouin M, Saloux E, et al. Cardiac sources of embolism should be routinely screened in ischemic colitis. Am J Gastroenterol 2003;98:1573–7. 10.1111/j.1572-0241.2003.07483.x [DOI] [PubMed] [Google Scholar]
- 36. Favier C, Bonneau HP, Minh T, et al. Endoscopic diagnosis of regressive ischemic colitis. Endoscopic, histologic and arteriographic correlations]. Nouv Presse Med 1976;5:77–9. [PubMed] [Google Scholar]
- 37. Houe T, Thorböll JE, Sigild U, et al. Can colonoscopy diagnose transmural ischaemic colitis after abdominal aortic surgery? An evidence-based approach. Eur J Vasc Endovasc Surg 2000;19:304–7. 10.1053/ejvs.1999.1005 [DOI] [PubMed] [Google Scholar]
- 38. Moszkowicz D, Trésallet C, Mariani A, et al. Ischaemic colitis: indications, extent, and results of standardized emergency surgery. Dig Liver Dis 2014;46:505–11. 10.1016/j.dld.2014.02.013 [DOI] [PubMed] [Google Scholar]
- 39. Mosele M, Cardin F, Inelmen EM, et al. Ischemic colitis in the elderly: predictors of the disease and prognostic factors to negative outcome. Scand J Gastroenterol 2010;45:428–33. 10.3109/00365520903513225 [DOI] [PubMed] [Google Scholar]
- 40. Reissfelder C, Sweiti H, Antolovic D, et al. Ischemic colitis: who will survive? Surgery 2011;149:585–92. 10.1016/j.surg.2010.11.008 [DOI] [PubMed] [Google Scholar]
- 41. Kim KH. Clinical characteristics of ischemic colitis according to the localization. J Korean Soc Coloproctol 2011;27:275 10.3393/jksc.2011.27.6.275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Genstorfer J, Schäfer J, Kettelhack C, et al. Surgery for ischemic colitis: outcome and risk factors for in-hospital mortality. Int J Colorectal Dis 2014;29:493–503. 10.1007/s00384-013-1819-1 [DOI] [PubMed] [Google Scholar]
- 43. Cosme A, Montoro M, Santolaria S, et al. Prognosis and follow-up of 135 patients with ischemic colitis over a five-year period. World J Gastroenterol 2013;19:8042–6. 10.3748/wjg.v19.i44.8042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paterno F, McGillicuddy EA, Schuster KM, et al. Ischemic colitis: risk factors for eventual surgery. Am J Surg 2010;200:646–50. 10.1016/j.amjsurg.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 45. Glauser PM, Wermuth P, Cathomas G, et al. Ischemic colitis: clinical presentation, localization in relation to risk factors, and long-term results. World J Surg 2011;35:2549–54. 10.1007/s00268-011-1205-5 [DOI] [PubMed] [Google Scholar]
- 46. Flobert C, Cellier C, Berger A, et al. Right colonic involvement is associated with severe forms of ischemic colitis and occurs frequently in patients with chronic renal failure requiring hemodialysis. Am J Gastroenterol 2000;95:195–8. 10.1111/j.1572-0241.2000.01644.x [DOI] [PubMed] [Google Scholar]
- 47. Gilshtein H, Hallon K, Kluger Y. Ischemic colitis caused increased early and delayed mortality. World J Emerg Surg 2018;13:31 10.1186/s13017-018-0193-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Castleberry AW, Turley RS, Hanna JM, et al. A 10-year longitudinal analysis of surgical management for acute ischemic colitis. J Gastrointest Surg 2013;17:784–92. 10.1007/s11605-012-2117-x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
flgastro-2019-101204supp001.pdf (61.1KB, pdf)