Abstract
Chickpea seed proteins are alleged source of nutraceuticals. These seed proteins were subjected to different proteases to produce peptides. The efficacy of these peptides was confirmed using six diverse human cancer cell lines (PA-1, Ishikawa cells, A549, MCF-7, HepG2, MDA-MB-231). Alcalase generated peptides exhibited the highest antagonistic inhibition of Ishikawa cells. Flow cytometric analysis revealed that chickpea peptide induced S and G2 phase arrest of cell cycle in a dose dependent manner. DNA fragmentation and apoptosis occurred by down regulation of Bcl-2 expression, upregulation of Bax expression and promotion of caspase-3 initiation. Chickpea peptides ascertain potential antiproliferative molecule that can be deployed in cancer treatment regimes.
Keywords: Alcalase, Bioactive, Chickpea peptide, Human cancer cells
Introduction
Cell proliferation is essential to maintain normal growth of the body but, if this process is disturbed then cell bypasses the cellcycle check-points and becomes cancerous (Koul 2020). Cancer is one of the main causes of human mortality worldwide and predicted to prevail in coming years (Bray and Soerjomataram 2015; WHO 2018). Understanding cancer signals leading to mutation have been challenging and a point of concern for the oncologists and medical practioners. The various cancer treatment regimes (chemotherapy, radiotherapy, surgery etc.) deployed now a days does not guarantee full-recovery, safety and put forth long-term side-effects (Xu et al. 2009; Kamshad et al. 2019). Therefore, the scientific community has been crusading long for developing safe natural anticancer drugs (Kuete et al. 2016; Mokaberi et al. 2019). It was rightly said by Hippocrates, the father of medicine “Let food be thy medicine and medicine be thy food”.
Functional foods and nutraceuticals sustain the body’s health and play a pivotal role in preventing carcinogenesis (Reddy et al. 2003; De Mejia and Dia 2010; Aluko 2018; Tahmineh et al. 2018; Papandreou et al. 2019). Functional foods are known for the prevention and alterations of cancer related pathologies hence are the subjects of investigation (Cragg et al. 2009; Shakibapour et al. 2019). Legumes afford proteins, peptides, fibers and phytochemicals and thus limelight the arena of functional foods. During digestion of pulse proteins, bioactive peptides ranging between 2–20 amino acids residues are released (Cavazos and Gonzalez de Mejia 2013; Mokaberi et al. 2020). Some of these peptides can proficiently control the physiological processes of cancer cells. Published reports described various biological activities of food peptides vis-a-vis, antioxidant, antimicrobial, immunomodulatory, antihypertensive, anticancer and apoptotic (Giron-Calle et al. 2010; Li-Chan 2017).
Chickpea (Cicer arietinum L.) of Fabaceae family is the third most vital food legume of the world (FAO 2013). Chickpea seeds are predominantly consumed due to its protein composition. One of the seed proteins albumin is reported to possess medicinal properties including antioxidant, antihyperlipidemic and antiproliferative (Xue et al. 2012, 2015). It is a source of functional peptides too. Consequently, we chose to work on chickpea seed peptides. Therefore, present study deals with the comparative assessment of the anticancer and apoptotic activity of chickpea seed peptides. This seems to be the maiden report and thus open a new vista for cancer therapeutics.
Materials and methods
Seed material and cancercell lines
Healthy seeds of chickpea variety ‘Digvijay’ were procured from Agricultural Research Station, Badnapur Maharastra, India. The human cancer cell lines were used for comparative determination of antiproliferative activities of six different human cancer cell lines viz. A549, HepG2, Ishikawa, MCF-7, MDA-MB-231 and PA-1. These cell lines were procured from National Centre for Cell Science (NCCS), Pune, Maharashtra, India. ATCC protocol was followed for cell-culture maintenance.
Chickpea seed extract preparation
Chickpea seed powder suspended in distilled water (1:10 w/v) was stirred for 8 h at 4 °C (pH 8.0), followed by centrifugation at 10,000×g for 15 min. The pH of the supernatant was adjusted to 4.5 and stirred for 30 min in cold and re-centrifuged at 10,000×g for 15 min (Yust et al. 2003). Protein concentration was estimated through Bradford’s method (Bradford 1976) and later lyophilized and stored at − 20 °C for further use.
Synthesis of bioactive peptides, ultrafiltration and purification by FPLC
Initially, chickpea seed extract was subjected to digestion using six different proteases viz. alcalase, flavorozyme, chymotrypsin, trypsin, pepsin and papain. The protein hydrolysate/peptides obtained through digestion was subsequently tested for their antiproliferative activity against above mentioned cell lines.
Alcalase generated peptides gave significant activity out of six proteases and therefore, further fractionated (Chale et al. 2014) using ultrafiltration membranes of different molecular weight cut-offs (30, 10, 5 and 3 kDa). The 3 kDa peptide fraction revealed highest antiproliferative activity and for that reason subjected to FPLC system (AKTA Systems, Canada) equipped with a HiTrap Q XL column. Unbound proteins were washed with the Tris buffer (pH 8). A linear salt gradient was used to elute the bound peptides. Two peaks were obtained. The peak fraction showing highest antiproliferative activity was lyophilized and used for further analysis.
Sulphorhodamine B (SRB) assay
The SRB assay was performed as reported earlier by Skehan et al. (1990). The cells were fixed with 10% chilled trichloroacetic acid (TCA) for 1 h at 4ºC. The supernatant from cell suspension was aspirated and microplates were washed thrice with chilled deionized water and subsequently air-dried. Then, 100 μl of 0.4% (w/v) SRB in 1% acetic acid was added to each well and incubated for 30 min. Unbound SRB was removed by three washes with 1% acetic acid and 200 μl of 10 mM tris base, pH (10.5) was added to extract the bound stain. The absorbance was read at 560 nm in a microplate reader (Bio-Rad, USA). The % cell inhibition was calculated using the following relation.
where, As—absorbance of sample; Ac—absorbance of control.
Lactate dehydrogenase leakage assay
Lactate dehydrogenase (LDH) cytotoxicity assay kit was used to measure the cell membrane integrity (Sigma-Aldrich, USA). The assay takes the advantage of LDH leakage measurement in the medium by damaged cells (Kim et al. 2008). To the plated cells (1 × 104 cells per well), different concentrations of chickpea bioactive peptide (5–100 µg/ml) was added and re-incubated for 24 h. 1% triton was added to control cell (not treated with peptide) serving as positive control. The LDH leakage (% of positive control) is expressed as the percentage of (ODtest−ODblank) / (ODpositive−ODblank). Where, ODtest is the optical density of chickpea bioactive peptide exposed cells, ODpositive is the optical density of the positive control cells and ODblank is the optical density of the wells without cells.
Reactive oxygen species assay (ROS assay)
The ROS was done using the oxidant-sensitive Dichloro-dihydro-fluorescein diacetate (DCFH-DA). Increasing doses of chickpea peptide to a uniform cell aggregation was added with DCFH. The fluorescence thus generated by DCFH oxidation was assessed by fluorescence microplate reader. Excitation at 480 nm and emission at 530 nm wavelengths were used to express ROS level (Ma et al. 2016).
Assay of caspase-3 activity
Caspase-3 reagent kit (Sigma-Aldrich, USA) was used for caspase-3 activity assay. Cells (1 × 106 cells/well) were treated separately with 5–500 μg/ml of chickpea peptide and tamoxifen or DMSO as a vehicle control. Caspase-3 reagent (100 µl) was added to each well after 24 h of incubation. The plates were then incubated for 2 h after thorough shaking and luminescence was measured (Flanagan et al. 2016).
DNA ladder assay
Chickpea peptides were used for assessing DNA fragmentation in Ishikawa cells. An apoptotic DNA ladder kit (Roche, USA) was used for isolation of genomic DNA. DNA Integrity was investigated using 1% (w/v) agarose gel electrophoresis.
Cell cycle analysis
Ishikawa cells (5 × 105 cells per well) were incubated with 5–500 μg/ml of chickpea peptide and/or DMSO as a control, for 24 h. Following trypsinization, the cells were washed, centrifuged and the pellet was resuspended in phosphate buffer saline (PBS; pH 8). The cells were fixed by adding 70% chilled ethanol for 2 h. RNAase was added to the cell suspension (0.3 ml in PBS) and incubated for 1 h and later subjected to a flow-cytometer for cell-cycle analysis. Propidium iodide staining facilitates the detection of apoptotic cells (Pozarowski and Darzynkiewicz 2004).
Western blot assay
The Ishikawa cell line was nurtured for 48 h with a medium holding chickpea peptide (250 and 500 µg). RIPA buffer containing 50 mM Tris HCl (pH 8.0) + 150 mM NaCl + 1% Triton X-100 + 0.1% SDS + 0.5% deoxycholic acid, was mixed with protease and phosphatase inhibitor and used for extracting proteins for western blotting. The membrane was probed with appropriate primary antibodies against caspase-3, Bcl-2, Bax and β-actin and visualized by Pierce ECL plus western blotting kit.
Identification of peptide by ESI–MS/MS
The purified peptide was analyzed by Agilent 1260 Infinity Capillary Pump and coupled to Q-TOF mass spectrometer (Agilent Technologies, USA). The ionization of sample was achieved in the positive ESI mode with the mass/charge ratio (m/z) ranges of 100–700. The molecular weight of purified peptide was detected by a charged (M + H)+1 state analysis in the mass spectrum. The amino acid sequence was identified by tandem MS analysis and manual interpretation of the ion series in the spectra.
Statistical analysis
All the tests were performed in triplicates and represented as mean ± SD. Statistical analysis of the data was done using Graph pad prism (version 5) software. Chickpea peptide concentration giving 50% inhibition was calculated by non-linear regression analysis to generate the curve and IC50 value. Dunnett’s compare test was applied with 95% confidence with difference considered as significant at p < 0.05.
Results and discussion
The available anticancer drugs have numerous side-effects and are costly; therefore, there is a need for searching safe alternatives (Wang et al. 2012). In recent years, herbal extracts/peptides have gained much attention of the scientific community due to their efficacy in cancer inhibition (Luna et al. 2014). The plant extracts/peptides recognize receptors present on cancer cell membrane and cause cytotoxicity and apoptosis. Therefore, complex formulations of cancer therapeutic medicines typically contain the use of whole plant extracts/mixtures. Combination of different cell lines are crucial to understand competitive binding of drugs (Rashidipour et al. 2016). Subsequently, several cell lines were employed in the present study. We report herein that chickpea seed peptides significantly inhibit human cancer cells proliferation with 48 h of incubation, in a dose-dependent manner.
Antiproliferative assay
Sulforhodamine B (SRB) based cell viability assay on human cancer cell lines was performed to analyze the cytotoxic impact of chickpea protein. SRB assay primarily uses the potential of the sulforhodamine B dye to bind electrostatically to amino acid residues of TCA-fixed cells (Skehan et al. 1990). The effect (inhibition of cell proliferation) of chickpea protein concentrations (5–500 µg/ml) on six different human cancer cell lines (A549, HepG2, Ishikawa, MCF-7, MDA-MB-231, and PA-1) have been examined using SRB assay (Figs. 1 and 2). The dose related inhibitory effects of chickpea peptide and tamoxifen (anticancer drug, used positive control) on the human cancer cells after 48 h of incubation was observed (Fig. 3) Among the cell-lines, Ishikawa cells produced noteworthy results (IC50 101.5 µg/ml), that was comparable to tamoxifen (IC50 85.79 µg/ml). A549, HepG2 and PA-1 cells too exerted subordinate antiproliferative effect with IC50 values of 126.4, 113.7 and 133.4 µg/ml, respectively (Table 1). The inhibitory ability observed in case of MCF-7 and MDA-MB-231 cells was almost similar as evidenced by the IC50 values (110.3 and 107.2 µg/ml). The chickpea peptide mediated inhibition of proliferation in Ishikawa cells was 19.38% at 5 µg/ml, which reached to 42.90% at 500 µg/ml. Hence, these results advocate the putative role of chickpea peptide as an anticancer agent.
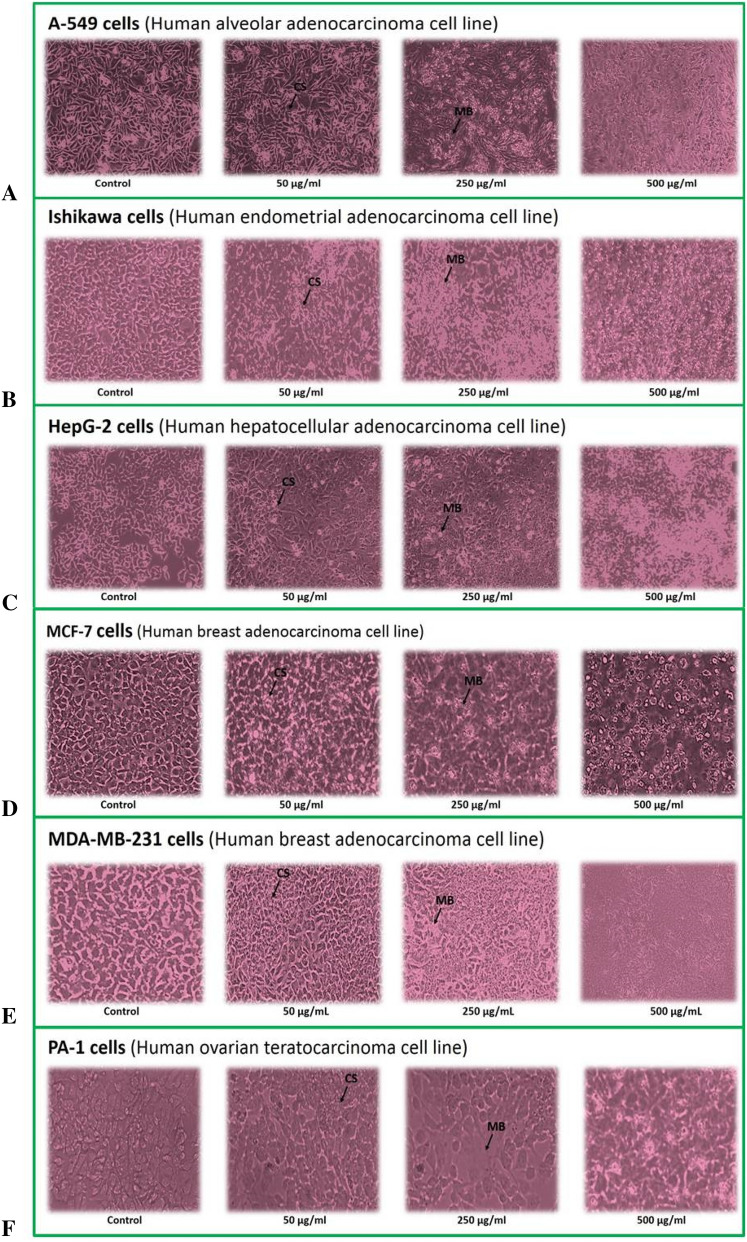
Fig.1.
Morphological changes in different human cancer cell lines after 48 h of chickpea protein treatment. Magnification: 10X. CS Cellular shrinkage, BL Membrane blebbing
Fig. 2.

Effect of chickpea protein on % cell inhibition
Fig. 3.
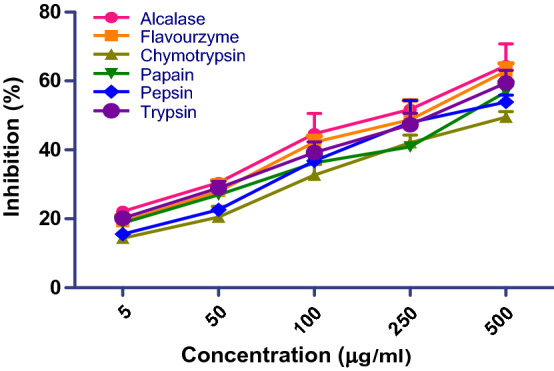
Antiproliferative effect (SRB assay) of chickpea peptide prepared using six proteases on Ishikawa cell lines
Table 1.
IC50 values towards different cell lines
| Cell lines | IC50 (µg/ml) |
|---|---|
| A-549 | 126.4 ± 1.98** |
| HepG-2 | 113.7 ± 0.99** |
| Ishikawa | 101.5 ± 1.83** |
| MCF-7 | 110.3 ± 2.97** |
| MDA-MB-231 | 107.2 ± 1.62** |
| PA-1 | 133.4 ± 0.70** |
| Tamoxifen (anticancer drug) | 85.79 ± 1.97 |
**means Level of significance p < 0.001
Prior to the treatment (with peptide and tamoxifen), cancer cells morphology was flat and spreading, while post-treatment the cells exhibited shrinkage and breakage in cell membrane and showed detachment with culture plate (floating in the medium). Based on the outcome of SRB assay, Ishikawa cells were, therefore, selected to examine anticancer effect of chickpea peptides (with different MWs) prepared by six proteases. The cell progression, Caspase-3 activity, ROS production and LDH leakage were further investigated to study chickpea peptide induced cell apoptosis in Ishikawa cells.
Antiproliferative effect of chickpea bioactive peptide
Alcalase generated peptides displayed maximum antiproliferation with lowest IC50 value 100.03 µg/ml (Table 2). The cell morphology and viable cell number was also deteriorated. Earlier studies have revealed that alcalase cleaves the peptide bond at the interior of polypeptide chain as an endo-peptidase (Yust et al. 2003). The proteases differ in their ability to hydrolyze various peptide bonds. Alcalase generated peptide was initially separated into four fractions with MW of 10, 5–10, 3–5 and 3 kDa, respectively, to evaluate their ant-proliferative effects. The lower 3 kDa fractions exhibited the highest proliferative inhibition as compared to the higher MW fractions. The % inhibition was 27.30, 34.13, 48.66, 54.44 and 63.24% at 5, 50, 100, 250 and 500 µg/ml concentrations, respectively (Fig. 4b). The 3–5 and 10 kDa fractions showed lower proliferative effect, followed by 5–10 kDa. The amino acids sequence of enzyme specifies the structure that determines the catalytic activity. Although structure determines the function, a novel enzymatic activity cannot yet be predicted from structure alone. Proteases cleave the protein to yield an active peptide and eventually enhance the turnover number towards diverse reactions.
Table 2.
Antiproliferative (IC50 values) effect of chickpea peptide proteases on Ishikawa cell lines
| Enzymes | IC50 µg/ml |
|---|---|
| Alcalase | 100.3 ± 1.06 |
| Flavourzyme | 102.4 ± 0.99 |
| Chymotrypsin | 197.2 ± 2.33 |
| Papain | 111.5 ± 1.81 |
| Pepsin | 148.8 ± 1.10 |
| Trypsin | 110.1 ± 0.91 |
The values were expressed as the mean ± SD
Fig. 4.
Antiproliferative effect of chickpea peptide with different molecular weight
Peptides with low MW shows high mobility and diffusivity than their high MW counterparts, to interact with cancer cell components and, therefore, exhibit enhanced antiproliferation (Jumeri and Kim 2011). Figure 4a, b fractions show a time- and dose-dependent effect on cells of Ishikawa. The % inhibition was in a range of 13.86–36.41% at 5 µg/ml, which reached to 47.24–68.90% at 500 µg/ml, with the increasing time (12–48 h). Plant seeds synthesize specific proteins that functions as storage and/or play a role in defense and exhibit certain biochemical and functional properties (Diaz et al. 2017; Gautam et al. 2018). Vicilin is one such major plant storage protein with known health benefits. In a separate study, enzymatic hydrolysate of mungbean vicilin protein (MBVP) under in vitro conditions exhibited angiotensin converting enzyme (ACE) inhibitory and antiproliferative activities (Gupta et al. 2018a, b). As mentioned before, compared to large proteins, small molecule peptides exhibit better inhibition properties. Our study is in consonance with the previous report of Kim et al. (2000), wherein soya protein-peptide fractions having low molecular weight exhibited notable antiproliferative activity. This may be due to protein alterations to an intermediate state (Moosavi-Movahedi et al. 2003, 2004). Finally, the purified peptide from chickpea protein hydrolysate was analyzed and its amino acid sequence was determined by ESI–MS/MS spectroscopy.
LDH release assay
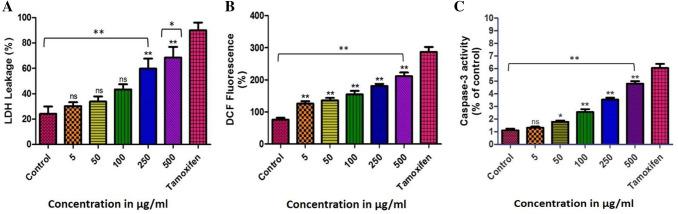
The extent of LDH levels in cell culture is a major indicator of cytotoxicity (Wang et al. 2014). Being an intracellular enzyme, LDH leakage from the cells to cell-culture medium projects an unhealthy condition leading to loss of cell membrane integrity (Karlsson et al. 2013). In the current study, LDH leakage from the chickpea peptide treated cells got increased after 24 h of treatment with chickpea peptide (5–500 µg/ml), as compared to the untreated cells (Fig. 5a). The pattern of LDH released from Ishikawa cell line treated with chickpea hydrolysate suggests that the cytotoxic effect of the hydrolysate is concentration-dependent. Furthermore, the percentage range of LDH generated from cells after 24 h treatment was noticeable (30.3 ± 2.9% to 68.5 ± 8.4%), compared to tamoxifen (89.9 ± 6.1%) and control cell (24.1 ± 5.8%). This result highlighted chickpea peptide as punitive marker for measuring the extent of cellular damage.
Fig. 5.
Effects of chickpea peptide on Ishikawa cells. Caspase-3 activity, ROS generation and LDH leakage significantly increased after treating cells for 24 h compared to control. Level of significance *p < 0.05, **p < 0.01 and ns for not significance
LDH is one of the significant markers of cytotoxicity. The cytotoxic agents exert damage to cell membranes and permit LDH leakages into culture medium (Decker and Lohmann-Matthes 1988). Prolonged incubation with anticancer agent, damages and fragments the cells and culminate in cell death. Some of the plant extract/peptides induce varied response on LDH leakage in different cell types. This includes the example of Euphorbia hirta whole plant extract (Kwan et al. 2015). In the present study, LDH leakage in Ishikawa cells got elevated due to presence of high dose of chickpea-peptide, compared to control cells. This may be due to the cytotoxic nature of chickpea bioactive peptide, thus confirming its anticancer nature.
ROS generation
Being a marker of cellular metabolism/apoptosis, ROS plays major role in mediating cytotoxicity and cell cycle arrest and enhances cellular apoptosis. The antagonistic action (ROS generation) of chickpea peptide on Ishikawa cells growth has, therefore, been studied (Fig. 5b). Intracellular ROS production (during 24 h) was measured by DCFH oxidation. The dose dependent treatment with chickpea peptide (5–500 µg/ml) exhibited an increase in the ROS generation (range: 125.8 ± 7.6% to 211.6 ± 11.1%), compared to negative control (untreated cells: 76.11 ± 5.8%) and positive control (tamoxifen: 287.6 ± 14.7%). The present findings indicate that chickpea peptide concentrations exhibited pro-oxidant activity through activation of intracellular ROS, compared to tamoxifen. Thus, suggesting its participation in modulating the redox reactions in Ishikawa cells.
ROS generate in those cells that have a tendency to proliferative and contribute in discrimination of the normal and abnormal cytology (Fridovich 1978). Increased intracellular ROS generation occurs due to reaction of several anticancer agents/treatments such as anthracyclines, cisplatin, bleomycin and irradiation that are currently used in cancer treatment regimes (Hug et al. 1997; Miyajima et al. 1997; Serrano et al. 1999; Chan and Yu 2000). Present study reveals chickpea peptide (concentration dependent) enhances the intracellular ROS levels and induces cell death (Rosenkranz et al. 1992).
Caspase-3 activation
The apoptotic pathways are significantly mediated by caspase-3 which is a hallmark of caspase cascade. Initiation and activation of caspases occurs in mitochondria (Dia and Gonzalez 2010). Caspase-3 activation by chickpea peptide was performed to confirm the progression of apoptosis in Ishikawa cells (Janicke et al. 1998). The chickpea peptide being a putative pharmacological molecule expressively increased the activities of caspase-3 in a dose dependent manner. Ishikawa cells treated with 5, 50, 100, 250 and 500 µg/ml of chickpea peptide resulted in the activation of caspases-3 by 1.3, 1.7, 2.5, 3.5 and 4.8 fold, compared to tamoxifen (positive control) and control (negative control), respectively (Fig. 6c). These findings suggest that chickpea peptide could induce apoptosis via caspase-dependent pathways.
Fig. 6.
Induction of cell cycle arrest and apoptosis in Ishikawa cells due to chickpea peptide
The process of apoptosis is correlated to increase in caspase 3 activity. Caspases gets activated by proteolytic cleavage and participate in the apoptosis (Salvesan and Dixit 1999). Chickpea peptide treated cells exhibited the mechanism of cell death involved in apoptosis. Many extracts of plant origin such as sweet potato protein hydrolysates and bacaba phenolic extract activate caspase mediated apoptosis (Finco et al. 2016; Zhang and Mu 2017).
Cell cycle assay
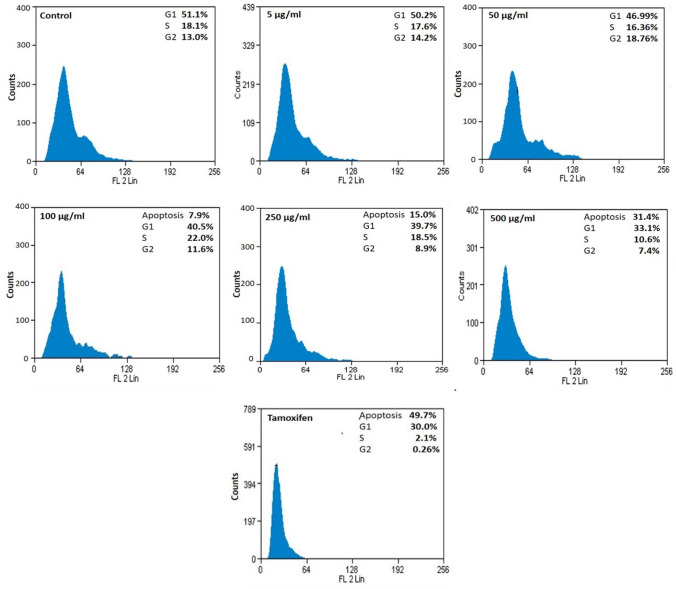
Cell cycle progression vis-a-vis induction of apoptosis was studied in the present investigation. The effect of chickpea peptide on cell cycle progression through propidium iodide staining is shown in Fig. 7. The chickpea peptide induced dose-dependent enhancement of S and G2 cell cycle arrest in Ishikawa cells and further maintained the arrest of cells in the S and G2 phase. The percentage of cells in S and G2 phase decreased (18.1 and 13.0%) in the control (untreated cells) and for treated cells 10.6 and 7.4% at 500 µg/ml, respectively. In parallel to tamoxifen, chickpea peptide resulted considerable decline of cell count in S and G2 phase. In addition, peptide treatment reduced the cell numbers compared to untreated cells resulting in damage of 31.4% cell proportion by apoptosis.
Fig.7.
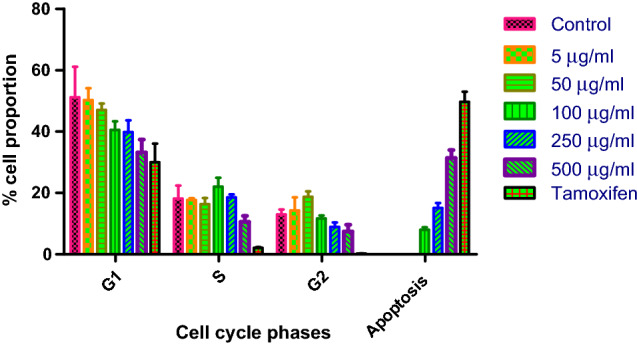
Cell cycle analysis output, cells in G1, S, G2 and apoptosis phases after 24 h of treatment
The inhibitory concentration of peptide blocked the cell cycle at S-phase and stopped the cells from entering into proliferative phase. Moreover, S phase transition provides an efficient check point in cell-cycle progression that can severely affect the proliferation of cell population. In the present study, flow cytometric analysis exhibited that chickpea peptides induce cell cycle arrest and apoptosis in Ishikawa cells. The mechanism for the interconnection between cell cycle regulation and apoptosis rely on the up-regulation of p21 upon treatment with therapeutic agents (Porter and Janicke 1999). A proposed mechanism of chickpea peptide induced apoptosis signaling pathway in Ishikawa cancer cells is shown in Fig. 8.
Fig. 8.
Schematic representation of possible mechanism involved in the apoptotic pathway in chickpea peptide treated Ishikawa cells
Apoptosis causes programmed cell death and equates the balance between cell death and cell renewal in animals (Alenzi 2004). Chickpea peptide induced arrest of S- and G2-phase of cell cycle resulted in 31.4% apoptosis in the cell population. Our previous studies on MCF-7 cells revealed that chickpea lectin inhibits the proliferation breast cancer cells and brings apoptosis via cell-cycle arrest (Gupta et al. 2018a, b). Recent studies by Ali et al. (2019) on seeds of Adenium obesum has shown cyto-genotoxic effects on breast cancer cells by increasing the count of early and late apoptotic cells.
DNA ladder assay
DNA fragmentation can be correlated to apoptosis (Aluko 2018). Significant DNA fragmentation and damage of cancer cells was observed with chickpea peptide treated cells (500 µg/ml) than the untreated control cells thus, mediating apoptosis (Fig. 9a). DNA fragmentation in treated cancer cells occurred due to peptide-mediated-inhibition of cellular activity. Peptide binding to DNA takes place through outside interaction by an exothermic reaction (Moosavi-Movahedi et al. 2004) It is, therefore, predicted that chickpea peptides can work as putative antioxidant and anticancer agents. An advantage of exploring such peptides is their reduced toxicity, as they are speedily eliminated from the blood stream (Ishida et al. 2011; Aluko 2018).
Fig. 9.
DNA fragmentation analysis of Ishikawa cells after treated with chickpea peptide. a Lane 1, marker; Lane 2, control; Lane 3, 250 μg/ml chickpea peptide; Lane 4, 500 μg/ml chickpea peptide, b Western blot demonstrated that chickpea peptide enhanced the activity of Caspase 3 in Ishikawa cells and also shows downregulation of Bcl-2 and upregulation of Bax
Expression of apoptosis-related proteins
After knowing the fact that Ishikawa cells undergo apoptosis due to the treatment of chickpea peptide, the expression levels of apoptosis related proteins (Bcl-2 and Bax) was checked through Western blot. The β-actin was used as positive control. The expression of Bcl-2 was decreased (down-regulation Bcl-2) and expression of Bax increased (up-regulation of Bax) progressively through chickpea peptide induced apoptosis (Fig. 9b). In framework with chickpea peptide induced Bax expression, we also investigated caspase-3 activation. The expression level of caspase-3 was significantly raised in chickpea peptide treated Ishikawa cells, which further proves caspase-3 participation in the proposed aptotic apoptotic pathway (Fig. 8).
Apoptosis is a genetically controlled characteristic (Dia and Gonz alez 2010). Bcl-2, an antiapoptotic protein, is potentially used as a predictive biomarker of cancer (Hector and Prehn 2009). ‘Lunasin polypeptide’ from soya bean activates apoptosis in colon cancer cells by reducing Bcl-2 to Bax ratio (Dia and Gonz alez 2010). Caspase family members play influential role in mediating apoptosis (Lavrik et al. 2005). Molecular and structural changes in apoptosis are directly related to caspase activation. Among the identified caspases, caspase 3 is an indicator enzyme that induces apoptosis. In our study, the inhibition of Ishikawa cells by chickpea peptide followed the intrinsic pathway that damaged the mitochondria and promoted caspase-3 mediated apoptosis. However, these studies need to be supported using animal models also.
Amino acid sequence of the purified peptide
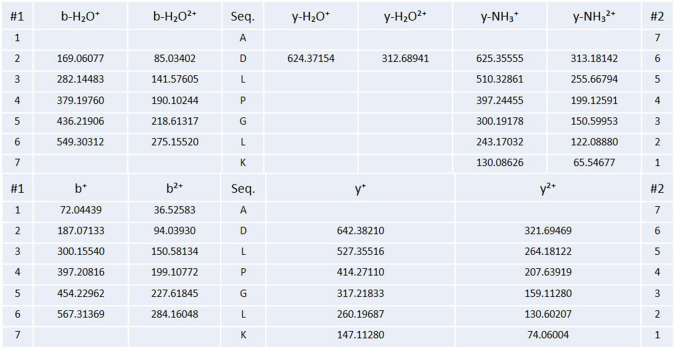
The amino acid sequence of the purified peptide was identified. Ion peaks with doubly or higher charges were selected for processing. The sequence of amino acids ADLPGLK derived from the spectra has high signal-to-noise ratio and showed complete or near-complete backbone fragmentation and indicating low error (< 0.8 Da) in the data (Figs. 10 and 11).
Fig. 10.
Identification of molecular mass and amino acid sequence of the chickpea purified peptide
Fig. 11.
Ion table of amino acid sequence of the chickpea purified peptide
According to the results of HR-LC–MS, the molecular mass of the purified peptide was 713.41 D. It was analyzed using a peptide sequencer and was identified as an heptapeptide, Ala–Asp–Leu–Pro–Gly–Leu–Lys. To the best of our knowledge, this was the first report on the natural peptide ADLPGLK with high antiproliferative activities derived from legume. As known, composition, structure, and hydrophobicity of peptides plays a critical role in their physicochemical properties and bioactivities. In the present study, the identified peptide ADLPGLK contain 40% hydrophobic residues, i.e., Ala (A) and Leu (L) which might be responsible for its antiproliferative activity.
Huang et al. reported that the hydrophobic property of the peptides might be a key for its antiproliferative activity, which provided an important way to synthesize anticancer peptides as potential therapeutics in clinical practices using a de novo design approach (Huang et al. 2011). Xue et al. (2015) identified a chickpea peptide (CPe-III) (RQSHFANAQP) with antioxidant activity and antiproliferative effect which contains 40% hydrophobic residues. It was also reported that CPe-III-S exerted antiproliferative activity by increasing the amounts of p53 protein in the MCF-7 and MDA-MB-231 cells.
Conclusion
Chickpea seed peptides harbor pharmacological activities. Foods containing chickpea peptides can be supplemented in diets to prevent the occurrence pre-cancerous lesions. A discrete cytotoxic activity shown that chickpea peptide induced apoptosis through down-regulation of Bcl-2 and up-regulation of Bax directed activation of caspase-3. However, more in-depth and meticulous in vivo studies on the anticancer potential of chickpea peptides shall bring greater insights into the mechanisms involved.
Acknowledgements
The authors are thankful to the Jiwaji University, Gwalior for the infrastructural support. We are also thankful to National Centre for Cell Science (NCCS), Pune repository for supplying cell lines and Jawaharlal Nehru University (JNU) Advanced Instrumentation Research Facility (AIRF) for providing FACS facility.
Compliance with ethical standards
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Neha Gupta, Email: ng.biotech.ng@gmail.com.
Sameer Suresh Bhagyawant, Email: sameerbhagyawant@gmail.com.
References
- Ajji PK, Binder MJ, Walder K, Puri M. Balsamin induces apoptosis in breast cancer cells via DNA fragmentation and cell cycle arrest. Mol Cellular Biochem. 2017;432:189–198. doi: 10.1007/s11010-017-3009-x. [DOI] [PubMed] [Google Scholar]
- Alenzi FQB. Links between apoptosis, proliferation and the cell cycle. Brit J Biomed Sci. 2004;61:1–4. doi: 10.1080/09674845.2004.11732652. [DOI] [PubMed] [Google Scholar]
- Ali AQ, Farah MA, Abou-Tarboush FM, Al-Anazi KM, Ali MA, Lee J, Waleed AQH, Mahmoud AH. Cytogenotoxic effects of Adenium obesum seeds extracts on breast cancer cells. Saudi J Biol Sci. 2019;26:547–553. doi: 10.1016/j.sjbs.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluko RE (2018) Food protein-derived peptides: production, isolation, and purification. In: Proteins in food processing. Woodhead Publishing Series in Food Science, Technology and Nutrition, pp 389–412
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bray F, Soerjomataram I (2015) The changing global burden of cancer: transitions in human development and implications for cancer prevention and control. In: Gelband H, Jha P, Sankaranarayanan R, Horton S (eds), 3rd edn. Cancer Disease Control Priorities. The World Bank, Washington, pp 23–44. [PubMed]
- Cavazos A, Gonzalez de Mejia E. Identification of bioactive peptides from cereal storage proteins and their potential role in prevention of chronic diseases. Comp Rev Food Sci Food Safety. 2013;12(4):364–380. doi: 10.1111/1541-4337.12017. [DOI] [PubMed] [Google Scholar]
- Chale FGH, Ruiz JCR, Fernandez JJA, Ancona DAB, Campos MRS. ACE inhibitory hypotensive and antioxidant peptide fractions from (Mucuna pruriens) proteins. Proc Biochem. 2014;49:1691–1698. [Google Scholar]
- Chan WH, Yu JS. Inhibition of UV irradiation-induced oxidative stress and apoptotic biochemical changes in human epidermal carcinoma A431 cells by genistein. J Biomed Sci. 2000;78:73–84. doi: 10.1002/(sici)1097-4644(20000701)78:1<73::aid-jcb7>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Cragg GM, Grothaus PG, Newman DJ. Impact of natural products on developing new anti-cancer agents. Chem Rev. 2009;109:3012–3043. doi: 10.1021/cr900019j. [DOI] [PubMed] [Google Scholar]
- De Mejia EG, Dia VP. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metastasis Rev. 2010;29:511–528. doi: 10.1007/s10555-010-9241-4. [DOI] [PubMed] [Google Scholar]
- Decker T, Lohmann-Matthes ML. A quick and simple method for the quantitation of lactate dehydrogenase release in measurements of cellular cytotoxicity and tumor necrosis factor (TNF) activity. J Immunol Methods. 1988;115:61–69. doi: 10.1016/0022-1759(88)90310-9. [DOI] [PubMed] [Google Scholar]
- Dia VP, Gonz alez de Mejıa E. Lunasin promotes apoptosis in human colon cancer cells by mitochondrial pathway activation and induction of nuclear cluster in expression. Cancer Lett. 2010;295:44–53. doi: 10.1016/j.canlet.2010.02.010. [DOI] [PubMed] [Google Scholar]
- Diaz IL, Partida ANG, Moreno LV. Legume lectins: proteins with diverse applications. Int J Mol Sci. 2017;18:1242. doi: 10.3390/ijms18061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finco FDBA, Kloss L, Graeve L. Bacaba (Oenocarpus bacaba) phenolic extract induces apoptosis in the MCF-7 breast cancer cell line via the mitochondria-dependent pathway. NFS J. 2016;5:5–15. [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Sci. 1978;201:875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gautam AK, Srivastava N, Nagar DP, Bhagyawant SS. Biochemical and functional properties of a lectin purified from the seeds of Cicer arietinum L. 3 Biotech. 2018;8:272. doi: 10.1007/s13205-018-1272-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron-Calle J, Alaiz M, Vioque J. Effect of chickpea protein hydrolysates on cell proliferation and in vitro bioavailability. Food Res Int. 2010;43:1365–1370. [Google Scholar]
- Gupta N, Bisen PS, Bhagyawant SS. Chickpea lectin inhibits human breast cancer cell proliferation and induces apoptosis through cell cycle arrest. Prot Pept Lett. 2018;25:492–499. doi: 10.2174/0929866525666180406142900. [DOI] [PubMed] [Google Scholar]
- Gupta N, Srivastava N, Bhagyawant SS. Vicilin-A major storage protein of mungbean exhibits antioxidative potential, anti-proliferative effects and ACE inhibitory activity. PLoS ONE. 2018;13:e0191265. doi: 10.1371/journal.pone.0191265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector S, Prehn JHM. Apoptosis signaling proteins as prognostic biomarkers in colorectal cancer: a review. Biochem Biophys Acta. 2009;1795:117–129. doi: 10.1016/j.bbcan.2008.12.002. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang X, Wang H, Liu Y, Chen Y. Studies on mechanism of action of anticancer peptides by modulation of hydrophobicity within a defined structural framework. Mol Cancer Therapy. 2011;10:416–426. doi: 10.1158/1535-7163.MCT-10-0811. [DOI] [PubMed] [Google Scholar]
- Hug H, Strand S, Grambihler A, et al. Reactive oxygen intermediates are involved in the induction of CD95 ligand mRNA expression by cytostatic drugs in hepatoma cells. J Biol Chem. 1997;272:28191–28193. doi: 10.1074/jbc.272.45.28191. [DOI] [PubMed] [Google Scholar]
- Ishida Y, Shibata Y, Fukuhara I, Yano Y, Takehara I, Kaneko K. Effect of an excess intake of casein hydrolysate containing Val-Pro-Pro and Ile-Pro-Pro in subjects with normal blood pressure, high-normal blood pressure or mild hypertension. Biosci Biotech Biochem. 2011;75:427–433. doi: 10.1271/bbb.100560. [DOI] [PubMed] [Google Scholar]
- Janicke RU, Sprengart ML, Wati MR, et al. Caspase-3 is required for DNA fragmentation and morphological changes associated with apoptosis. J Biol Chem. 1998;273:9357–9360. doi: 10.1074/jbc.273.16.9357. [DOI] [PubMed] [Google Scholar]
- Jumeri KSM. Antioxidant and anti-cancer activities of enzymatic hydrolysates of solitary tunicate (Styela clava) Food Sci Biotech. 2011;20:1075–1085. [Google Scholar]
- Kamshad M, Jahanshah Talab M, Beigoli S, Sharifirad A, Chamani J. Use of spectroscopic and zeta potential techniques to study the interaction between lysozyme and curcumin in the presence of silver nanoparticles at different sizes. J Biomol Struct Dyn. 2019;37(8):2030–2040. doi: 10.1080/07391102.2018.1475258. [DOI] [PubMed] [Google Scholar]
- Karlsson HL, Cronholm P, Hedberg Y, Tornberg M, Battice LD, Svedhem S, et al. Cell membrane damage and protein interaction induced by copper containing nanoparticles-importance of the metal release process. Toxicol. 2013;313:59–69. doi: 10.1016/j.tox.2013.07.012. [DOI] [PubMed] [Google Scholar]
- Kim H, Choi JY, Lee JM, et al. Dexamethasone increases angiopoietin-1 and quiescent hematopoietic stem cells: A novel mechanism of dexamethasone-induced hematoprotection. FEBS Lett. 2008;582:3509–3514. doi: 10.1016/j.febslet.2008.09.021. [DOI] [PubMed] [Google Scholar]
- Kim SE, Kim HH, Kim JY, Kang YI, Woo HJ, Lee HJ. Anti-cancer activity of hydrophobic peptides from soy proteins. BioFactors. 2000;12:151–155. doi: 10.1002/biof.5520120124. [DOI] [PubMed] [Google Scholar]
- Koul B. Herbs for cancer treatment. Singapore: Springer Nature; 2020. [Google Scholar]
- Kuete V, Tchinda CF, Mambe FT, Beng VP, Efferth T. Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement Altern Med. 2016;16:267. doi: 10.1186/s12906-016-1253-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan YP, Saito T, Ibrahim D, Saleh Al-Hassan FM, Oon CE, Chen Y, Jothy SL, Kanwar JR, Sasidharan S. Evaluation of the cytotoxicity, cell-cycle arrest, and apoptotic induction by Euphorbia hirta in MCF-7 breast cancer cells. Pharma Biol. 2015;54(7):1223–1236. doi: 10.3109/13880209.2015.1064451. [DOI] [PubMed] [Google Scholar]
- Lavrik IN, Golks A, Krammer PH. Caspases: pharmacological manipulation of cell death. J Clin Invest. 2005;15:2665–2672. doi: 10.1172/JCI26252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Chan ECY. Bioactive peptides and protein hydrolysates: research trends and challenges for application as nutraceuticals and functional food ingredients. Curr Opin Food Sci. 2017;1:28–37. [Google Scholar]
- Luna Vital DA, Gonz alez de Mejıa E, Dia VP, Loarca-Pina G. Peptides in common bean fractions inhibit human colorectal cancer cells. Food Chem. 2014;157:347–355. doi: 10.1016/j.foodchem.2014.02.050. [DOI] [PubMed] [Google Scholar]
- Miyajima A, Nakashima J, Yoshioka K, et al. Role of reactive oxygen species in cis-dichlorodiammine platinum-induced cytotoxicity on bladder cancer cells. Br J Cancer. 1997;76:206–210. doi: 10.1038/bjc.1997.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokaberi P, Babayan-Mashhadi F, Amiri Tehrani Zadeh Z, Saberi MR, Chamani J. Analysis of the interaction behavior between Nano-Curcumin and two human serum proteins: combining spectroscopy and molecular stimulation to understand protein-protein interaction. J Biomol Struct Dyn. 2020;20:1–20. doi: 10.1080/07391102.2020.1766570. [DOI] [PubMed] [Google Scholar]
- Mokaberi P, et al. New insights into the binding behavior of lomefloxacin and human hemoglobin using biophysical techniques: binary and ternary approaches. New J Chem. 2019;43:8132–8145. [Google Scholar]
- Moosavi-Movahedi AA, Chamani J, Ghourchian H, Shafiey H, Sorenson CM, Sheibani N. Electrochemical evidence for the molten globule states of cytochrome c induced by N-alkyl sulfates at low concentrations. J Protein Chem. 2003;22(1):23–30. doi: 10.1023/a:1023011609931. [DOI] [PubMed] [Google Scholar]
- Moosavi-Movahedi AA, Golchin AR, Nazari K, Chamani J, Saboury AA, Bathaie SZ, Tangestani-Nejad S. Microcalorimetry, energetics and binding studies of DNA–dimethyltin dichloride complexes. Thermochim Acta. 2004;414(2):233–241. [Google Scholar]
- Papandreou C, Becerra-Tomas N, Bullo M, Martínez-Gonzalez MA, Corella D, Estruch R, Ros E, Aros F, Schroder H, Fito M, Serra-Majem L, Lapetra J, Fiol M, Ruiz-Canela M, Sorli JV, Salas-Salvado J. Legume consumption and risk of all-cause, cardiovascular, and cancer mortality in the PREDIMED study. Clin Nutr. 2019;38:348–356. doi: 10.1016/j.clnu.2017.12.019. [DOI] [PubMed] [Google Scholar]
- Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Rashidipour S, Naeeminejad S, Chamani J. Study of the interaction between DNP and DIDS with human hemoglobin as binary and ternary systems: spectroscopic and molecular modeling investigation. J Biomol Struct Dyn. 2016;34(1):57–77. doi: 10.1080/07391102.2015.1009946. [DOI] [PubMed] [Google Scholar]
- Rosenkranz AR, Schmaldienst S, Stuhlmeier KM, Chen W, Knapp W, Zlabinger GJ. A microplate assay for the detection of oxidative products using 2′,7′- dichlorofluorescin-diacetate. J Immunol Methods. 1992;156(1):39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- Salvesan GS, Dixit VM. Caspase activation: the induced-proximity model. Proc Natl Aca Sci USA. 1999;96:10964–10967. doi: 10.1073/pnas.96.20.10964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano J, Palmeira CM, Kuehl DW, et al. Cardioselective and cumulative oxidation of mitochondrial DNA following subchronic doxorubicin administration. Biochem Biophys Acta. 1999;1411:201–205. doi: 10.1016/s0005-2728(99)00011-0. [DOI] [PubMed] [Google Scholar]
- Shakibapour N, Dehghani Sani F, Beigoli S, Sadeghian H, Chamani J. Multi-spectroscopic and molecular modeling studies to reveal the interaction between propyl acridone and calf thymus DNA in the presence of histone H1: binary and ternary approaches. J Biomol Struct Dyn. 2019;37(2):359–371. doi: 10.1080/07391102.2018.1427629. [DOI] [PubMed] [Google Scholar]
- Skehan P, et al. New colorimetric cytotoxicity assay for anti-cancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- Tahmineh S, Maral H, Sima B, Mohammad RS, Jamshidkhan C. Probing the binding of lomefloxacin to a calf thymus DNA-histone H1 complex by multi-spectroscopic and molecular modeling techniques. J Mol Liquids. 2018;256:127–138. [Google Scholar]
- Wang H, Khor TO, Shu L, Su Z, Fuentes F, Lee JH, Kong ANT. Plants against cancer: a review on natural phytochemicals in preventing and treating cancers and their druggability. Anticancer Agents Med Chem. 2012;12:1281–1305. doi: 10.2174/187152012803833026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Deng X, Zhang F, Chen D, Ding W. ZnO nanoparticle-induced oxidative stress triggers apoptosis by activating JNK signaling pathway in cultured primary astrocytes. Nanoscale Res Lett. 2014;9:117. doi: 10.1186/1556-276X-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Liu X, Zhou S, Wei M. Combination of immunotherapy with anaerobic bacteria for immunogene therapy of solid tumors. Gene Therapy Mol Biol. 2009;13:36–52. [Google Scholar]
- Xue Z, Gao J, Zhang Z, Yu W, Wang H, Kou X. Antihyperlipidemic and antitumor effects of chickpea albumin hydrolysate. Plant Foods Human Nutr. 2012;67:393–400. doi: 10.1007/s11130-012-0311-3. [DOI] [PubMed] [Google Scholar]
- Xue Z, Wen H, Zhai L, Yu Y, Li Y, Yu W, Cheng A, Wang C, Kou X. Antioxidant activity and anti-proliferative effect of a bioactive peptide from chickpea (Cicer arietinum L.) Food Res Int. 2015;77:75–81. [Google Scholar]
- You L, Zhao M, Liu RH, Regenstein JM. Antioxidant and anti-proliferative activities of loach (Misgurnus anguillicaudatus) peptides prepared by papain digestion. J Agric Food Chem. 2011;59:7948–7953. doi: 10.1021/jf2016368. [DOI] [PubMed] [Google Scholar]
- Yust MM, Pedroche J, Girón-Calle J, Alaiz M, Millán F, Vioque J. Production of ace inhibitory peptides by digestion of chickpea legumin with alcalase. Food Chem. 2003;81:363–369. [Google Scholar]
- Zhang M, Mu TH. Contribution of different molecular weight fractions to anti-cancer effect of sweet potato protein hydrolysates by six proteases on HT-29 colon cancer cells. Int J Food Sci Tech. 2017;53:525–532. [Google Scholar]