ABSTRACT
Spermatogonial stem cell (SSC) transplantation is an alternative reproductive method to achieve conservation and production of elite animals in livestock production. Creating a recipient animal without endogenous germ cells is important for effective SSC transplantation. However, natural mutants with depletion of SSCs are difficult to obtain, and drug ablation of endogenous germ cells is arduous to perform for practical use. In this study, we used mouse models to study the preparation of recipients with congenital germ cell ablation. We knocked out (KO) Ets-variant gene 5 (Etv5) in mice using the CRISPR/Cas9 system. The testicular weight of Etv5−/− mice was significantly lower than that of wild-type (WT) mice. The germ cell layer of the seminiferous tubules gradually receded with age in Etv5−/− mice. At 12 weeks of age, the tubules of Etv5−/− mice lacked almost all spermatogenic cells with a Sertoli cell-only phenotype, and sperm were completely absent in the epididymis. We subsequently transplanted allogeneic SSCs with enhanced green fluorescent protein (EGFP) into 3- (immature) or 7-week-old (mature) Etv5−/− mice. Partially restoration of germ cell layers in the seminiferous tubules and spermatogenesis was observed in all immature testes but not in mature adult testes at 2 months post-transplantation. The presence of heterologous genes Etv5 and EGFP in recipient testicular tissue and epididymal sperm by PCR indicated that sperm originated from the transplanted donor cells. Our study demonstrates that, although Etv5−/− mice could accommodate and support foreign germ cell transplantation, this process occurs in a quite low efficiency to support a full spermatogenesis of transplanted SSCs. However, using Etv5−/− mice as a recipient model for SSC transplantation is feasible, and still needs further investigation to establish an optimized transplantation process.
KEY WORDS: Etv5, Spermatogonial stem cells, Gene knockout, Transplantation, Spermatogenesis
Summary: Spermatogonial stem cell transplantation in mice with germline ablation by genetic modification of a specific gene.
INTRODUCTION
Spermatogonial stem cells (SSCs) are male germline stem cells that reside in the basement membrane of the seminiferous tubule in the testis (de Rooij, 2017; Kubota and Brinster, 2018). SSCs are capable of self-renewing to maintain the stem cell pool throughout the lifespan and differentiating into spermatozoa after puberty (Yoshida, 2012). SSCs form the foundation of spermatogenesis and male fertility. SSCs share some common identities with other adult stem cells, but they also harbor an unique and important function by transmitting genetic information from the paternal generation to the descendants (Komeya and Ogawa, 2015). SSCs can be isolated, propagated in vitro, cryopreserved, and transplanted into the recipient testis to generate SSC-derived progeny (Kubota and Brinster, 2018). The potential to manipulate or transplant SSC has offered a new approach to repopulate sterile testis and restore spermatogenesis in animal models or patients. In 1994, Brinster and colleagues first reported that transplantation of mouse SSCs into the seminiferous tubules of infertile recipient mice reinitiates donor-derived spermatogenesis to produce viable offspring (Brinster and Avarbock, 1994). Since then, SSC transplantation has also been demonstrated in many species, including rat, goat, sheep, pig, and non-human primate (Ogawa et al., 1999; Honaramooz et al., 2003; Rodriguez-Sosa et al., 2009; Mikkola et al., 2006; Hermann et al., 2012). This technique opens new avenues for the treatment of male infertility, development of alternative livestock reproduction technology, and generation of transgenic animals for biomedical and agricultural purposes.
SSC transplantation is routinely performed in both mice and rats but has not yet been well-established in large mammals, such as pigs and cattle (Mikkola et al., 2006; Herrid et al., 2006). SSC transplantation in large farm animals is a promising technique for fast multiplication of elite or genetically desired individuals to benefit agricultural outputs (Giassetti et al., 2019). In addition, translation of this technology into human clinics has not been realized (Nagamatsu and Hayashi, 2017). Full implementation of the transplant relies largely on effective derivations of germline-ablated recipient and high-quality donor cells. Various approaches, such as drug treatment (Brinster and Avarbock, 1994), irradiation (Herrid et al., 2009), and heat shock (Ma et al., 2011), have been adopted to generate a sterile recipient for SSC transplantation. The most common and effective strategies for germ cell ablation in mouse models are injection of chemotoxic drugs (busulfan) and use of mutant W mice lacking endogenous germ cells. However, these strategies are not easily reproduced in large animals, as an optimal busulfan dose to balance an adequate ablation of endogenous germ cells and whole-body side effect is difficult to control; moreover, no other species has a W genetic background, for which its mutation information remains unclear (Kubota and Brinster, 2018). The issue of recipient preparation prompted us to develop a common and simple method to generate germline-ablated recipients. This model should feature (i) congenital germ cell deficiency; (ii) intact Sertoli cells and testicular structure to support foreign SSC residence, growth, and differentiation; (iii) transmissibility of phenotype to progeny; and (iv) easy application to various animal species. On the basis of the above considerations, specific gene mutations that are associated with the developmental deficit of SSCs without affecting testicular somatic support cells can be used to create such a recipient model. In this regard, the currently developed genome editing technology can engineer any genomic regions to generate desired genotypes and phenotypes in many species, therefore offering a universal platform for genome engineering in various animals.
Here, we establish an Ets-variant gene 5 (Etv5) gene-targeted mouse model and investigate its ability to support allotransplantation of SSCs. Genetic variants in the human Etv5 gene were believed to be associated with nonobstructive azoospermia associated with Sertoli cell-only syndrome (O'Bryan et al., 2012). Etv5 homozygous mutant (Etv5−/−) male mice are sterile due to the progressive loss of germ cells. Etv5 heterozygous mutant (Etv5+/−) mice are fertile and healthy, so they can be used as parental generation to maintain the mutant strain. Our study explored the possibility of using Etv5−/− mice as the recipient model with congenital germline ablation to facilitate SSC transplantation study. If Etv5−/− mice are applicable for SSC transplantation, this strategy can be extended to large farm animals by specific gene targeting to create transplant recipients serving SSC-based reproduction and transgenesis.
RESULTS
Generation of Etv5−/− mice by using CRISPR/Cas9
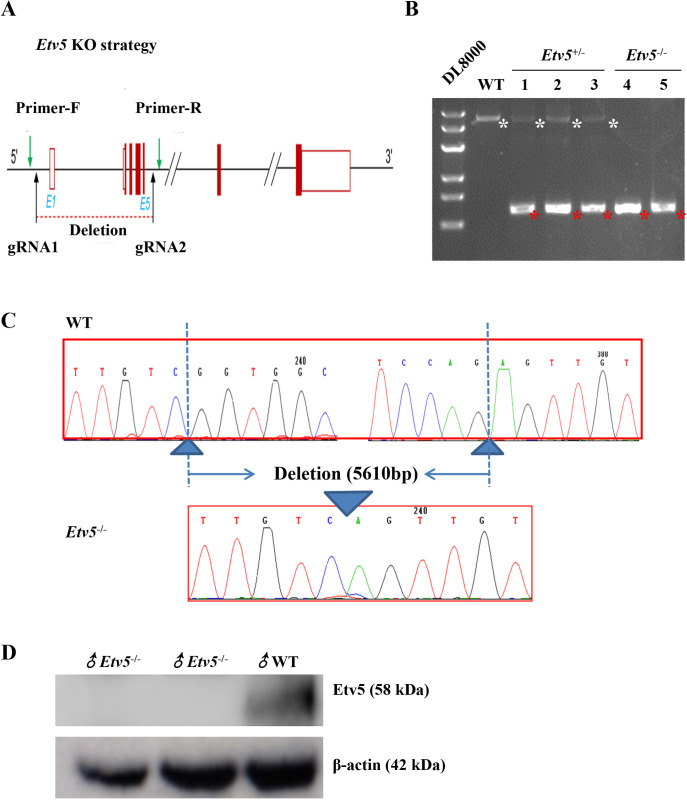
We designed two gRNAs targeting the introns on both sides of exon 1 (gRNA1) to exon 5 (gRNA2) to delete a ∼5610 bp fragment of the Etv5 gene to generate Etv5−/− mice by embryo injection of Cas9 mRNA and gRNAs (Fig. 1A). The efficiency of CRISPR-induced founder mice is shown in Table 1. The genotypes of the founder and their offspring were identified using primers F/R (Fig. 1A). DNA gel electrophoresis results demonstrated that homozygous knockout (KO) mice generated a 710 bp band, heterozygotes had two bands (710 and 6326 bp), and wild-type (WT) mice had a single band of 6326 bp (Fig. 1B). PCR and DNA sequencing demonstrated that homozygous KO mice had a 5610 bp deletion (Fig. 1C). In addition, western blot analysis confirmed a lack of Etv5 expression in homozygous Etv5−/− mice (Fig. 1D). Interestingly, Etv5−/− male mice at 3 weeks of age were obviously smaller than WT male mice, and this difference was more pronounced at 12 weeks of age (Fig. S1A). The body weights of Etv5−/− male mice were markedly lower at each point from 14 postnatal days to 84 days (P<0.05) compared with WT controls, with the magnitude of body weight decline between 34.3% and 45.7% (Fig. S1B).
Fig. 1.
Generation of Etv5−/− mice by CRISPR/Cas9. (A) Schematic depicting the strategy used for the generation of Etv5−/− mice, which included gRNA1 and gRNA2 targeting sites. Primer-F and primer-R were used to genotype the mice. (B) Genotype determined by PCR for newborn male mice littermates. WT, Etv5+/−, and Etv5−/− represent the WT, heterozygote, and homozygote, respectively. White asterisks indicate WT Etv5 genomic sequence (6326 bp), and red asterisks indicate the truncated Etv5 sequence after CRISPR editing (710 bp). (C) Sequencing of the PCR products of Etv5−/− mice and WT controls shown in B. (D) Etv5 protein expression in WT and Etv5−/− mice measured by western blot.
Table 1.
Generation of CRISPR-edited founder mice with Etv5 deletion
Lack of SSCs in the seminiferous tubules of Etv5−/− mice
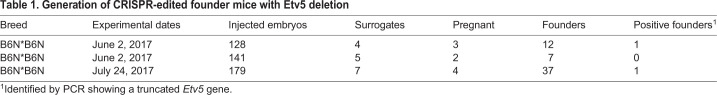
We measured the weight of testes in Etv5−/− mice from day 4 to 12 weeks of age and compared them with that in WT mice of the same age. The weights were almost similar in mice aged before 1 week. However, the testis weights of Etv5−/− mice were markedly lower from 3 to 12 weeks of age (P<0.01) compared with those of WT controls, with the magnitude of testis weight declining between 43.1% and 67.4% (Fig. 2A). The testis-to-body-weight ratios were also significantly lower in Etv5−/− mice than in WT mice after 3 weeks of age (Fig. 2B). The difference was also reflected in the sizes of testes; the testes of Etv5−/− mice were much smaller than those of WT mice at 3 weeks of age, and the difference became more evident at 12 weeks of age (Fig. 2C). Hematoxylin and Eosin (H&E) staining demonstrated a remarkable decrease in the number and type of testicular cells in the seminiferous tubules of Etv5−/− mice at 3 weeks of age. Spermatogenic cells were absent in the tubules of Etv5−/− mice, and Sertoli cell-only tubules were observed at 12 weeks of age (Fig. 2D). Additionally, we measured the protein expression levels of PLZF, which is a marker for SSCs. We observed that PLZF was still expressed in some spermatogonia in seminiferous tubules of Etv5−/− mice at 3 weeks of age. However, by 12 weeks of age, PLZF-positive cells in Etv5−/− testis had completely disappeared but were present in WT (Fig. 2E). The numbers of premeiotic germ cells, meiotic germ cells, and round spermatids significantly declined in Etv5−/− mice and were undetectable by 12 weeks of age (Fig. 2F). Moreover, the percentage of vacuolation observed in seminiferous tubules in both 3- and 12-week-old Etv5−/− mice were higher than that in WT control mice, reaching 100% at 12 weeks of age (Fig. 2G). The diameter of seminiferous tubules of Etv5−/− mice was significantly smaller than that of WT controls at both 3 and 12 weeks of age (P<0.01; Fig. 2H).
Fig. 2.
Absence of SSCs in the seminiferous tubules of Etv5−/− mice. (A) Weight of testes in Etv5−/− mice compared with WT littermates at periodic points during development. For all time points, n=4 per group for both WT and Etv5−/− mice. (B) Testis-to-body-weight ratio of Etv5−/− mice and WT littermates (n=4). (C) Comparison of size of testes of WT and Etv5−/− mice at 3 and 12 weeks of age. Scale bars: 3 mm. (D,E) Representative images for H&E staining and PLZF immunohistochemistry of testes in WT and Etv5−/− mice littermates at 3 and 12 weeks. Red arrows indicate SSCs with brown positive staining by PLZF antibody. Pentacles reveal vacuolation in some seminiferous tubules in Etv5−/− mice. Scale bars: 50 μm. (F) The numbers of premeiotic germ cells, meiotic germ cells, round spermatids, and elongated spermatids in seminiferous tubules in WT and Etv5−/− mice littermates at 3 and 12 weeks. The cell numbers were counted from the cross sections of seminiferous tubules in a 200× visual field. Samples from three mice were prepared, and data were collected from three slices per mice. (G) The percentage of vacuolation of seminiferous tubules. (H) Diameter of seminiferous tubules from WT and Etv5−/− mice at 3 and 12 weeks of age. n=3 per group shown in F, G, and H for both WT and Etv5−/− mice. Nine slices (three for each mouse) per group were prepared, and data from each slice were collected from at least three different regions. Data are means±standard deviation (s.d.). *represents P<0.05, **represents P<0.01, determined by unpaired t-test.
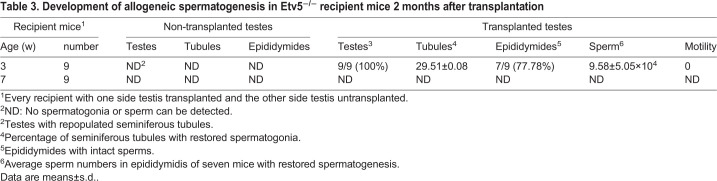
We next examined sperm production in KO mice at different ages. The results were consistent with the findings of H&E staining and immunohistochemistry. At the time of sexual maturity (6 weeks of age), there were numerous motile sperm in WT mice compared with age-matched Etv5−/− mice, whose sperm numbers were extremely low (Table 2 and Fig. S2). At 12 weeks of age, numerous motile sperm were observed in the epididymis WT mice, but there was a lack of spermatozoa in age-matched Etv5−/− mice (Table 2 and Fig. S2). In addition, we found that the expression levels of the Etv5 target gene Ccl9, spermatid-specific gene Prm2, and interstitial gland-specific gene Cyp17a1 were significantly decreased (P<0.01, P<0.01, and P<0.05, respectively) in Etv5−/− mice (Fig. S3A–C). We also tested testosterone concentrations in WT and Etv5−/− littermates at 6, 8, and 12 weeks. A significant reduction in testosterone concentrations was observed in Etv5−/− mice compared with WT mice, and a maximum difference was reached at 12 weeks of age (P<0.01; Fig. S3D).
Table 2.
Number and motility of sperm in one side epididymis between WT and Etv5−/− mice
Transplantation of allogeneic SSCs through the efferent duct
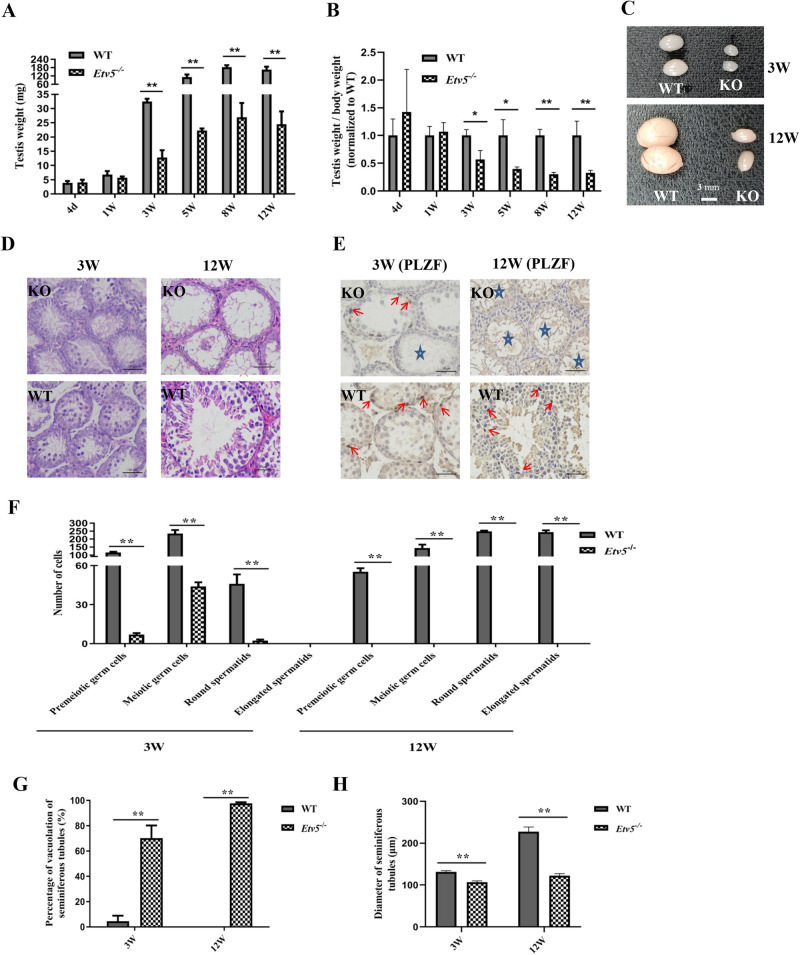
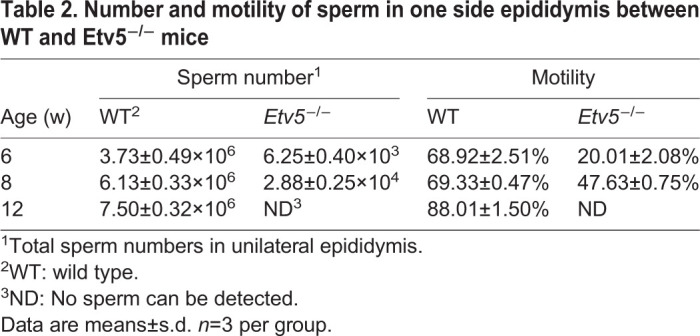
To determine whether the Etv5−/− male can serve as a recipient model for SSC transplantation, we transplanted allogeneic SSCs (expressing EGFP by lentiviral transduction) through the efferent duct into immature (3 weeks of age) and mature (7 weeks of age) mice (Fig. 3A and Table 3). Our preliminary experiments showed that almost all lentiviral transduced SSCs were positive for EGFP expression at a multiplicity of infection of 5:1 (viruses to cells). In nine immature recipients, all had repopulated seminiferous tubules with different degrees and seven harbored spermatozoa in epididymis at 2 months post-transplantation (Table 3; Fig. S4). The average sperm number of the seven restored mice was 9.58±5.05×104 (Table 3). Morphological analysis of the transplanted testes found that the proportion of tubules with restored spermatogonia was 29.51±0.08% in the seven restored testes (Table 3). Reconstruction of germ cell layers (Fig. 3B, left lower) and regeneration of elongated spermatozoa (Fig. 3B, right) could be observed in these tubules, and spermatogonia were positive for PLZF protein expression (Fig. 3C, transplanted groups) in Etv5−/− mice after SSC transplantation. No spermatozoa and germ cells were detected in non-transplanted age-matched testes (Fig. 3B,C). To determine the origin of the sperm from post-transplanted immature testes, we examined them under a fluorescence microscope. The regenerated sperm emitted green light, indicating they were of donor SSC origin (Fig. 3D). Furthermore, the presence of heterologous genes Etv5 and EGFP was measured in recipient epididymal sperm by PCR. Etv5 and EGFP were not detected in non-transplanted testes; however, they were present in spermatozoa of transplanted Etv5−/− mice (Fig. 3E). This finding further indicated that the sperm were derived from donor cells. In addition, no spermatozoa were detected in all nine mature recipients with SSC transplantation (Table 3).
Fig. 3.
Transplantation of allogeneic SSCs through the efferent duct. (A) Transplantation process of allogeneic SSCs through the efferent duct. (B) H&E staining of non-transplanted and transplanted testes. Red arrows indicate restored germ cell layers in the seminiferous tubules, and red stars indicate regenerated immature sperm. Scale bars: 50 μm. (C) PLZF immunohistochemistry of testes showing restored spermatogonia with brown positive staining (red arrows) in the seminiferous tubules. The insets represent high magnification of pointed cells in break line boxed areas. Scale bars: 50 μm. (D) Sperm collected from epididymis 2 months post-transplantation and age and breed-matched WT mice. Red boxes point to regenerated sperm with EGFP expression in Etv5−/− mice and WT sperm which are EGFP negative in the same conditions. Scale bars: 20 μm. (E) Heterologous Etv5 and EGFP DNA were measured to determine the origin of epididymal sperm. NT, non-transplanted testes; 1, transplanted testes; 2, sperm collected from epididymis of SSC-transplanted testes; SSCs, donor cells used for transplantation.
Table 3.
Development of allogeneic spermatogenesis in Etv5−/− recipient mice 2 months after transplantation
DISCUSSION
Etv5 is essential for SSC self-renewal and KO of Etv5 severely impairs SSC development and results in male infertility (Chen et al., 2005; Hofmann, 2008; and Ishii et al., 2012). Chen et al. demonstrated that ERM−/− (Etv5−/−) mice undergo a progressive germ cell depletion with a gradual loss of spermatogonia in the seminiferous tubules staring from 4 weeks of age. ERM−/− tubules lost most germ cells, but containing morphologically normal Sertoli cells at the basement membrane by 10 weeks of age (Chen et al., 2005). In our study, we successfully generated Etv5−/− mice by using CRISPR/Cas9 system for embryo injection. The generated KO mice gradually lost SSCs but preserved an intact structure of seminiferous tubules with a similar time frame as previous report, indicating that they could be potential germ cell-free models for SSC transplantation.
Our KO manipulation revealed that undifferentiated and differentiated spermatogonia were lost in the majority of seminiferous tubules, and only a small region of the tubules retained multilayer germ cells in immature mice. Etv5−/− mice at 3 weeks old had partial germ cell loss in the seminiferous tubules and their testicular sizes were slightly smaller than those of age-matched WT controls. By 12 weeks of age, the testicular sizes of Etv5−/− mice were much smaller than those of WT controls, with a severe germ cell lost in the tubules. Interestingly, 3-week-old Etv5−/− mice were smaller in body size and weight compared with WT mice. At 12 weeks of age, this trend was even more obvious. The reduced body weights in Etv5−/− mice indicated that Etv5 had an influence on overall growth. Etv5 mRNA has been detected in a variety of tissues, including the heart, lungs, thymus, lymphocytes, kidneys, and skeletal muscles (Liu et al., 2003; T'Sas et al., 2005). The widespread expression of Etv5 during development may be crucial for growth. In a previous study that investigated the viability of Etv5−/− mice, Etv5 mRNA was found to be abundantly expressed in the brain, lungs, and colon, but it was most abundantly expressed in the testes (Schlesser et al., 2008).
We next investigated whether our Etv5−/− mouse model would support donor-derived spermatogenesis and sperm generation after allogeneic SSC transplantation. Previous report demonstrated that Etv5−/− mice showed serious defects in Sertoli cells, which could not form an optimal testicular environment to support spermatogenesis (Chen et al., 2005). However, the overall structure of seminiferous tubule remains intact in spite of severe loss of germ cells and other somatic cells. Also, testicular cell preparation and transplantation process is usually accompanied by existence of lots of testicular somatic cells, which could supplement the defects of Sertoli cells in Etv5−/− mice. In the present study, we definitely obtained donor-derived spermatozoa in transplanted Etv5−/− mice. Although spermatogenesis was visible in transplanted immature Etv5−/− mouse testes, the number and motility of regenerated sperm in the epididymis were not ideal. We guess multiple reasons could contribute to the inefficiency in SSCT transplantation in Etv5−/− models, including immunological rejection between donor and recipient, impaired Sertoli cells constitution or stem cell microenvironment by absence of Etv5, or inadequate quality and number of implanted SSCs. Also, recoverable spermatogenesis following SSC transplantation seems to be slower than endogenous spermatogenesis. Complete spermatogenesis takes approximately 34.5 days from type A single (As) spermatogonia to mature spermatozoa in WT mice (Oakberg, 1956). Transplanted SSCs usually need 2 weeks for a complete colonization. Meiosis is usually initiated within the second month, with several spermatids being observed after 2-month transplantation. The degree of germ cell differentiation will continue to increase, with normal spermatogenesis being observed 3 months post-transplantation (Nagano et al., 1999). Therefore, the time point of 2 months in our experiment could not be enough for detection of a full round of spermatogenesis following SSC transplantation.
Our results showed SSC transplantation effect between immature and mature Etv5−/− testis is significantly different. SSC transplantation in immature Etv5−/− testis is more feasible than mature Etv5−/− testis, which is in line with previous studies which showed that SSC transplantation in immature pup testes is more efficient than adult testis (Shinohara et al., 2001; Ishii et al., 2013). As a lack a fully formed blood-testis barrier, transplantation of testis cells into pup testis has a five- to tenfold increased colonization efficiency compared with mature testis. The area for colonization per donor stem cell is also four times larger in recipient pups than adults (Shinohara et al., 2001). These factors facilitate a more efficient restoration of fertility by SSC transplantation in infertile immature recipients. Furthermore, immature Etv5−/− testis at the age of 3 weeks harbor residual endogenous spermatogonia that could facilitate maintenance of testicular function and help restoration of fertility after transplantation (Kanatsu-Shinohara et al., 2016).
In summary, we generated Etv5−/− mice with a genetic sterility. SSC donors transplanted into recipient testes could partially restore heterologous spermatogenesis and produce donor-derived sperm, even though their quantity and vitality were not optimal. SSC transplantation in these genetically modified mouse models is possible but remains at a low efficiency in the present report. Further investigations are required to optimize the SSC transplantation process in the genetically modified models to obtain ideal transplantation outcomes.
MATERIALS AND METHODS
Ethics statement
All animal experiments were performed in accordance with the guidelines of the National Institutes of Health's Guide for the Care and Use of Laboratory Animals. Our study was approved by the IACUC at South China Agricultural University.
Embryo injections for KO mice production
CRISPR-gRNA targeted sequences are shown in Fig. 1A. The mouse (C57BL/6) Etv5 gene (GenBank accession number: NM_023794.2; Ensembl: ENSMUSG00000013089) is located on chromosome 16, with 13 identifiable exons, with an ATG start codon on exon 2 and a TAA stop codon on exon 13. The introns on both sides of exons 1–5 were selected as the target sites for CRISPR/Cas9-mediated genome editing. A pair of gRNA targeting vectors (pRP[CRISPR]-hCas9-U6) was constructed and confirmed by sequencing: gRNA1 (matching the forward strand of the gene): GAACGGCCATTGTCGGTGGCAGG and gRNA2 (matching the forward strand of the gene): CTTCTATGCTAATAACGGGTGGG were selected. These two targeting vectors were used for embryo injections, together with Cas9 mRNA. gRNA generated by in vitro transcription was then co-injected into fertilized eggs for the generation of KO mice.
Etv5 genotyping and expression
For CRISPR-induced mutation assays, female and male heterozygous edited mice were bred to generate homozygous edited offspring. DNA was extracted from the toes of pups from the same litter at day 2 post-birth for PCR analysis and sequencing. The following two primers were used for genotyping by PCR: mouse Etv5 primer-Forward: 5′-CAACTGGTGCCCTTCCCAGTCT-3′, mouse Etv5 primer-Reverse: 5′-GCCGCTCTTAAACCTGTTCATTCG-3′. WT mice were designated as the control group, and Etv5−/− mice comprised the experimental group. Polyclonal anti-ERM/Etv5 antibody (1:100 dilution; ab102010, Abcam, Cambridge, MA, USA) recognizing amino acids 244 of the human ERM/Etv5 was used to measure Etv5 protein expression in Etv5−/− male and WT mice by western blotting. The anti-β-actin monoclonal antibody was used as the loading control.
H&E staining and immunohistochemistry
Whole testes were fixed in 4% paraformaldehyde and then paraffin sectioned for H&E staining and immunohistochemistry. For H&E staining, paraffin sections were counterstained with H&E and then observed under an electron microscope to compare spermatogonium between Etv5−/− mice and WT littermates. Immunohistochemistry was performed after antigen retrieval with EDTA (pH 9.0), 3% hydrogen peroxide was used for blocking endogenous peroxidase, and 3% BSA was used to block non-specific binding. Sections were incubated with specific antibodies. Anti-promyelocytic leukemia zinc-finger (PLZF) mouse antibody (sc-28319, Santa Cruz Biotechnology, Dallas, TX, USA), a marker of undifferentiated spermatogonia, was used at 1:100 dilutions. HRP-labeled goat anti-mouse IgG diluted at 1:200 was used as the secondary antibody. The sections were then washed and developed using DAB color rendering and nuclear staining. Hematoxylin-stained nucleus that was a blue and brownish yellow color indicated positive expression of DAB.
Real time PCR
Total RNA samples were extracted using Eastep Super Total RNA Extraction Kit (LS1040, Promega, Madison, WI, USA) according to the manufacturer's instructions. Total RNA was converted to cDNA using PrimeScript RT reagent Kit with gDNA Eraser (RR047A, Takara, Dalian, China). The mRNA expression levels of the Ccl9, Prm2, and Cyp17a1 genes were then measured by quantitative PCR using PowerUp™ SYBR™ Green Master Mix (A25742, Thermo Fisher Scientific, Austin, TX, USA). β-Actin served as an internal control. Each gene from control and experimental samples was tested in triplicate. Relative gene expression was calculated using the 2−ΔΔCT method.
Measurement of testosterone concentrations
Blood was collected from mice via the orbital vein and then centrifuged for serum collection. Testosterone concentrations in Etv5−/− male and WT control littermates at 12 weeks of age were determined using the mouse testosterone ELISA kit (E05101m, CUSABIO, Wuhan, China). In brief, 50 μl of standards or samples were added to each well, with blank wells left empty. About 50 μl of HRP conjugate was added to each well except for the blank well. Subsequently, 50 μl of antibody was added to each well. The wells were mixed and then incubated for 1 h at 37°C. Contents from each well were aspirated and washed three times. The assay plate was blotted dry, and 50 μl of substrate A and 50 μl of substrate B were added to each well and incubated for 15 min at 37°C in the dark. After incubation, 50 μl of stop solution was added to each well, and the optical density (OD) of each well was recorded within 10 min using a microplate reader set to 450 nm.
Semen collection and analysis
Mice were euthanized by cervical dislocation. The unilateral epididymis was surgically removed, cut into pieces, and immersed in 1 ml of SpermRinse™ (Vitrolife, Göteborg, Sweden). After incubation at room temperature, 10 μl of sperm solution was transferred to a hemocytometer to determine the number and vitality of sperm. The average of three data records from different mice in each group was then determined. The sperm smear test was used to compare the sperm concentrations between Etv5−/− male and WT mice. In brief, approximately 1 ml of sperm solution was centrifuged at 2000 rpm for 10 min. The resulting precipitate was re-suspended with 500 μl of SpermRinse™, followed by the addition of Trypan Blue (1:2 in volume). Slides were sealed using glycerol gelatin after 10 μl of the sample was placed onto glass slides.
SSC preparation
Testes were harvested from 6 to 8 days postpartum C57BL/6 male pups and then digested using a two-step enzymatic digestion protocol. In brief, testes were washed in DPBS with 2% penicillin–streptomycin, and the tunica albuginea and convoluted epididymis were removed. The seminiferous tubules were digested in 5 ml of DPBS solution I consisting of 1 mg/ml collagenase type IV (17104019, Gibco, Grand Island, NY, USA) and 20 U/ml DNase I (2212, Takara, Dalian, China) at 37°C for 10 min with intermittent agitation every 5 min. Samples were then centrifuged at 300 g for 5 min, and the supernatants were discarded. The precipitates were washed in DPBS and incubated in 5 ml of solution II consisting of 20 U/ml DNase I and 0.25% trypsin/EDTA at 37°C for 5 min until cells were completely dispersed. Digestion was terminated using 10% fetal bovine serum in DMEM/F12 (11320082, Gibco, Grand Island, NY, USA). The samples containing single cells and clumps were filtered through a nylon cell strainer with 40 μm pore size. Single-cell suspensions were collected in the filtrate and then centrifuged. Supernatants were discarded, and the cell pellet was washed three times with DPBS and then resuspended in complete medium. The number and viability of the resulting dissociated single cells had a density of 1.06×107 cells/ml with viability greater than 99%.
Lentivirus infection
SSCs were infected with LPP-EGFP-Lv156-400 lentivirus expressing EGFP prior to transplantation to generate EGFP-transgenic SSCs. In brief, 30 μl of lentivirus (1×108 TU/ml) was added into 1 ml of cell suspension and then seeded into 12-well plates. The cells were incubated at 37°C and 5% CO2 atmosphere for 12 h to allow lentivirus infection and EGFP transgene incorporation in SSC genome. Subsequently, cells were washed once in DPBS and re-suspended gently in DPBS using a Pasteur pipette. Cell suspensions were then transferred into a new 15 ml centrifuge tube and centrifuged. Supernatants were discarded and cell pellet were re-suspended in 300 μl of Hanks' Balanced Salt Solution (HBSS, 14170112, Gibco, Grand Island, NY, USA). The cell suspension was transferred to a designated transplantation suite for transplantation.
SSC transplantation
Mice with homozygous mutations of the Etv5 gene were used as recipients for transplantation. Every recipient was transplanted into only one side of the testis, with the other side of the testis used as the non-transplanted control. Mice were anesthetized by an intraperitoneal injection of anesthetic. An appropriate amount of 1.25% 2,2,2-tribromoethanol (M2910, Easycheck, Nanjing, China; 0.2 ml/10 g body weight) was absorbed in a 1 ml sterile syringe. The needle tip of the syringe was faced upward and then pierced the abdominal cavity at a 45° angle. The drug was slowly injected when the tip part could be moved handily. The mouse's toe or paw was stimulated, and we waited for it to faint but ensured that it could keep breathing steadily. At this moment, the mouse was placed dorsally under a stereomicroscope for the transplantation procedure.
The cuticular layer at the midline of the abdomen, not far from the genitals, was lifted using small forceps. A transverse 0.3 cm incision was made, followed by an incision to the peritoneum and abdominal muscle layer until the peritoneal cavity was visible. The lateral fat pad attached to the testis was gently pulled until the testis was exteriorized. The efferent duct that connected the testis to the epididymis was identified, and fat tissues around the duct were gently removed. The SSC suspension was then carefully transferred to a 100 µl volume microinjection syringe. The syringe was connected into a capillary glass tube with an inner diameter of 40–50 µm at the tip. The cell suspension was gently forced into the seminiferous tubules of the testis via the efferent duct by applying pressure to the syringe. The injection pipette was held parallel to the ordinate axis of the efferent duct. The injection rate and cell suspension flow rate were controlled manually by monitoring the movement of the cell suspension in the tubules. Approximately 5–10 μl of the donor cell suspension was injected into each recipient testis. The other testis was not surgically manipulated and served as a control. After transplantation, the testis was placed back into the peritoneal cavity. The incision was closed with one stitch of a 7-0 absorbable suture from the inside out, with the abdominal muscle layer first, followed by the peritoneum layer, and finally the cuticular layer. Mice were returned to their cages, monitored for distress, and assessed regularly until testis samples were collected.
Identification of allogeneic SSCs
Testes of recipient mice were harvested 2 months after transplantation to determine the functional recovery of spermatogenesis. Recipient testes were fixed in 4% paraformaldehyde and embedded in paraffin wax for histomorphology and immunohistochemistry analyses. Heterologous spermatogenesis was observed under a fluorescence stereomicroscope by using EGFP-positive sperm obtained from the epididymis of recipient mice. Presence of exogenous genes including Etv5 and EGFP in the testes and spermatozoa were measured by PCR.
Statistical analysis
PASW Statistics 21 (IBM SPSS, Chicago, IL, USA) was used to determine statistical significance and standard deviation. Body weight, testis weight, gene expression level, testosterone concentrations, and cell counts between WT and KO groups were analyzed using two-tailed unpaired t-test. Differences were considered significant at P<0.05 (*) and P<0.01(**).
Supplementary Material
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Formal analysis: X. Zhang, G.L., Z.L., H.Y.; Investigation: X. Zhang, X. Zhao, M.Z., P.X.; Writing - original draft: X. Zhang, H.Y.; Project administration: H.Y.; Funding acquisition: H.Y., B.C., Z.W.
Funding
This work was supported by the National Natural Science Foundation of China (31772555).
Supplementary information
Supplementary information available online at https://bio.biologists.org/lookup/doi/10.1242/bio.056804.supplemental
References
- Brinster R. L. and Avarbock M. R. (1994). Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl Acad. Sci. USA 91, 11303-11307. 10.1073/pnas.91.24.11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Ouyang W., Grigura V., Zhou Q., Carnes K., Lim H., Zhao G.-Q., Arber S., Kurpios N., Murphy T. L. et al. (2005). ERM is required for transcriptional control of the spermatogonial stem cell niche. Nature 436, 1030-1034. 10.1038/nature03894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giassetti M. I., Ciccarelli M. and Oatley J. M. (2019). Spermatogonial stem cell transplantation: insights and outlook for domestic animals. Annu. Rev. Anim. Biosci. 7, 385-401. 10.1146/annurev-animal-020518-115239 [DOI] [PubMed] [Google Scholar]
- Hermann B. P., Sukhwani M., Winkler F., Pascarella J. N., Peters K. A., Sheng Y., Valli H., Rodriguez M., Ezzelarab M., Dargo G. et al. (2012). Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell 11, 715-726. 10.1016/j.stem.2012.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrid M., Vignarajan S., Davey R., Dobrinski I. and Hill J. R. (2006). Successful transplantation of bovine testicular cells to heterologous recipients. Reproduction 132, 617-624. 10.1530/rep.1.01125 [DOI] [PubMed] [Google Scholar]
- Herrid M., Olejnik J., Jackson M., Suchowerska N., Stockwell S., Davey R., Hutton K., Hope S. and Hill J. R. (2009). Irradiation enhances the efficiency of testicular germ cell transplantation in sheep. Biol. Reprod. 81, 898-905. 10.1095/biolreprod.109.078279 [DOI] [PubMed] [Google Scholar]
- Hofmann M.-C. (2008). Gdnf signaling pathways within the mammalian spermatogonial stem cell niche. Mol. Cell. Endocrinol. 288, 95-103. 10.1016/j.mce.2008.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A., Behboodi E., Blash S., Megee S. O. and Dobrinski I. (2003). Germ cell transplantation in goats. Mol. Reprod. Dev. 64, 422-428. 10.1002/mrd.10205 [DOI] [PubMed] [Google Scholar]
- Ishii K., Kanatsu-Shinohara M., Toyokuni S. and Shinohara T. (2012). FGF2 mediates mouse spermatogonial stem cell self-renewal via upregulation of Etv5 and Bcl6b through MAP2K1 activation. Development 139, 1734-1743. 10.1242/dev.076539 [DOI] [PubMed] [Google Scholar]
- Ishii K., Kanatsu-Shinohara M. and Shinohara T. (2013). Cell-cycle-dependent colonization of mouse spermatogonial stem cells after transplantation into seminiferous tubules. J. Reprod. Dev. 60, 37-46. 10.1262/jrd.2013-083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M., Morimoto H. and Shinohara T. (2016). Fertility of male germline stem cells following spermatogonial transplantation in infertile mouse models. Biol. Reprod. 94, 112-111. 10.1095/biolreprod.115.137869 [DOI] [PubMed] [Google Scholar]
- Komeya M. and Ogawa T. (2015). Spermatogonial stem cells: progress and prospects. Asian J. Androl. 17, 771-775. 10.4103/1008-682X.154995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H. and Brinster R. L. (2018). Spermatogonial stem cells. Biol. Reprod. 99, 52-74. 10.1093/biolre/ioy077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Jiang H., Crawford H. C. and Hogan B. L. M. (2003). Role for ETS domain transcription factors Pea3/Erm in mouse lung development. Dev. Biol. 261, 10-24. 10.1016/S0012-1606(03)00359-2 [DOI] [PubMed] [Google Scholar]
- Ma W., An L., Wu Z., Wang X., Guo M., Miao K., Ma W. and Tian J. (2011). Efficient and safe recipient preparation for transplantation of mouse spermatogonial stem cells: pretreating testes with heat shock. Biol. Reprod. 85, 670-677. 10.1095/biolreprod.110.089623 [DOI] [PubMed] [Google Scholar]
- Mikkola M., Sironen A., Kopp C., Taponen J., Sukura A., Vilkki J., Katila T. and Andersson M. (2006). Transplantation of normal boar testicular cells resulted in complete focal spermatogenesis in a boar affected by the immotile short-tail sperm defect. Reprod. Domest. Anim. 41, 124-128. 10.1111/j.1439-0531.2006.00651.x [DOI] [PubMed] [Google Scholar]
- Nagamatsu G. and Hayashi K. (2017). Stem cells, in vitro gametogenesis and male fertility. Reproduction 154, F79-F91. 10.1530/REP-17-0510 [DOI] [PubMed] [Google Scholar]
- Nagano M., Avarbock M. R. and Brinster R. L. (1999). Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol. Reprod. 60, 1429-1436. 10.1095/biolreprod60.6.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakberg E. F. (1956). Duration of spermatogenesis in the mouse and timing of stages of the cycle of the seminiferous epithelium. Am. J. Anat. 99, 507-516. 10.1002/aja.1000990307 [DOI] [PubMed] [Google Scholar]
- O'Bryan M. K., Grealy A., Stahl P. J., Schlegel P. N., McLachlan R. I. and Jamsai D. (2012). Genetic variants in the ETV5 gene in fertile and infertile men with nonobstructive azoospermia associated with Sertoli cell–only syndrome. Fertil. Steril. 98, 827-835. 10.1016/j.fertnstert.2012.06.013 [DOI] [PubMed] [Google Scholar]
- Ogawa T., Dobrinski I. and Brinster R. L. (1999). Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell 31, 461-472. 10.1054/tice.1999.0060 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa J. R., Silvertown J. D., Foster R. A., Medin J. A. and Hahnel A. (2009). Transduction and transplantation of spermatogonia into the testis of ram lambs through the extra-testicular rete. Reprod. Domest. Anim. 44, 612-620. 10.1111/j.1439-0531.2007.01030.x [DOI] [PubMed] [Google Scholar]
- de Rooij D. G. (2017). The nature and dynamics of spermatogonial stem cells. Development 144, 3022-3030. 10.1242/dev.146571 [DOI] [PubMed] [Google Scholar]
- Schlesser H. N., Simon L., Hofmann M.-C., Murphy K. M., Murphy T., Hess R. A. and Cooke P. S. (2008). Effects of ETV5 (ets variant gene 5) on testis and body growth, time course of spermatogonial stem cell loss, and fertility in mice. Biol. Reprod. 78, 483-489. 10.1095/biolreprod.107.062935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Orwig K. E., Avarbock M. R. and Brinster R. L. (2001). Remodeling of the postnatal mouse testis is accompanied by dramatic changes in stem cell number and niche accessibility. Proc. Natl. Acad. Sci. 98, 6186-6191. 10.1073/pnas.111158198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- T'Sas F., Brenner C., Mauen S., Putmans P., Monté D., Van Lint C., Moser M., Baert J.-L. and De Launoit Y. (2005). Expression of the Ets transcription factor Erm is regulated through a conventional PKC signaling pathway in the Molt4 lymphoblastic cell line. FEBS Lett. 579, 66-70. 10.1016/j.febslet.2004.11.052 [DOI] [PubMed] [Google Scholar]
- Yoshida S. (2012). Elucidating the identity and behavior of spermatogenic stem cells in the mouse testis. Reproduction 144, 293-302. 10.1530/REP-11-0320 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.