Abstract
The emergence of the coronavirus disease 2019 (COVID-19) worldwide pandemic has been associated with a new constellation of cutaneous features in children. Among the unusual dermatologic presentations are the so-called COVID toes, inflammatory nodules of the feet and toes, sometimes involving the hands and fingers. These lesions mimic acral pernio, the synonym being chilblains. Unlike adult patients with COVID toes, children are less likely to manifest symptomatic COVID-19. Although a few studies have found some linkage to COVID-19 through the serum IgA or IgG severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein, other studies have no demonstrable linkage suggesting that barefoot children in cold weather develop such lesions. It appears that the chilblain-like lesions related to the period of the COVID-19 pandemic may reflect a brisk immune response portending a good prognosis and perhaps some form of innate immunity. The possible need to screen for coagulopathy is unclear, but this has been suggested in one report. Until we fully understand the pattern of immune response to COVID-19, questions may persist as to how disease manifestations are linked to SARS-CoV-2 exposures.
Introduction
The novel coronavirus, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was reported in China in December and has rapidly developed into a global pandemic and public health emergency.1 The disease, coronavirus disease 2019 (COVID-19), primarily produces fever and cough, but it can progress into acute respiratory distress syndrome and multiorgan failure.2 Although minimal dermatologic findings were reported at the initial outbreak, more recently, a variety of cutaneous manifestations associated with COVID-19 have been described in the literature, including urticarial, maculopapular, papulovesicular, purpuric eruptions, livedo reticularis, and thrombotic ischemic lesions.3 On March 8, 2020, 5 weeks after the emergence of COVID-19 in Italy, the first case of an acral chilblain-like (synonym pernio) lesion in a child with suspected COVID-19 appeared.4 Soon after, dermatologists around the world in areas hard-hit by the pandemic began reporting surges of chilblain-like lesions, especially in children and adolescents, and often in siblings of the same family. Given the increase in chilblain-like cases in the springtime and the temporal association with the spreading pandemic, it likely related to COVID-19 infection.5
Dermatologists around the world have been attempting to identify a causal relationship between COVID-19 and chilblain-like lesions. Literature related to the cutaneous manifestations of COVID-19 in the pediatric population has increased in recent months. Our goal is to systematically review the current literature of pediatric chilblains and its possible association with COVID-19.
Methods
A literature search was carried out using PubMed and included the terms “COVID-19,” “SARS-CoV-2,” “chilblains,” “pernio,” “perniosis,” “acral,” “COVID toes,” “pediatric,” “dermatology,” “dermatologic,” and “cutaneous.” Searches were limited to publications before July 8, 2020, and to the English language. Case reports, case series, preliminary studies, and research letters describing pediatric chilblain-like lesions were included. Contributions reporting chilblain-like lesions in exclusively adults were excluded. Presentations reporting a mean age that was not under 18 or without specifying the age of individual patients in the study were also excluded. Publications that were summaries and not original case reports or studies were excluded. Our initial search identified 32 records. After assessment for eligibility criteria, 15 publications were excluded, permitting 17 studies to be included in our qualitative analysis.
Results
The 17 records reviewed include 12 case series, 2 case reports, 2 research letters, and 1 registry study. We have italicized the results linking COVID toes to COVID-19 infection.
A Belgian prospective observational case series reported 31 patients with chilblain-like lesions, including 13 teenagers and 1 child.6 The remainder were young adults. Patients presented with erythematous or purple macules, localized to the feet and/or hands. Mild clinical manifestations possibly due to COVID-19 were reported in 20 (64%) of patients. Reverse transcription polymerase chain reaction (RT-PCR) analysis failed to detect SARS-CoV-2 RNA on nasopharyngeal swabs, and a serologic test with 100% specificity and sensitivity for IgM and IgG antibodies against SARS-CoV-2 was nonreactive in all patients. The authors were unable to verify a direct association between COVID-19 and chilblains using RT-PCR and serology (IgG and IgM).
A series of 14 cases of perniosis-like skin lesions in 11 children (mean age 14.4, range 13-18) and 3 young adults (mean age 29, range 23-29) in Italy.14 Both nasopharyngeal and rectal swabs for COVID-19 yielded negative results. The cutaneous manifestations consisted of acral eruption of erythematous-violaceous papules and macules with possible bullous evolution or digital swelling. Lesions were localized on the feet in eight patients, the hands in four, and the feet and hands in two. Three patients complained of lesional itching, and the remaining patients were asymptomatic. No systemic findings were reported, and lesions resolved after 2 to 4 weeks without treatment. This study emphasizes the lesional appearance as papules and nodules that can blister but clear in a few weeks.
In a prospective case series at the University of Bologna, 8 pediatric patients aged 11 to 15 years presented to an outpatient dermatology clinic or emergency room with acral lesions.15 The authors identified associated clinical manifestations primarily of itching (5/8) and pain (3/8) and noted two patterns, specifically a blistering type and a patchy or nodular type. No patient showed signs of systemic illness, and evidence for SARS-CoV-2 was negative in all cases (via RT-PCR nasopharyngeal swab, serum IgG and IgM antibody levels 3 weeks later, and PCR assay of the skin biopsy). The authors help to identify that pain and itch management are needed for chilblain-like lesions in the COVID-19 pandemic, that is, COVID toes.
Multiple Spanish studies have described chilblain-like lesions in young patients during the COVID-19 pandemic,7 , 10 the first examined skin biopsies from 7 pediatric patients (4 boys, 3 girls) 11 to 17 years old. Biopsies of chilblains acral lesions showed lymphocytic vascular damage. Immunohistochemistry showed the SARS-CoV-2 spike protein in endothelial cells in all patients, which was confirmed by electron microscopy showing viral particles within endothelial cells in one case. SARS-CoV-2 PCR from nasopharyngeal and oropharyngeal swab was negative in all cases tested; however, the immunohistochemistry and electron microscopy showed a definitive association with COVID-19 infection. Two teenagers reported in a separate study did not have a biopsy of the chilblain's lesions. One had a nonreactive serology and PCR and the other a positive contact without serology.10 A retrospective review in Barcelona described 12 patients with acral lesions, occurring during the COVID-19 pandemic.19 Ten out of the 12 patients were children and adolescents aged 7 to 14. Of the children and adolescents, four were boys, and six were girls. The authors observed two morphologic types that were present alone or in combination in some patients. The first pattern was “acral erythematous purpuric lesions” with edema, sometimes evolving into blisters, on the fingers and toes. These resembled chilblains. The second pattern involved “papular or macular purpuric round-shaped lesions” on the palmar or plantar surfaces and resembled vasculitis or erythema multiforme. No systemic clinical manifestations consistent with COVID-19 infection were reported. PCR for SARS-CoV-2 was nonreactive in all cases. Three patients had normal chest X rays. Biopsies from two patients showed nonspecific findings. All patients cleared with topical steroids alone or in combination with topical antibiotics within 2 to 3 weeks.19 Twenty-two children and adolescents (13 boys and 9 girls) aged 6 to 17 with acral lesions presented to the emergency department during the COVID-19 pandemic.18 The lesions were on the feet, specifically the toes and lateral aspect of the feet and heels. Three patients also had lesions on the fingers. These appeared as “erythemato-violaceous or purpuric macules” and sometimes as “dark ischemic areas with superficial blisters.” Skin biopsies were taken in six patients and revealed edema and a lymphocytic infiltrate. SARS-CoV-2 RT-PCR was performed in 19 patients, only one of which was reactive. One patient reported a household contact with confirmed COVID-19 infection, whereas 12 patients had a household contact with probable COVID-19. These studies show that in the absence of a biopsy and immunohistochemical staining, COVID-19 infection is hard to show in children with COVID toes. Clues such as household contacts may be the only relevant information.
The Italian pandemic yielded many case of COVID toes. In May 2020, a 16-year-old boy presented to the emergency department in Italy with a 20-day history of multiple asymptomatic erythemato–edematous macules and plaques. The lesions appeared mostly on the fingers, with one plaque on the toe. The patient reported mild diarrhea and dysgeusia 3 days before the appearance of lesions. Days after his lesions appeared, the patient's mother was hospitalized for COVID-19. Histologic examination showed edema with a deep lymphocytic infiltrate and perieccrine involvement. RT-PCR for SARS-CoV-2 via nasopharyngeal swab was positive. A 13-year-old Italian boy had documented probable COVID-19 exposure (sister) and symptomatology including fever of 38.5°C, myalgia, and headache. The patient responded to macrolides orally, with lesional resolution at 2 weeks.4 In a second study of 63 patients with chilblain-like lesions in Italy,11 the median age was 14 years. Feet alone were the most commonly affected area (85.7%), followed by feet and hands together (7%) and hands alone (6%). Thirty-one patients presented with erythematous–edematous lesions and 23 with blistering lesions. COVID-19 PCR was performed in 11 patients (17.5%), and serology was available in 6 (9.5%). Both tests were reactive in 2 cases (3.2%). An Italian prospective study described the clinical findings in 19 adolescent patients (mean age 14 years) presenting with chilblain-like lesions.8 Eleven (56%) patients reported flulike clinical manifestations 1 to 2 months before the onset of skin lesions. Lesions were localized to toes, heels, and soles. Nasopharyngeal swab and IgG serology for SARS-CoV-2 nucleoprotein were negative. Antibodies against the S1 domain of the SARS-CoV-2 spike protein were positive or borderline positive for IgA in the sera of 9 patients, and for IgG in the sera of four patients. The authors noted that IgG and IgA antibodies have been shown to be secreted in patients with COVID-19, with a suspicion of a relationship between COVID-19 and chilblain-like lesions.25 Once again the Italian data showed that COVID-19 infection can be hard to detect in children and adolescents. IgA screening may be an important clue in confirming infection in these patients. RT-PCR from nasopharyngeal swabs may be positive in symptomatic cases.
An international registry documenting 505 cases of dermatologic manifestations associated with confirmed or suspected cases of COVID-19 found that a majority of cases (65%) had pernio-like lesions. These patients (318) included 93 children and adolescents. The mean age of the patients was 25 years,9 and 45% of patients reported mild flulike clinical manifestations, which typically preceded their skin manifestations. Pernio-like lesions affected only the feet in 84% of cases, only the hands in 5.1% of cases, and a combination of hands and feet in 10% of cases. Of the 318 cases of pernio-like lesions, 23 cases (7%) were laboratory confirmed as COVID-19 positive. Although the majority of cases were not tested, 46 patients had confirmed negative PCR and 14 patients were confirmed to be antibody negative. The registry data suggest that in the absence of detectable disease, physicians may use exposure and symptomatology as a proxy for positive testing to COVID-19.
A brief study in four children (aged 11, 11, 6, and 5) during the COVID-19 pandemic in Italy,11 all of whom had nonreactive tests for the infection, described lesions as being erythematous macules over the dorsal and plantar aspects of the feet and toes, along with edematous macules and a central area of cyanosis. The cases were similar to prior reports. The cutaneous manifestations appeared several days after mild flulike clinical manifestations, including cough rhinitis, and fever. The same authors described chilblain-like lesions in 45 pediatric patients.13 A personal or family history of mild systemic clinical manifestations frequently preceded the onset of cutaneous manifestations. All patients tested negative for SARS-CoV-2 with nasopharyngeal swab aspirate, similar to these authors’ earlier report. The introduction of serologic tests yielded a positive IgG antibody result in one patient. Additionally, two patients had an elevated D-dimer and fibrinogen degradation products, prompting the authors to suggest a comprehensive laboratory evaluation including coagulation panels as part of the workup for suspected COVID-19 infection.
Thirty-three Italian children and adolescents with chilblain-like lesions between February 14 and April 10, 2020.16 Age ranged from 8 to 19 years, excluding one 54-year-old patient, and consisted of 11 girls and 22 boys. The lesions were described as “circumscribed erythematous edematous elements with a purplish red color” and appeared most often on the feet,29 followed by the hands,5 and rarely on the face.2 Most patients had no local clinical manifestations (19/33), and the most common complaint was swelling (9 cases), followed by itching,8 pain,6 and burning. Only three patients complained of systemic clinical manifestations, such as cough, sore throat, and fever, and none were tested for COVID-19 infection. Complete resolution was noted in 17 patients in 8 to 16 days. The patients responded well to management with analgesics (oral paracetamol), antibiotics, antihistamines, and topical steroids.
In the United States, a two-case series from New York21 reported one pediatric case in a 9-year-old boy, presenting with tender, warm, and erythematous nodules on his toes, present bilaterally for 3 weeks. There was no history of a recent illness. His parents were first responders working in hospitals during the New York City COVID-19 pandemic. His daily walks in March and April 2020 often took place in cold weather. He was treated with a 10-day course of oral cephalexin (40 mg/kg/day), and his lesions resolved within 4 weeks.
In Northern California,17 six children (five boys and one girl, aged 12 to 17) with acral lesions were described in early April. The children came from two separate families (three siblings in each family), and each family member developed acral lesions within 1 week of a sibling. Most patients had lesions appearing on the toes and complained of itching, and a few described tenderness at the lesions. All lesions appeared as “red to violaceous macules and dusky purpuric plaques” with some digits presenting with “edematous with overlying superficial bullae and focal hemorrhagic crust.” Biopsies from all six patients showed lymphocytic inflammatory infiltrate mimicking primary perniosis. Three (50%) of the patients showed additional cutaneous manifestation of livedo reticularis on the forearms and the dorsal surfaces of the hands and feet. In one family, two of the three siblings had mild upper respiratory clinical manifestations, including rhinorrhea, congestion, fevers, and sore throat, which began 1 week before the onset of cutaneous manifestations. The remainder of the children reported no associated clinical manifestations. One family had traveled to Europe, Africa, and Hawaii in the 3 weeks preceding the development of acral lesions. All were found to be SARS-COV-2 negative via PCR, and SARS-CoV-2 antibodies were negative. The authors noted that temperatures in Northern California were within the average monthly range for April, and not unseasonably cold. They concluded with an additional report of 24 cases of acral lesions appearing in adolescents aged 10 to 19 in the Bay Area, 13 of whom tested negative for COVID-19 infection via PCR. These authors add to the knowledge of the COVID toes by demonstrating the spread among siblings and the delay of infection by 1 week. There is some suggestion from these data that COVID toes may relate to a genetically transmissible response to COVID-19.
Discussion
Chilblains (synonyms: pernio or perniosis) have been discussed as a possible cutaneous manifestation of COVID-19. Idiopathic or primary chilblains are a dermatologic disorder believed to result from an abnormal vascular response to damp and cold but nonfreezing environments. The disease typically appears between the months of November to March. Proposed risk factors for the development of idiopathic chilblains include living in colder climates, low BMI, female sex, and smoking.22 Chilblains associated with systemic diseases, including autoimmune conditions, connective tissue diseases, hematologic malignancies, and Epstein-Barr Virus, are termed secondary pernio, and usually develop in older patients.5 Pernio classically presents as erythematous or violaceous papules on the toes and fingers bilaterally, sometimes with blistering or ulceration (Figure 1 ). Histologically, pernio has no pathognomonic features but may show a perivascular lymphocytic infiltrate with dermal edema.23 Clinical manifestations include pruritus, burning, and itching, and resolution usually occurs within 1 to 3 weeks.8
Fig. 1.
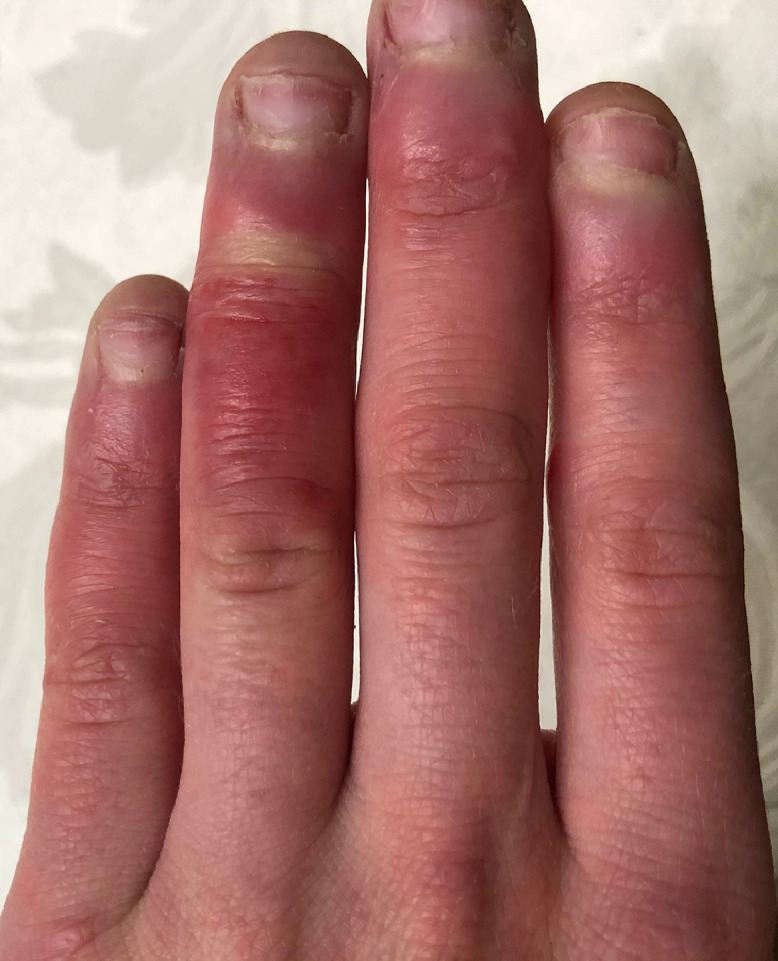
Chilblain-like lesions of the fingers.
The epidemiology of chilblains is not well known; however, studies indicate that it is relatively rare. One of the largest retrospective case series found only 104 patients over an 11-year period.22 Idiopathic pernio is supposedly rarer in children, as it predominantly affects young to middle-aged women.24 One recent 2-year prospective study found that half of chilblains cases presented in children under age 18, indicating that childhood chilblains do occur.23
Chilblain-like lesions may present as purpuric/erythematous macules, papules, nodules, or plaques (Figure 2 ).4 , 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 Some studies reported pustules and bullous lesions with central vesicular or necrotic areas.4 , 6 , 8 , 11 , 14 , 15 Pernio-like lesions appeared predominantly on the feet, localized mostly to the toes. Some patients showed additional lesions on the heels, soles, and lateral margins of the feet.13 Four studies included some patients with evidence of hand involvement, mostly of the fingers, in addition to the toes.10 , 12 , 15 , 16 , 18 , 20 Three studies showed cases with reports of lesions exclusively on hands,9 , 14 and one study reported facial lesions in addition to the foot.16 This distribution and morphology mimics the pattern typically seen in pernio. One of the largest retrospective studies of pernio showed localization to the toes in 82% of patients, and the fingers in 30%.22
Fig. 2.
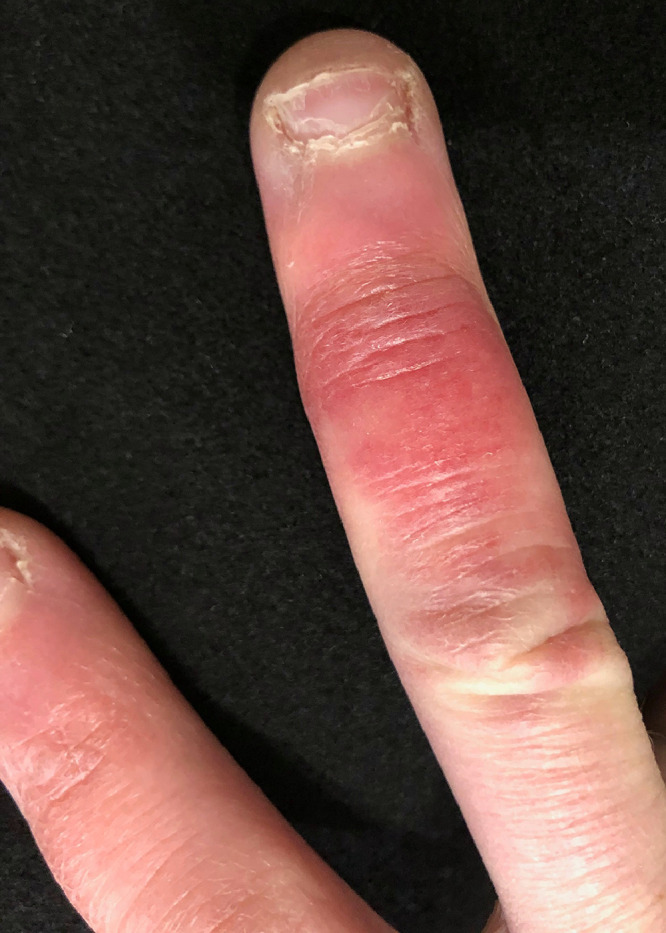
Periungual chilblain-like lesions.
Lesions were generally symptomatic with patients reporting itching, swelling, pain, tenderness, and burning. In the largest study of COVID-19 associated chilblains, which included both pediatric and adult patients, the most common clinical manifestations were pain and burning (66%) of total patients and pruritus (43%) of patients.9 On the contrary, seven studies discussed here reported a large number of patients without any localized clinical manifestations.8 , 9 , 14 , 16 , 18, 19, 20 This is consistent with true pernio, in which about a quarter of patients are not symptomatic.22
Histopathologic examination of skin biopsy specimens reported in 10 of 17 contributions revealed patterns consistent with typical chilblain lesions, showing variable degrees of lymphocytic vasculitis ranging from endothelial swelling and endothelialitis to fibrinoid necrosis and thrombosis with red blood cell extravasation.6 , 7 , 9 , 12 , 14 , 15 , 17 , 18 , 20 Some biopsies were nonspecific.19 One study reported positive SARS-CoV-2 immunohistochemistry in endothelial and epithelial cells of eccrine glands as well as coronavirus particles in the cytoplasm of endothelial cells on electron microscopy, leading the authors to believe that endothelial damage induced by the virus could be a key mechanism in the pathogenesis of COVID-19 chilblains.7 As this was the only pediatric report with electron microscopy, it highlights that serology alone may be inadequate to show the association of skin lesions with COVID-19 exposure and/or infection.
Authors studying the association of COVID-19 and chilblains looked for systemic clinical manifestations of illness in patients with pernio to suggest infection with SARS-CoV-2. Most children in these studies were asymptomatic14 , 15 , 21 or had mild flulike clinical manifestations, including mild fever, gastrointestinal clinical manifestations, upper respiratory clinical manifestations, and myalgias.6, 7, 8, 9 , 11 , 12 , 16, 17, 18 Some patients reported contact with family members with mild flulike clinical manifestations,4 , 7 , 13 and two reported contact with a family member who had confirmed COVID-19.10 , 20 Of those with flulike clinical manifestations, the findings largely preceded the onset of pernio.9 , 11 , 13 In studies that included a timeline, patients had flulike clinical manifestations, or contact with household members with flulike clinical manifestations, 1 to 2 weeks before the onset of pernio.10 , 12 , 17 , 18 , 20 In two studies, patients reported personal or household contact with family members having flulike clinical manifestations 1 to 2 months before the development of chilblains.8 , 12 Interestingly, two patients reported pernio preceding their illness by 2 days to 1 week.4 , 8 Notably, one patient was asymptomatic at the time of presentation with pernio, but was found to have pneumonia on chest X ray; however, PCR and rapid antibody test were negative in this patient.10 There were no reports of hospitalizations or mortalities in these studies, supporting COVID toes to be a positive prognostic indicator of mild disease in COVID-19 infection.
Treatment of lesions was generally supportive and consisted of topical steroids, oral paracetamol, and antihistamines for pain and itch.16 , 18 Three studies reported use of oral or topical antibiotics,16 , 19 including one patient who received a 10-day course of oral cephalexin (40 mg/kg/day).21 One patient required oral gabapentin for severe pain.19 Many patients received no treatment.7 , 8 , 10 , 12 , 14 All studies reported complete or near-complete resolution after several weeks, ranging from 1 to 8. It was difficult to establish the overall duration of pernio-like lesions given that patients did not present for care on the day the lesions appeared, and time to follow-up varied between studies.11 Notably, one patient showed improvement after 4 days of once daily mometasone furoate cream and once daily heparin gel (unknown dose), leading the authors to point out a potential useful therapy for chilblain-like lesions associated with COVID-19.16
Most studies included a thorough medical history and laboratory workup to exclude autoimmune or infectious causes of chilblains. In one study, three patients had an elevated D-dimer, two patients had fibrinogen degradation products, and one family showed slight reductions in fibrinogen; however, these were believed to be of minimal clinical significance.12 , 17 , 18 High antinuclear antibody titers and a confirmed history of perniosis6 and elevated ASO and DNAse B related to a recent group A strep infection17 were each reported once. In a study of 63 patients, one was positive for Mycoplasma pneumonia and six reported a history of autoimmune disorders.11 Some patients showed laboratory evidence of past infections with parvovirus B-19 and M pneumonia.15 Overall, no significant laboratory abnormalities were found on workup, and medical histories were unremarkable except for flulike clinical manifestations and COVID-19 exposures in many patients. The surge in chilblain-like lesions appears to have occurred in young, otherwise healthy individuals.
The link between chilblain-like lesions and COVID-19 is still under evaluation. In the studies discussed here, little laboratory evidence exists to show a current or prior COVID-19 infection in children with chilblains. In 10 of the 17 studies discussed here, PCR for SARS-CoV-2 was negative for all patients who were swabbed. One case report described chilblain-like lesions in an adolescent with PCR-confirmed COVID-19.20 In the remaining studies, PCR was not performed, or a positive PCR result was found in a select few patients (under 10% of study subjects in those studies). PCR on skin biopsy was also negative.8 In most studies that performed serology for IgG or IgM antibodies, the antibodies to SARS-CoV-2 were undetectable. Two additional patients were reported with positive PCR result for COVID-19.11 A single case has been reported with a reactive serology (IgG antibody to anti-S1/S2 protein).13 A larger series showed universally negative IgG nucleoprotein testing, but some reactive serology for S1 domain of SARS-Co-V-2.8
Authors performing these studies have hypothesized as to why PCR for COVID-19 is undetectable. It has been proposed that children with chilblains may have very mild cases of COVID-19 and that PCR sensitivity is lower due to a low viral load.18 It has been alternatively hypothesized that pernio represents an effective immune response against COVID-19 in patients with mild clinical manifestations.26 Scientists have speculated that chilblains are a result of an early interferon response in young patients, similar to chilblain lupus, whereby interferon type I dampens viral replication and portends an indolent course. These hypotheses are supported by the fact that most children and adolescents in the studies reviewed were asymptomatic or reported mild clinical manifestations before onset of lesions. Many authors agree that chilblains appear late in the disease progression of COVID-19 and presume that PCR is no longer positive, because the virus has cleared by the time patients are tested. Chilblain-like lesions as a late manifestation of mild COVID-19 infection are supported by the fact that lesions appeared in various countries at a similar time point in each country's curve, specifically weeks after a country reached its peak of infection.10 , 18
Some authors have proposed an epiphenomenon, in which the virus is not directly inducing pernio, but that children forced indoors during lockdown have increased cold floor exposure and are walking barefoot, resulting in increased cases of primary chilblains6; for example,15 personal behaviors and characteristics linked to primary chilblains include cold and sweaty extremities (8/8), BMI under 25th percentile (4/8), walking barefoot or using thin socks (8/8), prolonged poor posture including legs bent or crossed (8/8), cold floors (7/8), and home heating turned off (5/8). Additionally, (6) surveys of patients have shown that most patients reported not wearing shoes most of the day (80%), decreased physical activity (65%), and a low median BMI of 19.3. Given the lack of COVID-19 infection via laboratory findings and the evidence of risk factors for primary chilblains, the authors concluded that these cases represent primary chilblains resulting from a lockdown forcing children indoors with increased cold exposure.
Limitations inherent in these studies include small sample size and no controls. PCR was not performed in many patients due to limited availability of testing in asymptomatic or mildly symptomatic patients.9, 10, 11 , 16 , 18 Not all patients received follow-up with antibody testing.10 Additionally, there has been variability in the sensitivities and specificities of tests for COVID-19. The individual reliability of the test used and the timing of PCR and antibody tests could significantly affect outcomes. The specific assays used were unknown in some patients.9 Authors note that false-negative PCR testing has been reported and may be higher in minimally symptomatic patients.17 Many, but not all studies included here, eliminated the possibility of secondary chilblains due to an autoimmune or viral condition via laboratory testing and a thorough history; however, it is possible that some patients had other viral infections underlying their skin findings.
Although we do not yet have a confirmed association between COVID-19 infection and chilblains, several authors maintain the possibility of a correlation due to the following reasons: the rapid surge in pernio lesions in countries inundated by the virus, the temporal relationship with the pandemic, the appearance in springtime as opposed to the typical winter season, the occurrence in children without a history of prior chilblains, the occurrence in children in whom chilblains is typically uncommon, the occurrence in family units, and the equal distribution of boys to girls as opposed to idiopathic pernio, which is predominantly seen in woman. One study not reviewed here, because it included adults, showed that 41% of 71 pseudo-chilblains cases had laboratory confirmation of COVID-19, providing the strongest evidence of a correlation. Further studies with PCR and antibody testing are needed to identify an association between COVID-19 and pediatric chilblains.
Conclusions
Our review has summarized the currently available literature about the clinical presentation of chilblain-like lesions in adolescents and children and its possible association with COVID-19. Because the surge in pernio cases has been predominantly in children and children are often asymptomatic or mildly symptomatic carriers of COVID-19,16 the association can only be inferred at this time from secondary data, such as exposure to COVID-19 patients. Expanded studies with EM of skin lesions are needed to further the understanding of cutaneous manifestations of COVID-19. Detection of pernio may be a useful tool for the early diagnosis of COVID-19 in otherwise asymptomatic carriers and should be considered in contact tracing, thereby having an important public health implication for preventing disease spread.
Conflict of interest
None.
References
- 1.Gorbalenya A.E., Baker S.C., Baric R.S. The species severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elmas Ö.F., Demirbaş A., Özyurt K., Atasoy M., Türsen Ü. Cutaneous manifestations of COVID-19: A review of the published literature. Dermatol Ther. 2020:e13696. doi: 10.1111/dth.13696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mazzotta F., Troccoli T. Acute acro-ischemia in the child at the time of COVID-19. Eur J Pediatr Dermatol. 2020 [Google Scholar]
- 5.Massey P.R., Jones K.M. Going viral: A brief history of chilblain-like skin lesions (“COVID toes”) amidst the COVID-19 pandemic. Semin Oncol. 2020 doi: 10.1053/j.seminoncol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herman A., Peeters C., Verroken A. Evaluation of chilblains as a manifestation of the COVID-19 pandemic. JAMA Dermatol. 2020 doi: 10.1001/jamadermatol.2020.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colmenero I., Santonja C., Alonso-Riaño M. SARS-CoV-2 endothelial infection causes COVID-19 chilblains: Histopathological, immunohistochemical and ultraestructural study of 7 paediatric cases. Br J Dermatol. 2020 doi: 10.1111/bjd.19327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.El Hachem M., Diociaiuti A., Concato C. A clinical, histopathological and laboratory study of 19 consecutive Italian paediatric patients with chilblain-like lesions: Lights and shadows on the relationship with COVID-19 infection. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freeman E.E., McMahon D.E., Lipoff J.B. Pernio-like skin lesions associated with COVID-19: A case series of 318 patients from 8 countries. J Am Acad Dermatol. 2020 doi: 10.1016/j.jaad.2020.05.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Landa N., Mendieta-Eckert M., Fonda-Pascual P., Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 pandemic. Int J Dermatol. 2020;59:739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piccolo V., Neri I., Filippeschi C. Chilblain-like lesions during COVID-19 epidemic: A preliminary study on 63 patients. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colonna C., Monzani N.A., Rocchi A. Chilblain-like lesions in children following suspected COVID-19 infection. Pediatr Dermatol. 2020;37:437–440. doi: 10.1111/pde.14210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colonna C., Spinelli F., Monzani N.A., Ceriotti F., Gelmetti C. Chilblains in children in the time of COVID-19: New evidence with serology assay. Pediatr Dermatol. 2020 doi: 10.1111/pde.14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recalcati S., Barbagallo T., Frasin L.A. Acral cutaneous lesions in the time of COVID-19. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neri I., Virdi A., Corsini I. Major cluster of paediatric ‘true’ primary chilblains during the COVID-19 pandemic: a consequence of lifestyle changes due to lockdown. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruggiero G., Arcangeli F., Lotti T. Therapy for probable COVID-19 associated erythema pernio-like lesions in pediatric age. Case report. Dermatol Ther. 2020:e13616. doi: 10.1111/dth.13616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordoro K.M., Reynolds S.D., Wattier R., McCalmont T.H. Clustered cases of acral perniosis: Clinical features, histopathology, and relationship to COVID-19. Pediatr Dermatol. 2020;37:419–423. doi: 10.1111/pde.14227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andina D., Noguera-Morel L., Bascuas-Arribas M. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romaní J., Baselga E., Mitjà O. Chilblain and acral purpuric lesions in Spain during COVID confinement: Retrospective analysis of 12 cases. Actas Dermosifiliogr. 2020 doi: 10.1016/j.ad.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Locatelli A.G., Robustelli Test E., Vezzoli P. Histologic features of long-lasting chilblain-like lesions in a paediatric COVID-19 patient. J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin J., Rivera R.D., Lomiguen C. COVID toes: Digital vascular changes in patients with a COVID-19 infection. Fron Med Case Rep. 2020;1:1–8. [Google Scholar]
- 22.Cappel J.A., Wetter D.A. Clinical characteristics, etiologic associations, laboratory findings, treatment, and proposal of diagnostic criteria of pernio (chilblains) in a series of 104 patients at Mayo Clinic, 2000 to 2011. Mayo Clin Proc. 2014;89:207–215. doi: 10.1016/j.mayocp.2013.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Takci Z., Vahaboglu G., Eksioglu H. Epidemiological patterns of perniosis, and its association with systemic disorder. Clin Exp Dermatol. 2012;37:844–849. doi: 10.1111/j.1365-2230.2012.04435.x. [DOI] [PubMed] [Google Scholar]
- 24.Simon T.D., Soep J.B., Hollister J.R. Pernio in pediatrics. Pediatrics. 2005;116:e472–e475. doi: 10.1542/peds.2004-2681. [DOI] [PubMed] [Google Scholar]
- 25.Padoan A., Sciacovelli L., Basso D. IgA-Ab response to spike glycoprotein of SARS-CoV-2 in patients with COVID-19: A longitudinal study. Clin Chim Acta. 2020;507:164–166. doi: 10.1016/j.cca.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lesort C., Kanitakis J., Villani A. COVID-19 and outbreak of chilblains: Are they related? J Eur Acad Dermatol Venereol. 2020 doi: 10.1111/jdv.16779. [DOI] [PMC free article] [PubMed] [Google Scholar]