Abstract
The purpose of this study is to explore the effects of Diplectria barbata (Wall. Ex C.B. Clarke) Franken & Roons (DFR) on wound healing, antioxidant and aging in Normal Human Dermal Fibroblast cell (NHDF) cells and mouse skin models. We investigated the effects of the aging process in vitro and in vivo. DFRtreated NHDF cells showed a concentration-dependent increase in the expression of extracellular matrix (ECM) proteins (Collagen-2.5-fold increase at 50 μg/ml, Elastin-1.5-fold increase at 1μg/ml) as well as an increase in proteins related to cell survival, differentiation, and development, while expression of aging proteins such as matrix metalloproteinase 3 (MMP-3) was decreased (5-fold decrease at 50 μg/ml). DFR treatment also led to enhanced expression of antioxidant proteins such as nuclear factor erythroid 2-related factor 2 (10-fold increase at 50 μg/ml) and heme oxygenase 1 (1.5-fold increase at 25 μg/ml). To further investigate the antioxidative effects of DFR extracts, the 2,2-diphenyl-1-picrylhydrazyl radical scavenging activities were also evaluated. DFR extracts improved wound healing and resulted in increased expression of ECM proteins, while enzymes involved in collagen degradation, including MMP-3, were decreased in NHDF cells as well as in a mouse model. This study demonstrates the anti-aging, antioxidant, and wound healing properties of DFR extracts. Therefore, DFR extracts present may facilitate skin protection and care.
Keywords: Anti-aging, Anti-oxidant, Wound healing, Diplectria barbata (Wall. Ex C.B. Clarke) Franken & Roos, Extracts, NHDF cells
Introduction
Aging of human skin is characterized by a decline in fibrous tissue, a slow cellular regeneration, and reduction of the vascular and glandular network [1]. Intrinsic or chronological aging of the skin is an uncontrollable process characterized by smooth, dry, pale, and finely wrinkled skin [2]. A well-balanced array of extracellular matrix (ECM) components supports the skin both structurally and functionally. The dermal ECM is synthesized from fibroblasts and is primarily composed of fibrillar collagen bundles and elastic fibers, which provide skin with its resiliency and mechanical strength, respectively [3]. Collagen is the primary structural protein in the extracellular space of the various varieties of animal connective tissues and constitutes approximately 25–35% of all the protein content in the body [4]. Matrix metalloproteinases (MMPs) also play an important role in the loss of collagen in the ECM of connective tissues.
AKT, known as protein kinase B (PKB), is also important in cell proliferation and differentiation [5]. Mitogen-activated protein kinase kinases (MEKs) phosphorylate p44 MAPK and p42 MAPK (Erk1/2), increasing their enzymatic activity [6]. The activated ERKs translocate to the nucleus and promote changes in gene expression, growth, differentiation, or mitosis [7]. AKT plays an important role in the signaling pathways that regulate a variety of cellular functions including nutrient metabolism, cell growth, survival, proliferation, and apoptosis. Phosphorylation of AKT at serine 473 (S473) and threonine 308 (T308) fully activates AKT [8].
Many reports have demonstrated that nuclear factor erythroid 2-related factor 2 (NRF2) can help protect against the oxidative damage that is common in several diseases through the activation of antioxidant enzymes. NRF2 increases detoxification pathways and antioxidant potentials and protects cardiac fibroblasts and cardiomyocytes against oxidative stress [9, 10]. So, NRF2 is a major player of the antioxidant and anti-inflammatory response signaling pathways [11].
Commonly used in the pharmaceutical and cosmetic industries, plant extracts with antioxidant properties decrease the production of reactive oxygen species (ROS). It is well known that hydrogen peroxide and other ROS are involved in the pathogenesis of several skin conditions including aging, wrinkles, photosensitivity, and malignancy [12]. As is already known, antioxidant capacity is closely related to total phenolic content. Ingredients with this antioxidant capacity also activate the cell’s antioxidant defense system [13, 14].
Diplectria barbata (Wall. ex C. B. Clarke) Franken et Roos (DFR) is a plant produced in Vietnam. The DFR is distributed in Thanh Hoa, Quang Tri, Da Nang, Quang Nam, Kon Tum, Gia Lai, Lam Dong and Dong Nai regions of Vietnam. This plants grow naturally scattered along the forest or in deciduous, at an altitude of about 1200 m. It has potential skin protective effects, but these effects and their mechanism of action have not yet been elucidated. In this study, we investigated the anti-aging, antioxidant, and wound healing properties of extracts of the stem and leaves of DFR in normal human dermal fibroblast (NHDF) cells as well as in a mouse model.
Materials and methods
Plant materials
Diplectria barbata (Wall. Ex C.B. Clarke) Franken & Roos (DFR) extract provided from KRIBB (Korea Research Institute of bioscience and Biotechnology). Methanol extracts of DFR leaves were prepared as previously described [15]. The plants were dissolved in 99.9% methyl alcohol and incubated at 45 °C for 3 days to give an extract, filtered and concentrated. The extract was dried for 24 hours at – 70 °C and 45 °C using biotron corporation Modul spin 40. All of the above extraction and drying procedures were performed at KRIBB.
DFR extract was dissolved in 99.9% ethyl alcohol at concentration of 10, 25 and 50 ug/ml.
Cell culture
Normal Adult Human Primary Dermal Fibroblasts (NHDF) cells were purchased from ATCC (PCS-201-012, Manassas, VA, USA). NHDF cells were maintained in cultures in Dulbecco’s Modified Eagle’s Media (1:1) containing 10% fetal bovine serum and 1% antibiotic. NHDF cells were grown at 37 °C in humidified 5% CO2.
Ethics approval and consent to participate
The experiments were approved by the ethical committee for the use of experimental animals of the Institute of Laboratory Animal Sciences in Chungnam National University. (CNU-00821).
Cell viability
Cell viability were performed as previously described [15]. Briefly, NHDF cells were treated with DFR at concentrations of 10, 25 and 50 µg/mL for 24 h. The results were expressed as the relative cell viability of treated cells against those of the controls.
Cell wound healing
For the cell migration assay, the monolayers were carefully scratched using a 10 µl pipette tip. Positive controls were treated with DMSO. The cells were then treated with the DFR at concentrations of 10, 25 and 50 µg/mL for 24 h then wounded area were photographed. The recovery rate was calculated as the mean value after selecting the longest 5 lengths of wound area.
Western blot analysis
Western blot analysis was performed as previously described [16]. NHDF cells were plated at a density of 3 × 105 cells/well in 6-well culture plates for complete attachment at 37 °C in humidified 5% CO2. The cells were treated with DFR extract at doses of 10, 25 and 50 µg/mL for 24 hours. Briefly, cells were placed on ice and extracted with lysis buffer containing 50 mM Tris-HCl, pH 7.5, 1% v/v Nonidet P-40, 120 mM NaCl, 25 mM sodium fluoride, 40 mM β-glycerol phosphate, 0.1 mM sodium orthovanadate, 1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM benzamidine, and 2 µM microcystin-LR. Lysates were centrifuged for 30 min at 13,000 rpm. The cell extracts were resolved by 10.0–7.5% SDS-PAGE, and transferred to Immobilon-P membranes (Millipore, MA, USA). The filters were blocked for 1 h in 1 X tri-buffered saline buffer (TBS; 140 mM NaCl, 2.7 mM KCl, 250 mM Tris-HCl, pH 7.4), containing 5% skimmed milk and 0.2% Tween-20. The detection of protein expression was visualized by enhanced chemiluminescence, according to the manufacturer’s instructions (GE Healthcare, IL, USA). GAPDH was used as a loading control.
Antibodies and reagents
The following antibodies were used: collagen(ab138492)—(AbCam, Cambridge, UK), Elastin(sc-374,638), MMP-3(sc-21,732), pERK(sc-7383), ERK(sc-1647), NRF2(#13,032)-Santa Cruz, GAPDH(#5174s), pPKB(#3787s), PKB(#9272s), HO-1(#5853)—(Cell Signaling Tech, MA, USA) and secondary antibodies (anti-mouse or anti-rabbit) were from Komabiotech, Seoul, Republic of Korea. Followed by an overnight incubation with each protein’s primary antibodies diluted 1000-fold at 4 °C. The secondary antibody was diluted 2000-fold in the blocking buffer.
DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) assay
The antioxidant efficacy of DFR in the samples was determined using the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assay. The percentage of antioxidant activity (AA%) of each substance was assessed by DPPH (Sigma, MO, USA, Cat. D9132) free radical assay according to the manufacturer’s instructions. The samples were reacted with the stable DPPH radical in an ethanol solution. Standard BHT (butylayed hydroxytoluene-Sigma, MO, USA, Cat. B1215000) at different concentration (10, 25, 50 µg/mL) was mixed with Ethanol. The reaction mixture consisted of adding 100 µL of sample, 100 µL of 200 µM DPPH radical solution against methanol as blank. The changes in color were read [Absorbance (Abs)] at 517 nm after 30 min of reaction at room temperature using a Fluoroskan Ascent FL spectrophotometer (Thermoscientific, CA, USA). The concentration of the sample required to inhibit 50% of the DPPH free radical was calculated as IC50 and the value was determined using Log dose inhibition curve which was performed by using PRISM version 5.0 software.
Total antioxidant capacity assay
Cells were washed twice in ice-cold PBS and homogenized with 100 µL 60 mM NaOH at 4 °C. Homogenates were centrifuged at 1000xg for 10 min and supernatants were used for assay (Cell-biolabs, CA, USA, Cat. STA-360). Added 20 µL of the diluted Uric Acid Standards or samples to the 96-well microtiter plate and added 180 µL of the 1X Reaction Buffer to each well mix thoroughly. Plates were read [Absorbance (Abs)] at 490 nm and were initiated the reaction, add 50 µL of the 1X Copper Ion Reagent into each well then incubate 5 min on an orbital shaker. Add 50 µL of 1X Stop Solution to each well to terminate the reaction and read the plate again at 490 nm. Each uric acid standard and sample should be assayed in triplicate.
Anesthesia in mice
Avertin used in anesthetics dissolve 2.5 g in 5 ml amylene hydrate. And adding distilled water, up to a final volume of 200 ml. Next, this material was filtered through a 0.22 µm size filter. The material is given by intraperitoneal injection at a dose of 250 mg/kg.
Euthanasia in mice
The sacrifice method of mice used in the experiment was cervical dislocation. In addition, it was confirmed whether the heart stopped after euthanasia.
Mouse wound healing
Experiment was performed to demonstrate the effect of DFR on skin wounds in mice. Mice were obtained from DooYeol biotech (Seoul, Korea) with 7 week old Male C57BL/6 mice (n = 04). They were first anaesthetized, and the area assigned for wounding was shaved. The area of 0.36 cm2 (0.6 cm × 0.6 cm) formed by the biopsy punch was treated negative control and 10, 25, 50 µg/mL DFR extract. The treatment of wounded skin with DFR extracts was done with a cotton swab for 6 days (once a day). Mice were housed in standard mouse cages with a 12-h light/12-h dark cycle. All animal protocols were provided Chungnam National University.
Histological and immunohistochemistry analysis
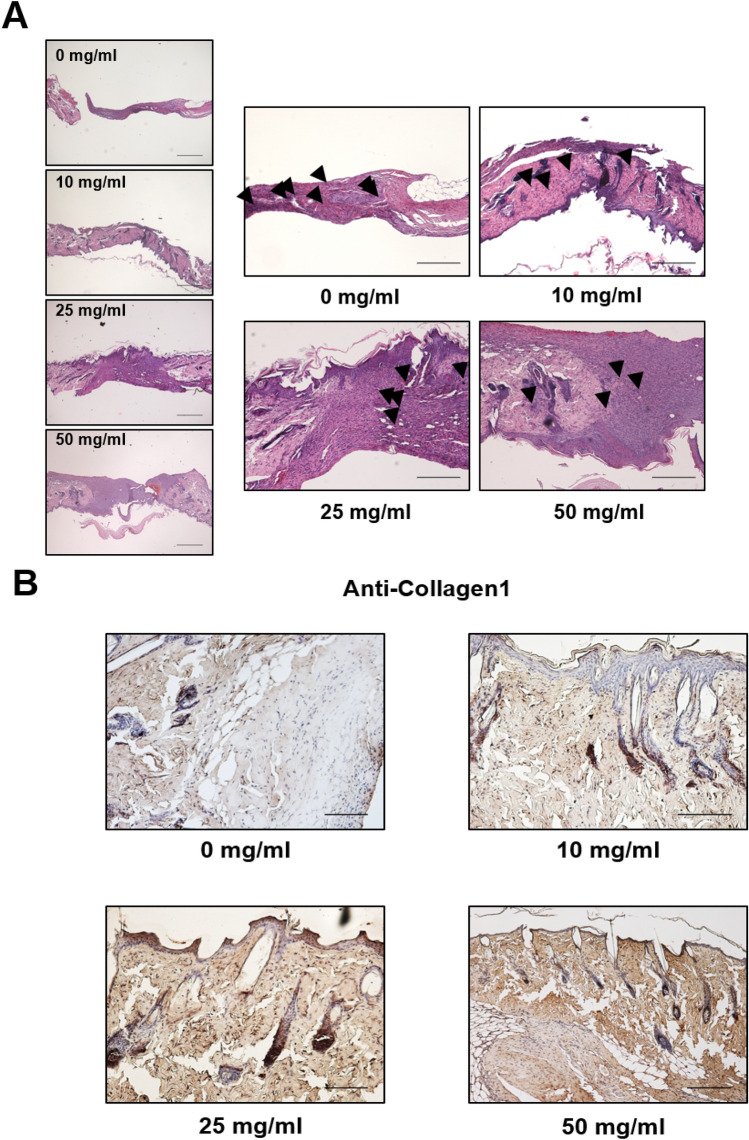
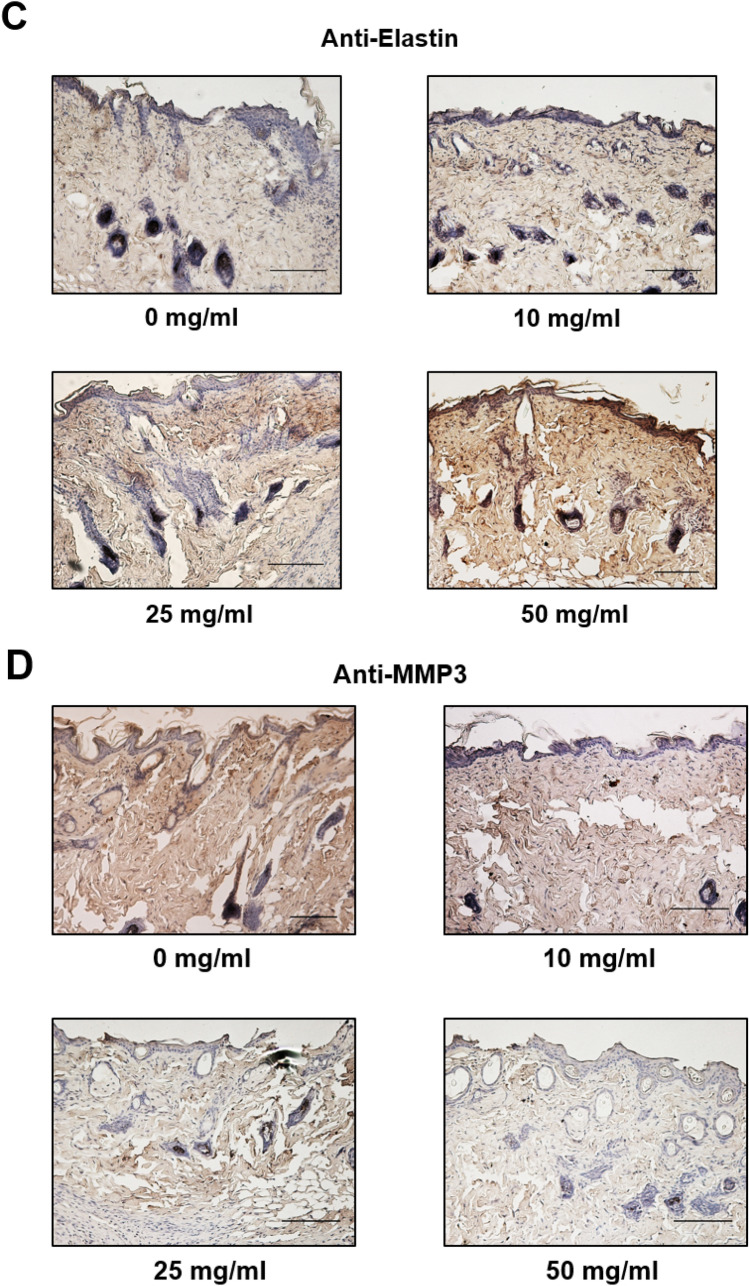
Mice were sacrificed and skin tissues were excised from the mice by surgical procedure. Tissues were fixed in formalin, dehydrated in ethanol, made transparent with xylene, sandwiched with liquid paraffin, and sliced with an automatic slicing machine. Place skin tissue pieces fixed in 10% neutral buffered formalin between paraffins and cut 4 µm thick sections for morphological analysis. Sectioned (4 µm) skin tissues were used for histological analysis (H&E staining) and immunohistological detection of Collagen 1, MMP-3 and Elastin expression. For immunohistochemistry skin sections were deparaffinized in xylene, treated with with 3% H2O2 (Samchun pure chemical CO., LTD, Seoul, Republic of Korea) for 15 min to block endogenous peroxidases. Slides were blocked in 10% normal horse or swine serum (Vector Laboratories, Burlingame, CA, USA) for 1 h at RT. Primary antibodies: rabbit anti-Collagen (1:100, Abcam, Cambridge, UK, Cat. ab138492), rabbit anti-MMP-3 (1:200 Santa Cruz, TX, USA, Cat. sc-21,732) or mouse-Elastin (1:200 Santa Cruz, TX, USA, Cat. sc-374,638) were applied (o/n; 4 °C). Antibody binding was detected with the ABC complex (Vectastain ABC kit from Vector Laboratories, CA, USA). Peroxidase activity was revealed using 3,3′-diaminobenzidine tetrahydrochloride (Vectastain from Vector Laboratories, CA, USA) as a substrate. Tissue sections were mounted undercoverslips with Permount mounting medium (Fisher Chemical, USA).
This section was stained with hematoxylin and eosin (H & E) as previously described. All tissue samples were evaluated by independent investigators without prior knowledge of the group to which the mouse belongs. All skin and all microscopic areas of each specimen were examined.
Statistical analysis
Quantification of Western blot analysis was carried out using the Image J program. Data are presented as means ± SD. The results were analyzed by Student’s unpaired t-test (SPSS version 12.0 software, SPSS Inc.). p < 0.05 (*) was considered significant, and p < 0.01 (**) was highly significant compared with corresponding control values.
Results
Cytotoxicity of DFR extracts and wound healing in NHDF cells
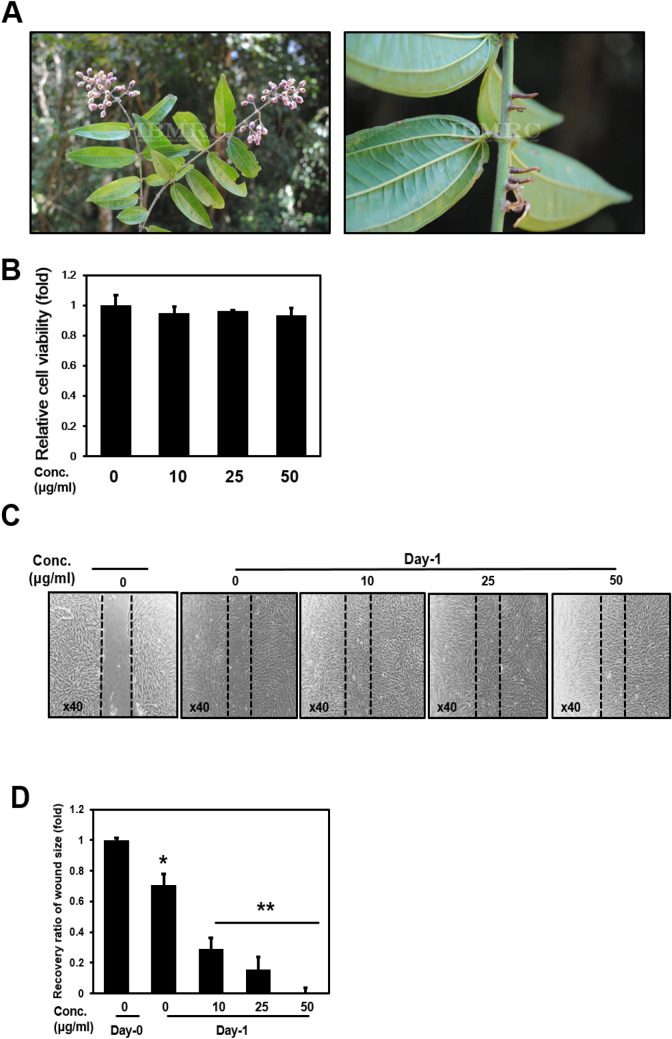
We have performed the screening experiment with a large number of plant extracts. After performing the MTT assay on the basis of the uniform concentration, extracts were selected that did not affect the cell viability. Then, to confirm that the selected extract has a positive effect on collagen expression, the experiment was performed by RT-PCR to finally select DFR. Photographs of leaves and stems of DFR used in this study (Fig. 1a). NHDF cells were treated with 10, 25, and 50 µg/mL of DFR extracts for 24 h. When compared with the untreated control, cells treated with DFR extracts at 10, 25, and 50 µg/mL exhibited no cytotoxicity (Fig. 1b). When confluent monolayers of cells were treated with DFR extracts for 24 h in a wound healing assay, cells closed readily when treated with doses from 10 to 50 µg/mL in a statistically significant concentration-dependent manner (Fig. 1c, d).
Fig. 1.
Cell viability assay and Cell wound healing assay. a Picture of Stem and leafs of DFR. b DFR extracts were no cytotoxicity in 10 µg/ml, 25 µg/ml and 50 µg/ml treatment by EZ-Cyto assay. Cell viability was calculated as ratio to control. c Cell wound healing assay was imaged 24hr after scratch and treat DFR extracts at the indicated dose manner. d Relative ratio of wound size. The results are presented as the means ± SD of three independent experiments. *p < 0.05, **p < 0.01
DFR extracts treatment increased the expression of differentiation, development, proliferation, and ECM proteins in NHDF cells
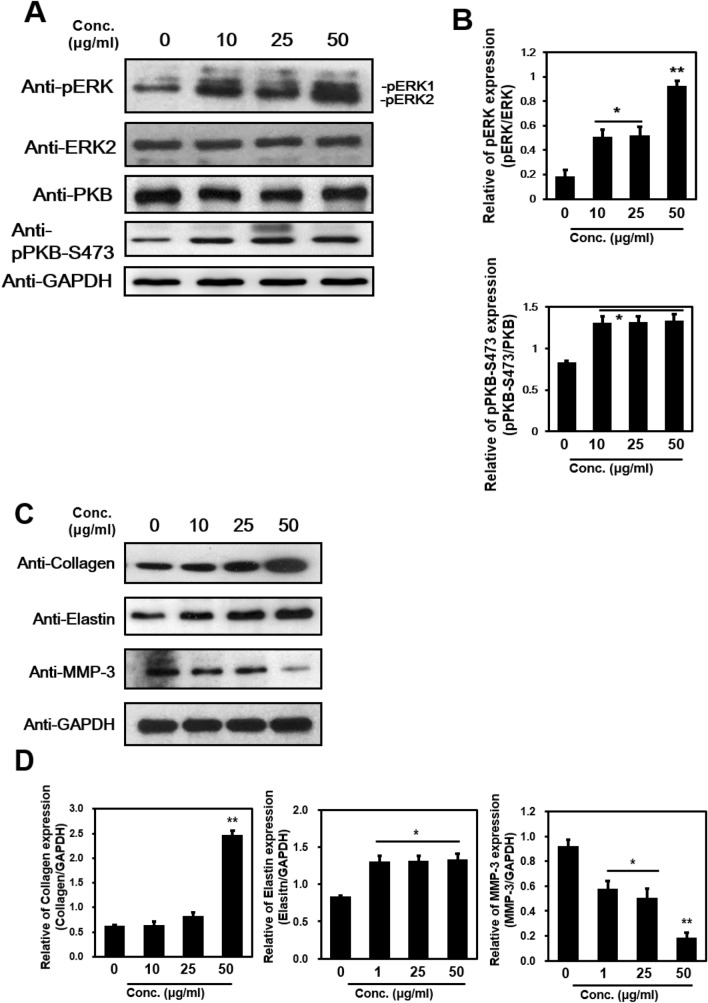
Protein extracts of NHDF cells either untreated or treated with DFR extracts for 24 h were analyzed by western blot. The ERK signaling pathway has been shown to regulate type I collagen gene expression in human fibroblasts [17]. Our data showed that expression of phosphor-ERK (T44/T42) and PKB (S473) was significantly increased in a concentrations-dependent manner with statistical differences (Fig. 2a, b).
Fig. 2.
Cell differentiation, development, proliferation protein and extracellular matrix protein expression by Western Blot. a, c Total protein extracted from non-treated or treated DFR extracts were analyzed by western blot with pERK, ERK2, pPKB-S473, anti-Collagen, anti-Elastin and anti-MMP-3 specific antibodies. b, d ERK and pERK, PKB and pPKB-S473 GAPDH and Collagen, Elastin, MMP-3 in the immunoprecipitates were quantified by Western analyses, as described in Methods. Bar heights are means ± SD of three independent experiments. *p < 0.05, **p < 0.01
Elastin and collagen maintain the elasticity and structure of human skin. Collagen is the primary component of connective tissue. It plays a role in tissue regeneration through its regulation of structure, permeability, and hydrophilicity in mammals [18]. Elastin is an insoluble elastic protein that constitutes 2–4% of the dermis matrix in connective tissue and allows it to resume its shape after stretching or contracting. It allows the skin to return to its original position when pressed [19]. We examined whether treatment with DFR extracts changed the expression of ECM proteins and found an increase in the levels of collagen and elastin compared with the untreated control (Collagen-2.5-fold increase at 50 µg/ml, Elastin-1.5-fold increase at 1 µg/ml; Fig. 2c, d). After treatment with the various doses of DFR extracts for 24 h, the expression of genes encoding ECM components such as collagen and elastin was significantly increased in a concentrations-dependent manner.
Proteins of the MMP family contribute to the degradation of ECM proteins during normal tissue remodeling processes [20]. In particular, the MMP-3 enzyme degrades ECM proteins such as collagen (type II, III, IV, IX, and X), proteoglycans, fibronectin, laminin, and elastin. MMP-3 is also known as an interstitial and fibroblast collagenase and is involved in wound repair, progression of atherosclerosis, and tumor initiation [21]. We demonstrated that the protein levels of MMP-3, which specifically destroys interstitial type II, III collagen, and I were significantly decreased (5-fold decrease at 50 µg/ml; Fig. 2c, d). These data showed that the anti-aging effects of DFR extracts in NHDF cells are related to the translocation of ECM molecules.
DFR extracts exhibit antioxidant properties
The production of ROS occurs in cellular metabolism, physiological processes, and in a number of pathological conditions such as aging and apoptosis. In some cases, the increase in oxidants and decrease in antioxidants cannot be prevented and the oxidative or antioxidative balance shifts towards the oxidative status leading to oxidative stress, which is relevant in more than 100 disorders, develops [22–24].
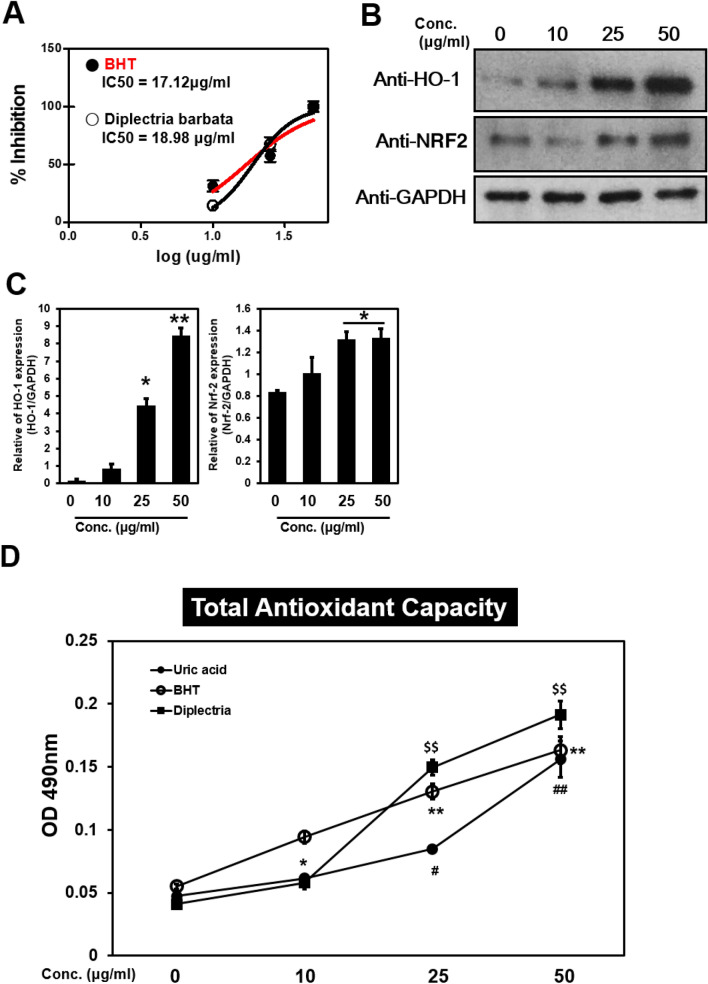
The main cause of exogenous skin aging is due to the negative action of free radicals [25, 26]. The redox system is assessed through evaluation of ROS generation and the level of antioxidants, whose activity is evaluated by the level of phospholipid metabolites in human skin fibroblasts. Antioxidant activity in the skin following topical application may represent an effective mechanism to decrease free radical exposure [27, 28]. We conducted an experiment using an excess of 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radicals to measure the anti-radical capacity of the DFR extracts. The results showed that for DFR extract has similar DPPH inhibition effect as the control BHT. The percentage of inhibition for IC50 value suggested that DFR extracts exhibit potent antioxidant activity (Fig. 3a). Upon exposure to ROS, NRF2 is released from the KEAP1-NRF2 complex and migrates from the cytoplasm to the nucleus and binds to the antioxidant response element (ARE), a regulatory enhancer region present in the promoter. This results in the translation of a number of phase II detoxifying and antioxidant enzymes such as the oxidative stress protein heme oxygenase 1 (HO-1) [29, 30]. The expression of NRF2, as well as molecules downstream of NRF2, including HO-1, was increased in DFR-treated samples in a concentrations-dependent manner. Our results suggest that treatment with DFR extracts activates the expression of NRF2, which upregulates HO-1 expression following inflammation and oxidative stress (Fig. 3b, c).
Fig. 3.
DFR extracts effects anti-oxidant. a The antioxidant properties of DFR extract were calculated based on the DPPH radical scavenging activities. Dose-response curves were generated from which IC50 values were deduced. b NHDF cells were non-treated or treated with DFR for 24 h. Cells were harvested, lysates were analyzed by immunoblotting using anti-HO-1, Anti-NRF2 antibodies. Results are representative of three independent experiments. c GAPDH and HO-1, NRF2 in the immunoprecipitates were quantified by Western analyses, as described in Methods. Bar heights are means ± SD of three independent experiments. d The total antioxidant capacity was plotted by measuring the absorbance at 490 nm after treating DFR extract. Uric acid and BHT (butylayed hydroxytoluene) were used as controls. *p < 0.05; **p < 0.01, versus between BHT samples; #p < 0.05, ##p < 0.01, versus between Uric acid samples; $$p < 0.01, versus between Diplectria samples
Various assays have been described to measure free radical damage or antioxidant status and as such, a single test that could show total antioxidant capacity (TAC) would be useful. TAC is commonly decreased in conditions associated with oxidative stress and the application of chain breaking antioxidants enhances antioxidant capacity [31]. We demonstrated that the TAC level following treatment with DFR extracts was increased in a dose-dependent manner (Fig. 3d). These results demonstrated that DFR extracts retained their antioxidant activity, as indicated by the increase in the percentage of DPPH inhibition.
Macroscopic monitoring of skin wounds in mouse model
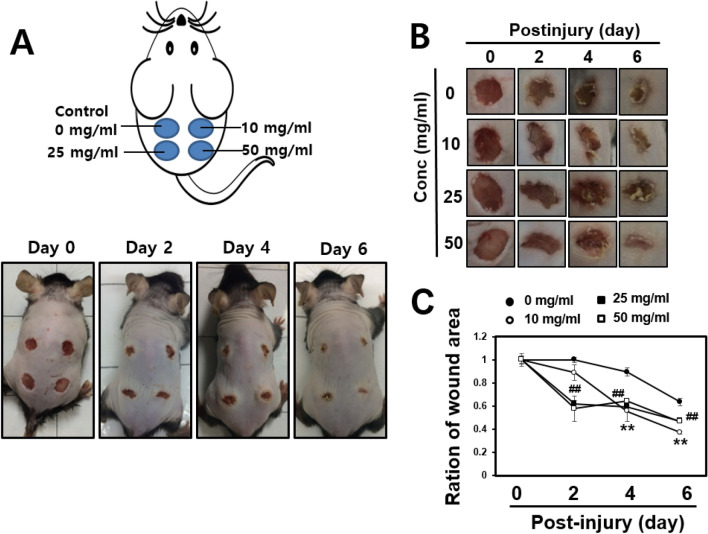
After a wound was generated, the area was left untreated or treated with 10, 25 or 50 mg/mL of DFR extract and the wound area was measured every 2 days. Six days after the incision was made, the area surrounding the wound in DFR extract-treated mice had disappeared, with a level of redness comparable to the untreated control (Fig. 4). In addition, the DFR extract-treated wound areas were smaller than the untreated control. After 2 days, the entire wound area was covered by new epithelium and the rate of coverage occurred in a dose-dependent manner (Fig. 4a, b). The ratio of the wound area 6 days post-incision to the initial wound area at 0 days was calculated (Fig. 4c). Hematoxylin and eosin-stained skin tissues were analyzed at 6 days post-incision. Analysis of the skin tissues from the wounds at 6 days post-incision in the untreated control group indicated that epithelialization was not complete, but wound tissues from DFR extract-treated mice showed an advanced re-epithelialization and dermal regeneration with a significant decrease in cell infiltration compared to the control (magnification × 200; Fig. 5a). Collagen is an element of skin tissue that is produced near the wound bed, allowing closure to occur [32]. Immunohistochemistry results demonstrated that the protein levels of collagen and elastin were increased but MMP-3 was decreased compared with the untreated control (Fig. 5b-d). These results demonstrate that DFR extract has the effect of increasing the expression of ECM-related genes in vitro as well as in vivo.
Fig. 4.
Wound healing effects on impaired of mouse skin. a Wounds of 5 mm diameter were created on the dorsal skin of 7 weeks of age Male C57Bl/6 mice. Before the procurement of wound biopsies at specific time points (non-treated and post-treated mg/ml 10, 25, 50). b Wound area was determined by measuring the diameter of open wounds. c Wound area was calculated using the distance of open wounds. **p < 0.01, 0 mg/ml versus 10 mg/ml samples; ##p < 0.01, 0 mg/ml versus 50 mg/ml samples
Fig. 5.
Extracellular matrix protein expression of the wounds with surrounding mouse skin. a Histological analysis of hematoxylin and eosin (H&E) stained epidermis and dermis from wounds on 6 days post incision. b–d Expression of Extracellular matrix protein (Collagen, Elastin, MMP3) the wounds with surrounding mouse tissues with immunohistochemistry
Discussion
DFR plants have been investigated for different pharmacological properties, but no such study has been done with DFR extracts. Here, we analyzed DFR extracts, which showed several beneficial activities, including anti-aging, antioxidant, and wound healing properties.
This study assessed the wound healing ability of DFR extracts using an in vivo incision wound mouse model. Wound healing re-establishes the integrity of damaged tissue and includes inflammatory, proliferation, and remodeling stages. The skin remodeling stage is characterized by reformation of the components of the collagen fibers that improve tensile strength [33]. Collagen is a major component of skin tissue and is important for all stages of wound healing or repair. The healing process is mostly regulated by the deposition of new collagen and its subsequent maturation [34]. In our wound incision study, treatment with DFR extracts enhanced wound healing compared with the untreated control. This effect may be caused by an increase in collagen expression and stabilization of the fibers.
The skin is constantly exposed to oxidative stress induced by ROS generated by endogenous or exogenous sources [25]. Recently, an increasing body of research has focused on antioxidants since they are able to counteract oxidative stress-induced pathological conditions. However, the studies have been primarily conducted on commercial molecules that are thought to exhibit antioxidant activity [35]. In another study, it was demonstrated that PKB/ERK signaling has an effect on wound healing. In particular, it was found that it is effective for diabetic wound healing [36, 37]. When treated with DFR extracts, the expression levels of PKB and ERK were increased in a concentration-dependent manner. Accordingly, in our study, treatment with DFR extracts resulted in increased wound healing and increased levels of ECM proteins such as collagen and elastin, but decreased the function of inhibition collagenases such as MMP-3.
The DPPH radical assay revealed that DFR extracts showed the similar IC50 (18.98 µg/ml) for anti-radical efficacy, compared with IC50 of BHT (17.12 µg/ml). And pre-treatment with DFR extracts increased the TAC and protected against free radical damage. These results indicate the antiradical activity of DFR extracts. Thus, the inhibition of radical production of DFR extract was demonstrated. The antiradical activity of DFR extracts following topical application was demonstrated, along with its antiradical capacity in the skin. These results suggest that DFR extracts have promising antioxidant capacities and may be a potential natural preservative agent for the food and pharmaceutical industries.
In the tissue repair process, inflammatory cells enhance the migration and proliferation of endothelial cells, extending neovascularization of connective tissue cells that synthesize collagen and of keratinocytes resulting in remodeling of the wounded tissue [20, 38, 39]. In the wound incision model, DFR extracts showed faster wound healing compared with the control group and increased levels of ECM proteins such as collagen and elastin, but decreased the function of inhibition collagenases such as MMP-3.
This study explored the anti-aging and wound healing effects of DFR stem and leaf extracts in NHDF cells and in an in vivo mouse model of wound healing. The treatment of NHDF cells with DFR extract not only improves ECM genes expression, but also promotes recovery of scratched areas in a concentration-dependent manner. In additions, DFR extracts exhibited antioxidant properties, which suggested they may provide protection by reducing the production of ROS. These observations were confirmed in the in vivo mouse model for wound healing. Consequently, DFR extracts represent a promising new material for anti-aging, antioxidant, and wound healing treatments. These results suggest that the extract and fractions of DFR have strong potential for use in anti-aging cosmetic formulations because of their strong antioxidant capacity and wound healing effects.
Acknowledgements
We would like to thank KRIBB (Korea Research Institute of bioscience & Biotechnology) for providing to DFR Extracts. This work was financially supported by a research fund from Chungnam National University (grant to J. Park) and by the Brain Korea 21 PLUS Project for Medical Science, Chungnam National University School of Medicine.
The English in this document has been checked by at least two professional editors, both native speakers of English. For a certificate, please see: http://www.textcheck.com/certificate/LL0V6r.
Abbreviations
- ARE
Antioxidant response element
- DFR
Diplectria barbata (Wall. Ex C.B. Clarke) Franken & Roos
- DPPH
2,2-Diphenyl-1-picryl-hydrazyl-hydrate
- ECM
Extracellular matrix
- HO-1
Heme oxygenase 1
- MMP-3
Matrix metalloproteinase 3
- MEK
Mitogen-activated protein kinase kinase
- NHDF
Normal human dermal fibroblast
- NRF2
Nuclear factor erythroid 2-related factor 2
- PKB
Protein kinase B
- ROS
Reactive oxygen species
- TAC
Total antioxidant capacity
Compliance with ethical standards
Conflict of Interest
The authors declare that they have no competing interests.
Footnotes
Youngeun Hong and Hyunji Lee equally have contributed to this work.
Contributor Information
Jongsun Park, Email: insulin@cnu.ac.kr.
Jisoo Park, Email: basel97@dju.kr.
References
- 1.You J, et al. The antiaging properties of Andrographis paniculata by activation epidermal cell stemness. Molecules. 2015;20(9):17557–17569. doi: 10.3390/molecules200917557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58(2):85–90. doi: 10.1016/j.jdermsci.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 3.Teti A. Regulation of cellular functions by extracellular matrix. J Am Soc Nephrol. 1992;2(10 Suppl):S83–S87. doi: 10.1681/ASN.V210s83. [DOI] [PubMed] [Google Scholar]
- 4.Di Lullo GA, et al. Mapping the ligand-binding sites and disease-associated mutations on the most abundant protein in the human, type I collagen. J Biol Chem. 2002;277(6):4223–4231. doi: 10.1074/jbc.M110709200. [DOI] [PubMed] [Google Scholar]
- 5.Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9(1):59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearson G, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22(2):153–183. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 7.Woodgett JR, Avruch J, Kyriakis J. The stress activated protein kinase pathway. Cancer Surv. 1996;27:127–138. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 8.Stephens L, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279(5351):710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 9.Purdom-Dickinson SE, et al. Induction of antioxidant and detoxification response by oxidants in cardiomyocytes: evidence from gene expression profiling and activation of Nrf2 transcription factor. J Mol Cell Cardiol. 2007;42(1):159–176. doi: 10.1016/j.yjmcc.2006.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu H, et al. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579(14):3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 11.Li W, et al. Activation of Nrf2-antioxidant signaling attenuates NFkappaB-inflammatory response and elicits apoptosis. Biochem Pharmacol. 2008;76(11):1485–1489. doi: 10.1016/j.bcp.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rinnerthaler M, et al. Oxidative stress in aging human skin. Biomolecules. 2015;5(2):545–589. doi: 10.3390/biom5020545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su ZY, et al. A perspective on dietary phytochemicals and cancer chemoprevention: oxidative stress, nrf2, and epigenomics. Top Curr Chem. 2013;329:133–162. doi: 10.1007/128_2012_340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 15.Lee H, et al. Anti-aging effects of Piper cambodianum P. Fourn. extract on normal human dermal fibroblast cells and a wound-healing model in mice. Clin Interv Aging. 2016;11:1017–1026. doi: 10.2147/CIA.S107734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park J, et al. Recognition of transmembrane protein 39A as a tumor-specific marker in brain tumor. Toxicol Res. 2017;33(1):63–69. doi: 10.5487/TR.2017.33.1.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Westermarck J, Kahari VM. Regulation of matrix metalloproteinase expression in tumor invasion. FASEB J. 1999;13(8):781–792. doi: 10.1096/fasebj.13.8.781. [DOI] [PubMed] [Google Scholar]
- 18.Romagna-Genon C. Comparative clinical study of guided tissue regeneration with a bioabsorbable bilayer collagen membrane and subepithelial connective tissue graft. J Periodontol. 2001;72(9):1258–1264. doi: 10.1902/jop.2000.72.9.1258. [DOI] [PubMed] [Google Scholar]
- 19.Popescu MC, et al. Biomaterials based on new polyurethane and hydrolyzed collagen, k-elastin, hyaluronic acid and chondroitin sulfate. Int J Biol Macromol. 2010;47(5):646–653. doi: 10.1016/j.ijbiomac.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care (New Rochelle) 2015;4(3):119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banik D, et al. MMP3-mediated tumor progression is controlled transcriptionally by a novel IRF8-MMP3 interaction. Oncotarget. 2015;6(17):15164–15179. doi: 10.18632/oncotarget.3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rahal A, et al. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hanson KM, Clegg RM. Observation and quantification of ultraviolet-induced reactive oxygen species in ex vivo human skin. Photochem Photobiol. 2002;76(1):57–63. doi: 10.1562/0031-8655(2002)076<0057:oaqoui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 24.Sakurai H, et al. Detection of reactive oxygen species in the skin of live mice and rats exposed to UVA light: a research review on chemiluminescence and trials for UVA protection. Photochem Photobiol Sci. 2005;4(9):715–720. doi: 10.1039/b417319h. [DOI] [PubMed] [Google Scholar]
- 25.Bickers DR, Athar M. Oxidative stress in the pathogenesis of skin disease. J Invest Dermatol. 2006;126(12):2565–2575. doi: 10.1038/sj.jid.5700340. [DOI] [PubMed] [Google Scholar]
- 26.Briganti S, Picardo M. Antioxidant activity, lipid peroxidation and skin diseases. What’s new. J Eur Acad Dermatol Venereol. 2003;17(6):663–669. doi: 10.1046/j.1468-3083.2003.00751.x. [DOI] [PubMed] [Google Scholar]
- 27.Almeida IF, et al. Protective effect of C. sativa leaf extract against UV mediated-DNA damage in a human keratinocyte cell line. J Photochem Photobiol B. 2015;144:28–34. doi: 10.1016/j.jphotobiol.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Hseu YC, et al. Dermato-protective properties of ergothioneine through induction of Nrf2/ARE-mediated antioxidant genes in UVA-irradiated human keratinocytes. Free Radic Biol Med. 2015;86:102–117. doi: 10.1016/j.freeradbiomed.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 29.Radjendirane V, et al. Disruption of the DT diaphorase (NQO1) gene in mice leads to increased menadione toxicity. J Biol Chem. 1998;273(13):7382–7389. doi: 10.1074/jbc.273.13.7382. [DOI] [PubMed] [Google Scholar]
- 30.Srisook K, Kim C, Cha YN. Molecular mechanisms involved in enhancing HO-1 expression: de-repression by heme and activation by Nrf2, the “one-two” punch. Antioxid Redox Signal. 2005;7(11–12):1674–1687. doi: 10.1089/ars.2005.7.1674. [DOI] [PubMed] [Google Scholar]
- 31.Woodford FP, Whitehead TP. Is measuring serum antioxidant capacity clinically useful? Ann Clin Biochem. 1998;35(1):48–56. doi: 10.1177/000456329803500105. [DOI] [PubMed] [Google Scholar]
- 32.Seo MY, et al. Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J Biosci Bioeng. 2009;107(3):266–271. doi: 10.1016/j.jbiosc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Krafts KP. Tissue repair: the hidden drama. Organogenesis. 2010;6(4):225–233. doi: 10.4161/org.6.4.12555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo S, Dipietro LA. Factors affecting wound healing. J Dent Res. 2010;89(3):219–229. doi: 10.1177/0022034509359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apak R, et al. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12(7):1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jere SW, Houreld NN, Abrahamse H. Role of the PI3K/AKT (mTOR and GSK3beta) signalling pathway and photobiomodulation in diabetic wound healing. Cytokine Growth Factor Rev. 2019;50:52–59. doi: 10.1016/j.cytogfr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 37.Huang H, et al. Impaired wound healing results from the dysfunction of the Akt/mTOR pathway in diabetic rats. J Dermatol Sci. 2015;79(3):241–251. doi: 10.1016/j.jdermsci.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 38.Coussens LM, Werb Z. Inflammation cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen L, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One. 2008;3(4):e1886. doi: 10.1371/journal.pone.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]