Abstract
The stringent response is characterized by the synthesis of the messenger molecules pppGpp, ppGpp or pGpp (here collectively designated (pp)pGpp). The phenotypic consequences resulting from (pp)pGpp accumulation vary among species and can be mediated by different underlying mechanisms. Most genome-wide analyses have been performed under stress conditions, which often mask the immediate effects of (pp)pGpp-mediated regulatory circuits. In Staphylococcus aureus, (pp)pGpp can be synthesized via the RelA-SpoT-homolog, RelSau upon amino acid limitation or via one of the two small (pp)pGpp synthetases RelP or RelQ upon cell wall stress. We used RNA-Seq to compare the global effects in response to induction of the synthetase of rel-Syn (coding for the enzymatic region of RelSau) or relQ without the need to apply additional stress conditions. Induction of rel-Syn resulted in changes in the nucleotide pool similar to induction of the stringent response via the tRNA synthetase inhibitor mupirocin: a reduction in the GTP pool, an increase in the ATP pool and synthesis of pppGpp, ppGpp and pGpp. Induction of all three enzymes resulted in similar changes in the transcriptome. However, RelQ was less active than Rel-Syn and RelP, indicating strong restriction of its (pp)pGpp-synthesis activity in vivo. (pp)pGpp induction resulted in the downregulation of many genes involved in protein and RNA/DNA metabolism. Many of the (pp)pGpp upregulated genes are part of the GTP sensitive CodY regulon and thus likely regulated through lowering of the GTP pool. New CodY independent transcriptional changes were detected including genes involved in the SOS response, iron storage (e.g. ftnA, dps), oxidative stress response (e.g., perR, katA, sodA) and the psmα1–4 and psmß1-2 operons coding for cytotoxic, phenol soluble modulins (PSMs). Analyses of the ftnA, dps and psm genes in different regulatory mutants revealed that their (pp)pGpp-dependent regulation can occur independent of the regulators PerR, Fur, SarA or CodY. Moreover, psm expression is uncoupled from expression of the quorum sensing system Agr, the main known psm activator. The expression of central genes of the oxidative stress response protects the bacteria from anticipated ROS stress derived from PSMs or exogenous sources. Thus, we identified a new link between the stringent response and oxidative stress in S. aureus that is likely crucial for survival upon phagocytosis.
Author summary
Most bacteria make use of the second messenger (pp)pGpp to reprogram bacterial metabolism under nutrient-limiting conditions. In the human pathogen Staphylococcus aureus, (pp)pGpp plays an important role in virulence, phagosomal escape and antibiotic tolerance. Here, we analyzed the immediate consequences of (pp)pGpp synthesis upon transcriptional induction of the (pp)pGpp-producing enzymes Rel, RelP or RelQ. (pp)pGpp synthesis provokes immediate changes in the nucleotide pool and severely impacts the expression of hundreds of genes. A main consequence of (pp)pGpp synthesis in S. aureus is the induction of ROS-inducing toxic phenol soluble modulins (PSMs) and simultaneous expression of the detoxifying system to protect the producer. This mechanism is likely of special advantage for the pathogen after phagocytosis.
Introduction
The stringent response is characterized by the synthesis of the alarmones pGpp, ppGpp and pppGpp, here collectively named (pp)pGpp. (pp)pGpp interferes with many cellular processes, including transcription, replication and translation [1,2,3,4,5,6,7,8,9,10]. Depending on the species, the stringent response is crucial for diverse biological processes, including differentiation, biofilm formation, antibiotic tolerance, production of secondary metabolites or virulence [8,11]. It is now clear that there are fundamental differences between the stringent response initially characterized in Eschericia coli and the stringent response in Firmicutes [7,9]. Differences have been observed in the enzymes involved in the synthesis and degradation of the messengers and in the downstream effects of (pp)pGpp.
(pp)pGpp is synthesized by RelA-SpoT-homologs (RSHs) by transferring pyrophosphate originating from ATP to the 3´ OH group of GTP, GDP or GMP. Long RSH enzymes are present in nearly all bacteria and show a conserved molecular architecture composed of a C-terminal sensory region and an N-terminal enzymatic region with distinct (pp)pGpp hydrolase and synthetase domains [12]. Firmicutes, such as Staphylococcus aureus, possess one long RSH enzyme, RelSau and in addition two small alarmone synthetases (SAS), RelP and RelQ. Amino acid limitation is the only condition known to induce a RelSau-mediated stringent response phenotype [13]. Under non-inducing conditions, RelSau is primarily in a hydrolase-On/synthetase-Off conformation even when the C-terminal sensory region is deleted [14]. The strong hydrolase activity of RelSau makes the enzyme essential for the detoxification of (pp)pGpp produced by RelP or RelQ [13].
RelP and RelQ are part of the VraRS cell-wall stress regulon [15] and are thus transcriptionally induced, e.g., after vancomycin treatment [16]. Thereby, they contribute to tolerance towards cell-wall active antibiotics such as ampicillin or vancomycin. Recently, structural and mechanistic characterization revealed that RelQ from Bacillus subtilis and Enterococcus faecalis form tetramers [17,18]. RelQ activity is strongly inhibited through the binding of single-stranded RNA. pppGpp binding leads to disassociation of the RelQ:RNA complex and its activation [18]. In contrast, RelP activity is inhibited by both pppGpp and ppGpp, activated by Zn2+ and insensitive to inhibition by RNA [19,20]. For the S. aureus enzymes it could be shown that RelQ, but not RelP are allosterically stimulated by the addition of pppGpp, ppGpp or pGpp [21]. Thus, although highly homologous, RelP and RelQ seem to have different functions within the cell. One can assume that different post-translational regulatory mechanisms are in play to fine-tune (pp)pGpp synthesis under different growth conditions.
In S. aureus, the stringent response plays important roles in virulence [13], phagosomal escape [22] and antibiotic tolerance [8,16,23,24,25,26,27]. The enzymes HprT and Gmk involved in GTP synthesis, putative GTPases (RsgA, RbgA, Era, HflX, and ObgE) and DNA primase were identified as (pp)pGpp target proteins [23,28]. (pp)pGpp binding inhibits enzyme activities, resulting in lowering of the GTP pool and inhibition of the translation apparatus and replication. Of note, in contrast to E. coli, (pp)pGpp from Firmicutes does not interfere with RNA polymerase activity [10]. Instead, in these organisms, (pp)pGpp regulates transcription via an indirect mechanism that strongly relies on the lowering of the intracellular GTP pool [22,28,29,30]. A decrease in the GTP level leads to the repression of nucleotide-sensitive, GTP-initiating promoters, e.g., those of rRNA genes [30,31]. Low GTP levels also affect the CodY regulon. The transcription factor CodY, when loaded with GTP and branched-chain amino acids, acts mainly as a repressor of many genes involved in amino acid synthesis and virulence [32,33]. The global transcriptional effects of (pp)pGpp have been examined previously in several Firmicutes, such as B. subtilis [34], Streptococcus pneumoniae [35], Enterococcus faecalis [36], Streptococcus mutans [37] and S. aureus [22]. These studies were based on the comparison of the wild-type and rel mutant strains under conditions mimicking amino acid starvation. Of note, these stress conditions are accompanied by profound physiological changes, which are only partially mediated by (pp)pGpp. For instance, amino acid limitation leads to the stabilization of many transcripts independent of (pp)pGpp [13]. Thus, from these analyses, it is difficult to draw firm conclusions on the primary transcriptional changes imposed by (pp)pGpp synthesis. Recently, one study tried to circumvent this drawback by transcriptional induction of (pp)pGpp synthetase in E. coli and gained major new insights [38].
Here, we aimed to compare the Rel-, RelQ- and RelP-mediated effects on nucleotide pools, transcription and functional consequences without imposing nutrient starvation or stress. Therefore, the Rel synthetase (Rel-Syn, N-Terminal region of Rel with mutated hydrolase), RelP and RelQ were expressed from an anhydrotetracycline (ATc)-inducible promoter in a (pp)pGpp0 strain in which the enzymatic domains of all three synthetases were mutated. Through RNA-Seq analyses, we identified new (pp)pGpp-regulated genes, many of which are involved in the oxidative stress response, iron storage and the synthesis of phenol-soluble modulins (PSMs). Thus, (pp)pGpp synthesis contributes not only to PSM-derived ROS production but also to protection from these toxic molecules.
Results
Changes in the nucleotide pools after transcriptional induction of rel-Syn and relQ
We first compared the stringent response imposed by mupirocin (isoleucyl-tRNA synthase inhibitor [39]) with the genetic induction of (pp)pGpp synthetases. To analyse Rel dependent (pp)pGpp synthesis without stress, we cloned the N-terminal region of Rel with inactivated hydrolase domain [14] designated as Rel-Syn. Rel-Syn or relQ were expressed using an ATc-inducible expression system in a (pp)pGpp0 strain background. Strain (pp)pGpp0 is unable to synthesize (pp)pGpp due to mutations in all three (pp)pGpp synthetases (full deletion of rel, synthetase mutations in relP and relQ [14]). Strains were grown to an early exponential growth phase and gene expression was induced by ATc for 30 min. Sub-inhibitory concentration of ATc resulted in similar induction of rel-Syn or relQ, (7.0 and 6,7 log2 fold increase compared to un-induced, respectively, S1 Data). ATc treatment did not influence gene expression in strains containing the empty control plasmid pCG248 (S1 Fig).
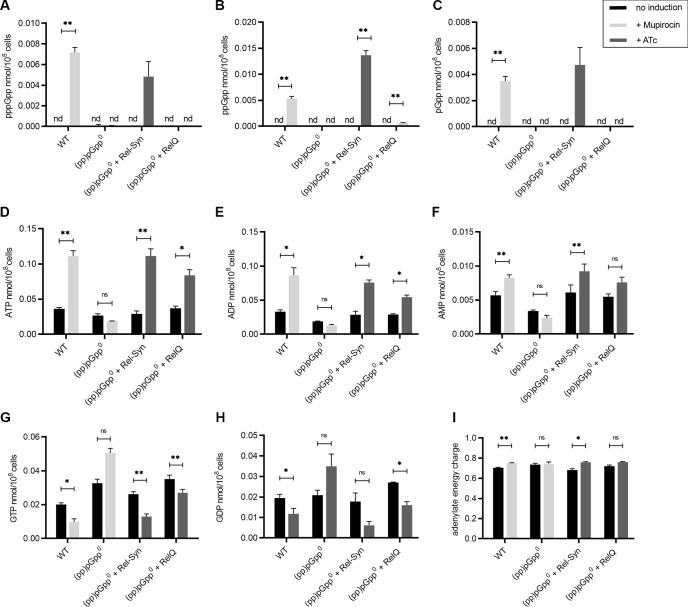
Quantification of the nucleotide pools after mupirocin treatment or induction of rel-syn or relQ revealed that induction of rel-syn resulted in similar levels of pppGpp, ppGpp, and pGpp as induction of the stringent response in the wild type by mupirocin (Fig 1). In Firmicutes, (pp)pGpp inhibit several enzymes involved in purine nucleotide synthesis and transport [7]. Accordingly, the concentrations of guanine nucleotides GTP and GDP was negatively correlated to (pp)pGpp synthesis (Fig 1). Of note, (pp)pGpp synthesis leads to concomitant increase of adenine nucleotides (ATP, ADP and AMP) and a slight increase in the adenylate energy charge. In the (pp)pGpp0 strain, mupirocin treatment resulted in significant increase of GTP and decrease ATP concordant with previous results [30]. In summary, Rel-mediated (pp)pGpp synthesis imposed by mupirocin or genetic rel-syn induction resulted in similar changes of the nucleotide pool characterised by reduction of guanine nucleotides and accumulation of adenine nucleotides. After induction of relQ, similar changes in the nucleotide pools were detectable. However, the effect of relQ induction on the nucleotide pools was significantly lower than that of rel-syn induction or mupirocin treatment. Thus, rel-syn induction probably mirrors stress conditions (e.g. upon mupirocin treatment), whereas relQ expression more likely reflect basal low level (pp)pGpp synthesis.
Fig 1. Changes in the nucleotide pool after mupirocin treatment or transcriptional induction of rel-Syn or relQ.
Strain HG001 and derivatives were grown to OD600 = 0.3 and treated for 30 min with or without 0.125 μg/ml mupirocin (lightgrey) or 0.1 μg/ml ATc (darkgrey). Nucleotide analyses were performed using mass spectrometry (ESI-TOF) in negative ion mode. Error bars represent SEM (n = 3) from three biological replicates. The adenylate energy charge was calculated by [ATP] + 0.5 [ADP]/[ATP] + [ADP] + [AMP]. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
Impact of (pp)pGpp synthesis on the transcriptome
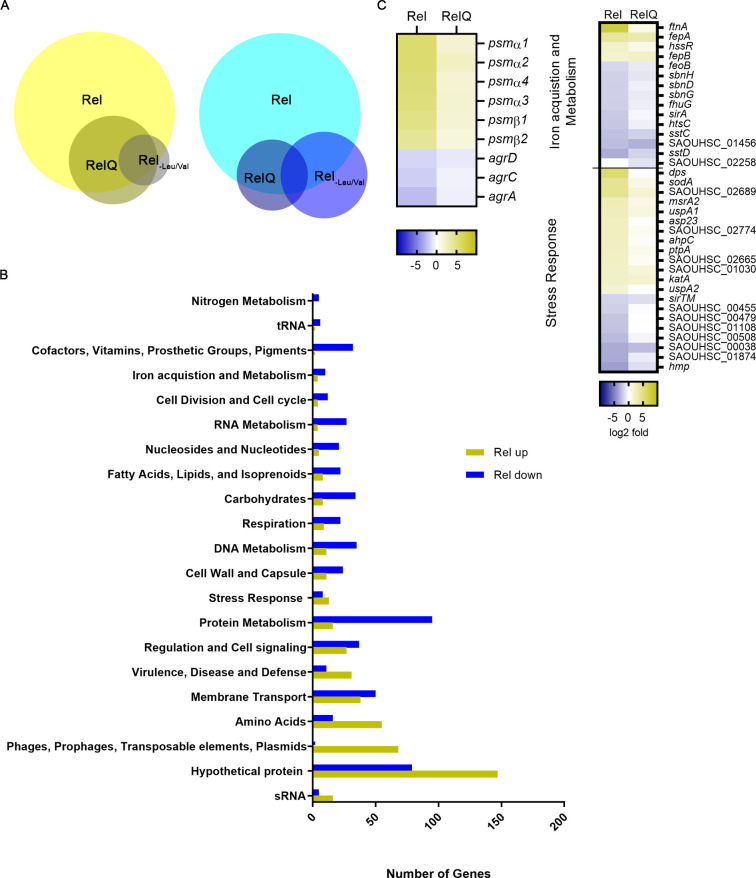
We next analysed the impact of (pp)pGpp synthesis after induction of rel-syn or relQ on mRNA abundance. RNA-Seq data revealed that 1074 genes or sRNAs were significantly affected by either Rel-Syn (total: 478 up, 551 down) or RelQ (total: 155 up, 97 down) with a large overlap in genes affected by Rel-Syn and RelQ (Fig 2A and S1 Data). However, consistent with the nucleotide measurements (Fig 1), the effect of relQ induction was less prominent (S1 Table). We compared the RNA-Seq data with previous microarray analyses obtained after stringent response imposed by transferring bacteria to amino acid limiting conditions (-Leu, -Val) [22]. Most of the previously identified stringent response genes were confirmed by the RNA-Seq analysis (Fig 2A). Of note, in the present analysis, only genes with at least three-fold differences and a significance level of p < 0.001 were included in the analysis shown in Fig 2 and S2 Data. Despite the higher stringency in the analysis, the present analysis revealed far more (pp)pGpp-regulated genes. Genes were classified into functional categories using the SEED annotation (http://pubseed.theseed.org). (pp)pGpp induction resulted in the downregulation of many genes involved in protein synthesis (e.g ribosomal proteins RplA-T) and RNA/DNA (e.g. purine biosynthesis, gyrase) metabolism consistent with previous results that the stringent response mainly leads to the shutdown of translation and replication [22] (Fig 2B). Many of the genes upregulated by Rel-Syn and RelQ are part of the CodY regulon (S1 Data) and thus likely regulated by lowering of the GTP pool. The sRNA, RsaD was recently described to be directly repressed by CodY [40]. Concordantly, rsaD was found strongly up-regulated upon rel-Syn and relQ induction (8.5 and 6.0 log2 fold change, respectively) and used as read-out for a prototypic CodY target is subsequent experiments. AlsS was identified as RsaD repressed target gene [40] and as expected downregulated upon rel-Syn expression. Thus, the genetic approach proved to gain reliable, physiologically relevant results as validated by comparison with previous studies on (pp)pGpp mediated transcriptional changes. We further focused on so far unknown genes/phenotypes which were induced during stringent conditions. Therefore, we concentrated on genes that were strongly affected by rel-syn induction (Fig 2C and S1 Data) but not known to be under the control of CodY. Interestingly, many of these genes were assigned to iron acquisition/metabolism (upregulation of genes involved in iron storage; downregulation of genes involved in siderophore biosynthesis and iron transport), stress response (dps, sodA, katA ahpC, uspA1/2, asp23, ptpA, and msrA2), and virulence (upregulation of psmsα/ß; downregulation of agr). Phage-encoded genes were also upregulated, indicating phage-inducing conditions. This is in line with the upregulation of recA and lexA. For further analysis, we selected ftnA, dps, agr and psmα as read-out for (pp)pGpp-mediated CodY independent activities.
Fig 2. Global changes in gene expression upon transcriptional induction of rel-Syn or relQ.
(pp)pGpp0 with inducible rel-Syn or relQ was grown to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc. A. Venn diagrams showing genes or sRNAs upregulated (yellow) or downregulated (blue) after induction in comparison to uninduced cultures (< 3-fold difference, p< 0.001). Previously, described stringent genes [22] are indicated as Rel-Leu/Val. B. Genes with significant changes after induction of rel-syn (< 3-fold difference, p< 0.001) according to functional categories. C. Heatmap representing Rel-Syn-dependent up- and downregulated genes assigned to the functional categories iron acquisition and metabolism, stress response and Agr-related genes.
Comparison of the mupirocin-induced stringent response and induction of rel-Syn
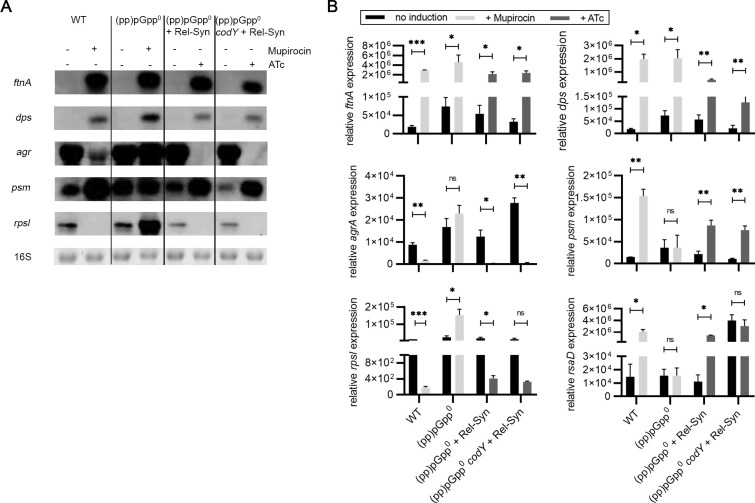
We compared the expression of the selected genes after induction of the stringent response via mupirocin and after rel-Syn induction by Northern blot analysis (Fig 3A) and qRT-PCR (Fig 3B). We verified the upregulation of ftnA, dps, psm and the sRNA rsaD under both conditions. Mupirocin also resulted in ftnA and dps activation in the (pp)pGpp0 strain indicating additional (pp)pGpp-independent effects of mupirocin on the expression of these genes. (pp)pGpp-mediated activation of psm expression is clearly not correlated to agr expression. Agr is the main activator required for psms expression [41]. Notably, the expression of the agr operon was even lower upon (pp)pGpp synthesis (S1 Data and Fig 3A and 3B), indicating that (pp)pGpp induces psm expression independent of Agr. Induction of rel-Syn in wild type background resulted in minor changes in gene expression compared to induction in the (pp)pGpp0 strain (S2 Fig). This emphasizes the strong hydrolase activity of Rel as present in the wild type leading to rapid hydrolysis of (pp)pGpp [14].
Fig 3. Correlation of mupirocin-induced stringent response and transcriptional induced rel-syn for selected CodY-independent genes.
Strain HG001 and derivatives were grown to OD600 = 0.3 and treated for 30 min with or without 0.125 μg/ml mupirocin or 0.1 μg/ml ATc (mutant strains with inducible rel-Syn). A. For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psm, agrA or rpsl. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane. B. Quantification of mRNA by qRT-PCR based on three biological replicates. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
CodY-independent activation of gene expression upon rel-Syn induction
(pp)pGpp synthesis leads to the lowering of the GTP pool and subsequently to de-repression of CodY target genes. Indeed, many of the genes that were upregulated in response to rel-Syn induction belong to the CodY regulon (S1 Data). However, the selected marker genes are not supposed to be regulated via CodY. For further validation we induced rel-Syn in a codY negative (pp)pGpp0 strain (Fig 3). The CodY target rsaD was confirmed to be de-repressed in the codY negative background [40]. However, other selected genes showed similar expression patterns in codY-positive and codY-negative backgrounds (Fig 3A and 3B). Thus, (pp)pGpp impacts the expression of these genes independent of CodY.
Induction of rel-Syn, relQ and relP in S. aureus USA300
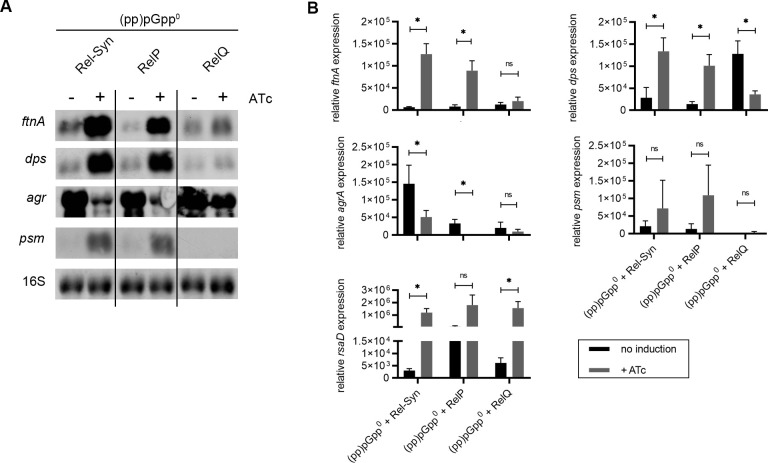
To confirm that the observed gene expression pattern is not restricted to strain HG001 and not due to potential second site mutations in the (pp)pGpp0 strain we repeated key experiments in strain USA300. Therefore a (pp)pGpp0 strain was constructed by sequential mutation of relP, relQ and rel. rel-Syn, relQ and relP were induced from the ATc inducible expression vectors as described. The transcriptional changes imposed by rel-Syn induction recapitulated the findings of rel-Syn induction in strain HG001 (Fig 4). We also verified that induction of relQ only slightly effects marker gene expression (Fig 4). However, induction of relP was highly effective, resulting in an expression pattern comparable to induction of rel-Syn. The strong effect of relP induction could be due to the fact that in contrast to relP it does not require pppGpp activation [20]
Fig 4. Gene expression following induction of rel-Syn, relQ or relP in USA300 strain background.
A. Strain USA300 and derivatives were grown to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc (mutant strains with inducible rel-Syn, relP or relQ). For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psmα or agrA. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane. B. Quantification of mRNA by qRT-PCR based on three biological replicates. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
Rel-Syn induction influences the oxidative stress response and virulence independent of PerR, Fur or SarA
Some of the prominent (pp)pGpp-activated genes are known to be under the control of other global regulators, such as PerR, Fur and SarA [42]. We speculated that (pp)pGpp dependent gene expression may somehow be mediated via these global regulators. Therefore, we analysed rel-Syn induction in per, fur and sarA mutants (Figs 5A and S3A).
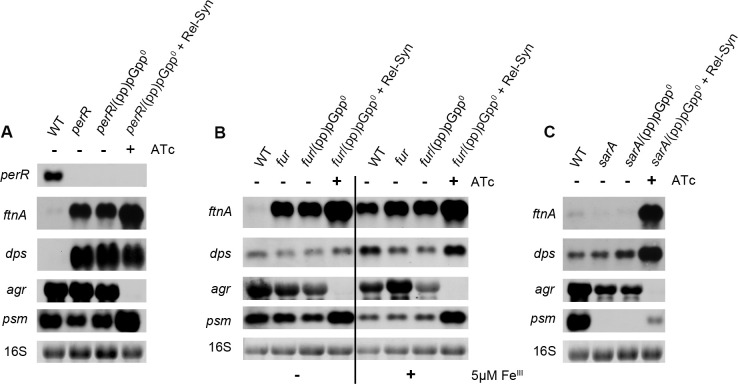
Fig 5. (pp)pGpp-dependent transcriptional changes independent of PerR, Fur or SarA.
Strain HG001 and derivatives were grown to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc (mutant strains with inducible rel-Syn). For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psmα or agrA. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane. For quantitative results see S3 Fig.
The (pp)pGpp controlled genes ftnA, dps, ahpC, katA and perR (S1 Data) are likely controlled via binding of PerR to a conserved PerR-binding motif (based on the public databases RegPrecise [43] and Aureowiki [44]). As expected, ftnA and dps were both upregulated in the perR mutants. Inducing rel-Syn in perR/(pp)pGpp0 strain showed further increase in ftnA, supporting that (pp)pGpp acts in addition and independent of PerR. For dps, the perR mutation also resulted in high expression, which was slightly decreased by (pp)pGpp. PerR deletion resulted in a slight decrease in psm expression, which was compensated by rel-syn induction. Thus, (pp)pGpp also affects gene expression in a per-negative background.
We found that many of the genes affected by Rel-Syn are indicative of iron overload conditions (e.g. upregulation of ftnA and dps (Fig 2D). We induced rel-Syn in a fur/(pp)pGpp0 background under low and high iron conditions (Figs 5B and S3). Independent of the availability of iron, ftnA, dps and psm were upregulated and agr was downregulated after rel-Syn induction in the fur-negative background indicating that (pp)pGpp regulation is not determined by iron availability or fur regulation.
SarA was shown to activate transcription of the agr operon [45,46] and proposed to be involved in oxidative stress sensing via a single Cys9 residue [47,48,49]. sarA was found to be significantly upregulated by Rel-Syn (S1 Data). ftnA and dps expression was not influenced by sarA mutation (Figs 5C and S3). agr and psm expression was downregulated in the sarA mutant, consistent with the proposed activation of the agr system by SarA [45]. Induction of rel-syn in the sarA mutant again showed the typical induction of ftnA, dps and repression of agr. Interestingly, psm expression remained hardly detectable in the SarA mutant. In the sarA mutant the very low Agr activity is likely not sufficient to allow for psm expression. Taken together, the results do not support the hypothesis that any of the candidate regulators function as a central hub for the observed (pp)pGpp dependent gene alterations.
(pp)pGpp is involved in oxidative stress resistance
Recently, it was shown that PSMs lead to the production of reactive oxygen species (ROS) [50]. One might speculate that under stringent conditions PSM-mediated ROS production triggers the expression of the oxidative stress genes. In this case, induction of rel-Syn should not result in the induction of these genes under anaerobic conditions, where ROS cannot be produced. However, rel-Syn induction resulted in the same transcriptional pattern regardless of whether bacteria were grown with or without oxygen (Fig 6A). We also analysed whether ROS would result in activation of the stringent response. However, H2O2 treatment did not affect transcription of stringent response genes such as fntA, dps, psm or rpsl (S5 Fig). Thus, (pp)pGpp-mediated gene alterations of the selected marker genes are not a consequence of ROS formation.
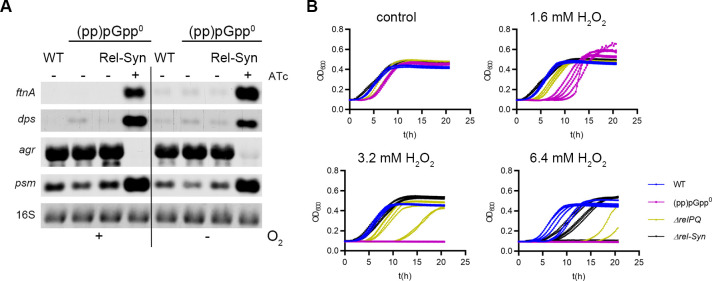
Fig 6. Functional link between stringent response and oxidative stress.
A. Strain HG001 and derivatives were grown with shaking aerobically or anaerobically to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc (mutant with inducible rel-Syn). For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psm or agrA. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane. B WT, (pp)pGpp0, ΔrelPQ and rel-Syn mutants were diluted from overnight culture to an OD = 0.1, challenged with different H2O2 concentrations, and growth was monitored over time.
These data indicate that (pp)pGpp simultaneously activates ROS-producing PSMs as well as ROS defence systems to prepare cells to withstand oxidative stress. To verify this hypothesis, we challenged wild type and mutant strains deficient in (pp)pGpp synthesis with H2O2. The (pp)pGpp0 strain was indeed more sensitive to oxidative stress. The minimal inhibitor concentration (MIC) of H2O2 to inhibit growth of the wild type was 6.4 mM and 3.2 mM for the (pp)pGpp0 strain. Moreover, the (pp)pGpp0 strain was more efficiently killed after 1 h or 2 h incubation with H202 (S4 Fig). Under the assay conditions, the basal (pp)pGpp might be derived from any of the (pp)pGpp synthetases. We analyzed a relPQ mutant and a rel-Syn mutant in which the synthetase domain of Rel was mutated. Both strains showed an intermittent phenotype in which the MIC varied between 3.2 and 6.4 mM when biological replicates were analyzed. To follow up on these ambiguities, we monitored growth after addition of H2O2 (Fig 6C). There was high variation in the lag time between biological replicates. Replicates of the relPQ or rel-Syn mutant showed a delayed lag phase, and some of the replicates could not grow. In cases where no growth was detectable the cultures were sterile as determined by colony counting. The delay of the lag phase was more prominent for the relPQ mutant than for the rel-Syn mutant. Nevertheless, none of the (pp)pGpp0 replicates could resume growth, and this result was consistent with the reproducible lowered MIC of this strain, indicating that (pp)pGpp indeed protects against oxidative stress.
We next speculated that under H202 conditions may induce stringent response in wild type bacteria. However, treatment of bacteria with H2O2 even at MIC concentrations did not induce stringent marker genes (S5 Fig). This indicates that the basal level of (pp)pGpp produced in wild type bacteria is sufficient to confer resistance.
Discussion
We chose a genetic approach to define the early transcriptional response upon (pp)pGpp synthesis without the need to apply additional stress conditions. As expected from previous studies, [22,51] (pp)pGpp synthesis resulted in severe downregulation of the translational machinery and de-repression of CodY target genes. Many additional (pp)pGpp-regulated genes that are presumably important for the survival of S. aureus during starvation conditions were identified. Regulation of these genes might occur indirectly through other so far ill-defined regulatory circuits or via changes in the nucleotide pool. Here, we focused mainly on genes that were found to be activated upon (pp)pGpp synthesis in a CodY-independent manner, particularly psm, ftnA and dps. The (pp)pGpp-dependent activation of these genes can also occur in strains missing the prototypic proteinaceous transcriptional regulators PerR, Fur, or SarA. These regulators are well known to be involved in the regulation of the selected genes. However, they need to be activated through oxidative stress and/or iron [42]. The RNAseq data revealed that transcription of the repressor perR is also upregulated upon (pp)pGpp synthesis indicating a feedback regulation to dampen the response. In summary, (pp)pGpp functions as a complementary, immediate message, allowing cells to react to adverse conditions such as amino acid starvation or cell wall stress. Under these conditions, upcoming oxidative stress seems to be anticipated, and (pp)pGpp prepares the cells for survival, e.g. ROS challenges (Fig 7). Indeed, a (pp)pGpp0 strain is more sensitive to H2O2. Both Rel and RelP/RelQ contribute to the protective effect.
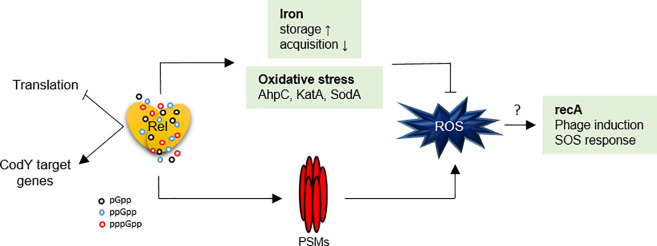
Fig 7. (pp)pGpp protects from oxidative stress.
(pp)pGpp leads to upregulation of oxidative stress and iron storage genes. These upregulated genes are beneficial to counteract endogenous (PSMs) or exogenous (e.g., H2O2) ROS. Upregulation of SOS and phage genes might be a consequence of ROS accumulation, e.g., by PSMs.
(pp)pGpp leads to psm activation
One of the most prominent effects of (pp)pGpp synthesis is the upregulation of psmα and psmβ, confirming previous results [22,52]. PSMs are a family of amphipathic, alpha-helical peptides that have multiple roles in staphylococcal pathogenesis and contribute to a large extent to the pathogenic success of virulent staphylococci [53,54]. (pp)pGpp-dependent psm expression within neutrophils was shown to be crucial for survival after phagocytosis [22]. However, PSMs also interact with the membrane of the producer, promote the release of membrane vesicles from the cytoplasmic membrane via an increase in membrane fluidity [55,56], reduce persister formation [57,58] and are involved in self-toxicity via ROS formation [50]. Interestingly, (pp)pGpp-dependent psm activation was not correlated with the activation of the quorum sensing Agr system, the main regulator required for psm expression [41]. Agr was even repressed under (pp)pGpp-inducing conditions. Previously, analysis of a clinical isolate overproducing (pp)pGpp also indicated that (pp)pGpp leads to agr inhibition [24]. Thus, (pp)pGpp-mediated psm activation is clearly uncoupled from agr expression. Recently, the sRNA Teg41 (S131) [59] and the transcriptional regulator MgrA [60], Rsp [61] or Rbf [62] were found to interfere with psm expression. However, it is unlikely that they mediate the (pp)pGpp regulatory effect because transcription of these regulators was unaltered based on our RNA-Seq analysis (S1 Data). Thus, the molecular mechanism by which (pp)pGpp leads to psm activation has to be elucidated. It is likely that the accompanying changes in the ATP/GTP ratios are crucial for this activation pattern. psm promoters might be sensitive to the concentration of the initiating nucleoside triphosphate (iNTP). The +1 position (e.g., G or A) of sensitive genes dictates whether transcriptional initiation/elongation requires high GTP or ATP levels, respectively [29]. Various sequence combinations determine whether a promoter is sensitive to iNTP [63]. Such sequence motifs are hard to predict within psm promoters. However, both the psmα and psmß operons start with an A at the +1 position [41], which might explain the higher expression due to the increased ATP levels following (p)ppGpp synthesis.
(pp)pGpp and oxidative stress response
Genes whose expression is indicative of iron overload conditions were also highly affected by (pp)pGpp. We recently, could confirm that the (pp)pGpp0 strain of strain USA300 has elevated free iron levels contributing to oxidative stress and increased ROS production [64]. A similar effect was reported for Vibrio cholera [65]. Here, the expression of the iron transporter FbpA was repressed via (pp)pGpp, resulting in a reduction of intracellular free iron required for the ROS-generating Fenton reaction. This contributed to reducing antibiotic-induced oxidative stress and thus tolerance, and this is also true for S. aureus [64]. In addition to interfering with iron metabolism, other genes involved in oxidative stress were activated by (pp)pGpp. A link between the stringent response and oxidative stress response has been observed in different organisms, although the underlying mechanisms and outcome might be highly diverse. (pp)pGpp-dependent upregulation of superoxide dismutase (SOD) was described in B. suis [66] and P. aeruginosa [67]. SOD was shown to be the key factor responsible for (pp)pGpp-mediated multidrug tolerance in P. aeruginosa [67]. Moreover, (pp)pGpp-deficient strains are often found to be more sensitive to oxidative stress [68,69,70]. However, in E. faecalis, a (pp)pGpp0 strain grew faster and to a higher growth yield than its parent in the presence of H2O2 [71].
Here, we show that the stringent response in S. aureus leads to the activation of ROS-inducing toxins and simultaneous expression of the detoxifying system to protect the producer. This is likely a special advantage for the pathogen once it encounters neutrophils and elevated ROS. (pp)pGpp-dependent PSM synthesis is required to escape from within cells after phagocytosis [22,72]. The upregulation of the oxygen stress programme will help protect the cell from endogenous as well as exogenous ROS.
Comparison of Rel-Syn, RelQ and RelP activity
We compared the in vivo activity of Rel-Syn, RelQ and RelP. RelP showed similar effect on gene expression as Rel-Syn, whereas RelQ was far less active (Fig 4). Thus, under our growth conditions RelQ activity seems to be restricted in vivo. Comparison of RelP and RelQ from other organisms revealed that RelQ is inhibited through RNA binding and auto-activated by (pp)pGpp [19,20]. We analyzed RelQ activity in an (pp)pGpp background under non-stress conditions where RelQ activity is likely restricted via RNA binding and/or the missing basal (pp)pGpp provided by other synthetases. The conditions that would relieve this restriction remain to be determined. A recent biochemical analysis of purified RelP and RelQ from S. aureus confirmed that RelQ, but not RelP, was allosterically-stimulated by the addition of pppGpp, ppGpp or pGpp [21]. Moreover, in vitro both enzymes were shown to efficiently synthesize pGpp in addition to pppGpp and ppGpp. In our in vivo analysis we could detect considerable quantities of pGpp derived from Rel activity (Fig 1). However, in vitro Rel seems to preferentially synthesize pppGpp [21]. One may speculate that pGpp detected in vivo might be enzymatically derived from NuDiX hydrolase, NahA, an enzyme that in B. subtilis efficiently produces pGpp by hydrolyzing (p)ppGpp [73]. In this organism, pGpp potently regulates the purine biosynthesis pathway but in contrast to (p)ppGpp does not interact with the GTPases. It was proposed that the different nucleotides may fulfill different roles through fine-tuning in signal transduction. Moreover, the different levels of (pp)pGpp in the cell may also dictate different outcomes. There is now growing evidence that basal level of (pp)pGpp (often below detection limit) are involved to maintain balanced growth whereas high levels are indicative for stress conditions resulting in severe reprogramming of the cell and growth arrest [74,75]. In this context, the induction of rel-Syn likely mirrors stress conditions, whereas relQ induction with low detectable (pp)pGpp synthesis more resembles the proposed basal level. From the RNA-Seq analysis we found no clear quantitative difference between both conditions. However, many of the genes affected by RelQ could be assigned to the CodY regulon. This illustrates the high sensitivity of CodY towards subtle changes of the GTP pool imposed by (pp)pGpp synthesis.
Different (pp)pGpp mode of actions result in similar outcome
Recently, genome-wide direct effects on transcription from ppGpp binding to its two sites on RNA polymerase were assessed in E. coli [38]. Similar to our approach, ppGpp was produced without concurrent starvation by conditional expression of a RelA variant lacking its autoinhibitory domain. The Rel variant was induced for 5–10 min in strains with or without the two binding sites for ppGpp on RNAP. It could be shown that transcriptional changes are in large part due to ppGpp-RNAP interference. However, (pp)pGpp does not bind to RNAP in Firmicutes. We anticipated that for full stringent response in S. aureus significant changes of the nucleotide pool have to occur and that transcriptional changes are not directly linked to (pp)pGpp in this organism. Therefore, we analysed transcriptional changes after 30 min of rel-Syn induction to allow the adjustment of the nucleotide pool. We assume that many transcriptional changes are caused by the observed changes in the nucleotide pool. Alternatively, at least some of them might be linked to secondary effects such as inhibition of growth or translation. In the future, time course experiments and concurrent proteome analyses will help to further clarify this issue. Nevertheless, despite major difference in the experimental set-up and the different underlying regulatory mechanisms between E. coli and S. aureus both approaches revealed a surprisingly similar outcome: First, in both organism the genetic approaches revealed many more (pp)pGpp-regulated genes in comparison to traditional analyses applying concurrent starvation. Second, observed (pp)pGpp-mediated transcriptional changes are highly similar: i.) large number of genes related to nucleotide, protein, and RNA metabolism, translation, and DNA synthesis are negatively regulated by ppGpp; ii) amino acid biosynthesis genes are highly upregulated and iii.) genes for the responses to DNA damage, general stress and oxidants responded to ppGpp in both organisms. Thus, stringent response has evolved in different organisms to fulfil similar functions by use of highly different mechanisms.
Materials and methods
Strains and growth conditions
Strains and plasmids are listed in S1 Table. For strains carrying a resistance gene a concentration of 10 μg/ml chloramphenicol, 10 μg/ml erythromycin or 100 μg/ml ampicillin was used only for overnight cultures. S. aureus strains were grown overnight in chemical defined medium (CDM) [32], diluted to an optical density (OD600) of 0.05 and grown until the early exponential phase OD600 = 0.3 with shaking (220 rpm, 37°C). Gene expression in strains carrying a plasmid with an ATc-inducible promoter was induced at OD600 = 0.3 with 0.1 μg/ml ATc for 30 min. We chose 30 min induction to allow adjustment of the anticipated changes of the nucleotide pool. For anaerobic growth the strains were diluted to an OD600 = 0.05 in hungate tubes (Chemglass), completely filled with CDM. ATc was applied using a syringe at OD600 = 0. 3. For OD measurements and RNA isolation, aliquots were drawn with a syringe.
Generation of (pp)pGpp0 mutant in USA300 JE2
For the USA300 (pp)pGpp0 mutant (USA300-229-230-263), lysates were prepared from RN4220 strains containing the mutagenesis vectors pCG229, pCG230 and pCG263, respectively (S1 Table). After plasmid transduction of USA300 JE2, mutagenesis was performed as previously described [76]. To avoid toxic accumulation of (pp)pGpp the genes were mutated in the order relP, relQ and finally rel. Mutations were verified by PCR using oligonucleotides listed in S2 Table.
Generation of perR, fur, sarA and psmα/β (pp)pGpp0 mutants in HG001
Φ11 lysates were generated from transposon mutants NE665 (perR), NE99 (fur) and NE1193 (sarA) from the NARSA transposon library [77] to transduce S. aureus strains HG001 and (pp)pGpp0. psmα or psmβ mutations were transduced using Φ11 lysates from previously described mutants [22]. All transductants were verified by PCR using oligonucleotides listed in S2 Table.
RNA isolation, Northern Blot analysis and qRT-PCR analysis
RNA isolation and Northern blot analysis were performed as described previously [78]. Briefly, bacteria were pelleted and resuspended in 1 ml TRIzol (Thermo Fisher Scientific) and lysed using zirconia/silica beads (0.1mm diameter) and a high speed homogenizer. RNA was isolated following the recommended procedure by TRIzol manufacturer. For RNA-Seq analysis RNA from the aqueous phase was further purified following the RNA-isolation protocol by Amp Tech ExpressArt RNA ready. Transcripts on the Northern blot were detected by dioxigenin-labeled probes, which were generated by a DNA-labelling PCR-Kit (Roche Life Science). Relative quantification of ftnA, dps, agrA, α-type psms, rsaD and rpsl transcripts by qRT-PCR was performed using the Quantstudio3 (Applied Biosystems) and the QuantiFast SYBR Green RT-PCR Kit (Qiagen). Briefly, 5 μg of total RNA were DNase-treated and diluted 1:10 for qRT-PCR. Relative quantities of transcripts were calculated by a standard curve for each gene generated using 6-fold serial dilution of HG001 wild type RNA. Primers for qRT-PCR are listed inS2 Table.
In vivo nucleotide extraction
Nucleotides were isolated based on published protocol [79]. Briefly, strains were grown in CDM overnight, diluted to an OD600 = 0.05 and grown in CDM until an OD600 = 0.3. Strains were split and treated with or without 0.1 μg/ml ATc for 30 min at 37°C and 220 rpm shaking. 100 ml bacterial cultures were harvested and transferred into 50 ml centrifuge tubes half filled with ice and centrifuged (5 min, 5000 x g, 4°C). Pellets were immediately frozen in liquid nitrogen and stored at -80°C until usage. Samples were thawed on ice and resuspended in 2M formic acid and incubated for 30 min. Resuspended bacteria were lysed by high speed homogenizer using zirconia/silicia beads (0.1 mm diameter) and kept on ice for 30 min. The aqueous phase was collected and mixed with 3 ml 50 mM NH4OAc (pH 4.5), loaded on columns (OASIS Wax cartridge 3xcc) and centrifuged (5000 x g, 5min, 4°C). Columns were pre-treated first with pure 3 ml methanol and then with 3 ml 50 mM NH4OAc (pH 4.5). Samples were washed first with 3 ml 50 mM NH4OAc (pH 4.5) followed by a washing step with 3 ml methanol. Elution was performed with 1 ml of 20% methanol, 10% NH4OH. Eluted nucleotides were flash frozen in liquid nitrogen and lyophilized overnight. Lyophilized nucleotides were resuspended in 100 μl ddH2O and analyzed via HPLC-MS. pppGpp and ppGpp standard molecule were purchased Jena Biosciences. pGpp was synthesized starting from conveniently protected guanosine and employing both phosphoramidite and phosphotriester methods (S1 Methods).
In vivo and in vitro analysis of (pp)pGpp via HPLC-MS
Nucleotide quantification was performed as described [14]. Briefly, nucleotides were analyzed using ESI-TOF (micrO-TOF II, Bruker) mass spectrometer connected to an UltiMate 3000 High-Performance Liquid Chromatography (HPLC). 5 μl of standards or samples were injected onto SEQuant ZIC-pHILIC column (Merck, PEEK 150 x 2.1 mm, 5 μm). MS analysis was performed in negative-ion mode over the mass range from 200 to 1,000 m/z. MS calibration was done by using a sodium formate solution as the tune mix. Nucleotide standards of AMP (346.0552 m/z), ATP (426.0215 m/z), GTP (521.9828 m/z), pGpp (521.9828m/z), ppGpp (601.9491 m/z) and pppGpp (681.9155 m/z) were diluted 10 times 1:1 from 1 mM until a concentration of 1.95 μM and analyzed by HPLC-MS as previously described [14]. Extracted ion chromatogram (EIC) spectra of all standards were presented in DataAnalysis (Bruker) and the area under the curve (AUC) of the respective EICs was calculated in GraphPad Prism 5 (baseline was set to 150). The obtained AUC values of the diluted standards were used to generate a calibration curve. For absolute nucleotide quantification, the AUC of the samples was plugged into the AUC values of the calibration curve and the concentration of the respective nucleotides in the samples was determined. Nucleotide identification was verified by matching the retention times and m/z values of detected peaks in the samples to the measured nucleotide standards. To separate pGpp from GTP (S6 Fig) we used an expectation–maximization (EM). The relative amount of the first chemical component in the mixture is calculated as . The second component was expressed as I2 = 1−I1.
The obtained concentrations of the adenosine nucleotides ATP, ADP and AMP in each sample were used to calculate the adenylate energy charge as described [80].
H2O2 killing assay
Strains were grown over night in CDM, diluted in fresh CDM to an OD600 = 0.1 and growth followed for 24 hours with different H2O2 concentrations in a microplate reader (Infinite M200, Tecan). H2O2 killing was determined by incubation of bacteria grown from an overnight culture to OD600 = 0.3 followed by incubation with 80 mM H202 for 1 or 2 h. MIC determination was performed according to European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines using CDM medium (at least three biological replicates for each strain).
RNA-Seq analysis
Strains were grown in triplicates to OD600 = 0.3, split into treated (ATc, 0.1 μg/ml) and untreated control and grown for 30 min, 37°C. Purified RNA was sent to Vertis Biotechnologie AG Freiburg for RNA Sequencing based on Illumina Next Seq 500 system. RNA was examined by a capillary electrophoresis on a Shimadzu MultiNA microchip followed by rRNA depletion using Ribo-Zero rRNA removel Kit from Illumina. RNA was converted to cDNA by fragmenting RNA samples by ultrasound and ligating an oligonucleotide adapter to the 3’end of the RNA. Using M-MLV reverse transcriptase first strand cDNA was created using 3’ adapter as primer. The 5’Illumina TruSeq sequencing adapter was ligated to the 3’end of the purified (Agencourt AMPure XP kit) cDNA and PCR was performed. Samples were pooled in equimolar amounts and fractionated in a size range of 200–500 bp using a preparative agarose gel and Illumina sequencing was performed using 75 bp reads. RNA-Seq analysis was performed using CLC Genomic Workbench (Qiagen). Reads were trimmed (TrueSeq-Antinsense Primer AGATCGGAAGAGCACACGTCTGAACTCCAGTCA) and mapped to the reference genome of HG001 (NZ_CP018205.1). Differential gene expression was performed comparing Rel-Syn or RelQ versus the (pp)pGpp0 mutant. Venn diagrams were performed comparing Rel-Syn vs. control and RelQ vs. control. Genes with at least 3-fold difference and a p-value ≥0.001 were defined as differentially regulated compared to the untreated control. Annotation of genes are according to recent “Aureowiki” annotation of strain 8325 (http://aureowiki.med.uni-greifswald.de/Main_Page) [44]. Of the previously identified RNA segments [81] those annotated as indep, s-indep, and indep-NT were regarded as potential regulatory RNAs (sRNAs) and included in the analysis.
Supporting information
Strain HG001 and derivatives were grown to OD600 = 0.3 and treated for 30 min with or without 0.1 μg/ml ATc (mutant strains with inducible rel-Syn, relQ or empty vector). For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for sodA, ftnA, dps, per, agrA or psm. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control.
(TIF)
Gene expression following rel-Syn induction in HG001 wild type. HG001 was grown to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc. Transcript were quantified by qRT-PCR on equal amount of total RNA. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001
(TIF)
Quantification of mRNA by qRT-PCR based on three biological replicates. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
(TIF)
Strains were grown to OD600 = 0.3 and then treated with 80mM H2O2 for 1 or 2 h.
(TIF)
Strains were grown to OD600 = 0.3 and treated with mupirocin or H202 for 30 min. RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psm, agrA or rpsl. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane.
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Role categories and regulons.
(XLSX)
(XLSX)
Acknowledgments
We thank Isabell Samp for excellent technical assistance and Ulrich Schoppmeier for support in data analyses. Mutants from the Nebraska library were obtained through the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) program.
Data Availability
All data are available from the GEO database https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE145144 (accession GSE145144).
Funding Statement
The study received the following grants from Deutsche Forschungsgemeinschaft (https://www.dfg.de): TR34 to C.W. and U.M.; SPP1879 to C.W.; TR261 and SFB766 to C.M. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Irving SE, Corrigan RM. Triggering the stringent response: signals responsible for activating (p)ppGpp synthesis in bacteria. Microbiology. 2018;164(3):268–76. 10.1099/mic.0.000621 [DOI] [PubMed] [Google Scholar]
- 2.Dozot M, Boigegrain RA, Delrue RM, Hallez R, Ouahrani-Bettache S, Danese I, et al. The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB. Cell Microbiol. 2006;8(11):1791–802. 10.1111/j.1462-5822.2006.00749.x [DOI] [PubMed] [Google Scholar]
- 3.Steinchen W, Bange G. The magic dance of the alarmones (p)ppGpp. Mol Microbiol. 2016;101(4):531–44. 10.1111/mmi.13412 [DOI] [PubMed] [Google Scholar]
- 4.Wu J, Xie J. Magic spot: (p) ppGpp. J Cell Physiol. 2009;220(2):297–302. 10.1002/jcp.21797 [DOI] [PubMed] [Google Scholar]
- 5.Zhu M, Pan Y, Dai X. (p)ppGpp: the magic governor of bacterial growth economy. Curr Genet. 2019. 10.1007/s00294-019-00973-z [DOI] [PubMed] [Google Scholar]
- 6.Gaca AO, Colomer-Winter C, Lemos JA. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol. 2015;197(7):1146–56. 10.1128/JB.02577-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu K, Bittner AN, Wang JD. Diversity in (p)ppGpp metabolism and effectors. Curr Opin Microbiol. 2015;24:72–9. 10.1016/j.mib.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs JK, Boraston AB. (p)ppGpp and the Stringent Response: An Emerging Threat to Antibiotic Therapy. ACS Infect Dis. 2019;5(9):1505–17. 10.1021/acsinfecdis.9b00204 [DOI] [PubMed] [Google Scholar]
- 9.Wolz C, Geiger T, Goerke C. The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int J Med Microbiol. 2010;300(2–3):142–7. 10.1016/j.ijmm.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 10.Hauryliuk V, Atkinson GC, Murakami KS, Tenson T, Gerdes K. Recent functional insights into the role of (p)ppGpp in bacterial physiology. Nat Rev Microbiol. 2015;13(5):298–309. 10.1038/nrmicro3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74(2):171–99. 10.1128/MMBR.00046-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atkinson GC, Tenson T, Hauryliuk V. The RelA/SpoT homolog (RSH) superfamily: distribution and functional evolution of ppGpp synthetases and hydrolases across the tree of life. PLoS One. 2011;6(8):e23479 10.1371/journal.pone.0023479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geiger T, Goerke C, Fritz M, Schafer T, Ohlsen K, Liebeke M, et al. Role of the (p)ppGpp synthase RSH, a RelA/SpoT homolog, in stringent response and virulence of Staphylococcus aureus. Infect Immun. 2010;78(5):1873–83. 10.1128/IAI.01439-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gratani FL, Horvatek P, Geiger T, Borisova M, Mayer C, Grin I, et al. Regulation of the opposing (p)ppGpp synthetase and hydrolase activities in a bifunctional RelA/SpoT homologue from Staphylococcus aureus. PLoS Genet. 2018;14(7):e1007514 10.1371/journal.pgen.1007514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuroda M, Kuroda H, Oshima T, Takeuchi F, Mori H, Hiramatsu K. Two-component system VraSR positively modulates the regulation of cell-wall biosynthesis pathway in Staphylococcus aureus. Mol Microbiol. 2003;49(3):807–21. 10.1046/j.1365-2958.2003.03599.x [DOI] [PubMed] [Google Scholar]
- 16.Geiger T, Kastle B, Gratani FL, Goerke C, Wolz C. Two small (p)ppGpp synthases in Staphylococcus aureus mediate tolerance against cell envelope stress conditions. J Bacteriol. 2014;196(4):894–902. 10.1128/JB.01201-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steinchen W, Schuhmacher JS, Altegoer F, Fage CD, Srinivasan V, Linne U, et al. Catalytic mechanism and allosteric regulation of an oligomeric (p)ppGpp synthetase by an alarmone. Proc Natl Acad Sci U S A. 2015;112(43):13348–53. 10.1073/pnas.1505271112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beljantseva J, Kudrin P, Andresen L, Shingler V, Atkinson GC, Tenson T, et al. Negative allosteric regulation of Enterococcus faecalis small alarmone synthetase RelQ by single-stranded RNA. Proc Natl Acad Sci U S A. 2017;114(14):3726–31. 10.1073/pnas.1617868114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manav MC, Beljantseva J, Bojer MS, Tenson T, Ingmer H, Hauryliuk V, et al. Structural basis for (p)ppGpp synthesis by the Staphylococcus aureus small alarmone synthetase RelP. J Biol Chem. 2018;293(9):3254–64. 10.1074/jbc.RA117.001374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steinchen W, Vogt MS, Altegoer F, Giammarinaro PI, Horvatek P, Wolz C, et al. Structural and mechanistic divergence of the small (p)ppGpp synthetases RelP and RelQ. Sci Rep. 2018;8(1):2195 10.1038/s41598-018-20634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang N, Xie S, Tang NY, Choi MY, Wang Y, Watt RM. The Ps and Qs of alarmone synthesis in Staphylococcus aureus. PLoS One. 2019;14(10):e0213630 10.1371/journal.pone.0213630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger T, Francois P, Liebeke M, Fraunholz M, Goerke C, Krismer B, et al. The stringent response of Staphylococcus aureus and its impact on survival after phagocytosis through the induction of intracellular PSMs expression. PLoS Pathog. 2012;8(11):e1003016 10.1371/journal.ppat.1003016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corrigan RM, Bellows LE, Wood A, Grundling A. ppGpp negatively impacts ribosome assembly affecting growth and antimicrobial tolerance in Gram-positive bacteria. Proc Natl Acad Sci U S A. 2016;113(12):E1710–9. 10.1073/pnas.1522179113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao W, Chua K, Davies JK, Newton HJ, Seemann T, Harrison PF, et al. Two novel point mutations in clinical Staphylococcus aureus reduce linezolid susceptibility and switch on the stringent response to promote persistent infection. PLoS Pathog. 2010;6(6):e1000944 10.1371/journal.ppat.1000944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dordel J, Kim C, Chung M, Pardos de la Gandara M, Holden MT, Parkhill J, et al. Novel determinants of antibiotic resistance: identification of mutated loci in highly methicillin-resistant subpopulations of methicillin-resistant Staphylococcus aureus. MBio. 2014;5(2):e01000 10.1128/mBio.01000-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matsuo M, Hiramatsu M, Singh M, Sasaki T, Hishinuma T, Yamamoto N, et al. Genetic and Transcriptomic Analyses of Ciprofloxacin-Tolerant Staphylococcus aureus Isolated by the Replica Plating Tolerance Isolation System (REPTIS). Antimicrob Agents Chemother. 2019;63(2):e02019–18. 10.1128/AAC.02019-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katayama Y, Azechi T, Miyazaki M, Takata T, Sekine M, Matsui H, et al. Prevalence of Slow-Growth Vancomycin Nonsusceptibility in Methicillin-Resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2017;61(11). 10.1128/AAC.00452-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kriel A, Bittner AN, Kim SH, Liu K, Tehranchi AK, Zou WY, et al. Direct regulation of GTP homeostasis by (p)ppGpp: a critical component of viability and stress resistance. Molecular Cell. 2012;48(2):231–41. 10.1016/j.molcel.2012.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krasny L, Tiserova H, Jonak J, Rejman D, Sanderova H. The identity of the transcription +1 position is crucial for changes in gene expression in response to amino acid starvation in Bacillus subtilis. Mol Microbiol. 2008;69(1):42–54. 10.1111/j.1365-2958.2008.06256.x [DOI] [PubMed] [Google Scholar]
- 30.Kastle B, Geiger T, Gratani FL, Reisinger R, Goerke C, Borisova M, et al. rRNA regulation during growth and under stringent conditions in Staphylococcus aureus. Environ Microbiol. 2015;17(11):4394–405. 10.1111/1462-2920.12867 [DOI] [PubMed] [Google Scholar]
- 31.Krasny L, Gourse RL. An alternative strategy for bacterial ribosome synthesis: Bacillus subtilis rRNA transcription regulation. EMBO J. 2004;23(22):4473–83. 10.1038/sj.emboj.7600423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pohl K, Francois P, Stenz L, Schlink F, Geiger T, Herbert S, et al. CodY in Staphylococcus aureus: a regulatory link between metabolism and virulence gene expression. J Bacteriol. 2009;191(9):2953–63. 10.1128/JB.01492-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Majerczyk CD, Dunman PM, Luong TT, Lee CY, Sadykov MR, Somerville GA, et al. Direct targets of CodY in Staphylococcus aureus. J Bacteriol. 2010;192(11):2861–77. 10.1128/JB.00220-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eymann C, Homuth G, Scharf C, Hecker M. Bacillus subtilis functional genomics: global characterization of the stringent response by proteome and transcriptome analysis. J Bacteriol. 2002;184(9):2500–20. 10.1128/jb.184.9.2500-2520.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kazmierczak KM, Wayne KJ, Rechtsteiner A, Winkler ME. Roles of rel(Spn) in stringent response, global regulation and virulence of serotype 2 Streptococcus pneumoniae D39. Mol Microbiol. 2009;72(3):590–611. 10.1111/j.1365-2958.2009.06669.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaca AO, Abranches J, Kajfasz JK, Lemos JA. Global transcriptional analysis of the stringent response in Enterococcus faecalis. Microbiology. 2012;158(Pt 8):1994–2004. 10.1099/mic.0.060236-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nascimento MM, Lemos JA, Abranches J, Lin VK, Burne RA. Role of RelA of Streptococcus mutans in global control of gene expression. J Bacteriol. 2008;190(1):28–36. 10.1128/JB.01395-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanchez-Vazquez P, Dewey CN, Kitten N, Ross W, Gourse RL. Genome-wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc Natl Acad Sci U S A. 2019;116(17):8310–9. 10.1073/pnas.1819682116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes J, Mellows G. Interaction of pseudomonic acid A with Escherichia coli B isoleucyl-tRNA synthetase. Biochem J. 1980;191(1):209–19. 10.1042/bj1910209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Augagneur Y, King AN, Germain-Amiot N, Sassi M, Fitzgerald JW, Sahukhal GS, et al. Analysis of the CodY RNome reveals RsaD as a stress-responsive riboregulator of overflow metabolism in Staphylococcus aureus. Mol Microbiol. 2020;113(2):309–25. 10.1111/mmi.14418 [DOI] [PubMed] [Google Scholar]
- 41.Queck SY, Jameson-Lee M, Villaruz AE, Bach TH, Khan BA, Sturdevant DE, et al. RNAIII-independent target gene control by the agr quorum-sensing system: insight into the evolution of virulence regulation in Staphylococcus aureus. Mol Cell. 2008;32(1):150–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gaupp R, Ledala N, Somerville GA. Staphylococcal response to oxidative stress. Front Cell Infect Microbiol. 2012;2:33 10.3389/fcimb.2012.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novichkov PS, Kazakov AE, Ravcheev DA, Leyn SA, Kovaleva GY, Sutormin RA, et al. RegPrecise 3.0—a resource for genome-scale exploration of transcriptional regulation in bacteria. BMC Genomics. 2013;14:745 10.1186/1471-2164-14-745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs S, Mehlan H, Bernhardt J, Hennig A, Michalik S, Surmann K, et al. AureoWiki The repository of the Staphylococcus aureus research and annotation community. Int J Med Microbiol. 2018;308(6):558–68. 10.1016/j.ijmm.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 45.Heinrichs JH, Bayer MG, Cheung AL. Characterization of the sar locus and its interaction with agr in Staphylococcus aureus. J Bacteriol. 1996;178(2):418–23. 10.1128/jb.178.2.418-423.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zielinska AK, Beenken KE, Joo HS, Mrak LN, Griffin LM, Luong TT, et al. Defining the strain-dependent impact of the Staphylococcal accessory regulator (sarA) on the alpha-toxin phenotype of Staphylococcus aureus. J Bacteriol. 2011;193(12):2948–58. 10.1128/JB.01517-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sun F, Ding Y, Ji Q, Liang Z, Deng X, Wong CC, et al. Protein cysteine phosphorylation of SarA/MgrA family transcriptional regulators mediates bacterial virulence and antibiotic resistance. Proc Natl Acad Sci U S A. 2012;109(38):15461–6. 10.1073/pnas.1205952109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ballal A, Manna AC. Control of thioredoxin reductase gene (trxB) transcription by SarA in Staphylococcus aureus. J Bacteriol. 2010;192(1):336–45. 10.1128/JB.01202-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Grosser MR, Weiss A, Shaw LN, Richardson AR. Regulatory Requirements for Staphylococcus aureus Nitric Oxide Resistance. J Bacteriol. 2016;198(15):2043–55. 10.1128/JB.00229-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.George SE, Hrubesch J, Breuing I, Vetter N, Korn N, Hennemann K, et al. Oxidative stress drives the selection of quorum sensing mutants in the Staphylococcus aureus population. Proc Natl Acad Sci U S A. 2019;116(38):19145–54. 10.1073/pnas.1902752116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reiss S, Pane-Farre J, Fuchs S, Francois P, Liebeke M, Schrenzel J, et al. Global analysis of the Staphylococcus aureus response to mupirocin. Antimicrob Agents Chemother. 2012;56(2):787–804. 10.1128/AAC.05363-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li L, Bayer AS, Cheung A, Lu L, Abdelhady W, Donegan NP, et al. The stringent response contributes to persistent methicillin-resistant Staphylococcus aureus endovascular infection through the purine biosynthetic pathway. J Infect Dis. 2020. 10.1093/infdis/jiaa202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheung GY, Joo HS, Chatterjee SS, Otto M. Phenol-soluble modulins—critical determinants of staphylococcal virulence. FEMS Microbiol Rev. 2014;38(4):698–719. 10.1111/1574-6976.12057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peschel A, Otto M. Phenol-soluble modulins and staphylococcal infection. Nat Rev Microbiol. 2013;11(10):667–73. 10.1038/nrmicro3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schlatterer K, Beck C, Hanzelmann D, Lebtig M, Fehrenbacher B, Schaller M, et al. The Mechanism behind Bacterial Lipoprotein Release: Phenol-Soluble Modulins Mediate Toll-Like Receptor 2 Activation via Extracellular Vesicle Release from Staphylococcus aureus. MBio. 2018;9(6). 10.1128/mBio.01851-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang X, Thompson CD, Weidenmaier C, Lee JC. Release of Staphylococcus aureus extracellular vesicles and their application as a vaccine platform. Nat Commun. 2018;9(1):1379 10.1038/s41467-018-03847-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bojer MS, Lindemose S, Vestergaard M, Ingmer H. Quorum Sensing-Regulated Phenol-Soluble Modulins Limit Persister Cell Populations in Staphylococcus aureus. Front Microbiol. 2018;9:255 10.3389/fmicb.2018.00255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu T, Wang XY, Cui P, Zhang YM, Zhang WH, Zhang Y. The Agr Quorum Sensing System Represses Persister Formation through Regulation of Phenol Soluble Modulins in Staphylococcus aureus. Front Microbiol. 2017;8:2189 10.3389/fmicb.2017.02189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zapf RL, Wiemels RE, Keogh RA, Holzschu DL, Howell KM, Trzeciak E, et al. The Small RNA Teg41 Regulates Expression of the Alpha Phenol-Soluble Modulins and Is Required for Virulence in Staphylococcus aureus. MBio. 2019;10(1). 10.1128/mBio.02484-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang Q, Jin Z, Sun B. MgrA Negatively Regulates Biofilm Formation and Detachment by Repressing the Expression of psm Operons in Staphylococcus aureus. Appl Environ Microbiol. 2018;84(16). 10.1128/AEM.01008-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu B, Sun B. Rsp promotes the transcription of virulence factors in an agr-independent manner in Staphylococcus aureus. Emerg Microbes Infect. 2020:1–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang B, Liu B, Sun B. Transcriptional regulation of virulence factors Hla and phenol-soluble modulins α by AraC-type regulator Rbf in Staphylococcus aureus. Int J Med Microbiol. 2020;310(5):151436 10.1016/j.ijmm.2020.151436 [DOI] [PubMed] [Google Scholar]
- 63.Sojka L, Kouba T, Barvik I, Sanderova H, Maderova Z, Jonak J, et al. Rapid changes in gene expression: DNA determinants of promoter regulation by the concentration of the transcription initiating NTP in Bacillus subtilis. Nucleic Acids Res. 2011;39(11):4598–611. 10.1093/nar/gkr032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fritsch VN, Loi VV, Busche T, Tung QN, Lill R, Horvatek P, et al. The alarmone (p)ppGpp confers tolerance to oxidative stress during the stationary phase by maintenance of redox and iron homeostasis in Staphylococcus aureus. Free Radic Biol Med. 2020. 10.1016/j.freeradbiomed.2020.10.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim HY, Go J, Lee KM, Oh YT, Yoon SS. Guanosine tetra- and pentaphosphate increase antibiotic tolerance by reducing reactive oxygen species production in Vibrio cholerae. J Biol Chem. 2018;293(15):5679–94. 10.1074/jbc.RA117.000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanna N, Ouahrani-Bettache S, Drake KL, Adams LG, Kohler S, Occhialini A. Global Rsh-dependent transcription profile of Brucella suis during stringent response unravels adaptation to nutrient starvation and cross-talk with other stress responses. BMC Genomics. 2013;14:459 10.1186/1471-2164-14-459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Martins D, McKay G, Sampathkumar G, Khakimova M, English AM, Nguyen D. Superoxide dismutase activity confers (p)ppGpp-mediated antibiotic tolerance to stationary-phase Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2018;115(39):9797–802. 10.1073/pnas.1804525115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yan X, Zhao C, Budin-Verneuil A, Hartke A, Rince A, Gilmore MS, et al. The (p)ppGpp synthetase RelA contributes to stress adaptation and virulence in Enterococcus faecalis V583. Microbiology. 2009;155(Pt 10):3226–37. 10.1099/mic.0.026146-0 [DOI] [PubMed] [Google Scholar]
- 69.Holley C, Gangaiah D, Li W, Fortney KR, Janowicz DM, Ellinger S, et al. A (p)ppGpp-null mutant of Haemophilus ducreyi is partially attenuated in humans due to multiple conflicting phenotypes. Infect Immun. 2014;82(8):3492–502. 10.1128/IAI.01994-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang J, Tian Y, Zhou Z, Zhang L, Zhang W, Lin M, et al. Identification and Functional Analysis of RelA/SpoT Homolog (RSH) Genes in Deinococcus radiodurans. J Microbiol Biotechnol. 2016;26(12):2106–15. 10.4014/jmb.1601.01017 [DOI] [PubMed] [Google Scholar]
- 71.Abranches J, Martinez AR, Kajfasz JK, Chavez V, Garsin DA, Lemos JA. The molecular alarmone (p)ppGpp mediates stress responses, vancomycin tolerance, and virulence in Enterococcus faecalis. J Bacteriol. 2009;191(7):2248–56. 10.1128/JB.01726-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Surewaard BG, de Haas CJ, Vervoort F, Rigby KM, DeLeo FR, Otto M, et al. Staphylococcal alpha-phenol soluble modulins contribute to neutrophil lysis after phagocytosis. Cell Microbiol. 2013;15(8):1427–37. 10.1111/cmi.12130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yang J, Anderson BW, Turdiev A, Turdiev H, Stevenson DM, Amador-Noguez D, et al. The nucleotide pGpp acts as a third alarmone in Bacillus, with functions distinct from those of (p) ppGpp. Nat Commun. 2020;11(1):5388 10.1038/s41467-020-19166-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fernández-Coll, Chashel m,. Possible roles for basal levels of (p)ppGpp: growth efficiency vs surviving stress. Front Microbiol. in press. 10.3389/fmicb.2020.592718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gaca AO, Kajfasz JK, Miller JH, Liu K, Wang JD, Abranches J, et al. Basal levels of (p)ppGpp in Enterococcus faecalis: the magic beyond the stringent response. MBio. 2013;4(5):e00646–13. 10.1128/mBio.00646-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55(1):58–63. 10.1016/j.plasmid.2005.05.005 [DOI] [PubMed] [Google Scholar]
- 77.Fey PD, Endres JL, Yajjala VK, Widhelm TJ, Boissy RJ, Bose JL, et al. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4(1):e00537–12. 10.1128/mBio.00537-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Goerke C, Campana S, Bayer MG, Doring G, Botzenhart K, Wolz C. Direct quantitative transcript analysis of the agr regulon of Staphylococcus aureus during human infection in comparison to the expression profile in vitro. Infect Immun. 2000;68(3):1304–11. 10.1128/iai.68.3.1304-1311.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Juengert JR, Borisova M, Mayer C, Wolz C, Brigham CJ, Sinskey AJ, et al. Absence of ppGpp Leads to Increased Mobilization of Intermediately Accumulated Poly(3-Hydroxybutyrate) in Ralstonia eutropha H16. Appl Environ Microbiol. 2017;83(13). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meyer H, Liebeke M, Lalk M. A protocol for the investigation of the intracellular Staphylococcus aureus metabolome. Anal Biochem. 2010;401(2):250–9. 10.1016/j.ab.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 81.Mader U, Nicolas P, Depke M, Pane-Farre J, Debarbouille M, van der Kooi-Pol MM, et al. Staphylococcus aureus Transcriptome Architecture: From Laboratory to Infection-Mimicking Conditions. PLoS Genet. 2016;12(4):e1005962 10.1371/journal.pgen.1005962 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Strain HG001 and derivatives were grown to OD600 = 0.3 and treated for 30 min with or without 0.1 μg/ml ATc (mutant strains with inducible rel-Syn, relQ or empty vector). For Northern blot analysis, RNA was hybridized with digoxigenin-labelled probes specific for sodA, ftnA, dps, per, agrA or psm. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control.
(TIF)
Gene expression following rel-Syn induction in HG001 wild type. HG001 was grown to OD600 = 0.3 and treated for 30 min without or with 0.1 μg/ml ATc. Transcript were quantified by qRT-PCR on equal amount of total RNA. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001
(TIF)
Quantification of mRNA by qRT-PCR based on three biological replicates. Statistical significance was determined by two-tailed Student´s T-test, *p ≤ 0.05, **p ≤ 0.01, ***p ≤ 0.001 and ****p ≤ 0.0001.
(TIF)
Strains were grown to OD600 = 0.3 and then treated with 80mM H2O2 for 1 or 2 h.
(TIF)
Strains were grown to OD600 = 0.3 and treated with mupirocin or H202 for 30 min. RNA was hybridized with digoxigenin-labelled probes specific for ftnA, dps, psm, agrA or rpsl. The 16S rRNA detected in ethidium bromide-stained gels is indicated as a loading control in the bottom lane.
(TIF)
(TIF)
(DOCX)
(DOCX)
(DOCX)
Role categories and regulons.
(XLSX)
(XLSX)
Data Availability Statement
All data are available from the GEO database https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE145144 (accession GSE145144).