Abstract
Background:
Most known risk factors for preterm birth, a leading cause of infant morbidity and mortality, are not modifiable. Advanced molecular techniques are increasingly being applied to identify biomarkers and pathways important in disease development and progression.
Aim of review:
We review the state of the literature and assess it from an epidemiologic perspective.
Key Scientific Concepts of Review:
PubMed, Embase, CINAHL, and Cochrane Central were searched on January 31, 2019 for original articles published after 1998 that utilized an untargeted metabolomic approach to identify markers of preterm birth. Eligible manuscripts were peer-reviewed and included original data from untargeted metabolomics analyses of maternal tissue derived from human studies designed to determine mechanisms and predictors of preterm birth. Of 2,823 results, 14 articles met the inclusion requirements. There was little consistency in study design, outcome definition, type of biospecimen, or the inclusion of covariates and confounding factors, and few consistent associations with metabolites were identified in this review. Studies to date on metabolomic predictors of preterm birth are highly heterogeneous in both methodology and resulting metabolite identification. There is an urgent need for larger studies in well-defined populations, to determine biomarkers predictive of preterm birth, and to reveal mechanisms and targets for development of intervention strategies.
Keywords: Metabolomics, metabolite, preterm birth, biomarker
1. Introduction
At least 8,500 infants die each year in the United States due to preterm birth (PTB), yet the underpinning mechanisms and causes remain unclear (Matthews and MacDorman, 2013). PTB has strong and potentially lifelong effects on children, mothers, and families. Infants born preterm are at increased risk for neurological and other morbidities (2007; Khan et al., 2015; Mwaniki et al., 2012), and the multiple pathologic processes leading to PTB may affect maternal health and well-being (Henderson et al., 2016; Romero et al., 2014). PTB also has a strong financial impact on families and communities with an average medical cost of $215,000 compared to $3,200 for a typical birth in 2011(Rankings, 2018). Known risk factors for PTB include low and very high maternal BMI, smoking, previous preterm delivery, conception via in vitro fertilization, a short cervix, and infections, including those of the uterus, cervix, or placenta (Hassan et al., 2011; Romero et al., 2014). Many of these risk factors are not modifiable, nor clearly linked to biological pathways. Numerous biomarkers have been identified but have not been validated, or have not proven to be adequately predictive for use in screening or diagnosis (Halscott et al., 2014; Lucaroni et al., 2018). Because of the urgent need for understanding the risk factors and the biological pathways of PTB, as well as the need for clinically useful predictive measures, advanced molecular techniques are increasingly being applied to the problem.
Meaningful exposures are mediated in the internal chemical environment by endogenous signaling molecules and exogenous chemicals, known as metabolites, that communicate with cells, tissues and organs via enzymes, transcription factors, and receptors (Beger et al., 2016; Brodsky and Medzhitov, 2009; Dennis et al., 2017; Liebler, 2008; Menon and Manning, 2013; Rappaport and Smith, 2010). An individual’s health is affected by both perturbations in endogenous metabolism and exogeneous factors (Beger et al., 2016). Metabolomics is the study of metabolites and how they reflect an individual’s state of health and wellness (Beger et al., 2016). Blood transports chemicals to and from tissues and represents a reservoir of all endogenous and exogenous chemicals in the body at a given time, sometimes summarized in the term “blood exposome”(Rappaport, 2012). This internal exposome includes metabolites derived from endogenous metabolic pathways, as well as those derived from exposures to medications, drugs, environmentally relevant chemicals, and ingestion of foods. Exposome-wide association studies (EWAS) can be conducted with untargeted analysis of small molecules from disease cases and controls using the mass-spectrometric methodologies employed for metabolomics (Matthews and MacDorman, 2013; Rappaport, 2012; Rappaport et al., 2014; Rappaport and Smith, 2010). Early applications of EWAS have revealed unexpected causes of heart disease that involve microbial metabolism of choline and other nutrients (Tang et al., 2013; Wang et al., 2011). Such untargeted analyses may be promising in identifying new exposures and new pathways of analyses for outcomes that are poorly understood.
Limited peer-reviewed literature describes the use of untargeted metabolomics approaches in studies of PTB. Some early metabolomic studies of reproductive outcomes employed targeted analyses of small sets of small molecules (Maitre et al., 2014; Menon et al., 2014). In contrast to targeted investigations, the untargeted design permits thousands of small-molecule features to be interrogated, thereby opening the door to discovery of unanticipated causal exposures arising from both exogenous and endogenous sources. We assessed the current state of the metabolomic literature with respect to PTB, examining studies in humans that aim to discover metabolites associated with PTB. We limited our analysis to measures taken from mothers and not infants to minimize the possibility of reverse causality and maximize the likelihood of identifying possibly clinically useful measures. In addition, we focus our consideration on the epidemiologically and clinically relevant components of these studies. To do so we followed key topics from the STROBE guidelines for observational studies: study design, participants, variable selection, data sources/measurement, and statistical methods (von Elm E, 2008).
2. Methods
2.1. Literature Search
A systematic review of the literature was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al., 2009). Studies were eligible if they included original data from an untargeted metabolomics analysis with pregnant women as the study population, biological samples derived from humans, designed to determine mechanisms of PTB, and were published after 1998. This year was selected because it is the year that the term metabolomics was introduced and has been used in other published systematic review protocols exploring metabolites (Leite et al., 2018). Details of the studies, such as gestational age, preterm phenotype (labor, rupture of membranes, etc.), and biological sample type studied were abstracted but not used as inclusion/exclusion criteria. No relevant non-English abstracts were found.
PubMed, Embase, the Cumulative Index to Nursing and Allied Health Literatures (CINAHL), and Cochrane Central Databases were searched for articles using the search terms (metabolom* OR metabolomics OR metabolome OR metabonom* OR metabonomics OR metabonome OR exposome OR “high resolution mass spectrometry” OR LC-HRMS) AND (birthweight OR preterm OR preterm birth OR premature OR premature birth). The search term exposome was included as it is one way of conceptualizing untargeted metabolomics (Rappaport, 2018). The reference list of these articles and the “Cited by” in both PubMed and Web of Science were used to identify additional articles on the topic. Articles were excluded that a) were conducted in animals or cells; b) were reviews, methods, conference abstracts, or comments; c) did not use PTB as an outcome; d) measured metabolites postnatally or only in the infant; e) examined metabolites as an outcome rather than a predictor; f) presented results where individual metabolites were not explicitly identified; or g) used a targeted analysis with a priori hypotheses about specific metabolites. Authors KP and RAC reviewed the search results by these criteria first by title, then by abstract, and finally by full text. If the investigators disagreed on a paper, they discussed until consensus was reached. If no consensus was reached, then author EH acted as a tiebreaker. Due to the varied nature of the metabolomics results, a meta-analysis was not able to be performed.
Significant metabolites from each of the included studies were extracted to identify potential patterns. The online tool, MetaboAnalyst4.0, was used to identify HMDB/KEGG IDs and sub-pathways (https://www.metaboanalyst.ca) (Chong et al., 2018). HMDB and KEGG databases were used to identify metabolites that were not recognized by MetaboAnalyst4.0 to obtain HMDB/KEGG IDs (Kanehisa, 2019; Wishart et al., 2018). Identified sub-pathways were then grouped into super pathways using KEGG PATHWAY Database (https://www.genome.jp/kegg/pathway.html#metabolism) (Kanehisa, 2019).
2.2. Study Assessment
Studies included were assessed according to key epidemiologic components with reference to the STROBE guidelines for assessing observational studies: study design, participants, variable selection, data sources/measurement, and statistical methods (von Elm E, 2008). Variable selection includes exposure definition, outcome definition, and inclusion of covariates. In epidemiology, “exposure” is used to represent any factor associated with, and usually hypothesized to be a cause of, the outcome under study. Metabolomic studies therefore usually address many exposures simultaneously.
These aspects of study methods affect broader validity: causal inference, generalizability, bias, and confounding. Basic causal inference requires, for example, an exposure to occur prior to the outcome, which case-control and cross-sectional study designs may not be able to assess. Lack of generalizability occurs when a study sample examined is not representative of a larger population, while selection bias can occur if participants are selected in a way that the association between exposure and outcome in the study sample differs relative to the source population. Information bias occurs when exposure, outcome, or covariate measurement is imprecise or differential. Confounding occurs when a third variable or set of variables distorts the relationship between exposure and outcome. We therefore examined the study parameters--study design, participants, variable selection (e.g., exposure definition, outcome definition, and covariates), data sources/measurement, and statistical methods--and how they might affect the conclusions that could be drawn.
3. Results
Our PubMed search on January 31st, 2019 resulted in 2,823 studies with 278 duplicates (Figure 1). Article titles were used to narrow the remaining 2,545 articles to 202 articles for full text review. Upon full text review, 188 additional articles were excluded; 87 were not about metabolites, 45 were conference abstracts, 27 were targeted analyses, 16 had an outcome other than PTB, five used samples from newborns not mothers, three had data that was not available for extraction, four were review, methods or protocol papers, and one was a non-human study. The three that with not available for extraction were studies with no individual level measurement of the exposure (n=2) or a study with mixed outcomes (n=1). Review of full text citations and “Cited by” literature in both PubMed and Web of Science identified no additional articles. Fourteen articles were included in the final review.
Figure 1.
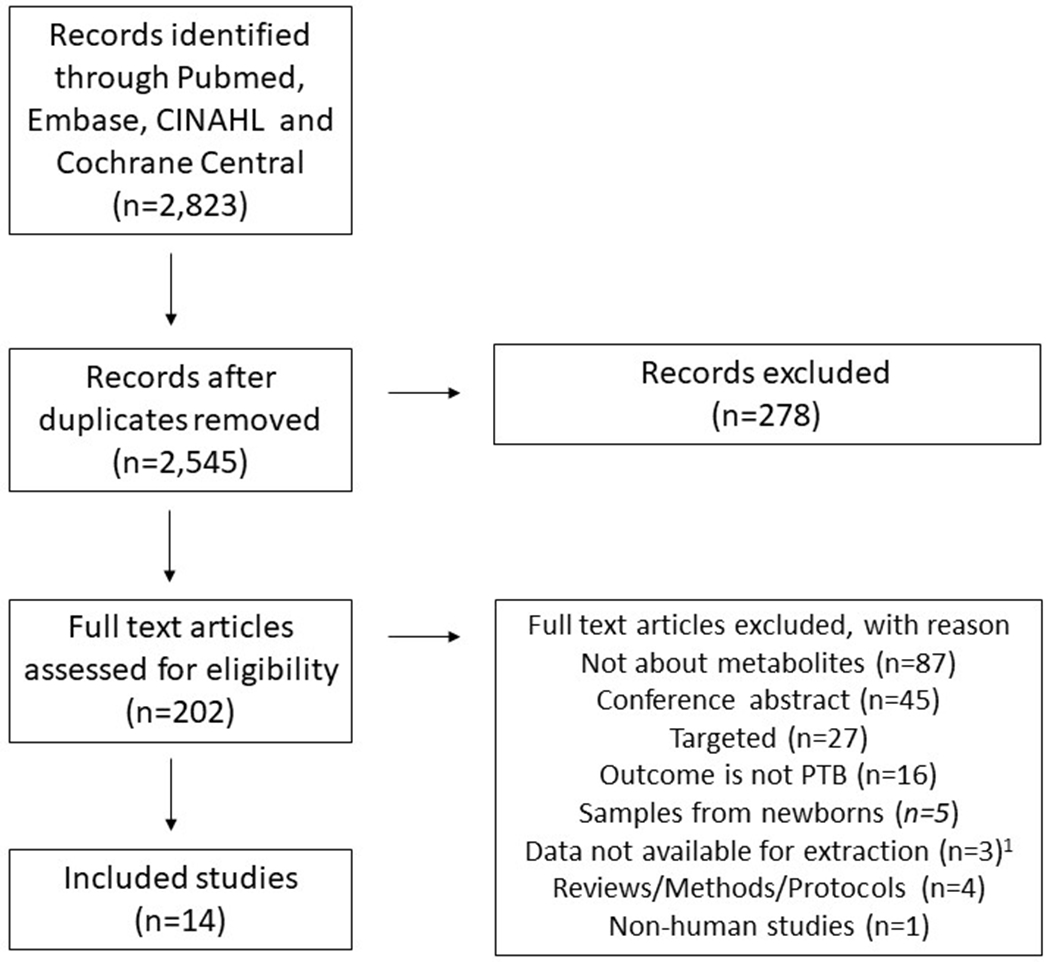
Flowchart outlining the protocol adopted in this systematic review
1Data not available for extraction is a combination of studies with no individual level measurement of exposure (n=2) or studies with combined outcomes (n=1)
3.1. Metabolites
There were no discernable patterns in metabolite regulation across the 14 studies included. Table 2 outlines the statistically significant metabolites from the included studies. No single threshold for significance was used across all studies, but specific significance levels are noted in the third column of Table 2. When authors did not specify a significance level in the text, this was noted as N/A.
Table 2.
Significant metabolites reported in included articles.
| Metabolites | ||||||
|---|---|---|---|---|---|---|
| Sample Source | Author (Year) | Significance level; Multiple Comparisonsa | # of metabolites extracted | Increased | Decreased | No difference in Metabolites |
| Cervicovaginal Fluid | Ghartey (2015)1 | p≤ 0.05 were significant, p>0.05 and p<0.10 were explored further; false discovery rate | 313 |
PTB vs. Term 20-23 g.w. Xanthine, nicotinamide adenine dinucleotide reduced (NADH) PTB vs. Term, 24-27 g.w. Glucose, glucose-6-phospate, N-acetylneuraminate, palmitoleate (16:1n7), 10-heptadecenoate (17:1n7), 1-palmitylglycerol (1-monopalmitin), nicotinamide, 2-pyrrolidinone PTB 24-27 g.w. vs. 20-23 g.w. 2-pyrrolidinone, tryptamine Term delivery 24-27 g.w. vs. 20-23 g.w. Methyl-4-hydroxybenzoate |
PTB vs. Term 20-23 g.w. Lysine, indolelactate, cystine, creatinine, 4-Guanidinobutanoate, 5-Oxoproline, dimethylarginine (SDMA +ADMA), alanylphenylalanine, arginylproline, glycylisoleucine, glyclleucine, isoleucylalanine, isoleucylglutamate, isoleucylglycine, isoleucylserine, isoleucylthreonine, leucylglutamate, leucylleucine, phenylalanylisoleucine, penylalanylleucine, phenylalanylphenylalanine, tyrosylglutamine, tyrosylleucine, valylalanine, valylaspartate, valylglycine, valylleucine, valylphenylalanine, isobar, N-acetylglucosamine, N-acetylgalactosamine, phosphate, myoinositol, 1-stearoylglycerophosphoserine, 1-palmitoglycerol (1-monopalmitin), cytosine, erythritol, glycolate (hydroxyacetate) PTB vs. Term, 24-27 g.w. 3-(4-hydroxyphenyl) lactate, ornithine, creatinine, isoleucylglycine, isoleucylthreonine, threonylproline, valylthreonine, myoinositol, 1-palmitoylglycerol (1-monopalmitin), dehydroisoandrosterone sulfate (DHEA-S) Term delivery 24-27 g.w. vs. 20-23 g.w. Phenylalanylphenylalanine, glucose, glucose-6-phosphate, xylulose, xylose, maltohexaose, maltopentaose, maltose, N-acetylglucosamine, N-acetyl galactosamine, myo-inositol, 1-palmitoylglycerol (1-monopalmitin), dehydroisoandrosterone sulfate (DHEA-S), nicotinamide, 3-Hydroxyhippurate, erythritol PTB 24-27 g.w. vs. 20-23 g.w. Phenylacetylglutamine, p-cresol sulfate, 3-indoxyl sulfate, urea, catechol sulfate |
|
| Ghartey (2017) | p≤0.05; false discovery rate | 301 |
PTB vs. term Mannitol/sorbitol, methylphosphate, |
PTB vs. term Suberate, azelate, sebacate, undecanedioate, tetradecanedioate, hexadecanedioate, propionylcarnitine, palmitoylcarnitine, glycerol, glycerol 3-phosphate, glycerophsphoglycerol, sedoheptulose, mannose, N-acetylserine, threonine, glutamine, P-Citrylglutamate, 6-Oxopiperidine-2-carboxylate, homoarginine, trans-4-hydroxyproline, creatinine, 5-Oxoproline, β-Alanine, 3-Aminoisobutyrate, pyridoxate, methyl-4-hyroxybenzoate, propyl-4-hydroxybenzoate, acesulfame |
PTB vs. term Glucose, glucose-1-phosphate, sialic acid |
|
| Thomas (2015) | p≤0.05; false discovery rate | 112 |
sPTB vs. Term No significant differences after adjustment for false discovery rate |
|||
| Amniotic Fluid | Baraldi (2016) | N/A; N/A | 1369 negative and 1742 positive RT_mass variables |
PTB vs. Term Muconic dialdehyde, Dicarboxylic unsaturated fatty acid, amino acid chain, 3-methoxybenzenepropanoic acid, 4-hyrpoxynonenal alkyne, hydropyridine |
PTB vs. Term Phosphatidylcholine |
|
| Graca (2010)b | p<0.05; N/A | N/A |
PTB vs. Term Allantoin |
PTB vs. Term Alanine, citrate, myo-inositol, unassigned 3.12 ppm |
||
| Menon (2014)2 | p<0.05; false discovery rate |
PTB vs. Term Acetaminophen metabolites (4-Acetamidophenol, 2-methoxyacetaminopehn sulfate, 3-(cysteine-S-yl) acetaminophen, 3-(N-acetyl-L-cysten-S-yl0 acetaminophen, p-acetamidopheylglucuronide), progesterone, glycocholate, taurocholate, taurochenodeoxycholate, taurodeoxycholate, and glycodeoxycholate, bilirubin [Z,Z], bilirubin [E,E], biliverdin, dexapantehenol, 1,2-Propanediol |
PTB vs. Term P-cresol sulfate, phenol sulfate, glycocholenate sulfate, 3-indoxyl sulfate, squalene, lathosterol, cortisol, cortisone, theobromine, theophylline, 1-methylurate, 1,7-dimethylurate, 1,3,7-trimethylurate, and 7-methylxanthine, 3-hydroxybutyrate (BHBA), arachidonate, mead acid, 13,14-Dihydro-15-keto-prostaglandin A2, 12-HETE |
|||
| Romero (2010)c,d | N/A; N/A |
Term delivery Galactose, hexose cluster 5, hexose cluster 3, mannose, hexose cluster 2, hexose cluster 6, fructose, urea, 3-hyroxybutanois acid, unknown 286, palmitate, threo-isocitric acid, glycerol, citric acid. PTB without IAI Hexose cluster 6, dulcitol, urocanic acid, possible N-acetyl glutamine, 1-methyladenine, butanoic acid, beta hydroxyphenylethyamine, vitamin B6, salicylamide, oleic acid, 6 unknowns (128, 276, 285, 283, 132, 344) PTB with IAI Alanine, pyroglutamic acid, glutamine, leucine, proline, isoleucine, valine, glutamic acid, glycine, tyrosine, palmitate, urea, inositol, octadecanoic acid, possible heptanedioic acid, possible alpha aminoadipic acid, possible butanoic acid 3oxy, butanedioic acid, 3 unknowns (270, 8, 121) |
Term delivery Alanine, glutamine, pyroglutamic acid, isoleucine, glutamic acid, serine, tyrosine, possible heptanedioic acid, possible alpha aminoadipic acid, pentanedioic acid, normetanephrine, unknown 8, unknown 121, unknown 221 PTB without IAI Alanine, pyroglutamic acid, proline, glycine, glutamine, galactose, hexose cluster 5, hexose cluster 3, mannose, inositol, urea, 3-hydroxybutanoic acid, palmitate, octadecanoic acid, butanedioic acid PTB with IAI Galactose, hexose cluster 3, hexose cluster 5, mannose, hexose cluster 6, hexose cluster 2, hexose cluster 1, fructose, glycerol, gluconic acid, threo-isocitric acid, unknown 221, unknown 286. |
|||
| Urine | Diaz (2013)b | p<0.05; N/A | Slightly more than 5700 |
PTB vs. Term 4 unassigned spectral regions (0.66-0.73, 1.37-1.45, 1.45-1.47, 1.60-1.65 ppm) |
PTB vs. Term 3-methylhistidine, 4-hydroxyphenylacetate, 2 unassigned spectral regions (5.33-5.34, 6.73-6.76 ppm) |
|
| Maitre (2014) | p<0.05; false discovery rate | 34 |
PTB, spontaneous vs Term Steroid conjugate −0.63 (s), lysine, N-methyl-2-pyridone-5-carboxamide PTB, induced vs. Term Steroid conjugate −0.63 (s), N-acetyl glycoprotein fragments |
PTB, spontaneous vs Term Trimethylamine-N-oxide (TMAO), glycine, formate PTB, induced vs. Term Phenylacetylglutamine |
||
| Blood | Li (2016) | p<0.05; N/A | 163 |
PTB vs. Term3 PCaaC38:6 (a Diacyl-PC) |
||
| Lizewska (2018) | p<0.05; false discovery rate | 374 (out of 73,649) positive features and 375 (out of 57,411) negative features; 51 identified using databases |
PTB vs. term symptomatic
4 Palmotoleic acid*, linolenic acid (C18:3w3)*, linoleic acid (C18:2w6)*, oleic acid*, (4E,8E,10E,-d18:3)Sphingosine* PTB within 1 week of PTL diagnosis vs. term symptomati4 Lauric*, myristic*, palmotoleic acid**, linolenic acid (C18:3w3)**, linoleic acid (C18:2w6)**, oleic acid***, arachidonic acid*, eicosadienoic (C20:2)*, docosahexaenoic acid (DHA)*, docosapentaenoic acid (C22:5w3)*, TG (triglyceride)*, 2-Hydroksybutyric acid/3-hydroksybutyricacid*, (4E,8E10E-d18:3) Sphingosine**, Anandamide (20:I, n-9)* PTB vs. asymptomatic term4 LysoPE (20:5)*, PS (O-18:0/0:0)*, Glycochenodeoxycholate***, Chenodeoxycholate/glycoursodeoxycholate/glycodeoxycholate*, TUDCA/TUDCA isomer/taurodeoxycholic acid* Term symptomatic vs. term asymptomatic4 Lysine*, tryptophan*, Lyso PC (14:0)*, Lyso PC/PC (15:0)*, Lyso PE (18:1)*, Lyso PE (18:2*), Lyso PC or PC (18:3)*, Lyso PE (20:5)*, PS (O-18:0/0:0)***, Deoxycholic acid*, glycochenodeoxycholate****, chenodeoxycholate/glycoursodeoxycholate/glycodeoxycholate***, glycocholic acid*, TUDCA/TUDCA isomer/taurodeoxycholic acid**, malonaldehyde*, carnitine*, D-fructose/D-glucose* |
PTB vs. term symptomatic
4 Tryptophan*, pregnenolone sulfate*, L-2-amino-3-(1-pyrazolyl) propanoic acid* PTB within 1 week of PTL diagnosis vs. term symptomatic 4 Histidine*, tryptophan**, pregnenolone sulfate**, Chenodeoxycholate/glycoursodeoxycholate/glycodeoxycholate*, L-2-amino-3-(1-pyrazolyl) propanoic acid* PTB vs. asymptomatic term 4 C16 sphingosine-1-phosphate*, progesterone****, pregnenolone/bolasterone***, pregnenolone sulfate*, 17-Hydroxypregnenolone sulfate**, 11-Beta-hydroxyandrosterone-3-Glucuronide**, cortisone/aldosterone/prednisolone**, malic acid**, N,N’-Dicyclohexyl urea****, Biliverdin* Term symptomatic vs. term g asymptomatic4 Lauric*, myristic*, hydroxymyristic acid*, palmotoleic acid***, linolenic acid (C18:3w3)**, linoleic acid (C18:2w6)**, oleic acid**, arachidonic acid*, eicosadienoic (C20:2)*, Docosapentaenoic acid (C22:5w3)*, TG (triglyceride)***, progesterone***, pregnenolone/bolasterone***, 17-hydroxypregnenolone sulfate*, 11-Beta-Hydroxyandrosterone-3-Glucuronide*, cortisone/aldosterone/prednisolone***, cortisol/hydroxychorticosterone*, 3b,16a-Dihydroxyandrosterone*, testosterone metabolite*, 2-Hydroksybutyric acid/3-Hydroksybutyric acid*, malic acid**, N,N’-Dicyclohexyl urea****, Sphingolipid: obscuraminol A/curcigasterin 277*, Biliverdin* |
||
| Amniotic Fluid and Urine | Graca (2012)b | p<0.05 for Mann-Whitney-Wilcoxon tests, p<0.001 for SHY correlation analysis; N/A |
Amniotic Fluid PTB vs. Term Hexose, 16 unassigned features (m/z: 331.12, 353.10, 309.16, 226.12, 597.09, 390.98, 202.18. 749.29, 247.16, 316.21, 920.87, 361.20, 765.55, 812.68, 785.66, 787.67) |
Amniotic Fluid PTB vs. Term Methionine, valine, Isoleucine/leucine, phenylalanine, histidine, 12 unassigned features (m/z: 192.03, 222.04, 247.14, 371.06, 248.06, 397.20, 136.08, 147.07, 260.19, 169.99, 246.17, 527.25) |
Urine PTB vs. Term Sample size too small |
|
| Amniotic Fluid and Blood | Virgiliou (2017)f | p<0.05; Bonferroni adjustment | Maternal Serum: 2018 (+ESI) and 294 (−ESI); 1371 +ESI and 218 −ESI for statistical analysis; Amniotic fluid: a set of 3074 and 997 features was obtained for +ESI and −ESI. |
Maternal Serum PTB vs. Term5: PI-Cer (d18:0/16:0) [M+NH4]+, C16 sulfatide [M+NA]+, (3’-sulfo)Galbeta-Cer(d18:0/18:0(2OH))([M + Na]+) PI (O-43:0) [M +NA]+ (or PI(O-34:2) [M +H]+ or PI (P-34:2) [M + H]+), PE (42:9)[M +Na]+ (or PE(44:12) [M +Na]+ ), PI (o-36:2 [M +Na]+ (or PI(O-38:5 [M +Na]+ or PI(P-38:4) [M +Na]+), PS(38:2) [M +Na]+ , PI-Cer (d18:0/16:0) [M +Na]+ (or PG (O-27:1) [M +Na]+, PG (P-27:0) [M +Na]+ ), m/z_rt: 844.54_8.42, 804.54_8.43, 804.55_8.77, 812.61_11.44 Amniotic Fluid PTB vs. Term: m/z_rt: 745.7_599 |
Amniotic Fluid PTB vs. Term: m/z_rt: 94.9_68, 113_63, 427.2_760, 323_79, 264.2_728, 435.2_769 |
|
| Blood and Urine | Diaz (2011)b | p<0.05; N/A |
Urine PTB vs. Term Unassigned 2, 2-hydroxyisobutyrate, choline |
Blood PTB vs. Term Sample size too small |
Not all data is presented. The table listing metabolites only provided significant metabolites at p<0.05 and distinguishing between increase or decrease could only be done when the paper was retrieved through Elsevier. Metabolites with a significance p<0.10 were mentioned in text as well as no changes, but these are not reflected in the table.
Some metabolites were identified as having strong p-values that contributed to differentiating between groups, but due to the layout of the papers we were unable to determine the direction of their impact. These metabolites were histidine metabolites (cis-urocanate, trans-urocanate, and 1-methylimidazoleacetate), steroid metabolite conjugates (androsterone, androsteroid, estriol, and cortisone, 21-hydroxypregnenolone, pregnen-diol, 5α-pregnan-3β,20α-diol, and 4-andtrosten-3β,17β-diol). Acetaminophen metabolites were also compared via t-test but there was no annotation to determine what was statistically significant or not.
Bivariate analyses identified the following as significantly, negatively correlated with gestational age: C16:2, C2, xLeu, PCaaC36:4, PCaaC38:4, PCaaC38:5, PCaaC38:6, PCaaC40:4, PCaaC40:5, PCaaC40:6, PCaaC42:4, PCaeC40:5.
*p value ⩽ 0.05; **p value ⩽ 0.01; ***p value ⩽ 0.001; ****p value ⩽ 0.0001; all metabolites with p<0.05 were listed in the table, metabolites with p<0.01 and p<0.001.
While false discovery rates (FDR) were commonly reported as a method for adjustment for multiple comparisons, other studies use false discovery rates for other metrics which reflects the discordance between the statistical analysis and multiple comparisons columns
Denotes a study where PTD was not the primary outcome or was part of a sub-analysis including multiple adverse pregnancy outcomes
This included 2 studies with the same groups, the first was an exploratory study to see if metabolites could classify by phenotype and the second a validation study; only the results of the validation study are presented
Significance was not noted, but, the listed metabolites were reported as differentially regulated.
A trend analysis; p-values and 95% CI are not reported
Only results from the untargeted analysis are presented here
Possible annotations were noted in the table of the text along with significance and trend. A total of 13 lipids were reported to significantly differentiate between groups in the text of the results, but only twelve with an increased trend were presented in the table. Possible annotations are provided when available, otherwise the m/z_rt is listed.
Across studies, a total of 163 non-duplicated metabolite names were significantly different between groups. MetaboAnalyst recognized 118 of the 163 metabolites with HMDB/KEGG IDs and 43 metabolites were recognized through HMDB and KEGG databases. Only 80 of these 161 metabolites were able to be grouped into sub-pathways (Table 3). The 56 sub-pathways that were identified belong to superpathways of carbohydrates, lipids, amino acids, energy, nucleotides, cofactors & vitamins, and biosynthesis of other secondary metabolites. Overall, individuals who gave birth preterm had decreases in metabolites associated with numerous sub-pathways related to the super-pathway of vitamins and co-factors (Table 3). B vitamins (e.g., B1, Thiamine; B5, pantothenic acid; B6, pyridoxine; and B7, Biotin) are co-factors to enzymes involved in a wide range of pathways that were perturbed in PTB including amino acid metabolism, Krebs cycle metabolism, fatty acids synthesis, pyruvate metabolism, formation of ketone bodies, and tryptophan metabolism (Table 3).
Table 3.
Super pathway and sub-pathways
| Superpathwa y reference | Sub-pathway name | Increased/Decreased* | Metabolite name | HMDB ID | Kegg ID | Reference study |
|---|---|---|---|---|---|---|
| Carbohydrate | Amino sugar and nucleotide sugar metabolism | Increased | Glucose | HMDB0000122 | C00031 | Ghartey 2015 |
| Increased | glucose-6-phospate | HMDB0001401 | C00668 | Ghartey 2015 | ||
| Decreased | N-acetylglucosamine | HMDB0000215 | C00140 | Ghartey 2015 | ||
| Decreased | Mannose | HMDB0000169 | C00159 | Ghartey 2017 | ||
| Pentose phosphate pathway | Increased | Glucose | HMDB0000122 | C00031 | Ghartey 2015 | |
| Increased | Glucose-6-phospate | HMDB0001401 | C00668 | Ghartey 2015 | ||
| Pyruvate metabolism | Decreased | Formate | HMDB0000142 | C00058 | Maitre 2014 | |
| Fructose and mannose metabolism | Decreased | Mannose | HMDB0000169 | C00159 | Ghartey 2017 | |
| Galactose metabolism | Increased | Glucose-6-phospate | HMDB0001401 | C00668 | Ghartey 2015 | |
| Increased | Glucose | HMDB0000122 | C00031 | Ghartey 2015 | ||
| Decreased | Mannose | HMDB0000169 | C00159 | Ghartey 2017 | ||
| Decreased | Glycerol | HMDB0000131 | C00116 | Ghartey 2017 | ||
| Decreased | Myoinositol# | HMDB0000211 | C00137 | Ghartey 2015/Graca 2010 | ||
| Butanoate metabolism | Increased | 2-Hydroksybutyric acid/3-hydroksybutyricacid | HMDB0000008/HMDB0000357 | C00195/C05984 | Lizewska 2018 | |
| Decreased | 3-hydroxybutyrate | HMDB0000357 | C01089 | Menon 2014 | ||
| Ascorbate and aldarate metabolism | Decreased | Myoinositol# | HMDB0000211 | C00137 | Ghartey 2015/Graca 2010 | |
| Citrate cycle (TCA cycle) | Decreased | Citrate | HMDB0000094 | C00158 | Graca 2010 | |
| Glycolysis or Gluconeogenesis | Increased | Glucose | HMDB0000122 | C00031 | Ghartey 2015 | |
| Increased | glucose-6-phospate | HMDB0001401 | C00668 | Ghartey 2015 | ||
| Starch and sucrose metabolism | Increased | Glucose | HMDB0000122 | C00031 | Ghartey 2015 | |
| Increased | Glucose-6-phospate | HMDB0001401 | C00668 | Ghartey 2015 | ||
| Glyoxylate and dicarboxylate metabolism | Decreased | glycolate (hydroxyacetate) | HMDB0000115 | Ghartey 2015 | ||
| Propanoate metabolism | Increased | 2-Hydroksybutyric acid/3-hydroksybutyricacid | HMDB0000008/HMDB0000357 | C00195/C05984 | Lizewska 2018 | |
| Decreased | Valine | HMDB0000883 | C00183 | Graca 2012 | ||
| Decreased | β-Alanine | HMDB0000056 | C00099 | Ghartey 2017 | ||
| Lipid | Fatty acid biosynthesis | Increased | Oleic acid | HMDB0000207 | C00712 | Lizewska 2018 |
| Fatty acid metabolism | Increased | 10-heptadecenoate (17:1n7) | HMDB0060038 | Ghartey 2015 | ||
| Increased | 1-palmitylglycerol (1-monopalmitin) | HMDB0031074 | Ghartey 2015 | |||
| Increased | Palmitoleate (16:1n7) | HMDB0003229 | C08362 | Ghartey 2015 | ||
| Increased | myristic | HMDB0000806 | C06424 | Lizewska 2018 | ||
| Increased | Palmotoleic acid | HMDB0003229 | C08362 | Lizewska 2018 | ||
| Increased | Lauric | HMDB0000638 | C02679 | Lizewska 2018 | ||
| Decreased | Palmitoylcarnitine | HMDB0000222 | C02990 | Ghartey 2017 | ||
| Glycerolipid metabolism | Increased | TG (triglyceride) | C02737 | Lizewska 2018 | ||
| Decreased | Glycerol | HMDB0000131 | C00116 | Ghartey 2017 | ||
| Decreased | Glycerol 3-phosphate | HMDB0000126 | C00093 | Ghartey 2017 | ||
| Glyoxylate and dicarboxylate metabolism | Decreased | Citrate | HMDB0000094 | C00158 | Graca 2010 | |
| Decreased | Formate | HMDB0000142 | C00058 | Maitre 2014 | ||
| Inositol phosphate metabolism | Decreased | Myoinositol# | HMDB0000211 | C00137 | Ghartey 2015/Graca 2010 | |
| Steroid hormone biosynthesis | Increased | Progesterone& | HMDB0001830 | C00410 | Menon 2014 | |
| Decreased | Dehydroisoandrosterone sulfate (DHEA-S) | HMDB0001032 | C04555 | Ghartey 2015 | ||
| Decreased | Progesterone& | HMDB0001830 | C00410 | Lizewska 2018 | ||
| Decreased | Cortisol | HMDB0000063 | C00735 | Menon 2014 | ||
| Decreased | Cortisone/aldosterone/prednisolone | HMDB0002802/HMDB0000037/HMDB0014998 | C00762/C01780/C07369 | Lizewska 2018 | ||
| Decreased | Pregnenolone/bolasterone | HMDB0000253/HMDB0006048 | C01953/C14475 | Lizewska 2018 | ||
| Decreased | Cortisone | HMDB0002802 | C00762 | Menon 2014 | ||
| Glycerophospholipid metabolism | Increased | Choline | HMDB0000097 | C00114 | Diaz 2011 | |
| Increased | LysoPE (20:5) | HMDB0011519 | C05464 | Lizewska 2018 | ||
| Increased | PS (O-18:0/0:0) | HMDB0012378 | C04438 | |||
| Decreased | Phosphatidylcholine | C00157 | Baraldi 2016 | |||
| Decreased | Glycerol 3-phosphate | HMDB0000126 | C00093 | Ghartey 2017 | ||
| Linoleic acid metabolism | Decreased | Linoleic acid (C18:2w6) | HMDB0000673 | C01595 | Lizewska 2018 | |
| Primary bile acid biosynthesis | Increased | Glycochenodeoxycholate | HMDB0000637 | C05466 | Lizewska 2018 | |
| Increased | Glycocholate | HMDB0000138 | C01921 | Menon 2014 | ||
| Increased | Taurochenodeoxycholate | HMDB0000951 | C05465 | Menon 2014 | ||
| Increased | Taurocholate | HMDB0000036 | C05122 | Menon 2014 | ||
| Increased | Chenodeoxycholate/glycoursodeoxycholate/glycodeoxycholate | HMDB0000518/HMDB0000708/HMDB0000631 | C02528 | Lizewska 2018 | ||
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Synthesis and degradation of ketone bodies | Increased | 2-Hydroksybutyric acid/3-hydroksybutyricacid | HMDB0000008/HMDB0000357 | C00195/C05984 | Lizewska 2018 | |
| Decreased | 3-hydroxybutyrate | HMDB0000357 | C01089 | Menon 2014 | ||
| Alpha-Linolenic acid metabolism | Increased | linolenic acid (C18:3w3) | HMDB0001388 | C06427 | Lizewska 2018 | |
| Sphingolipid metabolism | Decreased | C16 sphingosine-1-phosphate | HMDB0000277 | C06124 | Lizewska 2018 | |
| Increased | (4E,8E,10E,-d18:3)Sphingosine | C00422 | Lizewska 2018 | |||
| Arachidonic acid metabolism | Increased | Arachidonic acid | HMDB0001043 | C00219 | Lizewska 2018 | |
| Amino Acid | Alanine, aspartate and glutamate metabolism | Decreased | Alanine | HMDB0000161 | C00041 | Graca 2010 |
| Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | ||
| Arginine and proline metabolism | Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | |
| Decreased | 4-Guanidinobutanoate | HMDB0003464 | C01035 | Ghartey 2015 | ||
| Decreased | Creatinine# | HMDB0000562 | C00791 | Ghartey 2015/Ghartey 2017 | ||
| Decreased | Trans-4-hydroxyproline | HMDB0000725 | C01157 | Ghartey 2017 | ||
| Decreased | dimethylarginine (SDMA +ADMA) | HMDB0001539/HMDB03334 | C21188/C21189 | Ghartey 2015 | ||
| Decreased | Ornithine | HMDB0000214 | C00077 | Ghartey 2015 | ||
| Cysteine and methionine metabolism | Decreased | Cystine | HMDB0000192 | C00491 | Ghartey 2015 | |
| Decreased | Alanine | HMDB0000161 | C00041 | Graca 2010 | ||
| Decreased | Methionine | HMDB0000696 | C00073 | Graca 2012 | ||
| Lysine degradation | Increased | Lysine& | HMDB0000182 | C00047 | Maitre 2014 | |
| Decreased | Lysine& | HMDB0000182 | C00047 | Ghartey 2015 | ||
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Phenylalanine metabolism | Decreased | Phenylacetylglutamine | HMDB0006344 | C04148 | Maitre 2014 | |
| Decreased | Phenylalanine | HMDB0000159 | C00079 | Graca 2012 | ||
| Decreased | 4-hydroxyphenylacetate | HMDB0000020 | C00642 | Diaz 2013 | ||
| Phenylalanine, tyrosine and tryptophan biosynthesis | Decreased | Tryptophan | HMDB0000929 | C00078 | Lizewska 2018 | |
| Decreased | Phenylalanine | HMDB0000159 | C00079 | Graca 2012 | ||
| Tryptophan metabolism | Decreased | Indolelactate | HMDB0000671 | C02043 | Ghartey 2015 | |
| Decreased | Valine | HMDB0000883 | C00183 | Graca 2012 | ||
| Tyrosine metabolism | Decreased | 4-hydroxyphenylacetate | HMDB0000020 | C00642 | Diaz 2013 | |
| Decreased | 3-(4-hydroxyphenyl)lactate | HMDB0000755 | C03672 | Ghartey 2015 | ||
| Glycine, serine and threonine metabolism | Increased | Choline | HMDB0000097 | C00114 | Diaz 2011 | |
| Decreased | Tryptophan | HMDB0000929 | C00078 | Lizewska 2018 | ||
| Decreased | Threonine | HMDB0000167 | C00188 | Ghartey 2017 | ||
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Histidine metabolism | Decreased | 3-methylhi stidine | HMDB0000479 | C01152 | Diaz 2013 | |
| Decreased | Histidine# | HMDB0000177 | C00135 | Graca 2012/Lizewska 2018 | ||
| Lysine biosynthesis | Increased | Lysine& | HMDB0000182 | C00047 | Maitre 2014 | |
| Decreased | Lysine& | HMDB0000182 | C00047 | Ghartey 2015 | ||
| Decreased | Isoleucine/leucine | HMDB0000172/HMDB0000687 | C00407/C00123 | Graca 2012 | ||
| Valine, leucine and isoleucine biosynthesis | Decreased | Threonine | HMDB0000167 | C00188 | Ghartey 2017 | |
| Decreased | Valine | HMDB0000883 | C00183 | Graca 2012 | ||
| Valine, leucine and isoleucine degradation | Decreased | Alanine | HMDB0000161 | C00041 | Graca 2010 | |
| Metabolism of other amino acids | beta-Alanine metabolism | Decreased | Histidine# | HMDB0000177 | C00135 | Graca 2012/Lizewska 2018 |
| Decreased | β-Alanine | HMDB0000056 | C00099 | Ghartey 2017 | ||
| Cyanoamino acid metabolism | Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | |
| D-Glutamine and D-glutamate metabolism | Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | |
| D-Arginine and D-ornithine metabolism | Decreased | Ornithine | HMDB0000214 | C00077 | Ghartey 2015 | |
| Synthesis and degradation of ketone bodies | Decreased | 5-Oxoproline# | HMDB0000267 | C01879 | Ghartey 2015/Ghartey 2017 | |
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Decreased | Ornithine | HMDB0000214 | C00077 | Ghartey 2015 | ||
| Taurine and hypotaurine metabolism | Increased | Taurocholate | HMDB0000036 | C05122 | Menon 2014 | |
| Decreased | Alanine | HMDB0000161 | C00041 | Graca 2010 | ||
| Energy | Methane metabolism | Decreased | Trimethylamine-N-oxide (TMAO) | HMDB0000925 | C01104 | Maitre 2014 |
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Decreased | Formate | HMDB0000142 | C00058 | Maitre 2014 | ||
| Nitrogen metabolism | Decreased | Formate | HMDB0000142 | C00058 | Maitre 2014 | |
| Decreased | Tryptophan | HMDB0000929 | C00078 | Lizewska 2018 | ||
| Decreased | Histidine# | HMDB0000177 | C00135 | Lizewska 2018/Graca 2012 | ||
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Decreased | Phenylalanine | HMDB0000159 | C00079 | Graca 2012 | ||
| Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | ||
| Nucleotide | Purine metabolism | Increased | Xanthine | HMDB0000292 | C00385 | Ghartey 2015 |
| Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | ||
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Pyrimidine metabolism | Decreased | Cytosine | HMDB0000630 | C00380 | Ghartey 2015 | |
| Decreased | 3-Aminoisobutyrate | HMDB0003911 | C05145 | Ghartey 2017 | ||
| Decreased | β-Alanine | HMDB0000056 | C00099 | Ghartey 2017 | ||
| Decreased | Glutamine | HMDB0000641 | C00064 | Ghartey 2017 | ||
| Cofactors and Vitamins | Pantothenate and CoA biosynthesis | Decreased | β-Alanine | HMDB0000056 | C00099 | Ghartey 2017 |
| Decreased | Valine | HMDB0000883 | C00183 | Graca 2012 | ||
| Porphyrin and chlorophyll metabolism | Decreased | Threonine | HMDB0000167 | C00188 | Ghartey 2017 | |
| Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | ||
| Decreased | Biliverdin | HMDB0001008 | C00500 | Lizewska 2018 | ||
| Thiamine metabolism | Decreased | Glycine | HMDB0000123 | C00037 | Maitre 2014 | |
| Ubiquinone and other terpenoid-quinone biosynthesis | Decreased | 3-(4-hydroxyphenyl)lactate | HMDB0000755 | C03672 | Ghartey 2015 | |
| Nicotinate and nicotinamide metabolism | Increased | nicotinamide | HMDB0001406 | C00153 | Ghartey 2015 | |
| Increased | N-methyl-2-pyridone-5-carboxamide | HMDB0004193 | C05842 | Maitre 2014 | ||
| Vitamin B6 metabolism | Decreased | Pyridoxate | HMDB0000017 | C00847 | Ghartey 2017 | |
| Biotin metabolism | Increased | Lysine& | HMDB0000182 | C00047 | Maitre 2014 | |
| Decreased | Lysine& | HMDB0000182 | C00047 | Ghartey 2015 | ||
| Biosynthesis of other secondary metabolites | Caffeine metabolism | Increased | Xanthine | HMDB0000292 | C00385 | Ghartey 2015 |
| Decreased | 7-methylxanthine | HMDB0001991 | C16353 | Menon 2014 | ||
| Decreased | 1-methylurate | HMDB0003099 | C16359 | Menon 2014 | ||
| Decreased | theophylline | HMDB0001889 | C07130 | Menon 2014 |
Increased/decreased in PTB groups compared to control groups
Same direction;
Opposite direction
Four metabolites were reported that were lower among women with PTB compared to term births across multiple studies according to their HMDB/KEGG IDs: 5-oxoproline (Ghartey et al., 2017; Ghartey et al., 2015), creatinine (Ghartey et al., 2017; Ghartey et al., 2015), histidine (Graca et al., 2012; Lizewska et al., 2018), and myoinositol (Ghartey et al., 2015; Graca et al., 2010). No single metabolite was present in more than two included studies.
Two metabolites were reported that were significantly associated with PTB in opposite directions across multiple studies according to their HMDB/KEGG IDs: progesterone and lysine. One study found that, compared to women with a term birth, the level of lysine was higher in urine samples collected at 7.5-16 gestational weeks among women who gave birth preterm, but another found lower levels in cervicovaginal fluid samples collected at 20-23 gestational weeks among women who gave birth preterm (Ghartey et al., 2015; Maitre et al., 2014). Adding to the complexity, in a third study, lysine was higher in blood serum samples among term deliveries with preterm labor symptoms (symptomatic) compared to term deliveries without preterm labor symptoms (asymptomatic) with samples collected at different gestational weeks. (Lizewska et al., 2018). Progesterone was higher among women with preterm compared to term births in amniotic fluid samples collected during labor (Menon et al., 2014) but lower in both preterm compared to asymptomatic term births and term symptomatic compared to term asymptomatic births (though sample collection times varied (Lizewska et al., 2018). Finally, one study found no statistically significant difference in any metabolites identified in cervicovaginal fluid samples between PTB and low-risk term women after controlling for false discovery rates (Thomas et al., 2015).
3.2. Metabolomic techniques
There was little variability in sample storage. Most samples were frozen at −70 to −80 degrees Celsius until they were extracted and analyzed. The analytic process was less homogeneous; four studies used nuclear magnetic resonance imaging (NMR) spectroscopy, nine used a form of mass spectroscopy, and one used another technique (full details in Table 1). 1H-NMR was the only method used among those who used NMR (Diaz et al., 2011; Graca et al., 2010; Maitre et al., 2014). There was variability among studies who used mass spectroscopy, with authors often using more than one technique, including Liquid Chromatography (Ultra/High Performance) and Gas Chromatography (Baraldi et al., 2016; Ghartey et al., 2017; Ghartey et al., 2015; Graca et al., 2012; Lizewska et al., 2018; Menon et al., 2014; Romero et al., 2010; Thomas et al., 2015; Virgiliou et al., 2017). One author used an Absolute Targeted Metabolite Identification and Quantification (IDQTM) kit p-150 with LC-MS (Li et al., 2016).
Table 1.
Study Characteristics of included articles.
| Sample Source | Author (Year), Country | Study Design, Analytic Technique b | Sample Population |
Participant Characteristics (timing of sample collection): PTB: Sample size Term: Sample size |
PTB definition, gestational age diagnostic criteria | Covariates included in analysis |
|---|---|---|---|---|---|---|
| Cervicovaginal Fluid | Ghartey (2015), United States | Nested Case-control [PREDICT cohort], UPLC-MS/MS, GC/MS | Tertiary care institution |
High-risk women (20-23 g.w. and 24-27 g.w.): PTB:10 Term:10 |
<37 g.w.; N/A | No |
| Ghartey (2017), United States | Nested Case-control [STOP cohort], UPLC-MS/MS, GC/MS | Tertiary care institution |
Preterm labor symptoms (22-366/7g.w.): PTB:20 Term:30 |
<38 g.w.; N/A | No | |
| Thomas (2015), New Zealand | Nested Case-control [SCOPE study], GC/MS |
Low-risk women (20 g.w.): PTB:30 Term:30 |
<37 g.w.; N/A | No | ||
| Amniotic Fluid | Baraldi (2016), Italy | Case-control, UPLC/MS | General hospital |
Amniocentesis due to spontaneous preterm labor with intact membranes (21-28 g.w.): PTB: 13 Term:11 |
<37 g.w.; N/A | Yes |
| Graca (2010)d | Case-control, 1H-NMR | N/A |
Amniocentesis for cytogenic based diagnostics (14-25 g.w.): PTB: 12 Term: 82 |
<37 g.w.; Clinical information and questionnaire at time of collection. | No | |
| Menon (2014), United States | Nested case-control [Nashville Birth Cohort], UPLC/MS, GC/MS | Tertiary care |
(during labor dilation <6cm): PTB:25 Term: 25 |
<340/7 g.w.; LMP verified by ultrasound | No | |
| Romero (2010)e, Chile and United States | Nested Case-control; 2 studies, UPLC/MS, GC/MS |
Amniocentesis due to spontaneous preterm labor with intact membranes (22-35 g.w.): PTB with IAI: 40 PTB without IAI: 33 Term without IAI: 40 |
<37 g.w.; N/A | No | ||
| Diaz (2013)d, Portugal | Nested case-control, 1H-NMR |
(14-26 g.w.): PTB: 26 Term: 84 |
<37 g.w.; Clinical information and questionnaire | No | ||
| Maitre (2014), Greece | Nested case-control [RHEA cohort], 1H NMR | Maternity clinics |
(7.5-16 g.w.)g: PTB, spontaneous: 88 PTB, medically induced: 26 Term: 275 |
<37 g.w.; LMP verified by ultrasound and corrected by crown-rump length | Yes | |
| Li (2016), Germany | Cross-sectional, Absolute IDQTM-kit | Obstetrics Department |
(at delivery before administration of oxytocin): Ran gestational age as continuous outcome, also divided into two groups PTB: 57 Term:466 |
<37 g.w.; LMP | Yes | |
| Lizewska (2018), Poland | Case-control; HPLC | Three tertiary centers |
Spontaneous preterm labor symptoms (times vary): Preterm: 57 (24-37 g.w.) Preterm, deliver within 7 days of diagnosis: 37 (24-37 g.w.) Term, symptomatic: 49 (23-27 g.w.) No spontaneous preterm labor symptoms (38-41 g.w.) Term: 25 |
<37 g.w.; First trimester ultrasound | No | |
| Graca (2012)d | Case-control, UPLC/MS1 | N/A |
Amniocentesis (15-25 g.w.): Amniotic Fluid PTB: 11 Term: 26 Urine PTB: 6 Term: 21 |
<37 g.w.; Clinical information and questionnaire | No | |
| Amniotic Fluid and Blood | Virgiliou, (2017), Greece | Case-Control, UHPLC/MS (untargeted), HILIC UHPLC-MS/MS (targeted) | University General Hospital |
Amniocentesis (14-23 g.w.): PTB: 35 Term: 35 |
29 g.w.-365/7g.w.; N/A | No |
| Blood and Urine | Diaz (2011)d | Prospective Cohort, 1H-NMR | N/A |
Amniocentesis (14-25 g.w.): Urine PTB:17 Term: 25 Blood PTB: 4 Term: 20 |
<37 g.w.; N/A | No |
The same data was used from the Graca (2010) article, but run using UPLC/MS and was compared to the 1H NMR results from the 2010 paper via SHY analysis (results and methods outlined in Table 2 and supplemental table 1).
Timing of sample collection is measured in gestation weeks (g.w.)
When the study is nested, the name of the parent cohort study is provided in brackets if available.
The covariates were used as part of a preliminary, exploratory data analysis by PCA and PLS-DA to exclude any confounding effects.
This included 2 studies with the same groups. The first was an exploratory study to see if metabolites could classify by phenotype and the second a validation study; only the results of the validation study are presented
Denotes a study where PTB was not the primary outcome or was part of a sub-analysis including multiple adverse pregnancy outcomes
Insufficient number of blood plasma samples for the study.
Timing of sample selection was calculated through the mean +/− 3 standard deviations provided in text.
Analytic Techniques: NMR=Nuclear Magnetic Resonance Imaging; MS=Mass Spectrometry; UPLC=Ultra Performance Liquid Chromatography; ELISA=Enzyme-Linked Immunosorbent Assay; IAI= Intra-Amniotic Infection; GC= Gas Chromatography; PTL= Preterm Labor; MIR=Mid-Infrared Spectroscopy; LMP=Last menstrual period; LC= Liquid Chromatography; NIR= Near-Infrared Spectroscopy; HPLC= High Performance Liquid Chromatography; UHPLC= Ultra-High-Performance Liquid Chromatography, LMP=Last Menstrual Period
Quality control (QC) processes for statistical analysis included the use of measured data from standard reference materials and quality control samples to address the veracity of experimental data as well as other processes (Beger et al., 2019). Detailed quality control information is outlined in the supplemental table. Most studies included used normalization and scaling (Baraldi et al., 2016; Diaz et al., 2013; Diaz et al., 2011; Graca et al., 2010; Graca et al., 2012; Maitre et al., 2014; Menon et al., 2014; Romero et al., 2010; Thomas et al., 2015). Six studies explicitly mentioned imputing missing data (Baraldi et al., 2016; Ghartey et al., 2017; Ghartey et al., 2015; Li et al., 2016; Menon et al., 2014) and logarithmic transformation (Baraldi et al., 2016; Ghartey et al., 2017; Ghartey et al., 2015; Graca et al., 2012; Menon et al., 2014). Five studies included quality control samples (Baraldi et al., 2016; Ghartey et al., 2017; Lizewska et al., 2018; Menon et al., 2014; Virgiliou et al., 2017).
3.3. Epidemiologic characteristics of included studies
3.3.1. Study design
Among the fourteen articles that were included for review, there was one cross-sectional study (Li et al., 2016), one prospective cohort study (Diaz et al., 2011), five case-control studies (Baraldi et al., 2016; Graca et al., 2010; Graca et al., 2012; Lizewska et al., 2018; Virgiliou et al., 2017), and seven nested case-control studies (Diaz et al., 2013; Ghartey et al., 2017; Ghartey et al., 2015; Maitre et al., 2014; Menon et al., 2014; Romero et al., 2010; Thomas et al., 2015) (Table 1). Over half of the nested case-control studies listed their parent cohort study by name (n=5) (Ghartey et al., 2017; Ghartey et al., 2015; Maitre et al., 2014; Menon et al., 2014; Thomas et al., 2015).
3.3.2. Participants
Six studies used a study sample from Europe (Baraldi et al., 2016; Diaz et al., 2013; Li et al., 2016; Lizewska et al., 2018; Maitre et al., 2014; Virgiliou et al., 2017), one from South America (Romero et al., 2010), four from the United States (Ghartey et al., 2017; Ghartey et al., 2015; Menon et al., 2014; Romero et al., 2010), and one from New Zealand (Thomas et al., 2015). South America was solely represented by Chile; one study collected samples from both the United States and Chile (Romero et al., 2010). Of the studies conducted in Europe, one was in Italy (Baraldi et al., 2016), one in Portugal (Diaz et al., 2013), one in Germany (Li et al., 2016), one in Poland (Lizewska et al., 2018), and two in Greece (Maitre et al., 2014; Virgiliou et al., 2017). Three articles did not provide information on where the study was conducted (Diaz et al., 2011; Graca et al., 2010; Graca et al., 2012). It appeared that all studies were hospital- or clinic-based.
Many of the studies had either multiple case or multiple control groups. Three categories of cases were used: women at risk for PTB (e.g., intraamniotic infection, preterm labor symptoms, or previous history of PTB) (Baraldi et al., 2016; Ghartey et al., 2017; Romero et al., 2010), medically induced PTB (Maitre et al., 2014), and women who delivered before 37 gestational weeks without other comorbidities (Diaz et al., 2013; Diaz et al., 2011; Ghartey et al., 2015; Graca et al., 2010; Graca et al., 2012; Li et al., 2016; Lizewska et al., 2018; Maitre et al., 2014; Menon et al., 2014; Romero et al., 2010; Thomas et al., 2015; Virgiliou et al., 2017). Among women at risk for PTB, cases were defined by the presence, absence, or combination of either intraamniotic infection or spontaneous preterm labor (Baraldi et al., 2016; Ghartey et al., 2017). There were two broad categories of controls used in the included studies: healthy or “normal” pregnant women as defined by the authors (Diaz et al., 2013; Diaz et al., 2011; Ghartey et al., 2015; Graca et al., 2010; Graca et al., 2012; Li et al., 2016; Lizewska et al., 2018; Maitre et al., 2014; Menon et al., 2014; Thomas et al., 2015; Virgiliou et al., 2017) and women with preterm labor symptoms who delivered at term (Baraldi et al., 2016; Ghartey et al., 2017; Lizewska et al., 2018; Romero et al., 2010). Table 1 contains specific PTB and comparison group classifications.
Information on control selection was only provided in the four studies that used a matched design (Graca et al., 2010; Maitre et al., 2014; Thomas et al., 2015; Virgiliou et al., 2017). One article matched only on gestational age (Graca et al., 2010); a second matched by country of origin, maternal age, and parity (Maitre et al., 2014); the third matched on maternal age and the gestational week the amniocentesis was performed (Virgiliou et al., 2017); and the fourth matched on maternal age and ethnicity (Thomas et al., 2015).
3.3.3. Variables
3.3.3.1. Outcome definition: Gestational age
Eleven articles (79%) defined PTB as delivery prior to 37 weeks gestational age (Baraldi et al., 2016; Diaz et al., 2013; Diaz et al., 2011; Ghartey et al., 2017; Ghartey et al., 2015; Graca et al., 2010; Graca et al., 2012; Li et al., 2016; Maitre et al., 2014; Romero et al., 2010; Thomas et al., 2015), with the others defining PTB as <34 weeks (Menon et al., 2014), 29–37 weeks (Virgiliou et al., 2017), and 24–37 gestational weeks or delivering preterm within seven days of diagnosis of threatened premature labor (Lizewska et al., 2018). Half of the included articles (n=7) did not state how gestational age was determined (Baraldi et al., 2016; Diaz et al., 2011; Ghartey et al., 2017; Ghartey et al., 2015; Romero et al., 2010; Thomas et al., 2015; Virgiliou et al., 2017). Those who did either collected unspecified clinical information from obstetrical and neonatal medical records and questionnaire at time of sample collection (Diaz et al., 2013; Graca et al., 2010; Graca et al., 2012), used some combination of last menstrual period (LMP) and ultrasound (Maitre et al., 2014; Menon et al., 2014), used first trimester ultrasound only (Lizewska et al., 2018), or used LMP from the medical record at the first pregnancy exam (Li et al., 2016).
3.3.3.2. Covariate choice and definition
While all articles collected basic demographic information, only three articles included confounders in their models (Baraldi et al., 2016; Li et al., 2016; Maitre et al., 2014). The most commonly assessed confounders were maternal age (Baraldi et al., 2016; Maitre et al., 2014) and maternal weight or BMI (Baraldi et al., 2016; Li et al., 2016; Maitre et al., 2014). Other covariates include: parity (Maitre et al., 2014), smoking (Maitre et al., 2014), maternal education (Maitre et al., 2014), gestational age (Baraldi et al., 2016), previous miscarriages (Baraldi et al., 2016), previous PTB (Li et al., 2016), systolic blood pressure at third trimester (Li et al., 2016), sex of the newborn (Baraldi et al., 2016), maternal therapy at amniocentesis (e.g., nifedipine, betamethasone, atosiban, progesterone) (Baraldi et al., 2016), and gestational age at amniocentesis (Baraldi et al., 2016). Two studies included metabolites that were initially statistically significant in subsequent multivariable analyses (Li et al., 2016; Maitre et al., 2014).
3.3.4. Data sources/measurement: Type and timing of sample collection
The most common type of sample collected in the included articles was amniotic fluid (n=6) (Baraldi et al., 2016; Graca et al., 2010; Graca et al., 2012; Menon et al., 2014; Romero et al., 2010; Virgiliou et al., 2017). Three studies collected blood (Diaz et al., 2011; Li et al., 2016; Lizewska et al., 2018), three collected urine (Diaz et al., 2013; Diaz et al., 2011; Maitre et al., 2014), and three collected cervico-vaginal fluid (Ghartey et al., 2017; Ghartey et al., 2015; Thomas et al., 2015). Three studies collected multiple biological samples: one study collected both amniotic fluid and urine, another amniotic fluid and blood, and a third collected both blood and urine (Diaz et al., 2011; Graca et al., 2012; Virgiliou et al., 2017). Samples were collected at various times during pregnancy and often at a single time measurement. One article provided no information on when samples were collected (Romero et al., 2010). Eleven (78.5%) of the included articles collected their samples at a single time point (Baraldi et al., 2016; Diaz et al., 2013; Diaz et al., 2011; Ghartey et al., 2017; Graca et al., 2010; Graca et al., 2012; Li et al., 2016; Lizewska et al., 2018; Maitre et al., 2014; Menon et al., 2014; Thomas et al., 2015; Virgiliou et al., 2017), and one study used multiple time points (see Table 1 for details)(Ghartey et al., 2015). Only two articles collected samples during the same gestational window (14-25 weeks) (Diaz et al., 2011; Graca et al., 2010); otherwise, the intervals for sample collection often overlapped but were not identical (i.e., one used 14-25 weeks and others 14-26 or 14-23).
If the first trimester was defined as 0-136/7 weeks, the second trimester as 14-276/7 weeks and the third trimester as the 28th week and beyond, the distribution across trimesters was the same for both cases and controls. There were no studies that collected samples exclusively in the first trimester; one study collected samples spanning the first and second trimester as calculated through the mean gestational weeks reported +/− 3 standard deviations (Maitre et al., 2014). Seven studies collected samples in the second trimester (Diaz et al., 2013; Diaz et al., 2011; Ghartey et al., 2015; Graca et al., 2010; Graca et al., 2012; Thomas et al., 2015; Virgiliou et al., 2017), three studies collected samples spanning both the second and third trimesters (Baraldi et al., 2016; Ghartey et al., 2017; Lizewska et al., 2018), and two collected samples at delivery (Li et al., 2016; Menon et al., 2014)
3.3.5. Statistical methods
The sample sizes of the 14 included studies ranged from 20 to 523 (Ghartey et al., 2015; Li et al., 2016). Bivariate analysis was often first used (Wilcoxon rank-sum test, ANOVA, Chi-square, Fisher exact test, Mann Whitney U test, volcano plots) to gain an overview of potentially important metabolites. Multivariate approaches were used to analyze metabolites individually, as well as the relationships among the individual metabolites (Bartel et al., 2013). Common multivariate analyses used among the 14 included studies were Principal Component Analysis (PCA), (Orthogonally) Projection to Latent Structures-Discriminant Analysis (PLS-DA/OPLS-DA, random forests, and multivariate linear regression models, used for high-dimensional feature selection or classification. Statistics are outlined by study in the supplementary table.
4. Discussion
This review aims to analyze the current state of the literature on metabolites and PTB with an emphasis on epidemiologic methods. There is little consistency in metabolite perturbation by disease status across the fourteen included studies, and there was very little overlap in significant metabolites when matched by their HMDB/KEGG IDs. Four metabolites (myoinositol, creatinine, histidine, and 5-oxoproline) were negatively associated with PTB across multiple studies (Ghartey et al., 2017; Ghartey et al., 2015; Graca et al., 2010; Graca et al., 2012; Lizewska et al., 2018). Additionally, some metabolites have conflicting results across studies. The four consistent metabolites were identified in studies that either include women who have previous PTB, had preterm labor symptoms in the current pregnancy, or who underwent amniocentesis, which may indicate that the difference in the metabolomic profile among women with preterm and term birth might be more evident in women who have a baseline high risk of PTB. Furthering this argument, one study included in this review found no significant difference in any metabolites between low-risk women with normal pregnancies who delivered preterm compared to women who delivered at term (Thomas et al., 2015). The authors concluded that future research should concentrate on high-risk pregnancies (e.g., those with intraamniotic infections). However, inconsistencies in the metabolomic findings could be a result of the variability in the study design, participants, outcome or exposure definition, inclusion of covariates, and statistical methods. For instance, the opposing direction of associations between progesterone ((Lizewska et al., 2018; Menon et al., 2014) or lysine (Ghartey et al., 2015; Maitre et al., 2014) with PTB in different studies might be due to the different biological samples (cervicovaginal fluid vs amniotic fluid), different timing of sample collection (second-third trimester vs during labor), or variation due to small sample sizes (especially Ghartey 2015 and Menon 2014).
4.1. Metabolomic results across studies
While a majority of these studies did not conduct pathway analyses, this is likely a product of the time in which the studies were conducted and the current standards of analysis rather than errors in the study methods.
Four metabolites were identified across multiple studies with effects in the same direction: histidine, 5-oxoproline, creatinine, and myoinositol. No previous studies were found with information relevant to 5-oxoproline or histidine. Creatinine and 5-oxoproline were identified by the same authors using two different nested case-control studies from the same hospital (Ghartey et al., 2017; Ghartey et al., 2015). While the two cohorts that the cases and controls were selected from are different, they both recruited from the University of Pennsylvania and both collected samples from cervicovaginal fluid. Both studies showed that creatinine is lower among women who gave birth preterm compared to women who delivered at term. Creatinine has an anti-oxidative function, reduces inflammatory responses, and improves glucose tolerance in humans (Wu et al., 2009). Creatinine has previously been shown to be higher among women who have premature rupture of membranes (PROM) and complain of vaginal leakage compared to women with vaginal leakage but no PROM (Begum et al., 2017; Kariman et al., 2013). However, while PROM is a risk factor for PTB, PROM may have a different set of causes.
A -7% variation in myoinositol in second trimester amniotic fluid was found among women who delivered before 37 gestational weeks (p<0.05) and a .42-fold change in cervicovaginal fluid myoinositol levels in preterm women with samples collected at 20 to 23 gestational weeks compared to term deliveries (p<0.05) (Ghartey et al., 2015; Graca et al., 2010). Myoinositol is important in normal fetal growth and development because it participates in membrane phospholipid synthesis and specifically seems to promote hormone-induced lung maturation (Hallman et al., 1985).
4.2. Epidemiologic Characteristics
4.2.1. Study design
One possible explanation for the heterogeneous results is different study designs. Both case-control and cross-sectional studies must be examined carefully to determine any risk for reverse causality: metabolites could be the result of PTB rather than a cause. This becomes a concern especially when metabolomic samples are collected during labor (Menon et al., 2014). An additional critical component to case-control studies is the selection of the control group. In order to provide valid estimates of effect, case-control studies should be conceptualized as arising from a source population and should not eliminate controls with causal intermediates (Poole, 1999). A majority of the included studies were nested case-control studies, though for many studies the underlying cohort and control selection method are not well defined. A well-defined underlying cohort is necessary to ensure both external and internal validity for nested case-control studies (Rothman et al., 2008); generally, it appears that for most of the included studies, samples were collected until enough from the desired groups were available, meaning there is no clear source population. If this method of participant selection resulted in cases and controls not coming from comparable populations, results would be biased. The most obvious way this could happen is if the groups were selected over different time frames and an exposure or the population served by the hospital had shifted over time. Without a specifically hypothesized source of selection bias, it is difficult to assess this issue directly, but it remains a concern. Additionally, the appropriateness of matching designs generally depends on the topic and population under study (Rothman et al., 2008). Given the limited matching criteria of most of the included studies, overmatching is unlikely to be a concern. Still, it is possible that matching on ethnicity, for instance, might eliminate differences in metabolites along pathways that contribute to disparities.
4.2.2. Participants
Heterogeneous patterns may also be due to differences in study populations and/or selection bias that was introduced during the case and control selection process. Recruitment and follow-up methods can lead to selection bias—if participants are selected in a way that their exposure and outcome association differs from the source population, study results will be biased. For example, in one study with 94 preterm deliveries compared to 74 term deliveries selected from three tertiary centers (Lizewska et al., 2018), it is possible that controls are relatively healthier than cases (i.e. the younger maternal age) in ways not captured by the exposures measured, which may result in overestimating the effect size of some exposures on PTB. Future studies should provide more transparency on the selection process to allow assessment of selection bias and comparison across studies.
In addition, thought should be put into the exclusion criteria and focusing on the metabolome of either exclusively low-risk (e.g., “normal”) pregnancies, or high-risk pregnancies (Supplemental Table 1). Studies that use women with intraamniotic infection as either cases or control groups may identify metabolites synthesized from the bacterial infection, independent of preterm delivery. Conversely, metabolites associated with intraamniotic infection may trigger PTB. For example, one study where all participants had spontaneous preterm labor with intact membranes (three groups: term birth, PTB without intraamniotic infection, and PTB with intraamniotic infection) found that when using a combination of the other two groups as the comparison group alanine was lower among term birth women and PTB without intraamniotic infection, but higher among PTB women with intraamniotic infection compared to the other two groups (Romero et al., 2010). It is not clear whether the higher level of alanine among PTB women was due to intraamniotic infection or preterm labor.
4.3.3. Variables
4.3.3.1. Outcome definition: Gestational age
While different methods of identifying gestational age were used across the studies, they were the same for the case and control groups within studies. Therefore, concern would not be for internal validity or differential ascertainment of exposure, but whether results are comparable across studies. As gestational age was generally determined clinically (albeit with slightly different methods), this seems unlikely to be a major source of variation across studies.
4.3.3.2. Covariate choice and definition
Metabolic profiles vary by age and lifestyle factors (Sumner et al., 2018). Among the three studies that mentioned covariates, one study considered covariates but did not include them in the final analysis because they believed there were no confounding effects (Baraldi et al., 2016). Two studies adjusted for lifestyle and behavioral confounders (Li et al., 2016; Maitre et al., 2014), although the change in effect size after adjustment could not be evaluated because they either used a different measure for effect size before and after adjustment (Maitre et al., 2014) or did not show the unadjusted effect size (Li et al., 2016). This makes it difficult to assess the degree of confounding by these covariates. However, steroid conjugate at 0.63 ppm was significantly associated with both medically induced and spontaneous PTB in unadjusted analysis, but became non-significant across groups after adjusting for maternal education, maternal age, parity and smoking habits (Maitre et al., 2014). In another study, several metabolites were identified as associated with PTB prior to adjusting for confounders, but only PCaaC38:6 was negatively associated with PTB after adjusting for PTB history, maternal pre-pregnancy BMI before pregnancy, systolic blood pressure, and maternal weight at the third trimester (Li et al., 2016). This suggests that confounding can be present in metabolomic studies and must be addressed both methodologically and analytically. Potential confounders should be conceptualized carefully in the context of pregnancy because women can become pregnant multiple times. For example, the underlying causes of a previous PTB might be the same as those of the present PTB. If so, controlling for the previous PTB would result in omitting the common causes of previous and present PTB events (Howards et al., 2007).
4.4. Data sources and measurement
4.4.1. Type and timing of sample collection
In order to understand complicated pregnancies, we need to also understand the changes in the metabolome that occur naturally throughout normal pregnancies (Luan et al., 2015). No articles discuss the common metabolite changes over the course of pregnancy, largely because samples are taken at a single time point. Therefore, it is difficult to assess normal metabolomic levels compared to what might be an early warning signal of PTB (Diaz et al., 2013). The existing studies of metabolomic changes in pregnancy are almost as heterogeneous as those reviewed here. Studies comparing metabolites during pregnancy to women who are not pregnant have found differing results. For example, some studies showed higher levels of alanine in pregnant women’s urine or blood (Diaz et al., 2013; Orczyk-Pawilowicz et al., 2016), while another found no significant difference (Luan et al., 2015). This makes the inconsistent findings relative to PTB even harder to interpret: one study identified no change in alanine between preterm and normal pregnancy groups (Thomas et al., 2015) while another study found alanine was lower among pre-PTD women (Graca 2010). Yet another study found that alanine was lower among term birth women compared to PTB women with or without intraamniotic infection; they also found that the change in direction of the association with alanine among women with preterm labor was dependent upon the presence or absence of intraamniotic infection (Romero et al., 2010). These examples highlight the difficulty in interpretation when there is no consensus as to what constitutes a “true” baseline (Luan et al., 2015). It would be advantageous for future studies to collect samples over the course of pregnancy, and not be limited to a specific trimester or small window of pregnancy.
An additional complexity is that the interpretation of metabolomic data depends on the biological sample being studied. To effectively utilize within- and between-group comparisons, we must take multiple standardized measurements and compare across both analytic techniques and sample types (e.g., blood, amniotic fluid, urine). The heterogeneity of metabolites seen in this review may be due to differences across types of samples, although heterogeneity within types of samples was large as well. One recent article attempting to distinguish differences in the metabolomic profile between amniotic fluid and blood samples among normal term pregnancies found that, even though samples were not matched, there was a high rate of agreement between amniotic fluid and blood samples (Orczyk-Pawilowicz et al., 2016). This supports the idea that metabolic predictors of PTB can be analyzed using different sample types. However, given the small number of studies demonstrating this and the lack of studies comparing cervicovaginal fluid and other biological samples, more baseline research is needed.
4.4.2. Sample variability
The metabolome is continuously changing, with a large part of this change due to both short- and long-term dietary intake (Townsend et al., 2016). The metabolite profile is different among individuals who fast more than 13 hours compared to individuals who fast less than four hours (Townsend et al., 2016). Collecting samples at different times after the last meal introduces variation in metabolites in the datasets, and lack of fasting was mentioned in every paper reviewed but was not taken into consideration analytically or discussed in any of the limitations. Adjustment for time since last meal in the analysis phase may address this issue to some extent, but was not done in any of papers reviewed. Maternal diet profiles impact even amniotic fluid metabolic profiles (Fotiou et al., 2018), which highlights the importance of obtaining dietary information in metabolomic studies of PTB. If diet is associated with the outcomes being studied, then non-fasting samples, which capture some of this information, may be useful. However, this requires sufficient sample size. If differences in diet are confounding but not causal, then use of non-fasting samples will lead to inaccurate interpretations. Future research should address nutritional metabolites specifically. Since it is not always possible to collect fasting samples among pregnant women, it would be advantageous to collect diet information to reduce the effects of diet as a potential confounder.
4.4.3. Variability among Analytical and Statistical Methods
QC processes are reported in the included studies, but there is heterogeneity among which processes were used. QC processes are critically important to ensure that the data acquired and reported in scientific publications and housed in data repositories are of high quality and are analytically reproducible. Standard operating procedures with quality control assessments should be established for each aspect of the metabolomics study including sample receipt and storage, instrument tune and calibration, chromatographic conditions (e.g., columns and gradients), randomization of study samples, biospecimen preparation (e.g., extraction solvents, internal standards), instrument settings for data acquisition, spectral alignment and formatting processes, normalization, and methods used in signal filtering, handling missing data, and in statistical and multivariate analysis. Deposition of these QC procedures into public repositories, together with the data, would facilitate understanding differences in results between laboratories. Without well-defined QC procedures, harmonization across laboratories and multi-laboratory studies become nearly impossible (Beger et al., 2019).
4.4.3.1. Metabolomics Technologies
The two main metabolomics technologies used in the studies reviewed were Nuclear Magnetic Resonance (NMR) Spectroscopy and chromatography-coupled Mass Spectroscopy (MS). NMR provides a stable, highly reproducible platform which is ideal for longitudinal studies involving multiple instruments within or across laboratories (Sumner et al., 2018). However, NMR has considerably lower sensitivity and resolution compared with MS. Untargeted MS metabolomics methods detect tens of thousands of signals which can be assigned to hundreds of metabolites, while most NMR studies report fewer than 100 metabolites. MS and NMR approaches are complementary. They can be used for cross-platform comparisons: when an analyte is detected on both platforms, NMR can detect metabolites that do not ionize well on MS platforms, and MS can detect low-concentration endogenous metabolites, as well as low-concentration exogenous metabolites such as those derived from environmentally relevant compounds. However, in the studies included for review authors used one analytic technique and did not compare across platforms. This may have resulted in metabolites being identified on one platform and not another, thus decreasing our ability to compare metabolites identified across studies.
4.4.3.2. Analytical Sources of variability
The 14 studies presented have varying results that could be related to differences in a) sample preparation and chromatographic conditions, b) parameters used in data acquisition and data processing, c) metabolite identifications based on comparison with authentic standards versus hypothesized metabolites identifications based on database matches. The inclusion of blanks, internal standards, quality control pools, and reference material have become increasingly used in metabolomics investigations, as a means to filter data and remove signals that vary within and between studies. These 14 studies do not all provide sufficient detail in the manuscripts to determine why results are different between studies. However, it would be feasible to combine and reanalyze the raw data for each platform type, which addresses another reason for deposition of the raw data and standard operating procedures. While no Standard Reference Materials for untargeted metabolomics studies are commercially available at this time, many laboratories are creating reference material by pooling biospecimens from healthy human subjects and analyzing aliquots of the reference material with each batch of samples. Inclusion of such reference material will help future investigators agglomerate datasets retrieved from repositories to not only reveal why different results are obtained for studies within or across laboratories, but also to increase the power since many studies published to date have been conducted with small sample sizes
4.5. Statistical methods
The 14 studies included in this review had sample sizes ranging between 4 individuals in a phenotypic group to 466 individuals in a phenotypic group. The statistical methods used included t-test, random forest models, intra quartile trends, fold change, and multivariate statistics to obtain variable importance to projection (VIP). Information regarding standards used for data processing and how missing data was handled was not detailed enough to make comparisons between the studies. Considering these sample sizes, all studies may not have had sufficient power to identify all biologically important metabolites. Difference in statistical methods could also impact the consistency across studies. For example, in these 14 studies, some investigations used VIP to determine signals important to differentiating study groups (even when the Q2 was low)(Baraldi et al., 2016; Diaz et al., 2011; Graca et al., 2012; Lizewska et al., 2018; Virgiliou et al., 2017), while other investigations relied on hypothesis testing (p<0.05) with or without correction for multiple testing. Small sample sizes or underpowered studies may contribute to contradictory findings, which are likely to represent chance associations or false positives.
4.6. Future Directions
The field is moving away from listing metabolites that differ between groups and towards examining pathways and causal models (Johnson et al., 2016). Some of the earlier work included for this review is methodologically limited by what was available at the time. Future studies that rely on existing data should incorporate more complex analyses, including pathway analyses of metabolites. Researchers who are designing new studies should pay careful attention to study design, study source population, participant selection, timing and repeated collection of exposure samples, and covariates to control for confounding. These methodological considerations can be aided through the development and use of consortia to promote cross-discipline collaboration and dialogue. Consortia promote the development and utilization of more uniform outcome definitions as well as inclusion and exclusion criteria that allow for accurate assessment of the relationship between exposure and outcome. Consortia expand the study population, which increases generalizability, and provide a larger sample size, which allows meaningful subgroup analyses. For example, investigating differences by race or comparing low-risk and high-risk pregnancies. Metabolomics consortia are developing in many fields, but in perinatal epidemiology they remain relatively limited. While examples of perinatal consortia exist, for example SAMBA/SCOPE groups and a potential extension of COMet to perinatal outcomes, efforts in this direction could be stronger (Cecatti et al., 2016; Sumner et al., 2018). Another possibility is to add pregnancy outcomes to existing metabolomic studies, particularly those in children or young adults.
5. Conclusion
These papers clearly highlight the promise of metabolomic analysis, including the possibility of better predictive models. However, perhaps not surprisingly given the youth of the field, few conclusions can be drawn. Some studies reach opposite conclusions with respect to the same metabolites; this may be the result of differing biological fluids, gestational age, populations, or may indicate random variation due to small sample size. At the same time, molecular analysis also has its limitations. Proximal metabolites (those produced by the body and generally already identified as risk markers) are present in much larger quantities than distal exposures (such as low-dose environmental exposures). Metabolomic analysis may lead us to re-discovering known risk factors (such as lipids or inflammation), rather than developing truly novel markers, or identifying chemical pollutants that might be triggering these pathways, a goal of exposome-wide analysis (Vineis et al., 2017). Each article included for review ends with a discussion of the feasibility of metabolomics and how metabolites can be a powerful tool for identifying PTB risk. Greater attention to epidemiological components outlined in this review, as well as the formation of perinatal metabolite consortia, would improve the ability to identify novel metabolomic biomarkers that improve our understanding of the etiology of PTB.
Supplementary Material
Acknowledgments
We would like to thank Elaine Hicks, Tulane Science Library Resource Librarian for her help in forming the search terms for this review.
Funding
This work was funded by NICHD grant R21HD087878 (Harville, PI), NIDDK Grant U24DK097193-01 (Sumner, PI), and NIEHS grant U19 ES019525-01 (Sumner, Co-I)
Footnotes
Protocol and Registration
No review protocol or registration information exists
Conflict of Interest
The authors declare they have no conflict of interest
Ethics
This article does not contain any studies with human and/or animal participants performed by any of the authors
Data Availability
The data reported in this review is freely available through database search using the included search terms. No dataset is available online.
References
- (2007) Societal Costs of Preterm Birth in Behrman RE and Butler AS (Eds), Preterm Birth: Causes, Consequences, and Prevention, National Academies Press (US), Washington (DC) pp. 298–429. [PubMed] [Google Scholar]
- Baraldi E, Giordano G, Stocchero M, Moschino L, Zaramella P, Tran MR, Carraro S, Romero R and Gervasi MT (2016) Untargeted Metabolomic Analysis of Amniotic Fluid in the Prediction of Preterm Delivery and Bronchopulmonary Dysplasia. PLoS One 11, e0164211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel J, Krumsiek J and Theis FJ (2013) Statistical methods for the analysis of high-throughput metabolomics data. Comput Struct Biotechnol J 4, e201301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger RD, Dunn W, Schmidt MA, Gross SS, Kirwan JA, Cascante M, Brennan L, Wishart DS, Oresic M, Hankemeier T, Broadhurst DI, Lane AN, Suhre K, Kastenmuller G, Sumner SJ, Thiele I, Fiehn O, Kaddurah-Daouk R, for “Precision, M. and Pharmacometabolomics Task Group”-Metabolomics Society, I. (2016) Metabolomics enables precision medicine: “A White Paper, Community Perspective”. Metabolomics 12, 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beger RD, Dunn WB, Bandukwala A, Bethan B, Broadhurst D, Clish CB, Dasari S, Derr L, Evans A, Fischer S, Flynn T, Hartung T, Herrington D, Higashi R, Hsu PC, Jones C, Kachman M, Karuso H, Kruppa G, Lippa K, Maruvada P, Mosley J, Ntai I, O’Donovan C, Playdon M, Raftery D, Shaughnessy D, Souza A, Spaeder T, Spalholz B, Tayyari F, Ubhi B, Verma M, Walk T, Wilson I, Witkin K, Bearden DW and Zanetti KA (2019) Towards quality assurance and quality control in untargeted metabolomics studies. Metabolomics 15, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum J, Samal SK, Ghose S and Niranjan G (2017) Vaginal Fluid Urea and Creatinine in the Diagnosis of Premature Rupture of Membranes in Resource Limited Community Settings. J Family Reprod Health 11, 43–49. [PMC free article] [PubMed] [Google Scholar]
- Brodsky IE and Medzhitov R (2009) Targeting of immune signalling networks by bacterial pathogens. Nature Cell Biology 11, 521–6. [DOI] [PubMed] [Google Scholar]
- Cecatti JG, Souza RT, Sulek K, Costa ML, Kenny LC, McCowan LM, Pacagnella RC, Villas-Boas SG, Mayrink J, Passini R Jr., Franchini KG, Baker PN, Preterm S and groups S.s. (2016) Use of metabolomics for the identification and validation of clinical biomarkers for preterm birth: Preterm SAMBA. BMC Pregnancy Childbirth 16, 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong J, Soufan O, Li C, Caraus I, Li S, Guillaume Bourque, Wishart DS. and Xia J (2018) MetaboAnalyst 4.0: towards more transparent and integrative metabolomics analysis. Nucleic Acids Research 46, W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis KK, Marder E, Balshaw DM, Cui Y, Lynes MA, Patti GJ, Rappaport SM, Shaughnessy DT, Vrijheid M and Barr DB (2017) Biomonitoring in the Era of the Exposome. Environmental Health Perspectives 125, 502–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz SO, Barros AS, Goodfellow BJ, Duarte IF, Galhano E, Pita C, Almeida Mdo C, Carreira IM and Gil AM (2013) Second trimester maternal urine for the diagnosis of trisomy 21 and prediction of poor pregnancy outcomes. Journal of Proteome Research 12, 2946–57. [DOI] [PubMed] [Google Scholar]
- Diaz SO, Pinto J, Graca G, Duarte IF, Barros AS, Galhano E, Pita C, Almeida Mdo C, Goodfellow BJ, Carreira IM and Gil AM (2011) Metabolic biomarkers of prenatal disorders: an exploratory NMR metabonomics study of second trimester maternal urine and blood plasma. Journal of Proteome Research 10, 3732–42. [DOI] [PubMed] [Google Scholar]
- Fotiou M, Fotakis C, Tsakoumaki F, Athanasiadou E, Kyrkou C, Dimitropoulou A, Tsiaka T, Chatziioannou AC, Sarafidis K, Menexes G, Theodoridis G, Biliaderis CG, Zoumpoulakis P, Athanasiadis AP and Michaelidou AM (2018) (1)H NMR-based metabolomics reveals the effect of maternal habitual dietary patterns on human amniotic fluid profile. Sci Rep 8, 4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghartey J, Anglim L, Romero J, Brown A and Elovitz MA (2017) Women with Symptomatic Preterm Birth Have a Distinct Cervicovaginal Metabolome. American Journal Perinatology 34, 1078–1083. [DOI] [PubMed] [Google Scholar]
- Ghartey J, Bastek JA, Brown AG, Anglim L and Elovitz MA (2015) Women with preterm birth have a distinct cervicovaginal metabolome. American Journal of Obstetrics and Gynecology 212, 776.e1–776.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graca G, Duarte IF, Barros AS, Goodfellow BJ, Diaz SO, Pinto J, Carreira IM, Galhano E, Pita C and Gil AM (2010) Impact of prenatal disorders on the metabolic profile of second trimester amniotic fluid: a nuclear magnetic resonance metabonomic study. Journal of Proteome Research 9, 6016–24. [DOI] [PubMed] [Google Scholar]
- Graca G, Goodfellow BJ, Barros AS, Diaz S, Duarte IF, Spagou K, Veselkov K, Want EJ, Lindon JC, Carreira IM, Galhano E, Pita C and Gil AM (2012) UPLC-MS metabolic profiling of second trimester amniotic fluid and maternal urine and comparison with NMR spectral profiling for the identification of pregnancy disorder biomarkers. Molecular BioSystems 8, 1243–54. [DOI] [PubMed] [Google Scholar]
- Hallman M, Saugstad OD, Porreco RP, Epstein BL and Gluck L (1985) Role of myoinositol in regulation of surfactant phospholipids in the newborn. Early Hum Dev 10, 245–54. [DOI] [PubMed] [Google Scholar]
- Halscott TL, Ramsey PS and Reddy UM (2014) First trimester screening cannot predict adverse outcomes yet. Prenatal Diagnosis 34, 668–76. [DOI] [PubMed] [Google Scholar]
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, Vijayaraghavan J, Trivedi Y, Soma-Pillay P, Sambarey P, Dayal A, Potapov V, O’Brien J, Astakhov V, Yuzko O, Kinzler W, Dattel B, Sehdev H, Mazheika L, Manchulenko D, Gervasi MT, Sullivan L, Conde-Agudelo A, Phillips JA, Creasy GW and Trial P (2011) Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol 38, 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson J, Carson C and Redshaw M (2016) Impact of preterm birth on maternal well-being and women's perceptions of their baby: a population-based survey. BMJ Open 6, e012676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Schisterman EF and Heagerty PJ (2007) Potential confounding by exposure history and prior outcomes: an example from perinatal epidemiology. Epidemiology 18, 544–51. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Ivanisevic J and Siuzdak G (2016) Metabolomics: beyond biomarkers and towards mechanisms. Nature Reviews Molecular Cell Biology 17, 451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Furumichi M, Morishima K, and Tanabe M (2019) New approach for understanding genome variations in KEGG. Nucleic Acids Research 47, D590–D595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariman N, Afrakhte M, Hedayati M, Fallahian M and Alavi Majd H (2013) Diagnosis of premature rupture of membranes by assessment of urea and creatinine in vaginal washing fluid. Iran J Reprod Med 11, 93–100. [PMC free article] [PubMed] [Google Scholar]
- Khan KA, Petrou S, Dritsaki M, Johnson SJ, Manktelow B, Draper ES, Smith LK, Seaton SE, Marlow N, Dorling J, Field DJ and Boyle EM (2015) Economic costs associated with moderate and late preterm birth: a prospective population-based study. BJOG 122, 1495–505. [DOI] [PubMed] [Google Scholar]
- Leite DFB, Morillon AC, Melo Junior EF, Souza RT, Khashan AS, Baker PN, Kenny LC and Cecatti JG (2018) Metabolomics for predicting fetal growth restriction: protocol for a systematic review and meta-analysis. BMJ Open 8, e022743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lu YP, Reichetzeder C, Kalk P, Kleuser B, Adamski J and Hocher B (2016) Maternal PCaaC38:6 is Associated With Preterm Birth - a Risk Factor for Early and Late Adverse Outcome of the Offspring. Kidney Blood Press Res 41, 250–7. [DOI] [PubMed] [Google Scholar]
- Liebler DC (2008) Protein damage by reactive electrophiles: targets and consequences. Chemical Research in Toxicology 21, 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizewska B, Teul J, Kuc P, Lemancewicz A, Charkiewicz K, Goscik J, Kacerovsky M, Menon R, Miltyk W and Laudanski P (2018) Maternal Plasma Metabolomic Profiles in Spontaneous Preterm Birth: Preliminary Results. Mediators Inflamm 2018, 9362820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan H, Meng N, Liu P, Fu J, Chen X, Rao W, Jiang H, Xu X, Cai Z and Wang J (2015) Non-targeted metabolomics and lipidomics LC-MS data from maternal plasma of 180 healthy pregnant women. Gigascience 4, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucaroni F, Morciano L, Rizzo G, F DA., Buonuomo E, Palombi L and Arduini D (2018) Biomarkers for predicting spontaneous preterm birth: an umbrella systematic review. Journal of Maternal-Fetal & Neonatal Medicine 31, 726–734. [DOI] [PubMed] [Google Scholar]
- Maitre L, Fthenou E, Athersuch T, Coen M, Toledano MB, Holmes E, Kogevinas M, Chatzi L and Keun HC (2014) Urinary metabolic profiles in early pregnancy are associated with preterm birth and fetal growth restriction in the Rhea mother-child cohort study. BMC Medicine 12, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews TJ and MacDorman MF (2013) Infant mortality statistics from the 2010 period linked birth/infant death data set. National Vital Statistics Reports 62, 1–26. [PubMed] [Google Scholar]
- Menon R, Jones J, Gunst PR, Kacerovsky M, Fortunato SJ, Saade GR and Basraon S (2014) Amniotic fluid metabolomic analysis in spontaneous preterm birth. Reproductive Sciences 21, 791–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon S and Manning BD (2013) Cell signalling: nutrient sensing lost in cancer. Nature 498, 444–5. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG and Group P (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62, 1006–12. [DOI] [PubMed] [Google Scholar]
- Mwaniki MK, Atieno M, Lawn JE and Newton CR (2012) Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet 379, 445–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orczyk-Pawilowicz M, Jawien E, Deja S, Hirnle L, Zabek A and Mlynarz P (2016) Metabolomics of Human Amniotic Fluid and Maternal Plasma during Normal Pregnancy. PLoS One 11, e0152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole C (1999) Controls who experienced hypothetical causal intermediates should not be excluded from case-control studies. American journal of epidemiology 150 6, 547–51. [DOI] [PubMed] [Google Scholar]
- Rankings A.s.H. (2018) Public Health Impact: Preterm Birth, America’s Health Rankings. [Google Scholar]
- Rappaport SM (2012) Biomarkers intersect with the exposome. Biomarkers 17, 483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM (2018) Redefining environmental exposure for disease etiology. NPJ Syst Biol Appl 4, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM, Barupal DK, Wishart D, Vineis P and Scalbert A (2014) The blood exposome and its role in discovering causes of disease. Environmental Health Perspectives 122, 769–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport SM and Smith MT (2010) Environment and disease risks. Science 330, 460–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Dey SK and Fisher SJ (2014) Preterm labor: one syndrome, many causes. Science 345, 760–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Mazaki-Tovi S, Vaisbuch E, Kusanovic JP, Chaiworapongsa T, Gomez R, Nien JK, Yoon BH, Mazor M, Luo J, Banks D, Ryals J and Beecher C (2010) Metabolomics in premature labor: a novel approach to identify patients at risk for preterm delivery. Journal of Maternal-Fetal & Neonatal Medicine 23, 1344–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ, Greenland S and Lash TL (2008) Modern Epidemiology, Third Edition edn. Wolters Kluwer, Philadelphia, PA. [Google Scholar]
- Sumner S, Pathmasiri W, Carlson JE, McRitchie SL and Fennell TR (2018) Metabolomics in Smart, R (Ed), Molecular and Biochemical Toxicology, John Wiley, Hoboken: pp. 181–199. [Google Scholar]
- Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y and Hazen SL (2013) Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. New England Journal of Medicine 368, 1575–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MM, Sulek K, McKenzie EJ, Jones B, Han TL, Villas-Boas SG, Kenny LC, McCowan LM and Baker PN (2015) Metabolite Profile of Cervicovaginal Fluids from Early Pregnancy Is Not Predictive of Spontaneous Preterm Birth. International Journal of Molecular Sciences 16, 27741–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend MK, Bao Y, Poole EM, Bertrand KA, Kraft P, Wolpin BM, Clish CB and Tworoger SS (2016) Impact of Pre-analytic Blood Sample Collection Factors on Metabolomics. Cancer Epidemiol Biomarkers Prev 25, 823–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vineis P, Chadeau-Hyam M, Gmuender H, Gulliver J, Herceg Z, Kleinjans J, Kogevinas M, Kyrtopoulos S, Nieuwenhuijsen M, Phillips DH, Probst-Hensch N, Scalbert A, Vermeulen R, Wild CP and Consortium EX (2017) The exposome in practice: Design of the EXPOsOMICS project. International Journal of Hygiene and Environmental Health 220, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virgiliou C, Gika HG, Witting M, Bletsou AA, Athanasiadis A, Zafrakas M, Thomaidis NS, Raikos N, Makrydimas G and Theodoridis GA (2017) Amniotic Fluid and Maternal Serum Metabolic Signatures in the Second Trimester Associated with Preterm Delivery. Journal of Proteome Research 16, 898–910. [DOI] [PubMed] [Google Scholar]
- von Elm E AD, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. (2008) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE)statement: guidelines for reporting observational studies. J Clin Epidemiol 61, 344–9. [DOI] [PubMed] [Google Scholar]
- Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ and Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472, 57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D, Feunang Y, Marcu A, Guo A and Liang K (2018) HMDB 4.0 — The Human Metabolome Database for 2018. Nucleic Acids Research 46(D1), D608–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Davis TA, Kim SW, Li P, Marc Rhoads J, Carey Satterfield M, Smith SB, Spencer TE and Yin Y (2009) Arginine metabolism and nutrition in growth, health and disease. Amino Acids 37, 153–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


