Abstract
Importance:
A significant proportion of COVID-19 transmission occurs silently during the pre-symptomatic and asymptomatic stages of infection. Children, while being important drivers of silent transmission, are not included in COVID-19 vaccination campaigns given their exclusion from clinical trials thus far.
Objective:
To investigate the impact of a targeted approach to identifying silent infections among children as a proxy for their vaccination.
Design:
This study used an age-structured disease transmission model to simulate the synergistic impact of interventions in reducing attack rates over the course of one year.
Setting:
A synthetic population representative of the demographics of the United States (US).
Participants:
Six age groups of 0–4, 5–10, 11–18, 19–49, 50–64, 65+ years old, stratified for their population size based on US census data.
Exposures:
Vaccination of adults, self-isolation of all symptomatic cases within 24 hours of symptom onset, and detection of silent infections.
Main Outcomes and Measures:
Vaccination of adults was implemented to reach a 40% coverage over the course of one year with a vaccine efficacy of 95% against symptomatic and severe COVID-19. Without vaccination of children, we determined the proportion and speed that would be required for identifying silent infections among this age group to suppress future attack rates below 5%.
Results:
A targeted approach that identifies 20.6% and 28.6% of silent infections among children within 2 or 3 days post-infection, respectively, would be required to bring attack rates under 5% with vaccination of adults. If silent infections among children remained undetected, achieving the same attack rates would require an unrealistically high vaccination coverage (at least 82%) of this age group, in addition to the base-case 40% vaccination coverage of adults. The results were robust in sensitivity analyses with respect to vaccine efficacy against infection and reduced susceptibility of children to infection.
Conclusions and Relevance:
In the absence of vaccine availability for children, a targeted approach to rapid identification of silent COVID-19 infections in this age group can significantly mitigate disease burden. Without measures to interrupt transmission chains from silent infections, vaccination of adults is unlikely to contain the outbreaks in the near term.
Introduction
The ongoing COVID-19 pandemic has caused significant global morbidity and mortality.1 Public health interventions, including social-distancing, testing and contact tracing, and isolation of cases, have substantially reduced the spread of SARS-CoV-2.2–4 However, enhanced viral transmissibility in cold weather and the erosion of support for mitigation measures have led to a resurgence of cases, particularly in Europe, Canada, and the United States (US).5–7
Global efforts to contain this deadly disease have led to the development of several vaccine candidates in different stages of clinical trials.8 The efficacy results of two vaccines (Pfizer-BioNTech, Moderna)9,10 have exceeded the World Health Organization preferred population-based efficacy of 70% for a COVID-19 vaccine.11 The US Food and Drug Administration (FDA) has authorized emergency use of these vaccines in the US population. Most clinical trials have followed FDA guidelines,12 prioritizing the evaluation of vaccine safety and efficacy in adults, as this population group has borne the majority of reported infections, severe illnesses, and deaths.13–15 Given the lack of vaccine safety and efficacy data for children,16 vaccination campaigns have been targeted towards adults and those at high risk of infection and severe outcomes. Thus, non-pharmaceutical interventions will still be required for mitigating disease transmission among children.
Given that children are more likely to develop asymptomatic infection compared to other age groups,17–20 they can be important drivers of silent transmission.21 We developed an age-stratified SARS-CoV-2 transmission model to investigate the impact of a targeted strategy for identifying silent infections among this age group when only adults are vaccinated. We then calculated the proportion and the speed of identification required to suppress future attack rates below 5% and, alternatively, the vaccination coverage among children which could achieve the same goal.
Methods
We modelled the transmission of SARS–CoV-2 by developing an age-structured compartmental model taking into account the natural history of disease as well as self-isolation and vaccination dynamics. The population was stratified into six age groups: 0–4, 5–10, 11–18, 19–49, 50–64, 65+ years, parametrized from US census data.22 Model parameterization was based on age-specific data regarding asymptomatic rates of infection and relative transmissibilities during different stages of infection.23,24 Contact rates between and within age groups were heterogeneous and derived from empirical studies of social mixing.25,26 Newly infected individuals moved from the susceptible stage to the latent stage, and proceeded to a communicable silent infection stage (i.e., either asymptomatic or pre-symptomatic). A proportion of infected individuals remain asymptomatic until recovery,17–20 while others develop symptoms following the pre-symptomatic stage. The average duration of these epidemiological stages and other age-specific relevant parameters are derived from published estimates (Appendix Table 2). For the base-case, susceptibility to infection was constant across ages, but as a sensitivity analysis, we reduced susceptibility by half for children under 10 years of age.27–29
In our model, all symptomatic cases were identified and isolated within 24 hours following symptom onset. For isolation of silent infections, we varied the proportion identified and the time from infection to identification in the range 2 to 5 days, reflecting observed delays in testing and contact tracing. Isolated individuals limited their daily contacts to the age-specific rates reported during COVID-19 lockdown25,26 until the end of their infectious period.
In vaccination scenarios, we distributed vaccines over time among individuals older than 18 years of age from the onset of simulations. We assumed that 80% of individuals 50 years and older, and 22% of adults aged 18–49 years would be vaccinated, resulting in an overall vaccine coverage of 40% among adults within 1 year.30 The vaccine efficacy against developing symptomatic or severe disease post-vaccination was 95%, based on the results of phase III clinical trials.9,10 We also assumed that vaccine efficacy against infection was 50% lower than the efficacy against disease, but also considered a scenario with the same efficacy of 95% as a sensitivity analysis (Appendix).
We calibrated the transmission rate to an effective reproduction number Re = 1.5, accounting for the effect of current non-pharmaceutical interventions and 10% pre-existing immunity in the population.31 We assumed that the transmission rate was identical for pre-symptomatic and symptomatic cases, but reduced by 74% on average for asymptomatic cases based on recent estimates of asymptomatic COVID-19 infectivity,24 and that recovered individuals were not susceptible to reinfection. We then conducted model simulations independently for each intervention scenario and calculated the attack rate as the proportion of individuals infected within 1 year. For the scenario without vaccination, we considered identification of silent infection among all age groups. When vaccination of adults was implemented, identification of silent infection was targeted towards only children with delays of 2–5 days post-infection. For each scenario of a time delay to identification, we calculated the vaccine coverage of children that would be required in addition to vaccination of adults, in order to achieve a similar attack rate if efforts to identify silent infections were completely halted.
Results
Identification of silent infections in the population
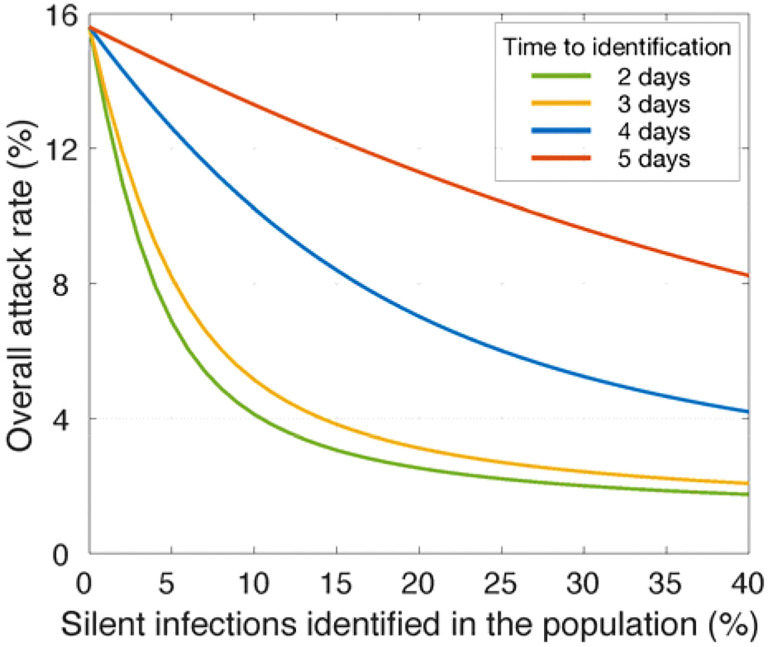
In the absence of vaccination and with Re = 1.5, an overall attack rate of 15.6% would be expected when no silent infections in the population are detected (Figure 1). If silent infections are identified within 2 or 3 days post-infection, a rapid decline in the attack rate can be achieved with isolation of a relatively small (≤15%) percent of silent infections, with diminishing returns as identification rates rise above 20% (Figure 1). However, with a further delay in identification, a significantly larger proportion of silent infections need to be detected to have a similar impact in reducing the attack rate. For instance, with 10% of silent infections identified in the population and isolated within 2 days of infection, the attack rate can be reduced to 4.1%. To achieve the same attack rate with delays of 3, 4, and 5 days, detection rates of 13.5%, 40.8%, and 97% for silent infections would be required, respectively.
Figure 1.
Reduction of attack rate in the population achieved with different rates of silent infections (i.e., asymptomatic and pre-symptomatic) identified and isolated in the absence of vaccination. Colour curves indicate the average time from infection to identification.
Targeted identification of silent infections among children
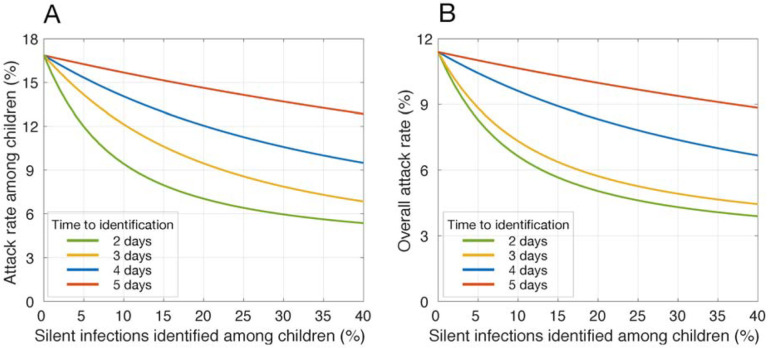
With vaccines distributed to only adults, attack rates among children and the overall population would be reduced to 16.9% and 11.4%, respectively, without identification of silent infections (Figure 2). We simulated the effect of a targeted strategy for identification of silent infections only among children on reducing attack rates. Attack rates declined rapidly with increasing identification of silent infections within 2 and 3 days post-infection (Figure 2). For example, identification of 20.6% and 28.6% would be required to suppress the overall attack rate below 5% (Figure 2). With a delay of 4 days, over 81% identification of children with silent infections would be required to suppress attack rates under 5%. With a further delay, attack rates would remain above 5% with identification of silent infections only among children.
Figure 2.
Reduction of attack rate achieved with different rates of silent infections (i.e., asymptomatic and pre-symptomatic) identified and isolated among children, when only adults were vaccinated. Colour curves indicate the average time from infection to identification. Vaccination coverage of adults reached 40% within 1 year.
If silent infections among children remained undetected, an unrealistically high vaccination coverage (at least 82%) of this age group, in addition to 40% vaccination coverage of adults, must be achieved within 1 year to suppress attack rates below 5%. These results suggest that, even when vaccines do become available for children, rapid identification of their silent infections is still essential to mitigate disease burden in the population.
Sensitivity analyses
We evaluated whether reduced susceptibility to infection among children or higher vaccine efficacy against infection would affect the results. If susceptibility among children under 10 years old is reduced by half, then less contact-tracing is necessary to control COVID-19 with vaccination of adults (Appendix). For instance, 15%, 20.4%, and 59.7% identification of silent infections within 2, 3, or 4 days following infection would suppress the overall attack rate below 5%, or alternatively, vaccination coverage among children would need to reach 78% within 1 year. We observed qualitatively similar results when vaccine protection against infection was the same as efficacy against disease (Appendix).
Discussion
A substantial proportion of COVID-19 cases are attributed to silent transmission from individuals in the pre-symptomatic and asymptomatic stages of infection.32–35 Children are particularly likely to have mild or asymptomatic infections,18,36 increasing the likelihood that they will serve as unidentified links between more severe cases. Although vaccines against COVID-19 now have emergency use authorization, these products have not yet been tested in children and it will be several months before children are widely vaccinated. In the absence of their vaccination, augmenting symptom-based screening with identification of silent infections is essential to control outbreaks.23,37 Our results suggest that the proportion of silent infections being identified among children is secondary to the speed of identification. For example, if the time from infection to identification was reduced from 4 days to 2 days post-infection without reduction of susceptibility for children under 10 years old, the same overall attack rate of 5% could be achieved with identifying over 3.9-fold (from 81% to 20.6%) lower proportion of silent infections. Therefore, enhancing the capacity for rapid tracing of contacts of symptomatic individuals is critical to mitigating disease transmission.
The recent resurgence of COVID-19 cases has overwhelmed the healthcare system in many jurisdictions, hampering the ability of public health to conduct effective contact tracing.38–40 Vaccination can alleviate the current upward pressure of COVID-19 outbreaks, and may allow for resource reallocation towards targeted contact tracing in settings where unvaccinated individuals congregate, such as schools and daycares In a scenario where vaccines are only available for adults, our results show that if only 1 in 5 infected children were identified within 2 days post-infection (e.g., by contact tracing and routine testing), the overall attack rate could be reduced to below 5%. With recent advances in non-invasive testing modalities, such as saliva tests,41 routine testing in settings like schools could feasibly achieve this identification target.
Our results should be interpreted within the context of model limitations. First, we did not explicitly include the effects of other interventions, but instead calibrated the model to current estimates of the effective reproduction number which implicitly account for these effects.31 The relaxation of such measures would increase the need for vigilant contact-tracing among unvaccinated populations. Our sensitivity analyses confirm that rapid contact-tracing will still be an important dimension of control even if child susceptibility is half that of adults. For the effect of vaccination, we parameterized the model with results of phase III clinical trials for vaccine efficacy.9,10 Given the uncertainty around distribution capacity and uptake of vaccines, we simulated the model with a vaccination rate to achieve a 40% vaccine coverage of adults within one year. If vaccines are distributed more rapidly or with much higher uptake, it is possible that the rapid rise of population-level immunity could reduce the need for a targeted strategy to identify silent infections in children. However, given the current limitations in initial vaccine supplies and challenges with cold-chain distribution of mRNA vaccines,42,43 it is unlikely that vaccination will remove the need for non-pharmaceutical interventions in the near-term.
As the spread of COVID-19 intensifies, early interruption of transmission chains becomes critical to outbreak control. Contact tracing at the time of symptom onset or testing, as opposed to at the time of testing results, could have a large impact on suppressing disease transmission, especially in the context of delays in turnaround time for COVID-19 test results.
Supplementary Material
Key Points.
Question:
What is the impact of a targeted strategy for identification of silent COVID-19 infections among children in the absence of their vaccination?
Findings:
In this modelling study, we found that identifying 20–30% of silent infections among children within three days post-infection would bring attack rates below 5% if only adults were vaccinated. If silent infections among children remained undetected, achieving the same attack rate would require an unrealistically high vaccination coverage (at least 82%) of this age group, in addition to vaccination of adults.
Meaning:
Rapid identification of silent infections among children can replicate effects of their vaccination.
Funding/Support:
Canadian Institutes of Health Research [OV4 - 170643, COVID-19 Rapid Research]; the National Institutes of Health [1RO1AI151176-01; 1K01AI141576-01], and the National Science Foundation [RAPID 2027755; CCF-1918784].
Role of the Funder/Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None declared.
References
- 1.Johns Hopkins University. COVID-19 Map. Johns Hopkins Coronavirus Resource Center. Accessed September 1, 2020. https://coronavirus.jhu.edu/map.html [Google Scholar]
- 2.Lau H, Khosrawipour V, Kocbach P, et al. The positive impact of lockdown in Wuhan on containing the COVID-19 outbreak in China. Journal of Travel Medicine. 2020;27(3):taaa037. doi: 10.1093/jtm/taaa037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaxman S, Mishra S, Gandy A, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584(7820):257–261. doi: 10.1038/s41586-020-2405-7 [DOI] [PubMed] [Google Scholar]
- 4.Zhang K, Vilches TN, Tariq M, Galvani AP, Moghadas SM. The impact of mask-wearing and shelter-in-place on COVID-19 outbreaks in the United States. International Journal of Infectious Diseases. 2020;101:334–341. doi: 10.1016/j.ijid.2020.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pollet M. Coronavirus second wave: Which countries in Europe are experiencing a fresh spike in COVID-19 cases? Euronews. Accessed November 15, 2020. https://www.euronews.com/2020/11/13/is-europe-having-a-covid-19-second-wave-country-by-country-breakdown [Google Scholar]
- 6.Zafar A. Canadian ICUs brace for COVID-19 resurgence on top of the flu. CBC News. Accessed November 15, 2020. https://www.cbc.ca/news/health/covid-19-icu-canada-fall-1.5780468 [Google Scholar]
- 7.Maxouris C, Levenson E, Waldrop T. Some US states return to previous restrictions to slow surge of coronavirus cases. CNN. Accessed November 15, 2020. https://www.cnn.com/2020/06/29/health/us-coronavirus-monday/index.html [Google Scholar]
- 8.Corum J, Wee S-L, Zimmer C. Coronavirus Vaccine Tracker. The New York Times. Accessed November 15, 2020. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html [Google Scholar]
- 9.Polack FP, Thomas SJ, Kitchin N, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. Published online December 10, 2020. doi: 10.1056/NEJMoa2034577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anderson EJ, Rouphael NG, Widge AT, et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N Engl J Med. 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. WHO Target Product Profiles for COVID-19 Vaccines. Accessed November 15, 2020. https://www.who.int/publications/m/item/who-target-product-profiles-for-covid-19-vaccines
- 12.Food and Drug Administration. Development and Licensure of Vaccines to Prevent COVID-19; Guidance for Industry. [Google Scholar]
- 13.Centers for Disease Control and Prevention. COVID-19 Provisional Counts - Weekly Updates by Select Demographic and Geographic Characteristics. Accessed November 16, 2020. https://www.cdc.gov/nchs/nvss/vsrr/covid_weekly/index.htm
- 14.Our World in Data. Mortality Risk of COVID-19. Accessed November 16, 2020. https://ourworldindata.org/mortality-risk-covid
- 15.Government of Canada. Epidemiological summary of COVID-19 cases in Canada - Canada.ca. Accessed November 16, 2020. https://health-infobase.canada.ca/covid-19/epidemiological-summary-covid-19-cases.html
- 16.Dunn A. Drugmakers still haven’t started testing their coronavirus vaccines in children, putting kids at the end of the line for a potential shot. Business Insider. Accessed November 16, 2020. https://www.businessinsider.com/coronavirus-vaccine-for-kids-moderna-plans-pediatric-trial-2020-9 [Google Scholar]
- 17.Poline J, Gaschignard J, Leblanc C, et al. Systematic Severe Acute Respiratory Syndrome Coronavirus 2 Screening at Hospital Admission in Children: A French Prospective Multicenter Study. Clinical Infectious Diseases. Published online July 25, 2020:ciaa1044. doi: 10.1093/cid/ciaa1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. Ford N, ed. PLoS Med. 2020;17(9):e1003346. doi: 10.1371/journal.pmed.1003346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Xu W, Dozier M, He Y, Kirolos A, Theodoratou E. The role of children in transmission of SARS-CoV-2: A rapid review. Journal of Global Health. 2020;10(1):011101. doi: 10.7189/jogh.10.011101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeBiasi RL, Delaney M. Symptomatic and Asymptomatic Viral Shedding in Pediatric Patients Infected With Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Under the Surface. JAMA Pediatr. Published online August 28, 2020. doi: 10.1001/jamapediatrics.2020.3996 [DOI] [PubMed] [Google Scholar]
- 21.Hyde Z. COVID-19, children and schools: overlooked and at risk. Medical Journal of Australia. 2020;213(10):444. doi: 10.5694/mja2.50823 [DOI] [PubMed] [Google Scholar]
- 22.U.S. Census Bureau QuickFacts: United States. Population Demographics. Published 2020. Accessed April 16, 2020. https://www.census.gov/quickfacts/fact/table/US/PST045219
- 23.Moghadas SM, Fitzpatrick MC, Sah P, et al. The implications of silent transmission for the control of COVID-19 outbreaks. PNAS. 2020;117(30):17513–17515. doi: 10.1073/pnas.2008373117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sayampanathan AA, Heng CS, Pin PH, Pang J, Leong TY, Lee VJ. Infectivity of asymptomatic versus symptomatic COVID-19. The Lancet. Published online December 2020:S0140673620326519. doi: 10.1016/S0140-6736(20)32651-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mossong J, Hens N, Jit M, et al. Social Contacts and Mixing Patterns Relevant to the Spread of Infectious Diseases. Riley S, ed. PLoS Med. 2008;5(3):e74. doi: 10.1371/journal.pmed.0050074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jarvis CI, Van Zandvoort K, Gimma A, et al. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18(1):124. doi: 10.1186/s12916-020-01597-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies NG, Klepac P, Liu Y, et al. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26(8):1205–1211. doi: 10.1038/s41591-020-0962-9 [DOI] [PubMed] [Google Scholar]
- 28.Steinman JB, Lum FM, Ho PP-K, Kaminski N, Steinman L. Reduced development of COVID-19 in children reveals molecular checkpoints gating pathogenesis illuminating potential therapeutics. Proc Natl Acad Sci USA. 2020;117(40):24620–24626. doi: 10.1073/pnas.2012358117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tosif S, Neeland MR, Sutton P, et al. Immune responses to SARS-CoV-2 in three children of parents with symptomatic COVID-19. Nat Commun. 2020;11(1):5703. doi: 10.1038/s41467-020-19545-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moghadas SM, Vilches TN, Zhang K, et al. The Impact of Vaccination on COVID-19 Outbreaks in the United States. medRxiv; 2020. doi: 10.1101/2020.11.27.20240051 [DOI] [Google Scholar]
- 31.Rt COVID-19. Rt: Effective Reproduction Number. Accessed November 16, 2020. https://rt.live/ [Google Scholar]
- 32.Wei WE, Li Z, Chiew CJ, Yong SE, Toh MP, Lee VJ. Presymptomatic Transmission of SARS-CoV-2 — Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):411–415. doi: 10.15585/mmwr.mm6914e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.He X, Lau EHY, Wu P, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nature Medicine. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5 [DOI] [PubMed] [Google Scholar]
- 34.Mizumoto K, Kagaya K, Zarebski A, Chowell G. Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID-19) cases on board the Diamond Princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance. 2020;25(10). doi: 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nishiura H, Kobayashi T, Miyama T, et al. Estimation of the asymptomatic ratio of novel coronavirus infections (COVID-19). International Journal of Infectious Diseases. 2020;94:154–155. doi: 10.1016/j.ijid.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King JA, Whitten TA, Bakal JA, McAlister FA. Symptoms associated with a positive result for a swab for SARS-CoV-2 infection among children in Alberta. CMAJ. Published online January 1, 2020. doi: 10.1503/cmaj.202065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang K, Shoukat A, Crystal W, Langley JM, Galvani AP, Moghadas SM. Routine Saliva Testing for the Identification of Silent COVID-19 Infections in Healthcare Workers. medRxiv; 2020. doi: 10.1101/2020.11.27.20240044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Devlin M. Contact tracing suspended in Toronto as new cases overwhelm teams. Daily Hive. Accessed November 16, 2020. https://dailyhive.com/toronto/toronto-suspends-contact-tracing [Google Scholar]
- 39.Reuters. Irish COVID-19 “test and trace” system overwhelmed by case surge. Accessed November 16, 2020. https://fr.reuters.com/article/us-health-coronavirus-ireland/irish-covid-19-test-and-trace-system-overwhelmed-by-case-surge-idINKBN27614V
- 40.Kraker D. Overwhelmed by cases, health departments struggle to trace virus’ spread. MPR News. Accessed November 16, 2020. https://www.mprnews.org/story/2020/11/13/overwhelmed-by-crush-of-cases-health-departments-struggle-to-contact-trace [Google Scholar]
- 41.Public Health Ontario. The use of saliva as an alternate specimen for SARS-CoV-2 (COVID-19) PCR testing. https://www.publichealthontario.ca/-/media/documents/ncov/main/2020/09/saliva-alternate-specimen-sars-cov2-pcr-testing.pdf?la=en
- 42.Doucleff M. COVID-19 Vaccine Race Pits Wealthy Countries Against Poor Countries. NPR. Accessed November 16, 2020. https://www.npr.org/sections/goatsandsoda/2020/11/05/931397094/poor-countries-fall-behind-in-race-to-reserve-covid-19-vaccine [Google Scholar]
- 43.Khemlani A. Coronavirus vaccine: Cold storage remains hurdle for Pfizer vaccine distribution. Yahoo Finance. Accessed November 16, 2020. https://ca.finance.yahoo.com/news/cold-chain-throws-cold-water-over-pfizer-vaccine-hopes-133716649.html [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.