Key Points
Question
In freshly retrieved donor oocyte cycles, are fresh embryo transfers more likely to result in live birth than cryopreserved-thawed embryo transfers?
Findings
In this retrospective cohort study that included 33 863 women undergoing assisted reproduction using freshly retrieved donor oocytes, with 51 942 embryo transfer cycles, fresh embryo transfers compared with cryopreserved-thawed embryo transfers resulted in a live birth rate of 56.6% vs 44.0%; the adjusted difference was statistically significant.
Meaning
In cycles using fresh donor oocytes, fresh embryo transfers compared with cryopreserved-thawed embryo transfers were associated with a higher live birth rate, but interpretation of the findings is limited by the potential for selection and confounding bias.
Abstract
Importance
In in vitro fertilization cycles using autologous oocytes, data have demonstrated higher live birth rates following cryopreserved-thawed embryo transfers compared with fresh embryo transfers. It remains unknown if this association exists in cycles using freshly retrieved donor oocytes.
Objective
To test the hypothesis that in freshly retrieved donor oocyte cycles, a fresh embryo transfer is more likely to result in a live birth compared with a cryopreserved-thawed embryo transfer.
Design, Setting, and Participants
Retrospective cohort study using national data collected from the Society for Assisted Reproductive Technology for 33 863 recipients undergoing fresh donor oocyte cycles in the US between January 1, 2014 and December 31, 2017.
Exposures
Fresh embryo transfer and cryopreserved-thawed embryo transfer.
Main Outcomes and Measures
The primary outcome was live birth rate; secondary outcomes were clinical pregnancy rate and miscarriage rate. Analyses were adjusted for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching.
Results
Recipients of fresh and cryopreserved-thawed embryos had comparable median age (42.0 [interquartile range {IQR}, 37.0-44.0] years vs 42.0 [IQR, 36.0-45.0] years), gravidity (1 [IQR, 0-2] vs 1 [IQR, 0-3]), parity (0 [IQR, 0-1] vs 1 [IQR, 0-1]), and body mass index (24.5 [IQR, 21.9-28.7] vs 24.4 [IQR, 21.6-28.7]). Of a total of 33 863 recipients who underwent 51 942 fresh donor oocyte cycles, there were 15 308 (29.5%) fresh embryo transfer cycles and 36 634 (70.5%) cryopreserved-thawed embryo transfer cycles. Blastocysts were transferred in 92.4% of fresh embryo transfer cycles and 96.5% of cryopreserved-thawed embryo transfer cycles, with no significant difference in the mean number of embryos transferred. Live birth rate following fresh embryo transfer vs cryopreserved-thawed embryo transfer was 56.6% vs 44.0% (absolute difference, 12.6% [95% CI, 11.7%-13.5%]; adjusted relative risk [aRR], 1.42 [95% CI, 1.39-1.46]). Clinical pregnancy rates were 66.7% vs 54.2%, respectively (absolute difference, 12.5% [95% CI, 11.6%-13.4%]; aRR, 1.34; [95% CI, 1.31-1.37]). Miscarriage rates were 9.3% vs 9.4%, respectively (absolute difference, 0.2% [95% CI, −0.4% to 0.7%]); aRR, 0.98 [95% CI, 0.91-1.07]).
Conclusions and Relevance
In this retrospective cohort study of women undergoing assisted reproduction using freshly retrieved donor oocytes, the use of fresh embryo transfers compared with cryopreserved-thawed embryo transfers was associated with a higher live birth rate. However, interpretation of the findings is limited by the potential for selection and confounding bias.
This cohort study uses US national registry data to compare the association of fresh vs cryopreserved-thawed embryo transfer and live birth rates among women undergoing assisted reproduction technology (ART) procedures using freshly retrieved donor oocytes between 2014 and 2017.
Introduction
The use of donor oocytes to achieve pregnancy has been steadily increasing in the US since its inception in 1984, accounting for 9.2% (24 300/263 577) of all nationally reported cycles in 2016.1,2,3 According to data from the Society for Assisted Reproductive Technology (SART), the live birth rate for donor oocyte cycles approached 40% to 50% by 2017.4
Data from the Centers for Disease Control and Prevention (CDC) indicated a marked decline in fresh embryo transfers from fresh donor oocytes, from a peak of approximately 11 000 cycles per year in 2007 to 5600 cycles in 2016, with a steady increase in the use of cryopreserved-thawed embryos from donor oocytes, approaching 13 500 cycles.2 In addition, frozen donor oocytes purchased through oocyte banks have become increasingly available, with a 60% increase (from 905 to 1447) in frozen donor oocyte cycles from 2016 to 2017.2 However, retrospective SART data have suggested that fresh donor oocytes perform better than frozen donor oocytes.5,6
The increasing popularity of cryopreserved-thawed embryo transfers in donor oocyte cycles may be partly driven by convenience, elimination of the need for synchronization between donor and recipient cycles, and/or increasing utilization of preimplantation genetic testing for aneuploidy (PGT-A).
In autologous in vitro fertilization (IVF) cycles, data from randomized trials and meta-analyses have demonstrated higher live birth rates following cryopreserved-thawed embryo transfers compared with fresh embryo transfers among women with high oocyte yield at retrieval.7,8 However, to date, no comparison of pregnancy outcomes has been made between transfers of fresh embryos and cryopreserved-thawed embryos derived exclusively from fresh donor oocytes.9 The hypothesis of this study was that, conversely, in cycles using embryos derived exclusively from freshly retrieved donor oocytes, a fresh embryo transfer is more likely to result in a live birth compared with a cryopreserved-thawed embryo transfer.
Methods
The Partners Human Research Committee, the institutional review board of Partners HealthCare, and the SART Research Committee approved this study (protocol number 2018P002386). Informed consent was not required from participants because this was a retrospective study in which no personally identifying information was collected.
Donor oocyte cycles from years 2014-2017 in the SART database were included. SART collects cycle information from 370 IVF clinics in the US, accounting for more than 95% of all assisted reproduction volume nationwide. Data from 2014-2017 are the most recent data available as there is always a 2-year lag in clinic reporting to capture live birth data for all cycles in the specified time frame. Significant advances, such as use of vitrification rather than slow-freezing techniques for embryo cryopreservation, and trophectoderm biopsy rather than cleavage-stage blastomere biopsy for preimplantation genetic testing, had already been widely adopted throughout SART-affiliated IVF clinics in the US by the year 2014. Thus, data from 2014-2017 reflect current clinical practice.
Participants
Women undergoing IVF cycles using freshly retrieved donor oocytes from an anonymous donor, without prior oocyte freezing, were included (eFigure 1 in the Supplement). Cycles in which the recipient’s age at the time of transfer was over 55 years were excluded.
Only cycles in which at least 1 embryo was available to transfer at either the cleavage or blastocyst stage were included. Cycles with and without PGT-A were included in the overall cohort.
Demographic characteristics of recipients, including age, gravidity, parity, infertility diagnosis, use of a gestational carrier, and race/ethnicity (which have been shown to affect IVF outcomes), were self-reported by patients to individual clinics, based on fixed categories, and reported by individual clinics to SART.10,11 Recipient body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) was recorded and reported by participating clinics. Use of assisted hatching (in which a small hole is made in the zona pellucida to biopsy a blastocyst for PGT or to potentially facilitate implantation of a day 3 embryo) and use of intracytoplasmic sperm injection for fertilization were recorded for each group. The day of embryo transfer and number of embryos transferred were recorded for each cycle.
Demographic characteristics of oocyte donors reported in the SART database were limited to age, when reported. Anti-Müllerian hormone levels, gravidity, parity, BMI, and race/ethnicity of donors were not included in the SART database. For this reason, only anonymous oocyte donors, recommended by the American Society for Reproductive Medicine (ASRM) to be young, healthy, and with sufficient ovarian reserve, were included in the study.12
Data on the type of recipient uterine preparation for the embryo transfer (ie, a programmed cycle in which exogenous estrogen and progesterone are given vs a natural cycle in which the embryo transfer is determined by spontaneous ovulation) were not available in the SART database. However, all fresh embryo transfers using freshly retrieved donor oocytes are obligate programmed cycles because of the necessity to synchronize recipient endometrium with donor stimulation. Similarly, programmed cycles are commonly used in cryopreserved-thawed embryo transfer cycles for women using donor oocytes, as the majority have diminished ovarian reserve and/or ovulatory dysfunction.
Exposures
The exposures were fresh embryo transfer vs cryopreserved-thawed embryo transfer in recipients of embryos derived from freshly retrieved donor oocytes.
Outcomes
The primary outcome was live birth, defined as the delivery of 1 or more live-born infants. The secondary outcomes were clinical pregnancy rate and miscarriage rate. Clinical pregnancy was defined as an intrauterine gestational sac visualized on ultrasound, or documentation of a live birth, miscarriage, or termination if ultrasound data were missing. Miscarriage rate was defined as loss of an intrauterine gestation prior to 20 weeks. These outcome definitions have been previously established by SART and the CDC.2,4,13
Statistical Analyses
Medians and interquartile ranges (IQRs) were calculated for baseline and cycle characteristics that were not normally distributed. Using log binomial regression models, relative risks (RRs) and 95% confidence intervals were calculated for the primary and secondary outcomes. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Crude and adjusted analyses were performed, controlling for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching. Missing data for baseline characteristics are documented herein. Cycles with missing data for variables included in the regression models were removed from those analyses. Sensitivity analyses for PGT-A were performed for all outcomes. A post hoc subgroup analysis was performed using the initial transfer for each recipient included in the cohort. Using log binomial regression, interactions were tested for fresh and cryopreserved-thawed cycles and use of PGT-A, as well as fresh and cryopreserved-thawed cycles and first embryo transfer or subsequent embryo transfer. SART data protect clinic anonymity; thus, statistical models were not able to account for site effect.
A significance level was set at α = .05. All appropriate tests were 2-sided. Analyses were performed using SAS version 9.4 (SAS Institute Inc).
Results
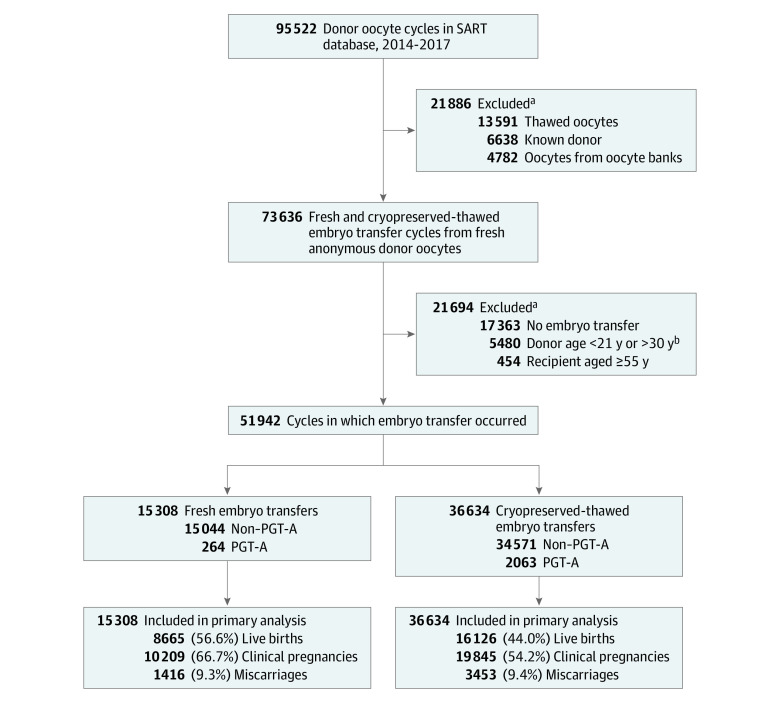
Recipients of fresh and cryopreserved-thawed embryos had comparable median age (42.0 [IQR], 37.0-44.0] years vs 42.0 [IQR, 36.0-45.0] years), gravidity (1 [IQR, 0-2] vs 1 [IQR, 0-3]), parity (0 [IQR, 0-1] vs 1 [IQR, 0-1]), and BMI (24.5 [IQR, 21.9-28.7] vs 24.4 [IQR, 21.6-28.7]). A total of 51 942 embryo transfer cycles from 33 863 women undergoing assisted reproduction with freshly retrieved donor oocytes were identified. Of these, 15 308 (29.5%) were fresh embryo transfer cycles and 36 634 (70.5%) were cryopreserved-thawed embryo transfer cycles, as shown in Figure 1.
Figure 1. Selection, Transfer Cycle Type, PGT-A, and Outcomes of Embryo Transfer Cycles Evaluated Through the Study.
PGT-A indicates preimplantation genetic testing for aneuploidy; SART, Society for Assisted Reproductive Technology.
aExclusions were not mutually exclusive.
bThe American Society for Reproductive Medicine recommends that anonymous donors be between 21 and 34 years of age. All included cycles used only anonymous oocyte donors. Cycles that were missing documentation of donor age were included. A total of 337 (2.2%) fresh embryo transfer cycles and 17 694 (48.3%) cryopreserved-thawed embryo transfer cycles were missing donor age data.
Donor age was missing in 34.7% of all cycles (2.2% of fresh embryo transfer cycles and 48.3% of cryopreserved-thawed embryo transfer cycles). Among cycles that recorded donor age, the mean donor age was 25.6 years in the fresh embryo transfer group and 25.5 years in the cryopreserved-thawed embryo transfer group. Demographic characteristics of recipients of fresh embryo transfers and cryopreserved-thawed embryo transfers are reported in Table 1. Cycles using donor oocytes along with a gestational carrier represented 14.9% of the cohort, including 1133 (7.4%) of the 15 308 cycles in the fresh embryo transfer group and 6624 (18.1%) of the 36 634 cycles in the cryopreserved-thawed embryo transfer group.
Table 1. Demographics and Cycle Characteristics of Recipientsa.
| Characteristics | Fresh embryo transfer (n = 15 308) | Cryopreserved-thawed embryo transfer (n = 36 634) |
|---|---|---|
| Age, y | n = 15 290 | n = 36 602 |
| Overall, median (IQR) | 42.0 (37.0-44.0) | 42.0 (36.0-45.0) |
| Age group, No. (%) | ||
| <35 | 2349 (15.4) | 7516 (20.5) |
| 35-37 | 1566 (10.2) | 3445 (9.4) |
| 38-40 | 2502 (16.4) | 4646 (12.7) |
| 41-42 | 2411 (15.8) | 4539 (12.4) |
| ≥43 | 6462 (42.3) | 16 456 (45.0) |
| Gravidity, median (IQR) | 1 (0-2) [n = 15 129] | 1 (0-3) [n = 34 622] |
| Parity, median (IQR) | 0 (0-1) [n = 8946] | 1 (0-1) [n = 25 320] |
| BMI | n = 11 321 | n = 22 365 |
| Overall, median (IQR) | 24.5 (21.9-28.7) | 24.4 (21.6-28.7) |
| Category, No. (%) | ||
| <18.5 | 235 (2.1) | 590 (2.6) |
| 18.5-24.9 | 5894 (52.1) | 11 608 (51.9) |
| 25-29.9 | 2946 (26.0) | 5744 (25.7) |
| 30.0-39.9 | 1976 (17.5) | 3891 (17.4) |
| ≥40 | 270 (2.4) | 532 (2.4) |
| Infertility diagnosis, No. (%)b | n = 15 308 | n = 36 634 |
| Diminished ovarian reserve | 11 722 (76.6) | 26 763 (73.1) |
| Male factor | 2556 (16.7) | 5302 (14.5) |
| Tubal factor | 965 (6.3) | 1967 (5.4) |
| Uterine factor | 853 (5.6) | 2729 (7.5) |
| Endometriosis | 793 (5.2) | 1709 (4.7) |
| Ovulatory | 385 (2.5) | 663 (1.8) |
| Unexplained | 663 (4.3) | 1392 (3.8) |
| Other | 2718 (17.8) | 9129 (24.9) |
| Gestational carrier, No. (%)c | 1133 (7.4) [n = 15 308] | 6624 (18.1) [n = 36 634] |
| Race/ethnicity, No. (%)d | n = 9831 | n = 22 367 |
| White | 6909 (70.3) | 14 852 (66.4) |
| Asian | 1318 (13.4) | 4333 (19.4) |
| Black/African American | 860 (8.7) | 1715 (7.7) |
| Hispanic/Latina | 703 (7.2) | 1347 (6.0) |
| Other | 41 (0.4) | 120 (0.5) |
| Day of transfer, No. (%)e | n = 14 071 | n = 28 622 |
| Day 3 | 1076 (7.7) | 1000 (3.5) |
| Day 3 and day 5/6 | 0 | 5 (0.02) |
| Day 5/6 | 12 995 (92.4) | 27 617 (96.5) |
| No. of embryos transferred, median (IQR) | 2 (1-2) [n = 15 308] | 1 (1-2) [n = 36 634] |
| Assisted hatching, No. (%)f | 3509 (22.9) [n = 15 301] | 25 701 (70.2) [n = 36 594] |
| Intracytoplasmic sperm injection, No. (%)g | 12 803 (83.6) [n = 15 308] | NA [n = 36 634] |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); IQR, interquartile range; NA, not available.
Percentages may not total to 100% on account of rounding.
Infertility diagnosis categories13 (multiple diagnoses can be made for 1 patient): “unexplained” includes patients who have completed an evaluation with no obvious explanation for their infertility; male factor refers to reduced sperm concentrations or other issues related to sperm function that make it difficult for a sperm to fertilize an oocyte under normal conditions; tubal factor refers to the fallopian tubes being blocked or damaged; ovulatory infertility refers to conditions in which the ovaries are not producing oocytes normally; infertility due to endometriosis is a medical condition that involves presence of tissue similar to the uterine lining in locations outside the uterus; diminished ovarian reserve refers to a reduced ability of the ovary to produce oocytes; uterine factor infertility refers to a structural or functional disorder of the uterus that results in reduced fertility; “other” incorporates diagnoses that do not meet criteria for any other category of infertility, such as women with a known chromosomal structural rearrangement or women with a history of malignancy that has led to infertility.
A gestational carrier is a woman who carries a pregnancy for intended parents when carrying a pregnancy is medically contraindicated or impossible for the intended mother.
Race and ethnicity categories were self-reported by patients to individual clinics. The category of “other” includes Native Hawaiian or other Pacific Islander, American Indian, and Alaska Native individuals as well as patients who reported “part” for their race/ethnicity.
Embryo transfer can occur at the cleavage stage of embryo development (day 3) or at the blastocyst stage of development (days 5/6). Rarely, a patient may have a transfer of a day 3 embryo followed by a transfer of a day 5/6 embryo. Pregnancy rates are higher with blastocyst transfer (day 5/6). Because only 30% to 50% of embryos develop into blastocysts, embryo transfer may be performed on day 3 for women with fewer available embryos to reduce the chance of cycle cancellation if no embryos survive to the blastocyst stage.
Assisted hatching involves creating a small opening in the zona pellucida to biopsy a blastocyst or to potentially facilitate implantation of a day 3 embryo. Within the time frame of the study, assisted hatching was commonly performed prior to transferring cryopreserved-thawed embryos.
The use of intracytoplasmic sperm injection, in which the sperm is directly injected into the oocyte for fertilization, was reported only in fresh embryo transfer cycles and was not available for cryopreserved-thawed embryo transfer cycles.
Recipient age data were missing in 18 (0.1%) fresh and 32 (0.1%) cryopreserved-thawed embryo transfer cycles. Recipient BMI data were missing in 3987 (26.0%) fresh and 14 269 (39.0%) cryopreserved-thawed embryo transfer cycles. Race/ethnicity data were missing in 5477 (35.8%) fresh and 14 267 (38.9%) cryopreserved-thawed transfer cycles. Day-of-transfer data were missing in 1237 (8.1%) fresh and 8012 (21.9%) cryopreserved-thawed embryo transfer cycles.
Blastocysts were transferred in 92.4% of fresh embryo transfer cycles and 96.5% of cryopreserved-thawed embryo transfer cycles. There was no significant difference in the mean number of embryos transferred in the fresh embryo transfer group (1.5 [SD, 0.5]) and cryopreserved-thawed embryo transfer group (1.4 [SD, 0.5]). The absolute difference was 0.1% (95% CI, 0.09%-0.11%). These data were not normally distributed; the median number of embryos transferred was 2 (IQR, 1-2) for the fresh and 1 (IQR, 1-2) for the cryopreserved-thawed embryo transfer groups, as presented in Table 1.
Assisted hatching was performed more frequently in the cryopreserved-thawed embryo transfer group at 70.2% (25 701/36 594), compared with 22.9% (3509/15 301) in the fresh embryo transfer group, excluding the small proportion of cycles for which this variable was not reported (Table 1).
Data regarding use of intracytoplasmic sperm injection for fertilization of donor oocytes were available only among fresh embryo transfer cycles. Intracytoplasmic sperm injection was used in the majority of this group (83.6% [12 803/15 308]).
Primary Outcome
As shown in Table 2, the live birth rate was statistically significantly higher after fresh embryo transfer compared with cryopreserved-thawed embryo transfer (56.6% vs 44.0%, respectively; absolute difference, 12.6% [95% CI, 11.7%-13.5%]; unadjusted RR, 1.29 [95% CI, 1.26-1.31]; adjusted RR [aRR], 1.42 [95% CI, 1.39-1.46]), controlling for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching.
Table 2. Live Birth, Clinical Pregnancy, and Miscarriage Rates in Fresh vs Cryopreserved-Thawed Embryo Transfer Cycles Derived From Freshly Inseminated Donor Oocytes.
| Outcomes | No. (%) | Absolute difference, % (95% CI) | Relative risk (95% CI) | ||
|---|---|---|---|---|---|
| Fresh embryo transfer (n = 15 308) | Cryopreserved-thawed embryo transfer (n = 36 634) | Unadjusted | Adjusteda | ||
| Primary outcome | |||||
| Live birth | 8665 (56.6) | 16 126 (44.0) | 12.6 (11.7 to 13.5) | 1.29 (1.26-1.31) | 1.42 (1.39-1.46) |
| Secondary outcomes | |||||
| Clinical pregnancy | 10 209 (66.7) | 19 845 (54.2) | 12.5 (11.6 to 13.4) | 1.23 (1.21-1.25) | 1.34 (1.31-1.37) |
| Miscarriage | 1416 (9.3) | 3453 (9.4) | 0.2 (−0.4 to 0.7) | 0.98 (0.93-1.04) | 0.98 (0.91-1.07) |
Adjusted for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching.
Secondary Outcomes
The clinical pregnancy rate of 66.7% after a fresh embryo transfer was statistically significantly higher than the 54.2% rate after a cryopreserved-thawed embryo transfer (absolute difference, 12.5% [95% CI, 11.6%-13.4%]; RR, 1.23 [95% CI, 1.21-1.25]; aRR, 1.34 [95% CI, 1.31-1.37]).
There was no statistically significant difference in the miscarriage rate between the 2 groups, at 9.3% following fresh embryo transfer and 9.4% following cryopreserved-thawed embryo transfer (absolute difference, 0.2% [95% CI, −0.4% to 0.7%]; aRR, 0.98 [95% CI, 0.91-1.07]).
Cycles Using PGT-A
Only 4.5% of cycles (2327/51 942) underwent PGT-A. Of those, 89% (2063/2327) produced embryos included in the cryopreserved-thawed embryo transfer group and 11% (264/2327) produced embryos included in the fresh embryo transfer group.
As shown in Figure 2, when comparing fresh non–PGT-A embryo transfers with cryopreserved-thawed PGT-A embryo transfers, the fresh non–PGT-A embryo transfer group demonstrated a statistically significantly higher live birth rate (56.5% vs 50.0%, respectively; absolute difference, 6.6% [95% CI, 4.3%-8.9%]; aRR, 0.74 [95% CI, 0.68-0.81]), adjusted for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching), although the absolute difference in outcomes was smaller than that seen when comparing all fresh vs all cryopreserved-thawed embryo transfers.
Figure 2. Live Birth, Clinical Pregnancy, and Miscarriage Rates in Fresh and Cryopreserved-Thawed Embryo Transfers Derived From Freshly Inseminated Donor Oocytes With and Without PGT-A.
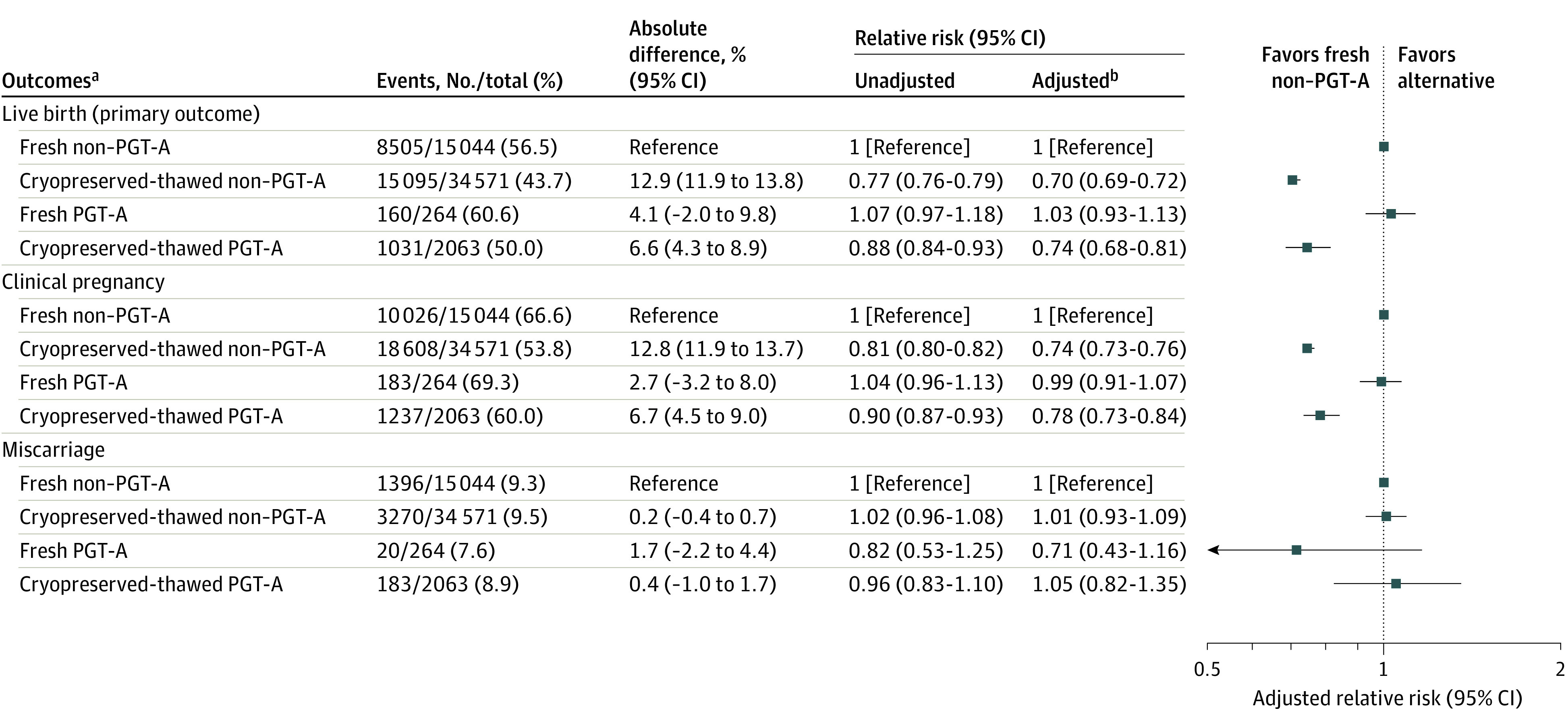
These analyses were tested for the presence of an interaction, and the results for all outcomes were found not to be significant (live birth, P = .72; clinical pregnancy, P = .30; miscarriage, P = .17).
aPreimplantation genetic testing (PGT-A) is the removal of 3 to 5 trophectoderm cells from a blastocyst to test the embryo for chromosomal aneuploidy prior to transfer.
bAdjusted for donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching.
The fresh non–PGT-A embryo transfer group also had a statistically significantly higher clinical pregnancy rate compared with the cryopreserved-thawed PGT-A embryo transfer group (66.6% vs 60.0%, respectively; absolute difference, 6.7% [95% CI, 4.5%-9.0%]; aRR, 0.78 [95% CI, 0.73-0.84]). The miscarriage rate was not significantly different between fresh non–PGT-A cycles and cryopreserved-thawed PGT-A cycles (9.3% vs 8.9%, respectively; absolute difference, 0.4% [95% CI, −1.0% to 1.7%]; aRR, 1.05 [95% CI, 0.82-1.35]).
Figure 2 also shows the comparison between fresh non–PGT-A embryo transfers and the small group of fresh PGT-A embryo transfers (n = 264), with no statistically significant differences identified in live birth, clinical pregnancy, or miscarriage rates.
Fresh non–PGT-A embryo transfers were associated with a statistically significantly higher live birth rate than cryopreserved-thawed non–PGT-A embryo transfers (56.5% vs 43.7%, respectively; absolute difference, 12.9% [95% CI, 11.9%-13.8%]; aRR, 0.70 [95% CI, 0.69-0.72]).
eFigure 2 in the Supplement depicts live birth rates stratified by year (2014-2017) for all 4 groups: fresh non–PGT-A, cryopreserved-thawed non–PGT-A, fresh PGT-A, and cryopreserved-thawed PGT-A embryo transfer cycles.
Subgroup Analysis of Initial Embryo Transfers
A post hoc subgroup analysis was performed including only the initial embryo transfer from each cycle captured in the specified time frame, to account for the possibility of multiple cryopreserved-thawed embryo transfers in the same recipient in the study population. This subgroup analysis was tested for the presence of an interaction, and the results for all outcomes were found not to be significant (live birth, P = .44; clinical pregnancy, P = .29; miscarriage, P = .63). The findings were similar to the initial analyses. The live birth rate remained statistically significantly higher following initial fresh embryo transfer compared with initial cryopreserved-thawed embryo transfer (58% vs 49.2%, respectively; absolute difference, 8.8% [95% CI, 7.7%-9.9%]; aRR, 1.29 [95% CI, 1.25-1.32]). The clinical pregnancy rate was similarly significantly higher in the initial fresh embryo transfer group compared with the initial cryopreserved-thawed embryo transfer group (67.9% vs 59.3%, respectively; absolute difference, 8.7% [95% CI, 7.6%-9.7%]; aRR, 1.23 [95% CI, 1.21-1.26]). The miscarriage rate was not significantly different at 9.0% after initial fresh embryo transfer vs 9.2% after initial cryopreserved-thawed embryo transfer (absolute difference, 0.2% [95% CI, −0.5% to 0.8%]; aRR, 0.96 [95% CI, 0.87-1.05]).
Discussion
This study demonstrated that in cycles using fresh donor oocytes, live birth rates were statistically significantly higher following fresh embryo transfer compared with cryopreserved-thawed embryo transfer, even when PGT-A was performed. This finding was in contrast to previous investigations of cycles using autologous oocytes that demonstrated higher live birth rates following cryopreserved-thawed embryo transfers.
The proposed mechanisms for improved pregnancy rates with the use of cryopreserved-thawed embryos over fresh embryos in autologous oocyte cycles have included the possibility that endometrial receptivity is improved in a cryopreserved-thawed embryo transfer cycle, compared with one in which the uterus has been exposed to exogenous gonadotropins at supraphysiologic levels, resulting in supraphysiologic estrogen levels and disordered endometrium.8,14 Live birth rates following fresh embryo transfer or cryopreserved-thawed embryo transfer, with autologous or donor oocytes, have been shown to be higher when using a gestational carrier, which suggests the uterus does have a significant effect.15
However, in donor oocyte cycles, the recipient uterus has not been exposed to supraphysiologic levels of gonadotropins or estrogen. Typical uterine preparations include a programmed cycle using exogenous estrogen and progesterone (aiming for physiologic levels of both) to synchronize the recipient’s endometrium with the stage of embryo development, or, less commonly, performing the transfer on a natural cycle, timing the embryo transfer with spontaneous ovulation, again synchronizing with the stage of embryo development. Most recipients of donor oocytes are not candidates for a natural-cycle uterine preparation due to a high incidence of diminished ovarian reserve and/or ovulatory dysfunction in this population. Furthermore, among women using freshly retrieved donor oocytes with a fresh embryo transfer, programmed cycles are obligatory to synchronize the recipient endometrium with donor stimulation. Thus, a disordered endometrium does not explain the differences observed in the study population.
Another possible explanation for the advantage of autologous cryopreserved-thawed embryo transfer over fresh embryo transfer has been the possibility that cryopreserved-thawed embryos self-select as the highest-quality embryos because they have met criteria for freezing as well as survived the cryopreserve-thaw cycle.16 However, a contrasting argument has been made that the best embryo would have been preferentially selected for the fresh transfer, with the remaining high-quality embryos cryopreserved. A recent retrospective review of SART data attempted to control for embryo quality by including only initial fresh or cryopreserved-thawed embryo transfers and demonstrated that a freeze-all strategy benefits only high responders (women with ≥15 oocytes retrieved), with intermediate responders (6-14 oocytes retrieved) and low responders (1-5 oocytes retrieved) having better success with a fresh embryo transfer.17 In the current study, the transfer of cryopreserved-thawed embryos was associated with a lower likelihood of live birth compared with fresh embryo transfers in the donor oocyte patient population. The subgroup analysis of only the initial transfer in either group attempted to address the question of embryo quality and was consistent with the initial findings, demonstrating a significantly higher live birth rate in the fresh rather than cryopreserved-thawed embryo transfer group.
When the PGT-A cycles were compared with the non–PGT-A cycles, the association of higher live birth rate following non–PGT-A fresh embryo transfers remained, highlighting the fact that in young, healthy oocyte donors, the risk of aneuploidy is low. Data from this study suggested that PGT-A was not associated with higher clinical pregnancy or live birth rates in donor oocyte cycles. This finding corroborated recent data from a randomized study that showed no benefit of PGT-A in autologous cycles for women younger than 35 years and supported the guidelines published by the ASRM.18,19
Because miscarriage rates were similar following fresh or cryopreserved-thawed embryo transfers, the differences in clinical pregnancy and live birth rates were accounted for by differences in implantation and cannot be explained by pregnancy loss. Thus, these data suggest that the cryopreservation-thaw process may lower the implantation potential of an embryo derived from a fresh donor oocyte. Although no difference was identified in miscarriage rates between the fresh non–PGT-A embryo transfer group and either the fresh or cryopreserved-thawed PGT-A embryo transfer groups, the confidence intervals were wide, reflecting variability of an uncommon outcome in a large cohort.
The findings of this study have several implications for clinical practice. First, preparing for a fresh transfer of an embryo derived from a fresh donor oocyte necessitates a complex process of synchronizing the preparation of the recipient’s endometrial lining with the donor’s follicular development.20 This is substantially more challenging than thawing and transferring a cryopreserved embryo. This study may provide helpful counseling regarding the decision to pursue a fresh vs a cryopreserved-thawed embryo transfer when using freshly retrieved donor oocytes.
Second, the expense of a fresh donor oocyte cycle is substantial, costing as much as $30 000 to $40 000.21,22 Given the considerable financial investment, these data may influence patient decision-making regarding transferring a fresh embryo derived from a fresh donor oocyte vs cryopreserving all embryos a priori for convenience. Future work investigating the cost-effectiveness of fresh vs cryopreserved-thawed embryo transfer in this population would be helpful in guiding practice.
Despite the fact that fresh embryo transfers were associated with a higher live birth rate, a theoretical concern remains regarding obstetrical outcomes. This is a potential concern due to the increased risk of preeclampsia and macrosomia seen in programmed cycles with autologous oocytes.8,16 It is theoretically possible that these same obstetrical risks may apply to a fresh donor oocyte embryo transferred into a programmed recipient uterus, although this was outside of the scope of the current study. Use of donor oocytes independently increases the risk of preeclampsia, and whether this is in part due to the type of uterine preparation needs to be elucidated in future studies.23,24
Major strengths of this study include the large sample size and the ability to capture live birth rate as the primary outcome. More than 95% of all IVF cycles in the US report to SART and thus the study captures most of the donor oocyte cycles taking place nationwide. These data allowed an investigation of the differences in outcomes between PGT-A and non–PGT-A cycles. The adjusted analyses controlled for the most important confounders (donor age, day of embryo transfer, use of a gestational carrier, and assisted hatching).
Limitations
This study has several limitations. First, the retrospective design allows associations to be detected between the exposures and the outcomes but cannot address causality.
Second, because of substantial missing data from the SART database, the analyses were unable to control for potential residual confounders, which may bias the results. Important information was unavailable in the SART database, including donor age, which was missing in 38.7% of all cycles. Data for donors including anti-Müllerian hormone levels, race and ethnicity, gravidity, parity, BMI, smoking status, and several other factors that might affect success rates were not available. In addition, information regarding use of intracytoplasmic sperm injection in the cryopreserved-thawed embryo transfer group was not reported by clinics. The type of PGT-A testing used (comparative genomic hybridization vs next-generation sequencing) was not available in the SART database. Given the time frame of the study, it is likely that the majority of reporting clinics used vitrification for embryo freezing, rather than slow-freezing techniques, although this information was also not available.
Third, it was not possible to link cycles from the same donor across different recipients. Because oocyte donors may cycle more than once at different clinics, it is not possible to ensure that embryos generated from the same donor are not disproportionately represented in the cohort. However, this is unlikely to have had a significant effect given the size of the sample population.
Fourth, a recipient may have undergone more than 1 embryo transfer within the specified time frame or had embryo transfers outside of the specified time frame. The subgroup analysis of only the initial embryo transfer in each recipient was therefore performed to control for the possibility of multiple embryo transfers into the same recipient, and the results were not meaningfully different from the main analysis.
Conclusions
In this retrospective cohort study of women undergoing assisted reproduction using freshly retrieved donor oocytes, the use of fresh embryo transfers compared with cryopreserved-thawed embryo transfers was associated with a higher live birth rate. However, interpretation of the findings is limited by the potential for selection and confounding bias.
eFigure 1. Progression From Fresh Donor Oocyte Retrieval to Fresh or Cryopreserved-Thawed Embryo Transfer
eFigure 2. Live Birth Rates by Year: Embryo Transfer Cycles Using Freshly Retrieved Donor Oocytes
References
- 1.Lutjen P, Trounson A, Leeton J, Findlay J, Wood C, Renou P. The establishment and maintenance of pregnancy using in vitro fertilization and embryo donation in a patient with primary ovarian failure. Nature. 1984;307(5947):174-175. doi: 10.1038/307174a0 [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention 2016 Assisted Reproductive Technology National Summary Report Published 2016. Accessed January 27, 2020. https://www.cdc.gov/art/reports/
- 3.Kawwass JF, Monsour M, Crawford S, et al. ; National ART Surveillance System Group . Trends and outcomes for donor oocyte cycles in the United States, 2000-2010. JAMA. 2013;310(22):2426-2434. doi: 10.1001/jama.2013.280924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Society for Assisted Reproductive Technology Final National Summary Report for 2017. Accessed January 27, 2020. https://www.sartcorsonline.com/rptCSR_PublicMultYear.aspx?reportingYear=2017#donor-fresh-egg
- 5.Kushnir VA, Darmon SK, Barad DH, Gleicher N. New national outcome data on fresh versus cryopreserved donor oocytes. J Ovarian Res. 2018;11(1):2. doi: 10.1186/s13048-017-0378-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kushnir VA, Gleicher N. Fresh versus cryopreserved oocyte donation. Curr Opin Endocrinol Diabetes Obes. 2016;23(6):451-457. doi: 10.1097/MED.0000000000000290 [DOI] [PubMed] [Google Scholar]
- 7.Roque M, Haahr T, Geber S, Esteves SC, Humaidan P. Fresh versus elective frozen embryo transfer in IVF/ICSI cycles: a systematic review and meta-analysis of reproductive outcomes. Hum Reprod Update. 2019;25(1):2-14. doi: 10.1093/humupd/dmy033 [DOI] [PubMed] [Google Scholar]
- 8.Wei D, Liu J-Y, Sun Y, et al. . Frozen versus fresh single blastocyst transfer in ovulatory women: a multicentre, randomised controlled trial. Lancet. 2019;393(10178):1310-1318. doi: 10.1016/S0140-6736(18)32843-5 [DOI] [PubMed] [Google Scholar]
- 9.Shi Y, Sun Y, Hao C, et al. . Transfer of fresh versus frozen embryos in ovulatory women. N Engl J Med. 2018;378(2):126-136. doi: 10.1056/NEJMoa1705334 [DOI] [PubMed] [Google Scholar]
- 10.McQueen DB, Schufreider A, Lee SM, Feinberg EC, Uhler ML. Racial disparities in in vitro fertilization outcomes. Fertil Steril. 2015;104(2):398-402. doi: 10.1016/j.fertnstert.2015.05.012 [DOI] [PubMed] [Google Scholar]
- 11.Humphries LA, Chang O, Humm K, Sakkas D, Hacker MR. Influence of race and ethnicity on in vitro fertilization outcomes: systematic review. Am J Obstet Gynecol. 2016;214(2):212.e1-212.e17. doi: 10.1016/j.ajog.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Practice Committee of the American Society for Reproductive Medicine and Practice Committee of the Society for Assisted Reproductive Technology Recommendations for gamete and embryo donation: a committee opinion. Fertil Steril. 2013;99(1):47-62. doi: 10.1016/j.fertnstert.2012.09.037 [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention Assisted Reproductive Technology Manual 2017;17(2):105-116. [Google Scholar]
- 14.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: a prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril. 2011;96(2):344-348. doi: 10.1016/j.fertnstert.2011.05.050 [DOI] [PubMed] [Google Scholar]
- 15.Murugappan G, Farland LV, Missmer SA, Correia KF, Anchan RM, Ginsburg ES. Gestational carrier in assisted reproductive technology. Fertil Steril. 2018;109(3):420-428. doi: 10.1016/j.fertnstert.2017.11.011 [DOI] [PubMed] [Google Scholar]
- 16.Ginström Ernstad E, Wennerholm UB, Khatibi A, Petzold M, Bergh C. Neonatal and maternal outcome after frozen embryo transfer: increased risks in programmed cycles. Am J Obstet Gynecol. 2019;221(2):126.e1-126.e18. doi: 10.1016/j.ajog.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 17.Acharya KS, Acharya CR, Bishop K, Harris B, Raburn D, Muasher SJ. Freezing of all embryos in in vitro fertilization is beneficial in high responders, but not intermediate and low responders: an analysis of 82,935 cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril. 2018;110(5):880-887. doi: 10.1016/j.fertnstert.2018.05.024 [DOI] [PubMed] [Google Scholar]
- 18.Munné S, Kaplan B, Frattarelli JL, et al. ; STAR Study Group . Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071-1079. doi: 10.1016/j.fertnstert.2019.07.1346 [DOI] [PubMed] [Google Scholar]
- 19.Penzias A, Bendikson K, Butts S, et al. ; Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology . The use of preimplantation genetic testing for aneuploidy (PGT-A): a committee opinion. Fertil Steril. 2018;109(3):429-436. doi: 10.1016/j.fertnstert.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 20.Melnick AP, Rosenwaks Z. Oocyte donation: insights gleaned and future challenges. Fertil Steril. 2018;110(6):988-993. doi: 10.1016/j.fertnstert.2018.09.021 [DOI] [PubMed] [Google Scholar]
- 21.Donor Egg Bank USA Egg donation: should you choose fresh or frozen? Published June 3, 2018. Accessed January 28, 2020. https://donoreggbankusa.com/resources/blog/egg-donation-should-you-choose-fresh-or-frozen-infographics
- 22.Quaas AM, Melamed A, Chung K, Bendikson KA, Paulson RJ. Egg banking in the United States: current status of commercially available cryopreserved oocytes. Fertil Steril. 2013;99(3):827-831. doi: 10.1016/j.fertnstert.2012.10.047 [DOI] [PubMed] [Google Scholar]
- 23.Masoudian P, Nasr A, de Nanassy J, Fung-Kee-Fung K, Bainbridge SA, El Demellawy D. Oocyte donation pregnancies and the risk of preeclampsia or gestational hypertension: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;214(3):328-339. doi: 10.1016/j.ajog.2015.11.020 [DOI] [PubMed] [Google Scholar]
- 24.Schwarze JE, Borda P, Vásquez P, et al. . Is the risk of preeclampsia higher in donor oocyte pregnancies? a systematic review and meta-analysis. JBRA Assist Reprod. 2018;22(1):15-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Progression From Fresh Donor Oocyte Retrieval to Fresh or Cryopreserved-Thawed Embryo Transfer
eFigure 2. Live Birth Rates by Year: Embryo Transfer Cycles Using Freshly Retrieved Donor Oocytes