Abstract
In this work, cobalt phosphide nanoparticles (Co2P NPs) were prepared by simple and mild hydrothermal method without the use of harmful phosphorous source. The morphological structure and surface component of Co2P were characterized by transmission electron microscopy, X-ray diffraction and X-ray photoelectron spectroscopy measurements. Considering the excellent electrocatalytic reduction activity and good electrical conductivity of transition-metal phosphide, we fabricated Co2P NPs on indium tin oxide (ITO) substrate (Co2P/ITO) for H2O2 detection. The Co2P/ITO transducer displayed a rapid amperometric response less than 5 s, a broader response range from 0.001 to 10.0 mM and a low detection limit of 0.65 μM. In addition, the non-enzymatic Co2P/ITO sensor showed outstanding selectivity, reproducibility, repeatability and stability, all of which qualified the Co2P/ITO electrode for quite a reliable and promising biosensor for H2O2 sensing.
Keywords: Cobalt phosphide, Hydrogen peroxide, Non-enzymatic, Amperometric sensor
Introduction
Hydrogen peroxide (H2O2) is a representative reactive oxygen species in living organisms, and it plays a critical role in normal physiologic function [1]. The concentration of H2O2 in living cells is related closely with the cell physiological balance [2]. Numerous studies have also been reported that cancer, Alzheimer’s diseases, Parkinson’s diseases and some severe diseases may be caused by abnormal concentration of H2O2 [3–5]. Developing accurate, sensitive, rapid and selective methods to detect the concentration of H2O2, a normal oxidative stress biomarker, will be undoubtedly beneficial to the early diagnosis. Up to now, a host of analytical methods such as spectroscopy [6], colorimetry [7], fluorescence [8, 9] and electrochemical methods [10–12] have been applied in H2O2 determination. Electrochemical method, especially amperometric test is gradually becoming one of the most simple and effective detection methods for H2O2 biological analysis among diverse sensing methods due to its advantages such as high sensitivity, outstanding selectivity and low cost.
Enzymatic electrochemical sensors have been proved to be effective instruments for detecting H2O2. However, the large-scale practical application of enzyme-based sensors is limited by complicated immobilization, environmental instability and low reproducibility. Therefore, developing non-enzymatic electrochemical H2O2 sensors is highly indispensable.
In recent years, a growing number of sensors based on noble metal [13–15], non-noble metal and their corresponding compounds [16–19] or carbon materials [20, 21] have been used for electrochemical H2O2 detection. As electrochemical active materials for fabricating non-enzymatic biosensors, transition metal compounds have been received increasing interests. Transition-metal phosphides (TMPs) are a class of newly developed materials with excellent electrocatalytic activity, good electrical conductivity and a plenty of outstanding properties. Thus, they have been extensively studied for applications in water splitting [22, 23], hydrodesulfurization [24], and supercapacitor electrodes [25]. Recent research indicates that CoP, Ni2P and Cu3P [26–28] can also be used as efficient electrocatalyst for non-enzymatic H2O2 detection. However, the number of researches about the application of TMPs in bioanalysis is still limited nowadays. Besides, the use of triphenylphosphine [29, 30], white phosphorous [31, 32] or other environmental hazardous phosphorous source [33] can increase the operational risk in the preparation of TMPs. Therefore, some research work for developing green method in TMP preparation is worth being supplemented in this area.
In this work, cobalt phosphide nanoparticles (Co2P NPs) were prepared by one-step hydrothermal method utilizing cobalt acetate and red phosphorous as raw materials. Herein, we fabricated Co2P NPs on indium tin oxide (ITO) substrate by drop-casting method for H2O2 detection. Co2P displayed excellent electrocatalytic activity toward H2O2 reduction. Moreover, it revealed favorable selectivity, excellent reproducibility and good stability, which therefore exhibited its potential application as a sensitive platform for H2O2 detection.
Experimental Section
Reagents and Materials
All reagents were analytical grade and used without further purification. Cobalt (II) acetate tetrahydrate (Co(Ac)2·4H2O), cobalt chloride hexahydrate (CoCl2·6H2O), D-(+)-glucose, L-Glycine (L-Gly), ascorbic acid (AA), uric acid (UA), urea, NaCl, KCl, NaH2PO4, Na2HPO4, hydrogen peroxide (30% H2O2), ethanol and acetone were purchased from Sinopharm Chemical Reagent Co., Ltd. China. D-(–)-fructose, L-arginine (L-Arg), L-lysine (L-Lys), dopamine (DA), acetaminophen (APAP), amino trimethylene phosphonic acid (ATMP, 50 wt%) were purchased from Aladdin Ltd. Commercial red phosphorous (98.5%, 100 mesh) were purchased from Energy Chemical Technology (Shanghai) Co., Ltd. Nafion PFSA polymer dispersion (5%) were purchased from Beijing Honghaitian technology Co., Ltd. Deionized water was used in all the experiments. The indium tin oxide (ITO) glass (10 × 20 × 1.1 mm with an ITO film of 185 ± 2 nm and a sheet resistance of 6.6 ± 0.1 Ω) was supplied from Shenzhen South Xiangcheng Technology Co., Ltd.
Synthesis of Co2P Nanoparticles
Commercial red phosphorous (2 g) was dispersed in 15 mL H2O under sonification and hydrothermally treated at 200 °C for 12 h in a 50 mL Teflon-lined stainless autoclave to clear oxide layers [34]. Then, the hydrothermal treated red phosphorous was dried in a vacuum oven. After finishing the pretreatment of red phosphorous, 1 mmol Co(Ac)2·4H2O was dissolved in 30 mL distilled water to obtain an aqueous solution. Then, the hydrothermal treated red phosphorous was added into the solution under ultrasonication for 15 min with the molar ratio of Co/P 1/10. The prepared suspension was rapidly poured into a 50 mL Teflon-lined autoclave. Then, the autoclave was placed in an electronic oven and hydrothermally treated at 160, 200, 240 °C for 12 h, respectively. Then, the product was collected by centrifugation and washed three times with distilled water and ethanol, respectively. Finally, Co2P NPs were dried at 60 °C for 3 h in air.
Synthesis of Co(PO3)2
The preparation method of Co(PO3)2 was referred to the previous report [35]. 0.1 M CoCl2·6H2O methanol solution was prepared firstly. Then, 2 mL ATMP (50 wt%) was added dropwise into 20 mL the above purple solution and stirred for 30 min. The insoluble cobalt-metaphosphate coordination polymer formed in the solution subsequently. The obtained pink powder was further heated to 900 °C under Ar flow with a heating rate of 5 °C·min−1 and then held for 2 h. After cooling down to room temperature, the black product was collected and reheated at 650 °C for 4 h in air to remove the carbonized organic ligand. Finally, the light-purple powder of Co(PO3)2 was obtained.
Fabrication of Co2P/ITO Electrode
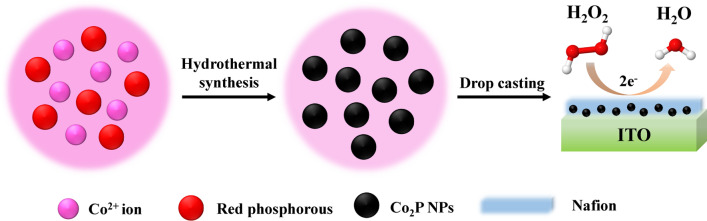
Firstly, the ITO glass (1 cm × 2 cm) was cleaned in acetone, ethanol and deionized water for 10 min, respectively, by sonication. After that, the treated ITO was dried under nitrogen sweeping. For the modification of the electrode, 5 mg of the Co2P NPs was dispersed in 1 mL deionized water to form 5 mg mL−1 Co2P suspension. Then, 5 μL 5% Nafion solution was added into the suspension and the mixture was ultrasonicated for 15 min to obtain uniform ink-like suspension. The Co2P/ITO electrode was prepared by drop-casting 100 μL of Co2P suspension on the ITO surface, and dried in air as working electrode. The schematic preparation process of Co2P/ITO electrode is shown in Scheme 1.
Scheme 1.
Schematic preparation of Co2P/ITO electrode and H2O2 sensing
Characterizations
The X-ray diffraction (XRD) data were analyzed by a D8 ADVANCE diffractometer with Cu Kα radiation. The transmission electron microscopy (TEM) measurement was conducted using a Tecnai G2 F20 with energy dispersed spectrum detector. X-ray photoelectron spectroscopy (XPS) spectra were measured on a Thermo ESCALAB 250XI spectrometer.
Electrochemical Measurements
Voltammetry measurements were accomplished by CHI 660E electrochemical workstation in a three-electrode system, employing Co2P/ITO electrode as working electrode, a platinum foil (1 cm × 1 cm) as counter electrode and Ag/AgCl with 3 M KCl solution as reference electrode to study the electrochemical activities of the synthesized samples for H2O2 detection. Phosphate buffer saline (PBS; 0.1 M, pH 7.4) was used as the electrolyte to simulate the physiological medium in human body. The sensing performances of Co2P/ITO electrode toward H2O2 detection were investigated by cyclic voltammetry (CV) and amperometry (I-t). All the detection experiments were performed under 100 rpm stirring at room temperature. Electrochemical impedance tests were performed on VersaSTAT 3F electrochemical workstation and ferricyanide solution was used as the electrolyte for impedance measurement.
Results and Discussion
Characterization of Co2P NPs
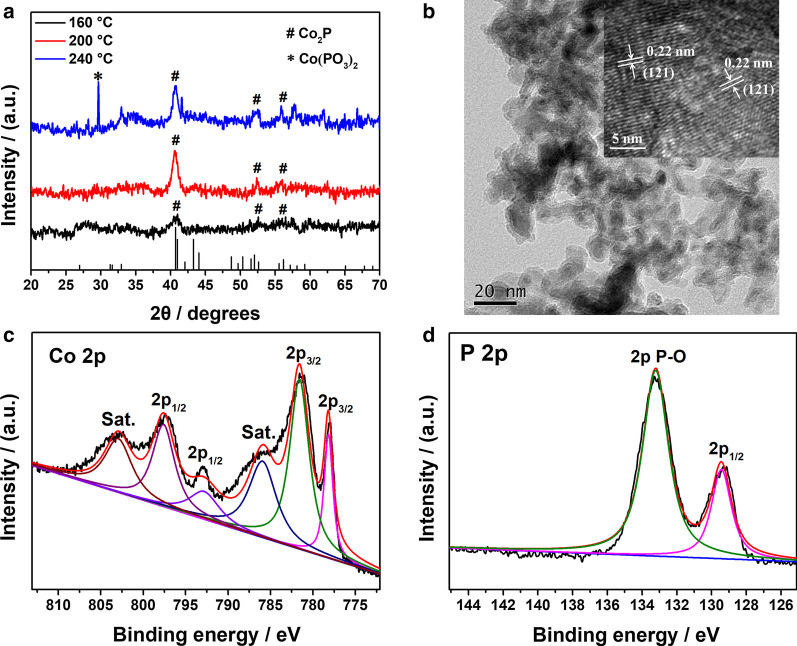
The crystal structure of Co2P NPs was confirmed by XRD measurement. Figure 1a shows the XRD patterns of Co2P samples prepared at 160, 200 and 240 °C for 12 h. The Co2P sample prepared at 200 °C shows diffraction peaks at around 40.7°, 40.9°, 52.0° and 56.2° which correspond to the characteristic diffraction planes at (121), (201), (002) and (320) for the orthorhombic phase of Co2P (JCPDS no. 32-0306). When temperature varied from 160 to 200 °C, the intensities of diffraction peaks increased and the peaks became narrower and sharper, indicating that the products had a higher crystallinity at 200 °C. However, when temperature reached 240 °C, some impurities were formed and the diffraction peaks at 29.7° was attributed to the diffraction plane at (-222) of Co(PO3)2 (JCPDS no. 27-1120). The influence of synthetic time on the preparation of Co2P under 200 °C is shown in Additional file 1: Fig. S1. When the time duration was controlled within 12 h, the obtained Co2P NPs displayed the lowest value of full width at half maximum of (121) peak, suggesting better crystallinity. Besides, none of impurities existed in the sample when the reaction time varied from 6 to 24 h. According to the Scherrer formula, the calculated grain size of Co2P NPs prepared at 200 °C for 12 h was 14.2 nm.
Fig. 1.
a XRD patterns of Co2P NPs prepared at different temperatures for 12 h. b Transmission electron microscopic image and high-resolution transmission electron microscopic image (inset) of Co2P NPs. XPS spectra of Co2P in the c Co 2p region and d P 2p region
The morphology of Co2P NPs was assessed by TEM measurements. As shown in Fig. 1b, the product prepared at 200 °C is composed of irregular nanoparticles with the diameter around 10–20 nm and two lattice fringes can be clearly seen in the high-resolution TEM (HRTEM) image (inset in Fig. 1b). The distance between the neighboring planes is 0.22 nm, corresponding to the (121) facets of Co2P, which further confirms that the formation of TMP is Co2P.
The XPS technique was employed in analyzing the chemical compositions on the surface of the Co2P. Additional file 1: Fig. S2 shows the XPS survey spectrum of Co2P. Co, P and O elements are detected in the sample, confirming the existence of Co2P and some oxidized products. Energy-dispersive X-ray spectroscopy (EDX) spectra of Co2P (Additional file 1: Fig. S3) further confirms the co-existence of three elements (Co, P, O) in the sample. The high-resolution XPS spectra of Co 2p and P 2p are shown in Fig. 1c, d, respectively. In Co 2p spectrum, the peaks at 781.1 and 797.6 eV can be ascribed to the binding energies (BEs) of Co2+ 2p3/2 and Co2+ 2p1/2, respectively [26, 36]. The peaks at 786.0 and 803.1 eV are two apparent shake-up satellite peaks. The Co 2p BE of 778.2 eV shifts positively from that of metallic Co (777.9 eV), which suggests that Co in Co2P has a partial positive charge (δ+) with a small value (0 < δ < 2) [37]. On the contrary, the P 2p BE of 129.4 eV shifts negatively from that of elemental P (130.2 eV) so that the P has a partial negative charge (δ−) in Co2P. The changes of BE in Co and P element compared with their elementary substance, respectively, reveal that the transfer direction of electron density in Co2P is from Co to P [38]. Superficial oxidation of Co2P generates a few of oxidized P species in the sample. Therefore, the peaks at 133.2 eV in high BE range are assigned to the oxides [39].
Electrochemical Detection of H2O2 at Co2P/ITO Electrode
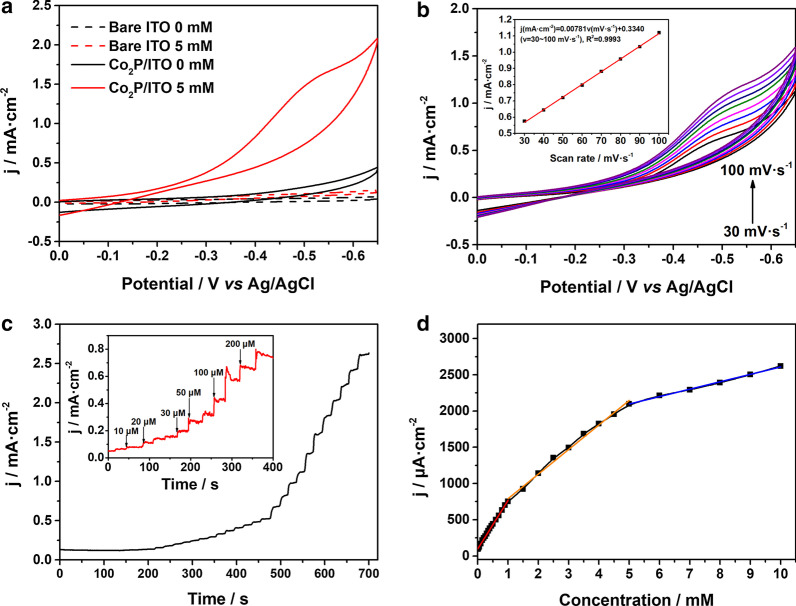
To investigate the electrocatalytic activity of Co2P NPs in H2O2 reduction, we designed a non-enzymatic H2O2 electrode by drop-casting Co2P NPs suspension on a bare ITO surface. Figure 2a shows the CV curves of bare ITO and Co2P/ITO in 0.1 M PBS at pH 7.4 with and without 5.0 mM H2O2, respectively. The dash lines indicate that the response of bare ITO to H2O2 reduction is negligible. However, the Co2P/ITO electrode exhibits a remarkable reduction peak at − 0.5 V in the presence of H2O2, which demonstrates the prominent electrocatalytic activity of Co2P NPs toward H2O2 reduction. Figure 2b presents the CV curves of Co2P/ITO at different scan rates (from 30 to 100 mV s−1) with 2.5 mM H2O2. When increasing the scan rate, the reduction peak current increased and the peak potential shifted to the more negative potential side, indicating the reduction in H2O2 on Co2P/ITO was an irreversible reaction. The corresponding calibration curve (inset, Fig. 2b) shows that the reduction peak current densities increase linearly proportional to the scan rate, suggesting that the electrochemical reduction of H2O2 on the surface of Co2P/ITO electrode is a surface-controlled process [40].
Fig. 2.
a CV curves of bare ITO and Co2P/ITO electrode in 0.1 M PBS with and without 5.0 mM H2O2 at a scan rate of 100 mV s−1. b CV curves of Co2P/ITO electrode in 2.5 mM H2O2 at scan rates from 30 to 100 mV s−1. Inset: The corresponding plot of current versus the scan rate. c Amperometric responses of Co2P/ITO electrode with successive addition of H2O2 in 0.1 M PBS. d The calibration curve of steady current versus the concentration of H2O2
Figure 2c, d show the amperometric response and the calibration curve of Co2P/ITO electrode upon the successive addition of H2O2 into the 0.1 M PBS at − 0.5 V with stirring. The Co2P/ITO electrode exhibited quick response to the addition of H2O2 and achieved the steady-state current within 5 s. The calibration curve in Fig. 2d shows that the transducer displays a multi-linear range of H2O2 concentration from 0.001 to 1.0 mM, 1.0–5.0 mM and 5.0–10.0 mM. The sensitivity of the sensor alters with the increasing concentration of H2O2, due to the change of electrocatalytic reduction kinetics of H2O2 on the electrode surface. According to previous reports, the rate-determining step of H2O2 reduction is dominated by H2O2 adsorption at low concentration, whereas the activation of H2O2 is the major determinant at high concentration. In the middle region, the reduction kinetics of H2O2 is controlled by adsorption and activation at the same time [10]. A multitude of analysts will adsorb on the surface of Co2P and cover the active sites in the high concentration, which lead to the decrease in sensitivity [41].
The comparison on H2O2 sensing performances of the prepared Co2P sample at various reaction temperature and time is shown in Additional file 1: Fig. S4, S5 and Table S1, indicating that the Co2P sample prepared at 200 °C for 12 h displays the best H2O2 sensing performances. When reaction temperature raised to 240 °C, the formed Co(PO3)2 in Co2P could be regarded as impurity. To further clarify the influence of Co(PO3)2 on H2O2 detection, the electrochemical properties of Co(PO3)2 were investigated. As shown in Additional file 1: Fig. S6, Co(PO3)2 displays negligible electrochemical response toward H2O2 and its conductivity is inferior to Co2P, which declines the current signal of Co2P/ITO in amperometric test. Therefore, the higher purity and better crystallinity of Co2P sample may contribute to the improvement of sensing performances. Thus, we choose the Co2P sample prepared at 200 °C and 12 h as the best H2O2 sensing material. The calibration I–t curve also presents a good linear relationship in the concentration of 1.0–50 μM, the physiological range of H2O2 concentration in biosystem (Fig. S7) [28], which could be helpful to improve the possibility of practical applications of this sensor. In addition, the limit of detection (LOD) of the H2O2 sensor can be calculated to be 0.65 μM at a signal-to-noise ratio of 3. Compared with the previously reported H2O2 sensor, the comprehensive electrochemical performances of our Co2P/ITO transducer are superior to those with favorable sensitivity, linear range and LOD, as shown in Table 1.
Table 1.
Sensing performances on comparison of Co2P/ITO with other cobalt-based non-enzymatic H2O2 sensors
| Materials | Linear range | Sensitivity (μA mM−1 cm−2) | Detection limit (μM) | References |
|---|---|---|---|---|
| Co3O4–rGO | 15–675 μM | 1140 | 2.4 | [42] |
| Co3O4 nanowire/N-carbon foam | 0.01–1.4 mM | 230 | 1.4 | [16] |
| Co3O4/MWCNTs | 0.02–0.43 mM | 1000 | 2.46 | [43] |
| Co3O4/rGO | 1–18.5 mM | – | 0.5 | [44] |
| CoS | 0.005–14.82 mM | 459 | 1.5 | [45] |
| CoP NWs | 0.001–12 mM | – | 0.48 | [26] |
| Hb/CoP-CC (carbon cloth) | 2.0–2670 μM | 56.2 | 0.67 | [40] |
| Co2P/ITO | 0.0001–1.0 mM | 668.6 | 0.65 | This work |
| 1.0–5.0 mM | 339.0 | |||
| 5.0–10.0 mM | 102.3 |
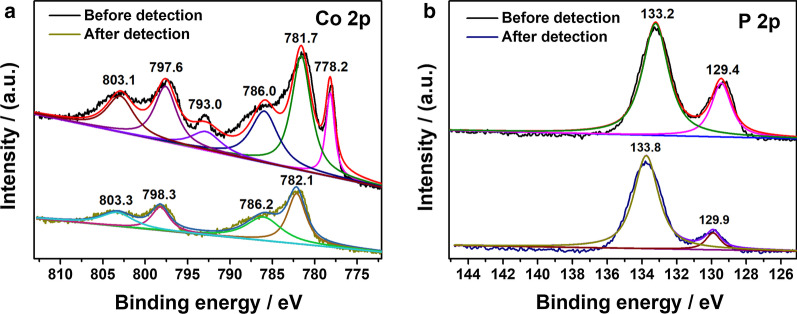
After detecting 1.0 mM H2O2 repeatedly for 35 times (Fig. 3a, b), the XPS spectra in Co 2p and P 2p region of Co2P are analyzed to further investigate the sensing mechanism. There is no significant change in the position of the peaks in P 2p region before and after H2O2 detection. However, the peaks at 778.2 and 793.0 eV in Co 2p spectrum disappeared after multiple measurements. As the peak at 778.2 eV indicates the existence of reduced Co species in Co2P sample [37], the disappearance of these two peaks demonstrates that the reduced Co species with low valence in Co2P may be oxidized by H2O2 during the detection process, especially with high concentration of H2O2. The remnant peaks in Co 2p region (782.1 and 798.3 eV) are attributed to Co2+ 2p3/2 and Co2+ 2p1/2, respectively, suggesting the exclusive existence of Co(II) species in Co2P after multiple measurements. According to previous reports about the utilization of cobalt-based electrocatalyst in H2O2 detection, Co2+ species are demonstrated as the catalytic active sites for H2O2 reduction [46–48]. Generally, the electrochemical reduction in H2O2 goes through two steps in PBS [49, 50], as shown below.
| 1 |
| 2 |
| 3 |
Fig. 3.
The comparison of XPS spectra in a Co 2p region and b P 2p region of Co2P before and after detection
In the first step, H2O2 obtains an electron to form adsorbed OH− (OHad). When the intermediate OHad obtains an additional electron, the final reduction product of H2O2, H2O, is generated. As the redox potential of H2O2/H2O is higher than Co3+/Co2+, the Co(II) species in Co2P can be oxidized to Co(III) in the electron transfer process and H2O2 is reduced to H2O irreversibly. During the amperometric test, the applied bias is − 0.5 V versus Ag/AgCl (equals to 0.14 V vs. NHE), which is lower than the standard redox potential of Co3+/Co2+. As a result, the oxidized Co(III) can be reduced to Co(II) and theses catalytic active sites of Co(II) are regenerated again. Therefore, it can be concluded that the catalytic cycle of Co (II) species takes place during electrochemical detection of H2O2 and the reduced Co species with low valence are oxidized by H2O2 after repetitive measurements.
Selectivity, Stability, Reproducibility and Repeatability of Co2P/ITO Electrode
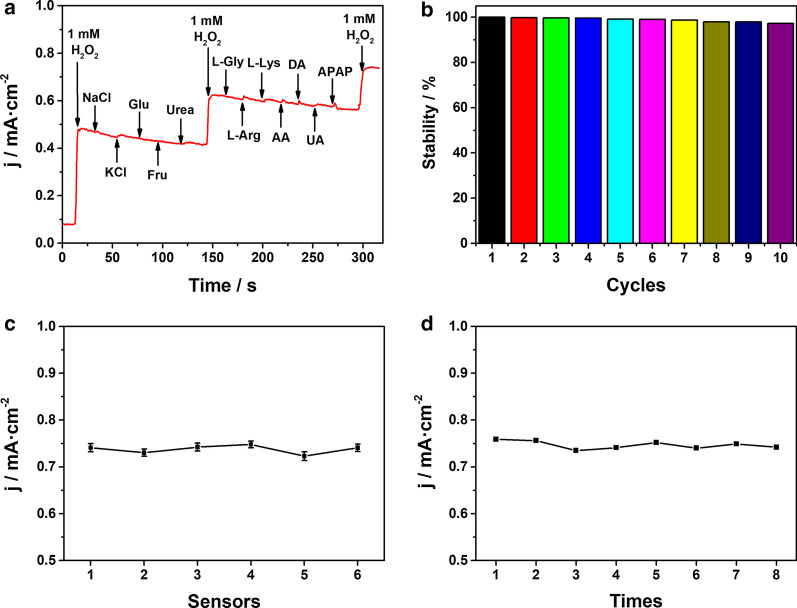
Anti-interference performance is another important property of biosensor. High purity nitrogen was utilized to avoid the influence by dissolved oxygen in solution because oxygen could be reduced at similar potential which was applied in amperometric test [51]. Comparing the CV curves of Co2P/ITO in 0.1 M PBS with or without nitrogen purging, the reduction potential and the current response of 2.5 mM H2O2 are similar, as shown in Fig. S8, which therefore suggests that the interference of dissolved oxygen can be neglected. Selectivity of Co2P/ITO was also tested with common substances and other small molecules in body fluid, such as some inorganic salts, saccharides, amino acids and reductive biomolecules. As shown in Fig. 4a, the current response after adding the above interferents can be neglected compared with the response of 1.0 mM H2O2. As both two O atoms of H2O2 could be bonded with one or two Co atoms [52], the H2O2 molecule would chemically adsorb on Co(II) species in Co2P specifically. In addition, the interference from indiscriminate oxidation of some reductive compounds in real biological samples at high potential can be also reduced significantly at lower bias potential [53]. Therefore, the favorable selectivity of Co2P toward H2O2 mainly benefits from the Co(II) species as specific adsorption sites and the applied negative bias potential during sensing process.
Fig. 4.
a Amperometric responses of Co2P/ITO electrode with the addition of 1 mM H2O2 and other interfering species (10 mM NaCl, KCl, Glu, Fru, urea, L-Gly, L-Arg, L-Lys, AA; 1 mM DA, UA; 0.5 mM APAP) in 0.1 M PBS. b The cathodic peak currents of ten successive scanning CV curves in 50 μM H2O2. c Reproducibility of six Co2P/ITO electrodes for detecting 1.0 mM H2O2. d Repeatability of Co2P/ITO electrode for detecting 1.0 mM H2O2 eight times
Moreover, the stability, reproducibility and repeatability of the Co2P/ITO transducer were also evaluated. The reduction peak currents of ten successive scanning CV curves in 50 μM H2O2 is shown in Fig. 4b. After ten cycles, the peak current of the electrode only fell by 2.7%. In addition, the sensor remained about 98.2% of its initial current response after being stored in air for one month (Fig. S9), demonstrating ideal detecting stability and outstanding long-term durability. The electrode-to-electrode reproducibility is investigated by calculating the relative standard deviation (RSD) of H2O2 current responses. To eliminate the potential error from electrode fabrication as far as possible, the steady current density in the presence of H2O2 is subtracted by the initial background signal of individual electrode and the obtained difference value is regarded as the electrochemical response of each electrode. Six Co2P/ITO electrodes were fabricated under the same conditions for controlled experiments and the RSD of current responses was 1.24%, as shown in Fig. 4c, indicating the relatively excellent reproducibility of Co2P/ITO. Meanwhile, repeatability was measured in one electrode by detecting 1.0 mM H2O2 eight times, and the RSD of 1.14% was achieved (Fig. 4d). The above results illustrate the satisfactory stability, reproducibility and repeatability of the electrode for non-enzymatic electrochemical detection of H2O2.
Conclusion
In summary, Co2P NPs were successfully synthesized by hydrothermal method. Furthermore, the Co2P NPs prepared at 200 °C for 12 h have been proved as an efficient catalyst toward electrochemical reduction of H2O2 in pH 7.4 PBS. As a non-enzymatic H2O2 sensor, the Co2P/ITO electrode displayed a rapid amperometric response less than 5 s, a broader response range from 0.001 to 10.0 mM and a low detection limit of 0.65 μM, as well as satisfactory selectivity, reproducibility and stability. This work aims to broaden the research about the application of transition metal phosphide in electrochemical detection of small biomolecules and our Co2P/ITO sensor could be designed as a new non-enzymatic platform for H2O2 detection.
Supplementary information
Additional file 1. Fig. S1. XRD patterns of Co2P NPs synthesized with different reaction times at 200 °C. Fig. S2. XPS survey spectrum of Co2P. Fig. S3. EDX spectra of Co2P NPs. Fig. S4. Amperometric responses of Co2P/ITO electrodes prepared at (a) different temperatures and (c) different times with successive addition of H2O2 in 0.1 M PBS. (b), (d) The calibration curve of steady current versus the concentration of H2O2. Fig. S5. The linear relationship between current density and concentration of H2O2 in different concentration ranges (a) 0.0001–1.0 mM, (b) 1.0–5.0 mM, (c) 5.0–10.0 mM. Fig. S6. Comparison of electrochemical properties between Co2P and Co(PO3)2. (a) LSV curves of Co2P and Co(PO3)2 modified electrode in 0.1 M PBS with and without 2.5 mM H2O2 at a scan rate of 100 mV s−1. (b) Nyquist plots of bare ITO, Co2P/ITO and Co(PO3)2/ITO electrode (electrolyte: 5.0 mM K3[Fe(CN)6]/ K4[Fe(CN)6] and 0.1 M KCl; bias: open circuit potential, amplitude: 5 mV, frequency range: 100 kHz ~ 0.01 Hz). Fig. S7. The linear relationship between current density and concentration of H2O2 in the physiological range. Fig. S8. CVs for Co2P/ITO electrode in 0.1 M PBS with or without N2 purging at a scan rate of 100 mV s−1. Fig. S9. CV responses at a scan rate of 100 mV s−1 in 0.1 M PBS containing 0.1 mM H2O2 of a Co2P/ITO electrode before and after being stored in air for one month. Table S1. The comparison on H2O2 sensing performance of the bare ITO electrode and the prepared Co2P sample at various reaction temperature.
Acknowledgements
Not applicable.
Abbreviations
- NPs
Nanoparticles
- ITO
Indium tin oxide
- TEM
Transmission electron microscopy
- HRTEM
High-resolution transmission electron microscopy
- XRD
X-ray diffraction
- XPS
X-ray photoelectron spectroscopy
- EDX
Energy-dispersive X-ray spectroscopy
- CV
Cyclic voltammetry
- I-t
Amperometry
- Gly
Glycine
- AA
Ascorbic acid
- UA
Uric acid
- Arg
Arginine
- Lys
Lysine
- DA
Dopamine
- APAP
Acetaminophen
- ATMP
Trimethylene phosphonic acid
- PBS
Phosphate buffer
- LOD
Detection limit
- RSD
Relative standard deviation
Authors' contributions
The manuscript was written through contributions of all authors. DHY, JYT and RBB designed and performed the experiments. JYT and SYY analyzed the experiment data and wrote the manuscript. MNJ and HML assisted with interpretation of the data from electrochemical test. ZGK, FW and CLL helped to check the manuscript before submission. CLL conceived of the study and all authors read and approved the final version of the manuscript.
Funding
We appreciate the financial support by the National Natural Science Foundation of China (81660708), National Key Research and Development Project (2019YFC0312602), Science and Technology Support Program of Jiangsu Province (BE2018389) and the Key Project of Tibetan Medical Administration of Tibet (2017005). The work is also supported by “Double First-Class” University Project (CPU2018GY25), the Qinglan Project of Young Academic Leaders of Jiangsu Province and Fundamental Research Funds for the Central Universities (2632019YX01).
Availability of data and materials
All data and materials are fully available without restriction.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Donghang Yin, Email: cpu2013y@163.com.
Junyan Tang, Email: 2324512118@qq.com.
Rongbiao Bai, Email: 1048758901@qq.com.
Shuyi Yin, Email: cpu_daisy_yin@163.com.
Mengnan Jiang, Email: 18551737730@163.com.
Zigui Kan, Email: ziguik@cpu.edu.cn.
Hongmei Li, Email: lihongm2001@hotmail.com.
Fei Wang, Email: feiwang@cpu.edu.cn.
Caolong Li, Email: licl@cpu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1186/s11671-020-03469-9.
References
- 1.Rhee SG. H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 2.Lippert AR, De Bittner GCV, Chang CJ. Boronate oxidation as a bioorthogonal reaction approach for studying the chemistry of hydrogen peroxide in living systems. Acc Chem Res. 2011;44:793–804. doi: 10.1021/ar200126t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finkel T, Serrano M, Blasco MA. The common biology of cancer and ageing. Nature. 2007;448:767–774. doi: 10.1038/nature05985. [DOI] [PubMed] [Google Scholar]
- 4.Lux CD, Joshi-Barr S, Nguyen T, Mahmoud E, Schopf E, Fomina N, Almutairi A. Biocompatible polymeric nanoparticles degrade and release cargo in response to biologically relevant levels of hydrogen peroxide. J Am Chem Soc. 2012;134:15758–15764. doi: 10.1021/ja303372u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi DJ, Jamieson CHM, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Gasser C, Kilgus J, Harasek M, Lendl B, Brandstetter M. Enhanced mid-infrared multi-bounce ATR spectroscopy for online detection of hydrogen peroxide using a supercontinuum laser. Opt Express. 2018;26:12169–12179. doi: 10.1364/OE.26.012169. [DOI] [PubMed] [Google Scholar]
- 7.Guo JL, Wang Y, Zhao M. 3D flower-like ferrous(II) phosphate nanostructures as peroxidase mimetics for sensitive colorimetric detection of hydrogen peroxide and glucose at nanomolar level. Talanta. 2018;182:230–240. doi: 10.1016/j.talanta.2018.01.080. [DOI] [PubMed] [Google Scholar]
- 8.Feng CC, Wang FY, Dang YJ, Xu ZA, Yu HJ, Zhang W. A self-assembled ratiometric polymeric nanoprobe for highly selective fluorescence detection of hydrogen peroxide. Langmuir. 2017;33:3287–3295. doi: 10.1021/acs.langmuir.7b00189. [DOI] [PubMed] [Google Scholar]
- 9.Lampard EV, Sedgwick AC, Sun XL, Filer KL, Hewins SC, Kim G, Yoon J, Bull SD, James TD. Boronate-based fluorescence probes for the detection of hydrogen peroxide. Chemistryopen. 2018;7:262–265. doi: 10.1002/open.201700189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang JF, Zhu YH, Zhong H, Yang XL, Li CZ. Dispersed CuO nanoparticles on a silicon nanowire for improved performance of nonenzymatic H2O2 detection. ACS Appl Mater Interfaces. 2014;6:7055–7062. doi: 10.1021/am501799w. [DOI] [PubMed] [Google Scholar]
- 11.Li DP, Liu XY, Yi R, Zhang JX, Su ZQ, Wei G. Electrochemical sensor based on novel two-dimensional nanohybrids: MoS2 nanosheets conjugated with organic copper nanowires for simultaneous detection of hydrogen peroxide and ascorbic acid. Inorg Chem Front. 2018;5:112–119. doi: 10.1039/C7QI00542C. [DOI] [Google Scholar]
- 12.Liu J, Bo XJ, Yang J, Yin DD, Guo LP. One-step synthesis of porphyrinic iron-based metal-organic framework/ordered mesoporous carbon for electrochemical detection of hydrogen peroxide in living cells. Sens Actuators B Chem. 2017;248:207–213. doi: 10.1016/j.snb.2017.03.117. [DOI] [Google Scholar]
- 13.Bai ZH, Li GY, Liang JT, Su J, Zhang Y, Chen HZ, Huang Y, Sui WG, Zhao YX. Non-enzymatic electrochemical biosensor based on Pt NPs/RGO-CS-Fc nano-hybrids for the detection of hydrogen peroxide in living cells. Biosens Bioelectron. 2016;82:185–194. doi: 10.1016/j.bios.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Wang XJ, Guo XL, Chen J, Ge C, Zhang HY, Liu YY, Zhao L, Zhang Y, Wang ZM, Sun LT. Au nanoparticles decorated graphene/nickel foam nanocomposite for sensitive detection of hydrogen peroxide. J Mater Sci Technol. 2017;33:246–250. doi: 10.1016/j.jmst.2016.11.029. [DOI] [Google Scholar]
- 15.Liu W, Hiekel K, Hubner R, Sun HJ, Ferancova A, Sillanpaa M. Pt and Au bimetallic and monometallic nanostructured amperometric sensors for direct detection of hydrogen peroxide: Influences of bimetallic effect and silica support. Sens Actuators B Chem. 2018;255:1325–1334. doi: 10.1016/j.snb.2017.08.123. [DOI] [Google Scholar]
- 16.Dong JK, Xu HY, Zhang FJ, Chen C, Liu L, Wu GT. Synergistic effect over photocatalytic active Cu2O thin films and their morphological and orientational transformation under visible light irradiation. ApplCatal A Gen. 2014;470:294–302. doi: 10.1016/j.apcata.2013.11.010. [DOI] [Google Scholar]
- 17.Sherino B, Mohamad S, Halim SNA, Manan NSA. Electrochemical detection of hydrogen peroxide on a new microporous Ni-metal organic framework material-carbon paste electrode. Sens Actuators B Chem. 2018;254:1148–1156. doi: 10.1016/j.snb.2017.08.002. [DOI] [Google Scholar]
- 18.Xue B, Li KZ, Gu SY, Zhang LL, Lu JH. Ni foam-supported ZnO nanowires and Co3O4/NiCo2O4 double-shelled nanocages for efficient hydrogen peroxide detection. Sens Actuators B Chem. 2018;262:828–836. doi: 10.1016/j.snb.2018.02.091. [DOI] [Google Scholar]
- 19.Yuan RM, Li HJ, Yin XM, Zhang LL, Lu JH. Stable controlled growth of 3D CuO/Cu nanoflowers by surfactant-free method for non-enzymatic hydrogen peroxide detection. J Mater Sci Technol. 2018;34:1692–1698. doi: 10.1016/j.jmst.2017.11.030. [DOI] [Google Scholar]
- 20.Fu L, Wang AW, Lai GS, Lin CT, Yu JH, Yu AM, Liu Z, Xie KF, Su WT. A glassy carbon electrode modified with N-doped carbon dots for improved detection of hydrogen peroxide and paracetamol. Microchim Acta. 2018;185:87. doi: 10.1007/s00604-017-2646-9. [DOI] [PubMed] [Google Scholar]
- 21.Huang B, Wang Y, Lu ZW, Du HJ, Ye JS. One pot synthesis of palladium-cobalt nanoparticles over carbon nanotubes as a sensitive non-enzymatic sensor for glucose and hydrogen peroxide detection. Sens Actuators B Chem. 2017;252:1016–1025. doi: 10.1016/j.snb.2017.06.038. [DOI] [Google Scholar]
- 22.Yan LT, Cao L, Dai PC, Gu X, Liu DD, Li LJ, Wang Y, Zhao XB. Metal-organic frameworks derived nanotube of nickel-cobalt bimetal phosphides as highly efficient electrocatalysts for overall water splitting. Adv Funct Mater. 2017;27:1703455. doi: 10.1002/adfm.201703455. [DOI] [Google Scholar]
- 23.Xin YM, Kan X, Gan LY, Zhang ZH. Heterogeneous bimetallic phosphide/sulfide nanocomposite for efficient solar-energy-driven overall water splitting. ACS Nano. 2017;11:10303–10312. doi: 10.1021/acsnano.7b05020. [DOI] [PubMed] [Google Scholar]
- 24.Song LM, Zhang SJ, Ma QY. Synthesis of an iron phosphide catalyst based on sulfides and hydrodesulfurization property. Chem Eng J. 2015;281:281–285. doi: 10.1016/j.cej.2015.06.069. [DOI] [Google Scholar]
- 25.Elshahawy AM, Guan C, Li X, Zhang H, Hu YT, Wu HJ, Pennycook SJ, Wang J. Sulfur-doped cobalt phosphide nanotube arrays for highly stable hybrid supercapacitor. Nano Energy. 2017;39:162–171. doi: 10.1016/j.nanoen.2017.06.042. [DOI] [Google Scholar]
- 26.Liu DN, Chen T, Zhu WX, Cui L, Asiri AM, Lu Q, Sun XP. Cobalt phosphide nanowires: an efficient electrocatalyst for enzymeless hydrogen peroxide detection. Nanotechnology. 2016;27:33. doi: 10.1088/0957-4484/27/33/33LT01. [DOI] [PubMed] [Google Scholar]
- 27.Xiong XL, You C, Cao XQ, Pang LF, Kong RM, Sun XP. Ni2P nanosheets array as a novel electrochemical catalyst electrode for non-enzymatic H2O2 sensing. Electrochim Acta. 2017;253:517–521. doi: 10.1016/j.electacta.2017.09.104. [DOI] [Google Scholar]
- 28.Li ZZ, Xin YM, Wu WL, Fu BL, Zhang ZH. Topotactic conversion of copper(I) phosphide nanowires for sensitive electrochemical detection of H2O2 release from living cells. Anal Chem. 2016;88:7724–7729. doi: 10.1021/acs.analchem.6b01637. [DOI] [PubMed] [Google Scholar]
- 29.Chen XJ, Cheng M, Chen D, Wang RM. Shape-controlled synthesis of Co2P nanostructures and their application in supercapacitors. ACS Appl Mater Interfaces. 2016;8:3892–3900. doi: 10.1021/acsami.5b10785. [DOI] [PubMed] [Google Scholar]
- 30.Pan Y, Lin Y, Chen YJ, Liu YQ, Liu CG. Cobalt phosphide-based electrocatalysts: synthesis and phase catalytic activity comparison for hydrogen evolution. J Mater Chem A. 2016;4:4745–4754. doi: 10.1039/C6TA00575F. [DOI] [Google Scholar]
- 31.Ni Y, Li J, Zhang L, Yang S, Wei X. Urchin-like Co2P nanocrystals: synthesis, characterization, influencing factors and photocatalytic degradation property. Mater Res Bull. 2009;44:1166–1172. doi: 10.1016/j.materresbull.2008.09.041. [DOI] [Google Scholar]
- 32.Liu SL, Yan L, Li HL. Solvothermal synthesis of flower-like Co2P nanostructures and its electrochemical performance. Sci Adv Mater. 2014;6:746–750. doi: 10.1166/sam.2014.1763. [DOI] [Google Scholar]
- 33.Pradhan B, Kumar GS, Dalui A, Khan AH, Satpati B, Ji QM, Shrestha LK, Ariga K, Acharya S. Shape-controlled cobalt phosphide nanoparticles as volatile organic solvent sensor. J Mater Chem C. 2016;4:4967–4977. doi: 10.1039/C6TC00949B. [DOI] [Google Scholar]
- 34.Xia DH, Shen ZR, Huang GC, Wang WJ, Yu JC, Wong PK. Red Phosphorus: an earth-abundant elemental photocatalyst for "green" bacterial inactivation under visible light. Environ Sci Technol. 2015;49:6264–6273. doi: 10.1021/acs.est.5b00531. [DOI] [PubMed] [Google Scholar]
- 35.Xu LH, Zhang SL, Guo SY, Zhang XJ, Cosnier S, Marks RS, Wang WJ, Zeng HB, Shan D. ATMP derived cobalt-metaphosphate complex as highly active catalyst for oxygen reduction reaction. J Catal. 2020;387:129–137. doi: 10.1016/j.jcat.2020.04.014. [DOI] [Google Scholar]
- 36.Liang F, Huang L, Tian L, Li JY, Zhang HJ, Zhang SW. Microwave-assisted hydrothermal synthesis of cobalt phosphide nanostructures for advanced supercapacitor electrodes. CrystEngComm. 2018;20:2413–2420. doi: 10.1039/C8CE00054A. [DOI] [Google Scholar]
- 37.Huang ZP, Chen ZZ, Chen ZB, Lv CC, Humphrey MG, Zhang C. Cobalt phosphide nanorods as an efficient electrocatalyst for the hydrogen evolution reaction. Nano Energy. 2014;9:373–382. doi: 10.1016/j.nanoen.2014.08.013. [DOI] [Google Scholar]
- 38.Burns AW, Layman KA, Bale DH, Bussell ME. Understanding the relationship between composition and hydrodesulfurization properties for cobalt phosphide catalysts. ApplCatal A Gen. 2008;343:68–76. doi: 10.1016/j.apcata.2008.03.022. [DOI] [Google Scholar]
- 39.Liu YW, Cao XQ, Kong RM, Du G, Asiri AM, Lu Q, Sun XP. Cobalt phosphide nanowire array as an effective electrocatalyst for non-enzymatic glucose sensing. J Mater Chem B. 2017;5:1901–1904. doi: 10.1039/C6TB02882A. [DOI] [PubMed] [Google Scholar]
- 40.Dong SY, Yang QX, Fu YL, Zhang DD, Huang TL. Carbon cloth-supported cobalt phosphide as an active matrix for constructing enzyme-based biosensor. J Solid State Electr. 2018;22:1689–1696. doi: 10.1007/s10008-017-3864-0. [DOI] [Google Scholar]
- 41.Wang L, Yu J, Zhang YY, Yang H, Miao LF, Song YH. Simple and large-scale strategy to prepare flexible graphene tape electrode. ACS Appl Mater Interfaces. 2017;9:9089–9095. doi: 10.1021/acsami.6b14624. [DOI] [PubMed] [Google Scholar]
- 42.Kong LJ, Ren ZY, Zheng NN, Du SC, Wu J, Tang JL, Fu HG. Interconnected 1D Co3O4 nanowires on reduced graphene oxide for enzymeless H2O2 detection. Nano Res. 2015;8:469–480. doi: 10.1007/s12274-014-0617-6. [DOI] [Google Scholar]
- 43.Heli H, Pishahang J. Cobalt oxide nanoparticles anchored to multiwalled carbon nanotubes: synthesis and application for enhanced electrocatalytic reaction and highly sensitive nonenzymatic detection of hydrogen peroxide. Electrochim Acta. 2014;123:518–526. doi: 10.1016/j.electacta.2014.01.032. [DOI] [Google Scholar]
- 44.Zhang XM, Li KZ, Li HJ, Lu JH, Fu QG, Zhang LL. Hydrothermal synthesis of cobalt oxide porous nanoribbons anchored with reduced graphene oxide for hydrogen peroxide detection. J Nanopart Res. 2016;18:232. doi: 10.1007/s11051-016-3544-5. [DOI] [Google Scholar]
- 45.Wu WQ, Yu BB, Wu HM, Wang SF, Xia QH, Ding Y. Synthesis of tremella-like CoS and its application in sensing of hydrogen peroxide and glucose. Mater Sci Eng C Mater. 2017;70:430–437. doi: 10.1016/j.msec.2016.08.084. [DOI] [PubMed] [Google Scholar]
- 46.Chen Q, Ding R, Liu H, Zhou LX, Wang Y, Zhang Y, Fan GY. Flexible active-site engineering of monometallic Co-layered double hydroxides for achieving high-performance bifunctional electrocatalyst toward oxygen evolution and H2O2 reduction. ACS Appl Mater Interfaces. 2020;12:12919–12929. doi: 10.1021/acsami.0c01315. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Xu H, Zhao X, Hui ZY, Yu CY, Wang LM, Xue JL, Zhao Y, Zhou RC, Dai HH, Miao CY, Chen Q, Zhou JY, Sun Z, Huang W. Identifying the active site of ultrathin NiCoLDH as an efficient peroxidase mimic with superior substrate affinity for sensitive detection of hydrogen peroxide. J Mater Chem B. 2019;7:6232–6237. doi: 10.1039/C9TB01652J. [DOI] [PubMed] [Google Scholar]
- 48.Wang L, Wu T, Wu H, Zhong J, Wang N, Wang RM. A novel non-enzymatic hydrogen peroxide sensor based on Co:ZnO modified electrodes. Prog Nat Sci Mater. 2018;28:24–27. doi: 10.1016/j.pnsc.2017.12.001. [DOI] [Google Scholar]
- 49.Zhang W, Fan GZ, Yi H, Jia G, Li ZS, Yuan CW, Bai YF, Fu DG. Interfacial engineering of hierarchical transition metal oxide heterostructures for highly sensitive sensing of hydrogen peroxide. Small. 2018;14:1703713. doi: 10.1002/smll.201703713. [DOI] [PubMed] [Google Scholar]
- 50.Zhang CM, Li L, Ju J, Chen W. Electrochemical sensor based on graphene-supported tin oxide nanoclusters for nonenzymatic detection of hydrogen peroxide. Electrochim Acta. 2016;210:181–189. doi: 10.1016/j.electacta.2016.05.151. [DOI] [Google Scholar]
- 51.Meichtry JM, Dillert R, Bahnemann DW, Litter MI. Application of the stopped flow technique to the TiO2-heterogeneous photocatalysis of hexavalent chromium in aqueous suspensions: comparison with O2 and H2O2 as electron acceptors. Langmuir. 2015;31:6229–6236. doi: 10.1021/acs.langmuir.5b00574. [DOI] [PubMed] [Google Scholar]
- 52.Lu JT, Zhang HW, Li S, Guo SS, Shen L, Zhou TT, Zhong H, Wu L, Meng QG, Zhang YX. Oxygen-vacancy-enhanced peroxidase-like activity of reduced Co3O4 nanocomposites for the colorimetric detection of H2O2 and glucose. Inorg Chem. 2020;59:3152–3159. doi: 10.1021/acs.inorgchem.9b03512. [DOI] [PubMed] [Google Scholar]
- 53.Jia WZ, Guo M, Zheng Z, Yu T, Rodriguez EG, Wang Y, Lei Y. Electrocatalytic oxidation and reduction of H2O2 on vertically aligned Co3O4nanowalls electrode: toward H2O2 detection. J Electroanal Chem. 2009;625:27–32. doi: 10.1016/j.jelechem.2008.09.020. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Fig. S1. XRD patterns of Co2P NPs synthesized with different reaction times at 200 °C. Fig. S2. XPS survey spectrum of Co2P. Fig. S3. EDX spectra of Co2P NPs. Fig. S4. Amperometric responses of Co2P/ITO electrodes prepared at (a) different temperatures and (c) different times with successive addition of H2O2 in 0.1 M PBS. (b), (d) The calibration curve of steady current versus the concentration of H2O2. Fig. S5. The linear relationship between current density and concentration of H2O2 in different concentration ranges (a) 0.0001–1.0 mM, (b) 1.0–5.0 mM, (c) 5.0–10.0 mM. Fig. S6. Comparison of electrochemical properties between Co2P and Co(PO3)2. (a) LSV curves of Co2P and Co(PO3)2 modified electrode in 0.1 M PBS with and without 2.5 mM H2O2 at a scan rate of 100 mV s−1. (b) Nyquist plots of bare ITO, Co2P/ITO and Co(PO3)2/ITO electrode (electrolyte: 5.0 mM K3[Fe(CN)6]/ K4[Fe(CN)6] and 0.1 M KCl; bias: open circuit potential, amplitude: 5 mV, frequency range: 100 kHz ~ 0.01 Hz). Fig. S7. The linear relationship between current density and concentration of H2O2 in the physiological range. Fig. S8. CVs for Co2P/ITO electrode in 0.1 M PBS with or without N2 purging at a scan rate of 100 mV s−1. Fig. S9. CV responses at a scan rate of 100 mV s−1 in 0.1 M PBS containing 0.1 mM H2O2 of a Co2P/ITO electrode before and after being stored in air for one month. Table S1. The comparison on H2O2 sensing performance of the bare ITO electrode and the prepared Co2P sample at various reaction temperature.
Data Availability Statement
All data and materials are fully available without restriction.