Abstract
Introduction
Individuals who enroll in intensive behavioral therapy (IBT) programs are asked to make several lifestyle changes simultaneously. However, few studies have examined the relative effects of adherence to different treatment components on weight loss.
Objective
This secondary analysis of the SCALE IBT trial assessed adherence to the medication regimen, dietary self-monitoring, and physical activity recommendations and their relative contributions to weight change in individuals with obesity who were provided with IBT combined with either liraglutide 3.0 mg or placebo.
Methods
SCALE IBT was a double-blinded, multicenter, randomized controlled trial comparing 56-week weight losses in individuals with obesity who received liraglutide 3.0 mg (n = 142) or placebo (n = 140), as an adjunct to IBT. Adherence to dietary self-monitoring, physical activity, and medication usage (liraglutide or placebo) were measured during the 56-week treatment period. A regression model was used to estimate the relative contribution of adherence to each treatment component to weight loss at week 56.
Results
The proportion of individuals who adhered to each intervention component decreased over time. Compared with non-adherence, complete adherence to dietary self-monitoring and physical activity recommendations were associated with estimated weight changes of −7.2% (95% CI −10.4 to −4.0; p < 0.0001) and −2.0% (95% CI −3.2 to −0.8; p = 0.0009), respectively. Complete adherence to liraglutide predicted an additional weight loss of −6.5% (95% CI −10.2 to −2.9; p = 0.0005) relative to individuals who did not adhere to the medication regimen, while adherence to placebo did not have a statistically significant effect on weight loss (p = 0.33).
Conclusions
High adherence to dietary self-monitoring and use of liraglutide 3.0 mg was associated with clinically relevant weight loss with IBT and adjunctive pharmacotherapy. The effect of adherence to physical activity was significant but smaller.
Keywords: Adherence, Weight loss, Diet, Exercise, Liraglutide
Introduction
Obesity treatment guidelines recommend that patients who would benefit from weight loss participate in a comprehensive lifestyle modification program with the goal of losing 5–10% of their initial weight [1, 2, 3]. Individuals who are enrolled in these programs are instructed to make several changes to their lifestyle simultaneously. These behaviors typically include consuming a reduced calorie diet, increasing physical activity, and engaging in supportive behaviors such as regularly attending counseling visits and self-monitoring dietary intake, weight, and physical activity [1, 4, 5]. Implementing meaningful change in any one of these behaviors, in turn, requires that participants establish and maintain multiple secondary lifestyle changes. For example, increasing physical activity may necessitate waking up earlier, navigating scheduling conflicts, buying equipment or memberships, or sacrificing leisure time. Establishing and sustaining the behavior changes that comprise a weight-management intervention is therefore a complex undertaking, requiring considerable effort.
Most obesity treatment guidelines also now include recommendations for the adjunctive use of pharmacotherapy alongside lifestyle modification approaches [1, 2, 3]. Several anti-obesity medications have been approved by the Food and Drug Administration (FDA), including the glucagon-like peptide 1 receptor agonist, liraglutide 3.0 mg [6]. These medications increase average weight loss [7, 8, 9], but require individuals to make additional behavioral changes in order to adhere to a long-term medication regime.
The relationship between behavior change adherence and weight loss has been investigated in a number of lifestyle modification trials. Frequency of dietary self-monitoring is well established as a predictor of short-term weight loss (i.e., at 24 weeks or less), though few studies have evaluated the long-term benefits of this behavior [10, 11]. Similarly, several studies have identified a positive association between adherence to physical activity goals and weight loss [12]. However, few studies have examined simultaneously the relative benefits of adherence to these different treatment components. In two such studies, dietary self-monitoring conferred a weight loss benefit that was independent of adherence to other treatment components [13, 14]. Only one of the studies found physical activity adherence to be independently associated with weight loss. To our knowledge, no studies have evaluated the relative importance of adherence to a medication regimen alongside self-monitoring and physical activity recommendations when pharmacotherapy is added to lifestyle modification.
SCALE IBT was a randomized clinical trial (ClinicalTrials.gov: NCT02963935) designed to assess the effects of liraglutide 3.0 mg versus placebo, both in combination with intensive behavioral therapy (IBT), on weight loss in individuals with obesity [9]. In the primary analysis, participants assigned to take liraglutide 3.0 mg as an adjunct to IBT lost a mean of 7.5% of their initial weight at week 56, compared with a significantly smaller 4.0% for those assigned to IBT with placebo (p = 0.0003) [9]. The present pre-specified secondary analysis evaluated adherence throughout the 56-week trial to three treatment components: dietary self-monitoring, physical activity, and medication usage (i.e., liraglutide 3.0 mg or placebo). We assessed simultaneously whether adherence to these treatment components predicted 56-week weight loss.
Materials and Methods
Study Design
SCALE IBT was a multi-site, randomized, controlled, double-blind trial comparing liraglutide 3.0 mg to placebo, both combined with IBT, on weight loss at 56 weeks in people with obesity (defined as a body mass index [BMI] of ≥30). Participants were randomly assigned to either once-daily subcutaneous liraglutide 3.0 mg or placebo (1:1 ratio). For full details of the SCALE IBT trial design, please refer to the primary publication, Wadden et al. [9], 2019.
All participants received 23 brief (∼15 min) IBT counseling sessions over 56 weeks following a visit schedule based on the Centers for Medicare and Medicaid Services (CMS) recommendations for the treatment of obesity in primary care settings [15]. Visits occurred weekly for the first month, every 2 weeks in months 2–6, and once monthly in months 7–13. This schedule was followed regardless of whether participants lost ≥3 kg during the first 6 months, the CMS requirement for continued treatment after that point.
The CMS-based IBT program followed an abbreviated lifestyle counseling protocol based on the Diabetes Prevention Program, including recommendations for reduced caloric intake, increased physical activity, and behavior change [16, 17]. Participants' daily caloric target throughout the trial was based on their body weight at randomization and ranged from 1,200 to 1,800 kcal/day. All participants were initially prescribed 100 min of physical activity per week (e.g., walking or similar aerobic activity). They were encouraged to be physically active for bouts of 10 min or more at a moderate intensity and to spread their physical activity equally across 4–5 days each week. Their target activity goal increased by 25 min every 4 weeks, up to a prescription of 250 min per week.
All participants were instructed to record their daily food and calorie intake using paper or electronic records. They were asked to wear electronic activity trackers (Polar® Loop 2) to provide objective data on levels of physical activity. Participants were asked to report at treatment visits whether or not they had administrated the trial product each week.
Measures
Weight change was measured as the percent reduction in body weight from baseline to week 56.
Adherence variables were measured continuously as a percentage, calculated by dividing the number of weeks in which a participant adhered to a given intervention by 56 weeks. At each IBT session, interventionists assessed and recorded weekly adherence to each treatment component using the following prespecified definitions:
Dietary self-monitoring adherence was assessed via participants' completion of food diaries and was defined as completing at least one food diary entry on 5 days per week.
Physical activity adherence was assessed using activity recorded by the electronic activity trackers and was defined as completing at least 50% of the target minutes of physical activity per week. In the primary analysis, physical activity was scored as the mean percentage fulfillment of total physical activity goals summed at the end of trial, rather than on a weekly basis.
Medication (trial product) adherence was recorded on a weekly basis by study interventionists based on participants' self-report and was defined as taking at least one administration of the trial product per week. Taking at least one dose per week was considered to represent taking the medication on most days because missing >3 consecutive days required reinitiation of the dose escalation (i.e., restarting at 0.6 mg).
Statistical Methods
Associations among Adherence to the Intervention Components
We first sought to determine whether a participant, who is adherent to one component of the weight loss intervention, is more likely to be adherent to the other components. Spearman correlation coefficients were used to assess bivariate associations between adherence to each intervention component and the other two components.
Differences in Adherence between Treatment Arms
Next, we investigated whether participants randomized to liraglutide were more or less likely to be adherent to the different intervention components than those assigned to placebo. The number of weeks meeting the adherence criteria for each intervention component was analyzed using a negative binomial model, with treatment as the primary predictor and baseline BMI, sex, and baseline body weight as covariates. Outcomes were expressed as comparative treatment ratios for liraglutide versus placebo. A treatment ratio of 1 denotes no difference in adherence between the treatment arms, values >1 favor liraglutide, and values <1 favor placebo.
Effect of Adherence on Weight Loss
The primary analysis evaluated the effect of adherence to the intervention components on change in body weight (%) at week 56. This was tested using a linear regression model that included dietary self-monitoring, physical activity, and medication adherence, and their interactions with randomized treatment group. This regression model was reduced by removing nonsignificant interactions with treatment group (p > 0.05). The resulting regression coefficients were used to estimate the independent contribution of adherence to each component on 56-week weight loss.
Results
Trial Participants and Mean Adherence
A total of 282 participants were randomized; 142 were assigned to liraglutide 3.0 mg and 140 to placebo, both in combination with IBT. The liraglutide and placebo-treated groups had mean (SD) BMIs at baseline of 39.3 (6.8) and 38.7 (7.2), respectively. Their mean ages were 45.5 (11.6) and 49.0 (11.2) years, 83.8 and 82.9% were female, and 78.9 and 82.1% were identified as white (19.0 and 15.7% as black), respectively. All other baseline demographics also were well matched between the treatment groups [9].
A high proportion of participants completed the trial (99.3% in the liraglutide 3.0 mg group and 92.9% in the placebo group) and remained on trial product at week 56 (80.3 and 73.6%, respectively). As shown in Table 1, a total of 81 participants discontinued the trial product at least once, including 36 in the liraglutide group and 45 in the placebo group. Of those patients, 29 later resumed taking the trial product and 16 also went on to be treatment completers (8 participants in each group).
Table 1.
Trial disposition
| Liraglutide 3.0 mg + IBT | Placebo + IBT | Total | |
|---|---|---|---|
| Randomized, n (%) | 142 (100) | 140 (100) | 282 (100) |
| Exposed, n (%) | 142 (100) | 140 (100) | 282 (100) |
| On drug at week 56, n (%) | 114 (80.3) | 103 (73.6) | 217 (77.0) |
| Participants who discontinued trial product at least once, n (% of randomized) | 36 (25.4) | 45 (32.1) | 81 (28.7) |
| Participants who resumed treatment having discontinued at least once, n (% of discontinued) | 13 (36.1) | 16 (35.6) | 29 (35.8) |
| Participants who were treatment completers having discontinued at least once and then resumed treatment, n (% of resumed) | 8 (61.5) | 8 (50.0) | 16 (55.2) |
| Discontinued trial product, n (%) | 28 (19.7) | 37 (26.4) | 65 (23.0) |
| Participants who discontinued but returned for final study visit (retrieved), n (%) | 27 (19.0) | 27 (19.3) | 54 (19.1) |
| Withdrawals, n (%) | 1 (0.7) | 10 (7.1) | 11 (13.9) |
IBT, intensive behavioral therapy.
Intercorrelations in Adherence to the Intervention Components
Adherence to the weight loss intervention components had small to moderate positive correlations with each other. The size of these correlations ranged from 0.27 to 0.41 (Table 2).
Table 2.
Correlations among different adherence measures
| Total number of weeks adherent to physical activity | Total number of weeks adherent to dietary self-monitoring | |
|---|---|---|
| Total number of weeks adherent to dietary self-monitoring | 0.41 (p < 0.0001) | N/A |
| Total number of weeks adherent to trial product | 0.32 (p < 0.0001) | 0.27 (p < 0.0001) |
Data are rho values based on Spearman correlation coefficients.
Adherence over 56 Weeks
Table 3 shows the mean number of weeks that participants adhered to each intervention component during the 56-week study period. Summed across the groups, participants adhered to the dietary self-monitoring, physical activity, and trial product goals on 37.2, 29.5, and 48.2 of the 56 weeks, respectively.
Table 3.
Number of weeks that patients adhered to each intervention component during the 56-week IBT program
| Liraglutide 3.0 mg + IBT (n = 142) | Placebo + IBT (n = 140) | |
|---|---|---|
| Weeks adherent to dietary self-monitoring | 38.4 (16.0) | 36.1 (17.3) |
| Weeks adherent to physical activity | 29.0 (17.1) | 30.0 (17.2) |
| Weeks adherent to the trial product | 49.5 (14.0) | 46.8 (16.1) |
Data are means (SD). The maximum number of weeks that a participant could have adhered to the intervention was 56 for all treatment components. IBT, intensive behavioral therapy.
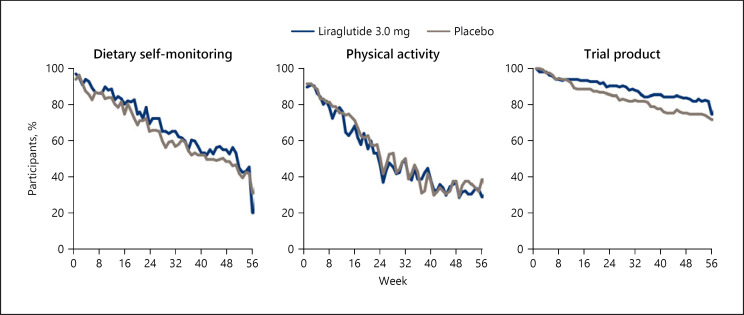
Figure 1 shows the proportion of participants in each treatment group who were adherent to the dietary self-monitoring, physical activity, and trial product goals as a function of the week since randomization. As seen in the figure, the proportion of participants who were adherent to each intervention decreased steadily over the 56-week follow-up period across both treatment groups. For example, at week 4 (the end of the weekly treatment and dose titration period), 91.1% of participants adhered to dietary self-monitoring, 87.6% to the physical activity recommendation, and 98.2% to trial product. By week 24, this had declined to 67.7, 49.3, and 88.3%, respectively, and at week 56, 26.7, 34.3, and 73.3%, respectively, were adherent to these recommendations.
Fig. 1.
Proportion of participants adhering to dietary self-monitoring, physical activity, and trial product over time. Data are observed mean proportions over time.
There were no significant differences between the liraglutide and placebo groups in adherence to any of the intervention components: dietary self-monitoring (treatment ratio [TR] 1.07 [95% CI 0.94 to 1.22]; p = 0.2762), physical activity (TR 0.99 [95% CI 0.84 to 1.16]; p = 0.8827), or medication usage (TR 1.06 [95% CI 0.95 to 1.17]; p = 0.3042).
Adherence and Body Weight
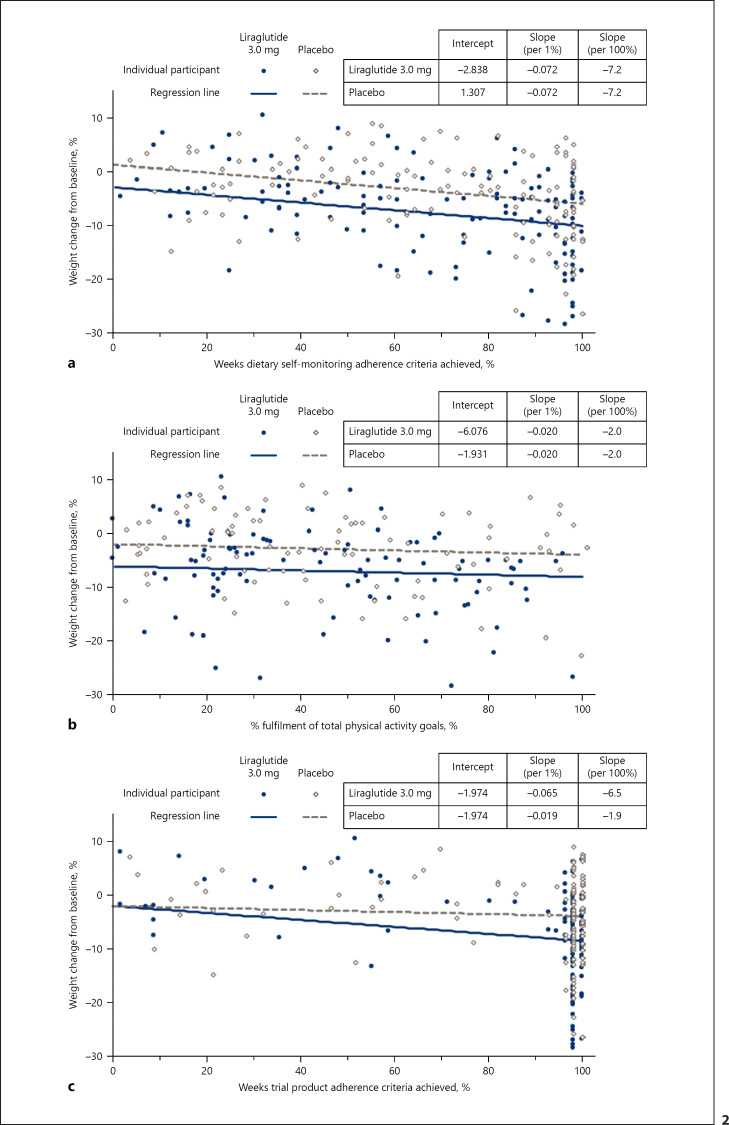
The regression model estimated the effects of adherence on body weight for each component throughout the 56-week treatment period. Figure 2a–c demonstrates the relationship between participants' attainment of the adherence criteria on the x axis, and the estimated weight loss achieved in the trial on the y axis. The placebo-adjusted effect of liraglutide 3.0 mg on weight loss is reflected in the differences between regression lines.
Fig. 2.
Weight change (%) from baseline to week 56 as function of weeks adherent to (a) dietary self-monitoring, (b) physical activity, and (c) the medication regimen. Data are estimated means using a regression model that included dietary self-monitoring, physical activity, and medication adherence, and the interaction between treatment condition and trial product (all other interaction terms were not statistically significant). Regression lines correspond to mean adherence to (a) physical activity, (b) dietary self-monitoring, (c) liraglutide 3.0 mg and placebo. Scatter plots were created using estimated data for individuals' data points. The regression lines in each panel assume mean adherence for the two other components. The difference in weight loss between the mean estimates at 0 and 100% adherence is described in the text.
The effects of dietary self-monitoring and physical activity adherence on weight loss did not differ significantly between the treatment groups, and interaction terms were removed from the final model. Participants who adhered to dietary self-monitoring on all 56 weeks were predicted to lose 7.2 percentage points more in baseline weight (95% CI −10.4 to −4.0; p < 0.0001) than participants who did not self-monitor on any of the weeks. Participants who adhered to the physical activity recommendations for all 56 weeks had an estimated reduction of 2.0 percentage points more of initial body weight (95% CI −3.2 to −0.8; p = 0.0009), when compared to those with zero weeks of adherence.
There were significant differences between the liraglutide and placebo groups in the effect of adhering to the medication regimen. Participants who were adherent to administering liraglutide 3.0 mg on all 56 weeks were predicted to achieve an additional mean weight loss of 6.5 percentage points (95% CI −10.2 to −2.9; p = 0.0005) when compared to 0 weeks of adherence. For the placebo-treated participants, full adherence did not result in a significantly larger weight loss (mean contribution of −1.9% [95% CI −5.6 to 1.9]; p = 0.33) when compared to no adherence. These treatment effects corresponded to a statistically significant, placebo-subtracted weight loss of 4.6% (95% CI −6.5 to −2.8; p < 0.0001) for patients fully adherent to the medication regimen.
We used this model to compare the estimated weight effects at 56 weeks between hypothetical individuals who were 100% adherent to all components of the intervention with counterparts who were 0% adherent. Hypothetical individuals with 0% adherence to any of the treatment components were estimated to have gained 4.6% (95% CI +1.5 to +7.7) of their initial weight at week 56. Individuals who were 100% adherent for the full 56 weeks to all three treatment components would have estimated weight losses of 11.1% (95% CI −12.6 to −9.6) and 6.5% (95% CI −8.0 to −4.9) of their initial weight in the liraglutide and placebo groups, respectively. Thus, the total estimated weight difference between 0 and 100% adherence would be −15.7% (95% CI −19.5 to −11.9) with liraglutide and −11.1% (95% CI −14.9 to −7.2) with placebo.
Discussion
The results of this pre-specified secondary analysis from the SCALE IBT trial demonstrated that greater adherence to dietary self-monitoring, physical activity, and liraglutide 3.0 mg were all associated with larger weight losses at 56 weeks. Only the effect of adherence to placebo did not reach statistical significance. Full adherence to dietary self-monitoring and liraglutide 3.0 mg were associated with the largest changes in weight from baseline. Although statistically significant, full adherence to the physical activity recommendation was not associated with a clinically meaningful weight loss at week 56 (i.e., ≥5% of body weight) relative to participants who did not meet the target activity goal at any point during the study period. Of note, treatment with liraglutide (versus placebo) did not result in a detectable difference in adherence to any component of the IBT program.
The present findings are consistent with those of previous studies that have reported bivariate associations between treatment adherence and weight loss with IBT [13, 14, 18]. The relatively smaller benefit of physical activity adherence in our study is also consistent with evidence suggesting that physical activity plays a larger role in weight maintenance than with initial weight loss [19]. Our results extend previous findings by measuring behavioral adherence and weight loss over a longer duration and by evaluating simultaneously the role of adherence to three different treatment components. They also provide an estimate of the additional weight loss associated with adherence to each component.
This is one of the first studies to test the relationship between medication adherence and weight loss when pharmacotherapy is added to IBT [20]. Adherence to the medication regimen was independently associated with larger weight losses for participants assigned to liraglutide plus IBT but not for those assigned to placebo plus IBT. This finding is likely consistent with the relative benefit of the active medication over placebo.
The placebo-subtracted effect on body weight of being fully adherent to trial product was also estimated as part of SCALE IBT's prespecified analysis plan using a different methodology (as reported with the primary outcomes) [8]. Using a mixed model repeated measures (MMRM) approach, weight change was estimated under the if-all-adhered-to-treatment principle by using data from individuals still on drug to predict effects in those who had discontinued. The mean placebo-subtracted weight change observed with the MMRM technique was very similar to the estimate achieved with the regression model in the present study; −4.59% (95% CI −6.54 to −2.64) and −4.63% (95% CI −6.45 to −2.81), respectively. This degree of concordance indicates that the underlying assumptions of the different statistical models used were robust.
The steady decreases in the percentage of participants who adhered to each component over the course of the 56-week trial may partly explain why weight loss typically slows after the initial months of an IBT program, though that relationship was not evaluated directly in the present data. It is unclear why more individuals struggle to achieve behavioral goals later in the program despite receiving ongoing support (e.g., accountability, problem solving) from an interventionist. In the present study, treatment visits occurred less frequently as the study progressed (weekly in month 1, every other week in months 2–6, and monthly in months 7–13). However, similar decreases in adherence were observed in a previous study that provided weekly visits for 6 months [14]. This suggests that visit frequency is not the only determinant of adherence, although this hypothesis would best be tested by an RCT that provided different visit schedules.
The relatively small correlations between adherence to the different intervention components may reflect that consistent adherence to diet, physical activity, and pharmacotherapy recommendations each require different skill sets, approaches, support systems, and tools. Determining predictors of adherence to these different components could inform the development of treatment methods to improve weight loss outcomes.
Although the present analyses were pre-specified, the approaches used to define weekly and overall adherence to the different treatment components were novel and thus preliminary in nature. The definitions used for adherence in the present study were relatively permissive and were scored as binary variables (yes/no) when characterizing adherence across each study week. It is possible that these criteria were insufficiently sensitive to capture differences between the treatment groups, intercorrelations among the adherence measures, or to detect more nuanced relationships between adherence and weight loss. However, it is notable that even using these permissive criteria, adherence to dietary self-monitoring and liraglutide usage were associated with clinically meaningful relative weight losses.
Additionally, the criterion for evaluating medication adherence relied on participants' self-report. It is possible that recall biases affected the accuracy of this data. The use of observed records from the food diaries and electronic activity trackers to assess adherence to the IBT components represents a relative strength.
We also note that estimates of the total weight loss that would be achieved by an individual who was 0 vs. 100% adherent to all treatment components were exploratory, and caution should be exercised when interpreting these data. No participants actually had zero adherence to all three intervention components; therefore the plausibility of the estimated total weight change of such individuals could not be verified with the data. However, there were data for individuals who were close to 100% adherent to all three components, and the observed total weight losses of those subjects (data not shown) were in line with the model estimates, which lends support to the validity of the model.
We chose to include in our analyses representative measures of adherence to each of the core components of an IBT program with adjunctive pharmacotherapy: diet, physical activity, and medication. However, additional aspects of treatment adherence were not reported here, including adherence to the prescribed calorie targets and to session attendance. Most previous studies also have used dietary self-monitoring as a proxy for overall dietary adherence [18, 20, 21, 22], and one study found that dietary monitoring predicts weight loss when controlling for adherence to the calorie goal [14]. However, the relative importance of these additional behaviors could not be determined in the present study.
The present results also cannot be used to determine a causal relationship between adherence and weight loss, or the direction of causality if present. Although this study was designed to investigate whether adherence influences weight loss, weight loss also may influence adherence. For example, amount of weight change could affect participants' motivation to continue complying with components of the intervention or the accuracy of their retrospective self-report of these behaviors (for medication adherence).
Despite these limitations, these findings do support there being a strong association between treatment adherence and weight loss with IBT and adjunctive liraglutide 3.0 mg. The notable decline in the number of participants who adhered to these behaviors during the trial is therefore concerning, particularly when considering that these data come from a clinical trial setting in which individuals were selected from the general patient population based, in part, on their motivation and willingness to participate. Further research that helps to identify barriers to ongoing adherence as they develop could help to inform strategies for improving long-term adherence to intervention components.
The results of this secondary analysis of data from SCALE IBT demonstrated that treatment adherence is not a unitary construct and is specific to individual intervention components. Although medication adherence remained high in both treatment groups, adherence to all treatment components decreased over time. Overall, these findings suggested that adherence to dietary self-monitoring and liraglutide 3.0 mg were associated with clinically relevant weight losses with IBT and adjunctive pharmacotherapy, whereas adherence to the physical activity recommendation was associated with a significant but smaller added weight loss.
Statement of Ethics
The trial protocol was approved by the Copernicus Group Independent Review Board® (NOV1-16-503) for 16 of the 17 sites, and by the local Institutional Review Board-III − Medical University of South Carolina (Pro00061921) for the remaining site. The study was conducted in accordance with the principles of the Declaration of Helsinki and International Council for Harmonisation (ICH) Good Clinical Practice guidelines. All trial participants formally agreed to publication of the trial results as part of their Informed Consent Forms.
Conflict of Interest Statement
J.S.T. has received consulting fees and/or honoraria for speaking for Novo Nordisk. A.N.F. is an employee of Novo Nordisk, Inc. T.A.W. has received grants, on behalf of the University of Pennsylvania, from Novo Nordisk, as well as honoraria from Novo Nordisk and WW (formerly Weight Watchers) for serving on scientific advisory boards. P.A. is a full-time employee of Novo Nordisk and owns stocks in the company. L.E. holds stocks in and is an employee of Novo Nordisk. D.S. has received research grants from Novo Nordisk. D.R. has received grants from Obesinov SARL, and consulting fees/honoraria for speaking from Novo Nordisk.
Funding Sources
The study was sponsored by Novo Nordisk, which developed the study protocol, supplied the trial drugs, planned and performed the statistical analyses, and funded writing assistance.
Author Contributions
J.S.T., A.N.F., T.A.W., P.A., L.E., D.S., and D.R. were involved in the design or conduct of the study, the preparation of the manuscript, and the decision to submit it for publication, and all verify the accuracy and completeness of the data and analyses.
Acknowledgements
We thank Adam Dagnall, DPhil, and Beverly La Ferla, MRes, of Watermeadow Medical, an Ashfield Company, for editorial and medical writing services, which were funded by Novo Nordisk.
References
- 1.Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Obesity Society 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014 Jun;129((25 Suppl 2)):S102–38. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Endocrine Society Pharmacological management of obesity: an endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2015 Feb;100((2)):342–62. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 3.Garvey WT, Mechanick JI, Brett EM, Garber AJ, Hurley DL, Jastreboff AM, et al. Reviewers of the AACE/ACE Obesity Clinical Practice Guidelines American Association of Clinical Endocrinologists and American College of Endocrinology Comprehensive Clinical Practice Guidelines for Medical Care of Patients with Obesity. Endocr Pract. 2016 Jul;22(Suppl 3):1–203. doi: 10.4158/EP161365.GL. [DOI] [PubMed] [Google Scholar]
- 4.Wadden TA, Webb VL, Moran CH, Bailer BA. Lifestyle modification for obesity: new developments in diet, physical activity, and behavior therapy. Circulation. 2012 Mar;125((9)):1157–70. doi: 10.1161/CIRCULATIONAHA.111.039453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Webb VL, Wadden TA. Intensive Lifestyle Intervention for Obesity: Principles, Practices, and Results. Gastroenterology. 2017 May;152((7)):1752–64. doi: 10.1053/j.gastro.2017.01.045. [DOI] [PubMed] [Google Scholar]
- 6.Novo Nordisk A/S Saxenda Prescribing Information®. [cited 27 May 2020]. Available from: https://www.novo-pi.com/saxenda.pdf.
- 7.Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of Pharmacological Treatments for Obesity With Weight Loss and Adverse Events: A Systematic Review and Meta-analysis. JAMA. 2016 Jun;315((22)):2424–34. doi: 10.1001/jama.2016.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wadden TA, Walsh OA, Berkowitz RI, Chao AM, Alamuddin N, Gruber K, et al. Intensive Behavioral Therapy for Obesity Combined with Liraglutide 3.0 mg: A Randomized Controlled Trial. Obesity (Silver Spring) 2019 Jan;27((1)):75–86. doi: 10.1002/oby.22359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wadden TA, Tronieri JS, Sugimoto D, Lund MT, Auerbach P, Jensen C, et al. Liraglutide 3.0 mg and Intensive Behavioral Therapy (IBT) for Obesity in Primary Care: The SCALE IBT Randomized Controlled Trial. Obesity (Silver Spring) 2020 Mar;28((3)):529–36. doi: 10.1002/oby.22726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helsel DL, Jakicic JM, Otto AD. Comparison of techniques for self-monitoring eating and exercise behaviors on weight loss in a correspondence-based intervention. J Am Diet Assoc. 2007 Oct;107((10)):1807–10. doi: 10.1016/j.jada.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 11.Ingels JS, Misra R, Stewart J, Lucke-Wold B, Shawley-Brzoska S. The Effect of Adherence to Dietary Tracking on Weight Loss: Using HLM to Model Weight Loss over Time. J Diabetes Res. 2017;2017:6951495. doi: 10.1155/2017/6951495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conroy MB, Yang K, Elci OU, Gabriel KP, Styn MA, Wang J, et al. Physical activity self-monitoring and weight loss: 6-month results of the SMART trial. Med Sci Sports Exerc. 2011 Aug;43((8)):1568–74. doi: 10.1249/MSS.0b013e31820b9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollis JF, Gullion CM, Stevens VJ, Brantley PJ, Appel LJ, Ard JD, et al. Weight Loss Maintenance Trial Research Group Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008 Aug;35((2)):118–26. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acharya SD, Elci OU, Sereika SM, Music E, Styn MA, Turk MW, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Patient Prefer Adherence. 2009 Nov;3:151–60. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services (CMS) ADecision memo for intensive behavioral therapy for obesity (CAG-00423N) [cited 27 May 2020]. Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=253.
- 16.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002 Feb;346((6)):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wadden TA, Volger S, Sarwer DB, Vetter ML, Tsai AG, Berkowitz RI, et al. A two-year randomized trial of obesity treatment in primary care practice. N Engl J Med. 2011 Nov;365((21)):1969–79. doi: 10.1056/NEJMoa1109220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burke LE, Conroy MB, Sereika SM, Elci OU, Styn MA, Acharya SD, et al. The effect of electronic self-monitoring on weight loss and dietary intake: a randomized behavioral weight loss trial. Obesity (Silver Spring) 2011 Feb;19((2)):338–44. doi: 10.1038/oby.2010.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaput JP, Klingenberg L, Rosenkilde M, Gilbert JA, Tremblay A, Sjödin A. Physical activity plays an important role in body weight regulation. J Obes. 2011;2011:360257. doi: 10.1155/2011/360257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tronieri JS, Wadden TA, Walsh O, Berkowitz RI, Alamuddin N, Chao AM. Measures of adherence as predictors of early and total weight loss with intensive behavioral therapy for obesity combined with liraglutide 3.0 mg. Behav Res Ther. 2020 Aug;131:103639. doi: 10.1016/j.brat.2020.103639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wadden TA, Berkowitz RI, Womble LG, Sarwer DB, Phelan S, Cato RK, et al. Randomized trial of lifestyle modification and pharmacotherapy for obesity. N Engl J Med. 2005 Nov;353((20)):2111–20. doi: 10.1056/NEJMoa050156. [DOI] [PubMed] [Google Scholar]
- 22.Laitner MH, Minski SA, Perri MG. The role of self-monitoring in the maintenance of weight loss success. Eat Behav. 2016 Apr;21:193–7. doi: 10.1016/j.eatbeh.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]