Abstract
Background
CPAP effectiveness is limited by suboptimal adherence. Prior studies of adherence have focused on middle-aged men.
Research Question
Does CPAP adherence vary by age and sex?
Study Design and Methods
Telemonitoring data from a CPAP manufacturer database were used to assess adherence in patients initiating CPAP therapy between November 2015 and October 2018. Analyses were restricted to patients in the United States aged 18 to 90 years.
Results
Across 789,260 patients initiated on CPAP (mean age, 55 ± 14 years; 58.2% male), overall adherence by US Centers of Medicare & Medicaid Services criteria was 72.6%, but it varied dramatically by age and sex, ranging from 51.3% in 18- to 30-year-old women to 80.6% in 71- to 80-year-old men. Patterns of use over the first 90 days demonstrated that younger age groups had peak CPAP use by the 2nd night, with a subsequent decay in use, including abandonment of CPAP, which was greatest among 18- to 30-year-old women. In contrast, older patients steadily increase use, taking more than a week to maximize usage, and then they have much slower decays in use over time. Younger, but not older, patients have lower use of CPAP on weekends compared with weekday nights.
Interpretation
CPAP adherence rates vary substantially by demographics, with 18- to 30-year-old women having the lowest adherence. The pattern of use over the first 90 days also varies substantially by age and sex. Further research to understand and address the causes of disparities will be crucial to maximizing the benefits of CPAP therapy.
Key Words: adherence, CPAP, disparity, sleep apnea
Abbreviations: CMS, Centers for Medicare & Medicaid Services; DME, durable medical equipment
OSA is a common disorder that adversely impacts sleep quality and daytime alertness as well, resulting in increased risk for motor vehicle accidents and cardiovascular disease.1 The most common OSA therapy, CPAP, is highly efficacious in normalizing breathing, but its effectiveness in improving health outcomes is limited by adherence.
Adherence to CPAP among research participants varies from 17% to 71%.2 The cause of this heterogeneity is unclear, and evidence is conflicting regarding whether demographic factors such as age and sex influence adherence. A systematic review over a 20-year period reported mean CPAP usage across studies of 4.5 h/night.3 These data are limited in generalizability, however, because all of the CPAP users were participating in clinical research.
In 2008, the US Centers for Medicare & Medicaid Services (CMS) instituted a policy whereby long-term coverage for CPAP is denied if patients do not meet an adherence threshold of ≥4 hours of use on 70% of nights in a consecutive 30-day period within the first 90 days. This policy has been subsequently adopted by most private US insurers, leading durable medical equipment (DME) providers to implement widespread telemonitoring of CPAP adherence with early troubleshooting to achieve CMS adherence thresholds. Recent data from a large clinical cohort suggest that 74.6% of patients initiated on CPAP now meet CMS adherence criteria.4 How adherence varies across demographic groups remains unclear. The goal of this work was to understand the distribution of adherence in a contemporary clinical population of patients initiating CPAP in the United States, including how adherence varies by age and sex.
Methods
Our sample consisted of individuals who had been registered in a large cloud-based database of CPAP therapy, Encore Anywhere (Philips Respironics). Demographic data were entered by the DME company caring for each patient. We limited our analyses to patients who began using a Philips machine between November 1, 2015 and October 31, 2018, because this is a time frame during which DME companies were regularly using Encore Anywhere to monitor all patients as standard of care. To the extent possible, we excluded those who had previously used positive airway pressure based on duplicate patient records. We limited the study population to those who were initiated on either fixed or auto-titrating CPAP, so as to prevent inclusion of patients with diseases other than OSA. We included only patients with at least 30 seconds of usage to prevent inclusion of accounts created as a demonstration or in error. We further limited the study population to patients who had a valid date of birth, sex, and zip code, and restricted to those aged 18 to 90 years and in a zip code within the 50 US states plus the District of Columbia.
A deidentified dataset was generated by Philips and transmitted to the University of Pittsburgh for statistical analysis. Because of the deidentified nature of the dataset, the University of Pittsburgh institutional review board deemed this research to be exempt from human subjects research review.
Nightly usage was used to calculate mean adherence across the first 90 days as well as to determine whether each individual met CMS adherence criteria. Dates with missing usage were input as 0 hours. A weekly trend was assessed by categorizing each day of use by day of the week. Age was categorized by decade and sex as men and women. Mean adherence levels as well as proportion meeting minimal adherence thresholds were estimated for each age and sex stratum where results were standardized to the overall sex distribution for each age stratum and to the overall age distribution for each sex.5
Summary adherence data were calculated for each individual and then mixed-effects linear regression was performed to assess the impact of age and sex on mean adherence levels, where DME provider was modeled as a random effect. Similarly, mixed-effects logistic regression was used to assess the impact of age and sex on achieving CPAP adherence based on CMS criteria, with DME provider modeled as a random effect. We used the sandwich variance estimator for this random effect because it is robust to distributional assumptions.6 For temporal data, multilevel modeling was used to model daily usage as a function of day of the week, clustering on individual to account for within-subject correlation. Effect modification was evaluated both by assessing the magnitude of age-by-sex and age-by-day-of-week interaction terms as well as by conducting analyses stratified by age, by sex, and by day of the week. Likelihood ratio tests were used to formally test the statistical significance of interaction comparing models with and without interaction terms. Because the amount of missing demographic data varied greatly by DME provider, sensitivity analyses were conducted, limited to patients of those DME providers where fewer than 10% of patient accounts had missing date of birth, sex, or zip code. Given the very large sample size, we focus on effect sizes and CIs rather than P values in interpreting differences.7 All analyses were conducted in Stata 16.0 (StataCorp; College Station, TX).
Results
Between November 1, 2015 and October 31, 2018, 1,666,927 patients in the United States had a first CPAP account in the Encore database with at least 30 seconds of use. Of these, 161,859 accounts were excluded because of missing or out-of-range age, 521,585 because of missing sex, and 194,223 because of missing or invalid zip code. Thus, data from 789,260 patients cared for by 1,523 DME providers were included in this analysis. Mean (SD) age of this cohort was 55 (14) years, with 58.3% men. Sensitivity analyses focused on the 221,471 patients from 323 DME providers where the rate of any missing demographic data was below 10% (mean missingness in these providers was 4.3%).
Overall, mean (SD) nightly usage of CPAP at 7 days and 90 days was 4.8 (2.6) hours and 4.7 (2.6) hours, respectively. A total of 72.6% of patients met CMS adherence criteria. Among those patients cared for by DME providers with low rates of missing demographic data, the CMS adherence rate was 73.7%.
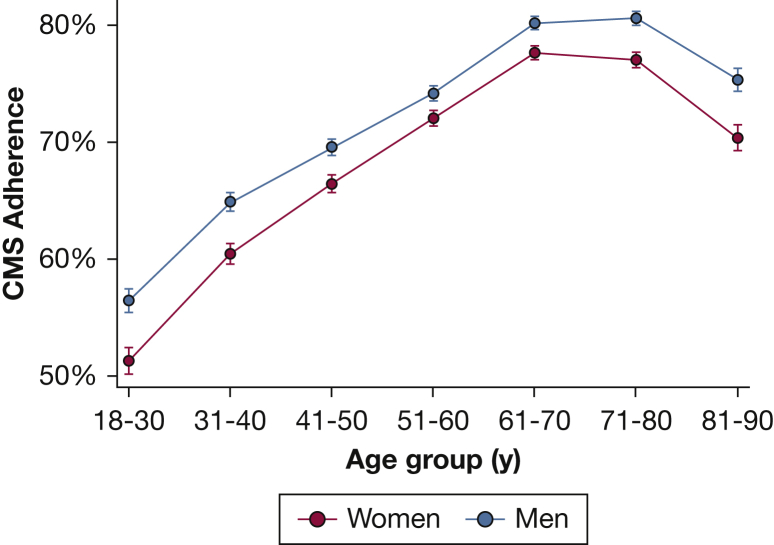
Figure 1 displays the proportion of patients achieving CMS adherence criteria as a function of age and sex, with adherence rates ranging from 80.6% in 71- to 80-year-old men to 51.3% in 18- 30-year-old women. The proportion of individuals meeting CMS adherence criteria increases substantially from 54.7% in those aged 18 to 30 years to 79.0% in those aged 61 to 70 years, and then falls slightly to 73.1% in those aged 81 to 90 years. In age-standardized analyses, the proportion of women achieving CMS adherence was lower than that for men (71.3% vs 73.2%). This difference existed across ages but was more marked at younger ages (P < .001 for age-by-sex interaction). Among 51- to 60-year-olds, the absolute difference in the proportion achieving CMS adherence was 2.2% (72.0% in women vs 74.2% in men) but in those younger than 30 years of age, the absolute difference was 5.2% (51.3% in women vs 56.5% in men). A very similar pattern of adherence by age, sex, and age by sex interaction was observed in the sensitivity analysis (e-Fig 1), in which the sex difference in the proportion of 51- to 60-year-olds achieving CMS adherence was 2.1% (73.4% in women vs 75.5% in men) compared with 9.3% in those younger than 30 (50.7% in women vs 60.0% in men).
Figure 1.
CPAP adherence rates by age and sex. The proportion of patients meeting CMS criteria (4 or more hours of use per night on 70% of nights in a consecutive 30-day period within the first 90 days) for long-term CPAP coverage by age group plotted separately for women and men. Error bars display 95% CIs. Results displayed are the output from mixed-effects logistic regression models accounting for durable medical equipment provider. CMS = Centers for Medicare & Medicaid Services.
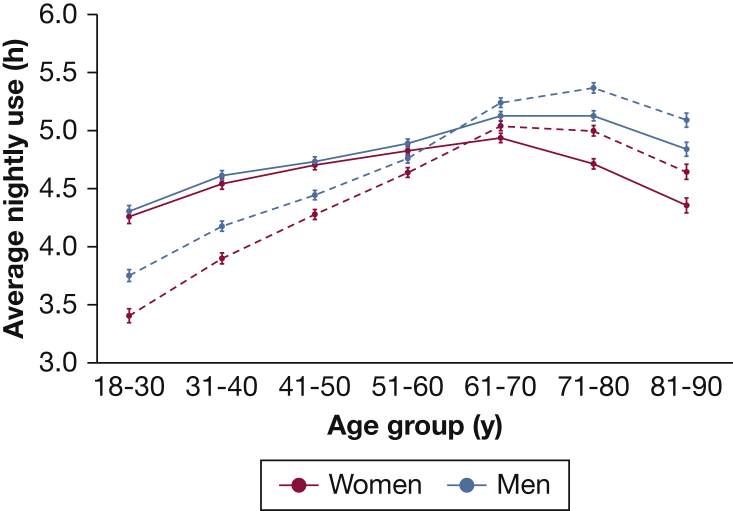
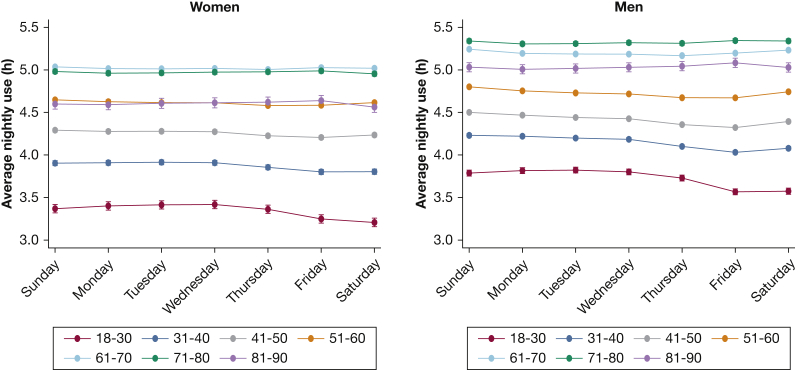
Mean hours of CPAP use also varied substantially by age and sex, with 90-day nightly average ranging from 5.4 hours in 71- to 80-year-old men to 3.4 hours in 18- to 30-year-old women (Fig 2). Average nightly use evolved in substantially different patterns by age and sex, going from the initial 7 days to 90 days. Both younger and older age groups had lower nightly CPAP use at 7 days compared with 61- to 70-year-olds. Over 90 days, however, the differences between 61- to 70-year-olds and older age groups diminished, suggesting that those older than age 70 take longer than a week to become fully proficient with CPAP. In contrast, the reduced usage in younger age groups at 7 days further declined over time, leading to even larger differences at 90 days relative to 61- to 70-year-olds. In the initial 7 days, sex differences in usage were minimal up to age 60, but in older age groups, women had substantially lower CPAP use than men (4.4 h in women vs 4.8 h in men aged 81 to 90 years). By 90 days, both older women and men have improved usage, although the sex disparity persisted (mean 90-day use among 81- to 90-year-olds was 4.6 h in women vs 5.1 h in men). In contrast, among younger age groups, where only small sex differences existed at 7 days, there was a widening gap between women and men by 90 days. Among 18- to 30-year-olds, mean use at 7 days was 4.3 hours in both women and men, whereas mean use at 90 days was 3.4 hours in women vs 3.8 hours in men.
Figure 2.
Evolution of CPAP use from 7 days to 90 days by age and sex. Average CPAP use (h/night) by age group plotted separately for men and women over the first 7 days (solid line) and 90 days (dashed line). Error bars display 95% CIs. Results displayed are the output from mixed-effects linear regression models, accounting for durable medical equipment provider.
To better understand demographic differences in CPAP usage, we also explored the proportion of low adherers (<1 h/night), which represents people effectively abandoning CPAP. A strong U-shaped age relationship was observed in the likelihood of being a low adherer at 90 days, with the risk lowest in 61- to 70-year-olds (e-Fig 2). Young women in particular were the most likely to be low adherers (25.2% of 18- to 30-year-old women at 90 days), and in general, the sex disparity in being a low adherer was greater at both ends of the age spectrum.
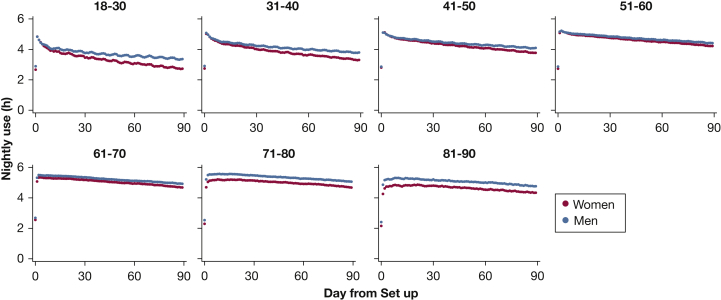
Figure 3 displays mean nightly CPAP use over the first 90 days by age and sex. In those younger than age 60, CPAP use increases rapidly, peaking by night 2 and then decaying to give a concave contour. The most rapid decay was observed in women aged 18 to 30 years. In older age groups, CPAP use trajectory has a convex contour, increasing more gradually than in younger age groups, peaking as late as a week after initiation, and then declining much more gradually than for younger age groups as well. The observed decline in nightly usage between day 7 and day 90 ranged from 1.4 (95%CI, 1.4-1.5) hours in 18- to 30-year-old women to only 0.4 (95%CI, 0.3-0.5) hours in 81- to 90-year-old women.
Figure 3.
Average nightly CPAP use over the first 90 days plotted by age and sex. Nightly use (h) of CPAP over each of the first 90 days for each age and sex category. Results displayed are unadjusted.
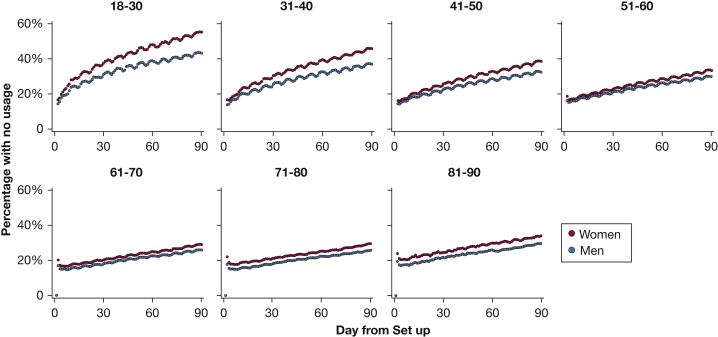
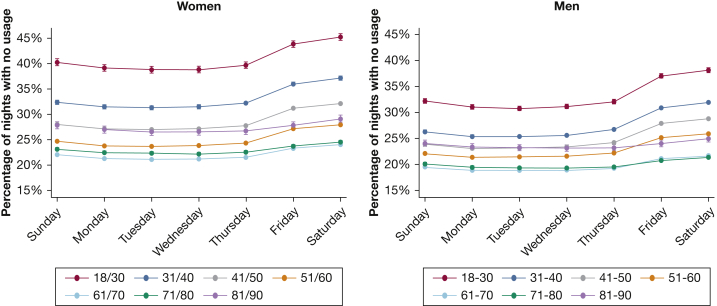
Figure 4 displays the proportion of individuals in each age and sex category with no use of CPAP on each night over the first 90 days. Across all groups, a steady rise in abandonment of CPAP occurs with time, but the rate of increase in non-use varies considerably, being steepest in younger age groups. Furthermore, the disparity between women and men in slopes is also much greater in younger ages. Only 44.8% of women aged 18 to 30 years turned on their CPAP machine by day 90.
Figure 4.
Non-use of CPAP over the first 90 days plotted by age and sex. Proportion with no use of CPAP over each night of the first 90 days for each age and sex category. Results displayed are unadjusted.
CPAP use was lower on weekends than on weeknights over the first 90 days, with highest use on Sunday nights and lowest use on Friday nights (Fig 5). These effects varied significantly by age (P < .001 for age-by-day of week interaction), which is also evident by observing the scalloped pattern in Figures 3 and 4 that is more pronounced in 18- to 30-year-olds and becomes gradually less obvious with increasing age. There was virtually no variation across the week among those older than age 60 years. In contrast, among those aged 18 to 30 years, use varied from 3.7 hours on Tuesdays to 3.4 hours on Saturdays. Weekend effects were similar in women and men. A similar pattern was observed for likelihood of no CPAP use, which varied from 33.6% on Tuesday nights to 40.6% on Saturday nights in those aged 18 to 30 years (Fig 6). These patterns were virtually identical in sensitivity analyses restricting to those DME providers with low missing data rates (e-Figs 3, 4).
Figure 5.
Average nightly CPAP use by day of week. Mean nightly use (h) of CPAP over the first 90 days by day of the week plotted by age and stratified by sex. Error bars display the 95% CIs. Results displayed are model estimates accounting for within-subject clustering.
Figure 6.
Non-use of CPAP by day of week. Percentage of nights where CPAP was not used over the first 90 days by day of the week plotted by age and stratified by sex. Error bars display the 95% CIs. Results displayed are model estimates accounting for within-subject clustering.
Discussion
Overall, in this analysis of 789,260 patient records, we found that CPAP adherence over the first 90 days varies substantially by age and sex, ranging from 51.3% in 18- to 30-year-old women up to 80.3% in 71- to 80-year-old men. Given insurance coverage policies regarding CPAP, this suggests the proportion of young women being denied long-term CPAP therapy is more than double the proportion among older men.
Interestingly, the patterns of use over the first 90 days also vary by demographics. Younger age groups quickly achieve their maximal CPAP use by the second night but then have relatively steep declines in adherence. In contrast, older age groups take up to a week to master regular use of CPAP. From that point, usage remains fairly consistent over time with only slight declines out to 90-days. Prior research on how CPAP adherence varies by age has been mixed8, 9, 10; however, these past studies have been limited in reporting results from single academic medical centers with relatively small sample sizes. The largest prior study, including 4,281 patients across multiple sites in Germany, reported greater long-term adherence in patients older than age 60 years but did not examine patterns over time.11
Our current understanding of CPAP adherence suggests that adherence can be conceived as the interplay between self-efficacy, treatment expectancy, and risk perception.12 One of the potential explanations for reduced usage at younger ages is the lower adherence to CPAP on weekends vs weeknights. This variability in younger patients may be related to employment, whereby retired individuals have more regular sleep schedules across the week. Because working adults are more likely to sleep longer on weekends, one might hypothesize that CPAP use would be longer on weekends; however, we find the opposite result. This suggests that the decisional balance to use CPAP shifts substantially toward nonadherence on weekends in younger age groups. Potential reasons for this change include the salience attached to socializing and staying out late on weekends, resulting in not having a regular bedtime and bedtime routine, sleeping away from home, and increased partner intimacy on weekends.
Similarly, decisional balance on using CPAP may differ because of age differences in the presence of a supportive bed partner. Younger age groups are more likely to be in less secure relationships, in which use of CPAP may be embarrassing. In contrast, older patients are more likely to be in stable relationships with a partner who is supportive and encouraging of CPAP use.13 This social support may be lost at the oldest ages, where patients are more likely to be widowed. Other possibilities for decreased use at the oldest age groups are difficulties in operating or caring for CPAP. Our finding that peak CPAP usage happens much later in older patients is congruent with the notion that older patients may have lower self-efficacy with CPAP use, taking longer to master the technology in a modern CPAP device. Possibly the weaker relationship between OSA and sleepiness at older ages14 may result in decreased symptomatic responses to CPAP therapy, leading to decreased positive feedback to continued use. In addition, not only may actual symptom response differ but outcome expectations may differ. To the extent that clinicians frame treatment benefits around sleepiness or other symptoms that are most salient to middle-aged men, expectations of the benefits to be gained from CPAP may vary widely by age and sex and contribute to the adherence differences observed.
In addition to age differences, we found a small but consistently reduced level of adherence in women compared with men across all ages, but this difference was magnified at extremes of age. Interestingly, the time course of the sex disparity also varies by age. In those younger than age 30, women have rates of use nearly identical to those of men during the first week, but their usage declines much more steeply than that for men over time. This steeper decline is associated with a larger proportion of young women abandoning CPAP use. The pattern seen in those older than age 70 years was much different. Women had substantially reduced usage of CPAP in the first week, and this disparity remained fairly constant out to 90 days.
Prior research has not found a consistent disparity in CPAP use by sex.8, 9, 10 This may be due to relatively small sample sizes as well as a narrow age range focused on middle-aged populations, whereas our data suggest that the sex disparity is magnified at younger and older ages. Nevertheless, the largest prior study examining sex differences in CPAP did find a similar reduction in CPAP adherence among women compared with men.11 Reasons for the sex disparity cannot be fully elucidated from this analysis, but a number of explanations are possible. To the extent that OSA is viewed as a male disease, women may have greater reluctance to admit they have the disease and so may be less accepting of treatment. Societal expectations that place a greater emphasis on the appearance of women also may adversely impact the decisional balance for women such that they are less accepting of a treatment that may be viewed as unattractive. Concerns about image change while wearing CPAP have been identified as a barrier to CPAP adherence.15 Specific challenges related to CPAP use such as claustrophobia may be more common in women than men.16 Another possibility is that the symptoms more commonly associated with OSA in women, such as insomnia and fatigue, may be less responsive to CPAP therapy, preventing the positive feedback from symptom resolution. Again, this difference in symptoms also may lead to differences in outcome expectations if clinicians frame treatment benefits around male symptoms. Differences in OSA pathophysiology also may explain our findings, because the OSA phenotype differs by sex, with women not only tending to have lower severity as assessed by the apnea-hypopnea index, but also greater evidence of rapid eye movement predominant disease as well as more flow-limited events that do not meet standard criteria for scoring hypopneas.17,18 These differences may lead to disparities in the ability of clinicians to identify the optimal CPAP settings to normalize breathing during sleep in women as well as differences in efficacy of auto-titrating algorithms.19
One important finding of our work was that women made up over 40% of those initiated on CPAP in this large database. This is a far more balanced sex distribution compared with that reported in patients referred for clinical evaluation in studies of patients 20 to 30 years ago,20,21 suggesting that attempts to increase clinician awareness about the existence of OSA in women have been effective. Unfortunately, suboptimal adherence likely results in continued disparities in OSA-related health outcomes.
Our findings provide novel insights into the epidemiology of CPAP adherence by identifying demographic groups who have systematically lower adherence rates. These groups—both younger and elderly age groups as well as women—have been traditionally underrepresented in OSA and CPAP adherence research. This work highlights the need to understand the specific challenges faced by these groups and develop strategies designed to address those obstacles. For example, the steep decline in CPAP use in younger patients suggests that interventions to increase motivation need to occur almost immediately after initiation in this group. Similarly, our finding of decreased adherence on weekends among younger patients suggests that troubleshooting interventions that operate only on weekdays are less likely to be effective in this age group. Just as behavioral weight loss interventions include specific strategies to address the challenges of eating out,22 behavioral interventions for CPAP may benefit by explicitly addressing the challenges of using CPAP when going out.
The overall level of adherence observed in this study matches closely the proportion reported in a contemporaneous large national cohort.4 These rates above 70% contrast substantially with data from earlier periods. A recently published analysis reported a CMS adherence rate of only 43% of patients initiating positive airway pressure therapy from 2000 to 2016.23 This timeframe is before or during the period of institution and enforcement of CPAP adherence requirements by CMS and then adoption by private insurers. In addition, before 2015, the use of remote monitoring was not uniform practice, with many DME providers reserving this technology only in higher-risk patients, creating biases that would decrease observed adherence rates.
Although there are many strengths to this work, including the large, nationally representative population that allows for adequate assessment of relatively small subgroups, limitations should be noted. Chief among these is the limited information regarding factors such as disease severity, symptoms, prior OSA treatments such as surgery, race, and socioeconomic status, which all may impact CPAP adherence. Future research merging electronic health records with CPAP datasets would allow for a more comprehensive understanding of the factors associated with adherence. In addition, our findings are limited to users of only one brand of CPAP, although it should be noted that a recent report of 90-day adherence from a competing manufacturer reported very similar population-level adherence rates.4 Furthermore, our analyses were limited to the first 90 days of CPAP use, because this timeframe corresponds to CMS criteria for long-term coverage determination. Modeling adherence patterns over extended timeframes would be helpful in understanding predictors of long-term adherence but this is beyond the scope of this analysis. Although potential for selection bias exists in the substantial portion of individuals missing demographic data, this missingness is due to procedures in place at the level of the DME provider rather than individual patient factors, lessening the potential for bias unless DME providers served different population demographics. In fact, our sensitivity analyses restricting to those DME providers with minimal missing data demonstrate the same overall patterns, suggesting that our findings are robust.
In summary, short-term adherence rates to CPAP vary widely by age and sex, with the lowest rates observed in young women. Substantial differences in patterns of use are seen over the first weeks by age and sex, suggesting the need for individualizing interventions to maximize adherence.
Acknowledgments
Author contributions: S. R. P. conceived the study. S. M. N. did the statistical analysis. S. R. P. wrote the first draft of the manuscript. M. S. A., J. P. B., and C. J. S. were involved with study design, data interpretation, and manuscript preparation. All authors critically reviewed this draft for important intellectual content and read and approved the final version. S. R. P. and S. M. N. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: S. R. P. has received grant funding through his university from Bayer Pharmaceuticals, Philips Respironics, and Respicardia. J. P. B., C. J. S., and M. S. A. are employees of Philips Respironics. None declared (S. M. N.).
Role of sponsors: This work was funded by a grant from Philips Respironics as well as the National Institutes of Health (NIH) HL127307. Philips Respironics collected the data used in this report as part of its business operations but was not involved in the study design, interpretation of data, manuscript preparation, or decision to submit for publication. Drs Aloia and Bakker and Ms Stitt, all employees of Philips Respironics, were involved with study design, interpretation of data, and manuscript preparation. Philips Respironics did review and approve the final manuscript prior to publication. NIH had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Additional information: The e-Figures can be found in the Supplemental Materials section of the online article.
Footnotes
FUNDING/SUPPORT: This work was funded by a grant from Philips Respironics and National Institutes of Health grant HL127307.
Supplementary Data
References
- 1.Veasey S.C., Rosen I.M. Obstructive sleep apnea in adults. N Engl J Med. 2019;380(15):1442–1449. doi: 10.1056/NEJMcp1816152. [DOI] [PubMed] [Google Scholar]
- 2.Weaver T.E., Grunstein R.R. Adherence to continuous positive airway pressure therapy: the challenge to effective treatment. Proc Am Thorac Soc. 2008;5(2):173–178. doi: 10.1513/pats.200708-119MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rotenberg B.W., Murariu D., Pang K.P. Trends in CPAP adherence over twenty years of data collection: a flattened curve. J Otolaryngol Head Neck Surg. 2016;45(1):43. doi: 10.1186/s40463-016-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cistulli P.A., Armitstead J., Pepin J.L. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med. 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tripepi G., Jager K.J., Dekker F.W., Zoccali C. Stratification for confounding—part 2: direct and indirect standardization. Nephron Clin Pract. 2010;116(4):c322–c325. doi: 10.1159/000319591. [DOI] [PubMed] [Google Scholar]
- 6.Zeger S.L., Liang K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42(1):121–130. [PubMed] [Google Scholar]
- 7.Lederer D.J., Bell S.C., Branson R.D. Control of confounding and reporting of results in causal inference studies: guidance for authors from editors of respiratory, sleep, and critical care journals. Ann Am Thorac Soc. 2019;16(1):22–28. doi: 10.1513/AnnalsATS.201808-564PS. [DOI] [PubMed] [Google Scholar]
- 8.Sin D.D., Mayers I., Man G.C., Pawluk L. Long-term compliance rates to continuous positive airway pressure in obstructive sleep apnea: a population-based study. Chest. 2002;121(2):430–435. doi: 10.1378/chest.121.2.430. [DOI] [PubMed] [Google Scholar]
- 9.Pelletier-Fleury N., Rakotonanahary D., Fleury B. The age and other factors in the evaluation of compliance with nasal continuous positive airway pressure for obstructive sleep apnea syndrome: a Cox's proportional hazard analysis. Sleep Med. 2001;2(3):225–232. doi: 10.1016/s1389-9457(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 10.Budhiraja R., Parthasarathy S., Drake C.L. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 11.Woehrle H., Graml A., Weinreich G. Age- and gender-dependent adherence with continuous positive airway pressure therapy. Sleep Med. 2011;12(10):1034–1036. doi: 10.1016/j.sleep.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 12.Weaver T.E., Maislin G., Dinges D.F. Self-efficacy in sleep apnea: instrument development and patient perceptions of obstructive sleep apnea risk, treatment benefit, and volition to use continuous positive airway pressure. Sleep. 2003;26(6):727–732. doi: 10.1093/sleep/26.6.727. [DOI] [PubMed] [Google Scholar]
- 13.Gentina T., Bailly S., Jounieaux F. Marital quality, partner's engagement and continuous positive airway pressure adherence in obstructive sleep apnea. Sleep Med. 2019;55:56–61. doi: 10.1016/j.sleep.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 14.Morrell M.J., Finn L., McMillan A., Peppard P.E. The impact of ageing and sex on the association between sleepiness and sleep disordered breathing. Eur Respir J. 2012;40(2):386–393. doi: 10.1183/09031936.00177411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye L., Antonelli M.T., Willis D.G., Kayser K., Malhotra A., Patel S.R. Couples' experiences with continuous positive airway pressure treatment: a dyadic perspective. Sleep Health. 2017;3(5):362–367. doi: 10.1016/j.sleh.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edmonds J.C., Yang H., King T.S., Sawyer D.A., Rizzo A., Sawyer A.M. Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung. 2015;44(2):100–106. doi: 10.1016/j.hrtlng.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anttalainen U., Tenhunen M., Rimpila V. Prolonged partial upper airway obstruction during sleep: an underdiagnosed phenotype of sleep-disordered breathing. Eur Clin Respir J. 2016;3:31806. doi: 10.3402/ecrj.v3.31806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Connor C., Thornley K.S., Hanly P.J. Gender differences in the polysomnographic features of obstructive sleep apnea. Am J Respir Crit Care Med. 2000;161(5):1465–1472. doi: 10.1164/ajrccm.161.5.9904121. [DOI] [PubMed] [Google Scholar]
- 19.McArdle N., King S., Shepherd K. Study of a novel APAP algorithm for the treatment of obstructive sleep apnea in women. Sleep. 2015;38(11):1775–1781. doi: 10.5665/sleep.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guilleminault C., Quera-Salva M.A., Partinen M., Jamieson A. Women and the obstructive sleep apnea syndrome. Chest. 1988;93(1):104–109. doi: 10.1378/chest.93.1.104. [DOI] [PubMed] [Google Scholar]
- 21.Redline S., Kump K., Tishler P.V., Browner I., Ferrette V. Gender differences in sleep disordered breathing in a community-based sample. Am J Respir Crit Care Med. 1994;149(3 Pt 1):722–726. doi: 10.1164/ajrccm.149.3.8118642. [DOI] [PubMed] [Google Scholar]
- 22.Diabetes Prevention Program Research G. The Diabetes Prevention Program (DPP): description of lifestyle intervention. Diabetes Care. 2002;25(12):2165–2171. doi: 10.2337/diacare.25.12.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pandey A., Mereddy S., Combs D. Socioeconomic inequities in adherence to positive airway pressure therapy in population-level analysis. J Clin Med. 2020;9(2) doi: 10.3390/jcm9020442. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.